Key Points
Question
Are low levels of vitamin D independently associated with the risk of SARS-CoV-2 seropositivity?
Findings
In this cohort study of 18 148 individuals whose vitamin D levels were measured before the COVID-19 pandemic, low levels of vitamin D were associated with SARS-CoV-2 seropositivity in unadjusted univariable analysis. However, after adjusting for potentially confounding factors, including age, sex, race/ethnicity, education, body mass index, blood pressure, smoking status, and geographical location, vitamin D level was not associated with SARS-CoV-2 seropositivity.
Meaning
Although SARS-CoV-2–seropositive individuals did have lower vitamin D levels than seronegative individuals, low vitamin D levels were not independently associated with the risk of seropositivity.
This cohort study examines whether low levels of vitamin D are associated with SARS-CoV-2 seropositivity, an indicator of previous infection.
Abstract
Importance
Low vitamin D levels have been reported to be associated with increased risk of SARS-CoV-2 infection. Independent, well-powered studies could further our understanding of this association.
Objective
To examine whether low levels of vitamin D are associated with SARS-CoV-2 seropositivity, an indicator of previous infection.
Design, Setting, and Participants
This is a cohort study of employees and spouses who elected to be tested for SARS-CoV-2 IgG as part of an annual employer-sponsored health screening program conducted in August to November 2020. This program includes commonly assessed demographic, biometric, and laboratory variables, including total vitamin D measurement. Baseline (prepandemic) levels of vitamin D and potential confounders were obtained from screening results from the previous year (September 2019 to January 2020). Data analysis was performed from December 2020 to March 2021.
Exposures
Low total serum 25-hydroxyvitamin D, defined as either less than 20 ng/mL or less than 30 ng/mL.
Main Outcomes and Measures
The main outcome was SARS-CoV-2 seropositivity, as determined with US Food and Drug Administration emergency use–authorized assays. The association of SARS-CoV-2 seropositivity with vitamin D levels was assessed by multivariable logistic regression analyses and propensity score analyses.
Results
The 18 148 individuals included in this study had test results for SARS-CoV-2 IgG in 2020 and vitamin D levels from the prepandemic and pandemic periods. Their median (interquartile range) age was 47 (37-56) years, 12 170 (67.1%) were women, 900 (5.0%) were seropositive, 4498 (24.8%) had a vitamin D level less than 20 ng/mL, and 10 876 (59.9%) had a vitamin D level less than 30 ng/mL before the pandemic. In multivariable models adjusting for age, sex, race/ethnicity, education, body mass index, blood pressure, smoking status, and geographical location, SARS-CoV-2 seropositivity was not associated with having a vitamin D level less than 20 ng/mL before (odds ratio [OR], 1.04; 95% CI, 0.88-1.22) or during (OR, 0.93; 95% CI, 0.79-1.09) the pandemic; it was also not associated with having a vitamin D level less than 30 ng/mL before (OR, 1.09; 95% CI, 0.93-1.27) or during (OR, 1.05; 95% CI, 0.91-1.23) the pandemic. Similar results were observed in propensity score analyses. SARS-CoV-2 seropositivity was associated with obesity (OR, 1.26; 95% CI, 1.08-1.46), not having a college degree (OR, 1.40; 95% CI, 1.21-1.62), and Asian (OR, 1.46; 95% CI, 1.13-1.87), Black (OR, 2.74; 95% CI, 2.25-3.34), Hispanic (OR, 2.65; 95% CI, 2.15-3.27), American Indian or Alaska Native, and Native Hawaiian or other Pacific Islander (OR, 2.01; OR, 1.54-2.62) race/ethnicity, and was inversely associated with high blood pressure (OR, 0.82; 95% CI, 0.70-0.96), smoking (OR, 0.60; 95% CI, 0.47-0.78), and residing in the US Northeast (OR, 0.75; 95% CI, 0.62-0.92) and West (OR, 0.54; 95% CI, 0.44-0.67).
Conclusions and Relevance
In this cohort study, SARS-CoV-2 seropositivity was not associated with low levels of vitamin D independently of other risk factors.
Introduction
The COVID-19 pandemic has motivated efforts to understand the factors associated with risk of infection with SARS-CoV-2 and the progression of the disease.1,2,3 For example, recognized factors associated with the risk of contracting COVID-19 in the US include Black race and Hispanic ethnicity. Of particular interest are potentially modifiable risk factors, such as low levels of vitamin D, given the urgent need for effective tools to ameliorate the impact of the pandemic.
Low levels of vitamin D have been reported to be associated with increased risk of SARS-CoV-2 infection and progression of COVID-19. For example, a retrospective study4 of 489 patients tested for SARS-CoV-2 (71 tested positive) found that COVID-19 infection was more common in those with vitamin D deficiency (25-hydroxycholecalciferol <20 ng/mL or 1,25-dihydroxycholecalciferol <18 pg/mL) than in those with higher levels (odds ratio [OR], 1.77). Similar results were observed in a retrospective database analysis5 of laboratory results for patients tested for SARS-CoV-2 RNA and vitamin D levels: individuals with vitamin D deficiency or insufficiency were more likely to have positive SARS-CoV-2 results. For example, the positivity rate was 54% higher in patients with circulating 25-hydroxyvitamin D levels less than 20 ng/mL than in those with circulating levels of 30 to 34 ng/mL. Another retrospective study6 involving 7807 patients tested for COVID-19 (782 tested positive) found low vitamin D levels (ie, 25-hydroxyvitamin D <30 ng/mL) to be independently associated with risk of COVID-19 (OR, 1.50).
However, vitamin D level is inversely associated with several other factors associated with the risk of COVID-19; for example, compared with non-Hispanic White individuals, Black individuals have lower levels of 25-hydroxyvitamin D7 and are more likely to be infected with SARS-CoV-2.1 Consequently, the reported association between vitamin D and COVID-19 could be confounded by other risk factors, confounding that could be explored in well-powered studies. We, therefore, investigated the association of vitamin D level with the presence of IgG antibodies against SARS-CoV-2 in a cohort study of participants in an employer-sponsored biometric screening program who were offered no-cost testing for SARS-CoV-2 IgG antibodies. We examined whether low levels of vitamin D, measured just months before the COVID-19 pandemic and during the COVID-19 pandemic, were associated with SARS-CoV-2 seropositivity in this generally healthy working population, while adjusting for potentially confounding risk factors.
Methods
This population-based analysis of a deidentified data set was intended to improve the health of the group health plan members, an operations use of data permitted by the Health Insurance Portability and Accountability Act Privacy Rule (45 CFR 164.506). The Western Institutional Review Board determined that this study was exempt from review and the need for informed consent because it was a retrospective analysis of deidentified data. Reporting of this analysis follows the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) reporting guideline.
Study Setting and Design
This cohort study analyzed deidentified results from an employer-sponsored biometric screening program. This annual program is provided at no cost to employees and spouses of Quest Diagnostics, a clinical laboratory with workforce members in every state of the US. Participants are eligible for a reduced-cost employer-sponsored health plan. The annual screening program collects biometric (body mass index [BMI; calculated as weight in kilograms divided by height in meters squared], waist circumference, and blood pressure), demographic (sex, age, race/ethnicity, and state of residence), and laboratory testing (eg, lipid panel and total vitamin D) data. In 2020, participants could also opt in to be tested for SARS-CoV-2 IgG. All tests were conducted at the Quest Diagnostics laboratories, and the test results were not linked to other databases such as electronic health records.
The analysis included results from participants who opted to be tested for SARS-CoV-2 IgG during the 2020 screening event, conducted in August 2020 to November 2020 (pandemic period), and had also participated in the previous year’s screening program, conducted in September 2019 to January 2020 (ie, prepandemic baseline). Prepandemic results used for multivariable analyses included vitamin D levels, BMI, blood pressure, and smoking status. Pandemic-period results were limited to vitamin D levels and SARS-CoV-2 IgG status. Given the infectious nature of SARS-CoV-2, if more than 1 member of a household had participated in the biometric screening program, only 1 household member was selected into the study using a random number generator. This was done by assigning each participant a number that was randomly drawn from a standard normal distribution, and for each household, the participant assigned the lowest random number was selected.
Measurements
SARS-CoV-2 seropositivity was determined with assays for IgG antibodies to SARS-CoV-2, including the Ortho Clinical VITROS anti-SARS-CoV-2 IgG test (with 100% specificity and 90% sensitivity) and the Abbott Architect SARS-CoV-2 IgG test (with 99.6% specificity and 100% sensitivity). These assays had received US Food and Drug Administration emergency use authorization. Total 25-hydroxyvitamin D, the major circulating form of vitamin D, was measured using a chemiluminescent immunoassay (DiaSorin LIAISON1XL 25-hydroxyvitamin D, total) or a laboratory-developed test based on liquid chromatography or tandem mass spectrometry. Vitamin D level was considered low for those with a level below 20 or 30 ng/mL (to convert to nanomoles per liter, multiply by 2.496), which represent threshold values for vitamin D deficiency and insufficiency, respectively.8 Race/ethnicity was self-reported and categorized as Asian, Black, Hispanic, White, or other; other included individuals who self-reported as American Indian or Alaska Native, Native Hawaiian or other Pacific Islander, or 2 or more races or ethnicities. Educational attainment was self-reported and categorized as having a college degree or not. Blood pressure was measured with an automatic upper-arm monitor before blood sample collection. Normal blood pressure was defined as having a systolic blood pressure of less than 120 mm Hg and a diastolic blood pressure of less than 80 mm Hg. Elevated blood pressure was defined as having a systolic blood pressure of 120 to 129 mm Hg and a diastolic blood pressure of less than 80 mm Hg. High blood pressure was defined as having a systolic blood pressure of greater than or equal to 130 mm Hg or a diastolic blood pressure of greater than or equal to 80 mm Hg. Current smoking status was determined with a serum cotinine assay, and current smokers were defined as those with greater than or equal to 10 ng/mL (to convert to nanomoles per liter, multiply by 5.68) serum cotinine. Obesity was defined as having a BMI greater than or equal to 30. All tests and measurements were performed by Quest Diagnostics.
Statistical Analysis
Differences in the biochemical and demographic characteristics according to SARS-CoV-2 seropositivity were assessed with Wilcoxon rank-sum test for continuous variables and with the χ2 test for discrete variables. These tests were 2-sided. QQ-plots and Kolmogorov-Smirnov tests were used to assess normality of continuous variables. The association of study variables with SARS-CoV-2 seropositivity was assessed in logistic regression models that adjusted for the baseline covariates (Table 1). Sex, race/ethnicity, education, blood pressure, smoking status, and geographical location were coded as categorical variables, and age and BMI were treated as categorical (in forest plots) or continuous variables in logistic regression models. Assessment of the association between SARS-CoV-2 seropositivity and vitamin D level status was also performed by propensity score analyses to adjust for potential confounding. The propensity score was the estimated probability for vitamin D level status based on a logistic regression model that included age, sex, race/ethnicity, education, BMI, smoking status, blood pressure, and geographical location (age and BMI were coded as a continuous variable). On the basis of the propensity score, those with vitamin D levels less than 20 ng/mL were matched 1:1 to those with vitamin D levels greater than or equal to 20 ng/mL using a caliper of 0.1 and a greedy matching algorithm.9 These with vitamin D levels less than 30 ng/mL and vitamin D levels greater than or equal to 30 ng/mL were matched similarly. The association between SARS-CoV-2 seropositivity and vitamin D level status was then assessed by logistic regression in the matched sample set.
Table 1. Characteristics of the Individuals in the Study According to SARS-CoV-2 Serology Test Results.
| Characteristica | Participants, No. (%) | P valueb | Standardized difference | ||
|---|---|---|---|---|---|
| Total (N = 18 148) | SARS-CoV-2 seropositive (n = 900) | SARS-CoV-2 seronegative (n = 17 248) | |||
| Age, y | |||||
| Median (IQR) | 47 (37-56) | 45 (36-54) | 48 (37-56) | <.001 | 0.18 |
| ≥60 | 2926 (16.1) | 112 (12.4) | 2814 (16.3) | .002 | 0.12 |
| <60 | 15 222 (83.9) | 788 (87.6) | 14 434 (83.7) | ||
| Sex | |||||
| Male | 5978 (32.9) | 252 (28.0) | 5726 (33.2) | .001 | 0.11 |
| Female | 12 170 (67.1) | 648 (72.0) | 11 522 (66.8) | ||
| Race/ethnicity | |||||
| Asian | 3026 (17.0) | 104 (11.7) | 2922 (17.3) | <.001 | 0.54 |
| Black | 3035 (17.1) | 284 (32.1) | 2751 (16.3) | ||
| Hispanic | 2439 (13.7) | 192 (21.7) | 2247 (13.3) | ||
| Otherc | 1482 (8.3) | 82 (9.3) | 1400 (8.3) | ||
| White | 7785 (43.8) | 224 (25.3) | 7561 (44.8) | ||
| Education | |||||
| No college degree | 8246 (46.6) | 513 (58.6) | 7733 (46.0) | <.001 | 0.25 |
| College degree | 9452 (53.4) | 363 (41.4) | 9089 (54.0) | ||
| Body mass index | |||||
| Median (IQR) | 28 (24-33) | 30 (26-35) | 28 (24-33) | <.001 | 0.24 |
| ≥30 | 6861 (37.9) | 426 (47.3) | 6435 (37.4) | <.001 | 0.20 |
| <30 | 11 265 (62.1) | 474 (52.7) | 10 791 (62.6) | ||
| Blood pressure | |||||
| High | 7886 (43.5) | 368 (40.9) | 7518 (43.6) | .17 | 0.06 |
| Elevated | 2818 (15.5) | 137 (15.2) | 2681 (15.6) | ||
| Normal | 7427 (41.0) | 395 (43.9) | 7032 (40.8) | ||
| Smoking | |||||
| Yes | 2238 (12.3) | 74 (8.2) | 2164 (12.6) | <.001 | 0.14 |
| No | 15 895 (87.7) | 826 (91.8) | 15 069 (87.4) | ||
| Geographical location | |||||
| Northeast | 3937 (21.8) | 155 (17.2) | 3782 (22.1) | <.001 | 0.32 |
| Midwest | 2835 (15.7) | 139 (15.5) | 2696 (15.7) | ||
| West | 4195 (23.3) | 132 (14.7) | 4063 (23.7) | ||
| South | 7057 (39.2) | 473 (52.6) | 6584 (38.4) | ||
| Vitamin D level in the prepandemic period, ng/mL | |||||
| Median (IQR) | 27 (20-35) | 24 (18-32) | 27 (20-35) | <.001 | 0.23 |
| <30 | 10 876 (59.9) | 611 (67.9) | 10 265 (59.5) | <.001 | 0.17 |
| ≥30 | 7272 (40.1) | 289 (32.1) | 6983 (40.5) | ||
| <20 | 4498 (24.8) | 290 (32.2) | 4208 (24.4) | <.001 | 0.17 |
| ≥20 | 13 650 (75.2) | 610 (67.8) | 13 040 (75.6) | ||
| Vitamin D level in the pandemic period, ng/mL | |||||
| Median (IQR) | 27 (20-36) | 25 (18-33) | 27 (20-36) | <.001 | 0.16 |
| <30 | 10 595 (58.4) | 586 (65.1) | 10 009 (58.0) | <.001 | 0.15 |
| ≥30 | 7553 (41.6) | 314 (34.9) | 7239 (42.0) | ||
| <20 | 4424 (24.4) | 260 (28.9) | 4164 (24.1) | .001 | 0.11 |
| ≥20 | 13 724 (75.6) | 640 (71.1) | 13 084 (75.9) | ||
Abbreviation: IQR, interquartile range.
SI conversion factor: To convert vitamin D level to nanomoles per liter, multiply by 2.496.
Body mass index (calculated as weight in kilograms divided by height in meters squared), blood pressure, and smoking status were determined during a biometric screening program that took place from September 2019 to January 2020.
Unadjusted results from the Wilcoxon rank-sum test for continuous variables and from the χ2 test for discrete variables.
Other includes American Indian or Alaska Native, Native Hawaiian or other Pacific Islander, or 2 or more races or ethnicities.
A simulation study was performed to assess how loss of detecting SARS-CoV-2 seropositivity among some individuals who had COVID-19 but subsequently tested SARS-CoV-2 seronegative could have affected the association outcomes. A fraction of the participants in the SARS-CoV-2 seronegative group were randomly switched to the SARS-CoV-2 seropositive group by Bernoulli distribution with the probability of the loss of seropositivity. All covariates were kept intact for the participants whose status was changed and the logistic regression model as performed for the primary analysis was repeated on the simulated data set. This process was repeated 1000 times to allow differing sets of participants to switch groups. The OR and P value for the association of vitamin D with SARS-CoV-2 seropositivity were recorded in each of the 1000 simulations, and the median of the ORs was reported.
A P < .05 indicated statistical significance. Statistical analyses were performed using SAS statistical software version 9.4 (SAS Institute). Data analysis was performed from December 2020 to March 2021.
Results
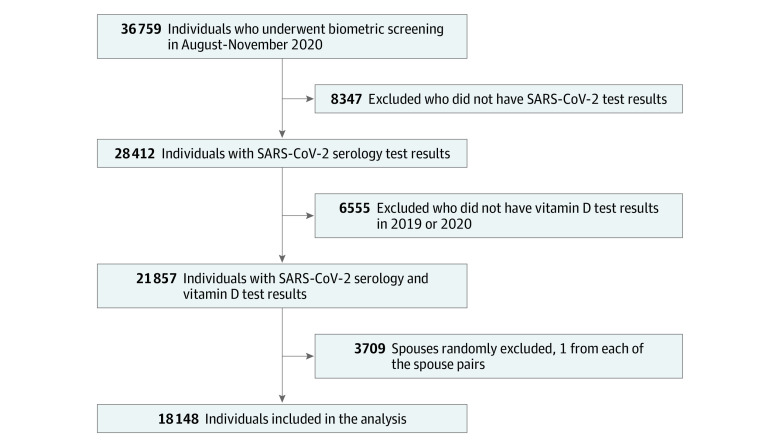
A total of 18 148 employees and spouses (median [interquartile range] age, 47 [37-56] years; 12 170 women [67.1%]) were included in this study following the selection process shown in Figure 1. Of these, 900 (5.0%) were SARS-CoV-2 seropositive, 10 876 (59.9%) had insufficient (<30 ng/mL) levels of vitamin D in the prepandemic period, and 4498 (24.8%) had deficient (<20 ng/mL) levels of vitamin D in the prepandemic period. Table 1 and eTable 1 and eTable 2 in the Supplement show the characteristics of the individuals in the study according to COVID-19 serology test results and vitamin D levels. eTable 3 in the Supplement shows the characteristics of the individuals who did not have SARS-CoV-2 serology testing and the individuals included in this study; the standardized differences in baseline covariates between these 2 groups were all less than 0.2, an effect size considered to be small.10
Figure 1. Study Flow Diagram.
Selection of the individuals in the study is presented.
SARS-CoV-2–seropositive individuals had lower median (interquartile range) vitamin D levels than did SARS-CoV-2–seronegative individuals, both before (24 [18-32] vs 27 [20-35] ng/mL) and during (25 [18-33] vs 27 [20-36] ng/mL) the pandemic (Table 1). In univariable analyses, SARS-CoV-2 seropositivity was significantly associated with low levels of vitamin D measured in 2019 as well as in 2020 (Table 2). This association remained whether low vitamin D level was defined as less than 20 ng/mL (2019, OR, 1.47 [95% CI, 1.28-1.70]; 2020, OR, 1.28 [95% CI, 1.10-1.48]) or less than 30 ng/mL (2019, OR, 1.44 [95% CI, 1.25-1.66]; 2020, OR, 1.35 [95% CI, 1.17-1.55]). SARS-CoV-2 seropositivity was also associated with age, sex, race/ethnicity, educational attainment, BMI, smoking status, and geographical location (Table 1). Of note, 1 in 3 seropositive individuals were Black, whereas only 1 in 6 seronegative individuals were Black. Low levels of vitamin D were associated with age, race/ethnicity, educational attainment, BMI, blood pressure, smoking status, and geographical location (eTable 1 and eTable 2 in the Supplement).
Table 2. Unadjusted Association Between Vitamin D Levels and SARS-CoV-2 Seropositivity.
| Vitamin D level comparison and year | OR (95% CI) | P value |
|---|---|---|
| <30 vs ≥30 ng/mL | ||
| 2019 | 1.44 (1.25-1.66) | <.001 |
| 2020 | 1.35 (1.17-1.55) | <.001 |
| <20 vs ≥20 ng/mL | ||
| 2019 | 1.47 (1.28-1.70) | <.001 |
| 2020 | 1.28 (1.10-1.48) | .001 |
Abbreviation: OR, odds ratio.
SI conversion factor: To convert vitamin D level to nanomoles per liter, multiply by 2.496.
In multivariable regression analyses with adjustment for age, sex, race/ethnicity, educational attainment, BMI, blood pressure, smoking status, and geographical location, SARS-CoV-2 seropositivity was not significantly associated with low levels of vitamin D measured in 2019 or in 2020 (Table 3). The ORs of SARS-CoV-2 seropositivity were 1.04 (95% CI, 0.88-1.22) for having a vitamin D level less than 20 ng/mL before the pandemic, 0.93 (95% CI, 0.79-1.09) for having a vitamin D level less than 20 ng/mL during the pandemic, 1.09 (95% CI, 0.93-1.27) for having a vitamin D level less than 30 ng/mL before the pandemic, and 1.05 (95% CI, 0.91-1.23) for having a vitamin D level less than 30 ng/mL during the pandemic. Propensity score analyses were also used to adjust for potential confounding between the SARS-CoV-2 seropositivity and vitamin D level status. In models with matching vitamin D status by propensity score (eFigure in the Supplement), there was no association between SARS-CoV-2 seropositivity and low levels of vitamin D level (Table 3).
Table 3. Association Between Vitamin D Levels and SARS-CoV-2 Seropositivity.
| Vitamin D level comparison and year | Multivariable regression analysisa | Propensity score analysisb | ||
|---|---|---|---|---|
| OR (95% CI) | P value | OR (95% CI) | P value | |
| <30 vs ≥30 ng/mL | ||||
| 2019 | 1.09 (0.93-1.27) | .29 | 1.12 (0.91-1.36) | .28 |
| 2020 | 1.05 (0.91-1.23) | .49 | 1.07 (0.88-1.29) | .52 |
| <20 vs ≥20 ng/mL | ||||
| 2019 | 1.04 (0.88-1.22) | .66 | 1.04 (0.84-1.27) | .74 |
| 2020 | 0.93 (0.79-1.09) | .36 | 0.99 (0.81-1.21) | .93 |
Abbreviation: OR, odds ratio.
SI conversion factor: To convert vitamin D level to nanomoles per liter, multiply by 2.496.
Adjusted for age (continuous), sex, race/ethnicity, education, body mass index (continuous), blood pressure, smoking status, and geographical location.
Based on matching pairs (see the eFigure in the Supplement).
Because individuals who were infected with SARS-CoV-2 may not test seropositive and, therefore, may be placed in the seronegative group, we conducted simulations to investigate how this might affect the association between vitamin D and seropositivity. We assumed that 10% of those infected with SARS-CoV-2 would not test seropositive, which would mean that 900 individuals would test seropositive after 1000 individuals were infected. The 100 individuals who did not test seropositive would be 0.58% of the 17 248 individuals in the SARS-CoV-2–seronegative group. Therefore, in the simulation, random draws from a Bernoulli distribution with 0.58% probability were used to switch some SARS-CoV-2–seronegative participants to the SARS-CoV-2–seropositive group. Of 1000 simulations where SARS-CoV-2–seronegative participants were randomly assigned SARS-CoV-2–seropositive status with 0.58% probability, the median OR was 1.08 for the association of vitamin D level less than 30 vs greater than or equal to 30 ng/mL with SARS-CoV-2 seropositivity and the P value was not significant (≥.05) in 998 of the 1000 simulations. The median OR for the association of vitamin D level less than 20 vs greater than or equal to 20 ng/mL with SARS-CoV-2 seropositivity was 1.04 and the P value was not significant (≥.05) in any of the 1000 simulations.
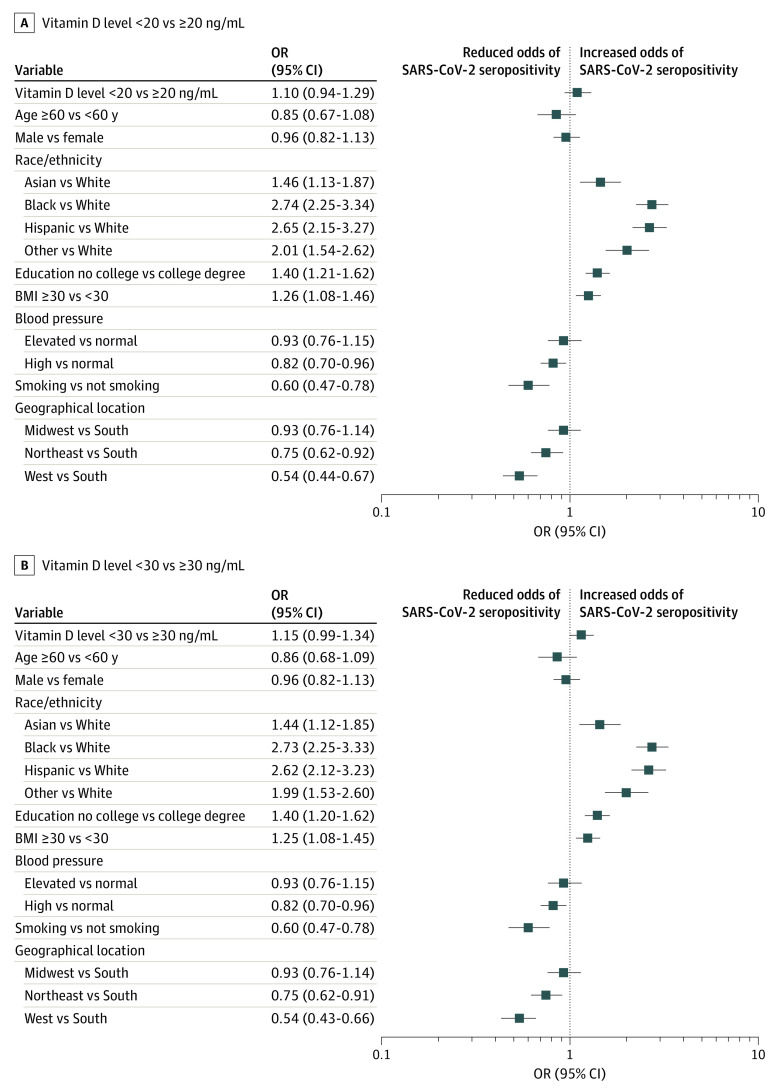
Finally, in multivariable regression analyses, SARS-CoV-2 seropositivity was independently associated with Asian (OR, 1.46; 95% CI, 1.13-1.87), Black (OR, 2.74; 95% CI, 2.25-3.34), Hispanic (OR, 2.65; 95% CI, 2.15-3.27), and American Indian or Alaska Native, and Native Hawaiian or other Pacific Islander (OR, 2.01; OR, 1.54-2.62) race/ethnicity, not having a college degree (OR, 1.40; 95% CI, 1.21-1.62), and obesity (OR, 1.26; 95% CI, 1.08-1.46) (Figure 2). SARS-CoV-2 seropositivity was also independently (inversely) associated with high blood pressure (OR, 0.82; 95% CI, 0.70-0.96), smoking (OR, 0.60; 95% CI, 0.47-0.78), and residing in the US Northeast (OR, 0.75; 95% CI, 0.62-0.92) and West (OR, 0.54; 95% CI, 0.44-0.67) (Figure 2).
Figure 2. Odds Ratios (ORs) of SARS-CoV-2 Seropositivity.
A, Vitamin D level less than 20 vs greater than or equal to 20 ng/mL. B, Vitamin D level less than 30 vs greater than or equal to 30 ng/mL. Association of SARS-CoV-2 seropositivity with each variable was adjusted for all other variables shown (covariates were treated categorically). The analysis was based on the 2019 data for vitamin D levels, body mass index (BMI; calculated as weight in kilograms divided by height in meters squared), blood pressure, and smoking status. Other race/ethnicity included American Indian or Alaska Native, Native Hawaiian or other Pacific Islander, or 2 or more races or ethnicities.
Discussion
In this study, low vitamin D levels were not independently associated with SARS-CoV-2 seropositivity in a cohort of working-age adults. SARS-CoV-2–seropositive individuals did have lower vitamin D levels than seronegative individuals, both before and during the pandemic, which is consistent with other reports.6,11 However, low levels of vitamin D were not associated with SARS-CoV-2 seropositivity after adjusting for age, sex, race/ethnicity, education, BMI, blood pressure, smoking status, and geographical location. As expected, vitamin D deficiency and insufficiency were more common in individuals who had a BMI greater than or equal to 30, who did not have a college degree, or who were younger, current smokers, hypertensive, or Asian, Black, Hispanic, American Indian or Alaska Native, and Native Hawaiian or other Pacific Islander race/ethnicity.
A major strength of this study is the large number of individuals with vitamin D levels (18 148 participants) and seropositivity for SARS-CoV-2 (900 participants). This study also differs in several ways from previous studies of the association between vitamin D levels and the risk of COVID-19. First, individuals in our study comprised working-age (and generally healthy) adults, whereas previous studies4,5,6,12 involved symptomatic patients who had been tested for SARS-CoV-2 RNA or seroconversion. Because the proportion of asymptomatic infection ranges from 20% to 75% for the general population,13 by including individuals who had asymptomatic SARS-CoV-2 infections, our study provides a more general evaluation of the risk of SARS-CoV-2 infection. Second, the vitamin D levels in our study were measured only a few months before the COVID-19 pandemic, whereas in some previously reported studies,14 vitamin D levels were measured more than a decade before the pandemic. Third, our study made use of self-reported race/ethnicity and educational attainment data, which may be more accurate or complete than that available to other studies.4,5,6 The lack of individual-level race/ethnicity data in some other studies may have hindered an accurate assessment of the association between vitamin D level and COVID-19 infection.
The variables that remained associated with SARS-CoV-2 seropositivity in our multivariable analyses may be markers for causative factors rather than themselves being causative biological or social factors. For example, consistent with previous reports,1 our study found that Black and Hispanic participants were more likely to be SARS-CoV-2 seropositive (1 in 3 seropositive individuals were Black, whereas only 1 in 6 seronegative individuals were Black). Lower educational attainment was independently associated with higher risk of COVID-19 infection in our study cohort, a finding that could be associated with job function. For example, roles such as phlebotomy do not require a college degree but do require constant contact with patients, which has been reported to be a factor associated with increased risk of SARS-CoV-2 seropositivity.15 In addition, individuals with hypertension and smokers appeared to have lower risk of being SARS-CoV-2 seropositive. The latter is consistent with findings from a meta-analysis by Simons and colleagues,16 which reported a reduced risk of SARS-CoV-2 infection in current compared with never smokers. The reduced risk of SARS-CoV-2 seropositivity in these populations could be associated with risk-reduction in behavior driven by the knowledge that smoking and hypertension can lead to worse COVID-19 outcomes.
Limitations
A limitation of this study is that the risk of SARS-CoV-2 seropositivity is not a perfect measurement of the risk of SARS-CoV-2 infection. Some seronegative individuals could have been infected with the SARS-CoV-2 virus but not identified by the serology assay used (the serology assay has a reported sensitivity of 90% to 100%). Newly infected individuals could have also been missed because they had not had enough time to develop IgG antibody, and infected individuals could have undergone seroreversion. However, sensitivity analysis suggests that SARS-CoV-2 seropositivity continued to not be associated with low levels of vitamin D after accounting for the loss of detecting seropositivity among some individuals who had COVID-19. Another limitation of this study is that, because vitamin D status was not randomized, there might be residual confounders or unobserved variable bias. A further limitation was the potential selection bias due to the inclusion of only individuals who chose to participate in the screening and opted to be tested for SARS-CoV-2 antibodies; however, the characteristics of those who did not opt for serology testing appeared to be similar to those included in the study.
Conclusions
In this cohort study, we found no evidence for an independent association between low levels of vitamin D and SARS-CoV-2 seropositivity. These findings do not support the hypothesis that vitamin D plays a role in susceptibility to SARS-CoV-2 infection.
eTable 1. Characteristics of the Individuals in the Study According to Vitamin D Levels Before COVID-19 Pandemic (September 2019 to January 2020): <30 vs. ≥30 ng/mL
eTable 2. Characteristics of the Individuals in the Study According to Vitamin D Levels Before COVID-19 Pandemic (September 2019 to January 2020): <20 vs. ≥20 ng/mL
eTable 3. Characteristics of the Individuals Included in the Study vs. Those Excluded From the Study Because of No SARS-CoV-2 Serology Testing (August 2020 to November 2020)
eFigure. Distributions of Propensity Scores Before and After Matching
References
- 1.Ogedegbe G, Ravenell J, Adhikari S, et al. Assessment of racial/ethnic disparities in hospitalization and mortality in patients with COVID-19 in New York City. JAMA Netw Open. 2020;3(12):e2026881. doi: 10.1001/jamanetworkopen.2020.26881 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Price-Haywood EG, Burton J, Fort D, Seoane L. Hospitalization and mortality among black patients and white patients with Covid-19. N Engl J Med. 2020;382(26):2534-2543. doi: 10.1056/NEJMsa2011686 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wadhera RK, Wadhera P, Gaba P, et al. Variation in COVID-19 hospitalizations and deaths across New York City boroughs. JAMA. 2020;323(21):2192-2195. doi: 10.1001/jama.2020.7197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Meltzer DO, Best TJ, Zhang H, Vokes T, Arora V, Solway J. Association of vitamin D status and other clinical characteristics with COVID-19 test results. JAMA Netw Open. 2020;3(9):e2019722. doi: 10.1001/jamanetworkopen.2020.19722 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kaufman HW, Niles JK, Kroll MH, Bi C, Holick MF. SARS-CoV-2 positivity rates associated with circulating 25-hydroxyvitamin D levels. PLoS One. 2020;15(9):e0239252. doi: 10.1371/journal.pone.0239252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Merzon E, Tworowski D, Gorohovski A, et al. Low plasma 25(OH) vitamin D level is associated with increased risk of COVID-19 infection: an Israeli population-based study. FEBS J. 2020;287(17):3693-3702. doi: 10.1111/febs.15495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yetley EA. Assessing the vitamin D status of the US population. Am J Clin Nutr. 2008;88(2):558S-564S. doi: 10.1093/ajcn/88.2.558S [DOI] [PubMed] [Google Scholar]
- 8.Holick MF, Binkley NC, Bischoff-Ferrari HA, et al. ; Endocrine Society . Evaluation, treatment, and prevention of vitamin D deficiency: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2011;96(7):1911-1930. doi: 10.1210/jc.2011-0385 [DOI] [PubMed] [Google Scholar]
- 9.Faries DE, Leon AC, Haro JM, Obenchain RL. Analysis of Observational Health Care Data Using SAS. SAS Institute; 2010. [Google Scholar]
- 10.Cohen J. Statistical Power Analysis for the Behavioral Sciences. 2nd ed. Lawrence Erlbaum Associates Publishers; 1988. [Google Scholar]
- 11.D’Avolio A, Avataneo V, Manca A, et al. 25-Hydroxyvitamin D concentrations are lower in patients with positive PCR for SARS-CoV-2. Nutrients. 2020;12(5):1359. doi: 10.3390/nu12051359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Faniyi AA, Lugg ST, Faustini SE, et al. Vitamin D status and seroconversion for COVID-19 in UK healthcare workers. Eur Respir J. 2020;2004234:2004234. doi: 10.1183/13993003.04234-2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yanes-Lane M, Winters N, Fregonese F, et al. Proportion of asymptomatic infection among COVID-19 positive persons and their transmission potential: a systematic review and meta-analysis. PLoS One. 2020;15(11):e0241536. doi: 10.1371/journal.pone.0241536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hastie CE, Mackay DF, Ho F, et al. Vitamin D concentrations and COVID-19 infection in UK Biobank. Diabetes Metab Syndr. 2020;14(4):561-565. doi: 10.1016/j.dsx.2020.04.050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sims MD, Maine GN, Childers KL, et al. COVID-19 seropositivity and asymptomatic rates in healthcare workers are associated with job function and masking. Clin Infect Dis. Published online November 5, 2020. doi: 10.1093/cid/ciaa1684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Simons D, Shahab L, Brown J, Perski O. The association of smoking status with SARS-CoV-2 infection, hospitalization and mortality from COVID-19: a living rapid evidence review with Bayesian meta-analyses (version 7). Addiction. Published online October 2, 2020. doi: 10.1111/add.15276 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eTable 1. Characteristics of the Individuals in the Study According to Vitamin D Levels Before COVID-19 Pandemic (September 2019 to January 2020): <30 vs. ≥30 ng/mL
eTable 2. Characteristics of the Individuals in the Study According to Vitamin D Levels Before COVID-19 Pandemic (September 2019 to January 2020): <20 vs. ≥20 ng/mL
eTable 3. Characteristics of the Individuals Included in the Study vs. Those Excluded From the Study Because of No SARS-CoV-2 Serology Testing (August 2020 to November 2020)
eFigure. Distributions of Propensity Scores Before and After Matching