Key Points
Question
Is perioperative systemic therapy feasible and safe in patients with resectable colorectal peritoneal metastases?
Findings
In this phase 2 clinical trial analyzing 79 patients randomized to perioperative systemic therapy (experimental arm) or cytoreductive surgery and hyperthermic intraperitoneal chemotherapy alone (control arm), the trial arms did not differ significantly regarding the proportions of macroscopic complete cytoreductive surgery (experimental arm, 89%; control arm, 86%) and major postoperative morbidity (experimental arm, 22%; control arm, 33%).
Meaning
Results of this phase 2 trial suggest that perioperative systemic therapy seems feasible and safe in patients diagnosed with resectable colorectal peritoneal metastases, justifying a phase 3 trial.
Abstract
Importance
To date, no randomized clinical trials have investigated perioperative systemic therapy relative to cytoreductive surgery and hyperthermic intraperitoneal chemotherapy (CRS-HIPEC) alone for resectable colorectal peritoneal metastases (CPM).
Objective
To assess the feasibility and safety of perioperative systemic therapy in patients with resectable CPM and the response of CPM to neoadjuvant treatment.
Design, Setting, and Participants
An open-label, parallel-group phase 2 randomized clinical trial in all 9 Dutch tertiary centers for the surgical treatment of CPM enrolled participants between June 15, 2017, and January 9, 2019. Participants were patients with pathologically proven isolated resectable CPM who did not receive systemic therapy within 6 months before enrollment.
Interventions
Randomization to perioperative systemic therapy or CRS-HIPEC alone. Perioperative systemic therapy comprised either four 3-week neoadjuvant and adjuvant cycles of CAPOX (capecitabine and oxaliplatin), six 2-week neoadjuvant and adjuvant cycles of FOLFOX (fluorouracil, leucovorin, and oxaliplatin), or six 2-week neoadjuvant cycles of FOLFIRI (fluorouracil, leucovorin, and irinotecan) and either four 3-week adjuvant cycles of capecitabine or six 2-week adjuvant cycles of fluorouracil with leucovorin. Bevacizumab was added to the first 3 (CAPOX) or 4 (FOLFOX/FOLFIRI) neoadjuvant cycles.
Main Outcomes and Measures
Proportions of macroscopic complete CRS-HIPEC and Clavien-Dindo grade 3 or higher postoperative morbidity. Key secondary outcomes were centrally assessed rates of objective radiologic and major pathologic response of CPM to neoadjuvant treatment. Analyses were done modified intention-to-treat in patients starting neoadjuvant treatment (experimental arm) or undergoing upfront surgery (control arm).
Results
In 79 patients included in the analysis (43 [54%] men; mean [SD] age, 62 [10] years), experimental (n = 37) and control (n = 42) arms did not differ significantly regarding the proportions of macroscopic complete CRS-HIPEC (33 of 37 [89%] vs 36 of 42 [86%] patients; risk ratio, 1.04; 95% CI, 0.88-1.23; P = .74) and Clavien-Dindo grade 3 or higher postoperative morbidity (8 of 37 [22%] vs 14 of 42 [33%] patients; risk ratio, 0.65; 95% CI, 0.31-1.37; P = .25). No treatment-related deaths occurred. Objective radiologic and major pathologic response rates of CPM to neoadjuvant treatment were 28% (9 of 32 evaluable patients) and 38% (13 of 34 evaluable patients), respectively.
Conclusions and Relevance
In this randomized phase 2 trial in patients diagnosed with resectable CPM, perioperative systemic therapy seemed feasible, safe, and able to induce response of CPM, justifying a phase 3 trial.
Trial Registration
ClinicalTrials.gov Identifier: NCT02758951
This randomized clinical trial compares the use of perioperative (neoadjuvant and adjuvant) systemic therapy with cytoreductive surgery and hyperthermic intraperitoneal chemotherapy (HIPEC) alone in patients with resectable colorectal peritoneal metastases.
Introduction
Although the additional benefit of hyperthermic intraperitoneal chemotherapy (HIPEC) after cytoreductive surgery (CRS) has been debated,1,2 CRS-HIPEC has been recommended for selected patients with resectable colorectal peritoneal metastases (CPM) in most national and international guidelines.3 Addition of perioperative (ie, neoadjuvant and adjuvant) systemic therapy to CRS-HIPEC may improve oncologic outcomes by preoperative tumor downstaging, eradication of systemic micrometastases, and postoperative elimination of microscopic residual disease. However, perioperative systemic therapy could induce toxic effects; may increase postoperative morbidity, especially when including bevacizumab4; and could result in preoperative intraperitoneal progression and consequent inoperability given its assumed relative inefficacy for CPM.5 To our knowledge, there have been no randomized trials investigating the value of perioperative systemic therapy for resectable CPM,6,7 leading to a wide variety in its administration and timing among countries and hospitals.3,6,8 To address this evidence gap, the CAIRO6 trial has been designed to assess whether perioperative systemic therapy improves overall survival of patients diagnosed with resectable CPM relative to CRS-HIPEC alone, the latter being standard of care in the Netherlands.9 Because CAIRO6 appears to be the first randomized trial investigating perioperative systemic therapy in this setting, a randomized phase 2 trial, reported herein, was incorporated to assess the feasibility and safety of perioperative systemic therapy, the response of CPM to neoadjuvant treatment, and the trial’s accrual before proceeding to a phase 3 trial.
Methods
Design
CAIRO6 is an investigator-initiated, open-label, parallel-group, phase 2-3, randomized superiority trial conducted in all 9 Dutch tertiary centers for the surgical treatment of CPM. The trial was approved by a central ethics committee (MEC-U) in the Netherlands and the institutional review boards of all centers. Patients gave written informed consent; no financial compensation was provided. The protocol is presented in Supplement 1 and is publicly available,10 with a summary published elsewhere.11 This study followed the Consolidated Standards of Reporting Trials (CONSORT) reporting guideline.
Patients
A complete list of eligibility criteria is presented in the eMethods in Supplement 2. Briefly, eligible patients were adults with a World Health Organization performance status of 0 or 1 (fully active or restricted in strenuous activity but ambulatory and able to carry out light work), pathologically proven isolated nonappendiceal CPM, resectable disease and a peritoneal cancer index of 20 or lower at diagnostic laparoscopy or laparotomy,12 and no systemic therapy within 6 months prior to enrollment. Enrollment was allowed for patients who underwent previous surgery for CPM and for patients who underwent diagnostic laparoscopy or laparotomy in a referring center, provided that the peritoneal cancer index was appropriately scored.
Randomization
Patients were randomized (1:1) to receive perioperative systemic therapy (experimental arm) or CRS-HIPEC alone (control arm) using randomization software with minimization stratified by peritoneal cancer index (≤10, >10), onset of CPM (synchronous, metachronous), previous systemic therapy for colorectal cancer (yes, no), and planned HIPEC regimen (oxaliplatin, mitomycin C).
Procedures
In the experimental arm, perioperative systemic therapy had to start within 4 weeks after randomization. In the control arm, upfront CRS-HIPEC had to be scheduled within 6 weeks after randomization.
Perioperative Systemic Therapy
The protocol summary includes a rationale for the trial’s perioperative systemic therapy regimens,11 with details on doses, routes, and schedules of different regimens presented in the eMethods in Supplement 2. Briefly, at the physician’s discretion, perioperative systemic chemotherapy comprised either four 3-week neoadjuvant and four 3-week adjuvant cycles of CAPOX (capecitabine and oxaliplatin), six 2-week neoadjuvant and six 2-week adjuvant cycles of FOLFOX (fluorouracil, leucovorin, and oxaliplatin), or six 2-week neoadjuvant cycles of FOLFIRI (fluorouracil, leucovorin, and irinotecan) followed by either four 3-week adjuvant cycles of capecitabine or six 2-week adjuvant cycles of fluorouracil with leucovorin. Bevacizumab was added to the first 3 (CAPOX) or 4 (FOLFOX or FOLFIRI) neoadjuvant cycles. In case of unacceptable toxic effects associated with oxaliplatin or irinotecan during neoadjuvant treatment, CAPOX or FOLFOX could have been switched to FOLFIRI and vice versa. In case of unacceptable toxic effects of oxaliplatin during adjuvant treatment, CAPOX or FOLFOX could have been switched to capecitabine or fluorouracil with leucovorin. Dose reductions were left to the discretion of the treating physician. Perioperative systemic therapy was terminated in case of disease progression, unacceptable toxic effects, or at the physician’s or patient’s decision.
Restaging thoracoabdominal computed tomography was performed after 3 (CAPOX-bevacizumab) or 4 (FOLFOX-bevacizumab or FOLFIRI-bevacizumab) neoadjuvant cycles or earlier if neoadjuvant treatment was prematurely terminated. In case of systemic progression, trial treatment was stopped. Patients with peritoneal progression without systemic progression intentionally underwent CRS (if deemed resectable) and HIPEC (if completely resected) without further perioperative systemic therapy. Patients with stable disease or response intentionally completed neoadjuvant treatment and underwent CRS (if deemed resectable) and HIPEC (if completely resected) followed by adjuvant treatment. CRS-HIPEC had to be scheduled within 6 weeks after completion of the last neoadjuvant cycle and at least 6 weeks after the last administration of bevacizumab to minimize the risk of bevacizumab-related postoperative morbidity.13 As recommended in international guidelines in colon cancer,14 adjuvant systemic chemotherapy had to start within 12 weeks after macroscopic complete CRS-HIPEC.
Surgical Procedure
CRS-HIPEC was performed according to the standardized Dutch protocol in all centers (eMethods in Supplement 2).15,16,17 In case of unresectable disease or macroscopic incomplete CRS, trial treatment was stopped. Patients undergoing macroscopic complete CRS-HIPEC were followed up according to a standardized schedule.11
Trial Accrual
After completion of the present phase 2 trial, investigators of all participating centers reviewed a list of all patients scheduled for CRS-HIPEC for treatment of CPM during recruitment in their center. Using the trial’s eligibility criteria, patients potentially eligible for trial participation were identified, and numbers of patients not approached or refusing to participate (including main reasons) were documented.
Outcomes
Primary feasibility and safety outcomes of the present phase 2 trial were the proportion of patients undergoing macroscopic complete CRS-HIPEC and the proportion of patients with Clavien-Dindo grade 3 or higher postoperative morbidity up to 3 months postoperatively.18 Key secondary outcomes were centrally assessed rates of objective radiologic response (using the radiologic peritoneal cancer index and Response Evaluation Criteria in Solid Tumors [RECIST])12,19 and major pathologic response (using Mandard tumor regression grading [TRG] and the Peritoneal Regression Grading Score)20,21 of CPM to neoadjuvant treatment (eMethods in Supplement 2). Objective radiologic response of CPM was defined as a 30% or more decrease in radiologic peritoneal cancer index. Major pathologic response of CPM was defined as Mandard TRG1-2. Other comparative outcomes were intraoperative characteristics (eg, peritoneal cancer index), length of hospital stay, readmissions, reoperations, and Clavien-Dindo grade 2 postoperative morbidity. Other descriptive outcomes were the proportion of patients with Common Terminology Criteria for Adverse Events (CTCAE, version 4.022) grade 3 or higher and grade 2 systemic therapy–related toxic effects up to a month after last administration of the therapy, and the ability to administer trial treatment within predefined time frames. Patient-reported outcomes will be separately reported.
Statistical Analysis
The aim of CAIRO6 is to randomize 358 patients to detect a 15% increase in 3-year overall survival (experimental arm, 65%; control arm, 50%) with a 5% dropout rate and 80% power at P < .05. Given the absence of prospective data regarding the feasibility and safety of perioperative systemic therapy for resectable CPM, the investigators and the ethics committee agreed upon an a priori–determined sample size of 80 patients for the present phase 2 trial as a sufficient number to assess the phase 2 trial’s primary outcomes and their corresponding prespecified stopping criteria: less than 50% of patients in the experimental arm undergoing macroscopic complete CRS-HIPEC or a more than 20% higher proportion of Clavien-Dindo grade 3 or higher postoperative morbidity in the experimental arm relative to the control arm.11 In addition, the trial was stopped if these 80 patients were not enrolled within 1 year after the last center started recruiting.11 Because the stopping criteria of the present phase 2 trial were not met, the independent data monitoring committee advised proceeding to a phase 3 trial, also including patients of the present phase 2 trial. Analyses were performed 2-sided with findings considered significant at P < .05, using SAS, version 9.4 (SAS Institute Inc). Continuous data were compared using the Mann-Whitney test or an unpaired t test. Categorical data were compared using a χ2 test or Fisher exact test. Missing data were not imputed. Because the primary aim of the present phase 2 trial was to assess the feasibility and safety of the treatment received rather than treatment assignment, outcomes were analyzed in a modified intention-to-treat population of all patients starting neoadjuvant treatment (experimental arm) or undergoing upfront surgery (control arm). Intraoperative and postoperative characteristics were separately assessed in patients undergoing surgery (surgical population) and macroscopic complete CRS-HIPEC (CRS-HIPEC population).
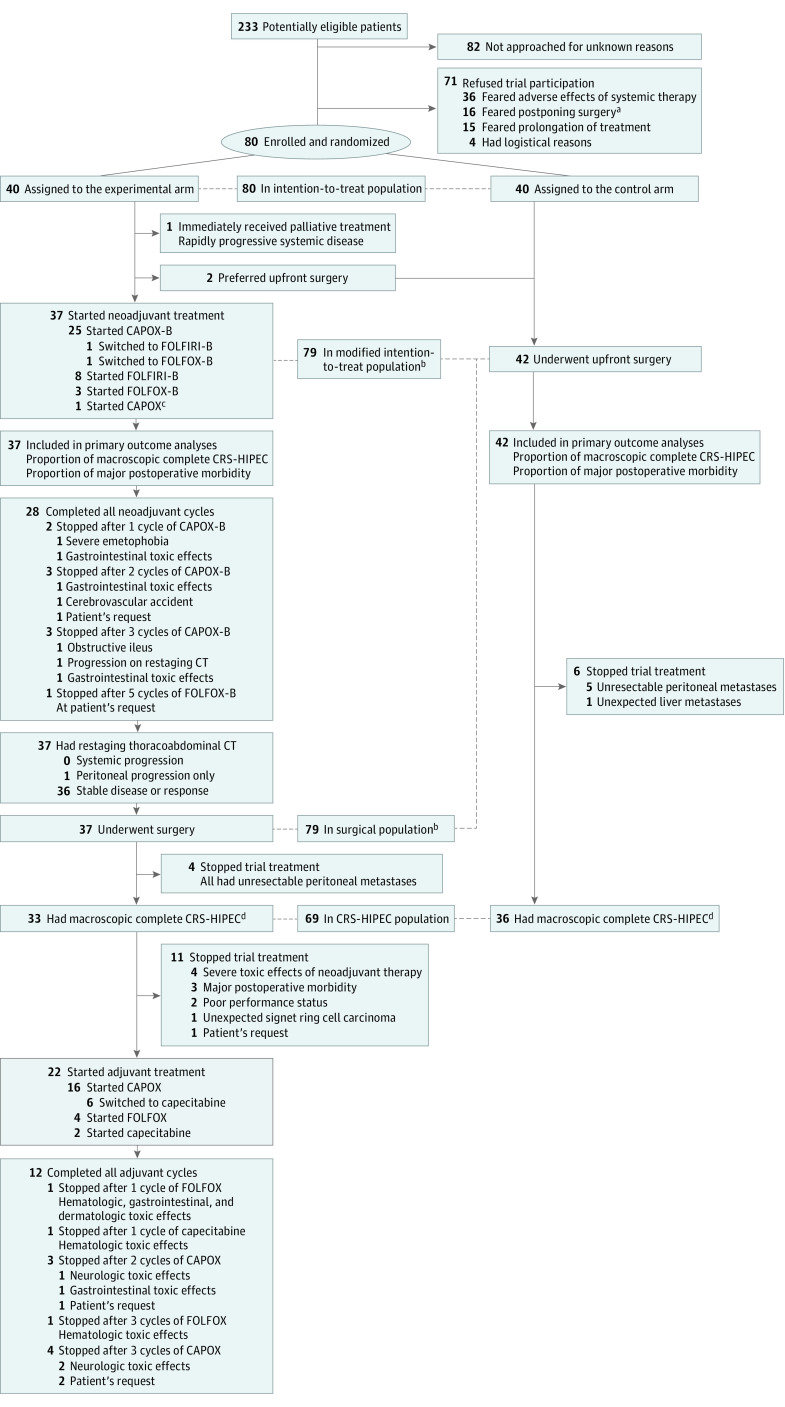
Results
Between June 15, 2017, and January 9, 2019, 233 patients were potentially eligible for trial participation; of these, 82 patients (35%) were not approached for unknown reasons, 71 (30%) refused trial participation for various reasons (Figure), and 80 (34%) were randomized (40 to each arm) in 9 centers within 1 year after the last center started recruiting in March 2018. The last patient completed trial treatment on October 1, 2019. The modified intention-to-treat population comprised 79 patients (43 [54%] men, 36 [46%] women; mean [SD] age, 62 [10] years), of whom 37 (47%) started neoadjuvant treatment and 42 (53%) underwent upfront surgery (Figure). Intention-to-treat and modified intention-to-treat populations had comparable distributions of baseline characteristics (eTable 1 in Supplement 2). Table 1 presents the baseline characteristics of the modified intention-to-treat population. Of 37 patients starting neoadjuvant treatment, 28 (76%) completed all neoadjuvant cycles, all 37 underwent surgery, 33 (89%) had macroscopic complete CRS-HIPEC, 22 (59%) started adjuvant treatment, and 12 (32%) completed all adjuvant cycles. Of 42 patients undergoing upfront surgery, 36 (86%) had macroscopic complete CRS-HIPEC. The surgical population consisted of the same 79 patients as the modified intention-to-treat population. The CRS-HIPEC population included 69 patients (baseline characteristics reported in eTable 2 in Supplement 2).
Figure. Trial Flow Diagram.
CAPOX indicates capecitabine and oxaliplatin; CAPOX-B, capecitabine, oxaliplatin, and bevacizumab; CRS-HIPEC, cytoreductive surgery and hyperthermic intraperitoneal chemotherapy; CT, computed tomography; FOLFIRI-B, fluorouracil, leucovorin, irinotecan, and bevacizumab; FOLFOX, fluorouracil, leucovorin, and oxaliplatin; FOLFOX-B, fluorouracil, leucovorin, oxaliplatin, and bevacizumab.
aPatients were concerned about disease progression during neoadjuvant treatment and subsequent inoperability.
bIncluded the same patients.
cDid not receive bevacizumab because of a wound dehiscence that developed after enrollment.
dMacroscopic complete cytoreductive surgery was defined as a completeness of cytoreduction score of 0 or an R-1 resection depending on the local classification used.
Table 1. Baseline Characteristics of the Modified Intention-to-Treat Population.
| Variable | Treatment arm, No. (%) | ||
|---|---|---|---|
| Experimental (n = 37) | Control (n = 42) | Total (n = 79) | |
| Sex | |||
| Male | 18 (49) | 25 (60) | 43 (54) |
| Female | 19 (51) | 17 (40) | 36 (46) |
| Age, mean (SD), y | 59 (11) | 64 (10) | 62 (10) |
| WHO Performance Statusa | |||
| 0 | 27 (73) | 35 (83) | 62 (78) |
| 1 | 9 (24) | 7 (17) | 16 (20) |
| 2 | 1 (3)b | 0 | 1 (1)b |
| Primary tumor location | |||
| Proximal colonc | 16 (43) | 15 (36) | 31 (39) |
| Distal colond | 19 (51) | 26 (62) | 45 (57) |
| Rectum | 1 (3) | 1 (2) | 2 (3) |
| Multiple | 1 (3) | 0 | 1 (1) |
| Histologic characteristics | |||
| Nonmucinous adenocarcinoma | 34 (92) | 39 (93) | 73 (92) |
| Mucinous adenocarcinoma | 3 (8) | 3 (7) | 6 (8) |
| Primary tumor status | |||
| Resected | 27 (73) | 25 (60) | 52 (66) |
| In situ | 10 (27) | 17 (40) | 27 (34) |
| T category of primary tumore | |||
| 0-3 | 16 (43) | 23 (55) | 39 (49) |
| 4 | 21 (57) | 19 (45) | 40 (51) |
| N category of primary tumore | |||
| 0 | 13 (35) | 16 (38) | 29 (37) |
| 1 | 16 (43) | 12 (29) | 28 (35) |
| 2 | 8 (22) | 14 (33) | 22 (28) |
| Previous systemic chemotherapy for colorectal cancer | |||
| No | 27 (73) | 32 (76) | 59 (75) |
| Adjuvant: a fluoropyrimidine with oxaliplatin | 9 (24) | 9 (21) | 18 (23) |
| Adjuvant: fluoropyrimidine monotherapy | 1 (3) | 1 (2) | 2 (3) |
| For metastatic disease | 0 | 0 | 0 |
| Previous resection of extraperitoneal colorectal metastases | |||
| No | 34 (92) | 42 (100) | 76 (96) |
| Yes | 3 (8) | 0 | 3 (4) |
| Onset of peritoneal metastases | |||
| Synchronous | 20 (54) | 24 (57) | 44 (56) |
| Metachronous | 17 (46) | 18 (43) | 35 (44) |
| Months from primary diagnosis to synchronous peritoneal metastases, median (range) | 1 (0-2) | 1 (0-2) | 1 (0-2) |
| Months from primary diagnosis to metachronous peritoneal metastases, median (range) | 14 (4-44) | 21 (7-48) | 19 (4-48) |
| Months from diagnosis of peritoneal metastases to trial enrollment, median (range) | 1 (0-4) | 1 (0-4) | 1 (0-4) |
| Baseline peritoneal cancer index,f median (range) | 3 (0-15)g | 5 (0-18)h | 5 (0-18) |
| Modality of determining baseline peritoneal cancer index | |||
| Laparoscopy | 22 (59)i | 36 (86) | 58 (73) |
| Laparotomy | 14 (38)i | 6 (14) | 20 (25) |
| Center of determining baseline peritoneal cancer index | |||
| Trial center | 18 (49)i | 25 (60) | 43 (54) |
| Referring center | 18 (49)i | 17 (40) | 35 (44) |
| Planned HIPEC regimen | |||
| Mitomycin C | 30 (81) | 34 (81) | 64 (81) |
| Oxaliplatin | 7 (19) | 8 (19) | 15 (19) |
Abbreviations: HIPEC, hyperthermic intraperitoneal chemotherapy; WHO, World Health Organization.
WHO Performance Status of 0 indicates able to carry out all normal activity without restriction; 1, restricted in strenuous activity but ambulatory and able to carry out light work; and 2, ambulatory and capable of all self-care but unable to carry out any work activities and are up and about for greater than 50% of waking hours.
Owing to severe obesity.
Cecum, ascending colon, hepatic flexure, and transverse colon.
Splenic flexure, descending colon, sigmoid, and rectosigmoid.
Pathologic category used for patients whose primary tumor was previously resected or patients in the control arm whose primary tumor was resected during upfront cytoreductive surgery; clinical category used for patients in the experimental arm whose primary tumor was still in situ or patients in the control arm whose primary tumor was not resected during upfront cytoreductive surgery.
The peritoneal cancer index assesses the extent of peritoneal disease in all 13 regions of the peritoneal cavity and ranges from 0 (no tumor visualized) to 39 (lesions larger than 5 cm or confluence of multiple small tumor nodules in all 13 regions).12
Two patients (5%) had a baseline peritoneal cancer index of 0.
Three patients (7%) had a baseline peritoneal cancer index of 0.
In 1 patient in the experimental arm, resectability was determined only by radiology.
The proportion of patients undergoing macroscopic complete CRS-HIPEC did not differ significantly between the trial arms (experimental: 33 of 37 patients [89%]; control: 36 of 42 patients [86%]; risk ratio, 1.04; 95% CI, 0.88-1.23; P = .74). Intraoperative and postoperative characteristics of the modified intention-to-treat (ie, surgical) population are presented in Table 2 and those of the CRS-HIPEC population are presented in eTable 3 in Supplement 2. In the modified intention-to-treat population, the proportion of patients with Clavien-Dindo grade 3 or higher postoperative morbidity did not differ significantly between the trial arms (experimental: 8 of 37 patients [22%]; control: 14 of 42 patients [33%]; risk ratio, 0.65; 95% CI, 0.31-1.37; P = .25). No surgery-related mortality occurred. Other intraoperative and postoperative characteristics did not differ significantly between the trial arms, except for the peritoneal cancer index (median [range], experimental: 5 [0-34] vs control: 12 [0-28]), ostomy formations (experimental: 7 of 37 patients [19%] vs control: 18 of 42 patients [43%]; P = .02), and length of hospital stay (median [interquartile range], experimental: 8 [6-16] days vs control: 11 [8-16] days) (Table 2). Details of Clavien-Dindo grade 2 postoperative morbidity are reported in eTable 4 in Supplement 2.
Table 2. Intraoperative and Postoperative Characteristics of the Modified Intention-to-Treat Population.
| Variable | Treatment arm, No. (%) | P value | ||
|---|---|---|---|---|
| Experimental (n = 37) | Control (n = 42) | Total (n = 79) | ||
| Intraoperative characteristics | ||||
| Cytoreductive surgery | ||||
| Macroscopic complete | 33 (89) | 36 (86) | 69 (87) | .74 |
| Not performed (open-close) | 4 (11)a | 6 (14)b | 10 (13) | |
| Peritoneal cancer index, median (range)c | 5 (0-34) | 12 (0-28) | 9 (0-34) | .01 |
| Primary tumor resection | ||||
| Yes | 8 (22) | 14 (33) | 22 (28) | .25 |
| No | 29 (78) | 28 (67) | 57 (72) | |
| Bowel anastomosis | ||||
| Yes | 17 (46) | 25 (60) | 42 (53) | .23 |
| No | 20 (54) | 17 (40) | 37 (47) | |
| Ostomy formation | ||||
| Yes | 7 (19) | 18 (43) | 25 (32) | .02 |
| No | 30 (81) | 24 (57) | 54 (68) | |
| Operating time, mean (SD), min | 317 (122) | 334 (155) | 326 (140) | .58 |
| HIPEC regimen | ||||
| Mitomycin C | 28 (85) | 29 (81) | 57 (83) | .64 |
| Oxaliplatin | 5 (15) | 7 (19) | 12 (17) | |
| No HIPEC performed | 4 | 6 | 10 | |
| Postoperative characteristics | ||||
| Initial length of hospital stay, median (IQR), d | 8 (6-16) | 11 (8-16) | 10 (7-16) | .04 |
| Readmission | ||||
| Yes | 8 (22) | 16 (38) | 24 (30) | .11 |
| No | 29 (78) | 26 (62) | 55 (70) | |
| Reoperation | ||||
| Yes | 7 (19) | 8 (19) | 15 (19) | .99 |
| No | 30 (81) | 34 (81) | 64 (81) | |
| Any Clavien-Dindo grade ≥2 postoperative morbidity | ||||
| Yes | 19 (51) | 30 (71) | 49 (62) | .07 |
| No | 18 (49) | 12 (29) | 30 (38) | |
| Any Clavien-Dindo grade ≥3 postoperative morbidity | ||||
| Yes | 8 (22) | 14 (33) | 22 (28) | .25 |
| No | 29 (78) | 28 (67) | 57 (72) | |
| Any Clavien-Dindo grade 4 postoperative morbidity | ||||
| Yes | 3 (8) | 2 (5) | 5 (6) | .54 |
| No | 34 (92) | 40 (95) | 74 (94) | |
| Details of Clavien-Dindo grade ≥3 postoperative morbidity d | ||||
| Adverse event | ||||
| Anastomotic leakage | ||||
| Grade 3 | 1 (3e)/(6f) | 3 (7e)/(12f) | 4 (5e)/(10f) | NAg |
| Grade 4 | 1 (3e)/(6f) | 0 | 1 (1e)/(2f) | |
| Intra-abdominal abscess | ||||
| Grade 3 | 0 | 2 (5) | 2 (3) | NAg |
| Grade 4 | 2 (5) | 1 (2) | 2 (3) | |
| Asystole, grade 4 | 0 | 1 (2) | 1 (1) | |
| Fascia dehiscence, grade 3 | 1 (3) | 2 (5) | 3 (4) | |
| Ileus, grade 3 | 2 (5) | 1 (2) | 3 (4) | |
| Gastroparesis, grade 3 | 1 (3) | 2 (5) | 3 (4) | |
| Pneumothorax, grade 3 | 0 | 1 (2) | 1 (1) | |
| Postoperative hemorrhage, grade 3 | 0 | 1 (2) | 1 (1) | |
| Colonic fistula, grade 3 | 0 | 1 (2) | 1 (1) | |
| Bowel perforation, grade 3 | 0 | 1 (2) | 1 (1) | |
| Hydronephrosis, grade 3 | 0 | 1 (2) | 1 (1) | |
| Luxation double J catheter, grade 3 | 1 (3) | 0 | 1 (1) | |
| Reoperations h | ||||
| Adverse event | ||||
| Anastomotic leakage | ||||
| Grade 3 | 1 (3e)/(6f) | 3 (7e)/(12f) | 4 (5e)/(10f) | NAg |
| Grade 4 | 1 (3e)/(6f) | 0 | 1 (1e)/(2f) | |
| Intra-abdominal abscess | ||||
| Grade 3 | 0 | 1 (2) | 1 (1) | NAg |
| Grade 4 | 2 (5) | 1 (1) | 3 (4) | |
| Fascia dehiscence, grade 3 | 1 (3) | 2 (5) | 3 (4) | |
| Ileus, grade 3 | 1 (3) | 0 | 1 (1) | |
| Postoperative hemorrhage, grade 3 | 0 | 1 (1) | 1 (1) | |
| Bowel perforation, grade 3 | 0 | 1 (1) | 1 (1) | |
| Tumor obstruction, grade 3 | 1 (3)i | 0 | 1 (1)i | |
Abbreviations: HIPEC, hyperthermic intraperitoneal chemotherapy; IQR, interquartile range; NA, not applicable.
All because of unresectable peritoneal metastases.
Five because of unresectable peritoneal metastases and 1 because of unexpected liver metastases.
The peritoneal cancer index assesses the extent of peritoneal disease in all 13 regions of the peritoneal cavity and ranges from 0 (no tumor visualized) to 39 (lesions larger than 5 cm or confluence of multiple small tumor nodules in all 13 regions).12
Because multiple Clavien-Dindo grade 3 or higher adverse events could have occurred in a single patient, numbers may not add up to the total number of patients with any Clavien-Dindo grade 3 or higher postoperative morbidity.
Percentage of all patients.
Percentage of patients with a bowel anastomosis.
Owing to low numbers, no comparison was made between both arms.
Because multiple reoperations could have been performed in a single patient, numbers may not add up to the total number of patients with a reoperation.
Patient had an early reoperation because of tumor obstruction due to progressive disease after an open-close procedure, which was not considered a postoperative complication.
Table 3 presents the systemic therapy–related toxic effects in the experimental arm. CTCAE grade 3 or higher systemic therapy–related toxic effects occurred in 13 of 37 patients (35%) without systemic therapy–related deaths. Details of CTCAE grade 2 systemic therapy–related toxic effects are reported in eTable 5 in Supplement 2. eTable 6 in Supplement 2 presents details of the central review of radiologic and pathologic response to neoadjuvant treatment. Using RECIST, 13 patients were evaluable, of whom 1 (8%) had a complete response, 1 (8%) had a partial response, and 11 (85%) had stable disease. Using the radiologic peritoneal cancer index, 32 patients were evaluable, of whom 1 (3%) had a complete response, 8 (25%) had a partial response, 23 (72%) had stable disease, and 2 (6%) had progressive disease. Thereby, the objective radiologic response rate of CPM to neoadjuvant treatment was 28% (9 of 32 evaluable patients). Table 4 shows the pathologic response. A total of 13 of 34 evaluable patients (38%) had a major pathologic response (TRG1-2) of CPM to neoadjuvant treatment and 8 (24%) had a complete pathologic response (TRG1).
Table 3. Systemic Therapy-Related Toxic Effects.
| Variable | Experimental (n = 37), No. (%) |
|---|---|
| Systemic therapy–related toxic effects | |
| CTCAE grade ≥2 | |
| Anya | 30 (81) |
| During neoadjuvant treatment | 22 (59) |
| During adjuvant treatment | 13 (59)b |
| CTCAE grade ≥3 | |
| Anya | 13 (35) |
| During neoadjuvant treatment | 12 (32) |
| During adjuvant treatment | 2 (9)b |
| CTCAE grade 4 | |
| Anya | 5 (14) |
| During neoadjuvant treatment | 3 (8) |
| During adjuvant treatment | 2 (9)b |
| Details of CTCAE grade ≥3 systemic therapy–related toxic effectsc | |
| Adverse event | |
| Ileus | |
| Grade 3 | 1 (3) |
| Grade 4 | 2 (5) |
| Diarrhea, grade 3 | 3 (8) |
| Nausea/vomiting, grade 3 | 2 (5) |
| Mucositis, grade 3 | 2 (5) |
| Infectious enterocolitis, grade 3 | 1 (3) |
| Abdominal infection (abscess), grade 3 | 1 (3) |
| Visceral arterial ischemia (due to intestinal stent), grade 3 | 1 (3) |
| Hypertension, grade 3 | 2 (5) |
| Catheter-related infection, grade 3 | 1 (3) |
| Peripheral sensory neuropathy, grade 3 | 1 (3) |
| Anemia, grade 3 | 1 (3) |
| Platelet count decreased, grade 3 | 1 (3) |
| Neutrophil count decreased | |
| Grade 3 | 2 (5) |
| Grade 4 | 1 (3) |
| γ-Glutamyl transferase level increased, grade 3 | 2 (5) |
| Hypokalemia, grade 3 | 1 (3) |
| Leukemia, grade 4 | 1 (3) |
Abbreviation: CTCAE, common terminology criteria for adverse events.
Either during neoadjuvant or adjuvant treatment. Because multiple toxic effects could have occurred in a single patient, the sum of toxic effects during neoadjuvant and adjuvant treatment may not add up to the Any category.
Twenty-two patients received adjuvant treatment.
Because multiple CTCAE grade 3 or higher adverse events could have occurred in a single patient, the numbers may not add up to the total number of patients with any CTCAE grade 3 or higher systemic therapy–related toxic effects.
Table 4. Centrally Assessed Pathologic Response to Neoadjuvant Treatment.
| Category | Classification system, No. (%)a | |
|---|---|---|
| Mandard TRG | PRGS | |
| Peritoneal metastases, evaluable, No.b | 34 | 34 |
| Grade 1 | 8 (24) | 8 (24) |
| Grade 2 | 5 (15) | 16 (47) |
| Grade 3 | 11 (32) | 5 (15) |
| Grade 4 | 5 (15) | 5 (15) |
| Grade 5 | 5 (15) | NA |
| Primary tumor, evaluable, No.b | 8 | 8 |
| Grade 1 | 1 (13) | 1 (13) |
| Grade 2 | 1 (13) | 5 (63) |
| Grade 3 | 4 (50) | 2 (25) |
| Grade 4 | 2 (25) | 0 (0) |
| Grade 5 | 0 | NA |
| Locoregional lymph nodes, evaluable, No.b | 8 | 8 |
| Grade 1 | 1 (13) | 1 (13) |
| Grade 2 | 0 | 4 (50) |
| Grade 3 | 4 (50) | 3 (38) |
| Grade 4 | 3 (38) | 0 |
| Grade 5 | 0 | NA |
| Overall, evaluable, No.b | 35 | 35 |
| Grade 1 | 9 (26) | 9 (26) |
| Grade 2 | 4 (11) | 16 (46) |
| Grade 3 | 12 (34) | 6 (17) |
| Grade 4 | 6 (17) | 4 (11) |
| Grade 5 | 4 (11) | NA |
Abbreviations: NA, not applicable; PRGS, peritoneal regression grading score; TRG, tumor regression grade.
Classification systems are explained in the eMethods in Supplement 2.
Reasons for nonevaluability are presented in eTable 6 in Supplement 2.
The eFigure in Supplement 2 shows violin plots of the ability to administer trial treatments within predetermined time frames. In the control arm, 39 of 42 patients (93%) underwent surgery within 6 weeks after randomization. In the experimental arm, 35 of 37 patients (95%) started neoadjuvant treatment within 4 weeks after randomization, 25 of 37 patients (68%) underwent surgery within 6 weeks after the last neoadjuvant cycle, and 21 of 22 patients (95%) started adjuvant treatment within 12 weeks after macroscopic complete CRS-HIPEC.
Discussion
In the present multicenter randomized phase 2 trial, perioperative systemic therapy seemed feasible and safe in patients diagnosed with resectable CPM. Neoadjuvant treatment induced objective radiologic (28%) and major pathologic (38%) responses of CPM.
Two previous single-arm phase 2 studies investigated perioperative systemic therapy for isolated CPM: one study23 evaluated six 3-week cycles of CAPOX before CRS-HIPEC in 14 patients and another study24 investigated perioperative doublet chemotherapy with cetuximab in 25 patients intentionally undergoing CRS-HIPEC for KRAS wild-type tumors. Both studies revealed lower rates of macroscopic complete CRS-HIPEC after neoadjuvant treatment (56%-57%) than the present trial (89%), probably because determination of resectability by laparoscopy or laparotomy was not required before enrollment in both previous studies. Other relevant literature consists of observational studies describing outcomes of neoadjuvant or perioperative systemic therapy for resectable CPM.6 However, because these studies included only patients undergoing CRS-HIPEC, nothing is known about the proportion and (probably poor) outcomes of patients not proceeding to CRS-HIPEC after neoadjuvant treatment because of, for example, toxic effects or progression. Hence, to our knowledge, the present trial provides the first prospective randomized comparison between perioperative systemic therapy and CRS-HIPEC alone for resectable CPM. The proportion of grade 3 or higher systemic therapy–related toxic effects in the present trial was comparable to that reported during 3 to 6 months of adjuvant systemic chemotherapy following resection of stage 3 colon cancer.25
Although accrual of the present trial was considered feasible, approximately half of the patients refused trial participation after being informed, mainly because they were concerned about toxic effects of and progression during neoadjuvant treatment leading to inoperability. However, although patients with CPM are generally considered to have relatively poor performance status and less response to systemic therapy,5 all trial participants who started neoadjuvant treatment underwent surgery. This finding suggests that fear of missing a window to operate due to progression or toxic effects may not be justified. Moreover, proportions of macroscopic complete CRS-HIPEC and major postoperative morbidity did not differ significantly between the arms. Together, these results may be used to inform clinicians and patients about the feasibility and safety of perioperative systemic therapy in this setting and justify a phase 3 trial. Patient-reported outcomes of the present trial,11 which will be separately reported, may increase insight into the burden of perioperative systemic therapy in this setting.
Few observational studies retrospectively assessed radiologic response26,27 and pathologic response27 of resected CPM to neoadjuvant treatment, but the investigators of these studies did not describe their methods of radiologic response assessment and used a slightly different pathologic classification, impeding comparison with the present trial. RECIST appears to be of limited use in this setting because radiologic response according to RECIST was nonevaluable in almost two-thirds of the patients in the present trial. Using the radiologic peritoneal cancer index may represent an alternative requiring validation in the phase 3 trial. Even though CPM had to be pathologically proven before enrollment, 24% of the patients in the present trial had no residual cancer cells in resected CPM after neoadjuvant treatment (TRG1). The proportion with TRG1-2 (38%) in the present trial is comparable to that in resected colorectal liver metastases after neoadjuvant doublet chemotherapy with bevacizumab (40%).28 Although this relevant proportion may suggest activity of systemic chemotherapy with bevacizumab in CPM, the investigators could not completely exclude false TRG1 owing to removal of all peritoneal disease before enrollment (eg, complete biopsy of a single peritoneal metastasis) or complete electrosurgical destruction of small peritoneal nodules during CRS that were tumor-positive but not sent for pathologic examination. This uncertainty highlights the importance of correlating these pathologic findings to survival in the phase 3 trial. The present trial revealed a lower intraoperative peritoneal cancer index in the experimental arm than in the control arm, which was also suggested by a recent retrospective US multi-institutional study.29 This finding may indicate activity of neoadjuvant treatment. Because the intraoperative peritoneal cancer index is one of the most important prognostic factors for survival in this setting,30 it will be interesting to see whether the observed difference in the intraoperative peritoneal cancer index translates into survival differences between the trial arms in the phase 3 trial.
After completion of the present phase 2 trial, a randomized clinical trial (PRODIGE7) revealed no overall survival benefit of complete CRS with oxaliplatin-based HIPEC compared with complete CRS alone in patients with extensively pretreated CPM.31 The investigators of the present trial are currently evaluating the results of PRODIGE7 to decide whether oxaliplatin-based HIPEC will be omitted in the phase 3 trial. Regardless of HIPEC, it remains important to assess the value of perioperative systemic therapy for resectable CPM.
Limitations
The present trial has several limitations. First, allowing 3 different perioperative systemic regimens may be considered a missed opportunity to standardize the perioperative regimen for resectable CPM. However, because eligible patients with metachronous CPM following primary resection could have had various adjuvant regimens, toxic effects of adjuvant treatment, and periods between adjuvant treatment and trial enrollment, the availability of 3 perioperative systemic regimens contributed to the accrual of these patients and the feasibility of the present trial, and may strengthen the trial’s applicability and external validity. Second, because the pragmatic sample size of 80 patients was agreed upon a priori owing to an absence of guiding data, the present trial may have not been adequately powered to detect potential statistically significant differences in the proportions of macroscopic complete CRS-HIPEC and major postoperative morbidity between the trial arms. Nevertheless, the experimental arm’s higher absolute proportion of macroscopic complete CRS-HIPEC and lower absolute proportion of major postoperative morbidity point toward the feasibility and safety of perioperative systemic therapy for resectable CPM and did not come close to the trial’s prespecified stopping criteria. Third, although resectability had to be confirmed by a peritoneal cancer index of 20 or lower during laparoscopy or laparotomy before enrollment, 5 of 42 patients scheduled for upfront surgery had unresectable CPM after explorative laparotomy, probably often due to an underestimation of the baseline peritoneal cancer index. This issue seems inevitable in pragmatic trials such as the present trial, as slightly higher open-close rates were reported by Dutch institutional series.32,33 Fourth, 35% of all potentially eligible patients were not approached for trial participation for unknown reasons. Efforts will be undertaken to decrease this number to minimize uncertainty about the phase 3 trial’s external validity. Fifth, after randomization, 2 patients assigned to the experimental arm preferred upfront surgery instead of starting neoadjuvant treatment. This issue highlights the importance of optimal informed consent to minimize pretreatment crossovers in the phase 3 trial. Sixth, not all trial treatments were given within predetermined time frames, and some patients did not meet all eligibility criteria: one patient was categorized as having a World Health Organization performance status of 2 and had determination of resectability by radiologic examination only, and central pathologic review revealed that another patient did not have pathologically proven CPM before enrollment. Because these issues could impair the homogeneity of the trial treatment and the trial population, these issues are points of consideration in the phase 3 trial.
Conclusions
In this randomized phase 2 trial in patients diagnosed with resectable CPM, perioperative systemic therapy seemed feasible, safe, and able to induce response of CPM. These results justify a phase 3 trial.
Trial Protocol.
eMethods. Detailed Methods
eTable 1. Comparison of Baseline Characteristics in the Intention-to-Treat and the Modified Intention-to-Treat Populations
eTable 2. Baseline Characteristics of the CRS-HIPEC Population
eTable 3. Intraoperative and Postoperative Characteristics of the CRS-HIPEC Population, Including Details of Clavien-Dindo grade ≥3 Postoperative Morbidity and Reoperations
eTable 4. Details of Clavien-Dindo Grade 2 Postoperative Morbidity in the Modified Intention-to-Treat (ie, Operated) and the CRS-HIPEC Population
eTable 5. Details of CTCAE Grade 2 Systemic Therapy–Related Toxicity in the Experimental Arm
eTable 6. Details of Central Review of Radiological and Pathological Response to Neoadjuvant Treatment
eFigure. Violin Plots of the Ability to Give Trial Treatments Within Predetermined Time Frames
Data Sharing Statement
Nonauthor Collaborators. The Dutch Peritoneal Oncology Group and the Dutch Colorectal Cancer Group
References
- 1.Evrard S. Autopsy of an expert consensus: end of hyperthermic intraperitoneal chemotherapy in colorectal carcinomatosis. Eur J Surg Oncol. 2018;44(12):1845-1846. doi: 10.1016/j.ejso.2018.07.061 [DOI] [PubMed] [Google Scholar]
- 2.Ceelen W. HIPEC with oxaliplatin for colorectal peritoneal metastasis: the end of the road? Eur J Surg Oncol. 2019;45(3):400-402. doi: 10.1016/j.ejso.2018.10.542 [DOI] [PubMed] [Google Scholar]
- 3.Klaver CEL, Groenen H, Morton DG, Laurberg S, Bemelman WA, Tanis PJ; research committee of the European Society of Coloproctology . Recommendations and consensus on the treatment of peritoneal metastases of colorectal origin: a systematic review of national and international guidelines. Colorectal Dis. 2017;19(3):224-236. doi: 10.1111/codi.13593 [DOI] [PubMed] [Google Scholar]
- 4.Eveno C, Passot G, Goéré D, et al. Bevacizumab doubles the early postoperative complication rate after cytoreductive surgery with hyperthermic intraperitoneal chemotherapy (HIPEC) for peritoneal carcinomatosis of colorectal origin. Ann Surg Oncol. 2014;21(6):1792-1800. doi: 10.1245/s10434-013-3442-3 [DOI] [PubMed] [Google Scholar]
- 5.Franko J, Shi Q, Meyers JP, et al. ; Analysis and Research in Cancers of the Digestive System (ARCAD) Group . Prognosis of patients with peritoneal metastatic colorectal cancer given systemic therapy: an analysis of individual patient data from prospective randomised trials from the Analysis and Research in Cancers of the Digestive System (ARCAD) database. Lancet Oncol. 2016;17(12):1709-1719. doi: 10.1016/S1470-2045(16)30500-9 [DOI] [PubMed] [Google Scholar]
- 6.Rovers KP, Simkens GA, Punt CJ, van Dieren S, Tanis PJ, de Hingh IH. Perioperative systemic therapy for resectable colorectal peritoneal metastases: sufficient evidence for its widespread use? a critical systematic review. Crit Rev Oncol Hematol. 2017;114:53-62. doi: 10.1016/j.critrevonc.2017.03.028 [DOI] [PubMed] [Google Scholar]
- 7.Chicago Consensus Working Group . The Chicago consensus on peritoneal surface malignancies: management of colorectal metastases. Ann Surg Oncol. 2020;27(6):1761-1767. doi: 10.1245/s10434-020-08315-x [DOI] [PubMed] [Google Scholar]
- 8.Bushati M, Rovers KP, Sommariva A, et al. The current practice of cytoreductive surgery and HIPEC for colorectal peritoneal metastases: results of a worldwide web-based survey of the Peritoneal Surface Oncology Group International (PSOGI). Eur J Surg Oncol. 2018;44(12):1942-1948. doi: 10.1016/j.ejso.2018.07.003 [DOI] [PubMed] [Google Scholar]
- 9.Rovers KP, Bakkers C, van Erning FN, et al. Adjuvant systemic chemotherapy vs active surveillance following up-front resection of isolated synchronous colorectal peritoneal metastases. JAMA Oncol. 2020;6(8):e202701. doi: 10.1001/jamaoncol.2020.2701 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dutch Colorectal Cancer Group . CAIRO 6. Accessed December 1, 2020. https://dccg.nl/trial/cairo-6
- 11.Rovers KP, Bakkers C, Simkens GAAM, et al. ; Dutch Peritoneal Oncology Group (DPOG); Dutch Colorectal Cancer Group (DCCG) . Perioperative systemic therapy and cytoreductive surgery with HIPEC versus upfront cytoreductive surgery with HIPEC alone for isolated resectable colorectal peritoneal metastases: protocol of a multicentre, open-label, parallel-group, phase II-III, randomised, superiority study (CAIRO6). BMC Cancer. 2019;19(1):390. doi: 10.1186/s12885-019-5545-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jacquet P, Sugarbaker PH. Clinical research methodologies in diagnosis and staging of patients with peritoneal carcinomatosis. Cancer Treat Res. 1996;82:359-374. doi: 10.1007/978-1-4613-1247-5_23 [DOI] [PubMed] [Google Scholar]
- 13.Hompes D, Ruers T. Review: incidence and clinical significance of bevacizumab-related non-surgical and surgical serious adverse events in metastatic colorectal cancer. Eur J Surg Oncol. 2011;37(9):737-746. doi: 10.1016/j.ejso.2011.06.004 [DOI] [PubMed] [Google Scholar]
- 14.Schmoll HJ, Van Cutsem E, Stein A, et al. ESMO Consensus guidelines for management of patients with colon and rectal cancer. a personalized approach to clinical decision making. Ann Oncol. 2012;23(10):2479-2516. doi: 10.1093/annonc/mds236 [DOI] [PubMed] [Google Scholar]
- 15.Kuijpers AMJ, Mirck B, Aalbers AGJ, et al. Cytoreduction and HIPEC in the Netherlands: nationwide long-term outcome following the Dutch protocol. Ann Surg Oncol. 2013;20(13):4224-4230. doi: 10.1245/s10434-013-3145-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Verwaal VJ, Bruin S, Boot H, van Slooten G, van Tinteren H. 8-Year follow-up of randomized trial: cytoreduction and hyperthermic intraperitoneal chemotherapy versus systemic chemotherapy in patients with peritoneal carcinomatosis of colorectal cancer. Ann Surg Oncol. 2008;15(9):2426-2432. doi: 10.1245/s10434-008-9966-2 [DOI] [PubMed] [Google Scholar]
- 17.Wisselink DD, Braakhuis LLF, Gallo G, et al. Systematic review of published literature on oxaliplatin and mitomycin C as chemotherapeutic agents for hyperthermic intraperitoneal chemotherapy in patients with peritoneal metastases from colorectal cancer. Crit Rev Oncol Hematol. 2019;142:119-129. doi: 10.1016/j.critrevonc.2019.06.014 [DOI] [PubMed] [Google Scholar]
- 18.Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. 2004;240(2):205-213. doi: 10.1097/01.sla.0000133083.54934.ae [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer. 2009;45(2):228-247. doi: 10.1016/j.ejca.2008.10.026 [DOI] [PubMed] [Google Scholar]
- 20.Mandard AM, Dalibard F, Mandard JC, et al. Pathologic assessment of tumor regression after preoperative chemoradiotherapy of esophageal carcinoma: clinicopathologic correlations. Cancer. 1994;73(11):2680-2686. doi: [DOI] [PubMed] [Google Scholar]
- 21.Solass W, Sempoux C, Detlefsen S, Carr NJ, Bibeau F. Peritoneal sampling and histological assessment of therapeutic response in peritoneal metastasis: proposal of the Peritoneal Regression Grading Score (PRGS). Pleura Peritoneum. 2016;1(2):99-107. doi: 10.1515/pp-2016-0011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Common Terminology Criteria for Adverse Events (CTCAE), version 4.0. Updated June 14, 2010. Accessed December 1, 2020. https://www.eortc.be/services/doc/ctc/CTCAE_4.03_2010-06-14_QuickReference_5x7.pdf
- 23.Leimkühler M, Hemmer PHJ, Reyners AKL, et al. Neoadjuvant chemotherapy followed by cytoreductive surgery and hyperthermic intraperitoneal chemotherapy for colorectal cancer: a feasibility and safety study. World J Surg Oncol. 2019;17(1):14. doi: 10.1186/s12957-018-1554-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Glockzin G, Zeman F, Croner RS, et al. Perioperative systemic chemotherapy, cytoreductive surgery, and hyperthermic intraperitoneal chemotherapy in patients with colorectal peritoneal metastasis: results of the prospective multicenter phase 2 COMBATAC trial. Clin Colorectal Cancer. 2018;17(4):285-296. doi: 10.1016/j.clcc.2018.07.011 [DOI] [PubMed] [Google Scholar]
- 25.Grothey A, Sobrero AF, Shields AF, et al. Duration of adjuvant chemotherapy for stage III colon cancer. N Engl J Med. 2018;378(13):1177-1188. doi: 10.1056/NEJMoa1713709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Passot G, Vaudoyer D, Cotte E, et al. Progression following neoadjuvant systemic chemotherapy may not be a contraindication to a curative approach for colorectal carcinomatosis. Ann Surg. 2012;256(1):125-129. doi: 10.1097/SLA.0b013e318255486a [DOI] [PubMed] [Google Scholar]
- 27.Passot G, You B, Boschetti G, et al. Pathological response to neoadjuvant chemotherapy: a new prognosis tool for the curative management of peritoneal colorectal carcinomatosis. Ann Surg Oncol. 2014;21(8):2608-2614. doi: 10.1245/s10434-014-3647-0 [DOI] [PubMed] [Google Scholar]
- 28.Klinger M, Tamandl D, Eipeldauer S, et al. Bevacizumab improves pathological response of colorectal cancer liver metastases treated with XELOX/FOLFOX. Ann Surg Oncol. 2010;17(8):2059-2065. doi: 10.1245/s10434-010-0972-9 [DOI] [PubMed] [Google Scholar]
- 29.Beal EW, Suarez-Kelly LP, Kimbrough CW, et al. Impact of neoadjuvant chemotherapy on the outcomes of cytoreductive surgery and hyperthermic intraperitoneal chemotherapy for colorectal peritoneal metastases: a multi-institutional retrospective review. J Clin Med. 2020;9(3):748. doi: 10.3390/jcm9030748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hallam S, Tyler R, Price M, Beggs A, Youssef H. Meta-analysis of prognostic factors for patients with colorectal peritoneal metastasis undergoing cytoreductive surgery and heated intraperitoneal chemotherapy. BJS Open. 2019;3(5):585-594. doi: 10.1002/bjs5.50179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Quénet F, Elias D, Roca L, et al. ; UNICANCER-GI Group and BIG Renape Group . Cytoreductive surgery plus hyperthermic intraperitoneal chemotherapy versus cytoreductive surgery alone for colorectal peritoneal metastases (PRODIGE 7): a multicentre, randomised, open-label, phase 3 trial. Lancet Oncol. 2021;22(2):256-266. doi: 10.1016/S1470-2045(20)30599-4 [DOI] [PubMed] [Google Scholar]
- 32.Hentzen JEKR, Constansia RDN, Been LB, et al. Diagnostic laparoscopy as a selection tool for patients with colorectal peritoneal metastases to prevent a non-therapeutic laparotomy during cytoreductive surgery. Ann Surg Oncol. 2020;27(4):1084-1093. doi: 10.1245/s10434-019-07957-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.van Oudheusden TR, Braam HJ, Luyer MDP, et al. Peritoneal cancer patients not suitable for cytoreductive surgery and HIPEC during explorative surgery: risk factors, treatment options, and prognosis. Ann Surg Oncol. 2015;22(4):1236-1242. doi: 10.1245/s10434-014-4148-x [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Trial Protocol.
eMethods. Detailed Methods
eTable 1. Comparison of Baseline Characteristics in the Intention-to-Treat and the Modified Intention-to-Treat Populations
eTable 2. Baseline Characteristics of the CRS-HIPEC Population
eTable 3. Intraoperative and Postoperative Characteristics of the CRS-HIPEC Population, Including Details of Clavien-Dindo grade ≥3 Postoperative Morbidity and Reoperations
eTable 4. Details of Clavien-Dindo Grade 2 Postoperative Morbidity in the Modified Intention-to-Treat (ie, Operated) and the CRS-HIPEC Population
eTable 5. Details of CTCAE Grade 2 Systemic Therapy–Related Toxicity in the Experimental Arm
eTable 6. Details of Central Review of Radiological and Pathological Response to Neoadjuvant Treatment
eFigure. Violin Plots of the Ability to Give Trial Treatments Within Predetermined Time Frames
Data Sharing Statement
Nonauthor Collaborators. The Dutch Peritoneal Oncology Group and the Dutch Colorectal Cancer Group