Abstract
Background
Whether advances in identification and management of atrial fibrillation and atrial flutter (collectively, AF) have led to improved outcomes is unclear. We sought to study trends in clinical outcomes selected as quality indicators for nonvalvular AF in Canada.
Methods
We identified hospitalized patients with a first diagnosis of nonvalvular AF between April 2006 and March 2015, in all of Canada except Quebec. We assessed trends in 1-year incidence of stroke/systemic embolism (SSE), major bleeding, and initial heart failure (HF) hospitalization.
Results
The cohort included 466,476 patients. The median age was 77 years (interquartile range, 68-84 years), 46% were female, and 68% had a Congestive Heart Failure, Hypertension, Age (≥75 years), Diabetes, Stroke/Transient Ischemic Attack, Vascular Disease, Age (65-74 years), Sex (Female) (CHA2DS2-VASc) score > 3. Within 1 year of discharge, 3.5% were hospitalized for stroke or SSE, 1.6% for major bleeding, and 8.6% for new HF. Over the study period, the crude rate of SSE declined from 3.6% to 3.3% (P = 0.002), whereas the rates of hospitalization for new HF and for major bleeding did not significantly change. After adjustment for CHA2DS2-VASc score, the yearly rates of incident SSE (risk ratio, 0.99; 95% confidence interval [CI], 0.98-0.99; P = 0.002) and HF (risk ratio, 0.99; 95% CI, 0.99-1.00; P = 0.001) declined ≤ 1% absolute, whereas major bleeding remained unchanged (risk ratio, 1.00; 95% CI, 0.99-1.00; P = 0.28).
Conclusions
Among hospitalized patients with nonvalvular AF in Canada, the rate of SSE and new HF decreased modestly over a 10-year period, with no significant change in major bleeding. Efforts to study process-based quality indicators, with increased focus on HF prevention, are needed.
Résumé
Contexte
On ne sait pas si les avancées en matière de détection et de prise en charge de la fibrillation auriculaire et du flutter auriculaire (collectivement appelés « FA » ci-après) ont permis d’améliorer les résultats pour les patients. Nous avons donc étudié les tendances à l’égard de résultats cliniques particuliers pris comme indicateurs de qualité relatifs à la FA non valvulaire au Canada.
Méthodologie
Nous avons recensé les patients hospitalisés au Canada (sauf au Québec) entre avril 2006 et mars 2015 en raison d’une FA non valvulaire nouvellement diagnostiquée. Nous avons évalué les tendances quant à la survenue d’un accident vasculaire cérébral ou d’une embolie systémique (AVC/ES), d’une hémorragie majeure et d’une première hospitalisation pour insuffisance cardiaque.
Résultats
La cohorte comprenait 466 476 patients, dont l’âge médian était de 77 ans (intervalle interquartile : 68 à 84 ans); 46 % des patients étaient des femmes, et 68 % avaient un score CHA2DS2-VASc (Congestive Heart Failure [insuffisance cardiaque congestive], hypertension, âge [≥ 75 ans], diabète, Stroke/Transient Ischemic Attack [AVC/accident ischémique transitoire] – maladie vasculaire, âge [65-74 ans], sexe [femmes]) supérieur à 3. Dans l’année suivant la sortie de l’hôpital, 3,5 % des patients ont été hospitalisés en raison d’un AVC ou d’un AVC/ES, 1,6 %, en raison d’une hémorragie majeure et 8,6 %, en raison d’une nouvelle insuffisance cardiaque. Au cours de la période étudiée, le taux brut d’AVC/ES est passé de 3,6 % à 3,3 % (p = 0,002), tandis que les taux d’hospitalisation en raison d’une nouvelle insuffisance cardiaque ou d’une hémorragie majeure n’ont pas changé de manière significative. Après correction pour tenir compte du score CHA2DS2-VASc, les taux annuels de survenue d’un AVC/ES (rapport des risques de 0,99; intervalle de confiance [IC] à 95 % : de 0,98 à 0,99; p = 0,002) et d’une insuffisance cardiaque (rapport des risques de 0,99; IC à 95 % : de 0,99 à 1,00; p = 0,001) ont diminué de ≤ 1 % en valeur absolue, tandis que le taux de survenue d’une hémorragie majeure n’a pas changé (rapport des risques : 1,00; IC à 95 % : de 0,99 à 1,00; p = 0,28).
Conclusions
Parmi les patients hospitalisés au Canada en raison d’une FA non valvulaire, les taux d’AVC/ES et de nouvelle insuffisance cardiaque ont affiché une réduction modeste sur une période de 10 ans, tandis que le taux d’hémorragie majeure n’a pas changé de manière significative. D’autres études évaluant les indicateurs de qualité fondés sur les procédés, notamment en matière de prévention de l’insuffisance cardiaque, s’imposent.
Atrial fibrillation and the related arrhythmia atrial flutter (collectively, AF) are the most common heart rhythm disorders encountered in clinical practice. AF has a global prevalence approaching 1%, with increasing incidence and prevalence because of population aging and changing patterns of risk factors.1, 2, 3 AF leads to debilitating symptoms, and is associated with important clinical sequelae: notably a fivefold increase in the risk of ischemic stroke or systemic embolism (SSE), a threefold increased risk of developing heart failure (HF), and a near doubling in mortality.4, 5, 6 Furthermore, the costs of AF care have been estimated at > CAD$5400 annually per patient.7
These facts have prompted several cardiovascular professional societies to develop and monitor a series of quality indicators (QIs) designed to track adherence to evidence-based processes as well as the outcomes of AF care.8, 9, 10 In Canada, this effort has been led by the Canadian Cardiovascular Society. As part of this work, the AF Quality Working Group published an initial set of QIs in 2016.11 In that report, priority QIs were identified and defined in 3 distinct categories: access to care, treatment, and outcomes. After completing an environmental scan of available data assets, the working group published a second report that included an expanded list of QIs and proposed methods to exploit currently available data sets to report on national trends for each QI category.12 In this article, we report national temporal trends over a 10-year period for 3 outcome QIs among hospitalized patients with nonvalvular AF: stroke or SSE, major hemorrhage, and new-onset HF.
Methods
Data source and cohort identification
The Canadian Institute for Health Information (CIHI) is a federally funded agency that collects health administrative data from provincial health authorities. For this analysis, CIHI supplied data from the Discharge Abstract Database (DAD), which contains complete data for all inpatient hospitalizations and surgical procedures, covering all Canadian provinces and territories except Quebec. Primary and secondary diagnoses are recorded in the DAD using the International Classification of Diseases, Tenth Revision, Clinical Modification (ICD10-CM). As previously published,12 Supplemental Table S1 includes the ICD10-CM diagnosis and Canadian Classification of Health Interventions codes we used to define the study population, identify comorbidities, and assess outcomes. The University of Alberta Health Research Ethics Board approved this project, as well as the waiver of individual informed consent.
The study cohort included all patients aged 20 years or older with ≥ 1 hospital discharges with a diagnosis of AF (ICD10-CM code I48.x) in any position in the discharge record, between April 2006 and March 2015. In Canada, there is no population-based national data set for outpatient or emergency care, so patients with AF not requiring hospitalization could not be identified. This widely accepted case definition for AF identification within the DAD has been validated against chart review in a primary care setting, and was reported to be insensitive (44.8%) but highly specific (99.8%).13 We excluded patients with a hospital discharge diagnosis of AF in the 2 years before the study period. We also excluded patients with a history of valvular heart disease or of valve surgery, and patients who died during the index hospitalization. In the case of multiple hospitalizations over the study period, the first hospitalization with nonvalvular AF was defined as the index hospitalization.
For risk adjustment and stratification we calculated baseline Congestive Heart Failure, Hypertension, Age, Diabetes, Stroke/Transient Ischemic Attack (CHADS2) and Congestive Heart Failure, Hypertension, Age (≥75 years), Diabetes, Stroke/Transient Ischemic Attack, Vascular Disease, Age (65-74 years), Sex (Female) (CHA2DS2-VASc) scores (C, congestive HF: 1 point; H, hypertension: 1 point; A, age: 1 point for age ≥ 75 years in CHADS2, 2 points for age ≥ 75 and 1 point for age 65-74 years in CHA2DS2-VASc; D, diabetes mellitus: 1 point; S, stroke or transient ischemic attack: 2 points; V, vascular disease, defined as a history of coronary artery disease or peripheral vascular disease: 1 point; Sc, sex category female: 1 point) using algorithms validated for identifying these conditions from administrative data.14,15
Study outcomes
All patients were followed for 1 year from the index discharge date for the occurrence of a hospitalization for SSE, major bleeding, or HF, using the definitions applied in a recent publication from our group.12,15 Supplemental Table S1 includes the ICD10-CM codes used to define these outcomes. Primary and secondary diagnoses were included. Stroke was subclassified as ischemic or hemorrhagic, and transient ischemic attacks (ICD10-CM G45, H34.0) were excluded as outcomes because lack of specificity.14 Hemorrhagic stroke was also counted as a major bleeding event.
Statistical analysis
Baseline characteristics are presented as median and interquartile range for continuous variables and absolute number with proportion for categorical variables. Cochrane Armitage trend or χ2 tests were performed for categorical data and nonparametric Kruskal-Wallis test was done for continuous data. We used a Poisson regression model to assess outcome rates over time and across provinces. Crude incidence was calculated as the number of patients who developed the given outcome within 1-year of the index discharge divided by the number of index AF patients. Crude incidences of outcomes were stratified according to sex. To assess temporal trends in outcomes over time after adjustment for risk scores, 2 separate Poisson regression models were performed; one included CHADS2 and fiscal year as covariates and the other model included CHA2DS2-VASc score and fiscal years. For assessment of the risk-adjusted temporal trend in outcomes, patients with previous HF were excluded from the analysis of this outcome. All analyses were performed using SAS version 9.4 (SAS Institute Inc, Cary, NC).
Results
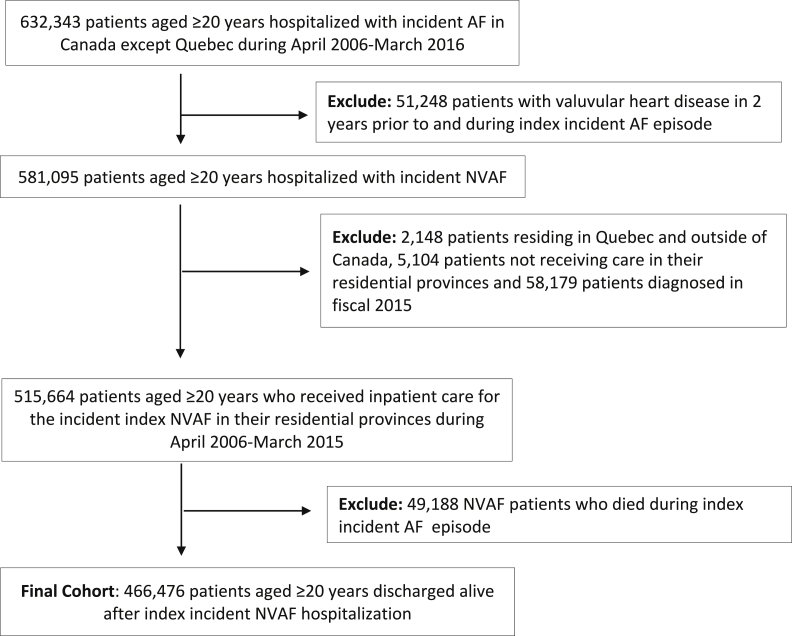
The formation of the study cohort is shown in Figure 1. After applying exclusion criteria, the final cohort consisted of 466,476 patients with a first hospitalization with a diagnosis of AF between April 2006 and March 2015 who were followed for 1 year. Although total mortality was not included in the data set, 33,034 (7.1%) patients died in-hospital during follow-up.
Figure 1.
Description of the study cohort. AF, atrial fibrillation; NVAF, nonvalvular atrial fibrillation.
Table 1 includes a summary of the baseline characteristics of the cohort. The median age was 77 years and 46% were female. The median CHADS2 score was 2 and the median CHA2DS2-VASc score was 3. The most common stroke risk factors were hypertension (43.8%), vascular disease (28.0%), and HF (25.5%), whereas 7.5% had a history of SSE. There were significant differences in all baseline characteristics between women and men.
Table 1.
Baseline characteristics of study cohort
| Variable | Female | Male | Total |
|---|---|---|---|
| Total | 213,475 (45.8) | 253,001 (54.2) | 466,476 |
| Median age (IQR), years | 80.0 (72.0-86.0) | 74.0 (65.0-82.0) | 77 (68-84) |
| Age < 65 years | 25,578 (12.0) | 63,111 (24.9) | 88,689 (19.0) |
| Age 65-74 years | 41,617 (19.5) | 67,032 (26.5) | 108,649 (23.2) |
| Age 75-84 years | 78,291 (36.7) | 81,988 (32.4) | 160,279 (34.3) |
| Age ≥ 85 years | 67,989 (31.8) | 40,870 (16.2) | 108,859 (23.3) |
| CHADS2 | |||
| 0 | 26760 (12.5) | 50492 (20.0) | 77252 (16.6) |
| 1 | 63442 (29.7) | 79194 (31.3) | 142636 (30.6) |
| 2 | 66573 (31.2) | 69275 (27.4) | 135848 (29.1) |
| 3 | 36746 (17.2) | 36456 (14.4) | 73202 (15.7) |
| 4 | 15598 (7.3) | 13760 (5.4) | 29358 (6.3) |
| 5 | 3736 (1.8) | 3320 (1.3) | 7056 (1.5) |
| 6 | 620 (0.3) | 504 (0.2) | 1124 (0.2) |
| Median CHADS2 (IQR) | 2 (1-3) | 1 (1-2) | 2 (1-2) |
| CHA2DS2-VASc | |||
| 0 | 0 (0.0) | 24,456 (9.7) | 24,456 (5.2) |
| 1 | 11,422 (5.4) | 33,930 (13.4) | 45,352 (9.7) |
| 2 | 19,781 (9.3) | 59,773 (23.6) | 79,554 (17.1) |
| 3 | 53,085 (24.9) | 59,893 (23.7) | 112,978 (24.2) |
| 4 | 58,326 (27.3) | 43,245 (17.1) | 101,571 (21.8) |
| 5 | 40,185 (18.8) | 22,429 (8.9) | 62,614 (13.4) |
| 6 | 21,892 (10.3) | 7548 (3.0) | 29,440 (6.3) |
| 7 | 7144 (3.3) | 1414 (0.6) | 8558 (1.8) |
| 8 | 1351 (0.6) | 313 (0.1) | 1664 (0.4) |
| 9 | 289 (0.1) | 0 (0.0) | 289 (0.1) |
| Median CHA2DS2-VASc (IQR) | 4 (3-5) | 3 (2-4) | 3 (2-4) |
| Pre-existing conditions | |||
| Diabetes | 49,707 (23.3) | 69,894 (27.6) | 119,601 (25.6) |
| Hypertension | 97,384 (45.6) | 106,784 (42.2) | 204,168 (43.8) |
| Coronary artery disease | 31,880 (14.9) | 67,496 (26.7) | 99,376 (21.3) |
| Vascular disease | 46,247 (21.7) | 84,304 (33.3) | 130,551 (28.0) |
| Heart failure | 56,541 (26.5) | 62,288 (24.6) | 118,829 (25.5) |
| Stroke/TIA | 20,853 (9.8) | 19,976 (7.9) | 40,829 (8.8) |
| Stroke/embolism | 17,805 (8.3) | 17,092 (6.8) | 34,897 (7.5) |
Data are presented as n (%) except where otherwise noted. All comparisons according to sex were statistically significant (P < 0.0001).
CHADS2, Congestive Heart Failure, Hypertension, Age, Diabetes, Stroke/Transient Ischemic Attack; CHA2DS2-VASc, Congestive Heart Failure, Hypertension, Age (≥75 years), Diabetes, Stroke/Transient Ischemic Attack, Vascular Disease, Age (65-74 years), Sex (Female); IQR, interquartile range; TIA, transient ischemic attack.
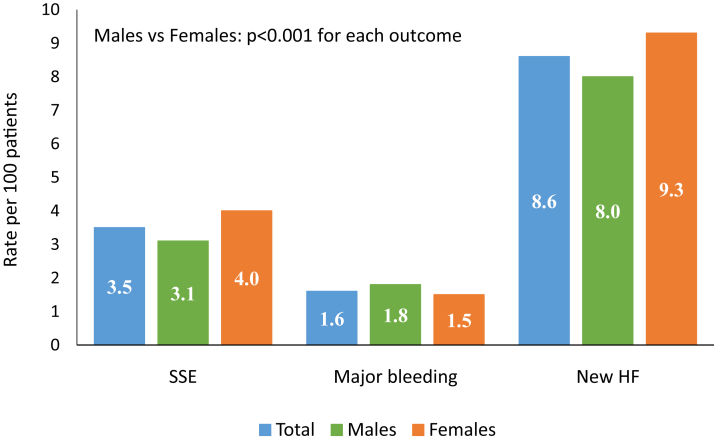
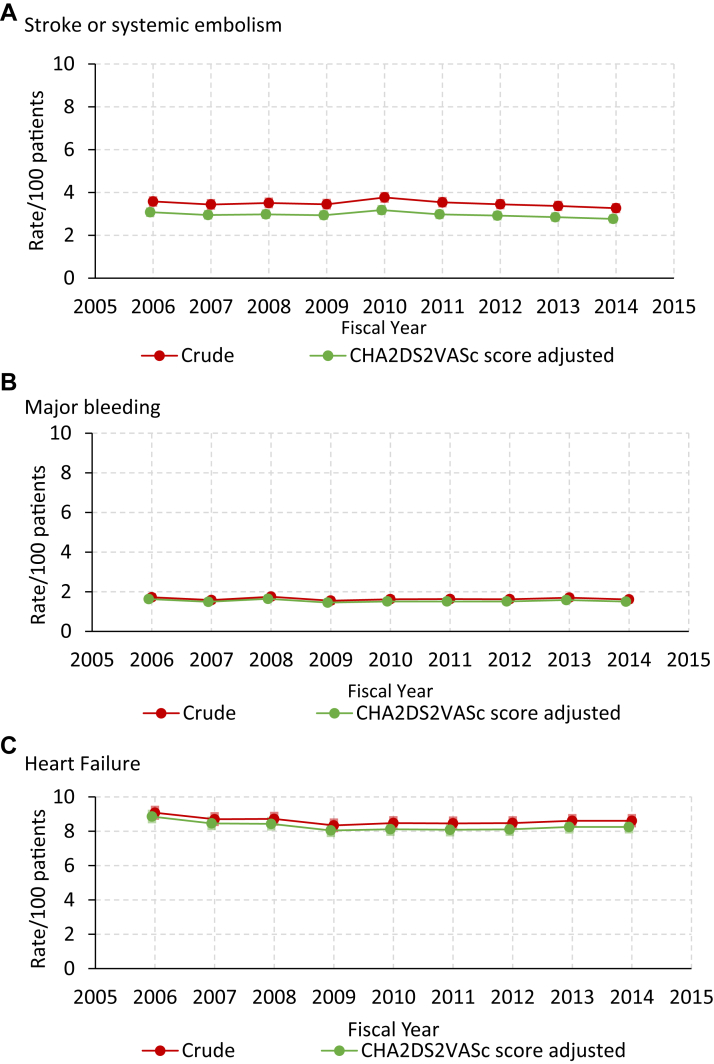
Figure 2 shows the unadjusted 1-year outcomes for the entire cohort stratified according to sex. Overall, the incidence of SSE was 3.5%, including 12,549 (2.7%) ischemic strokes, and 3062 (0.7%) hemorrhagic strokes. A total of 7661 patients (1.6%) had a hospital discharge for major bleeding. Finally, of 347,647 patients without a previous hospitalization for HF 29,929 (8.6%), had a first hospitalization for HF. Compared with men, women had a higher crude incidence of SSE and HF, but a slightly lower incidence of major bleeding. The crude national trends in each outcome are shown in Table 2. Over the study period, there were modest but statistically significant reductions in the rate of SSE, ischemic stroke, and HF hospitalization, but no measurable change in the rate of hemorrhagic stroke, or major bleeding. Figure 3 shows trends in the incidence of SSE (panel A), major bleeding (panel B), and HF hospitalization (panel C), before and after adjustment for the components of the CHA2DS2-VASc scores. Crude and adjusted analyses showed an absolute decline in the rate of SSE ≤ 1.0% between 2006 and 2014 (all P < 0.01). Incident admissions for HF also declined by ≤ 1.0% after adjustment for CHADS2 (P < 0.001) and CHA2DS2-VASc scores (P < 0.001). Trends in SSE and bleeding outcomes were similar in women and men (Supplemental Table S2). However, there appeared to be differences in trends for SSE according to age. The rate of SSE for those aged < 65 years rose significantly during the study period, whereas it fell in those aged ≥ 75 years (Supplemental Table S3 and Supplemental Fig. S1).
Figure 2.
One-year outcomes among patients discharged after hospitalization with a new diagnosis of nonvalvular atrial fibrillation. HF, heart failure; SSE, systemic embolism.
Table 2.
One-year crude outcome incidence according to fiscal year
| Variable | 2006 | 2007 | 2008 | 2009 | 2010 | 2011 | 2012 | 2013 | 2014 | Total | P∗ |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Patient n | 53,544 | 50,494 | 50,178 | 50,033 | 50,505 | 51,998 | 52,803 | 53,066 | 53,855 | 466,476 | |
| Stroke/embolism, n (%) | 1917 (3.6) | 1737 (3.4) | 1761 (3.5) | 1728 (3.5) | 1904 (3.8) | 1843 (3.5) | 1822 (3.5) | 1790 (3.4) | 1760 (3.3) | 16,262 (3.5) | 0.002 |
| Ischemic stroke, n (%) | 1498 (2.8) | 1341 (2.7) | 1357 (2.7) | 1329 (2.7) | 1468 (2.9) | 1403 (2.7) | 1405 (2.7) | 1389 (2.6) | 1359 (2.5) | 12,549 (2.7) | 0.016 |
| Hemorrhagic stroke, n (%) | 352 (0.7) | 311 (0.6) | 359 (0.7) | 320 (0.6) | 357 (0.7) | 358 (0.7) | 335 (0.6) | 341 (0.6) | 329 (0.6) | 3062 (0.7) | 0.32 |
| Major bleeding, n (%) | 923 (1.7) | 798 (1.6) | 880 (1.8) | 774 (1.5) | 820 (1.6) | 845 (1.6) | 856 (1.6) | 900 (1.7) | 865 (1.6) | 7661 (1.6) | 0.16 |
| Patient n† | 39,069 | 37,180 | 36,918 | 37,252 | 37,886 | 39,297 | 39,973 | 39,887 | 40,185 | 347,647 | |
| New HF, n (%) | 3547 (9.1) | 3235 (8.7) | 3221 (8.7) | 3106 (8.3) | 3214 (8.5) | 3326 (8.5) | 3388 (8.5) | 3433 (8.6) | 3459 (8.6) | 29,929 (8.6) | 0.021 |
AF, atrial fibrillation; HF, heart failure.
P value from Cochrane Armitage trend test.
Excluding patients with previous 2-year history of HF/HF during the index AF episode.
Figure 3.
Temporal trends in the 1-year incidence of hospitalization for (A) stroke or systemic embolism, (B) major bleeding, and (C) congestive heart failure. Patients with history of heart failure admission were excluded from heart failure analysis. CHADS2 adjusted incidence was nearly identical to CHA2DS2-VASc-adjusted incidence, is not displayed. CHADS2, Congestive Heart Failure, Hypertension, Age, Diabetes, Stroke/Transient Ischemic Attack; CHA2DS2-VASc, Congestive Heart Failure, Hypertension, Age (≥75 years), Diabetes, Stroke/Transient Ischemic Attack, Vascular Disease, Age (65-74 years), Sex (Female);
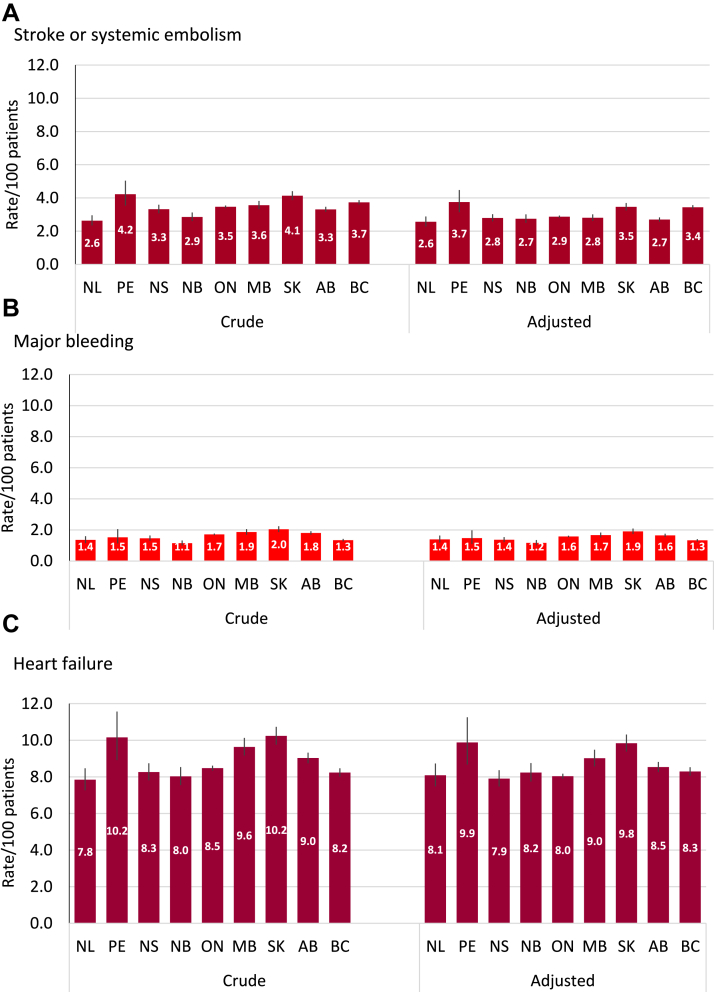
Supplemental Table S4 shows the interprovincial comparison in the crude 1-year incidence of each study outcome. Sample size ranged from 261,632 (50.7%) for Canada’s most populous province of Ontario, to 847 (0.16%) for the combined Territories of Nunavut, Northwest Territories, and Yukon. There were statistically significant differences between jurisdictions for each outcome, although absolute differences between provinces were not marked. For example, the 1-year rate of SSE ranged from 2.3% (281 events) in Newfoundland and Labrador to 3.9% (122 events) in Prince Edward Island. The incidence of major bleeding ranged from 1.1% (188 events) in New Brunswick to 2.0% (17 events) in the Territories, and incident HF hospitalizations ranged from 7.8% (652 events) in Newfoundland and Labrador to 10.2% (1706 events) in Saskatchewan. Adjustment for CHA2DS2-VASc score attenuated some of the interprovincial differences in these outcomes (Fig. 4).
Figure 4.
Provincial rates (crude and CHA2DS2-VASc-adjusted) for (A) stroke or systemic embolism, (B) major bleeding, and (C) heart failure. AB, Alberta; BC, British Columbia; CHA2DS2-VASc, Congestive Heart Failure, Hypertension, Age (≥75 years), Diabetes, Stroke/Transient Ischemic Attack, Vascular Disease, Age (65-74 years), Sex (Female); MB, Manitoba; NB, New Brunswick; NL, Newfoundland and Labrador; NS, Nova Scotia; ON, Ontario; PE, Prince Edward Island; SK, Saskatchewan.
Discussion
To our knowledge, this analysis is the first to report on QIs related to outcomes of hospitalized Canadians with a diagnosis of nonvalvular AF at a national level. We included almost half a million patients discharged from hospital for the first time with AF as a primary or secondary diagnosis over a 10-year period from 2006 to 2015, and report on trends for 3 patient-important outcomes identified as QIs: stroke or SSE, major bleeding, and new-onset HF. The main findings are: (1) that the risk-adjusted 1-year rate of SSE declined slightly during the study period, but remains at approximately 3%; (2) that incidence of major hemorrhage have not changed significantly; (3) that the rate of incident HF is more than twice that of SSE, and also declined modestly over the study period; and (4) that there is statistically significant but clinically modest interprovincial variation in these outcomes.
Contemporary trends in stroke, SSE, and bleeding in patients with nonvalvular AF
Although we did observe a statistically significant decline in the rate of SSE within 1 year of index hospitalization, its magnitude is perhaps less than might have been expected because of the important changes in AF management that occurred during the study period. First, stroke risk stratification has been transformed by the 2010 publication of the CHA2DS2-VASc and Hypertension, Abnormal Renal/Liver Function, Stroke, Bleeding History or Predisposition, Labile INR, Elderly (> 65 Years), Drugs/Alcohol Concomitantly (HAS-BLED) scores, which have subsequently been widely adopted.16, 17, 18, 19 Compared with the older CHADS2 score,20 use of the CHA2DS2-VASc score results in classification of > 60% of previously low- or intermediate-risk patients as having a definite indication for oral anticoagulation.21 The second and even more important change has been the introduction of direct-acting oral anticoagulants (DOACs), beginning with dabigatran in 2010. These agents overcome many of the barriers that historically limited uptake of vitamin K antagonists, and in clinical trials have been shown to be either superior or noninferior to warfarin for reducing the risk of ischemic stroke or SSE in patients with nonvalvular AF, with reduced risk of hemorrhagic stroke.22, 23, 24, 25 Combining these factors, we would expect to see a larger pool of patients with AF being treated with safer and potentially more effective agents, which would in theory result in a larger effect on outcomes than we have observed in this analysis. In fact, more pronounced positive trends in SSE outcomes have been observed over this time frame in other jurisdictions. For example, in a recent Swedish population-based study patients newly diagnosed with AF between 2011 and 2013 showed that the 1-year rate of ischemic stroke declined by 1% absolute (from 2.9% to 1.9%),26 compared with a change from 2.7% to 2.6% over those years in our sample. Of note, that study included outpatients and inpatients, and it is possible that changes in stroke risk stratification and therapy are more pronounced and rapid in a healthier outpatient population. Contradicting this hypothesis is our finding of a rising risk-adjusted incidence of SSE in those aged younger than 65 years. Furthermore, the study population was from a single county that is home to 13% of Sweden’s population, so generalizability to the Canadian populace is not assured.
The proportion of at-risk patients receiving oral anticoagulant therapy and the quality of therapy delivery are important factors likely mediating the trends in SSE and bleeding outcomes. Although we did not have access to prescription data for this analysis, there are recent data from Canada and elsewhere that can provide insight into our findings. In a population-based study conducted in the province of Alberta, Canada over a similar time period (2009-2015), in which pharmacy data were available, we found a rapid increase in prescriptions for DOACs that was counterbalanced by a decline in prescriptions for warfarin, with the result that there was a < 5% absolute increase in the overall proportion of patients at high risk of stroke who were anticoagulated.27 In that study we also did not observe significant reductions in the rates of stroke or bleeding events with time. It is important to recognize that despite their benefits, there are practical barriers to uptake of DOACs. Inclusion of these new drugs on public formularies and coverage by major insurers did not begin until 2012, with coverage decisions differing somewhat between provinces. An analysis of the overall Canadian market for oral anticoagulants showed that by 2014 warfarin continued to account for approximately 70% of prescriptions (for all indications), with substantial variability across medical specialties.28 Thus, it will be important to update our analysis to determine if further uptake of DOACs has now more substantially begun to influence outcomes.
Dosing of DOACs is also important: population-based and registry studies have consistently reported higher proportions of patients receiving reduced doses of dabigatran, rivaroxaban, or apixaban than would be expected on the basis of drug monographs. For example, a 2019 claims-based study from France showed that although the introduction of direct oral anticoagulants was associated with an overall increase in prescriptions for oral anticoagulants and a decline in the use of acetyl salicylic acid, 40% of prescriptions for DOAC were for a low dose.29 A similar pattern was observed in a Danish population-based study.30 Furthermore, a US multi-centre registry classified DOAC dosing according to established parameters, and showed that a significant but small percentage of patients were either under- or over-dosed, which in either case was associated with adverse clinical events.31 Unfortunately the current data set does not allow us to conclude whether, in the Canadian context, the significant costs borne by patients and payers associated with the shift from warfarin to DOACs over this time period have been worthwhile. Ongoing work by our group incorporating patient-level prescription data in a national sample will help to address this issue.
Factors other than the quality of stroke prevention therapy might also have affected our results. Although our analysis of trends adjusted for covariates contained in the CHA2DS2-VASc score, we could not account for shifts in population burden of other covariates or clinical frailty phenotypes.
Prevention of HF as a QI for AF?
Although preventing SSE has rightly long been a focus of AF management, the bidirectional association between AF and HF is receiving increasing attention. These 2 common cardiovascular conditions often coexist, and an extensive literature now documents that each can cause and/or exacerbate the other.32,33 The finding in this study that > 8% of patients discharged from hospital with AF will have a first admission for HF within 1 year, a rate that is almost double that of hospitalization for SSE, is cause for concern. Hospitalization for HF carries important prognostic implications—such as a twofold or greater increment in near-term mortality34,35—and is an adverse event that has importance to patients as well as to clinicians, and health care administrators.36 Furthermore, at least some HF hospitalizations among patients with AF are likely preventable with early identification of ventricular or valvular dysfunction and careful attention to rate and/or rhythm control. Partly for this reason, current AF guidelines recommend performing echocardiography as part of the initial evaluation of patients with AF.18,19,37 However, this measure was not included in the final list of QIs or performance measures in either Canada or the United States.8,11 On the basis of these findings, we propose that surveillance of rates of HF hospitalization among patients with AF continue at a national level, and that future iterations of national QIs include process measures that focus on prevention and management of HF and its consequences.
Limitations
A number of limitations need to be considered in the interpretation of this report. First, our case definition of AF required a hospital discharge, as this is the only definition that can currently be applied at a national level in Canada and that permits interprovincial comparisons. Although a DAD diagnosis of AF is highly specific, it is not sensitive, and biases our sample toward an older, more comorbid subset of the entire population with AF.13 This will somewhat inflate the absolute event rates observed, and could underlie differences between trends in events observed in this cohort and the recent Swedish one, as described previously. Second, for administrative reasons we lacked data from Quebec, Canada’s second most populous province. Third, we did not have access to prescription data, or other important clinical detail such as laboratory or cardiac imaging data, which were not available at a national level. Fourth, CIHI data sets do not include deaths that occur outside of hospital, so we are unable to comment on trends in overall mortality, which is a competing event for our outcomes of interest. A recent population-based study from Ontario showed no significant change in the risk of all-cause death within 12 months of an initial hospitalization or emergency room visit for AF between 2007 and 2015.38 This suggests that the trends we observed might not have been influenced by changes in mortality. Fifth, some patients might have been misclassified as free from HF at the time of index discharge if they had pre-existing HF not leading to hospitalization. Finally, we caution that the findings of the interprovincial comparisons should be considered exploratory. As expected, we did find some statistically significant differences in outcomes measures between provinces. However, the reported differences in crude and adjusted rates are all small in absolute terms. Furthermore, the highest and lowest event rates tended to be found in the provinces with the smallest populations and number of events, limiting the precision of these estimates.
Conclusions
Over a 10-year period spanning the introduction of new oral anticoagulant medications in Canada, we observed a modest decrease in the incidence of SSE, with no significant change in major bleeding, among hospitalized patients with nonvalvular AF. However, of concern was the observation of the 1-year risk of new HF hospitalization being more than twice as high as that of SSE. Future QIs work will focus on addressing barriers to implementing effective stroke prevention therapy, and on preventing HF in this vulnerable population.
Acknowledgements
The authors thank Dr Paul Dorian, Chair of the Canadian Cardiovascular Society Quality Project, for supporting this initiative and providing input into this report. We are also indebted to the other members of the Atrial Fibrillation Quality Working Group: Dr Noah Ivers, Derek Lefebvre, Garth Oakes, and Dr Rodney Zimmerman.
Funding Sources
Logistical support was provided by the Canadian Cardiovascular Society Quality Project.
Disclosures
Dr Wilton received research grants from Medtronic Canada, Abbott, and Boston Scientific, and consultancy fees from Arca Biopharma. Dr Deyell received research grants and honoraria from Biosense Webster, and honoraria from Abbott, BMS, and Servier. Dr Cox has received research grant funding and consultancy fees from Bayer. The remaining authors have no conflicts of interest to disclose.
Footnotes
Ethics Statement: The University of Alberta Health Research Ethics Board approved this project, as well as the waiver of individual informed consent. The research was conducted in accordance with the principles of the Declaration of Helsinki.
See page 617 for disclosure information.
To access the supplementary material accompanying this article, visit CJC Open at https://www.cjcopen.ca/ and at https://doi.org/10.1016/j.cjco.2021.01.003.
Supplementary Material
References
- 1.Chugh S.S., Havmoeller R., Narayanan K., et al. Worldwide epidemiology of atrial fibrillation: a Global Burden of Disease 2010 Study. Circulation. 2014;129:837–847. doi: 10.1161/CIRCULATIONAHA.113.005119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lloyd-Jones D.M., Wang T.J., Leip E.P., et al. Lifetime risk for development of atrial fibrillation: the Framingham Heart Study. Circulation. 2004;110:1042–1046. doi: 10.1161/01.CIR.0000140263.20897.42. [DOI] [PubMed] [Google Scholar]
- 3.Miyasaka Y., Barnes M.E., Gersh B.J., et al. Secular trends in incidence of atrial fibrillation in Olmsted County, Minnesota, 1980 to 2000, and implications on the projections for future prevalence. Circulation. 2006;114:119–125. doi: 10.1161/CIRCULATIONAHA.105.595140. [DOI] [PubMed] [Google Scholar]
- 4.Wolf P.A., Abbott R.D., Kannel W.B. Atrial fibrillation as an independent risk factor for stroke: the Framingham Study. Stroke. 1991;22:983–988. doi: 10.1161/01.str.22.8.983. [DOI] [PubMed] [Google Scholar]
- 5.Benjamin E.J., Wolf P.A., D’Agostino R.B., et al. Impact of atrial fibrillation on the risk of death: the Framingham Heart Study. Circulation. 1998;98:946–952. doi: 10.1161/01.cir.98.10.946. [DOI] [PubMed] [Google Scholar]
- 6.Stewart S., Hart C.L., Hole D.J., McMurray J.J.V. A population-based study of the long-term risks associated with atrial fibrillation: 20-year follow-up of the Renfrew/Paisley study. Am J Med. 2002;113:359–364. doi: 10.1016/s0002-9343(02)01236-6. [DOI] [PubMed] [Google Scholar]
- 7.Wodchis W.P., Bhatia R.S., Leblanc K., Meshkat N., Morra D. A review of the cost of atrial fibrillation. Value Health. 2012;15:240–248. doi: 10.1016/j.jval.2011.09.009. [DOI] [PubMed] [Google Scholar]
- 8.Heidenreich P.A., Solis P., Mark Estes N.A., et al. 2016 ACC/AHA clinical performance and quality measures for adults with atrial fibrillation or atrial flutter. Circ Cardiovasc Qual Outcomes. 2016;9:443–488. doi: 10.1161/HCQ.0000000000000018. [DOI] [PubMed] [Google Scholar]
- 9.Kirchhof P., Breithardt G., Bax J., et al. A roadmap to improve the quality of atrial fibrillation management: proceedings from the fifth Atrial Fibrillation Network/European Heart Rhythm Association consensus conference. Europace. 2016;18:37–50. doi: 10.1093/europace/euv304. [DOI] [PubMed] [Google Scholar]
- 10.Lee E., Choi E.K., Han K.D., et al. Mortality and causes of death in patients with atrial fibrillation: a nationwide population-based study. PLoS One. 2018;13 doi: 10.1371/journal.pone.0209687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cox J.L., Dai S., Gong Y., et al. The development and feasibility assessment of Canadian Quality Indicators for atrial fibrillation. Can J Cardiol. 2016;32:1566–1569. doi: 10.1016/j.cjca.2016.02.059. [DOI] [PubMed] [Google Scholar]
- 12.Sandhu R.K., Wilton S.B., Cruz J., et al. An update on the development and feasibility assessment of Canadian Quality Indicators for atrial fibrillation and atrial flutter. CJC Open. 2019;1:198–205. doi: 10.1016/j.cjco.2019.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tu K., Nieuwlaat R., Cheng S.Y., et al. Identifying patients with atrial fibrillation in administrative data. Can J Cardiol. 2016;32:1561–1565. doi: 10.1016/j.cjca.2016.06.006. [DOI] [PubMed] [Google Scholar]
- 14.Kokotailo R.A., Hill M.D. Coding of stroke and stroke risk factors using International Classification of Diseases, revisions 9 and 10. Stroke. 2005;36:1776–1781. doi: 10.1161/01.STR.0000174293.17959.a1. [DOI] [PubMed] [Google Scholar]
- 15.Quan H., Sundararajan V., Halfon P., et al. Coding algorithms for defining comorbidities in ICD-9-CM and ICD-10 administrative data. Med Care. 2005;43:1130–1139. doi: 10.1097/01.mlr.0000182534.19832.83. [DOI] [PubMed] [Google Scholar]
- 16.Lip G.Y., Nieuwlaat R., Pisters R., Lane D.A., Crijns H.J. Refining clinical risk stratification for predicting stroke and thromboembolism in atrial fibrillation using a novel risk factor-based approach: the Euro Heart Survey on Atrial Fibrillation. Chest. 2010;137:263–272. doi: 10.1378/chest.09-1584. [DOI] [PubMed] [Google Scholar]
- 17.Pisters R., Lane D.A., Nieuwlaat R., et al. A novel user-friendly score (HAS-BLED) to assess 1-year risk of major bleeding in patients with atrial fibrillation: the Euro Heart Survey. Chest. 2010;138:1093–1100. doi: 10.1378/chest.10-0134. [DOI] [PubMed] [Google Scholar]
- 18.January C.T., Wann L.S., Alpert J.S., et al. 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the Heart Rhythm Society. J Am Coll Cardiol. 2014;64:e1–76. doi: 10.1016/j.jacc.2014.03.022. [DOI] [PubMed] [Google Scholar]
- 19.Kirchhof P., Benussi S., Kotecha D., et al. 2016 ESC guidelines for the management of atrial fibrillation. Developed in collaboration with EACTS. Eur Heart J. 2016;37:2893–2962. doi: 10.1093/eurheartj/ehw210. [DOI] [PubMed] [Google Scholar]
- 20.Gage B.F., Waterman A.D., Shannon W., et al. Validation of clinical classification schemes for predicting stroke. JAMA. 2001;285:2864–2870. doi: 10.1001/jama.285.22.2864. [DOI] [PubMed] [Google Scholar]
- 21.Katz D.F., Maddox T.M., Turakhia M., et al. Contemporary trends in oral anticoagulant prescription in atrial fibrillation patients at low to moderate risk of stroke after guideline-recommended change in use of the CHADS2 to the CHA2DS2-VASc score for thromboembolic risk assessment: analysis from the National Cardiovascular Data Registry’s Outpatient Practice Innovation and Clinical Excellence Atrial Fibrillation Registry. Circ Cardiovasc Qual Outcomes. 2017;10 doi: 10.1161/CIRCOUTCOMES.116.003476. [DOI] [PubMed] [Google Scholar]
- 22.Connolly S.J., Ezekowitz M.D., Yusuf S., et al. Dabigatran versus warfarin in patients with atrial fibrillation. N Engl J Med. 2009;361:1139–1151. doi: 10.1056/NEJMoa0905561. [DOI] [PubMed] [Google Scholar]
- 23.Patel M.R., Mahaffey K.W., Garg J., et al. Rivaroxaban versus warfarin in nonvalvular atrial fibrillation. N Engl J Med. 2011;365:883–891. doi: 10.1056/NEJMoa1009638. [DOI] [PubMed] [Google Scholar]
- 24.Granger C.B., Alexander J.H., McMurray J.J., et al. Apixaban versus warfarin in patients with atrial fibrillation. N Engl J Med. 2011;365:981–992. doi: 10.1056/NEJMoa1107039. [DOI] [PubMed] [Google Scholar]
- 25.Giugliano R.P., Ruff C.T., Braunwald E., et al. Edoxaban versus warfarin in patients with atrial fibrillation. N Engl J Med. 2013;369:2093–2104. doi: 10.1056/NEJMoa1310907. [DOI] [PubMed] [Google Scholar]
- 26.Mochalina N., Isma N., Svensson P.J., et al. Ischemic stroke rates decline in patients with atrial fibrillation as anticoagulants uptake improves: a Swedish cohort study. Thromb Res. 2017;158:44–48. doi: 10.1016/j.thromres.2017.08.004. [DOI] [PubMed] [Google Scholar]
- 27.Yu A.Y.X., Malo S., Svenson L.W., Wilton S.B., Hill M.D. Temporal trends in the use and comparative effectiveness of direct oral anticoagulant agents versus warfarin for nonvalvular atrial fibrillation: a Canadian population-based study. JAMA. 2017;6 doi: 10.1161/JAHA.117.007129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Weitz J.I., Semchuk W., Turpie A.G., et al. Trends in prescribing oral anticoagulants in Canada, 2008-2014. Clin Ther. 2015;37:2506–2514.e2504. doi: 10.1016/j.clinthera.2015.09.008. [DOI] [PubMed] [Google Scholar]
- 29.Maura G., Billionnet C., Drouin J., et al. Oral anticoagulation therapy use in patients with atrial fibrillation after the introduction of non-vitamin K antagonist oral anticoagulants: findings from the French healthcare databases, 2011-2016. BMJ Open. 2019;9 doi: 10.1136/bmjopen-2018-026645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Staerk L., Gerds T.A., Lip G.Y.H., et al. Standard and reduced doses of dabigatran, rivaroxaban and apixaban for stroke prevention in atrial fibrillation: a nationwide cohort study. J Intern Med. 2018;283:45–55. doi: 10.1111/joim.12683. [DOI] [PubMed] [Google Scholar]
- 31.Steinberg B.A., Shrader P., Thomas L., et al. Off-label dosing of non-vitamin K antagonist oral anticoagulants and adverse outcomes: the ORBIT-AF II registry. J Am Coll Cardiol. 2016;68:2597–2604. doi: 10.1016/j.jacc.2016.09.966. [DOI] [PubMed] [Google Scholar]
- 32.Carlisle M.A., Fudim M., DeVore A.D., Piccini J.P. Heart failure and atrial fibrillation, like fire and fury. JACC Heart Fail. 2019;7:447–456. doi: 10.1016/j.jchf.2019.03.005. [DOI] [PubMed] [Google Scholar]
- 33.Wang T.J., Larson M.G., Levy D., et al. Temporal relations of atrial fibrillation and congestive heart failure and their joint influence on mortality: the Framingham Heart Study. Circulation. 2003;107:2920–2925. doi: 10.1161/01.CIR.0000072767.89944.6E. [DOI] [PubMed] [Google Scholar]
- 34.Ahmed A., Allman R.M., Fonarow G.C., et al. Incident heart failure hospitalization and subsequent mortality in chronic heart failure: a propensity-matched study. J Card Fail. 2008;14:211–218. doi: 10.1016/j.cardfail.2007.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Solomon S.D., Dobson J., Pocock S., et al. Influence of nonfatal hospitalization for heart failure on subsequent mortality in patients with chronic heart failure. Circulation. 2007;116:1482–1487. doi: 10.1161/CIRCULATIONAHA.107.696906. [DOI] [PubMed] [Google Scholar]
- 36.Lichstein E., Sharma A. Changing endpoints for heart failure studies. J Am Coll Cardiol. 2018;71:2653–2655. doi: 10.1016/j.jacc.2018.03.518. [DOI] [PubMed] [Google Scholar]
- 37.Healey J.S., Parkash R., Pollak T., Tsang T., Dorian P. Canadian Cardiovascular Society atrial fibrillation guidelines 2010: etiology and initial investigations. Can J Cardiol. 2011;27:31–37. doi: 10.1016/j.cjca.2010.11.015. [DOI] [PubMed] [Google Scholar]
- 38.Singh S.M., Abdel-Qadir H., Pang A., et al. Population trends in all-cause mortality and cause specific-death with incident atrial fibrillation. J Am Heart Assoc. 2020;9 doi: 10.1161/JAHA.120.016810. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.