Abstract
Digital health encompasses the use of information and communications technology and the use of advanced computing sciences in healthcare. This review covers the application of digital health in heart failure patients, focusing on teleconsultation, remote monitoring and apps and wearables, looking at how these technologies can be used to support care and improve outcomes. Interest in and use of these technologies, particularly teleconsultation, have been accelerated by the coronavirus disease 2019 pandemic. Remote monitoring of heart failure patients, to identify those patients at high risk of hospitalisation and to support clinical stability, has been studied with mixed results. Remote monitoring of pulmonary artery pressure has a consistent effect on reducing hospitalisation rates for patients with moderately severe symptoms and multiparameter monitoring shows promise for the future. Wearable devices and apps are increasingly used by patients for health and lifestyle support. Some wearable technologies have shown promise in AF detection, and others may be useful in supporting self-care and guiding prognosis, but more evidence is required to guide their optimal use. Support for patients and clinicians wishing to use these technologies is important, along with consideration of data validity and privacy and appropriate recording of decision-making.
Keywords: Digital health, e-health, m-health, cardiology, heart failure, telemedicine, wearables, machine learning, apps, COVID-19
Digital health is defined by the WHO as “a broad umbrella term encompassing eHealth, as well as emerging areas, such as the use of advanced computing sciences in ‘big data’, genomics and artificial intelligence”, and it defines eHealth as “the use of information and communications technology in support of health and health-related fields” (Figure 1 and Table 1).[1]
Figure 1: Domains of Digital Health and eHealth.
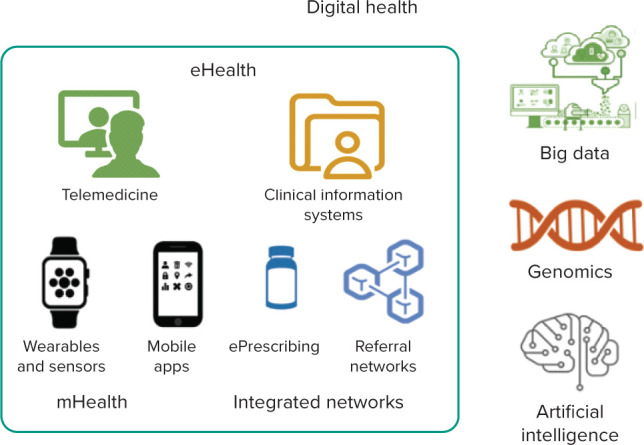
Table 1: Definitions.
| Term | Definition |
|---|---|
| Digital health | A broad umbrella term encompassing e-Health (which includes m-Health), as well as emerging areas, such as the use of advanced computing sciences in big data, genomics and artificial intelligence.[1] |
| eHealth | The use of information and communications technology in support of health and health-related fields.[1] |
| mHealth | Medical and public health practice supported by mobile devices, such as mobile phones, patient monitoring devices, personal digital assistants and other wireless devices.[64] |
| Telehealth | Delivery of health care services, where patients and providers are separated by distance.[65] Often used interchangeably with telemedicine. |
| Teleconsultation | The use of information and communications technology to consult with patients or other providers separated by distance. |
| Remote monitoring | A subset of telehealth that facilitates patient monitoring as well as the timely transfer of patient-generated data from patient to care team and back to the patient.[66] |
Cardiologists have been integrating basic forms of digital health into their practice for decades, from telephone consultations to electronic health records (EHRs). The traditional model of heart failure (HF) outpatient care, with scheduled face-to-face visits often many months apart, was largely unchanged by digital technology; remote monitoring (RM) and teleconsultation were the exception rather than the rule.
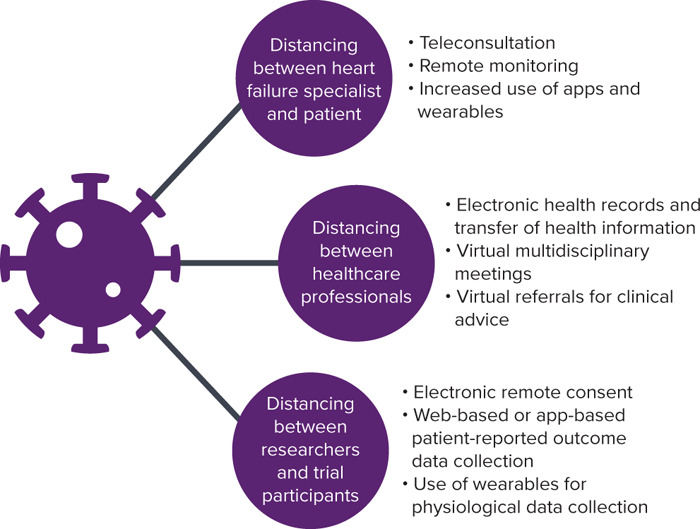
The coronavirus disease 2019 (COVID-19) pandemic necessitated a rapid and dramatic change to this approach, with government stay-at-home orders, shielding guidelines and health service reorganisation strongly discouraging face-to-face appointments. The focus of service delivery rapidly shifted to teleconsultation, with an increase in RM where this was possible (Figure 2). This short review discusses a variety of digital technologies that increasingly play a role in HF management for both healthcare professionals and patients.
Figure 2: Challenges Posed to Heart Failure Care by COVID-19 and Digital Health Solutions.
The Digital Landscape
The smartphone is now a portable, powerful personal computer and communications device sitting in most people’s pocket. Smartphone penetration ranges from 80% of adults in high-income countries, such as the US, UK, France and Germany, to 60% in China and 37% in India.[2]
A review by the cardiologist Eric Topol identified several key priorities in digital transformation in healthcare, including increased use of telemedicine, smartphone apps and sensors and wearables for diagnostic and RM purposes.[3] Artificial intelligence and machine learning techniques were also identified as likely to play an increasing role in image interpretation and pattern recognition, clinical decision support, disease and treatment monitoring and more rapid discovery of new therapeutics. However, several barriers exist to the widespread implementation of digital health programmes, as identified in the EU e-Health action plan 2012–2020 (Table 2).[4] The European Society of Cardiology (ESC) e-Cardiology Working Group proposed several solutions to tackle some of these issues, including redesign of workflows and assurance of interoperability of services.[5]
Table 2: Barriers and Solutions to the Large-scale Deployment of Digital Health-based Care in Cardiology.
| Barriers[4] | Solutions[5] | |
|---|---|---|
| Stakeholder resistance to adopt digital care |
|
|
| Legal, ethical and technical barriers |
|
|
| Lack of reimbursement |
|
|
ICT = Information and communications technology.
A particular challenge is that patient groups with the greatest healthcare need, such as the elderly or those with low incomes, may have the least access to digital technologies, creating a so-called digital divide. Lack of health literacy (the ability of patients to understand and use written healthcare information) and digital literacy (the ability to use information and communication technologies to find, evaluate, create and communicate information) must be tackled within the healthcare workforce and the general population if the digital revolution is to succeed at scale.[6]
Teleconsultation
Teleconsultation is used in this article to mean the use of information and communications technology to consult with patients at a distance, although teleconsultation can also refer to communication between healthcare professionals. Teleconsultation had been identified as a possible solution to overburdened outpatient departments before the COVID-19 pandemic.[7] Nevertheless, it has not been widely used in cardiology outpatient services until recently. A recent Organisation for Economic Co-operation and Development (OECD) report into telemedicine found that teleconsultations represented just 0.1–0.2% of the number of face-to-face consultations in selected OECD countries.[8] A lack of clear reimbursement mechanisms was cited as the most frequent barrier to adopting telemedicine services.
In March 2020, many healthcare organisations across Europe and the US rapidly moved to teleconsultation for routine outpatient appointments to minimise the risk of COVID-19 transmission to patients and staff, and reimbursement mechanisms were amended to incentivise this. For example, in the US, temporary measures allowed telehealth services to be billed as if they were in-person services for patients enrolled in Medicare or Medicaid.[9] State medical licensure within the US was also a barrier to providing teleconsultations for patients who lived in different states to the healthcare professional; typically physicians are only licensed to practise in the state in which they work, whereas telehealth consultations were deemed to take place in the patient’s location. To tackle this issue during COVID-19, most states issued waivers to allow telehealth provided by out-of-state physicians.[10]
Video consultations have been well received in primary care but have so far had limited evaluation in HF care.[11,12] A small study in selected HF patients found video consultations to be broadly acceptable but challenges included establishing a good connection, communication difficulties due to latency and connection degradation and inability to conduct physical examinations.[13] Another small study of video consultations in HF patients explored examinations in more detail. Although in all appointments a basic form of examination was possible, communicating instructions to patients and carers was particularly challenging for clinicians.[14]
Remote Monitoring for Decompensation of Heart Failure
HF is a long-term condition with episodic deterioration (decompensation).[15] Decompensation is a critical event; European registry data show that hospitalisation carries a 24% risk of death within 1 year.[16] Improvement in disease monitoring – identifying when patients may be deteriorating and when intervention may restabilise the syndrome – has obvious attractions. Traditional periodic clinical assessment suffers from the limitation that early signs of decompensation are unlikely to coincide with a clinic appointment and patients tend to seek professional advice only when their symptoms are advanced.[17] Self-monitoring, where patients track their symptoms, weight and other physiological variables (such as blood pressure), has been a mainstay of HF management for several years.[15] However, it relies on patients being motivated, having access to technology (such as scales and automatic sphygmomanometers), and having access to clinical advice, often through a HF nurse telephone service. Unfortunately, many HF patients lack the requisite knowledge for effective self-care, so effective interaction with clinicians – at the right time – is crucial for self-monitoring to translate into improved outcomes.[18,19]
RM is the use of telecommunication technologies to monitor patient status at a distance. Structured telephone support (STS) offers a basic level of RM. Patients are called by a member of the HF team to discuss symptoms, drug therapy, compliance with lifestyle measures and they may be asked to provide measurements such as weight and blood pressure or pulse rate. The evidence for STS is mixed, but a 2015 meta-analysis reported a marginal mortality benefit (risk ratio [RR] 0.87 for all-cause mortality; 95% CI [0.77–0.98]) and reduction in HF hospitalisations (RR 0.85; 95% CI [0.77–0.93]), but no effect on all-cause hospitalisations.[20] STS is relatively labour-intensive and costs vary according to the intensity of monitoring.
Non-invasive standalone systems involve the regular transmission of physiological data to HF teams. Teams may either review this data regularly or be alerted when a variable is outside a specified range.[21] The exact choice of measurements transmitted varies between different systems. The evidence base is mixed, with randomised trials failing to show consistent benefit.
One randomised trial of telemonitoring in 1,571 HF patients with New York Heart Association (NYHA) Class II–III symptoms and a HF hospitalisation in the preceding 12 months compared a wireless system, transmitting daily readings of weight, blood pressure, oxygen saturations, heart rate and a health status questionnaire, with usual care.[22] The composite outcome of all-cause mortality and percentage days hospitalised was reduced (RR 0.8; 95% CI [0.65–1.00]), although crucially this study excluded patients with depression or NYHA Class I or IV. A meta-analysis of smaller randomised telemonitoring trials showed a small mortality benefit (RR 0.80 for all-cause mortality; 95% CI [0.68–0.94]).[20] However the heterogeneity of interventions, health service structures, patients studied and definitions of ‘usual care’ make it difficult to make specific recommendations based on these data.
The ESC and American College of Cardiology HF guidelines, written before the COVID-19 pandemic, do not recommend routine use of RM (Table 3), but during the COVID-19 pandemic RM of chronic conditions was recommended by many organisations, including the Centers for Disease Control and Prevention, in order to maintain continuity of care in the absence of face-to-face contact.[15,23,24]
Table 3: Existing Guidelines on Remote Monitoring for Heart Failure Events (Pre-COVID-19).
|
ESC: 2016 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure[15] Recommendations for exercise, multidisciplinary management and monitoring of patients with heart failure:
|
|
HFA of the ESC: Clinical practice update on heart failure 2019[66] Telemedicine:
|
|
ACCF/AHA: 2013 ACCF/AHA Guideline for the Management of Heart Failure[23] Systems of care to promote care coordination for patients with chronic HF:
|
|
AHA: Using Remote Patient Monitoring Technologies for Better Cardiovascular Disease Outcomes (2019)[67] HF:
|
ACCF = American College of Cardiology Foundation; AHA = American Heart Association; COVID-19 = coronavirus disease 2019; CV = cardiovascular; ESC = European Society of Cardiology; HF = heart failure; HFA = Heart Failure Association; HFrEF = heart failure with reduced ejection fraction; IN-TIME = INfluence of Home Monitoring on The clinical Management of heart failurE patients with impaired left ventricular function; LVEF = left ventricular ejection fraction.
Invasive Device Remote Monitoring
Patients with symptomatic HF with severely reduced ejection fraction may have an implantable cardiac device such as an ICD or CRT as part of their HF management.[15,23] Such devices require regular checks to monitor device performance, longevity and detection of arrhythmia, but most modern devices can wirelessly connect with home monitors that transmit relevant data and alerts, allowing a device check to be performed remotely.[25] Home monitoring is safe and effective for routine device checks, with earlier detection of arrhythmia and technical issues.[26] Centres using home monitoring of implanted devices have reported reduced face-to-face contact.[27]
Implantable devices can also collect physiological data that may correlate with HF status. Intrathoracic impedance correlates well with pulmonary fluid content, but device-based impedance alerts resulted in a 79% increase in HF hospitalisation in one randomised trial, due to the low specificity of alerts when measuring impedance alone, and perhaps also the anxiety triggered by an audible alert from the device.[28,29] Multiparametric monitoring, incorporating intrathoracic impedance with other variables such as heart rate, heart rate variability, physical activity and heart sounds, has shown more potential. Routine remote multi-parametric monitoring of 1,650 patients with an implantable device at nine English hospitals over an average of 2.8 years failed to show an improvement over usual care in a randomised trial, but this study depended on human interpretation of the data trends.[30] Using similar parameters, the HeartLogic (Boston Scientific) algorithm was able to identify HF decompensation with a sensitivity of 70% and an unexplained alert rate of only 1.47 per patient-year, with a median lead time of 34 days before the HF event.[31] There is currently no specific evidence-based intervention to a HeartLogic alert, and it is therefore currently unclear whether the algorithm improves hospitalisation or mortality when used in routine practice. The MANAGE-HF randomised trial is due to report these outcomes in 2025 (NCT03237858).
Implantable haemodynamic monitors have shown promise at preventing HF hospitalisation. Pulmonary artery pressure (PAP) increases in response to increasing intracardiac pressure or fluid volume, with rises in pressure typically preceding symptoms by some weeks.[32] A randomised trial showed that remote daily PAP monitoring, (via a CardioMEMS device; Abbott) and titration of medications in response to rises in pressure, reduced subsequent HF hospitalisation by 30% in NYHA Class III patients who had been admitted for HF in the previous year.[33] Data from NYHA Class III patients outside the US confirm this benefit.[34,35] The GUIDE HF study (NCT03387813) is examining the impact in a broader spectrum of patients, including those with milder symptoms.
Apps and Wearables
While RM systems are generally ‘prescribed’ by clinicians and often reimbursed by healthcare systems or insurance companies, apps and wearables are largely marketed directly to consumers as tools for health and lifestyle maintenance. The last decade has seen a rapid proliferation of health apps. In 2017 it was estimated that 325,000 health apps were available on smartphones.[36] Despite this, very few of them have been designed specifically for HF patients; a 2019 review identified 10 apps focused on HF self-care available on the Apple App Store and Google Play store.[37] Four of these were developed by scientific societies (including the American Heart Association and the Swiss Federation of Cardiology) and they were predominantly aimed at patient education, symptom tracking and prompting users to seek early care for symptoms in order to address low health literacy and poor understanding of self-care in HF patients.[38,39]
However, few HF apps have been evaluated in randomised controlled trials, and those that have been are not yet commercially available. Without good quality evidence and clear app standards it is challenging for clinicians to know what to recommend, though there is a growing understanding of the importance of assessment and regulation in this field. Governments and organisations have adopted their own regulatory approaches to apps. For example, the Catalan government has created a public library of accredited health apps with the ICT Social Health Foundation, while other independent organisations such as the Organisation for the Review of Care and Health Apps work with health service providers internationally to assess healthcare apps. The National Institute for Health and Care Excellence in England has developed an evidence standards framework for digital health technologies, which provides a path to reimbursement for technologies that demonstrate effectiveness and value.[40]
Consumer wearables are devices that record and transmit physiological signals that can be worn, such as activity trackers and smartwatches, and these are becoming increasingly popular. Some products now offer irregular pulse detection, lead-1 ECG, blood pressure and oxygen saturation monitoring capabilities. These devices are, therefore, potentially useful tools in HF self-care and even RM, but patients bringing physiological data to clinicians poses several new dilemmas:
Are the data valid?
How should clinicians use the data in managing HF patients?
How are data regulated and incorporated into electronic health records?
Validity
Activity monitors using accelerometry, often in the form of a wristband/watch, are the most common form of wearable device. Most modern devices show high accuracy in measuring step count in controlled conditions, but devices were found to be less accurate at low ambulation speeds, which is of relevance to HF patients.[41,42] Wearable heart rate monitors use photoplethysmography (PPG), which is the illumination of a capillary bed and measurement of pulsatile changes in light absorption.[43]
Performance of PPG-based devices degrades with exertion, and a study of HF patients using Fitbit and Apple Watch showed poor accuracy in measuring dynamic heart rate changes.[44,45] PPG is, therefore, best used for measuring resting heart rate. Analysis of PPG waveforms, however, can detect irregular pulses and therefore potentially be used for AF screening[46] – the presence of AF has therapeutic implications in HF patients, such as decisions regarding rate or rhythm control, and potential need for lifelong anticoagulation. PPG alone cannot differentiate between AF and other causes of irregular pulse, but can be combined with ECG patch recording for confirmation in patients with an irregular pulse.[47]
Such an approach was used in the Apple Heart Study, a large-scale AF screening study using a PPG-based smartwatch algorithm.[48] In patients who had an irregular pulse notification and agreed to apply and send back an ECG patch recording, 34% had confirmed AF during the subsequent 2-week recording period and 77% of irregular pulse notifications with simultaneous recording were confirmed to be AF, with atrial ectopy making up the majority of the remainder.[48] Newer versions of the Apple Watch can also generate a lead 1 ECG. The HEARTLINE study, recruiting 150,000 patients aged over 65, is investigating whether the irregular pulse detection algorithm and ECG feature leads to a reduction in stroke and death in a real-world setting (NCT04276441).
Although theoretically PPG could be used to detect paroxysmal tachycardias, it is less well studied, and no PPG-based technologies are licensed outside of AF detection.[49] In addition to PPG and ECG features, miniature wrist oscillometric sphygmomanometers can now be incorporated into a smartwatch for blood pressure monitoring; the first such device to be licensed showed high accuracy when compared with manual blood pressure measurement at rest.[50]
Using Data From Wearables in Heart Failure Patients
Physical activity is an important prognostic parameter in HF. Six-minute walk test performance is a strong predictor of subsequent cardiac death in HF patients but is rarely used outside of research as it is cumbersome to measure.[51] Activity monitoring could provide a simple, objective measure of functional limitation. A retrospective study of 189 American patients with self-reported HF showed a significant negative association between physical activity and mortality, and a prospective Japanese study of 170 HF patients showed a step count of <4,889 steps/day was a stronger predictor of mortality than VO2 max (peak oxygen consumption, an important marker of cardiopulmonary fitness) on exercise testing.[52,53] However, prospective evidence of using activity monitors to guide therapy, for example instead of NYHA Class, is lacking. Activity monitors could be used to monitor and encourage adherence to exercise therapy in HF, but evidence from large randomised controlled trials in HF patients is lacking. Patient acceptability of wearables, including a watch or other wrist-worn device, is also likely to be variable.[54,55]
Regulation and Integration
Two key questions arise from wearable devices and apps: are they medical devices and who is responsible for the data generated? The EU Medical Device Regulation in Europe and the Food and Drug Administration in the US regulate medical devices in those geographies. Technologies such as apps and wearables fall under their remit if they are intended for medical purposes (i.e. for the diagnosis, prevention, monitoring or treatment of disease), make claims of health benefit, or pose potential risk of harm to patients.[56] Therefore, health technology companies are often careful to avoid medical claims and often market devices as ‘health and wellness’ products rather than tools for disease management.
As the information collected on users becomes more identifiable and medically relevant it can become subject to data protection regulations. In the EU, the General Data Protection Regulation regulates data that can be used to directly or indirectly identify a person, and applies to all companies processing the personal data of subjects residing in the EU. Privacy policies of many medical apps and wearables fail to meet these standards set for data storage, access, control and processing; this is an essential requirement before clinicians can recommend these products.[57] Incorporating wearable data into electronic health records currently requires manual input in most cases. Improvements in automatic upload of validated data may remove a barrier to clinicians using these data in clinical decision-making.
Machine Learning
Machine learning involves computers training themselves on large sample datasets to build predictive mathematical models. Most implanted devices can only provide short daily samples of data, but non-invasive monitors linked with smartphones can transmit continuously and allow for larger, richer datasets for analysis. The LINK-HF study investigated the use of a multisensor patch continuously measuring ECG signals, thoracic impedance, body temperature and accelerometry in 100 HF patients. Data were uploaded to the cloud via a smartphone, and machine learning was used to create a personalised baseline for patients. A prognostic machine learning algorithm was able to predict impending decompensation with a sensitivity of 88% and a specificity of 86% a median of 6.5 days before the HF event.[58] Further research is required to determine whether such algorithms can be used to trigger an appropriate intervention that can help restabilise the HF syndrome, and thus prevent the need for HF hospitalisation.
Other investigational products are taking a similar approach. The µCor patch (Zoll), equipped with an ECG monitor and radiofrequency transmitter measuring pulmonary fluid content, is under investigation for its ability to predict HF events (NCT03476187), and a smart-textile vest with multiple electrodes measuring similar parameters to HeartLogic (heart rate, heart rate variability, respiratory rate and thoracic impedance) is also being studied (NCT03719079).
Machine learning algorithms may pick up subtle ECG changes not detectable by human observers. They have shown promise in predicting future episodes of AF from sinus rhythm ECGs and even at identifying left ventricular systolic dysfunction from ECGs.[59] A recent study from the Mayo Clinic in the US retrospectively analysed the ECGs of 1,606 patients without known left ventricular systolic dysfunction (LVSD) who had a subsequent echocardiogram within 30 days. A machine learning algorithm was able to predict LVSD (defined as an ejection fraction <35%) with a sensitivity and specificity of 74% and 87% respectively.[60] The area under the receiver operating characteristic curve was 0.89, outperforming N-terminal pro-brain natriuretic peptide (0.80) at predicting LVSD. Such algorithms are not yet in clinical practice, and would need to be certified as a medical device before they would be able to be used, but they may form part of decision and diagnostic aids in the near future.
Machine learning analysis of cardiac imaging is a rapidly advancing field, with encouraging early results in image acquisition, interpretation in echocardiography and MRI.[61] As systems are able incorporate these data with electronic health records, the resulting rich datasets offer the potential of precision medicine and diagnostics, and improved access to key diagnostic testing.
Sustainability of New Technology
The COVID-19 pandemic has resulted in a rapid disruption and digital transformation of HF services, with greatly increased use of telehealth. The extent to which these changes will be sustained after social distancing measures are relaxed will depend on evidence of patient outcomes, patient and clinician acceptability, and use of healthcare resources during the pandemic, which is currently lacking. However, it is unlikely that healthcare practice will return to a state of business as usual. Teleconsultation is associated with a significant start-up cost for equipment, training and software licences, but many organisations have made substantial investments during the pandemic, thus overcoming a major barrier for on-going use. RM technologies need more detailed health technology assessments to judge their clinical and cost-effectiveness in different healthcare settings; use of the CardioMEMS device outside the US will depend on how it is priced and whether this remains cost-effective in a post-COVID world.
Conclusion
As citizens become digitally empowered, patients will increasingly be able to use technology to manage their own health and disease.
Self-management of type 1 diabetes using blood glucose sensors has meant that physician contact is the exception rather than the rule for well-controlled patients with that condition, and self-management of blood pressure in hypertension is effective and increasingly common.[62] Such a model would certainly be attractive in HF, but further research is needed to determine which physiological data and interventions can be used to reduce the risk of major HF decompensation events such as hospitalisation, or even to reduce mortality. PAP monitoring systems are effective at reducing HF hospitalisations, but demonstrating cost-effectiveness will be essential for widespread adoption outside of the US setting. Reimbursement mechanisms are a key driver and enabler for change, but often trail behind innovation. The COVID-19 pandemic has forced healthcare systems to re-evaluate reimbursement for digital technologies and has broken down many of the barriers to more widespread adoption of digital approaches to HF diagnosis and care.
References
- 1.WHO. WHO guideline: Recommendations on digital interventions for health system strengthening. Geneva: WHO, 2019. https://www.who.int/reproductivehealth/publications/digital-interventions-health-system-strengthening/en/ (accessed 19 January 2021) [PubMed]
- 2.Newzoo. Newzoo Global Mobile Market Report 2019. Newzoo, 2019. https://newzoo.com/insights/trend-reports/newzoo-global-mobile-market-report-2019-light-version (accessed 19 January 2021)
- 3.NHS Health Education England. The Topol review: Preparing the healthcare workforce to deliver the digital future. Health Education England; 2019. https://topol.hee.nhs.uk (accessed 19 January 2021)
- 4.European Commission. eHealth Action Plan 2012-2020: Innovative healthcare for the 21st century. Brussels: European Commission, 2012. https://ec.europa.eu/digital-single-market/en/news/ehealth-action-plan-2012-2020-innovative-healthcare-21st-century (accessed 19 January 2021)
- 5.Frederix I, Caiani EG, Dendale P et al. ESC e-Cardiology Working Group position paper: overcoming challenges in digital health implementation in cardiovascular medicine. Eur J Prev Cardiol. 2019;26:1166–77. doi: 10.1177/2047487319832394. [DOI] [PubMed] [Google Scholar]
- 6.American Library Association Digital literacy. ALA, 2021. https://literacy.ala.org/digital-literacy (accessed 19 January 2021)
- 7.Royal College of Physicians. Outpatients: The future – adding value through sustainability. London: RCP, 2018. https://www.rcplondon.ac.uk/projects/outputs/outpatients-future-adding-value-through-sustainability (accessed 19 January 2021)
- 8.Cravo Oliveira Hashiguchi T. Bringing health care to the patient: An overview of the use of telemedicine in OECD countries. Paris: OECD, 2020. [DOI]
- 9.U.S. Department of Health & Human Services. Telehealth: delivering care safely during COVID-19. Washington, DC: HHS, 2020. https://www.hhs.gov/coronavirus/telehealth/index.html (accessed 19 January 2021)
- 10.Federation of State Medical Boards. U.S. states and territories modifying requirements for telehealth in response to COVID-19. FSMB, 2021. https://www.fsmb.org/siteassets/advocacy/pdf/states-waiving-licensure-requirements-for-telehealth-in-response-to-covid-19.pdf (accessed 19 January 2021)
- 11.Thiyagarajan A, Grant C, Griffiths F et al. Exploring patients’ and clinicans’ experiences of video consultations in primary care: a systematic scoping review. BJGP Open. 2020;4:bjgpopen20X101020. doi: 10.3399/bjgpopen20X101020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Donaghy E, Atherton H, Hammersley V et al. Acceptability, benefits, and challenges of video consulting: a qualitative study in primary care. Br J Gen Pract. 2019;69:e58–94. doi: 10.3399/bjgp19X704141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shaw SE, Seuren LM, Wherton J et al. Video consultations between patients and clinicians in diabetes, cancer, and heart failure services: linguistic ethnographic study of video-mediated interaction. J Med Internet Res. 2020;22:e18378. doi: 10.2196/18378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Seuren LM, Wherton J, Greenhalgh T et al. Physical examinations via video for patients with heart failure: qualitative study using conversation analysis. J Med Internet Res. 2020;22:e16694. doi: 10.2196/16694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ponikowski P, Voors AA, Anker SD et al. 2016 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur Heart J. 2016;37:2129–200. doi: 10.1093/eurheartj/ehw128. [DOI] [PubMed] [Google Scholar]
- 16.Crespo-Leiro MG, Anker SD, Maggioni AP et al. European Society of Cardiology Heart Failure Long-Term Registry (ESC-HF-LT): 1-year follow-up outcomes and differences across regions. Eur J Heart Fail. 2016;18:613–25. doi: 10.1002/ejhf.566. [DOI] [PubMed] [Google Scholar]
- 17.Schiff GD, Fung S, Speroff T. Decompensated heart failure: symptoms, patterns of onset and contributing factors. Am J Med. 2003;114:625–30. doi: 10.1016/s0002-9343(03)00132-3. [DOI] [PubMed] [Google Scholar]
- 18.Rogers A, Addington-Hall J, Abery A et al. Knowledge and communication difficulties for patients with chronic heart failure: qualitative study. BMJ. 2000;321:605–7. doi: 10.1136/bmj.321.7261.605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Clark AM, Freydberg CN, McAlister FA et al. Patient and informal caregivers’ knowledge of heart failure: necessary but insufficient for effective self-care. Eur J Heart Fail. 2009;11:617–21. doi: 10.1093/eurjhf/hfp058. [DOI] [PubMed] [Google Scholar]
- 20.Inglis SC, Clark RA, Dierckx Ret al. Structured telephone support or non-invasive telemonitoring for patients with heart failure. Cochrane Database Syst Rev. 2015;10:CD007228. doi: 10.1002/14651858.CD007228.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Riley JP, Cowie MR. Telemonitoring in heart failure. Heart. 2009;95:1964–8. doi: 10.1136/hrt.2007.139378. [DOI] [PubMed] [Google Scholar]
- 22.Koehler F, Koehler K, Deckward O et al. Efficacy of telemedical interventional management in patients with heart failure (TIM-HF2): a randomised, controlled, parallel-group, unmasked trial. Lancet. 2018;392:1047–57. doi: 10.1016/S0140-6736(18)31880-4. [DOI] [PubMed] [Google Scholar]
- 23.Yancy CW, Jessup M, Bozkurt B et al. 2013 ACCF/AHA guideline for the management of heart failure. Circulation. 2013;128:e240–327. doi: 10.1161/CIR.0b013e31829e8776. [DOI] [PubMed] [Google Scholar]
- 24.Centers for Disease Control and Prevention. Using telehealth to expand access to essential health services during the COVID-19 pandemic. Atlanta, GA: CDC, 2020. https://www.cdc.gov/coronavirus/2019-ncov/hcp/telehealth.html (accessed 19 January 2021)
- 25.Wilkoff BL, Auricchio A, Brugada J et al. HRS/EHRA expert consensus on the monitoring of cardiovascular implantable electronic devices (CIEDs): description of techniques, indications, personnel, frequency and ethical considerations. Heart Rhythm. 2008;5:907–25. doi: 10.1016/j.hrthm.2008.04.013. [DOI] [PubMed] [Google Scholar]
- 26.Varma N, Epstein AE, Irimpen A et al. Efficacy and safety of automatic remote monitoring for implantable cardioverter-defibrillator follow-up: the Lumos-T safely reduces routine office device follow-up (TRUST) trial. Circulation. 2010;122:325–32. doi: 10.1161/CIRCULATIONAHA.110.937409. [DOI] [PubMed] [Google Scholar]
- 27.Hernández-Madrid A, Lewalter T, Proclemer A et al. Remote monitoring of cardiac implantable electronic devices in Europe: results of the European Heart Rhythm Association survey. Europace. 2014;16:129–32. doi: 10.1093/europace/eut414. [DOI] [PubMed] [Google Scholar]
- 28.Abraham WT. Intrathoracic impedance monitoring for early detection of impending heart failure decompensation. Congest Heart Fail. 2007;13:113–5. doi: 10.1111/j.1527-5299.2007.06255.x. [DOI] [PubMed] [Google Scholar]
- 29.Van Veldhuisen DJ, Braunschweig F, Conraads V et al. Intrathoracic impedance monitoring, audible patient alerts, and outcome in patients with heart failure. Circulation. 2011;124:1719–26. doi: 10.1161/CIRCULATIONAHA.111.043042. [DOI] [PubMed] [Google Scholar]
- 30.Morgan JM, Kitt S, Gill J et al. Remote management of heart failure using implantable electronic devices. Eur Heart J. 2017;38:2352–60. doi: 10.1093/eurheartj/ehx227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Boehmer JP, Hariharan R, Devecchi FG A multisensor algorithm predicts heart failure events in patients with implanted devices: results from the MultiSENSE Study. JACC Heart Fail. 2017. pp. 216–25. [DOI] [PubMed]
- 32.Adamson PB, Gold MR, Bennett T et al. Continuous hemodynamic monitoring in patients with mild to moderate heart failure: results of the Reducing Decompensation Events Utilizing Intracardiac Pressures in Patients With Chronic Heart Failure (REDUCEhf) trial. Congest Heart Fail. 2011;17:248–54. doi: 10.1111/j.1751-7133.201100247.x. [DOI] [PubMed] [Google Scholar]
- 33.Abraham WT, Stevenson LW, Bourge RC et al. Sustained efficacy of pulmonary artery pressure to guide adjustment of chronic heart failure therapy: complete follow-up results from the CHAMPION randomised trial. Lancet. 2016;387:453–61. doi: 10.1016/S0140-6736(15)00723-0. [DOI] [PubMed] [Google Scholar]
- 34.Angermann CE, Assmus B, Anker SD et al. Pulmonary artery pressure-guided therapy in ambulatory patients with symptomatic heart failure: the CardioMEMS European Monitoring Study for Heart Failure (MEMS-HF). Eur J Heart Fail. 2020;22:1891–901. doi: 10.1002/ejhf.1943. [DOI] [PubMed] [Google Scholar]
- 35.Cowie MR, de Groote P, McKenzie S et al. Rationale and design of the CardioMEMS Post-Market Multinational Clinical Study: COAST. ESC Heart Fail. 2020;7:865–72. doi: 10.1002/ehf2.12646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.US Food & Drug Administration. Device software functions including mobile medical applications. Washington, DC: FDA, 2019. https://www.fda.gov/medical-devices/digital-health-center-excellence/device-software-functions-including-mobile-medical-applications (accessed 19 January 2021)
- 37.Mortara A, Vaira L, Palmieri V et al. Would you prescribe mobile health apps for heart failure self-care? An integrated review of commercially available mobile technology for heart failure patients. Card Fail Rev. 2020;6:e13. doi: 10.15420/cfr.2019.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cajita MI, Cajita TR, Han HR. Health literacy and heart failure: a systematic review. J Cardiovasc Nurs. 2016;31:121–30. doi: 10.1097/JCN.0000000000000229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Clark AM, Freydberg CN, McAlister FA et al. Patient and informal caregivers’ knowledge of heart failure: necessary but insufficient for effective self-care. Eur J Heart Fail. 2009;11:617–21. doi: 10.1093/eurjhf/hfp058. [DOI] [PubMed] [Google Scholar]
- 40.Evidence standards framework for digital health technologies. National Institute for Health and Care Excellence (2021). https://www.nice.org.uk/about/what-we-do/our-programmes/evidence-standards-framework-for-digital-health-technologies (accessed 23 April 2021)
- 41.Kooiman TJM, Dontje ML, Sprenger SR et al. Reliability and validity of ten consumer activity trackers. BMC Sports Sci Med Rehabil. 2015;7:24. doi: 10.1186/s13102-015-0018-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Allen J. Photoplethysmography and its application in clinical physiological measurement. Physiol Meas. 2007;28:R1–39. doi: 10.1088/0967-3334/28/3/R01. [DOI] [PubMed] [Google Scholar]
- 43.Feehan LM, Geldman J, Sayre EC et al. Accuracy of Fitbit devices: systematic review and narrative syntheses of quantitative data. JMIR Mhealth Uhealth. 2018;6:e10527. doi: 10.2196/10527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cadmus-Bertram L, Gangnon R, Wirkus EJ et al. The accuracy of heart rate monitoring by some wrist-worn activity trackers. Ann Intern Med. 2017;166:610–2. doi: 10.7326/L16-0353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Moayedi Y, Abdulmajeed R, Duero Posada J et al. Assessing the use of wrist-worn devices in patients with heart failure: feasibility study. JMIR Cardio. 2017;1:e8. doi: 10.2196/cardio.8301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Paradkar N, Chowdhury SR. Cardiac arrhythmia detection using photoplethysmography. Annu Int Conf IEEE Eng Med Biol Soc. 2017;2017:113–6. doi: 10.1109/EMBC.2017.8036775. [DOI] [PubMed] [Google Scholar]
- 47.Chan PH, Wong CK, Poh YC et al. Diagnostic performance of a smartphone-based photoplethysmographic application for atrial fibrillation screening in a primary care setting. J Am Heart Assoc. 2016;5:e003428. doi: 10.1161/JAHA.116.003428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Perez M V., Mahaffey KW, Hedlin H et al. Large-scale assessment of a smartwatch to identify atrial fibrillation. N Engl J Med. 2019;381:1909–17. doi: 10.1056/NEJMoa1901183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ip JE. Wearable devices for cardiac rhythm diagnosis and management. JAMA. 2019;321:337–8. doi: 10.1001/jama.2018.20437. [DOI] [PubMed] [Google Scholar]
- 50.Kuwabara M, Harada K, Hishiki Y, Kario K. Validation of two watch-type wearable blood pressure monitors according to the ANSI/AAMI/ISO81060-2:2013 guidelines: Omron HEM-6410T-ZM and HEM-6410T-ZL. J Clin Hypertens (Greenwich) 2019;2:853–8. doi: 10.1111/jch.13499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rostagno C, Olivo G, Comeglio M et al. Prognostic values of 6-minute walk corridor test in patients with mild to moderate heart failure: comparison with other methods of functional evaluation. Eur J Heart Fail. 2003;5:247–52. doi: 10.1016/s1388-9842(02)00244-1. [DOI] [PubMed] [Google Scholar]
- 52.Loprinzi PD. The effects of free-living physical activity on mortality after congestive heart failure diagnosis. Int J Cardiol. 2016;203:598–9. doi: 10.1016/j.ijcard.2015.11.017. [DOI] [PubMed] [Google Scholar]
- 53.Izawa KP, Watanabe S, Oka K et al. Usefulness of step counts to predict mortality in Japanese patients with heart failure. Am J Cardiol. 2013;111:1767–71. doi: 10.1016/j.amjcard.2013.02.034. [DOI] [PubMed] [Google Scholar]
- 54.Alharbi M, Straiton N, Gallagher R. Harnessing the potential of wearable activity trackers for heart failure self-care. Curr Heart Fail Rep. 2017;14:23–9. doi: 10.1007/s11897-017-0318-z. [DOI] [PubMed] [Google Scholar]
- 55.Thorup C, Hansen J, Grønkjær M et al. Cardiac patients’ walking activity determined by a step counter in cardiac telerehabilitation: data from the intervention arm of a randomized controlled trial. J Med Internet Res. 2016;18:e69. doi: 10.2196/jmir.5191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Fraser AG, Byrne RA, Kautzner J et al. Implementing the new European Regulations on medical devices – clinical responsibilities for evidence-based practice: a report from the Regulatory Affairs Committee of the European Society of Cardiology. Eur Heart J. 2020;41:2589–96. doi: 10.1093/eurheartj/ehaa382. [DOI] [PubMed] [Google Scholar]
- 57.Jensen MT, Treskes RW, Caiani EG ESC working group on e-cardiology position paper: use of commercially available wearable technology for heart rate and activity tracking in primary and secondary cardiovascular prevention. Eur Heart J Digital Health. 2021. epub ahead of press. [DOI] [PMC free article] [PubMed]
- 58.Stehlik J, Schmalfuss C, Bozkurt B et al. Continuous wearable monitoring analytics predict heart failure hospitalization: the LINK-HF Multicenter Study. Circ Heart Fail. 2020;13:e006513. doi: 10.1161/CIRCHEARTFAILURE.119.006513. [DOI] [PubMed] [Google Scholar]
- 59.Attia ZI, Noseworthy PA, Lopez-Jimenez F et al. An artificial intelligence-enabled ECG algorithm for the identification of patients with atrial fibrillation during sinus rhythm: a retrospective analysis of outcome prediction. Lancet. 2019;394:861–7. doi: 10.1016/S0140-6736(19)31721-0. [DOI] [PubMed] [Google Scholar]
- 60.Adedinsewo D, Carter RE, Attia Z et al. Artificial intelligence-enabled ECG algorithm to identify patients with left ventricular systolic dysfunction presenting to the emergency department with dyspnea. Circ Arrhythm Electrophysiol. 2020;13:e008437. doi: 10.1161/CIRCEP.120.008437. [DOI] [PubMed] [Google Scholar]
- 61.Dey D, Slomka PJ, Leeson P et al. Artificial intelligence in cardiovascular imaging: JACC state-of-the-art review. J Am Coll Cardiol. 2019;73:1317–35. doi: 10.1016/j.jacc.2018.12.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Tucker KL, Sheppard JP, Stevens R et al. Self-monitoring of blood pressure in hypertension: a systematic review and individual patient data meta-analysis. PLoS Med. 2017;14:e1002389. doi: 10.1371/journal.pmed.1002389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Cowie MR, Bax J, Bruining N et al. e-Health: a position statement of the European Society of Cardiology. Eur Heart J. 2016;37:63–6. doi: 10.1093/eurheartj/ehv416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.WHO. mHealth: new horizons for health through mobile technologies. Geneva: WHO, 2011. https://www.who.int/goe/publications/goe_mhealth_web.pdf (accessed 19 January 2021)
- 65.WHO. Telehealth. 2016. https://www.who.int/gho/goe/telehealth/en/ (accessed 19 January 2021)
- 66.Seferovic PM, Ponikowski P, Anker SD et al. Clinical practice update on heart failure 2019: pharmacotherapy, procedures, devices and patient management. An expert consensus meeting report of the Heart Failure Association of the European Society of Cardiology. Eur J Heart Fail. 2019;21:1169–86. doi: 10.1002/ejhf.1531. [DOI] [PubMed] [Google Scholar]
- 67.American Heart Association. Using remote patient monitoring technologies for better cardiovascular disease outcomes: guidance. AHA, 2019. https://www.heart.org/-/media/files/about-us/policy-research/policy-positions/clinical-care/remote-patient-monitoring-guidance-2019.pdf (accessed 19 January 2021)


