Abstract
Background
Atrial fibrillation (AF) is frequently reported as a complication of noncardiac surgery. It is unknown whether new-onset perioperative AF is associated with an increased risk of stroke and death beyond the perioperative period. We performed a systematic review and meta-analysis to assess the long-term risks of stroke and mortality associated with new-onset perioperative AF after noncardiac surgery.
Methods
MEDLINE and EMBASE were searched from inception to March 2020 for studies reporting on the association between perioperative AF and the risk of stroke and death occurring beyond 30 days after noncardiac surgery. Reference screening, study selection, data extraction, and quality assessment were performed in duplicate. Data were pooled using inverse variance-weighted random-effects models and presented as risk ratios (RRs).
Results
From 7344 citations, we included 31 studies (3,529,493 patients). The weighted mean incidence of perioperative AF was 0.7%. During a mean follow-up of 28.1 ± 9.4 months, perioperative AF was associated with an increased risk of stroke (1.5 vs 0.9 strokes per 100 patient-years; RR: 2.9, 95% confidence interval [CI]: 2.1-3.9, I2 = 78%). Perioperative AF was also associated with a significantly higher risk of all-cause mortality (21.0 vs 7.6 deaths per 100 patient-years; RR: 1.8, 95% CI: 1.5-2.2, I2 = 94%). The pooled adjusted hazard ratios for stroke and all-cause mortality were 1.9 (95% CI: 1.6-2.2, I2 = 31%) and 1.5 (95% CI: 1.3-1.7, I2 = 20%), respectively.
Conclusions
Patients who had perioperative AF after noncardiac surgery had a higher long-term risk of stroke and mortality compared with patients who did not. Whether this risk is modifiable with oral anticoagulation therapy should be investigated.
Résumé
Contexte
La fibrillation atriale (FA) est une arythmie fréquemment attribuée à une complication d’une chirurgie non cardiaque. On ne sait toutefois pas si l’apparition d’une FA périopératoire est associée à un risque accru d’accident vasculaire cérébral et de décès au-delà de la période périopératoire. Nous avons donc procédé à un examen et à une méta-analyse systématiques dans le but d’évaluer les risques à long terme d’accident vasculaire cérébral et de décès associés à l’apparition d’une FA périopératoire à la suite d’une chirurgie non cardiaque.
Méthodologie
Des recherches ont été effectuées dans MEDLINE et EMBASE depuis leur création jusqu’à mars 2020 pour y relever les études signalant l’association entre la FA périopératoire et le risque d’accident vasculaire cérébral et de décès survenant au-delà de 30 jours à la suite d’une chirurgie non cardiaque. Le tri des références, la sélection des études, l’extraction des données et l’évaluation de la qualité ont été effectués en double. Les données ont été regroupées à l’aide de modèles à effets aléatoires pondérés par l’inverse de la variance et présentées sous forme de rapports de risques relatifs (RR).
Résultats
Parmi 7 344 références, nous avons inclus 31 études (3 529 493 patients). L’incidence moyenne pondérée de FA périopératoire était de 0,7 %. Dans le cadre d’un suivi moyen de 28,1 ± 9,4 mois, la FA périopératoire était associée à un risque accru d’accident vasculaire cérébral (1,5 vs 0,9 accident vasculaire cérébral par 100 années-patients; RR de 2,9; intervalle de confiance [IC] à 95 %; de 2,1 à 3,9; I2 = 78 %). La FA périopératoire était également associée à un risque considérablement plus élevé de décès toutes causes confondues (21,0 vs 7,6 décès par 100 années-patients; RR de 1,8; IC à 95 %; de 1,5 à 2,2; I2 = 94 %). Les rapports de risques instantanés ajustés regroupés d’accident vasculaire cérébral et de décès toutes causes confondues étaient de 1,9 (IC à 95 %; de 1,6 à 2,2; I2 = 31 %) et de 1,5 (IC à 95 %; de 1,3 à 1,7; I2 = 20 %), respectivement.
Conclusions
Les patients qui souffraient de FA périopératoire à la suite d’une chirurgie non cardiaque présentaient un risque accru à long terme d’accident vasculaire cérébral et de décès par rapport aux patients qui n’en souffraient pas. Il serait approprié d’examiner la possibilité de modifier ce risque par une anticoagulothérapie orale.
Atrial fibrillation (AF) is the most common cardiac arrhythmia and an independent risk factor for stroke and death.1, 2, 3, 4 Oral anticoagulation (OAC) can reduce the risk of stroke by 64% and the risk of mortality by 26%.5 In some cases, AF is newly detected during a hospitalization for surgery. It is unclear whether AF encountered in this setting is associated with an increased long-term risk of stroke. Thus, uncertainty exists as to whether perioperative AF associated with noncardiac surgery poses a long-term risk of stroke and whether this risk can be reduced by long-term OAC therapy.6,7 The paucity of recommendations regarding the management of perioperative AF after noncardiac surgery in clinical practice guidelines reflects the lack of definitive data in this area.8,9
Estimates of the incidence of postoperative AF in patients undergoing noncardiac surgery have ranged from 0.4% to 35%, depending on the type of surgery, patient population, and the method of detection.10, 11, 12, 13, 14, 15, 16, 17, 18, 19, 20 Perioperative AF has been associated with longer hospital stays, increased in-hospital mortality, and a short-term increase in the risk of stroke.12,20,21 However, the long-term implications of this arrhythmia are less well defined. Recent studies have shown that a large proportion of patients with postoperative AF may have recurrent AF episodes detected in the months and years after their index hospitalization,14,22, 23, 24 and an ongoing prospective study is addressing rates of AF recurrence.25 If perioperative AF is the first episode or first presentation of paroxysmal AF, then these patients may have an increased long-term risk of stroke and death. No systematic evaluation of the data of the long-term risks of stroke and death in patients with new-onset perioperative AF after noncardiac surgery is available.
Our objective was to perform a systematic review and meta-analysis to estimate the absolute and relative long-term risks of stroke and death for patients in whom perioperative AF was detected after noncardiac surgery in comparison with those without perioperative AF.
Methods
The protocol was preregistered with PROSPERO (CRD42017054309). Differences between the registered and final protocol appear in Supplemental Appendix S1. This study is reported according to the Meta-analysis of Observational Studies in Epidemiology (MOOSE) criteria (Supplemental Appendix S2).26
Search strategy
We conducted electronic searches in MEDLINE and EMBASE from inception to March 2020 (SupplementalAppendices S3 and S4). The search was reviewed by a librarian. We used controlled vocabulary including Medical Subject Heading terms and keywords for perioperative AF, noncardiac surgery, stroke, and mortality. Reference lists of eligible records were screened for additional studies. Abstracts were also included in the meta-analysis.
Eligibility criteria
We included studies that reported on the association between perioperative AF detected after noncardiac surgery and the risk of stroke and/or death, occurring either after the index hospitalization or more than 30 days after the date of surgery. Perioperative AF was defined as AF that occurred during a hospitalization for noncardiac surgery. We excluded studies with ≤10 patients.
Outcomes
The primary outcome was stroke and the secondary outcome was all-cause mortality. We included haemorrhagic, ischemic, and unspecified strokes—as defined by study authors.
Data collection and analysis
Pairs of reviewers independently screened study titles and abstracts for eligibility; if either reviewer thought that the abstract was potentially eligible, it was selected for full-text review. Full papers of potentially eligible studies were retrieved. Pairs of reviewers then independently screened full texts in duplicate and recorded the main reason for exclusion. Disagreements were resolved through discussion or third-party arbitration. The studies were then assessed independently and in duplicate for risk of bias using the CLARITY tool.27
Data extraction and management
Independently, 2 reviewers abstracted data on study outcomes. They also recorded study and patient characteristics including age, sex, cardiovascular comorbidities and risk factors, operation type, and use of OAC. Results were then compared and disagreements were resolved by discussion or a third reviewer if needed. Authors were contacted to clarify ambiguities and to request data on additional data from primary reports. Data in studies published in foreign languages were extracted by reviewers who could understand the language.
Data synthesis
We used Review Manager 5.3 (Cochrane Collaboration) to perform the meta-analysis. We calculated risk ratios (RRs) from the dichotomous data using the inverse variance method. We assessed all data pertaining to the identified outcomes for clinical and statistical heterogeneity. We also assessed statistical heterogeneity using I2.28 We considered an I2 greater than 50% as showing substantial heterogeneity.29 We then analyzed data quantitatively using a random-effects model and presented them as RRs with 95% confidence intervals (CI). We pooled hazard ratios (HRs) using the generic inverse variance method with data from studies that reported adjusted HRs. The data were then analyzed using a random-effects model and presented with 95% CI.
Subgroup analyses
We performed prespecified subgroup analyses, assessing the risks of stroke and death according to type of surgery (ie, based on the major body system under operation). We could not perform prespecified subgroup analyses according to use of OAC therapy and age (<65 vs ≥65 years) as these data were not available.
Assessment of the quality of the evidence
We evaluated the quality of evidence using the Grades of Recommendation, Assessment, Development and Evaluation (GRADE) approach.30 In this framework, observational studies begin as low-quality evidence. GRADE appraises the confidence in estimates of effect by considering within-study risk of bias, directness of the evidence, heterogeneity of the data, precision of effect estimates, and risk of publication bias. We inspected the funnel plots of standard errors vs effect estimates for publication bias and small-study effects.
Results
Search results and study selection
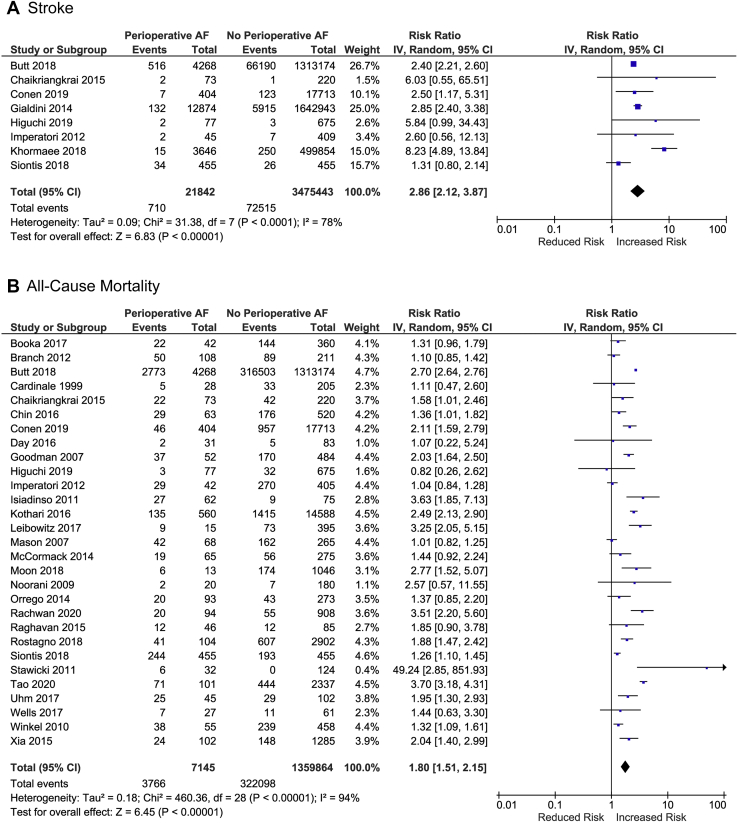
We identified a total of 7356 unique citations, from which 193 full texts were screened (Supplemental Fig. S1). Thirty-one cohort studies met inclusion criteria and were included in the meta-analysis (Supplemental Table S1): 25 retrospective14,18,19,31, 32, 33, 34, 35, 36, 37, 38, 39, 40, 41, 42, 43, 44, 45, 46, 47, 48, 49, 50, 51, 52, 53 and 6 prospective.54, 55, 56, 57, 58 Six cohort studies included all major noncardiac surgeries, whereas the others focused on 1 subtype of surgery: thoracic,15 orthopaedic,4 gastrointestinal,3 and vascular.3 Data on the occurrence of outcomes of interest were available for 3,529,727 patients over a mean follow-up of 28.1 ± 9.4 months. Previously unpublished data were obtained from 3 studies.14,47,50 The weighted mean incidence of perioperative AF was 0.7%; rates in individual studies ranged from 0.3% to 46%. Baseline Congestive Heart Failure, Hypertension, Age, Diabetes, Stroke/Transient Ischemic Attack (CHADS2) scores of included patients were available in 6 studies.14,43,47,48,50,58 Figure 1 summarizes the key findings from the meta-analysis.
Figure 1.
Risk of stroke and all-cause mortality in patients with and without perioperative atrial fibrillation after noncardiac surgery. AF, atrial fibrillation; CI, confidence interval.
Stroke
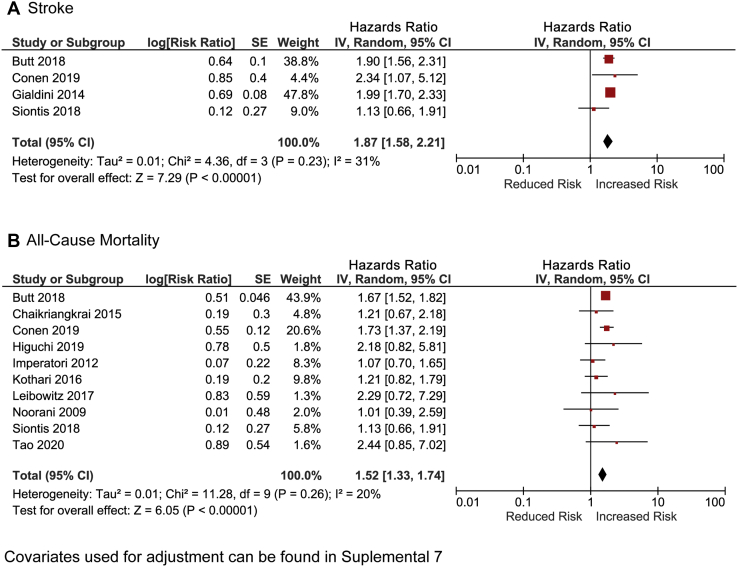
Eight studies, including 3,497,285 patients, reported long-term incidence of stroke (Fig. 1).14,34,47,48,50,52,56,58 Over a mean follow-up of 28.2 ± 9.7 months, more patients in whom perioperative AF was detected experienced a stroke as compared with patients without perioperative AF (1.5 vs 0.9 per 100 patient-years; unadjusted RR: 2.9, 95% CI: 2.1-3.9, I2 = 78%). The absolute risk increase for stroke in patients with perioperative AF was 0.6 per 100 patient-years. Pooled results from the 4 studies including 2,992,286 patients who reported adjusted HRs were adjusted HR: 1.9, 95% CI: 1.6-2.2, I2 = 31% (Fig. 2; Supplemental Table S2).14,34,47,50
Figure 2.
Pooled adjusted hazard ratio for stroke and all-cause mortality in patients with and without perioperative atrial fibrillation after noncardiac surgery. AF, atrial fibrillation; CI, confidence interval.
Data on 48,417 patients who underwent thoracic surgery were available from 4 studies with a mean follow-up of 25.3 ± 1.6 months (Table 1 and Supplemental Fig. S2).14,47,48,56 The absolute risk increase of stroke was 0.1 per 100 patient-years for patients with new onset-AF (0.15 vs 0.03 per 100 patient-years; RR: 1.4, 95% CI: 1.0-1.9, I2 = 0%). Data on 957,317 patients who underwent orthopaedic surgery were available from 2 studies with a mean follow-up of 24.5.1 ± 18.6 months.47,52 The absolute risk increase of stroke was 1.0 per 100 patient-years for patients with perioperative AF (2.4 vs 1.4 per 100 patient-years; RR: 4.1, 95% CI: 1.1-15.4, I2 = 96%).
Table 1.
Summary of meta-analysis findings of perioperative atrial fibrillation vs no perioperative atrial fibrillation after noncardiac surgery
| Outcome | Surgery subtype | Number of studies | Total number of patients | Events Perioperative AF, n (%) |
Events No perioperative AF, n (%) |
Absolute risk (%) | Mean follow-up (mo) ± SD | RR (95% CI) | I2 (%) |
|---|---|---|---|---|---|---|---|---|---|
| Stroke | Any noncardiac | 8 | 3,497,285 | 710 (3.3) | 72,515 (2.1) | 1.2 | 28.2 ± 9.7 | 2.9 (2.1-3.9) | 78 |
| Thoracic surgery | 4 | 48,417 | 45 (3.0) | 996 (2.1) | 0.9 | 25.3 ± 1.6 | 1.4 (1.0-1.9) | 0 | |
| Orthopaedic surgery | 2 | 957,317 | 201 (3.9) | 26,942 (2.8) | 1.1 | 24.5 ± 18.6 | 4.1 (1.1-15.4) | 96 | |
| All-cause mortality | Any noncardiac | 29 | 1,367,009 | 3766 (52.7) | 322,098 (23.7) | 29.0 | 37.6 ± 4.9 | 1.8 (1.5-2.2) | 94 |
| Esophageal surgery | 7 | 253,218 | 843 (59.0) | 59,484 (23.6) | 35.4 | 38.4 ± 1.9 | 1.7 (1.1-2.7) | 89 | |
| Liver transplantation | 3 | 3,448 | 50 (23.9) | 377 (11.6) | 12.3 | 9.6 ± 3.6 | 2.6 (1.9-3.7) | 37 | |
| Orthopaedic surgery | 4 | 459,671 | 1203 (70.7) | 127,717 (27.9) | 42.8 | 38.1 ± 3.4 | 2.8 (2.1-3.5) | 89 | |
| Thoracic surgery | 10 | 20,889 | 536 (47.2) | 7659 (38.8) | 8.4 | 37.7 ± 7.7 | 1.3 (1.1-1.6) | 68 | |
| Vascular surgery | 4 | 46,928 | 429 (43.8) | 17,498 (38.1) | 5.7 | 29.6 ± 14.2 | 1.7 (1.2-2.4) | 93 |
AF, atrial fibrillation; CI, confidence interval; RR, risk ratio; SD, standard deviation.
All-cause death
Twenty-nine studies, including 1,367,009 patients, reported data on mortality beyond 30 days after surgery (Fig. 1).18,19,31, 32, 33, 34, 35, 36, 37, 38, 39, 40, 41, 42, 43, 44, 45, 46, 47, 48, 49, 50, 51,53, 54, 55, 56, 57, 58 The mortality rate over a mean follow-up of 37.6 ± 4.9 months was higher in patients with perioperative AF compared with those without perioperative AF (21.0 vs 7.6 per 100 patient-years; unadjusted RR: 1.8, 95% CI: 1.5-2.2, I2 = 94%). The absolute risk increase for mortality in patients who experienced perioperative AF was 13.4 per 100 patient-years. Pooled results from the 10 studies including 1,356,157 patients who reported adjusted HRs were similar (adjusted HR: 1.5; 95% CI: 1.3-1.7, I2 = 20%) (Fig. 2).19,34,41,45,47,48,50,53,56,58
Data on 253,218 patients who underwent oesophageal surgery were available from 7 studies with a mean follow-up of 38.4 ± 1.9 months (Supplemental Fig. S3).31,35,36,38,40,47,49 The absolute risk increase for mortality was 10.9 per patient 100 patient-years for patients with perioperative AF (18.3 vs 7.4 per 100 patient-years; RR: 1.7, 95% CI: 1.1-2.7, I2 = 89%). Data on 3448 patients who underwent liver transplantation were available from 3 studies with a mean follow-up of 9.6 ± 3.6 months.32,37,43 The absolute risk increase for mortality was 17.1 per 100 patient-years for patients with perioperative AF (31.6 vs 14.5 per 100 patient-years; RR: 2.6, 95% CI: 1.9-3.7, I2 = 37%). Data from 459,671 patients who underwent orthopaedic surgery were available from 4 studies with a mean follow-up of 38.1 ± 3.4 months.44,45,47,53 The absolute risk increase for mortality was 15.5 per 100 patient-years for patients with perioperative AF (24.2 vs 8.8 per 100 patient-years; RR: 2.8, 95% CI: 2.1-3.5, I2 = 89%). Data from 20,889 patients who underwent thoracic surgery were available from 10 studies over a mean follow-up of 37.7 ± 7.7 months.18,33,39,42,46, 47, 48,51,54,56 The absolute risk increase for mortality was 3.8 per 100 patient-years for patients with perioperative AF (16.1 vs 12.3 per 100 patient-years; RR: 1.3, 95% CI: 1.1-1.6, I2 = 68%). Data from 46,928 patients who underwent vascular surgery were available from 4 studies over a mean follow-up of 29.6 ± 14.2 months.19,41,47,57 The absolute risk increase for mortality was 8.5 per 100 patient-years for patients with perioperative AF (23.8 vs 15.3 per 100 patient-years; RR: 1.7, 95% CI: 1.2-2.4, I2 = 93%).
Methodological quality of individual studies
The overall methodological quality (risk of bias) of included studies was moderate (Supplemental Table S3).59 The incidence of perioperative AF was significantly higher in prospective studies compared with retrospective studies (2.9% vs 0.7%, P < 0.001); however, the RR for stroke and all-cause mortality did not differ significantly between study designs (Supplemental Table S4). There were 13 studies that either included pre-existing AF or did not mention whether patients with pre-existing AF were excluded.18,19,31, 32, 33,37, 38, 39,42,46,51,55,56 However, event rates between studies did not differ significantly (Supplemental Table S5). Seven studies reported on treatment with OAC. One study excluded patients on OAC and 2 adjusted for use of OAC in their analyses.31,45,47,50,53,57,58
Quality of evidence
Visual inspection of funnel plots did not lead to concerns about publication bias (Supplemental Fig. S3). For both stroke and all-cause mortality, we judged that the quality of evidence according to the GRADE framework was low (Table 2). We made this judgement because the evidence consisted exclusively of observational studies and we did not detect any other major issues.
Table 2.
Rating quality of evidence using the GRADE framework59
| Outcome | Quality assessment |
Summary of findings |
Quality | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Number of studies (design) | Limitations (risk of bias) | Inconsistency | Indirectness | Imprecision | Publication bias | Number of patients with perioperative AF | Number of patients without perioperative AF | Relative risk (95% CI) | Absolute risk (%) | ||
| Stroke > 30 d or after discharge | 8 (Observational) | No serious limitations | No serious inconsistency | No serious indirectness | No serious imprecision | None detected | 710/21,842 | 72,515/3,475,443 | 2.9 (2.1-3.9) | 1.2 | Low |
| Death > 30 d or after discharge | 29 (Observational) | No serious limitations | No serious inconsistency | No serious indirectness | No serious imprecision | None detected | 3766/7145 | 322,098/1,359,864 | 1.8 (1.5-2.2) | 29.0 | Low |
AF, atrial fibrillation; CI, confidence interval; GRADE: Grades of Recommendation, Assessment, Development and Evaluation.
Discussion
In this systematic review and meta-analysis of 31 studies including 3,529,727 individuals, perioperative AF after noncardiac surgery was associated with significant increases in the long-term risks of stroke and death beyond 30 days after surgery. These observations were consistent across all surgical subtypes. These results support the need for trials to definitely assess the risks and benefits of OAC treatment in this population.
Perioperative AF after noncardiac surgery was consistently associated with a significant increase in the risk of stroke. The recommendations regarding management of AF after noncardiac surgery in clinical practice guidelines reflect uncertainty in this area, but the findings from this meta-analysis contribute to our growing understanding of the risks of postoperative AF.9,60 However, our findings do not necessarily mean that patients with perioperative AF after noncardiac surgery should receive long-term OAC. We found that the absolute risk of stroke in patients with perioperative AF was 0.6 per 100 patient-years over a mean follow-up of 28.2 ± 9.7 months. In studies that reported CHADS2 scores,47,48,50 these scores ranged from 0 to 4; this corresponds to a predicted annual risk of stroke between 2.8% and 4.0%, as shown in previous population-based studies of patients with clinical AF who were not taking OAC.61 Therefore, the incidences of stroke observed in this review are lower than one might expect, even after accounting for mortality and for the potential use of OAC in some patients. Depending on the type of surgery and the presence of other risk factors, this absolute risk of stroke may be too low to justify initiation of OAC. An annual stroke risk of 1.5% is the threshold above which the Canadian Cardiovascular Society recommends initiating OAC.62 In contrast, the American College of Cardiology/American Heart Association recommend to initiate OAC for a Congestive Heart Failure, Hypertension, Age (≥ 75 years), Diabetes, Stroke/Transient Ischemic Attack, Vascular Disease, Age (65-74 years), Sex (Female) (CHA2DS2-VASc) score ≥ 2 (equivalent to a 2.5% annual stroke risk).8 We must note that the lack of information about the use of OAC in the included studies limits our interpretation of stroke risk. As additional consideration, the risk of bleeding may be different in the postoperative population.63, 64, 65
Mortality was significantly higher in patients with perioperative AF after noncardiac surgery as a whole and in all subgroups. This is consistent with previous literature for AF in general and after noncardiac surgery specifically.4,20 The risk of mortality in this population is striking. It highlights the vulnerability of this population; AF occurring after noncardiac surgery may be a marker of frailty. It also suggests that death is an important competing risk that needs to be considered in randomized control trials in this population.
In the end, there is a pressing need to evaluate the risks and benefits of OAC in this population. The randomized Anticoagulation for Stroke Prevention in Patients with Recent Episodes of Perioperative Atrial Fibrillation after Noncardiac Surgery (ASPIRE-AF, NCT03968393) trial is ongoing. Although awaiting the results of this trial, perioperative AF after noncardiac surgery should not be dismissed, and patients who experience this arrythmia likely need close follow-up and shared decision-making on whether to initiate OAC.
Strengths and limitations
This systematic review and meta-analysis is, to the best of our knowledge, the largest and most comprehensive on the topic, looking at study-level data. Although the meta-analysis conducted by Lin et al.7 also included a large population of 2,458,010 patients, it also included those who underwent cardiac surgery. In fact, it had only included 7 papers studying patients who underwent noncardiac surgery.7 In addition, strict inclusion criteria for studies were applied, and the authors of included studies were contacted for missing data. The study protocol was preregistered, and we used validated tools to evaluate the methodological quality of individual studies and our confidence in the body of evidence.
The major limitation of this review is that all included studies were subject to residual confounding, with little adjustment for important covariates in most studies. We also found important methodological differences between studies in this review. Associations presented in this study were mainly estimated based on aggregated study-level rather than patient-level data, which precludes assessing the relationship between many prognostic variables and our outcome of interest. Furthermore, this may have led to ecological bias. In addition, most studies did not report the number of patients who were taking OAC, and although we collected data on stroke and death, we did not collect data on bleeding events. In addition, many studies included patients with pre-existing AF, which may increase the rates of perioperative AF and bias the association between AF and stroke.33,51,55,56
Conclusions
The detection of perioperative AF after noncardiac surgery is significantly associated with increased risk of stroke and all-cause mortality persisting well beyond the immediate follow-up period. The absolute risk of strokes in these patients is relatively low. These findings highlight the need for a large, randomized clinical trial investigating the benefits of OAC therapy on long-term stroke risk in this patient population.
Acknowledgements
We wish to thank Tetsuya Isayama, Morten Hylander, Gera Kisselman, and Alexandra Lengyel for help with extracting data in foreign languages.
Funding Sources
D.C. has a McMaster University Department of Medicine Mid-Career Research Award; his work is supported by the Hamilton Health Sciences RFA Strategic Initiative Program. W.F.M. holds personnel awards from the Canadian Institutes for Health Research (CIHR) and the Canadian Stroke Prevention Intervention Network (C-SPIN).
Disclosures
D.C. and W.F.M. have received speaker fees from Servier Canada. HK serves on an endpoint adjudication committee for a trial of empagliflozin for Boehringer Ingelheim. The other authors have no conflicts of interest to declare.
Footnotes
Ethics Statement: This systematic review and meta-analysis complies with the Declaration of Helsinki. Research Ethics approval was not required because anonymized aggregate data was used to conduct the meta-analysis.
See page 672 for disclosure information.
To access the supplementary material accompanying this article, visit CJC Open at https://www.cjcopen.ca/ and at https://doi.org/10.1016/j.cjco.2020.12.025.
Supplementary Material
References
- 1.Kannel W.B., Wolf P.A., Benjamin E.J., Levy D. Prevalence, incidence, prognosis, and predisposing conditions for atrial fibrillation: population-based estimates. Am J Cardiol. 1998;82:2–9. doi: 10.1016/s0002-9149(98)00583-9. [DOI] [PubMed] [Google Scholar]
- 2.Feinberg W.M., Blackshear J.L., Laupacis A., Kronmal R., Hart R.G. Prevalence, age distribution, and gender of patients with atrial fibrillation. Arch Intern Med. 1995;155:469–473. [PubMed] [Google Scholar]
- 3.Wolf P. a., Abbott R.D., Kannel W.B. Atrial fibrillation: a major contributor to stroke in the elderly. The Framingham Study. Arch Intern Med. 1987;147:1561–1564. [PubMed] [Google Scholar]
- 4.Benjamin E.J., Wolf P.A., D’Agostino R.B., et al. Impact of atrial fibrillation on the risk of death: the Framingham Heart Study. Circulation. 1998;98:946–952. doi: 10.1161/01.cir.98.10.946. [DOI] [PubMed] [Google Scholar]
- 5.Hart R.G., Pearce L.A., Aguilar M.I. Meta-analysis: antithrombotic therapy to prevent stroke in patients who have nonvalvular atrial fibrillation. Ann Intern Med. 2007;146:857–867. doi: 10.7326/0003-4819-146-12-200706190-00007. [DOI] [PubMed] [Google Scholar]
- 6.McIntyre W.F., Connolly S.J., Healey J.S. Atrial fibrillation occurring transiently with stress. Curr Opin Cardiol. 2018;33:58–65. doi: 10.1097/HCO.0000000000000475. [DOI] [PubMed] [Google Scholar]
- 7.Lin M.-H., Kamel H., Singer D.E., et al. Perioperative/postoperative atrial fibrillation and risk of subsequent stroke and/or mortality. Stroke. 2019;50:1364–1371. doi: 10.1161/STROKEAHA.118.023921. [DOI] [PubMed] [Google Scholar]
- 8.January C.T., Wann L.S., Alpert J., et al. 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation. J Am Coll Cardiol. 2014;64:e1–76. doi: 10.1016/j.jacc.2014.03.022. [DOI] [PubMed] [Google Scholar]
- 9.Kirchhof P., Benussi S., Zamorano J.L., et al. 2016 ESC guidelines for the management of atrial fibrillation developed in collaboration with EACTS. Russ J Cardiol. 2017;147:7–86. doi: 10.1016/j.rec.2016.11.033. [DOI] [PubMed] [Google Scholar]
- 10.Danelich I.M., Lose J.M., Wright S.S., et al. Practical management of postoperative atrial fibrillation after noncardiac surgery. J Am Coll Surg. 2014;219:831–841. doi: 10.1016/j.jamcollsurg.2014.02.038. [DOI] [PubMed] [Google Scholar]
- 11.Bessissow A., Khan J., Devereaux P.J., Alvarez-Garcia J., Alonso-Coello P. Postoperative atrial fibrillation in non-cardiac and cardiac surgery: an overview. J Thromb Haemost. 2015;13:S304–S312. doi: 10.1111/jth.12974. [DOI] [PubMed] [Google Scholar]
- 12.Bhave P.D., Goldman L.E., Vittinghoff E., Maselli J., Auerbach A. Incidence, predictors, and outcomes associated with postoperative atrial fibrillation after major noncardiac surgery. Am Heart J. 2012;164:918–924. doi: 10.1016/j.ahj.2012.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chelazzi C., Villa G., De Gaudio A.R. Review article: postoperative atrial fibrillation. ISRN Cardiol. 2011;2011:203179. doi: 10.5402/2011/203179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gialdini G., Nearing K., Bhave P.D., et al. Perioperative atrial fibrillation and the long-term risk of ischemic stroke. J Am Med Assoc. 2014;312:616–622. doi: 10.1001/jama.2014.9143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Amar D., Roistacher N., Burt M., et al. Clinical and echocardiographic correlates of symptomatic tachydysrhythmias after noncardiac thoracic surgery. Chest. 1995;108:349–354. doi: 10.1378/chest.108.2.349. [DOI] [PubMed] [Google Scholar]
- 16.Curtis J.J., Parker B.M., McKenney C.A., et al. Incidence and predictors of supraventricular dysrhythmias after pulmonary resection. Ann Thorac Surg. 1998;66:1766–1771. doi: 10.1016/s0003-4975(98)00942-4. [DOI] [PubMed] [Google Scholar]
- 17.Materazzo C., Piotti P., Mantovani C., Miceli R., Villani F. Atrial fibrillation after non-cardiac surgery: P-wave characteristics and Holter monitoring in risk assessment. Eur J Cardiothorac Surg. 2007;31:812–816. doi: 10.1016/j.ejcts.2007.02.007. [DOI] [PubMed] [Google Scholar]
- 18.Raghavan D., Gao A., Ahn C., et al. Contemporary analysis of incidence of post-operative atrial fibrillation, its predictors, and association with clinical outcomes in lung transplantation. J Hear Lung Transplant. 2015;34:563–570. doi: 10.1016/j.healun.2014.09.032. [DOI] [PubMed] [Google Scholar]
- 19.Noorani A., Walsh S.R., Tang T.Y., et al. Atrial fibrillation following elective open abdominal aortic aneurysm repair. Int J Surg. 2009;7:24–27. doi: 10.1016/j.ijsu.2008.09.002. [DOI] [PubMed] [Google Scholar]
- 20.Brathwaite D., Weissman C. The new onset of atrial arrhythmias following major noncardiothoracic surgery is associated with increased mortality. Chest. 1998;114:462–468. doi: 10.1378/chest.114.2.462. [DOI] [PubMed] [Google Scholar]
- 21.Alonso-Coello P., Cook D., Xu S.C., et al. Predictors, prognosis, and management of new clinically important atrial fibrillation after noncardiac surgery: a prospective cohort study. Anesth Analg. 2017;125:162–169. doi: 10.1213/ANE.0000000000002111. [DOI] [PubMed] [Google Scholar]
- 22.Lubitz S.A., Yin X., Rienstra M., et al. Atrial fibrillation after lung transplantation. Circulation. 2015;131:1648–1655. doi: 10.1161/CIRCULATIONAHA.114.014058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.McIntyre W.F., Um K.J., Cheung C.C., et al. Atrial fibrillation detected initially during acute medical illness: a systematic review. Eur Hear J Acute Cardiovasc Care. 2019;8:130–141. doi: 10.1177/2048872618799748. [DOI] [PubMed] [Google Scholar]
- 24.Walkey A.J., Hammill B.G., Curtis L.H., Benjamin E.J. Long-term outcomes following development of new-onset atrial fibrillation during sepsis. Chest. 2014;146:1187–1195. doi: 10.1378/chest.14-0003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.McIntyre W., Mendoza P., Belley-Cote E., et al. Design and rationale of the atrial fibrillation occurring transiently with stress (AFOTS) follow-up cohort study. Clin Cardiol. 2018;41:1273–1280. doi: 10.1002/clc.23053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Stroup D.F., Berlin J.A., Morton S.C., et al. Meta-analysis of observational studies in epidemiology. J Am Med Assoc. 2000;283:2008–2012. doi: 10.1001/jama.283.15.2008. [DOI] [PubMed] [Google Scholar]
- 27.Guyatt G., Busse J. Tool to assess risk of bias in cohort studies. http://www.clarityresearch.ca Available at:
- 28.Higgins J.P.T., Thompson S.G. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21:1539–1558. doi: 10.1002/sim.1186. [DOI] [PubMed] [Google Scholar]
- 29.Higgins J., Green S. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0. The Cochrane Collaboration. 2011. http://handbook-5-1.cochrane.org/ Available at: Accessed November 8, 2020.
- 30.Guyatt G., Oxman A., Kunz R., et al. What is “quality of evidence” and why is it important to clinicians? Br Med J Clin Res Ed. 2008;336:995–998. doi: 10.1136/bmj.39490.551019.BE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.McCormack O., Zaborowski A., King S., et al. New-onset atrial fibrillation post-surgery for esophageal and junctional cancer. Ann Surg. 2014;260:772–778. doi: 10.1097/SLA.0000000000000960. [DOI] [PubMed] [Google Scholar]
- 32.Moon Y.J., Kwon H.M., Park Y.S., Kim S.H., Hwang G.S. Brief episodes of newly developed intraoperative atrial fibrillation predicts worse outcomes in adult liver transplantation. Transplant Proc. 2018;50:1142–1146. doi: 10.1016/j.transproceed.2018.01.039. [DOI] [PubMed] [Google Scholar]
- 33.Orrego C.M., Cordero-Reyes A.M., Estep J.D., et al. Atrial arrhythmias after lung transplant: inderlying mechanisms, risk factors, and prognosis. J Hear Lung Transplant. 2014;33:734–740. doi: 10.1016/j.healun.2014.02.032. [DOI] [PubMed] [Google Scholar]
- 34.Siontis K., Chamberlain A., Gersh B., et al. Abstract 13038: association of post-operative atrial fibrillation after non-cardiac surgery with stroke and mortality : a community study. Electrophysiol Arrhythm. 2018;138(Suppl 1):A13038. [Google Scholar]
- 35.Stawicki S.P., Prosciak M., Gerlach A., et al. Atrial fibrillation after oesophagectomy: an indicator of postoperative morbidity. Gen Thorac Cardiovasc Surg. 2011;59:399–405. doi: 10.1007/s11748-010-0713-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wells C.I., Robertson J.P., Campbell S., Al-Herz F., Rhind B., Young M. Impact of atrial fibrillation on long-term survival following oesophagectomy: a 21-year observational study. ANZ J Surg. 2018;88:E268–E272. doi: 10.1111/ans.14054. [DOI] [PubMed] [Google Scholar]
- 37.Xia V.W., Worapot A., Huang S., et al. Postoperative atrial fibrillation in liver transplantation. Am J Transplant. 2015;15:687–694. doi: 10.1111/ajt.13034. [DOI] [PubMed] [Google Scholar]
- 38.Booka E., Takeuchi H., Fukuda K., et al. The impact of new-onset atrial fibrillation on short- and long-term outcomes after esophagectomy for esophageal cancer. J Clin Oncol. 2017;35(4_suppl):183. [Google Scholar]
- 39.Branch K.R., Parmar B.R., Mulligan M.S., et al. 727 negative clinical outcomes associated with postoperative atrial arrhythmias after lung transplantation. J Hear Lung Transplant. 2012;31:S249. [Google Scholar]
- 40.Day R.W., Jaroszewski D., Chang Y.H.H., et al. Incidence and impact of postoperative atrial fibrillation after minimally invasive esophagectomy. Dis Esophagus. 2016;29:583–588. doi: 10.1111/dote.12355. [DOI] [PubMed] [Google Scholar]
- 41.Kothari A.N., Halandras P.M., Drescher M., et al. Transient postoperative atrial fibrillation after abdominal aortic aneurysm repair increases mortality risk. J Vasc Surg. 2016;63:1240–1247. doi: 10.1016/j.jvs.2015.12.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mason D.P., Marsh D.H., Alster J.M., et al. Atrial fibrillation after lung transplantation: timing, risk factors, and treatment. Ann Thorac Surg. 2007;84:1878–1884. doi: 10.1016/j.athoracsur.2007.07.014. [DOI] [PubMed] [Google Scholar]
- 43.Rachwan R.J., Kutkut I., Hathaway T.J., et al. Postoperative atrial fibrillation and flutter in liver transplantation: an important predictor of early and late morbidity and mortality. Liver Transpl. 2020;26:34–44. doi: 10.1002/lt.25631. [DOI] [PubMed] [Google Scholar]
- 44.Rostagno C., Cartei A., Ranalli C., et al. Postoperative atrial fibrillation in patients undergoing surgery for hip fracture. J Interv Card Electrophysiol. 2018;51(Suppl 1):S1–147. [Google Scholar]
- 45.Tao L., Xiaodong X., Fan L., Gang D., Jun D. Association between new-onset postoperative atrial fibrillation and 1-year mortality in elderly patients after hip arthroplasty. Aging Clin Exp Res. 2020;32:921–924. doi: 10.1007/s40520-019-01271-x. [DOI] [PubMed] [Google Scholar]
- 46.Uhm J.-S., Kim B.K., Yu H.T., et al. Contemporary analysis of incidence of post-operative atrial arrhythmia, its predictors, and clinical impact in lung transplantation. Eur Heart J. 2017;38(suppl 1):164. [Google Scholar]
- 47.Butt J.H., Olesen J.B., Havers-Borgersen E., et al. Risk of thromboembolism associated with atrial fibrillation following noncardiac surgery. J Am Coll Cardiol. 2018;72:2027–2036. doi: 10.1016/j.jacc.2018.07.088. [DOI] [PubMed] [Google Scholar]
- 48.Chaikriangkrai K., Jyothula S., Jhun H.Y., et al. Incidence, risk factors, prognosis, and electrophysiological mechanisms of atrial arrhythmias after lung transplantation. J Am Coll Cardiol Clin Electrophysiol. 2015;1:296–305. doi: 10.1016/j.jacep.2015.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chin J.H., Moon Y.J., Jo J.Y., et al. Association between postoperatively developed atrial fibrillation and long-term mortality after esophagectomy in esophageal cancer patients: an observational study. PLoS One. 2016;11 doi: 10.1371/journal.pone.0154931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Conen D., Alonso-Coello P., Douketis J., et al. Risk of stroke and other adverse outcomes in patients with perioperative atrial fibrillation one year after noncardiac surgery. Eur Heart J. 2020;41:645–651. doi: 10.1093/eurheartj/ehz431. [DOI] [PubMed] [Google Scholar]
- 51.Isiadinso I., Meshkov A.B., Gaughan J., et al. Atrial arrhythmias after lung and heartlung transplant: effects on short-term mortality and the influence of amiodarone. J Hear Lung Transplant. 2011;30:37–44. doi: 10.1016/j.healun.2010.07.006. [DOI] [PubMed] [Google Scholar]
- 52.Khormaee S., Do H.T., Mayr Y., et al. Risk of ischemic stroke after perioperative atrial fibrillation in total knee and hip arthroplasty patients. J Arthroplasty. 2018;33:3016–3019. doi: 10.1016/j.arth.2018.04.009. [DOI] [PubMed] [Google Scholar]
- 53.Leibowitz D., Abitbol C., Alcalai R., Rivkin G., Kandel L. Perioperative atrial fibrillation is associated with increased one-year mortality in elderly patients after repair of hip fracture. Int J Cardiol. 2017;227:58–60. doi: 10.1016/j.ijcard.2016.11.067. [DOI] [PubMed] [Google Scholar]
- 54.Cardinale D., Martinoni A., Cipolla C.M., et al. Atrial fibrillation after operation for lung cancer: clinical and prognostic significance. Ann Thorac Surg. 1999;68:1827–1831. doi: 10.1016/s0003-4975(99)00712-2. [DOI] [PubMed] [Google Scholar]
- 55.Goodman S., Shirov T., Weissman C. Supraventricular arrhythmias in intensive care unit patients: short and long-term consequences. Anesth Analg. 2007;104:880–886. doi: 10.1213/01.ane.0000255759.41131.05. [DOI] [PubMed] [Google Scholar]
- 56.Imperatori A., Mariscalco G., Riganti G., et al. Atrial fibrillation after pulmonary lobectomy for lung cancer affects long-term survival in a prospective single-center study. J Cardiothorac Surg. 2012;7:4. doi: 10.1186/1749-8090-7-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Winkel T.A., Schouten O., Hoeks S.E., et al. Risk factors and outcome of new-onset cardiac arrhythmias in vascular surgery patients. Am Heart J. 2010;159:1108–1115. doi: 10.1016/j.ahj.2010.03.035. [DOI] [PubMed] [Google Scholar]
- 58.Higuchi S., Kabeya Y., Matsushita K., et al. Perioperative atrial fibrillation in noncardiac surgeries for malignancies and one-year recurrence. Can J Cardiol. 2019;35:1449–1456. doi: 10.1016/j.cjca.2019.07.008. [DOI] [PubMed] [Google Scholar]
- 59.Langer G., Meerpohl J.J., Perleth M., et al. GRADE Guidelines: 1. Introduction—GRADE evidence profiles and summary of findings tables. J Clin Epidemiol. 2012;106:357–368. doi: 10.1016/j.zefq.2012.05.017. [DOI] [PubMed] [Google Scholar]
- 60.January C.T., Wann L.S., Calkins H., et al. 2019 AHA/ACC/HRS focused update of the 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation. Circulation. 2019;140:e125–e151. doi: 10.1161/CIR.0000000000000665. [DOI] [PubMed] [Google Scholar]
- 61.Gage B., Waterman A.D., Shannon W., et al. Validation of clinical classification schemes for predicting stroke: results from the National Registry of Atrial Fibrillation. J Am Med Assoc. 2001;285:2864–2870. doi: 10.1001/jama.285.22.2864. [DOI] [PubMed] [Google Scholar]
- 62.Macle L., Cairns J., Leblanc K., et al. 2016 focused update of the Canadian Cardiovascular Society Guidelines for the management of atrial fibrillation. Can J Cardiol. 2016;32:1170–1185. doi: 10.1016/j.cjca.2016.07.591. [DOI] [PubMed] [Google Scholar]
- 63.Roshanov P.S., Eikelboom J.W., Crowther M., et al. Bleeding impacting mortality after noncardiac surgery: a protocol to establish diagnostic criteria, estimate prognostic importance, and develop and validate a prediction guide in an international prospective cohort study. Can Med Assoc J Open. 2017;5:E594–603. doi: 10.9778/cmajo.20160106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Devereaux P., Duceppe E., Guyatt G., et al. Dabigatran in patients with myocardial injury after non-cardiac surgery (MANAGE): an international, randomised, placebo-controlled trial. Lancet. 2018;391:2325–2334. doi: 10.1016/S0140-6736(18)30832-8. [DOI] [PubMed] [Google Scholar]
- 65.McIntyre W., Healey J.S., Devereaux P., Conen D. A Call for randomized trials of oral anticoagulation for patients with post-operative atrial fibrillation. J Am Coll Cardiol. 2019;73:1105. doi: 10.1016/j.jacc.2018.11.057. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.