Abstract
Non-tuberculous mycobacteria (NTM) is a collective name given to a group of more than 190 species of Mycobacterium. The clinical presentation for most NTM infections is non-specific, often resulting in delayed diagnosis. Further complicating matters is that NTM organisms can be difficult to isolate. Medications used to treat NTM infection can be difficult for patients to tolerate, and prolonged courses of anti-mycobacterial therapy are often required for adequate suppression or eradication. Herein, we review different NTM syndromes, appropriate diagnostic tests, and treatment regimens.
Abbreviations: ADR, adverse drug reactions; AFB, acid fast bacilli; AST, antimicrobial-susceptibility testing; ATS, American Thoracic Society; BCG, Bacille Calmette-Guerin; CLSI, Clinical and Laboratory Standards Institute; COPD, chronic obstructive pulmonary disease; ECG, electrocardiogram; EMB, ethambutol; Erm, erythromycin ribosomal methylase; FDA, Food and Drug Administration; HIV, human immunodeficiency virus; HRCT, high resolution computed tomography; IDSA, Infectious Disease Society of America; INF-γ, interferon- γ; INH, isoniazid; MAC, Mycobacterium avium complex; MIC, minimum inhibitory concentrations; MALDI-TOF, matrix-assisted laser desorption ionization time-of-flight mass spectrometry; MGIT, mycobacteria growth indicator tube; NTM, non-tuberculous mycobacteria; PCR, polymerase chain reaction; PFT, pulmonary function test; TB, tuberculosis; TDM, therapeutic drug monitoring
Keywords: Non-tuberculous mycobacteria, Mycobacterium avium, Mycobacterium abscessus
1. Introduction
Non-tuberculous mycobacteria (NTM) is a collective name given to a group of more than 190 species of Mycobacterium other than Mycobacterium tuberculosis and Mycobacterium leprae [1]. These organisms are ubiquitous in the environment, in particular in soil and water sources, and are increasingly recognized as a cause of human disease. Non-tuberculous mycobacteria cause infection not only in immunocompromised populations, but also in otherwise healthy individuals. A substantial number of challenges are associated with the diagnosis and management of NTM infections.
The clinical presentation for most NTM infections is non-specific, often resulting in delayed diagnosis. Once NTM is suspected, growing NTM from biological specimens for identification can be difficult secondary to variable growth time and requirements. Furthermore the actual identification of the organism often requires molecular methods that may not be widely available. Identification of NTM from biological specimens is not equivalent to a diagnosis of NTM infection; the ubiquitous presence of NTM in the environment creates the dilemma of distinguishing between colonization and true infection.
The treatment of NTM infections can also be challenging. Antimicrobial-susceptibility testing (AST) is not available for all NTM species and drugs of interest. Where it is available, it is not always reliable and the clinical applicability is uncertain. Optimal dosing of recognized anti-mycobacterial drugs is unknown, and guidelines for the optimal number of drugs and treatment duration are largely opinion-based. Furthermore, current guidelines for NTM infections lump NTM into a small number of categories. Thus differences among NTM species factors such as virulence, relevant host factors, resistance profile, and response to treatment are inadequately addressed. Herein, we present our approach to the diagnosis and treatment of NTM infections. We do not intend for this paper to serve as a detailed guidance for the management of individual NTM infections or species, but rather as a description of a higher level overall approach to the recognition, diagnosis, and management of these infections.
2. When should one suspect NTM infections?
The clinical manifestations of NTM infections are typically non-specific. In general, NTM infection is suspected in an individual with persistent symptoms (for example fever, respiratory symptoms, skin lesions) that do not respond to traditional anti-bacterial therapy. Alternatively, an understanding of common NTM infection syndromes and the context/clinical scenario in which they occur, can heighten the suspicion for the presence of an NTM infection. Pulmonary NTM infection continues to be the most common clinical presentation [2], but there are several other distinct clinical syndromes described with NTM. These are classified by organ system of involvement and summarized in Table 1. Additionally, several NTM species have unique clinical syndromes associated with them. These should be considered in appropriate clinical scenarios and are summarized in Table 2.
Table 1.
Common non-tuberculous mycobacterial infection syndromes.
| Clinical Syndrome | Most Common Species | Other Species | Risk Factors/ Associations |
|---|---|---|---|
| Pulmonary44, [110] | MAC M. kansasii |
M. abscessus M. fortuitum M. szulgai M. xenopi M. celatum M. asiaticum M. shimoidei |
α1-Antitrypsin deficiency Ciliary dyskinesia Cystic fibrosis GERD Bronchiectasis Pulmonary histoplasmosis Low BMI with scoliosis, pectus excavatum, mitral valve prolapse Smoking or heavy alcohol use (M. malmoense) Lymphopenia |
| M. malmoense (Scandinavian Peninsula; Northern Europe) | |||
| Cervical Lymphadenitis3, [4], [111] | MAC M. scrofulaceum |
M. malmoense | Children; 1–5 yrs Men with lung cancer |
| Skin, soft tissue infection |
M. ulcerans M. kansasii |
M. szulgai MAC |
Bathing in water from bore holes insect bites Possible association with pedicures |
| Tenosynovitis |
M. marinum* MAC M. abscessus |
M. terrae M. szulgai M. malmoense M. xenopi |
*Exposure to fish tanks or other contaminated marine water sources |
| Bone disease [112], [113] |
M. kansasii M. fortuitum |
MAC M. xenopi M. chelonae M. abscessus |
May occur via direct trauma or hematogenous seeding |
| Disseminated disease [114], [115] FUO BSI Abscesses Peritonitis |
MAC |
M. kansasii M. chelonae M. abscessus M. haemophilum M. scrofulaceum M. malmoense |
Severe Immunosuppression: HIV infection/AIDS (CD4 count <50 cells) SOT or stem cell transplant long-term, high dose steroids |
Abbreviations: BMI – Body Mass Index, BSI – Blood Stream Infection, FUO – Fever of Unknown Origin, HIV – Human Immunodeficiency Virus, MAC – Mycobacterium Avium Complex, SOT – Solid Organ Transplant.
Table 2.
Clinical syndromes associated with non-tuberculous mycobacteria.
| Clinical Syndrome | Common Species | Features | Risk Factors/Associations |
|---|---|---|---|
| Prosthetic valve endocarditis [116], [117] | M. chimaera | Presents months to years after index surgery | Cardiac surgery or LVAD |
| Buruli Ulcer (cutaneous/subcutaneous granulomatous lesion) | M. ulcerans | Areas of body with skin temperatures <30 °C | Common in Central Africa, Asia, South America |
| Fish tank or swimming pool granuloma (Fish Fancier’s Finger) | M. marinum | Preferential growth in cooler temperatures (27–32 °C) | Exposure to fish tanks or other contaminated marine water sources from direct inoculation |
| Lady Windermere Syndrome [44] (fibronodular pulmonary disease) | MAC M. fortuitum M. chelonae M. abscessus |
“Tree-in-bud” pattern preferentially affecting RML or lingula on CT chest | Women over 50 years; Non-smokers |
| Hot tub lung [35] (Hypersensitivity lung disease) | MAC M. fortuitum M. immunogenum |
CT chest mimics hypersensitivity pneumonitis | Indoor hot tub use |
| CLABSI [118] | M. mucogenicum | Long term central catheters; contaminated water source | |
| Postoperative wound infection [119] |
M. fortuitum M. chelonae M. abscessus |
Cosmetic surgery | |
| Peritoneal dialysis associated infection [120] |
M. fortuitum M. chelonae M. abscessus |
Abbreviations: CLABSI – Central Line Associated Blood Stream Infections, CT – Computed Tomography, LVAD – Left Ventricular Assist Device, MAC – Mycobacterium Avium Complex, RML – Right Middle Lobe.
3. Diagnosing non-tuberculous mycobacteria infections
There are no pathognomonic tests for diagnosis of NTM infections. Diagnosis requires correlation of the clinical syndrome with radiographic findings, and ultimately microbiologic confirmation of the etiologic agent. Specimen collection and testing may need to be repeated on several occasions to confirm or exclude a diagnosis of NTM infection. In view of the complexities involved, tests should be interpreted in consultation with specialists experienced in management of NTM infections, especially if the microbiologic results are not congruent with the clinical picture.
3.1. Microbiologic diagnosis
NTM are ubiquitous in the environment and are commonly found in soil and water, including municipal and household water supply systems. Therefore, caution must be exercised to avoid environmental contamination of biologic specimens to prevent false positive results. Specimens can be obtained from nearly any tissue or organ suspected to be infected.
The guidelines provided by American Thoracic Society (ATS) and Infectious Disease Society of America (IDSA) for diagnosis of pulmonary NTM infection require positive sputum culture results on at least two separate occasions [3], [4]. Alternatively, a single positive culture from a lower respiratory specimen such as bronchial lavage or washing is considered adequate for diagnosis. If clinical suspicion for pulmonary NTM infection is high and initial tests remain non-diagnostic, then additional sputum cultures should be obtained [3], [4]. Induction of sputum or bronchoscopy may be necessary in patients unable to expectorate sputum. The optimal interval between sample collections is unknown. Separating specimen collection by at least a week may be a reasonable timeframe to consider as the interval between sample collections, such that transient environmental contamination does not confuse the diagnosis [5]. Lung biopsy is rarely described as necessary for diagnosis, but may provide additional information beyond positive stains and cultures, demonstrating histopathology findings of mycobacterial infections, such as granuloma formation.
In non-pulmonary disease, source of culture is determined by site of involvement. For example, lymphadenitis may require excisional biopsy or fine needle aspiration for microscopy and mycobacterial culture. When NTM disease is disseminated, mycobacterial blood cultures may prove diagnostic. In the presence of cytopenias, bone marrow or liver biopsy may be useful for diagnosis [6]. It is important to note that skin infections are best evaluated with biopsy rather than superficial swabs since swabs may represent environmental exposure rather than infection [6].
When clinical suspicion exists, the requisite biologic samples should be obtained and sent to the microbiology laboratory, with notification of the suspicion for NTM infection, so that the laboratory personnel process these specimens appropriately. Different staining and culture techniques may be required to maximize yield. If NTM species with special culture requirements are suspected, such as growth at lower temperatures for skin related pathogens or nutritional supplementation of media, these should be informed to the laboratory personnel as well. Time to growth and colony counts should be recorded as well.
Some clinical specimens, such as sputum, have a high potential for contamination or bacterial overgrowth. When processing these specimens, concentration and decontamination techniques are applied to improve the microbiologic yield [3], [4]. Decontamination with N-acetyl-l-cysteine-NaOH-oxalic acid (NALC-NaOH-OxA) is commonly utilized, though some reports have expressed concern that it may affect the viability of mycobacteria [7], [8]. Another decontamination method that does not affect mycobacterial viability uses chlorhexidine [9]. Both of these methods are approved by the Clinical Laboratory Standards Institute (CLSI).
There are no established recommendations for staining of NTM; most recommendations were derived from staining methods established for M. tuberculosis. The cell walls of NTM, similar to other mycobacteria, have high lipid content and are designated as acid-fast bacilli (AFB) because of a unique ability to bind a carbol-fuchsin dye that cannot be decolorized by acid-alcohol. Several staining methods have been used for staining AFB (Table 3). It is not possible to distinguish tuberculous and non-tuberculous Mycobacterium species on the basis of staining alone. Sensitivity of staining likely depends on numerous factors, including burden of pathogen and tissue processing methods. Processing of sputum with bleach and concentrating the specimen before staining has been shown to increase sensitivity of detection of AFB in some studies [10], [11]. However, no data is available to accurately quantify minimum NTM pathogen burden to yield a positive AFB sputum smear.
Table 3.
Commonly used stains for detection of acid fast bacilli.
| Stain | Specimen type | Appearance | Other Findings | Mycobacteria |
|---|---|---|---|---|
| Ziehl-Neelson [121] | Any clinical specimen | Bright red with blue background | All | |
| Modified Kinyoun [121] | Cytospin or solid culture media | Bright red | Weakly acid fast organisms do not appear bright red |
M. fortuitum M. chelonae M. abscessus |
| All organisms appear identical with variable staining | MAC | |||
| Filament-like or with slight branching | Dry colonies of MAC | |||
| Rapid Modified Auramine O Fluorescent [122] | Any clinical specimen | Yellow to orange or bright green with dark background | Some rapid growing mycobacteria may not fluoresce well | All |
| Fite [123] | Preferred for tissue specimens | Pink with blue background | Preferred stain for M. leprae | |
| Gomori-methenamine Silver Stain [124] | AFB in tissue specimens | Brown to black |
M. kansasii MAC |
|
| Periodic Acid Schiff Stain [124] | AFB in tissue specimens | Pink |
M. ulcerans M. chelonei M. kansasii MAC from skin |
*Reduced with formalin.
Abbreviations: AFB – Acid Fast Bacilli, MAC – Mycobacterium Avium Complex.
All clinical specimens should be cultured and then further testing modalities applied to identify species. Establishing the diagnosis of NTM infection to the species level needs to be pursued as treatment varies depending on species. Mycobacteria are fastidious organisms, often requiring special growth media and prolonged incubation in a carbon dioxide rich atmosphere. Specimens should be inoculated in liquid (Mycobacteria Growth Indicator Tube (MGIT)) and/or solid media (Middlebrook 7H10 or 7H11 agar or Lowenstein-Jensen agar (L-J)) and incubated for a variable period of time to allow isolation of these organisms. Several organisms need special culture conditions to improve yield. Time to growth is usually earlier in liquid media than solid media.
NTM are commonly classified into rapid and slow growers based on time to growth of mature colonies on solid culture media [12]. Rapid growing NTM grow in less than seven days; slow growing NTM take more than seven days to grow. The characteristics of rapid (RGM) and slow-growing NTM species are summarized in Table 4.
Table 4.
Classification of non-tuberculous mycobacteria based on time to growth.
| Classification | Time to Growth | Major Groups | Common Species | Growth Requirements | Common Clinical Syndromes |
|---|---|---|---|---|---|
| Rapidly Growing | <7 days | M. fortuitum[125] |
M. fortuitum M. peregrinum M. senegalense M. setense M. porcinum M. houstonense M. boenickei M. brisbanense M. neworleansense |
35°–37 °C | SSTI Skeletal infection |
| M. chelonae/abscessus[126] |
M. chelonae M. salmoniphilum Subspecies of M. abscessus: M. bolleti M. massiliense M. abscessus |
SSTI Skeletal infection Disseminated infection Pulmonary disease |
|||
| M. mucogenicum[127] |
M. mucogenicum M. aubagnense M. phocaicum |
Bacteremia in ICH | |||
| Late pigmenting RGM |
M. smegmatis M. goodii |
Pneumonia Prosthetic valve endocarditis |
|||
| Early pigmenting RGM |
M. flavescens M. neoaurum M. vaccae M. canariasense M. cosmeticum M. monacense M. thermoresistibile M. bacteremicum M. iranicum |
BSI Prosthetic valve endocarditis SSTI Skeletal infection |
|||
| Non-pigmenting RGM |
M. mageritense M. wolinskyi |
SSTI CIED Infection |
|||
| Slow Growing [128] | >7 days | MAC |
M. intracellulare M. avium |
35°–37 °C | Pulmonary disease Lymphadenitis Skeletal infection Disseminated infection Hypersensitivity pneumonitis |
|
M. szulgai M. malmoense M. scrofulaceum |
SSTI Pulmonary disease Skeletal infection Disseminated infection Cervical lymphadenopathy |
||||
| M. kansasii | Skeletal infections Disseminated infection Pulmonary disease |
||||
| M. simiae |
M. simiae M. lentiflavum |
Disseminated infection | |||
| M. terrae/nonchromogenicum |
M. arupense M. kumamotonense M. heraklionense M. longobardum |
Skeletal infections | |||
| M. haemophilum | 28°–30 °C requires hemin or iron | SSTI Lymphadenitis |
|||
| M. genavense | Colitis in ICH | ||||
| M. xenopi | 42°–45 °C | Pulmonary disease Skeletal infection |
|||
| M. ulcerans | SSTI: “Buruli ulcer” | ||||
| M. marinum | 28°–30 °C | SSTI Skeletal infection “Fish tank Granuloma” |
|||
| M. gordonae | 35°–37 °C |
Abbreviations: BSI – Blood Stream Infection, CIED – Cardiovascular Implantable Electronic Device, ICH – Immunocompromised Host, MAC – Mycobacterium Avium Complex, RGM – Rapid Growing Mycobacteria, SSTI – Skin and Soft Tissue Infection.
Slow growing mycobacteria colonies are typically first seen at 2 weeks and mature colonies are expected at 3 weeks on solid media such as L-J agar [13] and the Middlebrook agars [12]. In contrast, the rapid growing mycobacteria colonies are typically seen by 4 days. Middlebrook 7H10 is a non-selective medium that can be utilized for quantitative reporting and to differentiate chromogenic from nonchromogenic mycobacteria (Table 5) [14]. MGIT is a liquid broth medium that allows automated detection of mycobacterial growth based on emission of fluorescence (MGIT 960 instrument; BD) [15], [16]. The sensitivity and specificity for detection of NTM for this system is 98.8% and 100%, respectively [17].
Table 5.
Reporting of non-tuberculous mycobacterial growth on middlebrook 7H10 medium.
| Colony Count | Interpretation |
|---|---|
| None | Negative |
| <50 | Actual count |
| 50–100 | + |
| 100–200 | ++ |
| 200–500 (Almost confluent growth) | +++ |
| >500 (Confluent growth) | ++++ |
| Appearance of Colonies | |
| White, Cream, Buff | Non-chromogenic |
| Lemon, Yellow, Orange, Red | Chromogenic |
Identification of NTM species is essential in the evaluation of a patient with NTM disease. Species identification allows for assessment of clinical significance, prognosis, and expected antimicrobial resistance. Unlike M. tuberculosis, no widely adopted techniques for species identification directly from clinical specimens exist. Therefore, culture remains the first step in identification. Once an organism has been isolated in culture, a number of diagnostic modalities can be employed to identify the organism to species level. Matrix-assisted laser desorption ionization time-of-flight mass spectrometry (MALDI-TOF MS) is very effective at identifying most NTM. It is usually cheaper and simpler than commercial nucleic acid amplification assays or whole genome sequencing. However, it requires pure isolates and may not be able to differentiate between closely related mycobacteria such as M. chimaera and M. intracellulare. It is also limited by the breadth of the spectra library available to the instrument [18].
Molecular diagnostics has revolutionized the practice of species identification, but can be applied only after identification of growth on culture media [6]. A variety of Food and Drug Administration (FDA)-approved DNA probes for species specific RNA is available. If MALDI-TOF and species-specific probes are unable to identify an organism, sequencing of the 16s RNA may be useful. Two regions of the 16s RNA can be helpful in identifying NTM species. Several commercially available systems that offer out-of-the box sequencing are available. However, several species may not be differentiated as there is not enough genomic divergence to allow for accurate diagnosis beyond species complex level. Similar to MALDI-TOF, test characteristics depend on the library of available sequences [19].
Identifying organisms directly from clinical specimen is an area of ongoing research. Polymerase chain reaction (PCR)-based assays of respiratory samples for rapid identification of NTM species are currently in development. Assays used to diagnose TB such as Xpert MTB/RIF Ultra and TrueNAT (MolBio) may have some cross reactivity with NTM species, but they are not a primary means for diagnosis of NTM disease [20]. Line probe assays do have the ability to identify some species of NTM and test for aminoglycoside and macrolide resistance, but they are not widely available. MALDI-TOF may also be effective at identifying NTM species directly from clinical specimens [18]. However, these techniques are at present experimental, but hold the promise for a more rapid identification of NTM infections in the future.
Histology. In the majority of patients, diagnosis of pulmonary NTM disease can be made without a tissue sample. When other microbiological criteria are met, biopsies are not required [3], [4], [21]. In contrast to this, the diagnosis of extra-pulmonary NTM infections typically requires obtaining a tissue sample. The histologic hallmark of mycobacterial disease is necrotizing or non-necrotizing granulomatous inflammation with the presence of an NTM organism. A single tissue sample with these features is sufficient to establish NTM disease [22]. In immunocompetent patients, NTM histologic findings can mirror that of tuberculosis [23]. Granulomatous inflammation alone is not specific for NTM. Lung biopsy may be culture negative due to the small tissue sample, and an NTM diagnosis can still be made with evidence of granulomatous inflammation and one or more cultures with NTM growth [3], [4], [24].
Immunocompromised patients may not show granulomas or detectable organisms on tissue biopsy. In cases of disseminated NTM infection, the lack of hallmark histologic findings does not exclude the diagnosis [24]. Histologic findings in this patient population may include foamy histioctyes containing mycobacteria, poorly formed granulomas, or no clear inflammatory response [23].
3.2. Antimicrobial susceptibility testing
CLSI and ATS/IDSA have published criteria for antimicrobial susceptibility testing (AST) [25]. The gold standard for determining antimicrobial susceptibility is culture and growth via broth microdilution [3], [26]. In general, NTM AST should be performed only for clinically significant isolates. CLSI recommends against susceptibility testing for NTM species that are rarely pathogenic, such as M. gordonae, M. mucogenicum, or M. terrae [25], [26].
Clinicians should be cognizant of the fact that correlation of clinical response to in-vitro susceptibility patterns has not been established for each species and anti-mycobacterial drug. For example, for Mycobacterium avium complex (MAC) isolates, there is no correlation of clinical response with in vitro minimum inhibitory concentrations (MIC) except for the macrolides and amikacin [27]. Thus, AST prior to initiation of treatment for MAC should be limited to clarithromycin and amikacin susceptibility testing. Similarly, failure of treatment for Mycobacterium kansasii is generally associated with rifampin resistance; MIC values for ethambutol (EMB) and isoniazid (INH) do not correlate well with clinical response [25], [26]. For this reason, AST prior to initiation of treatment for M. kansasii -include rifampin susceptibility testing first and expanded to other agents only if rifampin resistance is present. For these two NTM organisms, AST against a wider panel of antimicrobials is recommended only in the presence of macrolide-resistance (for MAC) and rifampin resistance (for M. kansasii).
For rapidly growing mycobacteria, susceptibility testing against a wider panel of antimicrobials upfront is recommended. However, it is important to note that the purpose of this testing is “to guide rather than dictate therapy”, as there is no established MIC cutoff for susceptibility or resistance for most of these agents [25], [26]. This wider panel of antimicrobials includes clarithromycin, amikacin, tobramycin, cefoxitin, ciprofloxacin, clarithromycin, doxycycline (or minocycline), imipenem, linezolid, moxifloxacin, tigecycline, clofazimine, and trimethoprim-sulfamethoxazole. Additionally, all M. abscessus and M. fortuitum isolates should be routinely tested for inducible macrolide resistance due to the presence of erythromycin ribosomal methylase (erm) gene [25], [28]. Testing for inducible macrolide resistance involves incubating the organism with sub-inhibitory concentrations of clarithromycin for 14 days [29].
4. Radiographic diagnosis of NTM infections
No radiographic pattern is pathognomonic for pulmonary NTM disease. Two radiographic patterns are commonly encountered in pulmonary NTM disease: fibro-cavitary disease and nodular bronchiectasis. A combination of these two radiographic patterns or a ‘mixed’ pattern may also be seen.
Fibro-cavitary NTM pulmonary disease: Fibro-cavitary disease usually occurs in middle age or older men with underlying structural lung disease such as COPD or pneumoconiosis. Fibro-cavitary disease can be identified on chest X-ray and is difficult to distinguish from tuberculosis (TB) and endemic fungal infections with X-ray alone (Fig. 1). This presentation is characterized by upper lobe predominant, thin or thick walled cavities with surrounding pleural thickening [30]. Whereas TB usually has calcified lymphadenopathy and associated parenchymal infiltrates, NTM is not typically associated with these findings [30]. Nonetheless radiographic findings alone should not be used to discern between TB and NTM infections. Pulmonary malignancies (particularly squamous cell carcinoma), fungal infections and pulmonary vasculitides may have similar radiographic findings making clinical, radiographic, microbiologic, and possibly histopathologic correlation necessary.
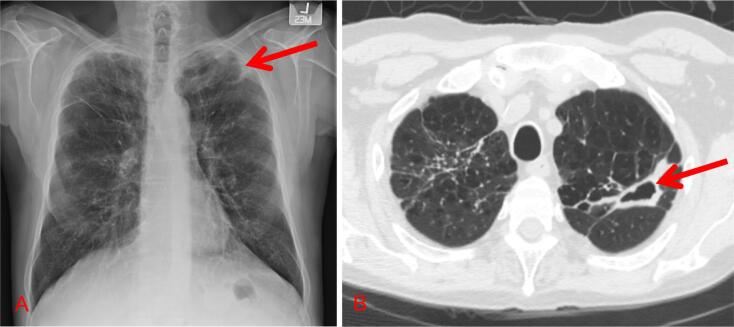
Fig. 1.
Radiographic images of patients with fibro-cavitary MAC. A. Chest roentgenogram demonstrating a left apical thin walled cavity with surrounding pleural reaction. The background lung architecture has increased reticular markings consistent with scarring. B. Chest computed tomography, axial section, demonstrating a thick-walled cavitary lesion in the left upper lobe.
Nodular bronchiectatic NTM pulmonary disease: Nodular bronchiectasis usually manifests in elderly, thin women and is associated with pectus excavatum, scoliosis, and mitral valve prolapse [31]. Nodular bronchiectasis may be visualized as ‘tram tracking’ with micro nodularity on chest roentgenogram but is best observed on high resolution computed tomography of the chest (HRCT). On HRCT, this appears as cylindrical bronchiectasis with branching centrilobular nodules (i.e. “tree-in-bud”) (Fig. 2) [32]. These changes are usually bilateral but preferentially involve the lingula and right middle lobe. Overall these radiographic findings are non-specific and can be seen in aspiration and other bacterial infections. When the cause of tree-in-bud opacities can be determined, less than half are attributed to NTM infection [33].
Fig. 2.
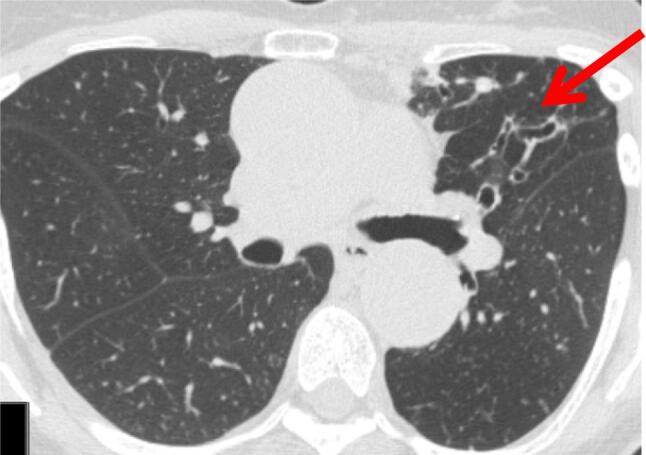
High resolution chest computed tomography of nodular bronchiectasis affecting the lingula. Lingula and right middle lobe are thought to be more often involved in nodular bronchiectatic NTM.
Hypersensitivity pneumonitis: Hypersensitivity pneumonitis secondary to inhalation of MAC is another variation of NTM associated lung disease that is worth mentioning. This entity is better known as “hot tub” lung disease [34] and is presumed to be secondary to a hypersensitivity reaction to aerosolized MAC rather than true infection since clinical symptoms and radiographic changes improve with antigen avoidance and/or glucocorticoids [35]. However, NTM infection and hypersensitivity features can coexist [35], [36]. HRCT findings are consistent with acute hypersensitivity pneumonitis: upper lobe predominant, bilateral ground glass opacities with air trapping on expiration and centrilobular nodularity.
Extra-pulmonary NTM disease: Radiographic features of extra-pulmonary NTM infection are non-specific and variable, depending on the site of infection. For skin and soft tissue NTM infections, radiographic imaging studies are not routinely required for diagnosis. However, imaging studies such as sonography, CT, and MR can be useful to localize an abscess or determine if infection extends into deeper soft tissues or adjacent bone, in particular in situations where the response to treatment is sub-optimal. Radiographic features of NTM lymphadenitis include decreased echogenicity on ultrasound, corresponding to intranodal liquefactive/cystic necrosis, as well as an enlarged nodal mass with central necrosis on CT or MRI [37], [38]. Common radiographic features of NTM osteomyelitis include permeative osteolysis, bone sequestration, and periostitis. Articular NTM infections manifest radiographic findings ranging from regional osteopenia to marginal and subchondral osseous erosions [39].
5. Checking interleukin-2 and interferon gamma pathways
Certain patients are more susceptible to NTM disease than others. This is illustrated in other mycobacterial diseases by the large number of patients with positive tuberculin skin testing who never progress to active disease, and the small number of patients who receive bacille Calmette-Guerin (BCG) vaccination and develop disseminated BCG-osis [42]. Protection from mycobacterial infection is provided by the type I cytokine pathway; investigations have shown that defects in the genes encoding IL-12, IL-23, and interferon- γ (INF-γ) receptors lead to reduced immunity to mycobacteria [43]. Patients with these primary immunodeficiencies are said to have ‘Mendelian susceptibility to mycobacterial disease’.
While not addressed in the 2020 or prior ATS/IDSA guidelines [4], [44], it is reasonable to assess patients for primary defects in cell mediated immunity who present with disseminated NTM infection without known secondary cause, especially if family members are also ill. This information informs prognosis and may affect treatment decisions [45]. These patients are also susceptible to other infections including cytomegalovirus, human herpes virus-8, herpes simplex virus, Listeria, and Histoplasma infections [46].
6. Distinguishing infection from colonization
Non-tuberculous mycobacteria are ubiquitous in the environment; isolation of NTM species from non-sterile samples does not equate to infection. Most NTM isolated from a respiratory source may not meet currently established criteria for NTM infection [47]. The respiratory tract can be colonized with NTM, especially if underlying structural airway and/or lung disease are present. MAC is the most commonly described NTM airway “colonizer” [48], but it is unclear if this is true colonization or low grade infection. Colonization without infection is an unproven concept in pulmonary NTM disease; however, this does not imply that all patients with NTM respiratory isolates require antimicrobial treatment. The current criteria for NTM pulmonary infections were established by a joint guideline from ATS and IDSA in 2007 but were more recently updated in 2020 [3], [4]. These guidelines by necessity focus on the most commonly encountered and best described NTM species—MAC, M. kansasii, and M. abscessus. Presumably these guidelines apply to other less common and lesser-known NTM species, but data and experience with these NTM species are limited. The diagnosis of pulmonary NTM disease requires clinical, radiographic, and microbiologic correlation. These criteria are of equal importance, and all must be met to diagnose pulmonary NTM disease [3].
7. Clinical manifestations of NTM infections
Pulmonary disease is the most common manifestation of NTM infections [48], but NTM can also cause localized infection of the skin, bones and soft tissues; disseminated infections in patients with severely compromised immune systems can also occur. The current ATS/IDSA guidelines review these alternate manifestations but do not specifically address the diagnosis and treatment of extra-pulmonary NTM [3], [44]. Common NTM infection syndromes are summarized in Table 1.
The clinical manifestations of NTM infections are variable and non-specific. Most patients with pulmonary NTM disease will have chronic or recurring cough with or without sputum production [49]. This may be accompanied by constitutional symptoms (e.g. fever, fatigue, weight loss, and night sweats), chest pain, or shortness of breath. These latter symptoms may only be present with advanced disease. Because many patients with pulmonary NTM disease have underlying lung disease such as bronchiectasis or chronic obstructive pulmonary disease (COPD), it can be very challenging to determine if NTM infection is the cause of the presenting symptoms.
Patients with skin, soft tissue, and musculoskeletal NTM infections may present with skin nodules, ulcers, wound drainage or abscesses in addition to the constitutional symptoms noted above. Musculoskeletal NTM infections may present with joint pain, stiffness, and swelling as well as muscle wasting and draining sinuses. Mycobacterial lymphadentis presents typically with enlarged lymph nodes, often times non-tender. Disseminated NTM infection may present with scattered skin lesions. In patients with advanced HIV infection, disseminated MAC infection presents as a systemic febrile illness with abdominal pain and diarrhea.
Physical findings reflect the system or organs affected but are variable and nonspecific. For example, features of skin and soft-tissue NTM include or pain, erythema, warmth, local pain, swelling, ulcers, and abscesses. It should be noted that cosmetic surgeries are common precipitant for NTM skin and soft tissue infections. A firm, painless mass with overlying skin changes is a common physical examination finding in NTM lymphadenitis. Hepatosplenomegaly is a common physical examination finding in disseminated MAC disease.
8. Antimicrobial treatment of non-tuberculous mycobacterial infections
As described in the preceding sections, the treatment of NTM infections is complex and limited by several issues, including the need for multidrug regimens, prolonged treatment durations, and the use of drugs that are often associated with adverse reactions and not well-tolerated. Co-morbidities that are often present in patients with NTM infections add another layer of complexity to the management. The optimal drug doses, drug combinations, and treatment durations have not been reliably established for most, if not all, NTM infections. It should be noted that several new medications used for the treatment of multi-drug resistant TB such as, bedaquiline, pretomanid and delamanid have in-vitro activity against NTM species but in vivo data is lacking at this time [50], [51]. Consensus guidelines for the management of NTM infections exist. However, they are based on limited data and expert opinion rather than evidence from adequately powered controlled trials with sufficiently long follow-up period. This notwithstanding, the following general considerations can be noted.
-
•
In general, anti-mycobacterial treatment should be offered only when the clinical, radiographic, and microbiologic criteria for the diagnosis of NTM infections are met.
-
•
The optimal management of NTM infections requires a well-coordinated multidisciplinary approach, involving infectious diseases physicians, pulmonologists, pharmacists, microbiologists, radiologist, nurses, respiratory therapists, and dietitians.
-
•
The antimicrobial drugs currently used for treating NTM infections were not specifically developed for that purpose. Their utility in treating NTM infections was derived from demonstration of activity in-vitro or in animal models, drug susceptibility testing, and observations from clinical practice. Antimicrobials that have demonstrated activity against NTM species and/or infections in vitro are highlighted in Table 6.
-
•
Treatment of NTM infections, even treatment of limited skin or soft tissue infection, typically requires the use of at least 2 drugs in combination. The decision of which drugs and how many drugs to use, while guided by susceptibility testing, is often based on clinical experience and expert opinion rather than evidence from controlled trials. The typical dosing for anti-mycobacterial drugs is reviewed in Table 7. The number of antimicrobials needed to treat an NTM infection is dependent on the severity of illness. Using macrolide-susceptible MAC pulmonary disease as an example, initial therapy for nodular bronchiectatic disease is a three-drug therapy including a macrolide, ethambutol, and rifamycin. However, severe bronchiectactic or fibro-cavitary disease would generally warrant the addition of an injectable aminoglycoside as a fourth agent for the first two to three months of therapy. Due to difficulties with adverse effects in certain target populations (i.e. elderly, low body weight pulmonary NTM patients), the drugs are often initiated and titrated to full doses in a staggered format to enhance tolerability and compliance to the drug therapy regimen.
-
•
Treatment of NTM is often provided in 2 phases: An initial phase with more drugs (often including intravenous antimicrobials), followed by a maintenance phase with fewer drugs, and when possible, an all oral drug regimen. The duration of the initial phase of therapy is variable, often in the range of 1–3 months. This recommended duration is not evidence based; in clinical practice, it is often shortened because of intolerance or treatment-emergent drug adverse events.
-
•
Empiric therapy: Species level identification is necessary to determine the most appropriate treatment regimen. For most NTM infections, susceptibility-based treatment is preferred over empiric therapy. Notwithstanding the poor in vitro susceptibility correlation with clinical outcomes, baseline susceptibility testing, to guide but not to dictate treatment, is recommended for NTM isolates from patients believed to have definite disease. However in disseminated and or severe infections, waiting for the full microbiologic results prior to initiation of anti-mycobacterial therapy may endanger patients’ lives. In such cases, empiric therapy based on the prevailing understanding of the usual antimicrobial susceptibility profile of the putative causative organism is recommended. For example, for infections by slow-growing NTM, a regimen consisting of an oral macrolide, rifamycin, ethambutol, and amikacin may be considered as empiric therapy; whereas, a regimen comprising of amikacin, imipenem, and a macrolide may serve as appropriate empiric therapy for infections presumed to be caused by rapid-growing NTMs. Empiric therapy should be tailored once the pathogen has been fully identified and AST results are available.
-
•
For some patient populations, initiating a full dose multi-drug regimen all at once may be intolerable. In such cases, clinicians may wish to consider initiating therapy sequentially, adding a drug every few days until the desired multidrug regimen is achieved. Sequential drug addition with dose titration during therapy initiation (Table 8) often facilitates tolerance of a difficult drug regimen. This approach affords minimal risk of drug resistance due to the slow replication of NTM compared to other bacterial species. This approach can be used for both pulmonary and non-pulmonary NTM infections. Moreover given the slow replication of NTM species in general and prolonged duration of therapy, this approach can also be used to improve tolerance even in severe infections.
-
•
The optimal duration of treatment for individual NTM species and infections is not known. In general, treatment is prolonged, ranging from 3 to 4 months for limited skin and soft tissue infections to 12 months and longer for severe lung and disseminated infections. Factors that guide the decision to discontinue antimicrobials include clinical, microbiologic, and radiologic response to treatment, as well as the tolerability of the treatment regimen. For example, drug therapy for pulmonary NTM disease should generally continue for 12 months after confirmed culture conversion.
-
•
Chronic suppressive therapy: For some NTM infections, no antimicrobial regimen has been shown to effect long-term microbiologic or clinical cure. In situations where curative treatment is not possible, the goal of therapy shifts from definitive eradication of infection to minimization of symptoms. For example, if source control is not possible (e.g. infected retained prosthetic material, severe structural lung abnormalities) then chronic suppressive therapy may be warranted. Non-pharmacologic adjunctive interventions (discussed below) such as aggressive pulmonary hygiene should be optimized in these patients.
Table 6.
Drugs with microbiologic activity for NTM demonstrated in human clinical disease or in vitro.
| Isolate (s) | Drugs |
|---|---|
| Slow Growing NTM | |
| MAC | Human: AMK [129] AZ [130] CFZ [131] CLR [132] BDQ [133] EMB [130] RIF [130] RFB [130] SM [134] LZD [135] MOX [136] Micro: CTZ/AV [137] ETO [138] TIG [139] TZD [140] |
| M. kansasii | Human: CLR [141] EMB [142] INH [142] LFX [143] RIF [142] RFB [144] SM [142] Micro: AMK [145] AZ [146] BDQ [146] CFZ [146] CIP [145] DLM [146] LFX [146] LZD [146] MOX [146] SMX [145] TIG [146] TZD [140] |
| M. marinum | Human: AMK [147] CLR [148] DOX [148] EMB [149] MIN [148] RIF [149] RFB [150] SMX/TMP [151] Micro: CPX [152] IMI [152] INH [152] LVX [152] LZD [153] MOX [152] TZD [140] |
| M. leprae | Human: CFZ [154] CLR [155] DAP [156] MIN [154] MOX [157] OFX [154] RIF [158] Micro: LFX [159] |
| Rapid Growing NTM | |
| MABC | Human: AMK [160] AZ [160] BDQ [133] CFX [160] CFZ [161] CLR [160] CPX [162] DOX [163] IMI [161] LZD [120] MOX [162] TIG [164] Micro: ERA [165] OMA [165] LZD [166] TZD [140] |
| M. chelonae | Human: AMK [167] AZ [168] CFZ [169] CLR [170] CPX [171] DOX [172] IMI [173] LFX [174] LZD [175] SMX [176] TIG [164] TOB [177] Micro: MIN [125] OMA [178] TDZ [140] |
| MFCa | Human: AZ [179] AMK [180] CLR [181] DOX [182] IMI [173] LFX [183] MIN [184] OFX [180] SMX/TMP [185] Micro: CFX [186] MOX [187] LZD [166] OMA [178] TIG [188] TOB [189] TZD [140] |
| MSGa | Human: AMK [190] CFX [190] DOX [190] IMI [190] SMX/TMP [190] TOBb[190] Micro: CLR [191] CPX [191] EMB [191] CFZ [192] TZD [140] |
| M. immunogenum | Human: AMK [193] CLR [194] Micro: AMK [195] CLR [195] LZD [140] TIG [188] TZD [140] |
| M. mucogenicum | Human: AMK [196] AZ [196] CIP [196] CLR [196] LFX [197] MFX [198] Micro: AMX [127] AMX/CLV [197] CFX [199] CPX [199] DOX [199] IMI [199] LZD [198] MIN [199] SMX/TMP [199] |
Abbreviations: AMK – amikacin, AMX/CLV – amoxicillin/clavulanate, AZ – azithromcyin, BDQ – bedaquiline, CFX – cefoxitin, CFZ – clofazimine, CLR – clarithromycin, CPX – ciprofloxacin, CTZ/AV – ceftazidime/avibactam, DAP – dapsone, DOX – doxycycline, DLM – delaminid, EMB – ethambutol, ERA-eravacycline, ETO – ethionamide, IMP – imipenem, INH – isoniazid, LFX – levofloxacin, LZD – linezolid, MIN – minocycline, MFX – moxifloxacin, OFX – oflaxacin, OMA-omadacycline, RFB – rifabutin, RIF – rifampin, SM – streptomycin, SMX – sulfamethoxazole, TIG – tigecycline, TOB – tobramycin, TMP – trimethoprim, TZD – tedizolid.
MABC – Mycobacterium Abscessus Complex (M. abscessus, M. massiliense, M. bolettii), MAC – Mycobacterium Avium Complex (M. avium, M. intrellulare, M. chimaera), MFC – Mycobacterium Fortuitum Complex (M. alvei, M. boenickei, M. conceptionense, M. farcinogenes, M. fortuitum, M. mageritense, M. neworleans, M. peregrinum, M. porcinum group (includes M. porcinum, M. bonickei, M. houstonense, M. neworleansense), M. senegalense, M. septicum)), MSG – Mycobacterium Smegmatis Group (M. smegmatis, M. wolinski, M. goodii).
aM. fortuitum, M. abscessus subspecies abscessus, and M. abscessus subspecies bolletii, and M. smegmatis have the erm gene for inducible macrolide resistance which should be tested for if considering macrolide [200].
bM. wolinksy isolates intrinsically resistant to tobramycin [191].
Table 7.
Typical drug dosing for NTM disease.
| Drug | Standard dose | Renal adjustment |
|---|---|---|
| Amikacin | 15 mg/kg1 IV daily | Est crcl < 30 ml/min:12–15 mg/kg1 three times weekly |
| Azithromycin | 250 mg once daily | No adjustment |
| Bedaquiline Fumarate | Week 1–2: 400 mg once daily with food Week 3–24: 200 mg three times weekly with food |
No adjustment |
| Cefoxitin | 200 mg/kg/day IV in 3 divided doses2 (maximum 12 g/24 h) |
Est crcl 30–49 ml/min: 1–2 g q 8–12 h Est crcl 10–29 ml/min: 1–2 g q12-24 h Est crcl < 10 ml/min: 1–2 g q24-48 h |
| Ciprofloxacin | 500–750 mg twice daily |
Est crcl 10–49 ml/min: 250–500 mg twice daily Est crcl < 10 ml/min: 250–500 mg daily |
| Clarithromycin | 500 mg twice daily or 1000 mg once daily (extended release) |
Est crcl < 30 ml/min: 1) 500 mg once daily -OR- 2) 500 mg once, then 250 mg twice daily |
| Clofazimine | 100 mg daily | No adjustment |
| Delaminid | 100 mg twice daily |
Est crcl < 30 ml/min: Not recommended |
| Doxycycline | 100 mg twice daily | No adjustment |
| Ethambutol | 15–20 mg/kg (maximum 1600 mg) daily |
Est crcl < 30 ml/min: 20–25 mg/kg three times weekly |
| Ethionamide3 | 15–20 mg/kg/day (max 1000 mg) daily in divided doses | No adjustment |
| I/C | Imipenem 1000 mg/Cilastin 1000 mg given intravenously twice daily |
Est crcl 20–40 ml/min: 750 mg −750 mg (I/C) every 12 h. Est crcl < 20 ml/min: 500 mg −500 mg (I/C) every 12 h) |
| Isoniazid3 | 5 mg/kg (max 300 mg) daily | No dose adjustment |
| Levofloxacin | 750 mg daily |
Est crcl 20–49 ml/min: 500–750 mg every 48 h Est crcl < 20 ml/min: 250–500 mg every 48 h |
| Linezolid3 | 600 mg daily | No adjustment4 |
| Minocycline | 200 mg once, then 100 mg twice daily |
Est crcl < 10 ml/min: Standard dose -OR- 200 mg once, then 100 mg once daily |
| Moxifloxacin | 400 mg daily | No dose adjustment |
| Rifabutin | 5 mg/kg (max 300 mg) daily |
Est crcl < 30 ml/min: Consider standard dose4 |
| Rifampin | 10 mg/kg (max 600 mg) daily | No dose adjustment |
| Streptomycin | 15 mg/kg1 daily |
Est crcl < 30 ml/min: 12–15 mg/kg1 3 times weekly |
| SMX/TMP | SMX/TMP 800 mg/160 mg twice daily |
Est crcl 15–29 ml/min: SMX/TMP 800 mg/160 mg once daily |
| Tedizolid | 200 mg once daily | No adjustment |
| Tigecycline | 100 mg IV once, then 50 mg twice daily2 | No adjustment |
Abbreviations: Est crcl – Estimate creatinine clearance, I/C – Imipenem/ Cilastin, IV – intravenous, kg – Kilogram, mg – Milligram, SMX/TMP – Sulfamethoxazole/Trimethoprim.
1Use an adjusted body weight (IBW + 0.4(ABW − IBW) for empiric dosing in the setting of obesity and follow serum levels.
2Some providers use reduced dose or schedule to optimize long-term tolerability.
3Consider Vitamin B6 25–50 mg daily to decrease risk of toxicity.
4Renally-cleared metabolite accumulation can occur. Monitor closely for toxicity.
aRifabutin: May consider decreasing to 50% of standard dose and monitoring serum drug levels to avoid toxicity. Standard dosing appropriate for patients on dialysis.
Table 8.
Example of MAC medication titration schedule.
| DAY 1 | DAY 2 | DAY 3 |
|---|---|---|
| Ethambutol 400 mg (1 tablet) once daily | Ethambutol 800 mg (2 tablets) once daily | Ethambutol 1200 mg (3 tablets) once daily |
| DAY 4 | DAY 5 | DAY 6 |
| Ethambutol 1200 mg (3 tablets) once daily Rifampin 300 mg (1 capsule) once daily |
Ethambutol 1200 mg (3 tablets) once daily Rifampin 300 mg (1 capsule) once daily |
Ethambutol 1200 mg (3 tablets) once daily Rifampin 600 mg (2 capsules) once daily |
| DAY 7 | DAY 8 | DAY 9 and ongoing |
| Ethambutol 1200 mg (3 tablets) once daily Rifampin 600 mg (2 capsules) once daily Azithromycin 125 mg (0.5 tablet) once daily |
Ethambutol 1200 mg (3 tablets) once daily Rifampin 600 mg (2 capsules) once daily Azithromycin 125 mg (0.5 tablet) once daily |
Ethambutol 1200 mg (3 tablets) once daily Rifampin 600 mg (2 capsules) once daily Azithromycin 250 mg (1 tablet) once daily |
9. Monitoring for clinical response to treatment
Clinical, radiographic, and microbiologic assessments are typically used to determine if a patient is responding to therapy.
Clinical symptoms should be assessed at each clinical encounter. Improvement in symptoms can be a key indicator of response to treatment, but clinical symptoms do not always correlate with changes in radiographic or microbiologic status. Furthermore, complete resolution of symptoms at the end of treatment is not universal given the multifaceted nature of NTM disease and comorbid illness that can contribute to symptomatology. Oftentimes clinical monitoring is dictated by the need to assess for the presence of drug toxicities in order to determine if drug and dose changes are necessary.
For pulmonary NTM infections, serial sputum cultures serve as a marker for treatment response. Current guidelines recommend monitoring sputum cultures every 1–2 months; confirmed sputum culture conversion (subsequent negative cultures one month apart) provides a basis for microbiologic cure and is a key factor in determining treatment duration. In situations where spontaneous expectorated sputum specimens cannot be collected, induced sputum should be obtained; obtaining deep respiratory samples via bronchoscopy for the purpose of monitoring response to therapy is not usually required.
For extra-pulmonary NTM infections, treatment response monitoring based on serial collection of microbiologic data is less well proscribed and is usually performed at the discretion of clinicians based on clinical progress.
Radiographic imaging studies are also surrogates for treatment response. Imaging is often performed during therapy and at the end of a planned treatment period to confirm treatment response. The optimal intervals for imaging performance are not well established.
10. Safety monitoring during anti-mycobacterial therapy
Adverse drug reactions (ADRs) are very common during the treatment of NTM infections and are associated with treatment interruption or early treatment cessation [27], [52], [53]. Not surprisingly, some evidence exists that ADRs also impact treatment outcome [54]. Therefore, educating patients about the likely ADRs that they may encounter with the drug-regimen that they have been prescribed, and monitoring them for treatment-emergent ADRs is a key component of the management of the treatment of NTM infections. This monitoring is conducted in a variety of ways; there is no evidence basis for the optimal frequency of monitoring of patients with NTM for adverse drug reactions. At a minimum, active assessment for the development of ADRs, through questions or physical examination, should be part of each clinical encounter. A high potential for drug interactions exists with many of the antimicrobials used to treat NTM infections; therefore, a close review of the medication list is warranted at every clinical encounter.
Safety monitoring for antimicrobial toxicity will vary based on multiple factors: co-morbid medical conditions, baseline functional status, liver function, kidney function, drug-drug interactions, concomitant medications with additive toxicity profile, intravenous versus oral therapy, drug dose, and the availability of laboratory resources. Nonetheless, it is important for clinicians to anticipate potential adverse reactions. Table 9 highlights the more notable adverse effects, while providing guidance on clinical and laboratory monitoring for extended therapy. These recommendations are meant to provide general guidance. More detailed monitoring may be warranted based on patient age, comorbidities, concurrent drugs, overlapping drug toxicities, and availability of healthcare resources.
Table 9.
| Drug | Adverse drug reactions | Laboratory/Clinical monitoring |
|---|---|---|
| Amikacin (parenteral) | Kidney toxicity, electrolyte abnormalities (K, Mg, Ca), vestibular, toxicity, hearing loss |
Clinical: consider audiometry, balance assessments Laboratory: BMP w/SCr, BUN, K (2x/weekly), TDM for durations >1 week |
| Amikacin (inhalation) | Sore mouth/throat, bronchospasm, cough, wheezing/SOB, dysphonia Rare: kidney/vestibular/auditory toxicity |
Clinical: pre-/post administration respiratory status Laboratory: SCr (monthly), serum trough level (to confirm lack of systemic accumulation) after 1 week of therapy |
| Amoxicillin- Clavulanate | Abdominal discomfort, diarrhea, nausea, vomiting Rare: hypersensitvity, rash |
Clinical: GI effects Laboratory: CBC w/differential, ALT and SCr (1 month, 3 months, then annually) |
| Azithromycin | GI (abdominal pain, nausea, vomiting, diarrhea, dyspepsia), headache, visual disturbance Rare: hearing loss, QT prolongation |
Clinical: consider audiometry, GI effects, consider ECGc Laboratory: CBC w/differential , ALT, SCr (1 month, 3 months, then annually) |
| Bedaquiline Fumarate | Nausea, arthralgia, headache, chest pain, QT prolongation, hepatotoxicity |
Clinical: ECG (baseline, 2 weeks, 12 weeks, and 24 weeks) Laboratory: serum Ca, Mag, and K (baseline); liver function tests: AST, ALT, bilirubins, alk phos (baseline, then monthly) |
| Cefoxitin | Injection site reactions, hypotension, transaminitis, leukopenia, thrombocytopenia Rare: aplastic anemia |
Clinical: rash and hypersensitivity symptoms Laboratory: CBC w/differential, ALT, and BMP w/SCr, BUN, K (weekly) |
| Ciprofloxacin | GI (abdominal pain, nausea, vomiting, diarrhea, dyspepsia), hypoglycemia, CNS effects (agitation, nervousness, disorientation, memory impairment), rash Rare: peripheral neuropathy, tendon rupture, QT prolongation |
Clinical: consider ECG, signs of hypoglycemia/ CNS effects Laboratory: CBC w/differential, ALT, and SCr (1 month, 3 months, then annually) |
| Clarithromycin | GI (taste disturbance, abdominal pain, nausea, vomiting, diarrhea, dyspepsia) Rare: hearing loss, QT prolongation, hepatotoxicity |
Clinical: consider audiometry, GI effects, consider ECGc, Monitor DDI Laboratory: CBC w/differential , ALT, SCr (1 month, 3 months, then annually) |
| Clofazimine | Orange/red discoloration of body fluids/feces, skin manifestations (hyperpigmentation ichythosis, xeroderma ,pruritis, phototoxicity, rash), GI disturbance (nausea, vomiting, diarrhea, anorexia, abdominal pain) Rare: QT prolongation, hepatitis, depression due to skin discoloration |
Clinical: ECG (baseline, 2 weeks, 12 weeks, and 24 weeks), GI adverse effects, psychological disturbances (depression/anxiety) related to skin effects Laboratory: liver function tests: AST, ALT, bilirubins, alk phos (baseline, then monthly) |
| Doxycycline | GI effects (nausea, vomitting, diarrea, dysphagia), photosensitivity, rash Rare: esophageal ulceration, benign intracranial hypertension, hepatitis |
Clinical: photosensitivity, esophageal discomfort Laboratory: CBC w/differential, ALT, and SCr (1 month, 3 months, then annually) |
| Ethambutol | optic neuritis, GI effects (nausea/vomitting) Rare: peripheral neuropathy |
Clinical: monthly visual acuity assessment and red-green color-discrimination assessment (Ishihara), fundoscopic exam (baseline and every 3 months) Laboratory: Scr (baseline and monthly) |
| Ethionamide | GI (nausea, vomiting, anorexia, taste disturbance, diarrhea), endocrine (gynecomastia, hair loss, acne, impotence, menstrual irregularity, reversible hypothyroidism), hepatotoxicity, neurotoxicity (peripheral neuropathy, optic neuritis, depression, and psychosis) |
Clinical: GI, endocrine, and psychiatric symptoms Laboratory: creatinine and LFTs: AST/ALT, alk phos, bilirubins (baseline and monthly), TSH (baseline and every 3 months) |
| Imipenem/Cilastin | GI effects (nausea, vomiting, diarrea), rash Rare: seizure, cytopenias |
Clinical: GI effects and rash Laboratory: CBC w/differential, ALT, and BMP w/SCr, BUN, K (weekly) |
| Isoniazid | Peripheral neuropathy, asymptomatic transaminase elevations, hepatitis Rare: rash, hypersensitivity reactions |
Clinical: clinical signs of hepatotoxicity (nausea, abdominal pain, jaundice) and neuropathy Laboratory: LFTs: AST/ALT, alk phos, bilirubins (baseline and monthly) if underlying liver compromise or concomitant hepatotoxic drugs |
| Levofloxacin | GI (nausea, diarrhea) hypoglycemia, CNS effects (dizziness, agitation, nervousness, disorientation, memory impairment, insomnia) Rare: peripheral neuropathy, tendon rupture, QT prolongation |
Clinical: Consider ECG, signs of hypoglycemia/CNS effects Laboratory: CBC w/differential, ALT, and SCr (1 month, 3 months, then annually) |
| Linezolid | GI effects (nausea, vomiting, diarrhea), headache, hematologic myelosuppression (thrombocytopenia, leukopenia, anemia) Rare: lactic acidosis, peripheral neuropathy, seizure, optic neuropathy |
Clinical: GI effects, clinical thrombocytopenia (unusual bleeding, bruising) and peripheral neuropathy Laboratory: CBC w/differential (weekly × 4 weeks, then bi-weekly), LFTs: AST/ALT, alk phos, bilirubins (baseline and monthly) |
| Minocycline | GI effects (nausea, vomiting, diarrea), skin effects (rash, photosensitivity, skin pigmentation), dizziness, headache |
Clinical: cutaneous, neurologic, and GI effects Laboratory: CBC w/differential, ALT, and SCr (1 month, 3 months, then annually) |
| Moxifloxacin | GI (nausea, diarrhea) hypoglycemia, CNS effects (dizziness, agitation, nervousness, disorientation, memory impairment, insomnia) Rare: peripheral neuropathy, tendon rupture, QT prolongation |
Clinical: consider ECG, signs of hypoglycemia/CNS effects Laboratory: CBC w/differential, ALT, and SCr (1 month, 3 months, then annually) |
| Rifabutin | Discoloration (orange/red) of body fluids, cutaneous effects (pruritus, rash), GI effects (nausea, vomitting), hepatitis, anterior uveítis, hematologic toxicity (leukopenia, neutropenia), arthralgias/myalgias |
Clinical: Monitor DDI, GI/ cutaneous effects Laboratory: CBC w/differential, ALT, and SCr (1 month then every 3 months) |
| Rifampin | Discoloration (orange/red) of body fluids, cutaneous effects (pruritus, rash), GI effects (nausea, vomitting), hepatitis, hematologic toxicity (leukopenia, hemolytic anemia) Rare: flu-like syndrome |
Clinical: Monitor DDI, GI/ cutaneous effects Laboratory: CBC w/differential, ALT, and SCr (1 month then every 3 months) |
| Streptomycin | Kidney toxicity, electrolyte abnormalities (K, Mg, Ca), vestibular, toxicity, hearing loss |
Clinical: consider audiometry, balance assessments Laboratory: BMP w/SCr, BUN, K (2x/weekly), TDMb for durations >1 week |
| Tigecycline | GI effects (nausea, vomiting, diarrea, anorexia, dyspepsia), dizziness, rash |
Clinical: nausea/vomitting, dizziness Laboratory: BMP w/SCr, BUN, K; LFTs: AST/ALT, alk phos, bilirubins; CBC w/differential (weekly) |
| Tobramycin | Kidney toxicity, electrolyte abnormalities (K, Mg, Ca), vestibular, toxicity, hearing loss |
Clinical: consider audiometry, balance assessments Laboratory: BMP w/SCr, BUN, K (2x/weekly), TDM for durations >1 week |
| Trimethoprim/Sulfamethoxazole | GI effects (nausea, vomiting, diarrea), cutaneous effects (pruritus, rash), |
Clinical: nausea/vomiting and rash Laboratory: CBC w/differential, ALT, and SCr (1 month, 3 months, then annually) |
Abbreviations: Alk Phos – alkaline phosphatase, ALT – alanine aminotransferase, AST – aspartate aminotransferase, BMP – basic metabolic panel, BUN – blood urea nitrogen, Ca – calcium, CBC – complete blood count, CNS – central nervous system, DDI – drug drug interaction, ECG – electrocardiogram, GI – gastrointestinal, K – potassium, Mag – magnesium, SCr – serum creatinine, SOB – shortness of breath, TDM – therapeutic drug monitoring, TSH – thyroid stimulating hormone.
Therapeutic drug monitoring (TDM) is the clinical practice of measuring drug concentrations in serum during a treatment course to ensure that target drug concentrations are achieved. The routine use of TDM other than to manage aminoglycoside therapy for NTM infections is not common. However, TDM of other drugs should be considered in situations where there are concerns for inappropriate drug exposure. Low therapeutic exposure could lead to treatment failure and/or resistance development while high exposure can lead to exposure dependent toxicity and intolerability of the drug program. Common reasons to consider TDM would include: sub-optimal treatment response, drug-drug interactions, exposure dependent toxicity development, renal/hepatic compromise, or malabsorption [4], [27].
We recommend 2 h and 6-h serum drug levels following the end of infusion for amikacin, streptomycin, and tobramycin to calculate a back-extrapolated Cmax. We recommend the following targets: goal amikacin or streptomycin Cmax of 35–45 mcg/ml (for 15 mg/kg once daily dosing) [27]. A Cmax of 65–80 mcg/ml may also be considered for a 25 mg/kg dose given three times weekly [55]. We recommend a goal tobramycin (for M. chelonae) Cmax of 20–30 mcg/ml on 7 mg/kg daily dosing. If serial levels are not feasible, consideration may be given to a single serum level taken 1-h following the end of infusion for amikacin or streptomycin targeting a serum range of 25–35 mcg/ml. For tobramycin, a single serum level taken 6–14 h following the start of infusion can be used, applying the Hartford nomogram [56]. After attainment of appropriate goal targets with stable renal function, we recommend weekly trough monitoring with a goal of ‘undetectable’ to ensure no drug accumulation.
11. Adjunctive therapies for NTM infections
Reduction of immunosuppression. Immunosuppressive therapies, such as oral and inhaled corticosteroids and TNF-alpha inhibitors, increase the risk for NTM disease. Prednisone equivalent doses of greater than 15 mg per day or inhaled fluticasone doses more than 800 mg per day are associated with a higher risk of NTM disease [57]. For patients taking TNF-alpha inhibitors, a United States’ study showed a 5–10 fold higher rate of NTM pulmonary disease as compared to the general population and rheumatoid arthritis patients on other therapies [58]. Infliximab showed the highest rates of NTM lung disease. However, Etanercept therapy was associated with more serious infections, including fatal MAC lung disease, fatal pulmonary M. abscessus infection, M. chelonae endophthalmitis, and M. xenopi spinal osteomyelitis [59].
Unlike with M. tuberculosis, routine screening for NTM disease prior to the initiation of TNF-alpha inhibitors is not recommended. However, certain patients, such as those with refractory, chronic cough may benefit from CT imaging and sputum testing for NTM disease prior to the start of immunosuppressive therapy [57]. When NTM disease is diagnosed, decreasing or discontinuing immunosuppressive medications can beneficial. For patients that need to continue on immunosuppressive medications, non-biologic agents such as methotrexate and low risks therapies such as abatacept should be considered [57].
Surgical resection. For extra-pulmonary NTM infections, such as skin/soft tissue, musculoskeletal, and lymphadenitis, surgical interventions such as debridement, excision, and drainage of abscesses are often a necessary and important component of the management of these infections [60], [61], [62], [63], [64], [65].
The situation is vastly different when it comes to pulmonary NTM infections. Here, surgical resection plays a role only in select situations and in facilities where a surgical and multidisciplinary team experienced in managing such interventions is available. Typical indications for surgical therapy include failure of medical therapy and alleviation of life-threatening symptoms such as hemoptysis. In areas of extensive lung damage, antimicrobial penetration is low leading to treatment failure [3], [4]. In a trial of 371 patients with mostly cavitary NTM disease, only 36% of patients achieved a cure after 24 months of multi-drug therapy [66]. In such cases, de-bulking surgery may slow disease progression as an adjunct to medical therapy. Drug therapy and medical interventions should precede surgery and continue afterward. Nutrition status should also be optimized prior to surgery. Studies reporting outcomes of surgical therapy in pulmonary NTM infections are mostly observational, heterogeneous in their patient and mycobacterial characteristics, and suffer from inherent selection bias. That notwithstanding, most reports indicate that adjunct surgical therapy can lead to improved patient outcomes with a low rate of complications [67], [68], [69], [70], [71].
Interferon-γ. INF-γ, a cytokine produced by CD4 lymphocytes, has been administered exogenously for the treatment of a variety of syndromes ranging from chronic granulomatous disease to hematologic malignancies [72], [73]. INF-γ plays a critical role in host defenses against mycobacteria through the activation of macrophages [74], [75]. The identification of low INF-γ production in certain individuals susceptible to NTM disease led to exploration of exogenous administration of various formulations of INF-γ for NTM treatment [76], [77], [78]. A randomized, double blind, placebo-controlled study performed in Cuba assessed 32 patients assigned to either placebo or recombinant, intramuscular INF-γ once daily for a month then three times per week up to 6 months as adjuvant to traditional antimicrobials. At the final evaluation, 72% of patients in the treatment group and 36% in the placebo group were deemed complete responders [78]. In contrast, a brief report from 2001 describes no sustained benefit of subcutaneous INF-γ therapy and an inability of INF-γ to improve the production of Th1 cytokines [79]. Flu-like symptoms including fevers, chills, and malaise are common with INF-γ [80]. Treatment with INF-γ may be a consideration in the treatment of select non-HIV patients with progressive, refractory NTM disease associated with a demonstrated INF-γ deficiency.
Rituximab. A specific syndrome of lymphadenitis associated with disseminated, rapid-growing NTM organisms has been identified in persons of Asian descent without HIV infection [81], [82]. Co-infection with endemic fungi, herpes viruses, salmonella as well as reactive skin diseases is common [81], [82]. High titers of antibodies neutralizing interferon-γ are seen resulting in increased susceptibility to these infections; thus, immune modulating therapies have been employed as adjunctive therapy to augment the body’s natural defenses [83], [84], [85], [86], [87], [88], [89].
Rituximab is an anti-CD20 monoclonal antibody that rapidly depletes circulating and tissue B-cells which has been used in the treatment of several autoantibody diseases including pemphigus vulgaris, pulmonary alveolar proteinosis, myasthenia gravis and red cell aplasia [90], [91], [92], [93], [94], [95]. Rituximab was employed to halt progressive NTM disease by neutralizing anti-interferon-γ antibodies in a small number of cases in Asian patients without HIV infection. In both reports, patient’s anti-interferon-γ levels were shown to be elevated at baseline. Subsequent to rituximab treatment, autoantibody titer was reduced and IFN-γ signaling was improved. In conjunction with laboratory marker improvement, all patients showed clinical improvement and none experienced significant adverse effect from rituximab [96], [97]. Rituximab dosing was based on lymphoma regimens at 375 mg/m2 given every 7 for at least 4 doses [90]. In one of the case reports, methylprednisolone was also given at 100 mg daily [96]. There is a small body of evidence that rituximab may serve as an important adjunctive to anti-mycobacterials for immune restoration purposes in patients with progressive, severe anti-interferon-γ associated NTM disease.
Non-pharmacological interventions. Beyond antimicrobial therapy, non-pharmacological interventions can contribute to the alleviation of symptoms and improvement in the quality of life of patients with NTM infections, and as such should be a component of the overall management of NTM infections [98]. These interventions include tobacco cessation (where applicable), pulmonary rehabilitation/pulmonary hygiene, exercise programs, and nutritional support.
Pulmonary hygiene. Many patients with NTM pulmonary disease also have bronchiectasis and signs of airway mucous plugging. In patients with concomitant bronchiectasis, bronchial hygiene regimens with active airway clearance interventions can be used as adjunctive therapy to theoretically facilitate clearance of NTM and prevent superimposed bacterial infections [4], [98]. Retrospective data suggests that pulmonary hygiene alone with active surveillance may be appropriate in patients with mild nodular bronchiectatic MAC pulmonary disease. Active airway clearance interventions have not been prospectively studied in NTM patients with bronchiectasis, but potential benefits are suspected based on expert opinion [99]. These regimens can include mucous clearance devices, such as flutter valves and chest vest, and inhaled medications such as, hypertonic saline nebulizations. An individualized approach and careful introduction of these treatments by a respiratory therapist or experienced nurse are recommended since these can cause bronchospasm and/or airway irritation. Short acting bronchodilators should precede administration of nebulized hypertonic saline in patients with airway hyper-reactivity.
Exercise training. The evidence supporting the benefit of exercise in the management of NTM infections is largely an extrapolation of studies conducted in patients with bronchiectasis, a common comorbidity in patients with NTM lung disease. These studies were of variable duration (3–8 weeks) and of variable design (inpatient or outpatient; with or without supervision; with or without respiratory physiotherapy). Overall, these programs resulted in improvements in exercise capacity and health-related quality of life, as well as decreases in the frequency of acute exacerbations [100], [101], [102], [103].
Nutrition. The linkage between infection and nutrition is not new. Poor nutrition can predispose to infection, infection in turn can contribute to malnutrition [104]. Weight loss is a common symptom in patients with NTM infections and may be further exacerbated by decreased appetite, nausea, early satiety, related to the treatment of the NTM infection itself [105], [106]. Poor nutritional status has been associated with poor outcomes in MAC lung disease [107], [108]. Vitamin A deficiency was strongly associated with the prevalence and risk for NTM pulmonary disease [109]. Dietitians and nutritionists are important members of the NTM management team. Nutrition assistance can ensure adequate protein and caloric intake, while providing guidance regarding other nutritional supplements, vitamins, micronutrients, and antioxidants.
12. Summary
Non-tuberculous mycobacterial infections are an increasingly important contributor to human disease, causing a wide range of infection from localized soft-tissue infiltration to disseminated life-threatening conditions. Having a ubiquitous presence in nature can make it challenging to discern NTM biological sample contamination from a true NTM infection. The clinical and radiographic features are non-specific; microbiologic diagnosis is cumbersome and insufficiently informative. Treatment is complex, requiring multi-drug regimens with substantial associated toxicity, administered for a prolonged period. Defining treatment milestones is difficult; clinical outcomes are often disappointing.
Given these considerable challenges, a thorough systematic approach to the diagnosis and treatment of NTM infections should be adopted to maximize the likelihood of positive treatment outcomes. Early diagnosis is key and requires a high index of suspicion and an understanding of the clinical scenarios suggestive of the presence of NTM infection. Identification of the causative organism to the species level should be performed; antimicrobial susceptibility should be tested on all isolates where suspicion of a true NTM infection exists. Treatment should be directed by AST results, in compliance with prevailing guidelines, and with careful attention paid to patient education and proactive monitoring for treatment responses as well as treatment-emergent adverse drug reactions. Antimicrobial therapy should be complemented by adjunct therapies such as pulmonary hygiene measures, nutritional support, and exercise training programs to improve clinical outcomes and quality of life.
Ethical statement
This paper has been written, revised, and reviewed by all co-authors.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- 1.Porvaznik I., Solovic I., Mokry J. Non-tuberculous mycobacteria: classification, diagnostics, and therapy. Adv Exp Med Biol. 2017;944:19–25. doi: 10.1007/5584_2016_45. [DOI] [PubMed] [Google Scholar]
- 2.Adzic-Vukicevic T., Barac A., Blanka-Protic A. Clinical features of infection caused by non-tuberculous mycobacteria: 7 years' experience. Infection. 2018;46(3):357–363. doi: 10.1007/s15010-018-1128-2. [DOI] [PubMed] [Google Scholar]
- 3.Griffith D.E., Aksamit T., Brown-Elliott B.A. An official ATS/IDSA statement: diagnosis, treatment, and prevention of nontuberculous mycobacterial diseases. Am J Resp Crit Care. 2007;175(4):367–416. doi: 10.1164/rccm.200604-571ST. [DOI] [PubMed] [Google Scholar]
- 4.Daley C.L., Iaccarino J.M., Lange C. Treatment of nontuberculous mycobacterial pulmonary disease: an official ATS/ERS/ESCMID/IDSA clinical practice guideline. Clin Infect Dis. 2020;71(4):905–913. doi: 10.1093/cid/ciaa1125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.van Ingen J. Microbiological diagnosis of nontuberculous mycobacterial pulmonary disease. Clin Chest Med. 2015;36(1):43–54. doi: 10.1016/j.ccm.2014.11.005. [DOI] [PubMed] [Google Scholar]
- 6.van Ingen J. Diagnosis of nontuberculous mycobacterial infections. Semin Respir Crit Care Med. 2013;34(1):103–109. doi: 10.1055/s-0033-1333569. [DOI] [PubMed] [Google Scholar]
- 7.Bange F.C., Bottger E.C. Improved decontamination method for recovering mycobacteria from patients with cystic fibrosis. Eur J Clin Microbiol Infect Dis. 2002;21(7):546–548. doi: 10.1007/s10096-002-0760-y. [DOI] [PubMed] [Google Scholar]
- 8.Bange F.C., Kirschner P., Bottger E.C. Recovery of mycobacteria from patients with cystic fibrosis. J Clin Microbiol. 1999;37(11):3761–3763. doi: 10.1128/jcm.37.11.3761-3763.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ferroni A., Vu-Thien H., Lanotte P. Value of the chlorhexidine decontamination method for recovery of nontuberculous mycobacteria from sputum samples of patients with cystic fibrosis. J Clin Microbiol. 2006;44(6):2237–2239. doi: 10.1128/JCM.00285-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Steingart K.R., Ng V., Henry M. Sputum processing methods to improve the sensitivity of smear microscopy for tuberculosis: a systematic review. Lancet Infect Dis. 2006;6(10):664–674. doi: 10.1016/S1473-3099(06)70602-8. [DOI] [PubMed] [Google Scholar]
- 11.Bonnet M., Ramsay A., Githui W., Gagnidze L., Varaine F., Guerin P.J. Bleach sedimentation: an opportunity to optimize smear microscopy for tuberculosis diagnosis in settings of high prevalence of HIV. Clin Infect Dis. 2008;46(11):1710–1716. doi: 10.1086/587891. [DOI] [PubMed] [Google Scholar]
- 12.Middlebrook G., Cohn M.L. Bacteriology of tuberculosis: laboratory methods. Am J Public Health Nations Health. 1958;48(7):844–853. doi: 10.2105/ajph.48.7.844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kassaza K., Orikiriza P., Llosa A. Lowenstein-Jensen selective medium for reducing contamination in Mycobacterium tuberculosis culture. J Clin Microbiol. 2014;52(7):2671–2673. doi: 10.1128/JCM.00749-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vestal A. Procedures for isolation and identification of mycobacteria DHEW publication No. 75-8530. In Centers for disease control and prevention. Georgia, USA; 1975.
- 15.Kontos F., Maniati M., Costopoulos C. Evaluation of the fully automated Bactec MGIT 960 system for the susceptibility testing of Mycobacterium tuberculosis to first-line drugs: a multicenter study. J Microbiol Methods. 2004;56(2):291–294. doi: 10.1016/j.mimet.2003.10.015. [DOI] [PubMed] [Google Scholar]
- 16.Shinnick T.M., Iademarco M.F., Ridderhof J.C. National plan for reliable tuberculosis laboratory services using a systems approach. Recommendations from CDC and the Association of Public Health Laboratories Task Force on Tuberculosis Laboratory Services. MMWR Recomm Rep. 2005;54(RR-6):1–12. [PubMed] [Google Scholar]
- 17.Yu M.C., Chen H.Y., Wu M.H. Evaluation of the rapid MGIT TBc identification test for culture confirmation of Mycobacterium tuberculosis complex strain detection. J Clin Microbiol. 2011;49(3):802–807. doi: 10.1128/JCM.02243-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mediavilla-Gradolph M.C., De Toro-Peinado I., Bermudez-Ruiz M.P. Use of MALDI-TOF MS for identification of nontuberculous mycobacterium species isolated from clinical specimens. Biomed Res Int. 2015 doi: 10.1155/2015/854078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Scoleri G.P., Choo J.M., Leong L.E. Culture-independent detection of nontuberculous mycobacteria in clinical respiratory samples. J Clin Microbiol. 2016;54(9):2395–2398. doi: 10.1128/JCM.01410-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Huh H.J., Song D.J., Ki C.S., Lee N.Y. Is Cross-reactivity with nontuberculous mycobacteria a systematic problem in the xpert MTB/RIF assay? Tuberc Respir Dis (Seoul) 2019;82(1):88–89. doi: 10.4046/trd.2018.0075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.McShane P.J., Glassroth J. Pulmonary disease due to nontuberculous mycobacteria: current state and new insights. Chest. 2015;148(6):1517–1527. doi: 10.1378/chest.15-0458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cowman S., Burns K., Benson S., Wilson R., Loebinger M.R. The antimicrobial susceptibility of non-tuberculous mycobacteria. J Infect. 2016;72(3):324–331. doi: 10.1016/j.jinf.2015.12.007. [DOI] [PubMed] [Google Scholar]
- 23.Mukhopadhyay S., Gal A.A. Granulomatous lung disease: an approach to the differential diagnosis. Arch Pathol Lab Med. 2010;134(5):667–690. doi: 10.5858/134.5.667. [DOI] [PubMed] [Google Scholar]
- 24.Jarzembowski J.A., Young M.B. Nontuberculous mycobacterial infections. Arch Pathol Lab Med. 2008;132(8):1333–1341. doi: 10.5858/2008-132-1333-NMI. [DOI] [PubMed] [Google Scholar]
- 25.Wayne P. Clinical and Laboratory Standards Institute (CLSI): Susceptibility testing …. 2011. Susceptibility testing of mycobacteria, nocardiae, and other aerobic actinomycetes; approved Standard. [PubMed] [Google Scholar]
- 26.Brown-Elliott B.A., Nash K.A., Wallace R.J., Jr. Antimicrobial susceptibility testing, drug resistance mechanisms, and therapy of infections with nontuberculous mycobacteria. Clin Microbiol Rev. 2012;25(3):545–582. doi: 10.1128/CMR.05030-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Egelund E.F., Fennelly K.P., Peloquin C.A. Medications and monitoring in nontuberculous mycobacteria infections. Clin Chest Med. 2015;36(1):55–66. doi: 10.1016/j.ccm.2014.11.001. [DOI] [PubMed] [Google Scholar]
- 28.Nash K.A., Andini N., Zhang Y., Brown-Elliott B.A., Wallace R.J. Intrinsic macrolide resistance in rapidly growing mycobacteria. Antimicrob Agents Chemother. 2006;50(10):3476–3478. doi: 10.1128/AAC.00402-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Maurer F.P., Castelberg C., Quiblier C., Bottger E.C., Somoskovi A. Erm(41)-dependent inducible resistance to azithromycin and clarithromycin in clinical isolates of Mycobacterium abscessus. J Antimicrob Chemother. 2014;69(6):1559–1563. doi: 10.1093/jac/dku007. [DOI] [PubMed] [Google Scholar]
- 30.Chu H.Q., Li B., Zhao L. Chest imaging comparison between non-tuberculous and tuberculosis mycobacteria in sputum acid fast bacilli smear-positive patients. Eur Rev Med Pharmacol Sci. 2015;19(13):2429–2439. doi: 10.1183/13993003.congress-2015.pa2674. [DOI] [PubMed] [Google Scholar]
- 31.Iseman M.D., Buschman D.L., Ackerson L.M. Pectus excavatum and scoliosis. Thoracic anomalies associated with pulmonary disease caused by Mycobacterium avium complex. Am Rev Respir Dis. 1991;144(4):914–916. doi: 10.1164/ajrccm/144.4.914. [DOI] [PubMed] [Google Scholar]
- 32.Jeong Y.J., Lee K.S., Koh W.J., Han J., Kim T.S., Kwon O.J. Nontuberculous mycobacterial pulmonary infection in immunocompetent patients: comparison of thin-section CT and histopathologic findings. Radiology. 2004;231(3):880–886. doi: 10.1148/radiol.2313030833. [DOI] [PubMed] [Google Scholar]
- 33.Miller W.T., Jr., Panosian J.S. Causes and imaging patterns of tree-in-bud opacities. Chest. 2013;144(6):1883–1892. doi: 10.1378/chest.13-1270. [DOI] [PubMed] [Google Scholar]
- 34.Rickman O.B., Ryu J.H., Fidler M.E., Kalra S. Hypersensitivity pneumonitis associated with Mycobacterium avium complex and hot tub use. Mayo Clin Proc. 2002;77(11):1233–1237. doi: 10.4065/77.11.1233. [DOI] [PubMed] [Google Scholar]
- 35.Hanak V., Kalra S., Aksamit T.R., Hartman T.E., Tazelaar H.D., Ryu J.H. Hot tub lung: presenting features and clinical course of 21 patients. Respir Med. 2006;100(4):610–615. doi: 10.1016/j.rmed.2005.08.005. [DOI] [PubMed] [Google Scholar]
- 36.Marras T.K., Wallace R.J., Jr., Koth L.L., Stulbarg M.S., Cowl C.T., Daley C.L. Hypersensitivity pneumonitis reaction to Mycobacterium avium in household water. Chest. 2005;127(2):664–671. doi: 10.1378/chest.127.2.664. [DOI] [PubMed] [Google Scholar]
- 37.Lindeboom J.A., Smets A.M., Kuijper E.J., van Rijn R.R., Prins J.M. The sonographic characteristics of nontuberculous mycobacterial cervicofacial lymphadenitis in children. Pediatr Radiol. 2006;36(10):1063–1067. doi: 10.1007/s00247-006-0271-6. [DOI] [PubMed] [Google Scholar]
- 38.Bagla S., Tunkel D., Kraut M.A. Nontuberculous mycobacterial lymphadenitis of the head and neck: radiologic observations and clinical context. Pediatr Radiol. 2003;33(6):402–406. doi: 10.1007/s00247-003-0884-y. [DOI] [PubMed] [Google Scholar]
- 39.Theodorou D.J., Theodorou S.J., Kakitsubata Y., Sartoris D.J., Resnick D. Imaging characteristics and epidemiologic features of atypical mycobacterial infections involving the musculoskeletal system. AJR Am J Roentgenol. 2001;176(2):341–349. doi: 10.2214/ajr.176.2.1760341. [DOI] [PubMed] [Google Scholar]
- 42.Dorman S.E., Holland S.M. Interferon-gamma and interleukin-12 pathway defects and human disease. Cytokine Growth Factor Rev. 2000;11(4):321–333. doi: 10.1016/s1359-6101(00)00010-1. [DOI] [PubMed] [Google Scholar]
- 43.Martinez-Barricarte R., Markle J.G., Ma C.S. Human IFN-gamma immunity to mycobacteria is governed by both IL-12 and IL-23. Sci Immunol. 2018;3(30) doi: 10.1126/sciimmunol.aau6759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Griffith D.E., Aksamit T.R. Bronchiectasis and nontuberculous mycobacterial disease. Clin Chest Med. 2012;33(2):283–295. doi: 10.1016/j.ccm.2012.02.002. [DOI] [PubMed] [Google Scholar]
- 45.Cottle L.E. Mendelian susceptibility to mycobacterial disease. Clin Genet. 2011;79(1):17–22. doi: 10.1111/j.1399-0004.2010.01510.x. [DOI] [PubMed] [Google Scholar]
- 46.Divangahi M., Khan N., Kaufmann E. Beyond killing mycobacterium tuberculosis: disease tolerance. Front Immunol. 2018;9:2976. doi: 10.3389/fimmu.2018.02976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Winthrop K.L., McNelley E., Kendall B. Pulmonary nontuberculous mycobacterial disease prevalence and clinical features: an emerging public health disease. Am J Respir Crit Care Med. 2010;182(7):977–982. doi: 10.1164/rccm.201003-0503OC. [DOI] [PubMed] [Google Scholar]
- 48.O'Brien R.J., Geiter L.J., Snider D.E., Jr. The epidemiology of nontuberculous mycobacterial diseases in the United States. Results from a national survey. Am Rev Respir Dis. 1987;135(5):1007–1014. doi: 10.1164/arrd.1987.135.5.1007. [DOI] [PubMed] [Google Scholar]
- 49.Field S.K., Escalante P., Fisher D.A., Ireland B., Irwin R.S., Panel C.E.C. Cough due to TB and other chronic infections: CHEST guideline and expert panel report. Chest. 2018;153(2):467–497. doi: 10.1016/j.chest.2017.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kim D.H., Jhun B.W., Moon S.M. In vitro activity of bedaquiline and delamanid against nontuberculous mycobacteria, including macrolide-resistant clinical isolates. Antimicrob Agents Chemother. 2019;63(8) doi: 10.1128/AAC.00665-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zhang F., Li S., Wen S. Comparison of in vitro susceptibility of mycobacteria against PA-824 to identify key residues of ddn, the deazoflavin-dependent nitroreductase from Mycobacterium tuberculosis. Infect Drug Resist. 2020;13:815–822. doi: 10.2147/IDR.S240716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Balavoine C., Lanotte P., Campana M., Ghanem M., Marchand-Adam S. Hypersensitivity pneumonitis and abscess reaction to nontuberculous mycobacteria acquired form jacuzzi aerosol. Rev Mal Respir. 2019;36(1):57–62. doi: 10.1016/j.rmr.2017.10.670. [DOI] [PubMed] [Google Scholar]
- 53.Kamii Y., Nagai H., Kawashima M. Adverse reactions associated with long-term drug administration in Mycobacterium avium complex lung disease. Int J Tuberc Lung Dis. 2018;22(12):1505–1510. doi: 10.5588/ijtld.18.0171. [DOI] [PubMed] [Google Scholar]
- 54.Kwon Y.S., Kwon B.S., Kim O.H. Treatment outcomes after discontinuation of ethambutol due to adverse events in mycobacterium avium complex lung disease. J Korean Med Sci. 2020;35(9) doi: 10.3346/jkms.2020.35.e59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Peloquin C.A., Berning S.E., Nitta A.T. Aminoglycoside toxicity: daily versus thrice-weekly dosing for treatment of mycobacterial diseases. Clin Infect Dis. 2004;38(11):1538–1544. doi: 10.1086/420742. [DOI] [PubMed] [Google Scholar]
- 56.Shulha J.A., Escalante P., Wilson J.W. Pharmacotherapy approaches in nontuberculous Mycobacteria infections. Mayo Clin Proc. 2019;94(8):1567–1581. doi: 10.1016/j.mayocp.2018.12.011. [DOI] [PubMed] [Google Scholar]
- 57.Henkle E., Winthrop K.L. Nontuberculous mycobacteria infections in immunosuppressed hosts. Clin Chest Med. 2015;36(1):91–99. doi: 10.1016/j.ccm.2014.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Winthrop K.L., Baxter R., Liu L. Mycobacterial diseases and antitumour necrosis factor therapy in USA. Ann Rheum Dis. 2013;72(1):37–42. doi: 10.1136/annrheumdis-2011-200690. [DOI] [PubMed] [Google Scholar]
- 59.Sexton P., Harrison A.C. Susceptibility to nontuberculous mycobacterial lung disease. Eur Respir J. 2008;31(6):1322–1333. doi: 10.1183/09031936.00140007. [DOI] [PubMed] [Google Scholar]
- 60.Uslan D.Z., Kowalski T.J., Wengenack N.L., Virk A., Wilson J.W. Skin and soft tissue infections due to rapidly growing mycobacteria: comparison of clinical features, treatment, and susceptibility. Arch Dermatol. 2006;142(10):1287–1292. doi: 10.1001/archderm.142.10.1287. [DOI] [PubMed] [Google Scholar]
- 61.Hogan J.I., Hurtado R.M., Nelson S.B. Mycobacterial Musculoskeletal Infections. Infect Dis Clin North Am. 2017;31(2):369–382. doi: 10.1016/j.idc.2017.01.007. [DOI] [PubMed] [Google Scholar]
- 62.Spinelli G., Mannelli G., Arcuri F., Venturini E., Chiappini E., Galli L. Surgical treatment for chronic cervical lymphadenitis in children. Experience from a tertiary care paediatric centre on non-tuberculous mycobacterial infections. Int J Pediatr Otorhinolaryngol. 2018;108:137–142. doi: 10.1016/j.ijporl.2018.02.042. [DOI] [PubMed] [Google Scholar]
- 63.Wi Y.M. Treatment of extrapulmonary nontuberculous mycobacterial diseases. Infect Chemother. 2019;51(3):245–255. doi: 10.3947/ic.2019.51.3.245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Goldstein N., St Clair J.B., Kasperbauer S.H., Daley C.L., Lindeque B. Nontuberculous mycobacterial musculoskeletal infection cases from a Tertiary Referral Center, Colorado, USA. Emerg Infect Dis. 2019;25(6):1075–1083. doi: 10.3201/eid2506.181041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Bollam R., Phan T. Mycobacterium marinum infection of the hand presenting as a nodular skin lesion. J Clin Tuberc Other Mycobact Dis. 2020;20 doi: 10.1016/j.jctube.2020.100166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.van Ingen J., Verhagen A.F., Dekhuijzen P.N. Surgical treatment of non-tuberculous mycobacterial lung disease: strike in time. Int J Tuberc Lung Dis. 2010;14(1):99–105. [PubMed] [Google Scholar]
- 67.Mitchell J.D. Surgical treatment of pulmonary nontuberculous mycobacterial infections. Thorac Surg Clin. 2019;29(1):77–83. doi: 10.1016/j.thorsurg.2018.09.011. [DOI] [PubMed] [Google Scholar]
- 68.Mitchell J.D., Bishop A., Cafaro A., Weyant M.J., Pomerantz M. Anatomic lung resection for nontuberculous mycobacterial disease. Ann Thorac Surg. 2008;85(6):1887–1892. doi: 10.1016/j.athoracsur.2008.02.041. discussion 1892–1883. [DOI] [PubMed] [Google Scholar]
- 69.Shiraishi Y., Katsuragi N., Kita H., Hyogotani A., Saito M.H., Shimoda K. Adjuvant surgical treatment of nontuberculous mycobacterial lung disease. Ann Thorac Surg. 2013;96(1):287–291. doi: 10.1016/j.athoracsur.2013.03.008. [DOI] [PubMed] [Google Scholar]
- 70.Asakura T., Hayakawa N., Hasegawa N. Long-term outcome of pulmonary resection for nontuberculous mycobacterial pulmonary disease. Clin Infect Dis. 2017;65(2):244–251. doi: 10.1093/cid/cix274. [DOI] [PubMed] [Google Scholar]
- 71.Aznar M.L., Zubrinic M., Siemienowicz M. Adjuvant lung resection in the management of nontuberculous mycobacterial lung infection: a retrospective matched cohort study. Respir Med. 2018;142:1–6. doi: 10.1016/j.rmed.2018.07.003. [DOI] [PubMed] [Google Scholar]
- 72.A controlled trial of interferon gamma to prevent infection in chronic granulomatous disease. The International Chronic Granulomatous Disease Cooperative Study Group. N Engl J Med 1991;324(8):509–16. [DOI] [PubMed]
- 73.Nathan C.F., Kaplan G., Levis W.R. Local and systemic effects of intradermal recombinant interferon-gamma in patients with lepromatous leprosy. N Engl J Med. 1986;315(1):6–15. doi: 10.1056/NEJM198607033150102. [DOI] [PubMed] [Google Scholar]
- 74.Chan J., Xing Y., Magliozzo R.S., Bloom B.R. Killing of virulent Mycobacterium tuberculosis by reactive nitrogen intermediates produced by activated murine macrophages. J Exp Med. 1992;175(4):1111–1122. doi: 10.1084/jem.175.4.1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Flynn J.L., Chan J., Triebold K.J., Dalton D.K., Stewart T.A., Bloom B.R. An essential role for interferon gamma in resistance to Mycobacterium tuberculosis infection. J Exp Med. 1993;178(6):2249–2254. doi: 10.1084/jem.178.6.2249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Hallstrand T.S., Ochs H.D., Zhu Q., Liles W.C. Inhaled IFN-gamma for persistent nontuberculous mycobacterial pulmonary disease due to functional IFN-gamma deficiency. Eur Respir J. 2004;24(3):367–370. doi: 10.1183/09031936.04.00036704. [DOI] [PubMed] [Google Scholar]
- 77.Holland S.M., Eisenstein E.M., Kuhns D.B. Treatment of refractory disseminated nontuberculous mycobacterial infection with interferon gamma. A preliminary report. N Engl J Med. 1994;330(19):1348–1355. doi: 10.1056/NEJM199405123301904. [DOI] [PubMed] [Google Scholar]
- 78.Milanes-Virelles M.T., Garcia-Garcia I., Santos-Herrera Y. Adjuvant interferon gamma in patients with pulmonary atypical Mycobacteriosis: a randomized, double-blind, placebo-controlled study. BMC Infect Dis. 2008;8:17. doi: 10.1186/1471-2334-8-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Lauw F.N., van Der Meer J.T., de Metz J., Danner S.A., van Der Poll T. No beneficial effect of interferon-gamma treatment in 2 human immunodeficiency virus-infected patients with Mycobacterium avium complex infection. Clin Infect Dis. 2001;32(4):e81–e82. doi: 10.1086/318705. [DOI] [PubMed] [Google Scholar]
- 80.Vial T., Descotes J. Clinical toxicity of the interferons. Drug Saf. 1994;10(2):115–150. doi: 10.2165/00002018-199410020-00003. [DOI] [PubMed] [Google Scholar]
- 81.Chetchotisakd P., Mootsikapun P., Anunnatsiri S. Disseminated infection due to rapidly growing mycobacteria in immunocompetent hosts presenting with chronic lymphadenopathy: a previously unrecognized clinical entity. Clin Infect Dis. 2000;30(1):29–34. doi: 10.1086/313589. [DOI] [PubMed] [Google Scholar]
- 82.Chetchotisakd P., Kiertiburanakul S., Mootsikapun P., Assanasen S., Chaiwarith R., Anunnatsiri S. Disseminated nontuberculous mycobacterial infection in patients who are not infected with HIV in Thailand. Clin Infect Dis. 2007;45(4):421–427. doi: 10.1086/520030. [DOI] [PubMed] [Google Scholar]
- 83.Patel S.Y., Ding L., Brown M.R. Anti-IFN-gamma autoantibodies in disseminated nontuberculous mycobacterial infections. J Immunol. 2005;175(7):4769–4776. doi: 10.4049/jimmunol.175.7.4769. [DOI] [PubMed] [Google Scholar]
- 84.Doffinger R., Helbert M.R., Barcenas-Morales G. Autoantibodies to interferon-gamma in a patient with selective susceptibility to mycobacterial infection and organ-specific autoimmunity. Clin Infect Dis. 2004;38(1):e10–e14. doi: 10.1086/380453. [DOI] [PubMed] [Google Scholar]
- 85.Hoflich C., Sabat R., Rosseau S. Naturally occurring anti-IFN-gamma autoantibody and severe infections with Mycobacterium cheloneae and Burkholderia cocovenenans. Blood. 2004;103(2):673–675. doi: 10.1182/blood-2003-04-1065. [DOI] [PubMed] [Google Scholar]
- 86.Kampitak T., Suwanpimolkul G., Browne S., Suankratay C. Anti-interferon-gamma autoantibody and opportunistic infections: case series and review of the literature. Infection. 2011;39(1):65–71. doi: 10.1007/s15010-010-0067-3. [DOI] [PubMed] [Google Scholar]
- 87.Kampmann B., Hemingway C., Stephens A. Acquired predisposition to mycobacterial disease due to autoantibodies to IFN-gamma. J Clin Invest. 2005;115(9):2480–2488. doi: 10.1172/JCI19316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Koya T., Tsubata C., Kagamu H. Anti-interferon-gamma autoantibody in a patient with disseminated Mycobacterium avium complex. J Infect Chemother. 2009;15(2):118–122. doi: 10.1007/s10156-008-0662-8. [DOI] [PubMed] [Google Scholar]
- 89.Poulin S., Corbeil C., Nguyen M. Fatal Mycobacterium colombiense/cytomegalovirus coinfection associated with acquired immunodeficiency due to autoantibodies against interferon gamma: a case report. BMC Infect Dis. 2013;13:24. doi: 10.1186/1471-2334-13-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Maloney D.G., Grillo-Lopez A.J., Bodkin D.J. IDEC-C2B8: results of a phase I multiple-dose trial in patients with relapsed non-Hodgkin's lymphoma. J Clin Oncol. 1997;15(10):3266–3274. doi: 10.1200/JCO.1997.15.10.3266. [DOI] [PubMed] [Google Scholar]
- 91.Ahmed A.R., Spigelman Z., Cavacini L.A., Posner M.R. Treatment of pemphigus vulgaris with rituximab and intravenous immune globulin. N Engl J Med. 2006;355(17):1772–1779. doi: 10.1056/NEJMoa062930. [DOI] [PubMed] [Google Scholar]
- 92.Illa I., Diaz-Manera J., Rojas-Garcia R. Sustained response to Rituximab in anti-AChR and anti-MuSK positive Myasthenia Gravis patients. J Neuroimmunol. 2008;201–202:90–94. doi: 10.1016/j.jneuroim.2008.04.039. [DOI] [PubMed] [Google Scholar]
- 93.Lindberg C., Bokarewa M. Rituximab for severe myasthenia gravis–experience from five patients. Acta Neurol Scand. 2010;122(4):225–228. doi: 10.1111/j.1600-0404.2010.01345.x. [DOI] [PubMed] [Google Scholar]
- 94.Borie R., Debray M.P., Laine C., Aubier M., Crestani B. Rituximab therapy in autoimmune pulmonary alveolar proteinosis. Eur Respir J. 2009;33(6):1503–1506. doi: 10.1183/09031936.00160908. [DOI] [PubMed] [Google Scholar]
- 95.Behler C.M., Terrault N.A., Etzell J.E., Damon L.E. Rituximab therapy for pure red cell aplasia due to anti-epoetin antibodies in a woman treated with epoetin-alfa: a case report. J Med Case Rep. 2009;3:7335. doi: 10.4076/1752-1947-3-7335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Czaja C.A., Merkel P.A., Chan E.D. Rituximab as successful adjunct treatment in a patient with disseminated nontuberculous mycobacterial infection due to acquired anti-interferon-gamma autoantibody. Clin Infect Dis. 2014;58(6):e115–e118. doi: 10.1093/cid/cit809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Koizumi Y., Sakagami T., Nishiyama N. Rituximab restores IFN-gamma-STAT1 function and ameliorates disseminated mycobacterium avium infection in a patient with anti-interferon-gamma autoantibody. J Clin Immunol. 2017;37(7):644–649. doi: 10.1007/s10875-017-0425-3. [DOI] [PubMed] [Google Scholar]
- 98.Lan C.C., Lai S.R., Chien J.Y. Nonpharmacological treatment for patients with nontuberculous mycobacterial lung disease. J Formos Med Assoc. 2020;119(Suppl 1):S42–S50. doi: 10.1016/j.jfma.2020.05.013. [DOI] [PubMed] [Google Scholar]
- 99.Griffith D.E., Philley J.V. The new “Hesitation Blues”: initiating Mycobacterium avium complex lung disease therapy. Eur Respir J. 2017;49(3) doi: 10.1183/13993003.00110-2017. [DOI] [PubMed] [Google Scholar]
- 100.Patel S., Cole A.D., Nolan C.M. Pulmonary rehabilitation in bronchiectasis: a propensity-matched study. Eur Respir J. 2019;53(1) doi: 10.1183/13993003.01264-2018. [DOI] [PubMed] [Google Scholar]
- 101.Lee A.L., Hill C.J., Cecins N. The short and long term effects of exercise training in non-cystic fibrosis bronchiectasis–a randomised controlled trial. Respir Res. 2014;15:44. doi: 10.1186/1465-9921-15-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Mandal P., Sidhu M.K., Kope L. A pilot study of pulmonary rehabilitation and chest physiotherapy versus chest physiotherapy alone in bronchiectasis. Respir Med. 2012;106(12):1647–1654. doi: 10.1016/j.rmed.2012.08.004. [DOI] [PubMed] [Google Scholar]
- 103.Zanini A., Aiello M., Adamo D. Effects of pulmonary rehabilitation in patients with non-cystic fibrosis bronchiectasis: a retrospective analysis of clinical and functional predictors of efficacy. Respiration. 2015;89(6):525–533. doi: 10.1159/000380771. [DOI] [PubMed] [Google Scholar]
- 104.Katona P., Katona-Apte J. The interaction between nutrition and infection. Clin Infect Dis. 2008;46(10):1582–1588. doi: 10.1086/587658. [DOI] [PubMed] [Google Scholar]
- 105.Wassilew N., Hoffmann H., Andrejak C., Lange C. Pulmonary disease caused by non-tuberculous mycobacteria. Respiration. 2016;91(5):386–402. doi: 10.1159/000445906. [DOI] [PubMed] [Google Scholar]
- 106.Wakamatsu K., Nagata N., Maki S. Patients with MAC lung disease have a low visceral fat area and low nutrient intake. Pulm Med. 2015;2015 doi: 10.1155/2015/218253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Kim S.J., Park J., Lee H. Risk factors for deterioration of nodular bronchiectatic Mycobacterium avium complex lung disease. Int J Tuberc Lung Dis. 2014;18(6):730–736. doi: 10.5588/ijtld.13.0792. [DOI] [PubMed] [Google Scholar]
- 108.Ikegame S., Maki S., Wakamatsu K. Nutritional assessment in patients with pulmonary nontuberculous mycobacteriosis. Intern Med. 2011;50(21):2541–2546. doi: 10.2169/internalmedicine.50.5853. [DOI] [PubMed] [Google Scholar]
- 109.Oh J., Park H.D., Kim S.Y., Koh W.J., Lee S.Y. Assessment of vitamin status in patients with nontuberculous mycobacterial pulmonary disease: potential role of vitamin a as a risk factor. Nutrients. 2019;11(2) doi: 10.3390/nu11020343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Lande L., George J., Plush T. Mycobacterium avium complex pulmonary disease: new epidemiology and management concepts. Curr Opin Infect Dis. 2018;31(2):199–207. doi: 10.1097/QCO.0000000000000437. [DOI] [PubMed] [Google Scholar]
- 111.Jindal N., Devi B., Aggarwal A. Mycobacterial cervical lymphadenitis in childhood. Indian J Med Sci. 2003;57(1):12–15. [PubMed] [Google Scholar]
- 112.Wood B.R., Buitrago M.O., Patel S., Hachey D.H., Haneuse S., Harrington R.D. Mycobacterium avium complex osteomyelitis in persons with human immunodeficiency virus: case series and literature review. Open forum. Infect Dis. 2015;2(3) doi: 10.1093/ofid/ofv090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Piccini P., Venturini E., Romano F. Osteomyelitis and arthritis caused by Mycobacterium intracellulare: an underestimated diagnosis. Int J Tuberc Lung Dis. 2017;21(10):1184–1185. doi: 10.5588/ijtld.17.0393. [DOI] [PubMed] [Google Scholar]
- 114.Varley C.D., Ku J.H., Henkle E., Schafer S.D., Winthrop K.L. Disseminated nontuberculous mycobacteria in HIV-infected patients, Oregon, USA, 2007–2012. Emerg Infect Dis. 2017;23(3):533–535. doi: 10.3201/eid2303.161708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Baldolli A., Daurel C., Verdon R., de La Blanchardiere A. High mortality in peritonitis due to Mycobacterium avium complex: retrospective study and systematic literature review. Infect Dis (Lond) 2019;51(2):81–90. doi: 10.1080/23744235.2018.1519639. [DOI] [PubMed] [Google Scholar]
- 116.Scriven J.E., Scobie A., Verlander N.Q. Mycobacterium chimaera infection following cardiac surgery in the United Kingdom: clinical features and outcome of the first 30 cases. Clin Microbiol Infect. 2018;24(11):1164–1170. doi: 10.1016/j.cmi.2018.04.027. [DOI] [PubMed] [Google Scholar]
- 117.Cappabianca G., Paparella D., D'Onofrio A. Mycobacterium chimaera infections following cardiac surgery in Italy: results from a National Survey Endorsed by the Italian Society of Cardiac Surgery. J Cardiovasc Med (Hagerstown) 2018;19(12):748–755. doi: 10.2459/JCM.0000000000000717. [DOI] [PubMed] [Google Scholar]
- 118.Ashraf M.S., Swinker M., Augustino K.L. Outbreak of Mycobacterium mucogenicum bloodstream infections among patients with sickle cell disease in an outpatient setting. Infect Control Hosp Epidemiol. 2012;33(11):1132–1136. doi: 10.1086/668021. [DOI] [PubMed] [Google Scholar]
- 119.Newman M.I., Camberos A.E., Clynes N.D., Ascherman J.A. Outbreak of atypical mycobacteria infections in U.S. Patients traveling abroad for cosmetic surgery. Plast Reconstr Surg. 2005;115(3):964–965. doi: 10.1097/01.prs.0000153818.99552.cc. [DOI] [PubMed] [Google Scholar]
- 120.Inoue H., Washida N., Morimoto K. Non-tuberculous mycobacterial infections related to peritoneal dialysis. Perit Dial Int. 2018;38(2):147–149. doi: 10.3747/pdi.2017.00172. [DOI] [PubMed] [Google Scholar]
- 121.Bennett J.E.D.R., Mandell Blaser M.J. Elsevier Health Sciences; 2014. Douglas, and Bennett's principles and practice of infectious diseases E-book. [Google Scholar]
- 122.Shea Y.R., Davis J.L., Huang L. High sensitivity and specificity of acid-fast microscopy for diagnosis of pulmonary tuberculosis in an African population with a high prevalence of human immunodeficiency virus. J Clin Microbiol. 2009;47(5):1553–1555. doi: 10.1128/JCM.00348-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Cocito C., Delville J. Biological, chemical, immunological and staining properties of bacteria isolated from tissues of leprosy patients. Eur J Epidemiol. 1985;1(3):202–231. doi: 10.1007/BF00234095. [DOI] [PubMed] [Google Scholar]
- 124.Schnietz R., Wilson M.L. Grocott methenamine silver and periodic acid-Schiff positivity in cutaneous Mycobacterium avium complex infection. J Cutan Pathol. 2018;45(7):551–553. doi: 10.1111/cup.13260. [DOI] [PubMed] [Google Scholar]
- 125.Brown-Elliott B.A., Wallace R.J., Jr. Clinical and taxonomic status of pathogenic nonpigmented or late-pigmenting rapidly growing mycobacteria. Clin Microbiol Rev. 2002;15(4):716–746. doi: 10.1128/CMR.15.4.716-746.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Brown-Elliott B.A., Vasireddy S., Vasireddy R. Utility of sequencing the erm(41) gene in isolates of Mycobacterium abscessus subsp. abscessus with low and intermediate clarithromycin MICs. J Clin Microbiol. 2015;53(4):1211–1215. doi: 10.1128/JCM.02950-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Adekambi T., Berger P., Raoult D., Drancourt M. rpoB gene sequence-based characterization of emerging non-tuberculous mycobacteria with descriptions of Mycobacterium bolletii sp. nov., Mycobacterium phocaicum sp. nov. and Mycobacterium aubagnense sp. nov. Int J Syst Evol Microbiol. 2006;56(Pt 1):133–143. doi: 10.1099/ijs.0.63969-0. [DOI] [PubMed] [Google Scholar]
- 128.Simner P.J. 11th ed. American Society of Microbiology; 2015. Mycobacterium: laboratory characteristics of slowly growing mycobacteria, in manual of clinical microbiology. [Google Scholar]
- 129.Olivier K.N., Griffith D.E., Eagle G. Randomized trial of liposomal amikacin for inhalation in nontuberculous mycobacterial lung disease. Am J Respir Crit Care Med. 2017;195(6):814–823. doi: 10.1164/rccm.201604-0700OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Griffith D.E., Brown B.A., Girard W.M., Griffith B.E., Couch L.A., Wallace R.J., Jr. Azithromycin-containing regimens for treatment of Mycobacterium avium complex lung disease. Clin Infect Dis. 2001;32(11):1547–1553. doi: 10.1086/320512. [DOI] [PubMed] [Google Scholar]
- 131.Field S.K., Cowie R.L. Treatment of Mycobacterium avium-intracellulare complex lung disease with a macrolide, ethambutol, and clofazimine. Chest. 2003;124(4):1482–1486. doi: 10.1378/chest.124.4.1482. [DOI] [PubMed] [Google Scholar]
- 132.Wallace R.J., Jr., Brown B.A., Griffith D.E., Girard W.M., Murphy D.T. Clarithromycin regimens for pulmonary Mycobacterium avium complex. The first 50 patients. Am J Respir Crit Care Med. 1996;153(6 Pt 1):1766–1772. doi: 10.1164/ajrccm.153.6.8665032. [DOI] [PubMed] [Google Scholar]
- 133.Philley J.V., Wallace R.J., Jr., Benwill J.L. Preliminary results of bedaquiline as salvage therapy for patients with nontuberculous mycobacterial lung disease. Chest. 2015;148(2):499–506. doi: 10.1378/chest.14-2764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Kobashi Y., Matsushima T., Oka M. A double-blind randomized study of aminoglycoside infusion with combined therapy for pulmonary Mycobacterium avium complex disease. Respir Med. 2007;101(1):130–138. doi: 10.1016/j.rmed.2006.04.002. [DOI] [PubMed] [Google Scholar]
- 135.Winthrop K.L., Ku J.H., Marras T.K. The tolerability of linezolid in the treatment of nontuberculous mycobacterial disease. Eur Respir J. 2015;45(4):1177–1179. doi: 10.1183/09031936.00169114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Koh W.J., Hong G., Kim S.Y. Treatment of refractory Mycobacterium avium complex lung disease with a moxifloxacin-containing regimen. Antimicrob Agents Chemother. 2013;57(5):2281–2285. doi: 10.1128/AAC.02281-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Deshpande D., Srivastava S., Pasipanodya J.G., Lee P.S., Gumbo T. A novel ceftazidime/avibactam, rifabutin, tedizolid and moxifloxacin (CARTM) regimen for pulmonary Mycobacterium avium disease. J Antimicrob Chemother. 2017;72(Suppl 2):i48–i53. doi: 10.1093/jac/dkx307. [DOI] [PubMed] [Google Scholar]
- 138.Guthertz L.S., Damsker B., Bottone E.J., Ford E.G., Midura T.F., Janda J.M. Mycobacterium avium and Mycobacterium intracellulare infections in patients with and without AIDS. J Infect Dis. 1989;160(6):1037–1041. doi: 10.1093/infdis/160.6.1037. [DOI] [PubMed] [Google Scholar]
- 139.Bax H.I., Bakker-Woudenberg I.A., Ten Kate M.T., Verbon A., de Steenwinkel J.E. Tigecycline potentiates clarithromycin activity against mycobacterium avium in vitro. Antimicrob Agents Chemother. 2016;60(4):2577–2579. doi: 10.1128/AAC.02864-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Brown-Elliott B.A., Wallace R.J., Jr. In vitro susceptibility testing of tedizolid against nontuberculous mycobacteria. J Clin Microbiol. 2017;55(6):1747–1754. doi: 10.1128/JCM.00274-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Griffith D.E., Brown-Elliott B.A., Wallace R.J., Jr. Thrice-weekly clarithromycin-containing regimen for treatment of Mycobacterium kansasii lung disease: results of a preliminary study. Clin Infect Dis. 2003;37(9):1178–1182. doi: 10.1086/378742. [DOI] [PubMed] [Google Scholar]
- 142.Ahn C.H., Lowell J.R., Ahn S.S., Ahn S.I., Hurst G.A. Short-course chemotherapy for pulmonary disease caused by Mycobacterium kansasii. Am Rev Respir Dis. 1983;128(6):1048–1050. doi: 10.1164/arrd.1983.128.6.1048. [DOI] [PubMed] [Google Scholar]
- 143.Yano K., Yoshida T., Minoda Y. Clinical outcome of the chronic flexor tenosynovitis in the hand caused by non-tuberculous mycobacterium treated by extensive tenosynovectomy and drugs. J Plast Surg Hand Surg. 2013;47(6):434–437. doi: 10.3109/2000656X.2013.776560. [DOI] [PubMed] [Google Scholar]
- 144.Cho J.H., Yu C.H., Jin M.K. Mycobacterium kansasii pericarditis in a kidney transplant recipient: a case report and comprehensive review of the literature. Transpl Infect Dis. 2012;14(5):E50–E55. doi: 10.1111/j.1399-3062.2012.00767.x. [DOI] [PubMed] [Google Scholar]
- 145.Wallace R.J., Jr., Dunbar D., Brown B.A. Rifampin-resistant Mycobacterium kansasii. Clin Infect Dis. 1994;18(5):736–743. doi: 10.1093/clinids/18.5.736. [DOI] [PubMed] [Google Scholar]
- 146.DeStefano M.S., Shoen C.M., Cynamon M.H. Therapy for mycobacterium kansasii infection: beyond 2018. Front Microbiol. 2018;9:2271. doi: 10.3389/fmicb.2018.02271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Huang Y., Xu X., Liu Y. Successful treatment of refractory cutaneous infection caused by Mycobacterium marinum with a combined regimen containing amikacin. Clin Interv Aging. 2012;7:533–538. doi: 10.2147/CIA.S36371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Aubry A., Chosidow O., Caumes E., Robert J., Cambau E. Sixty-three cases of Mycobacterium marinum infection: clinical features, treatment, and antibiotic susceptibility of causative isolates. Arch Intern Med. 2002;162(15):1746–1752. doi: 10.1001/archinte.162.15.1746. [DOI] [PubMed] [Google Scholar]
- 149.Wolinsky E., Gomez F., Zimpfer F. Sporotrichoid Mycobacterium marinum infection treated with rifampin-ethambutol. Am Rev Respir Dis. 1972;105(6):964–967. doi: 10.1164/arrd.1972.105.6.964. [DOI] [PubMed] [Google Scholar]
- 150.Caron J., Michot C., Fabre S., Godreuil S., Guillot B., Dereure O. Aggressive cutaneous infection with Mycobacterium marinum in two patients receiving anti-tumor necrosis factor-alfa agents. J Am Acad Dermatol. 2011;65(5):1060–1062. doi: 10.1016/j.jaad.2008.12.041. [DOI] [PubMed] [Google Scholar]
- 151.Lee M.W., Brenan J. Mycobacterium marinum: chronic and extensive infections of the lower limbs in south Pacific islanders. Aust J Dermatol. 1998;39(3):173–176. doi: 10.1111/j.1440-0960.1998.tb01276.x. [DOI] [PubMed] [Google Scholar]
- 152.Aubry A., Jarlier V., Escolano S., Truffot-Pernot C., Cambau E. Antibiotic susceptibility pattern of Mycobacterium marinum. Antimicrob Agents Chemother. 2000;44(11):3133–3136. doi: 10.1128/aac.44.11.3133-3136.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Braback M., Riesbeck K., Forsgren A. Susceptibilities of Mycobacterium marinum to gatifloxacin, gemifloxacin, levofloxacin, linezolid, moxifloxacin, telithromycin, and quinupristin-dalfopristin (Synercid) compared to its susceptibilities to reference macrolides and quinolones. Antimicrob Agents Chemother. 2002;46(4):1114–1116. doi: 10.1128/AAC.46.4.1114-1116.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Villahermosa L.G., Fajardo T.T., Jr., Abalos R.M. Parallel assessment of 24 monthly doses of rifampin, ofloxacin, and minocycline versus two years of World Health Organization multi-drug therapy for multi-bacillary leprosy. Am J Trop Med Hyg. 2004;70(2):197–200. [PubMed] [Google Scholar]
- 155.Ji B., Jamet P., Perani E.G., Bobin P., Grosset J.H. Powerful bactericidal activities of clarithromycin and minocycline against Mycobacterium leprae in lepromatous leprosy. J Infect Dis. 1993;168(1):188–190. doi: 10.1093/infdis/168.1.188. [DOI] [PubMed] [Google Scholar]
- 156.Lowe J. Treatment of leprosy with diamino-diphenyl sulphone by mouth. Lancet. 1950;1(6596):145–150. doi: 10.1016/s0140-6736(50)90257-1. [DOI] [PubMed] [Google Scholar]
- 157.Pardillo F.E., Burgos J., Fajardo T.T. Powerful bactericidal activity of moxifloxacin in human leprosy. Antimicrob Agents Chemother. 2008;52(9):3113–3117. doi: 10.1128/AAC.01162-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Freerksen E., Rosenfeld M., Depasquale G., Bonnici E., Gatt P. The Malta Project – a country freed itself of leprosy. A 27-year progress study (1972–1999) of the first successful eradication of leprosy. Chemotherapy. 2001;47(5):309–331. doi: 10.1159/000048539. [DOI] [PubMed] [Google Scholar]
- 159.Dhople A.M., Ibanez M.A. In vitro activity of levofloxacin, singly and in combination with rifamycin analogs, against Mycobacterium leprae. Antimicrob Agents Chemother. 1995;39(9):2116–2119. doi: 10.1128/aac.39.9.2116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Koh W.J., Jeong B.H., Kim S.Y. Mycobacterial characteristics and treatment outcomes in mycobacterium abscessus lung disease. Clin Infect Dis. 2017;64(3):309–316. doi: 10.1093/cid/ciw724. [DOI] [PubMed] [Google Scholar]
- 161.Jarand J., Levin A., Zhang L., Huitt G., Mitchell J.D., Daley C.L. Clinical and microbiologic outcomes in patients receiving treatment for Mycobacterium abscessus pulmonary disease. Clin Infect Dis. 2011;52(5):565–571. doi: 10.1093/cid/ciq237. [DOI] [PubMed] [Google Scholar]
- 162.Lyu J., Jang H.J., Song J.W. Outcomes in patients with Mycobacterium abscessus pulmonary disease treated with long-term injectable drugs. Respir Med. 2011;105(5):781–787. doi: 10.1016/j.rmed.2010.12.012. [DOI] [PubMed] [Google Scholar]
- 163.Jeon K., Kwon O.J., Lee N.Y. Antibiotic treatment of Mycobacterium abscessus lung disease: a retrospective analysis of 65 patients. Am J Respir Crit Care Med. 2009;180(9):896–902. doi: 10.1164/rccm.200905-0704OC. [DOI] [PubMed] [Google Scholar]
- 164.Wallace R.J., Jr., Brown-Elliott B.A., McNulty S. Macrolide/Azalide therapy for nodular/bronchiectatic mycobacterium avium complex lung disease. Chest. 2014;146(2):276–282. doi: 10.1378/chest.13-2538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Kaushik A., Ammerman N.C., Martins O., Parrish N.M., Nuermberger E.L. In vitro activity of new tetracycline analogs omadacycline and eravacycline against drug-resistant clinical isolates of Mycobacterium abscessus. Antimicrob Agents Chemother. 2019;63(6) doi: 10.1128/AAC.00470-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Wallace R.J., Jr., Brown-Elliott B.A., Ward S.C., Crist C.J., Mann L.B., Wilson R.W. Activities of linezolid against rapidly growing mycobacteria. Antimicrob Agents Chemother. 2001;45(3):764–767. doi: 10.1128/AAC.45.3.764-767.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Probst L.E., Brewer L.V., Hussain Z., Tokarewicz A.C. Treatment of Mycobacterium chelonae keratitis with amikacin, doxycycline and topical ciprofloxacin. Can J Ophthalmol. 1994;29(2):81–84. [PubMed] [Google Scholar]
- 168.Verghese S., Agrawal P., Benjamin S. Mycobacterium chelonae causing chronic wound infection and abdominal incisional hernia. Indian J Pathol Microbiol. 2014;57(2):335–337. doi: 10.4103/0377-4929.134736. [DOI] [PubMed] [Google Scholar]
- 169.Kullavanijaya P., Rattana-Apiromyakij N., Sukonthapirom-Napattalung P., Sirimachand S., Duangdeeden I. Disseminated Mycobacterium chelonae cutaneous infection: recalcitrant to combined antibiotic therapy. J Dermatol. 2003;30(6):485–491. doi: 10.1111/j.1346-8138.2003.tb00421.x. [DOI] [PubMed] [Google Scholar]
- 170.Wallace R.J., Jr., Tanner D., Brennan P.J., Brown B.A. Clinical trial of clarithromycin for cutaneous (disseminated) infection due to Mycobacterium chelonae. Ann Intern Med. 1993;119(6):482–486. doi: 10.7326/0003-4819-119-6-199309150-00006. [DOI] [PubMed] [Google Scholar]
- 171.Korres D.S., Papagelopoulos P.J., Zahos K.A., Kolia M.D., Poulakou G.G., Falagas M.E. Multifocal spinal and extra-spinal Mycobacterium chelonae osteomyelitis in a renal transplant recipient. Transpl Infect Dis. 2007;9(1):62–65. doi: 10.1111/j.1399-3062.2006.00178.x. [DOI] [PubMed] [Google Scholar]
- 172.Rajini M., Prasad S.R., Reddy R.R., Bhat R.V., Vimala K.R. Postoperative infection of laparoscopic surgery wound due to Mycobacterium chelonae. Indian J Med Microbiol. 2007;25(2):163–165. doi: 10.4103/0255-0857.32729. [DOI] [PubMed] [Google Scholar]
- 173.Yew W.W., Lau K.S., Tse W.K., Wong C.F. Imipenem in the treatment of lung infections due to Mycobacterium fortuitum and Mycobacterium chelonae: further experience. Clin Infect Dis. 1992;15(6):1046–1047. doi: 10.1093/clind/15.6.1046. [DOI] [PubMed] [Google Scholar]
- 174.Ichihara A., Jinnin M., Fukushima S., Inoue Y., Ihn H. Case of disseminated cutaneous Mycobacterium chelonae infection mimicking cutaneous vasculitis. J Dermatol. 2014;41(5):414–417. doi: 10.1111/1346-8138.12459. [DOI] [PubMed] [Google Scholar]
- 175.Brown-Elliott B.A., Wallace R.J., Jr., Blinkhorn R., Crist C.J., Mann L.B. Successful treatment of disseminated Mycobacterium chelonae infection with linezolid. Clin Infect Dis. 2001;33(8):1433–1434. doi: 10.1086/322523. [DOI] [PubMed] [Google Scholar]
- 176.Pring M., Eckhoff D.G. Mycobacterium chelonae infection following a total knee arthroplasty. J Arthroplasty. 1996;11(1):115–116. doi: 10.1016/s0883-5403(96)80170-7. [DOI] [PubMed] [Google Scholar]
- 177.Schneider P., Monsel G., Veziris N., Roujeau J.C., Bricaire F., Caumes E. Successful treatment of nodular lymphangitis due to Mycobacterium chelonae in two immunosuppressed patients. Dermatol Online J. 2011;17(3):8. [PubMed] [Google Scholar]
- 178.Shoen C., Benaroch D., Sklaney M., Cynamon M. In vitro activities of omadacycline against rapidly growing Mycobacteria. Antimicrob Agents Chemother. 2019;63(5) doi: 10.1128/AAC.02522-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Abbass K., Adnan M.K., Markert R.J., Emig M., Khan N.A. Mycobacterium fortuitum breast abscess after nipple piercing. Can Fam Physician. 2014;60(1):51–52. [PMC free article] [PubMed] [Google Scholar]
- 180.Sethi S., Arora S., Gupta V., Kumar S. Cutaneous Mycobacterium fortuitum infection: successfully treated with amikacin and ofloxacin combination. Indian J Dermatol. 2014;59(4):383–384. doi: 10.4103/0019-5154.135491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Matsumoto T., Otsuka K., Tomii K. Mycobacterium fortuitum thoracic empyema: a case report and review of the literature. J Infect Chemother. 2015;21(10):747–750. doi: 10.1016/j.jiac.2015.05.012. [DOI] [PubMed] [Google Scholar]
- 182.Dalovisio J.R., Pankey G.A., Wallace R.J., Jones D.B. Clinical usefulness of amikacin and doxycycline in the treatment of infection due to Mycobacterium fortuitum and Mycobacterium chelonei. Rev Infect Dis. 1981;3(5):1068–1074. doi: 10.1093/clinids/3.5.1068. [DOI] [PubMed] [Google Scholar]
- 183.Fujita N., Utani A., Matsumoto F. Levofloxacin alone efficiently treated a cutaneous mycobacterium fortuitum infection. J Dermatol. 2002;29(7):452–454. doi: 10.1111/j.1346-8138.2002.tb00305.x. [DOI] [PubMed] [Google Scholar]
- 184.Moriuchi R., Arita K., Ujiie H., Kodama K., Shimizu T., Shimizu H. Large subcutaneous abscesses caused by Mycobacterium fortuitum infection. Acta Derm Venereol. 2008;88(3):313–314. doi: 10.2340/00015555-0426. [DOI] [PubMed] [Google Scholar]
- 185.Pacht E.R. Mycobacterium fortuitum lung abscess: resolution with prolonged trimethoprim/sulfamethoxazole therapy. Am Rev Respir Dis. 1990;141(6):1599–1601. doi: 10.1164/ajrccm/141.6.1599. [DOI] [PubMed] [Google Scholar]
- 186.Wallace R.J., Jr., Brown B.A., Onyi G.O. Susceptibilities of Mycobacterium fortuitum biovar. fortuitum and the two subgroups of Mycobacterium chelonae to imipenem, cefmetazole, cefoxitin, and amoxicillin-clavulanic acid. Antimicrob Agents Chemother. 1991;35(4):773–775. doi: 10.1128/aac.35.4.773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Gillespie S.H., Billington O. Activity of moxifloxacin against mycobacteria. J Antimicrob Chemother. 1999;44(3):393–395. doi: 10.1093/jac/44.3.393. [DOI] [PubMed] [Google Scholar]
- 188.Wallace R.J., Jr., Brown-Elliott B.A., Crist C.J., Mann L., Wilson R.W. Comparison of the in vitro activity of the glycylcycline tigecycline (formerly GAR-936) with those of tetracycline, minocycline, and doxycycline against isolates of nontuberculous mycobacteria. Antimicrob Agents Chemother. 2002;46(10):3164–3167. doi: 10.1128/AAC.46.10.3164-3167.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Gayathri R., Therese K.L., Deepa P., Mangai S., Madhavan H.N. Antibiotic susceptibility pattern of rapidly growing mycobacteria. J Postgrad Med. 2010;56(2):76–78. doi: 10.4103/0022-3859.65278. [DOI] [PubMed] [Google Scholar]
- 190.Wallace R.J., Jr., Nash D.R., Tsukamura M., Blacklock Z.M., Silcox V.A. Human disease due to Mycobacterium smegmatis. J Infect Dis. 1988;158(1):52–59. doi: 10.1093/infdis/158.1.52. [DOI] [PubMed] [Google Scholar]
- 191.Brown B.A., Springer B., Steingrube V.A. Mycobacterium wolinskyi sp. nov. and Mycobacterium goodii sp. nov., two new rapidly growing species related to Mycobacterium smegmatis and associated with human wound infections: a cooperative study from the International Working Group on Mycobacterial Taxonomy. Int J Syst Bacteriol. 1999;49(Pt 4):1493–1511. doi: 10.1099/00207713-49-4-1493. [DOI] [PubMed] [Google Scholar]
- 192.Bopape M.C., Steel H.C., Cockeran R., Matlola N.M., Fourie P.B., Anderson R. Antimicrobial activity of clofazimine is not dependent on mycobacterial C-type phospholipases. J Antimicrob Chemother. 2004;53(6):971–974. doi: 10.1093/jac/dkh215. [DOI] [PubMed] [Google Scholar]
- 193.Band J.D., Ward J.I., Fraser D.W. Peritonitis due to a mycobacterium chelonei-like organism associated with intermittent chronic peritoneal dialysis. J Infect Dis. 1982;145(1):9–17. doi: 10.1093/infdis/145.1.9. [DOI] [PubMed] [Google Scholar]
- 194.Garcia-Zamora E., Sanz-Robles H., Elosua-Gonzalez M., Rodriguez-Vasquez X., Lopez-Estebaranz J.L. Cutaneous infection due to Mycobacterium immunogenum: an European case report and review of the literature. Dermatol Online J. 2017;23(10) [PubMed] [Google Scholar]
- 195.Wilson R.W., Steingrube V.A., Bottger E.C. Mycobacterium immunogenum sp. nov., a novel species related to Mycobacterium abscessus and associated with clinical disease, pseudo-outbreaks and contaminated metalworking fluids: an international cooperative study on mycobacterial taxonomy. Int J Syst Evol Microbiol. 2001;51(Pt 5):1751–1764. doi: 10.1099/00207713-51-5-1751. [DOI] [PubMed] [Google Scholar]
- 196.Abidi M.Z., Ledeboer N., Banerjee A., Hari P. Mycobacterium mucogenicum bacteremia in immune-compromised patients, 2008–2013. Diagn Microbiol Infect Dis. 2016;85(2):182–185. doi: 10.1016/j.diagmicrobio.2016.02.012. [DOI] [PubMed] [Google Scholar]
- 197.Hawkins C., Qi C., Warren J., Stosor V. Catheter-related bloodstream infections caused by rapidly growing nontuberculous mycobacteria: a case series including rare species. Diagn Microbiol Infect Dis. 2008;61(2):187–191. doi: 10.1016/j.diagmicrobio.2008.01.004. [DOI] [PubMed] [Google Scholar]
- 198.Pradier M., Boucher A., Robineau O., Chachaty E., Meybeck A., Senneville E. Mycobacterium mucogenicum bacteremia: major role of clinical microbiologists. BMC Infect Dis. 2018;18(1):646. doi: 10.1186/s12879-018-3545-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Han X.Y., De I., Jacobson K.L. Rapidly growing mycobacteria: clinical and microbiologic studies of 115 cases. Am J Clin Pathol. 2007;128(4):612–621. doi: 10.1309/1KB2GKYT1BUEYLB5. [DOI] [PubMed] [Google Scholar]
- 200.Nash K.A. Intrinsic macrolide resistance in Mycobacterium smegmatis is conferred by a novel erm gene, erm(38) Antimicrob Agents Chemother. 2003;47(10):3053–3060. doi: 10.1128/AAC.47.10.3053-3060.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Haworth C.S., Banks J., Capstick T. British Thoracic Society Guideline for the management of non-tuberculous mycobacterial pulmonary disease (NTM-PD) BMJ Open Respir Res. 2017;4(1) doi: 10.1136/bmjresp-2017-000242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Norris A.H., Shrestha N.K., Allison G.M. 2018 Infectious Diseases Society of America clinical practice guideline for the management of outpatient parenteral antimicrobial therapy. Clin Infect Dis. 2019;68(1):1–4. doi: 10.1093/cid/ciy867. [DOI] [PubMed] [Google Scholar]