Key Points
Question
Does the risk of stroke without anticoagulation change with age among patients aged 66 to 74 years with atrial fibrillation without non–sex-related CHA2DS2-VASc (congestive heart failure, hypertension, age ≥75 years, diabetes, stroke, vascular disease, age 65-74 years, female sex) risk factors?
Findings
In this population-based cohort study of 16 351 individuals, the overall 1-year risk of stroke without anticoagulation was 1.1% and did not differ significantly by sex. The 1-year stroke risk more than doubled as age increased, from 0.7% at 66 years to 1.7% at 74 years.
Meaning
Among patients aged 66 to 74 years with atrial fibrillation without non–sex-related CHA2DS2-VASc risk factors, anticoagulation therapy appears to be more likely to yield net clinical benefit in older rather than younger individuals.
Abstract
Importance
There are limited clinical trial data and discrepant recommendations regarding use of anticoagulation therapy in patients with atrial fibrillation (AF) aged 65 to 74 years without other stroke risk factors.
Objectives
To evaluate the risk of stroke without anticoagulation therapy in men and women with AF aged 66 to 74 years without other CHA2DS2-VASc (congestive heart failure, hypertension, age ≥75 years, diabetes, stroke, vascular disease, age 65-74 years, female sex) risk factors and examine the association of stroke incidence with patient age.
Design, Setting, and Participants
A population-based retrospective cohort study was conducted using linked administrative databases. The population included 16 351 individuals aged 66 to 74 years who were newly diagnosed with AF in Ontario, Canada, between April 1, 2007, and March 31, 2017. Exclusion criteria included long-term care residence, prior anticoagulation therapy, valvular disease, heart failure, hypertension, diabetes, stroke, and vascular disease. The cumulative incidence function was used to estimate the 1-year incidence of stroke in patients who did not receive anticoagulation therapy. Fine-Gray regression was used to study the association of patient characteristics with stroke incidence and derive estimates of stroke risk at each age. Death was treated as a competing risk and patients were censored if they initiated anticoagulation therapy. Inverse probability of censoring weights was used to account for patient censoring. Data analysis was performed from May 26, 2019, to December 9, 2020.
Exposures
Atrial fibrillation and age.
Main Outcomes and Measures
Hospitalizations for stroke.
Results
Of the 16 351 individuals with AF (median [interquartile range] age, 70 [68-72] years), 8352 (51.1%) were men; 6314 individuals (38.6%) started anticoagulation therapy during follow-up. The overall 1-year stroke incidence among patients who did not receive anticoagulation therapy was 1.1% (95% CI, 1.0%-1.3%) and the incidence of death without stroke was 8.1% (95% CI, 7.7%-8.5%). The incidence of stroke was not significantly associated with sex. The estimated 1-year stroke risk increased with patient age from 66 years (0.7%; 95% CI, 0.5%-0.9%) to 74 years (1.7%; 95% CI, 1.3%-2.1%).
Conclusions and Relevance
The risk of stroke more than doubled in this study as men and women with AF but no other CHA2DS2-VASc risk factors aged from 66 to 74 years. These data suggest that anticoagulation therapy is more likely to benefit older individuals within this group of patients, whereas younger individuals are less likely to gain net clinical benefit from anticoagulation therapy.
This cohort study examines whether the use of anticoagulation therapy is associated with the risk for stroke in individuals with atrial fibrillation aged 66 to 74 years without other CHA2DS2-VASc risk factors.
Introduction
Anticoagulation therapy with warfarin or direct oral anticoagulants (DOACs) reduces mortality and serious morbidity in patients with AF at higher risk of stroke.1,2 Most practitioners use the CHA2DS2-VASc (congestive heart failure, hypertension, age ≥75 years, diabetes, stroke, vascular disease, age 65-74 years, female sex)3 or CHADS24 scores to determine thresholds for initiating anticoagulation in patients with AF. The American College of Cardiology/American Heart Association (ACC/AHA)5 and the European Society of Cardiology (ESC)6 recommend anticoagulation for patients with CHA2DS2-VASc scores greater than or equal to 2 in men or greater than or equal to 3 in women, and the National Institute for Health and Care Excellence7 recommends anticoagulation at CHA2DS2-VASc scores greater than or equal to 2 for both sexes. In contrast, the American College of Chest Physicians (ACCP)8 recommends anticoagulation at CHA2DS2-VASc scores greater than or equal to 1 in men and greater than or equal to 2 in women, and the Canadian Cardiovascular Society9 recommends anticoagulation at age 65 years or older or with CHADS2 scores greater than or equal to 1. The National Institute for Health and Care Excellence guidelines suggest that anticoagulation therapy should be considered at a CHA2DS2-VASc score of 1, and the ESC and ACC/AHA guidelines do so at CHA2DS2-VASc scores of 1 in men and 2 in women. The discordance between guidelines is most prominent for patients aged 65 to 74 years without other stroke risk factors. Practitioners following the ACCP or Canadian Cardiovascular Society guidelines would frequently recommend anticoagulation therapy for patients in this age group. However, practitioners following other guidelines would prescribe anticoagulation therapy for such patients less frequently, with women receiving anticoagulation therapy more than men as per the National Institute for Health and Care Excellence guidelines. These divergent recommendations highlight the substantial clinical equipoise about using anticoagulants in this group of patients with AF.
The decision to initiate anticoagulation therapy for patients with AF implicitly assumes that their risk of stroke is high enough such that the benefits of anticoagulation outweigh the bleeding risks.10 It has been suggested that anticoagulation with warfarin is beneficial for patients whose stroke risk exceeds 1.7% per year, and DOACs are likely beneficial when patients’ annual stroke risk exceeds 0.9%.10 Although most guidelines use the CHA2DS2-VASc or CHADS2 scores to set thresholds for anticoagulation therapy, these scores were derived from data on patients diagnosed with AF before 2005.3,4 The stroke risk in patients with AF not receiving anticoagulation therapy has been steadily declining since then,11,12 possibly owing to better control of other stroke risk factors and identification of patients with lower burdens of AF. This decrease in stroke incidence highlights the need for contemporary estimates of the risk of stroke in AF.
Considering this equipoise, we sought to estimate the risk of stroke without anticoagulation therapy in individuals with AF aged 66 to 74 years without other CHA2DS2-VASc risk factors. We hypothesized that the risk of stroke without anticoagulation is less than or equal to 0.9% per year for younger individuals in this group. We also wanted to study the association of sex and other patient characteristics with stroke incidence in this group. Because decisions about anticoagulation are influenced by bleeding risk, an additional objective was to describe bleeding incidence in this patient group after starting anticoagulation therapy.
Methods
Data Sources
We conducted a population-based retrospective cohort study using linked administrative databases in Ontario, Canada, where all residents receive health coverage through the Ontario Health Insurance Plan. Prescription medication coverage is provided for residents older than 65 years through the Ontario Drug Benefit program. The Canadian Institute for Health Information Discharge Abstract Database records data on hospitalized patients, and the National Ambulatory Care Reporting System collects data on emergency department visits. The Ontario Health Insurance Plan physician claims database records billing data, and the Registered Persons Database maintains vital statistics data. These data sets were linked using unique encoded identifiers and analyzed at ICES (previously, Institute for Clinical Evaluative Sciences). Several algorithms have been validated for ascertainment of medical diagnoses using these databases.13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29 The use of data in this project was authorized under section 45 of Ontario’s Personal Health Information Protection Act,30 which does not require review by a research ethics board. This study followed the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) reporting guideline for cohort studies.
Cohort Creation
We created a cohort of community-dwelling individuals who were newly diagnosed with AF in Ontario between April 1, 2007, and March 31, 2017, but who had not started anticoagulation therapy. We identified patients for whom AF was documented on hospital or emergency department discharge records or if there were 4 physician billing claims within 365 days for AF (using International Classification of Disease, 9th Revision code 427). This algorithm has a specificity of 99.1% (95% CI, 98.9%-99.3%) for identifying AF.20 For individuals with AF diagnosed in the hospital or emergency department, the index date was that of hospital discharge. For individuals with AF diagnosed using physician claims, the index date was that of the first billing claim. We excluded patients younger than 66 years or older than 74 years and those with prior diagnoses of AF or valvular disease. We also excluded long-term care residents because they tend to be frail with high mortality rates, and our clinical question was focused on community-dwelling individuals. We further excluded individuals diagnosed with other CHA2DS2-VASc risk factors and those receiving warfarin or DOACs before the index date to form the primary (nonanticoagulation) study cohort.
Primary Outcome
The primary outcome was hospitalization with a most responsible diagnosis of stroke.31 Intracerebral bleeds were included in this outcome definition because their occurrence in patients with AF who are not receiving anticoagulation therapy likely represents hemorrhagic transformation of a cardioembolic stroke.32,33 Other intracranial bleeds outside the brain (eg, extradural and subdural bleeds) were excluded from the primary outcome definition. Death was treated as a competing risk. Diagnostic codes are provided in eTable 1 in the Supplement; validation studies reported that Ontario administrative databases have a sensitivity of 82% for detection of stroke.31 We limited follow-up to 1 year because patients frequently acquire additional stroke risk factors over time34 and to maintain consistency with the seminal CHADS24 and CHADS2-VASc3 studies.
Sensitivity Analyses
Because stroke hospitalizations may underestimate the incidence of serious strokes if they frequently lead to death before hospitalization, we studied a composite outcome of stroke or death from any cerebrovascular disease. Cause of death data were available only up to December 31, 2016, so follow-up for this sensitivity analysis was terminated on that date; patient accrual was limited to patients diagnosed with AF before January 1, 2016. We also studied an outcome definition that excluded intracerebral bleeds. Given concerns about confounding by indication (avoidance of anticoagulation in lower-risk patients), we examined stroke risk in patients diagnosed with AF before 2013 (ie, before DOACs were publicly covered in Ontario and before Canadian Cardiovascular Society guidelines recommended anticoagulation for patients aged >65 years). In addition, we estimated stroke incidence in patients whose AF was diagnosed in hospitals or emergency departments, because documentation from this setting may have higher specificity than AF ascertained using physician billing codes.35
Statistical Analysis
Baseline characteristics were summarized using the median (interquartile range) for continuous variables and counts (percentages) for categorical variables. We used the cumulative incidence function to estimate the incidence of stroke over the year following the index date while treating death as a competing risk (noncerebrovascular death was the competing risk when analyzing the composite outcome of stroke or cerebrovascular death). We estimated stroke risk separately for men and women, as well as the subset of patients younger than 70 years.
Patients were censored when they started anticoagulation therapy, because we were interested in estimating the risk of stroke had these patients not initiated anticoagulation. For all analyses of stroke incidence, we used inverse probability of censoring weights (IPCWs) to account for potential informative censoring related to anticoagulation initiation.36,37,38,39,40 The IPCWs were estimated using a Cox proportional hazards model in which the hazard of censoring due to anticoagulation initiation was regressed on age, sex, chronic obstructive pulmonary disease, chronic kidney disease, dementia, a history of cancer, and a frailty score derived using administrative data.26
We used Fine-Gray regression to study the association of stroke incidence with the variables used to estimate the IPCW while accounting for the competing risk of death. This model also incorporated IPCWs. Using the fitted model, we derived marginal estimates of the 1-year stroke risk at each age without anticoagulation therapy.41 The 95% CIs for stroke incidence were estimated by bootstrapping with 200 samples.
Incidence of Bleeding After Starting Anticoagulation
We identified patients who began anticoagulation therapy within 2 months following the index date without an intervening stroke. This subset constituted the anticoagulation cohort. The index date was when warfarin or DOAC agents were first dispensed. The outcome for this analysis was bleeding that led to hospitalization or was documented during hospitalization in any diagnostic field within the Canadian Institute for Health Information Discharge Abstract Database29 (henceforth referred to as hospital-diagnosed bleeding). Previous validation studies report that administrative data sets have a sensitivity of 94% for detection of major bleeding.29 We used the cumulative incidence function to estimate the risk of hospital-diagnosed bleeding over time while treating death as a competing risk. Fine-Gray regression was used to study the association of bleeding incidence with the patient characteristics described above, plus a history of bleeding, liver dysfunction, and anticoagulant category (warfarin vs DOAC). This fitted model was used to derive marginal estimates of the 1-year risk of hospital-diagnosed bleeding.41 In sensitivity analyses, we restricted the outcome definition to hospitalizations with a bleeding-related most responsible diagnosis; IPCW methods were not used in these analyses because individuals were not censored based on use of anticoagulants.
All analyses were performed using SAS Enterprise Guide, version 7.1 (SAS Institute Inc). Because we used administrative data sets from a universal health care system encompassing the entire population of Ontario, we assumed missing data were negligible unless otherwise stated. Statistical significance was defined as a 2-tailed P value <.05. Cells with fewer than 6 individuals were censored as per ICES contractual obligations with data providers. Data analysis was performed from May 26, 2019, to December 9, 2020.
Results
Cohort Characteristics
We identified 314 290 community-dwelling individuals with newly documented AF, of whom 20 464 (6.5%) were aged 66 to 74 years and had no other CHA2DS2-VASc factors (eFigure 1 in the Supplement). Of these, 4113 individuals were receiving anticoagulation therapy at baseline, leaving 16 351 individuals to form the primary study cohort. Baseline characteristics are provided in Table 1. The median age was 70 years (interquartile range, 68-72 years), 7999 individuals (48.9%) were women, and 8352 (51.1%) were men. A total of 6314 individuals (38.6%) started anticoagulation therapy during follow-up: 4145 (65.6%) within 60 days following the AF diagnosis and 2169 (34.4%) in the subsequent 10 months.
Table 1. Baseline Characteristics.
| Characteristic | No. (%) | |
|---|---|---|
| Full cohort | Anticoagulation within first 60 d | |
| No. | 16 351 | 4145 |
| Age, median (IQR), y | 70 (68-72) | 70 (68-72) |
| 66-70 | 9225 (56.4) | 2216 (53.5) |
| 71-74 | 7126 (43.6) | 1929 (46.5) |
| Sex | ||
| Female | 7999 (48.9) | 1993 (48.1) |
| Male | 8352 (51.1) | 2152 (51.9) |
| Income quintilea | ||
| 1 (lowest income) | 2811 (17.2) | 693 (16.7) |
| 2 | 3131 (19.1) | 786 (19.0) |
| 3 | 3290 (20.1) | 831 (20.0) |
| 4 | 3301 (20.2) | 850 (20.5) |
| 5 (highest income) | 3776 (23.1) | 977 (23.6) |
| Rural residence | 2422 (14.8) | 635 (15.3) |
| Chronic obstructive pulmonary disease | 883 (5.4) | 184 (4.4) |
| Kidney disease | 829 (5.1) | 200 (4.8) |
| Dementia | 298 (1.8) | 52 (1.3) |
| Cancer | 2035 (12.4) | 327 (7.9) |
| Hospital Frailty Risk score, median (IQR) | 1 (0-3) | 1 (0-3) |
| Excessive alcohol use | 390 (2.4) | 55 (1.3) |
| Prior bleeding | 1199 (7.3) | 265 (6.4) |
| Upper GI bleeding | 211 (1.3) | 36 (0.9) |
| Lower GI bleeding | 496 (3.0) | 126 (3.0) |
| Other bleeding | 547 (3.3) | 113 (2.7) |
Abbreviations: GI, gastrointestinal; IQR, interquartile range.
Data missing on some individuals.
Risk of Stroke
During 365 days following the index date, there were 183 (1.1%) hospitalizations for stroke (including 19 [0.1%] intracerebral bleeds), 347 (2.1%) hospital-diagnosed bleeds (excluding the 19 intracerebral bleeds), and 1484 (9.1%) deaths. None of the baseline characteristics was significantly associated with the hazard of being censored. The cumulative incidence of stroke during the year following the index date, with death treated as a competing risk, is illustrated in Figure 1. At 1 year, the risk of stroke among patients not receiving anticoagulation was 1.1% (95% CI, 1.0%-1.3%), and the incidence of death without stroke was 8.1% (95% CI, 7.7%-8.5%). The 1-year stroke risk was 1.1% in both men (95% CI, 0.9%-1.4%) and women (95% CI, 0.8%-1.4%). The stroke incidence in patients younger than 70 years was 0.7% (95% CI, 0.6%-0.9%). The stroke incidence was lower without inclusion of intracerebral bleeds in the outcome definition and higher in analyses that included any cerebrovascular death, and in analyses limited to patients with AF diagnosed before 2013 or diagnosed in the hospital or emergency department (Table 2). In all cases, the overall 1-year stroke risk in the subset of patients younger than 70 years did not exceed 1%.
Figure 1. Risk of Hospitalization for Stroke in Patients With Newly Diagnosed Atrial Fibrillation Not Receiving Anticoagulation Therapy.
Patients were censored when they started anticoagulation therapy and death was treated as a competing risk. Patient data were weighted by the inverse probability of censoring.
Table 2. Estimated Incidence of Stroke at 1 Year in the Full Cohort and Patients Aged Less Than 70 Yearsa.
| Variable | Stroke, % (95 CI) | |
|---|---|---|
| Full cohort | Patients aged <70 y | |
| Primary analysis | 1.1 (1.0-1.3) | 0.7 (0.6-0.9) |
| Including any cerebrovascular deaths | 1.3 (1.1-1.5) | 0.9 (0.7-1.1) |
| Excluding hemorrhagic stroke | 1.0 (0.9-1.2) | 0.7 (0.5-0.9) |
| Diagnosed before 2013 | 1.3 (1.0-1.6) | 0.7 (0.5-1.1) |
| Diagnosed in hospital or ED | 1.5 (1.3-1.8) | 1.0 (0.7-1.3) |
Abbreviation: ED, emergency department.
Data are reported from the primary analysis and the following sensitivity analyses: (1) with inclusion of cerebrovascular death (limited to patients diagnosed before 2016), (2) without inclusion of intracerebral bleeds in the outcome definition, (3) patients diagnosed before 2013, and (4) patients with atrial fibrillation diagnosed in hospitals or emergency departments. Death before the outcome of interest was treated as a competing risk. Patients were censored when they started anticoagulation therapy and were weighted by the inverse of the probability of being censored.
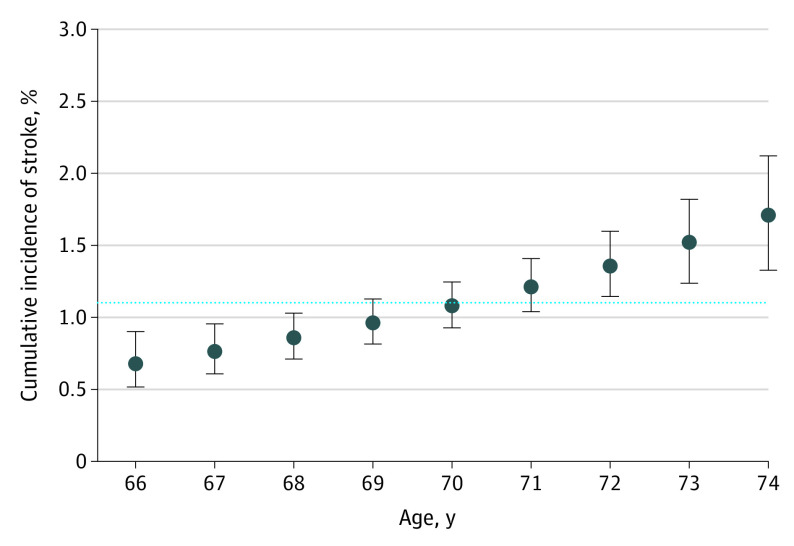
The results of the regression model examining the association of patient characteristics with the incidence of stroke in the cohort not receiving anticoagulation therapy are summarized in Table 3. The only baseline characteristic that was significantly associated with a higher stroke incidence was age. The associated subdistribution hazard ratio was 1.12 per year (95% CI, 1.06-1.18; P < .001). This finding translated to a greater than 2-fold increase in the estimated 1-year risk of stroke as patient age increased from 66 years (0.7%; 95% CI, 0.5%-0.9%) to 74 years (1.7%; 95% CI, 1.3%-2.1%) (Figure 2).
Table 3. Results of the Fine-Gray Regression Model Examining the Association of Patient Characteristics With the Incidence of Strokea.
| Variable | Hazard ratio (95% CI) | P value |
|---|---|---|
| Age | 1.12 (1.06-1.18) | <.001 |
| Female sex (vs male) | 1.01 (0.96-1.05) | .79 |
| Chronic obstructive pulmonary disease | 1.01 (0.76-1.35) | .94 |
| Chronic kidney disease | 1.11 (0.61-2.02) | .74 |
| Dementia | 1.22 (0.65-2.29) | .54 |
| Cancer | 1.53 (0.66-3.56) | .32 |
| Frailty | 0.74 (0.45-1.22) | .24 |
Death was treated as a competing risk. Patients were censored when they started anticoagulation therapy and data were weighted by the inverse of the probability of being censored.
Figure 2. Predicted 1-Year Incidence of Stroke at Each Age Between 66 and 74 Years Among Nonanticoagulated Patients Not Receiving Anticoagulation Therapy.
Death was treated as a competing risk. Patients were censored when they started anticoagulation and were weighted by the inverse of the probability of censoring. Error bars indicate 95% CI. The dotted line indicates 1-year stroke incidence in the full cohort (1.1%).
Risk of Bleeding After Anticoagulation
A total of 1936 individuals received warfarin and 2209 received a DOAC within 60 days after the index date. In the year after starting anticoagulation therapy, there were 89 (2.1%) hospital-diagnosed bleeds (eFigure 2 in the Supplement), including 24 (0.6%) upper gastrointestinal, less than 6 lower gastrointestinal, and 10 (0.2%) intracranial but extracerebral bleeds. The 1-year bleeding risk in the anticoagulated cohort was 2.1% (95% CI, 1.7%-2.6%); this risk did not change significantly with age (eFigure 3 and eTable 2 in the Supplement). We observed similar patterns when examining hospitalizations with bleeding-related most responsible diagnoses (eFigure 4, eFigure 5, and eTable 3 in the Supplement).
Discussion
This population-based observational cohort study sought to estimate the risk of stroke without anticoagulation in men and women with AF aged 66 to 74 years and no other CHA2DS2-VASc risk factors. This group accounted for 6.5% of all patients with newly diagnosed AF. The 1-year stroke risk among patients not receiving anticoagulation therapy was estimated at 1.1% (95% CI, 1.0%-1.3%) in the full cohort and 0.7% (95% CI, 0.6%-0.9%) for patients younger than 70 years. The estimated 1-year stroke risk without anticoagulation more than doubled as patient age increased from 66 to 74 years but did not differ by sex.
We believe these data can inform decision-making about using anticoagulant agents in this patient group; to our knowledge, there are no applicable randomized clinical trial data available for this patient group. Our observations suggest that the stroke risk among younger patients in this group may not meet thresholds at which anticoagulation is expected to yield net clinical benefit,10 being substantially lower than stroke incidence in trials that demonstrated the benefits of anticoagulation in AF. A meta-analysis of randomized clinical trials of antithrombotic therapy for patients with nonvalvular AF reported a stroke rate of 4.1% per year in the placebo/control arms.1 The mean CHADS2 score of the seminal randomized clinical trials of DOACs vs warfarin was 2.6, with a 3.8% incidence of thromboembolism in warfarin-treated patients over approximately 2 years’ median follow-up.2
Although several investigators have studied the risk of stroke in patients with AF who had lower CHA2DS2-VASc scores, most studies did not distinguish age from other stroke risk factors.12,42,43,44,45,46 Web-Table 1 of the 2016 ESC AF guidelines summarizes the reported event rate from 14 studies of AF patients with CHA2DS2-VASc scores less than or equal to 2.6 The risk per 100 patient-years varied widely (lowest, 0.1%-0.2%; highest, 2.8%), but most studies estimated the incidence to be less than 1.5% per year. Prominent exceptions were a study of patients with AF diagnosed between 1996 and 2011 in Taiwan (1-year risk, 2.8%; 95% CI, 2.6%-2.9% in men; 2.6%; 95% CI, 2.4%-2.7% in women)42 and another study of patients hospitalized with AF between 1997 and 2006 in Denmark (1-year risk, 2.01%; 95% CI, 1.70%-2.36%).43 An analysis of Danish data with extended accrual to 2012 reported a lower stroke risk (1.55 per 100 person-years).44 Another study in Denmark reported a 5-year stroke risk of 6.8% (95% CI, 5.7%-7.9%) in men and 8.2% (95% CI, 7.0%-9.5%) in women aged 70 years without other stroke risk factors who had AF diagnosed between 1997 and 2011.45
This variability in reported stroke risk may be associated with differences in study periods (lower risk in contemporary cohorts), geography, ethnicity, risk factor control (hypertension, diabetes, and dyslipidemia) and outcome definitions (stroke vs any thromboembolism).47,48,49,50,51 The variability may also relate to different age distributions within CHA2DS2-VASc age brackets (higher risk in right-skewed distributions) and follow-up duration (increasing risk with longer follow-up) because patients acquire additional stroke risk factors over time.34 In contrast, we studied stroke risk at yearly age increments with 1-year follow-up. Most studies did not account for the competing risk of death; another study reported that this oversight leads to overestimation of stroke risk, particularly when the patient with the index event is discharged from the hospital.52
Collectively, these studies underscore that patients with CHA2DS2-VASc scores less than or equal to 2 constitute a heterogeneous group with substantial differences in stroke risk. Increasing age is a major contributor to this heterogeneity, being an important but unmodifiable risk factor for stroke.34,46,53 We observed that the risk of stroke more than doubled as patients’ age increased from 66 to 74 years. However, the CHA2DS2-VASc model implies that these patients have similar stroke risk in the absence of other risk factors. This is an oversimplification; patients would be better served with an alternative approach that recognizes that the risk of stroke steadily increases with every passing year. Our data suggest that patients near the age of 66 years without other CHA2DS2-VASc risk factors may be less likely to derive net benefit from anticoagulation therapy, especially if they are treated with warfarin rather than DOACs.10 In contrast, older patients within the study population are more likely to derive net clinical benefit from anticoagulation therapy.10
The ACC/AHA and ESC guidelines recommend that practitioners consider anticoagulation therapy in men and women with 1 non–sex-related CHA2DS2-VASc risk factor but do not provide guidance on which patients in this gray zone are more likely to benefit from it. Our data suggest that age should be factored in risk-benefit assessments in these patients, with older individuals being more likely to benefit than younger ones. In addition to the age at which anticoagulation therapy begins, clinicians should consider patients’ medical comorbidities, risk of bleeding with the anticoagulant selected, and the patients’ values and preferences. Practitioners may also consider data that demonstrate that anticoagulation therapy may reduce the risk of nonembolic atherosclerotic events.54 In contrast, female sex was not independently associated with stroke or bleeding incidence in this lower-risk group, suggesting sex should not be used to influence anticoagulation decisions in this setting.55
Limitations
Our study has several limitations. We could not exclude residual confounding, particularly because patients receiving anticoagulants had fewer comorbidities than the overall cohort. We lacked data on race and other socioeconomic determinants of health; these factors can be important confounders if they differ between younger and older individuals. The algorithm to identify out-of-hospital AF introduces a potential immortal time bias (patients fulfilling criteria could not have died before the fourth claim); we adopted this approach because individuals remain at risk for stroke and bleeding between the first and fourth claims. Although the algorithm for identifying patients with AF has high specificity, we may have included patients who did not have AF or who only had transient AF. Our sensitivity for stroke detection was less than 85% and we did not account for aspirin use or nonfatal strokes in patients who were not admitted to the hospital. These factors would lead us to underestimate stroke risk. Conversely, the primary outcome definition captures intracerebral bleeds and nonembolic atherosclerotic strokes. Our sensitivity analysis included death due to any cerebrovascular disease rather than the narrower category of stroke-related death. These factors may have caused us to overestimate stroke risk. Moreover, patients' baseline characteristics were identified using algorithms that prioritize specificity over sensitivity and may underdiagnose comorbidities, leading to inclusion of patients who have unrecognized hypertension, diabetes, ventricular dysfunction, or valvular disease. Thus, the incidence of stroke in patients who do not have comorbidities may be lower than what was observed herein. We expect these limitations to similarly affect younger and older patients, making them unlikely to be associated with our primary observation that the risk of stroke increases with age.
Conclusions
The findings of this study suggest that there is a clinically relevant increase in the risk of stroke without anticoagulation associated with aging from 66 to 74 years in individuals with AF and no other CHA2DS2-VASc risk factors. Our observations suggest a greater likelihood of benefit from anticoagulation therapy among older patients in this group. Our data also suggest that the estimation of stroke risk in patients with AF may be enhanced by models that treat age as a continuous rather than a categorical variable.
eTable 1. Codes Used to Identify Stroke and Bleeding
eFigure 1. Study Flow Diagram
eFigure 2. The Incidence of Hospital-Diagnosed Bleeding in Patients Who Start Anticoagulation Within 60 Days of the Index Date (Death Treated as a Competing Risk)
eFigure 3. The Predicted One-Year Incidence of Bleeding at Each Age Between 66-74 Years Among Patients Who Started Anticoagulation Within 2 Months of AF Diagnosis
eTable 2. Results of the Fine-Gray Regression Model Examining the Association of Patient Characteristics With the Incidence of Hospital-Diagnosed Bleeding in Patients Who Start Anticoagulation Within the First 60 Days (With Death Treated as Competing Risk)
eFigure 4. Cumulative Incidence of Hospitalization for Bleeding in patients Who Start Anticoagulation Within 60 Days of the Index Date (Death Treated as a Competing Risk)
eFigure 5. One-Year Predicted Cumulative Incidence of Hospitalization for Bleeding at Each Age Between 66-74 Years in Patients Who Start Anticoagulation Within 60 Days of the Index Date (Death Treated as a Competing Risk)
eTable 3. Results of Fine-Gray Regression Models Examining Incidence of Bleeding Hospitalizations in Patients Who Start Anticoagulation Within 60 Days of the Index Date (Death Treated as a Competing Risk)
References
- 1.Hart RG, Pearce LA, Aguilar MI. Meta-analysis: antithrombotic therapy to prevent stroke in patients who have nonvalvular atrial fibrillation. Ann Intern Med. 2007;146(12):857-867. doi: 10.7326/0003-4819-146-12-200706190-00007 [DOI] [PubMed] [Google Scholar]
- 2.Ruff CT, Giugliano RP, Braunwald E, et al. Comparison of the efficacy and safety of new oral anticoagulants with warfarin in patients with atrial fibrillation: a meta-analysis of randomised trials. Lancet. 2014;383(9921):955-962. doi: 10.1016/S0140-6736(13)62343-0 [DOI] [PubMed] [Google Scholar]
- 3.Lip GY, Nieuwlaat R, Pisters R, Lane DA, Crijns HJ. Refining clinical risk stratification for predicting stroke and thromboembolism in atrial fibrillation using a novel risk factor-based approach: the euro heart survey on atrial fibrillation. Chest. 2010;137(2):263-272. doi: 10.1378/chest.09-1584 [DOI] [PubMed] [Google Scholar]
- 4.Gage BF, Waterman AD, Shannon W, Boechler M, Rich MW, Radford MJ. Validation of clinical classification schemes for predicting stroke: results from the National Registry of Atrial Fibrillation. JAMA. 2001;285(22):2864-2870. doi: 10.1001/jama.285.22.2864 [DOI] [PubMed] [Google Scholar]
- 5.January CT, Wann LS, Calkins H, et al. 2019 AHA/ACC/HRS focused update of the 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society in collaboration with the Society of Thoracic Surgeons. Circulation. 2019;140(2):e125-e151. doi: 10.1161/CIR.0000000000000665 [DOI] [PubMed] [Google Scholar]
- 6.Kirchhof P, Benussi S, Kotecha D, et al. ; ESC Scientific Document Group . 2016 ESC guidelines for the management of atrial fibrillation developed in collaboration with EACTS. Eur Heart J. 2016;37(38):2893-2962. doi: 10.1093/eurheartj/ehw210 [DOI] [PubMed] [Google Scholar]
- 7.National Institute for Health and Clinical Excellence . Atrial fibrillation: management, clinical guideline [CG 180]. Updated August 1, 2014. Accessed October 25, 2019. https://www.nice.org.uk/guidance/cg180
- 8.Lip GYH, Banerjee A, Boriani G, et al. Antithrombotic therapy for atrial fibrillation: CHEST guideline and expert panel report. Chest. 2018;154(5):1121-1201. doi: 10.1016/j.chest.2018.07.040 [DOI] [PubMed] [Google Scholar]
- 9.Andrade JG, Verma A, Mitchell LB, et al. ; CCS Atrial Fibrillation Guidelines Committee . 2018 Focused update of the Canadian Cardiovascular Society guidelines for the management of atrial fibrillation. Can J Cardiol. 2018;34(11):1371-1392. doi: 10.1016/j.cjca.2018.08.026 [DOI] [PubMed] [Google Scholar]
- 10.Eckman MH, Singer DE, Rosand J, Greenberg SM. Moving the tipping point: the decision to anticoagulate patients with atrial fibrillation. Circ Cardiovasc Qual Outcomes. 2011;4(1):14-21. doi: 10.1161/CIRCOUTCOMES.110.958108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Asinger RW, Shroff GR, Simegn MA, Herzog CA. Anticoagulation for nonvalvular atrial fibrillation: influence of epidemiologic trends and clinical practice patterns on risk stratification and net clinical benefit. Circ Cardiovasc Qual Outcomes. 2017;10(9):e003669. doi: 10.1161/CIRCOUTCOMES.117.003669 [DOI] [PubMed] [Google Scholar]
- 12.Friberg L, Skeppholm M, Terént A. Benefit of anticoagulation unlikely in patients with atrial fibrillation and a CHA2DS2-VASc score of 1. J Am Coll Cardiol. 2015;65(3):225-232. doi: 10.1016/j.jacc.2014.10.052 [DOI] [PubMed] [Google Scholar]
- 13.Schultz SE, Rothwell DM, Chen Z, Tu K. Identifying cases of congestive heart failure from administrative data: a validation study using primary care patient records. Chronic Dis Inj Can. 2013;33(3):160-166. doi: 10.24095/hpcdp.33.3.06 [DOI] [PubMed] [Google Scholar]
- 14.Lee DS, Donovan L, Austin PC, et al. Comparison of coding of heart failure and comorbidities in administrative and clinical data for use in outcomes research. Med Care. 2005;43(2):182-188. doi: 10.1097/00005650-200502000-00012 [DOI] [PubMed] [Google Scholar]
- 15.Vermeulen MJ, Tu JV, Schull MJ. ICD-10 adaptations of the Ontario acute myocardial infarction mortality prediction rules performed as well as the original versions. J Clin Epidemiol. 2007;60(9):971-974. doi: 10.1016/j.jclinepi.2006.12.009 [DOI] [PubMed] [Google Scholar]
- 16.Tu K, Mitiku T, Lee DS, Guo H, Tu JV. Validation of physician billing and hospitalization data to identify patients with ischemic heart disease using data from the Electronic Medical Record Administrative data Linked Database (EMRALD). Can J Cardiol. 2010;26(7):e225-e228. doi: 10.1016/S0828-282X(10)70412-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hux JE, Ivis F, Flintoft V, Bica A. Diabetes in Ontario: determination of prevalence and incidence using a validated administrative data algorithm. Diabetes Care. 2002;25(3):512-516. doi: 10.2337/diacare.25.3.512 [DOI] [PubMed] [Google Scholar]
- 18.Lipscombe LL, Hwee J, Webster L, Shah BR, Booth GL, Tu K. Identifying diabetes cases from administrative data: a population-based validation study. BMC Health Serv Res. 2018;18(1):316. doi: 10.1186/s12913-018-3148-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tu K, Wang M, Young J, et al. Validity of administrative data for identifying patients who have had a stroke or transient ischemic attack using EMRALD as a reference standard. Can J Cardiol. 2013;29(11):1388-1394. doi: 10.1016/j.cjca.2013.07.676 [DOI] [PubMed] [Google Scholar]
- 20.Tu K, Nieuwlaat R, Cheng SY, et al. Identifying patients with atrial fibrillation in administrative data. Can J Cardiol. 2016;32(12):1561-1565. doi: 10.1016/j.cjca.2016.06.006 [DOI] [PubMed] [Google Scholar]
- 21.Tu K, Campbell NR, Chen ZL, Cauch-Dudek KJ, McAlister FA. Accuracy of administrative databases in identifying patients with hypertension. Open Med. 2007;1(1):e18-e26. [PMC free article] [PubMed] [Google Scholar]
- 22.Gershon AS, Warner L, Cascagnette P, Victor JC, To T. Lifetime risk of developing chronic obstructive pulmonary disease: a longitudinal population study. Lancet. 2011;378(9795):991-996. doi: 10.1016/S0140-6736(11)60990-2 [DOI] [PubMed] [Google Scholar]
- 23.Fleet JL, Dixon SN, Shariff SZ, et al. Detecting chronic kidney disease in population-based administrative databases using an algorithm of hospital encounter and physician claim codes. BMC Nephrol. 2013;14:81. doi: 10.1186/1471-2369-14-81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jaakkimainen RL, Bronskill SE, Tierney MC, et al. Identification of physician-diagnosed Alzheimer’s disease and related dementias in population-based administrative data: a validation study using family physicians’ electronic medical records. J Alzheimers Dis. 2016;54(1):337-349. doi: 10.3233/JAD-160105 [DOI] [PubMed] [Google Scholar]
- 25.Robles SC, Marrett LD, Clarke EA, Risch HA. An application of capture-recapture methods to the estimation of completeness of cancer registration. J Clin Epidemiol. 1988;41(5):495-501. doi: 10.1016/0895-4356(88)90052-2 [DOI] [PubMed] [Google Scholar]
- 26.Gilbert T, Neuburger J, Kraindler J, et al. Development and validation of a hospital frailty risk score focusing on older people in acute care settings using electronic hospital records: an observational study. Lancet. 2018;391(10132):1775-1782. doi: 10.1016/S0140-6736(18)30668-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lapointe-Shaw L, Georgie F, Carlone D, et al. Identifying cirrhosis, decompensated cirrhosis and hepatocellular carcinoma in health administrative data: a validation study. PLoS One. 2018;13(8):e0201120. doi: 10.1371/journal.pone.0201120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tu JV, Chu A, Donovan LR, et al. The Cardiovascular Health in Ambulatory Care Research Team (CANHEART): using big data to measure and improve cardiovascular health and healthcare services. Circ Cardiovasc Qual Outcomes. 2015;8(2):204-212. doi: 10.1161/CIRCOUTCOMES.114.001416 [DOI] [PubMed] [Google Scholar]
- 29.Gomes T, Mamdani MM, Holbrook AM, Paterson JM, Hellings C, Juurlink DN. Rates of hemorrhage during warfarin therapy for atrial fibrillation. CMAJ. 2013;185(2):E121-E127. doi: 10.1503/cmaj.121218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Personal Health Information Protection Act, 2004, S.O. 2004, c. 3, Sched. A. Ontario.ca 2014.
- 31.Hall R, Mondor L, Porter J, Fang J, Kapral MK. Accuracy of administrative data for the coding of acute stroke and TIAs. Can J Neurol Sci. 2016;43(6):765-773. doi: 10.1017/cjn.2016.278 [DOI] [PubMed] [Google Scholar]
- 32.Sussman ES, Connolly ES Jr. Hemorrhagic transformation: a review of the rate of hemorrhage in the major clinical trials of acute ischemic stroke. Front Neurol. 2013;4:69. doi: 10.3389/fneur.2013.00069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Paciaroni M, Agnelli G, Corea F, et al. Early hemorrhagic transformation of brain infarction: rate, predictive factors, and influence on clinical outcome: results of a prospective multicenter study. Stroke. 2008;39(8):2249-2256. doi: 10.1161/STROKEAHA.107.510321 [DOI] [PubMed] [Google Scholar]
- 34.Chao TF, Lip GYH, Liu CJ, et al. Relationship of aging and incident comorbidities to stroke risk in patients with atrial fibrillation. J Am Coll Cardiol. 2018;71(2):122-132. doi: 10.1016/j.jacc.2017.10.085 [DOI] [PubMed] [Google Scholar]
- 35.Atzema CL, Austin PC, Chong AS, Dorian P. Factors associated with 90-day death after emergency department discharge for atrial fibrillation. Ann Emerg Med. 2013;61(5):539-548.e1. doi: 10.1016/j.annemergmed.2012.12.022 [DOI] [PubMed] [Google Scholar]
- 36.Hernán MA, Alonso A, Logan R, et al. Observational studies analyzed like randomized experiments: an application to postmenopausal hormone therapy and coronary heart disease. Epidemiology. 2008;19(6):766-779. doi: 10.1097/EDE.0b013e3181875e61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hernán MA, Hernández-Díaz S, Robins JM. A structural approach to selection bias. Epidemiology. 2004;15(5):615-625. doi: 10.1097/01.ede.0000135174.63482.43 [DOI] [PubMed] [Google Scholar]
- 38.Thomas LE, Yang S, Wojdyla D, Schaubel DE. Matching with time-dependent treatments: a review and look forward. Stat Med. 2020;39(17):2350-2370. doi: 10.1002/sim.8533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Robins JM, Finkelstein DM. Correcting for noncompliance and dependent censoring in an AIDS clinical trial with inverse probability of censoring weighted (IPCW) log-rank tests. Biometrics. 2000;56(3):779-788. doi: 10.1111/j.0006-341X.2000.00779.x [DOI] [PubMed] [Google Scholar]
- 40.Willems S, Schat A, van Noorden MS, Fiocco M. Correcting for dependent censoring in routine outcome monitoring data by applying the inverse probability censoring weighted estimator. Stat Methods Med Res. 2018;27(2):323-335. doi: 10.1177/0962280216628900 [DOI] [PubMed] [Google Scholar]
- 41.Austin PC. Absolute risk reductions and numbers needed to treat can be obtained from adjusted survival models for time-to-event outcomes. J Clin Epidemiol. 2010;63(1):46-55. doi: 10.1016/j.jclinepi.2009.03.012 [DOI] [PubMed] [Google Scholar]
- 42.Chao TF, Liu CJ, Wang KL, et al. Should atrial fibrillation patients with 1 additional risk factor of the CHA2DS2-VASc score (beyond sex) receive oral anticoagulation? J Am Coll Cardiol. 2015;65(7):635-642. doi: 10.1016/j.jacc.2014.11.046 [DOI] [PubMed] [Google Scholar]
- 43.Olesen JB, Lip GY, Hansen ML, et al. Validation of risk stratification schemes for predicting stroke and thromboembolism in patients with atrial fibrillation: nationwide cohort study. BMJ. 2011;342:d124. doi: 10.1136/bmj.d124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lip GY, Skjøth F, Rasmussen LH, Larsen TB. Oral anticoagulation, aspirin, or no therapy in patients with nonvalvular AF with 0 or 1 stroke risk factor based on the CHA2DS2-VASc score. J Am Coll Cardiol. 2015;65(14):1385-1394. doi: 10.1016/j.jacc.2015.01.044 [DOI] [PubMed] [Google Scholar]
- 45.Christiansen CB, Gerds TA, Olesen JB, et al. Atrial fibrillation and risk of stroke: a nationwide cohort study. Europace. 2016;18(11):1689-1697. doi: 10.1093/europace/euv401 [DOI] [PubMed] [Google Scholar]
- 46.Coppens M, Eikelboom JW, Hart RG, et al. The CHA2DS2-VASc score identifies those patients with atrial fibrillation and a CHADS2 score of 1 who are unlikely to benefit from oral anticoagulant therapy. Eur Heart J. 2013;34(3):170-176. doi: 10.1093/eurheartj/ehs314 [DOI] [PubMed] [Google Scholar]
- 47.Healey JS, Oldgren J, Ezekowitz M, et al. ; RE-LY Atrial Fibrillation Registry and Cohort Study Investigators . Occurrence of death and stroke in patients in 47 countries 1 year after presenting with atrial fibrillation: a cohort study. Lancet. 2016;388(10050):1161-1169. doi: 10.1016/S0140-6736(16)30968-0 [DOI] [PubMed] [Google Scholar]
- 48.Lip GYH, Brechin CM, Lane DA. The global burden of atrial fibrillation and stroke: a systematic review of the epidemiology of atrial fibrillation in regions outside North America and Europe. Chest. 2012;142(6):1489-1498. doi: 10.1378/chest.11-2888 [DOI] [PubMed] [Google Scholar]
- 49.Healey JS. At what age does stroke risk increase in patients with atrial fibrillation? does it depend on where you live? Can J Cardiol. 2016;32(12):1364-1365. doi: 10.1016/j.cjca.2016.09.002 [DOI] [PubMed] [Google Scholar]
- 50.Chao TF, Wang KL, Liu CJ, et al. Age threshold for increased stroke risk among patients with atrial fibrillation: a nationwide cohort study from Taiwan. J Am Coll Cardiol. 2015;66(12):1339-1347. doi: 10.1016/j.jacc.2015.07.026 [DOI] [PubMed] [Google Scholar]
- 51.Kabra R, Girotra S, Vaughan Sarrazin M. Refining stroke prediction in atrial fibrillation patients by addition of African-American ethnicity to CHA2DS2-VASc Score. J Am Coll Cardiol. 2016;68(5):461-470. doi: 10.1016/j.jacc.2016.05.044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Abdel-Qadir H, Fang J, Lee DS, et al. Importance of considering competing risks in time-to-event analyses: application to stroke risk in a retrospective cohort study of elderly patients with atrial fibrillation. Circ Cardiovasc Qual Outcomes. 2018;11(7):e004580. doi: 10.1161/CIRCOUTCOMES.118.004580 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Marinigh R, Lip GY, Fiotti N, Giansante C, Lane DA. Age as a risk factor for stroke in atrial fibrillation patients: implications for thromboprophylaxis. J Am Coll Cardiol. 2010;56(11):827-837. doi: 10.1016/j.jacc.2010.05.028 [DOI] [PubMed] [Google Scholar]
- 54.Eikelboom JW, Connolly SJ, Bosch J, et al. ; COMPASS Investigators . Rivaroxaban with or without aspirin in stable cardiovascular disease. N Engl J Med. 2017;377(14):1319-1330. doi: 10.1056/NEJMoa1709118 [DOI] [PubMed] [Google Scholar]
- 55.Nielsen PB, Skjøth F, Overvad TF, Larsen TB, Lip GYH. Female sex is a risk modifier rather than a risk factor for stroke in atrial fibrillation: should we use a CHA2DS2-VA score rather than CHA2DS2-VASc? Circulation. 2018;137(8):832-840. doi: 10.1161/CIRCULATIONAHA.117.029081 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eTable 1. Codes Used to Identify Stroke and Bleeding
eFigure 1. Study Flow Diagram
eFigure 2. The Incidence of Hospital-Diagnosed Bleeding in Patients Who Start Anticoagulation Within 60 Days of the Index Date (Death Treated as a Competing Risk)
eFigure 3. The Predicted One-Year Incidence of Bleeding at Each Age Between 66-74 Years Among Patients Who Started Anticoagulation Within 2 Months of AF Diagnosis
eTable 2. Results of the Fine-Gray Regression Model Examining the Association of Patient Characteristics With the Incidence of Hospital-Diagnosed Bleeding in Patients Who Start Anticoagulation Within the First 60 Days (With Death Treated as Competing Risk)
eFigure 4. Cumulative Incidence of Hospitalization for Bleeding in patients Who Start Anticoagulation Within 60 Days of the Index Date (Death Treated as a Competing Risk)
eFigure 5. One-Year Predicted Cumulative Incidence of Hospitalization for Bleeding at Each Age Between 66-74 Years in Patients Who Start Anticoagulation Within 60 Days of the Index Date (Death Treated as a Competing Risk)
eTable 3. Results of Fine-Gray Regression Models Examining Incidence of Bleeding Hospitalizations in Patients Who Start Anticoagulation Within 60 Days of the Index Date (Death Treated as a Competing Risk)