Abstract
Background
Adults born preterm (< 37 weeks’ gestation) exhibit altered cardiac growth and are susceptible to cardiac dysfunction. Sheep studies have shown that moderate preterm birth results in maladaptive structural remodelling of the cardiac ventricles. The aim of this study was to examine ventricular structure in lambs born at a greater severity of preterm birth and ventilated postnatally.
Methods
Former-preterm lambs delivered at 128 days’ gestation, and mechanically ventilated for a week after birth, were compared with unventilated lambs born at term (150 days’ gestation), at 2 months (term: n = 10, former-preterm: n = 8), and 5 months (term: n = 9, former-preterm: n = 8) term-equivalent age. The right ventricle and left ventricle plus septum were analysed using immunohistochemistry, histology, and stereology.
Results
Cardiomyocyte number, cross-sectional area, proliferation, and apoptosis were not affected by preterm birth or age. Left ventricle plus septum interstitial collagen levels increased with age (P = 0.0015) and were exacerbated by preterm birth (P = 0.0006; 2 months term: 0.57% ± 0.07%, former-preterm: 1.44% ± 0.18%; 5 months term: 1.37% ± 0.25%, former-preterm: 2.15% ± 0.31%). Right ventricle interstitial collagen levels increased with age (P = 0.012) but were not affected by preterm birth.
Conclusion
This study is the first to explore the effect of preterm birth combined with modern neonatal interventions on the ventricular myocardium in lambs. There was no adverse impact on cardiomyocyte growth in early postnatal life. Of concern, however, there was increased collagen deposition in the preterm hearts, which has the potential to induce cardiac dysfunction, especially if it becomes exaggerated with ageing.
Résumé
Introduction
Les adultes nés avant terme (< 37 semaines de grossesse) montrent une altération de la croissance cardiaque et sont exposés à une dysfonction cardiaque. Les études sur les moutons ont montré que la prématurité modérée entraîne un remodelage structurel inadapté des ventricules du cœur. L’objectif de la présente étude était d’examiner la structure ventriculaire des agneaux grands prématurés et oxygénés après la naissance.
Méthodes
Les agneaux anciens prématurés nés après 128 jours de gestation et sous respirateur durant une semaine ont été comparés aux agneaux nés à terme qui n’avaient pas été sous respirateur (150 jours de gestation) à l’âge du terme, soit deux mois (à terme : n = 10, anciens prématurés : n = 8) et cinq mois (à terme : n = 9, anciens prématurés : n = 8). Le ventricule droit et le ventricule gauche plus le septum ont été analysés par immunohistochimie, histologie et stéréologie.
Résultats
Le nombre de cardiomyocytes, la surface en coupe transversale, la prolifération et l’apoptose n’étaient pas affectés par la naissance prématurée ou l’âge. Les concentrations interstitielles en collagène du ventricule gauche plus le septum augmentaient avec l’âge (P = 0,0015) et étaient exacerbées par la naissance prématurée (P = 0,0006; âge du terme, deux mois : [à terme : 0,57 % ± 0,07 %, anciens prématurés : 1,44 % ± 0,18 % ]; âge du terme, cinq mois : [à terme : 1,37 % ± 0,25 %, anciens prématurés : 2,15 % ± 0,31 %]). Les concentrations interstitielles en collagène du ventricule droit augmentaient avec l’âge (P = 0,012), mais n’étaient pas affectées par la naissance avant terme.
Conclusion
Il s’agit de la première étude qui porte sur la combinaison des effets de la naissance avant terme aux interventions néonatales modernes sur le myocarde ventriculaire des agneaux. Aucune conséquence sur la croissance des cardiomyocytes dans la phase précoce de la vie postnatale n’a été observée. Toutefois, le dépôt accru de collagène dans le cœur des prématurés est préoccupant puisqu’il a le potentiel d’induire une dysfonction cardiaque, particulièrement s’il s’exacerbe avec le vieillissement.
Preterm birth is defined as delivery before 37 weeks of gestation. This definition is subdivided into 3 classifications according to the severity of prematurity: moderately preterm (birth between 32 and up to 37 weeks’ gestation), very preterm (birth between 28 and up to 32 weeks’ gestation), and extremely preterm (birth before 28 weeks’ gestation).1 Preterm birth necessitates the cardiopulmonary haemodynamic transition to occur at a time when many of the cardiomyocytes are undifferentiated and immature (with the level of cardiomyocyte maturity directly proportional to gestational age at birth).2,3 Therefore, the immature myocardium of former-preterm infants is often unable to cope with the marked increase in systemic arterial blood pressure and the subsequent acute increase in cardiac afterload that occurs soon after preterm birth.4 As a result, pathophysiological instability of systemic blood pressure is a challenge commonly faced by infants born preterm.5
Several clinical studies suggest that early remodelling of both cardiac ventricles after preterm birth occurs as a physiological adaptation to a premature increase in cardiac preload and afterload on the immature myocardium.6, 7, 8, 9 In addition, anatomic and functional differences in the heart after preterm birth, from early infancy through to mid-adulthood, have recently been reported. For example, an increase in left ventricle and septum (LV+S) and right ventricle (RV) mass relative to body size has been shown in former-preterm infants at 3 months of age, when compared with infants born at term.10 Similarly, magnetic resonance imaging studies in young adults born preterm report an increase in ventricular mass and wall thickness, reduced ventricular length and internal cavity diameter, and a displaced cardiac apex when compared with young adults born at term.11 However, not all studies report induction of cardiac hypertrophy in adulthood after preterm birth,12 thus implying variability in the long-term cardiac structural and/or functional consequences.
Individuals born preterm are also at increased risk of developing arterial and pulmonary hypertension later in life, with reports of high systolic blood pressure in childhood,8,13, 14, 15 adolescence,12,16 and in early11,17, 18, 19, 20, 21 and mid-adulthood.22,23 Decreasing gestational age at birth is associated with higher systolic blood pressure and greater systolic dysfunction. Both hypertension and ventricular hypertrophy (particularly in the LV) are major risk factors for cardiovascular disease. It is likely that the antecedents to cardiovascular disease in people born preterm arise in early postnatal life, due to maladaptive compensatory remodelling of the immature heart and blood vessels in response to premature exposure to postnatal haemodynamics. These structural changes may ultimately increase the vulnerability to long-term cardiovascular disease.24,25
In support of this idea, a study conducted in a sheep model of preterm birth (where the gestational timing of cardiomyocyte maturation closely resembles that in humans26, 27, 28, 29) showed increases in interstitial fibrosis and abnormalities in cardiomyocyte growth (increase in cardiomyocyte volume, nuclearity, and ploidy) in the hearts of lambs that were born moderately preterm (at 135 days’ gestation) without requiring assisted postnatal ventilation, examined at 9 weeks after term-equivalent age (TEA).30 It is expected that this structural remodelling of the preterm myocardium would be further exacerbated with decreasing gestational age at birth; this is addressed in the current study.
In contrast, no differences in myocardial fibrosis or in cardiomyocyte size, nuclearity, or ploidy were detected in an autopsy study of preterm neonates that died at an early time point between 1 and 42 days after birth.31 Of concern, however, there was a marked decrease in cardiomyocyte proliferation in the preterm neonates when compared with age-matched stillborn infants. This has the potential to adversely impact lifelong cardiomyocyte functional reserve. Given the low proliferative capacity of cardiomyocytes postnatally,26 it is conceivable that abnormal cardiomyocyte and fibrotic growth (as observed in the former-preterm sheep study) may subsequently manifest in the human heart in early childhood.
Over the past 3 decades there have been major advances in the clinical approaches aimed at increasing the survival rates of preterm neonates, particularly those born very and extremely preterm. The use of exogenous surfactant, gentler forms of assisted ventilation, and antenatal glucocorticoids in preterm neonates has markedly improved respiratory capacity, organ oxygenation, and lung development, respectively.32 However, these treatments are administered during a particularly vulnerable time when organ maturation is still ongoing, which may inadvertently affect the development of other vital organs, including the heart.33,34 Hence, as more clinically treated preterm individuals are now entering adulthood, it is imperative to determine how preterm birth combined with modern interventions impacts the cellular structure and growth of cardiomyocytes in the immature heart. This will allow for focussed therapeutic strategies to be developed to mitigate the abnormal postnatal cardiac development after preterm birth and subsequently reduce the risk of cardiovascular disease.
The aim of this preclinical study was to assess, at 2 and 5 months of age (equivalent to approximately 2 and 6 years of age in humans, respectively), the effects of preterm birth on cardiac structure in lambs born prematurely at a time point when lung development was at the saccular stage (equivalent to 28 weeks’ gestation in humans); these lambs required assisted ventilation after birth as well as continuous 24-hour intensive care monitoring. Our sheep model of preterm birth closely reflects the typical management of a scheduled caesarean delivery for medically indicated preterm birth in humans, including the administration of antenatal steroids, exogenous surfactant and caffeine citrate, and the use of neonatal ventilators.2 We chose to specifically look at the impact on both cardiac ventricles separately in this study, given their distinct haemodynamic roles. We hypothesized that preterm birth would result in abnormal cardiomyocyte growth and structural remodelling of the ventricular myocardium at 2 months’ TEA and that this would be further exacerbated at 5 months’ TEA. We expected that the adverse impacts of preterm birth would be greater than those observed previously in a sheep model of moderately preterm birth, given the increased severity of preterm birth in the present study.
Materials and Methods
Ethical approval
The sheep studies adhered to the American Physiology Society and National Institutes of Health guidelines for humane use of animals for research and were approved by the Institutional Animal Care and Use Committee at the University of Utah Health Sciences Center.
Sheep model and preterm delivery
Twenty-eight date-mated Rambouillet × Columbia ewes were randomly assigned to deliver preterm via caesarean section at 128 days’ gestation (n = 16) or to deliver spontaneously at term (approximately 150 days’ gestation) (n = 19). Healthy singleton lambs and lambs from multiple pregnancies were used in this study.
Details of the perinatal procedure and outcomes of this model (including the data related to weight gain, systematic blood pressure, and blood gas values) have been previously reported.35 Briefly, the ewes chosen for preterm delivery received intramuscular injections of dexamethasone phosphate (6 mg/kg; Vedco, Inc, St. Joseph, MO) 48 hours and again at 24 hours before caesarean delivery. At 128 days’ gestation, the ewes received an intramuscular injection of ketamine hydrochloride (10-20 mg/kg; KetaVed, Vedco, Inc, St. Joseph, MO), followed by inhalation anaesthesia with isoflurane (2.5% isoflurane; Piramal Healthcare, Bethlehem, PA). The foetuses were then exposed via midline hysterotomy and intubated with a cuffed endotracheal tube (3.0-4.0 French). Ten millilitres of lung liquid was aspirated and replaced with calfactant (6 mL, Infasurf; ONY Biotech, Amherst, NY), a nonpyrogenic pulmonary surfactant used to reduce surface tension within the lungs.
Lamb ventilation and nutrition
Once the umbilical cord was ligated and cut, the former-preterm lambs were manually resuscitated, using a Neopuff T-piece resuscitator (Fisher & Paykel, Auckland, New Zealand). The former-preterm lambs (n = 8 female, n = 8 male) were then weighed and placed on a heated bed.
All former-preterm lambs were treated intravenously, within 30 minutes of delivery, with a loading dose of caffeine citrate to stimulate ventilator drive (15 mg/kg, given over 90 minutes; Sagent Pharmaceuticals, Schaumburg, IL). Parenteral dextrose was infused to maintain plasma glucose between 60 and 90 mg/dL.
The former-preterm lambs were connected to an invasive mechanical ventilator (Babylog VN500; Dräger, Lübeck, Germany) configured to provide synchronized, intermittent, mandatory ventilation that was pressure controlled with warm and humidified oxygen. Initial ventilator settings were respiratory rate of 60 breaths/min, inspiratory time of 0.32 seconds, peak inspiratory pressure of 21 cm H2O, and positive end-expiratory pressure of 8 cm H2O. Tidal volume was kept at 5-7 mL/kg. Arterial blood gases were taken every 15 minutes for the first 90 minutes of postnatal life. The fraction of inspired oxygen was gradually lowered to attain a target oxygen saturation of 88%-94% by pulse oximetry (model SurgiVet V92001BP/Temp; Smith Medical ASD, Minneapolis, MN) to wean the lamb off mechanical ventilation.
The former-preterm lambs were mechanically ventilated for approximately 6 days and then transitioned onto noninvasive respiratory support, using nasal high-frequency oscillation. Once the former-preterm lambs were extubated, an uncuffed oral/nasal cannula (3.0 French; 13 cm length) was inserted approximately 5 cm into a nostril. Lidocaine (1% solution; Hospira, Inc, IL) was applied within the nostril to minimize pain and discomfort. The former-preterm lambs were placed on noninvasive respiratory support for approximately 3 days and then had the nasal tube removed. The former-preterm lambs received supplementary blow-by oxygen through a cone until fully stable. Term lambs (n = 13 female, n = 6 male) did not receive any form of respiratory support.
Orogastric feeding of ewe’s colostrum was started at approximately 3 hours of postnatal life (3 mL) for former-preterm lambs, and the volume was gradually increased as tolerated, with a target over the first week of postnatal life of approximately 60 kcal/kg/d. Term lambs stayed with their ewe for approximately 24 hours to let them take colostrum. Then, lambs born preterm (n = 16) and at term (n = 19) were bottle-fed milk (Sav A Lam milk replacer; Sav A Calf, Chilton, WI), and were given alfalfa pellets and water ad libitum.
Organ collection
At 2 months’ TEA (2 months after birth of term lambs, and 2 months after the day that TEA was reached for the former-preterm lambs), 8 former-preterm lambs (female n = 4, male n = 4) and 10 term lambs (female n = 7, male n = 3) were randomly assigned for euthanasia. At 5 months’ TEA, the remaining 8 former-preterm (female n = 4, male n = 4) and 9 term (female n = 6, male n = 3) lambs were also euthanized. At the end of each 2- or 5-month study period, the lambs were weighed then received an intramuscular injection of ketamine (10-20 mg/kg) followed by inhalation anaesthesia with 1.0%-2.5% isoflurane with oxygen. Lambs were intubated and ventilated with a tidal volume of 5-7 mL/kg and given heparin (1000 U iv). The anaesthetized lambs were then euthanized by barbiturate overdose with an intravenous injection of Beuthanasia solution containing pentobarbital sodium and phenytoin sodium (0.25 mL/kg; Intervet, NJ), followed by potassium chloride (10 mEq; Hospira, Inc) intravenously.
At necropsy, the chest cavity was opened and the heart was excised at the base of the great vessels and then weighed. The excised heart was immersed in a cold saline solution containing 20 mEq potassium chloride. The coronary vessels were perfused via their respective coronary ostia with cold phosphate buffered saline mixed with 20 mEq of potassium chloride and then with 10% buffered formalin. The heart was stored in 10% buffered formalin at 4°C.
Heart sampling
The LV+S were sampled together, separate from the RV. The smooth fractionator approach, as previously described in detail,36 was used to select 9 pieces of LV tissue (including the interventricular septum) and 9 pieces of RV tissue per lamb for glycol methacrylate embedding; a further 8 pieces per ventricle per lamb were selected for paraffin embedding.
Interstitial collagen
Paraffin-embedded tissue was sectioned at 5 μm, post-fixed in Bouin’s fixative, and stained with 1% Sirius red in saturated picric acid (picrosirius red). Fields of view of the myocardium were systematically sampled beginning at a random starting point. When a field of view landed on an area of myocardium that contained arteries or veins, these fields of view were not included in the image analysis and we moved onto the next sampling region until there was a total of 8 fields of view per tissue (8 pieces per ventricle per lamb). This resulted in a total of 64 fields of view per ventricle per lamb. The percentage of interstitial collagen within the myocardium was assessed using Image-Pro Plus (Version 6.2 for Windows; Media Cybernetics, Bethesda, MA).
Cardiomyocyte proliferation
Paraffin-embedded tissue sections at 5 μm were immersed in a Tris-EDTA buffer (10 mM Tris base, 1 mM EDTA solution, 0.05% Tween 20, pH 9.0) and microwaved for antigen retrieval. Endogenous peroxidase staining was prevented with a wash of hydrogen peroxide, and nonspecific background staining was prevented using CAS block (Invitrogen, Frederick, MD). The sections were incubated overnight with a primary Ki-67 antibody (1:100; M724001-2, MIB-1 Clone; Dako, Glostrup, Denmark), which detects the nuclei of proliferating cells, and subsequently incubated with a horseradish peroxidase (HRP)-conjugated goat antimouse secondary antibody (1:200; Invitrogen). A Dako EnVision + Dual Link HRP/DAB+ immunohistochemistry kit (Dako) was used to visualize the proliferating nuclei. The sections were then counterstained with haematoxylin. Paraffin-embedded tissue from a foetal sheep heart at 100 days’ gestation was used as a positive control, and sections excluding the primary antibody were used as negative controls.
The immuno-labelled cardiac tissue sections were randomly sampled (8 fields of view per 8 pieces of tissue per ventricle per lamb) using Image-Pro Plus software, and the average percentage of Ki-67-positive cardiomyocytes per field of view was quantified for each ventricle.
Cardiomyocyte apoptosis
A TUNEL (terminal deoxynucleotidyl transferase mediated deoxyuridine triphosphate nick end labeling) assay (S7110, ApopTag Fluorescein In Situ Apoptosis Detection Kit; Millipore, Billerica, MA) was used to determine the proportion of apoptotic cardiomyocytes within the LV+S and RV. Briefly, paraffin-embedded tissue, sectioned at 5 μm, was treated with proteinase K (21627; IHC Select; Merck, Germany), incubated with a terminal deoxynucleotidyl transferase enzyme in a humidified chamber at 37°C, and then subsequently incubated with fluorescein isothiocyanate-labelled anti-digoxigenin. The sections were counterstained with 4,6-diamidino-2-phenylindole hydrochloride (Invitrogen) to stain cell nuclei. They were mounted using ProLong Gold (Invitrogen). Rat breast tumour sections were used as a positive control.
Cardiomyocyte number
Cardiomyocyte number within the RV and LV+S was stereologically estimated using an optical disector–fractionator approach.37,38 To do this, the glycol methacrylate–embedded tissue blocks were serially sectioned at 20 μm thickness with every 20th section collected onto glass slides. Sections were stained with Harris’s haematoxylin (Amber Scientific, Queensland, Australia) in a 1000 W microwave oven at 50% power. The sections were then viewed with a light microscope (Olympus BX4, Japan) fitted with a ×100 objective oil immersion lens, a motorized stage, and a z-axis sensor. Using C.A.S.T software (Computer Aided Stereological Toolbox; Olympus, Denmark), the tissue sections were systematically sampled using a fixed x- and y-axis step length of 1500 μm, beginning at a random starting point. An unbiased counting frame (544.5 μm2) was superimposed over each field of view, and the number of cardiomyocyte nuclei within the middle 10 μm of each section was counted. The total number of cardiomyocyte nuclei counted per ventricle, using the optical disector approach, was then multiplied by the inverse of all tissue sampling fractions to estimate the total number of cardiomyocyte nuclei in the LV+S and RV. The total number of cardiomyocytes within each ventricle was subsequently calculated by halving the total number of cardiomyocyte nuclei to adjust for binucleation.
Cardiomyocyte cross-sectional area
Cardiomyocyte cross-sectional area was quantified using confocal microscopy. Paraffin-embedded sections of the LV+S and RV (8 per ventricle) were cut at 40 μm thickness. The sections were incubated with 4,6-diamidino-2-phenylindole hydrochloride (1:5000; Invitrogen) and Wheat Germ Agglutinin-Alexa Fluor 488 (1:20, W11261; Invitrogen) in phosphate buffered saline overnight and subsequently mounted using ProLong Gold (Invitrogen). Image acquisition, in random fields of view, was conducted using a Nikon C1 confocal microscope equipped with a ×40 objective oil immersion lens (Nikon, Japan). The cross-sectional area of 250 randomly selected cardiomyocytes was measured using Image J software (v6.2; National Institutes of Health, MD). Only cardiomyocytes with a nucleus in the plane of view and centrally located within the cell were measured. The average cardiomyocyte cross-sectional area per LV+S and RV was then determined.
Statistical analysis
Statistical analyses were conducted using GraphPad Prism version 7.01 for Windows (GraphPad Software, La Jolla, CA). Birth weights of the preterm and term lambs were analysed using a 2-way analysis of variance (ANOVA), with the factors preterm birth (P), sex (S), and their interaction (P∗S). Final body weight and heart weights were analysed using a 3-way ANOVA, with the factors preterm birth (P), sex (S), postnatal age (A), and their associated interactions. Differences between groups (preterm and term) and postnatal ages (2 and 5 months’ TEA) for all remaining data, where no significant sex effects were apparent (through initial analysis with 3-way ANOVA), were analysed using a 2-way ANOVA, with the factors preterm birth (P), postnatal age (A), and their interaction (P∗A). All data are presented as the mean ± standard error of the mean. P values less than 0.05 were considered statistically significant.
Results
Body and heart weights
Birth weight was significantly lower in the former-preterm lambs compared with the term lambs (preterm: 3.20 ± 0.19 kg, term: 4.74 ± 0.39 kg; P = 0.003). Sex did not have a significant effect on birth weight (P = 0.90), with no significant interaction effect between preterm birth and sex (P = 0.54). The average birth weight of female former-preterm lambs was 3.08 ± 0.18 kg, compared with 4.85 ± 0.47 kg in female term lambs. The average birth weight of male former-preterm lambs was 3.31 ± 0.35 kg, compared with 4.50 ± 0.75 kg in male term lambs.
Final body weight was significantly lower in former-preterm lambs compared with term lambs across the 2 time points, and the female lambs were lighter than male lambs overall (Table 1). As expected, final body weight in lambs at 5 months’ TEA was significantly greater compared with lambs at 2 months’ TEA (Table 1). There were no significant interaction effects between preterm birth and postnatal age (PP∗A = 0.30) or sex (PP∗S = 0.89) on body weight.
Table 1.
Body and heart weights of lambs born at term or preterm at 2 and 5 months’ term-equivalent age
| TERM |
FORMER-PRETERM |
P VALUE |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 2 mo | 5 mo | 2 mo | 5 mo | PP | PS | PA | |||||
| Sex | F | M | F | M | F | M | F | M | |||
| N | 7 | 3 | 6 | 3 | 4 | 4 | 4 | 4 | |||
| Singletons (N) | 1 | 1 | 3 | 0 | 2 | 1 | 0 | 1 | |||
| Body weight (kg) | 23.3 ± 1.9 | 29.5 ± 4.8 | 48.7 ± 3.8 | 56.4 ± 6.2 | 19.1 ± 1.4 | 23.5 ± 2.4 | 37.2 ± 5.6 | 45.2 ± 5.9 | 0.01 | 0.04 | < 0.0001 |
| Absolute heart weight (g) | 105.56 ± 9.93 | 142.63 ± 14.63 | 211.42 ± 13.27 | 254.70 ± 27.87 | 95.71 ± 6.47 | 121.27 ± 18.61 | 196.41 ± 12.41 | 241.73 ± 12.12 | 0.20 | 0.0031 | < 0.0001 |
| Heart weight to body weight ratio (g/kg) | 4.93 ± 0.42 | 4.93 ± 0.39 | 4.42 ± 0.32 | 4.53 ± 0.21 | 5.02 ± 0.16 | 5.33 ± 0.29 | 4.26 ± 0.12 | 4.80 ± 0.34 | 0.56 | 0.35 | 0.043 |
Data are shown as mean ± standard error of the mean. Data analysed by 3-way analysis of variance, with factors preterm birth (P), sex (S), and postnatal age (A).
Absolute heart weight and relative heart weight (heart weight to body weight ratio) did not differ between preterm and term lambs (Table 1). Female lambs had significantly lighter absolute heart weight compared with male lambs overall, but no sex differences were detected in relative heart weight. Across all lambs, absolute heart weight significantly increased with increasing postnatal age, whereas relative heart weight significantly decreased. There were no significant interaction effects associated with absolute (PP∗A = 0.94; PP∗S = 0.84) or relative (PP∗A = 0.72; PP∗S = 0.48) heart weights.
Cardiomyocyte proliferation and apoptosis
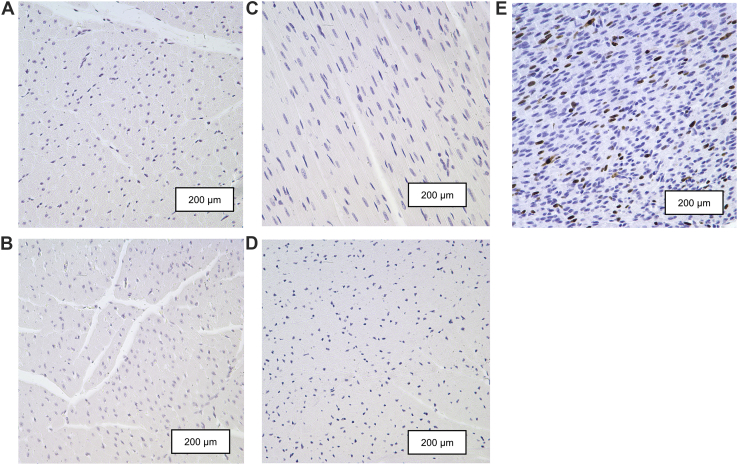
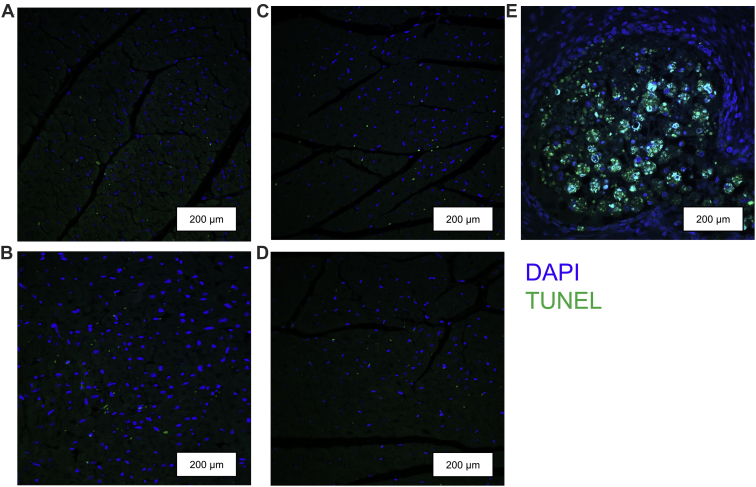
Levels of Ki-67 positive nuclei were extremely low (<0.001%) in the LV+S and RV of both former-preterm and term lambs at 2- and 5-month TEA (Fig. 1). Similarly, TUNEL assays revealed negligible levels of apoptotic cardiomyocytes in the LV+S and RV of both former-preterm and term lambs at 2- and 5-month TEA (Fig. 2).
Figure 1.
Representative photomicrographs of sections of myocardium from the left ventricles of both former-preterm lambs at (A) 2-month TEA and (B) 5-month TEA, and term lambs at (C) 2-month TEA and (D) 5-month TEA, which are absent of proliferating cardiomyocytes positive for Ki-67 antibody (brown). (E) The left ventricle from a foetal sheep heart at 100 days’ gestation was used as a positive control. Sections were counterstained with haematoxylin (purple). Scale bars = 200 μm. TEA, term-equivalent age.
Figure 2.
Representative photomicrographs of myocardium from the left ventricles of both former-preterm lambs at (A) 2-month TEA and (B) 5-month TEA, and term lambs at (C) 2-month TEA and (D) 5-month TEA, showing negligible levels of apoptotic cardiomyocyte nuclei (green) as assessed by the TUNEL assay. (E) Rat breast tumour sections were used as a positive control. Sections were counterstained with DAPI (blue). Scale bars = 200 μm. DAPI, 4,6-diamidino-2-phenylindole hydrochloride; TEA, term-equivalent age; TUNEL, terminal deoxynucleotidyl transferase mediated deoxyuridine triphosphate nick end labeling.
Cardiomyocyte growth
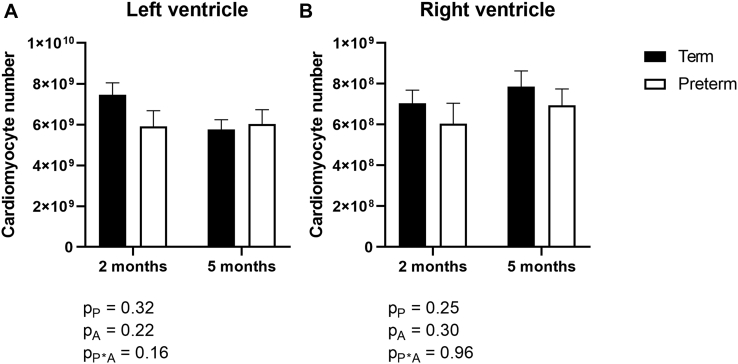
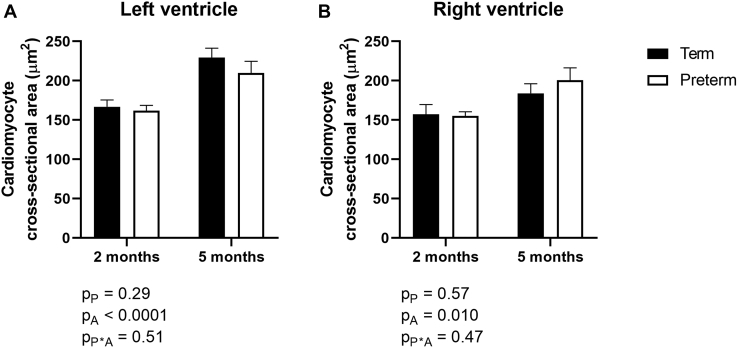
Total cardiomyocyte number in the LV+S and RV did not differ between former-preterm and term lambs, nor between lambs at 2- and 5-month TEA (Fig. 3). Overall, the cross-sectional area of cardiomyocytes in the LV+S and RV was significantly greater in lambs at 5 months’ TEA compared with lambs at 2 months’ TEA (Fig. 4). There were no significant differences in cardiomyocyte cross-sectional area between lambs born preterm compared with lambs born at term in either ventricle (Fig. 4).
Figure 3.
The total number of cardiomyocytes in the (A) left ventricle plus septum and (B) right ventricle of lambs born at term (150 days’ gestation; black) or preterm (128 days’ gestation; white) at 2- and 5-month term-equivalent age. Analysis by 2-way analysis of variance, with the factors preterm birth (P), postnatal age (A), and their interaction (P∗A). Data are shown as mean ± standard error of the mean. n = 8-10/group.
Figure 4.
Cross-sectional area of cardiomyocytes in the (A) left ventricle plus septum and (B) right ventricle of lambs born at term (150 days’ gestation; black) or preterm (128 days’ gestation; white) at 2- or 5-month term-equivalent age. Analysis by 2-way analysis of variance, with the factors preterm birth (P), postnatal age (A), and their interaction (P∗A). Data are shown as mean ± standard error of the mean. n = 8-10/group.
Myocardial interstitial collagen
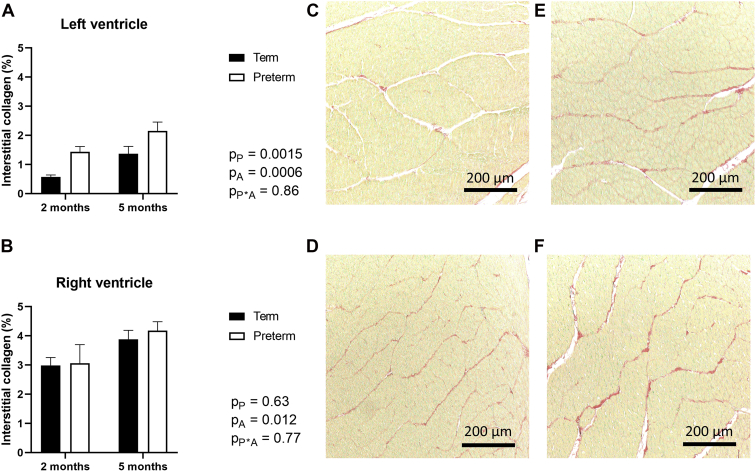
The percentage of interstitial collagen significantly increased in the LV+S of lambs between 2 and 5 months’ postnatal age and was exacerbated by preterm birth (2 months: term = 0.57% ± 0.07%, former-preterm = 1.44% ± 0.18%; 5 months: term = 1.37% ± 0.25%, former-preterm = 2.15% ± 0.31%) (Fig. 5A). Representative images of LV+S collagen staining are shown in Figure 5, C-F. In the RV, however, interstitial collagen levels increased with age but were not significantly affected by preterm birth (2 months: term = 2.99% ± 0.27%, former-preterm = 3.061% ± 0.64%; 5 months: term = 3.88% ± 0.31%, former-preterm = 4.18% ± 0.31%) (Fig. 5B).
Figure 5.
The percentage of interstitial collagen in the (A) left ventricle plus septum and (B) right ventricle of lambs born at term (150 days’ gestation) or preterm (128 days’ gestation) at 2- or 5-month term-equivalent age. Analysis by 2-way analysis of variance, with the factors preterm birth (P), postnatal age (A), and their interaction (P∗A). Data are shown as mean ± standard error of the mean. Representative photomicrographs of left ventricular myocardium stained with picrosirius red (collagen shown in red, and myocardium in yellow) from term-born lambs at (C) 2- and (E) 5-month, and former-preterm lambs at (D) 2- and (F) 5-month term-equivalent age. Scale bars = 200 μm. n = 8-10/group.
Discussion
Although the lambs in the present study were delivered considerably earlier than in previous studies of moderate preterm birth in unventilated lambs,30 we found a less severe adverse cardiac phenotype. We showed that preterm birth (and its associated clinical management), at the saccular stage of lung development, does not affect cardiomyocyte growth at 2- and 5-month TEA; of concern, however, there was greater myocardial collagen deposition within the extracellular compartment of the LV+S. This is likely a consequence of the premature haemodynamic transition occurring at the time of preterm birth, with a compensatory increase in collagen content providing the underdeveloped LV+S myocardium with structural stability. If this accelerated collagen deposition continues into adolescence and/or adulthood, the LV+S myocardium could become susceptible to cardiac fibrosis—an irreversible pathway that may render the LV with functional impairment later in life.
Preterm birth reduced body weight but did not affect heart growth
In this study, former-preterm lambs were lighter than term lambs at birth and remained lighter across 2- and 5-month TEA, findings consistent with those reported in children born preterm.39,40 Despite the lower body weights of the former-preterm lambs, we found that preterm birth combined with postnatal ventilation had no apparent effect on heart growth in lambs over the study period, a finding also seen in previous studies of moderately preterm and term-born lambs assessed at 9 weeks’ TEA.30 In a recent diffusion tensor imaging study by our laboratory, we showed that LV wall volume, cavity volume, length, and lateral wall width did not differ between former-preterm lambs and lambs born at term, by 5 months’ TEA.41
Similarly, an echocardiographic study showed that although body mass index and body surface area were reduced in 6-year-old children born extremely preterm compared with age-matched term controls, LV mass was unaffected after adjusting for body surface area.40 However, LV length, width, and aortic valve annulus diameter were 3-5% smaller in the preterm group; this was associated with impaired LV systolic function.40 This suggests that measurements of LV geometry, rather than LV mass alone, are important anatomic indicators of cardiac function.
Cardiomyocyte growth was not impacted by preterm birth
It is now well established that early life adverse events can negatively impact the growth of cardiomyocytes.42,43 To date, there are few studies that have explored the effects of preterm birth (either early or later in life) on the number and size of cardiomyocytes. Contrary to our initial hypothesis, we found no apparent differences in cardiomyocyte size (as assessed by measurements of cross-sectional area) between former-preterm and term-born lambs, at 2 or 5 months’ TEA. These findings were partly unexpected, given that previous studies in moderately preterm-born lambs at a similar postnatal time point found a marked increase in the size of mononucleated and binucleated cardiomyocytes in both the RV and LV. Although it was proposed that the increased severity of preterm birth in the present sheep study (birth at 128 days of gestation) would have a greater detrimental impact on cardiomyocyte growth than previously reported in former-preterm lambs (born moderately preterm at 133 days of gestation), this was not the case. In this regard, the postnatal clinical course of infants after preterm birth likely has an important influence on cardiomyocyte growth. The lambs examined in previous studies were of a different breed, were born moderately preterm, and did not require early postnatal respiratory support with oxygen-rich gas44 or neonatal intensive care and monitoring. It may be the case that the postnatal clinical interventions (such as mechanical ventilation with oxygen-rich gas, caffeine citrate, and exogenous surfactant therapy) used in the present study to maintain haemodynamic stability of the former-preterm lambs may have had a protective effect on cardiomyocyte growth. Studies exploring the long-term effects of each of these clinical interventions—and the doses of postnatal medications—are required to determine whether this is the case. In support of the current findings, no change in cardiomyocyte volume has previously been reported in human preterm neonates requiring mechanical ventilation after birth, compared with age-matched stillborn infants during gestation.31 However, no clinical studies to date have examined the effects of preterm birth on cardiomyocyte size in childhood or later in life.
The total number of cardiomyocytes within the LV+S and RV directly impact the functional capabilities and myocardial reserve of the respective ventricles. In human infants (and in sheep), there is a strong inverse correlation between gestational age and cardiomyocyte proliferation rate.2,3,45 At the time of preterm delivery, cardiomyocyte proliferation in lambs is still ongoing, but then decreases markedly around full-term age.2 Our study suggests that preterm birth combined with early postnatal mechanical ventilation in lambs does not adversely affect the trajectory of cardiomyocyte proliferation at an age equivalence of childhood, with no difference observed between groups in cardiomyocyte number or cell proliferation rate (almost negligible at this time point). Bensley et al.4 also showed that cardiomyocyte number within the LV+S and RV did not differ between lambs born preterm or at term, when examined at 9 weeks’ TEA. In contrast to these findings, a study in adult sheep born preterm (that received a higher clinical dosage of antenatal steroids and were not mechanically ventilated) showed a significant decrease in cardiomyocyte number within the RV compared with term-born sheep;44 the LV was not examined in that study. In addition, an autopsy study in deceased preterm neonates (at 1-42 days’ postnatal age) found that preterm birth greatly reduced the rate of cardiac cell proliferation.31 Differences in gestational age, postnatal age, and clinical care between studies likely account for the inconsistent findings. Given the importance of cardiomyocyte number on lifetime cardiovascular health, further controlled studies to examine the isolated effects of each of these factors on cardiomyocyte proliferation and growth are certainly required.
Increased myocardial collagen deposition after preterm birth
Excessive myocardial collagen is associated with impaired conductivity and contractility of the ventricles and is a hallmark feature of many cardiac diseases such as heart failure and cardiac hypertrophy.46 A major finding of this study is that myocardial interstitial collagen deposition within the LV+S was exacerbated by preterm birth (with early postnatal assisted ventilation) in lambs assessed at the human-equivalent ages of 2 and 6 years; increased LV+S collagen deposition is a finding consistent with other preterm lamb studies.30 Preterm birth, however, did not increase interstitial collagen content in the RV, in contrast to the previous report in lambs born moderately preterm and examined at a similar postnatal age.44
In the case of the preterm heart, if accelerated collagen deposition persists in the LV and is exacerbated over time, it is possible that this may adversely impact myocardial conductivity and contractility and lead to increased stiffness of the LV, thereby rendering individuals born preterm susceptible to impaired cardiac function.46 Although we did not assess myocardial conductivity and contractility, further studies in this animal model at later time points would help to elucidate this. Indeed, in support of this idea, young adults born preterm have been shown to have impaired LV systolic function11 and an impaired LV response to physiological stress when subjected to physical exercise47,48 compared with adults born at term; however, myocardial collagen content has not yet been assessed in adults born preterm to confirm this relationship.
Limitations
This study was conducted using former-preterm and term-born lambs from a mix of singleton and multiple pregnancies. Although previous studies suggest that cardiac development does not differ between twins and singletons,37,49 we were unable to determine in the current study whether this is also the case in the setting of preterm birth. As described above, all findings in this study relating to the impact of preterm birth on cardiac morphology also need to be evaluated in the context of the antenatal (dexamethasone exposure) and postnatal (eg, respiratory support) treatment of the lambs; the specific impact that each of these individual factors may have cannot be determined. The methodology we used in this study to determine the total number of cardiomyocytes within each ventricle assumes that all cardiomyocytes are binucleated by 2 months’ TEA in sheep. Two studies in term-born sheep found that approximately 98% of cardiomyocytes are binucleated by 9 weeks of age.30,50 However, there may be small differences in cardiomyocyte nuclearity between term and former-preterm lambs at this age, which may also be influenced by differences in antenatal and postnatal interventions. Bensley et al.30 found that in preterm-born lambs at 9 weeks after TEA, the ventricles contained significantly fewer binucleated and more mononucleated cardiomyocytes, as well as some trinucleated cardiomyocytes (LV: approximately 86% of cardiomyocytes were binucleated; RV: approximately 93% binucleated cardiomyocytes). In contrast, the majority of cardiomyocytes within the ventricles of age-matched lambs born at term were binucleated (LV: approximately 98% of cardiomyocytes were binucleated; RV: approximately 99% binucleated cardiomyocytes).30 Importantly, when we used these differing percentages for preterm and term lambs to determine total cardiomyocyte number (instead of halving the number of cardiomyocyte nuclei to determine total cardiomyocyte number for both groups), there remained no significant difference in cardiomyocyte number between term and former-preterm lambs.
Conclusion
As more survivors of preterm birth are now entering adulthood, the long-term detrimental health impacts continue to emerge. Adverse myocardial remodelling, and thus an increased susceptibility to the development of cardiac disease, is a biological phenomenon recently revealed in individuals born preterm and is becoming an increasingly important issue in public health. This study is the first to analyse the impact of preterm birth, at a time when the lungs were still very immature (and thus requiring neonatal interventions including respiratory ventilation), on cardiomyocyte growth and myocardial structure in juvenile life. Although cardiomyocyte growth was not affected, an increase in myocardial interstitial collagen was found in the LV of lambs born preterm with assisted ventilation. These findings add to the growing body of evidence that preterm birth results in early maladaptive structural remodelling of the heart, likely contributing to the functional deficits of the preterm heart in adulthood. Unexpectedly, the adverse impact of preterm birth on the ventricular myocardium was less than that previously reported in a sheep model of preterm birth where the lambs were delivered moderately preterm and did not require respiratory support after birth. The reasons for this are currently unknown and this is an important area for future research.
Acknowledgements
The authors wish to thank the staff at Monash Histology Platform and Monash Micro Imaging for their technical assistance.
Funding Sources
This work was supported by the National Institutes of Health grants R01-HL-110002 and R01-HL-062875 (to K.H.A.) and the Division of Neonatology. BL was the recipient of an MBio Postgraduate Discovery Scholarship from the Biomedicine Discovery Institute, Monash University.
Disclosures
The authors have no conflicts of interest to disclose.
Footnotes
Ethics Statement: The protocols of this study adhered to APS/NIS guidelines for the humane use of animals for research, and were prospectively approved by the IACUC at the University of Utah Health Sciences Center.
See page 583 for disclosure information.
References
- 1.Goldenberg R.L., Culhane J.F., Iams J.D., Romero R. Epidemiology and causes of preterm birth. Lancet. 2008;371:75–84. doi: 10.1016/S0140-6736(08)60074-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jonker S.S., Louey S., Giraud G.D., Thornburg K.L., Faber J.J. Timing of cardiomyocyte growth, maturation, and attrition in perinatal sheep. FASEB J. 2015;29:4346–4357. doi: 10.1096/fj.15-272013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Burrell J.H., Boyn A.M., Kumarasamy V. Growth and maturation of cardiac myocytes in fetal sheep in the second half of gestation. Anat Rec A Discov Mol Cell Evol Biol. 2003;274:952–961. doi: 10.1002/ar.a.10110. [DOI] [PubMed] [Google Scholar]
- 4.Bensley J.G., De Matteo R., Harding R., Black M.J. The effects of preterm birth and its antecedents on the cardiovascular system. Acta Obstet Gynecol Scand. 2016;95:652–663. doi: 10.1111/aogs.12880. [DOI] [PubMed] [Google Scholar]
- 5.Evans J.R., Lou Short B., Van Meurs K., Cheryl Sachs H. Cardiovascular support in preterm infants. Clin Ther. 2006;28:1366–1384. doi: 10.1016/j.clinthera.2006.09.006. [DOI] [PubMed] [Google Scholar]
- 6.Cox D.J., Bai W., Price A.N. Ventricular remodeling in preterm infants: computational cardiac magnetic resonance atlasing shows significant early remodeling of the left ventricle. Pediatr Res. 2019;85:807–815. doi: 10.1038/s41390-018-0171-0. [DOI] [PubMed] [Google Scholar]
- 7.Phad N.S., de Waal K., Holder C., Oldmeadow C. Dilated hypertrophy: a distinct pattern of cardiac remodeling in preterm infants. Pediatr Res. 2020;87:146–152. doi: 10.1038/s41390-019-0568-4. [DOI] [PubMed] [Google Scholar]
- 8.Mikkola K., Leipala J., Boldt T., Fellman V. Fetal growth restriction in preterm infants and cardiovascular function at five years of age. J Pediatr. 2007;151:494–499. doi: 10.1016/j.jpeds.2007.04.030. [DOI] [PubMed] [Google Scholar]
- 9.Schubert U., Muller M., Abdul-Khaliq H., Norman M. Preterm birth is associated with altered myocardial function in infancy. J Am Soc Echocardiogr. 2016;29:670–678. doi: 10.1016/j.echo.2016.03.011. [DOI] [PubMed] [Google Scholar]
- 10.Aye C.Y.L., Lewandowski A.J., Lamata P. Disproportionate cardiac hypertrophy during early postnatal development in infants born preterm. Pediatr Res. 2017;82:36–46. doi: 10.1038/pr.2017.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lewandowski A.J., Augustine D., Lamata P. Preterm heart in adult life: cardiovascular magnetic resonance reveals distinct differences in left ventricular mass, geometry, and function. Circulation. 2013;127:197–206. doi: 10.1161/CIRCULATIONAHA.112.126920. [DOI] [PubMed] [Google Scholar]
- 12.Kowalski R.R., Beare R., Doyle L.W., Smolich J.J., Cheung M.M., Victorian Infant Collaborative Study Group Elevated blood pressure with reduced left ventricular and aortic dimensions in adolescents born extremely preterm. J Pediatr. 2016;172:75–80.e72. doi: 10.1016/j.jpeds.2016.01.020. [DOI] [PubMed] [Google Scholar]
- 13.Bonamy A.K., Martin H., Jorneskog G., Norman M. Lower skin capillary density, normal endothelial function and higher blood pressure in children born preterm. J Intern Med. 2007;262:635–642. doi: 10.1111/j.1365-2796.2007.01868.x. [DOI] [PubMed] [Google Scholar]
- 14.Bayrakci U.S., Schaefer F., Duzova A., Yigit S., Bakkaloglu A. Abnormal circadian blood pressure regulation in children born preterm. J Pediatr. 2007;151:399–403. doi: 10.1016/j.jpeds.2007.04.003. [DOI] [PubMed] [Google Scholar]
- 15.Poon C.Y., Wilson D.G., Joshi S., Fraser A.G., Kotecha S. Longitudinal evaluation of myocardial function in preterm infants with respiratory distress syndrome. Echocardiography. 2019;36:1713–1726. doi: 10.1111/echo.14462. [DOI] [PubMed] [Google Scholar]
- 16.Evensen K.A., Steinshamn S., Tjonna A.E. Effects of preterm birth and fetal growth retardation on cardiovascular risk factors in young adulthood. Early Hum Dev. 2009;85:239–245. doi: 10.1016/j.earlhumdev.2008.10.008. [DOI] [PubMed] [Google Scholar]
- 17.Johansson S., Iliadou A., Bergvall N. Risk of high blood pressure among young men increases with the degree of immaturity at birth. Circulation. 2005;112:3430–3436. doi: 10.1161/CIRCULATIONAHA.105.540906. [DOI] [PubMed] [Google Scholar]
- 18.Lewandowski A.J., Davis E.F., Yu G. Elevated blood pressure in preterm-born offspring associates with a distinct antiangiogenic state and microvascular abnormalities in adult life. Hypertension. 2015;65:607–614. doi: 10.1161/HYPERTENSIONAHA.114.04662. [DOI] [PubMed] [Google Scholar]
- 19.Sipola-Leppanen M., Karvonen R., Tikanmaki M. Ambulatory blood pressure and its variability in adults born preterm. Hypertension. 2015;65:615–621. doi: 10.1161/HYPERTENSIONAHA.114.04717. [DOI] [PubMed] [Google Scholar]
- 20.Boardman H., Birse K., Davis E.F. Comprehensive multi-modality assessment of regional and global arterial structure and function in adults born preterm. Hypertens Res. 2016;39:39–45. doi: 10.1038/hr.2015.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Goss K.N., Beshish A.G., Barton G.P. Early pulmonary vascular disease in young adults born preterm. Am J Respir Crit Care Med. 2018;198:1549–1558. doi: 10.1164/rccm.201710-2016OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cooper R., Atherton K., Power C. Gestational age and risk factors for cardiovascular disease: evidence from the 1958 British birth cohort followed to mid-life. Int J Epidemiol. 2009;38:235–244. doi: 10.1093/ije/dyn154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Markopoulou P., Papanikolaou E., Analytis A., Zoumakis E., Siahanidou T. Preterm birth as a risk factor for metabolic syndrome and cardiovascular disease in adult life: a systematic review and meta-analysis. J Pediatr. 2019;210:69–80.e65. doi: 10.1016/j.jpeds.2019.02.041. [DOI] [PubMed] [Google Scholar]
- 24.Crump C., Howell E.A., Stroustrup A. Association of preterm birth with risk of ischemic heart disease in adulthood. JAMA Pediatr. 2019;173:736–743. doi: 10.1001/jamapediatrics.2019.1327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lewandowski A.J. The preterm heart: a unique cardiomyopathy? Pediatr Res. 2019;85:738–739. doi: 10.1038/s41390-019-0301-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ahuja P., Sdek P., MacLellan W.R. Cardiac myocyte cell cycle control in development, disease, and regeneration. Physiol Rev. 2007;87:521–544. doi: 10.1152/physrev.00032.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bergmann O., Bhardwaj R.D., Bernard S. Evidence for cardiomyocyte renewal in humans. Science. 2009;324:98–102. doi: 10.1126/science.1164680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Laflamme M.A., Murry C.E. Heart regeneration. Nature. 2011;473:326–335. doi: 10.1038/nature10147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mollova M., Bersell K., Walsh S. Cardiomyocyte proliferation contributes to heart growth in young humans. Proc Natl Acad Sci U S A. 2013;110:1446–1451. doi: 10.1073/pnas.1214608110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bensley J.G., Stacy V.K., De Matteo R., Harding R., Black M.J. Cardiac remodelling as a result of pre-term birth: implications for future cardiovascular disease. Eur Heart J. 2010;31:2058–2066. doi: 10.1093/eurheartj/ehq104. [DOI] [PubMed] [Google Scholar]
- 31.Bensley J.G., Moore L., De Matteo R., Harding R., Black M.J. Impact of preterm birth on the developing myocardium of the neonate. Pediatr Res. 2018;83:880–888. doi: 10.1038/pr.2017.324. [DOI] [PubMed] [Google Scholar]
- 32.Stoll B.J., Hansen N.I., Bell E.F. Trends in care practices, morbidity, and mortality of extremely preterm neonates, 1993-2012. JAMA. 2015;314:1039–1051. doi: 10.1001/jama.2015.10244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kim M.Y., Eiby Y.A., Lumbers E.R. Effects of glucocorticoid exposure on growth and structural maturation of the heart of the preterm piglet. PLoS One. 2014;9 doi: 10.1371/journal.pone.0093407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lewandowski A.J., Bradlow W.M., Augustine D. Right ventricular systolic dysfunction in young adults born preterm. Circulation. 2013;128:713–720. doi: 10.1161/CIRCULATIONAHA.113.002583. [DOI] [PubMed] [Google Scholar]
- 35.Dahl M.J., Bowen S., Aoki T. Former-preterm lambs have persistent alveolar simplification at 2 and 5 months corrected postnatal age. Am J Physiol Lung Cell Mol Physiol. 2018;315:L816–L833. doi: 10.1152/ajplung.00249.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Corstius H.B., Zimanyi M.A., Maka N. Effect of intrauterine growth restriction on the number of cardiomyocytes in rat hearts. Pediatr Res. 2005;57:796–800. doi: 10.1203/01.PDR.0000157726.65492.CD. [DOI] [PubMed] [Google Scholar]
- 37.Stacy V., De Matteo R., Brew N. The influence of naturally occurring differences in birthweight on ventricular cardiomyocyte number in sheep. Anat Rec (Hoboken) 2009;292:29–37. doi: 10.1002/ar.20789. [DOI] [PubMed] [Google Scholar]
- 38.Tang Y., Nyengaard J.R., Andersen J.B., Baandrup U., Gundersen H.J. The application of stereological methods for estimating structural parameters in the human heart. Anat Rec (Hoboken) 2009;292:1630–1647. doi: 10.1002/ar.20952. [DOI] [PubMed] [Google Scholar]
- 39.Odri Komazec I., Posod A., Schwienbacher M. Aortic elastic properties in preschool children born preterm. Arterioscler Thromb Vasc Biol. 2016;36:2268–2274. doi: 10.1161/ATVBAHA.116.308144. [DOI] [PubMed] [Google Scholar]
- 40.Mohlkert L.A., Hallberg J., Broberg O. The preterm heart in childhood: left ventricular structure, geometry, and function assessed by echocardiography in 6-year-old survivors of periviable births. J Am Heart Assoc. 2018;7 doi: 10.1161/JAHA.117.007742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Le B., Ferreira P., Merchant S. Vol. 304. Anat Rec; Hoboken: 2020. Microarchitecture of the hearts in term and former-preterm lambs using diffusion tensor imaging, pp. 803–817. [DOI] [PubMed] [Google Scholar]
- 42.Tappia P.S., Ramjiawan B. Developmental origins of myocardial abnormalities in postnatal life (1) Can J Physiol Pharmacol. 2019;97:457–462. doi: 10.1139/cjpp-2018-0446. [DOI] [PubMed] [Google Scholar]
- 43.Botting K.J., Wang K.C., Padhee M. Early origins of heart disease: low birth weight and determinants of cardiomyocyte endowment. Clin Exp Pharmacol Physiol. 2012;39:814–823. doi: 10.1111/j.1440-1681.2011.05649.x. [DOI] [PubMed] [Google Scholar]
- 44.Mrocki M.M., Nguyen V.B., Lombardo P. Moderate preterm birth affects right ventricular structure and function and pulmonary artery blood flow in adult sheep. J Physiol. 2018;596:5965–5975. doi: 10.1113/JP275654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lock M.C., Tellam R.L., Botting K.J. The role of miRNA regulation in fetal cardiomyocytes, cardiac maturation and the risk of heart disease in adults. J Physiol. 2018;596:5625–5640. doi: 10.1113/JP276072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Brower G.L., Gardner J.D., Forman M.F. The relationship between myocardial extracellular matrix remodeling and ventricular function. Eur J Cardiothorac Surg. 2006;30:604–610. doi: 10.1016/j.ejcts.2006.07.006. [DOI] [PubMed] [Google Scholar]
- 47.Huckstep O.J., Williamson W., Telles F. Physiological stress elicits impaired left ventricular function in preterm-born adults. J Am Coll Cardiol. 2018;71:1347–1356. doi: 10.1016/j.jacc.2018.01.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Huckstep O.J., Burchert H., Williamson W. Impaired myocardial reserve underlies reduced exercise capacity and heart rate recovery in preterm-born young adults. Eur Heart J Cardiovasc Imaging. 2021;22:572–580. doi: 10.1093/ehjci/jeaa060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Jonker S.S., Giraud M.K., Giraud G.D. Cardiomyocyte enlargement, proliferation and maturation during chronic fetal anaemia in sheep. Exp Physiol. 2010;95:131–139. doi: 10.1113/expphysiol.2009.049379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bensley J.G., De Matteo R., Harding R., Black M.J. Three-dimensional direct measurement of cardiomyocyte volume, nuclearity, and ploidy in thick histological sections. Sci Rep. 2016;6:23756. doi: 10.1038/srep23756. [DOI] [PMC free article] [PubMed] [Google Scholar]