Highlights
-
•
Exploration of neural basis of the short-interval intracortical inhibition (SICI) marker using paired-pulse TMS in individuals with schizophrenia.
-
•
Use of a combination of resting fMRI methods (ReHo and rsFC) to explore connectivity patterns associated with SICI.
-
•
Local insula connectivity was associated with SICI in schizophrenia.
-
•
Interhemispheric insula-sensorimotor connectivity mediates association between SICI and local insula connectivity.
Keywords: Schizophrenia, Transcranial magnetic stimulation (TMS), Short-interval intracortical inhibition (SICI), Resting fMRI, Regional homogeneity (ReHo)
Abstract
Short interval intracortical inhibition (SICI) is a biomarker for altered motor inhibition in schizophrenia, but the manner in which distant sites influence the inhibitory cortical-effector response remains elusive. Our study investigated local and long-distance resting state functional connectivity (rsFC) markers of SICI in a sample of N = 23 patients with schizophrenia and N = 29 controls. Local functional connectivity was quantified using regional homogeneity (ReHo) analysis and long-range connectivity was estimated using seed-based rsFC analysis. Direct and indirect effects of connectivity measures on SICI were modeled using mediation analysis. Higher SICI ratios (indicating reduced inhibition) in patients were associated with lower ReHo in the right insula. Follow-up rsFC analyses showed that higher SICI scores (indicating reduced inhibition) were associated with reduced connectivity between right insula and hubs of the corticospinal pathway: sensorimotor cortex and basal ganglia. Mediation analysis supported a model in which the direct effect of local insular connectivity strength on SICI is mediated by the interhemispheric connectivity between insula and left sensorimotor cortex. The broader clinical implications of these findings are discussed with emphasis on how these preliminary findings might inform novel interventions designed to restore or improve SICI in schizophrenia and deepen our understanding of motor inhibitory control and impact of abnormal signaling in motor-inhibitory pathways in schizophrenia.
1. Introduction
Rapid execution of appropriate motor responses and inhibition of inappropriate motor responses is a critical evolutionary trait linked to survival. Motor inhibition can be indexed behaviorally by examining reaction times to paradigms that require inhibition (e.g. “stop trials” in Stop-Signal task, “NoGo” trials on Go/NoGo task) (Aron and Poldrack, 2005, Eagle et al., 2008). Individuals with schizophrenia have abnormal performance across tasks indexing motor inhibitory control such as longer reaction times in the Stop-Signal task (Ettinger et al., 2018) . A consistent motor abnormality in schizophrenia is the short interval intracortical inhibition (SICI) measured using paired pulse transcranial magnetic stimulation (TMS). Conventionally applied to the motor cortex, SICI is measured as the ratio of the electromyographic (EMG) response in muscle to a pair of pulses separated by a short (1–4 ms) interval relative to a single, excitatory suprathreshold test pulse that can reliably elicit muscle EMG activity. While the paired-pulse SICI paradigm is conventionally applied to motor cortex, SICI has been applied to regions outside the motor cortex including the dorsolateral prefrontal cortex (DLPFC) (Noda et al., 2016). A major advantage of conventional SICI (applied at motor cortex) is that output of the paired-pulse TMS can be directly measured as evoked muscle EMG response. Conventional SICI serves as a robust biomarker for motor inhibition dysfunction as higher SICI ratios (indicating reduced inhibition) are readily replicated in schizophrenia (Bunse et al., 2014, Rogasch et al., 2014), including first-episode patients with limited antipsychotic medication exposure (Wobrock et al., 2008, Wobrock et al., 2009). However, SICI deficits have been reported in other brain regions such as the DLFPC (Noda et al., 2017), demonstrating inhibition dysfunction outside of the motor corticospinal pathway in schizophrenia. Yet the neural mechanisms underlying reduced intracortical inhibition in schizophrenia remain elusive.
Abnormal SICI was originally thought to be associated with the reduced connectivity localized to the descending corticospinal pathway (Di Lazzaro et al., 1999, Ferbert et al., 1992). Newer evidence suggests that brain networks outside the motor corticospinal pathway including frontal-basal ganglia interactions are likely involved in motor response inhibition (Wessel and Aron, 2017, Wiecki and Frank, 2013, Li et al., 2008). SICI score in healthy individuals was predicted by activation of distributed regions in frontal, parietal, occipital, temporal and cerebellar cortices (Dayan et al., 2018). Higher SICI scores (indexing reduced inhibition) in patients with schizophrenia were associated with lower resting state functional connectivity (rsFC) between the left motor cortex stimulation site and four distant regions spanning left and right middle frontal, left cerebellar and right insular cortex (Du et al., 2019). Taken together with the replicated finding of abnormal SICI in schizophrenia (Bunse et al., 2014, Rogasch et al., 2014), this raises the intriguing question of how functional communication between distant regions might influence SICI at the motor pathway in individuals with schizophrenia. This question is important from basic neuroscience (e.g. mapping the circuitry of motor inhibition) and translational (considering novel therapies for restoring or enhancing SICI in patients) perspectives.
To address this question, we studied how local and long-distance connectivity between five regions of interest identified in a prior analysis (left motor cortex, left middle frontal, right middle frontal, left cerebellar and right insular cortex) (Du et al., 2019) may contribute to SICI in schizophrenia. Long-distance connectivity was quantified using resting-state functional connectivity (rsFC) analysis, while strength of local connectivity within these areas was quantified using regional homogeneity (ReHo) analysis. Each analysis has its own strengths: ReHo measures the extent of correlated activity of a voxel and its nearest neighboring voxels (Zang et al., 2004), making it suitable to sensitively detect temporal homogeneity within local areas but not suitable to detect connections between spatially distant regions that rsFC analysis is designed to identify. Yet studies often employ only a single resting state fMRI analysis, making it difficult to map out a complete understanding of network interactions (within-network and cross-network) underlying a particular behavior or psychiatric disorder (Cole et al., 2010). Our primary aim was to map local and long-distance connectivity measures associated with SICI in a sample of 52 human participants (23 patients with schizophrenia, 29 controls), hypothesizing that strength of local connectivity at the motor cortex stimulation site would be associated with SICI score. We then tested multiple mediation models to explore the directional contribution of direct and indirect effects of local and long-distance connectivity on SICI score.
2. Materials and methods
2.1. Participants
The participants and the imaging data are the same as in our previous publication (Du et al., 2019). Twenty four patients with schizophrenia spectrum disorders (SSD) were recruited from outpatient clinics of the Maryland Psychiatric Research Center and neighboring outpatient clinics. Control participants (N = 30) were recruited using local media advertisements and were frequency-matched on age, sex and current smoking status with SSD participants. The Structured Clinical Interview for DSM-IV was performed on all participants (First et al., 2002). Exclusion criteria for control participants included a current Axis I diagnosis (including mood, anxiety, psychotic, substance-use and other Axis I diagnoses); controls were also required to have no family history of psychosis in two generations. Exclusion criteria for both groups included history of head injury with loss of consciousness, any major neurological, neurodevelopmental or medical illnesses, intellectual disability, and substance abuse or dependence within the past 6 months (except nicotine). In addition, we excluded women who were pregnant, because of the unknown effects of MRI scans on developing fetuses.
All SSD patients were taking antipsychotic medications with exception of four who were unmedicated at time of study. Nineteen patients were taking atypical antipsychotic medications, and two were taking typical antipsychotic medications; 1 patient was taking both atypical and typical medications. In addition to the above exclusion criteria, patients taking clozapine more than 400 mg/day were excluded. No patients took benzodiazepines at the time of scanning. Total daily dose of antipsychotic medication was calculated as chlorpromazine equivalent dose in milligrams per day (CPZ) (Woods, 2003).
Written informed consent was obtained from all participants as approved by the University of Maryland Institutional Review Board. Two participants were excluded due to excessive motion (see Imaging section below) such that the final sample for the current imaging analysis was N = 52 human subjects (N = 23 patients, N = 29 controls).
2.2. Imaging
All imaging scans were collected before the TMS sessions. Resting-state fMRI data were collected at the University of Maryland Center for Brain Imaging Research using a Siemens 3 T TRIO MRI (Erlangen, Germany) system equipped with a 32-channel phase array head coil. Resting-state functional T2*-weighted images were obtained (TR = 2000 ms, TE = 27 ms, flip angle = 90 degrees, FOV = 220 mm, 64 × 64 matrix, 1.94 × 1.94 in-plane resolution, 1.7-mm2 in-plane resolution, 4 mm slice thickness, 37 axial slices, 15-minute scan for 450 volumes). During the scan, participants were asked to keep their eyes closed, to relax, and not to think about anything in particular. We also acquired structural (T1-weighted) images for co-registration with the functional images during preprocessing; structural images were acquired using a fast spoiled gradient recalled sequence (TR = 11.08 ms, TE = 4.3 ms, flip angle = 45 degrees, field of view = 256 mm, 256 × 256 matrix, 172 slices, 1-mm3 spatial resolution).
Standard resting-state fMRI data preprocessing steps were performed using the Analysis of Functional NeuroImages (AFNI) software (Cox, 1996). After discarding the first two TRs for signal stabilization, data were spatially smoothed with a Gaussian filter (4 mm full width at half maximum). The linear trend, six motion parameters (three rotational and three translational directions), their six temporal derivatives (rate of change in rotational and translational motion), and time courses from the white matter and cerebrospinal fluid were removed as regressors of no interest. Time points with excessive motion (0.2 mm) and their neighboring time points were censored from statistical analysis. For group analysis, images were spatially normalized to the Talairach space. For each subject, the percent of censored time points was calculated. Two subjects (one patient, one control) had greater than 50% of time points censored and had clear motion artifacts (substantial “ringing” and blurring) on raw and processed rsfMRI scans and were omitted from the group fMRI analysis. Demographic and clinical data for the final imaging sample (N = 23 patients, N = 29 controls) are reported in Table 1.
Table 1.
Demographic and Clinical Characteristics.
| Characteristic | Schizophrenia Group (n = 23) | Healthy Control Group (n = 29) | Statistic (t, F, or (χ2) | P-value) |
|---|---|---|---|---|
| Age, Mean (SD) | 37.0 (13.5) | 41.6 (13.5) | 1.23 | 0.23 |
| Male/Female | 16/7 | 15/14 | 1.72 | 0.15 |
| BPRS Total, Mean (SD) | 40.2 (10.6) | – | – | – |
| CPZ equivalent dose, Mean (SD) | 583.8 (722.4) | – | – | – |
| Duration of Illness, Mean (SD)* | 14.9 (15.6) | – | – | – |
| RMT, Mean (SD) | 47.4 (8.5) | 47.4 (6.5) | 0.02 | 0.99 |
| SICI, Mean (SD) | 0.38 (0.28) | 0.25 (0.16) | −2.1 | 0.04 |
Abbreviations: BPRS = Brief Psychiatric Rating Scale; CPZ = chlorpromazine; RMT = resting motor threshold; SICI = short-interval intracortical inhibition; *Duration of illness was calculated as the participant’s present age minus the approximate age of persistent psychotic symptoms.
2.3. SICI and resting motor threshold calculations
Monophasic single TMS pulses and paired TMS pulses were given through a figure-of-eight coil (70 mm diameter) using Magstim 200 BiStim stimulators (Magstim Co., Whitland, UK). Surface EMG was recorded from right first dorsal interosseous (FDI) muscle using a NeuroScan synamp2 amplifier (Charlotte, NC) amplified (gain of 10) and sampled at 1000 Hz (Du et al., 2014, Du et al., 2015). Peak-to-peak amplitude of the motor-evoked potentials (MEP) was measured. The EMG root mean squared (RMS) value from 50 to 5 ms prior to TMS pulse was verified to ensure appropriate resting levels for each trial.
To determine the motor cortex hotspot, each participant’s structural images were imported into Brainsight™ Navigation system (Rogue Research Inc, Montreal, Canada) to allow individualized anatomic positioning of the coil. The stimulus target was left motor cortex where TMS induced the maximum response from right FDI muscle. Resting motor threshold (RMT) was defined as the minimum intensity needed to elicit a MEP of 0.50 mV in at least 5 of 10 consecutive stimuli (Du et al., 2014).
Paired-pulse TMS data were collected as a part of a larger study in which 14 different inter-stimulus intervals (ISIs) were tested on each session (1, 3, 6, 9, 12, 15, 18, 21, 30, 40, 80, 120, 200 and 500 ms). For paired-pulse TMS, a subthreshold conditioning stimulus (80% RMT) was followed by a suprathreshold stimulation (120% RMT) separated by a brief interval. For SICI, paired-pulse TMS trials with 1-ms and 3-ms interval were merged. Single-pulse TMS delivered at 120% RMT were administered in the same session, serving as the control (test) stimulation. SICI was defined as the ratio of peak-to-peak EMG response to paired-pulse TMS (numerator) to a single suprathreshold test stimulation pulse of 120% RMT (denominator). Trials were randomized and delivered in one session, with intertrial intervals jittered between 4 and 10 s. Participants were evaluated in two sessions about 4 weeks apart. High reproducibility of the paired-pulse effects between the two sessions was was observed and previously reported (Du and Hong, 2018). Therefore, we merged the data from the two sessions to obtain robust and reliable representation of SICI (i.e., 24 trials for test stimulation and 24 trials for SICI). The mean number of SICI trials retained was greater than 20 trials in both groups (mean number of trials for patients = 20.78 (5.03); mean number of trials for controls = 23.14 (2.59)); there was only a marginally significant difference in the number of trials between groups (t = 2.0, P = 0.05). Adding the number of trials as a covariate in our correlation and regression analyses did not significantly impact our reported findings. For this study, SICI was selected as a primary factor of interest because it reliably results in inhibition of the measured MEP (relative to the test pulse) by 50–90% in healthy individuals (Kujirai et al., 1993, Radhu et al., 2013) and is most consistently reduced in studies of Sz (Bunse et al., 2014, Rogasch et al., 2014, Wobrock et al., 2008, Wobrock et al., 2009) in comparison to other indices of inhibition (e.g. long-interval-intracortical inhibition; cortical silent period) and cortical excitability (e.g. intracortical facilitation, etc.).
2.4. ReHo calculation and analysis
We calculated ReHo within five clusters, guided by the findings of our previous study (Du et al., 2019). These regions included (1) the left motor cortex (TMS stimulation site), and four additional regions, which were shown to have reduced connectivity with the motor cortex associated with SICI in (Du et al., 2019): (2) left frontal cortex, (3) right frontal cortex, (4) right insular cortex and (5) left cerebellum. ReHo was defined as the Kendall’s coefficient of concordance score with the resting fMRI time series of neighboring voxels (Zang et al., 2004). By estimating synchronization of fMRI activation of a voxel to its neighboring voxels, ReHo provides an estimate of local functional connectivity. The number of time series within a given cluster was defined as 27 to account for face-wise, edge-wise, and node-wise neighbors per recommendations to cover all directions in 3D space (Jiang and Zuo, 2016). ReHo was calculated as the Kendall’s coefficient score at each voxel with its 26 nearest-neighboring voxels using the MATLAB function “y_reho.m” available in the DPABI_V3.0_171210 package. For each subject, a ReHo map was generated consisting of the collection of all voxels’ Kendall’s coefficient scores.
2.5. rsFC calculation and analysis
Clusters in which ReHo was found to be significantly associated with SICI were saved as binary masks and included as regions of interest in a follow-up rsFC analysis; whole brain rsFC analysis was performed between voxels in the mask and all other voxels in the brain. The average time series of each region of interest was correlated with all other voxels in the brain to generate whole-brain maps of rsFC. Fisher z transformation was applied to rsFC maps for further statistical analyses. Using AFNI’s 3dttest++ program, SICI was included as a regressor in a whole-brain voxelwise regression analysis; the average regression beta weight was calculated at each voxel and could be extracted for further analysis.
2.6. Statistical analysis
ReHo and rsFC analyses were performed in the whole sample (N = 52) and separately in the patient (N = 23) and control participants (N = 29). For the analyses of the five ReHo clusters, we specified our confidence as P < 0.05, and applied Bonferroni correction for the five tests (P = 0.05/5 = 0.01). For all voxelwise rsFC analyses, significant results were thresholded using Monte Carlo simulations by AFNI’s 3dClustSim with autocorrelation function option to yield corrected (whole-brain) results thresholded at alpha < 0.05, corresponding to cluster-size threshold of 49 voxels at voxelwise p = 0.001.
Subject-specific estimates of ReHo and rsFC in significant clusters were extracted to examine their contributions to SICI and a more general measure of motor cortical excitability: motor threshold. Bivariate Pearson correlation analyses were performed separately in SSD patient and control groups. Linear regression analysis was also performed to control for confounding effects of age, gender, and motion (quantified as the percentage of TRs censored during preprocessing). Mediation analyses explored whether and how ReHo measures might mediate the relationship between long-range functional connectivity and SICI score using Sobel test statistics. For post-hoc analyses, Bonferroni-corrections were applied to correct for the number of tests to yield corrected p < 0.05.
3. Results
3.1. Regional homogeneity and SICI
There were no significant patient vs. control differences in age, gender or smoking status; however, patients had higher SICI scores (indicating reduced inhibition) relative to controls (0.38 ± 0.28 vs. 0.25 ± 0.16, respectively, t = -2.1, p = 0.04). Patients had significantly increased resting ReHo in motor cortex relative to controls (t = 4.2, p < 0.001). No significant patient vs. control differences were observed in ReHo values of the remaining four regions.
In controls, SICI was not significantly associated with any of the ReHo measures (motor cortex, r = 0.07, p = 0.69; right insula, r = 0.04, p = 0.85; right middle frontal, r = -0.08, p = 0.68; left cerebellum r = -0.10, p = 0.60; left middle frontal, r = 0.03, p = 0.90). In SSD patients, SICI was significantly and negatively associated with ReHo in the right insula after Bonferroni correction for five tests of associations with ReHo (r = -0.52, p = 0.01) (Fig. 1), but not with ReHo in motor cortex (r = 0.13, p = 0.57), right middle prefrontal cortex (r = -0.35, p = 0.10), left cerebellum (r = -0.13, p = 0.56) or left middle prefrontal cortex (r = -0.42, p = 0.05). We applied a regression validation method to assess the reliability of our estimate of the correlation between right insula ReHo and SICI (Harrell, 2015). The results showed that the 95% bootstrap confidence interval of the adjusted correlation between SICI and ReHo is (-0.73, −0.19) based on 5000 bootstrap samples. Moreover, we confirmed that higher SICI (indicating lower inhibition) remained significantly associated with lower resting ReHo in right insula (t = -2.74, p = 0.01) in SSD patients after controlling for effects of age, sex, and motion (% TRs censored) covariates in linear regression analysis. Therefore, the association between SICI and ReHo is robust and significant.
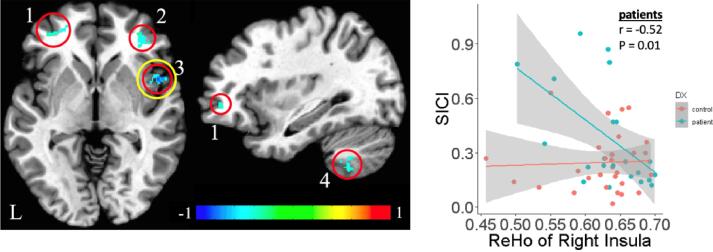
Fig. 1.
Strong Negative Association Between Local Connectivity of Right Insula and SICI Score in SSD Patients. A prior report from our group found that higher SICI scores in SSD patients (indicating reduced inhibition) were associated with lower resting functional connectivity between the left motor cortex and (1) left frontal cortex, (2) right frontal cortex, (3) right insular cortex and (4) left cerebellum (left; reproduced with minor modification from (Du et al., 2019) (left). Simple scatterplot of association between SICI and ReHo of right insula in SSD patients and controls (right). The cluster of the right insula is circled in yellow for emphasis and to show the location of the right insula cluster. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
In patients, motor threshold was significantly associated with ReHo in right insula (r = -0.58, p = 0.004), right middle frontal (r = -0.50, p = 0.01), and left middle frontal, r = -0.49, p = 0.02 but not the other two regions (motor cortex, r = 0.045, p = 0.84; left cerebellum r = -0.20, p = 0.37).
3.2. Resting state functional connectivity of right insula and SICI
To understand how the observed effect of insular local connectivity strength on SICI may be related to rsFC across distant regions, the right insula cluster was used as a seed for whole brain rsFC analysis. In patients, higher SICI scores (indicating reduced inhibition) were significantly associated with lower rsFC between the right insula seed and (1) left sensorimotor cortex (precentral and postcentral gyrus), (2) left basal ganglia (spanning caudate, putamen, globus pallidus), and (3) left superior temporal gyrus (Fig. 2).
Fig. 2.
Results of follow-up seed-based analysis with right insula as seed. In patients, higher SICI scores (indicating reduced inhibition) were associated with lower rsFC between right insula seed and (1) left sensorimotor cortex (top left) (2) left basal ganglia (middle left) and (3) left STG (bottom left). Scatterplots to the right show negative associations between SICI score and rsFC measures and positive associations between ReHo of right insula and rsFC measure in SSD patients.
3.3. Mediation analysis
Mediation analyses were performed using right insula ReHo and the three rsFC measures above (insula-sensorimotor, insula-basal ganglia, and insula-superior temporal) exploring all possible mediation model combinations with SICI as outcome measure, for a total of six models. In SSD patients, only one model using insula-sensorimotor rsFC as the mediator was significant, such that insula-sensorimotor rsFC significantly mediated the relationship between insula ReHo and SICI after Bonferroni correction for six tests (Fig. 3) (Sobel test statistic = -3.04, p = 0.002). Results for the rest of the models are in Table 2.
Fig. 3.
Significant result of mediation analyses. Insula-sensorimotor rsFC was found to significantly mediate the relationship between insula ReHo and SICI in patients after Bonferroni correction for the six tested mediation models. The t-value for each path is presented.
Table 2.
| Model |
Mediator (M) |
Independent Predictor (X) |
Outcome (Y) |
Sobel Test |
Permutation-Based Results |
||||
|---|---|---|---|---|---|---|---|---|---|
| Test stat | p-value | Effect | Std Error | Lower CI | Upper CI | ||||
| 1 | ReHo rt insula | insula-motor FC | SICI | 0.11 | 0.91 | 0.442 | 0.413 | −0.864 | 0.787 |
| 2 | ReHo rt insula | insula-basal ganglia FC | SICI | −0.53 | 0.60 | −0.175 | 0.339 | −0.880 | 0.532 |
| 3 | ReHo rt insula | insula-STG FC | SICI | −0.74 | 0.46 | −0.211 | 0.235 | −0.749 | 0.200 |
| 4* | Insula-motor FC | ReHo rt insula | SICI | −3.04 | 0.002 | −2.96 | 0.893 | −4.885 | −1.284 |
| 5 | Insula-basal ganglia FC | ReHo rt insula | SICI | −2.63 | 0.009 | −2.505 | 0.974 | −4.736 | −0.817 |
| 6 | Insula-STG FC | ReHo rt insula | SICI | −2.47 | 0.014 | −2.207 | 0.952 | −4.723 | −0.752 |
*significant after Bonferroni correction for six tests (p = 0.05/6 = 0.0083).
4. Conclusions
Of the four regions where rsFC with the left motor cortex TMS site was previously found to be associated with SICI (left frontal, left cerebellum, right frontal, right insula) (Du et al., 2019), only ReHo of right insula was associated with SICI score in SSD patients. Seed-based analysis of SICI effect on rsFC with the right insula confirmed that SICI score was strongly associated with right insula rsFC with two key hubs of the corticospinal pathway: sensorimotor cortex and basal ganglia. We then tested six mediation models to explore directional contribution of local and long-distance connectivity effects on SICI. In SSD, long-distance rsFC between right insula and left sensorimotor cortex significantly mediated the association between resting right insula ReHo and SICI. These preliminary findings suggest that reduced inhibition in SSD may be related to reduced connectivity with a distant brain region outside of the traditionally studied corticospinal pathway: the right insula.
Early studies on the mechanism of the SICI focused exclusively on TMS effects on the motor cortex stimulation site and the descending corticospinal pathway (Di Lazzaro et al., 1999, Ferbert et al., 1992). Consistent with this model of motor control, we hypothesized that that strength of local connectivity at the motor cortex stimulation site (indexed by ReHo) would be associated with SICI score. We did not find evidence favoring this hypothesis as SICI score was not associated with motor cortex ReHo in patients nor controls. However, our original hypothesis may have been based on an oversimplified model of motor control as SICI score was associated with activation of areas outside the corticospinal pathway including frontal, parietal, temporal and cerebellar regions in a recent study of healthy individuals (Dayan et al., 2018). Higher SICI scores (indicating reduced inhibition) are consistently reported across studies of schizophrenia (Bunse et al., 2014, Rogasch et al., 2014), raising the question of which of these distributed neural network interactions may contribute to these observed reductions in inhibition.
Few studies of schizophrenia have directly addressed this question, but one study reported that during “stop trials” of a Stop-Signal task, higher SICI scores (indicating reduced inhibition) in patients were linked with the higher activity in the middle and inferior frontal regions (Lindberg et al., 2016), which may reflect a strategy to compensate for abnormal signaling of frontal-basal ganglia pathways that play an essential role in regulating motor inhibition (Wessel and Aron, 2017, Wiecki and Frank, 2013, Li et al., 2008). This is consistent with our earlier observation of a significant association between higher SICI scores and lower resting connectivity between motor cortex and frontal regions in schizophrenia (Du et al., 2019). This study extends the knowledge by showing that local connectivity strength from a distant brain region – the right insula – affects SICI, further supporting the proposition that regions outside the traditionally studied corticospinal pathway may contribute to motor inhibitory deficits.
Higher SICI scores (indicating reduced inhibition) were associated with lower ReHo in the right insula in patients. While the insula is not considered part of the immediate motor pathways, there is evidence that it contributes to motor functions, as the motor cortex is directly connected to insular and perisylvian regions (including SII) by white matter association fiber pathways (Schmahmann and Pandya, 2006). In human subjects, insular cortex activation is demonstrated in studies of volitional movement (Fink et al., 1997), and TMS-induced movement (via motor cortex stimulation) (Bohning et al., 1999, Jung et al., 2020). Direct electrical stimulation of the monkey insular cortex – specifically the upper bank of the Sylvian fissure near area SII – resulted in motor inhibition of limb movement (Jezzini et al., 2012). Thus, it has been argued that standard cortical-basal ganglia circuit models of motor dysfunction might need to be expanded to include the insula as a major hub (Tinaz et al., 2018).
The significant association observed between right insula ReHo and SICI may be consistent with this expanded model, and this finding prompted a follow-up rsFC analysis selecting right insula as a seed. The purpose of this analysis was not to generate and report novel findings (as our group had previously performed a whole-brain rsFC analysis using motor cortex as a seed which identified the right insula as a hub of interest), but rather to lend ancillary support favoring (or refuting) our hypothesis that SICI score would be associated with right insula connectivity with hubs of the corticospinal pathway. Our findings were consistent with this hypothesis as higher SICI scores (indicating reduced inhibition) were associated with lower rsFC between right insula and both the left sensorimotor cortex and left basal ganglia. Dysfunction in the basal ganglia – particularly dopaminergic tone and signaling in the striatum – is often proposed as a primary site of brain dysfunction in schizophrenia (Perez-Costas et al., 2010, McCutcheon et al., 2019), and abnormal basal ganglia signaling with frontal and cerebellar regions may be related to altered motor inhibition in schizophrenia (Picazio and Koch, 2015). Patients with dystonia secondary to striatal brain lesions were shown to have higher SICI scores (indicating reduced inhibition) on both the affected and unaffected side, demonstrating the relevance of basal ganglia signaling for SICI (Trompetto et al., 2012). Together, these findings suggest that the basal ganglia can be involved in abnormal SICI motor control in certain disease conditions. Our findings highlight that abnormal signaling between basal ganglia and anterior insula may be relevant to the development of reduced motor intracortical inhibition in schizophrenia.
Interhemispheric connectivity between the right insula and left sensorimotor cortex significantly mediated the observed association between the insula ReHo and SICI. This finding may indicate that basic mechanisms of SICI and reduced intracortical inhibition in SSD may involve local connectivity strength from a distant brain region, the right insula, which may indirectly affect SICI through its long-distance resting functional connections with contralateral hubs of the motor corticospinal pathway. As shown in Fig. 2, local connectivity strength of right insula was positively correlated with long-distance rsFC with two corticospinal pathway locations in schizophrenia patients.
Results of pharmacological challenge studies suggest that SICI is predominately mediated by the function of the brain’s main inhibitory neurotransmitter, gamma aminobutyric acid (GABA) (Ziemann et al., 1996, Di Lazzaro et al., 2000). Dysfunction of GABAergic signaling has been consistently reported in postmortem studies of schizophrenia (Lewis et al., 1999, Lewis et al., 2012, Gonzalez-Burgos and Lewis, 2012), and also in EEG studies of schizophrenia that report abnormal gamma oscillations (an index of GABAergic signaling) (Gonzalez-Burgos and Lewis, 2012, McNally and McCarley, 2016). Our preliminary results lay out functional circuits that may be related to altered excitatory/inhibitory balance in schizophrenia indexed by SICI, but future research is needed to determine whether this functional circuitry can be replicated in other clinical datasets and how this circuitry relates to behavioral signs and symptoms including cognitive function and cognitive deficits, which rely heavily on appropriate excitatory/inhibitory balance (Gonzalez-Burgos and Lewis, 2012, John et al., 2018, Lesh et al., 2011, Murray et al., 2014).
Contrary to our expectation, motor threshold was significantly associated with ReHo in insula and both middle frontal regions. We anticipated that motor threshold would not be related to ReHo measures since this index of cortical excitability was not different between schizophrenia patient vs. control groups (see Table 1), and a prior study of schizophrenia reported that skull-to-cortex distance and the trajectory of diffusion direction of the corticospinal tract were both related to motor threshold in patients and controls, but schizophrenia diagnosis was not (Herbsman et al., 2009). Our results may indicate that functional brain communication (and perhaps integrity of the corticospinal tract) may be altered in the SSD patients and, thus, functional brain connectivity may have a larger than expected influence on motor threshold. This claim is largely speculative since our focus was not to analyze SSD case vs. control differences in connectivity, although we did observe that SSD patients had elevated ReHo in the motor cortex at rest, which may impact function of motor cortex during performance of tasks and/or TMS-evoked potentials.
The combined use of ReHo + rsFC is a novel approach. We used this combination of methods to test multiple mediation models, and our findings support a model in which the direct effect of local insular connectivity strength on SICI is mediated by the interhemispheric connectivity between insula and left sensorimotor cortex. The resting-state signal is complex: due to neurovascular coupling, resting fMRI fluctuations are linked to neuronal activity (Logothetis et al., 2001, Magri et al., 2012) , but other non-neuronal sources contribute (Nasrallah et al., 2015, Lu et al., 2019). Given this limitation and the complexity of the mechanisms underlying local and long-distance rsFC, the broader clinical implications of our findings remain unclear. We adopted a robust fMRI data preprocessing pipeline to control for confounding effects of motion on resting fMRI signal, in addition to removal of time courses from the white matter and cerebrospinal fluid as regressors of no interest. A prior research study showed that a regression model controlling for non-neural vascular activity related to breath holding resulted in significant reductions in the resting amplitudes of low frequency fluctuations (ALFF) in certain brain areas such as the anterior insula, which sits near the middle cerebral artery (Di et al., 1991. 2013). Keeping these limitations in mind, it is possible that these ReHo and rsFC measures may index an underlying mechanism of motor inhibition dysfunction that is partially determined by local connectivity of right insula and its long-distance connectivity to the sensorimotor target. Further evidence favoring involvement of the insula with SICI (versus an accidental finding related to vascular distortion effects) comes from the fact that the strongest associations observed between SICI and insula connectivity in a whole-brain voxelwise analysis was with the left motor cortex and left basal ganglia (two key regions in the corticospinal pathway) (Fig. 2), and the results of our regression validation method suggested the association between right insula ReHo and SICI was reliable. Thus, intervention attempts designed to improve local and/or long-distance connectivity strength with the right insula may strengthen inhibition in SSD. SICI is important as it was associated with other inhibitory tasks such as Go-No-Go and Stop-Signal task performance in healthy subjects (He et al., 2019), and processing speed performance in SSD (Du et al., 2017).
Additional limitations of this study including cross-sectional design limited to a single SICI measure and rsfMRI scan, in addition to a moderate sample size (N = 23 schizophrenia patients for correlation and regression analyses). Only four patients in our sample were not taking anti-psychotic medication at the time of the study, limiting our ability to conduct meaningful analyses in medication-naïve subgroup(s).
In conclusion, we used a combination of methods (ReHo and rsFC) to further our mechanistic understanding of the well-replicated phenomenon of reduced inhibition indexed by SICI in individuals with SSD. The right insula – a region that is not traditionally studied in the context of motor inhibitory control – may be a key region contributing to abnormal intracortical inhibition in SSD. Based on our results, we theorize that local connectivity within this region may indirectly affect SICI through its long-distance resting functional connections with contralateral hubs of the motor corticospinal pathway. Future research is needed to replicate the current pattern of findings in an independent sample and address important remaining questions pertinent to improving our understanding of motor inhibitory control and impact of abnormal signaling in motor-inhibitory pathways in schizophrenia and other clinical samples.
CRediT authorship contribution statement
Stephanie M. Hare: Conceptualization, Formal analysis, Visualization, Writing - original draft. Xiaoming Du: Conceptualization, Formal analysis, Investigation, Writing - review & editing. Bhim M. Adhikari: Formal analysis, Writing - review & editing. Shuo Chen: Formal analysis, Methodology, Resources, Writing - review & editing. Chen Mo: Formal analysis, Methodology, Resources, Writing - review & editing. Ann Summerfelt: Data curation, Project administration, Writing - review & editing. Mark D. Kvarta: Writing - review & editing. Laura Garcia: Project administration, Writing - review & editing. Peter Kochunov: Funding acquisition, Methodology, Resources, Writing - review & editing. L. Elliot Hong: conceptualization, Funding acquisition, Investigation, Resources, Supervision, Writing - review & editing.
Acknowledgments
Acknowledgments
Support was received from the National Institutes of Health grants: R01MH111671, R01MH112180, R01MH116948, R01EB01561, R01DC014085, R01MH121246-01, U01MH108148, UG3DA047685, P50MH103222, T32MH067533, 5T32MH073526, S10OD023696, and a State of Maryland contract (M00B6400091), and a University of Maryland Seed Grant (14-103).
Disclosures: Dr. Hong has received or is planning to receive research funding or consulting fees from Mitsubishi, Your Energy Systems LLC, Neuralstem, Taisho, Heptares, Pfizer, Luye Pharma, Sound Pharma, Takeda, and Regeneron. All other authors declare no financial interests that could represent a conflict of interest.
References
- Aron A.R., Poldrack R.A. The cognitive neuroscience of response inhibition: relevance for genetic research in attention-deficit/hyperactivity disorder. Biol. Psychiatry. 2005;57(11):1285–1292. doi: 10.1016/j.biopsych.2004.10.026. [DOI] [PubMed] [Google Scholar]
- Bohning DE, Shastri A, McConnell KA, et al. A combined TMS/fMRI study of intensity-dependent TMS over motor cortex. Biol Psychiatry. 1999;45(4):385-394. doi:10.1016/s0006-3223(98)00368-0. [DOI] [PubMed]
- Bunse T., Wobrock T., Strube W., Padberg F., Palm U., Falkai P., Hasan A. Motor cortical excitability assessed by transcranial magnetic stimulation in psychiatric disorders: a systematic review. Brain Stimulat. 2014;7(2):158–169. doi: 10.1016/j.brs.2013.08.009. [DOI] [PubMed] [Google Scholar]
- Cole D.M., Smith S.M., Beckmann C.F. Advances and pitfalls in the analysis and interpretation of resting-state FMRI data. Front Syst. Neurosci. 2010;4:8. doi: 10.3389/fnsys.2010.00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox R.W. AFNI: software for analysis and visualization of functional magnetic resonance neuroimages. Comput. Biomed. Res. Int. J. 1996;29(3):162–173. doi: 10.1006/cbmr.1996.0014. [DOI] [PubMed] [Google Scholar]
- Dayan E., López-Alonso V., Liew S.-L., Cohen L.G. Distributed cortical structural properties contribute to motor cortical excitability and inhibition. Brain Struct. Funct. 2018;223(8):3801–3812. doi: 10.1007/s00429-018-1722-1. [DOI] [PubMed] [Google Scholar]
- Di X., Kannurpatti S.S., Rypma B., Biswal B.B. Calibrating BOLD fMRI activations with neurovascular and anatomical constraints. Cereb. Cortex N Y N 1991. 2013;23(2):255–263. doi: 10.1093/cercor/bhs001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Lazzaro V., Insola A., Mazzone P., Tonali P., Rothwell J.C., Profice P., Oliviero A. Direct demonstration of interhemispheric inhibition of the human motor cortex produced by transcranial magnetic stimulation. Exp. Brain Res. 1999;124(4):520–524. doi: 10.1007/s002210050648. [DOI] [PubMed] [Google Scholar]
- Di Lazzaro V., Oliviero A., Meglio M., Cioni B., Tamburrini G., Tonali P., Rothwell J.C. Direct demonstration of the effect of lorazepam on the excitability of the human motor cortex. Clin. Neurophysiol Off. J. Int. Fed. Clin. Neurophysiol. 2000;111(5):794–799. doi: 10.1016/S1388-2457(99)00314-4. [DOI] [PubMed] [Google Scholar]
- Du X., Summerfelt A., Chiappelli J., Holcomb H.H., Hong L.E. Individualized brain inhibition and excitation profile in response to paired pulse TMS. J. Mot. Behav. 2014;46(1):39–48. doi: 10.1080/00222895.2013.850401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du X., Choa F.-S., Summerfelt A., Tagamets M.A., Rowland L.M., Kochunov P., Shepard P., Hong L.E. Neural summation in human motor cortex by subthreshold transcranial magnetic stimulations. Exp. Brain Res. 2015;233(2):671–677. doi: 10.1007/s00221-014-4146-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du X., Hong L.E. Test-retest reliability of short-interval intracortical inhibition and intracortical facilitation in patients with schizophrenia. Psychiatry Res. 2018;267:575–581. doi: 10.1016/j.psychres.2018.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du X., Kochunov P., Summerfelt A., Chiappelli J., Choa F.-S., Hong L.E. The role of white matter microstructure in inhibitory deficits in patients with schizophrenia. Brain. Stimulat. 2017;10(2):283–290. doi: 10.1016/j.brs.2016.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du X., Choa F.-S., Chiappelli J., Wisner K.M., Wittenberg G., Adhikari B., Bruce H., Rowland L.M., Kochunov P., Hong L.E. Aberrant middle prefrontal-motor cortex connectivity mediates motor inhibitory biomarker in schizophrenia. Biol. Psychiatry. 2019;85(1):49–59. doi: 10.1016/j.biopsych.2018.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eagle D.M., Bari A., Robbins T.W. The neuropsychopharmacology of action inhibition: Cross-species translation of the stop-signal and go/no-go tasks. Psychopharmacology (Berl). 2008;199(3):439–456. doi: 10.1007/s00213-008-1127-6. [DOI] [PubMed] [Google Scholar]
- Ettinger U., Aichert D.S., Wöstmann N., Dehning S., Riedel M., Kumari V. Response inhibition and interference control: Effects of schizophrenia, genetic risk, and schizotypy. J. Neuropsychol. 2018;12(3):484–510. doi: 10.1111/jnp.12126. [DOI] [PubMed] [Google Scholar]
- Ferbert A., Priori A., Rothwell J.C., Day B.L., Colebatch J.G., Marsden C.D. Interhemispheric inhibition of the human motor cortex. J. Physiol. 1992;453:525–546. doi: 10.1113/jphysiol.1992.sp019243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fink G.R., Frackowiak R.S., Pietrzyk U., Passingham R.E. Multiple nonprimary motor areas in the human cortex. J. Neurophysiol. 1997;77(4):2164–2174. doi: 10.1152/jn.1997.77.4.2164. [DOI] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon MG, Williams JBW. Structured Clinical Interview for DSM-IV-TR Axis I Disorders — Patient Edition (SCID-I/P, 11/2002 revision). Published online 2002.
- Gonzalez-Burgos G., Lewis D.A. NMDA receptor hypofunction, parvalbumin-positive neurons, and cortical gamma oscillations in schizophrenia. Schizophr. Bull. 2012;38(5):950–957. doi: 10.1093/schbul/sbs010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrell F.E. Springer; 2015. Regression Modeling Strategies: With Applications to Linear Models, Logistic and Ordinal Regression, and Survival Analysis. [Google Scholar]
- He J.L., Fuelscher I., Coxon J., Chowdhury N., Teo W.-P., Barhoun P., Enticott P., Hyde C. Individual differences in intracortical inhibition predict motor-inhibitory performance. Exp. Brain Res. 2019;237(10):2715–2727. doi: 10.1007/s00221-019-05622-y. [DOI] [PubMed] [Google Scholar]
- Herbsman T., Forster L., Molnar C., Dougherty R., Christie D., Koola J., Ramsey D., Morgan P.S., Bohning D.E., George M.S., Nahas Z. Motor threshold in transcranial magnetic stimulation: The impact of white matter fiber orientation and skull-to-cortex distance. Hum. Brain Mapp. 2009;30(7):2044–2055. doi: 10.1002/hbm.v30:710.1002/hbm.20649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jezzini A., Caruana F., Stoianov I., Gallese V., Rizzolatti G. Functional organization of the insula and inner perisylvian regions. Proc. Natl. Acad. Sci. U S A. 2012;109(25):10077–10082. doi: 10.1073/pnas.1200143109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang L., Zuo X.-N. Regional homogeneity. Neuroscientist. 2016;22(5):486–505. doi: 10.1177/1073858415595004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- John Y.J., Zikopoulos B., Bullock D., Barbas H. Visual attention deficits in schizophrenia can arise from inhibitory dysfunction in thalamus or cortex. Comput. Psychiatry Camb. Mass. 2018;2:223–257. doi: 10.1162/cpsy_a_00023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung J., Bungert A., Bowtell R., Jackson S.R. Modulating brain networks with transcranial magnetic stimulation over the primary motor cortex: A concurrent TMS/fMRI study. Front. Hum. Neurosci. 2020;14 doi: 10.3389/fnhum.2020.00031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- T. Kujirai M.D. Caramia J.C. Rothwell B.L. Day P.D. Thompson A. Ferbert S. Wroe P. Asselman C.D. Marsden Corticocortical inhibition in human motor cortex 471 1 1993 501 519 10.1113/jphysiol.1993.sp019912. [DOI] [PMC free article] [PubMed]
- Lesh T.A., Niendam T.A., Minzenberg M.J., Carter C.S. Cognitive control deficits in schizophrenia: Mechanisms and meaning. Neuropsychopharmacol Off Publ Am Coll Neuropsychopharmacol. 2011;36(1):316–338. doi: 10.1038/npp.2010.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis D.A., Pierri J.N., Volk D.W., Melchitzky D.S., Woo T.U. Altered GABA neurotransmission and prefrontal cortical dysfunction in schizophrenia. Biol. Psychiatry. 1999;46(5):616–626. doi: 10.1016/s0006-3223(99)00061-x. [DOI] [PubMed] [Google Scholar]
- Lewis D.A., Curley A.A., Glausier J.R., Volk D.W. Cortical parvalbumin interneurons and cognitive dysfunction in schizophrenia. Trends Neurosci. 2012;35(1):57–67. doi: 10.1016/j.tins.2011.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li C.R., Yan P., Sinha R., Lee T.-W. Sub-cortical processes of motor response inhibition during a stop signal task. NeuroImage. 2008;41(4):1352–1363. doi: 10.1016/j.neuroimage.2008.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindberg P.G., Térémetz M., Charron S., Kebir O., Saby A., Bendjemaa N., Lion S., Crépon B., Gaillard R., Oppenheim C., Krebs M.-O., Amado I. Altered cortical processing of motor inhibition in schizophrenia. Cortex J. Devoted Study Nerv. Syst. Behav. 2016;85:1–12. doi: 10.1016/j.cortex.2016.09.019. [DOI] [PubMed] [Google Scholar]
- Logothetis N.K., Pauls J., Augath M., Trinath T., Oeltermann A. Neurophysiological investigation of the basis of the fMRI signal. Nature. 2001;412(6843):150–157. doi: 10.1038/35084005. [DOI] [PubMed] [Google Scholar]
- Lu H., Jaime S., Yang Y. Origins of the resting-state functional MRI signal: Potential limitations of the “Neurocentric” model. Front. Neurosci. 2019;13 doi: 10.3389/fnins.2019.01136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magri C., Schridde U., Murayama Y., Panzeri S., Logothetis N.K. The amplitude and timing of the BOLD signal reflects the relationship between local field potential power at different frequencies. J. Neurosci. 2012;32(4):1395–1407. doi: 10.1523/JNEUROSCI.3985-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCutcheon R.A., Abi-Dargham A., Howes O.D. Schizophrenia, dopamine and the striatum: From biology to symptoms. Trends Neurosci. 2019;42(3):205–220. doi: 10.1016/j.tins.2018.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNally J.M., McCarley R.W. Gamma band oscillations: A key to understanding schizophrenia symptoms and neural circuit abnormalities. Curr. Opin. Psychiatry. 2016;29(3):202–210. doi: 10.1097/YCO.0000000000000244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray J.D., Anticevic A., Gancsos M., Ichinose M., Corlett P.R., Krystal J.H., Wang X.-J. Linking microcircuit dysfunction to cognitive impairment: Effects of disinhibition associated with schizophrenia in a cortical working memory model. Cereb. Cortex. 2014;24(4):859–872. doi: 10.1093/cercor/bhs370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nasrallah F.A., Yeow L.Y., Biswal B., Chuang K.-H. Dependence of BOLD signal fluctuation on arterial blood CO2 and O2: Implication for resting-state functional connectivity. NeuroImage. 2015;117:29–39. doi: 10.1016/j.neuroimage.2015.05.035. [DOI] [PubMed] [Google Scholar]
- Noda Y., Cash R.F.H., Zomorrodi R., Dominguez L.G., Farzan F., Rajji T.K., Barr M.S., Chen R., Daskalakis Z.J., Blumberger D.M. A combined TMS-EEG study of short-latency afferent inhibition in the motor and dorsolateral prefrontal cortex. J. Neurophysiol. 2016;116(3):938–948. doi: 10.1152/jn.00260.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noda Y., Barr M.S., Zomorrodi R., Cash R.F.H., Farzan F., Rajji T.K., Chen R., Daskalakis Z.J., Blumberger D.M. Evaluation of short interval cortical inhibition and intracortical facilitation from the dorsolateral prefrontal cortex in patients with schizophrenia. Sci. Rep. 2017;7(1) doi: 10.1038/s41598-017-17052-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez-Costas E., Melendez-Ferro M., Roberts R.C. Basal ganglia pathology in schizophrenia: dopamine connections and anomalies. J. Neurochem. 2010;113(2):287–302. doi: 10.1111/j.1471-4159.2010.06604.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Picazio S., Koch G. Is motor inhibition mediated by cerebello-cortical interactions? Cerebellum Lond. Engl. 2015;14(1):47–49. doi: 10.1007/s12311-014-0609-9. [DOI] [PubMed] [Google Scholar]
- Radhu N., de Jesus D.R., Ravindran L.N., Zanjani A., Fitzgerald P.B., Daskalakis Z.J. A meta-analysis of cortical inhibition and excitability using transcranial magnetic stimulation in psychiatric disorders. Clin. Neurophysiol. Off J. Int. Fed. Clin. Neurophysiol. 2013;124(7):1309–1320. doi: 10.1016/j.clinph.2013.01.014. [DOI] [PubMed] [Google Scholar]
- Rogasch N.C., Daskalakis Z.J., Fitzgerald P.B. Cortical inhibition, excitation, and connectivity in schizophrenia: a review of insights from transcranial magnetic stimulation. Schizophr. Bull. 2014;40(3):685–696. doi: 10.1093/schbul/sbt078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmahmann J., Pandya D. Oxford University Press; 2006. Fiber Pathways of the Brain. [Google Scholar]
- Tinaz S., Para K., Vives-Rodriguez A., Martinez-Kaigi V., Nalamada K., Sezgin M., Scheinost D., Hampson M., Louis E.D., Constable R.T. Insula as the interface between body awareness and movement: A neurofeedback-guided kinesthetic motor imagery study in parkinson’s disease. Front Hum. Neurosci. 2018;12 doi: 10.3389/fnhum.2018.00496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trompetto C., Avanzino L., Marinelli L., Mori L., Pelosin E., Roccatagliata L., Abbruzzese G. Corticospinal excitability in patients with secondary dystonia due to focal lesions of the basal ganglia and thalamus. Clin. Neurophysiol. Off J. Int. Fed. Clin. Neurophysiol. 2012;123(4):808–814. doi: 10.1016/j.clinph.2011.06.033. [DOI] [PubMed] [Google Scholar]
- Wessel J.R., Aron A.R. On the globality of motor suppression: Unexpected events and their influence on behavior and cognition. Neuron. 2017;93(2):259–280. doi: 10.1016/j.neuron.2016.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiecki T.V., Frank M.J. A computational model of inhibitory control in frontal cortex and basal ganglia. Psychol. Rev. 2013;120(2):329–355. doi: 10.1037/a0031542. [DOI] [PubMed] [Google Scholar]
- Wobrock T., Schneider M., Kadovic D., Schneider-Axmann T., Ecker U.K.H., Retz W., Rösler M., Falkai P. Reduced cortical inhibition in first-episode schizophrenia. Schizophr. Res. 2008;105(1-3):252–261. doi: 10.1016/j.schres.2008.06.001. [DOI] [PubMed] [Google Scholar]
- Wobrock T., Schneider-Axmann T., Retz W., Rösler M., Kadovic D., Falkai P., Schneider M. Motor circuit abnormalities in first-episode schizophrenia assessed with transcranial magnetic stimulation. Pharmacopsychiatry. 2009;42(05):194–201. doi: 10.1055/s-0029-1224137. [DOI] [PubMed] [Google Scholar]
- Woods S.W. Chlorpromazine equivalent doses for the newer atypical antipsychotics. J. Clin. Psychiatry. 2003;64(6):663–667. doi: 10.4088/jcp.v64n0607. [DOI] [PubMed] [Google Scholar]
- Zang Y., Jiang T., Lu Y., He Y., Tian L. Regional homogeneity approach to fMRI data analysis. NeuroImage. 2004;22(1):394–400. doi: 10.1016/j.neuroimage.2003.12.030. [DOI] [PubMed] [Google Scholar]
- Ziemann U., Lönnecker S., Steinhoff B.J., Paulus W. The effect of lorazepam on the motor cortical excitability in man. Exp. Brain Res. 1996;109(1):127–135. doi: 10.1007/BF00228633. [DOI] [PubMed] [Google Scholar]