Abstract
Liver cancer remains one of the leading causes of cancer deaths worldwide. The therapeutic effect of oxaliplatin on liver cancer is often limited by acquired resistance of the cancer cells. Abnormal activation of the PI3K/AKT pathway plays an important role in the acquired resistance of oxaliplatin. The present study investigated the effects of the PI3K inhibitor LY-294002 and AKT inhibitor MK2206 on the chemosensitivity of oxaliplatin-resistant liver cancer cells and the molecular mechanism involved. An oxaliplatin-resistant liver cancer cell line HepG2R was developed. MTT assay, clone formation experiments, flow cytometry and Annexin V-FITC/PI staining were used to determine the proliferation, cycle and apoptosis of HepG2R cells when oxaliplatin was combined with LY-294002 or MK2206 treatment. The effects of LY-294002 and MK-2206 on the abnormal activation of PI3K/AKT pathway and hypoxia inducible factor (HIF)-1α protein level in HepG2R cells were detected using western blotting. The results indicated that the PI3K/AKT pathway is stably activated in HepG2R cells. Compared with the AKT inhibitor MK2206, the PI3K inhibitor LY-294002 more effectively downregulated the phosphorylation levels of p85, p110α, p110β, p110γ and AKT in the PI3K/AKT pathway in HepG2R cells, and more effectively inhibited the proliferation of the cells. LY-294002 enhanced the chemotherapy sensitivity of HepG2R cells to oxaliplatin by inducing G0/G1 phase arrest and increasing the proportion of apoptotic cells. In addition, LY-294002 reduced the level of HIF-1α, which is highly expressed in HepG2R cells. It was concluded that LY-294002 enhanced the chemosensitivity of liver cancer cells to oxaliplatin by inhibiting the PI3K/AKT signaling pathway, which may be related to the inhibition of HIF-1α expression. These findings may have clinical significance for the treatment of oxaliplatin-resistant liver cancer.
Keywords: hepatocellular carcinoma, oxaliplatin, LY-294002, MK-2206, PI3K/AKT, hypoxia inducible factor-1α
Introduction
Liver cancer is a malignant tumor with high morbidity and mortality rate. Liver cancer is the sixth commonest cancer; its 2018 global mortality rate was ~8.2%, ranking fourth among all types of cancer mortality (1–3). Most liver cancer cases reach middle or late stages before they are diagnosed, and the best time for surgical treatment is lost (4,5). Therefore, chemotherapy is the main treatment for liver cancer. However, chemotherapy is prone to drug resistance, which has become a major problem faced by chemotherapy for liver cancer. Oxaliplatin has been approved for systemic chemotherapy in patients with liver cancer, but its efficacy is often reduced because of drug resistance (6,7). Therefore, it is necessary to explore the resistance mechanisms and find a more effective treatment strategy.
Resistance of liver cancer to chemotherapy is related to abnormal activation of the PI3K/AKT/hypoxia inducible factor (HIF)-1α signaling pathway (8–10). PI3K catalyzes the production of phosphatidylinositol- 3,4,5-triphosphate through phosphorylation of phosphatidy-linositol, phosphatidylinositol-4-phosphate and phosphatidylinositol- 4,5-bisphosphate (11,12). Chemotherapy drugs trigger this phosphorylation event, which in turn regulates cell proliferation, cell-cycle progression, cell migration and cell survival (13). In addition, several chemotherapy drugs can activate the serine/threonine kinase AKT (also known as protein kinase B). As a proto-oncogene, AKT plays an important role in regulating various cell functions, including metabolism, proliferation, survival, transcription and protein synthesis (14–17). HIF-1α, a functional subunit of HIF-1, can regulate angiogenesis, red blood cell production, cell cycle, metabolism and apoptosis. More importantly, HIF-1α can be induced by a variety of cytokines (including PTEN, SRC and P53) in an oxygen-independent manner through the PI3K/AKT pathway (18,19). However, whether the combined inhibition of the PI3K/AKT pathway and HIF-1α expression can increase the sensitivity of liver cancer cells to chemotherapy is not known.
LY294002 is a protein kinase inhibitor that can block the cell signaling pathway of phosphotidylinsitol-3-kinase and inhibit the expression of PI3Kα, PI3Kδ and PI3Kβ. LY294002 can enter cells and inhibit the PI3K and PI3K/Akt signaling pathways, including inhibition of Akt phosphorylation, thereby inhibiting cell division and induce G1 arrest of pancreatic cancer and other cells (20). MK-2206 is a highly selective AKT1/2/3 inhibitor, activated by the pleckstrin homology domain. MK-2206 inhibits the autophosphorylation of AKT at threonine 308 and serine 473 (21). Preclinical studies have shown that MK-2206 has an inhibitory effect on human cancer cell lines, such as breast, lung, colon and liver cancer (22–25). PI3K/AKT/HIF-1α reportedly induces chemoresistance in liver cancer cells (26,27), and LY-294002 can enhance the chemosensitivity of liver cancer (28). Therefore, the present study used LY-294002 in combination with oxaliplatin.
The present study explored the effect of LY-294002 or MK-2206 in combination with oxaliplatin on the abnormal activation of the PI3K/AKT pathway in oxaliplatin-resistant liver cancer. The focus of the study was the more significant inhibitory effect of LY-294002 and whether its effect is due to the inhibition of HIF-1α expression. It was proposed that HIF-1α may be a target to improve the sensitivity of chemotherapeutics, and that the combination of LY-294002 and oxaliplatin will be beneficial in the treatment of liver cancer and provide a basis for the development of targeted therapeutic strategies against liver cancer.
Materials and methods
Cell source and culture
The human liver cancer cell line HepG2 (control HepG2 cells) was purchased from Shanghai Mingjin Biological Technology Co, Ltd. The oxaliplatin-resistant HepG2 cell line, HepG2R, was induced in medium containing increasing concentration of oxaliplatin (0–40 µM) for a total of seven months. The two cell lines were cultured in RPMI-1640 medium (Hyclone; Cyvita) containing 10% fetal bovine serum (Hangzhou Sijiqing Biological Engineering Materials Co., Ltd.) and maintained in a 5% CO2 incubator at 37°C.
Chemicals, antibodies and reagents
LY-294002 (0.2 µM) and MK-2206 (50 nmol/l; MedChem Express) were dissolved in dimethyl sulfoxide (DMSO) to prepare a 2 mM stock solution. Oxaliplatin (Sigma-Aldrich; Merck KGaA) was dissolved in water to produce a 2 mM stock solution and stored at −20°C. Phosphorylated (p-) retinoblastoma [Rb; anti-Rb (phospho S807) antibody [EPR17732] (cat. no. ab184796)] and cyclin D1 [anti-Cyclin D1 antibody (SP4) cat. no. ab16663] antibodies were purchased from Abcam. Antibodies against caspase-3 (cat. no. 9662), cleaved caspase-3 (cat. no. 9661), caspase-9 (cat. no. 9502), cleaved caspase-9 (cat. no. 20750), PARP (cat. no. 9532), cleaved RARP (cat. no. 5625), PI3Kp110α (cat. no. 4249), PI3Kp110β (cat. no. 3011), PI3Kp110γ (cat. no. 5405), PI3Kp85 (cat. no. 4292), Akt (cat. no. 4691), p-Akt (Ser473; cat. no. 4060), Bad (cat. no. 9268), p-Bad (cat. no. 5284), Bax (cat. no. 14796) and Puma (cat. no. 24633) were purchased from Cell Signaling Technology, Inc. The p70S6K (cat. no. BM4240), p-S6K1 (T421+S424) (cat. no. BM4141) and eukaryotic translation initiation factor 4E-binding protein 1 (eIF4EBP1) antibodies (cat. no. BM4851) were from Wuhan Boster Biological Technology, Ltd. The p-eIF4EBP1 antibody (cat. no. bs-14550R) was from BIOSS. β-actin antibody (cat. no. AF5003) and BCA-200 protein detection kits were from Biosharp Life Sciences. All antibodies were diluted 1:1,000 before use. Phosphatase inhibitor mixture were provided by Beyotime Institute of Biotechnology.
Western blot analysis
HepG2 and HepG2R [Oxaliplatin (2 µM) treated 0, 6, 12, 24, 48, 72 and 96 h] cells (5.0×106) were added with lysis buffer (phosphatase inhibitor mixture) to extract protein. SDS-PAGE gels (10%) were placed at room temperature for 30 min, and 20 µl of protein samples were added to each well. The protein samples, separated by SDS-PAGE, were sequentially transferred on PVDF membrane (EMD Millipore) according to molecular weight. After being blocked with 5% skimmed milk at 37°C for 1 h, the corresponding primary antibodies were incubated at 4°C overnight After blocking with 5% skimmed milk at 37°C for 1 h, the corresponding primary antibody was incubated overnight at 4°C, and β-actin was used as a load control. The membrane was washed three times with TBST [10 M Tris, 150 mM NaCl, 0.05% Tween 20 (pH 8.3)], and secondary antibody was added and incubated at 37°C for 1 h. The membrane was washed again three times with TBST, luminescent solution (ECL Luminescence kit; EMD Millipore) was added for color development. ImageJ 1.44p software (National Institutes of Health) was used for immunoblotting quantitative analysis. The gray value of each imprinted signal was compared with the control group to analyze the expression of the target gene protein.
MTT assay
Cells were seeded in 96-well plates at a density of 5×103 cells/well and placed in an incubator for 24 h, and drugs were added. After 24 h, the medium was discarded, 10 µl of MTT medium was added to each well, and the plates were placed in an incubator. After culturing for 4 h, the culture solution was discarded, 100 µl of DMSO was added to each well and shaken for 10 min on a shaker. The absorbance at 490 nm was detected with an enzyme-linked immunoassay detector (ELx800; Omega Bio-Tek, Inc.).
Colony formation assay
The cells [HepG2R, plus oxaliplatin (2 µM) and/or LY-294002(0.2 µM)] were seeded in a six-well plate at a density of 1×103 cells/well. After the cells had adhered to the wall, drug was added. After 24 h, the drug-free medium was replaced, and the culture was continued for ~3 weeks. When the cell clones were clearly visible, the cell colonies were washed twice with pre-cooled physiological saline and fixed with 4% paraformaldehyde for 15 min at room temperature. After the fixing solution was removed, the cells were stained with crystal violet staining solution for 15 min at room temperature. The number of clones formed was counted to reflect the proliferation ability of cells under the action of each drug.
Cell cycle analysis
The cells were seeded in a six-well plate at a density of 1×105 cells/well and placed in an incubator for 24 h. Drugs were added for 24 h, and the cells were digested with trypsin for collection of the cells, which were fixed with 70% ethanol at room temperature for 1 h. Pre-cooled physiological saline (1 ml) was added to the cells, the cells were centrifuged (562.5 × g; room temperature; 5 min) and 100 µl of staining solution from the AnnexinV-FITC/PI staining kit (Jiangsu KGI Biotechnology Co., Ltd.) was added and incubated at 4°C in the dark for 30 min (staining solution is a mixture of 50 µg/ml PI, 100 µg/ml RNaseA and 0.2% Triton X-100 solution). In total, 1 ml of pre-cooled physiological saline was added to the staining solution, and the cells were centrifuged (562.5 × g; room temperature; 5 min) to obtain a cell pellet. Finally, the cells were resuspended in 300 µl of physiological saline, resuspended and analyzed with a flow cytometer (BD FACSCalibur; BD Biosciences). The results were analyzed with ModiFit LT 3.0 (Verity Software House, Inc.).
Annexin V FITC/PI and DAPI staining
The cells were seeded in a 24-well plate containing slides at a density of 1×104 cells/well, placed in an incubator for 24 h, and treated with drug. After 24 h, the slides were taken out, washed twice with normal saline, and stained with Annexin V FITC/PI (Jiangsu KGI Biotechnology Co., Ltd.) and DAPI (Shanghai Biyuntian Biotechnology Co., Ltd.) for 15 min at room temperature in the dark. Apoptotic rate was determined with a fluorescence microscope; 10 fields of view were evaluated at ×200 magnification. Apoptosis rate=(early apoptotic cells + late apoptotic cells)/total number of cells.
Measurement of mitochondrial membrane potential (JC-1)
The cells were inoculated into the well plates at a density of 1×104 cells/well, placed in an incubator for 24 h, and treated with drug for another 24 h. The cells were rinsed twice with saline, 500 µl JC-1 working solution was added to each well, and the plates were placed in an incubator (5% CO2, 37°C) and incubated for an additional 20 min. The staining solution was discarded, and the cells were rinsed with saline twice, then stained with DAPI solution at room temperature in dark for 15 min. As JC-1 aggregates in the mitochondria when the mitochondrial membrane potential is high, red fluorescence is emitted; conversely, when mitochondria are depolarized, JC-1 is in a monomer state and green fluorescence is emitted. Thus, the mitochondrial membrane potential of the cell can be judged according to the ratio of red and green fluorescence, and change of JC-1 fluorescence color can indicate change of the mitochondrial membrane potential. A decrease in mitochondrial membrane potential is considered a sign of early apoptosis (29). Red and green fluorescence were analyzed with a flow cytometer (BD FACSCalibur; BD Biosciences). The results were analyzed with CellQuest Pro 5.1.1 (Becton, Dickinson and Company).
Statistical analysis
The experimental data were obtained through at least three independent experiments. The data are expressed as mean ± SD, one-way ANOVA to measure significant differences between the means followed by Tukey's post hoc tests. P<0.05 was considered to indicate a statistically significant difference. SPSS 17.0 statistical software (SPSS Inc.) was used for all analyses.
Results
Stable activation of PI3K/AKT pathway in HepG2R cells induced by oxaliplatin
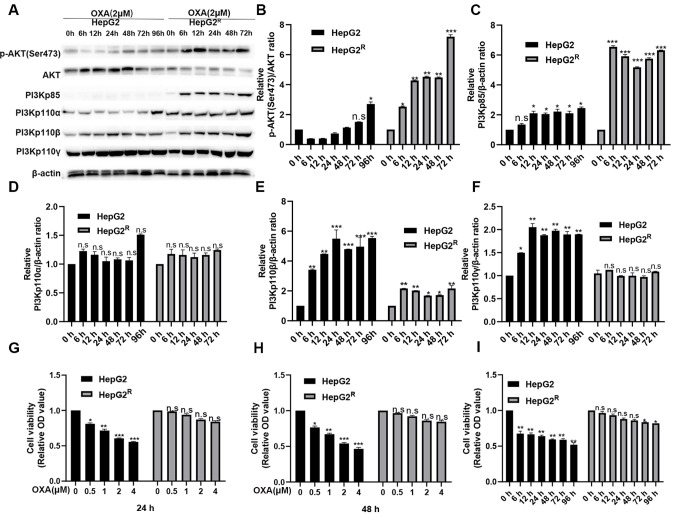
HepG2 and HepG2R cells were treated with 2 µM oxaliplatin. Protein was extracted after 0, 6, 12, 24, 48, 72 and 96 h and measured using western blotting. p-AKT (Ser473), PI3Kp85, PI3Kp110α, PI3Kp110β were found all strongly expressed in HepG2R but weakly expressed in HepG2 (Fig. 1A-F). However, the expression level of PI3Kp110γ was not different between the HepG2 and HepG2R cells; it is possible that PI3Kp110γ is not as effective as PI3Kp110α and PI3Kp110β in the PI3K/AKT signaling pathway. These results indicated that the PI3K/AKT signaling pathway activation level was higher in HepG2R compared with in HepG2 cells. The HepG2 and HepG2R cells were treated with various concentrations of oxaliplatin for 24 or 48 h, and cell viability of was analyzed using an MTT assay. As shown in Fig. 1G-H, oxaliplatin inhibited the cell viability of HepG2 in a time- and concentration-dependent manner, but the proliferation of HepG2R was almost unaffected. HepG2 and HepG2R cells were treated with 2 µmol/l oxaliplatin for the same time periods, and cell viability was determined. The viability of HepG2 cells was found gradually decreased over time, but the viability of HepG2R did not change significantly (Fig. 1I). Since the abnormal activation of the PI3K/AKT signaling pathway promoted the proliferation of HepG2R, it was hypothesized that the PI3K/AKT pathway inhibitors (LY-294002 and MK-2206) enhanced the sensitivity of liver cancer to oxaliplatin by targeting the PI3K/AKT pathway.
Figure 1.
In liver cancer cells, the PI3K/AKT signaling pathway in HepG2R cell line is abnormally activated compared with that in HepG2 cell line. (A) Cell lysates were collected and the designated protein detected using western blotting. Expression of (B) P13Kp85, (C) p-AKT(Ser473)/AKT, (D) PI3Kp110α, (E) PI3Kp110β and (F) PI3Kp110γ in HepG2 and HepG2R cells incubated with oxaliplatin (2 µmol/l) at various time points. (G) MTT analysis was used to determine the cell viability of HepG2 and HepG2R cells at different concentrations of oxaliplatin at 24 h. (H). MTT analysis was used to determine the cell viability of HepG2 and HepG2R cells at different concentrations of oxaliplatin at 48 h. (I) MTT analysis was used to determine the cell viability of HepG2 and HepG2R cells at 2 µM oxaliplatin at different time points. *P<0.05, **P<0.01 and ***P<0.001 vs. 0 h group. n.s, not significant; OXA, oxaliplatin; p-, phosphorylated; R, resistant.
LY-294002 can more effectively inhibit the PI3K/AKT pathway in the HepG2R compared with HepG2 cell line
To determine the effect of the PI3K site-specific inhibitor (LY-294002) or the AKT site-specific inhibitor (MK-2206) in combination with 2 µmol/l oxaliplatin on the PI3K/AKT pathway in HepG2R, the expression levels of PI3Kp110α, PI3Kp110β, PI3Kp110γ, AKT, p-AKT (Ser473) and PI3Kp85 were measured. It was found that LY-294002 (0.2 µM) and MK-2206 (50 nmol/l) downregulated the activation of the PI3K/AKT signaling pathway. The inhibitory effect for p-AKT(Ser473) was similar, but for PI3Kp110α, PI3Kp110β and PI3Kp110γ, the inhibitory effect of LY-294002 was greater (Fig. 2A-D). MTT assays were also conducted. As shown in Fig. 2E-H, the combination treatment more effectively inhibited HepG2R cell viability compared with single-drug treatment, and LY-294002 combined with oxaliplatin had a stronger inhibitory effect compared with MK-2206 with oxaliplatin. These results demonstrated that LY-294002 targeting the PI3K/AKT pathway can enhance the chemosensitivity of oxaliplatin.
Figure 2.
In the HepG2R cell line, PI3K/AKT pathway inhibitors downregulate the activation level of PI3K/AKT. (A-D) HepG2R cells were incubated with oxaliplatin (2 µmol/l) and/or LY-294002 (0.2 µmol/l) and MK-2206 (50 nmol/l) for 24 h. Cell lysates were collected, and the designated proteins were detected using western blotting. (E-H) Cytotoxicity of oxaliplatin and/or LY-294002 and MK-2206 to HepG2R by MTT analysis. *P<0.05, **P<0.01 and ***P<0.001 vs. single agent group. n.s, not significant; OXA, oxaliplatin; R, resistant; p-, phosphorylated.
LY-294002 combined with oxaliplatin effectively inhibits the proliferation of HepG2R cells
To improve our understanding of the molecular mechanism by which LY-294002 inhibits cell proliferation, HepG2R cells were treated with 2 µmol/l oxaliplatin and/or 0.2 µmol/l LY-294002. As illustrated in Fig. 3A and B, the combination of LY-294002 and oxaliplatin significantly downregulated the phosphorylation levels of the downstream proliferation signaling molecules S6K1 and eIF4EBP1 in the PI3K/AKT signaling pathway. The effect of LY-294002 and oxaliplatin on tumorigenesis was also evaluated in the HepG2R cell line with a colony formation assay. Compared with LY-294002 or oxaliplatin alone, the combination of LY-294002 and oxaliplatin induced the least number of clone formation of HepG2R cells and exerted a stronger inhibitory effect (Fig. 3C and D). These results indicated that LY-294002 inhibited the proliferation of HepG2R by blocking the PI3K/AKT/S6K1/eIF4EBP1 signaling pathway.
Figure 3.
LY-294002 enhances oxaliplatin induced cytotoxicity by blocking the PI3K/mTOR/S6K1/4EBP1 signaling pathway in HepG2R cells. (A and B) Treatment of HepG2R cells with oxaliplatin, LY-294002, or LY-294002 combined with oxaliplatin for 24 h. Direct effector response (S6K1 and eIF4EBP1) downstream of the PI3K/AKT pathway was illustrated. Cell lysates were collected, and the designated proteins were detected using western blotting. (C and D) Representative images of the colony formation assay in HepG2R cells. **P<0.01 and ***P<0.001. OXA, oxaliplatin; p-, phosphorylated; R, resistant; eIF4EBP1, eukaryotic translation initiation factor 4E-binding protein 1.
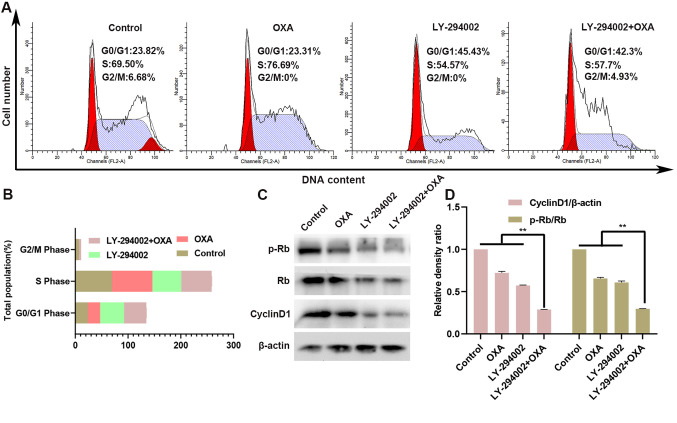
LY-294002 combined with oxaliplatin blocks the cell cycle of HepG2R
The HepG2R cell line was treated with oxaliplatin and/or LY-294002 (0.2 µM) for 24 h, and cell-cycle assays were performed. As shown in Fig. 4A and B, compared with the control group, the number of G0/G1 phase cells was increased in the LY-294002 group, while the number of G2/M phase and S phase cells was decreased. However, there was almost no change in the oxaliplatin-only treated group. Compared with treatment with oxaliplatin alone, treatment of the HepG2R cell line with LY-294002 and oxaliplatin for 24 h further increased the number of cells in G0/G1 phase, while the number of cells in G2/M phase and S phase was decreased. Thus, the combination of LY-294002 and oxaliplatin induced cell-cycle arrest in the G0/G1 phase. The expression levels of the downstream cycle molecules cyclin D1 and p-Rb in the PI3K/AKT signaling pathway were also measured in the HepG2R cell line. As seen in Fig. 4C and D, compared with oxaliplatin treatment alone, the treatment with the combination of LY-294002 and oxaliplatin downregulated the expression of cyclin D1 and the p-Rb/Rb ratio.
Figure 4.
LY-294002 inhibits oxaliplatin-induced S phase arrest and enhances G0/G1 phase cell arrest in HepG2R cells. (A and B) After 24 h of treatment with LY-294002, oxaliplatin, or LY-294002 combined with oxaliplatin, the cell cycle distribution of HepG2R cells was determined using flow cytometry. (C and D) Expression of cyclin D1 and p-Rb in HePG2R cells was determined using western blotting analysis. **P<0.01. OXA, oxaliplatin; p-, phosphorylated; R, resistant; Rb, retinoblastoma.
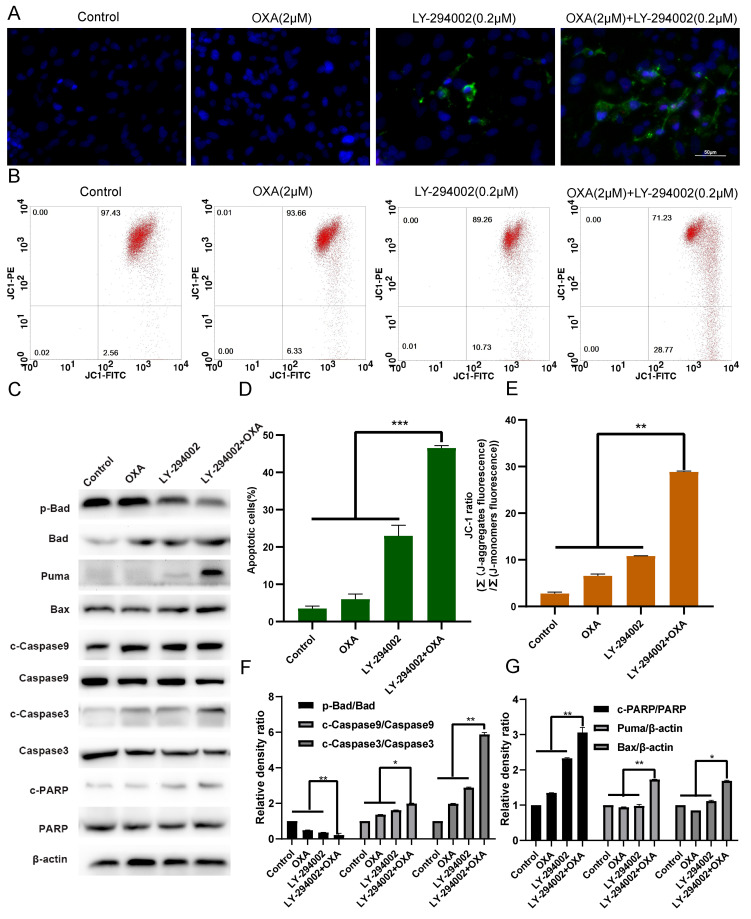
Combination of LY-294002 and oxaliplatin promotes the apoptosis of HepG2R cells
The effect of apoptosis on HepG2R cells was determined with Annexin V FITC/PI staining and DAPI kit after oxaliplatin and/or LY-294002 (0.2 µM) treatment for 24 h. As shown in Fig. 5A and D, compared with the oxaliplatin single-agent group, the LY-294002 and oxaliplatin combination group had a higher apoptosis rate. After treatment of the HepG2R cell line with oxaliplatin and/or LY-294002 for 24 h, the mitochondrial membrane potential was measured using JC-1 staining and flow cytometry. The results presented in Fig. 5B and E illustrate that, compared with the oxaliplatin single-agent group, the red fluorescence ratio of the combination-drug group was lower, indicating that the apoptosis rate of HepG2R cells was higher. At the same time, the results presented in Fig. 5C and F-G illustrate that, compared with the oxaliplatin single-agent group, LY-294002 combined with oxaliplatin inhibited the expression of downstream anti-apoptotic signaling molecules of the PI3K/AKT signaling pathway, such as p-Bad, and increased the expression of pro-apoptotic signaling molecules, such as Bad, Bax, Puma, PARP, cleaved caspase-3 and −9 and promoted apoptosis of the HepG2R cells.
Figure 5.
LY-294002 promotes HepG2R apoptosis induced by oxaliplatin. (A and D) LY-294002, oxaliplatin or LY-294002 combined with oxaliplatin for 24 h. Effect of LY-294002 on the apoptosis of liver cancer cells induced by oxaliplatin was determined with Annexin V FITC/PI staining. Magnification, ×400. (B and E) After 24 h treatment with LY-294002, oxaliplatin, or LY-294002 combined with oxaliplatin, the mitochondrial membrane potential was measured using JC-1 staining and flow cytometry. (C and F, G) After 24 h of treatment with LY-294002, oxaliplatin or LY-294002 combined with oxaliplatin, western blot analysis was used to determine the effect of LY-294002 on oxaliplatin-induced apoptosis. *P<0.05, **P<0.01 and ***P<0.001. OXA, oxaliplatin; p-, phosphorylated; R, resistant; c, cleaved.
LY-294002 inhibits HIF-1α expression through the PI3K/AKT signaling pathway
After HepG2 and HepG2R cells were treated with oxaliplatin for 24 h, the expression level of HIF-1α was increased in the HepG2 cell line, corresponding with the oxaliplatin concentration, as illustrated in Fig. 6A and D-E. In contrast, the expression level of HIF-1α in the HepG2R cell line was not significantly changed with increasing concentrations of oxaliplatin. The results of Fig. 6B and F show that, compared with the single oxaliplatin group, the combination of LY-294002 (0.2 µM) and oxaliplatin downregulated the expression of HIF-1α. The results of Fig. 6C and G show that, compared with the single oxaliplatin group, MK-2206 (50 nmol/l) combined with oxaliplatin also downregulated the expression of HIF-1α.
Figure 6.
LY-294002 downregulates the expression level of HIF-1α through the PI3K/AKT signaling pathway. (A and D, E) HepG2 and HepG2R cells were incubated with various concentrations of oxaliplatin for 24 h. Cell lysates were collected, and the designated proteins were detected using western blotting. (B and F) After 24-h treatment with LY-294002, oxaliplatin or LY-294002 combined with oxaliplatin, western blot analysis was performed to determine the expression level of HIF-1α. (C and G) After 24 h treatment with MK-2206, oxaliplatin, or MK-2206 combined with oxaliplatin, western blot analysis was used to determine that MK-2206 downregulated the expression level of HIF-1α combined with oxaliplatin. *P<0.05, **P<0.01 vs. 0 µM group or as indicated. OXA, oxaliplatin; n.s, not significant; HIF, hypoxia-inducible factor; R, resistant.
Discussion
Systemic chemotherapy is the main treatment of liver cancer in its middle and late stages (30). For the treatment of liver cancer, oxaliplatin has been approved as an effective systemic chemotherapeutic agent (31,32). Emerging resistance of oxaliplatin during chemotherapy; however, has become a problem in the treatment of liver cancer (33,34). The PI3K/AKT pathway is essential in a series of signal transduction cascades that regulate cell survival and apoptosis. Abnormal upregulation of the PI3K/AKT signaling pathway are observed in autoimmune and infectious diseases such as human immunodeficiency virus (HIV) and Covid-19. The PI3K/AKT signaling pathway plays an important role in regulating the progression of HIV and Covid-19 (35–38). In addition, abnormal upregulation of PI3K/AKT signaling pathway usually occurs in cancer, including liver cancer (39). For these reasons, the present study investigated the PI3K/AKT signaling pathway as a potential target for cancer treatment. PI3K/AKT/HIF-1α can reportedly induce chemoresistance of liver cancer cells (26,27). The current experimental results in HepG2R cells indicated abnormal activation of the PI3K/AKT/HIF-1α signaling pathway, which is consistent with previous research results (40). In the present study, the PI3K specific inhibitor LY-294002 was used, which can downregulate the level of PI3K/AKT/HIF1α alienation. LY-294002 can enhance the chemosensitivity of liver cancer (28). Therefore, the present use of LY-294002 in combination with oxaliplatin was well-founded. In the current study, the PI3K/AKT signaling pathway in HepG2R cells was in an abnormally activated state. LY-294002 and MK-2206 downregulated the activation of the pathway, with LY-294002 having a stronger effect compared with that of MK-2206. In addition, compared with MK-2206, LY-294002 more significantly inhibited the expression level of HIF-1α, which is highly expressed in HepG2R cells, reduced cell viability, promoted apoptosis, blocked the cell cycle and increased the sensitivity of the cells to oxaliplatin.
In various liver cancer cell lines, oxaliplatin activates the PI3K/AKT signaling pathway, which participates in the most important chemotherapy resistance mechanism by inhibiting apoptosis and promoting cell survival (41–43). We previously reported that oxaliplatin induces abnormal activation of the PI3K/AKT pathway to reduce the sensitivity of liver cancer cells to oxaliplatin, which is evidence of the importance of the PI3K/AKT pathway in anti-chemotherapy (44). The current study reported that the PI3K/AKT signaling pathway was stably activated in HepG2R cells, which may be the main reason for the resistance of liver cancer cells to oxaliplatin. MK-2206 or LY-294002 reduced the expression levels of p-AKT (Ser473) and PI3Kp85 and inhibited the proliferation of HepG2R; LY-294002 was more effective compared with MK-2206 in this activity. It was also demonstrated that inhibiting the abnormal activation of the PI3K/AKT signaling pathway increased the sensitivity of HepG2R to oxaliplatin.
Activation of the PI3K/AKT signaling pathway can promote the proliferation of liver cancer cells by promoting the progression of the cell cycle (45). Activation of the pathway also can activate the phosphorylation of the downstream effector molecules S6K1 and elF4EBP1, which is conducive to protein synthesis and proliferation of liver cancer cells (46). On the other hand, activation of the PI3K/AKT signaling pathway inhibits apoptosis by phosphorylation and inactivation of several targets (including Bad, c-Raf and caspase-9), leading to prolonged cell survival (47). Compared with LY-294002 or oxaliplatin alone, LY-294002 combined with oxaliplatin more effectively inhibited the proliferation and survival of HepG2R. In addition, the abnormal activation of the PI3K/AKT signaling pathway participates in cell cycle regulation by preventing GSK-3β-mediated phosphorylation and cyclin D1 and p-Rb degradation, and negatively regulates the expression levels of cyclin-dependent kinase inhibitors p27 and p21 (48). The present data revealed that the combination of LY-294002 and oxaliplatin can downregulate cyclin D1 and p-Rb and arrest liver cancer cells in the G0/G1 phase. This finding supports the view that LY-294002 enhances the ability of oxaliplatin to inhibit liver cancer proliferation.
Caspases are a family of cysteine proteases that are the central regulator of apoptosis. Promoter caspases (including caspase-2, −8, −9, −10, −11 and −12) are tightly integrated with pro-apoptotic signals. Once activated, these caspases cleave and activate downstream effector caspases (including caspase-3, −6, and −7), which in turn cleave cell proteins at specific Asp residues, thereby performing apoptosis. Members of the Bcl-2 family of proteins can protect the integrity of mitochondria by preventing damaged mitochondria from releasing cytochrome-c and the subsequent combination with caspase-9 to form a complex, resulting in the activation of caspase-9 (49,50). Abnormal activation of the PI3K/AKT signaling pathway can inhibit the activation of caspase-9 and activate pro-apoptotic Bcl-2 family members (including Bad, Bax, GSK-3 and FoxO1), resulting in anti-apoptosis (51,52). The current data showed that LY-294002 downregulated the activation of PI3K/AKT signaling pathway, upregulated the expression levels of Bad, Puma, Bax, cleaved caspase-9 and −3 and c-PARP, promoted apoptosis and increased the chemical sensitivity of liver cancer to oxaliplatin. Therefore, oxaliplatin will activate the PI3K/AKT signaling pathway when treating live cancer. HepG2R cells resistance to oxaliplatin will cause abnormal activation of the PI3K/AKT signaling pathway. LY-294002 downregulated the abnormal activation level of PI3K/AKT signaling pathway, and improved the chemical sensitivity of HepG2R to oxaliplatin. It was suspected that the combination with oxaliplatin is more effective compared with single drug treatment. The effects at the cell level have also confirmed the reliability of the conjecture, such as reducing cell viability, promoting apoptosis and blocking cell cycle progression. LY-294002 enhances the toxicity of oxaliplatin to HepG2R and plays a synergistic effect when combined with oxaliplatin.
HIF-1α expression is upregulated in liver tumors. HIF-1α can activate the downstream target gene VEGF, induce tumor cells to generate blood vessels, bring oxygen and nutrients to tumor cell and promote tumor cell proliferation (53). HIF-1α is highly expressed in colorectal cancer tissues in the early stage of colorectal cancer and is positively correlated with the progression of the disease (54). In liver cancer, HIF-1α can upregulate the expression of VEGF, cyclin D1, TGF-β, insulin-like growth factor 2 and other growth factors, promote the proliferation and differentiation of liver cancer cells and promote hyperproliferation (55). HIF-1α also initiates transcription of downstream target genes, which increases cell proliferation and decreases apoptosis (56). Since PI3K/AKT can regulate the expression of HIF-1α (11), the present study used PI3K/AKT signaling pathway inhibitors to evaluate the effect on the expression level of HIF-1α. HIF-1α was highly expressed in HepG2R cells, while MK-2206 and LY-294002 reduced the expression level of HIF-1α protein, with LY-294002 having the stronger effect. However, the current study did not explore the mutual regulation between PI3K/AKT and HIF-1α, which will be studied in the future. The downstream mTOR molecule of PI3K/AKT is upregulated in a variety of cancer types, including liver cancer. mTOR molecule is phosphorylated at Ser2448 and auto-phosphorylated at Ser2481 through the PI3K/AKT signaling pathway. The mTOR molecule plays an important role in cell proliferation and homeostasis and may be abnormally regulated in tumors (57). Besides, mTOR inhibitors have been approved for the treatment of certain types of cancer including gastrointestinal stromal tumors and breast cancer (58,59). However, because of time constraints, this was not investigated in the present study; it will be discussed in depth in our future work. In addition, lack of in vivo experimental data due to time restrictions and other factors mean that the reliability of the results have not been verified at the animal level. This is the limitation of this article and will be supplemented in the future.
In summary, the PI3K/AKT signaling pathway inhibitor LY-294002 increased the chemical sensitivity of liver cancer to oxaliplatin by blocking cell cycle progression, reducing cell viability and promoting apoptosis. These effects likely were achieved by inhibiting the abnormal activation of the PI3K/AKT signaling pathway in the cancer cells, thus reducing the expression of HIF-1α. These findings suggested that the combination of LY-294002 and oxaliplatin can be an effective treatment strategy for liver cancer.
Acknowledgements
Not applicable.
Funding Statement
The study was funded by The National Natural Science Fund of China (grant nos. 82071862, 81872017 and 81572431), The University Natural Science Research Project of Anhui Province (grant nos. KJ2018ZD011 and KJ2019A0093), The Anhui Provincial Science and Technology program (grant nos. 202004j07020053 and 1604a0802094), Research Foundation of the Institute of Environment-friendly Materials and Occupational Health (Wuhu), Anhui University of Science and Technology (grant no. ALW2020YF11) and The Huainan Science and Technology Project (grant no. 2017B41) funded this research.
Funding
The study was funded by The National Natural Science Fund of China (grant nos. 82071862, 81872017 and 81572431), The University Natural Science Research Project of Anhui Province (grant nos. KJ2018ZD011 and KJ2019A0093), The Anhui Provincial Science and Technology program (grant nos. 202004j07020053 and 1604a0802094), Research Foundation of the Institute of Environment-friendly Materials and Occupational Health (Wuhu), Anhui University of Science and Technology (grant no. ALW2020YF11) and The Huainan Science and Technology Project (grant no. 2017B41) funded this research.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Authors' contributions
RYX performed the experiments, analyzed the data and was the main contributor to writing the manuscript. YCZ analyzed the data. AML was responsible for the English revision work and performed some of the experiments. YFM, WPC, LS, YHX, SPZ and WYC performed the experiments. XLT was the project leader and was responsible for the design of the project, the revision of the manuscript and performed some of the experiments. RYX and XLT confirm the authenticity of all the raw data. All authors read and approved the final manuscript.
Ethics approval and consent to participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Feng RM, Zong YN, Cao SM, Xu RH. Current cancer situation in China: Good or bad news from the 2018 Global cancer statistics? Cancer Commun (Lond) 2019;39:22. doi: 10.1186/s40880-019-0368-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Miller KD, Goding Sauer A, Ortiz AP, Fedewa SA, Pinheiro PS, Tortolero-Luna G, Martinez-Tyson D, Jemal A, Siegel RL. Cancer statistics for Hispanics/Latinos, 2018. CA Cancer J Clin. 2018;68:425–445. doi: 10.3322/caac.21494. [DOI] [PubMed] [Google Scholar]
- 3.Li A, Zhang R, Zhang Y, Liu X, Wang R, Liu J, Liu X, Xie Y, Cao W, Xu R, et al. BEZ235 increases sorafenib inhibition of hepatocellular carcinoma cells by suppressing the PI3K/AKT/mTOR pathway. Am J Transl Res. 2019;11:5573–5585. [PMC free article] [PubMed] [Google Scholar]
- 4.Smith DK, Murphy BA. Lower levels of education and household income mediate lower dental care utilization among survivors of early life cancers. Prev Med Rep. 2019;14:100868. doi: 10.1016/j.pmedr.2019.100868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Liu X, Xie C, Li A, Zhang Y, Liu X, Zhou S, Shen J, Huo Z, Cao W, Ma Y, et al. BEZ235 enhances chemosensitivity of paclitaxel in hepatocellular carcinoma through inhibiting the PI3K/Akt/mTOR pathway. Am J Transl Res. 2019;11:7255–7271. [PMC free article] [PubMed] [Google Scholar]
- 6.Yang Y, Yao JH, Du QY, Zhou YC, Yao TJ, Wu Q, Liu J, Ou YR. Connexin 32 downregulation is critical for chemoresistance in oxaliplatin-resistant HCC cells associated with EMT. Cancer Manag Res. 2019;11:5133–5146. doi: 10.2147/CMAR.S203656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ye JZ, Yan SM, Yuan CL, Wu HN, Zhang JY, Liu ZH, Li YQ, Luo XL, Lin Y, Liang R. GP73 level determines chemotherapeutic resistance in human hepatocellular carcinoma cells. J Cancer. 2018;9:415–423. doi: 10.7150/jca.19185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhu YJ, Zheng B, Wang HY, Chen L. New knowledge of the mechanisms of sorafenib resistance in liver cancer. Acta Pharmacol Sin. 2017;38:614–622. doi: 10.1038/aps.2017.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhang Y, Liu X, Zhang J, Xu Y, Shao J, Hu Y, Shu P, Cheng H. Inhibition of miR-19a partially reversed the resistance of colorectal cancer to oxaliplatin via PTEN/PI3K/AKT pathway. Aging (Albany NY) 2020;12:5640–5650. doi: 10.18632/aging.102929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kang HG, Wang BZ, Zhang J, Liu MR, Li YX. Combination of temsirolimus and Adriamycin exhibits an enhanced antitumor effect in hepatocellular carcinoma. Clin Res Hepatol Gastroenterol. 2017;41:197–203. doi: 10.1016/j.clinre.2016.09.005. [DOI] [PubMed] [Google Scholar]
- 11.Sun X, Su Y, He Y, Zhang J, Liu W, Zhang H, Hou Z, Liu J, Li J. New strategy for in vitro activation of primordial follicles with mTOR and PI3K stimulators. Cell Cycle. 2015;14:721–731. doi: 10.1080/15384101.2014.995496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fruman DA, Chiu H, Hopkins BD, Bagrodia S, Cantley LC, Abraham RT. The PI3K pathway in human disease. Cell. 2017;170:605–635. doi: 10.1016/j.cell.2017.07.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Aoki M, Fujishita T. Oncogenic roles of the PI3K/AKT/mTOR axis. Curr Top Microbiol Immunol. 2017;407:153–189. doi: 10.1007/82_2017_6. [DOI] [PubMed] [Google Scholar]
- 14.Grosbois J, Demeestere I. Dynamics of PI3K and Hippo signaling pathways during in vitro human follicle activation. Hum Reprod. 2018;33:1705–1714. doi: 10.1093/humrep/dey250. [DOI] [PubMed] [Google Scholar]
- 15.Chang L, Graham PH, Ni J, Hao J, Bucci J, Cozzi PJ, Li Y. Targeting PI3K/Akt/mTOR signaling pathway in the treatment of prostate cancer radioresistance. Crit Rev Oncol Hematol. 2015;96:507–517. doi: 10.1016/j.critrevonc.2015.07.005. [DOI] [PubMed] [Google Scholar]
- 16.Brown JS, Banerji U. Maximising the potential of AKT inhibitors as anti-cancer treatments. Pharmacol Ther. 2017;172:101–115. doi: 10.1016/j.pharmthera.2016.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mehal W. NASH and HCC are driven by different signaling pathways with a common regulator. Cell Metab. 2019;29:3–4. doi: 10.1016/j.cmet.2018.12.012. [DOI] [PubMed] [Google Scholar]
- 18.Lee JH, Lee HJ, Sim DY, Jung JH, Kim KR, Kim SH. Apoptotic effect of lambertianic acid through AMPK/FOXM1 signaling in MDA-MB231 breast cancer cells. Phytother Res. 2018;32:1755–1763. doi: 10.1002/ptr.6105. [DOI] [PubMed] [Google Scholar]
- 19.Laughner E, Taghavi P, Chiles K, Mahon PC, Semenza GL. HER2 (neu) signaling increases the rate of hypoxia-inducible factor 1alpha (HIF-1alpha) synthesis: Novel mechanism for HIF-1-mediated vascular endothelial growth factor expression. Mol Cell Biol. 2001;21:3995–4004. doi: 10.1128/MCB.21.12.3995-4004.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Qu D, Weygant N, Yao J, Chandrakesan P, Berry WL, May R, Pitts K, Husain S, Lightfoot S, Li M, et al. Overexpression of DCLK1-AL increases tumor cell invasion, drug resistance, and KRAS activation and can be targeted to inhibit tumorigenesis in pancreatic cancer. J Oncol. 2019;2019:6402925. doi: 10.1155/2019/6402925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Simioni C, Martelli AM, Cani A, Cetin-Atalay R, McCubrey JA, Capitani S, Neri LM. The AKT inhibitor MK-2206 is cytotoxic in hepatocarcinoma cells displaying hyperphosphorylated AKT-1 and synergizes with conventional chemotherapy. Oncotarget. 2013;4:1496–1506. doi: 10.18632/oncotarget.1236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yu X, Liu J, Qiu H, Hao H, Zhu J, Peng S. Combined inhibition of ACK1 and AKT shows potential toward targeted therapy against KRAS-mutant non-small-cell lung cancer. Bosn J Basic Med Sci. 2021;21:198–207. doi: 10.17305/bjbms.2020.4746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li YL, Weng HC, Hsu JL, Lin SW, Guh JH, Hsu LC. The combination of MK-2206 and WZB117 exerts a synergistic cytotoxic effect against breast cancer cells. Front Pharmacol. 2019;10:1311. doi: 10.3389/fphar.2019.01311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhu Y, Zhong Y, Long X, Zhu Z, Zhou Y, Ye H, Zeng X, Zheng X. Deoxyshikonin isolated from Arnebia euchroma inhibits colorectal cancer by down-regulating the PI3K/Akt/mTOR pathway. Pharm Biol. 2019;57:412–423. doi: 10.1080/13880209.2019.1626447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang X, Wang X, Xu Y, Yan M, Li W, Chen J, Chen T. Effect of nicastrin on hepatocellular carcinoma proliferation and apoptosis through PI3K/AKT signalling pathway modulation. Cancer Cell Int. 2020;20:91. doi: 10.1186/s12935-020-01172-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jiao M, Nan KJ. Activation of PI3 kinase/Akt/HIF-1α pathway contributes to hypoxia-induced epithelial-mesenchymal transition and chemoresistance in hepatocellular carcinoma. Int J Oncol. 2012;40:461–468. doi: 10.3892/ijo.2011.1197. [DOI] [PubMed] [Google Scholar]
- 27.Ling S, Li J, Shan Q, Dai H, Lu D, Wen X, Song P, Xie H, Zhou L, Liu J, et al. USP22 mediates the multidrug resistance of hepatocellular carcinoma via the SIRT1/AKT/MRP1 signaling pathway. Mol Oncol. 2017;11:682–695. doi: 10.1002/1878-0261.12067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ma J, Xie SL, Geng YJ, Jin S, Wang GY, Lv GY. In vitro regulation of hepatocellular carcinoma cell viability, apoptosis, invasion, and AEG-1 expression by LY294002. Clin Res Hepatol Gastroenterol. 2014;38:73–80. doi: 10.1016/j.clinre.2013.06.012. [DOI] [PubMed] [Google Scholar]
- 29.Elefantova K, Lakatos B, Kubickova J, Sulova Z, Breier A. Detection of the mitochondrial membrane potential by the cationic Dye JC-1 in L1210 cells with massive overexpression of the plasma membrane ABCB1 drug transporter. Int J Mol Sci. 2018;19:1985. doi: 10.3390/ijms19071985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tang X, Li A, Xie C, Zhang Y, Liu X, Xie Y, Wu B, Zhou S, Huang X, Ma Y, et al. The PI3K/mTOR dual inhibitor BEZ235 nanoparticles improve radiosensitization of hepatoma cells through apoptosis and regulation DNA repair pathway. Nanoscale Res Lett. 2020;15:63. doi: 10.1186/s11671-020-3289-z. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 31.Devanabanda B, Kasi A. Treasure Island (FL): StatPearls Publishing Copyright© 2020, StatPearls Publishing LLC; 2020. Oxaliplatin. StatPearls. [Google Scholar]
- 32.Tang X, Chen L, Li A, Cai S, Zhang Y, Liu X, Jiang Z, Liu X, Liang Y, Ma D. Anti-GPC3 antibody-modified sorafenib-loaded nanoparticles significantly inhibited HepG2 hepatocellular carcinoma. Drug Deliv. 2018;25:1484–1494. doi: 10.1080/10717544.2018.1477859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shen JH, Chen PH, Liu HD, Huang DA, Li MM, Guo K. HSF1/AMPKα2 mediated alteration of metabolic phenotypes confers increased oxaliplatin resistance in HCC cells. Am J Cancer Res. 2019;9:2349–2363. [PMC free article] [PubMed] [Google Scholar]
- 34.Liao X, Song G, Xu Z, Bu Y, Chang F, Jia F, Xiao X, Ren X, Zhang M, Jia Q. Oxaliplatin resistance is enhanced by saracatinib via upregulation Wnt-ABCG1 signaling in hepatocellular carcinoma. BMC Cancer. 2020;20:31. doi: 10.1186/s12885-019-6480-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.He J, Ma J, Ren B, Liu A. Advances in systemic lupus erythematosus pathogenesis via mTOR signaling pathway. Semin Arthritis Rheum. 2020;50:314–320. doi: 10.1016/j.semarthrit.2019.09.022. [DOI] [PubMed] [Google Scholar]
- 36.Mammana S, Bramanti P, Mazzon E, Cavalli E, Basile MS, Fagone P, Petralia MC, McCubrey JA, Nicoletti F, Mangano K. Preclinical evaluation of the PI3K/Akt/mTOR pathway in animal models of multiple sclerosis. Oncotarget. 2018;9:8263–8277. doi: 10.18632/oncotarget.23862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nicoletti F, Fagone P, Meroni P, McCubrey J, Bendtzen K. mTOR as a multifunctional therapeutic target in HIV infection. Drug Discov Today. 2011;16:715–721. doi: 10.1016/j.drudis.2011.05.008. [DOI] [PubMed] [Google Scholar]
- 38.Fagone P, Ciurleo R, Lombardo SD, Iacobello C, Palermo CI, Shoenfeld Y, Bendtzen K, Bramanti P, Nicoletti F. Transcriptional landscape of SARS-CoV-2 infection dismantles pathogenic pathways activated by the virus, proposes unique sex-specific differences and predicts tailored therapeutic strategies. Autoimmun Rev. 2020;19:102571. doi: 10.1016/j.autrev.2020.102571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gong C, Ai J, Fan Y, Gao J, Liu W, Feng Q, Liao W, Wu L. NCAPG promotes the proliferation of hepatocellular carcinoma through PI3K/AKT signaling. Onco Targets Ther. 2019;12:8537–8552. doi: 10.2147/OTT.S217916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Xie Y, Zhong DW. AEG-1 is associated with hypoxia-induced hepatocellular carcinoma chemoresistance via regulating PI3K/AKT/HIF-1alpha/MDR-1 pathway. EXCLI J. 2016;15:745–757. doi: 10.17179/excli2016-694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Li XZ, Sun YL, Cao LQ, Li MJ. Oxaliplatin-rapamycin combination was superior to mono-drug in treatment of hepatocellular carcinoma both in vitro and in vivo. Neoplasma. 2016;63:880–887. doi: 10.4149/neo_2016_607. [DOI] [PubMed] [Google Scholar]
- 42.Liao B, Zhang Y, Sun Q, Jiang P. Vorinostat enhances the anticancer effect of oxaliplatin on hepatocellular carcinoma cells. Cancer Med. 2018;7:196–207. doi: 10.1002/cam4.1278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Fu X, Wen H, Jing L, Yang Y, Wang W, Liang X, Nan K, Yao Y, Tian T. MicroRNA-155-5p promotes hepatocellular carcinoma progression by suppressing PTEN through the PI3K/Akt pathway. Cancer Sci. 2017;108:620–631. doi: 10.1111/cas.13177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhang Y, Xie C, Li A, Liu X, Xing Y, Shen J, Huo Z, Zhou S, Liu X, Xie Y, et al. PKI-587 enhances chemosensitivity of oxaliplatin in hepatocellular carcinoma through suppressing DNA damage repair pathway (NHEJ and HR) and PI3K/AKT/mTOR pathway. Am J Transl Res. 2019;11:5134–5149. [PMC free article] [PubMed] [Google Scholar]
- 45.Wang Y, Nie H, Zhao X, Qin Y, Gong X. Bicyclol induces cell cycle arrest and autophagy in HepG2 human hepatocellular carcinoma cells through the PI3K/AKT and Ras/Raf/MEK/ERK pathways. BMC Cancer. 2016;16:742. doi: 10.1186/s12885-016-2767-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Li TT, Zhu D, Mou T, Guo Z, Pu JL, Chen QS, Wei XF, Wu ZJ. IL-37 induces autophagy in hepatocellular carcinoma cells by inhibiting the PI3K/AKT/mTOR pathway. Mol Immunol. 2017;87:132–140. doi: 10.1016/j.molimm.2017.04.010. [DOI] [PubMed] [Google Scholar]
- 47.Zhu F, Jiang D, Zhang M, Zhao B. 2,4-Dihydroxy- 3′-methoxy-4′-ethoxychalcone suppresses cell proliferation and induces apoptosis of multiple myeloma via the PI3K/akt/mTOR signaling pathway. Pharm Biol. 2019;57:641–648. doi: 10.1080/13880209.2019.1662814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Fang X, Yang D, Luo H, Wu S, Dong W, Xiao J, Yuan S, Ni A, Zhang KJ, Liu XY, Chu L. SNORD126 promotes HCC and CRC cell growth by activating the PI3K-AKT pathway through FGFR2. J Mol Cell Biol. 2017;9:243–255. doi: 10.1093/jmcb/mjw048. [DOI] [PubMed] [Google Scholar]
- 49.Zhou M, Zhang Q, Zhao J, Liao M, Wen S, Yang M. Phosphorylation of Bcl-2 plays an important role in glycochenodeoxycholate-induced survival and chemoresistance in HCC. Oncol Rep. 2017;38:1742–1750. doi: 10.3892/or.2017.5830. [DOI] [PubMed] [Google Scholar]
- 50.Pan W, Li W, Zhao J, Huang Z, Zhao J, Chen S, Wang C, Xue Y, Huang F, Fang Q, et al. lncRNA-PDPK2P promotes hepatocellular carcinoma progression through the PDK1/AKT/Caspase 3 pathway. Mol Oncol. 2019;13:2246–2258. doi: 10.1002/1878-0261.12553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Jiang S, Wang Q, Feng M, Li J, Guan Z, An D, Dong M, Peng Y, Kuerban K, Ye L. C2-ceramide enhances sorafenib-induced caspase-dependent apoptosis via PI3K/AKT/mTOR and Erk signaling pathways in HCC cells. Appl Microbiol Biotechnol. 2017;101:1535–1546. doi: 10.1007/s00253-016-7930-9. [DOI] [PubMed] [Google Scholar]
- 52.Sui Y, Zheng X, Zhao D. Rab31 promoted hepatocellular carcinoma (HCC) progression via inhibition of cell apoptosis induced by PI3K/AKT/Bcl-2/BAX pathway. Tumour Biol. 2015;36:8661–8670. doi: 10.1007/s13277-015-3626-5. [DOI] [PubMed] [Google Scholar]
- 53.Mendez-Blanco C, Fondevila F, Garcia-Palomo A, Gonzalez-Gallego J, Mauriz JL. Sorafenib resistance in hepatocarcinoma: Role of hypoxia-inducible factors. Exp Mol Med. 2018;50:1–9. doi: 10.1038/s12276-018-0159-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Gong J, Zhou S, Yang S. Vanillic Acid Suppresses HIF-1α expression via inhibition of mTOR/p70S6K/4E-BP1 and Raf/MEK/ERK pathways in human colon cancer HCT116 cells. Int J Mol Sci. 2019;20:465. doi: 10.3390/ijms20030465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Xu LF, Ni JY, Sun HL, Chen YT, Wu YD. Effects of hypoxia-inducible factor-1α silencing on the proliferation of CBRH-7919 hepatoma cells. World J Gastroenterol. 2013;19:1749–1759. doi: 10.3748/wjg.v19.i11.1749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Chen J, Bai M, Ning C, Xie B, Zhang J, Liao H, Xiong J, Tao X, Yan D, Xi X, et al. Gankyrin facilitates follicle-stimulating hormone-driven ovarian cancer cell proliferation through the PI3K/AKT/HIF-1α/cyclin D1 pathway. Oncogene. 2016;35:2506–2517. doi: 10.1038/onc.2015.316. [DOI] [PubMed] [Google Scholar]
- 57.Ferrin G, Guerrero M, Amado V, Rodriguez-Peralvarez M, De la Mata M. Activation of mTOR signaling pathway in hepatocellular carcinoma. Int J Mol Sci. 2020;21:1266. doi: 10.3390/ijms21041266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Duan Y, Haybaeck J, Yang Z. Therapeutic potential of PI3K/AKT/mTOR pathway in gastrointestinal stromal tumors: Rationale and progress. Cancers (Basel) 2020;12:2972. doi: 10.3390/cancers12102972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Steelman LS, Martelli AM, Cocco L, Libra M, Nicoletti F, Abrams SL, McCubrey JA. The therapeutic potential of mTOR inhibitors in breast cancer. Br J Clin Pharmacol. 2016;82:1189–1212. doi: 10.1111/bcp.12958. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.