Abstract
Mucosal tissues in the human female reproductive tract (FRT) are primary sites for both gynecological cancers and infections by a spectrum of sexually transmitted pathogens, including human immunodeficiency virus (HIV), that compromise women's health. While the regulation of innate and adaptive immune protection in the FRT by hormonal cyclic changes across the menstrual cycle and pregnancy are being intensely studied, little to nothing is known about the alterations in mucosal immune protection that occur throughout the FRT as women age following menopause. The immune system in the FRT has two key functions: defense against pathogens and reproduction. After menopause, natural reproductive function ends, and therefore, two overlapping processes contribute to alterations in immune protection in aging women: menopause and immunosenescence. The goal of this review is to summarize the multiple immune changes that occur in the FRT with aging, including the impact on the function of epithelial cells, immune cells, and stromal fibroblasts. These studies indicate that major aspects of innate and adaptive immunity in the FRT are compromised in a site‐specific manner in the FRT as women age. Further, at some FRT sites, immunological compensation occurs. Overall, alterations in mucosal immune protection contribute to the increased risk of sexually transmitted infections (STI), urogenital infections, and gynecological cancers. Further studies are essential to provide a foundation for the development of novel therapeutic interventions to restore immune protection and reverse conditions that threaten women's lives as they age.
Keywords: Dendritic cells, epithelial cells, female reproductive tract, fibroblasts, menopause, resident memory T cells, sex hormones, sexually transmitted infections, TGFβ
A review of immune changes in the female reproductive tract of women after menopause reveals that while there is a general decline in immune protection, specific immune aspects are upregulated or remain unchanged in a compartmentalized manner. Understanding how immune protection changes in the reproductive tract in the years following menopause is essential to protect women from sexually transmitted infections and gynecological cancers as they age.
1. INTRODUCTION: CHANGES IN SUSCEPTIBILITY TO GENITAL INFECTIONS AND GYNECOLOGICAL CANCER DUE TO AGING
The aged population (>60 years old) is increasing rapidly and projected to grow to 1.4 billion by 2030, with women accounting for approximately 2/3 of individuals in this age group (He et al., 2016). With age, genitourinary infections and gynecological cancers increase, with profound effects on the morbidity and mortality of women (Gavazzi & Krause, 2002). Epidemiological studies show that urinary tract infections (UTI) and sexually transmitted infection (STI) rates increase in older women (CDC, 2016a, 2016b), presenting a public health challenge that must be addressed. The incidence of STIs has increased by 38% since 2010 in the 50–70 year age group (CDC, 2016a, 2016b). UTIs are often caused by Escherichia coli (Hu et al., 2004), which colonize the FRT in older women prior to spreading to the urinary tract (Ghosh et al., 2014; Hummelen et al., 2011). Sexual activity is a risk factor for STIs and some UTIs, the prevalence of which is not widely recognized in older adults (CDC, 2016a; Hu et al., 2004; Taylor et al., 2017).
In addition to genitourinary infections, aged women have a high burden of comorbidities associated with endometrial, ovarian, and cervical cancers (CDC, 2019). Uterine cancer is the most common gynecological cancer worldwide and the sixth most common cause of cancer death which occurs primarily in postmenopausal women, with an average age of diagnosis of 60 years (Henley et al., 2020; Lu & Broaddus, 2020). Accompanying this is an increase in human papillomavirus (HPV) (types 16 and 18), the underlying cause of cervical cancer and precancerous lesions (Chan et al., 2019; Gonzalez et al., 2010; Gravitt et al., 2013; Rositch et al., 2012). Despite the burden of STIs and gynecological cancer in older women, they are not recognized as a clinical priority. Aged women are also generally excluded from STI prevention trials (Herrera et al., 2010), vaccination recommendations, and prevention advice (Granville & Pregler, 2018). Thus, there is a critical need to understand how, as women age, immune protection against STIs and cancer changes in the FRT—the primary mucosal surface where pathology initiates.
2. UNIQUENESS OF THE AGING PROCESS IN THE FRT: MENOPAUSE AND AGING IN WOMEN
The aging process in women is accompanied by the transition into menopause. Menopause marks the end of natural reproductive potential with the permanent secession of menstrual cycles, caused by the decline in ovarian sex hormone production (estradiol and progesterone) (Maruoka et al., 2014). Since the average age at menopause is 50 years (Palacios et al., 2010), and the average life expectancy of women in the USA is 78 years, women live for 30–40 years in a postmenopausal environment with low concentrations of sex hormones. How this hormone‐deprived environment affects immune function overtime is of great importance in understanding the mechanisms involved in immune protection in older women. Importantly, long‐term survival after menopause cannot be fully reproduced in animal models (Walker & Herndon, 2008), highlighting the importance of studying aging effects with human samples.
Not widely appreciated is that the immune system in the FRT is critical for reproductive success. Sex hormones tightly regulate immune function in the premenopausal FRT to ensure the balance between optimal conditions for pregnancy and protection against pathogens (Wira et al., 2015). To achieve this necessary balance, the FRT has evolved with distinct anatomical compartments consisting of the fallopian tubes, uterus (endometrium), endo‐ and ectocervix, and vagina (Figure 1). As reviewed elsewhere (Wira et al., 2015), each compartment contains adaptive and innate immune cells, but each site is separate and distinct regarding reproductive function and immune protection prior to menopause. Following menopause, immune cell populations and responses are dramatically altered. As women age, two interrelated processes overlap and contribute to changes in immune protection in the FRT: menopause and immunosenescence. While much is known about the effects of sex hormones on immune function in the FRT during the menstrual cycle, relatively little is known about the immunosenescent changes that occur after menopause and in the years that follow.
FIGURE 1.
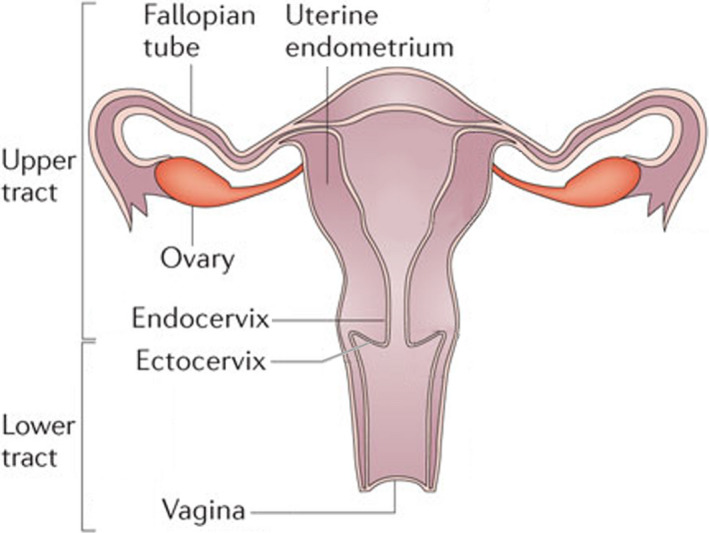
Diagram of the human female reproductive tract (FRT) showing the major tissue compartments. The upper FRT includes the fallopian tubes, endometrium, and endocervix, which are lined with columnar epithelial cells. The lower FRT consists of the ectocervix and vagina which is lined with squamous epithelial cells. The reproductive and immunological functions of each site are separate and distinct. Each site functions to optimize conditions for successful fertilization and implantation while protecting against sexually transmitted pathogens. Adapted from (Wira et al., 2015)
In this review, we consider immunosenescence throughout the FRT. We focus on changes in mucosal immune function following menopause and how they relate to potential changes on susceptibility to infections and the risk of gynecological cancers. Beyond the scope of this review are age‐related changes in the ovary, vulva, and other anatomically proximal organs, such as bladder and rectum, which contribute to morbidity in older women. Overall, following menopause, a growing body of evidence indicates that aging significantly alters adaptive and innate immunity, in ways that are distinct and site‐specific throughout the FRT.
3. CHANGES IN EPITHELIAL CELLS AND BARRIER PROTECTION INDUCED BY AGING
Epithelial cells line the surface of the FRT and are the first line of defense against incoming pathogens. They contribute to immune protection by (a) providing a physical barrier that separates the internal and external environments; (b) providing a chemical barrier composed of mucus, antimicrobials, cytokines, and chemokines that directly interact with pathogens and modulate the local immune system; and (c) mounting rapid innate immune responses to pathogens via pattern recognition receptors (PRRs). Little is known about how FRT epithelial functions change with age in postmenopausal women, since most studies focus on younger reproductive‐aged women.
3.1. Barrier protection
Epithelial cells form a physical barrier that protects underlying FRT tissues and its resident immune cells against potential pathogens and injuries. Epithelial cell phenotype varies with anatomical location in the FRT. The stratified squamous epithelium of the lower FRT (ectocervix and vagina) is 25–50 layers thick with superficial, parabasal, and basal layers (Patton et al., 2000). In contrast, the upper FRT (endocervix, endometrium, and fallopian tubes) is covered by a single layer of columnar epithelial cells. Whether increased barrier thickness correlates with increased protection against pathogens in the lower FRT is unclear. However, cervical ectopy, where columnar epithelium of the endocervix extrudes onto the surface of the ectocervix, is associated with increased transmission risk of HIV (Moss et al., 1991), human papillomavirus (HPV) (Rocha‐Zavaleta et al., 2004), Chlamydia trachomatis (Lee et al., 2006), and cytomegalovirus (CMV) (Critchlow et al., 1995).
Epithelial atrophy is common following menopause (Anderson et al., 1989; Farage & Maibach, 2006; Losif & Bekassy, 1984). Postmenopausal women have a thinner vaginal epithelium (21.4 vs 10.7 cell layers) compared to premenopausal women (Thurman et al., 2017), suggesting decreased barrier protection in the lower FRT. There is also a loss of hydration which leads to increased vaginal dryness, irritation, and inflammation. Loss of natural lubrication can lead to epithelial damage during sexual intercourse, potentially increasing pathogen access to the underlying tissue. Furthermore, epithelial wound healing is compromised following menopause in animal models (Ben Menachem‐Zidon et al., 2020; Shveiky et al., 2020), at other mucosal sites (Engeland et al., 2009; Horng et al., 2017) and in cell culture (Patel, M. Unpublished).
Tight junction and adherens junction protein complexes link adjacent epithelial cells and serve as a selectively permeable barrier that allows movement of proteins and solute across the epithelium (Anderson & Van Itallie, 2009; Blaskewicz et al., 2011). Tight junctions are primarily composed of ZO‐1, occludin, and multiple claudin proteins, while adherens junctions are composed of N‐, P‐ and O‐cadherin. Tight junctions are precisely regulated throughout the menstrual cycle by sex hormones in premenopausal women (Fahey et al., 2006; Gorodeski, 2001a, 2007; Gorodeski et al., 2005; Iwanaga et al., 1985; Murphy et al., 1992; Zeng et al., 2004). While the effect of aging on tight and adherens junction expression in the FRT is relatively unknown, vaginal epithelial E‐cadherin levels are lower in postmenopausal women than premenopausal women (Thurman et al., 2017). Furthermore, levels of paracellular permeability and transcellular resistance are lower in ectocervical cultures from postmenopausal women compared to premenopausal women (Gorodeski, 2001b). Since pathogens such as HIV can also decrease tight junction integrity between endometrial epithelial cells (Mukura et al., 2017; Nazli et al., 2010), aging may exacerbate the movement of pathogens into the underlying tissue. Thus, aging potentially leads to an overall decrease in FRT epithelial barrier protection (Figure 2).
FIGURE 2.
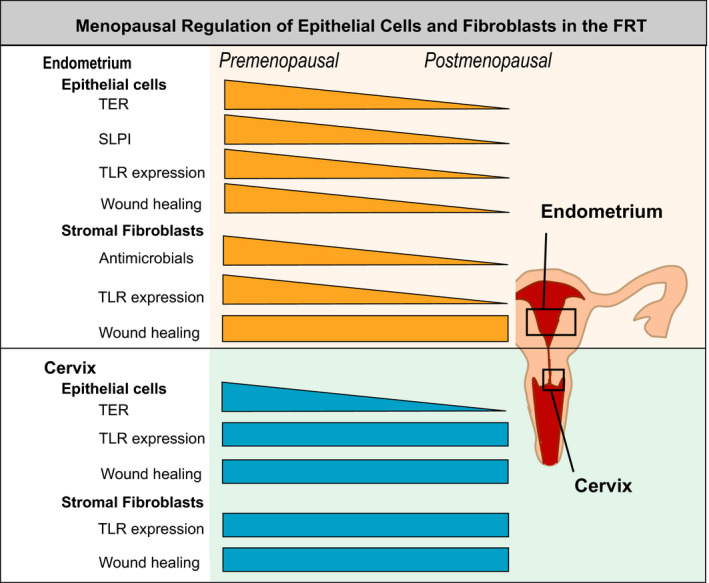
Regulation of epithelial cell and fibroblast function by menopausal status. This diagram shows key epithelial and stromal fibroblast functions and how they are modified after menopause. Triangles indicate a decline in cell function with menopause. A rectangle indicates no change following menopause. Effects are shown for the endometrium on the upper part and for the cervix (endocervix and ectocervix) on the lower part of the figure
3.2. Pattern Recognition Receptors (PRRs)
PRRs are essential for the recognition and response to pathogens. PRRs include Toll‐like receptors (TLR) and retinoic acid inducible gene (RIG)‐like receptors (RLR), which recognize conserved moieties known as pathogen‐associated molecular patterns (PAMPs) characteristic of broad classes of pathogens. PRR expression varies within the FRT by anatomical location and cell type (Pioli et al., 2004; Zarember & Godowski, 2002). Human endometrial epithelial cells express TLRs1‐9, with TLR1, 2, 3, and 5 being expressed at the highest levels (Schaefer et al., 2005). Vaginal epithelial cells express TLR1, 3, 5, and 6, but not TLR4 (Fichorova et al., 2002). At the tissue level, TLR4, RIG‐I, MDA5, NOD1, and NOD2 expressions are highest in the upper FRT and decline in the lower FRT (Ghosh et al., 2012; Pioli et al., 2004). TLR2 expression is highest in the fallopian tubes and cervix but lowest in the endometrium and ectocervix. In contrast, TLR7, 8, and 9 are consistently expressed from the fallopian tubes to ectocervix (Hart et al., 2009).
TLR expression varies with menstrual cycle stage and is lower in endometrial tissues at the proliferative phase compared to the secretory phase. Endometrial epithelial immune responses are partially regulated by sex hormones. Estradiol decreases secretion of IL‐6, IL‐8, and MIF by endometrial epithelial cells in response to TLR3 or TLR4 stimulation (Fahey et al., 2008; Lesmeister et al., 2005). How the decline in ovarian sex hormones affects epithelial innate immune responses to PRR ligands is unclear. In preliminary studies with endometrial epithelial cells, we discovered a trend toward decreased TLR3 expression in older women (>75 years) compared to younger women (50–59 years) (Figure 2). Since exposure to the TLR3 agonist poly(I:C) induces a proinflammatory antiviral response in endometrial and vaginal epithelial cells (Patel et al., 2012, 2018a; Schaefer et al., 2005; Trifonova et al., 2009), decreased responsiveness to viral pathogens could compromise epithelial cell‐mediated innate protection throughout the FRT. Whether PRR expression in general and responsiveness to TLR agonists decreases with age in the FRT is unknown. At other sites in the body, increased age is associated with decreased responsiveness to PRR stimulation (Dunston & Griffiths, 2010; Iram et al., 2012; Panda et al., 1950).
3.3. Mucus
FRT epithelial cells produce a protective mucus layer that reduces direct contact with pathogens, such as HIV, by trapping them and preventing access to the epithelium (Lai et al., 2009; Shukair et al., 2013). Major constituents of mucus are the negatively charged glycoproteins known as mucins which form a network of protein complexes that can bind incoming pathogens. In premenopausal women, mucin (MUC) gene expression varies with menstrual status leading to changes in the overall properties of mucus including consistency and permeability (Elstein, 1978; Gipson et al., 1997; Vigil et al., 2009). Following menopause, the expression of vaginal MUC4 and MUC5AC decreases (Moncla et al., 2016) potentially reducing mucus binding capacity and its ability to interact with pathogens.
In the lower FRT of premenopausal women, vaginal mucus has an acidic pH that reduces HIV infectivity (Tyssen et al., 2018). Some studies show that postmenopausal women have increased vaginal pH compared to premenopausal women (Thurman et al., 2017), while others show no difference in pH between the two populations (Murphy et al., 2019). Changes in pH could be mediated via altered composition of the vaginal microbiome in older women that in turn increases vaginal pH (Murphy et al., 2019).
3.4. Antimicrobials and cytokines
Antimicrobials and cytokines secreted by epithelial cells are key protective components of cervical‐vaginal fluids which bathe the entire FRT. Antimicrobials interact with pathogens both prior to contact with the epithelium and within the sub‐epithelial stromal compartment (Fahey et al., 2005; Wira et al., 2010). FRT epithelial cells secrete multiple antimicrobials and cytokines including human β‐defensins (HBDs), SLPI, lysozyme, tracheal antimicrobial peptide, TNFα, IL‐8, CCL20, elafin, and cathelicidin (Fahey et al., 2008; Schaefer et al., 2005; Wira et al., 2010, 2011). These have potent antiviral, antibacterial, and anti‐fungal activity and represent an immunological barrier that protects epithelial cells and other cells in the underlying stroma. Sex hormones directly regulate the secretion of antimicrobials by epithelial cells in vitro. Estradiol stimulates the secretion of SLPI, elafin, and HBD2 by endometrial epithelial cells (Fahey et al., 2008), but inhibits HBD2 and elafin secretion by vaginal epithelial cells (Patel et al., 2013). We showed that, due to the absence of SLPI secretion, apical secretions by endometrial epithelial cells in vitro from postmenopausal women are unable to inhibit Staphylococcus aureus growth in culture in contrast to those from premenopausal women (Fahey & Wira, 2002). Loss of antibacterial activity via decreased expression of epithelial antimicrobials could be one mechanism by which older women become more susceptible to bacterial infections.
Several studies have investigated how sex hormones affect antimicrobial and cytokine secretions in the FRT (Cortez et al., 2014; Fahey et al., 2008; Patel et al., 2014; Wira et al., 2010) using cervical‐vaginal lavage (CVL) fluid that consists of the combined secretions of epithelial cells and immune cells from the upper and lower FRT. We and others have found changes that correlate with stage of the menstrual cycle (Keller et al., 2007; Wira et al., 2010). At midcycle (days 13–14), IL‐8, Surfactant Protein A, SLPI, HBD2, α‐defensins 1–3, and lactoferrin in cervical‐vaginal lavage (CVL) fluids are depressed and remain so for 7–10 days (Keller et al., 2007). In contrast, total protein levels and TGFβ remained unchanged during this time. Similarly, Cortez et al. (Cortez et al., 2014) demonstrated that IL‐6, MIP1α, MIP1β, TNFα, GMCSF, IFNα2, and IL‐10 all decreased at midcycle compared to the proliferative and secretory phases.
Changes in the antimicrobial and cytokine profile in CVL after menopause remain unclear. Decreased levels of TNFα (Jais et al., 2016; Thurman et al., 2017), CCL20 (Ghosh et al., 2019; Jais et al., 2016), SLPI (Ghosh et al., 2019; Jais et al., 2016; Murphy et al., 2019; Thurman et al., 2017), and HBD2 (Ghosh et al., 2019; Jais et al., 2016; Murphy et al., 2019; Thurman et al., 2017) were observed in multiple CVL studies comparing pre‐ and postmenopausal women. Despite these differences in antimicrobial and cytokine levels, there were no differences in anti‐HSV‐2 activity between pre‐ and postmenopausal women (Chappell et al., 2015; Thurman et al., 2017). Intriguingly, several studies showed increased anti‐HIV activity in postmenopausal CVL (Jais et al., 2016; Murphy et al., 2019), while others showed no effect (Ghosh et al., 2019; Thurman et al., 2017). Similarly, there was no difference in Escherichia coli inhibition (Murphy et al., 2019; Thurman et al., 2017). Together, these conflicting studies demonstrate the complexity of epithelial‐mediated immune protection in the FRT and suggest that the mechanisms of immune protection against incoming pathogens vary considerably between pre‐ and postmenopausal women.
4. CHANGES IN FIBROBLASTS INDUCED BY AGING
Fibroblasts form a dense layer of cells in the sub‐epithelial stroma where they surround local immune cells. While primarily considered structural cells, they are key players in the innate immune response within the FRT (Wira et al., 2015). Fibroblasts express multiple PRRs, including TLR2, TLR3, TLR4, TLR5, TLR6, TLR9, RIG‐I, and MDA5, with endometrium fibroblasts generally expressing higher PRR levels than other sites in the FRT (Patel et al., 2018b; Patel Shen, & Wira, 2018). They mount innate immune responses against a broad range of incoming pathogens characterized by increased secretion of inflammatory cytokines and chemokines, leading to increased chemotaxis of immune cells (Patel et al., 2018b; Patel Shen, & Wira, 2018). FRT fibroblasts secrete Type I and Type III interferons (IFNs) and thus induce an antiviral state in adjacent cells (Patel et al., 2018b; Patel Shen, & Wira, 2018). Secretions from fibroblasts can also directly inhibit pathogen survival. For example, ovarian and endometrial fibroblast secretions can inhibit HIV infection of target cells (Patel et al., 2018b; Patel Shen, & Wira, 2018).
Similar to other FRT cells, fibroblasts are sensitive to the presence of sex hormones, particularly in the endometrium. In premenopausal women, endometrial fibroblasts decidualize during the secretory phase of the menstrual cycle due to increasing levels of progesterone. In vitro, estradiol stimulates the secretion of hepatocyte growth factor (HGF) and stromal‐derived factor‐1 (SDF‐1α) (Coleman et al., 2009, 2012) and potentiates the upregulation of IL‐27 in response to TLR3 stimulation (Patel et al., 2018a).
The phenotypic and functional changes that FRT fibroblasts undergo following menopause with reduced exposure to sex hormones, and subsequent aging are relatively unknown. Sensitivity to sex hormones is retained in postmenopausal fibroblasts suggesting that exogenous hormones can modulate the function of FRT fibroblasts as women age (Gibson et al., 2018). Endometrial fibroblasts from perimenopausal women have an altered transcriptome compared to premenopausal women characterized by changes in expression for cytoskeleton, proliferation, and survival genes (Erikson et al., 2017). However, studies with other tissues such as the skin demonstrate that with increased age, fibroblasts undergo senescence (Wang & Dreesen, 2018), reduced proliferative capacity (Bentov et al., 2014), decreased wound healing (Mahmoudi et al., 2019), transition to an activated inflammatory phenotype (Wolf et al., 2012), and promote epithelial growth and tumor development (Krtolica et al., 2001) (Figure 2).
5. CHANGES IN T‐CELL DISTRIBUTION AND FUNCTION IN THE FRT
T cells are the most abundant leukocytes in the FRT of pre‐ and postmenopausal women (Givan et al., 1997; Rodriguez‐Garcia et al., 2014; Wira et al., 2015). However, after menopause, T‐cell populations undergo changes in distribution, phenotype, and function in a site‐specific manner.
5.1. CD4+ T cells
More than 95% of CD4+ T cells in the FRT have a memory phenotype and can be found scattered throughout the FRT (Saba et al., 2010; Yeaman et al., 2001). CD4+ T cells recognize peptides presented on MHC class II molecules on antigen presenting cells and play a major role in regulating adaptive immune responses. CD4+ T cells represent 35%–50% of CD3+ T cells in the FRT and, after menopause, CD4+ T‐cell presence is significantly reduced in the endometrium compared to the endocervix and ectocervix (Rodriguez‐Garcia et al., 2014). Within the CD4+ T‐cell population, menopause alters Th17 cell distribution of in the FRT. In premenopausal women, Th17 cells represent a major proportion of the total CD4+ T‐cell population in the endocervix and ectocervix, but are a minor fraction in the endometrium (Joag et al., 2015; Ma et al., 2020; McKinnon et al., 2011; Rodriguez‐Garcia et al., 2014). After menopause, Th17 cells significantly increase in the endometrium, without changes in endocervix and ectocervix (Rodriguez‐Garcia et al., 2014). This compartmentalization of Th17 cell distribution may be relevant for reproductive success, given the studies indicating that Th17 cells in human blood and animal models correlate with early pregnancy loss (Abdolmohammadi Vahid et al., 2019; Fu et al., 2014; Lee et al., 2012; Wang et al., 2020). In addition, Th17 cells play a central role in maintenance of epithelial barrier function and protection against extracellular bacteria and fungi (Sandquist & Kolls, 2018; Stockinger & Omenetti, 2017). Changes in FRT Th17 cell distribution and function with aging, and the potential consequences for immune protection remain unknown.
Another significant change after menopause is the increased expression of CCR5 on FRT CD4+ T cells. Increased CCR5 expression has been demonstrated on CD4+ T cells from the cervix and endometrium, with CCR5 preferentially expressed on Th17 cells (Meditz et al., 2012; Rodriguez‐Garcia et al., 2014; Trifonova et al., 2014). CCR5 is a chemokine receptor with important roles in reproductive function and also the coreceptor used by HIV to infect genital tissues (Saba et al., 2010), representing a marker for susceptibility to HIV acquisition. Increased susceptibility to HIV infection in postmenopausal women has been demonstrated in epidemiological studies analyzing sero‐discordant couples and in ex vivo HIV infection studies using tissue explants (European Study Group on Heterosexual Transmission of HIV, 1992; Rollenhagen & Asin, 2011; Thurman et al., 2017).
5.2. Tissue‐resident memory T cells
Particularly relevant for mucosal surfaces is the presence of tissue‐resident memory T cells (TRMs), which remain in tissues without recirculating, thereby providing first line local defense against reinfection and reactivation (Masopust & Soerens, 2019). TRMs have been involved in protection against genital infections, such as HSV‐2 and HPV, and cancer in animal models (Cuburu et al., 2012; Shin & Iwasaki, 2012; Shin et al., 2016). TRMs can be identified by CD69 and CD103 expression (Gebhardt et al., 2009; Mueller & Mackay, 2016). We and others have demonstrated that a high proportion of human FRT T cells express the tissue residency markers CD69+ and CD103+ (Cantero‐Perez et al., 2019; Duluc et al., 2013; Joag et al., 2015; Ma et al., 2020; Moylan et al., 2016; Oja et al., 2017; Rodriguez‐Garcia et al., ,2017, 2018, 2020). TRMs remain constant throughout the life span in multiple organs and mucosal surfaces (Thome et al., 2014). However, in the FRT, CD103+ T‐cell presence significantly changes with menopause and aging in a site‐specific manner (Rodriguez‐Garcia et al., 2018). Endometrial CD103+ T cells increase after menopause and remain constant with postmenopausal aging. In contrast, in the cervix, CD103+ T cells progressively decline after menopause as women age. CD103 can be expressed on CD4+ and CD8+ T cells (Rosato et al., 2017); however, age‐related changes in the FRT are specific to CD8+ CD103+ T cells, with no modifications on CD4+ CD103+ T cells, which represent less than 10% of the CD103+ T‐cell population (Rodriguez‐Garcia et al., 2018).
5.3. CD8+ T cells
The proportion of CD8+ T cells increases in the endometrium after menopause (Rodriguez‐Garcia et al., 2014; Trifonova et al., 2014), accompanied by modifications in distribution, phenotype, and function. Cytotoxicity is a key function of CD8+ T cells to eliminate infected and cancerous cells. Additionally, uniquely important to the FRT, CD8+ T cells mediate allogeneic rejection, which results in infertility (Erlebacher, 2013a, 2013b). To prevent rejection of the semi‐allogeneic blastocyst, still unknown mechanisms control T‐cell function specifically in the endometrium to suppress CD8+ T‐cell cytotoxic activity. Cytotoxic activity of endometrial CD8+T cells, including direct killing of allogeneic target cells, is significantly suppressed in premenopausal women compared to postmenopausal women (Rodriguez‐Garcia et al., 2020; White et al., 1997). Within premenopausal women, cytotoxic activity was further suppressed during the secretory phase of the menstrual cycle, when implantation and pregnancy is likely to occur in the endometrium (Rodriguez‐Garcia et al., 2020; White et al., 1997). Importantly, cytotoxic activity is uniquely regulated in the endometrium, with no effect of menstrual cycle and menopausal status on cytotoxic activity by CD8+ T cells from the endocervix or ectocervix (Rodriguez‐Garcia et al., 2020; White et al., 1997).
TGFβ, produced by epithelial cells and fibroblasts (Omwandho et al., 2010; Wira & Rossoll, 2003), has been shown to suppress cytotoxic activity in animal and human experimental models, including the human endometrium (Lee & Rich, 1993; Rodriguez‐Garcia et al., 2020). Interestingly, TGFβ specifically suppressed cytotoxic function of CD8+ T cells from the endometrium but not from the cervix and ectocervix. Susceptibility to endometrial suppression by TGFβ decreased after menopause, highlighting the complex regulation of T cells in the FRT (Rodriguez‐Garcia et al., 2020). Additional major functional changes in CD8+ T cells after menopause include increased degranulation capacity and changes in the profile of granzymes produced, shifting from predominant granzyme B to granzyme A production in response to stimulation (Rodriguez‐Garcia et al., 2020). Granzyme A has proinflammatory properties (Arias et al., 2017; Wensink et al., 2015), and therefore, a modified granzyme A predominant profile after menopause may contribute to increased genital inflammation. Interestingly, while resident and non‐resident T cells have differential cytotoxic capacity (in vitro cytotoxicity and cytotoxic molecule content), both populations equally undergo changes in their granzyme profiles with increased degranulation after menopause (Rodriguez‐Garcia et al., 2020). Changes detected in T cells after menopause are summarized in Figure 3.
FIGURE 3.
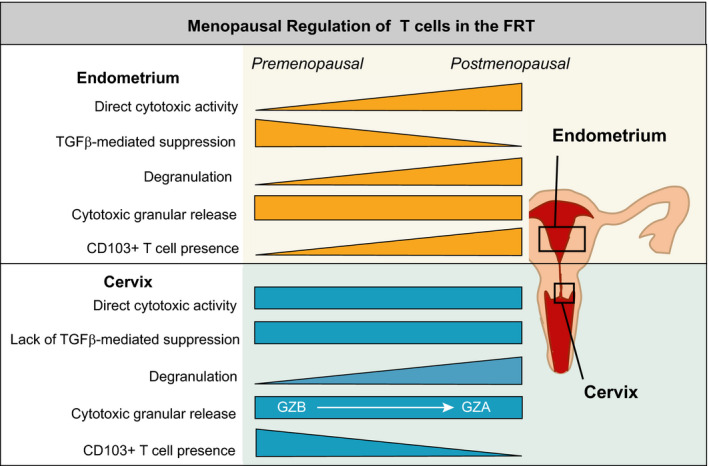
Regulation of CD8+ T‐cell function in the FRT by menopausal status. This diagram indicates key T‐cell functions that are modified after menopause. As indicated by the shape of each triangle, some functions decline while others increase after menopause. Rectangles indicate no change. Effects are shown for the endometrium on the upper part and for the cervix (endocervix and ectocervix) on the lower part of the figure
Sex hormones modify CD8+ T‐cell cytotoxic activity. Estradiol acts directly on CD8+ T cells, and progesterone indirectly by increasing epithelial cell TGFβ production, to suppress cytotoxicity of endometrial CD8+ T cells (Shen et al., 2021). These findings suggest hormonal suppression of endometrial cytotoxic function, essential for successful implantation and pregnancy. Following menopause, with marked reduction in hormone production, hormonal suppression is removed, and CD8+ T‐cell cytotoxicity may rebound to provide protection throughout the FRT. Potential changes in CD8+ T‐cell cytotoxic activity in the years after menopause are unknown.
6. CHANGES IN DISTRIBUTION AND FUNCTION OF DCS AND MACROPHAGES IN THE FRT
In addition to resident T cells, mucosal surfaces contain multiple subsets of resident DCs and macrophages essential for innate immune protection and the induction and maintenance of adaptive immune responses (Schlitzer et al., 2015). The phenotype and function of DCs and macrophages are known to be strongly influenced by the tissue environment (Schlitzer et al., 2015). In the FRT, DCs and macrophages play key roles in reproduction (Dekel et al., 2014; Gnainsky et al., 2015), and their presence in the premenopausal endometrium is regulated by sex hormone (Berbic et al., 2009; Evans & Salamonsen, 2012; Schulke et al., 2008). Therefore, as reproductive function ends, DC and macrophage presence and function in the FRT would be expected to be modified.
Diverse age‐dependent effects have been described for blood DCs, including increased secretion of proinflammatory cytokines, decreased secretion of type I and III IFN, increased responses against self‐antigens, and altered capacity to prime T cells (Agrawal et al., 2017). However, potential age‐dependent changes in DC populations in human tissues, and particularly in the FRT, are not well understood.
Several DC subsets are present throughout the FRT, including CD1c+, CD1a+, and CD14+ DCs. Others have compartmentalized distribution, such as CD103+ DCs, found exclusively in the endometrium, or Langerhans cells and epithelial DCs, found in the vagina (Bertram et al., 2019; Duluc et al., 2013; Hladik et al., 2007; Pena‐Cruz et al., 2018; Rodriguez‐Garcia et al., 2017; Mariani et al., 2002). In the FRT, DCs are found both in the sub‐epithelial compartment and within the epithelium (Iijima et al., 2008; Kaldensjo et al., 2011). We recently demonstrated that as women age, there is a progressive decline in CD11c+ DC number throughout the FRT (Figure 4) (Rodriguez‐Garcia et al., 2018). Decreased presence of CD1a+ antigen presenting cells have also been described in the vaginal mucosa from postmenopausal compared to premenopausal women (Thurman et al., 2017). The decline in DC numbers observed in the FRT contrasts with myeloid DCs in blood and in the intestinal and respiratory mucosae, which remain stable with age (Agrawal, 2017; Granot et al., 2017). Regarding phenotype, a trend toward increased maturation with age has been described in intestinal DCs (Granot et al., 2017), but whether this also applies to FRT DCs remains to be determined. PD‐L1 expression is increased on DCs in endometrium and cervix from postmenopausal compared to premenopausal women (Shen et al., 2016). PD‐L1 increases were specific to DCs and associated with decreased PD‐L1 expression on CD8+ T cells. The potential consequences of these changes on T‐cell activation and peripheral tolerance remain to be determined.
FIGURE 4.
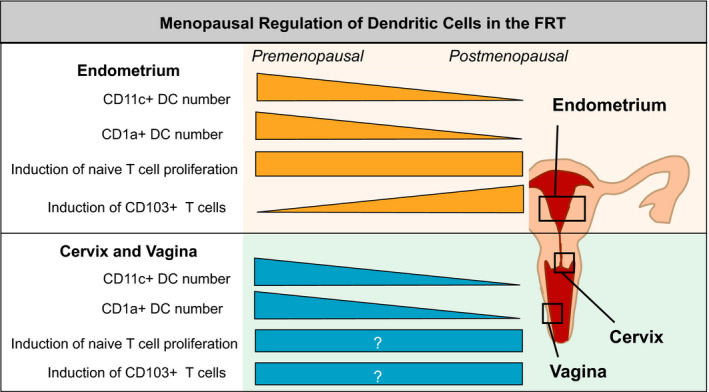
Regulation of DC distribution function in the FRT by menopausal status. This diagram shows key DC functions that are modified after menopause. Specific functions decline or increase after menopause as indicated. Rectangles indicate no change; those containing a question mark (?) indicate that changes are unknown. Effects are shown for the endometrium on the upper part and for the cervix (endocervix and ectocervix) and vagina on the lower part of the figure
A functional characteristic of DCs is the induction of CD103 expression on naïve CD8+ T cells, suggesting that DCs have the potential to control TRM presence in tissues (Yu et al., 2013). This ability has been demonstrated with human lung and FRT DCs (Duluc et al., 2013; Rodriguez‐Garcia et al., 2018; Yu et al., 2013). Importantly, this function is regulated by menopausal status in the endometrium, with postmenopausal DCs showing enhanced ability to induce CD103 expression on naïve CD8+ T cells when compared to premenopausal DCs (Rodriguez‐Garcia et al., 2018). The mechanism responsible for CD103 upregulation on CD8+ T cells is at least partly related to TGFβ signaling in a contact‐dependent manner (Rodriguez‐Garcia et al., 2018; Yu et al., 2013). Interestingly, this functional modification is highly selective, as it was not associated with changes in FRT DC capacity to induce T‐cell proliferation after menopause (Rodriguez‐Garcia et al., 2018). Potential menopausal regulation of DC function in other FRT compartments remains unknown (Figure 4).
Macrophages constitute 10%–20% of leukocytes in the FRT, with phenotypic differences between upper and lower tract (Givan et al., 1997; Trifonova et al., 2014). In the lower FRT, macrophages express high CD14 levels, while in the endometrium, macrophages express low levels of CD14, with a subset of endometrial macrophages expressing CD163 (Jensen et al., 2012; Quillay et al., 2015; Shen et al., 2009). While it is well known that macrophage numbers increase in the endometrium prior to menstruation (Evans & Salamonsen, 2012), little is known about changes after menopause. An early study reported significant differences in macrophage numbers between pre‐ and postmenopausal women in the fallopian tubes (Safwat et al., 2008), but whether that also applies to the endometrium or lower tract, or whether macrophage functional changes occur is unknown.
Functional changes in DC and macrophage throughout the FRT with aging, and the potential consequences for immune protection and induction of mucosal adaptive responses remain unknown.
7. CHANGES IN OTHER FRT CELLS
7.1. NK cells
Natural Killer (NK) cells represent 10–30% of FRT immune cells (Givan et al., 1997; Hunt, 1994; King et al., 1989; Wira et al., 2005). Their numbers in the endometrium change during the menstrual cycle, peaking prior to menstruation (Givan et al., 1997; Hunt, 1994; King et al., 1989; Wira et al., 2005). NK cell phenotype varies within the FRT. In the upper FRT, NK cells are CD56 BRIGHT, CD16‐, and CD94+, whereas in the lower FRT, NK cells are CD56 DIM CD16+ and CD94‐ (Eriksson et al., 2004; Kopcow et al., 2010; Mselle et al., 2007). FRT NK cells are essential for immune defense against pathogens such as HIV (Mselle et al., 2009; Quillay et al., 2016), control of FRT tumors (Degos et al., 2019), and tissue remodeling for reproduction (Jabrane‐Ferrat, 2019) via their cytotoxic effector functions and cytokine and chemokine secretion.
The extent to which phenotype and numbers of FRT NK cells change in the postmenopausal FRT remains unknown; however, blood NK cells undergo profound changes with aging (Hazeldine & Lord, 2013). Blood NK cell subsets change with age, with decreased CD56BRIGHT cells, increased CD56‐CD16+ cells (Solana et al., 2014), and increased CD57 expression, a marker of differentiated NK cells (Gayoso et al., 2011). The percentage and number of blood CD3‐CD56+ NK cells increases with age (Le Garff‐Tavernier et al., 2010; Lutz et al., 1950, 2005), but is accompanied by reduced proliferation capacity (Solana et al., 1999) suggesting accumulation as a result of increased longevity (Zhang et al., 2007). NK cells in postmenopausal women retain their sensitivity to sex hormones, since estradiol enhances proliferation of blood NK cells (Sho et al., 2017). With respect to cytotoxicity, the effects of age are unclear in that studies report decreased (Hazeldine et al., 2012), increased (Kutza & Murasko, 1994), or no change (Almeida‐Oliveira et al., 2011) in cytotoxic capacity of blood NK cells. NK cells from younger women upregulate IFNγ, MIP‐1α, and IL‐8 to a greater extent than cells from older women (Borrego et al., 1999; Krishnaraj & Bhooma, 1996; Mariani, Meneghetti, et al., 2002; Mariani et al., 2001; Mariani, Pulsatelli, et al., 2002; Solana et al., 1999).
7.2. B cells
The density and distribution of B cells varies within the premenopausal FRT, with IgA‐, IgG‐, or IgM‐producing cells predominantly found in the vagina, ectocervix, endocervix, and fallopian tubes, but minimal numbers in the endometrium and ovary (Crowley‐Nowick et al., 1995; Hurlimann et al., 1978; Kelly & Fox, 1979; Kutteh et al., ,1988, 1998; Rebello et al., 1975; Mariani et al., 2002). In the premenopausal endometrium, during secretory phase, B cells form the central core of endometrial lymphoid aggregates surrounded by CD8+ T cells (Yeaman et al., 1997), but are undetectable in smaller aggregates present during the proliferative phase of the menstrual cycle. In postmenopausal women, aggregates are absent and B cells sparsely distributed throughout endometrial tissue.
Endometrial secretions contain IgG and IgA, with IgG present at higher levels (Schumacher et al., 1980). IgA1 and IgA2 are present in approximately equal proportions (Kutteh et al., 1996). Endometrial secretion of IgA peaks shortly before ovulation (Kutteh et al., 1996; Schumacher et al., 1973, 1977, 1980), while stromal IgA peaks at ovulation (Kelly & Fox, 1979). IgA and IgG levels in cervical mucus also vary with stage of the menstrual cycle and are lowest at midcycle (Schumacher et al., 1973). However, in other studies, IgA and IgG were suppressed during secretory phase (Keller et al., 2007). There was no difference in IgG and IgA levels in cervico‐vaginal secretions between premenopausal, postmenopausal, and pregnant women (Jilanti & Isliker, 1977). In postmenopausal vaginal secretions, IgG and IgA levels were reduced by twofold and 15‐fold, respectively, following hysterectomy (Jilanti & Isliker, 1977), demonstrating significant endometrial contributions to FRT IgG and IgA levels.
Immunoglobulins present in FRT secretions are essential components of immune protection. IgG and IgA neutralize incoming pathogens and prevent their entry into target cells (Lamm et al., ,1978, 1995; Nedrud et al., 1987). For example, anti‐HIV IgM reduces infection of DCs in cervical‐vaginal explant tissues (Devito et al., 2018) while levels of anti‐HIV gp160 IgG antibodies in human CVL samples correlate with anti‐HIV activity and reduce HIV infection of target cells in vitro (Ghosh et al., 2010). Immunoglobulins also bind to mucus in FRT secretions via mucin proteins and trap pathogens within it. Anti‐HSV‐1 IgG, via its Fc component, traps HSV‐1 in human cervical‐vaginal mucus thus preventing contact with target cells (Wang et al., 2014). Intriguingly, while both IgA and IgG bind to cervical mucus, only IgG binds to cervical‐vaginal mucus (Fahrbach et al., 2013). Vaccination at peripheral sites elicits antibody‐mediated mucosal protection in the FRT. For example, vaccination against HPV16 in premenopausal women leads to increased titers of anti‐HPV IgG in cervical secretions that varies with menstrual cycle stage (Nardelli‐Haefliger et al., 2003). How aging affects the contribution of immunoglobulin‐mediated protection in the FRT is unknown.
8. CONCLUSIONS
The mucosal immune system in the human FRT has uniquely evolved to meet the challenges of an external environment as well as support new life. Across multiple anatomical compartments, mucosal immunity is precisely regulated to protect against sexually transmitted pathogens while accommodating allogeneic spermatozoa and an immunologically distinct semi‐allogeneic fetus. While much is known about the mucosal immune system in the FRT during the reproductive years, little is known about the changes that occur after menopause as women age. Limited studies into innate and adaptive immune functions in the FRT following menopause indicate that immune protection by epithelial cells, stromal fibroblasts, T cells, and DC in the FRT are compromised, with limited compensation. Much remains to be learned about the impact of age following menopause on immune protection in the FRT. Understanding the impact of age on mucosal immune protection in the FRT is crucial given the challenges women face in terms of urogenital infections, exposure to sexually transmitted pathogens, and gynecological cancers that threaten the lives of women worldwide. This review emphasizes the need for additional studies to provide a foundation for the development of age‐appropriate therapeutic interventions that increase protection in older women, the fastest growing segment of the population in developed countries.
CONFLICT OF INTEREST
The authors declare no conflict of interest.
AUTHOR CONTRIBUTIONS
MRG and CRW wrote the introduction section; MVP wrote epithelial cell, fibroblast, and other cells sections; MRG, CRW, and ZS wrote the T‐cell section; MRG wrote the DC section. All authors edited and approved the manuscript.
ACKNOWLEDGMENTS
This study was supported by NIH grants AG064794, AI117739 (CW), and AG060801 (MR‐G).
REFERENCES
- Abdolmohammadi Vahid, S. , Ghaebi, M. , Ahmadi, M. , Nouri, M. , Danaei, S. , Aghebati‐Maleki, L. , Mousavi Ardehaie, R. , Yousefi, B. , Hakimi, P. , Hojjat‐Farsangi, M. , Rikhtegar, R. , & Yousefi, M. (2019). Altered T‐cell subpopulations in recurrent pregnancy loss patients with cellular immune abnormalities. Journal of Cellular Physiology, 234, 4924–4933. [DOI] [PubMed] [Google Scholar]
- Agrawal, A. (2017). Dendritic cell‐airway epithelial cell cross‐talk changes with age and contributes to chronic lung inflammatory diseases in the elderly. International Journal of Molecular Sciences, 18, 1206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agrawal, A. , Agrawal, S. , & Gupta, S. (2017). Role of dendritic cells in inflammation and loss of tolerance in the elderly. Frontiers in Immunology, 8, 896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almeida‐Oliveira, A. , Smith‐Carvalho, M. , Porto, L. C. , Cardoso‐Oliveira, J. , Ribeiro, A. S. , Falcão, R. R. , Abdelhay, E. , Bouzas, L. F. , Thuler, L. C. S. , Ornellas, M. H. , & Diamond, H. R. (2011). Age‐related changes in natural killer cell receptors from childhood through old age. Human Immunology, 72, 319–329. [DOI] [PubMed] [Google Scholar]
- Anderson, D. J. , Marathe, J. , & Pudney, J. (1989). The structure of the human vaginal stratum corneum and its role in immune defense. American Journal of Reproductive Immunology (New York. N.Y.: 1989), 2014(71), 618–623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson, J. M. , & Van Itallie, C. M. (2009). Physiology and function of the tight junction. Cold Spring Harbor Perspectives in Biology, 1, a002584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arias, M. , Martinez‐Lostao, L. , Santiago, L. , Ferrandez, A. , Granville, D. J. , & Pardo, J. (2017). The untold story of granzymes in oncoimmunology: Novel opportunities with old acquaintances. Trends Cancer, 3, 407–422. [DOI] [PubMed] [Google Scholar]
- Ben Menachem‐Zidon, O. , Parkes, I. , Chill, H. H. , Reubinoff, B. , Sandberg, K. , Ji, H. , & Shveiky, D. (2020). Age‐associated differences in macrophage response in a vaginal wound healing rat model. International Urogynecology Journal, 31, 1803–1809. [DOI] [PubMed] [Google Scholar]
- Bentov, I. , Damodarasamy, M. , Plymate, S. , & Reed, M. J. (2014). Decreased proliferative capacity of aged dermal fibroblasts in a three dimensional matrix is associated with reduced IGF1R expression and activation. Biogerontology, 15, 329–337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berbic, M. , Schulke, L. , Markham, R. , Tokushige, N. , Russell, P. , & Fraser, I. S. (2009). Macrophage expression in endometrium of women with and without endometriosis. Human Reproduction, 24, 325–332. [DOI] [PubMed] [Google Scholar]
- Bertram, K. M. , Botting, R. A. , Baharlou, H. , Rhodes, J. W. , Rana, H. , Graham, J. D. , Patrick, E. , Fletcher, J. , Plasto, T. M. , Truong, N. R. , & Royle, C. (2019). Identification of HIV transmitting CD11c(+) human epidermal dendritic cells. Nature Communications, 10, 2759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blaskewicz, C. D. , Pudney, J. , & Anderson, D. J. (2011). Structure and function of intercellular junctions in human cervical and vaginal mucosal epithelia. Biology of Reproduction, 85, 97–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borrego, F. , Alonso, M. C. , Galiani, M. D. , Carracedo, J. , Ramirez, R. , Ostos, B. , Peña, J. , & Solana, R. (1999). NK phenotypic markers and IL2 response in NK cells from elderly people. Experimental Gerontology, 34, 253–265. [DOI] [PubMed] [Google Scholar]
- Cantero‐Perez, J. , Grau‐Exposito, J. , Serra‐Peinado, C. , Rosero, D. A. , Luque‐Ballesteros, L. , Astorga‐Gamaza, A. , Castellvi, J. , Sanhueza, T. , Tapia, G. , Lloveras, B. , Fernández, M. A. , Prado, J. G. , Solé‐Sedeno, J. M. , Tarrats, A. , Lecumberri, C. , Mañalich‐Barrachina, L. , Centeno‐Mediavilla, C. , Falcó, V. , Buzon, M. J. , & Genescà, M. (2019). Resident memory T cells are a cellular reservoir for HIV in the cervical mucosa. Nature Communications, 10, 4739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CDC (2016a). Sexually Transmitted Diseases Surveillance. [Google Scholar]
- CDC (2016b). Diagnoses of HIV infection among adults aged 50 years and older in the United States and dependent areas, 2010‐2014. In Surveillance Supplemental Report 2016;21(2). Accessed July 1, 2016. [Google Scholar]
- CDC (2019). Gynecologic Cancer Incidence, United States—2012–2016. USCS Data Brief 2019, 11. [Google Scholar]
- Chan, C. K. , Aimagambetova, G. , Ukybassova, T. , Kongrtay, K. , & Azizan, A. (2019). Human papillomavirus infection and cervical cancer: Epidemiology, screening, and vaccination‐review of current perspectives. Journal of Oncology, 2019, 3257939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chappell, C. A. , Isaacs, C. E. , Xu, W. , Meyn, L. A. , Uranker, K. , Dezzutti, C. S. , Moncla, B. J. , & Hillier, S. L. (2015). The effect of menopause on the innate antiviral activity of cervicovaginal lavage. American Journal of Obstetrics and Gynecology, 213, 204.e201–204.e2046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coleman, K. D. , Ghosh, M. , Crist, S. G. , Wright, J. A. , Rossoll, R. M. , Wira, C. R. , & Fahey, J. V. (2012). Modulation of hepatocyte growth factor secretion in human female reproductive tract stromal fibroblasts by poly (I:C) and estradiol. American Journal of Reproductive Immunology, 67, 44–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coleman, K. D. , Wright, J. A. , Ghosh, M. , Wira, C. R. , & Fahey, J. V. (2009). Estradiol modulation of hepatocyte growth factor by stromal fibroblasts in the female reproductive tract. Fertility and Sterility, 92, 1107–1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cortez, V. , Odem‐Davis, K. , Lehman, D. A. , Mabuka, J. , & Overbaugh, J. (2014). Quotidian changes of genital tract cytokines in human immunodeficiency virus‐1‐infected women during the menstrual cycle. Open Forum Infectious Diseases, 1, ofu002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Critchlow, C. W. , Wölner‐Hanssen, P. , Eschenbach, D. A. , Kiviat, N. B. , Koutsky, L. A. , Stevens, C. E. , & Holmes, K. K. (1995). Determinants of cervical ectopia and of cervicitis: Age, oral contraception, specific cervical infection, smoking, and douching. American Journal of Obstetrics and Gynecology, 173, 534–543. [DOI] [PubMed] [Google Scholar]
- Crowley‐Nowick, P. A. , Bell, M. , Edwards, R. P. , McCallister, D. , Gore, H. , Kanbour‐Shakir, A. , Mestecky, J. , & Partridge, E. E. (1995). Normal uterine cervix: Characterization of isolated lymphocyte phenotypes and immunoglobulin secretion. American Journal of Reproductive Immunology, 34, 241–247. [DOI] [PubMed] [Google Scholar]
- Cuburu, N. , Graham, B. S. , Buck, C. B. , Kines, R. C. , Pang, Y. Y. , Day, P. M. , Lowy, D. R. , & Schiller, J. T. (2012). Intravaginal immunization with HPV vectors induces tissue‐resident CD8+ T cell responses. Journal of Clinical Investigation, 122, 4606–4620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Degos, C. , Heinemann, M. , Barrou, J. , Boucherit, N. , Lambaudie, E. , Savina, A. , Gorvel, L. , & Olive, D. (2019). Endometrial tumor microenvironment alters human NK cell recruitment, and resident NK cell phenotype and function. Frontiers in Immunology, 10, 877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dekel, N. , Gnainsky, Y. , Granot, I. , Racicot, K. , & Mor, G. (2014). The role of inflammation for a successful implantation. American Journal of Reproductive Immunology, 72, 141–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devito, C. , Ellegård, R. , Falkeborn, T. , Svensson, L. , Ohlin, M. , Larsson, M. , Broliden, K. , & Hinkula, J. (2018). Human IgM monoclonal antibodies block HIV‐transmission to immune cells in cervico‐vaginal tissues and across polarized epithelial cells in vitro. Scientific Reports, 8, 10180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duluc, D. , Gannevat, J. , Anguiano, E. , Zurawski, S. , Carley, M. , Boreham, M. , Stecher, J. , Dullaers, M. , Banchereau, J. , & Oh, S. (2013). Functional diversity of human vaginal APC subsets in directing T‐cell responses. Mucosal Immunology, 6, 626–638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunston, C. R. , & Griffiths, H. R. (2010). The effect of ageing on macrophage Toll‐like receptor‐mediated responses in the fight against pathogens. Clinical & Experimental Immunology, 161, 407–416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elstein, M. (1978). Functions, and physical properties of mucus in the female genital tract. British Medical Bulletin, 34, 83–88. [DOI] [PubMed] [Google Scholar]
- Engeland, C. G. , Sabzehei, B. , & Marucha, P. T. (2009). Sex hormones and mucosal wound healing. Brain, Behavior, and Immunity, 23, 629–635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erikson, D. W. , Barragan, F. , Piltonen, T. T. , Chen, J. C. , Balayan, S. , Irwin, J. C. , & Giudice, L. C. (2017). Stromal fibroblasts from perimenopausal endometrium exhibit a different transcriptome than those from the premenopausal endometrium. Biology of Reproduction, 97, 387–399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eriksson, M. , Meadows, S. K. , Wira, C. R. , & Sentman, C. L. (2004). Unique phenotype of human uterine NK cells and their regulation by endogenous TGF‐beta. Journal of Leukocyte Biology, 76, 667–675. [DOI] [PubMed] [Google Scholar]
- Erlebacher, A. (2013). Immunology of the maternal‐fetal interface. Annual Review of Immunology, 31, 387–411. [DOI] [PubMed] [Google Scholar]
- Erlebacher, A. (2013). Mechanisms of T cell tolerance towards the allogeneic fetus. Nature Reviews Immunology, 13, 23–33. [DOI] [PubMed] [Google Scholar]
- European Study Group on Heterosexual Transmission of HIV (1992). Comparison of female to male and male to female transmission of HIV in 563 stable couples. BMJ: British Medical Journal. 304, 809–813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans, J. , & Salamonsen, L. A. (2012). Inflammation, leukocytes and menstruation. Reviews in Endocrine and Metabolic Disorders, 13, 277–288. [DOI] [PubMed] [Google Scholar]
- Fahey, J. V. , Schaefer, T. M. , Channon, J. Y. , & Wira, C. R. (2005). Secretion of cytokines and chemokines by polarized human epithelial cells from the female reproductive tract. Human Reproduction, 20, 1439–1446. [DOI] [PubMed] [Google Scholar]
- Fahey, J. V. , Schaefer, T. M. , & Wira, C. R. (2006). Sex hormone modulation of human uterine epithelial cell immune responses. Integrative and Computational Biology, 46, 1082–1087. [DOI] [PubMed] [Google Scholar]
- Fahey, J. V. , & Wira, C. R. (2002). Effect of menstrual status on antibacterial activity and secretory leukocyte protease inhibitor production by human uterine epithelial cells in culture. The Journal of Infectious Diseases, 185, 1606–1613. [DOI] [PubMed] [Google Scholar]
- Fahey, J. V. , Wright, J. A. , Shen, L. , Smith, J. M. , Ghosh, M. , Rossoll, R. M. , & Wira, C. R. (2008). Estradiol selectively regulates innate immune function by polarized human uterine epithelial cells in culture. Mucosal Immunology, 1, 317–325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fahrbach, K. M. , Malykhina, O. , Stieh, D. J. , & Hope, T. J. (2013). Differential binding of IgG and IgA to mucus of the female reproductive tract. PLoS One, 8, e76176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farage, M. , & Maibach, H. (2006). Lifetime changes in the vulva and vagina. Archives of Gynecology and Obstetrics, 273, 195–202. [DOI] [PubMed] [Google Scholar]
- Fichorova, R. N. , Cronin, A. O. , Lien, E. , Anderson, D. J. , & Ingalls, R. R. (2002). Response to Neisseria gonorrhoeae by Cervicovaginal epithelial cells occurs in the absence of toll‐like receptor 4‐mediated signaling. The Journal of Immunology, 168, 2424–2432. [DOI] [PubMed] [Google Scholar]
- Fu, B. , Tian, Z. , & Wei, H. (2014). TH17 cells in human recurrent pregnancy loss and pre‐eclampsia. Cellular & Molecular Immunology, 11, 564–570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gavazzi, G. , & Krause, K. H. (2002). Ageing and infection. The Lancet Infectious Diseases, 2, 659–666. [DOI] [PubMed] [Google Scholar]
- Gayoso, I. , Sanchez‐Correa, B. , Campos, C. , Alonso, C. , Pera, A. , Casado, J. G. , Morgado, S. , Tarazona, R. , & Solana, R. (2011). Immunosenescence of human natural killer cells. Journal of Innate Immunity, 3, 337–343. [DOI] [PubMed] [Google Scholar]
- Gebhardt, T. , Wakim, L. M. , Eidsmo, L. , Reading, P. C. , Heath, W. R. , & Carbone, F. R. (2009). Memory T cells in nonlymphoid tissue that provide enhanced local immunity during infection with herpes simplex virus. Nature Immunology, 10, 524–530. [DOI] [PubMed] [Google Scholar]
- Ghosh, M. , Fahey, J. V. , Shen, Z. , Lahey, T. , Cu‐Uvin, S. , Wu, Z. , Mayer, K. , Wright, P. F. , Kappes, J. C. , Ochsenbauer, C. , & Wira, C. R. (2010). Anti‐HIV activity in cervical‐vaginal secretions from HIV‐positive and ‐negative women correlate with innate antimicrobial levels and IgG antibodies. PLoS One, 5, e11366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh, M. , Jais, M. , Delisle, J. , Younes, N. , Benyeogor, I. , Biswas, R. , Mohamed, H. , Daniels, J. , Wang, C. , Young, M. , & Kassaye, S. (2019). Dysregulation in genital tract soluble immune mediators in postmenopausal women is distinct by HIV status. AIDS Research and Human Retroviruses, 35, 251–259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh, M. , Rodriguez‐Garcia, M. , & Wira, C. R. (2014). The immune system in menopause: Pros and cons of hormone therapy. The Journal of Steroid Biochemistry and Molecular Biology, 142, 171–175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh, M. , Shen, Z. , Fahey, J. V. , Crist, S. G. , Smith, J. M. , Patel, M. V. , & Wira, C. R. (2012). Pathogen recognition in the human female reproductive tract: Expression of intracellular cytosolic sensors NOD1, NOD2, RIG‐1, and MDA5. American Journal of Reproductive Immunology, 69(1), 41–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson, D. A. , Simitsidellis, I. , Collins, F. , & Saunders, P. T. K. (2018). Endometrial intracrinology: Oestrogens, androgens and endometrial disorders. International Journal of Molecular Sciences, 19, 3276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gipson, I. K. , Ho, S. B. , Spurr‐Michaud, S. J. , Tisdale, A. S. , Zhan, Q. , Torlakovic, E. , Pudney, J. , Anderson, D. J. , Toribara, N. W. , & Hill, J. A. (1997). Mucin genes expressed by human female reproductive tract epithelia. Biology of Reproduction, 56, 999–1011. [DOI] [PubMed] [Google Scholar]
- Givan, A. L. , White, H. D. , Stern, J. E. , Colby, E. , Gosselin, E. J. , Guyre, P. M. , & Wira, C. R. (1997). Flow cytometric analysis of leukocytes in the human female reproductive tract: Comparison of fallopian tube, uterus, cervix, and vagina. American Journal of Reproductive Immunology, 38, 350–359. [DOI] [PubMed] [Google Scholar]
- Gnainsky, Y. , Granot, I. , Aldo, P. , Barash, A. , Or, Y. , Mor, G. , & Dekel, N. (2015). Biopsy‐induced inflammatory conditions improve endometrial receptivity: The mechanism of action. Reproduction, 149, 75–85. [DOI] [PubMed] [Google Scholar]
- Gonzalez, P. , Hildesheim, A. , Rodriguez, A. C. , Schiffman, M. , Porras, C. , Wacholder, S. , Pineres, A. G. , Pinto, L. A. , Burk, R. D. , & Herrero, R. (2010). Behavioral/lifestyle and immunologic factors associated with HPV infection among women older than 45 years. Cancer Epidemiology, Biomarkers & Prevention, 19, 3044–3054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorodeski, G. I. (2001). Estrogen biphasic regulation of paracellular permeability of cultured human vaginal‐cervical epithelia. The Journal of Clinical Endocrinology & Metabolism, 86, 4233–4243. [DOI] [PubMed] [Google Scholar]
- Gorodeski, G. I. (2001). Vaginal‐cervical epithelial permeability decreases after menopause. Fertility and Sterility, 76, 753–761. [DOI] [PubMed] [Google Scholar]
- Gorodeski, G. I. (2007). Estrogen modulation of epithelial permeability in cervical‐vaginal cells of premenopausal and postmenopausal women. Menopause, 14, 1012–1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorodeski, G. I. , Hopfer, U. , Liu, C. C. , & Margles, E. (2005). Estrogen acidifies vaginal pH by up‐regulation of proton secretion via the apical membrane of vaginal‐ectocervical epithelial cells. Endocrinology, 146, 816–824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Granot, T. , Senda, T. , Carpenter, D. J. , Matsuoka, N. , Weiner, J. , Gordon, C. L. , Miron, M. , Kumar, B. V. , Griesemer, A. , Ho, S. H. , & Lerner, H. (2017). Dendritic cells display subset and tissue‐specific maturation dynamics over human life. Immunity, 46, 504–515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Granville, L. , & Pregler, J. (2018). Women's sexual health and aging. Journal of the American Geriatrics Society, 66, 595–601. [DOI] [PubMed] [Google Scholar]
- Gravitt, P. E. , Rositch, A. F. , Silver, M. I. , Marks, M. A. , Chang, K. , Burke, A. E. , & Viscidi, R. P. (2013). A cohort effect of the sexual revolution may be masking an increase in human papillomavirus detection at menopause in the United States. The Journal of Infectious Diseases, 207, 272–280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hart, K. M. , Murphy, A. J. , Barrett, K. T. , Wira, C. R. , Guyre, P. M. , & Pioli, P. A. (2009). Functional expression of pattern recognition receptors in tissues of the human female reproductive tract. Journal of Reproductive Immunology, 80, 33–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hazeldine, J. , Hampson, P. , & Lord, J. M. (2012). Reduced release and binding of perforin at the immunological synapse underlies the age‐related decline in natural killer cell cytotoxicity. Aging Cell, 11, 751–759. [DOI] [PubMed] [Google Scholar]
- Hazeldine, J. , & Lord, J. M. (2013). The impact of ageing on natural killer cell function and potential consequences for health in older adults. Ageing Research Reviews, 12, 1069–1078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He, W. , Goodkind, D. , & Kowal, P. (2016). An Aging World: 2015. United States Census Bureau. [Google Scholar]
- Henley, S. J. , Ward, E. M. , Scott, S. , Ma, J. , Anderson, R. N. , Firth, A. U. , Thomas, C. C. , Islami, F. , Weir, H. K. , Lewis, D. R. , Sherman, R. L. , Wu, M. , Benard, V. B. , Richardson, L. C. , Jemal, A. , Cronin, K. , & Kohler, B. A. (2020). Annual report to the nation on the status of cancer, part I: National cancer statistics. Cancer, 126, 2225–2249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrera, A. P. , Snipes, S. A. , King, D. W. , Torres‐Vigil, I. , Goldberg, D. S. , & Weinberg, A. D. (2010). Disparate inclusion of older adults in clinical trials: Priorities and opportunities for policy and practice change. American Journal of Public Health, 100, S105–S112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hladik, F. , Sakchalathorn, P. , Ballweber, L. , Lentz, G. , Fialkow, M. , Eschenbach, D. , & McElrath, M. J. (2007). Initial events in establishing vaginal entry and infection by human immunodeficiency virus type‐1. Immunity, 26, 257–270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horng, H. C. , Chang, W. H. , Yeh, C. C. , Huang, B. S. , Chang, C. P. , Chen, Y. J. , Tsui, K.‐H. , & Wang, P.‐H. (2017). Estrogen effects on wound healing. International Journal of Molecular Sciences, 18(11), 2325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu, K. K. , Boyko, E. J. , Scholes, D. , Normand, E. , Chen, C. L. , Grafton, J. , & Fihn, S. D. (2004). Risk factors for urinary tract infections in postmenopausal women. Archives of Internal Medicine, 164, 989–993. [DOI] [PubMed] [Google Scholar]
- Hummelen, R. , Macklaim, J. M. , Bisanz, J. E. , Hammond, J. A. , McMillan, A. , Vongsa, R. , Koenig, D. , Gloor, G. B. , & Reid, G. (2011). Vaginal microbiome and epithelial gene array in post‐menopausal women with moderate to severe dryness. PLoS One, 6, e26602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunt, J. S. (1994). Immunologically relevant cells in the uterus. Biology of Reproduction, 50, 461–466. [DOI] [PubMed] [Google Scholar]
- Hurlimann, J. , Dayal, R. , & Gloor, E. (1978). Immunoglobulins and secretory component in endometrium and cervix: Influence of inflammation and carcinoma. Virchows Archiv A Pathological Anatomy and Histology, 377, 211–223. [DOI] [PubMed] [Google Scholar]
- Iijima, N. , Linehan, M. M. , Zamora, M. , Butkus, D. , Dunn, R. , Kehry, M. R. , Laufer, T. M. , & Iwasaki, A. (2008). Dendritic cells and B cells maximize mucosal Th1 memory response to herpes simplex virus. The Journal of Experimental Medicine, 205, 3041–3052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iram, N. , Mildner, M. , Prior, M. , Petzelbauer, P. , Fiala, C. , Hacker, S. , Schöppl, A. , Tschachler, E. , & Elbe‐Bürger, A. (2012). Age‐related changes in expression and function of Toll‐like receptors in human skin. Development (Cambridge, England), 139, 4210–4219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwanaga, S. , Inokuchi, T. , Notohara, A. , Higashi, R. , Murakami, M. , & Kato, T. (1985). Alterations in tight junctions of human endometrial epithelial cells during normal menstrual cycle–freeze‐fracture electron microscopic study. Nihon Sanka Fujinka Gakkai Zasshi, 37, 2847–2852. [PubMed] [Google Scholar]
- Jabrane‐Ferrat, N. (2019). Features of human decidual NK cells in healthy pregnancy and during viral infection. Frontiers in Immunology, 10, 1397. 10.3389/fimmu.2019.01397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jais, M. , Younes, N. , Chapman, S. , Cu‐Uvin, S. , & Ghosh, M. (2016). Reduced levels of genital tract immune biomarkers in postmenopausal women: Implications for HIV acquisition. American Journal of Obstetrics and Gynecology, 215, 324.e321–324.e310. [DOI] [PubMed] [Google Scholar]
- Jensen, A. L. , Collins, J. , Shipman, E. P. , Wira, C. R. , Guyre, P. M. , & Pioli, P. A. (2012). A subset of human uterine endometrial macrophages is alternatively activated. American Journal of Reproductive Immunology, 68, 374–386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jilanti, R. , & Isliker, M. (1977). Immunoglobin in human cervico‐vaginal secretions. International Archives of Allergy and Immunology, 53, 402–408. [DOI] [PubMed] [Google Scholar]
- Joag, V. R. , McKinnon, L. R. , Liu, J. , Kidane, S. T. , Yudin, M. H. , Nyanga, B. , Kimwaki, S. , Besel, K. E. , Obila, J. O. , Huibner, S. , Oyugi, J. O. , Arthos, J. , Anzala, O. , Kimani, J. , Ostrowski, M. A. , & Kaul, R. (2015). Identification of preferential CD4 T‐cell targets for HIV infection in the cervix. Mucosal Immunology, 9, 1–12. [DOI] [PubMed] [Google Scholar]
- Kaldensjo, T. , Petersson, P. , Tolf, A. , Morgan, G. , Broliden, K. , & Hirbod, T. (2011). Detection of intraepithelial and stromal Langerin and CCR5 positive cells in the human endometrium: Potential targets for HIV infection. PLoS One, 6, e21344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller, M. J. , Guzman, E. , Hazrati, E. , Kasowitz, A. , Cheshenko, N. , Wallenstein, S. , Cole, A. L. , Cole, A. M. , Profy, A. T. , Wira, C. R. , Hogarty, K. , & Herold, B. C. (2007). PRO 2000 elicits a decline in genital tract immune mediators without compromising intrinsic antimicrobial activity. AIDS, 21, 467–476. [DOI] [PubMed] [Google Scholar]
- Kelly, J. K. , & Fox, H. (1979). The local immunological defense system of the human endometrium. Journal of Reproductive Immunology, 1, 39–45. [DOI] [PubMed] [Google Scholar]
- King, A. , Wellings, V. , Gardner, L. , & Loke, Y. W. (1989). Immunocytochemical characterization of the unusual large granular lymphocytes in human endometrium throughout the menstrual cycle. Human Immunology, 24, 195–205. [DOI] [PubMed] [Google Scholar]
- Kopcow, H. D. , Eriksson, M. , Mselle, T. F. , Damrauer, S. M. , Wira, C. R. , Sentman, C. L. , & Strominger, J. L. (2010). Human decidual NK cells from gravid uteri and NK cells from cycling endometrium are distinct NK cell subsets. Placenta, 31, 334–338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishnaraj, R. , & Bhooma, T. (1996). Cytokine sensitivity of human NK cells during immunosenescence. 2. IL2‐induced Interferon gamma secretion. Immunology Letters, 50, 59–63. [DOI] [PubMed] [Google Scholar]
- Krtolica, A. , Parrinello, S. , Lockett, S. , Desprez, P.‐Y. , & Campisi, J. (2001). Senescent fibroblasts promote epithelial cell growth and tumorigenesis: A link between cancer and aging. Proceedings of the National Academy of Sciences, 98, 12072–12077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kutteh, K. W. , Moldoveanu, Z. , & Mestecky, J. (1998). Mucosal immunity in the female reproductive tract: Correlation of immunoglobulins, cytokines, and reproductive hormones in human cervical mucus around the time of ovulation. AIDS Research and Human Retroviruses, 14, S51–55. [PubMed] [Google Scholar]
- Kutteh, W. H. , Hatch, K. D. , Blackwell, R. E. , & Mestecky, J. (1988). Secretory immune system of the female reproductive tract: I. Immunoglobulin and secretory component‐containing cells. Obstetrics and Gynecology, 71, 56–60. [PubMed] [Google Scholar]
- Kutteh, W. H. , Prince, S. J. , Hammond, K. R. , Kutteh, C. C. , & Mestecky, J. (1996). Variations in immunoglobulins and IgA subclasses of human uterine cervical secretions around the time of ovulation. Clinical and Experimental Immunology, 104, 538–542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kutza, J. , & Murasko, D. M. (1994). Effects of aging on natural killer cell activity and activation by interleukin‐2 and IFN‐α. Cellular Immunology, 155, 195–204. [DOI] [PubMed] [Google Scholar]
- Lai, S. K. , Hida, K. , Shukair, S. , Wang, Y.‐Y. , Figueiredo, A. , Cone, R. , Hope, T. J. , & Hanes, J. (2009). Human immunodeficiency virus type 1 is trapped by acidic but not by neutralized human cervicovaginal mucus. Journal of Virology, 83, 11196–11200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamm, M. E. , Mazaneca, M. B. , Nedrud, J. G. , & Kaetzel, C. S. (1995). New functions for mucosal IgA. Advances in Experimental Medicine and Biology, 371A, 647–650. [DOI] [PubMed] [Google Scholar]
- Lamm, M. E. , Weisz‐Carrington, P. , Roux, M. E. , McWilliams, M. , & Phillips‐Quagliata, J. M. (1978). In McGhee J. R., Mestecky J. R., & Babb J. L. (Eds.), Secretory immunity and infection: Proceedings of the international symposium on the secretory immune system and carries immunity (Vol. 35). Plenum Press. [Google Scholar]
- Le Garff‐Tavernier, M. , Béziat, V. , Decocq, J. , Siguret, V. , Gandjbakhch, F. , Pautas, E. , Debré, P. , Merle‐Beral, H. , & Vieillard, V. (2010). Human NK cells display major phenotypic and functional changes over the life span. Aging Cell, 9, 527–535. [DOI] [PubMed] [Google Scholar]
- Lee, H. M. , & Rich, S. (1993). Differential activation of CD8+ T cells by transforming growth factor‐beta 1. The Journal of Immunology, 151, 668–677. [PubMed] [Google Scholar]
- Lee, S. K. , Kim, J. Y. , Lee, M. , Gilman‐Sachs, A. , & Kwak‐Kim, J. (2012). Th17 and regulatory T cells in women with recurrent pregnancy loss. American Journal of Reproductive Immunology, 67, 311–318. [DOI] [PubMed] [Google Scholar]
- Lee, V. , Tobin, J. M. , & Foley, E. (2006). Relationship of cervical ectopy to chlamydia infection in young women. Journal of Family Planning and Reproductive Health Care, 32, 104–106. [DOI] [PubMed] [Google Scholar]
- Lesmeister, M. , Jorgenson, R. , Young, S. , & Misfeldt, M. (2005). 17beta‐estradiol suppresses TLR3‐induced cytokine and chemokine production in endometrial epithelial cells. Reproductive Biology and Endocrinology, 3, 74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Losif, C. S. , & Bekassy, Z. (1984). Prevalence of genitourinary symptoms in the late menopause. Acta Obstetricia Et Gynecologica Scandinavica, 63, 257–260. [DOI] [PubMed] [Google Scholar]
- Lu, K. H. , & Broaddus, R. R. (2020). Endometrial cancer. The New England Journal of Medicine, 383, 2053–2064. [DOI] [PubMed] [Google Scholar]
- Lutz, C. T. , Karapetyan, A. , Al‐Attar, A. , Shelton, B. J. , Holt, K. J. , Tucker, J. H. , & Presnell, S. R. (1950). Human NK cells proliferate and die in vivo more rapidly than T cells in healthy young and elderly adults. The Journal of Immunology, 2011(186), 4590–4598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lutz, C. T. , Moore, M. B. , Bradley, S. , Shelton, B. J. , & Lutgendorf, S. K. (2005). Reciprocal age related change in natural killer cell receptors for MHC class I. Mechanisms of Ageing and Development, 126, 722–731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma, T. , Luo, X. , George, A. F. , Mukherjee, G. , Sen, N. , Spitzer, T. L. , Giudice, L. C. , Greene, W. C. , & Roan, N. R. (2020). HIV efficiently infects T cells from the endometrium and remodels them to promote systemic viral spread. Elife, 9, e55487. 10.7554/eLife.55487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahmoudi, S. , Mancini, E. , Xu, L. , Moore, A. , Jahanbani, F. , Hebestreit, K. , Srinivasan, R. , Li, X. , Devarajan, K. , Prélot, L. , Ang, C. E. , Shibuya, Y. , Benayoun, B. A. , Chang, A. L. S. , Wernig, M. , Wysocka, J. , Longaker, M. T. , Snyder, M. P. , & Brunet, A. (2019). Heterogeneity in old fibroblasts is linked to variability in reprogramming and wound healing. Nature, 574, 553–558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mariani, E. , Meneghetti, A. , Neri, S. , Ravaglia, G. , Forti, P. , Cattini, L. , & Facchini, A. (2002). Chemokine production by natural killer cells from nonagenarians. European Journal of Immunology, 32, 1524–1529. [DOI] [PubMed] [Google Scholar]
- Mariani, E. , Pulsatelli, L. , Meneghetti, A. , Dolzani, P. , Mazzetti, I. , Neri, S. , Ravaglia, G. , Forti, P. , & Facchini, A. (2001). Different IL‐8 production by T and NK lymphocytes in elderly subjects. Mechanisms of Ageing and Development, 122, 1383–1395. [DOI] [PubMed] [Google Scholar]
- Mariani, E. , Pulsatelli, L. , Neri, S. , Dolzani, P. , Meneghetti, A. , Silvestri, T. , Ravaglia, G. , Forti, P. , Cattini, L. , & Facchini, A. (2002). RANTES and MIP‐1α production by T lymphocytes, monocytes and NK cells from nonagenarian subjects. Experimental Gerontology, 37, 219–226. [DOI] [PubMed] [Google Scholar]
- Maruoka, R. , Tanabe, A. , Watanabe, A. , Nakamura, K. , Ashihara, K. , Tanaka, T. , Terai, Y. , & Ohmichi, M. (2014). Ovarian estradiol production and lipid metabolism in postmenopausal women. Menopause, 21, 1129–1135. [DOI] [PubMed] [Google Scholar]
- Masopust, D. , & Soerens, A. G. (2019). Tissue‐resident T cells and other resident leukocytes. Annual Review of Immunology, 37, 521–546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKinnon, L. R. , Nyanga, B. , Chege, D. , Izulla, P. , Kimani, M. , Huibner, S. , Gelmon, L. , Block, K. E. , Cicala, C. , Anzala, A. O. , Arthos, J. (2011). Characterization of a human cervical CD4+ T cell subset coexpressing multiple markers of HIV susceptibility. The Journal of Immunology, 187, 6032–6042. [DOI] [PubMed] [Google Scholar]
- Meditz, A. L. , Moreau, K. L. , MaWhinney, S. , Gozansky, W. S. , Melander, K. , Kohrt, W. M. , Wierman, M. E. , & Connick, E. (2012). CCR5 expression is elevated on endocervical CD4+ T cells in healthy postmenopausal women. JAIDS Journal of Acquired Immune Deficiency Syndromes, 59, 221–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moncla, B. J. , Chappell, C. A. , Debo, B. M. , & Meyn, L. A. (2016). The effects of hormones and vaginal microflora on the glycome of the female genital tract: Cervical‐vaginal fluid. PLoS One, 11, e0158687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moss, G. B. , Clemetson, D. , D'Costa, L. , Plummer, F. A. , Ndinya‐Achola, J. O. , Reilly, M. , Holmes, K. K. , Piot, P. , Maitha, G. M. , Hillier, S. L. , Kiviat, N. C. , Cameron, C. W. , Wamola, I. A. , & Kreiss, J. K. (1991). Association of cervical ectopy with heterosexual transmission of human immunodeficiency virus: Results of a study of couples in Nairobi, Kenya. Journal of Infectious Diseases, 164, 588–591. [DOI] [PubMed] [Google Scholar]
- Moylan, D. C. , Goepfert, P. A. , Kempf, M. C. , Saag, M. S. , Richter, H. E. , Mestecky, J. , & Sabbaj, S. (2016). Diminished CD103 (alphaEbeta7) expression on resident T cells from the female genital tract of HIV‐positive women. Pathogens and Immunity, 1, 371–387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mselle, T. F. , Howell, A. L. , Ghosh, M. , Wira, C. R. , & Sentman, C. L. (2009). Human uterine natural killer cells but not blood natural killer cells inhibit human immunodeficiency virus type 1 infection by secretion of CXCL12. Journal of Virology, 83, 11188–11195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mselle, T. F. , Meadows, S. K. , Eriksson, M. , Smith, J. M. , Shen, L. , Wira, C. R. , & Sentman, C. L. (2007). Unique characteristics of NK cells throughout the human female reproductive tract. Clinical Immunology, 124, 69–76. [DOI] [PubMed] [Google Scholar]
- Mueller, S. N. , & Mackay, L. K. (2016). Tissue‐resident memory T cells: Local specialists in immune defence. Nature Reviews Immunology, 16, 79–89. [DOI] [PubMed] [Google Scholar]
- Mukura, L. R. , Hickey, D. K. , Rodriguez‐Garcia, M. , Fahey, J. V. , & Wira, C. R. (2017). Chlamydia trachomatis regulates innate immune barrier integrity and mediates cytokine and antimicrobial responses in human uterine ECC‐1 epithelial cells. American Journal of Reproductive Immunology, 78, e12764. [DOI] [PubMed] [Google Scholar]
- Murphy, C. R. , Rogers, P. A. , Hosie, M. J. , Leeton, J. , & Beaton, L. (1992). Tight junctions of human uterine epithelial cells change during the menstrual cycle: A morphometric study. Cells Tissues Organs, 144, 36–38. [DOI] [PubMed] [Google Scholar]
- Murphy, K. , Keller, M. J. , Anastos, K. , Sinclair, S. , Devlin, J. C. , Shi, Q. , Hoover, D. R. , Starkman, B. , McGillick, J. , Mullis, C. , Minkoff, H. , Dominguez‐Bello, M. G. , & Herold, B. C. (2019). Impact of reproductive aging on the vaginal microbiome and soluble immune mediators in women living with and at‐risk for HIV infection. PLoS One, 14, e0216049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nardelli‐Haefliger, D. , Wirthner, D. , Schiller, J. T. , Lowy, D. R. , Hildesheim, A. , Fo, P. , & De Grandi, P. (2003). Specific antibody levels at the cervix during the menstrual cycle of women vaccinated with human papillomavirus 16 virus‐like particles. JNCI: Journal of the National Cancer Institute, 95, 1128–1137. [DOI] [PubMed] [Google Scholar]
- Nazli, A. , Chan, O. , Dobson‐Belaire, W. N. , Ouellet, M. , Tremblay, M. J. , Gray‐Owen, S. D. , Arsenault, A. L. , & Kaushic, C. (2010). Exposure to HIV‐1 directly impairs mucosal epithelial barrier integrity allowing microbial translocation. PLoS Path, 6, e1000852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nedrud, J. G. , Liang, X. , Hague, N. , & Lamm, M. E. (1987). Combined oral/nasal immunization protects mice from sendai virus infection. The Journal of Immunology, 139, 3484–3492. [PubMed] [Google Scholar]
- Oja, A. E. , Piet, B. , Helbig, C. , Stark, R. , van der Zwan, D. , Blaauwgeers, H. , Remmerswaal, E. B. M. , Amsen, D. , Jonkers, R. E. , Moerland, P. D. , Nolte, M. A. , van Lier, R. A. W. , & Hombrink, P. (2017). Trigger‐happy resident memory CD4(+) T cells inhabit the human lungs. Mucosal Immunology, 11, 654–667. [DOI] [PubMed] [Google Scholar]
- Omwandho, C. O. , Konrad, L. , Halis, G. , Oehmke, F. , & Tinneberg, H. R. (2010). Role of TGF‐betas in normal human endometrium and endometriosis. Human Reproduction, 25, 101–109. [DOI] [PubMed] [Google Scholar]
- Palacios, S. , Henderson, V. W. , Siseles, N. , Tan, D. , & Villaseca, P. (2010). Age of menopause and impact of climacteric symptoms by geographical region. Climacteric, 13, 419–428. [DOI] [PubMed] [Google Scholar]
- Panda, A. , Qian, F. , Mohanty, S. , van Duin, D. , Newman, F. K. , Zhang, L. , Chen, S. , Towle, V. , Belshe, R. B. , Fikrig, E. , Allore, H. G. , Montgomery, R. R. , & Shaw, A. C. (1950). Age‐associated decrease in TLR function in primary human dendritic cells predicts influenza vaccine response. Journal of Immunology (Baltimore, Md.: 1950), 2010(184), 2518–2527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel, M. V. , Fahey, J. V. , Rossoll, R. M. , & Wira, C. R. (2013). Innate immunity in the vagina (part I): Estradiol inhibits HBD2 and elafin secretion by human vaginal epithelial cells. American Journal of Reproductive Immunology, 69, 463–474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel, M. V. , Ghosh, M. , Fahey, J. V. , Ochsenbauer, C. , Rossoll, R. M. , & Wira, C. R. (2014). Innate immunity in the vagina (Part II): Anti‐HIV activity and antiviral content of human vaginal secretions. American Journal of Reproductive Immunology, 72, 22–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel, M. V. , Ghosh, M. , Fahey, J. V. , & Wira, C. R. (2012). Uterine epithelial cells specifically induce interferon‐stimulated genes in response to polyinosinic‐polycytidylic acid independently of estradiol. PLoS One, 7, e35654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel, M. V. , Shen, Z. , Rossoll, R. M. , & Wira, C. R. (2018). IL‐27 expression and responsiveness in human uterine epithelial cells and fibroblasts in vitro and the role of estradiol. Journal of Interferon & Cytokine Research, 38, 101–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel, M. V. , Shen, Z. , Rossoll, R. M. , & Wira, C. R. (2018). Estradiol‐regulated innate antiviral responses of human endometrial stromal fibroblasts. American Journal of Reproductive Immunology, 80, e13042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel, M. V. , Shen, Z. , & Wira, C. R. (2018). Poly (I:C) and LPS induce distinct immune responses by ovarian stromal fibroblasts. Journal of Reproductive Immunology, 127, 36–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patton, D. L. , Thwin, S. S. , Meier, A. , Hooton, T. M. , Stapleton, A. E. , & Eschenbach, D. A. (2000). Epithelial cell layer thickness and immune cell populations in the normal human vagina at different stages of the menstrual cycle. American Journal of Obstetrics and Gynecology, 183, 967–973. [DOI] [PubMed] [Google Scholar]
- Pena‐Cruz, V. , Agosto, L. M. , Akiyama, H. , Olson, A. , Moreau, Y. , Larrieux, J. R. , Henderson, A. , Gummuluru, S. , & Sagar, M. (2018). HIV‐1 replicates and persists in vaginal epithelial dendritic cells. Journal of Clinical Investigation, 128, 3439–3444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pioli, P. A. , Amiel, E. , Schaefer, T. M. , Connolly, J. E. , Wira, C. R. , & Guyre, P. M. (2004). Differential expression of Toll‐like receptors 2 and 4 in tissues of the human female reproductive tract. Infection and Immunity, 72, 5799–5806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quillay, H. , El Costa, H. , Duriez, M. , Marlin, R. , Cannou, C. , Madec, Y. , de Truchis, C. , Rahmati, M. , Barre‐Sinoussi, F. , Nugeyre, M. T. , & Menu, E. (2016). NK cells control HIV‐1 infection of macrophages through soluble factors and cellular contacts in the human decidua. Retrovirology, 13, 39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quillay, H. , El Costa, H. , Marlin, R. , Duriez, M. , Cannou, C. , Chretien, F. , Fernandez, H. , Lebreton, A. , Ighil, J. , Schwartz, O. , Barré‐Sinoussi, F. , Nugeyre, M.‐T. , & Menu, E. (2015). Distinct characteristics of endometrial and decidual macrophages and regulation of their permissivity to HIV‐1 infection by SAMHD1. Journal of Virology, 89, 1329–1339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rebello, R. , Green, F. , & Fox, H. (1975). A study of the secretory immune system of the female genital tract. BJOG: an International Journal of Obstetrics and Gynaecology, 82, 812–816. [PubMed] [Google Scholar]
- Rocha‐Zavaleta, L. , Yescas, G. , Cruz, R. M. , & Cruz‐Talonia, F. (2004). Human papillomavirus infection and cervical ectopy. International Journal of Gynaecology and Obstetrics: the Official Organ of the International Federation of Gynaecology and Obstetrics, 85, 259–266. [DOI] [PubMed] [Google Scholar]
- Rodriguez‐Garcia, M. , Barr, F. D. , Crist, S. G. , Fahey, J. V. , & Wira, C. R. (2014). Phenotype and susceptibility to HIV infection of CD4+ Th17 cells in the human female reproductive tract. Mucosal Immunology, 7, 1375–1385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez‐Garcia, M. , Fortier, J. M. , Barr, F. D. , & Wira, C. R. (2018). Aging impacts CD103(+) CD8(+) T cell presence and induction by dendritic cells in the genital tract. Aging Cell, 17(3), e12733. 10.1111/acel.12733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez‐Garcia, M. , Shen, Z. , Barr, F. D. , Boesch, A. W. , Ackerman, M. E. , Kappes, J. C. , Ochsenbauer, C. , & Wira, C. R. (2017). Dendritic cells from the human female reproductive tract rapidly capture and respond to HIV. Mucosal Immunology, 10, 531–544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez‐Garcia, M. , Shen, Z. , Fortier, J. M. , & Wira, C. R. (2020). Differential cytotoxic function of resident and non‐resident CD8+ T cells in the human female reproductive tract before and after menopause. Frontiers in Immunology, 11, 1096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rollenhagen, C. , & Asin, S. N. (2011). Enhanced HIV‐1 replication in ex vivo ectocervical tissues from post‐menopausal women correlates with increased inflammatory responses. Mucosal Immunology, 4, 671–681. [DOI] [PubMed] [Google Scholar]
- Rosato, P. C. , Beura, L. K. , & Masopust, D. (2017). Tissue resident memory T cells and viral immunity. Current Opinion in Virology, 22, 44–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rositch, A. F. , Burke, A. E. , Viscidi, R. P. , Silver, M. I. , Chang, K. , & Gravitt, P. E. (2012). Contributions of recent and past sexual partnerships on incident human papillomavirus detection: Acquisition and reactivation in older women. Cancer Research, 72, 6183–6190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saba, E. , Grivel, J. C. , Vanpouille, C. , Brichacek, B. , Fitzgerald, W. , Margolis, L. , & Lisco, A. (2010). HIV‐1 sexual transmission: Early events of HIV‐1 infection of human cervico‐vaginal tissue in an optimized ex vivo model. Mucosal Immunology, 3, 280–290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Safwat, M. D. , Habib, F. A. , & Oweiss, N. Y. (2008). Distribution of macrophages in the human fallopian tubes: An immunohistochemical and electron microscopic study. Folia Morphologica, 67, 43–52. [PubMed] [Google Scholar]
- Sandquist, I. , & Kolls, J. (2018). Update on regulation and effector functions of Th17 cells. F1000Res, 7, 205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaefer, T. M. , Fahey, J. V. , Wright, J. A. , & Wira, C. R. (2005). Innate immunity in the human female reproductive tract: Antiviral response of uterine epithelial cells to the TLR3 agonist Poly(I:C). Journal of Immunology, 174, 992–1002. [DOI] [PubMed] [Google Scholar]
- Schlitzer, A. , McGovern, N. , & Ginhoux, F. (2015). Dendritic cells and monocyte‐derived cells: Two complementary and integrated functional systems. Seminars in Cell & Developmental Biology, 41, 9–22. [DOI] [PubMed] [Google Scholar]
- Schulke, L. , Manconi, F. , Markham, R. , & Fraser, I. S. (2008). Endometrial dendritic cell populations during the normal menstrual cycle. Human Reproduction, 23, 1574–1580. [DOI] [PubMed] [Google Scholar]
- Schumacher, G. F. B. (1973). Soluble proteins in cervical mucus. In Blandau R. J. & Moghissi K. (Ed.), The biology of the cervix (pp. 201–233). The University of Chicago Press. [Google Scholar]
- Schumacher, G. F. B. (1980). Humoral immune factors in the female reproductive tract and their changes during the cycle. In Dinsda D. & Schumacher G. (Ed.), Immunological aspects of infertility and fertility control (pp. 93–141). Elsevier. [Google Scholar]
- Schumacher, G. F. , Kim, M. H. , Hosseinian, A. H. , & Dupon, C. (1977). Immunoglobulins, proteinase inhibitors, albumin, and lysozyme in human cervical mucus. I. Communication: Hormonal profiles and cervical mucus changes–methods and results. American Journal of Obstetrics and Gynecology, 129, 629–636. [DOI] [PubMed] [Google Scholar]
- Shen, R. , Richter, H. E. , Clements, R. H. , Novak, L. , Huff, K. , Bimczok, D. , Sankaran‐Walters, S. , Dandekar, S. , Clapham, P. R. , Smythies, L. E. , & Smith, P. D. (2009). Macrophages in vaginal but not intestinal mucosa are monocyte‐like and permissive to human immunodeficiency virus type 1 infection. Journal of Virology, 83, 3258–3267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen, Z. , Rodriguez‐Garcia, M. , Patel, M. V. , Barr, F. D. , & Wira, C. R. (2016). Menopausal status influences the expression of programmed death (PD)‐1 and its ligand PD‐L1 on immune cells from the human female reproductive tract. American Journal of Reproductive Immunology, 76, 118–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen, Z. , Rodriguez‐Garcia, M. , Patel, M. V. , & Wira, C. R. (2021). Direct and Indirect endocrine‐mediated suppression of human endometrial CD8+T cell cytotoxicity. Scientific Reports, 11, 1773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin, H. , & Iwasaki, A. (2012). A vaccine strategy that protects against genital herpes by establishing local memory T cells. Nature, 491, 463–467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin, H. , Kumamoto, Y. , Gopinath, S. , & Iwasaki, A. (2016). CD301b+dendritic cells stimulate tissue‐resident memory CD8+T cells to protect against genital HSV‐2. Nature Communications, 7, 13346. 10.1038/ncomms13346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sho, T. , Hachisuga, T. , Koi, C. , Kurita, T. , Kagami, S. , Kawagoe, T. , Matsuura, Y. , Yoshimura, K. , & Hisaoka, M. (2017). 17β‐Estradiol induces proliferation of endometrial NK cells (CD56+) in postmenopausal women. Climacteric, 20, 571–576. [DOI] [PubMed] [Google Scholar]
- Shukair, S. A. , Allen, S. A. , Cianci, G. C. , Stieh, D. J. , Anderson, M. R. , Baig, S. M. , Gioia, C. J. , Spongberg, E. J. , Kauffman, S. M. , McRaven, M. D. , Lakougna, H. Y. , Hammond, C. , Kiser, P. F. , & Hope, T. J. (2013). Human cervicovaginal mucus contains an activity that hinders HIV‐1 movement. Mucosal Immunology, 6, 427–434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shveiky, D. , Iglesia, C. B. , Sarkar Das, S. , Ben Menachem‐Zidon, O. , Chill, H. H. , Ji, H. , & Sandberg, K. (2020). Age‐associated impairments in tissue strength and immune response in a rat vaginal injury model. International Urogynecology Journal, 31, 1435–1441. [DOI] [PubMed] [Google Scholar]
- Solana, R. , Alonso, M. C. , & Peña, J. (1999). Natural killer cells in healthy aging. Experimental Gerontology, 34, 435–443. [DOI] [PubMed] [Google Scholar]
- Solana, R. , Campos, C. , Pera, A. , & Tarazona, R. (2014). Shaping of NK cell subsets by aging. Current Opinion in Immunology, 29, 56–61. [DOI] [PubMed] [Google Scholar]
- Stockinger, B. , & Omenetti, S. (2017). The dichotomous nature of T helper 17 cells. Nature Reviews Immunology, 17, 535–544. [DOI] [PubMed] [Google Scholar]
- Taylor, T. N. , Munoz‐Plaza, C. E. , Goparaju, L. , Martinez, O. , Holman, S. , Minkoff, H. L. , Karpiak, S. E. , Gandhi, M. , Cohen, M. H. , Golub, E. T. , Levine, A. M. , Adedimeji, A. A. , Gonsalves, R. , Bryan, T. , Connors, N. , Schechter, G. , & Wilson, T. E. (2017). "The Pleasure Is Better as I've Gotten Older": Sexual health, sexuality, and sexual risk behaviors among older women living with HIV. Archives of Sexual Behavior, 46, 1137–1150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thome, J. J. , Yudanin, N. , Ohmura, Y. , Kubota, M. , Grinshpun, B. , Sathaliyawala, T. , Kato, T. , Lerner, H. , Shen, Y. , & Farber, D. L. (2014). Spatial map of human T cell compartmentalization and maintenance over decades of life. Cell, 159, 814–828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thurman, A. R. , Yousefieh, N. , Chandra, N. , Kimble, T. , Asin, S. , Rollenhagen, C. , Anderson, S. M. , Herold, B. C. , Freiermuth, J. L. , Starkman, B. S. , Mesquita, P. M. M. , Richardson‐Harman, N. , Cunningham, T. , Hillier, S. , Rabe, L. , Schwartz, J. L. , & Doncel, G. F. (2017). Comparison of mucosal markers of human immunodeficiency virus susceptibility in healthy premenopausal versus postmenopausal women. AIDS Research and Human Retroviruses, 33, 807–819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tourville, D. R. , Ogra, S. S. , Lippes, J. , & Tomasi, T. B. J. (1970). The human female reproductive tract: Immunohistological localization of gA, gG, gM, secretory piece and lactoferrin. American Journal of Obstetrics and Gynecology, 108, 1102–1108. [DOI] [PubMed] [Google Scholar]
- Trifonova, R. T. , Bollman, B. , Barteneva, N. S. , & Lieberman, J. (2018). Myeloid cells in intact human cervical explants capture HIV and can transmit it to CD4 T cells. Frontiers in Immunology, 9, 2719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trifonova, R. T. , Doncel, G. F. , & Fichorova, R. N. (2009). Polyanionic microbicides modify Toll‐like receptor‐mediated cervicovaginal immune responses. Antimicrobial Agents and Chemotherapy, 53, 1490–1500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trifonova, R. T. , Lieberman, J. , & van Baarle, D. (2014). Distribution of immune cells in the human cervix and implications for HIV transmission. American Journal of Reproductive Immunology, 71, 252–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyssen, D. , Wang, Y.‐Y. , Hayward, J. A. , Agius, P. A. , DeLong, K. , Aldunate, M. , Ravel, J. , Moench, T. R. , Cone, R. A. , & Tachedjian, G. (2018). Anti‐HIV‐1 activity of lactic acid in human cervicovaginal fluid. mSphere. 3, 10.1128/mSphere.00055-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vigil, P. , Cortés, M. E. , Zúñiga, A. , Riquelme, J. , & Ceric, F. (2009). Scanning electron and light microscopy study of the cervical mucus in women with polycystic ovary syndrome. Journal of Electron Microscopy, 58, 21–27. [DOI] [PubMed] [Google Scholar]
- Walker, M. L. , & Herndon, J. G. (2008). Menopause in nonhuman primates? Biology of Reproduction, 79, 398–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, A. S. , & Dreesen, O. (2018). Biomarkers of cellular senescence and skin aging. Frontiers in Genetics, 9, 247. 10.3389/fgene.2018.00247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, W. , Sung, N. , Gilman‐Sachs, A. , & Kwak‐Kim, J. (2020). T helper (Th) cell profiles in pregnancy and recurrent pregnancy losses: Th1/Th2/Th9/Th17/Th22/Tfh cells. Frontiers in Immunology, 11, 2025. 10.3389/fimmu.2020.02025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, Y. Y. , Kannan, A. , Nunn, K. L. , Murphy, M. A. , Subramani, D. B. , Moench, T. , Cone, R. , & Lai, S. K. (2014). IgG in cervicovaginal mucus traps HSV and prevents vaginal Herpes infections. Mucosal Immunology, 7, 1036–1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wensink, A. C. , Hack, C. E. , & Bovenschen, N. (2015). Granzymes regulate proinflammatory cytokine responses. The Journal of Immunology, 194, 491–497. [DOI] [PubMed] [Google Scholar]
- White, H. D. , Crassi, K. M. , Givan, A. L. , Stern, J. E. , Gonzalez, J. L. , Memoli, V. A. , Green, W. R. , & Wira, C. R. (1997). CD3+ CD8+ CTL activity within the human female reproductive tract: Influence of stage of the menstrual cycle and menopause. The Journal of Immunology, 158, 3017–3027. [PubMed] [Google Scholar]
- Wira, C. R. , Fahey, J. V. , Ghosh, M. , Patel, M. V. , Hickey, D. K. , & Ochiel, D. O. (2010). Sex hormone regulation of innate immunity in the female reproductive tract: The role of epithelial cells in balancing reproductive potential with protection against sexually transmitted pathogens. American Journal of Reproductive Immunology, 63, 544–565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wira, C. R. , Fahey, J. V. , Sentman, C. L. , Pioli, P. A. , & Shen, L. (2005). Innate and adaptive immunity in female genital tract: Cellular responses and interactions. Immunological Reviews, 206, 306–335. [DOI] [PubMed] [Google Scholar]
- Wira, C. R. , Ghosh, M. , Smith, J. M. , Shen, L. , Connor, R. I. , Sundstrom, P. , Frechette, G. M. , Hill, E. T. , & Fahey, J. V. (2011). Epithelial cell secretions from the human female reproductive tract inhibit sexually transmitted pathogens and Candida albicans but not Lactobacillus. Mucosal Immunology, 4, 335–342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wira, C. R. , Rodriguez‐Garcia, M. , & Patel, M. V. (2015). The role of sex hormones in immune protection of the female reproductive tract. Nature Reviews Immunology, 15, 217–230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wira, C. R. , & Rossoll, R. M. (2003). Oestradiol regulation of antigen presentation by uterine stromal cells: Role of transforming growth factor‐beta production by epithelial cells in mediating antigen‐presenting cell function. Immunology, 109, 398–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf, J. , Weinberger, B. , Arnold, C. R. , Maier, A. B. , Westendorp, R. G. J. , & Grubeck‐Loebenstein, B. (2012). The effect of chronological age on the inflammatory response of human fibroblasts. Experimental Gerontology, 47, 749–753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeaman, G. R. , Collins, J. E. , Fanger, M. W. , Wira, C. R. , & Lydyard, P. M. (2001). CD8+ T cells in human uterine endometrial lymphoid aggregates: Evidence for accumulation of cells by trafficking. Immunology, 102, 434–440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeaman, G. R. , Guyre, P. M. , Fanger, M. W. , Collins, J. E. , White, H. D. , Rathbun, W. , Orndorff, K. A. , Gonzalez, J. L. , Stern, J. E. , & Wira, C. R. (1997). Unique CD8+ T cell‐rich lymphoid aggregates in human uterine endometrium. Journal of Leukocyte Biology, 61, 427–435. [PubMed] [Google Scholar]
- Yu, C. I. , Becker, C. , Wang, Y. , Marches, F. , Helft, J. , Leboeuf, M. , Anguiano, E. , Pourpe, S. , Goller, K. , Pascual, V. , & Banchereau, J. (2013). Human CD1c+ dendritic cells drive the differentiation of CD103+ CD8+ mucosal effector T cells via the cytokine TGF‐beta. Immunity, 38, 818–830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zarember, K. A. , & Godowski, P. J. (2002). Tissue expression of human toll‐like receptors and differential regulation of toll‐like receptor mRNAs in leukocytes in response to microbes, their products, and cytokines. The Journal of Immunology, 168, 554–561. [DOI] [PubMed] [Google Scholar]
- Zeng, R. , Li, X. , & Gorodeski, G. I. (2004). Estrogen abrogates transcervical tight junctional resistance by acceleration of occludin modulation. The Journal of Clinical Endocrinology & Metabolism, 89, 5145–5155. [DOI] [PubMed] [Google Scholar]
- Zhang, Y. , Wallace, D. L. , de Lara, C. M. , Ghattas, H. , Asquith, B. , Worth, A. , Griffin, G. E. , Taylor, G. P. , Tough, D. F. , Beverley, P. C. L. , & Macallan, D. C. (2007). In vivo kinetics of human natural killer cells: The effects of ageing and acute and chronic viral infection. Immunology, 121, 258–265. [DOI] [PMC free article] [PubMed] [Google Scholar]


