Abstract
An I(III)-catalyzed oxidative cyclization-migration tandem reaction using Selectfluor as the oxidant was developed that converts unactivated anilines into 3H-indoles is reported herein. The reaction requires as little as 1 mol % of the iodocatalyst and is mild, tolerating pyridine- and thiophene functional groups, and the dependence of the diastereoselectivity of the process on the identity of the iodoarene- or iodoalkane pre-catalyst suggests that the catalyst is present for the stereochemical determining C–N bond forming step.
Graphical Abstract
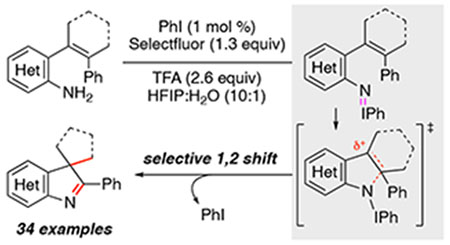
The ubiquitous nature of C–N bonds in non-planar N-heterocycles and materials continues to spur the development of efficient processes that generate electrophilic nitrogen catalytic intermediates to trigger C–N bond formation.1,2 While this intermediate can be accessed by oxidation, most methods require the presence of a strong electron-withdrawing N-substituent for a successful reaction outcome.3 In contrast, catalytic reactions that construct C–NAr bonds employ an aryl azide or nitroarene as the N-atom source require heat or superstoichiometric quantities of reductant to access the electrophilic N-aryl nitrogen intermediate.4,5 Oxidative methods that generate the electrophilic N-aryl intermediate are less common,6 and require an N-electron-withdrawing substituent on the aniline. We discovered that electrophilic N-aryl nitrenoids could be generated from I(III)-mediated oxidation of unactivated 2-substituted anilines and trapped to afford benzazepinones or 3H-indoles.7 At the conclusion of our study, we were curious if catalysis could be achieved by pairing iodobenzene with a superstoichiometric oxidant. Oxidative catalytic processes using an iodine catalyst have emerged recently as a powerful way to create new bonds through the dearomatization of electron-rich arenes.8,9 While phenols and aromatic ethers are common substrates, the development of I(III)-catalyzed oxidative methods of anilines remains nascent and requires a strong electron-withdrawing group on nitrogen for C–N bond formation.6 In 2011, Antonchick and co-workers reported that biaryl acetimides could be transformed into N-acetyl carbazoles using a di-iodobiaryl catalyst and acetic peroxide as the oxidant.6a Herein, we report that unactivated anilines can be converted into 3H-indoles, important bioactive scaffolds,10 through an I(III)-catalyzed oxidative cascade reaction using Selectfluor as the terminal oxidant.
To investigate if catalysis of an oxidative cyclization-migration reaction could be achieved, the reactivity of 2-aminostyrene 11a towards a substoichiometric amount of iodobenzene and a terminal oxidant was examined (Table 1).8h This substrate is readily available from the cross-coupling of 2-aminophenylboronic acid and the vinyl triflate of derived from 2-phenylcyclohexanone. While we had only modest success using the combination of 20 mol % of PhI with mCPBA as the oxidant,11 using Selectfluor as the oxidant resulted in 42% of 3H-indole 12a (entry 1).12,13 We found that a quantitative yield of 12a could be obtained if water was added as a co-solvent (entries 2 and 3); increasing the amount of water over 50%, however led to lower yields.14 Our investigations also determined that trifluoracetic acid additive could be replaced with trimethylsilyltrifluoroacetate (entry 4).15 To our delight, we found that the catalyst loading of PhI could be decreased to as little as 1 mol % without adversely affecting the yield (entries 5 and 6). The effect of changing the identity of the catalyst was also surveyed (entries 7 – 12). Biaryl 7 demonstrated that increasing the steric environment of the catalyst did not attenuate the yield of the oxidative cyclization process (entry 7). In contrast, changing the electronic nature of the ArI catalyst had a detrimental effect on the reaction outcome (entries 8 – 10): only 30% of 12a was formed using 4-nitroiodobenzene as the catalyst. The yield rebounded using either 4-CO2Me- or 4-MeO iodoarene but was lower than using iodobenzene. While alkyl iodine(III) species are established to decompose to I2 or I-OH upon exposure to an oxidant,16 we found 1-iodobutane afforded a quantitative yield of 12a (entry 11), and no reaction was observed when I2 was examined as a catalyst (entry 12).
Table 1.
Optimization of the I(III)-catalyzed oxidative cyclization-migration reaction.
 | ||||
|---|---|---|---|---|
| entry | RI | mol % | solvent | yield, %a |
| 1 | PhI | 20 | HFIP | 42 |
| 2 | PhI | 20 | HFIP/H2O (100:1) | 77 |
| 3 | PhI | 20 | HFIP/H2O (10:1) | 100 |
| 4c | PhI | 20 | HFIP/H2O (10:1) | 90 |
| 5 | PhI | 5 | HFIP/H2O (10:1) | 99 |
| 6 | PhI | 1 | HFIP/H2O (10:1) | 99b |
| 7 | 7 | 20 | HFIP/H2O (10:1) | 91 |
| 8 | 13 | 20 | HFIP/H2O (10:1) | 30 |
| 9 | 14 | 20 | HFIP/H2O (10:1) | 73 |
| 10 | 15 | 20 | HFIP/H2O (10:1) | 70 |
| 11 | n-BuI | 20 | HFIP/H2O (10:1) | 100 |
| 12 | I2 | 20 | HFIP/H2O (10:1) | n.r. |
As determined using 1H NMR spectroscopy using CH2Br2 as the internal standard.
98% isolated yield after silica gel chromatography.
Me3SiO2CCF3 used instead of TFA.
Using the combination of 1 mol % of PhI and Selectfluor as the stoichiometric oxidant, we surveyed the effect of changing the electronic- and steric nature of aniline 11 (Table 2). Irrespective of whether the para-R1-substituent was an electron-releasing- or electron-withdrawing group, nearly a quantitative yield of 3H-indole 12 was observed (entries 1 – 6). In contrast, the identity of the meta-R2-substituent impacted the efficiency of the oxidative cyclization-migration reaction requiring 5 mol % of PhI for conversion to 3H-indole. While a modest yield of 12g (R2 = OMe) was obtained (entry 7), changing its identity to a methyl-, halide- or even trifluoromethyl group resulted in a significantly greater yield of the 3H-indole with the latter requiring only 1 mol % of PhI (entries 8 – 11). In contrast to our earlier reports using aryl azides or nitrostyrenes,4b,5h the steric environment around the N-atom precursor can be increased with an additional ortho-substituent: high yields of 3H-indole was observed from anilines bearing an R3-methoxy-, methyl- or fluoro-group using only 1 mol % of PhI (entries 12 – 14).
Table 2.
Effect of changing the electronic nature of the aniline.
 | |||||
|---|---|---|---|---|---|
| entry | # | R1 | R2 | R3 | yield, %a |
| 1 | a | H | H | H | 98 (94)b |
| 2 | b | OMe | H | H | 99 |
| 3 | c | Me | H | H | 99 |
| 4 | d | Cl | H | H | 85 |
| 5 | e | F | H | H | 97 |
| 6 | f | OCF3 | H | H | 91 |
| 7 | g | H | OMe | H | 50c |
| 8 | h | H | Me | H | 88c |
| 9 | i | H | Cl | H | 82c |
| 10 | j | H | F | H | 76c |
| 11 | k | H | CF3 | H | 91 |
| 12 | l | H | H | OMe | 86 |
| 13 | m | H | H | Me | 92 |
| 14 | n | H | H | F | 90 |
Isolated after silica gel chromatography.
Reaction performed at 1.0 mmol scale with 20 mol % of PhI.
Reaction performed with 5 mol % of PhI.
The reaction scope was further explored using N-heteroaromatic substrates and by varying the identity of the ortho-alkenyl substituent using 20 mol % of PhI because lower catalyst loadings led to incomplete conversion unless otherwise noted (Scheme 2). We found that 2-, 3- or 4-aminopyridines were smoothly transformed into 3H-indoles 17a – 17c. While thiophenes were incompatible our stoichiometric reaction,7 they were tolerated using the catalytic conditions. Surprisingly, neither ring-contraction nor phenyl migration was observed from these electron-rich heteroarenes; instead cyclization only occurred to give 18d and 18e as the sole products.17 Next, the effect of modifying the structure of the ortho-alkenyl substituent was investigated. While no diastereoselectivity was observed in 17f with a homoallylic tert-butyl substituent using 1 mol % of PhI, substrates containing an ortho-heterocycle group could be transformed into spirocycles 17g and 17h although higher catalyst loading was required; the latter showing that this reaction can access a structural motif prevalent in biologically active molecules and alkaloids.2c Aniline 16i revealed that substrates bearing α-aryl substituents were effectively converted to 17i using only 1 mol % of PhI. Our survey showed that the identity of the β-substituent controlled the reaction outcome. While ring-contraction was observed with β-methyl substituent to afford 17j, when either a β-sulfone or β-carboxylate group was present, only [1,2] shift of the electron-poor group was observed to afford 18k and 18l. To further probe this phenomenon, the electronic nature of the β-aryl substituent of the ortho-cyclohexenyl substituent was varied in 16m and 16n: while the electron deficient 16m required higher catalyst loading, only ring-contraction was observed. Increasing the size of the ortho-cycloalkenyl substituent in 16o or acyclic 16p, however, triggered a [1,2] phenyl shift to afford only 18o or 18p.
Scheme 2.

Effect of heteroarenes and ortho-substituent identity on the reaction outcome.
The reversal in the migration aptitude that was observed for anilines 11a and 16o spurred us to investigate this phenomenon further with o-cycloheptenyl-substituted substrates (Scheme 2).
Submission of 16q to reaction conditions produced a 31:69 mixture of ring-contraction and aryl migration products. While reducing the electronic nature of the β-aryl group did not change the reaction outcome, only [1,2] aryl migration occurred with the electron-rich 16s.
In an attempt to gain more insight into the potential stereoselectivity of the process, the reactivity of allylic-substituted 16t was investigated (Scheme 3). Exposure of 16t to 20 mol % of PhI and 1.3 equiv of Selectfluor produced 17t as a 79:21 mixture of diastereomers. We were curious if changing the identity of the catalyst impacted the diastereoselectivity of the process. If the stereoselectivity of the process was affected, that would suggest the catalyst was present for the stereodetermining step.8e,18 Contrary to our expectations, increasing the steric nature of the aryl iodide by adding ortho-methyl- or ortho-isopropyl groups reduced the diastereoselectivity. This trend continued when the bulkier diodobiarene 7 was assayed to afford a nearly 1:1 mixture of diastereomers. The electronic nature of the iodoarene also affected the reaction outcome: a modest improvement in the diastereoselectivity was observed using 4-MeO-iodoarene; whereas, employing the electron-deficient 4-CO2Me-iodoarene resulted in a reduced stereoselectivity and yield of 3H-indole 17t. Iodoalkanes were also examined, and the diastereoselectivity inversely depended on the size and length of the alkyl chain.11 While the best diastereoselectivity was obtained using MeI, the yield of the transformation was significantly reduced.19 To determine if the lowered yield was a result of the acidic reaction conditions, trimethylsilyl trifluoroacetate was examined as the additive, and the yield of 17t was significantly improved without attenuation of the diastereoselectivity. Reducing the temperature of the reaction to 0 °C further improved stereoselectivity to 88:12 using MeI and Me3SiO2CCF3. Together these results suggest that the catalyst is present for the stereochemical defining C–N bond forming step (TS-19), and the inverse correlation of the steric nature with the selectivity implies that a shorted N–I bond is critical for achieving a selective reaction.
Scheme 3.

Investigation of the relationship between diastereoselectivity and the identity of the iodine catalyst.
We discovered an oxidative catalytic process for generating electrophilic N-aryl nitrenoids from unactivated indoles to produce non-planar N-heterocycles using the combination of an iodoarene- or iodoalkane catalyst and Selectfluor as the oxidant. The compatibility of pyridine- and thiofuran functionalities in this oxidative cyclization-migration reaction underscores the mildness of these conditions. The dependence of the diastereoselectivity with the identity of the catalyst indicates that it is present for the stereochemical defining C–N bond forming step. Our future studies will build on these results to probe the details of both the C–N and C–C bond forming steps as well as develop I(III)-catalyzed stereoselective oxidative reactions.
Supplementary Material
Scheme 1.

Development of I(III)-catalyzed C–N bond forming reactions.
ACKNOWLEDGMENT
We are grateful to the National Science Foundation CHE-1564959 and the National Institutes of Health (NIGMS) GM138388 for their generous financial support. We thank Mr. Furong Sun (UIUC) for high resolution mass spectrometry data.
Funding Sources
National Science Foundation, CHE-1564959
National Institutes of Health, NIGMS, GM138388
Footnotes
Dedicated to the memory of Professor Kilian Muñiz
ASSOCIATED CONTENT
Supporting Information
Experimental details and spectral data (PDF)
REFERENCES
- (1).(a) Dauban P; Sanière L; Tarrade A; Dodd RH Copper-catalyzed nitrogen transfer mediated by iodosylbenzene PhI=O. J. Am. Chem. Soc 2001, 123, 7707–7708. [DOI] [PubMed] [Google Scholar]; (b) Liang J-L; Yuan S-X; Huang JS; Yu W-Y; Che C-M Highly diastereo- and enantioselective intramolecular amidation of saturated C–H bonds catalyzed by ruthenium porphyrins. Angew. Chem. Int. Ed 2002, 41, 3465–3468. [DOI] [PubMed] [Google Scholar]; (c) Wehn PM; Du BJ, Enantioselective Synthesis of the Bromopyrrole Alkaloids Manzacidin A and C by Stereospecific C–H Bond Oxidation. J. Am. Chem. Soc 2002, 124, 12950–12951. [DOI] [PubMed] [Google Scholar]; (d) Espino CG; Fiori KW; Kim M; Du Bois J Expanding the scope of C–H amination through catalyst design. J. Am. Chem. Soc 2004, 126, 15378–15379. [DOI] [PubMed] [Google Scholar]; (e) Omura K; Uchida T; Irie R; Katsuki T Design of a robust Ru(salen) complex: aziridination with improved turnover number using N-arylsulfonyl azides as precursors. Chem. Common 2004, 2060–2061. [DOI] [PubMed] [Google Scholar]; (f) Lebel H. n.; Huard K; Lectard S N-To-syloxycarbamates as a source of metal nitrenes: rhodium-catalyzed C-H insertion and aziridination reactions. J. Am. Chem. Soc 2005, 127, 14198–14199. [DOI] [PubMed] [Google Scholar]; (g) Liang C; Robert-Peillard F; Fruit C; Müller P; Dodd RH; Dauban P Efficient diastereoselective intermolecular rhodium-catalyzed C–H amination. Angew. Chem, Int. Ed 2006, 45, 4641–4644. [DOI] [PubMed] [Google Scholar]; (h) Reddy RP; Davies HML Dirhodium Tetracarboxylates Derived from Adamantylglycine as Chiral Catalysts for Enantioselective C–H Aminations. Org. Lett 2006, 8, 5013–5016. [DOI] [PubMed] [Google Scholar]; (i) Milczek E; Boudet N; Blakey S Enantioselective C–H amination using cationic ruthenium(II)-pybox catalysts. Angew. Chem. Int. Ed 2008, 47, 6825–6828. [DOI] [PubMed] [Google Scholar]; (j) Thornton AR; Blakey SB Catalytic metallonitrene/alkyne metathesis: a powerful cascade process for the synthesis of nitrogen-containing molecules. J. Am. Chem. Soc 2008, 130, 5020–5021. [DOI] [PubMed] [Google Scholar]; (k) Stoll AH; Blakey SB Rhodium Catalyzed Allene Amination: Diastereoselective Synthesis of Aminocyclopropanes via a 2-Amidoallylcation Intermediate. J. Am. Chem. Soc 2010, 132, 2108–2109. [DOI] [PubMed] [Google Scholar]; (l) Hennessy ET; Betley TA Complex N-Heterocycle Synthesis via Iron-Catalyzed, Direct C–H Bond Amination. Science 2013, 340, 591–595. [DOI] [PubMed] [Google Scholar]; (m) Dolan NS; Scamp RJ; Yang T; Berry JF; Schomaker JM Catalyst-Controlled and Tunable, Chemoselective Silver-Catalyzed Intermolecular Nitrene Transfer: Experimental and Computational Studies. J. Am. Chem. Soc 2016,138, 14658–14667. [DOI] [PMC free article] [PubMed] [Google Scholar]; (n) Jiang H; Lang K; Lu H; Wojtas L; Zhang XP Intramolecular Radical Aziridination of Allylic Sulfamoyl Azides by Cobalt(II)-Based Metalloradical Catalysis: Effective Construction of Strained Heterobicyclic Structures. Angew. Chem. Int. Ed 2016,55, 11604–11608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (2).(a) Kochanowska-Karamyan AJ; Hamann MT Marine Indole Alkaloids: Potential New Drug Leads for the Control of Depression and Anxiety. Chem. Rev 2010, 110, 4489–4497. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Schmidt AW; Reddy KR; Knölker H-J Occurrence, Biogenesis, and Synthesis of Biologically Active Carbazole Alkaloids. Chem. Rev 2012, 112, 3193–3328. [DOI] [PubMed] [Google Scholar]; (c) James MJ; O’Brien P; Taylor RJK; Unsworth WP Synthesis of Spirocyclic Indolenines. Chem. Eur. J 2016, 22, 2856–2881. [DOI] [PubMed] [Google Scholar]
- (3).(a) Davies HML; Du Bois J; Yu J-Q C-H Functionalization in organic synthesis. Chem. Soc. Rev 2011, 40, 1855–1856. [DOI] [PubMed] [Google Scholar]; (b) Roizen JL; Harvey ME; Du Bois J Metal-Catalyzed Nitrogen-Atom Transfer Methods for the Oxidation of Aliphatic C–H Bonds. Acc. Chem. Res 2012, 45, 911–922. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Scamp RJ; Rigoli JW; Schomaker JM Chemoselective silver-catalyzed nitrene insertion reactions. Pure Appl. Chem. 2014, 86, 381. [Google Scholar]
- (4).For leading references on ArN3, see:; (a) Cenini S; Gallo E; Caselli A; Ragaini F; Fantauzzi S; Piangiolino C Coordination chemistry of organic azides and amination reactions catalyzed by transition metal complexes. Coord. Chem. Rev 2006, 250, 1234–1253. [Google Scholar]; (b) Shen M; Leslie BE; Driver TG Dirhodium(II)-Catalyzed Intramolecular C–H Amination of Aryl Azides. Angew. Chem. Int Ed 2008, 47, 5056–5059. [DOI] [PubMed] [Google Scholar]; (c) Driver TG Recent Advances in Transition Metal-Catalyzed N-Atom Transfer Reactions of Azides. Org. Biomol. Chem 2010, 8, 3831–3846. [DOI] [PMC free article] [PubMed] [Google Scholar]; (d) Jenkins DM Atom-Economical C2 + N1 Aziridination: Progress towards Catalytic Intermolecular Reactions Using Alkenes and Aryl Azides. Synlett 2012,23, 1267–1270. [Google Scholar]; (e) Ryu J; Shin K; Park SH; Kim JY; Chang S Rhodium-Catalyzed Direct C–H Amination of Benzamides with Aryl Azides: A Synthetic Route to Diarylamines. Angew. Chem., Int. Ed 2012, 51, 9904–9908. [DOI] [PubMed] [Google Scholar]; (f) Alt IT; Guttroff C; Plietker B Iron-Catalyzed Intramolecular Aminations of C(sp3)–H Bonds in Alkylaryl Azides. Angew. Chem. Int. Ed 2017, 56, 10582–10586. [DOI] [PubMed] [Google Scholar]
- (5).For leading references on ArNO2, see:; (a) Bartoli G; Palmieri G; Bosco M; Dalpozzo R The reaction of vinyl Grignard reagents with 2-substituted nitroarenes: A new approach to the synthesis of 7-substituted indoles. Tetrahedron Lett 1989, 30, 2129–2132. [Google Scholar]; (b) Cenini S; Ragaini F; Tollari S; Paone D Allylic Amination of Cyclohexene Catalyzed by Ruthenium Complexes. A New Reaction Involving an Intermolecular C–H Functionalization. J. Am. Chem. Soc 1996,118, 11964–11965. [Google Scholar]; (c) Akazome M; Kondo T; Watanabe Y Palladium complex-catalyzed reductive N-heterocyclization of nitroarenes: novel synthesis of indole and 2H-indazole derivatives. J. Org. Chem 1994, 59, 3375–3380. [Google Scholar]; (d) Söderberg BC; Shriver JA Palladium-Catalyzed Synthesis of Indoles by Reductive N-Heteroannulation of 2-Nitrostyrenes. J. Org. Chem 1997, 62, 5838–5845. [Google Scholar]; (e) S. Srivastava R; M. Nicholas K Iron-catalyzed allylic amination by nitroorganics. Chem. Commun 1998, 2705–2706. [Google Scholar]; (f) Gao H; Xu Q-L; Yousufuddin M; Ess DH; Kürti L Rapid Synthesis of Fused N-Heterocycles by Transition-Metal-Free Electrophilic Amination of Arene C–H Bonds. Angew. Chem. Int. Ed 2014, 53, 2701–2705. [DOI] [PubMed] [Google Scholar]; (g) Gui J; Pan C-M; Jin Y; Qin T; Lo JC; Lee BJ; Spergel SH; Mertzman ME; Pitts WJ; La Cruz TE; Schmidt MA; Darvatkar N; Natarajan SR; Baran PS Practical olefin hydroamination with nitroarenes. Science 2015, 348, 886–891. [DOI] [PubMed] [Google Scholar]; (h) Jana N; Zhou F; Driver TG Promoting Reductive Tandem Reactions of Nitrostyrenes with Mo(CO)6 and a Palladium Catalyst To Produce 3H-Indoles. J. Am. Chem. Soc 2015, 137, 6738–6741. [DOI] [PubMed] [Google Scholar]; (i) Tong S; Xu Z; Mamboury M; Wang Q; Zhu J Aqueous Titanium Trichloride Promoted Reductive Cyclization of o-Nitrostyrenes to Indoles: Development and Application to the Synthesis of Rizatriptan and Aspidospermidine. Angew. Chem. Int Ed 2015, 54, 11809–11812. [DOI] [PubMed] [Google Scholar]; (j) Zhou F; Wang D-S; Guan X; Driver TG Nitroarenes as the Nitrogen Source in Intermolecular Palladium-Catalyzed Aryl C–H Bond Aminocarbonylation Reactions. Angew. Chem. Int. Ed 2017, 56, 4530–4534. [DOI] [PubMed] [Google Scholar]
- (6).(a) Antonchick AP; Samanta R; Kulikov K; Lategahn J Organocatalytic, Oxidative, Intramolecular C–H Bond Amination and Metal-free Cross-Amination of Unactivated Arenes at Ambient Temperature. Angew. Chem. Int Ed 2011, 50, 8605–8608. [DOI] [PubMed] [Google Scholar]; (b) Cho SH; Yoon J; Chang S Intramolecular Oxidative C–N Bond Formation for the Synthesis of Carbazoles: Comparison of Reactivity between the Copper-Catalyzed and Metal-Free Conditions. J. Am. Chem. Soc 2011, 133, 5996–6005. [DOI] [PubMed] [Google Scholar]; (c) Fra L; Muñiz K Indole Synthesis through Sequential Electrophilic N–H and C–H Bond Activation Using Iodine(III) Reactivity. Chem. Eur. J 2016, 22, 4351–4354. [DOI] [PubMed] [Google Scholar]; (d) Bering L; Manna S; Antonchick AP Sustainable, Oxidative, and Metal-Free Annulation. Chem. Eur. J 2017, 23, 10936–10946. [DOI] [PubMed] [Google Scholar]
- (7).Deng T; Mazumdar W; Ford RL; Jana N; Izar R; Wink DJ; Driver TG Oxidation of Nonactivated Anilines to Generate N-Aryl Nitrenoids. J. Am. Chem. Soc 2020, 142, 4456–4463. [DOI] [PubMed] [Google Scholar]
- (8).(a) Dohi T; Maruyama A; Yoshimura M; Morimoto K; Tohma H; Kita Y Versatile Hypervalent-Iodine(III)-Catalyzed Oxidations with m-Chloroperbenzoic Acid as a Cooxidant. Angew. Chem. Int. Ed 2005, 44, 6193–6196. [DOI] [PubMed] [Google Scholar]; (b) Dohi T; Maruyama A; Takenaga N; Senami K; Minamitsuji Y; Fujioka H; Caemmerer SB; Kita Y A Chiral Hypervalent Iodine(III) Reagent for Enantioselective Dearomatization of Phenols. Angew. Chem. Int. Ed 2008, 47, 3787–3790. [DOI] [PubMed] [Google Scholar]; (c) Uyanik M; Okamoto H; Yasui T; Ishihara K Quaternary Ammonium (Hypo)iodite Catalysis for Enantioselective Oxidative Cycloetherification. Science 2010, 328, 1376–1379. [DOI] [PubMed] [Google Scholar]; (d) Martín RR; W. T. H; Kilian M Vicinal Difunctionalization of Alkenes with Iodine(III) Reagents and Catalysts. Chem. Asian J 2014, 9, 972–983. [DOI] [PubMed] [Google Scholar]; (e) Haubenreisser S; Wöste TH; Martínez C; Ishihara K; Muñiz K Structurally Defined Molecular Hypervalent Iodine Catalysts for Intermolecular Enantioselective Reactions. Angew. Chem. Int. Ed 2016, 55, 413–417. [DOI] [PMC free article] [PubMed] [Google Scholar]; (f) Uyanik M; Mutsuga T; Ishihara K 4,5-Dimethyl-2-Iodoxybenzenesulfonic Acid Catalyzed Site-Selective Oxidation of 2-Substituted Phenols to 1,2-Quinols. Angew. Chem. Int. Ed 2017, 56, 3956–3960. [DOI] [PubMed] [Google Scholar]; (g) Mennie KM; Banik SM; Reichert EC; Jacobsen EN Catalytic Diastereo- and Enantioselective Fluoroamination of Alkenes. J. Am. Chem. Soc 2018, 140, 4797–4802. [DOI] [PMC free article] [PubMed] [Google Scholar]; (h) Zhang D-Y; Zhang Y; Wu H; Gong L-Z Organoiodine-Catalyzed Enantioselective Alkoxylation/Oxidative Rearrangement of Allylic Alcohols. Angew. Chem. Int. Ed 2019, 58, 7450–7453. [DOI] [PubMed] [Google Scholar]
- (9).For recent reviews, see:; (a) Singh FV; Wirth T Hypervalent Iodine-Catalyzed Oxidative Functionalizations Including Stereoselective Reactions. Chem. Asian J 2014, 9, 950–971. [DOI] [PubMed] [Google Scholar]; (b) Yoshimura A; Zhdankin VV Advances in Synthetic Applications of Hypervalent Iodine Compounds. Chem. Rev 2016, 116, 3328–3435. [DOI] [PubMed] [Google Scholar]; (c) Muñiz K: Aminations with Hypervalent Iodine. In Hypervalent Iodine Chemistry, Wirth T, Ed.; Springer International Publishing: Switzerland, 2016; Vol. 373; pp 105–134. [DOI] [PubMed] [Google Scholar]; (d) Parra A Chiral Hypervalent Iodines: Active Players in Asymmetric Synthesis. Chem. Rev 2019, 119, 12033–12088. [DOI] [PubMed] [Google Scholar]
- (10).c.f.; (a) Lerchner A; Carreira EM Synthesis of (±)-Strychno-foline via a Highly Convergent Selective Annulation Reaction. Chem. Eur. J 2006,12, 8208–8219. [DOI] [PubMed] [Google Scholar]; (b) Indoles Part Four, The Monoterpenoid Indole Alkaloids. In Chemistry of Heterocyclic Compounds; Saxton JE, Ed.; John Wiley & Sons: Hoboken, 2008. [Google Scholar]; (c) Wang S; Sun W; Zhao Y; McEachern D; Meaux I; Barrière C; Stuckey JA; Meagher JL; Bai L; Liu L; Hoffman-Luca CG; Lu J; Shangary S; Yu S; Bernard D; Aguilar A; Dos-Santos O; Besret L; Guerif S; Pannier P; Gorge-Bernat D; Debussche L SAR405838: An Optimized Inhibitor of MDM2–p53 Interaction That Induces Complete and Durable Tumor Regression. Cancer Res. 2014, 74, 5855–5865. [DOI] [PMC free article] [PubMed] [Google Scholar]; (d) Gollner A; Rudolph D; Arnhof H; Bauer M; Blake SM; Boehmelt G; Cockroft X-L; Dahmann G; Ettmayer P; Gerstberger T; Karolyi-Oezguer J; Kessler D; Kofink C; Ramharter J; Rinnenthal J; Savchenko A; Schnitzer R; Weinstabl H; Weyer-Czernilofsky U; Wunberg T; McConnell DB Discovery of Novel Spiro[3H-indole-3,2′-pyrrolidin]-2(1H)-one Compounds as Chemically Stable and Orally Active Inhibitors of the MDM2–p53 Interaction. J. Med. Chem 2016, 59, 10147–10162. [DOI] [PubMed] [Google Scholar]
- (11).Please refer to the Supporting Information for more details.
- (12).For leading reports on the use of Selectfluor to oxidize aryl iodides, see:; (a) Ye C; Twamley B; Shreeve J. n. M. Straightforward Syntheses of Hypervalent Iodine(III) Reagents Mediated by Selectfluor. Org. Lett 2005, 7, 3961–3964. [DOI] [PubMed] [Google Scholar]; (b) Sarie JC; Thiehoff C; Mudd RJ; Daniliuc CG; Kehr G; Gilmour R Deconstructing the Catalytic, Vicinal Difluorination of Alkenes: HF-Free Synthesis and Structural Study of p-TolIF2. J. Org. Chem 2017, 82, 11792–11798. [DOI] [PubMed] [Google Scholar]
- (13).The unique reactivity of Selectfluor has been exploited in the development of environmentally sustainable processes. For example, see:; (a) (review) Yang L; Dong T; Revankar HM; Zhang C-P Recent progress on fluorination in aqueous media. Green Chem. 2017, 19, 3951–3992. [Google Scholar]; (b) Tian Q; Chen B; Zhang G Silver-initiated radical ring expansion/fluorination of ethynyl cyclobutanols: efficient synthesis of monofluoroethenyl cyclopentanones. Green Chem. 2016, 18, 6236–6240. [Google Scholar]; (c) Liang D; Li Y; Gao S; Li R; Li X; Wang B; Yang H Amide-assisted radical strategy: metal-free direct fluorination of arenes in aqueous media. Green Chem. 2017, 19, 3344–3349. [Google Scholar]; (d) Xie L-Y; Qu J; Peng S; Liu K-J; Wang Z; Ding M-H; Wang Y; Cao Z; He W-M Selectfluor-mediated regioselective nucleophilic functionalization of N-heterocycles under metal- and base-free conditions. Green Chem. 2018, 20, 760–764. [Google Scholar]
- (14).In the absence of PhI, 3H-indole 12a was obtained in only 22%.
- (15).For leading reports on the use of Me3SiO2CCF3 in I(III)-mediated oxidations, see:; (a) Hu B; Miller WH; Neumann KD; Linstad EJ; DiMagno SG An Alternative to the Sandmeyer Approach to Aryl Iodides. Chem. Eur. J 2015, 21, 6394–6398. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Qin L; Hu B; Neumann KD; Linstad EJ; McCauley K; Veness J; Kempinger JJ; DiMagno SG A Mild and General One-Pot Synthesis of Densely Functionalized Diaryliodonium Salts. Eur. J. Org. Chem 2015, 2015, 5919–5924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (16).Bosnidou AE; Muñiz K Alkyliodines in High Oxidation State: Enhanced Synthetic Possibilities and Accelerated Catalyst Turn-Over. Chem. Eur. J 2019,25, 13654–13664. [DOI] [PubMed] [Google Scholar]
- (17).The results of Shirinian and co-workers suggest that the 5H-thieno[3,2-b]pyrrole isomer is the thermodynamic product: see; Shimkin AA; Shirinian VZ; Nikalin DM; Krayushkin MM; Pivina TS; Troitsky NA; Vorontsova LG; Starikova ZA Isomerization of 3H- to 2H-[1]Benzothieno[3,2-b]pyrroles and Synthesis of the First Merocyanine Dyes Based on Them. Eur. J. Org. Chem 2006, 2087–2092. [Google Scholar]
- (18).For leading reports into the mechanism of iodine-mediated oxidative transformations, see:; (a) Richardson RD; Desaize M; Wirth T Hypervalent Iodine-Mediated Aziridination of Alkenes: Mechanistic Insights and Requirements for Catalysis. Chem. Eur. J 2007, 13, 6745–6754. [DOI] [PubMed] [Google Scholar]; (b) Harned AM Concerning the mechanism of iodine(iii)-mediated oxidative dearomatization of phenols. Org. Biomol. Chem 2018, 16, 2324–2329. [DOI] [PubMed] [Google Scholar]
- (19).While these data suggest that the alkyl group is present for the stereochemical determining C–N bond forming step, it is possible that an inorganic iodine species is the catalyst, see ref. 16.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


