Abstract
Accumulating evidence suggests that pulmonary expression of a disintegrin and metalloproteinase-33 (ADAM33) serves a key role in the pathogenesis of airway remodeling-related diseases, including asthma. Airway vascular proliferation has been recognized as a key feature of airway remodeling. Our previous study showed that ADAM33 is constitutively expressed in airway vascular smooth muscle cells in patients with asthma, suggesting a potential role of ADAM33 in regulating airway vascular remodeling. Using in vitro human aortic smooth muscle cells (HASMCs) and lentiviral vector carrying short hairpin RNA for ADAM33, the present study aimed to evaluate the influence of ADAM33 silencing on the proliferation and apoptosis of HASMCs and the underlying molecular pathways. Cellular proliferation was observed using the Cell Counting Kit-8 method. Cellular apoptosis was evaluated with Annexin V-PE/7-AAD staining and flow cytometry. Reverse transcription-quantitative PCR and western blotting were used to evaluate the changes in mRNA and protein levels of involved signaling molecules. It was found that silencing of ADAM33 expression in HASMCs significantly inhibited proliferation, but induced the apoptosis of HASMCs. These changes were accompanied by inhibition of the PI3K/AKT/ERK pathway and Bcl-2, but an increase in Bax expression. These results suggested that constitutive expression of ADAM33 may be important to maintain a proliferative phenotype in HASMCs. The influences of ADAM33 on proliferation and apoptosis of HASMCs may involve regulation of PI3K/AKT/ERK and Bax/Bcl-2 pathways. These findings suggested an important role of ADAM33 in airway vascular remodeling and potential therapeutic significance of ADAM33 inhibition in airway remodeling-related diseases.
Keywords: a disintegrin and metalloproteinase-33, airway remodeling, human aortic smooth muscle cells, proliferation, apoptosis
Introduction
Airway remodeling has been established as a key feature of a number of respiratory diseases, including asthma (1,2). Pathophysiologically, airway remodeling is characterized by a series of morphological changes of airway structures, including epithelium, basement membrane, smooth muscle and blood vessels (3,4). Among which, changes of airway vascular function and morphology have been observed as important components during the process of airway remodeling (5,6). An early study showed that vascular density and vascular area in airway submucosa and lamina propria of children with asthma are significantly increased (7), suggesting a potential important role of angiogenesis in the development of airway remodeling. A number of cellular changes have been involved in the pathogenesis of airway vascular remodeling. These changes include proliferation, hypertrophy, apoptosis and migration of endothelial and vascular smooth muscle cells (VSMCs), as well as the synthesis and degradation of extracellular matrix (8), among which phenotypic changes in VSMCs have been considered as an important feature (9,10). However, the potential molecular regulator of the change in VSMC phenotype in the pathogenesis of airway vascular remodeling remains to be elucidated.
A disintegrin and metalloproteinase-33 (ADAM33) is a member of the ADAM metalloproteinase family (11), which have been involved in the pathogenesis of airway remodeling-related diseases (12). Genetic polymorphisms of ADAM33 have been related to vulnerability to asthma (13). In addition, ADAM33 has been found to be expressed in the airway smooth muscles and basement membranes of almost all patients with asthma, but is absent in normal control subjects (14). Another study reported that the expression of ADAM33 in lung tissue is varied and is enriched in interstitial cells (including fibroblasts and smooth muscle cells) (15). The expression of ADAM33 in patients with asthma is related to the severity of the disease, the decline in respiratory function and the extent of airway hypersensitivity, inflammation and remodeling (16,17). Notably, our previous study in patients with asthma confirmed the expression of ADAM33 in airway VSMCs, which could be upregulated by IL-4 and −13 (18). Therefore, it could be hypothesized that expression of ADAM33 in airway VSMC may be important in regulating the proliferative phenotype of VSMCs in airway vascular remodeling.
Previous studies have shown that the PI3K/AKT pathways (19) and Bcl-2/Bax proteins (20) are key regulators for the proliferation and apoptosis of VSMCs. However, whether the role VSMCs ADAM33 in airway vascular remodeling may involve the regulation of PI3K/AKT and Bcl-2/Bax pathways remains to be elucidated. Since isolation and in vitro culture of human airway VSMCs are difficult, the present study used human aortic smooth muscle cells (HASMCs) to evaluate the influences of ADAM33 silencing on cellular proliferation and apoptosis. The present study aimed to provide the primary investigation into the role of ADAM33 expressed in airway VSMCs and pathogenic airway vascular remodeling.
Materials and methods
Patient characteristics and samples
Lung tissue samples were collected from five patients between February 2018 and December 2019 who received pneumonectomy for bronchiectasis or masses at The First Affiliated Hospital of Xinjiang Medical University (Urumqi, China). Patients included three men and two women, three with pulmonary massive lesions, two with bronchiectasis, aged 46–70 years old and all without smoking history. The lung tissues with complete cross section of bronchus >5 cm away from the pulmonary lesions were obtained for subsequent analysis. All the procedures were approved by the Ethics Committee of the First Affiliated Hospital of Xinjiang Medical University (approval no. 20180130-01). All the patients provided signed informed consent.
Immunohistochemical analysis
The lung tissues were fixed in 10% formalin for 6–8 h at 22–24°C. Briefly, after the specimens were washed with running water, they were dehydrated with a gradient series of alcohol (75% ethanol, 95% ethanol I, 95% ethanol II, absolute ethanol) in an oven at 60°C for 20 min at each step. Then, the specimens were transparentized in xylene for 20 min at each step. Finally, the samples were immersed in a wax soaking tank for 3 h. After cooling and solidification, they were trimmed to make wax blocks. Then paraffin-embedded sections were then prepared at 4-µm thickness and used for immunohistochemical analysis. After rehydration using a gradient alcohol solution, sections of lung tissues were blocked with 5% hydrogen peroxide for endogenous peroxidase and then underwent antigen repairmen (6 min, twice). Then, the sections were blocked with 5% BSA (Sangon Biotech Co., Ltd.) at room temperature for 20 min. After incubation with primary antibody for ADAM33 (1:100; cat. no. DF9166; Affinity Biosciences) at 4°C overnight, the slides were incubated with horseradish peroxidase-conjugated goat anti-rabbit IgG secondary antibody (1:200; cat. no. SP-9001; OriGene Technologies, Inc.) at 37°C for 30 min. Finally, the slides were stained with diaminobenzidine (DAB; OriGene Technologies, Inc.) for 5–30 sec and observed under a microscope (Olympus BX41TF; Olympus Corporation). Pulmonary tissues with expression of ADAM33 were stained as brown or brownish-yellow granules.
Cell culture
HASMCs were purchased from Procell Life Science & Technology Co., Ltd., and cultured with complete medium for HASMCs (cat. no. CM-H081; Procell Life Science & Technology Co., Ltd.). Briefly, HASMCs were taken out from liquid nitrogen and the thawed cells were cultured in a cell incubator at 37°C, saturated humidity and 5% CO2.
Immunofluorescence
After adjusting the cell concentration to 1×105 cells/ml, the cells were moved to slides and cultured for 24 h until the cell adhered. The cells were fixed with 4% paraformaldehyde for 20 min, incubated with 0.5% Txition-100 for 20 min and blocked with 1% BSA (Sangon Biotech Co., Ltd.) for 30 min at room temperature. Subsequently, cells were incubated with 80 µl anti-α-actin (smooth muscle) antibody (1:100; cat. no. ab124964; Abcam) for 2 h at 37°C, followed by incubation with 80 µl goat anti-rabbit IgG antibody (H&L, Alexa Fluor® 488; 1:500; cat. no. ab150081; Abcam) at 37°C in the dark for 1 h, and 80 µl DAPI (1 µg/ml; cat. no. D1306; Thermo Fisher Scientific, Inc.) at room temperature in the dark for 5 min. After washing with PBS three times, the slides were sealed with 50% glycerin and observed using laser confocal microscopy (magnification, ×400; Leica Microsystems GmbH).
Construction of lentiviral vector for ADAM33-shRNA and viral transfection
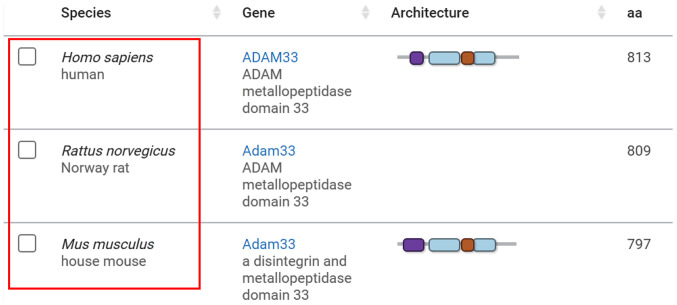
Previous studies have shown that ADAM33 is expressed in rats and mice (21,22) and its mRNA sequence has homology with human, as evidenced by information on the ADAM33 gene in the NCBI database (Fig. 1; http://www.ncbi.nlm.nih.gov/gene/80332/ortholog/?scope=32524). Lentiviral vectors carrying the short-hairpin (sh)RNA for silencing of ADAM33 targeting three different sequences of ADAM33 [lentiviral vector (LV)-ADAM33-shRNA1, LV-ADAM33-shRNA2 and LV-ADAM33-shRNA3]) and the negative control (NC) vectors with blanking sequence (LV-NC) were purchased from Hanbio Biotechnology Co., Ltd. The optimal transfection condition was confirmed with LV-NC when the transfection was performed with a Multiplicity of Infection (MOI) of 1×107 TU/ml, administration of HitransG P solution (Shanghai GeneChem Co., Ltd.) and maintained for 72 h. The mRNA sequences for ADAM33 used in the present study were: shRNA1, GCCACTACCAAGGGCGAGTAA; shRNA2, CAGCAGGAATGCCAGCTATTA; and shRNA3, GACTCTACCGTTCACCTAGAT.
Figure 1.
National Center for Biotechnology Information database shows the homology of ADAM33 in both rats, mice and humans. ADAM33, a disintegrin and metalloproteinase-33; aa, amino acids.
Cell proliferation test
The HASMCs were assigned to the following treatments: i) Blank control with no further treatment (control group); ii) negative control treated with LV-NC; and iii) ADAM33 silencing groups treated with LV-ADAM33-shRNA1. After digesting cells with trypsin, HASMCs with a confluence rate of 90% were prepared into a single cell suspension of 5×104 cells/ml in complete medium. The cells were inoculated into 96 well plates (100 µl/ well, i.e. 5×103 cells/well) and cultured in 5% CO2 at 37°C for 24 h. Cell Counting Kit-8 (CCK-8) solution (100 µl of 10%; Beijing Transgen Biotech Co., Ltd.) was added into each well. After incubation for 1 h, the optical density (OD) values at 450 nm of the adherent cell layer from each group were determined by enzyme-labeled instrument.
Cell apoptosis examination
The culture medium of cells from each group was moved into a centrifuge tube, washed with PBS twice, digested with trypsin and centrifuged at 173 × g for 5 min at room temperature. After washing with pre-cooled PBS, the supernatant was discarded and made into a single cell suspension. Then, 1X Binding Buffer (100 µl) was added to suspend the cells. According to the manufacturer's instructions of the Annexin V-PE/7-AAD Apoptosis Kit (cat. no. AP104; MultiSciences Biotech Co., Ltd.), 5 µl Annexin V-PE and 5 µl 7-AAD was added into each tube, and incubated in the dark at 4°C for 15 min. Following which, 100 µl 1X Binding Buffer was added to resuspend the cells, and then cells were passed through a 200-mesh sieve. Flow cytometry (BD LSRFortessa™; BD Biosciences) was then performed to determine the cellular apoptotic status. BD FACSDiva™ Software (version 8.0.2; BD Biosciences) was used to analyze the data. Both early apoptotic cells and late apoptotic cells were counted. The apoptosis rate was calculated using the following formula: (Q2 + Q4) cells/total cells.
Cell cycle analysis
The cells of each experimental group were collected and resuspended with 500 µl pre-cooled PBS. The cell suspension was added into 3.5 ml pre-cooled 80% ethanol and fixed at 4°C overnight. After centrifugation at 692 × g for 5 min, the cells were precipitated and the supernatant was discarded. After washing with pre-cooled PBS, 500 µl PI/RNase Stabilizing Buffer (cat. no. 550825; BD Pharmingen; BD Biosciences) was added to resuspend the cells, and then the single cell suspension was made by passing the sample through a 200-mesh nylon screen. Samples were incubated at 4°C for 30 min. Flow cytometry (BD LSRFortessa™; BD Biosciences) was used to detect red fluorescence and light scattering at 488 nm. The cell DNA content and light scattering analysis were carried out using Modfit LT3.1 analysis software (Verity Software House, Inc.).
Reverse transcription-quantitative (RT-q) PCR
Total RNA from HASMCs in each group was extracted using TRIzol® (Thermo Fisher Scientific, Inc.). Then, cDNA was synthesized from total RNA using 5X All-In-One RT MasterMix with AccuRT Genomic DNA Removal Kit (cat. no. G492; Applied Biological Materials, Inc.), according to the manufacturer's instructions. The mRNA expression levels were detected via qPCR. qPCR was performed with the SYBR premix Ex Taq (Takara Bio, Inc.) using a Bio-Rad CFX96 detection system (Bio-Rad Laboratories, Inc.) according to the manufacturer's instructions. The thermocycling conditions were as follows: Predenaturation at 95°C for 2 min, denaturation at 95°C for 30 sec for a total of 40 cycles, and annealing/extension at 60°C for 30 sec for a total of 40 cycles. The sequences of primers used for RT-qPCR to determine the expression levels of ADAM33, PI3K, AKT, ERK, Bcl-2, Bax and β-actin were are in Table I. The quantitative results were evaluated using the 2−∆∆Cq method (23) and the mRNA expression levels of the above genes were normalized against the mRNA expression level of β-actin.
Table I.
Primers for reverse transcription-quantitative PCR.
| Primer sequences (5′→3′) | ||||
|---|---|---|---|---|
| Gene | Forward | Reverse | Product size (bp) | Tm (°C) |
| ADAM33 | ATAGGCGTGGTGGCTCAT | TGCGGTGTCTTGCTGTG | 112 bp | 60 |
| PI3K | AAGAAGTTGAACGAGTGGTTGG | GCCCTGTTTACTGCTCTCCC | 192 bp | 60 |
| AKT | TCCTCCTCAAGAATGATGGCA | GTGCGTTCGATGACAGTGGT | 181 bp | 60 |
| ERK | TCTGGAGCAGTATTACGACCC | CTGGCTGGAATCTAGCAGTCT | 134 bp | 60 |
| Bcl-2 | GGTGGGGTCATGTGTGTGG | CGGTTCAGGTACTCAGTCATCC | 89 bp | 60 |
| Bax | CCCGAGAGGTCTTTTTCCGAG | CCAGCCCATGATGGTTCTGAT | 155 bp | 60 |
| β-actin | CATGTACGTTGCTATCCAGGC | CTCCTTAATGTCACGCACGAT | 250 bp | 60 |
ADAM33, a disintegrin and metalloproteinase-33; Tm, melting temperature.
Western blotting
The experimental cells were collected and 100 µl RIPA lysis buffer (cat. no. AR0105; Wuhan Boster Biological Technology, Ltd.) was added. Then, the supernatant was collected by centrifugation at 14,000 × g at 4°C for 60 min. The protein concentration was determined using the BCA method (cat. no. DQ111-01; Beijing Transgen Biotech Co., Ltd.) according to the manufacturer's instructions. Then, 30 µg protein sample was loaded per lane and resolved via 10% SDS-PAGE. Subsequently, the separated protein was transferred to a PVDF membrane, which was then blocked using 5% skimmed milk powder at room temperature for 1 h. The membrane was then rinsed with TBS with 0.1% Tween-20 (TBST; three times, 5 min/time). TBST was used to dilute primary antibodies against β-actin (1:3,000; cat. no. D110001; Sangon Biotech Co., Ltd.), PI3K (1:1,000; cat. no. ab191606; Abcam), Akt (1:1,000; cat. no. 4691T; Cell Signaling Technology, Inc.), phosphorylated (p)-Akt (Ser473; 1:1,000; cat. no. 9271T; Cell Signaling Technology, Inc.), ERK1/2 (1:1,000; cat. no. 9102S; Cell Signaling Technology, Inc.) and p-ERK (Thr202/Tyr204; 1:1,000; cat. no. 9101S; Cell Signaling Technology, Inc.). The membranes were incubated at 4°C overnight and rinsed with TBST (three times, 5 min/time). Then, after incubation with diluted goat anti-rabbit IgG H&L (HRP-conjugated; 1:5,000; cat. no. ab205718; Abcam) at room temperature for 2 h, membranes were washed with TBST again (three times, 5 min/time). The samples were detected and imaged by a ChemiScope 3000 Mini chemiluminescence instrument (Shanghai Qinxiang Scientific Instrument Co., Ltd.). Densitometry was performed by the supporting software (ChemiAnalysis; Shanghai Qinxiang Scientific Instrument Co., Ltd.).
Statistical analysis
Continuous data are expressed as means ± standard deviation from three independent repeats. Normally distributed data were compared with one-way ANOVA among multiple groups followed by LSD post hoc test. If the data did not conform to normality, they were converted to normal distribution between subsequent analyses. If the variance was not uniform, the Tamhane method (24) was used for comparison among multiple groups. SPSS 19.0 software (IBM Corp.) was used for statistical analysis. P<0.05 was considered to indicate a statistically significant difference.
Results
Expression of ADAM33 in human airway VSMCs
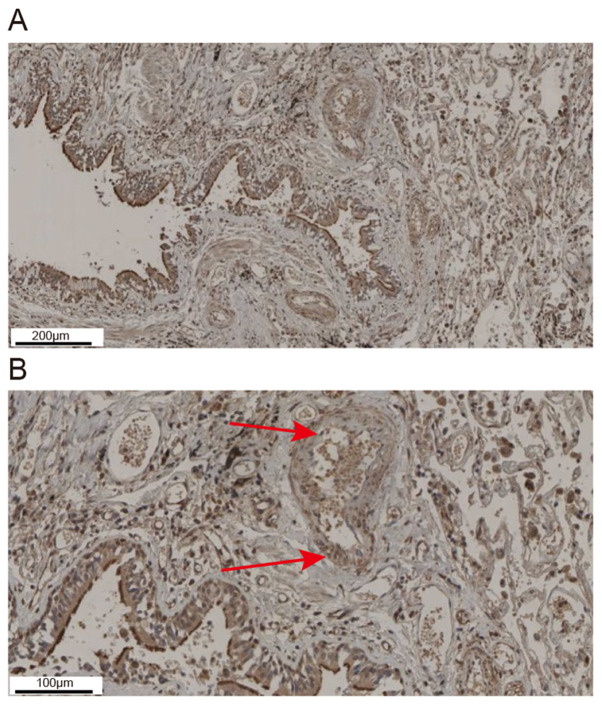
Results of immunohistochemical analysis showed that ADAM33 was mainly expressed in epithelial cells, smooth muscle cells, endothelial cells and interstitial inflammatory cells in the lung tissue of asthmatic patients (Fig. 2A). As indicated by the arrows, ADAM33 positive staining could also be seen in airway VSMCs (Fig. 2B).
Figure 2.
Immunohistochemical staining of ADAM33 in lung tissue of a patient with asthma. (A) Representative images showed that ADAM33 was mainly expressed in epithelial cells, smooth muscle cells, endothelial cells and interstitial inflammatory cells in the lung tissue of asthmatic patients. Scale bar, 200 µm. (B) Representative images showed that ADAM33 was also expressed in airway VSMCs in the lung tissue of asthmatic patients. Arrows indicate ADAM33 stained in brown in airway VSMCs in the lung tissue of asthmatic patients. Scale bar, 100 µm. ADAM33, a disintegrin and metalloproteinase-33; VSMCs, vascular smooth muscle cells.
Identification of HASMCs by immunofluorescence analysis of α-actin
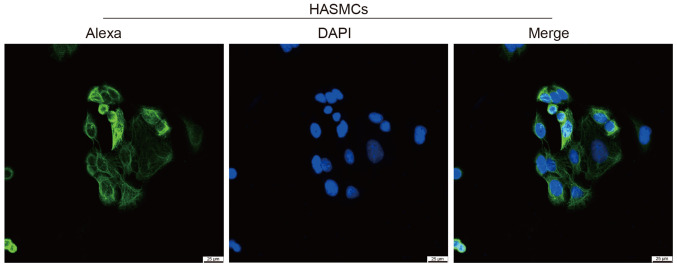
In the present study, HASMCs were identified by immunofluorescence analysis of α-actin. As shown in Fig. 3, α-actin (smooth muscle) protein is located in the cytoskeleton, cytoplasm and cytoplasm, which is consistent with the characteristics of smooth muscle cells.
Figure 3.
Identification of HASMCs by immunofluorescence analysis of α-actin. α-actin (smooth muscle) protein was located in the cytoskeleton, cytoplasm and cytoplasm, which is consistent with the characteristics of smooth muscle cells. Scale bar, 25 µm. HASMCs, human aortic smooth muscle cells.
Efficiency of LV-ADAM33-shRNA in silencing ADAM33 in HASMCs
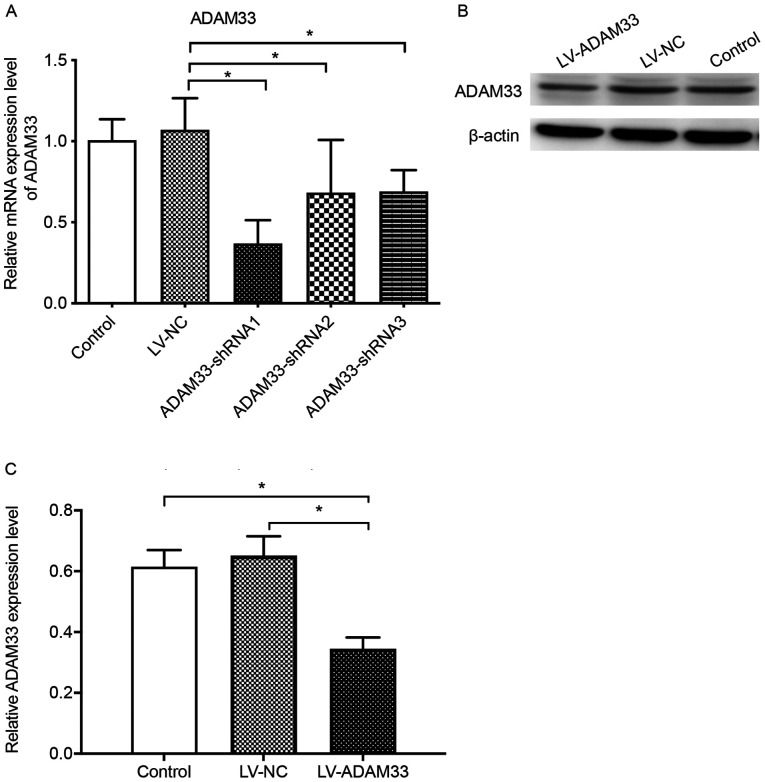
The influence of LV-ADAM33-shRNA on mRNA and protein expression of ADAM33 in HASMCs are shown in Fig. 4. Results of RT-qPCR showed that compared with the control group with no treatment, treatment with LV-NC did not significantly affect the mRNA expression of ADAM33 in HASMCs (P=0.616; Fig. 4A). Compared with the HASMCs transfected with LV-NC, HASMCs transfected with LV-ADAM33-shRNA1, LV-ADAM33-shRNA2 and LV-ADAM33-shRNA3 had significantly reduced mRNA levels of ADAM33 (P=0.000, P=0.006 and P=0.007; Fig. 4A). Among them, LV-ADAM33-shRNA1 was associated with most notable inhibitory effect on ADAM33 in HASMCs. Therefore, LV-ADAM33-shRNA1 was selected for subsequent studies. Further analyses with western blotting showed that LV-ADAM33-shRNA1 transfection significantly reduced the protein level of ADAM33 in HASMCs compared with the blank control group and negative control group with LV-NC (P=0.001 and P=0.000; Fig. 4B and C).
Figure 4.
Validation of lentivirus silencing effect of ADAM33 in HASMCs. (A) Comparison of ADAM33 mRNA levels among HASMCs in groups of blank control (no treatment), LV-NC and ADAM33 silencing with LV-ADAM33-shRNA 1–3 with reverse transcription-quantitative PCR. (B) Comparison of ADAM33 protein levels among HASMCs in groups of blank control (no treatment), LV-NC and ADAM33 silencing with LV-ADAM33-shRNA with western blotting. (C) Semi-quantitative analysis for the ADAM33 protein levels among HASMCs in blank control, LV-NC and LV-ADAM33-shRNA groups. *P<0.05. ADAM33, a disintegrin and metalloproteinase-33; HASMCs, human aortic smooth muscle cells; LV, lentiviral vector; NC, negative control; shRNA, short-hairpin RNA.
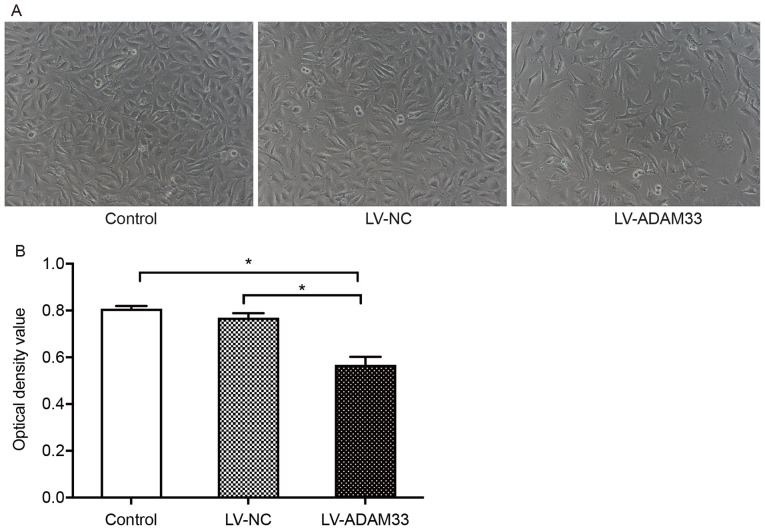
Influence of ADAM33 silencing on proliferation of HASMCs
The effects of ADAM33 silencing with LV-ADAM33-shRNA transfection on the proliferation of HASMCs were analyzed with the CCK-8 proliferation method. Under the microscope, HASMCs in the blank control group and negative control group were elongated and spindle shaped, arranged in bundles and covered the whole field of vision. However, in HASMCs with ADAM33 silencing by LV-ADAM33-shRNA, the cells clusters were dispersed and the density of the cellular distribution was notably reduced, suggesting that the proliferation of HASMCs was limited (Fig. 5A). Quantitative analyses showed that the OD value for cell growth of HASMCs after ADAM33 silencing was significantly reduced compared with those in the blank control and negative control groups (both P=0.000; Fig. 5B).
Figure 5.
Influence of ADAM33 silencing on the proliferation of HASMCs. (A) The proliferation status of HASMCs in the blank control, LV-NC and LV-ADAM33-shRNA groups under an optical microscope (magnification, ×100). (B) Quantitative evaluation of the ODs for cellular proliferation in HASMCs in the blank control, LV-NC and LV-ADAM33-shRNA groups. The proliferative ability of HASMCs was evaluated. OD value was measured at 450 nm. *P<0.05. ADAM33, a disintegrin and metalloproteinase-33; HASMCs, human aortic smooth muscle cells; LV, lentiviral vector; NC, negative control; shRNA, short-hairpin RNA; OD, optical density.
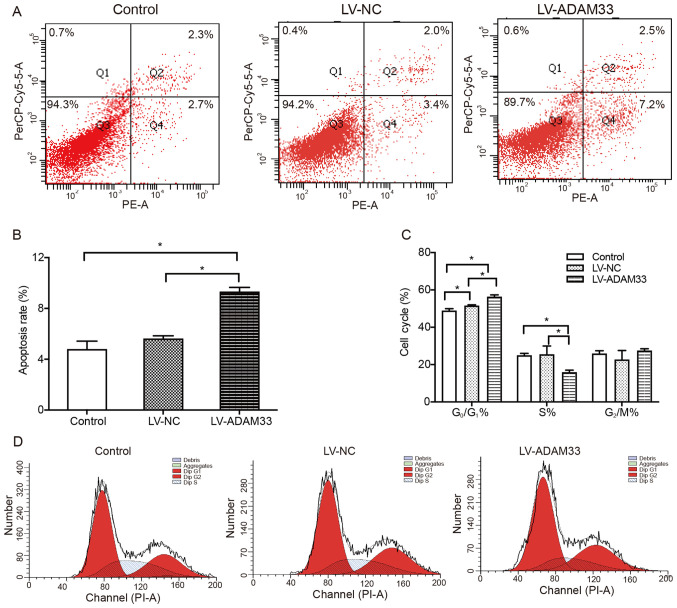
Influence of ADAM33 silencing on apoptosis and cell cycle distribution of HASMCs
Subsequently, the influence of ADAM33 silencing on apoptosis and cell cycle distribution in HASMCs was evaluated by flow cytometry. It was found that the apoptotic rates of HASMCs were similar between the blank control group and negative control group (P=0.356; Fig. 6A and B). However, ADAM33 silencing by LV-ADAM33-shRNA transfection significantly increased the rate of apoptotic cells (9.333±0.321 vs. 4.800±0.625% and 5.633±0.208%; P=0.001 and P=0.005; Fig. 6A and B) compared with HASMCs in the blank control group and negative control group. In addition, it was also found that the percentage of G0/G1 phase cells in the negative control group was higher than that in the blank control group (P=0.007; Fig. 6C and D). Compared with HASMCs in the blank control group and negative control group, the percentage of cells in the G0/G1 phase in the ADAM33 gene silencing group was significantly higher (P=0.000 and P=0.000; Fig. 6C), while the percentage of cells in S phase was lower (P=0.006 and P=0.005; Fig. 6C) and there was no significant difference in the percentage of cells in G2/M phase (P=0.523 and P=0.091; Fig. 6C). The results showed that ADAM33 gene silencing could inhibit the cell cycle transition from G0/G1 to S phase and promote cell apoptosis.
Figure 6.
Influence of ADAM33 silencing on apoptosis of HASMCs. (A) Flow cytometry plots of HASMC apoptosis in the blank control, LV-NC and LV-ADAM33-shRNA groups. The cells in the Q2 and Q4 quadrants are apoptotic HASMCs. (B) Quantitative analysis of the apoptosis rate of HASMCs in the blank control, LV-NC and LV-ADAM33-shRNA groups. (C) Quantitative analysis of the differences of cell cycle distributions of HASMCs in each group. (D) Flow cytometry of HASMC cell cycle distribution changes in the blank control, LV-NC and LV-ADAM33-shRNA groups. *P<0.05. ADAM33, a disintegrin and metalloproteinase-33; HASMCs, human aortic smooth muscle cells; LV, lentiviral vector; NC, negative control; shRNA, short-hairpin RNA.
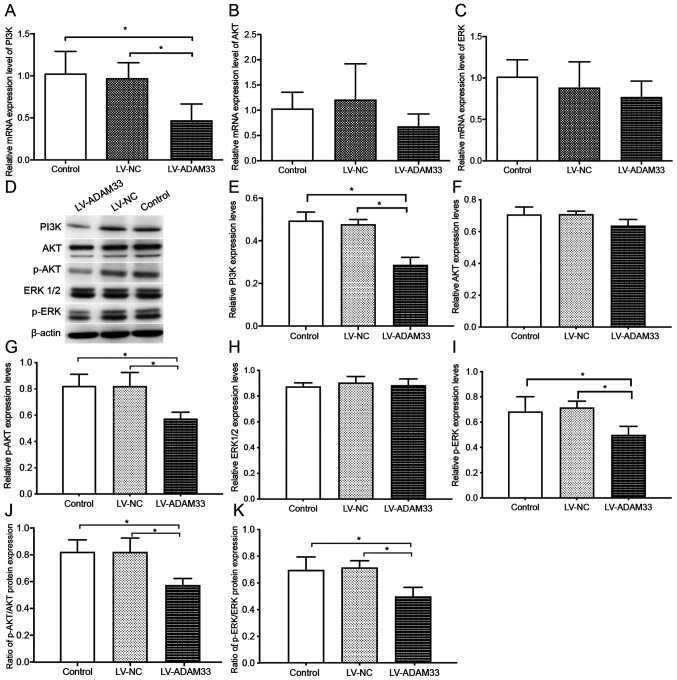
Influence of ADAM33 silencing on PI3K/AKT/ERK pathway singling molecules
To further understand the underlying molecular mechanisms of ADAM33 silencing on the proliferation of HASMCs, the changes of signaling molecules involved in PI3K/AKT/ERK pathway were evaluated following ADAM33 silencing. Results showed that ADAM33 silencing significantly reduced the mRNA level of PI3K (P=0.002 and P=0.003), while changes of mRNA levels of AKT and ERK were not significant compared with HASMCs in the blank control group and negative control group (P=0.259 and P=0.100; P=0.134 and P=0.470; Fig. 7A-C). Furthermore, western blotting analyses showed that ADAM33 silencing significantly reduced the protein level of PI3K compared with HASMCs in the blank control group and negative control group (P=0.000 and P=0.000; Fig. 7D and E). In addition, although protein levels of total AKT and ERK1/2 were not significantly changed, ADAM33 silencing significantly reduced the protein levels of p-AKT and p-ERK (P=0.011 and P=0.011; P=0.035 and P=0.019; Fig. 7F-I). The ratios of p-AKT/AKT and p-ERK/ERK1/2 were reduced (P=0.013 and P=0.020; P=0.044 and P=0.011; Fig. 7J and K). Taken together, these results suggested that ADAM33 silencing may attenuate the proliferation of HASMCs via inhibiting the PI3K/AKT/ERK pathways.
Figure 7.
Influence of ADAM33 silencing on the PI3K/AKT/ERK pathway singling molecules. Reverse transcription-quantitative PCR for the mRNA levels of (A) PI3K, (B) AKT and (C) ERK of HASMCs in the blank control, LV-NC and LV-ADAM33-shRNA groups. (D) Representative images of western blotting for the protein levels of PI3K, AKT, p-AKT, ERK1/2 and p-ERK of HASMCs in the blank control, LV-NC and LV-ADAM33-shRNA groups. Semi-quantitative analysis of the protein levels of (E) PI3K, (F) AKT, (G) p-AKT, (H) ERK1/2 and (I) p-ERK of HASMCs in the blank control, LV-NC and LV-ADAM33-shRNA groups evaluated by western blotting. Ratio of (J) p-AKT/AKT and (K) p-ERK/ERK1-2 of HASMCs in the blank control, LV-NC and LV-ADAM33-shRNA groups. *P<0.05. ADAM33, a disintegrin and metalloproteinase-33; HASMCs, human aortic smooth muscle cells; LV, lentiviral vector; NC, negative control; shRNA, short-hairpin RNA; p-, phosphorylated.
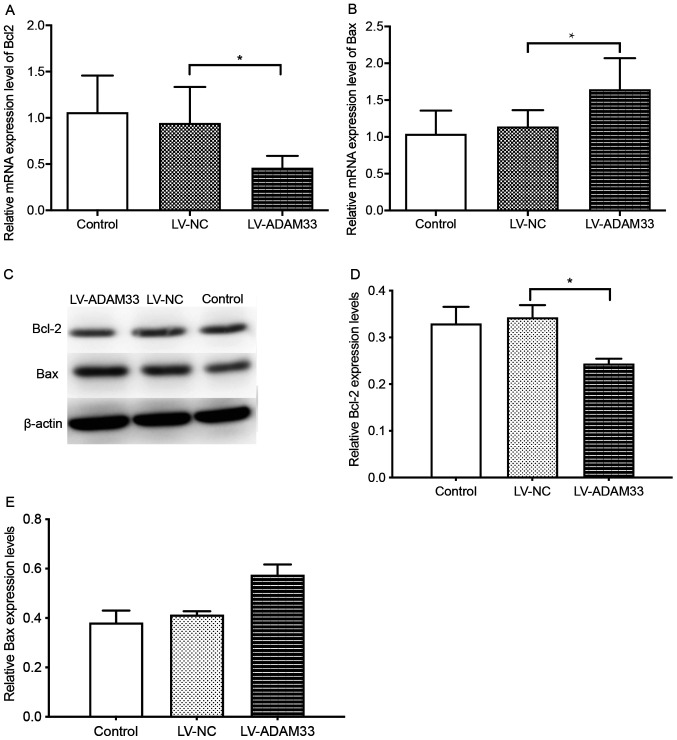
Influence of ADAM33 silencing on Bcl-2 and Bax apoptotic molecules
To elucidate the underlying mechanisms for the role of ADAM33 silencing on the apoptosis of HASMCs, the changes in Bcl-2 and Bax expression levels following ADAM33 silencing was evaluated. Results showed that ADAM33 silencing in HASMCs was associated with reduced mRNA expression of Bcl-2, but increased mRNA expression of Bax compared with HASMCs in the blank control group and negative control groups (P=0.014 and P=0.039; P=0.013 and P=0.032; Fig. 8A and B). Furthermore, results from western blotting also showed that ADAM33 silencing reduced the protein expression of Bcl-2, but increased the protein expression of Bax (P=0.007 and P=0.004; P=0.001 and P=0.002; Fig. 8C-E). Taken together, these results demonstrated that ADAM33 silencing may upregulate HASMCs apoptosis via regulation of Bax/Bcl-2 apoptotic-related protein expression.
Figure 8.
Influence of ADAM33 silencing on Bcl-2 and Bax apoptotic molecules. Reverse transcription-quantitative PCR for the mRNA levels of (A) Bcl-2 and (B) Bax of HASMCs in the blank control, LV-NC and LV-ADAM33-shRNA groups. (C) Representative images of western blotting of the protein levels of Bcl-2 and Bax of HASMCs in the blank control, LV-NC and LV-ADAM33-shRNA groups. Semi-quantitative analysis of the protein levels of (D) Bcl-2 and (E) Bax of HASMCs in the blank control, LV-NC and LV-ADAM33-shRNA groups evaluated by western blotting. *P<0.05. ADAM33, a disintegrin and metalloproteinase-33; HASMCs, human aortic smooth muscle cells; LV, lentiviral vector; NC, negative control; shRNA, short-hairpin RNA.
Discussion
The present study, using in vitro HASMCs and lentiviral vectors carrying shRNA for ADAM3, found that constitutive expression of ADAM33 in VSMCs is important to maintain a proliferative phenotype of VSMCs, probably via regulation of the PI3K/AKT/ERK pathways. In addition, silencing of ADAM33 inhibited the proliferation and induced the apoptosis of HASMCs, which was accompanied by the inhibition of PI3K/AKT/ERK pathways and regulation of the expression levels of Bcl-2 and Bax proteins towards a pro-apoptotic ratio. These findings, together with the findings of previous studies (25,26) and immunohistochemical result in this present study, which showed constitutive expression of ADAM33 in airway VSMCs in patients with asthma, suggested an important role of ADAM33 in airway vascular remodeling. These findings also suggested potential therapeutic significance of ADAM33 inhibition in airway remodeling-related diseases.
In the field of asthma research, ADAM33 is an important susceptibility gene, as revealed by whole genome scanning (27). Accordingly, further clarification of its function is important in translational medical research. Previous studies by our team have shown that cytokines of T cells, including IFN-γ, IL-4 and IL-13, may act on the airway wall vascular smooth muscle cells and affect airway wall vascular remodeling by regulating ADAM33 expression (18,28). The present study was designed for further understanding the molecular mechanism (signaling pathway) of abnormal ADAM33 gene expression on airway vascular remodeling. During the present study, a large amount of biopsy tissues of human airway VSMCs would be needed for cell isolation and culture and these technical difficulties greatly challenged the use of cells derived from biopsy. Therefore, HASMCs were selected.
Composed of 22 exons and 8 encoding regions, the biological functions of ADAM33 mainly involve protein hydrolysis, molecular modification, molecular release, intercellular and cellular matrix interaction, intracellular signal transduction and intercellular communication (29). Expression of ADAM33 in pulmonary tissues of patients with asthma has been confirmed (14,26). Subsequent studies have evaluated the role of ADAM33 in airway remodeling. A previous study in a human bronchial epithelial cell line showed that TGFβ1 is associated with enhanced expression of ADAM33, which subsequently induced epithelial-mesenchymal transition of airway epithelial cells that participate in airway remodeling in asthma (30). Notably, an early study showed that TGFβ1 suppresses the expression of ADAM33 mRNA in normal or asthmatic fibroblasts (31). By using an ovalbumin-induced asthma model in rats, silencing of ADAM33 has been shown to decrease the proliferation and increase the apoptosis of airway smooth muscle cells (ASMCs), suggesting that ADAM33 represents a potential investigative focus target aiding allergic asthma (22). Another study showed that vascular endothelial growth factor enhances ADAM33 expression and cell proliferation of ASMCs by activating the VEGFR2/ERK1/2 signaling pathway, which might be involved in the pathogenesis of airway remodeling (32). A subsequent study showed that 1,25-dihydroxyvitamin D3 can inhibit VEGF-induced ASMCs proliferation by suppressing VEGFR2 and ERK1/2 activation and downregulating ADAM33 (33). In addition, ADAM33 overexpression has been shown to alter the mechanical behavior of ASMCs in vitro, promoting a hypercontractile phenotype transition of ASMCs (34). A recent in vitro study in human embryonic lung Mrc-5 fibroblasts sensitized with Dermatophagoides farinae 1 showed that IFN-γ may participate in airway remodeling in asthma by regulating the expression of ADAM33 (35). It is evident that previous studies evaluating the role of ADAM33 in airway remodeling have focused on its role in bronchial epithelial cells, ASMCs and lung fibroblasts. It remains to be elucidated whether ADAM33 is expressed in airway VSMCs and whether it serves a role in the pathogenesis of airway remodeling. The present study confirmed that ADAM33 was expressed in VSMCs. Further in vitro experiments showed that silencing the constitutive expression of ADAM33 in HASMCs could significantly inhibit the proliferation of HASMCs and induced HASMC apoptosis. In view of the importance of vascular proliferation in the pathogenesis of airway remodeling-related diseases (5,7), the findings of the present study suggested that expression of ADAM33 in airway VSMCs may participate in the process of airway remodeling in the pathogenesis of asthma via stimulation of airway VSMC proliferation.
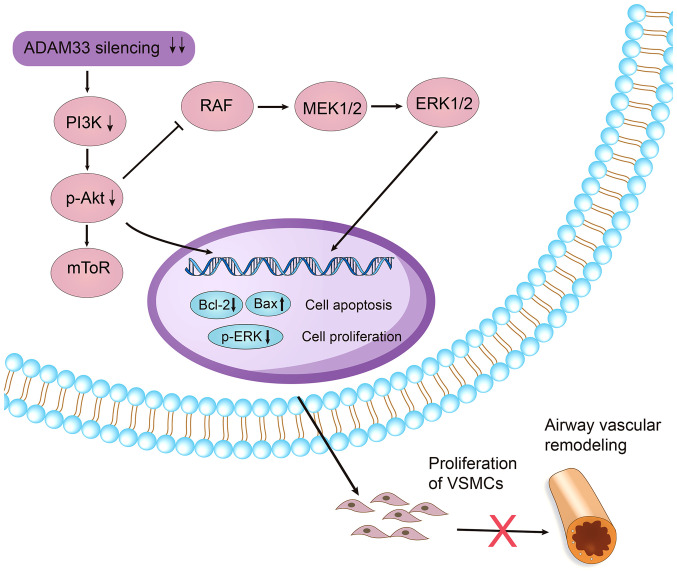
Activation of the PI3K/AKT signaling pathway has been extensively involved in the regulation of cell growth, proliferation and differentiation via phosphorylation of a variety of downstream enzymes, kinases and transcription factors of the pathway (36). A previous study showed that PI3K/AKT signaling mediated hyperinsulinemia-induced proliferation and collagen release of human ASMCs, therefore promoting airway remodeling in asthma (37). Another study showed that inhibition of the expression of p-PI3K and p-AKT was associated with attenuated angiogenesis and vascular remodeling and alleviated symptoms in a mouse model of asthma (38). The present study showed that silencing of ADAM33 in HASMCs was associated with inhibited cellular proliferation, which was accompanied by the inhibition of the PI3K/AKT/ERK pathways. These findings suggested that ADAM33 may stimulate proliferation of airway VSMCs via activation of the PI3K/AKT/ERK pathways. Bcl-2 and Bax proteins are universally expressed apoptosis regulatory proteins among various cells, which exert anti-apoptotic and pro-apoptotic activities, respectively (39). The present study found that silencing of ADAM33 in HASMCs was associated with induced cellular apoptosis, accompanied by the upregulation of Bax and downregulation of Bcl-2. These findings further indicated that constitutive expression of ADAM33 may inhibit the apoptosis of airway VSMCs via regulation of Bcl-2/Bax expression. Previous studies have also suggested the potential interactions between PI3K/AKT/ERK pathway and Bcl-2 and Bax apoptotic molecules (40,41). An early study in cultured airway SMCs showed that leptin can significantly inhibit apoptosis in airway SMCs apoptosis, at least partially via the activation of PI3K/AKT signaling pathway and subsequent upregulation of Bcl-2 and downregulation of Bax, towards an anti-apoptotic direction (40). Another study in VSMCs showed that overexpression of phosphatase and tensin homolog could inhibit the PI3K/AKT/ERK pathway, accompanied with upregulation of Bax and downregulation of Bcl-2, towards a pro-apoptotic direction (41). These findings, together with those of the present study, suggest that inhibition of PI3K/AKT/ERK pathway may lead to apoptosis in SMCs by upregulation of Bax and downregulation of Bcl-2, towards a pro-apoptotic direction. Taken together, by integrating the results of the aforementioned findings, it could be hypothesized that constitutive expression of ADAM33 in VSMCs may maintain a proliferative phenotype of VSMCs via regulation of the PI3K/AKT/ERK pathways. Accordingly, silencing of ADAM33 inhibited proliferation and induced the apoptosis of HASMCs via inhibition of PI3K/AKT/ERK pathways and subsequent regulation of Bcl-2 and Bax protein expression towards a pro-apoptotic ratio (Fig. 9). As we have previously shown constitutive expression of ADAM33 in airway VSMCs in patients with asthma, these findings may suggest the potential therapeutic significance of ADAM33 inhibition in airway remodeling-related diseases. It has to be mentioned that the findings of the present study are based on observations in HASMCs rather than in isolated airway VSMCs due to technical difficulties. These findings should be validated in future studies in airway VSMCs. Furthermore, although silencing ADAM33 appeared promising to inhibit the proliferation of HASMCs, the influences on proliferation of VSMCs, as well as in the process of airway vascular remodeling, should be investigated using preclinical asthma models.
Figure 9.
Graphical summary of the potential signaling pathways involved in the role of constitutive expression of ADAM33 in VSMCs for maintaining a proliferative phenotype of airway VSMCs. ADAM33, a disintegrin and metalloproteinase-33; VSMCs, vascular smooth muscle cells; p-, phosphorylated.
In conclusion, the present study demonstrated that constitutive expression of ADAM33 may be important to maintain a proliferative phenotype in HASMCs. The influences of ADAM33 on the proliferation and apoptosis of HASMCs may involve the regulation of PI3K/AKT/ERK and Bax/Bcl-2 pathways. These findings suggested an important role of ADAM33 in airway vascular remodeling and a potential therapeutic significance of ADAM33 inhibition in airway remodeling-related diseases.
Acknowledgements
Not applicable.
Funding Statement
This study was supported by the National Natural Science Foundation Regional Fund Project (grant no. 81760005).
Funding
This study was supported by the National Natural Science Foundation Regional Fund Project (grant no. 81760005).
Availability of data and materials
The datasets generated and analyzed during the current study are not publicly available due to patient privacy regulations of the different countries, but are available from the corresponding author on reasonable request.
Authors' contributions
JD and JW conceived and designed the experiments. FY, YH and HS performed the experiments. XG analyzed the data. FY and YH wrote the manuscript. All authors read and approved the final manuscript. JD, JW and FY confirmed the authenticity of all the raw data.
Ethics approval and consent to participate
The present study was approved by Ethics Committee of the First Affiliated Hospital of Xinjiang Medical University (approval no. 20180130-01; Urumqi, China). All the patients provided signed informed consent. The study complied with the Declaration of Helsinki. All methods were carried out in accordance with the ethical rules on clinical studies established by the World Health Organization guidelines.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Hough KP, Curtiss ML, Blain TJ, Liu RM, Trevor J, Deshane JS, Thannickal VJ. Airway remodeling in asthma. Front Med (Lausanne) 2020;7:191. doi: 10.3389/fmed.2020.00191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kardas G, Kuna P, Panek M. Biological therapies of severe asthma and their possible effects on airway remodeling. Front Immunol. 2020;11:1134. doi: 10.3389/fimmu.2020.01134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fehrenbach H, Wagner C, Wegmann M. Airway remodeling in asthma: What really matters. Cell Tissue Res. 2017;367:551–569. doi: 10.1007/s00441-016-2566-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhou-Suckow Z, Duerr J, Hagner M, Agrawal R, Mall MA. Airway mucus, inflammation and remodeling: Emerging links in the pathogenesis of chronic lung diseases. Cell Tissue Res. 2017;367:537–550. doi: 10.1007/s00441-016-2562-z. [DOI] [PubMed] [Google Scholar]
- 5.Harkness LM, Kanabar V, Sharma HS, Westergren-Thorsson G, Larsson-Callerfelt AK. Pulmonary vascular changes in asthma and COPD. Pulm Pharmacol Ther. 2014;29:144–155. doi: 10.1016/j.pupt.2014.09.003. [DOI] [PubMed] [Google Scholar]
- 6.Olivieri D, Chetta A. Therapeutic perspectives in vascular remodeling in asthma and chronic obstructive pulmonary disease. Chem Immunol Allergy. 2014;99:216–225. doi: 10.1159/000353307. [DOI] [PubMed] [Google Scholar]
- 7.Barbato A, Turato G, Baraldo S, Bazzan E, Calabrese F, Panizzolo C, Zanin ME, Zuin R, Maestrelli P, Fabbri LM, Saetta M. Epithelial damage and angiogenesis in the airways of children with asthma. Am J Respir Crit Care Med. 2006;174:975–981. doi: 10.1164/rccm.200602-189OC. [DOI] [PubMed] [Google Scholar]
- 8.Alagappan VK, de Boer WI, Misra VK, Mooi WJ, Sharma HS. Angiogenesis and vascular remodeling in chronic airway diseases. Cell Biochem Biophys. 2013;67:219–234. doi: 10.1007/s12013-013-9713-6. [DOI] [PubMed] [Google Scholar]
- 9.Bakakos P, Patentalakis G, Papi A. Vascular biomarkers in asthma and COPD. Curr Top Med Chem. 2016;16:1599–1609. doi: 10.2174/1568026616666150930121157. [DOI] [PubMed] [Google Scholar]
- 10.Rzucidlo EM. Signaling pathways regulating vascular smooth muscle cell differentiation. Vascular. 2009;17(Suppl 1):S15–S20. doi: 10.2310/6670.2008.00089. [DOI] [PubMed] [Google Scholar]
- 11.Klein T, Bischoff R. Active metalloproteases of the A Disintegrin and Metalloprotease (ADAM) family: Biological function and structure. J Proteome Res. 2011;10:17–33. doi: 10.1021/pr100556z. [DOI] [PubMed] [Google Scholar]
- 12.Dreymueller D, Uhlig S, Ludwig A. ADAM-family metalloproteinases in lung inflammation: Potential therapeutic targets. Am J Physiol Lung Cell Mol Physiol. 2015;308:L325–L343. doi: 10.1152/ajplung.00294.2014. [DOI] [PubMed] [Google Scholar]
- 13.Li HF, Yan LP, Wang K, Li XT, Liu HX, Tan W. Association between ADAM33 polymorphisms and asthma risk: A systematic review and meta-analysis. Respir Res. 2019;20:38. doi: 10.1186/s12931-019-1006-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lee JY, Park SW, Chang HK, Kim HY, Rhim T, Lee JH, Jang AS, Koh ES, Park CS. A disintegrin and metalloproteinase 33 protein in patients with asthma: Relevance to airflow limitation. Am J Respir Crit Care Med. 2006;173:729–735. doi: 10.1164/rccm.200409-1175OC. [DOI] [PubMed] [Google Scholar]
- 15.Poon AH, Houseman EA, Ryan L, Sparrow D, Vokonas PS, Litonjua AA. Variants of asthma and chronic obstructive pulmonary disease genes and lung function decline in aging. J Gerontol A Biol Sci Med Sci. 2014;69:907–913. doi: 10.1093/gerona/glt179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hur GY, Broide DH. Genes and pathways regulating decline in lung function and airway remodeling in asthma. Allergy Asthma Immunol Res. 2019;11:604–621. doi: 10.4168/aair.2019.11.5.604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kozlik P, Zuk J, Bartyzel S, Zarychta J, Okon K, Zareba L, Bazan JG, Kosalka J, Soja J, Musial J, Bazan-Socha S. The relationship of airway structural changes to blood and bronchoalveolar lavage biomarkers, and lung function abnormalities in asthma. Clin Exp Allergy. 2020;50:15–28. doi: 10.1111/cea.13501. [DOI] [PubMed] [Google Scholar]
- 18.Zhang L, Hi X, Yao L, J. W, W. Q. The effect of interleukin4 on ADAM33 gene expression in airway wall vascular smooth muscle cells. Journal of Xinjiang Medical University. 2016;39:265–269. [Google Scholar]
- 19.Isenovic ER, Kedees MH, Tepavcevic S, Milosavljevic T, Koricanac G, Trpkovic A, Marche P. Role of PI3K/AKT, cPLA2 and ERK1/2 signaling pathways in insulin regulation of vascular smooth muscle cells proliferation. Cardiovasc Hematol Disord Drug Targets. 2009;9:172–180. doi: 10.2174/187152909789007034. [DOI] [PubMed] [Google Scholar]
- 20.Chae IH, Park KW, Kim HS, Oh BH. Nitric oxide-induced apoptosis is mediated by Bax/Bcl-2 gene expression, transition of cytochrome c, and activation of caspase-3 in rat vascular smooth muscle cells. Clin Chim Acta. 2004;341:83–91. doi: 10.1016/j.cccn.2003.11.009. [DOI] [PubMed] [Google Scholar]
- 21.Lin F, Song A, Wu J, Jiang X, Long J, Chen J, Duan Y, Shi Y, Deng L. ADAM33 protein expression and the mechanics of airway smooth muscle cells are highly correlated in ovalbumin-sensitized rats. Mol Med Rep. 2013;8:1209–1215. doi: 10.3892/mmr.2013.1621. [DOI] [PubMed] [Google Scholar]
- 22.Zhou J, Bai W, Liu Q, Cui J, Zhang W. Silencing of ADAM33 restrains proliferation and induces apoptosis of airway smooth muscle cells in ovalbumin-induced asthma model. J Cell Biochem. 2018 Nov 18; doi: 10.1002/jcb.27263. (Epub ahead of print) [DOI] [PubMed] [Google Scholar]
- 23.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 24.Tamhane AC. Higher Education Press; Beijing: 2006. Statistical analysis of designed experiments: Theory and applications. [Google Scholar]
- 25.Dijkstra A, Postma DS, Noordhoek JA, Lodewijk ME, Kauffman HF, ten Hacken NH, Timens W. Expression of ADAMs (‘a disintegrin and metalloprotease’) in the human lung. Virchows Arch. 2009;454:441–449. doi: 10.1007/s00428-009-0748-4. [DOI] [PubMed] [Google Scholar]
- 26.Foley SC, Mogas AK, Olivenstein R, Fiset PO, Chakir J, Bourbeau J, Ernst P, Lemière C, Martin JG, Hamid Q. Increased expression of ADAM33 and ADAM8 with disease progression in asthma. J Allergy Clin Immunol. 2007;119:863–871. doi: 10.1016/j.jaci.2006.12.665. [DOI] [PubMed] [Google Scholar]
- 27.Van Eerdewegh P, Little RD, Dupuis J, Del Mastro RG, Falls K, Simon J, Torrey D, Pandit S, McKenny J, Braunschweiger K, et al. Association of the ADAM33 gene with asthma and bronchial hyperresponsiveness. Nature. 2002;418:426–430. doi: 10.1038/nature00878. [DOI] [PubMed] [Google Scholar]
- 28.Wen J, Muyesha P, Gong X, Hu X, Hao Y, Yan F, Zang L, Wang J. Effect of γ-interferon on the expression of ADAM33 gene in human airway wall vascular smooth muscle cells. Journal of Xinjiang Medical University. 2019;42:965–970. [Google Scholar]
- 29.Tripathi P, Awasthi S, Gao P. ADAM metallopeptidase domain 33 (ADAM33): A promising target for asthma. Mediators Inflamm. 2014;2014:572025. doi: 10.1155/2014/572025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fang L, Wu J, Huang T, Zhang P, Xin X, Shi Y. TGF-β1 stimulates epithelial-mesenchymal transition mediated by ADAM33. Exp Ther Med. 2018;15:985–992. doi: 10.3892/etm.2017.5478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yang Y, Wicks J, Haitchi HM, Powell RM, Manuyakorn W, Howarth PH, Holgate ST, Davies DE. Regulation of a disintegrin and metalloprotease-33 expression by transforming growth factor-β. Am J Respir Cell Mol Biol. 2012;46:633–640. doi: 10.1165/rcmb.2011-0030OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pei QM, Jiang P, Yang M, Qian XJ, Liu JB, Zheng H, Zhao LH, Kim SH. Upregulation of a disintegrin and metalloproteinase-33 by VEGF in human airway smooth muscle cells: Implications for asthma. Cell Cycle. 2016;15:2819–2826. doi: 10.1080/15384101.2016.1220462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kim SH, Pei QM, Jiang P, Yang M, Qian XJ, Liu JB. Effect of active vitamin D3 on VEGF-induced ADAM33 expression and proliferation in human airway smooth muscle cells: Implications for asthma treatment. Respir Res. 2017;18:7. doi: 10.1186/s12931-016-0490-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Duan Y, Long J, Chen J, Jiang X, Zhu J, Jin Y, Lin F, Zhong J, Xu R, Mao L, Deng L. Overexpression of soluble ADAM33 promotes a hypercontractile phenotype of the airway smooth muscle cell in rat. Exp Cell Res. 2016;349:109–118. doi: 10.1016/j.yexcr.2016.10.004. [DOI] [PubMed] [Google Scholar]
- 35.Li W, Liang R, Huang H, Wu B, Zhong Y. Effects of IFN-gamma on cell growth and the expression of ADAM33 gene in human embryonic lung Mrc-5 fibroblasts in vitro. J Asthma. 2018;55:15–25. doi: 10.1080/02770903.2017.1310226. [DOI] [PubMed] [Google Scholar]
- 36.Yu JS, Cui W. Proliferation, survival and metabolism: The role of PI3K/AKT/mTOR signalling in pluripotency and cell fate determination. Development. 2016;143:3050–3060. doi: 10.1242/dev.137075. [DOI] [PubMed] [Google Scholar]
- 37.Singh S, Bodas M, Bhatraju NK, Pattnaik B, Gheware A, Parameswaran PK, Thompson M, Freeman M, Mabalirajan U, Gosens R, et al. Hyperinsulinemia adversely affects lung structure and function. Am J Physiol Lung Cell Mol Physiol. 2016;310:L837–L845. doi: 10.1152/ajplung.00091.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Su X, Ren Y, Yu N, Kong L, Kang J. Thymoquinone inhibits inflammation, neoangiogenesis and vascular remodeling in asthma mice. Int Immunopharmacol. 2016;38:70–80. doi: 10.1016/j.intimp.2016.05.018. [DOI] [PubMed] [Google Scholar]
- 39.Edlich F. BCL-2 proteins and apoptosis: Recent insights and unknowns. Biochem Biophys Res Commun. 2018;500:26–34. doi: 10.1016/j.bbrc.2017.06.190. [DOI] [PubMed] [Google Scholar]
- 40.Liu WJ, Zhu SY, Chen YL, Wu X, Ni WJ, Chen YF, Zhao L. The effects of leptin on apoptosis of airway smooth muscle cells via the PI3K/Akt signaling pathway. Zhonghua Jie He He Hu Xi Za Zhi. 2012;35:915–918. (In Chinese) [PubMed] [Google Scholar]
- 41.Wang S, Cheng Z, Chen X. Promotion of PTEN on apoptosis through PI3K/Akt signal in vascular smooth muscle cells of mice model of coronary heart disease. J Cell Biochem. 2019;120:14636–14644. doi: 10.1002/jcb.28725. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated and analyzed during the current study are not publicly available due to patient privacy regulations of the different countries, but are available from the corresponding author on reasonable request.