Key Points
Question
What dynamic variables are associated with malignant progression in pathologically proven intraductal papillary mucinous neoplasms (IPMNs) of the pancreas kept under surveillance?
Findings
In this international cohort study of 292 patients with branch-duct IPMNs, the development of additional worrisome features and high-risk stigmata during surveillance were independently associated with the diagnosis of high-grade dysplasia at final pathological examination. Among high-risk stigmata, only jaundice was associated with the diagnosis of invasive cancer.
Meaning
These findings suggest that the management of IPMNs under surveillance should be based on the evaluation of dynamic variables assessed through repeated observations.
Abstract
Importance
The progression of intraductal papillary mucinous neoplasms (IPMNs) of the pancreas to malignant disease is still poorly understood. Observational and surgical series have failed to provide comprehensive information.
Objective
To identify dynamic variables associated with the development of malignant neoplasms by combining pathological features with data from preoperative repeated observations.
Design, Setting, and Participants
The Crossover Observational Multicentric Study included a retrospective cohort of patients with branch-duct IPMNs (BD IPMNs) enrolled in a surveillance program from January 1, 2000, to December 31, 2019. Patients were enrolled from 5 referral centers: the Pancreas Institute, Verona, Italy; Seoul National University Hospital, Seoul, South Korea; Singapore General Hospital, Singapore; Johns Hopkins School of Medicine, Baltimore, Maryland; and University of Texas MD Anderson Cancer Center, Houston. Patients underwent a minimum of 12 months of preoperative surveillance (median, 37 [interquartile range (IQR), 20-68] months).
Main Outcomes and Measures
Dynamic variables associated with malignant disease were explored to estimate the presence of high-grade dysplasia (HGD) and invasive cancer at final pathological examination.
Results
A total of 292 patients were included in the analysis (137 women [46.9%] and 155 men [53.1%]; median age, 64 [IQR, 56-71] years). During surveillance, 27 patients (9.2%) developed a worrisome feature after 5 years, and 46 of 276 (16.7%) developed high-risk stigmata (HRS). At final pathological evaluation, 107 patients (36.6%) had HGD or invasive cancer, and 16 (5.5%) had IPMNs with concomitant pancreatic ductal adenocarcinoma. Rates of HGD and invasive cancer at pathological evaluation significantly differed between those without worrisome features and those developing HRS from a previous worrisome feature (9 [27.3%] vs 13 [61.9%]; P < .001). Developing an additional worrisome feature during surveillance (odds ratio [OR], 3.24 [95% CI, 1.38-7.60]; P = .007) or an HRS from a baseline worrisome feature (OR, 2.87 [95% CI, 1.01-8.17]; P = .048) was associated with HGD at final pathological evaluation. Among HRS, development of jaundice on a low-risk cyst was independently associated with invasive cancer (OR, 16.04 [95% CI, 2.94-87.40]; P = .001).
Conclusions and Relevance
These findings suggest that in BD IPMNs under surveillance, harboring a stable worrisome feature carries the lowest risk of malignant disease. Development of additional worrisome features or HRS is associated with the presence of HGD, whereas the occurrence of jaundice is associated with invasive cancer.
This multicenter cohort study identifies dynamic variables associated with development of malignant neoplasms by combining pathological features with data from preoperative repeated observations among patients with intraductal papillary mucinous neoplasms.
Introduction
Pancreatic cancer is one of the leading causes of cancer-related death, and its burden is expected to increase in the near future owing to longer life expectancy.1 The extensive use of cross-sectional imaging has not only identified a plethora of pancreatic cystic neoplasms,2 but it has also offered the opportunity to focus efforts on a selected population at risk for pancreatic cancer in whom active surveillance could play an important role in improving survival.3 In fact, intraductal papillary mucinous neoplasms (IPMNs) with high-grade dysplasia (HGD), along with pancreatic intraepithelial neoplasia 3 and mucinous cystic neoplasia with HGD, are the precursor lesions of pancreatic cancer,4,5 and as reported by the International Cancer of the Pancreas Screening Consortium,6 they represent the ideal surgical target of a surveillance program.7
The ultimate goal in the surveillance of IPMNs is to understand which neoplasms will eventually evolve into malignant neoplasms and to avoid the risks of unnecessary pancreatic surgery for patients who will never develop malignant neoplasms in their lifetime. During the last 20 years, much research has been devoted to addressing this conundrum. The results have been remarkable, but at present, a major limitation with current treatment guidelines is the surgical overtreatment of IPMNs.7,8 Many surgical series have been published, and the findings have been condensed into guidelines.9,10,11 However, without considering the actual denominator at all (ie, those patients who are steered toward surveillance), the risk of developing malignant disease has always been overestimated.4,12
Recently, to minimize selection bias, researchers have broadened their point of view by starting to consider large observational series to describe the natural history of IPMNs.13,14,15 In the absence of pathological confirmation and given the risk of misdiagnosis, these series were burdened by the opposite problem. Expanding the denominator to patients who may have an IPMN will certainly underestimate the risk of malignant neoplasms.
The most appropriate study design to balance the risk of malignant disease for patients with IPMNs would be to combine both aspects: consider a population with IPMNs under surveillance to avoid the selection bias of surgical series and focus on those patients crossing over from surveillance to surgery to obtain a pathological confirmation. This was the aim of the present crossover observational multicentric study.
Methods
Study Design
This study was approved by the institutional review boards of the participating centers, which waived the need for informed consent for the use of retrospective deidentified data, and followed the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) reporting guideline. The study was performed at 5 referral centers on 3 continents: the Pancreas Institute, University of Verona Hospital Trust, Verona, Italy; Department of Surgery, Seoul National University Hospital, Seoul, South Korea; Department of Hepato-Pancreato-Biliary and Transplant Surgery, Singapore General Hospital, Singapore; Department of Surgery, Johns Hopkins School of Medicine, Baltimore, Maryland; and Department of Surgical Oncology, University of Texas MD Anderson Cancer Center, Houston, Texas.
All patients undergoing surgical resection for a branch-duct IPMN (BD IPMN) from January 1, 2000, to December 31, 2019, after an initial surveillance of at least 12 months were considered for inclusion in the present analysis. We defined BD IPMN as the presence of a pancreatic cyst with a connection with the main pancreatic duct (MPD) through a secondary duct, without MPD dilatation detected using abdominal magnetic resonance imaging with and without contrast enhancement and with cholangiopancreatography or with abdominal computerized tomographic imaging with and without contrast enhancement. Indications for surgery were more aggressive in the first years of the study, because no guidelines existed. From 2006, International Association of Pancreatology Sendai criteria were generally applied, and from 2012, the Fukuoka guidelines with their subsequent revision in 2017 were used.9 Patients with an initial diagnosis of presumed BD IPMN who developed high-risk stigmata (HRS), namely, obstructive jaundice and enhancing mural nodules of at least 5 mm, and those with an MPD measuring at least 10 mm, were considered for resection if they met requirements for surgery. In the presence of other possible variables associated with malignant disease and classified as worrisome features, namely, acute pancreatitis, cyst of at least 30 mm, thickened/enhancing cyst wall, MPD measuring 5.0 to 9.9 mm, enhancing mural nodules smaller than 5 mm, abrupt change in MPD caliber with distal atrophy of the pancreatic gland, an increased level of carbohydrate antigen 19-9, and cyst growth of at least 5 mm in 2 years, patients underwent further assessment with endoscopic ultrasonography. These patients were referred for surgery in the presence of mural nodules or solid components of at least 5 mm, malignant cytologic findings, or signs of direct involvement of the MPD. Because most patients were evaluated before 2017, the HRS and worrisome features of most patients were not defined according to the updated definitions. Specifically, mural nodule size was not available, and carbohydrate antigen 19-9 levels were not available for several patients. The growth rate was computed from the available size measures. The general policy for follow-up consisted of abdominal magnetic resonance imaging with and without contrast enhancement and with cholangiopancreatography 6 months after the diagnosis and then every 12 months in the absence of clinical or radiological signs of progression.
Pathological evaluation was performed by specialized pancreatic pathologists and reported as suggested in the Baltimore and Verona consensus meetings.5,28 Lesions harboring high-grade dysplasia (HGD) or invasive cancer at final pathological examination were considered malignant. Surveillance time was measured from the first observation to surgery. Follow-up was measured from surgery to the last postoperative follow-up.
The aim of the study was to analyze a selected cohort of patients with a final pathological diagnosis of BD IPMN who underwent preoperative surveillance. This approach allowed us to weigh the development of the different HRS and worrisome features with the presence of HGD or invasive cancer at the final pathological examination. Furthermore, the analysis could describe several different evolutionary patterns of pathologically proven BD IPMNs during an initial period of surveillance. Branch-duct IPMNs that did not present with HRS or worrisome features at baseline were defined as low-risk cysts. We defined the term progression to mean the development of a new worrisome feature or HRS regardless of the baseline status, whereas stability meant that a cyst did not develop any new worrisome feature or HRS compared with the baseline status.
Statistical Analysis
Continuous variables were expressed as means with SD or as medians with interquartile range (IQR) when appropriate. Categorical variables were expressed as frequencies with percentages. A binary logistic regression model was used to estimate the effect size of the association between the considered risk factors and the development of the pathological outcomes. Variables were selected with the backward selection method using the Wald statistic. χ2 Tests with Yates correction in 2 × 2 contingency tables were used for categorical data, whereas the Mantel-Haenszel test was used for ordinal variables. The 2-tailed t test was used to compare means, whereas the Mann-Whitney U test was used to compare medians. All tests were 2 tailed. Statistical analysis was performed with SPSS for Windows, version 25 (IBM Corp). Two-sided P < .05 indicated statistical significance.
Results
Baseline Study Population Characteristics
The final study population consisted of 292 patients (137 women [46.9%] and 155 men [53.1%]). The median age at diagnosis was 64 (IQR, 56-71) years, with a median surveillance time of 37 (IQR, 20-68) months. Baseline population characteristics are given in Table 1. Baseline status included worrisome features at diagnosis (116 [39.7%]), HRS at diagnosis (16 [5.5%]), and absence of HRS and worrisome features at diagnosis (160 [54.8%]) (Figure).
Table 1. Study Population Characteristics.
| Characteristic | Patient data (N = 292)a |
|---|---|
| Sex | |
| Male | 155 (53.1) |
| Female | 137 (46.9) |
| Age at diagnosis, median (IQR), y | 64 (58-61) |
| Surveillance duration, median (IQR), mo | 37 (20-68) |
| Growth rate ≥5 mm/y | 97 (33.2) |
| Size, mm | |
| >30 | 138 (47.3) |
| >40 | 79 (27.1) |
| Pancreatitis | 68 (23.3) |
| Jaundice | 7 (2.4) |
| Enhancing/thick walls (>2 mm) | 42 (14.4) |
| MPD, mm | |
| 5-9 | 117 (40.1) |
| ≥10 | 45 (15.4) |
| Nonenhancing mural nodules | 51 (17.5) |
| Enhancing mural nodules | 13 (4.5) |
| WF at diagnosis | 116 (39.7) |
| HRS at diagnosis | 16 (5.5) |
| Types of resection | |
| Pancreaticoduodenectomy | 159 (54.5) |
| Distal pancreatectomy | 111 (38.0) |
| Total pancreatectomy | 22 (7.5) |
| 30-D postoperative mortality | 1 (0.3) |
| Grade of progressionb | |
| Dysplasia | |
| Low-grade | 185 (63.4) |
| High-grade | 63 (21.6) |
| Invasive cancer | 44 (15.1) |
Abbreviations: HRS, high-risk stigmata; IQR, interquartile range; MPD, main pancreatic duct; WF, worrisome feature.
Unless otherwise indicated, data are expressed as number (%) of patients.
The highest grade of dysplasia is reported. Patients presenting with an intraductal papillary mucinous neoplasm with high-grade dysplasia and an invasive component are reported in the invasive cancer group.
Figure. Classification of Patients According to Their Evolutive Patterns by the Occurrence of a Worrisome Feature (WF) or High-Risk Stigmata (HRS).
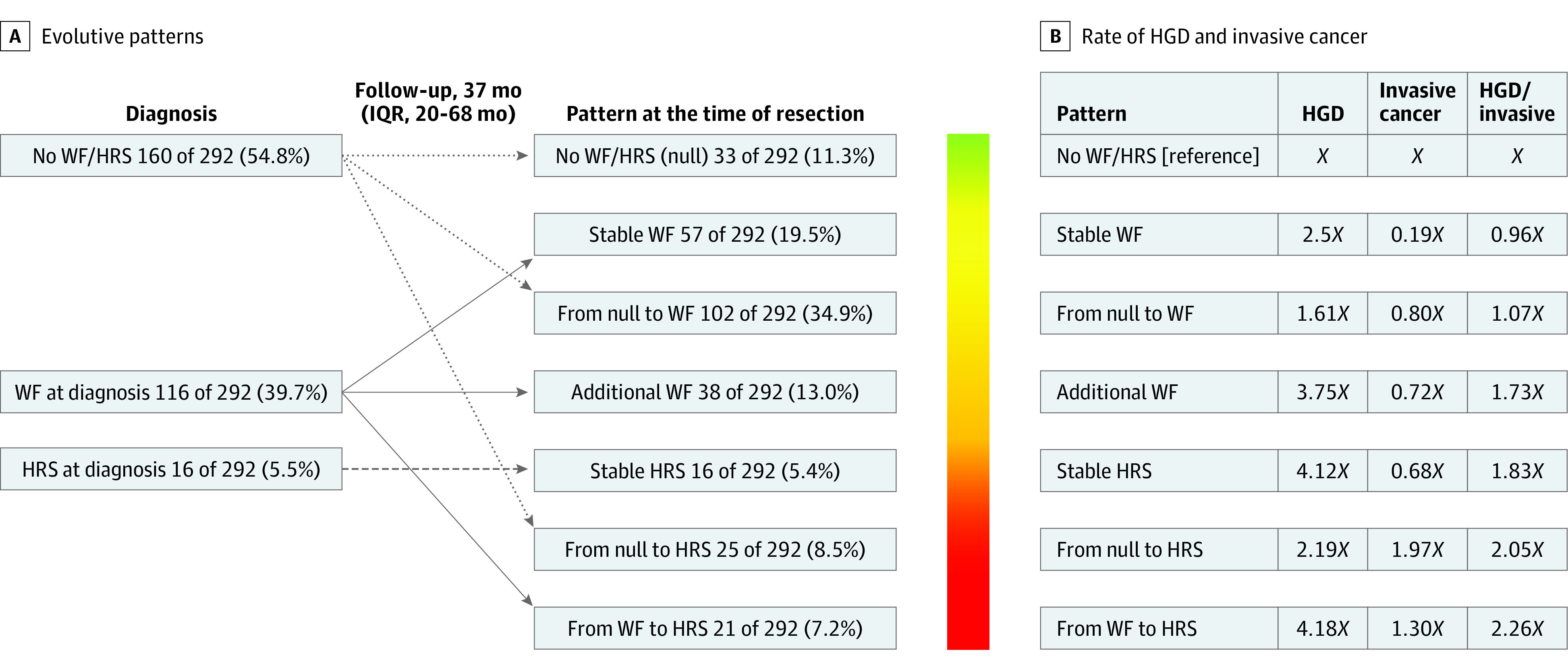
High-grade dysplasia (HGD) and invasive cancer (Inv) rates are reported as factors of the baseline category No WF/HRS (null) here represented by X. On the right, the combined rate of HGD/Inv is reported, showing a significant increase from category 1 to category 7 (P = .004, Mantel-Haenszel test). IQR indicates interquartile range.
Development of Worrisome Features and HRS
Of the 176 patients who did not have a worrisome feature or HRS at diagnosis, 102 (58.0%) developed a worrisome feature after a median surveillance of 33 (IQR, 15-62) months. Of note, 27 patients (9.2%) developed a worrisome feature after 5 years of surveillance. Of the 276 patients who did not have an HRS at diagnosis, 46 (16.7%) developed an HRS during surveillance after a median of 28 (IQR, 11-64) months from the baseline diagnosis. The Figure depicts the different patterns of progression from diagnosis to surgery from 3 different baseline clinical pictures to 7 different pathways of progression. The rate of HGD/invasive cancer increased significantly from 9 patients (27.3%) with low-risk IPMNs showing complete stability until surgery to 13 patients (61.9%) progressing from worrisome features to HRS during surveillance (P < .001). Progression from worrisome features at diagnosis to the development of an additional worrisome feature (odds ratio [OR], 3.24 [95% CI, 1.38-7.60]; P = .007) or to the development of an HRS during surveillance (OR, 2.87 [95% CI, 1.01-8.17]; P = .048) were the only 2 pathways associated with a significantly higher risk of harboring HGD at the final pathological diagnosis (Table 2). Only progression from a low-risk cyst to the development of an HRS was significantly associated with an increased risk of harboring invasive cancer (OR, 4.20 [95% CI, 1.61-10.93]; P = .003). In contrast, patients with a baseline worrisome feature showing cyst stability until surgical resection had a significantly lower risk of having invasive cancer (OR, 0.16 [95% CI, 0.03-0.87]; P = .03). Even considering only the 145 patients who had a minimum of 36 months of preoperative surveillance, the results did not significantly change. Table 3 shows the binary logistic regression exploring the role of each worrisome feature and HRS in estimating the development of HGD/invasive cancer. Among the different HRS, only the development of jaundice was found to be significantly associated with the development of invasive cancer, with an OR of 16.04 (95% CI, 2.94-87.40; P < .001). Considering the different worrisome features, no single worrisome feature was found to be significantly associated with the presence of HGD at final pathological examination (Table 3).
Table 2. Effect Size of Progression Patterns With Regard to Development of HGD or HGD/Invasive Cancer.
| Pattern | HGD | HGD/invasive cancer | ||
|---|---|---|---|---|
| OR (95% CI) | P value | OR (95% CI) | P value | |
| No WF/HRS (null) | 1 [Reference] | NA | 1 [Reference] | NA |
| Stable WF | 2.48 (0.64-9.57) | .19 | 0.95 (0.36-2.50) | .92 |
| New WF | 1.06 (0.44-2.54) | .90 | 1.14 (0.60-2.17) | .69 |
| Additional WF | 3.24 (1.38-7.60) | .007 | 2.36 (1.14-4.88) | .02 |
| Stable HRS | 2.79 (0.90-8.66) | .08 | 2.11 (0.76-5.90) | .15 |
| From null to HRS | 1.38 (0.45-4.24) | .58 | 2.32 (0.99-5.42) | .05 |
| From WF to HRS | 2.87 (1.01-8.18) | .048 | 2.57 (1.02-6.50) | .046 |
Abbreviations: HGD, high-grade dysplasia; HRS, high-risk stigmata; NA, not applicable; OR, odds ratio; WF, worrisome feature.
Table 3. Binary Logistic Regression Model of Single HRS or WF With Regard to Diagnosis of Invasive Cancer or HGD.
| Variable | OR (95% CI) | P value |
|---|---|---|
| Single HRS and invasive cancer | ||
| Jaundice | 16.04 (2.94-87.40) | .001 |
| MPD >10 mm | 1.43 (0.58-3.53) | .44 |
| Enhancing mural nodule | 2.39 (0.65-8.76) | .19 |
| Single WF and HGD | ||
| Growth rate ≥5 mm/y | 1.29 (0.65-2.56) | .48 |
| Size ≥30 mm | 1.11 (0.56-2.20) | .76 |
| Pancreatitis | 1.87 (0.93-3.76) | .08 |
| Thick walls | 0.73 (0.26-2.08) | .55 |
| MPD 5-9 mm | 1.73 (0.92-3.26) | .09 |
| Nonenhancing mural nodule | 1.43 (0.67-3.06) | .35 |
Abbreviations: HRS, high-risk stigmata; MPD, main pancreatic duct; OR, odds ratio; WF, worrisome feature.
Surgical and Pathological Data
Most patients underwent pancreaticoduodenectomy (159 [54.5%]) or distal pancreatectomy (111 [38.0%]). Postoperative pancreatic fistula was the most common postoperative complication (48 [16.4%]). Forty-one patients (14.0%) developed a severe complication (Clavien-Dindo grade ≥III). One patient (0.3%) died within 30 days of the operation (Table 1).
One hundred fifty-six patients (53.4%) were diagnosed with BD IPMN, 120 (41.1%) were diagnosed with mixed IPMN, and 16 (5.5%) were diagnosed with concomitant pancreatic ductal adenocarcinoma. The overall rate of malignant disease (HDG or invasive cancer) was 36.6% (107 patients). High-grade dysplasia was found in 63 patients (21.6%). Among patients with low-risk cysts at baseline that remained stable until surgical resection (33 [11.3%]), 6 were found to harbor an invasive lesion. Three of these patients (9.1%) had concomitant pancreatic ductal adenocarcinoma at the final pathological examination. With a median follow-up of 19 (IQR, 1-51) months, 47 patients (16.1%) developed recurrence after a median time of 17 (IQR, 10-54) months from surgery.
Risk Factors for HGD
Sixty-three patients (21.6%) were found to harbor HGD at the final pathological examination. These patients underwent resection after a median of 34 (IQR, 19-60) months from diagnosis compared with 38 (IQR, 22-72) months among patients who had a benign lesion at final pathological examination (P = .53). Comparing patients with HGD and those with benign lesions, age at diagnosis was similar (65 [IQR, 57-74] vs 63 [IQR, 58-70] years; P = 24). Moreover, there were no differences in terms of baseline median cyst diameter of 25 (IQR, 15-35) mm and 20 (IQR, 13-28) mm, respectively (P = .12), and regarding median growth rate of 1.6 (IQR, 0-4.3) mm/y and 1.3 (IQR, 0-3.2) mm/y, respectively (P = .68). In a univariate analysis, the presence of enhancing mucinous neoplasms (4 of 185 [2.1%] vs 5 of 63 [7.9%]; P = .046), worrisome features at diagnosis (70 of 185 [37.8%] vs 34 of 63 [54.0%]; P = .03), the development of additional worrisome features during surveillance (20 of 185 [10.8%] vs 13 of 63 [20.6%]; P = .047), the progression from worrisome features to HRS during surveillance (8 of 185 [4.3%] vs 8 of 63 [12.7%]; P = .02), or the development of a new worrisome feature from a baseline low-risk cyst (72 of 185 [38.9%] vs 15 of 63 [23.8%]; P = .03) were significantly associated with a higher rate of HGD compared with low-grade dysplasia at final pathological examination (Table 4). After a collinearity assessment, only the development of an additional worrisome feature during surveillance, the progression from worrisome features to HRS during surveillance, or the development of a new worrisome feature were evaluated through a binary logistic regression model. The development of additional worrisome features during surveillance in patients with worrisome features at diagnosis (OR, 2.43 [95% CI, 1.12-5.28]; P = .03) and the progression from worrisome features to HRS during surveillance (OR, 3.74 [95% CI, 1.33-10.55]; P = .01) were found to be independently associated with the presence of HGD at final pathological examination (Table 4).
Table 4. Univariate and Multivariate Analysis of Variables for Association With HGD.
| Variables | Patient groupa | P value | OR (95% CI)b | P value | |
|---|---|---|---|---|---|
| LGD (n = 185) | HGD (n = 63) | ||||
| Surveillance time, median (IQR), mo | 38 (20-70) | 34 (17-59) | .09 | NA | NA |
| Age at diagnosis, median (IQR), y | 63 (56-72) | 66 (58-74) | .23 | NA | NA |
| Diameter, median (IQR), mm | |||||
| Initial | 20 (13-28) | 25 (15-35) | .10 | NA | NA |
| Final | 30 (20-37) | 32 (21-45) | .09 | NA | NA |
| Growth rate, mm/y | |||||
| Median (IQR) | 1.32 (0-3.2) | 1.62 (0-4.3) | .62 | NA | NA |
| ≥5 | 58 (31.4) | 25 (39.7) | .28 | NA | NA |
| Size ≥30 mm | 89 (48.1) | 33 (52.4) | .47 | NA | NA |
| Pancreatitis | 39 (21.1) | 21 (33.3) | .06 | NA | NA |
| Jaundice | 1 (0.5) | 0 | NA | NA | NA |
| Thick walls | 28 (15.1) | 7 (11.1) | .53 | NA | NA |
| MPD, mm | |||||
| 5-9 | 67 (36.2) | 30 (47.6) | .10 | NA | NA |
| ≥10 | 23 (12.4) | 14 (22.2) | .06 | NA | NA |
| Nonenhancing mural nodule | 31 (16.8) | 13 (20.6) | .33 | NA | NA |
| Enhancing mural nodule | 4 (2.2) | 5 (7.9) | .046 | NA | NA |
| WF at diagnosis | 70 (37.8) | 34 (54.0) | .03 | NA | NA |
| WF developed during surveillance | |||||
| Additional | 20 (10.8) | 13 (20.6) | .047 | 2.43 (1.12-5.28) | .03 |
| New | 72 (38.9) | 15 (23.8) | .03 | 0.66 (0.32-1.33) | .24 |
| HRS at diagnosis | 9 (4.8) | 6 (9.5) | >.99 | ||
| From WF to HRS during surveillance | 8 (4.3) | 8 (12.7) | .02 | 3.74 (1.33-10.55) | .01 |
Abbreviations: HGD, high-grade dysplasia; HRS, high-risk stigmata; IQR, interquartile range; LGD, low-grade dysplasia; MPD, main pancreatic duct; NA, not applicable; OR, odds ratio; WF, worrisome feature.
Unless otherwise indicated, data are expressed as number (percentage) of patients.
After a collinearity assessment, only 3 variables were evaluated through a binary logistic regression model.
Discussion
This multicentric retrospective study focused on a selected population of patients with BD IPMNs of the pancreas who underwent initial surveillance after first observation and then surgery because they were judged to be at risk of incipient or ongoing malignant progression. Seven different pathways of progression from the baseline status have been identified and analyzed to assess the risk of malignant progression. Harboring a worrisome feature that remains stable from baseline was associated with the lowest risk of progression toward HGD. On the other hand, the development of an additional worrisome feature or HRS during surveillance was associated with a higher rate of HGD at final pathological evaluation. The development of obstructive jaundice was the only variable associated with the presence of invasive cancer.
Only patients with a minimum of 12 months of preoperative surveillance were included in this study. To evaluate the possible effect of this threshold, we also tested a different threshold of 36 months. The results did not significantly change considering only these 145 patients. The scientific literature of the last 2 decades has been characterized by numerous surgical series reporting a 10% mean rate of HGD (range, 0-26.3%) and a 13% mean rate of invasive cancer (range, 0-36.7%) in resected BD IPMNs.4 These findings have led to the definition of several guidelines9,10,11 with high sensitivity but low specificity in detecting malignant disease and subsequent surgical overtreatment for IPMNs.16 In fact, the surgical series did not include the entire population under surveillance in the denominator, which represents most patients affected by a BD IPMN.17,18,19 To overcome this major concern, several observational series from centers with a large caseload of pancreatic cystic disease have been published in the last few years.13,14,15,20,21 The point of view changed dramatically by considering patients from their initial evaluation and regardless of results of final pathological evaluation. This allowed us to present a more accurate picture of presumed BD IPMNs, recalibrating the rates of malignant disease to lower values and resizing the role of several factors known to be associated with development of malignant disease, such as cyst size, cyst growth rate, and main pancreatic duct dilatation.22,23,24,25 This new approach, however, raised several new concerns, such as the possibility of underestimating the risk of malignant progression, considering the substantial rate of misdiagnosis,26,27 and the absence of pathological confirmation for patients under surveillance.
The present study represents a junction point between the 2 different approaches, mitigating their biases. As expected for a highly selected population, the rate of malignant disease reported on final pathological evaluation (36.3%) was higher when compared with that of previous studies.4 Cyst stability from a baseline worrisome feature and cyst progression with a new worrisome feature from a baseline low-risk cyst were identified as the 2 pathways with the lowest risk. Cyst progression with an additional worrisome feature or with a new HRS starting from a baseline worrisome feature were the scenarios with higher risk of malignant progression. Of note, once an additional worrisome feature is found in addition to a previous one and eventually confirmed with endoscopic ultrasonography, surgery is indicated according to the present data. Interestingly, when considering each single factor, only the presence of obstructive jaundice was related to an increased incidence of an invasive component. This means that association of a single static feature cannot be calculated for incipient malignant progression except for obstructive jaundice, which is indeed a sign of overt cancer. However, it should be noted that a considerable number of patients (6 of 33 [18.2%]) presented with concomitant pancreatic ductal adenocarcinoma or with an invasive IPMN, even starting from a baseline low-risk cyst and remaining stable until surgery. Patients with low-risk disease were considered to require surgical resection even if their cysts did not develop any codified suspicious features during surveillance. This finding may be due to 2 factors: (1) the absence of specific terms to describe the subtle changes that those patients’ cysts underwent and (2) the retrospective nature of the study, which could have led to the loss of information regarding the development of worrisome features and HRS.
It is difficult to define the role of surgery for IPMNs, because it cannot be limited exclusively to the concept of cancer prevention. This aim would be easily pursued by having all patients with IPMNs undergo surgery. However, the cost in terms of morbidity and mortality would not be justified considering the rate of postoperative severe complications was 14.0% (Clavien-Dindo grade ≥III). At the same time, waiting to observe signs of malignant progression, such as mural nodules or obstructive jaundice, may negatively affect patient survival. The goal of surgery for IPMNs should be to identify those lesions that have the potential to progress to invasive cancer and to resect them before an invasive component develops. This goal will be reached as our understanding of the biology of IPMNs advances. However, although no definitive biomarkers are available, the use of the known worrisome features and HRS helps the clinician select patients who are at a higher risk of harboring HGD. The results reported in the present analysis confirm the need for an improved selection of patients for resection based on new biological markers. In fact, of the 185 patients (63.4%) who were selected using the available radiological and clinical variables, none harbored a cyst with either HGD or invasive cancer at final pathological examination. Subsequently, we found that dynamic variables outperformed the static presence of worrisome features and HRS at the time of surgery for association with the presence of HGD at final pathological examination. Therefore, the risk estimation performed through repeated observation seems to increase the accuracy in determining which patients need to undergo resection without increasing the rate of invasive cancers at final pathological examination. Finally, it seems reasonable to curb the term worrisome for features that do not add a substantial risk of harboring HGD or invasive cancer.
Limitations
This study has several limitations. First, we only included patients who were undergoing resection, and we excluded most patients who were affected by a presumed BD IPMN and are therefore kept under surveillance. This choice, based on the need to have a pathological diagnosis for all patients, would impair the implementation of our findings in the real world. Furthermore, it requires additional larger observational studies to validate our results. Second, although all patients came from recognized high-volume centers, the retrospective nature of the study precluded the collection of all parameters classified as worrisome features in the updated version of the International Association of Pancreatology guidelines. In particular, mural nodule size and carbohydrate antigen 19-9 level were not available for most patients in this series.
Conclusions
This cohort study of an extremely selected population of patients affected by IPMNs who crossed over from surveillance to surgery owing to an estimated increased risk of malignant disease suggests a role of well-known static risk factors when considered in a dynamic process. Patients harboring a stable worrisome feature carried the lowest risk of HGD, whereas developing an additional worrisome feature or an HRS from an already present worrisome feature carried the highest risk. When considering each risk factor as a static entity, obstructive jaundice was the only feature associated with an increased rate of invasive cancer at final pathological examination. The dynamic interpretation of risk factors over time seems to be the most effective way to select patients for surgery after surveillance before they develop an invasive component.
References
- 1.Rahib L, Smith BD, Aizenberg R, Rosenzweig AB, Fleshman JM, Matrisian LM. Projecting cancer incidence and deaths to 2030: the unexpected burden of thyroid, liver, and pancreas cancers in the United States. Cancer Res. 2014;74(11):2913-2921. doi: 10.1158/0008-5472.CAN-14-0155 [DOI] [PubMed] [Google Scholar]
- 2.Moris M, Bridges MD, Pooley RA, et al. Association between advances in high-resolution cross-section imaging technologies and increase in prevalence of pancreatic cysts from 2005 to 2014. Clin Gastroenterol Hepatol. 2016;14(4):585-593.e3. doi: 10.1016/j.cgh.2015.08.038 [DOI] [PubMed] [Google Scholar]
- 3.Konings ICAW, Canto MI, Almario JA, et al. ; International Cancer of the Pancreas Screening (CAPS) Consortium . Surveillance for pancreatic cancer in high-risk individuals. BJS Open. 2019;3(5):656-665. doi: 10.1002/bjs5.50180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tanaka M. Intraductal papillary mucinous neoplasm of the pancreas as the main focus for early detection of pancreatic adenocarcinoma. Pancreas. 2018;47(5):544-550. doi: 10.1097/MPA.0000000000001047 [DOI] [PubMed] [Google Scholar]
- 5.Basturk O, Hong SM, Wood LD, et al. ; Baltimore Consensus Meeting . A revised classification system and recommendations from the Baltimore consensus meeting for neoplastic precursor lesions in the pancreas. Am J Surg Pathol. 2015;39(12):1730-1741. doi: 10.1097/PAS.0000000000000533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Goggins M, Overbeek KA, Brand R, et al. ; International Cancer of the Pancreas Screening (CAPS) consortium . Management of patients with increased risk for familial pancreatic cancer: updated recommendations from the International Cancer of the Pancreas Screening (CAPS) Consortium. Gut. 2020;69(1):7-17. doi: 10.1136/gutjnl-2019-319352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Khoury RE, Kabir C, Maker VK, Banulescu M, Wasserman M, Maker AV. What is the incidence of malignancy in resected intraductal papillary mucinous neoplasms? an analysis of over 100 US institutions in a single year. Ann Surg Oncol. 2018;25(6):1746-1751. doi: 10.1245/s10434-018-6425-6 [DOI] [PubMed] [Google Scholar]
- 8.Walsh RM, Perlmutter BC, Adsay V, et al. Advances in the management of pancreatic cystic neoplasms. Published online August 1, 2020. Curr Probl Surg. doi: 10.1016/j.cpsurg.2020.100879 [DOI] [PubMed] [Google Scholar]
- 9.Tanaka M, Fernández-Del Castillo C, Kamisawa T, et al. Revisions of international consensus Fukuoka guidelines for the management of IPMN of the pancreas. Pancreatology. 2017;17(5):738-753. doi: 10.1016/j.pan.2017.07.007 [DOI] [PubMed] [Google Scholar]
- 10.Del Chiaro M, Besselink MG, Scholten L, et al. ; European Study Group on Cystic Tumours of the Pancreas . European evidence-based guidelines on pancreatic cystic neoplasms. Gut. 2018;67(5):789-804. doi: 10.1136/gutjnl-2018-316027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vege SS, Ziring B, Jain R, Moayyedi P; Clinical Guidelines Committee; American Gastroenterology Association . American Gastroenterological Association Institute guideline on the diagnosis and management of asymptomatic neoplastic pancreatic cysts. Gastroenterology. 2015;148(4):819-822. doi: 10.1053/j.gastro.2015.01.015 [DOI] [PubMed] [Google Scholar]
- 12.Lensing RJ, Bipat S. Incidences of pancreatic malignancy and mortality in patients with untreated branch-duct intraductal papillary mucinous neoplasms undergoing surveillance: a systematic review. Pancreas. 2017;46(9):1098-1110. doi: 10.1097/MPA.0000000000000907 [DOI] [PubMed] [Google Scholar]
- 13.Han Y, Lee H, Kang JS, et al. Progression of pancreatic branch duct intraductal papillary mucinous neoplasm associates with cyst size. Gastroenterology. 2018;154(3):576-584. doi: 10.1053/j.gastro.2017.10.013 [DOI] [PubMed] [Google Scholar]
- 14.Pergolini I, Sahora K, Ferrone CR, et al. Long-term risk of pancreatic malignancy in patients with branch duct intraductal papillary mucinous neoplasm in a referral center. Gastroenterology. 2017;153(5):1284-1294.e1. doi: 10.1053/j.gastro.2017.07.019 [DOI] [PubMed] [Google Scholar]
- 15.Lawrence SA, Attiyeh MA, Seier K, et al. Should patients with cystic lesions of the pancreas undergo long-term radiographic surveillance? results of 3024 patients evaluated at a single institution. Ann Surg. 2017;266(3):536-544. doi: 10.1097/SLA.0000000000002371 [DOI] [PubMed] [Google Scholar]
- 17.Sharib JM, Fonseca AL, Swords DS, et al. Surgical overtreatment of pancreatic intraductal papillary mucinous neoplasms: do the 2017 International Consensus Guidelines improve clinical decision making? Surgery. 2018;164(6):1178-1184. doi: 10.1016/j.surg.2018.07.014 [DOI] [PubMed] [Google Scholar]
- 18.Del Chiaro M, Beckman R, Ateeb Z, et al. Main duct dilatation is the best predictor of high-grade dysplasia or invasion in intraductal papillary mucinous neoplasms of the pancreas. Ann Surg. 2020;272(6):1118-1124. doi: 10.1097/SLA.0000000000003174 [DOI] [PubMed] [Google Scholar]
- 19.Sadakari Y, Ienaga J, Kobayashi K, et al. Cyst size indicates malignant transformation in branch duct intraductal papillary mucinous neoplasm of the pancreas without mural nodules. Pancreas. 2010;39(2):232-236. doi: 10.1097/MPA.0b013e3181bab60e [DOI] [PubMed] [Google Scholar]
- 20.Izumo W, Higuchi R, Furukawa T, et al. Importance of each high-risk stigmata and worrisome features as a predictor of high-grade dysplasia in intraductal papillary mucinous neoplasms of the pancreas. Pancreatology. 2020;20(5):895-901. doi: 10.1016/j.pan.2020.06.011 [DOI] [PubMed] [Google Scholar]
- 21.Oyama H, Tada M, Takagi K, et al. Long-term risk of malignancy in branch-duct intraductal papillary mucinous neoplasms. Gastroenterology. 2020;158(1):226-237.e5. doi: 10.1053/j.gastro.2019.08.032 [DOI] [PubMed] [Google Scholar]
- 22.Del Chiaro M, Ateeb Z, Hansson MR, et al. Survival analysis and risk for progression of intraductal papillary mucinous neoplasia of the pancreas (IPMN) under surveillance: a single-institution experience. Ann Surg Oncol. 2017;24(4):1120-1126. doi: 10.1245/s10434-016-5661-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Marchegiani G, Andrianello S, Pollini T, et al. “Trivial” cysts redefine the risk of cancer in presumed branch-duct intraductal papillary mucinous neoplasms of the pancreas: a potential target for follow-up discontinuation? Am J Gastroenterol. 2019;114(10):1678-1684. doi: 10.14309/ajg.0000000000000378 [DOI] [PubMed] [Google Scholar]
- 24.Marchegiani G, Andrianello S, Morbin G, et al. Importance of main pancreatic duct dilatation in IPMN undergoing surveillance. Br J Surg. 2018;105(13):1825-1834. doi: 10.1002/bjs.10948 [DOI] [PubMed] [Google Scholar]
- 25.Marchegiani G, Andrianello S, Perri G, et al. Vanishing pancreatic cysts during follow-up: another step towards de-emphasizing cyst size as a major clinical predictor of malignancy. Dig Surg. 2018;35(6):508-513. doi: 10.1159/000485199 [DOI] [PubMed] [Google Scholar]
- 26.Akahoshi K, Ono H, Akasu M, et al. Rapid growth speed of cysts can predict malignant intraductal mucinous papillary neoplasms. J Surg Res. 2018;231:195-200. doi: 10.1016/j.jss.2018.05.056 [DOI] [PubMed] [Google Scholar]
- 27.Correa-Gallego C, Ferrone CR, Thayer SP, Wargo JA, Warshaw AL, Fernández-Del Castillo C. Incidental pancreatic cysts: do we really know what we are watching? Pancreatology. 2010;10(2-3):144-150. doi: 10.1159/000243733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Salvia R, Malleo G, Marchegiani G, et al. Pancreatic resections for cystic neoplasms: from the surgeon’s presumption to the pathologist’s reality. Surgery. 2012;152(3)(suppl 1):S135-S142. doi: 10.1016/j.surg.2012.05.019 [DOI] [PubMed] [Google Scholar]
- 29.Adsay V, Mino-Kenudson M, Furukawa T, et al. ; Members of Verona Consensus Meeting, 2013 . Pathologic evaluation and reporting of intraductal papillary mucinous neoplasms of the pancreas and other tumoral intraepithelial neoplasms of pancreatobiliary tract: recommendations of Verona Consensus Meeting. Ann Surg. 2016;263(1):162-177. doi: 10.1097/SLA.0000000000001173 [DOI] [PMC free article] [PubMed] [Google Scholar]


