INTRODUCTION
In the early 2000s, a subcommittee of the American Clinical Neurophysiology Society (ACNS) set out to “standardize terminology of periodic and rhythmic EEG patterns in the critically ill to aid in future research involving such patterns.” The initial proposed terminology was published in 2005.1 This was presented at many meetings on several continents, subjected to multiple rounds of testing of interrater reliability, underwent many revisions, and was then published as an ACNS guideline in 2013.2 Interrater agreement of the 2012 version (published in early 2013) was very good, with almost perfect agreement for seizures, main terms 1 and 2, the +S modifier, sharpness, absolute amplitude, frequency, and number of phases.3 Agreement was substantial for the +F and +R modifiers (66% and 67%) but was only moderate for triphasic morphology (58%) and fair for evolution (21%, likely at least partly because of the short EEG samples provided).3 The authors concluded that interrater agreement for most terms in the ACNS critical care EEG terminology was high and that these terms were suitable for multicenter research on the clinical significance of these critical care EEG patterns.
With the help of infrastructure funding from the American Epilepsy Society and administrative and website support from the ACNS, a database that incorporated the ACNS terminology was developed for clinical and research purposes, tested during routine clinical care in multiple centers,4 and made available at no cost on the ACNS website (https://www.acns.org/research/critical-care-eeg-monitoring-research-consortium-ccemrc/ccemrc-public-database). This greatly enhanced the ability to complete multicenter investigations.
After the establishment of the standardized terminology and free access to a database incorporating these terms, there have been many investigations into the clinical significance of rhythmic and periodic patterns (RPPs) in critically ill patients. Patterns such as lateralized rhythmic delta activity (LRDA) were found to be highly associated with acute seizures,5,6 equivalent to the association found with lateralized periodic discharges (LPDs) in one study.5 The association of all the main patterns in the nomenclature with seizures was defined in a multicenter cohort of almost 5,000 patients, with seizure rates highest for LPDs, intermediate for LRDA and generalized periodic discharges (GPDs), and lowest for generalized rhythmic delta activity (GRDA).6 This and other studies have shown that several of the modifiers within the nomenclature do indeed have clinically relevant meaning. For example, studies have shown that higher frequency (especially >1.5 Hz), higher prevalence, longer duration, and having a “plus” modifier are all associated with a higher chance of acute seizures.6,7 On the other hand, whether a pattern was spontaneous or “stimulus-induced” did not seem to have a significant effect on its association with seizures.6 In other investigations, the “triphasic morphology” modifier was investigated blindly with multiple expert reviewers, calling into question its relationship with metabolic encephalopathy and its lack of a relationship with seizures.8,9 For patients with refractory status epilepticus treated with anesthetic-induced coma, the presence of “highly epileptiform” bursts suggested that an attempted wean off of anesthetics at that time was much more likely to lead to seizure recurrence than if the bursts were not highly epileptiform.10 Even long-term outcome seemed to be associated with some modifiers, with a higher risk of later epilepsy found if LPDs were more prevalent, had longer duration, or had a “plus” modifier.7
CHANGES IN THE 2021 VERSION OF THE TERMINOLOGY
Although the previous version of the terminology was easy to use, reliable, and valuable for both research and clinical care, new terms and concepts have emerged. In this version, we incorporate recent research findings, add definitions of several new terms, and clarify a few definitions of old terms. Most of the old terms remain unchanged, but there have been some important clarifications and corrections (such as the calculation of the number of phases) and multiple additions. All changes have been summarized in Table 1. One new main term 1 was added (Unilateral Independent), and main term 2 “Lateralized” was updated to include “bilateral asynchronous” patterns. Electrographic seizures (ESz), electrographic status epilepticus (ESE), electroclinical seizures (ECSz), and electroclinical status epilepticus (ECSE) have now been defined, largely based on the “Salzburg criteria.”11,12 Brief potentially ictal rhythmic discharges (BIRDs) have been added based on recent publications13,14, and a consensus definition of the ictal-interictal continuum (IIC) has been proposed. We also added definitions of identical bursts,15 state changes, cyclic alternating pattern of encephalopathy (CAPE), and extreme delta brush (EDB).16 To facilitate daily use, we are also providing the “ACNS Standardized Critical Care EEG Terminology 2021: Condensed Version” (see Supplemental Digital Content, http://links.lww.com/JCNP/A149) and the “ACNS Standardized Critical Care EEG Terminology 2021: Reference Chart” (see Supplemental Digital Content, http://links.lww.com/JCNP/A150). Finally, for educational purposes and conceptual clarity, we provided extensive schematic diagrams (Figures 1-42) of most patterns to quickly demonstrate the core features and principles. Supplemental figures include EEG examples from 30 cases and are available as Supplemental Digital Content at http://links.lww.com/JCNP/A134.
TABLE 1.
ACNS Standardized Critical Care EEG Terminology: Major and Minor Changes Between the 2012 and 2021 Versions
| Major changes |
EEG background
Ictal-Interictal Continuum (IIC) (new term: Section F, page 25) |
| Minor changes |
EEG background
|
FIG. 1.
A. Symmetric vs mild asymmetry in voltage. B. Symmetric vs mild asymmetry in frequency. C. Marked asymmetry in voltage and frequency.
FIG. 42.

The Ictal-Interictal Continuum (IIC). Does not qualify as an electrographic seizure or electrographic status epilepticus but can be considered with any of the following features: A. Any PD or SW pattern that averages >1.0 Hz and ≤2.5 Hz over 10 seconds (>10 and ≤25 discharges in 10 seconds). B and Any PD or SW pattern that averages ≥0.5 Hz and ≤1.0 Hz over 10 seconds (≥ 5 and ≤10 discharges in 10 seconds), AND has a plus modifier or fluctuation. D and E. Any lateralized RDA (LRDA, BIRDA, UIRDA, MfRDA) averaging >1 Hz for ≥10 seconds (at least 10 waves in 10 seconds) with a plus modifier or fluctuation.
METHODS
All the definitions are based on extensive discussions not only among the authors of this document but also among many others, both live and via email and questionnaires. There was not always complete consensus on some issues; electronic voting (with each voter blinded to the opinion of others for the first round) was used for most of these issues. We considered additional changes from previous versions or from the literature such as eliminating the 10-second cutoff for defining electrographic seizures but because no clear consensus was reached (it was close to a split decision), this was not changed.
2021 ACNS CRITICAL CARE EEG TERMINOLOGY
General Notes
NOTE: This terminology is intended to be used at all ages, excluding neonates, although some terms may not be ideal for infants. For the neonatal version of the terminology, please see https://www.acns.org/UserFiles/file/The_American_Clinical_Neurophysiology_Society_s.12.pdf.18
NOTE: This terminology is intended for use in the critically ill, although it can be applied in other settings as well. It is mostly compatible with the 2017 multinational revised glossary of terms most commonly used by clinical electroencephalographers.19
NOTE: Although any finding on EEG can be focal, regional, or hemispheric, such as an asymmetry or slowing, and this is a very important distinction in some circumstances such as epilepsy surgery, all of these are combined within the terms “lateralized” or “asymmetric” in this nomenclature. However, additional localizing information (e.g., where the pattern is maximal and which lobes are involved) can be provided and can also be applied to several modifiers and sporadic epileptiform discharges. This additional localizing information was built into the freely available Critical Care EEG Monitoring Research Consortium (CCEMRC) database that incorporated the previous version of this nomenclature (https://www.acns.org/research/critical-care-eeg-monitoring-research-consortium-ccemrc/ccemrc-public-database).4 A new database is being created with this 2021 nomenclature fully incorporated.
NOTE: In this section and throughout the document, the term “ictal” is used to refer to an EEG pattern seen during an epileptic seizure, whether clinical or electrographic-only, as the term is commonly used in EEG literature.
NOTE: “Hz” is used as an abbreviation for “per second” for all types of periodic or rhythmic patterns, even when referring to noncontinuous waveforms.
NOTE: All voltage measurements in this document are based on peak to trough (not peak to baseline) measurements in a standard 10–20 longitudinal bipolar recording. However, for assessing voltage symmetry, an appropriate referential recording is preferred.
NOTE: The term “consistent” or “consistently” refers to >80% of instances (e.g., >80% of discharges in a periodic pattern, >80% of cycles of a rhythmic pattern, or present >80% of the record for a background pattern).
A. EEG BACKGROUND
1. Symmetry
Symmetric.
Mild asymmetry (consistent asymmetry in voltage [Fig. 1A] on an appropriate referential recording of <50% or consistent asymmetry in frequency of 0.5 to 1 Hz [Fig. 1B]).
Marked asymmetry (≥50% voltage or >1 Hz frequency asymmetry [Fig. 1C]).
NOTE: When any of the following features (Section A2-A10) are asymmetric, they should be described separately for each hemisphere.
2. Predominant Background Frequency When Most Awake or After Stimulation
Beta (>13 Hz)
Alpha.
Theta.
Delta.
NOTE: If two or three frequency bands are equally prominent, report each one.
3. Posterior Dominant (“Alpha”) Rhythm (must be demonstrated to attenuate with eye opening; wait >1 second after eye closure to determine frequency to avoid “alpha squeak”)
Present: Specify frequency to the nearest 0.5 Hz.
Absent.
Unclear.
4. Continuity (Fig. 2)
FIG. 2.

Continuity. Percentages for each category refer to the percentage of the record that is “attenuated” or “suppressed.” How this percentage is derived is demonstrated in Fig. 4, page 6.
Continuous
- Nearly Continuous: continuous, but with occasional (1–9% of the record) periods of attenuation or suppression lasting ≥1 second. Describe typical duration of attenuation/suppression.
- Attenuation: periods of lower voltage are ≥10 μV but <50% of the higher voltage background.
- Suppression: periods of lower voltage are <10 μV.
NOTE: If attenuations/suppressions are stimulus-induced, this is referred to as “SI-attenuation” or “SI-suppression.”
NOTE: This voltage cutoff, as with other voltages, differs from the ACNS neonatal terminology.18
-
c.
Discontinuous: A pattern of attenuation/suppression alternating with higher voltage activity, with 10% to 49% of the record consisting of attenuation or suppression.
-
d.
Burst attenuation/Burst suppression: A pattern of attenuation/suppression alternating with higher voltage activity, with 50% to 99% of the record consisting of attenuation (see Supp EEG 1, Supplemental Digital Content 1, http://links.lww.com/JCNP/A134) or suppression (see Supp EEG 2, Supplemental Digital Content 1, http://links.lww.com/JCNP/A134).
NOTE: The term “suppression-burst” is synonymous with “burst-suppression.”
NOTE: Bursts must average ≥0.5 seconds and have at least 4 phases (i.e., at least 3 baseline crossings; see Section C 3d, page 13, for definition of number of phases); if shorter or fewer phases, they should be considered “discharges” (as defined under RPPs, main term 2, see Section C 2a, page 12) (Fig. 3). Bursts within burst-suppression or burst-attenuation can last up to 30 seconds.
FIG. 3.
Discharge vs. Burst. *Phase: an area under the curve on one side of the baseline (see Section C 3d, page 13, and Fig. 23, page 13).
For nearly continuous, discontinuous, and burst attenuation/burst suppression patterns, specify:
i. Attenuation Percent or Suppression Percent: the percent of the record/epoch that is attenuated or suppressed (Fig. 4). This can range from 1% to 99%. If <1%, it is considered continuous. If >99%, it is considered either suppressed or attenuated, but not burst-attenuation/burst-suppression or discontinuous. For example, a record with 2 second bursts alternating with 8 seconds of suppression would be burst-suppression with a suppression percent of 80%.
FIG. 4.

Attenuation percent or Suppression percent: the percent of the record/epoch that is attenuated or suppressed. This can range from 1% to 99%. If <1%, it is considered continuous. If >99%, it is considered either suppressed or attenuated, but not discontinuous. For example, a record with 2 second bursts alternating with 8 seconds of suppression, as shown here, would be Burst-Suppression with a suppression percent of 80%.
For burst attenuation/burst suppression patterns only, also specify the following:
Localization of bursts: Bursts can be described using the same terms in Main Term 1 that apply to rhythmic and periodic discharges: generalized (including with shifting predominance; see Section C 1a below, page 9), lateralized, bilateral independent, unilateral independent, or multifocal (Fig. 5).
Typical duration of bursts and interburst intervals.
Sharpest component of a typical burst using the sharpness categories defined under Section C 3e below, page 14.
The presence or absence of “Highly Epileptiform Bursts”: present if two or more epileptiform discharges (spikes or sharp waves) are seen within most (>50%) bursts and occur at an average of 1 Hz or faster within a single burst (frequency is calculated as the inverse of the typical interpeak latency of consecutive epileptiform discharges within a single burst) (see Supp EEG 3, Supplemental Digital Content 1, http://links.lww.com/JCNP/A134) (Fig. 6A); record typical frequency and location (G, L, BI, UI or Mf, as defined in RPP Section C1, page 9). Also present if a rhythmic, potentially ictal-appearing pattern occurs within most (>50%) bursts; record maximum frequency and location if this occurs (Fig. 6B).
The presence or absence of “Identical Bursts”: Present if the first 0.5 seconds or longer of each burst (Fig. 7A) or of each stereotyped cluster of 2 or more bursts (Fig. 7B) appears visually similar in all channels in most (>90%) bursts (see Supp EEG 4, Supplemental Digital Content 1, http://links.lww.com/JCNP/A134).
FIG. 5.

Localization of bursts. A. Generalized bursts, shifting predominance based on asynchrony. Symmetric bursts, at times starting on the left and others on the right, but never consistently the same side. This would be an example of generalized bursts, with shifting predominance based on asynchrony (rather than asymmetry, where they would sometimes be of greater amplitude on the left and other times the right). B. Lateralized bursts, bilateral asynchronous. Symmetric bursts consistently starting on the left with a lag before being seen on the right. This is an example of lateralized, bilateral asynchronous bursts. They are not Bilateral Independent (BI) bursts because there is a consistent relationship between the activity between hemispheres, i.e. the patterns are not independent.
FIG. 6.
A. Highly Epileptiform Bursts. --- dashed lines represent longer duration of suppression; ED epileptiform discharge. B. Highly Epileptiform Bursts. --- dashed lines represent longer duration of suppression.
FIG. 7.

A. Identical Bursts. The first 0.5 seconds or longer of each burst are visually similar in all channels (though only 1 channel shown) in most (>90%) bursts. B. Identical Bursts in a Stereotyped Cluster. The first 0.5 seconds or longer of each of 2 or more bursts in a stereotyped cluster are visually similar in all channels (though only 1 channel shown) in most (>90%) bursts.
-
e.
Suppression/attenuation: entirety or near-entirety (>99%) of the record consists of either suppression (all <10 μV, as defined above) or low voltage activity (all <20 μV but not qualifying as suppression). Specify whether attenuated or suppressed.
5. Reactivity
Change in cerebral EEG activity to stimulation: This may include change in voltage or frequency, including attenuation of activity. Strength and/or nature of stimulus should be noted, and a standard protocol of testing reactivity with multiple escalating stimuli is strongly encouraged.20,21 Appearance of muscle activity or eye blink artifacts does not qualify as reactive. Categorize as the following:
Reactive.
Unreactive.
NOTE: It is suggested that if an EEG is “unreactive” after one round of stimulation, a second round of standardized noxious stimulation should be performed to confirm the finding and should be applied with the patient in their nonstimulated state. If “unreactive” and the patient is on sedatives or paralytics, we suggest including this important caveat in the impression.
-
c.
SIRPIDs-only: when the only reactivity is stimulus-induced rhythmic, periodic, or ictal-appearing discharges (SIRPIDs).22 This includes SI-RDA, SI-PDs, SI-SW, SI-seizures, SI-bursts, SI-IIC, or SI-BIRDs (see multiple sections below).
-
d.
Unclear (typically used when testing may have not been adequate, there was too much artifact to assess the response, or there was a hint of a change in cerebral activity but not definite).
-
e.
Unknown (typically used when reactivity was not tested or patient was maximally alert throughout the EEG epoch).
6. State Changes
Present if there are at least 2 sustained types of background EEG related to the level of alertness or stimulation; each must persist at least 60 seconds to qualify as a “state” (Fig. 8). Stimulation should be able to transition the patient from the less alert to more alert/more stimulated state. State changes can also occur spontaneously. The more alert/stimulated pattern is considered the primary reported “background” EEG pattern for the patient. Categorize state changes as the following:
FIG. 8.
State changes. At least 2 sustained types of background EEG, where: 1. The background activity is related to level of alertness or stimulation. 2. Each must persist ≥60 seconds to qualify as a “state”. 3. Stimulation should be able to transition the patient from the less alert to more alert/more stimulated state. 4. The more alert/more stimulated state is considered the “reported background” EEG. 5. State changes can also occur spontaneously. STIM = stimulation, Spont. = spontaneous.
EEG background 1: stimulated/more awake: used for background feature description (“reported background”)
EEG background 2: unstimulated/less awake state; commonly lasts minutes to hours (minimum: 60 s)
Present with normal stage N2 sleep transients (K-complexes and spindles)
- Present but with abnormal stage N2 sleep transients
- Describe both K complexes and spindles separately as the following:
- Present and normal.
- Present but abnormal. Specify abnormality (e.g., asymmetry, location, frequency, poorly formed).
- Absent.
Present but without stage N2 sleep transients.
Absent
NOTE: The presence of state changes virtually always indicates the presence of reactivity; however, the presence of reactivity does not necessarily indicate the presence of state changes because the reactivity may last <60 seconds.
7. Cyclic Alternating Pattern of Encephalopathy (CAPE)
CAPE refers to changes in background patterns (which may include RPPs), each lasting at least 10 seconds, and spontaneously alternating between the 2 patterns in a regular manner for at least six cycles (but often lasts minutes to hours) (Fig. 9). A cycle refers to the period of time before the sequence repeats (i.e., includes both states once). Document whether seen in the patient’s more awake/stimulated state or less awake state if known. Describe each pattern and typical duration of each pattern. Optional: Describe if this pattern corresponds with cycling of other functions such as respirations, heart rate, blood pressure, movements, muscle artifact, and pupil size.
FIG. 9.
Cyclic Alternating Pattern of Encephalopathy (CAPE). Changes in EEG background between pattern 1 and pattern 2, where: 1. Each pattern lasts at least 10 seconds, 2. Spontaneously alternates between the two patterns in a regular manner, 3. For at least 6 cycles.
Present.
Absent.
Unknown/unclear.
NOTE: If each pattern of CAPE lasts >60 seconds, this would qualify as the presence of state changes. If CAPE is always present, cannot be interrupted with stimulation, and at least one of the states lasts <60 seconds, it remains possible for a patient to have CAPE and no state changes.
8. Voltage
High: most or all activity ≥150 μV in longitudinal bipolar with standard 10–20 electrodes (measured from peak to trough).
Normal.
Low: most or all activity <20 μV in longitudinal bipolar with standard 10–20 electrodes (measured from peak to trough), but not qualifying as suppressed.
Suppressed: all activity <10 μV.
NOTE: If the background is nearly continuous or discontinuous, EEG background voltage refers to the higher voltage portion.
9. Anterior-Posterior (AP) Gradient
An AP gradient is present if, at any point in the epoch, there is a clear and persistent (at least 1 continuous minute) anterior to posterior gradient of voltages and frequencies such that lower voltage, faster frequencies are seen in anterior derivations, and higher voltage, slower frequencies are seen in posterior derivations (Fig. 10). A reverse AP gradient is defined identically but with a posterior to anterior gradient of voltages and frequencies.
FIG. 10.

Anterior-posterior (AP) gradient.
Present.
Absent.
Present, but reversed.
10. Breach Effect
Breach effect refers to EEG activity over or nearby a skull defect and consists of activity of higher amplitude and increased sharpness, primarily of faster frequencies, compared with the rest of the brain, especially compared with the homologous region on the opposite side of the head.
Present (provide location).
Absent.
Unclear.
B. SPORADIC EPILEPTIFORM DISCHARGES
This refers to nonrhythmic and nonperiodic spikes, polyspikes, and sharp waves, as previously defined by Kane et al.19 in the 2017 revised glossary of terms most commonly used by clinical electroencephalographers. A “spike” is defined as “a transient, clearly distinguished from background activity, with pointed peak at a conventional time scale and duration from 20 to <70 ms,” with duration measured at the EEG baseline (Fig. 11). A “sharp wave” is defined identically, but with a duration of 70 to 200 ms. A spike or sharp wave is usually diphasic or triphasic, apiculate (i.e., pointed peak), asymmetric (typically with a steeper ascending slope than descending, but can be the opposite), and either followed by a slow wave or associated with some other disruption of the background. A “polyspike” refers to 2 or more spikes occurring in a row with no interdischarge interval and lasting <0.5 seconds (if ≥0.5 seconds, they would either qualify as BIRDs [see section E below, page 24] or, if alternating with suppression or attenuation, a highly epileptiform burst within burst suppression/attenuation [see section A 4d, page 5] [Fig. 12]). The prevalence of epileptiform discharges (combining spikes, polyspikes and sharp waves) should be categorized as follows:
FIG. 11.

Sporadic Epileptiform Discharges.
FIG. 12.

Polyspike versus BIRDs versus Highly Epileptiform Bursts.
Abundant: ≥1 per 10 seconds, but not periodic.
NOTE: It can be helpful to record the estimated average and maximum number of spikes per 10-second epoch when abundant epileptiform discharges are seen.
-
b.
Frequent: ≥1/minute but less than 1 per 10 seconds
-
c.
Occasional: ≥1/hour but less than 1/minute
-
d.
Rare: <1/hour
C. RHYTHMIC AND PERIODIC PATTERNS (RPPs)
All terms consist of two main terms, with modifiers added as appropriate. Main term 1 refers to the localization of the pattern and main term 2 specifies the type of pattern.
1. Main Term 1: G, L, BI, UI, or Mf
Generalized (G): any bilaterally synchronous and symmetric pattern (see Supp EEGs 5 and 6, Supplemental Digital Content 1, http://links.lww.com/JCNP/A134) (Fig. 13), even if it has a restricted field (e.g., bifrontal).
FIG. 13.

Generalized Periodic Discharges (GPDs). Generalized: Bilateral synchronous and symmetric periodic discharges. In this case, the pattern is “frontally predominant.”
NOTE: A pattern that is bilateral with shifting predominance based on asymmetry (i.e., amplitude, sometimes higher on left and sometimes right), OR based on asynchrony (i.e., timing, sometimes earlier on the left and sometimes right) but is not consistently (>80% of the time) lateralized to one side would still be considered “Generalized.” With shifting asynchrony, one should specify the typical time lag between sides.
NOTE: Some suggested that a more accurate term would be “bilateral synchronous,” but this was rejected for several reasons: 1. many lateralized patterns are also bilateral synchronous (see definition of “lateralized” immediately below); 2. this is more difficult to abbreviate (2 letters); and 3, the word “generalized” has been used widely to refer to patterns, discharges, seizures, and epilepsies that are not truly generalized. “Generalized” in this sense has also been used in many studies in the literature related to critical care EEG and in the previous version of this nomenclature. Thus, it was not changed.
NOTE: Additional localizing information for Generalized patterns:
“Frontally predominant”: Voltage in anterior derivations is at least 50% greater than that in posterior derivations on a common average, transverse bipolar, ipsilateral ear, or noncephalic referential recording (see Supp EEG 7, Supplemental Digital Content 1, http://links.lww.com/JCNP/A134).
“Occipitally predominant”: Voltage in posterior derivations is at least 50% greater than in anterior derivations on a common average, transverse bipoloar, ipsilateral ear, or noncephalic referential recording.
“Midline predominant”: Voltage in midline derivations is at least 50% greater than in parasagittal derivations on a common average or noncephalic referential recording.
- “Generalized, not otherwise specified”: Similar voltage in all regions and not qualifying as any one of the above three categories.
-
b.Lateralized (L): unilateral (Fig. 14); OR bilateral but clearly and consistently higher amplitude in one hemisphere (bilateral asymmetric) (see Supp EEG 8, Supplemental Digital Content 1, http://links.lww.com/JCNP/A134) (Fig. 15); OR bilateral but with a consistent lead-in from the same side (bilateral asynchronous) (Fig. 16). This includes focal, regional, and hemispheric patterns.
-
c.Bilateral Independent (BI): two independent (and therefore asynchronous) lateralized patterns with one in each hemisphere, with both patterns occurring simultaneously (see Supp EEG 9, Supplemental Digital Content 1, http://links.lww.com/JCNP/A134) (Fig. 17), i.e., two independent patterns occurring at the same time (overlapping in time) rather than sequentially (one starting after the other stops).
-
b.
FIG. 14.

Lateralized Periodic Discharges (LPDs, unilateral). Unilateral: Periodic discharges only seen in one hemisphere (in this case left).
FIG. 15.

Lateralized Periodic Discharges (LPDs, bilateral asymmetric). Bilateral asymmetric: Periodic discharges seen bilaterally but clearly and consistently (>80% of the time) higher amplitude over one hemisphere (in this case left).
FIG. 16.

Lateralized Periodic Discharges (LPDs, bilateral asynchronous). Bilateral asynchronous: Periodic discharges seen bilaterally but clearly and consistently (>80% of the time) earlier on one side (in this case left). These are not Bilateral Independent (BI) because the latency between hemispheres is fixed (i.e., they are not independent populations).
FIG. 17.

Bilateral Independent Periodic Discharges (BIPDs). In BIPDs, lateralized patterns occur in each hemisphere asynchronously and at different frequencies.
NOTE: If there are two independent lateralized patterns at different times (e.g., on the left for an hour and then later in the record on the right for an hour), these would be LPDs from the left and LPDs from the right, but not BIPDs because they are not simultaneous.
NOTE: The “I” in “BI” is capitalized because it stands for its own word, “Independent.”
-
d.
Unilateral Independent (UI): two independent (and therefore asynchronous) periodic (see Supp EEG 10, Supplemental Digital Content 1, http://links.lww.com/JCNP/A134) or rhythmic patterns (see Supp EEG 11, Supplemental Digital Content 1, http://links.lww.com/JCNP/A134) in the same hemisphere, with both patterns occurring simultaneously (Fig. 18), i.e., two independent patterns occurring at the same time (overlapping in time) rather than sequentially (one starting after the other stops).
FIG. 18.
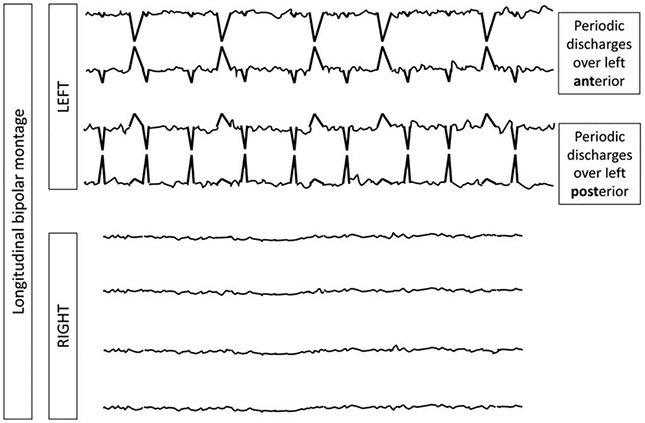
Unilateral Independent Periodic Discharges (UIPDs). In UIPDs, periodic discharges occur in two independent locations simultaneously with both populations within a single hemisphere (in this case left).
NOTE: If there are two independent lateralized patterns at different times (e.g., in the left frontal region for an hour and then later in the record the left temporal region for an hour), these would be two populations of LPDs on the left, but not unilateral independent periodic discharges because they are not simultaneous.
NOTE: Focal midline patterns can be deemed in the same hemisphere (ipsilateral) as an independent pattern in either the left or right hemisphere. For example, PDs at 1 Hz in the left hemisphere occurring simultaneously with independent focal midline PDs at 0.5 Hz would still qualify as unilateral independent periodic discharges.
-
e.
Multifocal (Mf): at least three independent lateralized patterns, with at least one in each hemisphere, with all three or more patterns occurring simultaneously (see Supp EEG 12, Supplemental Digital Content 1, http://links.lww.com/JCNP/A134) (Fig. 19).
FIG. 19.

Multifocal Periodic Discharges (MfPDs). In MfPDs, periodic discharges occur in three independent locations simultaneously with at least one in each hemisphere. If all three populations occurred within a single hemisphere this would remain UIPDs.
NOTE: Additional localizing information for Lateralized patterns:
- Specify unilateral versus bilateral; and if bilateral, whether asymmetric, asynchronous, or both:
- “Unilateral”: seen in only one hemisphere (Fig. 14). Side should be specified.
- “Bilateral asymmetric”: seen bilaterally but clearly and consistently higher amplitude in one hemisphere (Fig. 15). Referred to as “Lateralized, bilateral asymmetric.” For example, PDs seen bilaterally and synchronously but consistently greater on the left would be referred to as “Left LPDs, bilateral asymmetric” (see Supp EEG 13, Supplemental Digital Content 1, http://links.lww.com/JCNP/A134).
- “Bilateral asynchronous”: seen bilaterally but clearly and consistently with a lead-in from the same side (Fig. 16). Referred to as “Lateralized, bilateral asynchronous.” For example, bifrontal LPDs that are consistently earlier on the left. Specify the typical time lag between sides.
NOTE: A lateralized pattern can be both “bilateral asymmetric” and “bilateral asynchronous. ”
-
ii.
Specify the most involved lobe(s) (F, P, T, O, or hemispheric if more specific localization is not possible).
NOTE: For unilateral independent patterns, the above should be specified for each pattern separately.
NOTE: Additional localizing information for Bilateral Independent and Multifocal patterns:
- Specify symmetric versus asymmetric
- “Symmetric”: Approximately equal in both hemispheres or with no consistent asymmetry. Patterns that are bilateral, independent, and symmetric would be called “Bilateral Independent, symmetric,” or “Multifocal, symmetric.”
- “Asymmetric”: Clearly and consistently more prominent on one side. Patterns that are bilateral and independent but clearly more prominent on one side would be called “Bilateral Independent, asymmetric,” or “Multifocal, asymmetric,” followed by “L > R” or “R > L.”
Specify lobes most involved in both hemispheres (F, P, T, O, or “hemispheric” if more specific localization is not possible).
2. Main Term 2: PDs, RDA or SW
- Periodic Discharges (PDs):
- Periodic: Repetition of a waveform with relatively uniform morphology and duration with a clearly discernible interdischarge interval between consecutive waveforms and recurrence of the waveform at nearly regular intervals (Fig. 20). “Nearly regular intervals” is defined as having a cycle length (i.e., period) varying by <50% from one cycle to the next in most (>50%) cycle pairs.
- Discharges: Waveforms lasting <0.5 seconds, regardless of number of phases, or waveforms ≥0.5 seconds with no more than 3 phases. This is as opposed to Bursts, defined as waveforms lasting ≥0.5 seconds and having at least 4 phases. Discharges and bursts must clearly stand out from the background activity.
- Rhythmic Delta Activity (RDA):
- Rhythmic: Repetition of a waveform with relatively uniform morphology and duration and without an interval between consecutive waveforms (Fig. 21). The duration of one cycle (i.e., the period) of the rhythmic pattern should vary by <50% from the duration of the subsequent cycle for most (>50%) cycle pairs to qualify as rhythmic. An example of a rhythmic pattern would be a sinusoidal waveform, although there are other examples; a pattern can be sharp at the top and/or the bottom of the waveform and still be rhythmic (but would no longer be sinusoidal). Irregular or polymorphic delta should not be reported as RDA.
- RDA: Rhythmic activity 0.5 to ≤4.0 Hz.
Spike-and-wave or Sharp-and-wave (SW): Spike, polyspike, or sharp wave consistently followed by a slow wave in a regularly repeating and alternating pattern (spike-wave-spike-wave-spike-wave), with a consistent relationship between the spike (or polyspike or sharp wave) component and the slow wave for at least six consecutive cycles and with no interval between one spike-wave complex and the next (see Supp EEG 14, Supplemental Digital Content 1, http://links.lww.com/JCNP/A134) (Fig. 22) (if there is an interval, this would qualify as PDs, where each discharge is a spike-and-wave).
FIG. 20.
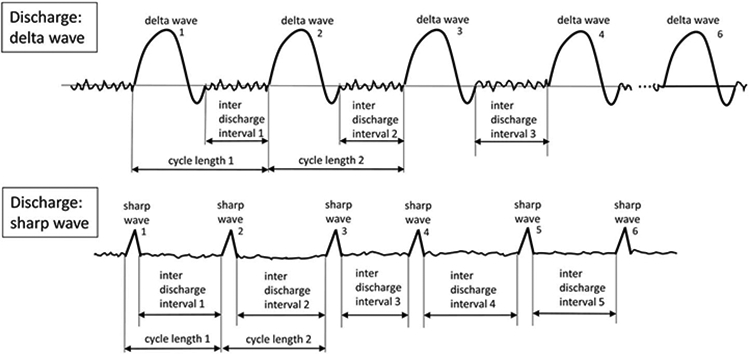
Periodic Discharges (PDs). 1. Repetition of a waveform with relatively uniform morphology and duration, 2. with a clearly discernable interdischarge interval between consecutive waveforms, and 3. recurrence of the waveform at nearly regular intervals: having a cycle length (i.e., period) varying by <50% from one cycle to the next in the majority (>50%) of cycle pairs. A pattern can qualify as rhythmic or periodic if and only if it continues for at least 6 cycles (e.g. 1 Hz for 6 seconds, or 3 Hz for 2 seconds).
FIG. 21.
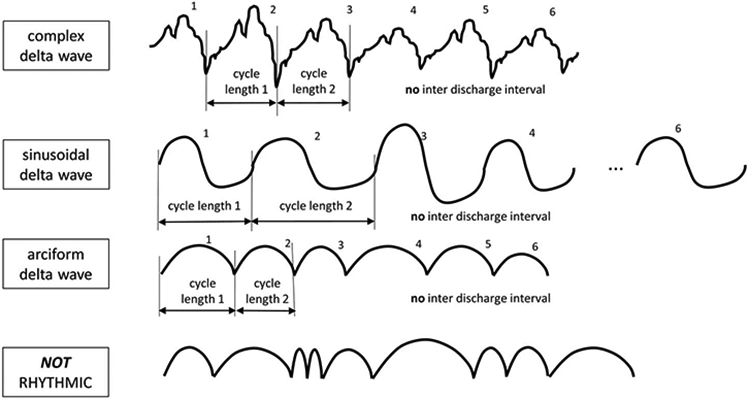
Rhythmic Delta Activity (RDA). 1. Repetition of a waveform with relatively uniform morphology and duration and 2. without an interval between consecutive waveforms. 3. The duration of one cycle (i.e., the period) of the rhythmic pattern should vary by <50% from the duration of the subsequent cycle for the majority (>50%) of cycle pairs to qualify as rhythmic. A pattern can qualify as rhythmic or periodic if and only if it continues for at least 6 cycles (e.g. 1 Hz for 6 seconds, or 3 Hz for 2 seconds).
FIG. 22.

“Spike-and-wave” or “Sharp-and-wave” (SW). Spike-and-wave or Sharp-and-wave (SW): Polyspike, spike, or sharp wave consistently followed by a slow wave in a regularly repeating and alternating pattern (spike-wave-spike-wave-spike-wave), with a consistent relationship between the spike (or polyspike or sharp wave) component and the slow wave for at least 6 cycles; and with no interval between one spike-wave complex and the next (if there is an interval, this would qualify as PDs, where each discharge is a spike-and-wave).
NOTE: A pattern can qualify as rhythmic or periodic if and only if it continues for at least six consecutive cycles (e.g., 1 Hz for 6 seconds, or 3 Hz for 2 seconds).
NOTE: If a pattern qualifies as both PDs and RDA simultaneously, it should be coded as PDs+R rather than RDA+S (see “plus” modifier below).
3. Main Modifiers (Most of the following section can be applied to any EEG phenomenon)
- Prevalence: Specify percent of record or epoch that includes the pattern. This should be based on the proportion of an epoch that includes or is within the pattern. The time between widely spaced PDs counts as part of the pattern duration. For example, 2-Hz PDs present for 1 minute every 10 minutes is 10% prevalence, and 0.25-Hz PDs present for 1 minute every 10 minutes is also 10% prevalence. When categorizing or using qualitative terms, follow the cutoffs listed below for each term. Suggested clinical terms are given as well. If two or more patterns are present, record the presence and prevalence of each one (e.g., ~20% GRDA, 20% GPDs, and 30% BIPDs).
- Continuous: ≥90% of record/epoch.
- Abundant: 50% to 89% of record/epoch.
- Frequent: 10% to 49% of record/epoch.
- Occasional: 1% to 9% of record/epoch.
- Rare: <1% of record/epoch.
- Duration: Specify typical duration of pattern if not continuous. When categorizing or using qualitative terms, follow the cutoffs listed below for each term. Also record the longest continuous duration.
- Very long: ≥1 hour.
- Long: 10 to 59 minutes.
- Intermediate duration: 1 to 9.9 minutes.
- Brief: 10 to 59 seconds.
- Very brief: <10 seconds.
Frequency = rate per second: Specify typical rate and range for all patterns (e.g., LPDs with typical frequency of 1 Hz and range of 0.5–2 Hz).
Record typical, minimum, and maximum frequency using the following categories: <0.5, 0.5, 1, 1.5, 2, and 2.5; and if very brief duration: 3, 3.5, and 4 Hz.
NOTE: For PDs and SW, typical frequencies >2.5 Hz can only be applied to RPPs <10 second duration (“very brief” by definition); if PDs or SW have a typical frequency >2.5 Hz and are ≥10 seconds, these would qualify as electrographic seizures (criterion A) and should be referred to as such rather than as PDs or SW.
NOTE: No RPP in this terminology can have a typical frequency of >4 Hz; if an RPP is >4 Hz and ≥0.5 seconds, it would always meet the criteria for either BIRDs (if <10 seconds) or electrographic seizure (if ≥10 seconds) (see definitions below). If <0.5 seconds, this would not qualify as any RPP but might qualify as a polyspike.
-
d.
Number of phases = 1 + number of baseline crossings of the typical discharge as assessed in longitudinal bipolar and in the channel in which it is most readily appreciated. A phase is that part of the signal that is on one side of (above or below) the imaginary baseline (Fig. 23). The start and end points do not count as baseline crossings. Applies to PDs and the entire spike-and-wave or sharp-and-wave complex of SW (including the slow wave). This does not apply to RDA. Categorize as 1, 2, 3 or >3.
-
e.Sharpness: Specify for both the dominant phase (phase with greatest voltage) and the sharpest phase if different. For both phases, describe the typical discharge. Applies only to PDs and the spike/sharp component of SW, not RDA. Categorize as
- Spiky: duration of that component, measured at the EEG baseline, is <70 ms
- Sharp: duration of that component is 70 to 200 ms
- Sharply contoured: used for waveforms that have a sharp morphology (steep slope to one side of the wave and/or pointy or apiculate at inflection point[s]) but are too long in duration to qualify as a sharp wave.
- Blunt: having smooth or sinusoidal morphology.
-
f.Voltage (amplitude) [of PDs, SW or RDA; not background EEG, which is in section A8, page 7, above]:
- Absolute: Typical voltage measured in standard longitudinal bipolar 10-20 recording in the channel in which the pattern is most readily appreciated. For PDs, this refers to the highest voltage component. For SW, this refers to the spike/sharp wave. Voltage should be measured from peak to trough (not peak to baseline). Specify for RDA as well. Categorize as
- Very low: <20 μV
- Low: 20 to 49 μV
- Medium: 50 to 149 μV
- High: ≥150 μV
- Relative: For PDs only (PDs require two voltages, absolute and relative). Typical ratio of voltage of the highest voltage component of the typical discharge to the voltage of the typical background between discharges, measured in the same channel and montage as absolute voltage. Categorize as ≤2 or >2.
-
g.Stimulus-Induced (SI−) or Stimulus-Terminated (ST−): SI− versus ST− versus spontaneous: Categorize as
- Stimulus-Induced (SI−): reproducibly brought about or exacerbated by an alerting stimulus, with or without clinical alerting, when a patient is in their less-stimulated state; an SI− pattern may also be seen spontaneously at other times (due to spontaneous alerting or arousal) (see Supp EEG 15, Supplemental Digital Content 1, http://links.lww.com/JCNP/A134). Even if most instances of the pattern are spontaneous, it can still qualify as “SI-” if it can be reproducibly brought about by an alerting stimulus.
- Stimulus-Terminated (ST−): reproducibly terminated or attenuated by an alerting stimulus, with or without clinical alerting, when a patient is in their less-stimulated state; an ST− pattern may also self-terminate at other times. Even if most instances of the pattern resolve or attenuate spontaneously, it can still qualify as “ST-” if it can be reproducibly terminated or attenuated by an alerting stimulus.
- Spontaneous: never clearly induced, exacerbated, improved, or terminated by stimulation.
- Unknown: includes unclear or untested.
FIG. 23.
The Number of Phases. Number of Phases = 1 + number of baseline crossings of the typical discharge. In this case there are a total of 2 baseline crossings, therefore the number of phases is 1 + 2 = 3 phases. A phase is the part of the signal above or below the imaginary baseline. In this case, phase 1 (pink) is above, phase 2 (blue) is below, and phase 3 (yellow) is above again.
NOTE: Specify type of stimulus (auditory; light tactile; patient care and other nonnoxious stimulations; or noxious: suction, sternal rub, nailbed pressure, nostril tickle, trapezius squeeze, or other).
NOTE: The term “SIRPIDs” refers to stimulus-induced (or stimulus-exacerbated) rhythmic, periodic, or ictal-appearing discharges and is a term that includes all SI− patterns together (SI-RDA, SI-PDs, SI-SW, SI-IIC, SI-BIRDs, or SI-seizures). In general, one should refer to the specific “SI−" pattern rather than using the general term “SIRPIDs,” especially for a given patient.
-
h.Evolution: Evolving, Fluctuating, or Static: terms refer to changes in frequency, location, or morphology.
- Evolving: At least two unequivocal, sequential changes in frequency, morphology, or location defined as follows: Evolution in frequency is defined as at least two consecutive changes in the same direction by at least 0.5 Hz, e.g., from 2 to 2.5 to 3 Hz, or from 3 to 2 to 1.5 Hz (Fig. 24); Evolution in morphology is defined as at least two consecutive changes to a novel morphology (Fig. 25); Evolution in location is defined as sequentially spreading into or sequentially out of at least two different standard 10-20 electrode locations (Fig. 26). The two consecutive changes must be in the same category (frequency, morphology or location) to qualify.
- To qualify as evolution in frequency, a single frequency must persist for at least three cycles (e.g., 1 Hz for 3 seconds, or 3 Hz for 1 second). Thus, the following pattern would qualify as evolving: 3 Hz for ≥1 second, then 2 Hz for ≥1.5 seconds (the first change), then 1.5 Hz for ≥2 seconds (the 2nd change) (see Supp EEG 16, Supplemental Digital Content 1, http://links.lww.com/JCNP/A134).
- To qualify as evolution in morphology, each different morphology or each morphology plus its transitional forms must last at least three cycles. Thus, the following example would qualify: Spiky 4-phase PDs for three cycles then sharp 2–3 phase PDs for three cycles then blunt diphasic PDs for three cycles.
- To qualify as evolution in location, the pattern must spread into or out of two standard 10-20 electrode locations and the involvement of each additional electrode must be present for at least three cycles, e.g., 1-Hz LPDs only at T7, spreading to include F7 for 3 seconds then F7, T7, and P7 for 3 seconds.
- The criteria for evolution must be reached without the evolving feature (frequency, morphology, or location) remaining unchanged for five or more continuous minutes. Thus, the following pattern would not qualify as evolving: 3 Hz for 1 minute, then 2 Hz for 7 minutes, then 1.5 Hz for 2 minutes.
- Evolution in voltage (amplitude) alone does not qualify as evolving and does not qualify as a different morphology.
FIG. 24.
Evolution of frequency. At least 2 unequivocal, sequential changes in frequency; defined as at least 2 consecutive changes in the same direction by at least 0.5 Hz. To qualify as present, a single frequency must persist for at least 3 cycles. The criteria for evolution must be reached without the evolving feature (frequency) remaining unchanged for 5 or more continuous minutes.
FIG. 25.
Evolution of morphology. At least 2 consecutive changes to a novel morphology. Each different morphology or each morphology plus its transitional forms must last at least 3 cycles.
FIG. 26.
Evolution of location. Defined as sequentially spreading into or sequentially out of at least two different standard 10–20 electrode locations. To qualify as present, a single location must persist for at least 3 cycles.
NOTE: Evolution of an RPP is now limited to patterns that are ≤4 Hz AND <10 seconds duration. Any >4-Hz RPP with evolution lasting <10 seconds would qualify as definite BIRDs (see Section E, page 24). Any RPP with evolution lasting ≥10 seconds meets criterion B of an electrographic seizure and should be coded as such.
-
ii.
Fluctuating: ≥3 changes, not more than 1 minute apart, in frequency (by at least 0.5 Hz) (Fig. 27), morphology (Fig. 28), or location (by at least 1 standard interelectrode distance) (Fig. 29), but not qualifying as evolving (see Supp EEG 17, Supplemental Digital Content 1, http://links.lww.com/JCNP/A134). This includes patterns fluctuating from 1 to 1.5 to 1 to 1.5 Hz; alternating between two morphologies repeatedly; or spreading in and out of a single additional electrode location repeatedly. To qualify as present, a single frequency, morphology, or location must persist at least three cycles (e.g., 1 Hz for 3 seconds, or 3 Hz for 1 second).
FIG. 27.
Fluctuating frequency. ≥3 changes, not more than one minute apart, in frequency (by at least 0.5 Hz), but not qualifying as evolving. This includes patterns fluctuating from 1 to 1.5 to 1 to 1.5 Hz. To qualify as present, a single frequency must persist at least 3 cycles (e.g. 1 Hz for 3 seconds, or 3 Hz for 1 seconds).
FIG. 28.
Fluctuating morphology. ≥3 changes, not more than one minute apart, in morphology, but not qualifying as evolving. This includes patterns alternating between 2 morphologies repeatedly. To qualify as present, a single morphology must persist at least 3 cycles.
FIG. 29.
Fluctuating location. ≥3 changes, not more than one minute apart, in location (by at least 1 standard interelectrode distance), but not qualifying as evolving. This includes patterns spreading in and out of a single electrode repeatedly. To qualify as present, a single location must persist at least 3 cycles.
The following would not qualify as fluctuating: 2 Hz for 30 seconds, then 1.5 Hz for 30 seconds, then 2 Hz for 3 minutes, then 1.5 Hz for 30 seconds, then 2 Hz for 5 minutes. The changes are too far apart (>1 minute).
The following would qualify as fluctuating: 2 Hz for 10 seconds, then 2.5 Hz for 30 seconds, then 2 Hz for 5 seconds, then 2.5 Hz for 5 seconds.
-
iii.
Static: Not qualifying as evolving or fluctuating.
NOTE: Change in voltage (amplitude) alone would not qualify as evolving or fluctuating.
NOTE: If evolving or fluctuating in frequency, a minimum and maximum frequency should be specified under the “frequency” modifier above. For nongeneralized patterns, specify degree of spread (none, unilateral, or bilateral).
Plus (+) = additional feature which renders the pattern more ictal-appearing (i.e., more closely resembling an EEG pattern seen during seizures) than the usual term without the plus. This modifier applies only to PDs and RDA, not SW.
Subtyping of “+”: all cases with “+” should be categorized as follows into +F, +R, +FS, or +FR:
“+F”: with superimposed (some prefer the synonyms of admixed or associated) fast activity, defined as theta or faster, whether rhythmic or not. “+F” can be applied to PDs (see Supp EEGs 18 and 19, Supplemental Digital Content 1, http://links.lww.com/JCNP/A134) (Fig. 30) or RDA (Figs. 31A and 31b).
“+R”: with superimposed rhythmic or quasi-rhythmic delta activity; can be applied to PDs only (Fig. 32).
“+S”: with associated sharp waves or spikes, or sharply contoured; can be applied to RDA only (see Supp EEG 20, Supplemental Digital Content 1, http://links.lww.com/JCNP/A134). The sharp contour, sharp waves, or spikes need to occur at least once every 10 seconds but not as part of an SW pattern (Fig. 33).
FIG. 30.
Lateralized Periodic Discharges PLUS fast activity (LPDs+F). Code as +F if the fast activity is part of the RDA or PDs pattern and not simply part of the background activity.  fast activity cycling with the periodic discharge.
fast activity cycling with the periodic discharge.
FIG. 31.
Rhythmic Delta Activity PLUS fast activity (RDA+F). If a pattern qualifying as RDA or PDs has associated continuous fast frequencies (theta or faster), this can and should be coded as +F if the fast activity is not present in the background activity when the RDA or PDs is not present.  fast activity cycling with the rhythmic delta and having a stereotyped relationship to the delta wave. EDB = Extreme Delta Brush.
fast activity cycling with the rhythmic delta and having a stereotyped relationship to the delta wave. EDB = Extreme Delta Brush.
FIG. 32.
Periodic Discharges PLUS RDA (PDs+R). RDA occurring at the same time as PDs but without time-locked association with the PDs would qualify as PDs+R.
FIG. 33.

Generalized Rhythmic Delta Activity PLUS Spikes (GRDA+S). Generalized rhythmic delta activity with associated spikes in one hemisphere only (RDA on one side and synchronous RDA +S on the other) would still qualify as GRDA+S.
NOTE: It is possible to have “+FR” for PDs or “+FS” for RDA.
NOTE: The wave within periodic spike-wave discharges (spike-wave-interval-spike-wave-interval-spike-wave-interval…) does not qualify a pattern as PD+R because the wave is simply part of the spike-wave complex (which is the periodic discharge itself). However, RDA occurring at the same time as PDs but without time-locked association with the PDs would qualify as PD+R.
NOTE: If a pattern qualifies as both PDs and RDA simultaneously with approximately equal prominence, it should be coded as PDs+R rather than RDA+S.
NOTE: Re: Bilateral “+” versus unilateral: If a pattern is bilateral and qualifies as plus on one side, but not on the other, the overall main term should include the plus (although one side does not warrant a plus). For example, bilateral independent periodic discharges with fast activity in one hemisphere only (PDs on one side, and independent PDs+F on the other) would qualify for BIPDs+F (see Supp EEG 21, Supplemental Digital Content 1, http://links.lww.com/JCNP/A134) (Fig. 34). Similarly, generalized rhythmic delta activity with associated spikes in one hemisphere only (RDA on one side and synchronous RDA+S on the other) would qualify for GRDA+S.
FIG. 34.

Bilateral Independent Periodic Discharges PLUS fast activity (BIPDs+F). BIPDs with fast activity in one hemisphere only (PD on one side, and PD +F on the other) would qualify for BIPDs+F.
NOTE: Re: +F: If a pattern qualifying as RDA or PDs has associated continuous fast frequencies (theta or faster), this can and should be coded as +F if the fast activity is not present in the background activity when the RDA or PDs is not present. In other words, code as +F if the fast activity is part of the RDA or PD pattern and not simply part of the background activity (Figs. 30B and 30C). When referring to PDs+F, the fast activity can either be continuous (as long as the fast activity was not present when the PDs were not present) or can occur with each discharge in a regular fashion (regardless of background).
NOTE: “Extreme Delta Brush (EDB)”: A specific subtype of +F (Table 2):
TABLE 2.
Relationship between RDA+F, PDs+F and Extreme Delta Brush (EDB)
| RDA+F; or PDs+F if (and only if) the PDs are blunt delta waves |
||
|---|---|---|
| Continuous/ Abundant (≥50% of record/epoch) |
Frequent/ Occasional (≥1 to 49% of record/epoch) |
|
| Fast activity WITH stereotyped relationship to delta wave | Definite EDB | Possible EDB |
| Fast activity WITHOUT stereotyped relationship to delta wave | Possible EDB | RDA+F or PDs+F, but NOT EDB |
- Definite EDB: Consists of either abundant or continuous:
- PDs+F, in which each PD consists of a single blunt delta wave with superimposed fast activity, and in which the fast activity has a stereotyped relationship to the delta wave (i.e., periodic delta brushes) (see Supp EEG 22, Supplemental Digital Content 1, http://links.lww.com/JCNP/A134) (Figs. 35A and 35C).
Possible EDB:
FIG. 35.
Extreme Delta Brush (EDB). A. This is a subset of +F, with abundant or continuous RDA+F or PDs+F (only if the PDs are blunt delta waves), where the fast activity has a stereotyped relationship to each delta wave. B. Extreme delta brush (EDB): RDA subtype. Examples of RDA that meet criteria for definite and possible EDB (A-C); with an example that does not (D), as the fast activity is part of the background (therefore this pattern does not count as RDA+F). C. Extreme Delta Brush (EDB): PD subtype. Examples of PDs (where the PDs are blunt delta waves) that meet criteria for definite and possible EDB. Example B is not EDB as the +F is not on the delta wave (i.e., it is not delta brushes). Example E is not +F as the fast activity is part of the background, therefore it can not be EDB.
Satisfying criterion A) or B) above EXCEPT either:
only occasional or frequent (rather than abundant or continuous) OR
the superimposed fast activity lacks a stereotyped relationship to the delta wave; continuous, invariant fast activity during RDA would fall into this category (Figs. 35B and 35C).
NOTE: EDB is a subtype of +F, therefore it must qualify as +F for it to also be considered as EDB. RDA with fast activity in the background and not associated with the pattern does not qualify as +F and therefore cannot qualify as EDB.
NOTE: The only periodic pattern that can qualify for EDB is periodic delta brushes. Any other periodic pattern with superimposed fast activity remains PD+F only. By similar notion PD+F with the fast in between periodic delta waves would also not qualify as EDB. This is because the fast activity is not associated with the wave (i.e., it is not periodic delta brushes).
NOTE: EDB can be in any location as any other form of RDA or PDs (i.e., generalized [Fig. 36A], lateralized [Fig. 36B], bilateral independent [Fig. 36C], unilateral independent or multifocal).
FIG. 36.

A. Generalized Extreme Delta Brush (GEDB). GRDA+F also qualifies as definite GEDB if the RDA+F is abundant or continuous; and as possible GEDB if the RDA+F is occasional or frequent. B. Lateralized Extreme Delta Brush (LEDB). LRDA+F also qualifies as definite LEDB if the LRDA+F is abundant or continuous; and as possible LEDB if the LRDA+F is occasional or frequent. C. Bilateral Independent Extreme Delta Brush (BIEDB). BIRDA+F also qualifies as definite BIEDB if the BIRDA+F is abundant or continuous; and as possible EDB if the BIRDA+F is occasional or frequent.
NOTE: There are multiple other features that may make a pattern more “ictal-appearing,” such as increased sharpness, higher voltage (amplitude), and fluctuation, but these are already accounted for in the other modifiers.
4. Minor Modifiers (Most of the following section can be applied to any EEG phenomenon)
“Sudden onset” versus “gradual onset” (“sudden onset” preferred over “paroxysmal”). Sudden onset is defined as progressing from absent to well-developed within 3 seconds.
“Triphasic morphology”: Three phases, negative-positive-negative, with each phase longer than the previous, and the second (positive) phase of highest voltage (see Supp EEG 23, Supplemental Digital Content 1, http://links.lww.com/JCNP/A134); or the same but with the first (negative) phase of sufficiently low voltage to be obscured by background activity, leaving a biphasic waveform, positive-negative in polarity. Note that a biphasic waveform may be categorized as “triphasic” by this definition. The phrase “with triphasic morphology” should be added to the appropriate term when this modifier applies. This modifier applies to PDs and SW, but not RDA; it can also be used to describe sporadic discharges.
“Anterior-posterior lag” or “posterior-anterior lag”: A lag is present if there is a consistent measurable delay of >100 ms from the most anterior to the most posterior derivation in which it is seen, or vice versa (see Supp EEG 23, Supplemental Digital Content 1, http://links.lww.com/JCNP/A134) (Fig. 37); specify typical delay in milliseconds from anterior to posterior (negative = posterior to anterior lag) in both a longitudinal bipolar and a referential montage, preferably with an ipsilateral ear reference. This applies to PDs or the spike/sharp wave component of SW.
- Polarity: Specify for the dominant phase (phase with the greatest voltage) only. Should be determined in a referential montage. Describe the typical discharge. Applies only to PDs and the spike/sharp component of SW, not RDA. Categorize as
- Positive
- Negative
- Dipole, tangential
- Unclear
FIG. 37.

Anterior-posterior (AP) lag.
D. ELECTROGRAPHIC AND ELECTROCLINICAL SEIZURES
1. Electrographic Seizures (ESz)
ESz (largely based on the Salzburg criteria)11,12 is defined as either:
Epileptiform discharges* averaging >2.5 Hz for ≥10 seconds (>25 discharges in 10 seconds), OR
Any pattern with definite evolution as defined above and lasting ≥10 seconds (see Supp EEG 24a, b, and c, Supplemental Digital Content 1, http://links.lww.com/JCNP/A134) (Fig. 38).
FIG. 38.

Electrographic seizure (ESz).
*NOTE: Electrographic seizures can consist of sharply contoured discharges that are not technically “epileptiform.” For example, >25 sharply contoured discharges in 10 seconds is still a seizure, although each discharge can be >200 ms duration (and therefore technically not “epileptiform”).
NOTE: Whether to maintain or eliminate the “10 second rule” (clearly an arbitrary cutoff) was a matter of significant debate among the authors and the greater EEG community surveyed during creation of this version of the nomenclature. However, because there was no consensus or convincing new literature to change this, we maintained the status quo in this matter. Hopefully, future investigations will help determine the proper minimum duration for defining a seizure, if there is one.
2. Electrographic Status Epilepticus (ESE)
ESE is defined as an ESz for ≥10 continuous minutes or for a total duration of ≥20% of any 60-minute period of recording. The 10 minute cutoff matches the definition of focal status epilepticus with impaired consciousness by the International League Against Epilepsy.17 The 20% cutoff, lowered from the previous 50%, is based on expert consensus and on one study in critically ill children in whom the risk of neurological decline was significantly greater when the maximum hourly seizure burden was >20%.23 A similar cutoff was identified in neonates with hypoxic-ischemic encephalopathy.24
NOTE: “Possible electrographic seizure” and “possible electrographic SE”: These terms are synonyms for patterns on the ictal-interictal continuum (IIC); see section F below, page 25. For the sake of standardized reporting, the pattern should be described using the RPP modifiers (section C) and identified as meeting the criteria for the IIC. For this reason, “possible ESz” and “possible ESE” have not been defined but can be used synonymously with IIC in EEG impressions or when communicating with referring clinicians.
3. Electroclinical Seizure (ECSz)
ECSz is defined as any EEG pattern with either:
Definite clinical correlate* time-locked to the pattern (of any duration) (see Supp EEG 25, Supplemental Digital Content 1, http://links.lww.com/JCNP/A134) (Fig. 39), OR
EEG AND clinical improvement with a parenteral (typically IV) antiseizure medication (see Supp EEG 26a and b, Supplemental Digital Content 1, http://links.lww.com/JCNP/A134) (Fig. 39).
FIG. 39.
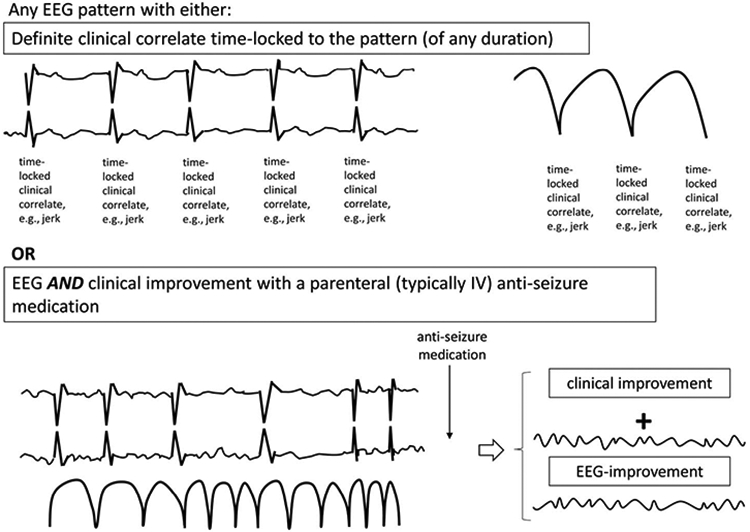
Electroclinical seizure (ECSz).
NOTE: The EEG pattern during an “electroclinical seizure” does not necessarily need to qualify as an “electrographic seizure.” For example, if static 1-Hz PDs have a clinical correlate, this would not qualify as an ESz but would qualify as an electroclinical seizure (ECSz). Many seizures would however qualify for both “electrographic” and “electroclinical” seizures, and these should be reported under both terms.
NOTE: An electroclinical seizure (ECSz) can be of any duration, including <10 seconds, if (and only if) there is a definite clinical correlate. By definition, electrographic seizures must be ≥10 seconds duration. An evolving electrographic pattern lasting <10 seconds qualifies as either an evolving RPP (e.g., “evolving RDA”) or “evolving BIRDs” (see section E, page 24).
*NOTE: A “definite clinical correlate” can be subtle, including face twitching, eye deviation, or nystagmus. Provided that the clinical sign is clearly time-locked to the EEG pattern (and absent when the pattern is absent), then it should be considered an electroclinical seizure.
NOTE: Any seizure or status epilepticus without prominent motor activity can also be referred to as “nonconvulsive.” The term “nonconvulsive” is preferred over “subclinical” because it is usually unclear if the electrographic activity is contributing to the patient’s impaired mental status; if it were contributing, it would still be nonconvulsive but would not be subclinical.
NOTE: The term “nonconvulsive” can be applied to both electrographic and electroclinical seizures. All ESz and ESE alone (without clear clinical correlate) would be nonconvulsive. However, any ECSz or ECSE without prominent motor activity could also be termed nonconvulsive.
4. Electroclinical Status Epilepticus (ECSE)
ECSE is defined as an electroclinical seizure for ≥10 continuous minutes or for a total duration of ≥20% of any 60-minute period of recording. An ongoing seizure with bilateral tonic-clonic (BTC) motor activity only needs to be present for ≥5 continuous minutes to qualify as ECSE. This is also referred to as “convulsive SE,” a subset of “SE with prominent motor activity.”17 In any other clinical situation, the minimum duration to qualify as SE is ≥10 minutes.
4b. Possible ECSE:
Possible ECSE is an RPP that qualifies for the IIC that is present for ≥10 continuous minutes or for a total duration of ≥20% of any 60-minute period of recording, which shows EEG improvement with a parenteral antiseizure medication BUT without clinical improvement. This remains largely in line with “possible NCSE” as defined by the Salzburg criteria.
NOTE: Possible ECSE cannot include patterns that already qualify as ESz/ESE.
NOTE: If parenteral antiseizure medication leads to resolution of ESz/ESE AND clinical improvement, then these should be reported as ESz/ESE AND ECSz/ECSE (similar to how an isolated seizure can be both an ESz and an ECSz).
NOTE: For patients with prior known epileptic encephalopathy, to qualify as ECSE, the EEG pattern needs to represent either:
an increase in prominence or frequency of epileptiform discharges compared with baseline, with an observable decline in clinical state, OR
EEG and clinical improvement with a parenteral (typically IV) antiseizure medication (Fig. 40).
FIG. 40.

Electroclinical seizure (ECSz)—for patients with previous known epileptic encephalopathy.
NOTE: As a principle, all EEG data have to be put into clinical (history, clinical presentation, physical examination) and paraclinical (laboratory, toxicology, cerebral imaging) context to help establish or reject the diagnosis of status epilepticus.
NOTE: If any of these phenomena (ESz, ECSz, ESE, ECSE) are stimulus-induced (reproducibly brought about or exacerbated by an alerting stimulus), then they warrant an “SI−” prefix, as described in section C 3g above, page 14.
E. BRIEF POTENTIALLY ICTAL RHYTHMIC DISCHARGES (BIRDs)
(Largely based on Yoo JY et al., JCN 201714)
BIRDs are defined as focal (including L, BI, UI or Mf) or generalized rhythmic activity >4 Hz (at least six waves at a regular rate) lasting ≥0.5 to <10 seconds, not consistent with a known normal pattern or benign variant, not part of burst-suppression or burst-attenuation, without definite clinical correlate, and that has at least one of the following features:
Evolution (“evolving BIRDs,” a form of definite BIRDs) (Fig. 41A)
Similar morphology and location as interictal epileptiform discharges or seizures in the same patient (definite BIRDs) (see Supp EEG 27, Supplemental Digital Content 1, http://links.lww.com/JCNP/A134) (Figs. 41B and 41C)
Sharply contoured but without (a) or (b) (possible BIRDs) (Fig. 41D)
FIG. 41.
Brief Potentially Ictal Rhythmic Discharges (BIRDs). A. BIRDs with evolution, aka "evolving BIRDs" (a form of definite BIRDs). B. BIRDs with a similar morphology and location as interictal epileptiform discharges in the same patient (definite BIRDs). C. BIRDs with a similar morphology and location as seizures in the same patient (definite BIRDs). D. BIRDs that are sharply contoured but without the above features (possible BIRDs).
NOTE: Paroxysmal fast activity lasting ≥0.5 to <10 seconds qualifies as BIRDs, whether generalized (also known as generalized paroxysmal fast activity, or GPFA) or focal.
NOTE: Although they are termed “brief,” technically all BIRDs are “very brief” because they are <10 seconds.
F. THE ICTAL-INTERICTAL CONTINUUM (IIC)
This term is synonymous with “possible ESz” or “possible electrographic SE.” The IIC is a purely electrographic term that is not a diagnosis; it requires careful interpretation in the full clinical context. A pattern on the IIC is a pattern that does not qualify as an ESz or ESE, but there is a reasonable chance that it may be contributing to impaired alertness, causing other clinical symptoms, and/or contributing to neuronal injury. Thus, it is potentially ictal in at least some sense and often warrants a diagnostic treatment trial, typically with a parenteral antiseizure medication. Although this is a concept under development and with no broad consensus, the following patterns can be considered to be on the IIC:
Any PD or SW pattern that averages >1.0 and ≤2.5 Hz over 10 seconds (>10 and ≤ 25 discharges in 10 seconds) (see Supp EEG 28, Supplemental Digital Content 1, http://links.lww.com/JCNP/A134) (Fig. 42A); or
Any PD or SW pattern that averages ≥0.5 Hz and ≤1.0 Hz over 10 seconds (≥5 and ≤10 discharges in 10 seconds) and has a plus modifier or fluctuation (see Supp EEG 29a-c and 30, Supplemental Digital Content 1, http://links.lww.com/JCNP/A134) (Figs. 42B and 42C); or
-
Any lateralized RDA averaging >1 Hz for at least 10 seconds (at least 10 waves in 10 seconds) with a plus modifier or fluctuation (Figs. 42D and 42E). This includes any LRDA, BIRDA, UIRDA, and MfRDA, but not GRDA.
AND
Does not qualify as an ESz or ESE (Section D above, page 22).
NOTE: If treatment of a pattern on the IIC with a parenteral antiseizure medication leads to improvement in the EEG AND definite clinical improvement, this would meet criterion B of an ECSz or ECSE. If treatment of an IIC pattern with a parenteral antiseizure medication leads to improvement in the EEG BUT NOT clinical improvement, this would be possible ECSE.
NOTE: If the IIC pattern is stimulus-induced (reproducibly brought about or exacerbated by an alerting stimulus), then it warrants an “SI−” prefix, as described in section C 3g above, page 14.
G. MINIMUM REPORTING REQUIREMENTS IN CLINICAL CARE
The recommendations from the ACNS Consensus Statement on Continuous EEG in Critically Ill Adult and Children, Part II,25 are repeated here for convenience:
First 30 to 60 minutes (equivalent to a “standard” or “routine” EEG). This should be reviewed as soon as possible and reported to the clinical team.
Each 24-hour period.
A written report should be completed at least once per day. If significant changes occur in the record during this period, then additional epochs should be reported separately as needed, either verbally or in writing.
NOTE: We recommend communicating updates to the clinical team at least twice per day except in unusually stable circumstances.
H. OTHER TERMS
Daily Pattern Burden is defined as the total duration of a pattern per 24 hours. For example, if GPDs were present for 33% of the record for 12 hours, then 10% of the record for 12 hours, the Daily GPD Burden would be 4 hours + 1.2 hours = 5.2 hours.
Daily Seizure Burden can be calculated similarly: e.g., six 30-second seizures in one day would have a Daily Seizure Burden of 3 minutes.
NOTE: Hourly pattern burden and hourly seizure burden can be calculated and reported in a similar manner as the daily burdens, as can maximal hourly burden of each.
-
3.
Daily Pattern Index is defined as Daily Burden X Mean Frequency (Hz). In the above example, if GPDs were at 1.5 Hz, the Daily GPD Index would be 5.2 h × 1.5 Hz = 7.8 Hz-hours. Similarly, an hourly pattern index can be described. For example, 1.5-Hz LPDs with a prevalence of 20% for 1 hour would have an hourly pattern index of 12 minutes × 1.5 Hz = 18 Hz-mins.
Supplementary Material
EEG 1 Burst-attenuation pattern
EEG 2 Burst-suppression pattern
EEG 3 Identical Highly-Epileptiform Bursts
EEG 4 Identical Non-Highly-Epileptiform Bursts
EEG 5 Generalized Periodic Discharges (GPDs)
EEG 6 Generalized Rhythmic Delta Activity (GRDA)
EEG 7 GPDs (frontal predominant)
EEG 8 Lateralized Periodic Discharges (LPDs)
EEG 9 Bilateral Independent Periodic Discharges (BIPDs)
EEG 10 Unilateral Independent Periodic Discharges (UIPDs)
EEG 11 Unilateral Independent Rhythmic Delta Activity (UIRDA)
EEG 12 Multifocal Periodic Discharges (MfPDs)
EEG 13 LPDs (bilateral asymmetric)
EEG 14 Generalized Spike-and-Wave (GSW)
EEG 15 Stimulus Induced - GRDA (SI-GRDA)
EEG 16 LRDA with evolution
EEG 17 LPDs with fluctuation
EEG 18 GPDs+F
EEG 19 LPDs+F
EEG 20 LRDA+S
EEG 21 BIPDs+F
EEG 22 Extreme Delta Brush (EDB)
EEG 23 GPDs with triphasic morphology and A-P lag
EEG 24a Electrographic seizure (ESz)
EEG 24b Electrographic seizure (ESz) cont.
EEG 24c Electrographic seizure (ESz) cont.
EEG 25 Electroclinical seizure (ECSz)
EEG 26a Electroclinical seizure (ECSz)
EEG 26b Electroclinical seizure (ECSz)
EEG 27 Brief Potentially Ictal Rhythmic Discharges (BIRDs)
EEG 28 Ictal-Interictal Continuum (IIC) – Focal
EEG 29a Ictal-Interictal Continuum (IIC) – Generalized
EEG 29b Ictal-Interictal Continuum (IIC) – Generalized
EEG 29c Ictal-Interictal Continuum (IIC) – Generalized
EEG 30 Ictal-Interictal Continuum (IIC) with Quantitative EEG (QEEG)
Acknowledgments
L. J. Hirsch received consultation fees from Aquestive, Ceribell, Marinus, Medtronic, Neuropace and UCB; received authorship royalties from Wolters Kluwer and Wiley; and received honoraria for speaking from Neuropace and Natus. S. M. LaRoche received royalties from Demos/Springer Publishing. S. Beniczky is consultant for Brain Sentinel & Epihunter and Philips; speaker for Eisai, UCB, GW Pharma, Natus, BIAL; and received research grants from Brain Sentinel, Philips, Eisai, UCB, GW Pharma, Natus, BIAL, Epihunter, Eurostars (EU), Independent Research Fund Denmark, Filadelfia Research Foundation, Juhl Foundation, Hansen Foundation. N. S. Abend received royalties from Demos; grants from PCORI and Epilepsy Foundation; and an institutional grant from UCB Pharma. J. W. Lee received grants from Bioserenity, Teladoc, Epilepsy Foundation; is co-founder of Soterya Inc; is a board member of the American Clinical Neurophysiology Society; does consulting for Biogen; and is site PI for Engage Therapeutics and NIH/NINDS R01-NS062092. C. J. Wustof does consulting for Persyst and PRA Health Care. C. D. Hahn received grants from Takeda Pharmaceuticals, UCB Pharma, Greenwich Biosciences. M. B. Westover is co-founder of Beacon Biosignals. E. E. Gerard received grants from Greenwich Pharmaceuticals, Xenon Pharmaceuticals, Sunovion, and Sage. S. T. Herman received grants from UCB Pharma, Neuropace, Sage. H. A. Haider receives author royalties from UpToDate and Springer; does consulting for Ceribell, and is on advisory board for Eisai. A. Rodriguez-Ruiz is co-owner of Rodzi LLC which has no relationship to this work. E. J. Gilmore received a grant from UCB Pharma. J. Claassen is a shareholder of iCE Neurosystems and received a grant from McDonnell Foundation. A, M. Husain received grants from UCB Pharma, Jazz Pharma, Biogen Idec; and received payment from Marinus Pharma, Eisai Pharma, Neurelis Pharma, Blackthorn Pharma, Demos/Springer and Wolters Kluwer publishers. J. Y. Yoo received grants from NIH NeuroNEXT, Zimmer Biomet, LVIS; and receives author royalties from Elsevier. P. W. Kaplan receives author royalties from Demos and Wiley publishers; does consulting for Ceribell; and is expert witness qEEG. M. R. Nuwer is a shareholder of Corticare. M. van Putten is co-founder of Clinical Science Systems. R. Sutter received grants from Swiss National Foundation (No 320030_169379), and UCB Pharma. F. W. Drislane received a grant from American Academy of Neurology. E. Trinka discloses fees received from UCB, Eisai, Bial, Böhringer Ingelheim,Medtronic, Everpharma, GSK, Biogen, Takeda, Liva-Nova, Newbridge, Novartis, Sanofi, Sandoz, Sunovion, GW Pharmaceuticals, Marinus, Arvelle; grants from Austrian Science Fund (FWF), Österreichische Nationalbank, European Union, GSK, Biogen, Eisai, Novartis, Red Bull, Bayer, and UCB; other from Neuroconsult Ges.m.b.H., has been a trial investigator for Eisai, UCB, GSK, Pfitzer. The remaining authors have no funding or conflicts of interest to disclose.
ABBREVIATION LIST
- ACNS
American Clinical Neurophysiology Society
- BI
Bilateral Independent
- BIRDs
Brief Potentially Ictal Rhythmic Discharges
- BTC
Bilateral Tonic-Clonic
- CAPE
Cyclic Alternating Pattern of Encephalopathy
- CCEMRC
Critical Care EEG Monitoring Research Consortium
- ECSz
Electroclinical seizure
- ECSE
Electroclinical status epilepticus
- EDs
Epileptiform Discharges
- EDB
Extreme Delta Brush
- EEG
Electroencephalography
- ESE
Electrographic status epilepticus
- ESz
Electrographic seizure
- G
Generalized
- GPFA
Generalized Paroxysmal Fast Activity
- Hz
Hertz (i.e., per second)
- IIC
Ictal-Interictal Continuum
- L
Lateralized
- Mf
Multifocal
- PDs
Periodic Discharges
- RDA
Rhythmic Delta Activity
- RPP
Rhythmic or Periodic Pattern (i.e., PDs, RDA or SW)
- SE
Status epilepticus
- SI
Stimulus-Induced
- SIRPIDs
Stimulus-Induced Rhythmic, Periodic or Ictal-Appearing Discharges
- ST
Stimulus-Terminated
- SW
Spike-and-wave or sharp-and-wave
- UI
Unilateral Independent
- +
Plus = Additional feature which renders the pattern more ictal-appearing than the usual term without the plus
- +F
Superimposed fast activity
- +R
Superimposed rhythmic activity
- +S
Superimposed sharp waves or spikes, or sharply contoured
Footnotes
SUPPLEMENTAL DIGITAL CONTENT
The “ACNS Standardized Critical Care EEG Terminology 2021: Condensed Version” is available at Supplemental Digital Content, http://links.lww.com/JCNP/A149, and the “ACNS Standardized Critical Care EEG Terminology 2021: Reference Chart” is available at Supplemental Digital Content, http://links.lww.com/JCNP/A150.
EEG LIST
Supplemental figures are available as Supplemental Digital Content at http://links.lww.com/JCNP/A134.
REFERENCES
- 1.Hirsch LJ, Brenner RP, Drislane FW, et al. The ACNS subcommittee on research terminology for continuous EEG monitoring: proposed standardized terminology for rhythmic and periodic EEG patterns encountered in critically ill patients. J Clin Neurophysiol 2005;22:128–135. [DOI] [PubMed] [Google Scholar]
- 2.Hirsch LJ, LaRoche SM, Gaspard N, et al. American clinical Neurophysiology Society’s Standardized Critical Care EEG Terminology: 2012 version. J Clin Neurophysiol 2013;30:1–27. [DOI] [PubMed] [Google Scholar]
- 3.Gaspard N, Hirsch LJ, LaRoche SM, Hahn CD, Westover MB. Interrater agreement for critical care EEG terminology. Epilepsia 2014;55:1366–1373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lee JW, LaRoche S, Choi H, et al. Development and feasibility testing of a critical care EEG monitoring database for standardized clinical reporting and multicenter collaborative research. J Clin Neurophysiol 2016;33:133–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gaspard N, Manganas L, Rampal N, Petroff OA, Hirsch LJ. Similarity of lateralized rhythmic delta activity to periodic lateralized epileptiform discharges in critically ill patients. JAMA Neurol 2013;70:1288–1295. [DOI] [PubMed] [Google Scholar]
- 6.Rodriguez Ruiz A, Vlachy J, Lee JW, et al. Association of periodic and rhythmic electroencephalographic patterns with seizures in critically ill patients. JAMA Neurol 2017;74:181. [DOI] [PubMed] [Google Scholar]
- 7.Pedersen GL, Rasmussen SB, Gyllenborg J, Benedek K, Lauritzen M. Prognostic value of periodic electroencephalographic discharges for neurological patients with profound disturbances of consciousness. Clin Neurophysiol 2013;124:44–51. [DOI] [PubMed] [Google Scholar]
- 8.Foreman B, Mahulikar A, Tadi P, et al. Generalized periodic discharges and ‘triphasic waves’: a blinded evaluation of inter-rater agreement and clinical significance. Clin Neurophysiol 2016;127:1073–1080. [DOI] [PubMed] [Google Scholar]
- 9.O’Rourke D, Chen PM, Gaspard N, et al. Response rates to anticonvulsant trials in patients with triphasic-wave EEG patterns of uncertain significance. Neurocrit Care 2016;24:233–239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Thompson SA, Hantus S. Highly epileptiform bursts are associated with seizure recurrence. J Clin Neurophysiol 2016;33:66–71. [DOI] [PubMed] [Google Scholar]
- 11.Beniczky S, Hirsch LJ, Kaplan PW, et al. Unified EEG terminology and criteria for nonconvulsive status epilepticus. Epilepsia 2013;54:28–29. [DOI] [PubMed] [Google Scholar]
- 12.Leitinger M, Trinka E, Gardella E, et al. Diagnostic accuracy of the Salzburg EEG criteria for non-convulsive status epilepticus: a retrospective study. Lancet Neurol 2016;15:1054–1062. [DOI] [PubMed] [Google Scholar]
- 13.Yoo JY, Rampal N, Petroff OA, Hirsch LJ, Gaspard N. Brief potentially ictal rhythmic discharges in critically ill adults. JAMA Neurol 2014;71:454–462. [DOI] [PubMed] [Google Scholar]
- 14.Yoo JY, Marcuse LV, Fields MC, et al. Brief potentially ictal rhythmic discharges [B(I)RDs] in noncritically ill adults. J Clin Neurophysiol 2017;34:222–229. [DOI] [PubMed] [Google Scholar]
- 15.Hofmeijer J, Tjepkema-Cloostermans MC, van Putten MJ. Burst-suppression with identical bursts: a distinct EEG pattern with poor outcome in postanoxic coma. Clin Neurophysiol 2014;125:947–954. [DOI] [PubMed] [Google Scholar]
- 16.Schmitt SE, Pargeon K, Frechette ES, et al. Extreme delta brush: a unique EEG pattern in adults with anti-NMDA receptor encephalitis. Neurology 2012;79:1094–1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Trinka E, Cock H, Hesdorffer D, et al. A definition and classification of status epilepticus—Report of the ILAE Task Force on Classification of Status Epilepticus. Epilepsia 2015;56:1515–1523. [DOI] [PubMed] [Google Scholar]
- 18.Shellhaas RA, Chang T, Tsuchida T, et al. The American Clinical Neurophysiology Society’s Guideline on Continuous Electroencephalography Monitoring in Neonates. J Clin Neurophysiol 2011;28:611–617. [DOI] [PubMed] [Google Scholar]
- 19.Kane N, Acharya J, Benickzy S, et al. A revised glossary of terms most commonly used by clinical electroencephalographers and updated proposal for the report format of the EEG findings. Revision 2017. Clin Neurophysiol Pract 2017;2:170–185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Admiraal MM, van Rootselaar AF, Horn J. Electroencephalographic reactivity testing in unconscious patients: a systematic review of methods and definitions. Eur J Neurol 2017;24:245–254. [DOI] [PubMed] [Google Scholar]
- 21.Admiraal MM, Van Rootselaar AF, Horn J. International consensus on EEG reactivity testing after cardiac arrest: towards standardization. Resuscitation 2018;131:36–41. [DOI] [PubMed] [Google Scholar]
- 22.Hirsch LJ, Claassen J, Mayer SA, Emerson RG. Stimulus-induced rhythmic, periodic, or ictal discharges (SIRPIDs): a common EEG phenomenon in the critically ill. Epilepsia 2004;45:109–123. [DOI] [PubMed] [Google Scholar]
- 23.Payne ET, Zhao XY, Frndova H, et al. Seizure burden is independently associated with short term outcome in critically ill children. Brain 2014;137:1429–1438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kharoshankaya L, Stevenson NJ, Livingstone V, et al. Seizure burden and neurodevelopmental outcome in neonates with hypoxic-ischemic encephalopathy. Dev Med Child Neurol 2016;58:1242–1248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Herman ST, Abend NS, Bleck TP, et al. Consensus statement on continuous EEG in critically ill adults and children, part II: personnel, technical specifications, and clinical practice. J Clin Neurophysiol 2015;32:96–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
EEG 1 Burst-attenuation pattern
EEG 2 Burst-suppression pattern
EEG 3 Identical Highly-Epileptiform Bursts
EEG 4 Identical Non-Highly-Epileptiform Bursts
EEG 5 Generalized Periodic Discharges (GPDs)
EEG 6 Generalized Rhythmic Delta Activity (GRDA)
EEG 7 GPDs (frontal predominant)
EEG 8 Lateralized Periodic Discharges (LPDs)
EEG 9 Bilateral Independent Periodic Discharges (BIPDs)
EEG 10 Unilateral Independent Periodic Discharges (UIPDs)
EEG 11 Unilateral Independent Rhythmic Delta Activity (UIRDA)
EEG 12 Multifocal Periodic Discharges (MfPDs)
EEG 13 LPDs (bilateral asymmetric)
EEG 14 Generalized Spike-and-Wave (GSW)
EEG 15 Stimulus Induced - GRDA (SI-GRDA)
EEG 16 LRDA with evolution
EEG 17 LPDs with fluctuation
EEG 18 GPDs+F
EEG 19 LPDs+F
EEG 20 LRDA+S
EEG 21 BIPDs+F
EEG 22 Extreme Delta Brush (EDB)
EEG 23 GPDs with triphasic morphology and A-P lag
EEG 24a Electrographic seizure (ESz)
EEG 24b Electrographic seizure (ESz) cont.
EEG 24c Electrographic seizure (ESz) cont.
EEG 25 Electroclinical seizure (ECSz)
EEG 26a Electroclinical seizure (ECSz)
EEG 26b Electroclinical seizure (ECSz)
EEG 27 Brief Potentially Ictal Rhythmic Discharges (BIRDs)
EEG 28 Ictal-Interictal Continuum (IIC) – Focal
EEG 29a Ictal-Interictal Continuum (IIC) – Generalized
EEG 29b Ictal-Interictal Continuum (IIC) – Generalized
EEG 29c Ictal-Interictal Continuum (IIC) – Generalized
EEG 30 Ictal-Interictal Continuum (IIC) with Quantitative EEG (QEEG)



















