Abstract
Cerebrotendinous xanthomatosis (CTX) is caused by autosomal recessive loss-of-function mutations in CYP27A1, a gene encoding cytochrome p450 oxidase essential for bile acid synthesis, resulting in altered bile acid and lipid metabolism. Here, we aimed to identify metabolic aberrations that drive ongoing neurodegeneration in some patients with CTX despite chenodeoxycholic acid (CDCA) supplementation, the standard treatment in CTX. Using chromatographic separation techniques coupled to mass spectrometry, we analyzed 26 sterol metabolites in serum and cerebrospinal fluid (CSF) of patients with CTX and in one CTX brain. Comparing samples of drug naive patients to patients treated with CDCA and healthy controls, we identified 7α,12α-dihydroxycholest-4-en-3-one as the most prominently elevated metabolite in serum and CSF of drug naive patients. CDCA treatment substantially reduced or even normalized levels of all metabolites increased in untreated patients with CTX. Independent of CDCA treatment, metabolites of the 27-hydroxylation pathway were nearly absent in all patients with CTX. 27-hydroxylated metabolites accounted for ∼45% of total free sterol content in CSF of healthy controls but <2% in patients with CTX. Metabolic changes in brain tissue corresponded well with findings in CSF. Interestingly, 7α,12α-dihydroxycholest-4-en-3-one and 5α-cholestanol did not exert toxicity in neuronal cell culture. In conclusion, we propose that increased 7α,12α-dihydroxycholest-4-en-3-one and lack of 27-hydroxycholesterol may be highly sensitive metabolic biomarkers of CTX. As CDCA cannot reliably prevent disease progression despite reduction of most accumulated metabolites, supplementation of 27-hydroxylated bile acid intermediates or replacement of CYP27A1 might be required to counter neurodegeneration in patients with progressive disease despite CDCA treatment.
Supplementry key words: bile acid metabolism; cholesterol metabolism; cytochrome P450; lipodystrophies; oxysterols, cerebrotendinous xanthomathosis; inborn errors of metabolism; cerebrospinal fluid; mass spec
Abbreviations: CTX, cerebrotendinous xanthomatosis; CSF, cerebrospinal fluid; CDCA, chenodeoxycholic acid; LDH, lactate dehydrogenase; 7α-HC, 7α-hydroxycholesterol; 7α-HCO, 7α-hydroxycholest-4-en-3-one; 7α,12α-diHCO, 7α,12α-dihydroxycholest-4-en-3-one; 27-HC, 27-hydroxycholesterol; 7α,25-diHCO, 7α,25-dihydroxycholest-4-en-3-one
Cerebrotendinous xanthomatosis (CTX) is a neurometabolic disease leading to progressive spastic-ataxic gait disorder, cognitive decline, and frequently premature death due to abnormal bile acid and cholesterol metabolism (1, 2, 3). It is caused by loss of function mutations in CYP27A1 which is a key enzyme of bile acid synthesis from cholesterol in the liver (4, 5). Lack of CYP27A1 results in accumulation of abnormal lipids including 5α-cholestanol which is widely used as a diagnostic biomarker in CTX (6). Chenodeoxycholic acid (CDCA) supplementation is established as a therapy in CTX (7). It primarily reduces the production of accumulated metabolites by end product inhibition of expression mainly of cholesterol 7α-hydroxylase (CYP7A1) (8), coding the rate-limiting enzyme of the classical bile acid synthesis pathway, and is also shown to often normalize serum levels of 5α-cholestanol (7, 9, 10). Despite this normalization of potentially neurotoxic metabolites and reports of clinical improvement in some patients after short-term treatment, many patients experience a progression of neurological deficits in long-term follow-up, especially when treatment is not initiated before neurological damage is apparent (9, 11, 12). To explore the metabolic background of neurodegeneration in CTX, we used mass spectrometry to perform in depth analyses of serum and cerebrospinal fluid (CSF) of patients with CTX. By comparing biosamples of treated and untreated patients, we aimed to identify metabolites that did not normalize upon CDCA treatment and may drive continuous progression of the disease. Metabolites that were highly elevated were assessed for neurotoxic effects in a neuronal cell culture model.
Materials and Methods
Patient cohort
Patients with CTX were recruited from the leukodystrophy outpatient clinic of the University Hospital Tübingen, Germany and the neurogenetics outpatient clinic at the Friedrich-Baur-Institute, University Hospital, LMU Munich, Germany, between 2011 and 2019. Biosamples were obtained from nine patients with CTX including four patients treated with 250 mg CDCA three times a day. Serum and CSF of healthy control individuals without CTX (n = 10) were provided by the Biobank of the Hertie Institute for Clinical Brain Research, Tübingen, Germany. Controls CO 1–5 were used as reference for free metabolite determination; samples CO 6–10 served as controls for the assessment of total amounts of cholesterol metabolites. Controls did not differ concerning age and sex from the patient group. Brain autopsy material derives from a male CTX patient who died at 26 years of age as described in Battacharyya et al. (13) (IRB00007099, Oregon Health and Science University). Brain material from a 56-year-old male without neurological symptoms was kindly provided as a control by the Thomas Willis Oxford Brain Bank with appropriate research ethics approval (South Wales REC 13/WA/0292). Biographic data and clinical features of all participants are detailed in Table 1. Ethical approval for liquid biopsies was given by the regulatory board of the Medical Faculty at the University of Tübingen, Germany (vote 199/2011BO1). Participants gave their written informed consent before inclusion in this study. The study was performed according to the declaration of Helsinki principles.
Table 1.
Clinical data of CTX patients included in this study and biosamples available for metabolic analyses
| Age at Sampling | Age of Onset | Gender | CYP27A1 Mutations | Clinical Features | Pretreatment |
Posttreatment |
|||
|---|---|---|---|---|---|---|---|---|---|
| Serum | CSF | Serum | CSF | ||||||
| CTX 1 | 54 | 43 | F | c.379C>T, p.Arg127Trp c.1016C>T, p.Thr339Met |
Cognitive deficits, cerebellar ataxia | - | - | X | X |
| CTX 2 | 47 | 41 | F | c.379C>T, p.Arg127Trp c.1016C>T, p.Thr339Met |
Cataracts, cognitive deficits, cerebellar ataxia, spastic tetraparesis | - | - | X | X |
| CTX 3 | 45 | 43 | F | unknown | Cataracts, cognitive deficits | - | - | X | - |
| CTX 4 | 61 | 30 | F | c.1183C>A, p.Arg395Ser hom | Tendon xanthomas | X | X | - | - |
| CTX 5 | 47 | 10 | M | c.808C>T, p.Arg270∗ c.1184+1G>A, p.? |
Cognitive deficits, cataracts, depression, polyneuropathy, cerebellar ataxia | X | X | X | X |
| CTX 6a | 60 | n.d. | M | c.379C>T, p.Arg127Trp; c.410G>A, p.Arg137Gln |
Spastic paraparesis, tendon xanthomas | X | X | - | - |
| CTX 7 | 35 | 7 m | M | c.646G>C, p.Ala216Pro c.1213C>T, p.Arg405Trp |
Epilepsy, diarrhea, cognitive deficits, cerebellar ataxia, spastic tetraparesis | X | X | - | - |
| CTX 8a | 29 | 26 | F | c.409C>T, p.Arg137Trp hom | Tendon xanthomas | X | X | - | - |
| CTX 9 | 26 | n.d. | M | unknown | Large tendon xanthoma, cataracts, ataxia, and cognitive deficits. | Brain autopsy | |||
| CO 1 | 61 | - | F | - | - | X | X | - | - |
| CO 2 | 45 | - | F | - | - | X | X | - | - |
| CO 3 | 53 | - | F | - | - | X | X | - | - |
| CO 4 | 46 | - | F | - | - | X | X | - | - |
| CO 5 | 32 | - | M | - | - | X | X | - | - |
| CO 6 | 57 | - | F | - | - | X | X | - | - |
| CO 7 | 53 | - | M | - | - | X | X | - | - |
| CO 8 | 27 | - | F | - | - | X | X | - | - |
| CO 9 | 48 | - | F | - | - | X | X | - | - |
| CO 10 | 30 | - | F | - | - | X | X | - | - |
| CO 11 | 56 | - | M | - | - | Brain autopsy | |||
CSF, cerebrospinal fluid; CTX, cerebrotendinous xanthomatosis; n.d., not determined.
Patients have low 5α-cholestanol levels.
Oxysterol analysis
Cholesterol and its downstream metabolites are mainly present either in their free form, which is biologically active or esterified to fatty acids for intracellular storage or transport in lipoproteins. Sulfated and glucuronidated forms are also found in serum and plasma. The usual determination of total amounts in biological samples requires a “harsh” saponification reaction to break the steryl ester bonds, which can lead to unintended alterations in molecule structures.
Therefore, we analyzed the free (nonesterified) fraction of oxysterols in serum and CSF by employing a charge-tagging approach using “enzyme-assisted derivatization for sterol analysis” followed by liquid chromatography (LC) and electrospray ionization mass spectrometry (MS) with multistage fragmentation (MSn) as previously described (14, 15, 16). In brief, oxysterols and C27 acids were separated from cholesterol and other hydrophobic sterols by solid phase extraction. The resulting oxysterol fraction was divided into two equal aliquots A and B. Fraction A was first oxidized using cholesterol oxidase and then derivatized with [2H5]Girard P reagent, whereas fraction B was derivatized with [2H0] Girard P reagent in the absence of cholesterol oxidase treatment. Using this approach, we were able to differentiate between metabolites that are either in a 3-oxo-4-ene form (Fraction B) or in the 3β-hydroxy-5-ene form (Fraction A minus Fraction B). To allow for simultaneous detection of both forms, immediately before LC-MSn the two fractions were combined. A detailed analysis scheme including exact masses, retention times and fragmentation spectra can be found in Griffiths et al. (17). Oxysterols in brain were analyzed using a similar approach as described in the same reference.
As detection of 5α-cholestanol in serum and CSF was not possible with LC-MS because of masking of the signal by excess cholesterol (18), we used gas chromatographic separation to determine total amounts (nonesterified plus esterified) of cholesterol, 5α-cholestanol, 7α-hydroxycholesterol (7α-HC), and 27-hydroxycholesterol (27-HC) as described before (19). Note that the systematic name for 27-HC is (25R)26-hydroxycholesterol (cholest-5-ene-3β,(25R)26-diol); however, as 27-HC is more commonly used, it will be adopted throughout the manuscript. For some metabolites, we were able to discriminate between (25R)26- and (25S)26-hydroxylated or carboxylated stereoisomers. For simplicity, only the latter were explicitly stated.
Toxicity assays
Mouse motor neuron-like hybrid cell line (NSC-34) cells were cultured in T-75 flasks (Corning) in DMEM/F12 medium supplemented with 10% fetal bovine serum (FBS, Gibco) and routinely passaged at ∼80–90% confluency using Trypsin/EDTA (Merck).
For cytotoxicity assays, 7,500 cells/well were seeded in black-walled CellCarier 96-well plates (PerkinElmer) coated with a 1:60 dilution of growth factor reduced Matrigel (Corning). Cells were cultivated for 3 days in DMEM/F12 + 10% FBS. Afterwards, FBS was withdrawn from the medium to induce differentiation for 4 days. Then, serial dilutions of 7α,12α-dihydroxycholest-4-en-3-one (TRC), 5α-cholestanol, and 27-HC (Avanti Polar lipids) were freshly prepared in DMEM/F12 from a stock solution of 12.5 mM (solved in EtOH) to yield final concentrations of 0.2 μM, 1 μM, 5 μM, 25 μM, and 100 μM and applied to the cells for 4 days without media change in between.
Cell proliferation reagent WST-1 (Roche) and LDH-Glo™ Cytotoxicity Assay (Promega) were performed according to the manufacturer’s guidelines, and absorption or luminescence were measured after 1 h of incubation on a SpectraMax M2e Microplate Reader (Molecular Devices).
Statistics
For statistical analysis of the oxysterol and sterol, concentrations data were log-transformed using the following equation: with added to prevent log values of 0. For each metabolite, normal distribution was assumed when absolute values for skewness and kurtosis were below 1. Equality of variances was assessed using Levene’s test (α = 0.05). If a data set passed both tests, it was further analyzed by one-way ANOVA, and pairwise comparisons were calculated with the least significant difference test, otherwise the nonparametric Kruskal-Wallis test followed by Mann-Whitney U tests was used. Data were Bonferroni corrected with m = 4 for the four primary pathways (7α-hydroxylation, 24-hydroxylation, 25-hydroxylation, and 27-hydroxylation) analyzed in this study assuming that different metabolites belonging to the same pathway are not independent variables. Significance was assumed for P < 0.0125. The data were analyzed using SPSS.
Results
Cholesterol
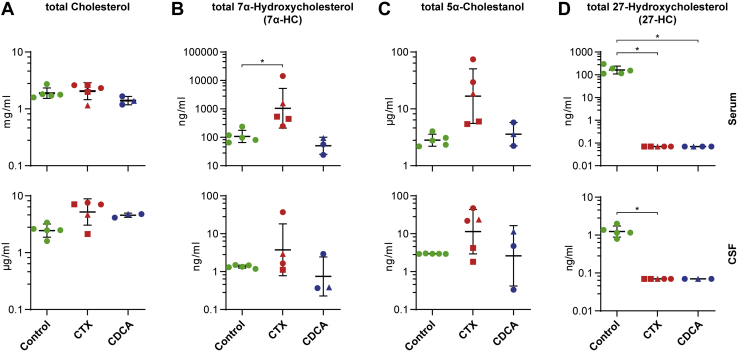
As cholesterol is the biosynthetic precursor of all primary bile acids (Fig. 1), we compared total cholesterol levels measured by GC-MS in the three groups (healthy controls, drug-naive CTX patients, and CDCA-treated patients with CTX). There were no significant differences in the amount of total cholesterol in serum (Table 2). In CSF, the mean total cholesterol concentration in untreated patients with CTX was 2-fold higher compared with controls, but the difference was not statistically significant (Fig. 2A, Table 3).
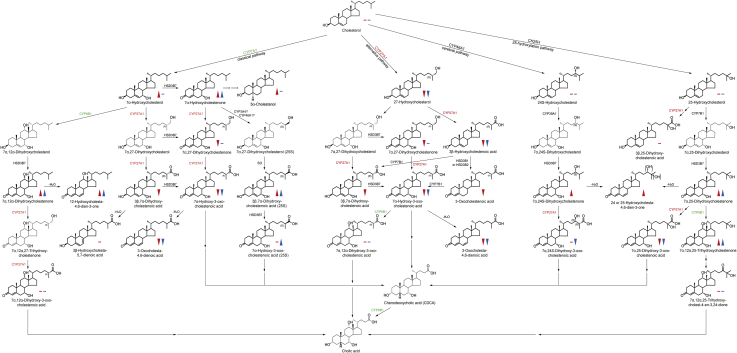
Fig. 1.
Overview of bile acid synthesis pathways. Important intermediates of the classical pathway of bile acid synthesis as the main route of cholesterol catabolism in the liver are displayed on the left. Next to it, intermediates of alternate pathways including the acidic pathway initiated by CYP27A1 (expressed in many tissues including the lung and brain), the cerebral pathway (CYP46A1; only expressed in brain), and the 25-hydroxylation pathway (CH25H) are shown. Reactions catalyzed by CYP27A1, the enzyme lacking in CTX, are highlighted by red lettering of CYP27A1. Reactions catalyzed by CYP7A1 and CYP8B1, the main enzymes upregulated in CTX, are highlighted by green lettering. Red arrowheads (significantly increased/decreased) and bars (unchanged) indicate differences measured in patients with CTX in relation to controls in serum. Blue arrowheads exhibit differences between the groups in CSF. Only metabolites with symbols were analyzed. CSF, cerebrospinal fluid; CTX, cerebrotendinous xanthomatosis.
Table 2.
Sterol concentrations in serum
| Systematic Name | Common Name (Abbreviation) | Control (n = 5) |
CTX (n = 5) |
CDCA (n = 4) |
Note | |||
|---|---|---|---|---|---|---|---|---|
| Mean | Range | Mean | Range | Mean | Range | |||
| GC-MS | ||||||||
| Total cholest-5-en-3β-ol | Total cholesterol | 1.86 mg/ml | 1.53–2.66 | 2.07 mg/ml | 1.08–2.55 | 1.34 mg/ml | 1.12–1.60 | |
| Total 5α-cholestan-3β-ol | Total 5α-cholestanol | 2.7 μg/ml | 2.0–3.9 | 26.7 μg/ml | 5.3–74.4 | 3.7 μg/ml | 0.21–0.56 | a |
| Total cholest-5-ene-3β,7α-diol | Total 7α-hydroxycholesterol (7α-HC) | 119.4 ng/ml | 65–237 | 3,413 ng/ml | 247–14,287 | 58 ng/ml | 24–94 | a |
| Total cholest-5-ene-3β,(25R)26-diol | Total 27-hydroxycholesterol (27-HC) | 174.2 ng/ml | 113–298 | 4.33 ng/ml | 0–13 | 0 | - | a,b |
| LC-MS (all values in ng/ml and for free oxysterols) | ||||||||
| Monohydroxycholesterols | ||||||||
| Cholest-5-ene-3β,7α-diol | 7α-Hydroxycholesterol (7α-HC) | 8.63 | 0.71–35.63 | 54.04 | 0–226.96 | 12.01 | 1.65–23.15 | |
| 7α-Hydroxycholest-4-en-3-one | 7α-Hydroxycholestenone (7α-HCO) | 4.63 | 0–8.52 | 205.96 | 18.58–584.19 | 6.99 | 2.3–9.1 | a,d |
| Cholest-5-ene-3β,24S-diol | 24S-Hydroxycholesterol (24S-HC) | 6.50 | 4.5–8.62 | 17.01 | 8.02–25.53 | 11.67 | 7.69–18.42 | |
| Cholest-5-ene-3β,25-diol | 25-Hydroxycholesterol (25-HC) | 0.97 | 0.53–2.2 | 0.56 | 0.0–1.21 | 1.29 | 0.51–3.13 | |
| Cholest-5-ene-3β,(25R)26-diol | 27-Hydroxycholesterol (27-HC) | 11.52 | 7.69–15.54 | 0.00 | - | 0.00 | - | a,b,d |
| Dihydroxycholesterols and dihydroxycholestenones | ||||||||
| 7α,12α-Dihydroxycholest-4-en-3-one | 7α,12α-Dihydroxycholestenone (7α,12α-diHCO) | 0.16 | 0.02–0.38 | 1614.9 | 49.21–4,602 | 35.64 | 3.44–50.07 | a,b,c |
| 7α,24-Dihydroxycholest-4-en-3-one | 7α,24-Dihydroxycholestenone (7α,24-diHCO) | 0.04 | 0–0.07 | 1.45 | 0.17–2.7 | 0.35 | 0.23–0.64 | a,d |
| 7α,25-Dihydroxycholest-4-en-3-one | 7α,25-Dihydroxycholestenone (7α,25-diHCO) | 1.12 | 0.6–1.43 | 9.31 | 3.29–14.36 | 3.35 | 2.08–4.46 | a,b |
| 7α,(25R)26-Dihydroxy-cholest-4-en-3-one | 7α,27-Dihydroxycholestenone (7α,27-diHCO) | 2.13 | 0.89–3.34 | 0.00 | - | 0.00 | - | a,b,d |
| Trihydroxycholestenones | ||||||||
| 7α,12α,25-Trihydroxy-cholest-4-en-3-one | 7α,12α,25-Trihydroxycholestenone (7α,12α,25-triHCO) | 0.34 | 0.17–0.47 | 33.38 | 4.1–53.55 | 1.89 | 1.05–2.9 | a,d,e |
| Monohydroxycholestenoic and oxocholestenoic acids | ||||||||
| 3β-Hydroxycholest-5-en-(25R)26-oic acid | 3β-Hydroxycholestenoic acid (3β-HCA) | 55.00 | 44.13–64.22 | 0.00 | - | 0.00 | - | a,b,d |
| 3-Oxocholest-4-en-(25R)26-oic acid | 3-Oxocholestenoic acid | 1.04 | 0.58–1.47 | 0.00 | - | 0.00 | - | a,b,d |
| Dihydroxycholestenoic and monohydroxyoxocholestenoic acids | ||||||||
| 3β,7α-Dihydroxycholest-5-en-(25R)26-oic acid | 3β,7α-Dihydroxycholestenoic acid (3β,7α-diHCA) | 10.15 | 5.58–18.26 | 0.00 | - | 0.00 | - | a,b,d |
| 7α-Hydroxy-3-oxocholest-4-en-(25R)26-oic acid | 7α-Hydroxy-3-oxocholestenoic acid (7αH,3O-CA) | 31.57 | 19.16–43.76 | 0.00 | - | 0.00 | - | a,b,d |
| 3β,7α-Dihydroxycholest-5-en-(25S)26-oic acid | 3β,7α-Dihydroxycholestenoic acid (25S) (3β,7α-diHCA (25S)) |
3.86 | 2.92–5.04 | 8.78 | 0.7–18.29 | 2.79 | 0.65–6.28 | |
| 7α-Hydroxy-3-oxocholest-4-en-(25S) 26-oic acid | 7α-Hydroxy-3-oxocholestenoic acid(25S) (7αH,3O-CA (25S)) | 4.18 | 3.31–5.38 | 1.87 | 0.25–4.42 | 0.47 | 0.25–0.87 | |
| 3β,25-Dihydroxycholest-5-en-26-oic acid and/or 3β,25,X-Trihydroxycholest-5-en-Y-one | 3β,25-Dihydroxy-cholestenoic acid and/or 3β,25,X-Trihydroxycholest-5-en-Y-one | 2.68 | 2.23–3.42 | 1.89 | 0.39–3.3 | 0.73 | 0.26–1.07 | d,f,g |
| Dihydroxyoxocholestenoic acids | ||||||||
| 7α,12α-Dihydroxy-3-oxocholest-4-en-26-oic acid and/or 7α,12α,25-Trihydroxycholest-4-ene-3,24-dione | 7α,12α-Dihydroxy-3-oxocholestenoic acid and/or 7α,12α,25-Trihydroxycholest-4-ene-3,24-dione | 0.71 | 0.24–1.07 | 7.26 | 0.00–24.03 | 0.00 | - | d,e,f,h |
| 7α,24-Dihydroxy-3-oxocholest-4-en-26-oic acid | 7α,24-Dihydroxy-3-oxocholestenoic acid | 0.07 | 0.02–0.1 | 0.36 | 0.00–0.92 | 0.01 | 0.00–0.03 | d,f |
| 7α,25-Dihydroxy-3-oxocholest-4-en-26-oic acid | 7α,25-Dihydroxy-3-oxocholestenoic acid | 0.20 | 0.07–0.41 | 0.00 | - | 0.00 | - | a,b,d |
| Dien-sterols | ||||||||
| 3β-Hydroxycholesta-5,7-dien-26-oic acid and/or 3β-Hydroxycholesta-5,Y-dien-26-oic acid | 3β-Hydroxycholest-5,7-dienoic acid and/or 3β-Hydroxycholesta-5,Y-dien-26-oic acid | 1.59 | 0.15–2.72 | 0.44 | 0.00–1.85 | 0.00 | - | d |
| 3-Oxocholesta-4,6-dien-26-oic acid and/or 3-Oxocholesta-4,Y-dien-26-oic acid | 3-Oxocholesta-4,6-dienoic acid and/or 3-Oxocholesta-4,Y-dien-26-oic acid | 6.73 | 3.61–9.23 | 0.16 | 0.00–0.54 | 0.08 | 0–0.14 | d,e,h |
| 12α-Hydroxycholesta-4,6-dien-3-one | 12α-Hydroxycholesta-4.6-dien-3-one (12α-HC-4,6-dien-3-one) | 0.45 | 0.00–0.78 | 306.98 | 35.63–636.95 | 11.45 | 2.34–19 | a,b,c |
| 24 or 25-Hydroxycholesta-4,6-dien-3-one and/or 24 or 25-Hydroxycholesta-4,8-dien-3-one | 24 or 25-Hydroxycholesta-4,6-dien-3-one and/or 24 or 25-Hydroxycholesta-4,8-dien-3-one | 0.26 | 0.12–0.48 | 1.6 | 0.72–2.13 | 0.77 | 0.63–0.95 | d,e,h |
CDCA, chenodeoxycholic acid; CTX, cerebrotendinous xanthomatosis.
CTX group: Patients CTX 4–8, CDCA group: Patients CTX 1–3 and CTX 5
P < 0.0125 for comparison between controls and untreated patients with CTX.
P < 0.0125 for comparison between controls and patients with CTX treated with 750 mg/day CDCA.
P < 0.0125 for comparison between drug-naive and CDCA-treated patients with CTX.
Values below limit of quantification of 0.1 ng/ml in CSF or 0.5 ng/ml in serum.
Identification based on exact mass retention time and MS3 spectrum in the absence of an authentic standard.
MS3 spectra do not allow the differentiation between the isomers/epimers.
The position of the additional hydroxy group is not determined.
In CTX, there is no direct synthesis of (25R)26-oic acids and most likely a 25-hydroxy-3,24-dione structure is present.
Fig. 2.
Total levels of key metabolic markers of cerebrotendinous xanthomatosis (CTX). A–D: Concentrations of the indicated metabolites measured by GC-MS in serum (upper panel) and CSF (lower panel) of controls (green), untreated patients with CTX (CTX, red) and patients treated with 750 mg/day CDCA (CDCA, blue). The data are presented as individual data points and mean ± SD on a logarithmic scale with antilog numbering. Absolute values of zero correspond to log-values of ∼0.7. Square-shaped data points highlight patients with CTX with low cholestanol levels and triangular points highlight the patient we obtained data from before and after CDCA treatment. ∗P < 0.0125 calculated by one-way ANOVA and Fisher’s LSD or by Kruskal-Wallis and Mann-Whitney-U tests. ∗ are only indicated for significant pair-wise comparisons. CDCA, chenodeoxycholic acid; CSF, cerebrospinal fluid.
Table 3.
Sterol concentrations in cerebrospinal fluid (CSF)
| Systematic Name | Common Name (Abbreviation) | Control (n = 5) |
CTX (n = 5) |
CDCA (n = 3) |
Note | |||
|---|---|---|---|---|---|---|---|---|
| Mean | Range | Mean | Range | Mean | Range | |||
| GC-MS | ||||||||
| Total cholest-5-en-3β-ol | Total cholesterol | 2.4 μg/ml | 1.5–3.3 | 5.6 μg/ml | 2.1–7.5 | 4.5 μg/ml | 4.1–4.7 | |
| Total 5α-cholestan-3β-ol | Total 5α-cholestanol | 2.9 ng/ml | 2.9–3.0 | 19.7 ng/ml | 1.7–47.2 | 5.4 ng/ml | 0.3–11.3 | |
| Total cholest-5-ene-3β,7α-diol | Total 7α-hydroxycholesterol (7α-HC) | 1.29 ng/ml | 1.12–1.42 | 10.62 ng/ml | 1.05–37 | 1.16 ng/ml | 0.3–2.87 | |
| Total cholest-5-ene-3β,(25R)26-diol | Total 27-hydroxycholesterol (27-HC) | 1.23 ng/ml | 0.73–1.94 | 0 | - | 0 | - | a |
| LC-MS (all values in ng/ml and for free oxysterols) | ||||||||
| Monohydroxycholesterols | ||||||||
| Cholest-5-ene-3β,7α-diol | 7α-Hydroxycholesterol (7α-HC) | 0.16 | 0.09–0.21 | 0.69 | 0.12–2.66 | 0.16 | 0.05–0.27 | |
| 7α-Hydroxycholest-4-en-3-one | 7α-Hydroxycholestenone (7α-HCO) | 0.00 | - | 1.48 | 0.14–6.09 | 0.04 | 0.02–0.07 | a,b,d |
| Cholest-5-ene-3β,24S-diol | 24S-Hydroxycholesterol (24S-HC) | 0.00 | 0–0.01 | 0.09 | 0–0.23 | 0.03 | 0.01–0.07 | d |
| Cholest-5-ene-3β,25-diol | 25-Hydroxycholesterol (25-HC) | 0.00 | - | 1.97 | 0–6.34 | 1.43 | 0–4.28 | d |
| Cholest-5-ene-3β,(25R)26-diol | 27-Hydroxycholesterol (27-HC) | 0.02 | 0.01–0.03 | 0.00 | - | 0.00 | - | a,d |
| Dihydroxycholesterols and dihydroxycholestenones | ||||||||
| 7α,12α-Dihydroxycholest-4-en-3-one | 7α,12α-Dihydroxycholestenone (7α,12α-diHCO) | 0.03 | 0–0.08 | 8.76 | 0.32–31.97 | 0.13 | 0.1–0.15 | a,d |
| 7α,24-Dihydroxycholest-4-en-3-one | 7α,24-Dihydroxycholestenone (7α,24-diHCO) | 0.00 | - | 0.00 | - | 0.00 | - | d |
| 7α,25-Dihydroxycholest-4-en-3-one | 7α,25-Dihydroxycholestenone (7α,25-diHCO) | 0.00 | - | 0.17 | 0.12–0.24 | 0.09 | 0.04–0.13 | a,b,d |
| 7α,(25R)26-Dihydroxy-cholest-4-en-3-one | 7α,27-Dihydroxycholestenone (7α,27-diHCO) | 0.01 | 0–0.03 | 0.01 | 0.00–0.04 | 0.00 | - | d |
| Trihydroxycholestenones | ||||||||
| 7α,12α,25-Trihydroxy-cholest-4-en-3-one | 7α,12α,25-Trihydroxycholestenone (7α,12α,25-triHCO) | 0.11 | 0.07–0.23 | 0.43 | 0.17–0.86 | 0.05 | 0.00–0.1 | a,c,d,e |
| Monohydroxycholestenoic and oxocholestenoic acids | ||||||||
| 3β-Hydroxycholest-5-en-(25R)26-oic acid | 3β-Hydroxycholestenoic acid (3β-HCA) | 0.61 | 0.41–0.87 | 0.00 | - | 0.01 | 0.00–0.02 | a,d |
| 3-Oxocholest-4-en-(25R)26-oic acid | 3-Oxocholestenoic acid | 0.00 | - | 0.00 | - | 0.00 | - | d |
| Dihydroxycholestenoic and monohydroxyoxocholestenoic acids | ||||||||
| 3β,7α-Dihydroxycholest-5-en-(25R)26-oic acid | 3β,7α-Dihydroxycholestenoic acid (3β,7α-diHCA) | 0.00 | - | 0.00 | - | 0.00 | - | d |
| 7α-Hydroxy-3-oxocholest-4-en-(25R)26-oic acid | 7α-Hydroxy-3-oxocholestenoic acid (7αH,3O-CA) | 13.21 | 6.93–30.36 | 0.10 | 0.00–0.17 | 0.00 | - | a,d |
| 3β,7α-Dihydroxycholest-5-en-(25S)26-oic acid | 3β,7α-Dihydroxycholestenoic acid (25S) (3β,7α-diHCA (25S)) | 0.00 | - | 0.00 | - | 0.00 | - | d |
| 7α-Hydroxy-3-oxocholest-4-en-(25S) 26-oic acid | 7α-Hydroxy-3-oxocholestenoic acid(25S) (7αH,3O-CA (25S)) | 2.85 | 1.59–7.21 | 0.20 | 0.12–0.32 | 0.11 | 0.08–0.17 | a |
| 3β,25-Dihydroxycholest-5-en-26-oic acid and/or 3β,25,X-Trihydroxycholest-5-en-Y-one | 3β,25-Dihydroxy-cholestenoic acid and/or 3β,25,X-Trihydroxycholest-5-en-Y-one | 0.00 | - | 0.00 | - | 0.00 | - | e,f,g |
| Dihydroxyoxocholestenoic acids | ||||||||
| 7α,12α-Dihydroxy-3-oxocholest-4-en-26-oic acid and/or 7α,12α,25-Trihydroxycholest-4-ene-3,24-dione | 7α,12α-Dihydroxy-3-oxocholestenoic acid and/or 7α,12α,25-Trihydroxycholest-4-ene-3,24-dione | 0.36 | 0.16–0.73 | 0.50 | 0.00–1.85 | 0.00 | - | e,d,f,h |
| 7α,24-Dihydroxy-3-oxocholest-4-en-26-oic acid | 7α,24-Dihydroxy-3-oxocholestenoic acid | 0.20 | 0.11–0.48 | 0.01 | 0.00–0.03 | 0.01 | 0.00–0.01 | a,d,f |
| 7α,25-Dihydroxy-3-oxocholest-4-en-26-oic acid | 7α,25-Dihydroxy-3-oxocholestenoic acid | 0.76 | 0.49–1.6 | 0.00 | - | 0.00 | - | a,d,f |
| Dien-sterols | ||||||||
| 3β-Hydroxycholesta-5,7-dien-26-oic acid and/or 3β-Hydroxycholesta-5,Y-dien-26-oic acid | 3β-Hydroxycholest-5,7-dienoic acid and/or 3β-Hydroxycholesta-5,Y-dien-26-oic acid | 0.00 | - | 0.00 | - | 0.00 | - | d |
| 3-Oxocholesta-4,6-dien-26-oic acid and/or 3-Oxocholesta-4,Y-dien-26-oic acid | 3-Oxocholesta-4,6-dienoic acid and/or 3-Oxocholesta-4,Y-dien-26-oic acid | 2.08 | 0.87–3.84 | 0.01 | 0.00–0.02 | 0.03 | 0.00–0.08 | a,b,d |
| 12α-Hydroxycholesta-4,6-dien-3-one | 12α-Hydroxycholesta-4.6-dien-3-one (12α-HC-4,6-dien-3-one) | 0.00 | - | 4.59 | 0.54–11.97 | 0.00 | - | a,d |
| 24 or 25-Hydroxycholesta-4,6-dien-3-one and/or 24 or 25-Hydroxycholesta-4,8-dien-3-one | 24 or 25-Hydroxycholesta-4,6-dien-3-one and/or 24 or 25-Hydroxycholesta-4,8-dien-3-one | 0.00 | - | 0.00 | - | 0.00 | - | d |
CTX, cerebrotendinous xanthomatosis; CDCA, chenodeoxycholic acid.
CTX group: Patients CTX 4–8, CDCA group: Patients CTX 1, CTX 2, and CTX 5
P < 0.0125 for comparison between controls and untreated patients with CTX.
P < 0.0125 for comparison between controls and patients with CTX treated with 750 mg/day CDCA.
P < 0.0125 for comparison between drug-naive and CDCA-treated patients with CTX.
Values below limit of quantification of 0.1 ng/ml in CSF or 0.5 ng/ml in serum.
Identification based on exact mass retention time and MS3 spectrum in the absence of an authentic standard.
MS3 spectra do not allow the differentiation between the isomers/epimers.
The position of the additional hydroxy group is not determined.
In CTX, there is no direct synthesis of (25R)26-oic acids and most likely a 25-hydroxy-3,24-dione structure is present.
7α-hydroxylation pathway
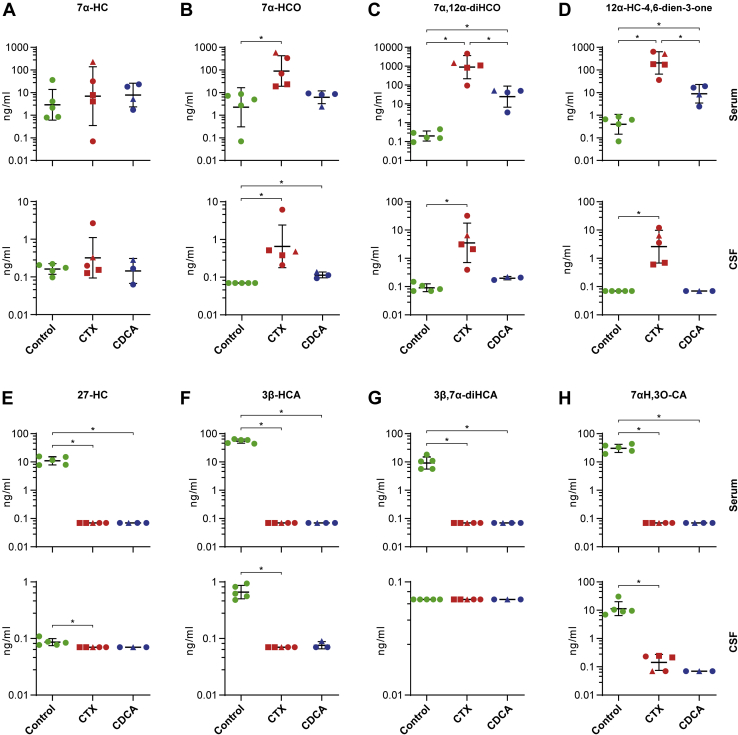
The classical CYP7A1-related pathway accounts for ∼90% of total bile acid synthesis and is initiated by 7α-hydroxylation of cholesterol (Fig. 1) (20). Total serum levels of 7α-HC as assessed by GC-MS were increased 14-fold in untreated patients with CTX compared with healthy controls (P < 0.0125, Fig. 2B), whereas the concentration of the free fraction of 7α-HC as determined by LC-MS was comparable between groups (Fig. 3A). However, the 3-oxo form, 7α-hydroxycholest-4-en-3-one (7α-HCO, also known by the trivial name C4 or 7α-C4), was elevated two orders of magnitude in serum of patients with untreated CTX (P < 0.0125, Fig. 3B, Table 2). Total 5α-cholestanol, the most commonly used diagnostic biomarker in CTX, was on average ∼10-fold elevated in serum and CSF, measured by GC-MS, but two patients showed 5α-cholestanol levels only slightly above control values (marked by red squares in Fig. 2C). Interestingly, these two patients had also the lowest amounts of 7α-HCO in serum and 7α-HC in CSF as measured by LC-MS (Fig. 3A, B).
Fig. 3.
Analysis of metabolites of the classical CYP7A1-dependent pathway and of the alternative CYP27A1-dependent pathway for bile acid synthesis. A–H: Serum concentrations of free oxysterols measured by LC-ESI-MS are presented in the upper panel and CSF findings in the lower panel. Healthy controls are indicated in green, untreated patients with CTX in red and patients treated with 750 mg/d CDCA in blue. Individual data points and mean ± SD are presented on a logarithmic scale with antilog numbering. Absolute values of zero correspond to log-values of ∼0.7. Square-shaped data points highlight patients with CTX with low cholestanol levels and triangular points highlight the patient we obtained data from before and after CDCA treatment. ∗P < 0.0125 calculated by one-way ANOVA and Fisher’s LSD or by Kruskal-Wallis and Mann-Whitney-U tests. ∗ are only indicated for significant pair-wise comparisons. CDCA, chenodeoxycholic acid; CTX, cerebrotendinous xanthomatosis.
The 12α-hydroxylated downstream metabolite 7α,12α-dihydroxycholest-4-en-3-one (7α,12α-diHCO) turned out to be the most highly elevated compound in our data set even in the two patients with almost normal 5α-cholestanol, 7α-HC, and/or 7α-HCO values (Fig. 3C), with mean levels about 11,000-fold higher in untreated patients with CTX compared with controls (1614.9 ng/ml [range: 49.21–4602.03] vs. 0.16 ng/ml [range: 0.02–0.38]; P < 0.0125). In CSF, the mean concentration of 7α,12α-diHCO was 350-fold higher in untreated patients with CTX compared with controls (8.76 ng/ml [range 0.32–31.97] vs. 0.03 ng/ml [range: 0–0.08], P < 0.0125, Fig. 3C). A similar pattern was found for 12α-hydroxycholesta-4,6-dien-3-one, the dehydration product of 7α,12α-diHCO (Fig. 3D, Table 3).
27-Hydroxylation pathway
The 27-hydroxylation pathway is depicted in Fig. 1. Owing to mutations in CYP27A1, the synthesis of 27-HC, 3β-hydroxycholestenoic acid, and other downstream metabolites of the alternative pathway of bile acid synthesis are absent (below the limit of detection/quantification) in patients with CTX (Fig. 3E–H). Of interest, 3β,7α-dihydroxycholestenoic acid, previously shown to be neuroprotective (21), was completely lacking in serum of the CTX group (Fig. 3G), as well as its downstream metabolite 7α-hydroxy-3-oxocholestenoic acid (Fig. 3H), one of the main oxysterols exported from brain which was not detectable in patients’ serum and CSF (22). Note that unless stated otherwise, these metabolites have 25R-stereochemistry.
24S-hydroxylation pathway
The cerebral pathway of bile acid synthesis is initiated by the 24S-hydroxylation of cholesterol by CYP46A1 (only expressed in brain tissue). In parallel, 24S-hydroxylation provides the major route for cholesterol removal from the brain (Fig. 1). 7α,24S-Dihydroxycholestenone and its dehydration product 24S-hydroxycholesta-4,6-dien-3-one were increased in serum of untreated patients with CTX (P < 0.0125, Table 2). The abovementioned metabolites were not detectable in CSF. Whereas 24S-hydroxycholesterol was unchanged, and 7α,24-dihydroxy-3-oxocholestenoic acid was reduced in CSF of the CTX group (P < 0.0125; Table 3).
25-hydroxylation pathway
The 25-hydroxylation pathway (Fig. 1) is responsible for only about 1–2% of bile acid production, at least in rat (23). Nevertheless, the concentrations of 7α,25-dihydroxycholest-4-en-3-one (7α,25-diHCO) and 7α,12α,25-trihydroxycholest-4-en-3-one were highly elevated about 9 to 30-fold in serum of untreated patients compared to controls (P < 0.0125, Table 2). Both metabolites are precursors of 25-hydroxylated bile alcohols that are established as biomarkers for CTX in blood, feces, and urine (24, 25). In CSF, the levels of these molecules are much lower compared with serum, and 7α,25-diHCO was not detectable in controls, but both metabolites were significantly elevated about 10-fold to 20-fold above the detection limit in CTX (P < 0.0125, Table 3). A detailed overview of all analyzed metabolites depicted in Fig. 1 is provided in Tables 2 and 3 for serum and CSF, respectively.
Sterol distribution in CTX and control brain
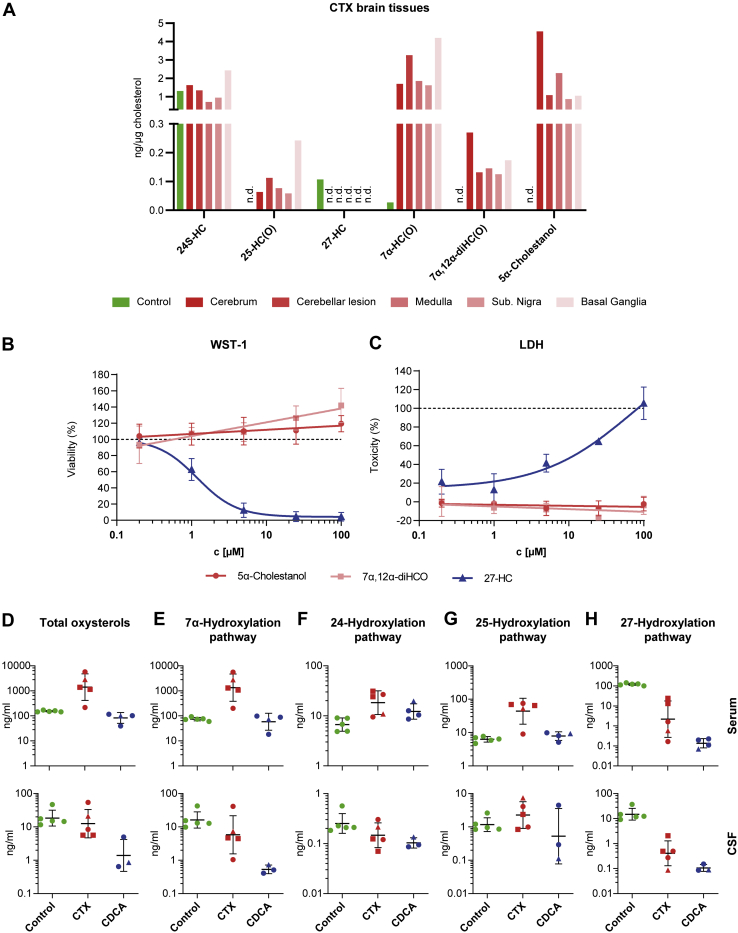
To compare biochemical findings in serum and CSF with concentrations in the brain, we assessed the oxysterol composition in the cerebrum, cerebellum, medulla oblongata, substantia nigra, and basal ganglia from an autopsy case with CTX and cerebrum from a control brain (Table 4, Fig. 4A). The patient with CTX was not treated with CDCA before death. Because of low concentrations of some of the analyzed metabolites, the brain levels of the respective cholest-5-en-3β-ol (HC) and cholest-4-en-3-one (HCO) forms were combined for Fig. 4A and abbreviated as HC(O). A separate listing of the two forms can be found in Table 4. After normalization for cholesterol levels to correct for differences in sample conditions, the mean concentration of 7α-HC(O) was more than 60-fold increased in CTX brain compared with control brain (Fig. 4A). The metabolites 7α,12α-diHC(O) and 25-HC(O) were not detected in control brain but were present in the CTX brain (Fig. 4A). Similarly, 5α-cholestanol was ascertainable only in the CTX brain. In contrast, 27-HC was only found in control brain. The concentration of 24S-hydroxycholesterol was comparable in control and CTX brain (Fig. 4A). In the CTX brain, levels of all metabolites were similar in cerebrum compared with medulla oblongata, substantia nigra, basal ganglia, and a cerebellar xanthoma. (Fig. 4A).
Table 4.
Sterol concentrations in different brain regions of one control and one CTX patient
| Common Name | Abbreviation | Control |
CTX |
||||
|---|---|---|---|---|---|---|---|
| Cerebrum | Cerebrum | Medulla Oblongata | Cerebellar Xanthoma | Substantia Nigra | Basal Ganglia | ||
| 24S-Hydroxycholesterol | 24S-HC | 1.31 | 1.63 | 0.73 | 1.35 | 0.95 | 2.43 |
| 25-Hydroxycholesterol | 25-HC | 0.00 | 0.06 | n.m. | n.m. | n.m. | n.m. |
| 25-Hydroxycholestenone | 25-HCO | 0.00 | 0.01 | n.m. | n.m. | n.m. | n.m. |
| 25-HC+25-HCO | 0.00 | 0.06 | 0.08 | 0.11 | 0.06 | 0.24 | |
| 7α-Hydroxycholesterol | 7α-HC | 0.03 | 1.35 | 1.41 | 2.86 | 1.18 | 3.81 |
| 7α-Hydroxycholestenone | 7α-HCO | 0.00 | 0.34 | 0.45 | 0.40 | 0.45 | 0.40 |
| 7α-HC+7α-HCO | 0.03 | 1.70 | 1.87 | 3.26 | 1.63 | 4.20 | |
| 27-Hydroxycholesterol | 27-HC | 0.11 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| 7α,12α-Dihydroxycholesterol | 7α,12α-HC | 0.00 | 0.08 | 0.01 | 0.02 | 0.01 | 0.00 |
| 7α,12α-Dihydroxycholestenone | 7α,12α-HCO | 0.00 | 0.19 | 0.14 | 0.11 | 0.11 | 0.18 |
| 7α,12α-HC+7α,12α-HCO | 0.00 | 0.27 | 0.15 | 0.13 | 0.13 | 0.18 | |
| 5α-Cholestanol | 5α-Cholestanol | 0.00 | 4.56 | 2.29 | 1.07 | 0.87 | 1.05 |
All values in ng/μg cholesterol and for free oxysterols measured by LC-MS.
CTX, cerebrotendinous xanthomatosis; n.m., not measured.
Fig. 4.
Sterol composition of brain, toxicity of CTX-related metabolites, and overall oxysterol composition. A: Oxysterol content (free, nonesterified) as measured by LC-ESI-MS of brain autopsies of an untreated CTX patient (red) and a control (green). Data are normalized to cholesterol content. B: WST-1 assay and (C) LDH release assay of NSC-34 cells treated with increasing concentrations (0.2 μM, 1 μM, 5 μM, 25 μM, and 100 μM) of the indicated sterol metabolites. N = 3–6. D: Sum of all oxysterols and (E–H) the individual pathways measured by LC-ESI-MS in serum (upper panel) and CSF (lower panel) of controls (green), untreated patients with CTX (red) and patients treated with 750 mg/day CDCA (blue). Individual data points and mean ± SD are presented on a logarithmic scale with antilog numbering. Absolute values of zero correspond to log values of ∼0.7. Square-shaped data points highlight patients with CTX with low cholestanol levels and triangular points highlight the patient we obtained data from before and after CDCA treatment. One-way ANOVA and Fisher’s LSD or Kruskal-Wallis and Mann-Whitney-U tests were performed and showed no statistical significance.
Toxicity of 7α,12α-dihydroxycholest-4-en-3-one and 5α-cholestanol in cell culture
To explore potential detrimental effects of the two most abundant abnormal oxysterols in CTX, we performed WST-1 cleavage and lactate dehydrogenase (LDH) release assays in neuronal NSC-34 cells. In addition to 7α,12α-diHCO and 5α-cholestanol, 27-HC was included in toxicity assessments as a positive control metabolite that has previously been shown to be neurotoxic (26). As expected, the addition of 27-HC was associated with dose-dependent decreases in WST-1 cleavage and increases in LDH release, with IC-50 values of 1.2 μM and 72.7 μM, respectively (Fig. 4B, C). In contrast, the addition of 7α,12α-diHCO and 5α-cholestanol did not change WST-1 cleavage or LDH release up to concentrations three orders of magnitude above levels found in CSF of patients with CTX.
CDCA effects on abnormal metabolites
To determine the metabolic effects of CDCA treatment, we analyzed sterols in serum and CSF of patients with CTX under supplementation therapy with CDCA (750 mg/day) for at least 8 months. We found CDCA to largely normalize the elevated levels of total 5α-cholestanol (Fig. 2C) and total 7α-HC in serum as well as in CSF (Fig. 2B), whereas it had no effect on total cholesterol levels (Fig. 2A). Concerning free 7α,12α-diHCO, the most extensively elevated metabolite in our analysis, CDCA treatment lowered the mean serum levels significantly from 1614.9 ng/ml (range: 49.21–4602.03) to 35.64 ng/ml (range: 3.44–50.07) (P < 0.0125), whereas mean levels were still 220-fold increased compared with controls (P < 0.0125, Fig. 3C). In CSF, CDCA supplementation reduced the mean levels of 7α,12α-diHCO from 8.76 ng/ml (range: 0.32–31.97) to 0.13 ng/ml (range: 0.1–0.15). Most other metabolites of the 7α-, 24S- and 25-hydroxylation pathways that were elevated in CTX were reduced to almost normal levels with CDCA treatment (for details see Table 2). In CSF, only the two metabolites 7α-HCO and 7α,25-diHCO were still significantly increased compared to controls after CDCA treatment although with very low absolute concentrations (Table 3). No effect of CDCA treatment was seen on metabolites of the 27-hydroxylation pathway. 27-Hydroxylated metabolites remained absent in serum and CSF upon treatment.
Overall levels of oxysterols
In total, seven metabolites in the free sterol fraction were significantly increased in serum of untreated patients with CTX compared with controls. On the other hand, eight metabolites were decreased, and nine metabolites were similar to controls (Table 2).
To compare total amounts of oxysterols present in the different samples, mean concentrations of all 24 metabolites determined by LC-MS in the free sterol fraction were assigned to the respective pathways as depicted in Fig. 1 (Fig. 4D–H). By this analysis, we found total serum oxysterols to be drastically increased from 154.55 ng/ml (range: 145.38–170.44) in controls to 2265.95 ng/ml (range: 217.76–5725.68) in untreated patients with CTX (Fig. 4D). Especially the 7α-hydroxylation pathway contributed to this increase, comprising 36% of total oxysterols in controls and 96% in the CTX group (Fig. 4E). More specifically, the two metabolites 7α,12α-diHCO and 12α-hydroxycholesta-4,6-dien-3-one alone were responsible for 90% of the difference. While the mean total amount of metabolites belonging to the 24S- and 25-hydroxylation pathways were only slightly increased compared with controls the 27-hydroxylation pathway was heavily reduced from 120.45 ng/ml (range: 110.55–143) in controls to only 7.86 ng/ml (range: 0.10–24.03) in drug-naive patients with CTX which corresponds to 0.34% of total oxysterols. CDCA treatment reduced mean total oxysterols in serum of patients with CTX to 89.49 ng/ml (range: 39.59–115.4) because of a normalization of the 7α-, 24S and 25-hydroxylation pathways (Fig. 4E–G). However, metabolites of the 27-hydroxylation pathway were still absent (Fig. 4H).
The relative contribution of the different pathways to total oxysterol levels was similar in serum and CSF in controls, although the overall levels were much lower in CSF (Fig. 4D).
In CSF, we could only detect 17 metabolites. Of these, the five metabolites with increased levels in the CTX group were all found in the 7α- and the 25-hydroxylation pathways, whereas seven metabolites of the 27-hydroxylation pathway were almost absent (Fig. 4E, F, H). Metabolites of the 24S-hydroxylation pathway were slightly reduced. Upon CDCA treatment, overall oxysterol levels in CSF were drastically reduced to only 2.09 ng/ml (range: 0.58–4.89) and even 90% lower than in controls (Fig. 4D). Thereby, both, metabolites of the 7α- and the 27-hydroxylation pathway were greatly reduced or almost absent with only 0.48 ng/ml (range: 0.34–0.66) and 0.04 ng/ml (range: 0.02–0.08), respectively (Fig. 4E, H).
Discussion
In this comprehensive analysis of lipid metabolites in CTX and controls, GC-MS and LC-MS were used to quantify 26 metabolites in serum and CSF from drug naive and CDCA-treated patients with CTX. These analyses revealed a dichotomic pattern with metabolites in the 7α- and 25-hydroxylation pathways being increased in CTX, whereas 27-hydroxylated and carboxylated cholesterol products were largely lacking. This finding is in good accordance with results from published studies (2, 17, 27) and generally applies to both serum and CSF, though with substantial differences in the concentration and composition of oxysterols between serum and CSF.
We further compared serum and CSF findings with the oxysterol profile in brain. Mass spectrometry analysis of a CTX brain confirmed oxysterol alterations that were similar to changes observed in CSF. The metabolites 25-hydroxycholesterol and 7α,12α-diHC(O) were absent in both CSF and cerebrum of controls, whereas they were detectable in CSF and brain of patients with CTX (Figs. 3C, 4A, Tables 3 and 4). Moreover, levels of 7α-HC(O) were highly increased in CSF and brain from patients with CTX (Figs. 2B, 3A, B, 4A), whereas 27-HC was only detectable in controls (Figs. 2D, 3E, 4A). The highly elevated 7α-HC(O) in brain of an untreated CTX patient is in accordance with the proposed mechanism of cerebral accumulation of 5α-cholestanol in CTX via flux of 7α-HC(O) across the blood-brain barrier into the brain in a CTX mouse model and subsequent conversion to 5α-cholestanol (28). It is noteworthy that CYP7A1, the enzyme that introduces the 7α-hydroxy group to cholesterol and sterol 12α-hydroxylase (CYP8B1) which is responsible for 12α-hydroxylation are liver specific and not expressed in brain (data available from v20.1.proteinatlas.org) (29). Hence, the identification of these metabolites in brain most likely reflects uptake from the circulation by crossing the blood-brain barrier or alternatively via nonenzymatic free radical oxidation reactions. Our study proves that key metabolic changes observed in brain tissue were also identified in CSF and serum of CTX patients and suggest that both, CSF and serum, are suitable for monitoring the effects of therapeutic approaches in CTX.
7α,12α-diHCO, 5α-cholestanol, and 7α-HC(O) were identified as the most abundant oxysterols in CTX with markedly increased levels in both serum and CSF. It has been hypothesized that increased levels of these metabolites in CSF and brain may contribute to neurodegeneration in patients with CTX. In our neuronal cell culture toxicity study, no toxic effect was identified for 7α,12α-diHCO and 5α-cholestanol in contrast to 27-HC at concentrations up to three orders of magnitude higher than levels found in CSF of patients (Fig. 4B, C). These results suggest no direct contribution of the tested compounds to neurotoxicity in CTX. Nevertheless, long-term exposure to neuronal cells may produce toxicity that would not be identified in our cell culture system. This possibility is in line with the observation that neurodegenerative complications from CTX typically develop slowly over decades. Moreover, it is possible that other cell types in the brain may be adversely affected by chronically elevated levels of oxysterols, leading to neurodegeneration.
In CTX, reduced amounts of bile acids and possibly bile alcohols induce a feedback mechanism mediated by farnesoid X receptor leading to upregulation of enzymes involved in bile acid synthesis including CYP7A1 and CYP8B1 (30). This leads to increased production of oxysterols that normally require 27-hydroxylation to be further processed into bile acids. As most CYP27A1 mutations cause an almost complete lack of sterol 27-hydroxylase, these metabolites and 5α-cholestanol accumulate in tissues resulting in tendon xanthomas, early onset cataracts, and cerebellar lipid deposits.
CDCA is established as an efficacious treatment for CTX that suppresses CYP7A1 and CYP8B1 activity to almost normal levels (31). In our study, this is reflected by the marked reduction or normalization after CDCA therapy of most metabolites which are elevated in untreated patients with CTX. For example, the overall oxysterol serum levels were elevated 10-fold in untreated patients with CTX compared with controls (2447 ng/ml vs. 207 ng/ml) but normalized to 163 ng/ml after CDCA treatment. In contrast, in CSF, the overall oxysterol levels were not increased in CTX, but their composition was altered. The CDCA treatment reduced the total levels of oxysterols in CSF far below controls. This is driven by the lack of 27-hydroxylated metabolites which constitute 45% of free sterols in CSF of healthy.
Consistent with results from earlier studies (6, 21), CDCA treatment did not alleviate impairment in the 27-hydroxylation pathway in CTX, resulting in severe lack of related metabolites like 27-HC, 3β-hydroxycholestenoic acid, and 3β,7α-diHCA. While 27-HC is the main metabolite imported from the circulation into the brain (32), 7α-hydroxy-3-oxocholestenoic acid together with 24S-hydroxycholesterol are the main export products (22). 27-HC has been reported to be a regulator of brain cholesterol synthesis and homeostasis mediated by its binding to nuclear liver X receptors and INSIG proteins (33, 34). Furthermore, 27-HC has been shown to potentially foster neuronal cholesterol efflux by increasing expression of the ABC transporters ABCA1, ABCG5, and ABCG8 and APOE (35). This suggests that the lack of CYP27A1 activity in CTX adversely affects both the import of cholesterol metabolites into the brain as well as a route for cholesterol removal. The lack of 27-HC may further stimulate cholesterol synthesis in the brain, which might increase the tendency to accumulate cholesterol and 5α-cholestanol in brain xanthomas. In addition, we have previously shown that the CYP27A1 metabolite 3β,7α-diHCA which is strongly reduced in CTX increases motor neuron survival and facilitates maturation into islet-1+ cells in a liver X receptors–dependent manner (21). Hence, the lack of these 27-hydroxylated and carboxylated metabolites in the brain of patients with CTX may play a critical role in neurodegenerative processes.
Taken together, there is strong evidence that impairment of the 27-hydroxylation pathway may have a crucial role in the pathogenesis of neurodegeneration in CTX. Although treatment with CDCA normalizes the levels of metabolites such as 5α-cholestanol in plasma and seems to be beneficial when treatment is started early in the course of CTX, many patients show progressive neurodegeneration in the long term. Such patients may benefit from treatment that improves sterol 27-hydroxylation. A potential strategy is the replacement of defective CYP27A1. In principle, this could be achieved either by direct enzyme replacement, liver transplantation, supplementation of CYP27A1 mRNA via nanoparticles, or by hepatocyte-specific viral vectors transferring a wildtype gene copy. Further studies are needed to determine the efficacy of these liver centered strategies in the brain. Treatment with adeno-associated viruses to target disease-relevant genes directly in the brain could be another option. Currently, several phase I/II experimental clinical trials for treatment of other neurodegenerative diseases such as Alzheimer’s disease, Parkinson’s disease, or spinal muscular atrophy with this method are ongoing (36).
From a diagnostic point of view, our results challenge the notion of 5α-cholestanol as the primary diagnostic biomarker for CTX. Previously, it has been shown that other diseases like familial hypercholesterolemia and sitosterolemia also present with increased levels of 5α-cholestanol (37). Although the diagnosis of CTX is now primarily genetically based, there are still variants of unknown significance on the one hand and mutations that are difficult to detect like deep intronic splice variants on the other hand that require metabolic confirmation to reach a definite diagnosis, historically performed by measuring 5α-cholestanol levels. In our series, we found two patients with only marginal increases in 5α-cholestanol levels compared with controls. Hence, we recommend using the increase in 7α,12α-diHCO and the lack of 27-HC or their ratio as primary diagnostic biomarkers in CTX as proposed in prior publications (37, 38). In addition, it is important to assess the oxysterol metabolism in CTX after diagnosis to ensure treatment efficacy. This study suggests to monitor apart from 5α-cholestanol other biomarkers like free 7α-HCO or 7α,12α-diHCO which may constitute more sensitive markers of sufficient suppression of the classical CYP7A1-initiated pathway and do not return to normal levels with CDCA treatment (Fig. 3B, C).
Early diagnosis and treatment appear to be of major importance according to the study of Stelten et al. (12) who found favorable responses when CDCA treatment started before the age of 24 years. In our study, treatment started late (between 45 and 60 years of age) and only after cognitive decline in all patients and after the development of additional neurological symptoms including cerebellar ataxia in three-fourths patients. Although it would be interesting to investigate the metabolic profile in patients who have been treated since young age and show favorable courses of the disease in response to CDCA treatment, we do not expect major metabolic differences in this group compared with the patients in later stages included in our study. All metabolites accumulating in CTX do almost normalize in serum and CSF with CDCA treatment also in late stages and metabolites that are missing in CTX because of a lack of 27-hydroxylation cannot be substituted independent how early CDCA treatment is started. However, early treatment may prevent deposition of large amounts of abnormal lipids in the brain that are potentially insoluble, especially in late stages of the disease.
There are several limitations of our study. Despite the extensive metabolic analyses performed in this study, there are other known sterol metabolites that could not be assessed by the mass spectrometric techniques applied in this study. These include bile alcohols that could not be detected by LC-MS, without polarity switching, in serum and CSF, but are known to be substantially increased in plasma, feces, and urine of CTX patients (39). However, the bile alcohol precursor 7α,12α,25-trihydroxycholest-4-en-3-one was elevated in serum and CSF of untreated patients with CTX. After CDCA treatment, the levels were reduced in both specimens (Tables 2 and 3).
A further limitation of our study is the small number of treated and untreated patients with CTX available for analyses. In the future, additional samples may be collected in international consortia like the European Reference Networks for Rare Neurological Diseases (ERN-RND; www.ern-rdn.eu) or Metabolic Diseases (MetabERN; https://metab.ern-net.eu). This would especially be of interest for longitudinal follow-up studies comparing neurological outcomes to measurements of baseline and posttreatment levels of oxysterols.
In summary, this comprehensive analysis of metabolic abnormalities in CTX indicates a dichotomic profile and suggests that quantification of sterol metabolites other than 5α-cholestanol may have increased utility for diagnosis of CTX. Because oxysterols that are increased in untreated CTX are substantially reduced in serum and CSF after CDCA therapy, it is less certain that elevated levels of toxic metabolites drive the progression of disease that is observed in some patients with CTX despite long-term CDCA treatment. In contrast, the lack of 27-hydroxylated sterol metabolites is a profound and persisting finding in patients with CTX before and after treatment with CDCA. This includes a deficiency of metabolites essential for lipid import and export in the brain, as well as potentially neuroprotective compounds. Therefore, we speculate that a curative approach may require complementation of 27-hydroxylated metabolites either pharmacologically or by restoration of sterol 27-hydroxylase activity. Additional studies are needed to define optimal therapeutic strategies for the management of patients with CTX.
Data availability
Anonymized data from the present study can be requested for well-defined research questions. Please contact the corresponding author Ludger Schöls, University Hospital Tübingen, ludger.schoels@uni-tuebingen.de.
Conflicts of interest
E. Y. is shareholder in CholesteniX Ltd.; Y. W. is listed as inventor on the patent Kit and method for quantitative detection of steroids US9851368B2 and is shareholder in CholesteniX Ltd.; P. B. D. is consultant at Retrophin and received an institutional grant from Retrophin; A. E. D. is consultant for Leadiant Biosciences and Retrophin, institutional grant recipient from Retrophin. The OHSU Foundation and Chemical Physiology and Biology Department have received gifts from Retrophin. These gifts, which have not been made specifically in connection with this research, have been reviewed by the OHSU integrity office; W. J. G. is listed as inventor on the patent Kit and method for quantitative detection of steroids US9851368B2 and is shareholder in CholesteniX Ltd. All other authors declare that they have no conflicts of interest with the contents of this article.
Acknowledgments
The authors heartily thank Stefanie Schuster for her advice on figure design and helpful discussions of data and statistics, also they appreciate Yvonne Schelling for her help with sample management. Anja Kerksiek, Institute of Clinical Chemistry and Clinical Pharmacology, University Hospital Bonn, Bonn, Germany, for GC-MS technical assistance in analysis of CSF sterols.
L.S., H.H., and F.R. are members of the European Reference Network for Rare Neurological Diseases—Project ID No 739510.
Author contributions
L. S. conceptualization; P. H., E. Y., L. G., O. W. H., Y. W., D. L., W. J. G. methodology; P. M. statistical methodology; P. H., P. M. formal analysis; P. H., E. Y., H. H., L. G. investigation; F. R., S. L. C., P. B. D., A. E. D. resources; P. H., L. S. writing - original draft; all authors writing - review & editing; P. H. visualization; S. H., W. J. G., L. S. supervision; S. H., L. S project administration.
Funding and additional information
This work was supported by the Charitable Hertie Foundation to support the group of L. S. and by the UK Biotechnology and Biological Sciences Research Council, grant numbers BB/I001735/1, BB/N015932/1, and BB/L001942/1.
Contributor Information
William J. Griffiths, Email: w.j.griffiths@swansea.ac.uk.
Ludger Schöls, Email: ludger.schoels@uni-tuebingen.de.
References
- 1.Lv Bogaert, Scherer H.J., Epstein É. Masson & Cie; Paris, France: 1937. Une forme cérébrale de la cholestérinose généralisée : (type particulier de lipidose à cholestérine) [Google Scholar]
- 2.Salen G., Grundy S.M. The metabolism of cholestanol, cholesterol, and bile acids in cerebrotendinous xanthomatosis. J. Clin. Invest. 1973;52:2822–2835. doi: 10.1172/JCI107478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nie S., Chen G., Cao X., Zhang Y. Cerebrotendinous xanthomatosis: a comprehensive review of pathogenesis, clinical manifestations, diagnosis, and management. Orphanet J. Rare Dis. 2014;9:179. doi: 10.1186/s13023-014-0179-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cali J.J., Hsieh C.L., Francke U., Russell D.W. Mutations in the bile acid biosynthetic enzyme sterol 27-hydroxylase underlie cerebrotendinous xanthomatosis. J. Biol. Chem. 1991;266:7779–7783. [PMC free article] [PubMed] [Google Scholar]
- 5.Bjorkhem I., Diczfalusy U., Lutjohann D. Removal of cholesterol from extrahepatic sources by oxidative mechanisms. Curr. Opin. Lipidol. 1999;10:161–165. doi: 10.1097/00041433-199904000-00010. [DOI] [PubMed] [Google Scholar]
- 6.Björkhem I., Hansson M. Cerebrotendinous xanthomatosis: an inborn error in bile acid synthesis with defined mutations but still a challenge. Biochem. Biophys. Res. Commun. 2010;396:46–49. doi: 10.1016/j.bbrc.2010.02.140. [DOI] [PubMed] [Google Scholar]
- 7.Berginer V.M., Salen G., Shefer S. Long-term treatment of cerebrotendinous xanthomatosis with chenodeoxycholic acid. N. Engl. J. Med. 1984;311:1649–1652. doi: 10.1056/NEJM198412273112601. [DOI] [PubMed] [Google Scholar]
- 8.Chiang J.Y., Kimmel R., Weinberger C., Stroup D. Farnesoid X receptor responds to bile acids and represses cholesterol 7alpha-hydroxylase gene (CYP7A1) transcription. J. Biol. Chem. 2000;275:10918–10924. doi: 10.1074/jbc.275.15.10918. [DOI] [PubMed] [Google Scholar]
- 9.Duell P.B., Salen G., Eichler F.S., DeBarber A.E., Connor S.L., Casaday L., Jayadev S., Kisanuki Y., Lekprasert P., Malloy M.J., Ramdhani R.A., Ziajka P.E., Quinn J.F., Su K.G., Geller A.S. Diagnosis, treatment, and clinical outcomes in 43 cases with cerebrotendinous xanthomatosis. J. Clin. Lipidol. 2018;12:1169–1178. doi: 10.1016/j.jacl.2018.06.008. [DOI] [PubMed] [Google Scholar]
- 10.Salen G., Meriwether T.W., Nicolau G. Chenodeoxycholic acid inhibits increased cholesterol and cholestanol synthesis in patients with cerebrotendinous xanthomatosis. Biochem. Med. 1975;14:57–74. doi: 10.1016/0006-2944(75)90020-4. [DOI] [PubMed] [Google Scholar]
- 11.Pilo de la Fuente B., Sobrido M.J., Giros M., Pozo L., Lustres M., Barrero F., Macarron J., Diaz M., Jimenez-Escrig A. [Usefulness of cholestanol levels in the diagnosis and follow-up of patients with cerebrotendinous xanthomatosis] Neurologia. 2011;26:397–404. doi: 10.1016/j.nrl.2010.12.009. [DOI] [PubMed] [Google Scholar]
- 12.Stelten B.M.L., Huidekoper H.H., van de Warrenburg B.P.C., Brilstra E.H., Hollak C.E.M., Haak H.R., Kluijtmans L.A.J., Wevers R.A., Verrips A. Long-term treatment effect in cerebrotendinous xanthomatosis depends on age at treatment start. Neurology. 2019;92:e83–e95. doi: 10.1212/WNL.0000000000006731. [DOI] [PubMed] [Google Scholar]
- 13.Bhattacharyya A.K., Lin D.S., Connor W.E. Cholestanol metabolism in patients with cerebrotendinous xanthomatosis: absorption, turnover, and tissue deposition. J. Lipid Res. 2007;48:185–192. doi: 10.1194/jlr.M600113-JLR200. [DOI] [PubMed] [Google Scholar]
- 14.Crick P.J., Bentley T.W., Wang Y., Griffiths W.J. Revised sample preparation for the analysis of oxysterols by enzyme-assisted derivatisation for sterol analysis (EADSA) Anal. Bioanal. Chem. 2015;407:5235–5239. doi: 10.1007/s00216-015-8609-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Crick P.J., William Bentley T., Abdel-Khalik J., Matthews I., Clayton P.T., Morris A.A., Bigger B.W., Zerbinati C., Tritapepe L., Iuliano L., Wang Y., Griffiths W.J. Quantitative charge-tags for sterol and oxysterol analysis. Clin. Chem. 2015;61:400–411. doi: 10.1373/clinchem.2014.231332. [DOI] [PubMed] [Google Scholar]
- 16.Griffiths W.J., Crick P.J., Wang Y., Ogundare M., Tuschl K., Morris A.A., Bigger B.W., Clayton P.T., Wang Y. Analytical strategies for characterization of oxysterol lipidomes: liver X receptor ligands in plasma. Free Radic. Biol. Med. 2013;59:69–84. doi: 10.1016/j.freeradbiomed.2012.07.027. [DOI] [PubMed] [Google Scholar]
- 17.Griffiths W.J., Crick P.J., Meljon A., Theofilopoulos S., Abdel-Khalik J., Yutuc E., Parker J.E., Kelly D.E., Kelly S.L., Arenas E., Wang Y. Additional pathways of sterol metabolism: Evidence from analysis of Cyp27a1-/- mouse brain and plasma. Biochim. Biophys. Acta Mol. Cell Biol. Lipids. 2019;1864:191–211. doi: 10.1016/j.bbalip.2018.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mackay D.S., Jones P.J., Myrie S.B., Plat J., Lutjohann D. Methodological considerations for the harmonization of non-cholesterol sterol bio-analysis. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2014;957:116–122. doi: 10.1016/j.jchromb.2014.02.052. [DOI] [PubMed] [Google Scholar]
- 19.Popp J., Lewczuk P., Kölsch H., Meichsner S., Maier W., Kornhuber J., Jessen F., Lütjohann D. Cholesterol metabolism is associated with soluble amyloid precursor protein production in Alzheimer's disease. J. Neurochem. 2012;123:310–316. doi: 10.1111/j.1471-4159.2012.07893.x. [DOI] [PubMed] [Google Scholar]
- 20.Smith L.P., Nierstenhoefer M., Yoo S.W., Penzias A.S., Tobiasch E., Usheva A. The bile acid synthesis pathway is present and functional in the human ovary. PLoS One. 2009;4 doi: 10.1371/journal.pone.0007333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Theofilopoulos S., Griffiths W.J., Crick P.J., Yang S., Meljon A., Ogundare M., Kitambi S.S., Lockhart A., Tuschl K., Clayton P.T., Morris A.A., Martinez A., Reddy M.A., Martinuzzi A., Bassi M.T. Cholestenoic acids regulate motor neuron survival via liver X receptors. J. Clin. Invest. 2014;124:4829–4842. doi: 10.1172/JCI68506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Meaney S., Heverin M., Panzenboeck U., Ekstrom L., Axelsson M., Andersson U., Diczfalusy U., Pikuleva I., Wahren J., Sattler W., Bjorkhem I. Novel route for elimination of brain oxysterols across the blood-brain barrier: conversion into 7alpha-hydroxy-3-oxo-4-cholestenoic acid. J. Lipid Res. 2007;48:944–951. doi: 10.1194/jlr.M600529-JLR200. [DOI] [PubMed] [Google Scholar]
- 23.Duane W.C., Bjorkhem I., Hamilton J.N., Mueller S.M. Quantitative importance of the 25-hydroxylation pathway for bile acid biosynthesis in the rat. Hepatology. 1988;8:613–618. doi: 10.1002/hep.1840080329. [DOI] [PubMed] [Google Scholar]
- 24.Batta A.K., Salen G., Shefer S., Tint G.S., Batta M. Increased plasma bile alcohol glucuronides in patients with cerebrotendinous xanthomatosis: effect of chenodeoxycholic acid. J. Lipid Res. 1987;28:1006–1012. [PubMed] [Google Scholar]
- 25.Vaz F.M., Bootsma A.H., Kulik W., Verrips A., Wevers R.A., Schielen P.C., DeBarber A.E., Huidekoper H.H. A newborn screening method for cerebrotendinous xanthomatosis using bile alcohol glucuronides and metabolite ratios. J. Lipid Res. 2017;58:1002–1007. doi: 10.1194/jlr.P075051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schols L., Rattay T.W., Martus P., Meisner C., Baets J., Fischer I., Jagle C., Fraidakis M.J., Martinuzzi A., Saute J.A., Scarlato M., Antenora A., Stendel C., Hoflinger P., Lourenco C.M. Hereditary spastic paraplegia type 5: natural history, biomarkers and a randomized controlled trial. Brain. 2017;140:3112–3127. doi: 10.1093/brain/awx273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Berginer V.M., Salen G., Patel S.B. Cerebrotendinous Xanthomatosis. In: Rosenberg R.N., Pascual J.M., editors. Rosenberg's Molecular and Genetic Basis of Neurological and Psychiatric Disease. Academic Press; Boston, MA: 2015. pp. 589–598. [Google Scholar]
- 28.Bavner A., Shafaati M., Hansson M., Olin M., Shpitzen S., Meiner V., Leitersdorf E., Bjorkhem I. On the mechanism of accumulation of cholestanol in the brain of mice with a disruption of sterol 27-hydroxylase. J. Lipid Res. 2010;51:2722–2730. doi: 10.1194/jlr.M008326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Uhlen M., Fagerberg L., Hallstrom B.M., Lindskog C., Oksvold P., Mardinoglu A., Sivertsson A., Kampf C., Sjostedt E., Asplund A., Olsson I., Edlund K., Lundberg E., Navani S., Szigyarto C.A. Proteomics. Tissue-based map of the human proteome. Science. 2015;347:1260419. doi: 10.1126/science.1260419. [DOI] [PubMed] [Google Scholar]
- 30.Chiang J.Y. Regulation of bile acid synthesis: pathways, nuclear receptors, and mechanisms. J. Hepatol. 2004;40:539–551. doi: 10.1016/j.jhep.2003.11.006. [DOI] [PubMed] [Google Scholar]
- 31.Ellis E., Axelson M., Abrahamsson A., Eggertsen G., Thörne A., Nowak G., Ericzon B.-G., Björkhem I., Einarsson C. Feedback regulation of bile acid synthesis in primary human hepatocytes: evidence that CDCA is the strongest inhibitor. Hepatology. 2003;38:930–938. doi: 10.1053/jhep.2003.50394. [DOI] [PubMed] [Google Scholar]
- 32.Heverin M., Meaney S., Lutjohann D., Diczfalusy U., Wahren J., Bjorkhem I. Crossing the barrier: net flux of 27-hydroxycholesterol into the human brain. J. Lipid Res. 2005;46:1047–1052. doi: 10.1194/jlr.M500024-JLR200. [DOI] [PubMed] [Google Scholar]
- 33.Li D., Long W., Huang R., Chen Y., Xia M. 27-Hydroxycholesterol Inhibits Sterol Regulatory Element-Binding Protein 1 Activation and Hepatic Lipid Accumulation in Mice. Obesity (Silver Spring) 2018;26:713–722. doi: 10.1002/oby.22130. [DOI] [PubMed] [Google Scholar]
- 34.Radhakrishnan A., Ikeda Y., Kwon H.J., Brown M.S., Goldstein J.L. Sterol-regulated transport of SREBPs from endoplasmic reticulum to Golgi: oxysterols block transport by binding to Insig. Proc. Natl. Acad. Sci. U.S.A. 2007;104:6511–6518. doi: 10.1073/pnas.0700899104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kim W.S., Chan S.L., Hill A.F., Guillemin G.J., Garner B. Impact of 27-hydroxycholesterol on amyloid-beta peptide production and ATP-binding cassette transporter expression in primary human neurons. J. Alzheimers Dis. 2009;16:121–131. doi: 10.3233/JAD-2009-0944. [DOI] [PubMed] [Google Scholar]
- 36.Piguet F., Alves S., Cartier N. Clinical Gene Therapy for Neurodegenerative Diseases: Past, Present, and Future. Hum. Gene Ther. 2017;28:988–1003. doi: 10.1089/hum.2017.160. [DOI] [PubMed] [Google Scholar]
- 37.Lutjohann D., Stellaard F., Bjorkhem I. Levels of 7alpha-hydroxycholesterol and/or 7alpha-hydroxy-4-cholest-3-one are the optimal biochemical markers for the evaluation of treatment of cerebrotendinous xanthomatosis. J. Neurol. 2020;267:572–573. doi: 10.1007/s00415-019-09650-0. [DOI] [PubMed] [Google Scholar]
- 38.DeBarber A.E., Luo J., Giugliani R., Souza C.F., Chiang J.P., Merkens L.S., Pappu A.S., Steiner R.D. A useful multi-analyte blood test for cerebrotendinous xanthomatosis. Clin. Biochem. 2014;47:860–863. doi: 10.1016/j.clinbiochem.2014.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bindl L., Lutjohann D., Lentze M.J., von Bergmann K. Cerebrotendinous xanthomatosis presenting as "chologenic diarrhoea". Acta Paediatr. 2001;90:828–829. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Anonymized data from the present study can be requested for well-defined research questions. Please contact the corresponding author Ludger Schöls, University Hospital Tübingen, ludger.schoels@uni-tuebingen.de.