Abstract
Introduction
Venous thromboembolism (VTE) is a serious adverse event associated with tamoxifen use, with a 2 to 3-fold increase incidence in users. We aimed to reduce the incidence of venous thromboembolism in patients undergoing breast related surgery by implementing a risk stratifying algorithm for the perioperative management of tamoxifen.
Methods
A retrospective control cohort was compared to a prospective interventional cohort to validate the algorithm which was created by a multidisciplinary team. The algorithm classed patients as low, moderate, high, or very high risk, based on patient factors, and then managed their tamoxifen accordingly during the perioperative period. Each case was then analysed for the presence of a symptomatic, diagnosed venous thromboembolic event up to 60 days post operatively.
Results
A total of 446 (n = 446) consecutive patients were analysed between May 2015 and July 2018 with a 3.36% (15/446) incidence of venous thromboembolism. The retrospective arm consisting of 306 cases, not subjected to the algorithm, showed a 4.58% (14/306) event rate while the prospective arm of 140 cases, managed with the algorithm, showed an event rate of 0.71% (1/140). Analysis with Fisher's exact test showed a significant reduction in VTE using the algorithm (p = 0.0447, CI = 0.95). The cessation of tamoxifen was more rationalised (no algorithm-18.1 days, low risk-0.125 days, moderate risk-14.988 days, high risk-29.6 days, very high risk-32.5 days) and stopped for 11.6% fewer days when using the algorithm.
Conclusion
The use of this algorithm significantly reduces the risk of venous thromboembolism in this population while reducing the number of omitted tamoxifen doses.
Keywords: Tamoxifen, Thromboembolism, Venous thrombosis, Pulmonary embolism, Breast, Surgery
Highlights
-
•
Risk stratifying algorithm aimed at reducing venous thromboembolism in peri-operative patients using tamoxifen.
-
•
Protocol driven management of perioperative tamoxifen reduces venous thromboembolism rates.
-
•
Use of algorithm reduces number of missed doses of tamoxifen.
-
•
Verified perioperative tamoxifen management algorithm.
1. Introduction
The use of tamoxifen, a Selective Oestrogen Receptor Modulator (SERM), is a known risk factor for Venous Thrombo-Embolism (VTE), namely Deep Vein Thrombosis (DVT) and Pulmonary Embolism (PE). Tamoxifen increases the risk of developing VTE by 2 to 3-fold [1], with an incidence of 1.3% in patients taking tamoxifen [2,3]. However, patients on tamoxifen often have multiple other significant risk factors for developing VTE, including cancer (4-fold risk increase with annual incidence of 0.48%) [4]; surgery (10-fold increase in cancer surgery vs no surgery, most occurring within 60 days post-operation) [5,6]; and chemotherapy (1% Risk of VTE in the 6 week post breast surgery in patients currently receiving chemotherapy) [7,8].
Breast cancer patients who remain on tamoxifen for adjuvant treatment often undergo revision surgery following tumour extirpation surgery or reconstruction, or other non-breast related surgery. However, there is a paucity of information in the literature with regards to the management of tamoxifen in this population, with merely a suggestion that tamoxifen should be stopped as the risk of developing VTE during the peri-operative period outweighs the risk of withdrawing the cytostatic benefits from tamoxifen, albeit temporarily [[9], [10], [11]]. The aim of this paper is to present a validated, risk stratifying algorithm for patients taking tamoxifen in the peri-operative period, in order to reduce the VTE risk in this susceptible group of patients undergoing surgery, without compromising their adjuvant treatment for breast cancer.
2. Methodology
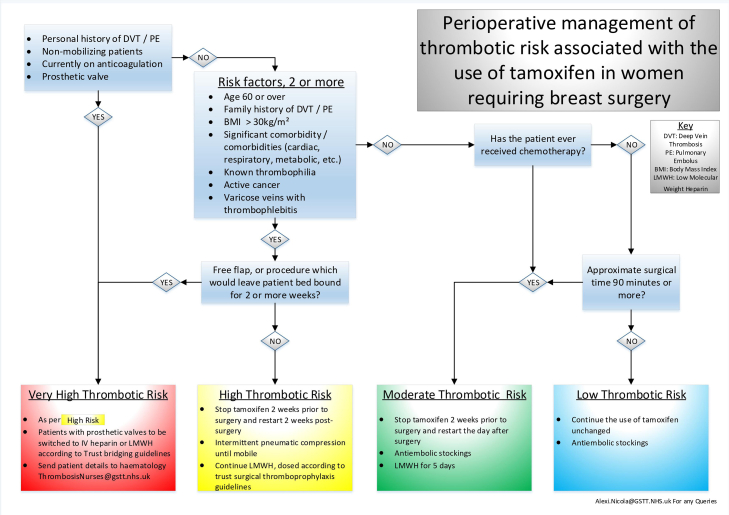
A multi-disciplinary team consisting of oncoplastic surgeons, anaesthetists, oncologists, haematologists and pharmacists developed an algorithm for standardizing the management of tamoxifen peri-operatively within a single institution. Factors taken into account included patient risk factors, cancer treatment history, duration and rehabilitation of the proposed surgery. The patient factors specifically included all the risk factors for VTE as listed by the National Clinical Guideline Centre Group [12]. The patients were then stratified into low, moderate, high, or very high-risk groups for developing thrombo-embolic events. Recommendations from local thromboprophylaxis protocols and the National Institute for Care and Health Excellence (NICE) guidelines were incorporated to complete the algorithm (Fig. 1).
Fig. 1.
Risk Stratifying Algorithm for the Peri-Operative management of Tamoxifen.
Prior to June 2017, there was no protocol in place for the dosage of peri-operative tamoxifen in our institution. A retrospective control cohort (labelled Pre-A) was compared with a prospective cohort (labelled Post-A). The Post-A cohort was prescribed tamoxifen according to the algorithm as set out in Fig. 1. All Post-A patients were consecutively recruited in the pre-operative nurse-led assessment clinics from June 2017 to reduce selection bias. The prevalence of VTE occurring within 60 days of surgery was recorded in both groups. Other data parameters included patient demographics, the duration whereby tamoxifen was withheld, and co-morbidities to account for other risk factors.
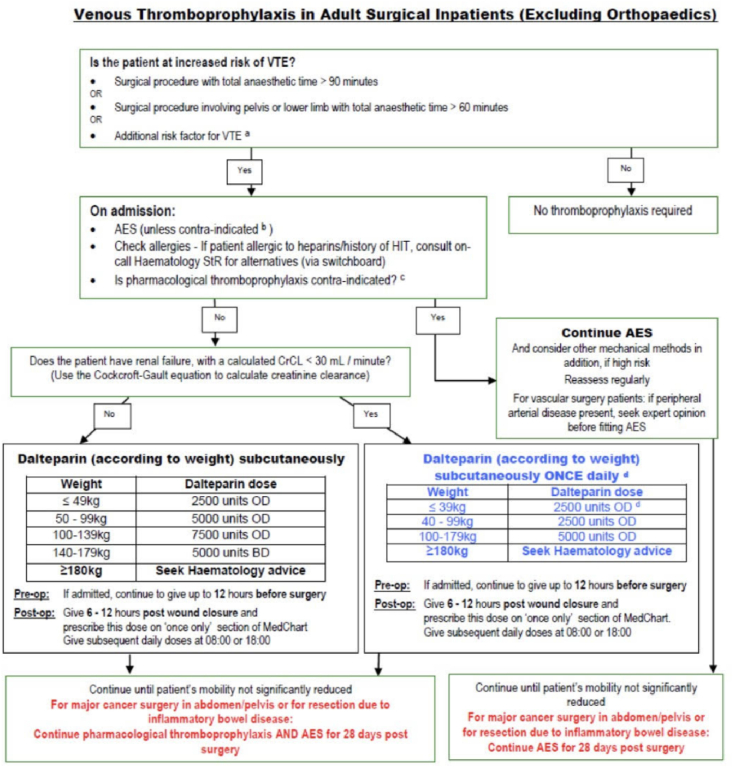
The Post-A group was stratified into risk groups, (Low, Moderate, High, and Very High) by the algorithm while the Pre-A group had the stratification applied to the participants retrospectively. This allowed for further comparison within the stratified groups. All patients were prescribed post-operative thromboprophylaxis according to the local protocol for our institution, with tamoxifen recognised as an additional risk factor for VTE (Fig. 2).
Fig. 2.
Venous Thromboprophylaxis in Adult Surgical Inpatients (Excluding Orthopaedics), Guy's and St. Thomas NHS Foundation trust.
The Fisher's exact Chi2 test was employed to compare the incidence of VTE between the two groups. Analysis of Variance test (ANOVA) was used to compare the stratified risk groups within the Pre-A and Post-A cohorts. All statistical calculations were performed in Excel 2016. Report was written to be STROBE adherent [13].
3. Results
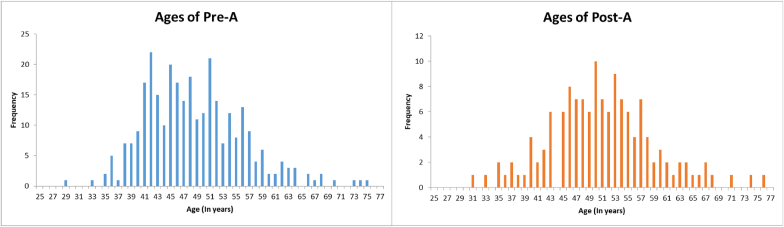
A total of 306 cases were recruited to the Pre-A group in the period between May 2015 to May 2017. From June 2017 to July 2018, a total of 140 patients were recruited to the Post-A group (Table 1). In the Pre-A group, the mean age was 49 years, with a range of 29–75 years. In the Post-A group, the mean age was 50 years, with a range of 31–76 years. (Fig. 3). In the Pre-A group, 14 cases of VTE were documented, i.e. 6 cases of Pulmonary Embolism (PE) and 8 cases of Deep Vein Thrombosis (DVT); thus, the Pre-A group VTE incidence was 4.79% (Table 2). The Post-A group had a single case of PE and no cases of DVT, resulting in a VTE incidence of 0.72%. When analysed with the Fisher's exact Chi2 test, this showed a significant decrease in the VTE rate with a p = 0.0447 and a CI = 95%. This also yielded an absolute risk reduction of 3.86%.
Table 1.
Number of Cases by Risk Stratifying Pre-A and Post-A with age Histogram.
| Risk Group | Pre-A (n) | Pre-A (%) | Post-A (n) | Post = A (%) |
|---|---|---|---|---|
| Low Risk | 51 | 16.67% | 24 | 17.14% |
| Moderate Risk | 182 | 59.48% | 81 | 57.86% |
| High Risk | 62 | 20.26% | 33 | 23.57% |
| Very High Risk | 11 | 3.59% | 2 | 1.43% |
Fig. 3.
Age distribution of patients in the Pre-A and Post-A cohorts.
Table 2.
Demographics of patients with VTE events.
| Age | VTE Event | Co-Morbidity | Risk Stratification | Procedure |
|---|---|---|---|---|
| Pre-A | ||||
| 47 | DVT | BMI, Metabolic, Cardiac, Cancer | High | >90 |
| 45 | DVT | Chemo | Moderate | >90 |
| 62 | PE | Age, Hx of DVT, Cardiac, Cancer | High | >90 |
| 59 | PE | Metabolic, Cancer | High | <90 |
| 64 | DVT | Age, Cancer, Cardiac | High | >90 |
| 68 | DVT | Age, Hx of DVT, Cardiac, Cancer, Anti-Coag | Very High | >90 |
| 62 | PE | Age, BMI, Cancer | High | >90 |
| 51 | DVT | MBI, Metabolic, Cardiac, Cancer | High | <90 |
| 54 | PE | BMI, Metabolic, Cancer | High | >90 |
| 51 | DVT | F Hx, Cardiac, BMI, Cancer | Very High | >90 |
| 57 | DVT | F Hx, Cancer | High | >90 |
| 62 | PE | Age, Cancer | High | >90 |
| 52 | PE | Cardiac, Metabolic, Cancer | High | >90 |
| 50 | DVT | Hx of DVT, Cancer, F Hx | Very High | >90 |
| Post-A | ||||
| 70 | PE | Age, Cardiac, Cancer | High | >90 |
The duration of tamoxifen cessation was compared between the Pre-A and Post-A groups (Table 3). However, there was incomplete data within the Pre-A group with data from only 262 of 306 (85.6%) patients available. The total number of doses withheld may also be considered as ‘days’ of tamoxifen withheld (as the regimes are prescribed once daily). In the Pre-A group, the average number of days of tamoxifen withheld was 18.1 days. In the Post-A group, the average number of days of tamoxifen withheld by risk stratification was as follows; low risk for 0.125 days, moderate risk for 14.988 days, high risk for 29.6 days, and very high risk for 32.5 for days. The average days of tamoxifen withheld in the Post-A group across the cohort is 16.8 days, which is a reduction of 11.6% compared to the Pre-A group.
Table 3.
Doses of tamoxifen withheld.
| Average number of doses withheld | Range | |
|---|---|---|
| Pre-A | ||
| No Stratification | 18.1 | 0–32 |
| Post-A | ||
| Low Risk | 0.125 | 0–5 |
| Moderate Risk | 14.988 | 14–27 |
| High Risk | 29.6 | 28–42 |
| Very High Risk | 32.5 | 28–37 |
4. Discussion
Tamoxifen is a selective oestrogen receptor modulator (SERM) that inhibits the growth of breast cancer cells by competitive antagonism of the oestrogen receptor. One of the serious and potentially life-threatening side effects of tamoxifen is venous thromboembolism (VTE). The risk of developing VTE with tamoxifen is two to three-fold [1], especially during the first 2 years after exposure [14].
In the event of further surgery in women taking tamoxifen, it is imperative to balance between the continuous cytostatic effect, by continuing tamoxifen; against the reduction of VTE risk, by stopping tamoxifen for a finite period peri-operatively. The half-life elimination of tamoxifen is between 5 and 7 days. A literature search performed in search of best practice with regards to the dosage of tamoxifen in the peri-operative period revealed a marked paucity of evidence. The NICE guidelines stipulate that tamoxifen may need to be stopped for up to 6 weeks prior to elective surgery, without further details on risk stratification nor specification of the duration of stopping tamoxifen [15]. While there are extensive guidelines on venous thromboprophylaxis for oestrogen containing contraceptives and hormone replacement therapy, there is no suggestion with regards to the management of SERMs [16].
The main aim of this study was to create and implement a protocol that can be used to help reduce the VTE rate in an at-risk population. In our institution, there was a lack of standardised practice amongst the clinicians (tamoxifen was withheld for a range of 7–28 days pre-operatively) which prompted the effort to produce this algorithm. Hussain et al. proposed a comprehensive protocol which takes into consideration all the risk factors for VTE and the pharmacokinetics of tamoxifen [11]. The resulting protocol which we devised is not dissimilar to that proposed by Hussain et al., and indeed our protocol takes into account all the risk factors for VTE as listed by the National Clinical Guideline Centre Group. Additionally, it advocates that the highest risk individuals to be referred to specialist thrombosis haematologists for individual review and management. In contrast, the protocol also omits the need to stop tamoxifen in the low-risk patient group. We then proceeded to evaluate the efficacy of this protocol with comparative studies and not only confirmed a statistically significant reduction in VTE events after its implementation, but also reduced the overall number of missed doses of tamoxifen due to segregation of the patients into low-risk and moderate risk pathways. In our study, 65% of the Post-A patients fell into these categories. These Results also suggest that patients in the Pre-A group who fell into the lower risk stratification groups were “over-managed” with regard to withholding tamoxifen while the higher risk groups were potentially “under-managed”.
The age distribution between the retrospective (Pre-A) and prospective (Post-A) groups were similar. To reduce selection bias, all 446 cases for both Pre -A and Post-A cohorts were consecutive cases recruited at the pre-admission clinics, with no exclusion criteria. An ANOVA test comparing pre-A and post-A cohorts showed no significant difference in the cohorts’ risk stratification, and by inference, their co-morbidities and type of surgery.
As mentioned earlier in the article, all patients were prescribed standardised post-operative thromboprophylaxis according to the local protocol for our institution, with tamoxifen recognised as an additional risk factor for VTE (Fig. 2). VTE diagnoses were identified as patients who presented to hospital with a symptomatic DVT or PE. There was no prophylactic screening for VTE implemented at any point during this study. We acknowledge that some VTE events may have been undiagnosed, leading to under-reporting. We do not think pre-operative discussion of VTE between the two groups differed and the protocol implementation is unlikely to have introduce a bias in VTE reporting.
We feel that despite the limitations of a retrospective study, we were able to eliminate selection bias and compare similar populations in both cohorts as detailed above. Our data supports a statistically significant decrease in VTE rate when the algorithm was applied, at a confidence interval of 95%, with an absolute risk reduction of 3.86% and a number needed to treat of 27. We believe that this is due to greater attention in the management of the high and very high-risk patients, in terms of the dosing of tamoxifen and post-operative thrombophylaxis. However, we accept that a bigger cohort of patients would be preferrable for greater statistical power.
5. Conclusion
This algorithm for the management of peri-operative tamoxifen has significantly reduced VTE events in our institution, and reduced the tamoxifen cessation period. It can be introduced by any member of the healthcare team, in particular the nurse-led pre-operative assessment team; reduces variability in practice, promotes efficacious adjuvant treatment of breast cancer and identifies high-risk patients who may require closer monitoring or bridging treatment in the peri-operative period.
Ethics
An application for ethics approval was submitted but deemed unnecessary by the Local Ethics Committee.
Declaration of competing interest
None of the authors have any disclosures.
Acknowledgements
Nil.
Footnotes
American Society for Reconstructive Microsurgery, Annual Meeting (ASRM) 1st – February 5, 2019.
International Society of Thrombosis and Haemostasis, Annual Congress (ISTH) 6th – July 10, 2019.
British Association of Plastic, Reconstructive and Aesthetic Surgeons, Winter Meeting (BAPRAS) 4th – December 6, 2019.
References
- 1.The risk of venous thromboembolic disease associated with adjuvant hormone therapy for breast carcinoma: a systematic review. Deitcher SR, Gomes MP. 2004;101:439–449. doi: 10.1002/cncr.20347. 3. Cancer. [DOI] [PubMed] [Google Scholar]
- 2.Selective oestrogen receptor modulators in prevention of breast cancer: an updated meta-analysis of individual participant data. Cuzick J, et al. 2013;381:1827–1834. doi: 10.1016/S0140-6736(13)60140-3. 9880. Lancet. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Adomaityte J. vol. 99. 2008. Effect of raloxifene therapy on venous thromboembolism in postmenopausal women. A meta-analysis; pp. 338–342. (Thrombosis and Haemostasis). 2. [PubMed] [Google Scholar]
- 4.Andtbacka R. Incidence and prevention of venous thromboembolism in patients undergoing breast cancer surgery and treated according to clinical pathways. Ann Surg. 2006;243:96–101. doi: 10.1097/01.sla.0000193832.40178.0a. 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Breast cancer and hormone-replacement therapy: the million women study. Bliss, J and Gray, R. 2003;362:1328–1329. doi: 10.1016/S0140-6736(03)14591-6. 9392. The Lancet. [DOI] [PubMed] [Google Scholar]
- 6.Sweetland S. Duration and magnitude of the postoperative risk of venous thromboembolism in middle aged women: prospective cohort study. Brittish Medical journal. 2010;340 doi: 10.1136/bmj.b4583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kirwan c. Platelet release of Vascular Endothelial Growth Factor (VEGF) in patients undergoing chemotherapy for breast cancer. Journal of Angiogenes Res. 2009;24:1–7. doi: 10.1186/2040-2384-1-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Thromboembolic complications after perioperative chemotherapy in women with early breast cancer: a European organization for research and treatment of cancer breast cancer cooperative group study. Clahsen P., editor. J Clin Oncol. 1994;12:1266–1271. doi: 10.1200/JCO.1994.12.6.1266. 6. [DOI] [PubMed] [Google Scholar]
- 9.Associations between tamoxifen, estrogens, and FSH serum levels during steady state tamoxifen treatment of postmenopausal women with breast cancer. Gjerde J. 2010;10:313. doi: 10.1186/1471-2407-10-313. BMC Cancer. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Peto R. Relevance of breast cancer hormone receptors and other factors to the efficacy of adjuvant tamoxifen: patient-level meta-analysis of randomised trials. Early Breast Cancer Trialists' Collaborative Group (EBCTCG) 2011;378:771–784. doi: 10.1016/S0140-6736(11)60993-8. 9793. Lancet. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Stopping tamoxifen peri-operatively for VTE risk reduction: a proposed management algorithm. Hussain T., Kneeshaw P., editors. Int J Surg. 2012;10:313–316. doi: 10.1016/j.ijsu.2012.05.001. 6. [DOI] [PubMed] [Google Scholar]
- 12.Venous thromboembolism: reducing the risk of venous thromboembolism (deep vein thrombosis and pulmonaryembolism) in patients admitted to hospital. Group, National Clinical Guideline Centre; London : National Clinical Guideline Centre Group: 2009. https://www.nice.org.uk/guidance/cg92/documents/venous-thromboembolism-reducing-the-risk-prepublication-check-full-guideline2 vol. s. [Google Scholar]
- 13.vol. 11. PLoS Med; 2014. Observational studies: getting clear about transparenc. (The PLOS medical. 8). s.l. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tamoxifen treatment and risk of deep venous thrombosis and pulmonary embolism: a Danish population-based cohort study. Hernandez RK, Lash TL, et al. 19, Boston : Cancer, 2009, Vol. vol. 15. [DOI] [PubMed]
- 15.Tamoxifen - managing adverse effects: scenario: Tamoxifen - prescribing information. Excellence. National Institute for Health and Care. s.l. : NICE; 2019. https://cks.nice.org.uk/topics/tamoxifen-managing-adverse-effects/management/tamoxifen-prescribing-information/ vol. s. [Google Scholar]
- 16.Venous thromboembolic diseases: diagnosis, management and thrombophilia testing. NICE, National Institute for Health and Care Excellence; 2020. https://www.nice.org.uk/guidance/ng158 National Institute for Health and Care Excellence. [PubMed] [Google Scholar]