Abstract
The European Parliament requested EFSA to develop a holistic risk assessment of multiple stressors in honey bees. To this end, a systems‐based approach that is composed of two core components: a monitoring system and a modelling system are put forward with honey bees taken as a showcase. Key developments in the current scientific opinion (including systematic data collection from sentinel beehives and an agent‐based simulation) have the potential to substantially contribute to future development of environmental risk assessments of multiple stressors at larger spatial and temporal scales. For the monitoring, sentinel hives would be placed across representative climatic zones and landscapes in the EU and connected to a platform for data storage and analysis. Data on bee health status, chemical residues and the immediate or broader landscape around the hives would be collected in a harmonised and standardised manner, and would be used to inform stakeholders, and the modelling system, ApisRAM, which simulates as accurately as possible a honey bee colony. ApisRAM would be calibrated and continuously updated with incoming monitoring data and emerging scientific knowledge from research. It will be a supportive tool for beekeeping, farming, research, risk assessment and risk management, and it will benefit the wider society. A societal outlook on the proposed approach is included and this was conducted with targeted social science research with 64 beekeepers from eight EU Member States and with members of the EU Bee Partnership. Gaps and opportunities are identified to further implement the approach. Conclusions and recommendations are made on a way forward, both for the application of the approach and its use in a broader context.
Keywords: agent‐based simulation, Apis mellifera, ApisRAM, bee biological agents, EU Bee Partnership, plant protection products, sentinel hives
Short abstract
This publication is linked to the following EFSA Supporting Publications articles: http://onlinelibrary.wiley.com/doi/10.2903/sp.efsa.2021.EN-6608/full
Summary
The introduction of this scientific opinion clarifies the background and Terms of Reference of this mandate (Section 1.1 ), but also provides background information on the MUST‐B project (‘EU efforts towards a holistic and integrated risk assessment approach of multiple stressors in bees’) and the EU Bee Partnership that are referred to in the mandate (Section 1.2 and Table 1 ) as well as to specific EU projects that are supporting MUST‐B (Appendix A ).
Table 1.
Overview of the key milestones achieved by EFSA in bee health, between 2012 and 2021
| Years | Key milestones (references) |
|---|---|
| 2012 | |
| 2013 | |
| 2014 | |
| 2015 |
|
| 2016 |
|
| 2017 |
|
| 2018 |
|
| 2019 |
|
| 2020 |
|
| 2021 |
|
The Coordinators of the ENVI committee of the European Parliament endorsed a request for a scientific opinion by the European Food Safety Authority (EFSA) on the science behind the development of an integrated holistic approach for the risk assessment of multiple stressors in managed honey bees (Apis mellifera). This request is composed of four Terms of Reference. Three of them are focused on the development of a methodology that takes into account multiple stressors including single or multiple chemicals (i.e. coming from the environment, whether regulated or not; see Appendix B for clarifications of the terminology) and their various types of effects on bees (i.e. additive, synergistic, acute, chronic and sublethal). This development needs to include the work carried out by MUST‐B and the EFSA Scientific Panels. The last term of reference is on the EU Bee Partnership and the guidance needed for harmonised data collection.
The MUST‐B project is a multidisciplinary and multi‐annual scientific project that was initiated as a self‐task of the EFSA Scientific Committee and Emerging Risks Unit (Section 1.2.1 ). MUST‐B is composed of a task force represented by several units from ESFA's Scientific and Communication departments, four working groups (from the Scientific Committee and Emerging Risks Unit, the Animal Health and Welfare Unit, the Pesticides Unit and the Communication Unit), a discussion group of stakeholders called the ‘EU Bee Partnership’ (composed of representative stakeholders) and several outsourcing activities (i.e. risk factors analysis on EPILOBEE data set; chemical mixtures risk assessment in managed and wild bees; modelling development for an agent‐based honey bee colony model; field data collection to calibrate the honey bee colony model; and development of a prototype platform for the EU Bee Partnership to collect and exchange standardised data on bee health and beekeeping). Ongoing research projects such as ‘PoshBee’ and ‘B‐GOOD’ from Horizon 2020 support specifically the MUST‐B project and other EU‐funded projects such as ‘INSIGNIA’, ‘IoBee’ and ‘SAMS’ that are relevant to MUST‐B. Further description on these research projects is detailed in Appendix A . Data play a central role in MUST‐B, and the FAIR principles (findability, accessibility, interoperability, reusability) are very relevant. In addition, MUST‐B promotes data quality, reliability, openness and transparency, and supports stakeholder engagement to increase trust and evidence‐based risk assessment (Section 1.2.2 ). A willingness to increase trust is demonstrated by the shared goal of the EU Bee Partnership on the collection and sharing of harmonised data on bee health and beekeeping in Europe (Section 1.2.2 ).
The request from the European Parliament and the EFSA MUST‐B project fit into the actions and measures that were outlined in the European Green Deal for the European Union and its citizens. These specifically seek to make the EU's economy sustainable by supporting Member States to improve and restore damaged ecosystems, by promoting an environment that is free from pollution including multiple pollutants such as chemicals, by reducing the use and risk of chemical pesticides and by promoting data access and interoperability with the support of artificial intelligence and digitalisation (see Section 1.2.3 ).
In the interpretations of the Terms of Reference (Section 1.3 ), it is clarified that this scientific opinion presents ideas and concepts for consideration and future development. This opinion is aligned to aspirations outlined in the EU Green Deal and the prospective EFSA strategy 2027, presenting ideas and facilitating discussion, leading to practical solutions, in this critical area of environmental risk assessment of multiple stressors in honey bees. Further clarification of the terminologies used in the mandate and in this opinion is provided in Appendix B .
To contextualise the need for the development of a holistic and integrated environmental risk assessment approach for honey bees suffering exposure to multiple stressors, two overviews are provided in Section 2 . The first one on the regulatory background of the current framework for the environmental risk assessment of plant protection products (Section 2.1 ) and the second on the environmental risk assessment of multiple stressors in honey bees (Section 2.2.1 ) with insights into the challenges met (Section 2.2.2 ). Those challenges are linked to the complexity of a honey bee colony (Section 2.2.2.1 and Appendix C ) and to the landscape in which colonies are located (Section 2.2.2.2 ).
The proposed holistic and integrated environmental risk‐assessment approach for honey bees is based on a ‘systems‐based approach’ (Section 3.1 ), which has two core components linked by data flows (modelling and monitoring) and that is further developed in this scientific opinion (Section 3.2 ).
The modelling system is based on the development of ApisRAM, the agent‐based simulation model that EFSA has outsourced to assess either a single or two chemicals, including plant protection products, in interaction with other stressors such as biological agents (Varroa, Nosema, deformed wing virus and acute bee paralysis virus), beekeeping management practices and environmental factors relevant to the colony, including weather and floral resources (Section 3.2.1 ). This agent‐based simulation was formalised by the MUST‐B working group to address some shortcomings identified by EFSA when evaluating the usefulness of BEEHAVE as a regulatory tool (Section 3.2.1.1 ). ApisRAM is built in ALMaSS (an Animal, Landscape and Man Simulation System) with landscapes being simulated in substantial spatial detail (10 × 10 km area at a resolution of 1 m2) (Section 3.2.1.2 ). ApisRAM integrates multiple stressors (Section 3.2.1.3 ) and will also include sublethal effects such as reproductive performance of queens, development of hypopharyngeal glands and homing ability (Section 3.2.1.4 ). Any future development of ApisRAM will include the possibility to assess multiple chemicals (i.e. more than two compounds) and several other additional features, including several colonies, additional biological agents such as predators, nutrition quality and interaction with other stressors (see Section 3.2.1.5 ).
Regarding the monitoring system of the systems‐based approach, it will be composed of a network of sentinel hives located in various regions of Europe and connected to a platform for data storage and analysis (Section 3.2.2 ). Data will be collected to inform ApisRAM on the status of the honey bee colony. This will be defined by the HEALTHY‐B toolbox and indicators (Section 3.2.2.1 and Appendix D for a description of the (digital) tools to assess honey bee colony status). Additional data will be collected on exposure to chemical residues both inside and outside the sentinel hives (Section 3.2.2.2 ), and about the structure, management and dynamics of the landscapes, including around sentinel beehives (Section 3.2.2.3 ).
The systems‐based approach would have applications in, and potential benefit to, beekeeping, farming, research, risk assessment, risk management and the broader society (NGOs, Industry and EU citizens) (Section 4 ). As a tool for risk assessment and risk management, each of the following attributes is relevant: holistic, protective, integrative, adaptive, responsive, inclusionary, transparent and by being effectively communicated (Section 4.3 ).
A societal outlook on the systems‐based approach is included to understand and integrate the views of beekeepers and stakeholders (Section 5 and Appendices E and F ). Targeted research was conducted with beekeepers in selected EU countries to assess their views on the proposed approach, their needs and expectations in terms of data for managing their colonies, digital advancements and requirements for communication of applied research. Several stakeholders could interact with the system, including the members of the EU Bee Partnership, to promote accessible and reliable data collection and management. This systems‐based approach could contribute to risk assessment of bee health that is more open, transparent and trustworthy, contributing to the long‐term prosperity of honey bee colonies and beekeeping in Europe (Section 5.1 and Appendix E ). This work addresses the first term of reference of the mandate on the inclusion of beekeeping management practices in the development of a holistic risk assessment approach. In addition, interviews were conducted with members of the EU Bee Partnership to determine their perceived involvement and collaboration, the efficiency of the partnership, their views on harmonised data collection and sharing as well as the future involvement of new partners and the wider community of beekeepers (resources and coordination) (Section 5.2 and Appendix F ). This work is aligned to the Term of Reference on the need for guidance to the EU Bee Partnership on harmonised data collection and sharing in bee health and beekeeping in Europe. This work was also published as a standalone report, providing more details on the material, methodologies and results (EFSA, 2021).
The scientific opinion identified gaps and opportunities for the implementation of the systems‐based approach (Section 6 ). These related to environmental risk assessment, including the risk assessment of combined exposure to chemical mixtures (Section 6.1 ), the systems‐based approach (Section 6.2 ) comprising the modelling and monitoring (Section 6.2.1 ) and data flows into the systems‐based approach (Section 6.2.2 ).
Finally, conclusions and recommendations are made (Section 7 ). The importance of the socio‐political context (EU Green Deal) of this work was highlighted, as was the support of social sciences in facilitating exchanges with stakeholders in this opinion. This work goes beyond the ‘one substance approach’ by adopting a ‘systems‐based approach for a holistic and integrated risk assessment of multiple stressors’. The opinion highlights the importance of data in the systems‐based approach, and the spectrum of beneficiaries and players (Section 7.1 ). It was recommended that a systems‐based approach be introduced on a phased basis, seeking a more holistic and integrated environmental risk assessment of multiple stressors in honey bees. That is, from the simpler assessment of single substances through to the more complex assessment of multiple chemicals, landscape and indirect effects. Additional recommendations highlight the need for a more systematic inclusion of social sciences in EFSA's risk assessments to take into account stakeholders’ perspectives and make more fit‐for‐purpose risk assessments; the development of a Pan‐EU data collection effort through the sentinel hive network; the establishment of specific indicators of the health status of a honey bee colony, including the fine tuning of appropriate tools; and research investigations to fill the gaps in environmental risk assessment, model testing and data harmonisation. It is recommended that the systems‐based approach reports relevant information in a manner that gives EU citizens a better understanding of the possible causes and underlying mechanisms of bee health decline in Europe and worldwide. The proposed approach developed in this opinion on honey bees as a showcase could be used more broadly to advance environmental risk assessments (i.e. on other bee types, non‐target arthropods and other terrestrial or aquatic organisms) (Section 7.2 ).
1. Introduction
1.1. Background and Terms of Reference as provided by the European Parliament
On 28 June 2018, the Coordinators of the ENVI committee endorsed a request for a scientific opinion by the European Food Safety Authority (EFSA) on the science behind the development of an integrated and holistic approach for the risk assessment of multiple stressors in managed honey bees (Apis mellifera).
This request was submitted in accordance with Article 29 of Regulation (EC) No 178/20021 on ‘laying down the general principles and requirements of food law, setting up the European Food Safety Authority and laying down procedures in matters of food safety’, which provides that the European Parliament may request the Authority to issue a scientific opinion on matters falling within the Authority's mission.
The request was introduced taking into account the world‐wide importance of bees and pollinators, as 84% of plant species and 76% of Europe's food production depend on pollination by bees and this represents an estimated economic value of EUR14.2 billion a year. However, the health of honey bee colonies has been declining and there have been intensive scientific efforts to better understand the reasons for this decline, which may be related to intensive agriculture and pesticides use, poor bee nutrition, viruses and attacks by invasive species, as well as environmental changes and habitat loss.
The ENVI committee, therefore, considered it opportune for Parliament to request that EFSA deliver a scientific opinion on the science behind the development of an integrated and holistic approach for the risk assessment of multiple stressors in managed honey bees (Apis mellifera). The following issues have been identified for the development of a scientific opinion on the risk assessment of multiple stressors from both the in‐hive environment and the surrounding landscape:
The development of a methodology to take into account not only cumulative and synergistic effects of plant protection products (PPPs) but also include issues related to bees’ genetic variety, bee pathogens, bee management practices and the colony environment (Term of Reference ToR 1).
The assessment of acute and chronic effects of multiple stressors on honey bees including colony survival and sublethal effects (Term of Reference ToR 2).
The work being developed by stakeholders to achieve harmonised data collection and sharing on bee health in EU and, by doing so, support the EU Bee Partnership initiative by providing guidance for harmonised data collection and evidence‐based risk assessments (Term of Reference ToR 3).
The work conducted by EFSA under its project MUST‐B that integrates the work being developed in EFSA's various panels (Term of Reference ToR 4).
This scientific opinion, to be delivered by June 2021, should integrate efforts already conducted and propose a framework for risk assessment that can ensure the protection of managed honey bees in Europe.
1.2. The MUST‐B project
1.2.1. Activities within EFSA
Honey bee colony health decline finds its origin in a complex socioecological system. It can be considered as a multifactorial and multistakeholder issue given the number of factors and stressors that affect honey bee colony health, and the number of stakeholders who are involved. However, the current risk assessment approaches remain compartmentalised and intradisciplinary, and more holistic, multidisciplinary and integrated approaches are required to resolve such complexity.
At EFSA, a multidisciplinary task force was set up to move towards the development of such a transversal and holistic approach for the risk assessment of multiple stressors in bees. The task force published a review of the work carried out on bee health by EFSA, the European Member States (MS) and the European Commission (EFSA, 2012, 2014a). The task force highlighted knowledge gaps and research needs that would assist the development of a harmonised environmental risk assessment scheme for bees. This work was further discussed with stakeholders (EFSA, 2013a) and the European Commission (EFSA and European Commission Directorate‐General for Agriculture and Rural Development, 2016) to support EFSA's efforts to develop a holistic risk assessment for bees and to determine research priorities on bee health under the H2020 framework. In this context, two EU research calls were launched and granted in 2018 and 2019. The first project, ‘PoshBee’ (‘Pan‐European assessment, monitoring and mitigation of stressors on the health of bees’) (Appendix A ), should prove useful for the further implementation of this holistic risk assessment approach by gathering more evidence on exposure and risk characterisation of multiple chemicals for bee health, taking into account interactions with other stressors and factors in the environment of bees, such as biological stressors, nutrition and the broader environment (weather, climate and landscape characteristics). Within this project, it is intended to gather data on lethal, chronic and sublethal effects including toxicokinetics and toxicodynamics, full dose relationships and to develop new protocols and markers based on ‘omics (transcriptomics, proteomics and metabolomics) of chemical and biological stressors exposure/effects to be used in monitoring plans. The second project, ‘B‐GOOD’ (‘Giving beekeeping guidance by computational‐assisted decision making’) (Appendix A ), focuses on the development of ready‐to‐use tools for operationalising the ‘health status index’ developed by EFSA (EFSA AHAW Panel, 2016) to enable data collection and return to beekeepers, while exploring various socioeconomic and ecological factors beyond bee health. Within this project, the plan is to create an EU platform to collect and share knowledge, science and practice related to honey bees, their environment and agricultural and beekeeping practices. The ultimate project goal is to validate technologies for monitoring colonies and indicators of bee health in an automated or semi‐automated way to facilitate standardised and accurate data collection and transfer. In addition to these two research projects that specifically refer to MUST‐B, there is a third project that was initiated in 2018 called ‘INSIGNIA’ (‘A citizen science protocol for honey bee colony as bio‐sampler for pesticides’) which is building on the knowledge gained from the COLOSS Citizen Scientist Initiative (CSI) – Pollen project. This citizen science project (using beekeepers) will provide innovative methods to sample and analyse pesticides residues and botanical origin of the pollen brought back by foragers to the colony and determine pesticides exposure across various landscapes in EU (Appendix A ).
In parallel with the above research, which will generate data, tools and methodologies supporting EFSA's initial work in bee health, EFSA initiated a large 5‐year project in 2015 called ‘MUST‐B’ (‘EU efforts towards a holistic and integrated risk assessment approach of multiple stressors in bees’) to move further towards the development of a more holistic and integrated approach to the risk assessment of multiple stressors in bees, focusing to start with on honey bees.
MUST‐B was rooted in EFSA's strategic objectives 2020 (EFSA, 2015), in particular on the prioritisation of public and stakeholder engagement in the process of scientific assessment, on the widening of EFSA's evidence base, on the optimisation of access to its data and on preparing for future risk assessment challenges. MUST‐B focuses on three pillars: (i) the development of tools and methodologies for the risk assessment of multiple stressors in bees at the landscape level, (ii) gathering robust data (i.e. harmonised and standardised) for evidence‐based risk assessment of bee health and (iii) the engagement of stakeholders for harmonised data collection and data sharing in EU on bee health.
The MUST‐B working group has developed a framework incorporating modelling, experimental and field‐monitoring approaches. These complementary approaches are being combined to extrapolate risks from individual to colony levels, to assess the complexity of co‐exposures from multiple stressors coming from both the hive environment and the landscape and to determine their relative contribution to colony losses and weakening. Modelling is at the core of the MUST‐B framework with the development of a predictive agent‐based simulation of a honey bee colony, ‘ApisRAM’, in real Geographic Information System (GIS) landscapes and that is currently outsourced by EFSA. This computer simulation is being developed as a quantitative tool for regulatory risk‐assessment purposes and as a predictive and explanatory tool to better understand the (relative) risks and impacts of multiple stressors on honey bee colonies, including the overall complexity of interactions. The conceptual model (a qualitative description of the system to be modelled, including insights into the environmental and biological processes and their interactions and interdependencies) was defined by a group of experts, the MUST‐B working group (EFSA, 2016a,b), who took account of recommendations on the usefulness and suitability of an existing model, BEEHAVE (Becher et al., 2014), for use in a regulatory context (EFSA PPR Panel, 2015). The working group followed the EFSA scientific opinion on good modelling practices for model development and testing (EFSA PPR Panel, 2014). Criteria, derived based on minimum requirements, were described to design a field data collection to be conducted in the three EU regulatory zones to validate and calibrate the model as a regulatory tool for the risk assessment of PPPs in interaction with other stressors and factors such as biological stressors (Varroa destructor, Nosema spp. and their associated viruses, deformed wing virus (DWV) and acute bee paralysis virus (ABPV)), resources in the landscape, beekeeping management practices and weather conditions, on honey bee colonies (EFSA, 2017a,b). This is further described in Section 4.2 . EFSA outsourced the data collection in two EU MS, Denmark and Portugal (validating two EU zones, the north and the south). When designing the field data collection, the working group took into account lessons learnt from the EPILOBEE project (Laurent et al., 2016), a programme of active surveillance conducted across 17 EU MS during 2012–2014 (Jacques et al., 2016), in particular the lack of data on monitoring chemicals in contact with bees and bee products (e.g. PPP and veterinary products) and the importance of the preparatory phase for the fine tuning of field protocols, training field operators and setting a database to promote, as much as possible, automatic and harmonised entry and reduced field operator bias. In this exercise, the working group considered the work of the Healthy‐B working group (EFSA AHAW Panel, 2016) who developed a toolbox of methods and indicators to assess colony health in a standardised way across the EU.
In the Scientific Opinion and Guidance Document on the risk assessment of PPPs in bees (Apis mellifera, Bombus spp. and solitary bees) (EFSA PPR Panel, 2012; EFSA, 2013b), EFSA highlighted some shortcomings regarding semi‐field and field studies. An obvious one for field studies is the lack of control in exposure of bees that can forage freely over large areas in the landscape, usually beyond the limits of the tested plots and sometimes across (control and tested) plots and can be exposed not only to the single tested chemical but also to others present in the landscape. Another is the intrinsic variability between colonies and plots, leading to a much larger sampling size than currently used in regulatory tests to reach a meaningful statistical power. In addition, the methodologies and technologies used are not always adequate to detect chronic, small or sublethal effects and long‐term effects, which in time can become detrimental at the colony level. Finally, the spatial and temporal diversity of the agro‐environmental conditions in the EU may not be covered by the regulatory studies currently performed (European Parliament, 2018). This is further described in Section 2.2 .
ApisRAM is a honey bee colony model that has been developed in support of the MUST‐B work. It could be used in a range of different contexts including risk assessment (support to field testing) and research (e.g. to understand the relative importance of different stressors on colony weakening and losses). ApisRAM has been built in ALMaSS (Topping et al., 2003), which is an existing simulation system used to model human and other impacts on animals at landscape scales using detailed agent‐based simulation (ABS). ALMaSS has been used extensively in pesticide risk assessment, relevant to each of the following species: a carabid beetle Bembidion lampros (Topping et al., 2009; Topping and Lagisz, 2012; Topping et al., 2014; EFSA PPR Panel, 2015; Topping et al., 2015), the Eurasian skylark, Alauda arvensis (Odderskaer et al., 2003, 2004; Sibly et al., 2005; Topping et al., 2005; Topping et al., 2005; Jacobsen et al., 2008; Topping and Luttik, 2017), the European Brown Hare, Lepus europaeus (Topping et al., 2016; Topping and Weyman, 2018), the field vole, Microtus agrestis (Topping et al., 2008; Dalkvist et al., 2009; Dalkvist et al., 2013; Schmitt et al., 2016), the linyphiid spider, Erigone atra (Thorbek and Topping, 2005; Topping et al., 2014) and the rabbit, Oryctolagus cuniculus (Topping and Weyman, 2018). In each case, ALMaSS includes a landscape model as an environment into which an animal model is placed (Topping et al., 2003).
The current project, as a show case, is focused on honey bees. However, key developments in the current scientific opinion (including systematic data collection from sentinel beehives and agent‐based modelling) have the potential to substantially contribute to the future development of risk assessments of multiple stressors in other organisms at larger spatial and temporal scales.
An overview of the activities conducted at EFSA on bee health, including the MUST‐B project is provided in Table 1.
1.2.2. The EU Bee Partnership
Since 2012, the European Parliament has coordinated the activities of the European Week of Bees and Pollination comprising a high‐level conference and a scientific symposium with beekeepers. Conscious that bee mortality observed across the world was likely to be due to a number of factors, stakeholders involved in EU Bee Week activities have stressed the need for better collaboration between all interested parties through greater dialogue. They have also called for the establishment of an operational technical platform to improve exchanges between beekeepers and scientists.
In 2017, the European Parliament's Apiculture and Bee Health Working Group tasked EFSA with coordinating the Bee Week's scientific symposium with a focus on the collection and sharing of harmonised bee health data in Europe. The event brought together around 130 stakeholders (i.e. beekeepers, farmers, industry, scientists, risk assessors and managers, the public and policy makers), and ended with a general agreement among all representative stakeholders to work towards setting up an EU Bee Partnership (EUBP). This Partnership has been facilitated by EFSA, and the European Commission (DG AGRI, SANTE, ENV, RTD, CONNECT) (EFSA, 2017a,b) has also participated. Further to recommendations by stakeholders, EFSA supported the establishment of a discussion group composed of representative stakeholders to define the Terms of Reference (ToR) of the EUBP (EFSA, 2018a). Progress has been reported back to the annual European Parliament Bee Week High Level Conference.
This 2017 event highlighted the need for harmonising data collection and collaborations across all involved stakeholders, underpinning improvements to the sharing, analysis and management of data on bee health throughout EU (EFSA, 2017a,b). Data standardisation was discussed, including the development of a common format (data models) and a common representation (terminologies, vocabularies, coding schemes). EFSA had prior expertise in this area, having developed harmonised and standardised field data collection to assess bee health (e.g. the Standard Sample Description for Food and Feed version 2.0, SSD2; DATA Unit, 2017) for the calibration and validation of the ApisRAM model (EFSA, 2013c) (see Appendix A in EFSA, 2017a,b). At this event, the need for increased data quality and reliability (with quality controls in place) was also emphasised, including electronic capture of data from laboratory information management systems and digital hives (e.g. hive sensors, hive scales, weather stations, etc.). These data need to be validated with proper business rules following the EFSA Guidance on Data Exchange version 2.0 (EFSA, 2014b) to ensure transparency and openness regarding the way in which the data are collected and analysed. A final theme of discussion was the need for efficient data exchange using specific files format for data transmission. In the beekeeping community, the XML standard for exchange about bees and beekeeping data and information (Cazier et al., 2018; Haefeker, 2018) was adopted by the EUBP when developing a prototype platform for harmonised data exchange in EU on bee health and beekeeping.
In 2019, with the knowledge gained from IoBee (Appendix A ), a European‐funded Fast‐Track‐to‐Innovation project coordinated by BeeLife, a proof of concept (PoC) was developed for the EUBP platform to store, exchange and analyse data from different sources on bee health. The PoC uses XML format for data interoperability, a standard that was endorsed by the EUBP as a self‐describing data format that can allow data exchange.
In 2020, EFSA supported the EUBP in developing the PoC into a prototype platform. This prototype forms the basis for an operational platform to be further developed and that would integrate all relevant information, knowledge and data to be collected by, and exchanged among, stakeholders on bee health and beekeeping. This operational platform would make relevant data accessible to end users such as beekeepers, beekeeping or farming associations, researchers, agencies and policymakers. The platform will ultimately be expanded to include other bee species and more widely to other pollinators both within and outside EU, where cheap and easy‐to‐use open source Information and Communications Technology (ICT) applications are being developed (e.g. see SAMS project in Appendix A ).
The ambition to develop the operational platform in the future to include data on wild bees (i.e. bumblebees and solitary bees) and other pollinators could lead to a number of synergies with the European Commission's EU Pollinators Initiative.
1.2.3. In the context of the EU Green Deal
In December 2019, the European Commission set out the European Green Deal for the European Union and its citizens to make the EU economy sustainable by turning climate and environmental challenges into opportunities (European Commission, 2019a). For this purpose, a new paradigm and specific Sustainable Development Goals were defined (European Commission, 2019b) with a roadmap of key actions and measures, including legislative ones, to achieve these goals, to be undertaken between 2020 and 2021 (European Commission, 2019c).
Many of these actions and measures are particularly relevant to the overall approach that has been developed under MUST‐B, including:
helping MSs to improve and restore damaged ecosystems by addressing the main drivers of biodiversity loss;
promoting a toxic‐free environment through better monitoring, reporting, preventing and remedying pollution and addressing combined effects from multiple pollutants (e.g. multiple chemicals);
significantly reducing the use and risk of chemical pesticides by encouraging innovation for the development of safe and sustainable alternatives;
promoting data access and interoperability with innovation, e.g. digitalisation and artificial intelligence for evidence‐based decisions regarding environmental challenges.
1.3. Interpretation of the Terms of Reference
This scientific opinion presents ideas and concepts for consideration and future development. The document is not prescriptive, nor is it constrained by or aligned to specific EU legislation. Rather, the opinion seeks to present a framework, and supporting rationale, that is robust and forward thinking, while acknowledging that some detail will require further elaboration, which in part will be reliant on new scientific discoveries. This scientific opinion is aligned to aspirations outlined in the EU Green Deal and the EFSA Strategy 2027 (EFSA, 2019a,b,c), presenting ideas and facilitating discussion, leading to practical solutions in this critical area of environmental risk assessment of multiple stressors in honey bees.
The ToR 1 and ToR 2 refer to the development of a holistic and integrated risk assessment methodology taking into account various factors (i.e. ‘bee genetic diversity’, ‘beekeeping management practices’, ‘resource providing unit’) and stressors (‘chemical’ and ‘biological’) as well as various effect types (‘acute’, ‘chronic’, ‘sublethal’, ‘cumulative’, ‘synergistic’, ‘antagonistic’) on colony health status. For one colony, this would mean an adequate size, demographic structure and behaviour; an adequate production of bee products (both in relation to the annual life cycle of the colony and the geographical location); and provision of pollination services (see Section 3.2.2.1 ). ToR 1 refers to ‘PPPs’ and ‘cumulative effects’. Clarifications on the definitions of those terms (i.e. ‘PPPs’ and ‘cumulative effects’) are provided in Appendix B . In their environment, bees are exposed to PPPs as well as other chemicals (e.g. biocides, veterinary products and contaminants), which are referred to in this scientific opinion as ‘multiple chemicals’ or ‘chemical stressors’. The term ‘cumulative effects’ is functionally synonymous to ‘cumulative impacts’ and frequently used in the area of environmental impact assessment under Annex IV of the Directive 2011/92/EU2 (see Appendix B for more details). In the context of this scientific opinion, which is focused on environmental risk assessment of multiple chemicals and stressors in honey bees, ‘cumulative effects’ refer to ‘combined effects from exposure to multiple chemicals’ at a given time (EFSA Scientific Committee, 2019) or to ‘combined effects from exposure to multiple chemicals and/or other multiple stressors’ at a single or multiple time points. E.g. foragers might be exposed in time and space to several and different types of stressors, resulting in complex (non‐linear) responses at the colony level.
To address these two ToRs, an integrated and holistic approach is presented in Section 3.1 ‘A proposal for a systems‐based approach to multiple stressors in honey bees. Findings from social research have informed the holistic approach by providing an understanding of the perspectives of the interested parties (e.g. beekeepers). This is further developed under Section 5.1 in which targeted research was conducted among beekeepers in EU to assess their understanding of the proposed approach, their needs and expectations in terms of data for managing their colonies, digital advancements and requirements for communication of applied research.
Furthermore, methodologies for risk assessment sought in ToR 1 and ToR 2 are addressed under Section 3.2 ‘the core components of the systems‐based approach’ and some clarifications on the terminology used under these ToRs are provided by the working group (Appendix B ). The proposed approach is in line with the recommendations for actions made under the EU Green Deal (see Section 1.2.3 ) and is based on the work achieved under the auspice of the MUST‐B project (see Section 1.2.1 ) and the knowledge gained on the risk assessment of combined exposure to multiple chemicals (EFSA Scientific Committee, 2019) applied to honey bee colonies (Spurgeon et al., 2016).
The third term of reference (ToR 3) refers to harmonised data collection and sharing among stakeholders as developed by the EUBP, which has the goal to improve data collection, management, sharing and communications to achieve a holistic assessment of bee health in EU and beyond (see Sections 1.2.1 and 1.2.2 ). EFSA supports this initiative which is aligned to its mission to facilitate discussion among stakeholders, by providing guidance for harmonised data collection in the context of bee health assessment (Jacques et al., 2016, 2017; EFSA, 2017a,b) and by promoting research in this field (EFSA, 2014a; EFSA and European Commission's Directorate‐General for Agriculture and Rural Development, 2016), with the support of developments under the research framework H2020. This Term of Reference (ToR 3) is addressed under Section 5.2 and supported by feedback obtained from members of the EUBP.
The inclusion of the need to take into account beekeeping management practices in the Terms of Reference of the request (ToR 1), as well as the work being developed to achieve harmonised data collection (ToR 3), have prompted EFSA to bring social science skills to the interdisciplinary mix of expertise working together on this project. In line with EFSA's social science roadmap, targeted social research was commissioned to provide an understanding of the perspectives of the interested parties and thus strengthen engagement and communication with target audiences.
The ToR 4 is on the inclusion of EFSA's work on bee health through the MUST‐B project. As highlighted in Section 1.2.1 and Table 1 , MUST‐B is rooted in EFSA's work, through the multidisciplinary Bee Task Force representing all Panels and Units involved in bee health (EFSA, 2012). This work is based on stakeholder engagement for a participative, inclusionary and integrated approach to environmental risk assessment, taking into account multiple chemical and biological stressors and combining modelling and monitoring strategies. This work might provide additional lines of evidence to risk assessors and may already have been implemented as part of the current predictive risk assessment that is under review through the EFSA Bee Guidance on the risk assessment of PPPs in bees (EFSA, in preparation). The main differences between the Guidance Document and this Scientific Opinion in terms of requestor, legislative status, purpose, scope and timeframe are summarised below (Figure 1). However, beyond those differences, the two documents each contribute, within their respective remits, to the future of environmental risk assessment (More et al., 2021).
Figure 1.
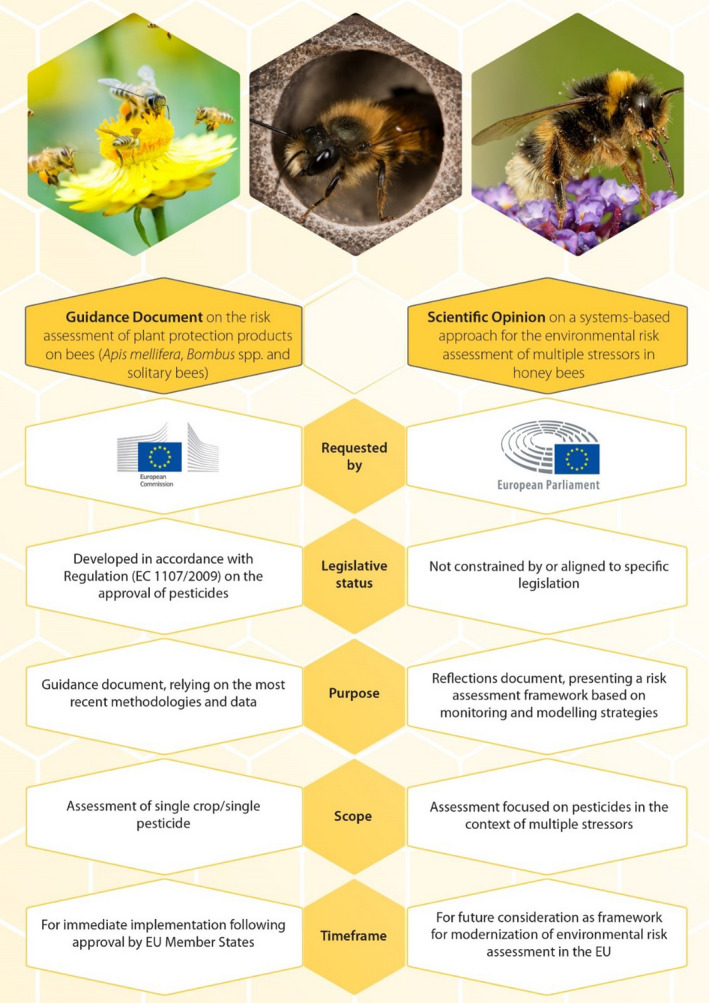
Main differences, in terms of requestor, legislative status, purpose, scope and timeframe, between the Guidance Document (the reviewed guidance document on the risk assessment of PPPs in bees; EFSA, in preparation) and this Scientific Opinion (on the systems‐based approach for the ERA of multiples stressors in honey bees) (figure from More et al. (2021))
Finally, a description of the terms in the mandate that require clarification to avoid any misinterpretation is presented in Appendix B . In addition, a glossary of the widely used terms and concepts in the multiple‐stressor research (e.g. ‘multiple‐stressor’, ‘cumulative effect’, ‘stressor interaction’, ‘additive’, ‘antagonist’) is provided by Orr et al. (2020).
2. The need for a holistic and integrated approach to the environmental risk assessment for honey bees
2.1. Environmental risk assessment of PPPs
The EU legal framework regarding placing on the market of PPPs (i.e. Regulation (EC) No 1107/20093) provides for a two‐phase procedure before they can be placed on the market: an EU‐level approval of the active substance used in PPPs and a national level authorisation of the PPPs. Directive 2009/128/EC4 (the Sustainable Use Directive) sets up a framework to achieve the sustainable use of pesticides. Any actions for PPP risk reductions, including some mitigation measures, belong to this Directive.
An environmental risk assessment of PPPs is required under Regulation (EC) No 1107/20093 to demonstrate that residues of a PPP, following its use according to good agricultural practices, under realistic conditions of use, does not pose any unacceptable effect on the environment. For the risk assessment for bees, a guidance document was developed by EFSA (EFSA, 2013b) and is currently under review (EFSA, in preparation). This guidance includes recommendations for conducting the risk assessment for bees (honey bees, bumble bees and solitary bees) that are exposed to residues of a PPP and its metabolites. In particular, the document provides guidance on how the exposure estimation for a specific use can be performed by considering the various routes of exposure (oral uptake of contaminated pollen/nectar and contact exposure) and the various sources of exposure. The document also outlines how the hazard characterisation will be conducted by considering acute, chronic, sublethal and accumulative effects. The risk assessment, based on the exposure and hazard characterisation, seeks to demonstrate whether the PPP under evaluation and its use have an unacceptable effect on bees (namely ‘has no unacceptable acute or chronic effects on colony survival and development, taking into account effects on honey bee larvae and honey bee behaviour’). Operationally, this is demonstrated by compliance of the risk assessments with the specific protection goals defined by the risk managers. The risk assessment of mixtures of PPPs regarding formulations or preparations that contain at least one active substance (see Appendix B for further definitions) is part of the evaluation of a PPP under Regulation (EC) No 1107/20093, and it is also covered by the EFSA Guidance Document (EFSA, 2013b).
Recently, EFSA published the MIXTOX Guidance on ‘harmonised methods for human health, animal health and ecological risk assessment of combined exposure to multiple chemicals’. MIXTOX provides stepwise approaches for problem formulation and for each step of the risk assessment process, i.e. exposure assessment, hazard assessment and risk characterisation using whole mixture and component‐based approaches as well as a reporting table to summarise the outcome of the risk assessment (EFSA Scientific Committee, 2019). This Guidance is relevant to bee health risk assessment of multiple chemicals (PPPs, biocides, veterinary products and contaminants) and provides an example to investigate combined toxicity in honey bees, regarding interactions between multiple chemicals, using component‐based approaches. In addition, these methodologies have been further adapted to the risk assessment of multiple stressors in a honey bee colony using the ApisRAM model (Sections 3.2.1.3 and 3.2.1.5 ). It is foreseen that these methodologies provide further opportunities to develop fit‐for‐purpose environmental risk assessment approaches for single and multiple PPPs applied at the same time, or in the same crops/fields applied at different time points, on bees. Indeed, the co‐exposure of honey bee colonies with multiple PPPs is the result of the complexity of landscapes, but also of the succession of crops on the same plot, taking into account the persistence of PPPs in the environment. With the complexity of the landscapes, this implies to consider the mobility of PPPs in the environment, especially by surface water and potential drift from spray applications. Co‐exposure to bees can also be explained by the large surface areas foraged by bees and the accumulation of residues in the hive (pollen/beebread, nectar/honey and wax).
Of all the areas in EFSA's remit that could contribute to the EU Green Deal, advancing the environmental risk assessment of PPPs is expected to have the highest possible impact. Although Regulation (EC) No 1107/20093 and the Sustainable Use Directive provide a robust framework for the environmental risk assessment of PPPs, several improvements are demanded by recent scientific evidence, increasingly complex environmental challenges and evolving societal concerns. The opportunity for enhancing the environmental risk assessment of PPPs is also driven by: (1) the new EFSA mandate given by the transparency regulation (EU) 2019/13815; and (2) European Commission's REFIT process of the Regulation (EC) No 1107/20093 (Group of Chief Scientific Advisers, 2018).
2.2. Environmental risk assessment of multiple stressors in honey bees
2.2.1. Overview
Managed colonies of honey bees (Apis mellifera spp.) represent an important source of goods and income. For the honey market, while Europe is the second world producer (2,80,000 tons), it is only 60% self‐sufficient in honey (European Commission, 2020a,b). Further, honey bee colony losses are reported globally, and particular in Europe and North America, reaching high mortality rates of about 30% (Laurent et al., 2016; Steinhauer et al., 2014). Such mortality rates are not specific to honey bee populations, but also affect other bee species, insects and biodiversity more widely (Potts et al., 2010; Dirzo et al., 2014; Hallmann et al., 2017; Wagner, 2019; Skarbek et al., 2021). Such concerns have been raised in the context of inefficiencies in the current environmental risk assessment framework (EFSA, 2012; Brühl and Zaller, 2019; Sgolastra et al., 2020; Topping et al., 2020).
Building on this body of evidence, there are several key issues related to bee health risk assessment of multiple chemicals and stressors, and broadly speaking for terrestrial organisms, that need to be addressed:
The environmental risk assessment of regulated products (e.g. PPPs, biocides, veterinary products) is following the ‘one substance’ approach even though data on formulations with multiple active substances are reviewed for the registration of formulated products (see previous Section 2.1). Further work is needed to take account of current practices that lead to the co‐occurrence, co‐exposure and potential combined effects (additive, synergistic or antagonistic) of multiple chemicals on organisms through time. E.g. current farming practices can lead to mixture applications (Fryday et al., 2011), multiple applications to single crops (Garthwaite et al., 2015; Luttik et al., 2017) and also the application of veterinary products inside the hives (Mullin et al., 2010; Lozano et al., 2019).
The risk assessments of regulated chemical products need to be conducted in the context of multiple stressors such as non‐regulated chemicals, biological agents and beekeeping management practices and potentially impacted by habitat, weather and climate.
There is a need for a link between the temporal dynamics of the exposed populations in the landscape and the desired level of protection of the organisms. A proper hazard and exposure assessment should be developed enabling a spatio‐temporal landscape for environmental risk assessment to be performed. E.g. given the lifespan of worker bees (~ 40 days, during the foraging season, approximately from spring to autumn), and an awareness that bees and their colonies can be continuously exposed to chemicals throughout all the beekeeping seasons (Tosi et al., 2018), a longer chronic timeframe would be more realistic to allow chronic lethal and sublethal effects occurring beyond 10‐days to be captured (OECD, 2017; Simon‐Delso et al., 2018; Tosi et al., in press).
There is a requirement for evidence regarding species‐specific traits in relation to chemical toxicity, in particular for surrogate species used to cover main taxa/guilds.
Sublethal effects are not fully addressed by the current regulatory risk assessment schemes because of the lack of knowledge of such effects on individual bees and at the colony level (for social bees such as honey bees) over a range of time scales, from relatively short to long (i.e. from a few hours to a few months).
Indirect effects that have been recognised as significant at the ecosystem level (e.g. effects on pollination services) are currently not accounted for.
A scientific rationale is needed for the derivation of assessment factors taking into account variability and uncertainty about extrapolations from laboratory experiments to field trials. While representativeness is higher in field than laboratory conditions, variability and uncertainty are likely higher in the field because of the foraging range of the bees (which covers larger surface areas than those covered in the higher tier studies, leading to underestimating the foragers’ exposure).
Context dependency should be addressed to quantify exposure and susceptibility at individual and population levels, considering both regulated and non‐regulated stressors. Furthermore, there is a scientific need for more data on realistic, context‐specific risk assessment scenarios, reflecting the choice and importance of landscape management, and the timing and frequency of PPPs (Topping et al., 2016).
Data gaps on pre‐existing conditions related to habitat, nutritional status as well as effects from other stressors on bee populations need to be filled. Such pre‐existing conditions might be the result of several carry over effects, as seen with pollen scarcity that influence colony development over the longer term (Requier et al., 2016) and therefore, long‐term monitoring will be needed to cover such effects.
There is limited quantitative information on the actual exposure and toxicity (including colony recovery and resilience) of pesticides in honey bees.
2.2.2. Current challenges to honey bee health risk assessment
There is a series of challenges faced with the risk assessment of PPPs for honey bee colony health in the context of multiple stressors. In particular, risk assessments need to consider:
-
The biological complexity of the honey bee colony, the role of individuals within the superorganism, the reliance on appropriate behaviours of many individual bees on the proper functioning and health of the colony and the factors that influence colony dynamics (noting that the current risk assessment guidelines cannot capture the complexity of the dynamics of a superorganism):
-
–
The challenge in seeking to connect individual bee responses to colony‐level impact.
-
–
The challenge in linking the specific protection goals to sublethal endpoints that affect individual bees and in turn the colony that is functioning as a superorganism.
-
–
The challenges faced in risk assessment, including multiple routes of exposure (i.e. acute vs. chronic, oral vs. contact), co‐exposure to multiple chemicals, delayed exposure through honey stores and comb wax and variability of chemical toxicity in relation to multiple factors (such as seasonality, bee age, bee genetics).
-
–
The genetic diversity of honey bees, including variations within and between colonies and between the subspecies present in EU that may be sufficient to influence a risk assessment.
-
–
-
The complex landscape in which the colony is situated and foraging, with implications for many aspects of the risk assessment, including PPP co‐exposure:
-
–
The temporal co‐occurrence of multiple stressors.
-
–
The spatial scale affecting potential recovery.
-
–
The interactions that occurs between these stressors (both regulated and non‐regulated).
-
–
Indirect effects due to loss in food/habitat.
-
–
Although many of these issues are relevant to environmental risk assessment more broadly, honey bee risk assessment presents several particular challenges that are considered further in Sections 2.2.2.1 and 2.2.2.2 . As examples, honey bee colonies are superorganisms with complex feedback loops, buffering effects and emergent properties. Stressor effects occur at the level of the individual bee, indirectly leading to effects at the colony level that can be difficult to predict. Environmental risk assessment in mammals and birds (EFSA, 2009) is conducted at the level of the individual for the acute effects and at the level of the population for the long‐term effects. In non‐target arthropods, the risk assessment is conducted at the level of the population (EFSA PPR Panel, 2014). Furthermore, foraging from a single honey bee colony can occur over a landscape that is both large (e.g. for a foraging distance of 5 km, the foraged surface area goes up to 80 km2) and complex, with the potential for combined exposure of the colony to multiple chemicals and other stressors. The complexity of landscapes varies in different agricultural contexts, from homogeneous (e.g. intensive agriculture presenting less diverse floral resources) to more heterogeneous (e.g. mixed cropping systems presenting more diverse floral resources and non‐cultivated areas such as gardens, field edges, roadside areas (ditchbanks, streambanks), urban/suburban lands, etc.).
2.2.2.1. The complexity of a honey bee colony
Honey bee colonies are biologically complex. Each colony is comprised of one fertile female queen, tens of thousands of unfertile female workers and hundreds of fertile male drones (Winston, 1987). Honey bees are eusocial insects, and colonies are characterised by cooperative brood care, overlapping generations within a colony of adults and a division of labour into reproductive and non‐reproductive groups (Seeley, 1985; Winston, 1987). The individuals have differing life stages, with differing diets and energy requirements across these life stages and castes (Michener, 1974). Although each individual has specialised tasks (nursing, foraging, etc.), the colony members work cooperatively as a ‘superorganism’ to support functions that are comparable with those of cells in a multicellular organism (Wilson and Sober, 1989; Moritz and Southwick, 1992; Page et al., 2016). The proper functioning and health of the colony is reliant on the appropriate behaviours of many individual bees.
The dynamics of honey bee colonies is influenced by a range of factors, given that the colony (e.g. demographic status) interacts closely with its environment (e.g. resource availability, weather conditions). As a result, the egg‐laying rate of the queen, a primary driver of colony dynamics, will be influenced by key feedback loops, including those between colony demography, colony size and egg laying. The number of nurse bees and food influx (from the environment) will each affect egg laying and brood survival, and hence the future colony structure (Robinson, 1992; Becher et al., 2013). A colony is able to control the ratio of in‐hive and forager bees through mechanisms of social feedback (Russell et al., 2013).
From individuals to colony effects
A key feature of honey bee colonies, as with other superorganisms, is the development of emergent properties, these being properties that emerge within a system that are different from those of the components (i.e. individual bees) that make up the system (Seeley, 1989; Wilson and Sober, 1989; Moritz and Southwick, 1992). Emergent system properties (e.g. colony size, brood production) are a function of long‐causal chains and feedback loops (positive and negative) between the interacting bees and their local environment. Examples of these emergent properties include thermoregulation (which is considered in further detail here), initiation of comb construction and colony population dynamics.
Thermoregulation of a colony is achieved through the actions of bees responding to temperature conditions in their immediate vicinity. As temperatures fall, bees cluster together with heat being generated by mature bees by shivering their flight muscles. Younger bees, which cannot generate heat in this way, are found at the centre of the cluster. In response to the surrounding environment, therefore, worker bees can warm up or cool the colony and brood combs by various mechanisms such as shielding and fanning (Stabentheiner et al., 2010). Thermoregulation in honey bee colonies is costly in terms of energy budget, particularly during the winter (EFSA PPR Panel, 2012). In addition, in Europe, winter mortalities in honey bee colonies are of concern (Laurent et al., 2016). A hypothetical and explanatory scenario was made to describe the possible complex mechanisms from the individual to colony level behind such mortalities (Figure 2). The example used the context of exposure to multiple stressors and included a failure in thermoregulation (Figure 2). These processes demonstrate the various mechanisms by which a colony may lose individual bees from spring to autumn (via intoxication and infection loops) and result in a colony of bees that is not large enough to thermoregulate efficiently in winter (first chilling loop), which in turn may lead to a much smaller swarm (second chilling loop; step 4) and ultimately lead to the loss of the colony. There could be several chilling loops by which the colony loses bees more or less rapidly over time, depending on the strength of the colony before wintering (initial population size).
Figure 2.
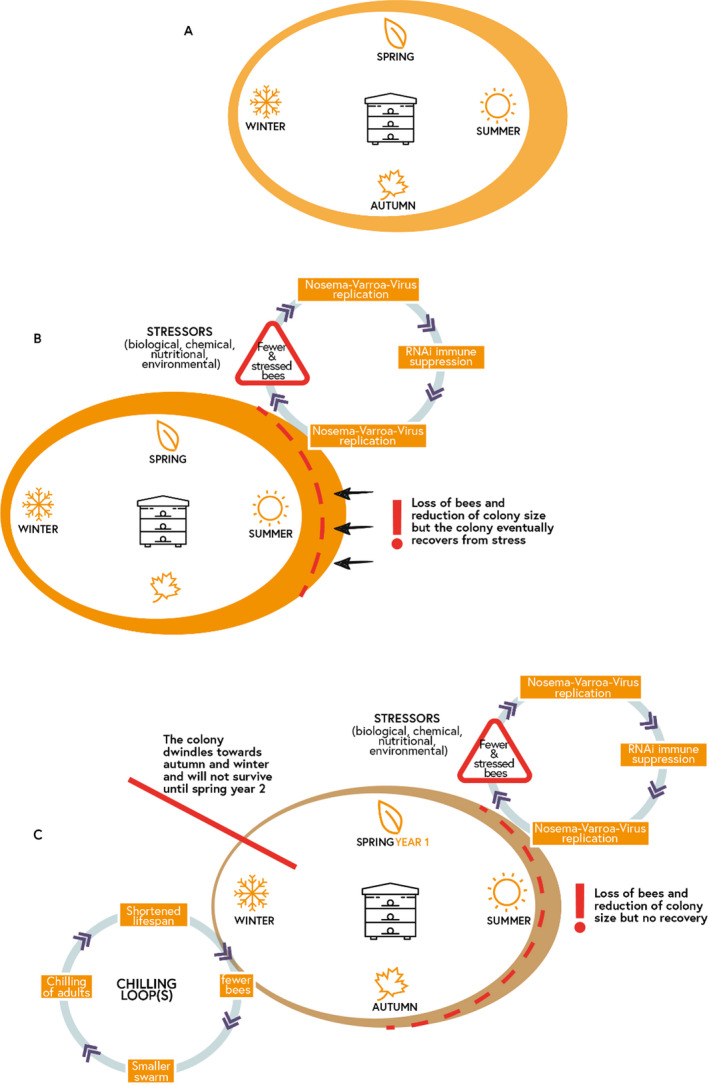
Development and size of a colony under different situations: from an average colony maintained by good beekeeping practices (A) and from a robust or weak colony (B and C, respectively). Exposure to chemical, biological and/or nutritional (i.e. food quality and quantity) stressors leads to either recovery (B) or winter colony mortality (C)
This example of emergent effects highlights the complex relationship between individual bees and colony effects. Responses are multifaceted and occur at different levels: at the level of the individual (e.g. thermoregulating bees, wax‐producing bees, nursing bees, foraging bees); at the level of the process (with associated feedback loops) (e.g. production of heat or ventilation, production of wax, egg laying and brood care); and through emergent properties (e.g. thermoregulation, comb construction, colony population dynamics). Furthermore, these systems are complex and adaptive (sensu Holland, 2006), and stressors can be multiple and interacting. The various internal and external aspects of a colony interact dynamically in both space and time and are composed of many components interacting with each other in non‐linear ways, including learning. In summary, therefore, the link between these factors and overall colony behaviour and characteristics is not simple, with the potential for an apparent disconnection between individual responses and colony effects.
Stressors that do not lead to direct mortality of individual bees can still substantially and adversely affect colony health. E.g. Varroa destructor, a parasitic mite that feeds on honey bees represents a major threat for the colonies (Bailey and Ball, 1991). Another example is neonicotinoid pesticides which can impair forager flight, locomotion, navigation and orientation (Tison et al., 2016; Tosi and Nieh, 2017; Tosi et al., 2017b), leading to a significant reduction in the number of foragers returning to the nest after a day's activity (Henry et al., 2012). In response, there are short‐term compensatory effects at a colony level, but at a cost to the colony. Nurse bees are assigned to forage at an earlier age, and drone brood production is delayed in favour of increased worker brood production (Henry et al., 2015). In the long term, however, adverse colony impacts can be substantial, leading to significantly reduced adult bee populations, a decrease in brood surface areas and average frame weights and reduced in‐hive temperature control (Meikle et al., 2016; Tsvetkov et al., 2017). As one example, in the mid and long term for the bee population in a given area, the reduction in the number of males produced could affect the fertilisation of the virgin queens, with possible negative consequences on the dynamics of bee populations in this area. Indeed, queens artificially inseminated with a smaller quantity of semen than normal present physiological disorders and exhibited lower rates of overwintering survival than normal (see the review by Brutscher et al., 2019).
Importance of sublethal effects for bee colony health
There is increasing evidence of adverse impacts on honey bee colony health from sublethal effects that may translate into lethal effects, including those affecting communication (Eiri and Nieh, 2012), memory (Decourtye et al., 2005), navigation/orientation (Fischer et al., 2014; Henry et al., 2015; Tison et al., 2016), locomotion such as bee activity and motor functions (Tosi and Nieh, 2017; Wu et al., 2017), thermoregulation (Vandame and Belzunces, 1998; Tosi et al., 2016), reproduction (Wu‐Smart and Spivak, 2016), development (i.e. HPGs) (Hatjina et al., 2013), social immunity (Brandt et al., 2016), food consumption (Blacquiere et al., 2012; Tosi and Nieh, 2017) and preference (Kessler et al., 2015), phototaxis (Tosi and Nieh, 2017) and detoxification (Han et al., 2018; Tarek et al., 2018). Some authors have highlighted the impact of sublethal effects (Desneux et al., 2007) on colony health when bees are exposed to neonicotinoids (e.g. Pisa et al., 2015; Meikle et al., 2016; Wu‐Smart and Spivak, 2016; Pisa et al., 2017), sulfoximine (Siviter et al., 2018) and butenolide (Tosi and Nieh, 2019) insecticides. While pesticides are the most studied factor to cause sublethal effects on honey bees (Thompson, 2012; ANSES, 2015), other stressors may be involved.
These studies highlighted the need to capture sublethal effects which, at the time of writing, are currently not considered in environmental risk assessment, and to link sublethal effects to specific protection goals (SPGs). The association between sublethal effects (measured at the level of the individual) and SPGs (defined at the level of the colony) has not previously been made. Furthermore, the current system cannot appropriately capture the impact of sublethal effects either in the laboratory (lower tiers) or in the field (higher tiers) due to the limitations of the current tests (Tosi and Nieh, 2019). Nonetheless, a solution specific for highly social honey bees should be found: standard SPGs and risk assessment guidelines are not designed for superorganisms with a complex behavioural repertoire fundamental for honey bee colony functioning and fitness (Berenbaum and Liao, 2019). This has become a major challenge, as the criteria for PPP approval as listed in Regulation (EC) No 1107/20093 specifically requires the impact of PPPs on colony survival and development to be considered, taking into account larvae and behaviour of honey bees. Because chemicals such as PPPs can jeopardise both individual and colony health via sublethal alterations, we propose to specifically protect behavioural and physiological traits via novel definition of SPGs. A specific assessment of the variety of sublethal alterations caused by chemicals such as PPPs, together with an assessment of the best methods to implement in risk assessment, are urgently needed. Procedures described by the Organisation for Economic Co‐operation and Development (OECD, 2005, 2009) for the development of novel methodologies could be implemented in risk assessments, allowing the assessment of sublethal effects that are relevant to bee health and applicable in risk assessment schemes. Possible candidates are tests in which the design and outcome are close to real world conditions, thus requiring little extrapolation. E.g. tests assessing bee locomotion ability using freely moving individuals to measure endpoints that can be relatively easy to connect to the real world in a quantitative manner (i.e. variation in distance travelled) would be preferred. Risk assessment should obtain high‐quality sublethal effect data that can feed both the classic risk assessment scheme and the novel (i.e. modelling) system‐based approach.
Multiple routes of exposures to chemicals
Honey bees are exposed to chemicals such as PPPs through multiple routes (EFSA PPR Panel, 2012). A honey bee colony is comprised of tens of thousands of bees who live inside their nest but also fly freely outside, with the potential for contact with chemicals both in their nest and in the field.
Honey bees can be exposed to chemicals through ingestion, contact and inhalation. The exposure can occur at a single point in time (acute) or over prolonged periods (chronic).
Bee exposure is influenced by multiple aspects:
Application methods: PPPs can reach the bees via multiple application methods, including e.g. spray, seed dressing, granules, fumigation and soil drenching.
Contaminated material: PPPs can contaminate the air (i.e. through spray treatments), the food (i.e. nectar, honeydew, pollen, water) and other materials (i.e. resin, water collected for thermoregulation purposes and in rare occasions guttation water) to which bees are exposed in the environment, as well as within their nest (i.e. wax, propolis, honey, beebread). Further information is provided in Section 3.2.2.2 .
Bee type: Exposure depends on the level of development (i.e. larvae, adults), the sex and caste (workers, queen, drones), the task performed (i.e. nurses, wax‐producing bees, foragers) and the season (i.e. summer vs. winter bees), as each one of these aspects underlies different nutritional and behavioural habits.
The genetic diversity of honey bees
Honey bees (Apis mellifera), both in managed and feral colonies, are represented by a complex of about 26 subspecies, of which 11 are present in EU, including the strain Buckfast that is distributed all over EU (Ruttner, 1988; Sheppard and Meixner, 2003; Appendix C ). In addition, there are some local populations that are well adapted to the local flora and area (Büchler et al., 2014; Hatjina et al., 2014). These locally adapted populations are referred to as ‘ecotypes’ (Strange et al., 2007; Meixner et al., 2013). E.g. the honey bee population from the Landes region, in France, has its annual brood cycle perfectly matched with the locally abundant floral source (Strange et al., 2007).
Apiculture in Europe is facing a rapid loss of biodiversity. Hybridisation due to increased movement of bees for honey production, pollination and overwintering in more favourable regions as well as trade of honey bee queens are currently the main threats to the diversity and conservation of the native and locally adapted populations (Meixner et al., 2010; Büchler et al., 2014; Momeni et al., 2021).
Under EU legislation, testing for approval of the use of an active substance is requested and conducted at the species level, without regard to the subspecies. The Western (A. m. mellifera), Italian (A. m. ligustica) and Carnolian (A. m. carnica) subspecies or the Buckfast strain are the most often studied, but rarely confirmed by DNA testing. These species cover mostly the Western and central zones of EU, under‐representing the southern part (e.g. Spain, Malta, Portugal, Sardinia, Greece, Cyprus). Before a PPP is authorised, its use needs to be tested and approved across three EU regulatory zones, where each zone corresponds to the natural range of distribution of some honey bee subspecies and ecotypes (Appendix C ). The South Zone is comprised of nine EU MS and is the most diverse (10 subspecies), whereas the North and Centre Zones, comprising six and 13 EU MS, respectively, have only one and two subspecies, respectively.
From a regulatory perspective, it is relevant to determine whether genetics (in terms of variations in behavioural and physiological responses to environmental changes as well as in terms of adaptation to local conditions and stressors) can influence the outcome of the risk assessment, e.g. are some subspecies more sensitive to pesticides than others and are some subspecies more resistant to other stressors, which could interact with pesticides, than others?
Knowledge on the influence of genetics on the sensitivity of honey bee subspecies to pesticides remains scarce. Too few studies have been conducted to allow robust conclusions to be drawn. Rinkevich et al. (2015) demonstrated different sensitivities to exposure to multiple chemicals in three honey bee subspecies, with toxicity to a mixture of acaricide and insecticides increasing sevenfold in A. m. primorski (a synthetic subspecies) and by five‐fold in A. m. ligustica, each compared with A. m. carnica. Further studies conducted on A. m. mellifera, A. m. ligustica and A. m. carnica found differences in sensitivity to neonicotinoids between colonies rather than between subspecies, highlighting the importance of the colony genetic pool (Laurino et al., 2013). Although such studies tend to demonstrate genetics as a significant factor to consider when assessing the toxicity of pesticides in honey bees, substantive evidence is currently missing.
In contrast, studies assessing resistance of honey bees to other stressors such as Varroa are more abundant. Survival to infestation by the mite was reported most notably in the African race A. m. scutellata in Brazil (Moretto et al., 1991; Rosenkranz, 1999; Locke, 2016), and in small subpopulations of European races as in Sweden (Gotland island) (Fries et al., 2003; Fries and Bommarco, 2007) and Russia (Danka et al., 1995; Rinderer et al., 2001, 2010) and others (review in Locke, 2016). However, to date, no specific bee subspecies in Europe has been shown to have more resistance to Varroa than others.
Finally, given the (hyper)polyandrous mating system of honey bees (a queen is inseminated by several males, supposedly on average 12, but most probably by dozens of males (Baudry et al., 1998; Withrow and Tarpy, 2018)), the genetic diversity of honey bees is translated into significant variability between and within colonies. Such diversity has an impact on risk assessments, as a considerable number of colonies would need to be tested in field conditions to detect expected field‐realistic effects such as sublethal effects (Cresswell, 2011).
2.2.2.2. The complexity of the environment of a honey bee colony
Bees operate in landscapes that are comprised of a range of stressors/factors, drivers and structures that determine the context for the expression of the exposure to PPPs. As noted in Section 2.2.2.1 , the colony is a complex adaptive system that is highly responsive to changes in this context. Therefore, the dynamism of the superorganism honey bee plays a central role in predictive models for multiple stressors and bees, influencing the time‐ and space‐specific conditions of individual bees, finally altering individual and societal decisions, resulting in emergent colony dynamics.
Context features of particular importance to the bee complex adaptive system include each of the following:
Landscape structure interacts with stressor exposure, resource availability, land use and bee decisions and dynamics through variation in landscape element structure. The composition of the landscape in terms of heterogeneity and structural pattern will interact with bee behaviour and ultimately result in colony variation. Landscape structure is therefore a complex confounding variable influencing the effect of land use and management.
Land use and management alters the landscape in complex ways. In many EU locations, agricultural land use predominates as a series of fields of differing sizes, usage and management, resource availability and patterns of PPP application (European Union, 2018). In complex landscapes, there will be co‐occurrence of PPPs, at a single point in time and/or sequentially, leading to co‐exposure of honey bee colonies to multiple chemicals throughout a single foraging season (Mullin et al., 2010; Tosi et al., 2018), including in honey stores and comb wax (Mitchell et al., 2017). Bee colonies living in complex landscapes are also co‐exposed to multiple stressors which prevalence and level varies dynamically, such as biological agents and nutritional stress.
Resource availability for bees varies depending on the landscape surrounding the colony. This variation depends on plant species and leads to spatial and temporal differences for each colony. Each plant has a peculiar blooming period with specific production of pollen and/or nectar in terms of quantity and quality (Couvillon et al., 2014; Donkersley et al., 2014; Baude et al., 2016). Thus, given the variability of both climate, weather and land use in EU, resource availability can vary greatly at smaller and broader geographic scales. Resource availability can also influence competition among bees, in particular between honey bees and bumble bees (Herbertsson et al., 2016). As a result of competition between honey bees and other plant‐pollinators, high‐density beekeeping in natural areas appears to have more serious negative, long‐term impacts on native pollinator biodiversity than was previously assumed (Valido et al., 2019).
Nutritional stress occurs in dearth of food that lead to suboptimal levels of food quality and quantity (i.e. decrease or lack of nutritional intake, also including its nutrients diversity), which can lead to an adverse impact on bee health (Naug, 2009).
Individual decisions made by honey bee foragers, including forager preference for particular fields, crops and non‐crops, are a key feature of bee colonies.
Climate and weather are crucial parameters driving bee health as they deeply influence their environment, e.g. leading to a seasonal sublethal and lethal impact of stressors (Tong et al., 2019) that can even impair winter colony survival.
-
Biological agents:
-
–
Infectious agents. Nosemosis is a disease in adult bees that affects the digestive tract and can cause acute diarrhoea, and in some cases mortality, in affected colonies (Fries, 1993). Nosema fungi are members of the microsporidia flora, a group of eukaryotic, obligate intracellular, single‐cell parasites. Two species of Nosema are found in honey bees: Nosema apis and N. ceranae (Fries, 1993; Higes et al., 2006).
-
–
Macroparasites. The mite Varroa destructor is a parasite of adult honey bees and their brood and the course of this parasitism is usually lethal (OIE, 2008). However, the role of V. destructor alone is not clear, because the mite is often a carrier and amplifier of viruses, in particular the DWV (Lanzi et al., 2006).
-
–
Bacteria. Paenibacillus larvae and Melissococcus plutonius are the agents responsible for American Foulbrood and European Foulbrood, respectively (Forsgren, 2010; Genersch, 2010).
-
–
Viruses. ABPV has been detected in several MS (Ribière et al., 2008; de Miranda et al., 2010). When fed, sprayed on or injected into, healthy bees, it makes them tremble and paralysed within a few days (Bailey, 1967). The virus can infect larvae, pupae and adult bees (de Miranda et al., 2010). ABPV commonly occurs at low levels in apparently healthy bee colonies and causes no reliable field symptoms (Aubert et al., 2008). However, several studies have reported that ABPV can be a major cause in several MS of mortality in colonies infected with V. destructor (Ribière et al., 2008; de Miranda et al., 2010). DWV is can be transmitted with V. destructor (Ribière et al., 2008). It is one of the most implicated predictors of honey bee health decline from various studies conducted in several MS (Budge et al., 2015). Individual infections with DWV may cause deformation of emerging bees and earlier death in adults, reducing the survival of winter honey bees (Dainat et al., 2012).
-
–
Predators. Vespa velutina (yellow‐legged hornet) is an Asian hornet species introduced in EU, reported for the first time in 2004 in France (Haxaire et al., 2006). It is an insect predator, in particular of honey bee colonies, and attacks foragers departing and returning to the hive (Arca et al., 2014; Monceau et al., 2014).
-
–
Beekeeping practices that deviate from best practices (Bee Research Institute, 2009; EFSA AHAW Panel, 2016) can substantially influence honey bee colony health.
Other stressors of anthropogenic origin, e.g. pollutants, roads and manicured parklands (etc.), may also be relevant (Winfree et al., 2009; Søvik et al., 2015; Bellucci et al., 2019).
3. A systems‐based approach of multiple stressors in honey bee colonies
3.1. A proposed systems‐based approach
The systems‐based approach has been developed in the context of both the key environmental risk assessment concerns (Section 2.2 ) and the specific challenges to honey bee health risk assessment (Sections 2.2.1 and 2.2.2 , respectively). It is aligned with the goals of the European Green Deal (von der Leyen, 2019), notably protecting the environment and preserving biodiversity as highlighted in both the Farm to Fork and Biodiversity Strategies. It is also in support of new strategic priorities for EFSA in the area of environmental risk assessment (EFSA, online a,b). EFSA recognises the need to advance environmental risk assessment, to address both current and new challenges and to respond to growing societal concerns and aspirations towards a more sustainable use of the environment for future generations.
The proposed systems‐based approach includes two core components, which are tightly linked, plus interacting components and applications, as described in Figure 3.
Figure 3.
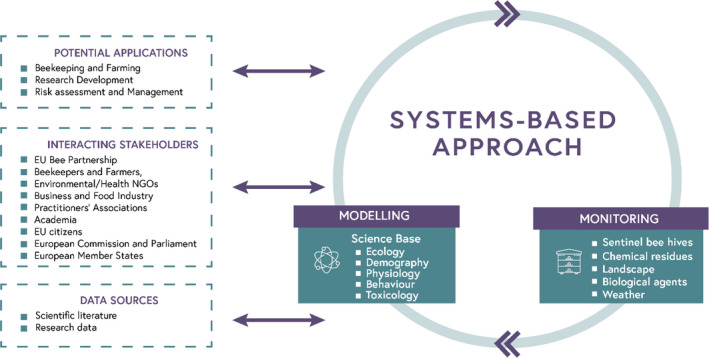
A systems‐based approach for the risk assessment of multiple stressors in honey bee colonies (the ‘modelling’ component refers to the ApisRAM model and the ‘monitoring’ component is related to the sentinel hive and the surrounding landscape to a radius of 3 km around the hive as well as the broader EU landscape)
The two core components include:
modelling to predict the impacts of stressors on honey bees and to support evidence‐based risk assessment of agrochemical use. The development and ongoing revision of the modelling component will be informed by current and future scientific knowledge. Further, the modelling component can inform the monitoring component with respect to hot spots of interest where sentinel hives could be placed;
monitoring to assess bee colony status, residues and levels of potential stressors, both within and outside bee colonies and also to provide ongoing calibration and validation of the modelling component.
The modelling component is constructed based on a fundamental understanding of the science base of the organism in its environment (ecology, demography, physiology, behaviour and toxicology). Knowledge gained from well‐designed experiments and studies, conducted in the laboratory and in the field, have and will continue to play a critical role in this regard. Contributing to the science base, there is a need to estimate the landscape‐specific use of agrochemical‐treated crops, attractive to bees and used by foragers, which depends on the dispersion and richness of flowers in the region, and of the impact of the prevailing stressors in the region, including both biological (such as infectious agents) and chemical stressors (e.g. several applications of PPPs in time and space) including cumulative and synergistic effects. There is also a need to continuously investigate bee health and the individual and combined effects of stressors, so that novel knowledge can be implemented in the model, therefore increasing its prediction accuracy. The science base provides fundamental data on multiple pesticide toxicological endpoints, population dynamics, as well as on the interrelationships and impact of multiple stressors.
The monitoring component focuses on ongoing, structured data collection, primarily from a series of sentinel hives positioned in different climatic zones and landscapes within the EU. The data to be collected include:
Colony data from the sentinel hives, including exposure to multiple stressors such as biological agents (see Section 3.2.2.1 ).
Chemical residue data, primarily from the sentinel hives. This will include chemical contaminants in honey and other bee‐related products, e.g. comb honey, pollen and royal jelly. The chemical residue data in pollen should come with palynological data, that would allow to link the contamination of pollen samples by PPP with the agricultural resources foraged by honey bees, and finally with PPP uses behind the contamination, as recommended in the field data collection conducted in Portugal and Denmark to calibrate the model ApisRAM (Dupont et al., 2021). Other bee products collected from the sentinel hives could be relevant, e.g. pollen that is used as feed and beeswax from honey bee colonies that may be used in honey pots and consumed in the form of honeycombs by humans (EFSA, 2020). In addition, other data will be useful, collected as part of existing monitoring schemes in the EU (European Parliament, 2018; Group of Chief Scientific Advisers, 2018), under Article 31 of Regulation (EC) No 396/20056 (EFSA, 2019), and from applicants for the registering of PPPs to assess exposure to chemicals in nectar and pollen foraged by bees. Furthermore, data on veterinary residues (which are related to specific beekeeping management practices) may be useful, specifically if these data include locational information.
Landscape data, relating both to sentinel hives and the general EU landscape, including the localisation of farms around beehives to assess the exposure of honey bee colonies to veterinary products (e.g. pastures, receiving cattle faeces contaminated with antiparasitic products, should be known). This will be collated from a wide range of sources, including GIS mapping sources, the CAP subsidy scheme data, i.e. Land‐Parcel‐Information‐System data, farm and crop management expert knowledge, models and published data on nectar and pollen sources and weather data. Into the future, it would be important that pesticide data are accessed electronically, under the Sustainability Directive.
As highlighted above, the modelling and monitoring components will be closely interlinked. Monitoring will be undertaken through a network of sentinel hives, allowing ongoing collection of harmonised, high‐quality data to be used for ongoing model validation. Following collation and interpretation, these data will also provide stakeholders with an understanding of honey bee colony health at different spatial scales. The modelling component will be used to simulate honey bee colony health at multiple locations throughout the EU (essentially a grid of simulated hives, but not limited to the location of the sentinel hives), including at locations with, e.g. differing environments, farming systems and bee genetics. The modelling component can be used as an early warning system. It can also be used to predict the future status of honey bee colonies, using a computer simulation whose inputs include monitoring and toxicological information. Some applications are envisaged, related to beekeepers and farmers, research development, risk assessment and risk management, as outlined further in Section 4 . The system would be connected to various stakeholders and organisations involved in monitoring (e.g. beekeepers, farmers, scientists, applicants, MS, European Commission, EP and EU citizens) and collecting and exchanging standardised and interoperable data (see Section 5.2 ).
The detailed specificities of this systems‐based approach are further described below.
3.2. The core components of the systems‐based approach
3.2.1. Modelling
3.2.1.1. Introduction
Modelling approaches are useful to simulate the behaviour of whole population dynamics as well as to focus on significant phenomena detrimental to bee‐life history traits (Devillers, 2014). Modelling approaches can also provide support to our understanding of bee mortality in the context of complex stressor interactions to which bees are continuously exposed in their environment (Barron, 2015). In particular, predictive system models may be used to investigate tipping points in bee populations under environmental stresses (Henry et al., 2017).
A number of bee mechanistic models have been developed for honey bee colonies (Schmickl and Crailsheim, 2007; Becher et al., 2013; Khoury et al., 2011, 2013; Torres et al., 2015), bumblebee colonies (Bryden et al., 2013) and solitary bees (Everaars and Dormann, 2014), including the BEEHAVE model (Becher et al., 2013) which was the first attempt to couple in‐hive dynamics and pathology with foraging dynamics in a spatially explicit landscape. In an EFSA review published in 2015 (EFSA PPR Panel, 2015), the early BEEHAVE model was found not to be suitable for use in a regulatory context, and therefore is not able to address the risk posed by multiple stressors at a landscape level. At the time of this review, a range of concerns were raised, including the absence of a pesticides module and other missing stressors. While NetLogo, which was the choice of modelling environment, provides an excellent user interface, it presents limited opportunities for model extension to include other built‐in modules. The model was found to be larger and more complex than could easily be accommodated by the NetLogo environment, which increased the difficulty of code testing and development. The review identified other constraints including limited consideration of exposure pathways, the need for more sophisticated and diverse environmental scenarios and the need for model validation. The original BEEHAVE model (Becher et al., 2013, 2014) was developed prior to the release of the EFSA PPR opinion on good modelling practice (EFSA PPR Panel, 2014) and is only in partial compliance. In the 2015 EFSA review (EFSA PPR Panel, 2015), points of non‐compliance were listed, including the lack of a formal model description, no clear separation of model variables into state and forcing variables and very limited referencing or documentation in support of model equations or algorithms.
The original BEEHAVE model (Becher et al., 2013, 2014) was used to simulate the impacts of foraging stress (Horn et al., 2015), pesticides and pharmaceuticals (Rumkee et al., 2015; Carter et al., 2020), the Asian hornet, Vespa velutina (Requier et al., 2020), sublethal effects (Thorbek et al., 2017a, Schmolke et al., 2019) and to evaluate overwintering success (Abi‐Akar et al., 2020) and pesticide protection goals (Thorbek et al., 2017b). There has been development of BEEHAVE relating to foraging (BEESCOUT; Becher et al., 2016), a pollen exposure‐effects module (explicitly linking exposure to pesticide residues in pollen with colony effects, Schmolke et al., 2019) and model evaluation using field data (Agatz et al., 2019; Schmolke et al., 2020), which addresses some of the issues raised previously (EFSA PPR Panel, 2015). However, BEEHAVE does not currently simulate a dynamic environment with fully differentiated forage resources and detailed farming and pesticide use. It also does not represent individual bees nor the spatial distribution of in‐hive resources, in‐hive pesticide fate or dynamic multiple stressor interactions and epidemiology, which together result in a superorganism with multiple interacting looping effects. BEEHAVE also lacks an ecotoxicological module of sufficient detail.
Following the PPR evaluation (EFSA PPR Panel, 2015), EFSA has evaluated the potential to extend BEEHAVE to incorporate the necessary complexity to support the systems approach. However, BEEHAVE remains in the NetLogo environment, which in the earlier review (EFSA PPR Panel, 2015) was not considered adequate to achieve future goals for honey bee environmental risk assessment (i.e. taking into account the complexity of a honey bee colony and its environment). Therefore, the MUST‐B working group defined specifications for a new colony model (EFSA, 2016a,b). Development of a model fulfilling these specifications was awarded to ApisRAM via an EFSA procurement open call. As such, ApisRAM seeks to overcome the limitations of BEEHAVE to be able to realistically represent a honey bee colony in a range of environmental contexts. This work was further supported by an extensive EFSA data collection project with the express purpose of calibrating and validating ApisRAM (EFSA, 2017a,b).
3.2.1.2. An agent‐based simulation for honey bee colonies: ApisRAM
ApisRAM is a honey bee colony model that was designed to assess risks posed to honey bees exposed to pesticides in the context of multiple existing stressors, including Varroa, viral diseases, Nosema, weather, beekeeping practices and the availability of resources in the landscape. The technical specifications of ApisRAM were developed within the MUST‐B project (EFSA, 2016a,b), with the following objectives:
integration of multistressor impacts (pesticides and other regulated or non‐regulated stressors),
simulation of interactions between stressors,
prediction of complex system‐dynamics, including the possibility to integrate mitigation options (e.g. width of sown field margins or restricted pesticide usage),
assistance in clarifying the relative importance of different stressors, including how the impact of a pesticide on colony health might change with changing context, e.g. climate, and/or farming.
ApisRAM is written in C++ and follows an object‐oriented programming design. Each bee is represented as an object in the simulation and the life of the bee and the tasks it performs are simulated in detail at 10‐min time intervals. The model design follows the specifications described by EFSA (2016a,b) and considers three basic sets of modules:
-
The «Foraging», «Colony» and «In‐Hive Product » modules. These modules are dynamic and comprise the following processes:
The «Foraging» and «Colony» modules, which are based on energy budgeting at the individual level. Biological processes are described in terms of demographic (development, fecundity and mortality), physiological and behavioural traits.
The «In‐Hive Products» module describes the processes of inflow, maturation, storage and outflow of in‐hive products. Within the hive, the resource is spatially realistic, being stored in simulated cells on simulated frames.
«Resource Providing Unit» and «Environmental Drivers» (RPU‐ED) modules. The RPU & ED modules handle hourly data for all time‐varying parameters relevant to key model output parameters, e.g. including hourly production of pollen and nectar. A function of the landscape module, the RPU & ED modules will provide input to the colony module on the spatio‐temporal availability of resources and weather conditions. ALMaSS (see Section 1.2.1 ) uses existing environmental fate models representing soil and vegetation compartments, daily redistribution and degradation and application.
-
Additional factors and stressors modules:
The «Pesticides» module comprises all the concepts involved with pesticides exposure and effects.
The «Biological Agents» module comprises the effects on colony and in‐hive products of Varroa destructor with its two associated viruses (DWV and ABPV) and Nosema.
The «Beekeeping Management Practices» (BMP) module comprises a selection of common EU beekeeping practices.
Within ApisRAM, ALMaSS provides the environment into which a colony model is placed, controlling the conditions under which the colony model is simulated. ALMaSS has existed for over 20 years and is currently undergoing expansion from its Danish roots to have the capability to represent conditions in 12 countries (Belgium, Denmark, Finland, Ireland, Italy, Portugal, Poland, France, Sweden, the Netherlands, UK and Germany). The underlying landscape simulations are provided by various projects including H2020 Ecostack, B‐GOOD and PoshBee. The ALMaSS landscapes are highly detailed environments, modelled usually on 10 × 10 km area at a resolution of 1 m2. The farming activities and vegetation grown are modelled in detail, with all farming activities that might influence the bees being modelled at field level in realistic time frames. Foraging/scouting bees can therefore interact with this landscape and be exposed to pesticides, forage/scout for resources and bring material back to the colony from the simulated environment.
Key design features separating this model from previous models are primarily rooted in the mechanistic detail with which the behaviour and biology of the bees is represented. This ABS is designed to incorporate local (to the individual) input information, including communication between bees and to represent both the positive and feedback situations that this creates. Key interactions are mediated through common «currencies», i.e. energy for driving many behaviours (foraging, thermoregulation), and a bee ‘vitality’ index. This vitality is directly affected by biological agents (infectious agents such as Nosema, DWV and ABPV and pests such as Varroa), pesticides, temperature and nutrition to individual bee performance and lifespan, thus acts as an integrative link. The strength and form of effect can be varied with the stressor. In the model, ‘vitality’ comprises the bee's immunocompetence status and a daily probability of dying, and therefore provides a method for integrating the effects of both direct and indirect stressors.
A reduction in vitality occurs based on the impact of any stressor. To implement this, the pesticide and biological agent stressors affect the bee immune system strength, which interacts with the vitality. Nutrition and temperature will also change the vitality parameter. Good nutrition (enough food) will increase vitality, whereas poor nutrition will also contribute to a decreasing vitality. Temperature extremes will also change the bee's vitality. When the immune system is strong and the bee has access to the required food to develop (larvae) and perform its daily activities (adults) and to thermoregulate to maintain brood temperature (for larval development) and to provide the heat needed to the nest (for adult survival) during winter, the bee's vitality is strengthened. Otherwise, it will decrease until death. By combining these stressors through the vitality parameter, the feedback between stressors can be modelled mechanistically. The model can create a non‐linear feedback linking different stressors e.g. automatically increasing the impact of pesticides for weakened bees due to biological agents and/or temperature/nutrition. This mechanism provides the basis for incorporating the effect of multiple pesticides into ApisRAM (see Section 3.2.1.3 ).
Each individual bee is characterised by a continuous and time‐varying body burden of PPPs. Pesticide modelling utilises the ALMaSS pesticide models for modelling pesticide spray application and seed coating and environmental fate and combines this with the ‘vitality’ index using a toxic unit (TU) approach. A TU is a measure of toxicity as determined by the acute toxicity units or chronic toxicity units with higher TUs indicating greater toxicity (EFSA Scientific Committee, 2019) (see Section 3.2.1.5 for more details). Using this approach, it will be possible to model multiple chemical stressors, although the current model only considers two chemicals at one time. ApisRAM also includes the immunosuppression action of Varroa and has a detailed representation of temperature control in the winter months, when the balance between resource use and temperature is critical to bee survival. In ABS such as ApisRAM, colony behaviours emerge from decisions and actions that are taken by individuals. The model is supported by a parallel EFSA project on field data collection that will provide data to improve and test the model from landscapes located in Denmark and Portugal (EFSA, 2017a,b) and, in the near future, from several other EU countries with additional data collection efforts generated from some EU research projects (i.e. B‐GOOD and PoshBee; see also Section 1.2.1 ).
Although the modelling approach developed under ApisRAM presents a lot of advantages for simulating the complex dynamics of a honey bee colony in its changing environment, it also presents some specific limitations in its current state of development and also some gaps that are further discussed below, in Sections 3.2.1.5 and 6.2 , respectively.
3.2.1.3. Integration of multiple‐stressor effects on individual bees in ApisRAM
To be able to dynamically model the complex interactions between stressors, a unifying approach is needed and requires linking the combined effects of PPPs or other chemicals with biological agents, environment and beekeeping practices described in Section 3.2.1 . This requires more data and assumptions than just the toxic response for PPPs and expands on the concept of applying a TU approach by also specifying what the combined toxicity has on the vitality component of the bee. As a starting point, the default assumption of dose addition is applied to lethal effects, and the associated impact on individual vitality is a proxy for the mortality rate. Thus, for each stressor, a common data structure needs to be provided based on the description of combined effects, their impact on vitality and associated dose–responses (Figure 4). To maximise flexibility two distinct components are considered for each stressor (e.g. changes in physiological/behavioural endpoints or in bee vitality/immune competence). This scheme works for both survival and sub‐lethal effects. The stressors combined and considered for a TU approach must be close enough in terms of toxicological effect to be considered as a single group.
Figure 4.
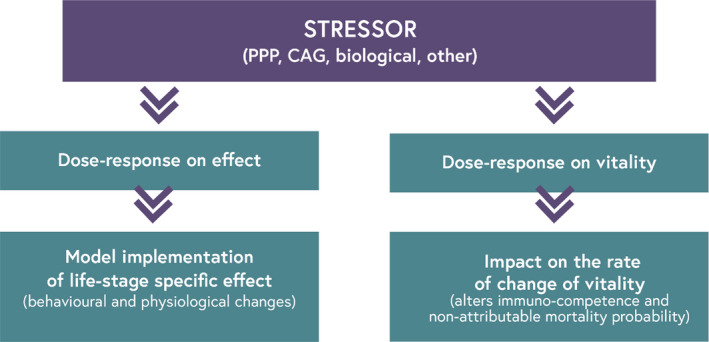
The basic data structure needed to specify stressor impacts on individual bees in ApisRAM. This requires the specification of stressor effect on physiology and behaviour as well as on vitality (CAG: cumulative assessment group; EFSA PPR Panel, 2013)
Overall, harmonised structured data for all these stressors expressed as TUs, effect descriptions and vitality impacts can be integrated into the ApisRAM model to model their overall impact on the colony viability.
For all cases, integration of stressor effects in ApisRAM requires the following steps to be taken:
The dose–response relationship between the stressor and the effect on the individual needs to be established. Ideally, a time‐dependency of such dose–response needs to be accounted for. However, such data are not often available and, in this case, only a simple threshold response value can be derived.
Regarding sublethal endpoints such as the Proboscis Extension Reflex (PER), the results of these tests need to be translated to a behavioural effect or a mode of action associated with exposure to chemicals or biological agents within the model. E.g. a sublethal effect measured in the laboratory could, if relevant, be translated as a dose–response at the individual level for the probability that foragers will not return to the colony. Similarly, other mechanisms, such as egg production, foraging range, temperature tolerance etc., could be integrated as necessary.
The contribution of different exposure routes are currently assumed to be additive and responses are calculated according to a time‐indexed body burden on an individual basis. In addition, individual effects need to be explicitly considered for each life‐stage in the model.
The model will then need to be modified to include the effects of each stressor through modification of the model code or parameters, as necessary, for their specific implementation.
3.2.1.4. Integration of sublethal effects in ApisRAM
ApisRAM is covering three types of sublethal effects:
Reproductive performance of queens
The exposure to pesticides is calculated for each individual in the hive, including the queen. A reduction in reproductive performance (e.g. egg‐laying rate) will be calculated from the dose to which the queen has been exposed. The reduction in fecundity (i.e. reduction of number of eggs laid) will be calculated from the effect threshold and a slope (Dai et al., 2010; Chaimanee et al., 2016; Martin et al., 2018).
Development of hypopharyngeal gland (HPG)
Jelly production for individual bees is dependent on the size of the HPG and it is calculated daily. E.g. if the best toxicological information is that there is a threshold response, then a reduction in HPG size will be applied when this threshold is reached (Hatjina et al., 2013; Zaluski et al., 2017).
Homing ability
When a foraging bee is exposed to pesticide, its homing ability might be affected, i.e. either it will fail to return to the hive or the duration of its return flight will be prolonged. To implement this, the bee's mortality rate will be incremented by a user defined value when it forages, based on experimental toxicological information. Homing flight ability was recently ring tested for the preparation of an OECD Test Guideline. The test evaluates the capacity of forager bees to return to the colony after single exposure to a test chemical using radio‐frequency identification (RFID) tagging technology (OECD, 2020). This means that if the bee's homing ability is affected by pesticides, based on toxicity testing, the bee will have a higher mortality when foraging.
3.2.1.5. Future development of ApisRAM
EFSA's guidance on harmonised methods for the risk assessment of combined exposure to multiple chemicals
In common with other areas of risk assessment, exposure to multiple chemicals (PPPs as tank mixtures/formulations, biocides, veterinary products, contaminants) is also relevant to honey bee colony health, and has implications for combined exposure, toxicity (with the potential of additive, synergistic or antagonistic effects) and overall risk assessment. The stepwise approaches in the MIXTOX Guidance (see Sections 2.1 and 2.2.2.1 ) can be applied to bee risk assessment of combined exposure to multiple chemicals using whole mixture and component‐based approaches (EFSA Scientific Committee, 2019). The whole mixture approach is applied when toxicity of the whole mixture or a similar mixture is known as if it was a single chemical. The component‐based approach is the preferred approach when individual components have been characterised and their exposure levels and hazard properties are known or partially known. The individual components are often organised into assessment groups using specific criteria that can include physico‐chemical properties, hazard properties and exposure considerations (EFSA Scientific Committee, 2019). When evidence demonstrates deviation from dose addition, i.e. the existence of interactions that may enhance (synergism, potentiation) or reduce (antagonism) combined toxicity, MIXTOX provides a means to integrate such information in the hazard and risk characterisation steps. These include the mean deviation ratio (MDR), a method to compare dose–response data from experimental toxicity studies on multiple chemicals with predictions generated using concentration addition models. The relative potency of the chemicals is normalised using the TU approach (EFSA Scientific Committee, 2019). Another method described elsewhere is the estimated mean ratio (EMR) (Carnesecchi et al., 2019). The EMR is defined as the ratio between the estimated mean of an end‐point (e.g. mortality; LD50, LC50, EC50 or sublethal effect) for the single stressor at the dose present and the estimated mean of the toxicological end‐point of the binary stressors (Carnesecchi et al., 2019). The EMR allows the magnitude of interactions to be quantified (as potency or synergism ratios) in the absence of dose–response data using each individual data set for binary stressors (e.g. single compounds and binary mixtures, biological or nutritional stressors).
Both the EMR and MDR approaches have been applied in a recent meta‐analysis to investigate interactions of PPPs and veterinary medicines on acute contact, acute oral, chronic contact and chronic oral toxicity data in bees (Carnesecchi et al., 2019). This analysis demonstrated that effects on bees result mostly from synergistic interactions (72%) and less from dose addition (17%) or antagonist (11%) interaction types. Such interactions are mostly observed with fungicides and insecticides/acaricides (55%) through the metabolic inhibition of the detoxification system via the cytochrome P450s enzymes (Carnesecchi et al., 2019) occurring in the bee mid‐gut (Hawthorne and Dively, 2011; Johnson, 2015).
For PPPs, synergistic effects result from either a PPP formulation with more than one active substance or from different PPPs applied as tank mixes. Regarding formulations, MS need to conduct pre‐market testing on bees (EC 284/20137) that represents a substantiated assessment of any potential synergistic effects that may occur. For tank mixes, specific data requirements have neither been laid down in Annex II of Directive 91/414/EEC nor are they provided in Regulation (EU) No 283/20138. MS provide general guidelines and recommendations.
Besides interactions among PPPs, interactions of those products with veterinary products applied to bees may impact the metabolism and immune system of the bees, e.g. by decreasing the bee's ability to detoxify xenobiotics and by increasing its susceptibility to infection by pathogens (Boncristiani et al., 2012). Regarding other stressors such as poor nutrition, synergistic effects can occur with chemicals and result in increased toxicity (Tosi et al., 2017a). Combined toxicity of multiple chemicals can be predicted and/or estimated through concentration addition or independent action (response addition) approaches, assuming similar or dissimilar mode of action, respectively (Van Gestel et al., 2011).
Developments related to combined exposure and effects from multiple chemicals in ApisRAM
In the original tender specifications, there was a requirement for the pesticides module to simulate in‐hive exposure and effects for at least two substances (e.g. two pesticides sprayed in the same tank mix or one parent substance and a metabolite). It is recommended that ApisRAM simulates exposure to multiple chemicals to more accurately reflect the field and in‐hive exposure of honey bees to multiple chemicals over time, reflecting recent scientific progress in this area, including the MIXTOX Guidance.
For the risk assessment of multiple stressors, risk is characterised while applying the sum of TU or sum of hazard quotients approach as the sum of individual exposure ratios and hazard quotients for each stressor at the individual level (i.e. mortality, sublethal effects for each stressor) using a dose addition assumption with exposure estimates (EFSA Scientific Committee, 2019). Possible refinements of these methods include the integration of the body burden information (elimination rate or bioaccumulation factor) that is described in the MIXTOX Guidance. An alternative method is to quantify the relative impact of each stressor on mortality or sublethal effects to determine the potentially affected fraction of the species. This can be applied using mortality data or a TU as a common metric for adverse effects (i.e. a hazard metric). It is noted that this approach assumes a similar mode of action and uses response addition models to quantify the combined effects (EFSA Scientific Committee, 2019).
The methods described above would need to be applied to multiple PPPs and other chemicals (e.g. biocides, veterinary products and contaminants) as well as other stressors (e.g. resource availability, biological agents), to make a dynamic multiple‐stressor risk assessment operational. The preferred methodology is the component‐based approach for which the chemicals are grouped into (cumulative) assessment groups, under the assumption that combined toxicity is additive. As a minimum, for each chemical or cumulative assessment group, an assumption regarding a dose–response relationship, such as the presence of a threshold, needs to be defined (EFSA Scientific Committee, 2019). Substances of a similar mode of action could be taken into account cumulatively in the model, as a compound stressor. It would improve the detection of dose‐effect links and allow for comparing between agricultural regions.
In ApisRAM, there is the need to identify ways to use the current ecotoxicological knowledge to integrate combine toxicity data in the model. Hazard data for acute contact, acute oral and acute chronic toxicity in honey bees are available in a number of databases mostly as LD50 values:
EFSA OpenFoodTox at https://zenodo.org/record/1252752#.X42a-mgzbD4
the US Computational toxicology dashboard at https://epa.figshare.com/
the OECD e‐chem portal at https://www.echemportal.org/echemportal/index.ac
These available LD50 values for chemicals reflect toxicity for the individuals and can be integrated into the ApisRAM to provide an overall assessment of the impact of multiple chemicals on colony survival. Therefore, it is proposed to simulate combined toxicity using the dose addition assumption while reporting toxicity of the individual chemicals using TUs. For interactions, and particularly synergistic effects, the recent meta‐analysis by Carnesecchi et al. (2019) provides a structured database with available evidence of the magnitude interactions for binary mixtures of chemicals as TUs (https://zenodo.org/record/3383713#.Xow7lMgzZeU). In addition, recent (Quantitative Structure–Activity Relationship (QSAR) models have been published to predict TUs for chemicals and for data‐poor compounds. These models can provide in silico predictions of TUs and further refinements can be achieved using available data and QSAR models to integrate information on body burden of chemicals in bees (Carnesecchi et al., 2020).
3.2.2.
3.2.2.1.
Other developments
To date, the development of ApisRAM has been guided by technical specifications as outlined by EFSA (2016a,b). This report has primarily focused on central model aspects, however there is the need, using a modular approach, for subsequent model expansion as outlined below:
The addition of new sublethal endpoints to reflect on possible impacts on key biological traits with quantitative effects (e.g. memory/learning capacity with the PER and locomotion ability).
The addition of new biological agents. ApisRAM currently models a restricted number of biological agents, including Varroa destructor and associated viruses (DWV, ABPV) and Nosema (N. ceranae and N. apis). Future development should include additional important biological agents, including predators like Vespa velutina (the Asian hornet), macroparasites including Aethina tumida (the small hive beetle) that emerged recently as an invasive species in EU, and additional microparasites such as fungi and protozoa.
The inclusion of multiple colonies in a complex landscape. ApisRAM currently represents a single colony in a complex landscape. With future development, it is recommended that the model includes multiple colonies to consider the potential for interaction between colonies, including the transmission and spread of infectious biological agents among the colonies.
The inclusion of dynamic processes for biological agents. In the technical specifications, biological agents were modelled as static processes, reflected as varying effects given their absence or presence. With future ApisRAM development, biological agents should be modelled using dynamic processes, to represent the complex population processes that influence the host–parasite relationship.
An expanded nutritional component. The current nutritional component of ApisRAM is relatively simplistic and will need to expand during future model development. In particular, there is a need for further modelling improvement of the effects of the quantity and quality of food in interaction with other stressors (e.g. chemical/contaminants) (EFSA, 2020; Duan et al., 2021).
Expanded modelling of beekeeping practices. In the technical specifications for ApisRAM, there is limited modelling of beekeeping practices. Currently, these include change in the number of workers, chemical control, replacement of combs with brood, replacement of combs with feed sources, supplementary feeding and beekeeper category and experience (EFSA, 2016a,b). It is recommended that modelling beekeeping practices be expanded to more realistically reflect the critical role of human management on honey bee colony health (e.g. Sperandio et al., 2019).
Expanded modelling at the subspecies level. In ApisRAM, the user has the possibility to select the honey bee subspecies on which the risk assessment is performed. Knowing that local populations of honey bees are better adapted to the local flora and specific area of origin (Hatjina et al., 2014; Büchler et al., 2014), more data on the influence of the local environment on the local subspecies are needed to be able to simulate effects at the subspecies and regional levels.
The inclusion of a resource modelling component predicting the production of pollen and nectar in specific context over time. This approach would be based on phenological information on individual key plants. In particular the project B‐GOOD (Giving Beekeeping Guidance by Computational‐Decision Making) (Appendix A ) could assist in this implementation by providing a dynamic landscape model across the EU, capturing the major floral resources for bees. The model will be incorporated into the ApisRAM model.
ApisRAM will develop over time into different versions to integrate calibration, testing and validation steps with incoming data and information from various sources (research, stakeholders, etc.). This is represented in Figure 5.
Figure 5.
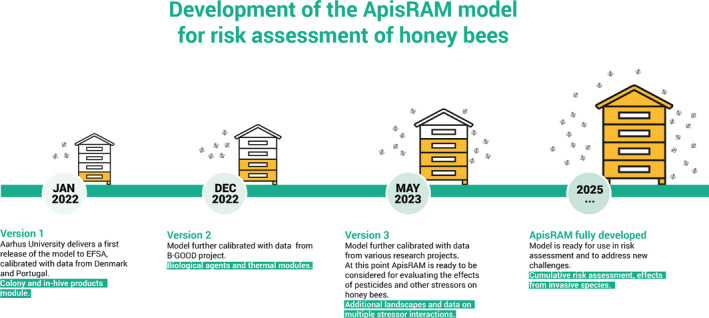
Development of the ApisRAM model for the environmental risk assessment of PPPs in combination with other stressors in honey bee colonies
3.2.3. Monitoring
Monitoring is an essential core component of the system‐based approach, and it is envisaged that there will be continuous data collection from sentinel beehives and surrounding landscapes. Data collection will be guided by the guidelines defined by HEALTHY‐B (which identified tools to assess the health status of managed honey bee colonies; EFSA AHAW Panel, 2016) and by relevant research (e.g. B‐GOOD project; Appendix A ). The monitoring programmes should be designed appropriately to inform the model and to validate the broader risk assessment process. This monitoring activity can provide a foundation to the pesticidovigilance (Milner and Boyd, 2017) EU network.
3.2.3.1. Sentinel beehives
Characteristics and attributes
As part of the MUST‐B project, a scientific opinion has produced a toolbox to facilitate harmonised data collection (EFSA AHAW Panel, 2016). It was concluded that the characteristics and attributes of a healthy managed honey bee colony are:
an adequate size (sufficient to complete the annual life cycle at a given location), demographic structure and behaviour in relation to the annual life cycle of the colony and geographical locations;
an adequate production of bee products in relation to the annual life cycle of the colony and geographical locations;
an adequate provision of pollination services.
Furthermore, five colony attributes have been distinguished that should be analysed when assessing the health status of a honey bee colony:
-
1
The queen presence and performance, because these may influence the size, structure and survival of the colony.
-
2
Brood demography and colony size are considered key indicators to describe the demography of the colony and its potential to produce honey and provide pollination services:
Brood demography (number of eggs, larvae and pupae) refers to the amount, survival and development of worker brood and queen brood in a hive. In particular, the amount of brood is a key indicator for the development and survival of the colony because it represents the future bee population of the colony.
Colony size: a reduced colony size (number of adult workers) in suitable environmental circumstances could indicate a possible health problem in the colony. The presence of dead bees in the vicinity of the hive, in the hive entrance and in the bottom of the hive may also be indicative of a health problem affecting the colony.
-
3
The behaviour and physiology, because these attributes influence the demography, defence against infectious agents, pests and predators, as well as the outputs of the colony:
Thermoregulation refers to the honey bees’ ability to regulate the in‐hive environment, specifically the temperature and humidity. This involves the maintenance of a stable temperature (~ 35°C) across the brood chamber whenever brood is being reared (late winter to early autumn). Thermal sensors make it possible to monitor the internal temperature of the colony.
Colony foraging activity is a key indicator to describe behaviour given its high relevance in relation to the health status of the colony.
Abnormal (ectopic or inappropriate) behaviours of workers, the queen or drones could be one of the first signs of adverse effect of one or multiple stressors, that can contribute to a decreased colony health.
-
4
The in‐hive products, because they influence the energy available to the colony for its development and functioning, including the provision of bee products and pollination services.
-
5
The disease, infection and infestation, because this influences its overall health condition:
Infectious and parasitic diseases are those conditions in which bees display clinical signs (as opposed to being healthy carriers of potentially pathogenic microbes). Infection refers to the invasion and multiplication of microorganisms, such as bacteria, viruses and parasites that are not normally present within a honey bee.
Infestation refers to the external invasion or colonisation of honey bees or their immediate surroundings by arthropods, which may cause disease or are potential vectors of infectious agents.
Variables and tools to assess bee health status (EFSA AHAW Panel, 2016 )
The following variables and tools (more details can be found in Appendix D ) were selected to assess the above characteristics and attributes of a honey bee colony:
Foraging rate is the total number of foraging trips per time unit. Video recordings can be used to assess foraging rate.
Forager1 mortality is the difference between the total number of foragers going out and the total number of foragers returning to the hive daily. Video recordings can be used to assess foragers’ mortality.
Brood development is the number of brood cells contained on the brood combs. The cells can contain eggs, larvae and pupae (drones and workers). One method to assess brood development is to make digital photographs of the combs without the adult bees. The equipment used is key in delivering the required level of accuracy (Dupont et al., 2021). Add‐ons are available for the automatic detection of eggs, young larvae, old larvae and pupae.
Colony size is the total number of adult bees in the colony (foragers and adult in‐hive bees). This can be assessed by weighing the whole hive with or without bees, in which the difference in weight equals the net weight of bees, or through image analysis approaches making it possible to count the total number of bees in a colony. This can also be an indirect measure of in‐hive bee mortality. This measurement induces stress for the colony and therefore, time between two measurements should be long enough to allow recovery of the colony to the normal operating range values as followed by Odoux et al. (2014) and Requier et al. (2015).
Honey production and food stores refers to the amount of honey in the body of the hive (honey for bees) and in supers (honey for beekeepers). The remaining food stores of interest for the model calibration and verification is the amount of pollen stored in the hive. The amounts of honey and nectar stored in the nest (hive body) can be assessed by weighting each comb separately. In addition, the honey stored in the super and harvested during the high season should be reported by the beekeeper. The amount of pollen stored in the hive (beebread) should be evaluated by digital photography. As for the colony size measurement, time between two measurements should be long enough to allow recovery of the colony to the normal operating range values.
Infectious agents (see Section 3.2.2.3 for a description of those variables and Appendix B.5 in EFSA AHAW Panel, 2016 for a description of the tools to measure those variables).
Abnormal behaviour may result from many potential causes and might indicate a problem at the colony level. Atypical worker behaviour (for description and video of abnormal behaviour of bees, see Tosi and Nieh, 2019) can be assessed inside the hive, in the vicinity of the hive or in the fields around the hive. Inside the hive, the recommended method to assess the level of atypical behaviour of the workers is by checking through the hive combs and carefully observing adult bees’ activities. The behaviours of honey bee workers are typically stereotyped given the well coded social structure and biology of their colony, for this reason experienced beekeepers and researchers can recognise atypical behaviours following appropriate and specifically‐designed training (see EFSA AHAW Panel, 2016, Appendix F for more details, Tosi and Nieh, 2019). A reliable measurement of bee behaviours should take in consideration the colony status in the framework of multiple biotic and abiotic factors.
The selection of appropriate locations for sentinel hives, including the number and spacing of hives needed to provide statistically and biologically meaningful data will require further investigation with the support of simulation modelling, field studies of several locations and spacing designs to test model results at various locations and then detailed statistical analysis of the field results. B‐GOOD which seeks to refine the HEALTHY‐B health status index will provide new scientific knowledge and tools to support the systems‐based approach, and the setting and operationalisation of the sentinel hives. This information should be considered when defining the monitoring systems based on sentinel hives.
3.2.3.2. Chemical residues within and outside sentinel beehives
Continuous monitoring of residues would allow a post‐market assessment of chemical contamination by PPPs, veterinary medicines for bees, heavy metals and other environmental contaminants. It also provides ‘ground truthing’ information that is required to properly develop, validate and calibrate the model, allowing comparisons between the predicted and the observed chemical levels over time. There is a technical challenge to have tools for high‐throughput, low detection threshold and multi‐residual analysis. One possible tool is under development in the PoshBee program through an ‘omics’ approach.
Bees are exposed to pesticides through multiple routes (Section 2.2.2.1 ). Like PPPs, veterinary products used by beekeepers could end up in the bee matrices and impact bees. In addition, heavy metals, which can be found in the environment and brought back by foragers to the hive, may accumulate in bee matrices.
For the registration of PPPs, it is not compulsory for applicants to collect data related to chemical residues contained in bee matrices. However, these data would be useful to determine their presence and dissipation over time following treatment in order to calibrate the model and assess effects on bee colonies, at different locations and in different EU countries.
Here is a list of the most common matrices for measuring PPP contamination inside and outside the bee colony, and which multi‐residual profiles (i.e. pesticide presence and concentration) would be needed before, during and after PPP exposure:
honey/nectar/beebread stored in the hive (a,b)
pollen and nectar collected from foragers returning to the hive (a,b)
pollen and nectar collected directly on the treated crops/plants (a,b)
bees (foragers and larvae) (a)
brood food (royal and worker jelly) (a)
beeswax (a)
propolis (c)
dust (produced by treated seed applications) (c)
soil on which the crops are grown (c)
water from puddles and surface water (c)
guttation water (c)
plant material from surrounding weeds in the field (c).
Given the prominent sampling and multi‐residual analysis costs, prioritisation is proposed that highlighting the matrices that are most relevant for bee health assessment (a), model calibration (b) or case‐by‐case sampling (e.g. for early warning signals or sudden excessive bee mortalities) (c).
A case‐by‐case approach should be followed based on time‐specific and space‐specific circumstances and observations. Standard case‐by‐case scenarios should be further developed by experts to facilitate the early understanding of adverse events for bee colonies (i.e. unexpected bee disappearance or mortality). Prioritisation could be discussed among experts, taking in consideration research and specific needs.
3.2.3.3. The landscape, including around sentinel beehives
A range of landscape scale data will be required to inform the ApisRAM model, both surrounding each sentinel hive and more broadly, including:
Landscape structure and management
The ‘structure’ includes data on land use (crop and forestry practices), land cover (polygon characteristics such as shape, surface and distances from colony and polygon content such as fields or urban/suburban areas and landmarks/barriers such as roads), crop and non‐crop/vegetation (including individual isolated trees and linear aspects) and soil (with associated characteristics) types. The ‘management’ includes the agricultural management, including pesticide use, as well as management of other non‐agricultural features (e.g. mowing grasses, roadside verges). There is a need for access to data collected under the Sustainable Directive on farm level PPP usage. Management feeds into the landscape dynamics below as a driver for dynamic changes in local conditions.
Landscape dynamics
It includes data on pollen/nectar/water availability and changes in time and space; concentration of PPP in relevant matrices (based on information collected from plant phenology for both crop and off‐crop areas, forestry and farm management events such as planting, rotation, fertilising, spraying or seeds coating, harvesting, ploughing and plant pest control actions such as application time, location and method), weather (temperature, solar radiation, relative humidity, total precipitation, wind direction and speed) and climate (thermal sums, average temperature, average precipitation and snow cover).
These data should be described and collected in GIS databases. In the model, different spatial scales are used, including the cell (1 m2 resolution), the ‘patch’ (series of cells in a unique habitat) and the resource providing unit (an area with a radius of 3 km around the colony comprised of a series of patches). For key time‐varying parameters, hourly data are needed.
4. Potential applications and benefits
The proposal is for an integrated systems‐based approach to the environmental risk assessment of multiple stressors in honey bee colonies, incorporating involvement and multidirectional information flows between all relevant stakeholders, including the broader society. Multiple applications are possible, with associated benefits, as illustrated below.
4.1. Beekeeping and farming
It is envisaged that there will be ongoing collection of harmonised, high‐quality data from the network of sentinel hives distributed across the EU. With these data, it will be possible to conduct ongoing regional, national and EU‐level surveillance of honey bee colony health. This could include insights into temporal and spatial trends in honey bee colony health, and of associated stressors and early detection of the emergence of the presence and impact of new PPPs, pathogens and other stressors. This information has relevance to governments and a range of industries, including those involved in honey production.
The proposed systems‐based approach has direct applicability to beekeepers. Data from the sentinel hive network could directly assist beekeepers through the dissemination of relevant information, with potential benefits including:
the potential for beekeepers to benchmark the performance of their colonies against summarised regional data;
contributing to an early warning system, including the early detection of new and emerging colony stressors in specific regions or more broadly;
a regional understanding of honey flow, both in time and space, to assist beekeepers when placing hives;
contributing to an understanding of the presence, or recent introduction, of specific PPPs, biological agents or other stressors in the region.
This information will support individual decision making, enabling beekeepers to adjust their actions as needed.
It is envisaged that the sentinel beehive data can contribute to a broader network of data collection conducted by individual participating beekeepers, for the broader benefit of the industry. However, this will require interoperability of data systems and processes within the beekeeping industry. The proposed systems‐based approach and the EUBP will each play a significant role to facilitate this key objective. It is important that sophisticated model outputs from ApisRAM can be linked to specific monitoring endpoints that beekeepers are able to measure and understand.
The monitoring data will inform farmers about the health of local bee colonies, providing ongoing assurance of good farming practice or early warning when bee health is being adversely impacted. For those farmers who rely on pollination services, the monitoring data will also provide an overview of pollination deficit, if present (EFSA, 2017a,b).
4.2. Research development
The systems‐based approach will contribute to ongoing research on honey bee colony health, complementing the large body of existing and ongoing work in this area. The data from the network of sentinel colonies will be particularly valuable, given the emphasis on data quality and harmonisation, and the ongoing collection of data from bee colonies in different regions and MS. ApisRAM, including future developments, offers insights into colony and broader processes, while also highlighting key knowledge gaps. Using a systems‐based approach, it should be possible to advance knowledge in a range of areas, including:
an understanding of the processes that impact honey bee colony health, including sublethal and indirect effects;
an understanding of the impact of multiple stressors on honey bee colony health;
the relative importance of different stressors on honey bee colony health, including any variation that occurs in time and space;
information about key endpoints, easily measured and understood by beekeepers, that are most closely linked to sophisticated model outputs;
the impact of changing climate parameters (such as air temperature and precipitation) on honey bee colony health.
Data from independent research will allow ongoing model calibration, maximising the validity of modelling over different regions of EU. It will be important that the systems‐based approach is both transparent and inclusionary to allow researchers to advance important research questions, including ongoing adaptation of environmental risk assessment in the constantly changing environment.
4.3. Risk assessment, risk management and the broader society
Using a systems‐based approach, computer‐based assessment tools will be used to integrate the wide‐ranging and evolving scientific understanding of hazards and exposure with monitoring data of PPP residues and in situ surveillance of sentinel honey bee colonies. However, this approach differs from the traditional methods used in risk assessment. Traditional approaches aim at identifying the most significant determinant of risk and therefore to tease apart the contributions of different components. The systems‐based approach seeks instead, to evaluate the impact on the colony of the combination of stressors in the context in which the bee colony exists.
The system provides an opportunity for both a predictive (i.e. at pre‐authorisation or approval/renewal levels) and post‐authorisation (i.e. at post‐marketing level) risk assessment, combining these two perspectives together rather than working independently or against each other. Monitoring of sentinel hives and chemical residues will provide data for ongoing model testing and calibration, to allow periodic assessment of PPP exposure, hazard and risk in the context of the multiple stressors that currently impact honey bee colony health. This means that known background levels of bee mortality, chemical residues and infectious agents in agricultural landscapes would also be considered. This approach to risk assessment offers flexibility, including the potential for a phased approach to risk assessment to be applied within a complex landscape (e.g. considering first a single substance, then multiple effects, indirect effects, multiple chemical applications over time and finally other stressors), or for the risk assessment to specifically focus on geographical areas of concern. The proposed systems‐based approach should produce efficiencies over the existing system, which relies principally on laboratory and field testing, and which only considers a single product and single use. Further, the proposed approach offers the potential for increased societal trust in environmental risk assessment for pesticides.
With respect to risk management, the systems‐based approach could be used to evaluate and prioritise potential mitigation measures, including integrated pest management, to reduce risks from pesticide exposure and from other stressors, both to managed honey bees and other insect pollinators. Mitigation strategies could be linked to local agricultural landscapes, where beekeepers and farmers would be integral to the system both as data providers and through the communication of information to inform local decision making. Using this approach, there is the potential for site‐specific assessments and critical evaluation of monitoring results. Reaction time will be delayed by 1 year due to the time required to collate and made available the farming data (i.e. based on subsidy data collected annually). The outcome of the systems‐based approach would be visualised which will help to build public trust. The stakeholders (e.g. EUBP) could support in collating and sharing such data through a common platform (the Bee Hub). Post‐market pesticidovigilance activities would inform regular review following initial authorisation (Milner and Boyd, 2017; Topping et al., 2020). From an industry perspective, this could allow safer intended use to be measured at smaller spatial scales, leading to the potential for differentiated pesticide authorisation and use across the EU. For citizens, a systems‐based approach may provide a means to explain the complexity of the system, including a better understanding of the impact of PPP application given the presence of multiple stressors. For risk managers, this approach could potentially be used to provide a better understanding of risk ranges with the potential to conduct impact analyses in different areas/regions, and as an exploratory tool to inform the setting of the level of protection needed. It could also provide the opportunity for linkages between, and cross‐compliance across, multiple European policy instruments and legislation, including Pesticides Regulations (EC) No 396/20055 and (EC) No 1107/20093, the Common Agricultural Policy and the Sustainable Use Directive 2009/128/EC4, the Water Framework Directive 2000/60/EC.
A systems‐based approach to risk assessment and risk management has the potential to offer each of the following attributes:
Holistic and protective approach for the health of honey bees (colony size, behaviour, structure), its products (nectar/honey, pollen/beebread, propolis, royal jelly) including for the benefit of beekeepers, the services provided to the ecosystem (crop and wild flowers pollination) and society (cultural heritage), collectively supporting sustainable apiculture, agriculture and environment in the EU. This will require socioeconomic perspectives to be considered to support risk assessment and decision making regarding the level of desired protection.
Integrative, offering a means to evaluate a focal PPP in the context of multiple stressors, whose levels depend on context‐specific attributes of the agroecosystem where the PPP is to be deployed. Therefore, the approved use of a particular PPP would depend on the local spectrum of threats to bee health, which could include, e.g. synergies with biological agents, nutrition and/or other agrochemicals.
Comprehensive, facilitating detailed evaluation of a range of risk mitigation strategies, including integrated pest management. Furthermore, the systems‐based approach has the potential to contribute to an improved understanding of the long‐term sustainability of current agricultural practices.
Adaptive, to allow a PPP to be approved for regional or local use based on the context‐specific levels of risk to honey bee health that are in the agroecosystem where the PPP is to be deployed. In other words, the approved use of a particular PPP could vary spatially across the EU.
Responsive, with the possibility that the approval status of a PPP could change based on changes in the risks to honey bee health in the particular agroecosystem. The approved use of a particular PPP would be subject to ongoing review across the EU and at any point of time, the scale of use could be changed or approval could be withdrawn if deemed necessary.
Facilitating, contributing to current efforts, including by the EUBP, to resolve a broad range of industry‐specific data issues, including data accessibility and sharing, data interoperability and quality control.
Inclusionary, seeking a central role for stakeholders (e.g. beekeepers, farmers, researchers). The participation of these stakeholders, including communications to the broader society, will be critical.
Transparent and effectively communicated, with the systems components, methods and approaches and all inputs and outputs (where legally possible) all being publicly available. This will require a user‐friendly platform to be set up for information exchange with stakeholders (e.g. beekeepers, farmers, NGOs, researchers, risk assessors and risk managers).
Aligned with EU and societal aspirations, as outlined in the European Green Deal. E.g. the Farm to Fork strategy emphasises sustainable food production, including a neutral or positive environmental impact and a 50% reduction in the overall use and risk of chemical pesticides and in the use of more hazardous pesticides by 2030 (European Commission, 2020a,b).
Through each of the above‐mentioned attributes, a systems‐based approach has the potential to the building of trust in risk assessment and risk management of PPPs in the context of honey bees in EU.
5. Societal perspectives from stakeholders in the area of bee health
5.1. An exchange with beekeepers on the proposed systems‐based approach
5.1.1. Focus groups with beekeepers
Involvement of beekeepers is fundamental for putting the systems‐based approach in place. With that in mind, a holistic approach must also consider evidence on the beekeepers’ understanding and views on success factors of implementing the approach. Using qualitative and quantitative methods to generate such evidence is among recognised contributions of social research and expertise to the entire risk analysis process (Wendling, 2014).
To provide a societal outlook to the approach, targeted research was conducted among beekeepers in EU to examine the understanding of the proposed approach as a development of the current regulation, the needs and expectations in terms of data for managing beehives, digital advancements and requirements for enhanced communication on the topic. A qualitative method – focus groups – was selected as it provides information on attitudes, perceptions and opinions of participants, obtained through an open discussion led by a facilitator (Krueger, 1988). Such purposive sample, rather than a statistically representative sample of a broader population, elicits more in‐depth views on a specific topic, which would be more challenging to obtain through a quantitative method (e.g. survey). A questionnaire was used to guide the discussion (see Appendix E ).
Focus groups of two to three hours were organised in eight countries. The selection of the countries considered: i) the number of registered beehives as an approximation of the size of the beekeeping activity; ii) representation of different beekeeping landscapes across EU; and iii) pertinence to ongoing efforts in the realm of bee health linked to the MUST‐B work (e.g. testing of the ApisRAM) and the systems‐based approach developed under the MUST‐B project and presented in detail in this scientific opinion. With these criteria in mind, countries selected for the study included Belgium, Bulgaria, Denmark, France, Germany, Greece, Slovenia and Spain.
Beekeepers selected for participation are members of the associations and unions active in each country. A total of 64 beekeepers participated in the discussions, with data collection facilitated through an external contractor (EFSA, 2021).
5.1.2. Broad perspectives
Regarding the regulatory system, beekeepers raised some concerns about the current PPP regulation, e.g. in terms of testing and marketing of new products, while appreciating the opportunity to participate in discussions on possible future approaches. They see the system‐based approach as ambitious, having to account for multiple factors, but however as a potentially useful system for providing alerts and better knowledge of field situations, regarding the use of pesticides and bee health. They also stressed the need to understand the combined effects of chemicals. Conditions for data sharing – fundamental to the functioning of the proposed approach – including the need to ensure two‐way flow of data between beekeepers and regulators, confidentiality provisions and possible introduction of incentives. The latter was also noted as a pre‐requisite for adoption of new technologies in bee management. Beekeepers called for the attention of the European institutions, to ensure bee health jointly, including through relevant research such as treatment of Varroa.
5.1.3. Specific feedback
5.1.3.1. Regulatory system
There was a positive sentiment towards EFSA's initiative to involve beekeepers in the development of the systems‐based approach and to elicit their views about current difficulties in beekeeping and opportunities for improving the health of honey bee colonies in EU.
Beekeepers perceived a misbalance between institutional consideration given to beekeeping compared with the chemical and agricultural industries and called to EU and national institutions to trust and pay attention to them, informing them better about regulations.
The overall regulatory system was often perceived as lacking capacity to protect honey bees. The pitfalls identified were related to both product toxicity and undue exposure of honey bees. In the beekeepers’ views, the first comes from suboptimal testing procedures, whereas the second is related to the unauthorised use of PPPs. Beekeepers argued that, in some cases, ‘non‐compliance’9 in the use of PPPs can even occur following national derogations for using EU‐level banned neonicotinoids. Importantly, opposing beekeepers and farmers was considered as neither a productive nor a desirable outcome.
Regarding the testing and marketing approval of pesticides, it was stated that this does not necessarily consider the long‐term effects of chronic toxicity on bee mortality, as well as sublethal effects. Laboratory conditions are perceived to be disconnected from reality and the protocols for field testing were seen as non‐representative, e.g. one spot testing cannot account for historical contaminations. In several groups, the lack of the application of the EFSA (2013a,b,c) Bee guidance was mentioned.
The health of honey bee colonies was reported as having been deteriorating over the past 30–40 years. The nature of the PPPs to which bees are exposed has also changed over the last decades in beekeepers’ opinions: whereas before mortality was more ‘visible’ (‘dead bees in front of the hive’), current products are perceived to lead to a progressive weakening of the colonies.
To support the beekeeping sector, political action was called for on the production side, by adapting subsidies to beekeepers’ needs, lowering or even removing taxes and providing a framework for better professional training. In focus group participants’ views, these measures need to be accompanied by strict controls on the honey market, to avoid fraud. Adulterated honey was mentioned as an example, some of which is imported from non‐EU countries.
5.1.3.2. Data exchange between beekeepers and institutions
For beekeepers, the most significant contribution of the systems‐based approach to the existing situation would be in terms of a better knowledge of the field situations, by accounting for the heterogeneity of landscapes in EU and the heterogeneity of different beekeeping situations. There is hope that the systems‐based approach could serve one day as an alert system, allowing beekeepers to continuously stay informed about the global situation of honey bees in their country and even in EU and get instant information about potential poisoning events in their vicinity. To respond to this need, the systems‐based approach should stay independent from any commercial or political interests.
Participants of the focus groups acknowledged that building a systems‐based approach to account for the heterogeneity of the situation in the European beekeeping sector is challenging, as: (i) there is a continuous variation of meteorological and landscape conditions; (ii) beekeeping practices might vary from one country to another; and (iii) new pesticides can be introduced onto the market. According to the focus groups, such a novel approach could certainly produce valuable knowledge, however they acknowledged the high demand of it becoming a practical, reactive decision‐aid tool. Therefore, a balance of collecting input data through digital monitoring systems and observations made by experienced beekeepers will have to be struck.
If the systems‐based approach could contribute to identifying cocktail effects when a new pesticide is marketed, this would potentially respond to one of the main challenges of the current regulatory framework.
Focus groups’ participants expressed high interest to have data both on the state of their hives and on the environment. The potential benefits highlighted included an early warning or alert system about possible toxic events and a post‐authorisation monitoring system. For some, producing knowledge to protect the environment and the biodiversity was enough to declare their support for the systems‐based approach.
If generalised in EU, beekeepers hope that the systems‐based approach and associated documentation will be accessible in national languages. It was unclear how the approach related to existing data, and if the very numerous projects and monitoring initiatives that had taken place in European apiaries over the last 20 years would be used or not.
Whereas all beekeepers confirmed their agreement, in principle, with the idea of providing data about their bees, several conditions need to be fulfilled and could be a determinant for effective data sharing: i) results should come back to those who provide the data; ii) business information should stay confidential as these are sensitive data that could potentially be used by financial services and associated taxation burden; and iii) anonymity should be a choice left open to those willing to share data.
5.1.3.3. Use of digital tools in beehives
The respondents felt that the usefulness of digital tools depends on the parameters measured. While scales and cameras are already used, more complex data provided by digital tools might not be easy to interpret. For now, complex measurements of colony activity seem to be useful rather than for research purposes.
The most useful data from digitalisation would be data that helped them know better the status of their hives in real time and detect health problems. In addition to these parameters, recording bee behaviour, such as sound, could be indicative of threatening events.
There was important concern expressed in several groups about potential effects of electromagnetic fields produced by such digital technologies on the health of the bee colonies.
Adoption of digital equipment raised, on the one hand, pragmatic considerations about convenience for beekeepers especially in terms of cost of new technologies and, on the other hand, the topic of beekeepers’ attitudes towards bees. Regarding costs, suggestion was made these be borne by the EU for setting a Union‐level assessment approach. On the topic of attitudes towards bees, beekeepers noted that bee colonies are the symbol of a way of living in agreement with nature, where humans rather accompany and facilitate the life of bees instead of trying to heavily intervene to adjust and modify it for their economic interest.
5.1.3.4. Research for bees
Research needs reported by focus groups’ participants included ways to better understand and deal with Varroa, investigating the reasons why queens are living for a shorter period than before and better characterisation of the chronic, sublethal effects of pesticides. There was also interest in research on the impacts of electromagnetic fields, including the developing 5G network and high voltage lines.
To make existing academic research easier to use in practice and more relevant for beekeepers’ needs, scientists and relevant institutions are asked to better communicate and diffuse existing research and involve beekeepers upstream in setting up research topics and protocols. Beekeepers welcome the creation of a ‘hub’, a sort of centralised platform collecting research findings, available in all languages and easy to understand for the wider audience.
Clearly, for all groups, all these research topics need significant financial investment, up to the ecological and agricultural challenges associated with continuously weakening bee colonies. Research should aim at large‐scale studies, ideally cohort studies involving a representative number of apiaries using different bee breeds. Research results, regardless of whether funding was from public or private money, should be freely available, according to the beekeepers.
5.2. An exchange with the members of the EU Bee Partnership
Prioritising public and stakeholder involvement in risk assessment is a key value of EFSA, which developed a framework to expand assess both for EFSA and stakeholders to the evidence base and expertise needed for high‐quality scientific risk assessment, combining to improve trust in and creating a sense of ownership over, the decisions made (EFSA, 2018b). The EUBP initiative (see Section 1.2.2 ) is a concrete example of EFSA's Stakeholder Engagement Approach (SEA) and the systems‐based approach that is promoting the inclusion of the various stakeholders’ groups involved in bee health and beekeeping (beekeepers and farmers, consumers, environmental/health NGOs and advocacy groups, business and food industry, practitioners’ associations and academia) and that supports the SEA.
In parallel with stakeholder engagement, increase in data accessibility, quality, openness and transparency are other important actions that EFSA supports to improve reproducibility and reusability of evidence in food and feed safety risk assessments (Cappè et al., 2019). In support of this and building on the efforts made under MUST‐B on standardised bee health data (see Section 1.2.3 ), the EUBP is developing an EU platform to gather and share such harmonised data among interested stakeholders. Through Application Processing Interface (API) technologies, the platform will be connected to the monitoring system (sentinel hives) to provide real‐time data and trend analysis on honey bee colonies status and dynamics. These data will support the maintenance and sustainability of healthy stocks of honey bee colonies, beekeeping activities and pollination services in the EU. The data produced from the monitoring and stored in the platform will also support the implementation of the model that needs to be calibrated against real world data collected under different EU landscapes, including the diversity of honey bees (various subspecies/ecotypes) and under different scenarios of beekeeping practices, land use and exposure to biological stressors.
Beyond the EUBP representatives, who are the current data providers of the EU platform, several other stakeholders are expected to benefit from, and interact with, the systems‐based approach (e.g. beekeepers, farmers, academia, risk assessors and risk managers from national and EU competent authorities).
At the European Commission level, within the new framework of the European Partnerships in Horizon EU, new initiatives are expected to emerge in areas that are relevant to bee health and beekeeping. These initiatives could support the system‐based approach by regularly communicating and informing the platform on the latest research and scientific development.
As for the European Parliament, it is important that all relevant stakeholders are involved in the evaluation process to understand the rationale for the decisions made by policy makers and to provide sustainable solutions to the EU beekeeping sector, and to halt the loss of biodiversity in EU.
The third Term of Reference of this scientific opinion is devoted to ‘The work being developed by stakeholders to achieve harmonised data collection and sharing on bee health in EU and by doing so, support the EUBP initiative by providing guidance for harmonised data collection and evidence‐based risk assessments’.
As outlined in the ToR for an EUBP (EFSA, 2018a), the Partnership was set up in 2017 with the objective of ‘improving data collection, management, sharing and communications to achieve a holistic approach to the assessment of bee health in EU and beyond’. The Partnership Synergy framework (Weiss et al., 2002) was adopted to gather partners’ views on the relationships formed and discuss expectations for the future.
Qualitative semi‐structured telephone interviews with eight representatives10 of the stakeholders being part of the EUBP were conducted (see Appendix F for more details on the questionnaire).
Perceived involvement and collaboration – The group was described as a composition of different perspectives and points of view, a characteristic that was seen as a strength of the Partnership. Terms like ‘committed’ and ‘constructive’ were adopted as definitions of the level of perceived participation of the members in the discussions. It was reported that the Partnership helps in creating a network, it is a place for information exchange and learning opportunities. It was mentioned that the involvement varies according to the expertise and the topic of discussion, reflecting the heterogeneous structure of the Partnership.
The Partnership plays a reconciling role between the political and technical aspects of the discussions, both of which need to be taken into account to ensure achievement of the planned results of the initiative.
Perceived efficiency – Partners stated that the final objective of the Partnership is clear, and the discussions held so far show a progress in that direction. The tasks and activities planned are ongoing and steps forward have been made on the PoC on data sharing. The effective contribution to the work differs based on the knowledge and competencies of the members – and may at times seem unbalanced, depending on the topic of conversation. Partners who own data provide support in developing tools and try to find solutions for data standardisation and are therefore much more active in relevant discussions. Partners not directly involved in potential data sharing are more active in the theoretical discussions. What is seen as crucial is that the Partnership sustains the exchange of information between members, regardless of the level of alignment of their viewpoints.
Views on harmonised data collection and sharing – The challenges raised by members can be categorised into three main areas. First, socioeconomic challenges related to the reasons for sharing data. The interviewees reported that each partner has a different interest in sharing data which needs to be taken into account. Some might wish to receive a feedback on the shared data, others might see value in receiving recognition for contributing to the common project. Regarding beekeepers, the ‘psychology of incentives and disincentives’ must be considered, i.e. the factors that contribute to affect beekeepers’ inclination to share data or not, including issues such as perceived taxation risks. Partners agreed that confidentiality of data is a fundamental pre‐requisite for sharing to avoid financial/juridical consequences and ensure compliance with the General Data Protection Regulation (GDPR) (EU) 2016/679. Other important requisites are mutual, transparent and open sharing considering copyright aspects.
Second, technical challenges regarding data format and harmonisation which are being worked on through the BeeXML initiative that would make it possible to share data in a standardised fashion. Some partners mentioned already existing large data sets such as COLOSS and EPILOBEE which would represent a starting source of knowledge when formatted.
Once data are gathered, the third challenge relates to data quality and analysis, e.g. data collected by beekeepers and agreement on information to extract.
Future involvement of partners and wider community of beekeepers – The interviewees stated that, after the constructive theoretical discussions, it is now time to move forward to put the knowledge of different EUBP members into practice. This means making data available and working to create an inventory of data on bee health, as highlighted in the EUBP ToR.
Some suggestions were made on the way to further exploit the breadth of resources of the Partnership. One might be to develop a concrete small‐scale project and test it to learn from it and have the possibility to show the results to the wider community. Another, more long term, suggestion was to work on building the Bee Hub, a collection of bee‐related data, a comprehensive and harmonised toolbox for beekeepers to find and access information, desirably in all EU languages. Accessibility to any tool set‐up by the partnership to a wider community of beekeepers is one of the key values that the Partnership can deliver to the beekeeping sector.
Trust among members was identified as a key pre‐requisite to progress with next steps, especially in terms of transparency and quality control of the data.
Resources and coordination – The partners stressed the fact that the work they are performing is carried out on a voluntary basis, therefore the resources that they can offer are limited. Supplementary resources would be welcome, including in terms of skills to be added to the partnership mix, namely:
a data scientist expert in IT and new technologies that could provide support in data aggregation, modelling and simulation;
an ecologist with focus on climate change to help understanding the environmental impact on bee health;
a social scientist for understanding the challenges linked to data sharing;
an expert who could provide juridical advice on privacy issues in data sharing;
a person in charge of organising events to make the Partnership more visible to the wider community of beekeepers and other stakeholders.
Regarding new partners, the decision to include other practitioners was appreciated and the potential inclusion of additional members from the agricultural sector was deemed favourable. The involvement of experts in wild bees and wild pollinators would also be encouraged, even as part of a subgroup of the Partnership.
The coordination by EFSA was praised by many interviewees who recognised the crucial role played in setting up and facilitating the discussions and providing technical support. According to the partners interviewed, the EUBP should become a permanent initiative, a long‐term project. A European authority such as EFSA is seen as well placed to a leadership role, to ensure a balanced representation and contribution of stakeholders’ categories. EFSA's broad expertise in a variety of areas – from toxicology to social sciences – would represent an asset for reaching the objectives that the Partnership set in its ToR.
Overall, the interviewees expressed a high level of awareness on the uniqueness of this discussion group, a place for information exchange and knowledge sharing. Therefore, the discourse of the interviews is reminiscent of the EU motto ‘United in diversity’, as partners showed a sense of belonging regardless of the diverse backgrounds and fields of expertise.
Three recommendations for the future of the Partnership can be drawn:
Outlook 1: Engage in practical research projects – Partners feel that it is time for translating theory into practice through: i) actual collection of bee health data; and ii) set‐up of a project to test the evidence acquired so far. This step is essential for demonstrating the beneficial results of the Partnership to the members involved and to the wider community of beekeepers.
Outlook 2: Add new skills to the partnership mix – As the contribution of the different perspectives coming from the diverse partners’ fields of expertise has proven successful, engaging further resources would be advisable. These include skills in the areas of data management and socioeconomic analysis and juridical aspects of data sharing, as well as participation of players from the agricultural sector.
Outlook 3: Define a sustainable, longer term plan – The Partnership should consider its presence in the long term, under the overall coordination of EFSA, in synergy with other relevant public authorities. A sustainable partnership model would guarantee a steady progress in areas such as data sharing in support of bee health, while the supervision by an independent public body would allow for an equal representation of the different viewpoints and support the work through both scientific advice and technical assistance. Dedicated financial resources should support these efforts.
6. Gaps and opportunities for implementation
6.1. Environmental risk assessment of multiple chemicals
The system‐based approach has the potential to address many of the issues raised in Sections 2.2.1 and 2.2.2 by the current environmental risk assessment for honey bees, as outlined in Table 2.
Table 2.
List of actions from proposed systems‐based approach to address the issues raised by the current environmental risk assessment for honey bees
| Issues raised by the current environmental risk assessment for honey bee colony health (see Section 2.2.1) | Actions from the proposed systems‐based approach |
|---|---|
| i) one substance’ approach | Assessment of co‐exposure to multiple chemicals will rely on the availability of toxicological data to be simulated (through the prioritisation of data to be generated on combined chemical toxicity) |
| ii) multiple stressors | A unifying approach is proposed to dynamically model the complex interactions between a complex array of chemical and non‐chemical stressors |
| iii) spatio‐temporal dynamics of exposed populations | Integrating exposure through time is an emergent property of the modelling approach used. Multiple‐stressor effects are combined in a realistic manner to reflect realistic exposures to bees in spatially and temporally explicit simulated landscapes |
| iv) species‐specific traits in relation to chemical toxicity | Regarding honey bees (and the corresponding modelling component: ApisRAM), research data on the influence of genetic diversity on toxicological sensitivity (subspecies level) are needed |
| v) sublethal effects | The measurement of sublethal effects, linked to the specific protection goals, is integrated and refined in the monitoring and modelling processes |
| vi) indirect effects | Integration of data for known interactions can be built in (e.g. indirect effects of herbicides on bee foraging), but full ecosystem interactions are not currently possible |
| vii) uncertainties linked to exposure assessments of foragers | With increasing model validity, the integration of realistic foraging behaviours and landscapes would lead to realistic exposures of single and multiple stressors |
| viii) context dependency | Context dependency is implicit in this approach |
| ix) pre‐existing conditions | Monitoring (colony health status, disease load, beehive stores and residue levels in the hive and outside, in the environment, as well as resources in the landscape) would bring the required information to determine the pre‐existing conditions |
While the systems‐based approach can overcome some of the issues raised regarding the current environmental risk assessment, there are also issues for which knowledge gaps remain and need to be filled, as highlighted in the EFSA Guidance Document on risk assessment of combined exposure to multiple chemicals (EFSA Scientific Committee, 2019). In particular, there is a need for:
combined toxicity data (lethal and sublethal effects) of multiple chemicals in honey bee subspecies. This is particularly relevant to chronic combined toxicity for which very limited data are available and would make it possible to identify potential interactions that may lead to deviation from dose addition (potentiation, synergism). For acute lethal effects, combined toxicity data are limited to a limited number of PPPs in managed honey bees while no data are available for sublethal effects (Carnesecchi et al., 2019);
toxicokinetic data for single and multiple chemicals in honey bee subspecies (bioaccumulation, half‐life, etc.);
data on combined exposure and effects of multiple stressors (chemicals, diseases, nutritional stress, etc.).
As more experimental data on combined toxicity and toxicokinetics of multiple chemicals become available, implementations of methodologies for risk assessment of multiple chemicals in honey bees include:
Development of open source curated databases for honey bee subspecies related to the use of chemicals, exposure and hazard assessment of multiple chemicals including plan protection products, veterinary drugs and contaminants. Such a database should include available information on lethal and sublethal effects of chemicals, toxicokinetic information and can also be further developed to include information on other stressors (nutrition, diseases? etc.).
Methodologies for risk assessment in open source tools including TU approaches using lethal and sublethal effects as well as validated in silico models (QSAR models; Carnesecchi et al., 2019, 2020) applying dose addition as the default model or models integrating synergistic effects. When data on synergistic effects are evidenced, these tools would make it possible to investigate the application of an extra uncertainty factor.
Landscape modelling for risk assessment of multiple chemicals in bees to integrate hazard and exposure information. It is recommended to develop specific case studies to test and validate the ApisRAM model and other available models.
6.2. The systems‐based approach
6.2.1. Modelling and monitoring
Modelling the effects of multiple stressors on honey bee health potentially offers substantial benefits in risk assessment of PPPs. In particular, modelling could produce forecasts of bee health that are specific to location and that integrate the interactive effects of multiple stressors. Additionally, in comparison with field trials, modelling is likely to be economically efficient and could be more specifically applicable to varied localities.
ApisRAM has been developed to meet these modelling requirements. By using detailed agent‐based simulation, ApisRAM seeks to overcome the shortcomings of the BEEHAVE model as identified by the EFSA PPR Panel (2015). There are multiple strengths from this approach. ApisRAM considers model inputs that are environmentally realistic, including localised landscapes and toxicant/pathogen profiles. Multiple stressors can be handled and both sublethal and chronic effects can be incorporated. ApisRAM allows visualisation and is testable at several levels of organisation, including individual level and through emergent dynamics. The model forms a framework to evaluate current knowledge (gaps) because it provides a complete system description. Furthermore, ApisRAM creates a system for hypothesis testing and prediction by combining mechanistic construction and emergent system properties. This allows this type of model to predict beyond the range of the data used to construct it (unlike e.g. multiple regression). This approach also offers multiple opportunities, including biological discovery (new understandings of exposure, of the impacts of individual‐based sublethal effects on the colony, and of the relative importance/impact of different stressors), credibility and conviction including through animation, the ‘digital twin’: sentinel hive concept that will allow real‐time validation and prediction, long‐term planning (effects/chronic) and a better understanding of the relative importance and impact of multiple stressors on honey bee colony health.
ApisRAM may meet the intended targets and produce great benefits, but there is a risk that it may not forecast the impacts of multiple stressors on hives to the degree of accuracy required for regulatory use. The agent‐based simulation has to transfer various environmental signals through a three‐layered network comprised of tens of thousands of individually differentiated components whose interconnection strengths are based on estimation to produce a single output, colony strength, that should be both accurate and precise. To achieve this, the model has a substantial demand for knowledge (relating both to biological and chemical areas) and many parameters need to be estimated, including individual‐level dose–responses on behaviour, and cumulative toxic load; chemical and non‐chemical stressor interactions. Furthermore, the model is complex in terms of software engineering and there are increased demands for professional approaches to code development, and the need for specialised expertise. Therefore, there are several threats, including the production of false or imprecise predictions and poor resolution (leading to the production of multiple predictions with similar certainty). This is a demanding task. To mitigate key risks associated with this proposal, the following recommendations are made:
Contingency plans cover the possibility that ApisRAM will not in the short term provide forecasting with the required levels of regulatory precision.
In the medium term, laboratory and field tests are not relinquished or downgraded.
A broad range of available data should be used for ongoing model validation, seeking robustness through comprehensive evaluation of the range of model outputs.
It is important to acknowledge that issues will arise as more models and information become available, requiring ongoing validation and comparison. This iterative approach to development will steadily reduce the degree of uncertainty associated with model use by both identifying and enumerating causes of variation (which are then no longer uncertainties), and thus increasing the domain of applicability of the model.
6.2.2. Data flows into the systems‐based approach
Substantial data have been generated relevant to the reporting of global bee health decline, but these data come from disparate sources and are heterogeneous in quality. They are not standardised and interoperable, and this poses constraints on their use. This issue is highlighted in Section 1.2.2 and addressed under Term of Reference ToR 3 of this scientific opinion in which the European Parliament requested EFSA to provide guidance to the EUBP on how to improve harmonised data collection and sharing on bee health in EU.
As part of initial discussions towards the development of the EUBP, a symposium was held in 2017 on challenges and opportunities for the collection and sharing of bee health data. It was organised by EFSA with 130 attendees including beekeepers, farmers, industry, scientists, risk assessors, policy makers and the public (EFSA, 2017a,b). There was general agreement among attendees of the overall benefits of data sharing and of the potential benefits to each stakeholder if data sharing was possible. The listing of benefits was similar to those outlined in the current scientific opinion (Section 5 ). However, multiple challenges were noted at the symposium, aligned to:
data availability and access (as one example, there is compulsory collection of subsidies data, however these are not accessible in all MS);
data collection (highlighting the need for non‐invasive monitoring tools to overcome current difficulties with data variability and uncertainty using current field methodologies);
data collation and management (with the need to facilitate data sharing by promoting open data access);
data analysis (with a particular priority being methodologies that allow simulation and prediction);
data communication (envisaging a flexible system to maximise usefulness for individual stakeholders).
In the years since this symposium, many of these data issues have been substantially addressed, for the benefit of the EUBP and other stakeholders wishing to participate in the data collection effort. These advances are also relevant to the concepts and methods outlined in this scientific opinion. In particular, substantial progress has been made in the areas of:
harmonised data collection using standardised structures for reporting beekeeping actions and apiary inspections. Electronic methodologies, such as e‐logbooks, should be preferred as they could guarantee a simpler management, archiving and transparency in the longer term;
data sharing capacity, which has improved overall interoperability between the data‐relevant tools;
stakeholder willingness to share data that is clearly linked to their needs and benefits;
trust between different parties using open source data and software that could, e.g. be transparently monitored by users;
privacy must (and can) be protected (i.e. names, locations), and valid strategies are now available for privacy protection including data aggregation strategies;
efficiency of, and success in, data collection efforts through the adoption of methods that have been already tested and proven useful.
Although multiple strategies and methods have been, or are being, developed and tested, further research on the topic should be pursued to enhance efficiency and reliability of the system, while ideally reducing costs.
Nonetheless, there are still unresolved issues related to data collection and sharing. The most significant concerns, as currently identified, include data quality and accuracy, and the validity of the currently available software applications. There is also a broad lack of information on the uncertainties around data collection measures:
Data accuracy and uncertainty. All scientific data measurements should be accompanied by an assessment of their associated uncertainty. While scientists normally know the levels of uncertainty in their data, this is rarely the case for most beekeepers. Therefore, user‐friendly methods that allow an assessment of data uncertainty should be available to beekeepers, as this is an essential parameter to estimate. With respect to bee colony health data, this is relevant to parameters such as the size of the population (adults and brood), the content of pesticide residues in the different substrates of the hive, etc.
Validity of software applications. The effectiveness, precision and reliability of most current tools and applications have not been scientifically evaluated. Current European projects (e.g. B‐GOOD) should assist in this regard. In the long term, valid tools and applications to assess the health of bee colonies (e.g. BEEP) offer the potential for near real‐time monitoring of bee health, and the possibility of timely intervention for problem resolution.
6.3. Timeline for implementation of the systems‐based approach
The implementation of the systems‐based approach relies on progress and new developments in the following areas:
Modelling: the first version of ApisRAM that was outsourced by ESFA can be implemented in time, with support from research & development, as described in Section 3.2.1.5 .
Monitoring: the sentinel hives will benefit from the rapid development made in the field of digitalisation and the internet of things. As mentioned in this opinion (Section 3.2.2.1 & Appendix A ) several ongoing projects are in the process of producing high‐tech devices for harmonised data collection and automatic recording of bee health.
Research and Development (R&D): new development made in R&D will be key, as highlighted in Section 3.1 and described in Appendix A , a few ongoing EU projects (H2020) will deliver scientific knowledge as of 2021 and until mid‐2023, to support MUST‐B approach, i.e. to support the operationalisation of the systems‐based approach.
Data exchange platform(s): as mentioned at several occasions in this opinion (Sections 1.2.2 , 4 , 5 and 6.2.2 ), data and stakeholders involvement have a central role in the success of the systems‐based approach. The development of platforms to promote harmonised data collection and sharing among stakeholders will strengthen data flows into the systems‐based approach.
Toolkits: to guide end‐users (beekeepers, farmers, scientists, risk assessors and risk managers) on the use of the new tools and methods, toolkits will be developed to enhance knowledge sharing.
The above developments are detailed in the table below (Table 3 ) with indicative times when these outputs will be made available. This information is provided to support risk managers who need to make informed decisions on the assessment of bee health in EU.
Table 3.
Timeline for finalisation of the elements of the systems‐based approach
| Elements | Specific outputs | Provider | Expected output |
|---|---|---|---|
| Modelling | Manuscript describing the development of a solitary bee population dynamics model | Jagiellonion University | August 2021 |
| EFSA procurement on the Honey bee colony dynamics (ApisRAM) Model for the RA of PPPs (calibrated with data from at least 2 EU countries, Denmark and Portugal) | EFSA/Aarhus University | January 2022 | |
| ApisRAM calibrated with data from B‐GOOD (biological agents and thermal modules) | Aarhus University | December 2022 | |
| ApisRAM model ready to be considered for evaluating the effects of pesticides and other stressors on honey bees | Various research projects | May 2023 | |
| Bumble bee population dynamics and solitary bee model for the RA of PPPs | PoshBee | May 2023 | |
| ApisRAM fully developed for use in RA and to address new challenges (cumulative risk assessment, effects from invasive species) | Various research projects | Beyond 2025 | |
| Monitoring | Automatic/semi‐automatic tools for digital hives (to allow real‐time monitoring of bee health): bee counters (foraging, field mortality, identify pests entering hive and differences between workers and drones), proof of concept for optoelectronic sensors (identify different species) | IoBee & SAMS | April 2020 and December 2020 |
| Verification of the quality of the CORINE database for landscape‐level exposure modelling | INSIGNIA | December 2020 | |
| Development of a non‐invasive environmental monitoring system with honey bee colonies in a CS setting, guided by a protocol to be agreed, which combines scientific, practical & methodological recommendations | INSIGNIA | December 2020 | |
| Air sensor tool: atmospheric agrochemicals exposure in/outside hives | PoshBee | January 2023 | |
| Haemolymph collection kit and tool | November 2018 | ||
| HB MALDI imaging method | May 2022 | ||
| BeeTyping | Poshbee & SmartBees | May 2023 Finalised in 2018 | |
| Synthesis of Omics approaches | PoshBee | Jan. 2023 | |
| Consolidated peptide/protein database and markers | May 2023 | ||
| Scientific knowledge from PoshBee H2020 project | Chronic and sublethal effects of chemicals1 and combinations (TK/TD of chemicals; acute/chronic effects) in HB, BB and SB | PoshBee | October‐November 2021 and February 2022 |
| Effects of chemical1 X pathogens in HB, BB, SB | Nov. 2021 & Jan. 2023 | ||
| Nutrition requirements in HB, BB, SB | Aug 2022 | ||
| Effects of pathogen X nutrition (SB) & chemical1 X nutrition in HB, BB, SB | Sept. 2022 & Jan. 2023 | ||
| Semi‐field level effects of multiple stressors (pesticide X fungicide & pesticide X nutrition) in HB, BB and SB | August 2021 | ||
| Field level effects of multiple stressors | May 2023 | ||
| Scientific knowledge from B‐GOOD H2020 project | Operationalisation of the Health Status Index (HIS) developed under the Healthy‐B toolbox (EFSA AHAW Panel, 2016): critical HIS components identified | B‐GOOD | Nov. 2021 |
| Logbook functionality (BEEP app) to register colony inspections in the field | Available2, new version in May 2021 | ||
| Automatic remote sensing of weight, temperature and sound (BEEP base) | Available3 | ||
| Monitoring devices to collect real‐time data on colony development (discrimination of workers with and without pollen, drones and queens) | May 2022 | ||
| Monitoring devices to collect real‐time data on colony exposure to chemical and biological stressors (including tool for genotyping) | May 2021 & May 2022 | ||
| Monitoring devices to collect real‐time data on colony health status under different environments and conditions in EU | May 2023 | ||
| Stakeholder platform for harmonised data exchange | Prototype platform of the EU Bee Partnership to exchange harmonised data on bee health and beekeeping in EU | EFSA/BeeLife | Feb. 2021 |
| B‐GOOD bee health data web portal (by BEEP and Pensoft) | B‐GOOD |
Phase 1: April 2021 Phase 2: Dec. 2022 |
|
| Toolkits: multi‐media knowledge exchange to enhance tool uptake and use | Impact strategy | PoshBee | May 2019 |
| Policy entry points and briefs | Aug. 2020 | ||
| Incentives and barriers to tool adoption | May 2022 | ||
| Overview of tools, protocols, guides | Jan. 2023 | ||
| Responses to multi stressors | March 2023 | ||
| extension, publicity and impact of the monitoring studies | INSIGNIA | Dec. 2020 | |
| Protocols for bee regulatory testing schemes | Risk mapping of honey bee pesticide exposure | INSIGNIA | Dec. 2020 |
| Analyses for authorised and non‐authorised pesticides and veterinary drugs | INSIGNIA | Dec. 2020 | |
| Plant diversity as food sources for bees | INSIGNIA | Dec. 2020 | |
| Evaluation of agricultural and pesticide legislation practice | INSIGNIA | Dec. 2020 | |
| Testing chemicals on life‐stages and castes/sexes of model species | PoshBee | Feb. 2021 | |
| Ground nesting model SB | Sept. 2022 | ||
| Chemical X pathogen/nutrition in HB, BB and SB | Sept. 2022 | ||
| Field testing | Aug. 2021 & May 2023 | ||
| Publication of a ring‐tested methodology for assessing the long‐term chronic toxicity of chemicals in honey bees (Tosi et al., in press) | University of Turin, COLOSS APITOX Task Force | June 2021 |
Tested chemicals: sulfoxaflor, azoxystrobin, glyphosate.
HB: honey bees (Apis mellifera); BB: bumble bees (Bombus terrestris); SB: solitary bees (Osmia bicornis).
7. Conclusions and Recommendations
7.1. Conclusions
This scientific opinion addresses a mandate from the European Parliament about global bee losses and colony weakening, with the particular focus on honey bee colonies in EU, and the need for holistic and integrated systems‐based approaches. The work developed in this scientific opinion falls into the prerogative of the European Green Deal for the EU and its citizens to make EU's economy sustainable by turning climate and environmental challenges into opportunities.
The environmental risk assessment of pesticides is an area in which there are high expectations for change (Group of Chief Scientific Advisers, 2018). To respond to such expectations, EFSA has identified gaps in terms of methodologies, data, knowledge and expertise in environmental risk assessment and, as a consequence, opportunities for implementation (EFSA available online a,b). The systems‐based approach as outlined in this scientific opinion is closely aligned with many of these goals. These include integrating new tools such as modelling to include spatio‐temporal dynamic aspects and to consider impacts from other stressors, whether regulated or not; connecting various EU regulation policies assessment such as the Sustainable Use Directive, the Common Agricultural Policy, the Water Framework Directive and the Habitat and Birds Directive; optimising and developing a new framework for the use of environmental monitoring/surveillance data; involving stakeholders in the gathering and sharing of data; integrating methodologies for combined risk assessment of multiple chemicals as well as cumulative effects; and continuous review of new scientific tools/methodologies/data and decisions, in order to make any necessary corrective actions. This scientific opinion offers a concrete example on how to advance environmental risk assessment of multiple chemicals (including pesticides) and other stressors (covering various types of interactions: e.g. between multiple chemicals, between chemicals and other stressors and/or between multiple non‐chemical stressors).
The EFSA Guidance Document on the risk assessment of PPPs for bees addresses the requirements of EU Regulation (EC) No 1107/20093 i.e. that an active substance or a PPP can be approved in EU if they do not pose unacceptable risks to the environment, including bees. Therefore, the risk assessment in the EFSA Guidance Document focuses on a single substance/PPP and their intended uses and does not consider the combined effects from exposures to multiple chemicals and/or other stressors. In contrast, the system‐based approach in the current scientific opinion offers the opportunity for risk assessors to complement their risk assessment with additional evidence on bee health from monitoring data and modelling simulations.
The systems‐based approach relies on interoperable data that flow from a monitoring system (sentinel honey bee colonies and hives) to a modelling system (ApisRAM, an agent‐based simulation model of a honey bee colony). The systems‐based approach has several applications, from beekeeping/farming, research to risk assessment and management (including the broader society: industry, NGOs and citizens), and it interacts with all stakeholders involved in bee health. The approach was developed for honey bees, but could be applied to bumblebees, solitary bees and other insect pollinators to strengthen their protection and the ecosystem services that they provide to the wider environment.
The support of the social sciences proved useful for this scientific opinion, which had the objective to include stakeholders’ perspectives in the development of a holistic and integrated approach to the risk assessment of multiple stressors in honey bees. Stakeholders were broadly supportive of the concepts and willing to participate to produce a more fit‐for‐purpose evaluation, and have called for concrete benefits and practical solutions. Support from institutions at all levels, as well as adequate resource commitment, was seen as crucial to successful implementation of the proposed approach.
This scientific opinion identified a number of gaps and opportunities for implementation related to environmental risk assessment and the systems‐based approach comprising ApisRAM, for monitoring and data flows into the system. The systems‐based approach can only be fully implemented when these gaps are filled.
7.2. Recommendations
The systems‐based approach is new and innovative, and a phased introduction is recommended. The modelling system can be implemented for risk assessment of pesticides in a phased manner, from the simpler assessment of single substances through to the more complex assessment of mixtures, landscape and indirect effects. The systems‐based approach would allow the risk assessment of pesticides in the context of multiple stressors. It would also strengthen current approaches towards pesticidovigilence.
This scientific opinion is focused on a systems‐based approach for environmental risk assessment of multiple stressors in honey bees. This opinion is a showcase for advancing environmental risk assessment. The approach and methodology presented in this opinion could be adapted for implementation in a broader context (e.g. environmental risk assessments in other bee types, insect pollinators, non‐target arthropods and other organisms from terrestrial and aquatic compartments).
This work is the first case in EFSA's history to integrate input from the social sciences, in the form of targeted research within current risk assessment approaches. The input provided from this field is significant, allowing the perspectives of interested parties to be considered. EFSA risk assessment methodologies should be extended to include stakeholder views when relevant, contributing to the building of trust in the science underpinning the risk analysis process in the EU. In addition, social research in support of risk assessment approaches may highlight the need for targeted communication to stakeholders in areas of their interest or where further information is needed to clarify the approaches.
To sustain the monitoring system and make it operational, the EUBP and platform for data exchange among stakeholders in EU on bee health and beekeeping would need to become one of EFSA's targeted platforms, similar to two other stakeholder consultative groups: the Group on Emerging Risks and the Group on Food Chemical Occurrence Data. This outcome emerged from interviews with EUBP members that were conducted as part of the social science contribution to the scientific opinion. Such a platform would need dedicated resources to allow bee specialists and data managers to develop, coordinate and sustain the platform, for the benefit of stakeholders. Key issues for attention include data quality and the usefulness to stakeholders of key outputs.
The (digital) tools and types of data (indicators) to be collected under the network of sentinel hives, to monitor and assess the health status of bees, was defined under the HEALTHY‐B toolbox. These tools and indicators are currently being fine‐tuned and prioritised under the research project B‐GOOD. Clarity on the quality of these tools (including their accuracy and reliability) and indicators is expected to provide support to the overall data collection effort.
The agent‐based ApisRAM simulation model needs continuous development, implementation and testing with accurate data from the field and from new research in the areas of bee ecology, demography, physiology and toxicology. These data are key to building confidence and trust in the model.
The European Parliament highlighted the lack of harmonised and standardised data in EU on bee health and beekeeping. This scientific opinion suggests several avenues for improving data collection and sharing for the benefit of bee health and beekeeping in EU, by promoting data accessibility, quality, reliability, openness and transparency. The network of sentinel hives should be used as a catalyst for these efforts.
The systems‐based approach should report relevant information in a manner that will allow EU citizens a better understanding of the causes and of underlying mechanisms of bee losses, both in EU and world‐wide.
Finally, the public consultation conducted on the draft opinion prior to its adoption for publication by the EFSA Scientific Committee has highlighted considerable interest, particularly with respect to potential applications, benefits and concerns. This scientific opinion has been developed as a reflections document, presenting ideas and concepts in a manner that is neither prescriptive nor constrained or aligned to specific EU legislation. It is recommended that a discussion is initiated with all relevant stakeholders to consider the issues raised and potential next steps.
Abbreviations
- ABPV
Acute bee paralysis virus
- ABS
Agent‐Based Simulation
- AHAW
EFSA Panel of Animal Health and Animal Welfare
- ALMaSS
An Animal, Landscape and Man Simulation System
- API
Application Processing Interface
- ApisRAM
An Agent‐based simulation for honey bee colony
- BEEHAVE
Model to simulate the development of a honey bee colony and its nectar and pollen foraging behaviour in different landscapes
- B‐GOOD
Giving Beekeeping Guidance by Computational‐Decision Making
- BMP
Beekeeping Management Practice
- C++
Programming language
- CAP
Common Agricultural Policy
- CS
Citizen Science
- CSI
Citizen Scientist Initiative
- DEFRA
Department of Environment, Food and Rural Affairs
- DG AGRI
Directorate‐General for Agriculture and Rural Development
- DG‐ENV
Directorate‐General for the Environment
- DG‐RTD
Directorate‐General for Research and Innovation
- DG‐Santé
Directorate‐General for Health and Food Safety
- DWV
Deformed Wing Virus
- ED
Environmental Drivers module
- EEIA
European Environmental Impact Assessment
- EMR
Estimated Mean Ratio
- EP
European Parliament
- ERA
Environmental Risk Assessment
- EUBP
European Bee Partnership
- EUNIS
European Nature Information System
- GDPR
General Data Protection Regulation
- GAP
Good Agricultural Practices
- GIS
Geographic Information System
- HEALTHY‐B
Assessing the health status of managed honey bee colonies (HEALTHY‐B): a toolbox to facilitate harmonised data collection
- HPG
Hypopharyngeal gland
- HIS
Health Status Index
- ICT
Information and Communications Technology
- IoBee
EC‐funded H2020 project on ‘The Internet of Bees’
- IoT
The Internet of Things
- LD50
Median Lethal Dose
- MDR
Model Deviation Ratio
- MIXTOX
Guidance on harmonised methodologies for human health, animal health and ecological risk assessment of combined exposure to multiple chemicals
- MS
Member State
- MUST‐B
EU efforts towards a holistic and integrated risk assessment approach of multiple stressors in bees
- OECD
The Organisation for Economic Co‐operation and Development
- PER
Proboscis Extension Reflex
- PoshBee
Pan‐European assessment, monitoring and mitigation of stressors on the health of bees
- PPP
Plant Protection Product
- PPR
EFSA Panel of Plant Protection Products and their Residues
- PoC
Proof of Concept
- QSAR
Quantitative Structure‐Activity Relationship
- RFID
Radio‐Frequency Identification
- RPU
Resource Providing Unit
- SAMS
International Partnership on Innovation in Smart Apiculture Management Services
- SC
EFSA Scientific Committee
- SDSS
Spatial Decision Support System
- SEA
Stakeholder Engagement Approach
- SOP
Standard Operating Procedure
- ToR
Term of Reference
- TM
Tank Mix
- TU
Toxic unit
- US
United States
- WIA
World Integrated Assessment
- XML
Extensible Markup Language
Appendix A – EU research projects relevant to MUST‐B
1.
In recent years, the EC has funded several research projects that have the ambition to provide validated reliable methods for automated monitoring of the health status of bee colonies and their activity, linked to pollination services and the honey harvest, a source of income for beekeepers.
Five main projects are concerned: B‐GOOD (until May 2023), PoshBee (until May 2023), INSIGNIA (until December 2020) IoBee (until April 2020) and SAMS (until December 2020). A brief summary of the objectives of these projects is presented below, as well as the expected results.
PoshBee (Pan‐European assessment, monitoring and mitigation of stressors on the health of bees)
PoshBee is the first attempt to quantify chemical exposure of bees at a Pan‐European level, encompassing the major biogeographic zones and two key crops for bees (apples and oilseed rape). At these sites, hives (honey bees), nests (bumble bees) and bee trap nests (solitary bees) were placed and a large sampling of various matrices (pollen, nectar, beebread, bees, wax, bee haemolymph) was conducted to determine exposure to agrochemicals, pathogens and nutritional stress for bees (on different life stages, sexes and castes). Effects from multiple stressors’ interactions (agrochemical, biological and nutritional) are tested in laboratory, semi‐field, field and landscape settings and new monitoring tools (e.g. air sensor to detect agrochemical exposure in beehives; a ‘health card’ based on the development of a molecular marker, i.e. the MALDI Beetyping® of bee haemolymph) will be developed for large‐scale monitoring. Key information and data in the field of bee toxicology and ecology will be generated to fill in the current gaps for the environmental risk assessment of multiple stressors in bees (EFSA, 2014b). For nutrition, the influence of sugar concentration in nectar, pollen diversity, quality and quantity are tested on the development and survival of bees exposed to agrochemicals. In honey bees, toxicological data (toxicokinetics and toxicodynamics) is gathered on four different subspecies and novel wild bee species are tested for their use in risk assessment testing. PoshBee builds on the MUST‐B approach to expand the modelling approach developed for honey bees (ApisRAM) to bumble bees and solitary bees. Those systems and agent‐based modelling approaches are developed to assess higher order interactions between multiple stressors on bee health.
B‐GOOD (Giving Beekeeping Guidance by Computational‐Decision Making)
B‐GOOD is committed to find solutions and to develop innovative technologies to help beekeepers keep their colonies healthy and to ensure sustainable beekeeping practices. Data from within and around beehives will be collected with innovative tools to perform risk assessment for colony management and to give guidance in decision making. To achieve this, B‐GOOD will seek for correlative relationships between complex data by machine learning and modelling, eventually leading to the operationalisation of the Health Status Index (HIS) developed under the Healthy‐B toolbox (EFSA AHAW Panel, 2016). The data will be collected using the open source BEEP system that comprises: (i) a logbook functionality (BEEP app) to register colony inspections in the field; and (ii) several devices for automatic remote sensing of weight, temperature and sound (BEEP base). Other new monitoring devices are under development to deliver real‐time data on colony development, colony exposure to chemical and biological stressors and ultimately to determine colony health status under different environments and conditions in EU. These new technologies will be tested at large scale in EU in a coordinated and harmonised way, carrying out a pilot and several field studies in different representative EU countries.
INSIGNIA (Citizen Science Investigation For Pesticides In Apicultural Products)
INSIGNIA is a pilot study on best practices for a European wide monitoring programme with honey bee colonies in an apiculturist citizen science (CS) setting to study pesticide use and exposure of honey bees and investigation of pollen food sources. Honey bees are regarded as one of the best bio‐indicators due to their sensitivity to chemicals, their dependence on the environment and the use of their products both for food and medicine.
Data resulting from this monitoring programme will enable risk assessment modelling, evaluation of the authorisation of pesticides and agricultural practices and evaluation of the available food sources for bees.
The objectives of the project are: (i) the development of a non‐invasive environmental monitoring system with honey bee colonies in a CS setting, guided by a protocol to be agreed, which combines scientific, practical and methodological recommendations; (ii) risk mapping of honey bee pesticide exposure; (iii) analyses for authorised and non‐authorised pesticides and veterinary drugs; (iv) indication of plant diversity as food sources for bees; (v) evaluation of agricultural and pesticide legislation practice; (vi) verification of the quality of the CORINE database for landscape‐level exposure modelling; and vii) extension, publicity and impact of the monitoring studies.
The INSIGNIA consortium aims for an innovative interpretation of the objectives by the introduction of sentinel apiaries, non‐biological sampling matrices, sociological evaluation and steering of field practitioner processes (CS) to obtain a robust sampling protocol for the non‐invasive environmental monitoring of honey bee colonies.
IoBee (Beehive health IoT application to fight Honey Bee Colony Mortality)
IoBee is a project concluded in April 2020 that initially aimed to pilot and commercialise a new Internet of Things (IoT) sensor application that can automatically assess the threat status of a colony. The developed system wirelessly sends results to a cloud server, making field data available for running prediction models, perform risk assessments, issue warnings and make historical analysis using the Spatial Decision Support System (SDSS). The IoBee consortium also developed pairing technologies to the initial monitoring systems. Partners developed new technological applications envisaging synergies between them, including the use of satellite imaging and data modelling. The initial goal of the project was to allow beekeepers to be active participants in colony surveillance programmes with unprecedented accuracy and responsiveness. As a result, it would enable the remote detection of unhealthy or threatened colonies with greater precision, saving millions of euros in potential losses.
The multidisciplinary consortium developed three essential technologies within the framework of this project: (i) bee counters, which allow beekeepers to assess the strength of the foraging force, determine mortality rates in the field, and identify deviations in flight duration and nectar availability. The counter also identifies pests entering and leaving the hive. At the same time, it should be able to classify and distinguish between drone and worker bee traffic. (ii) optoelectronic sensors that automatically provide insect counts and identifies different species. Therefore, opening the possibility to measure the overall health of pollinators in the field, without the use of traditional insect traps that harm insects. (iii) Data models using satellite imaging, providing phenology historical data and model predictions through the application of the SDSS. Additionally, IoBee was the basis for the development of the Bee Hub PoC, an integrative tool for the analysis, visualisation and communication of pollinator‐related data.
SAMS (International Partnership on Innovation in Smart Apiculture Management Services)
SAMS enhances international co‐operation of ICT and sustainable agriculture between EU and developing countries in pursuit of EU commitment to UN Sustainable Development Goal ‘End hunger, achieve food security and improved nutrition and promote sustainable agriculture’.
Beekeeping with small‐scale operations provides perfect innovation laboratories for demonstration and dissemination of cheap and easy‐to‐use open source ICT applications in developing countries. Bee health and sustainable beekeeping are a key for sustainable agriculture world‐wide.
SAMS allows active monitoring and remote sensing of bee health and beekeeping by developing appropriate ICT solution supporting management of bee health and bee productivity and a role model for effective international co‐operation.
The system contains three main functional groups: (i) the central computer unit where the sensors are connected; (ii) the sensor frame with temperature sensor and microphone placed in the beehive; and (iii) the scale unit placed beneath the beehive with outdoor temperature and humidity sensors.
In the coming months, precise information should be available on the precision and reliability of these different systems, both for beekeepers and researchers.
Appendix B – Clarifications on terminologies
1.
| Working Group interpretation | |
|---|---|
| Terms used in the mandate | Terms use in this opinion |
| Bees’ genetic variety | The term ‘genetic variety’ is more commonly referred to as ‘genetic diversity’. For this mandate, which is focused on managed honey bees (Apis mellifera) in EU, a total of 10 subspecies can be found over this range of distribution, namely, A. m. mellifera, A. m. carnica, A. m. cypria, A. m. cecropia, A. m. macedonica, A. m. adami, A. m. ligustica, A. m. sicula, A. m. rutneri and A. m. iberiensis, and the hybrid Buckfast. Other subspecies of A. mellifera can also be found in Africa and Middle East. Across EU, populations of A. mellifera spp. have varying degrees of introgressions, as determined using molecular markers. This information is also relevant when recording bees’ genetic diversity. |
| Bee management practices | When designing the specifications for the honey bee colony model, the MUST‐B WG described the main beekeeping management practices to be considered:
|
| Bee pathogens | The term ‘bee pathogens’ is commonly used, but is insufficiently precise. Rather, the term ‘biological stressors’, comprising infectious (bacteria, virus, microsporidia, etc.), parasites (Varroa) and predators (hornets), should be used. |
| Cumulative effects of PPPs |
The term ‘cumulative effects of PPPs’ can be defined as being ‘effects from exposure to multiple PPPs from multiple routes or from combined exposure to multiple PPPs by a single route’ (EFSA, 2013a). According to the Directive 2011/92/EU2, under Annex IV, ‘effects on the factors specified in Article 3(1) should cover the direct effects and any indirect, secondary, cumulative, transboundary, short‐term, medium‐term and long‐term, permanent and temporary, positive and negative effects of the project’. The cumulative impact assessment (including cumulative effects) is a project‐level assessment, carried out as part of a response to the requirements of the European Environmental Impact Assessment (EEIA), Habitats and Wild Bird Directives, designed to identify potentially significant impacts of developments and possible mitigation and monitoring measures. All proposals for projects that are subject to the EEIA Directive (85/337/EEC) as amended by the Council Directives 97/11/EC, 2003/35/EC, 2009/31/EC and 2011/92/EU, must be accompanied by an Environmental Statement describing the aspects of the environment likely to be significantly affected by the project. These are covered by a set of guiding principles. Finally, additional definitions can be found in the MIXTOX Guidance (EFSA Scientific Committee, 2019) related to:
|
| Synergistic effects of PPPs | The term ‘Synergistic effects of PPPs’ is defined as being ‘effects from toxicological interaction in which the combined biological effect of two or more substances is greater than expected on the basis of dose addition or response addition’ (EFSA, 2013a). |
| In‐hive environment and/or colony environment | In the EFSA scientific opinion on ‘assessing the health status of managed honey bee colonies (HEALTHY‐B): a toolbox to facilitate harmonised data collection’ (EFSA AHAW Panel, 2016), attributes related to the colony were identified and refer to the ‘in‐hive environment’ or ‘colony environment’:
|
| Monitoring | It is defined as checking regularly in order to perceive change in some quality or quantity (‘checking’ implies a measurement activity and ‘regularly in order to perceive change’ a measurement activity repeated over time). Monitoring techniques are techniques employed in the process of checking, observing and measuring events, processes or physical, chemical, biological and environmental phenomena. |
| Plant Protection Products (PPPs) |
The definition of Plant Protection Products (PPPs) is included in Article 2(1) of Regulation (EC) No 1107/20093. PPPs (may also be called as formulations or preparations) are often a mix of different chemicals and contain at least one active substance. For active substances, the same regulation gives the following definition: ‘This Regulation shall apply to substances, including microorganisms having general or specific action against harmful organisms or on plants, parts of plants or plant products, referred to as ‘active substances’’, which means that most often the constituent of a preparation, which is expected to trigger a stress to bees (e.g. toxic action) or interacting with other stressors, is the active substance. Most often, the constituents of the preparations which are not considered as active substances are inert materials or have weak activity compared to the active substance(s). However, some specific formulations may contain chemicals that are called as safeners or synergists. These constituents alone have low toxicity, but they are considered as components that can interact with the active substance(s) and change their toxicity. The properties of the PPPs that are relevant in the context of this scientific opinion are largely determined by the active substance(s) that it contains. Therefore, this scientific opinion, which is on the development of a risk assessment of multiple PPPs in honey bee colonies, focuses on the active substance(s) of the formulations. |
| Stressor and factor | A stressor is defined as any physical, chemical or biological entity that can adversely affect bee health (EFSA AHAW Panel, 2016). A factor is an attribute of the environment, colony or individual that modulates the adverse effect of stressors on bee health. |
| Surrounding landscape | According to EFSA (EFSA AHAW Panel, 2016), external drivers influencing honey bee colony health were identified and among them, the resource providing unit (RPU) refers to the ‘surrounding landscape’ and ‘is defined in terms of the environment components or units responsible for the genesis and regulation of the resources for a colony’. The shape and the area of the RPU are defined by the maximum foraging distance reached by the bees of a given colony in all the possible directions starting from the hive. The structural (e.g. position and dimension of different crops in the RPU) characteristics of the RPU provide information on the availability, type, amount and accessibility of the resource. The RPU can be divided into subunits or patches, which are considered homogeneous areas from a resource production point of view (EUNIS, 2007; cited in EFSA AHAW Panel, 2016). |
Appendix C – Honey bee subspecies in the three EU regulatory zones
1.
| EU zones | Countries | Representative subspecies |
|---|---|---|
| South | Bulgaria | A. m. carnica |
| Croatia | A. m. carnica | |
| Cyprus | A. m. cypria | |
| France | A. m. mellifera | |
| Greece | A. m. cecropia, A. m. macedonica & A. m. adami | |
| Italy | A. m. ligustica & A. m. sicula | |
| Malta | A. m. rutneri | |
| Portugal | A. m. iberiensis | |
| Spain | A. m. iberiensis | |
| North | Denmark | A. m. mellifera |
| Estonia | A. m. mellifera | |
| Latvia | A. m. mellifera | |
| Lithuania | A. m. mellifera | |
| Finland | A. m. mellifera | |
| Sweden | A. m. mellifera | |
| Centre | Belgium | A. m. mellifera & A. m. carnica |
| Czech | A. m. mellifera & A. m. carnica | |
| Germany | A. m. mellifera & A. m. carnica | |
| Ireland | A. m. mellifera & A. m. carnica | |
| Luxembourg | A. m. mellifera & A. m. carnica | |
| Hungary | A. m. mellifera & A. m. carnica | |
| Netherlands | A. m. mellifera & A. m. carnica | |
| Austria | A. m. mellifera & A. m. carnica | |
| Poland | A. m. mellifera & A. m. carnica | |
| Romania | A. m. mellifera & A. m. carnica | |
| Slovenia | A. m. mellifera & A. m. carnica | |
| Slovakia | A. m. mellifera & A. m. carnica | |
| UK | A. m. mellifera & A. m. carnica |
Appendix D – Automatic or semi‐automatic tools for digital hives
1.
To monitor the health of bee colonies in a non‐invasive way (i.e. by reducing disturbance to colonies, i.e. without opening the hives), various semi‐automatic monitoring tools have been developed over several years. Large numbers of commercial devices are now available on the market, sold by various small‐sized to medium‐sized enterprises (SMEs). There are usually connected tools that transfer measurement data to the user (generally a beekeeper) via computer or cell phone (SMS). An interesting use of these tools might be for monitoring apiaries in some national territories and in certain regions, which could provide scientific data on the state of European beekeeping in its different regions. Beforehand, it would be essential to carry out a comparative scientific study of the reliability and the durability of the commercial devices, under the conditions of hives in the field. The main types of devices currently used, as well as their main constraints, are described below.
Specific description of major characteristics and limitations of each tool are provided. Because a limited set of currently available tools is provided and since there is limited available evidence to determine their potential reliability, further assessments should be performed, including a cost–benefit analysis, before a tool can be recommended for use in large monitoring programmes.
Automatic scales
Automatic scales can be used to assess honey production and food stores and colony development (also used as a proxy of colony size) (Meikle and Holst, 2015).
Automatic scales are probably the most used devices. Their principle is to measure the weight of the hive at regular intervals (sometimes several times a day), and thus makes it possible to follow the development of weight as a function of time. The weight of the hive is the sum of the material of the hive (mainly wood), combs, bees (brood and adults) and food reserves (honey and pollen). The beekeeper can decide to intervene on a colony from remote based on colony weight, as this is a proxy for various important events. E.g. if there is a sudden and sharp decrease in weight, the colony could be swarming (the beekeeper can then try to recover the swarm) or seriously intoxicated (the beekeeper can then alert authorities). If the decrease in weight is lower, but regular, while the period is favourable to the activity of bees, the beekeeper can try to identify the anomaly. In contrast, if colony weight rapidly increases, it may be the sign of intense honey flow, leading the beekeeper to quickly add supers on his hives.
These scales are often associated with thermal and hygrometric sensors that make it possible to know the meteorological conditions in the apiary.
A major limitation is its cost.
Thermal sensors in the hive
Thermal sensors can be used to assess in‐hive temperature (and maintenance of brood temperature and thermoregulation efficiency).
These sensors make it possible to monitor the internal temperature of the colony. Under normal conditions, brood temperature is about 34°C. A sharp drop of temperature can be a sign of adult bee thermoregulation inability. As a consequence, brood health can be impaired, even causing larvae/nymph mortality. A bee thermoregulation impairment can also indicate additional bee health issues at colony level (i.e. foragers need to thermoregulate to efficiently fly and forage). The beekeeper, informed, can try to discover the causes and, possibly, a remedy. The sensors, being used inside the hive, can be covered in propolis and thus require maintenance. A major limitation is its invasiveness.
Sound and/or vibration sensors
Sound and vibration sensors can be used to assess the health status of the colony (e.g. Varroa infestation) and alert on the start of a swarming event (Ramsey et al., 2020).
In addition to chemical and dance communications, bees also communicate through sounds (emitted into the air) and vibrations (transmitted by the combs). Sensors placed outside or inside the hive can provide information on the sounds and/or vibrations coming from a colony. E.g. the initial stages of swarming, bee activity disorders and hornet attacks have specific patterns. The analysed parameters are the variations of the intensity and, possibly, the frequencies emitted. In some cases, the recordings are made by smartphones placed at the entrance of the hive for a short time.
Hive‐entrance video recordings
Video setting can be used to estimate foraging activity and foragers’ mortality.
Using the video to monitor the entrance of a hive allows recording the activity of the bees and, in particular, the activity of foragers who leave and return to the hive. In theory, the difference between the number of outgoing and incoming bees in a day is the number of bees that do not return to the hive, whether they died, drifted or got lost. The difference between the number of outgoing and incoming bees typically represents the number of missing bees. E.g. these data are important to know regarding intoxication by pesticides. Nonetheless, some missing bees could have drifted to other colonies.
This device also makes it possible to record swarming events, as well as the attacks of wasps and hornets. Given the large number of images recorded and the large number of bees to evaluate, automatic image analysis devices could be useful and even necessary at times for analysing big data.
In summary, the videos can be used for quantitative, semi‐quantitative or qualitative analyses depending of the desired level of accuracy and the quality of the device.
A major limitation is its high cost. Furthermore, regarding strong flight activity (i.e. during honey flow), the counting error could drastically increase. A mistaken measurement of incoming and outgoing bees would lead to a mistaken evaluation of eventually disappearing bees. The video quality can be reduced periodically also depending on weather conditions (dirt).
Bee counters
Bee counters can be used to estimate foraging activity and foragers’ mortality (Struye et al., 1994).
These devices are placed at the entrance of the hive to count incoming and outgoing bees and thus to evaluate the activity of the bees, typically foragers. Bee counters have small channels allowing the passage of one worker bee at the time, each one of them being counted via photocells (or possibly other technology). The direction of the bee (incoming or outgoing) is identified, as they use multiple sensors per channel. Regarding video recordings, the difference between outgoing and incoming bees represents the number of missing bees.
Major limitations are its cost and the invasiveness. Additionally, contrary to the video sensors, bee counters do not distinguish foragers from other bees entering and leaving the colony without foraging (guards, etc.). This device may disturb the normal functioning of the colony, because of the narrowness of its holes which, e.g. prevents the males from going out and can die in front of the channels, and block them. It seems therefore preferable to use the bee counters only for limited periods.
Photography image analysis software
In the process of collecting data in the field for the calibration of the model ApisRAM, the software DeepBee® (Alves et al., 2020) was developed and used to assess accurately brood development (eggs, capped and uncapped brood) and food stores (nectar, honey and pollen). A major limitation is its necessary dependency from an external software that can be costly (i.e. a paid subscription), as well as accuracy and invasiveness (hives should be opened for some time to take high‐quality photographs).
Appendix E – Beekeepers focus groups interview guide
1.
Package 1 – Regulatory system
Facilitator to give a short introduction on the current regulatory framework in EU.
Is the current legislation in EU, particularly the one on pesticides, protecting adequately bee health?
Which are the positive aspects?
How could it be improved?
Facilitator to give a short presentation on MUST‐B (this could include showing the video).
Now consider the Vision proposed by MUST‐B – would it support beekeeping as a sector by protecting bee health?
Package 2 – Data exchange between beekeepers and institutions
Providing data might be a reason of concern for some beekeepers who might fear administrative use of that data either for regulatory purposes or for imposing financial obligations.
What would be reasonable conditions for beekeepers to serenely share data with both national and EU authorities? Please share any data sharing concerns, if you have some (prompt if any difference for data sharing with national authorities as opposed to sharing with EU authorities).
Which confidentiality and data protection provisions would allow you to serenely share data?
The current regulatory system might be adapted in such a way that it produces useful data for managing beehives.
Thinking now about the data that the model would generate – which data would be useful for beekeepers?
(Examples of data to prompt discussion if needed: early warning system connected to selected apiaries (pilot monitoring in EU) in EU delivering real‐time data on bee health: personal communications to beekeepers on predictions on resource availability, intoxication probability, infectious agent prevalence, etc.)
Package 3 – Use of digital tools in beehives
Adoption of new technologies, referred to here as digital beehives, may include scales, counting/image processing, sensors (for vibration, temperature, humidity).
In your opinion, what are the advantages and disadvantages of adopting these technologies?
If there are concerns, what are they?
How would adopting these changes change beekeeping? E.g. would it affect the way you/beekeepers manage time? Would it disturb hives? Would it impact the quality of life of bees? And would it affect other aspects of your work?
And beyond your work, would this impact your way of life?
Is the adoption feasible? In your opinion, how would other beekeepers in <name of country> feel about adopting these technologies?
How do you see the role of EU in promoting the adoption of new technologies for beekeeping?
If you could influence innovation on how bee health is monitored, which kinds of IT/semi-automatic tools would you find most useful? What sort of innovation could these tools bring?
Package 4 – Research for bees
This package is meant to prompt individual views of participants on interaction of beekeepers with research rather than test knowledge of beekeepers about research.
How could the work of researchers help you in your activity? In your view, are there any important questions that have not yet been answered?
If you could influence applied research in the beekeeping sector – more specifically on healthy colonies – which areas would you prioritise?
(Examples to prompt the discussion if needed > ensuring bee‐friendly agriculture; assessing exposure to multiple chemicals; applications that give real‐time data on the status of colony health.)
How could researchers and beekeepers interact during research planning and development?
How can the EU ensure that the research projects it supports are relevant for beekeepers?
Through which channels could the EU communicate on research progress to beekeepers (examples of channels > events, newsletters, websites, mobile phone applications).
Appendix F – EUBP Interview Guide
1.
In the context of the scientific opinion on ‘A holistic approach to the risk assessment of multiple stressors in honey bees’, Term of Reference (ToR) 3 includes a description of the work being developed by stakeholders to achieve harmonised data collection and sharing on bee health in EU and to support the EU Bee Partnership initiative.
In this framework, EFSA is interested in gathering your views on the partnership and briefly discuss with you the future and the steps ahead. Instead of focusing on why the partnership formed, we would like to understand how this relationship works.
-
1
How would you describe the involvement of the partners within the EU Bee Partnership? (Would you define it as formal, informal? Does it lead to better ways of thinking? Is it collaborative? Is it a platform that effectively connects stakeholders and encourages discussion and networking?)
[This question is intended to understand the degree of perceived collaboration among the partners and the views on relationships set up.]
-
2
How do you perceive the contributions of different partners? (Is there a balanced contribution from all partners? Does the partnership value the efforts of participants? Are the planned activities on track?)
[This question is intended to gather insight on the perceived efficiency of the group.]
-
3
On the topic of data, what are the practicalities needed for harmonised data collection? What would be the conditions to comfortably share data between members of the EU Bee Partnership? (How important is the agreement on data format? Are there any concerns/challenges for data sharing? How important is to ensure anonymity?)
[This question is intended to gather partners’ views on the potential challenges for harmonised data collection and data sharing.]
-
4
What is the next big step for the EU Bee Partnership? Which are the main benefits for the beekeepers from the work the partnership intends to do? (What is the direction the Partnership is going in? How can it incorporate the perspectives of the wider community of beekeepers?)
[This question is intended to understand views on how to ensure beekeepers’ support to the partnership.]
-
5
On the topic of the future, what kind of additional resources would contribute to the success of the partnership? How would the partnership be best coordinated in the long term? (Examples of non‐financial resources: skills and expertise, data and information, connections to political decision makers, government agencies. Should the partnership be extended to other organisations or groups? Who has the influence and ability to bring people together for meetings or other activities and best coordinate the partnership?)
[This question is intended to gather information on partners’ expectations for the future.]
Supporting information
Technical report: Systems based approach honey bees
Suggested citation: EFSA Scientific Committee , More S, Bampidis V, Benford D, Bragard C, Halldorsson T, Hernández‐Jerez A, Bennekou SH, Koutsoumanis K, Machera K, Naegeli H, Nielsen SS, Schlatter J, Schrenk D, Silano V, Turck D, Younes M, Arnold G, Dorne J‐L, Maggiore A, Pagani S, Szentes C, Terry S, Tosi S, Vrbos D, Zamariola G and Rortais A, 2021. Scientific Opinion on a systems‐based approach to the environmental risk assessment of multiple stressors in honey bees. EFSA Journal 2021;19(5):6607, 75 pp. 10.2903/j.efsa.2021.6607
Requestor: European Parliament
Question numbers: EFSA‐Q‐2018‐00645 and EFSA‐Q‐2016‐00358
Declarations of interest: The declarations of interest of all scientific experts active in EFSA's work are available at https://ess.efsa.europa.eu/doi/doiweb/doisearch.
Acknowledgements: EFSA wishes to acknowledge the contribution to this opinion of Ana Afonso, Annette Aldrich, Matilde Garcia Gomez, Laura Maxim, Alexandra Papanikolaou, Silvia Pieper, Tobin Robinson, Marten Schoonman, Christopher Topping, and Giuseppe Triacchini.
Approved: 14 April 2021
This publication is linked to the following EFSA Supporting Publications articles: http://onlinelibrary.wiley.com/doi/10.2903/sp.efsa.2021.EN-6608/full
Notes
Regulation (EC) No 178/2002 of the European Parliament and of the Council of 28 January 2002 laying down the general principles and requirements of food law, establishing the European Food Safety Authority and laying down procedures in matters of food safety. OJ L 31, 1.2.2002, pp. 1–24.
Directive 2011/92/EU of the European Parliament and of the Council of 13 December 2011 on the assessment of the effects of certain public and private projects on the environment Text with EEA relevance. OJ L 26, 28 January 2012, pp. 1–21.
Regulation (EC) No 1107/2009 of the European Parliament and of the Council of 21 October 2009 concerning the placing of plant protection products on the market and repealing Council Directives 79/117/EEC and 91/414/EEC. OJ L 309, 24.11.2009, pp. 1–50.
Directive 2009/128/EC of the European Parliament and of the Council of 21 October 2009 establishing a framework for Community action to achieve the sustainable use of pesticides. OJ L 309, 24.11.2009, pp. 71–86.
Regulation (EU) 2019/1381 of the European Parliament and of the Council of 20 June 2019 on the transparency and sustainability of the EU risk assessment in the food chain and amending Regulations (EC) No 178/2002, (EC) No 1829/2003, (EC) No 1831/2003, (EC) No 2065/2003, (EC) No 1935/2004, (EC) No 1331/2008, (EC) No 1107/2009, (EU) 2015/2283 and Directive 2001/18/EC (Text with EEA relevance.) PE/41/2019/REV/1. OJ L 231, 6.9.2019, pp. 1–28.
Regulation (EC) No 396/2005 of the European Parliament and of the Council of 23 February 2005 on maximum residue levels of pesticides in or on food and feed of plant and animal origin and amending Council Directive 91/414/EECText with EEA relevance. OJ L 70, 16.3.2005, pp. 1–16.
Commission Regulation (EU) No 284/2013 of 1 March 2013 setting out the data requirements for plant protection products, in accordance with Regulation (EC) No 1107/2009 of the European Parliament and of the Council concerning the placing of plant protection products on the market Text with EEA relevance. OJ L 93, 3.4.2013, pp. 85–152.
Commission Regulation (EU) No 283/2013 of 1 March 2013 setting out the data requirements for active substances, in accordance with Regulation (EC) No 1107/2009 of the European Parliament and of the Council concerning the placing of plant protection products on the market Text with EEA relevance. OJ L 93, 3.4.2013, pp. 1–84.
In this context ‘non‐compliance’ describes the views of beekeepers when it comes to use of PPPs. It does not in any way refer to national derogations (be they for EU‐level banned/restricted neonicotinoids or for other EU‐level banned/restricted active substances) which are provided for in the regulatory system (Article 53 of 1107/209).
European Professional Beekeepers Association (EPBA), Apimondia (International Federation of Beekeepers’ Associations), Association of Veterinary Consultants (AVC), European Crop Protection Association (ECPA), International Confederation of European Beet Growers (CIBE), BeeLife European Beekeeping Coordination, Pesticide Action Network (PAN) – Europe, European Union Reference Laboratory for honey bee health (EURL).
References
- Abi‐Akar F, Schmolke A, Roy C, Galic N and Hinarejos S, 2020. Simulating honey bee large‐scale colony feeding studies using the BEEHAVE model—Part II: analysis of overwintering outcomes. Environmental Toxicology and Chemistry, 39, 2286–2297. 10.1002/etc.4844 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agatz A, Kuhl R, Miles M, Schad T and Preuss TG, 2019. An evaluation of the BEEHAVE model using honey bee field study data: insights and recommendations. Environmental Toxicology and Chemistry, 38, 2535–2545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alves T, Pinto AM, Ventura P, Neves CJ, Biron DG, Junior AC, De Paula Filho PL and Rodrigues PJ, 2020. Automatic detection and classification of honey bee comb using deep learning. Computers and Electronics in Agriculture, 170. [Google Scholar]
- ANSES , 2015. Co‐exposure of bees to stress factors. ANSES Opinion Request No 2012‐SA-0176. 242 pp.
- Arca M, Papachristoforou A, Mougel F, Rortais A, Monceau K, Bonnard O, Tardy P, Thiéry D, Silvain JF and Arnold G, 2014. Defensive behaviour of Apis mellifera against Vespa velutina in France: testing whether European honeybees can develop an effective collective defence against a new predator. Behavioural Processes, 106, 122–129. [DOI] [PubMed] [Google Scholar]
- Aubert M, Ball B, Fries I, Moritz RF, Milani N and Bernardinelli I, 2008. Virology and the honey bee. Office for Official Publications for the European Communities, Luxembourg. [Google Scholar]
- Bailey L and Ball BV, 1991. Honey Bee Pathology, 2nd Edition. Academic Press, London. [Google Scholar]
- Bailey L, 1967. Acute bee‐paralysis virus in adult honey bees injected with sacbrood virus. Virology, 33, 368.de. [DOI] [PubMed] [Google Scholar]
- Barron AB, 2015. Death of the bee hive: understanding the failure of an insect society. Current Opinion in Insect Science, 10, 45–50. 10.1016/j.cois.2015.04.004 [DOI] [PubMed] [Google Scholar]
- Baude M, Kunin WE, Boatman ND, Conyers S, Davies N, Gillespie MA, Morton RD, Smart SM and Memmott J, 2016. Historical nectar assessment reveals the fall and rise of floral resources in Britain. Nature, 530, 85–88. 10.1038/nature16532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baudry E, Solignac M, Garnery L, Gries M, Cornuet JM and Koeniger N, 1998. Relatedness among honeybees (Apis mellifera) of a drone congregation area. Proceedings of the Royal Society London B, 265, 2009–2014. [Google Scholar]
- Becher MA, Osborne JL, Thorbek P, Kennedy PJ and Grimm V, 2013. Towards a systems approach for understanding honeybee decline: a stocktaking and synthesis of existing models. Journal of Applied Ecology, 50, 868–880. 10.1111/1365-2664.12112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becher MA, Grimm V, Thorbek P, Horn J, Kennedy PJ and Osborne JL, 2014. BEEHAVE: a systems model of honeybee colony dynamics and foraging to explore multifactorial causes of colony failure. Journal of Applied Ecology, 51, 470–482. 10.1111/1365-2664.12222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becher MA, Grimm V, Knapp J, Horn J, Twiston‐Davies G and Osborne JL, 2016. BEESCOUT: a model of bee scouting behaviour and a software tool for characterizing nectar/pollen landscapes for BEEHAVE. Ecological Modelling, 340, 126–133. 10.1016/j.ecolmodel.2016.09.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bee Research Institute , 2009. Hygiene in the apiary (a manual for hygienic beekeeping). Written on the basis of research results of the Bee Research Institute, as a part of the EU FP6 research project. Available online: https://www.apiservices.biz/documents/articles-en/hygiene_in_the_apiary.pdf
- Bellucci V, Lucci S, Bianco P, Ubaldi A, Felicioli A, Porrini C, Mutinelli F, Battisti S, Spallucci V, Cersini A, Pietropaoli M and Formato G, 2019. Monitoring honey bee health in five natural protected areas in Italy. Veterinaria Italiana, 55, 15–25. 10.12834/VetIt.1209.6739.4 [DOI] [PubMed] [Google Scholar]
- Berenbaum MR and Liao LH, 2019. Honey bees and environmental stress: toxicologic pathology of a superorganism. Toxicologic Pathology, 47, 1076–1081. 10.1177/0192623319877154 [DOI] [PubMed] [Google Scholar]
- Blacquiere T, Smagghe G, van Gestel CAM and Mommaerts V, 2012. Neonicotinoids in bees: a review on concentrations, side‐effects and risk assessment. Ecotoxicology, 21, 973–992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boncristiani H, Underwood R, Schwarz R, Evans JD, Pettis J and vanEngelsdorp D, 2012. Direct effect of acaricides on pathogen loads and gene expression levels in honey bees Apis mellifera . Journal of Insect Physiology, 58, 613–620. [DOI] [PubMed] [Google Scholar]
- Brandt A, Gorenflo A, Siede R, Meixner M and Buchler R, 2016. The neonicotinoids thiacloprid, imidacloprid, and clothianidin affect the immunocompetence of honey bees (Apis mellifera L.). Journal of Insect Physiology, 86, 40–47. [DOI] [PubMed] [Google Scholar]
- Brühl CA and Zaller JG, 2019. Biodiversity decline as a consequence of an inappropriate environmental risk assessment of pesticides. Frontiers in Environmental Science, 7, 177. 10.3389/fenvs.2019.00177 [DOI] [Google Scholar]
- Brutscher LM, Baer B and Niño EL, 2019. Putative drone copulation factors regulating honey bee (apis mellifera) queen reproduction and health: a review. Insects, 10, 8. 10.3390/insects10010008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryden J, Gill RJ, Mitton RAA, Raine NE and Jansen VAA, 2013. Chronic sublethal stress causes bee colony failure. Ecology Letters, 16, 1463–1469.24112478 [Google Scholar]
- Büchler R, Costa C, Hatjina F, Andonov S, Meixner MD, Le Conte Y, Uzunov A, Berg S, Bienkowska M, Bouga M, Drazic M, Dyrba W, Kryger P, Panasiuk B, Pechhacker H, Petrov P, Kezić N, Korpela S and Wilde J, 2014. The influence of genetic origin and its interaction with environmental effects on the survival of Apis mellifera L. colonies in Europe. Journal of Apicultural Research, 53, 205–214. [Google Scholar]
- Budge GE, Pietravalle S, Brown M, Laurenson L, Jones B, Tomkies V and Delaplane KS, 2015. Pathogens as predictors of honey bee colony strength in England and Wales. PLoS ONE, 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cappè S, Gilsenan M, O'Dea E, Richardson J and Verloo D, 2019. Editorial: the future of data in EFSA. EFSA Journal 2019;17, e17011, 3 pp. 10.2903/j.efsa.2019.e17011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carnesecchi E, Svendsen C, Lasagni S, Grech A, Quignot N, Amzal B, Toma C, Tosi S, Rortais A, Cortinas‐Abrahantes J, Capri E, Kramer N, Benfenati E, Spurgeon D, Guillot G and Dorne JLD, 2019. Investigating combined toxicity of binary mixtures in bees: meta‐analysis of laboratory tests, modelling, mechanistic basis and implications for risk assessment. Environment International, 133(Pt B). 10.1016/j.envint.2019.105256 [DOI] [PubMed] [Google Scholar]
- Carnesecchi E, Toropov AA, Toropova AA, Kramer N, Svendsen C, Dorne JL and Benfenati E, 2020. Predicting acute contact toxicity of organic binary mixtures in honey bees (A. mellifera) through innovative QSAR models. Science of the Total Environment, 704. 10.1016/j.scitotenv.2019.135302 [DOI] [PubMed] [Google Scholar]
- Carter LJ, Agatz A, Kumar A and Williams M, 2020. Translocation of pharmaceuticals from wastewater into beehives. Environmental International, 134. 10.1016/j.envint.2019.105248 [DOI] [PubMed] [Google Scholar]
- Cazier J, Haefeker W and Hassler E, 2018. BXML Part 2 achieving the goal of standardized data. Bee Culture. The Magazine of American Beekeeping. October 2018. Available online: https://www.beeculture.com/bxml-part-2-achieving-the-goal-of-standardized-data/
- Chaimanee V, Evans JD, Chen Y, Jackson C and Pettis JS, 2016. Sperm viability and gene expression in honey bee queens (Apis mellifera) following exposure to the neonicotinoid insecticide imidacloprid and the organophosphate acaricide coumaphos. Journal of Insect Physiology, 89, 1–8. [DOI] [PubMed] [Google Scholar]
- Couvillon MJ, Schürch R and Ratnieks FLW, 2014. Waggle dance distances as integrative indicators of seasonal foraging challenges. PLoS ONE, 9. 10.1371/journal.pone.0093495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cresswell JE, 2011. A meta‐analysis of experiments testing the effects of a neonicotinoid insecticide (imidacloprid) on honey bees. Ecotoxicology, 20, 149–157. 10.1007/s10646-010-0566-0 [DOI] [PubMed] [Google Scholar]
- Dai PL, Wang Q, Sun JH, Liu F, Wang X, Wu YY and Zhou T, 2010. Effects of sublethal concentrations of bifenthrin and deltamethrin on fecundity, growth, and development of the honeybee Apis mellifera ligustica . Environmental Toxicology and Chemistry, 29, 644–649. [DOI] [PubMed] [Google Scholar]
- Dainat B, Evans JD, Chen YP, Gauthier L and Neumann P, 2012. Dead or alive: deformed wing virus and Varroa destructor reduce the life span of winter honeybees. Applied and Environmental Microbiology, 78, 981–987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalkvist T, Topping CJ and Forbes VE, 2009. Population‐level impacts of pesticide‐induced chronic effects on individuals depend more on ecology than toxicology. Ecotoxicology and Environmental Safety, 72, 1663–1672. 10.1016/j.ecoenv.2008.10.002 [DOI] [PubMed] [Google Scholar]
- Dalkvist T, Sibly RM and Topping CJ, 2013. Landscape structure mediates the effects of a stressor on field vole populations. Landscape Ecology, 28, 1961–1974. [Google Scholar]
- Danka RG, Rinderer TE, Kuznetsov VN and Delatte GT, 1995. A USDA‐ARS project to evaluate resistance to Varroa jacobsoni by honey bees of far‐eastern Russia. American Bee Journal, 135, 746–748. [Google Scholar]
- Data Unit , 2017. Harmonized terminology for scientific research [Data set]. Zenodo. 10.5281/zenodo.344473 [DOI] [Google Scholar]
- de Miranda JR, Cordoni G and Budge G, 2010. The acute bee paralysis virus–Kashmir bee virus–Israeli acute paralysis virus complex. Journal of Invertebrate Pathology, 103, 30–47. [DOI] [PubMed] [Google Scholar]
- Decourtye A, Devillers J, Genecque E, Le Menach K, Budzinski H, Cluzeau S and Pham‐Delègue MH, 2005. Comparative sublethal toxicity of nine pesticides on olfactory learning performances of the honeybee Apis mellifera . Archives of Environmental Contamination and Toxicology, 48, 242–250. 10.1007/s00244-003-0262-7 [DOI] [PubMed] [Google Scholar]
- Desneux N, Decourtye A and Delpuech JM, 2007. The sublethal effects of pesticides on beneficial arthropods. Annual Reviews of Entomology, 52, 81–106. 10.1146/annurev.ento.52.110405.091440 [DOI] [PubMed] [Google Scholar]
- Devillers J, 2014. In Silico Bees. CRC Press, Taylor, Francis Group, Boca Raton. 314 pp. [Google Scholar]
- Dirzo R, Young HS, Galetti M, Ceballos G, Isaac NJ and Collen B, 2014. Defaunation in the Anthropocene. Science, 345, 401–406. 10.1126/science.1251817 [DOI] [PubMed] [Google Scholar]
- Donkersley P, Rhodes G, Pickup RW, Jones KC and Wilson K, 2014. Honeybee nutrition is linked to landscape composition. Ecology and Evolution, 4, 4195–4206. 10.1002/ece3.1293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duan X, Hatjina F and Topping CJ, 2021. The Apis Risk Assessment Model (ApisRAM). OC/EFSA/SCER/2016/03. To be published as an EFSA External Scientific Report (in press).
- Dupont YL, Kryger P, Capela N, Axelsen JA, Bruus MB, Sørensen PB, Frederiksen J, Jeppesen AS, Balslev MG, Strandberg B, Castro S, Lichtenberg‐Kraag B, Groom GB, Silva A, Pinto A and Sousa JP, 2021. Field data collection for the evaluation of the honey bee colony model ‘ApisRAM’. EFSA External Scientific Report (in press). [Google Scholar]
- EFSA , online a. Available online: https://www.efsa.europa.eu/sites/default/files/2021-04/draft-strategy-2027-for-public-consultation_0.pdf
- EFSA , online b. Available online https://www.efsa.europa.eu/fr/call/public-consultation-draft-efsa-strategy-2027
- EFSA (European Food Safety Authority) and European Commission's Directorate General for Agriculture and Rural Development, 2016. EU scientific workshop on bee health and sustainable pollination. EFSA supporting publication 2016;EN‐1026, 75 pp. 10.2903/j.efsa.2016.EN-1026 [DOI]
- European Commission , 2020a. Honey market overview (Autumn 2020). Available online: https://ec.europa.eu/info/sites/info/files/food-farming-fisheries/animals_and_animal_products/documents/market-presentation-honey_autumn2020_en.pdf
- European Commission , 2019a. Communication from the Commission to the European Parliament, the European Council, the Council, the European Economic and Social Committee and the Committee of the Regions. The European Green Deal. COM(2019) 640 final. Brussels, 11 December 2019.
- European Commission , 2019b. Communication from the Commission to the European Parliament, the European Council, the Council, the European Economic and Social Committee and the Committee of the Regions. Annual Sustainable Growth Strategy 2020. COM(2019) 650 final. Brussels, 17 December 2019.
- European Commission , 2019c. Annex to the Communication from the Commission to the European Parliament, the European Council, the Council, the European Economic and Social Committee and the Committee of the Regions. The European Green Deal. COM(2019) 640 final ANNEX. Brussels, 11 December 2019.
- European Commission , 2020b. Communication from the Commission to the European Parliament, the Council, the European Economic and Social Committee and the Committee of the Regions. A Farm to Fork Strategy for a Fair, Healthy and Environmentally‐friendly Food System. COM(2020) 381, Brussels, 20 May 2020.
- EFSA (European Food Safety Authority), 2009. Guidance Document on risk assessment for birds & mammals on request from EFSA. EFSA Journal2009;7(12):1438. 10.2903/j.efsa.2009.1438 [DOI] [Google Scholar]
- EFSA (European Food Safety Authority), 2012. Inventory of EFSA's activities on bees. EFSA supporting publications 2012;EN‐358, 89 pp. 10.2903/sp.efsa.2012.EN-358 [DOI]
- EFSA (European Food Safety Authority), 2013a. EFSA's 18th Scientific colloquium on towards holistic approaches to the risk assessment of multiple stressors in bees. Available online:http://www.efsa.europa.eu/sites/default/files/corporate_publications/files/509e.pdf
- EFSA (European Food Safety Authority), 2013b. Guidance Document on the risk assessment of plant protection products on bees (Apis mellifera, Bombus spp. and solitary bees). EFSA Journal 2013;11(7):3295, 266 pp. 10.2903/j.efsa.2013.3295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA (European Food Safety Authority), 2013c. Standard Sample Description ver. 2.0. EFSA Journal 2013;11(10):3424, 114 pp. 10.2903/j.efsa.2013.3424 [DOI] [Google Scholar]
- EFSA (European Food Safety Authority), 2014a. Towards an integrated environmental risk assessment of multiple stressors on bees: review of research projects in Europe, knowledge gaps and recommendations. EFSA Journal 2014;12(3):3594, 102 pp. 10.2903/j.efsa.2014.3594 [DOI] [Google Scholar]
- EFSA (European Food Safety Authority), 2014b. Guidance on Data Exchange version 2.0. EFSA Journal 2014;12(12):3945, 173 pp. 10.2903/j.efsa.2014.3945 [DOI] [Google Scholar]
- EFSA (European Food Safety Authority), 2015. EFSA Strategy 2020 Trusted science for safe food. 10.2805/397609 Available online: http://www.efsa.europa.eu/en/corporate/pub/strategy2020 [DOI]
- EFSA (European Food Safety Authority), 2016a. A mechanistic model to assess risks to honeybee colonies from exposure to pesticides under different scenarios of combined stressors and factors. EFSA supporting publication 2016;EN‐1069, 116 pp. 10.2903/sp.efsa.2016.EN-1069 [DOI]
- EFSA (European Food Safety Authority), 2017a. Specifications for field data collection contributing to honey bee model corroboration and verification. EFSA supporting publication 2017:EN‐1234, 54 pp. 10.2903/sp.efsa.2017.EN-1234 [DOI]
- EFSA (European Food Safety Authority), 2018a. Terms of reference for an EU Bee Partnership. EFSA supporting publication 2018;EN‐1423, 18 pp. 10.2903/sp.efsa.2018.EN-1423 [DOI]
- EFSA (European Food Safety Authority), 2018b. Decision of the management board of the European Food Safety Authority on the criteria for establishing a list of stakeholders and the establishment of the stakeholder forum and stakeholder bureau. Available online: https://www.efsa.europa.eu/sites/default/files/Document18992.pdf
- EFSA (European Food Safety Authority), 2019a. 81st Management Board meeting: ESFA's strategy 2027; general food law implementation; annual accounts and social science roadmap. Parma Italy, 19 June 2019. Available online: https://www.efsa.europa.eu/en/events/event/81st-management-board-meeting-efsas-strategy-2027-general-food-law
- EFSA (European Food Safety Authority), 2019b. Scanning the Food Safety Environment. EFSA's Strategic Environmental Scan Report July 2019, 44 pp. Available online: http://www.efsa.europa.eu/sites/default/files/EFSA_Environmental_Scan_Report_2019.pdf
- EFSA (European Food Safety Authority), 2019c. 2027 strategy definition. Management Board Public session, 18 December 2019. Available online: https://www.efsa.europa.eu/sites/default/files/event/mb-82/mb191218-7-p.pdf
- EFSA (European Food Safety Authority), 2020. Risk assessment of beeswax adulterated with paraffin and/or stearin/stearic acid when used in apiculture and as food (honeycomb). EFSA supporting publication 2020;EN‐1859, 64 pp. 10.2903/sp.efsa.2020.EN-1859 [DOI]
- EFSA (European Food Safety Authority), 2021. Social research in support of a systems‐based approach to the environmental risk assessment of multiple stressors in honey bees. EFSA supporting publication 2021 (in press). [DOI] [PMC free article] [PubMed]
- EFSA (European Food Safety Authority), in preparation. Review of the Guidance Document on the risk assessment of plant protection products on bees (Apis mellifera, Bombus spp. and solitary bees). EFSA Journal. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA (European Food Safety Authority), Anastassiadou M, Brancato A, Brocca D, Carrasco Cabrera L, Ferreira L, Greco L, Jarrah S, Kazocina A, Leuschner R, Lostia A, Magrans JO, Medina P, Miron I, Pedersen R, Raczyk M, Reich H, Ruocco S, Sacchi A, Santos M, Stanek A, Tarazona J, Theobald A and Verani A, 2019. Guidance on the reporting data on pesticide residues in food and feed according to Regulation (EC) No 396/2005 (2018 data collection). EFSA Journal 2019;17(4):5655, 72 pp. 10.2903/j.efsa.2019.5655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA (European Food Safety Authority) and European Commission's Directorate General for Agriculture and Rural Development, 2016b. EU scientific workshop on bee health and sustainable pollination. EFSA supporting publication 2016;EN‐1026, 75 pp. 10.2903/j.efsa.2016.en-1026 [DOI]
- EFSA (European Food Safety Authority) and European Farmers and European Agri Cooperatives, European Professional Beekeepers Association, BeeLife the European Beekeeping Coordination and the European Crop Protection Association, 2017b. Collecting and sharing data on bee health: Towards a European Bee Partnership. EFSA supporting publication 2017:EN‐1299, 34 pp. 10.2903/j.efsa.2017.en-1299 [DOI]
- EFSA AHAW Panel (EFSA Panel on Animal Health and Welfare), 2016. Scientific Opinion on assessing the health status of managed honeybee colonies (HEALTHY‐B): a toolbox to facilitate harmonised data collection. EFSA Journal 2016;14(10):4578, 241 pp. 10.2903/j.efsa.2016.4578. Available online: http://onlinelibrary.wiley.com/doi/10.2903/j.efsa.2016.4578/epdf [DOI] [Google Scholar]
- EFSA PPR Panel (EFSA Panel on Plant Protection Products and their Residues), 2012. Scientific Opinion on the science behind the development of a risk assessment of Plant Protection Products on bees (Apis mellifera, Bombus spp. and solitary bees). EFSA Journal 2012;10(5):2668, 275 pp. 10.2903/j.efsa.2012.2668. Available online: www.efsa.europa.eu/efsajournal EFSA, 2013. [DOI] [Google Scholar]
- EFSA PPR Panel (EFSA Panel on Plant Protection Products and their Residues), 2013. Scientific Opinion on the identification of pesticides to be included in cumulative assessment groups on the basis of their toxicological profile (2014 update). EFSA Journal 2013;11(7):3293, 131 pp. 10.2903/j.efsa.2013.3293 [DOI] [Google Scholar]
- EFSA PPR Panel (EFSA Panel on Plant Protection Products and their Residues), 2014. Scientific Opinion on good modelling practice in the context of mechanistic effect models for risk assessment of plant protection products. EFSA Journal 2014;12(3):3589, 92 pp. 10.2903/j.efsa.2014.3589. Available online: www.efsa.europa.eu/efsajournal [DOI] [Google Scholar]
- EFSA PPR Panel (EFSA Panel on Plant Protection Products and their Residues), 2015. Statement on the suitability of the BEEHAVE model for its potential use in a regulatory context and for the risk assessment of multiple stressors in honeybees at the landscape level. EFSA Journal 2015;13(6):4125, 92 pp. 10.2903/j.efsa.2015.4125. Available online: www.efsa.europa.eu/efsajournal [DOI] [Google Scholar]
- EFSA Scientific Committee , More SJ, Hardy A, Bampidis V, Benford D, Bennekou SH, Bragard C, Boesten J, Halldorsson TI, Hernández‐Jerez AF, Jeger MJ, Knutsen HK, Koutsoumanis KP, Naegeli H, Noteborn H, Ockleford C, Ricci A, Rychen G, Schlatter JR, Silano V, Nielsen SS, Schrenk D, Solecki R, Turck D, Younes M, Benfenati E, Castle L, Cedergreen N, Laskowski R, Leblanc JC, Kortenkamp A, Ragas A, Posthuma L, Svendsen C, Testai E, Dujardin B, Kass GEN, Manini P, Zare Jeddi M, Dorne J‐LCM and Hogstrand C, 2019. Guidance on harmonised methodologies for human health, animal health and ecological risk assessment of combined exposure to multiple chemicals. EFSA Journal 2019;17(3):5634, 77 pp. 10.2903/j.efsa.2019.5634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA Scientific Committee , More S, Bampidis V, Benford D, Bragard C, Halldorsson T, Hernández‐Jerez A, Hougaard Bennekou S, Koutsoumanis X, Machera K, Hanspeter N, Nielsen SS, Schlatter J, Dieter Schrenk D, Silano V, Turck D, Younes M, Arnold G, Dorne JL, Maggiore A, Pagani S, Szentes C, Terry S, Tosi S, Vrbos D, Zamariola G and Rortais A, 2021. A systems‐based approach to the environmental risk assessment of multiple stressors in honey bees. EFSA Journal 2021 (in press). [DOI] [PMC free article] [PubMed]
- European Parliament , 2018. Report on the Union's authorization procedure for pesticides (2018/2153(INI)). Special Committee on the Union's authorization procedure for pesticides. Rapporteurs: Norbert Lins, Bart Staes. Plenary 18 December 2018.
- Eiri DM and Nieh JC, 2012. A nicotinic acetylcholine receptor agonist affects honey bee sucrose responsiveness and decreases waggle dancing. Journal of Experimental Biology, 215, 2022–2029. 10.1242/jeb.068718 [DOI] [PubMed] [Google Scholar]
- European Union , 2018. Agriculture, Forestry and Fishery Statistics. Edition 2018. Statistical book, Eurostat. [Google Scholar]
- EUNIS , 2007. Database of EUNIS habitat classification (revised descriptions 2012) amended in 2019. European Environmental Agency. Available online: http://www.eea.europa.eu/themes/biodiversity/eunis/eunis-habitat-classification
- Everaars J and Dormann CF, 2014. Simulation of solitary (non‐Apis) bees competing for pollen. In: Devillers J (ed). In Silico Bees. CRC Press, Taylor, Francis Group, Boca Raton. pp. 209–268. [Google Scholar]
- Fischer J, Müller T, Spatz A‐K, Greggers U, Grünewald B and Menzel R, 2014. Neonicotinoids interfere with specific components of navigation in honeybees. PLoS ONE, 9. 10.1371/journal.pone.0091364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forsgren E, 2010. European foulbrood in honey bees. Journal of invertebrate pathology, 103(Suppl), 5–9. [DOI] [PubMed] [Google Scholar]
- Fries I, 1993. Nosema apis – a parasite in the healthy bee colony. Bee World, 74, 5–19. [Google Scholar]
- Fries I and Bommarco R, 2007. Possible host–parasite adaptations in honey bees infested by Varroa destructor mites. Apidologie, 38, 525–533. [Google Scholar]
- Fries I, Hansen H, Imdorf A and Rosenkranz P, 2003. Swarming in honey bees (Apis mellifera) and Varroa destructor population development in Sweden. Apidologie, 34, 389–397. [Google Scholar]
- Fryday S, Thompson H and Garthwaite D, 2011. Background information for considering risk of exposure to multiple pesticides. Department of Environment Food and Rural Affairs (DEFRA) project PS2354 (DEFRA, 2011).
- Garthwaite D, Sinclair C, Glass R, Pote A, Trevisan M, Sacchettini G, Spanoghe P, Ngoc KD, Fevery D, Machera K, Charistou A, Nikolopoulou D, Aarapaki N, Tskirakis A, Gerritsen‐Ebben R, Spaan S, González FE, Stobiecki S, Śliwiński W, Stobiecki T and Hakaite P, 2015. Collection of pesticide application data in view of performing environmental risk assessments for pesticides. EFSA supporting publication 2015;EN‐846, 246 pp. 10.2903/sp.efsa.2015.en-846 [DOI] [Google Scholar]
- Genersch E, 2010. American Foulbrood in honeybees and its causative agent, Paenibacillus larvae. Journal of Invertebrate Pathology, 103, S10–S19. [DOI] [PubMed] [Google Scholar]
- Group of Chief Scientific Advisers , 2018. EU Authorisation processes of plant protection products from a scientific point of view. Scientific Opinion 5 (supported by SAPEA Evidence Review Report No. 3), Brussels, 4 June 2018. Available online: https://ec.europa.eu/research/sam/pdf/sam_ppp_report.pdf
- Haefeker W, 2018. Hive management systems for beekeeping. Veterinaria italiana Monografia, 27, 15. [Google Scholar]
- Hallmann CA, Sorg M, Jongejans E, Siepel H, Hofland N, Schwan H, Stenmans W, Muller A, Sumser H, Horren T, Goulson D and de Kroon H, 2017. More than 75 percent decline over 27 years in total flying insect biomass in protected areas. PLoS ONE, 12. 10.1371/journal.pone.0185809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han WS, Wang YJ, Gao JL, Wang SJ, Zhao S, Liu JF, Zhong YH and Zhao DX, 2018. Acute toxicity and sublethal effects of myclobutanil on respiration, flight and detoxification enzymes in Apis cerana. Pesticide Biochemistry and Physiology, 147, 133–138. [DOI] [PubMed] [Google Scholar]
- Hatjina F, Papaefthimiou C, Charistos L, Dogaroglu T, Bouga M, Emmanouil C and Arnold G, 2013. Sublethal doses of imidacloprid decreased size of hypopharyngeal glands and respiratory rhythm of honeybees in vivo. Apidologie, 44, 467–480. 10.1007/s13592-013-0199-4 [DOI] [Google Scholar]
- Hatjina F, Costa C, Buechler R, Uzunov A, Drazic M, Filipi J, Charistos L, Ruottinen L, Andonov S, Meixner MD, Bienkowska M, Dariusz G, Panasiuk B, Le Conte Y, Wilde J, Berg S, Bouga M, Dyrba W, Kiprijanovska H, Korpela S, Kryger P, Lodesani M, Pechhacker H, Petrov P and Kezic N, 2014. Population dynamics of European honey bee genotypes under different environmental conditions. Journal of Apicultural Research, 53, 233–247. [Google Scholar]
- Hawthorne DJ and Dively GP, 2011. Killing them with kindness? In‐hive medications may inhibit xenobiotic efflux transporters and endanger honey bees. PLoS ONE, 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haxaire J, Tamisier J‐P and Bouguet J‐P, 2006. Vespa velutina Lepeletier, 1836, une redoutable nouveauté pour la faune de France (Hym., Vespidae). Bulletin de la Société Entomologique de France, 111, 194. [Google Scholar]
- Henry M, Beguin M, Requier F, Rollin O, Odoux J‐F, Aupinel P, Aptel J, Tchamitchian S and Decourtye A, 2012. A common pesticide decreases foraging success and survival in honey bees. Science, 336, 348–350. 10.1126/science.1215039 [DOI] [PubMed] [Google Scholar]
- Henry M, Cerrutti N, Aupinel P, Decourtye A, Gayrard M, Odoux J‐F, Pissard A, Rüger C and Bretagnolle V, 2015. Reconciling laboratory and field assessments of neonicotinoid toxicity to honeybees. Proceedings of the Royal Society B, 282, 2110. 10.1098/rspb.2015.2110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henry M, Becher MA, Osborne JL, Kennedy PJ, Aupinel P, Bretagnolle V, Brun F, Grimm V, Horn J and Requier F, 2017. Predictive systems models can help elucidate bee declines driven by multiple combined stressors. Apidologie, 48, 328–339. 10.1007/s13592-016-0476-0 [DOI] [Google Scholar]
- Herbertsson L, Lindström SAM, Rundlöf M, Bommarco R and Smith HG, 2016. Competition between managed honeybees and wild bumblebees depends on landscape context. Basic and Applied Ecology, 17, 609–616. [Google Scholar]
- Higes M, Martın R and Meana A, 2006. Nosema ceranae, a new microsporidian parasite in honeybees in Europe. Journal of Invertebrate Pathology, 92, 93–95. [DOI] [PubMed] [Google Scholar]
- Holland JH, 2006. Studying complex adaptive systems. Journal of Systems Science and Complexity, 19, 1–8. 10.1007/s11424-006-0001-z [DOI] [Google Scholar]
- Horn J, Becher MA, Kennedy PJ, Osborne JL and Grimm V, 2015. Multiple stressors: using the honeybee model BEEHAVE to explore how spatial and temporal forage stress affects colony resilience. Oikos, 125, 1001–1016. 10.1111/oik.02636 [DOI] [Google Scholar]
- Jacobsen LB, Jensen JD, Hauch J, Topping CJ and Andersen MI, 2008. Pesticide reducing instruments – an interdisciplinary analysis of effectiveness and optimality. In Chalifour NJ, Milne JE, Ashiabor H, Deketelaere K and Kreiser L (eds.).Critical Issues in Environmental Taxation: International and Comparative Perspectives. Oxford University Press, Oxford. pp. 569–599. [Google Scholar]
- Jacques A, Laurent M, Ribière‐Chabert M, Saussac M, Bougeard S, Hendrikx P and Chauzat M‐P, 2016. Statistical analysis on the EPILOBEE dataset: explanatory variables related to honeybee colony mortality in EU during a 2‐year survey. EFSA supporting publication 2016;EN‐883, 228 pp. Available online: 10.2903/sp.efsa.2016.en-883 [DOI]
- Jacques A, Laurent M, EPILOBEE Consortium, Ribière‐Chabert M, Saussac M, Bougeard S, Budge GE, Hendrikx P and Chauzat M‐P, 2017. A pan‐European epidemiological study reveals honey bee colony survival depends on beekeeper education and disease control. PLoS ONE, 12, e0172591. 10.1371/journal.pone.0172591 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson RM, 2015. Honey bee toxicology. Annual Review of Entomology, 60, 415–434. 10.1146/annurev-ento-011613-162005 [DOI] [PubMed] [Google Scholar]
- Kessler SC, Tiedeken EJ, Simcock KL, Derveau S, Mitchell J, Softley S, Radcliffe A, Stout JC and Wright GA, 2015. Bees prefer foods containing neonicotinoid pesticides. Nature, 521, 74–76. 10.1038/nature14414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khoury DS, Myerscough MR and Barron AB, 2011. A quantitative model of honey bee colony population dynamics. PLoS ONE, 6. 10.1371/journal.pone.0018491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khoury DS, Barron AB and Myerscough MR, 2013. Modelling food and population dynamics in honey bee colonies. PLoS ONE, 8. 10.1371/journal.pone.0059084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krueger RA, 1988. Focus groups: a practical guide for applied research. Sage Publications Inc. [Google Scholar]
- Lanzi G, de Miranda JR, Boniotti MB, Cameron CE, Lavazza A, Capucci L, Camazine SM and Rossi C, 2006. Molecular and biological characterization of deformed wing virus of honeybees (Apis mellifera L.). Journal of Virology, 80, 4998–5009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laurent M, Hendrikx P, Ribière‐Chabert M and Chauzat M‐P, 2016. A pan‐European epidemiological study on honeybee colony losses 2012–2014. EPILOBEE – 2012–2014 Version 2. 44 pp.
- Laurino D, Manino A, Patetta A and Porporato M, 2013. Toxicity of neonicotinoid insecticides on different honey bee genotypes. Bulletin of Insectology, 66, 119–126. [Google Scholar]
- Locke B, 2016. Natural Varroa mite‐surviving Apis mellifera honeybee populations. Apidologie, 47, 467–482. [Google Scholar]
- Lozano A, Hernando MD, Uclés S, Hakme E and Fernández‐Alba AR, 2019. Identification and measurement of veterinary drug residues in beehive products. Food Chemistry, 274, 61–70. 10.1016/j.foodchem.2018.08.055 [DOI] [PubMed] [Google Scholar]
- Luttik R, Zorn MI, Brock TCM, Roex EWM and Van der Linden AMA, 2017. Multiple stress by repeated use of plant protection products in agricultural areas. National Institute for Public Health and the Environment of Netherlands. RIVM Report 2016‐0152. 70 pp.
- Martin J, Fine JD, Cash‐Ahmed A and Robinson GE, 2018. The effect of imidacloprid on honey bee queen fecundity. PRECS, 2018, 5. Available online: https://spark.parkland.edu/precs_2018/5/ [Google Scholar]
- Meikle WG and Holst N, 2015. Application of continuous monitoring of honeybee colonies. Apidologie, 46, 10–22. [Google Scholar]
- Meikle WG, Adamczyk JJ, Weiss M, Gregorc A, Johnson DR, Stewart SD, Zawislak J, Carroll MJ and Lorenz GM, 2016. Sublethal effects of imidacloprid on honey bee colony growth and activity at three sites in the US. PLoS ONE, 11. 10.1371/journal.pone.0168603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meixner MD, Costa C, Kryger P, Hatjina F, Bouga M, Ivanova E and Büchler R, 2010. Conserving diversity and vitality for honey bee breeding. Journal of Apicultural Research, 49, 85–92. 10.3896/IBRA.1.49.1.12 [DOI] [Google Scholar]
- Meixner MD, Pinto MA, Bouga M, Kryger P, Ivanova E and Fuchs S, 2013. Standard methods for characterising subspecies and ecotypes of Apis mellifera . Journal of Apicultural Research, 52, 1–28. 10.3896/IBRA.1.52.4.05 [DOI] [Google Scholar]
- Michener CD, 1974. The Social Behavior of the Bees. Harvard University Press. [Google Scholar]
- Milner AM and Boyd IL, 2017. Toward pesticidovigilance. Science, 357, 1232–1234. 10.1126/science.aan2683 [DOI] [PubMed] [Google Scholar]
- Mitchell EAD, Mulhauser B, Mulot M and Aebi A, 2017. A worldwide survey of neonicotinoids in honey. Science, 358, 109–111. 10.1126/science.aan3684 [DOI] [PubMed] [Google Scholar]
- Momeni J, Parejo M, Nielsen RO, Langa J, Montes I and Papoutsis L, 2021. Authoritative subspecies diagnosis tool for European honey bees based on ancestry informative SNPs. BMC Genomics, 22, 101. 10.1186/s12864-021-07379-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monceau K, Bonnard O and Thiéry D, 2014. Vespa velutina: a new invasive predator of honeybees in Europe. Journal of Pest Science, 87, 1–16. 10.1007/s10340-013-0537-3 [DOI] [Google Scholar]
- More S, Rortais A, Auteri D and Pagani S, 2021. Editorial: EFSA is working to protect bees and to shape the future of environmental risk assessment. EFSA Journal 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moretto G, Gonçalves LS, De Jog D and Bichuette MZ, 1991. The effects of climate and bee race on Varroa jacobsoni Oud infestations in Brazil. Apidologie, 22, 197–203. [Google Scholar]
- Moritz RF and Southwick EE, 1992. Bees as Superorganisms: An Evolutionary Reality. Springer‐Verlag, New York. [Google Scholar]
- Mullin CA, Frazier M, Frazier JL, Ashcraft S, Simonds R and Pettis JS, 2010. High levels of miticides and agrochemicals in North American apiaries: implications for honey bee health. PLoS ONE, 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naug D, 2009. Nutritional stress due to habitat loss may explain recent honeybee colony collapses. Biological Conservation, 142, 2369–2372. 10.1016/j.biocon.2009.04.007 [DOI] [Google Scholar]
- Odderskaer P, Tybirk K, Topping JA, Axelsen M and Pedersen CJB, 2003. Effects of reduced pesticide use on plants, insects and birds. DJF Rapport, Markbrug, 89, 199–211. [Google Scholar]
- Odderskær P, Topping C and Jepsen J, 2004. ALMaSS simulation of the impact of removing pesticides. [ALMaSS simulering af virkningen af at fjerne pesticider]. Danmarks Jordbrugsforskning. [Google Scholar]
- Odoux JF, Aupinel P, Gateff S, Requier F, Henry M and Bretagnolle V, 2014. ECOBEE: a tool for long‐term honey bee colony monitoring at the landscape scale in West European intensive agroecosystems. Journal of Apicultural Research, 53, 57‐6. [Google Scholar]
- OECD (Organisation for Economic Co‐operation and Development), 2005. Guidance document on the validation and international acceptance of new or updated test methods for hazard assessment. OECD Series on testing and assessment, 34, 1–96. [Google Scholar]
- OECD (Organisation for Economic Co‐operation and Development), 2009. Guidance document for the development of OECD guidelines for the testing of chemicals. the validation and international acceptance of new or updated test methods for hazard assessment. OECD Series on testing and assessment, 33, 1–16. [Google Scholar]
- OECD (Organisation for Economic Co‐operation and Development), 2017. Test No. 245: Honey Bee (Apis Mellifera L.), Chronic Oral Toxicity Test (10‐Day Feeding), OECD Guidelines for the Testing of Chemicals, Section 2, OECD Publishing, Paris, 10.1787/9789264284081-en [DOI]
- OECD (Organisation for Economic Co‐operation and Development), 2020. Proposal of a new OECD guideline for the testing of chemicals. Honey bee (Apis mellifera L.) homing flight test, using single oral exposure to sublethal doses of test chemical. Available online: https://www.oecd.org/chemicalsafety/testing/Draft%20OECD%20TG_Homing%20flight%20test_honeybees_18%20May.pdf [Google Scholar]
- OIE (World Organisation for Animal Health), 2008. Varroosis of honey bees. OIE Terrestrial Manual, Chapter 2.2.7. OIE, Paris. [Google Scholar]
- Orr JA, Vinebrooke RD, Jackson MC, Kroeker KJ, Kordas RL, Mantyka‐Pringle C, Van den Brink PJ, De Laender F, Stoks R, Holmstrup M, Matthaei CD, Monk WA, Penk MR, Leuzinger S, Schäfer RB and Piggott JJ, 2020. Towards a unified study of multiple stressors: divisions and common goals across research disciplines. Proceedings of the Royal Society B, 287, 0421. 10.1098/rspb.2020.0421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Page P, Lin Z, Buawangpong N, Zheng H, Hu F, Neumann P, Chantawannakul P and Dietemann V, 2016. Social apoptosis in honey bee superorganisms. Scientific Reports, 6, 27210. 10.1038/srep27210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pisa LW, Amaral‐Rogers V, Belzunces LP, Bonmatin JM, Downs CA, Goulson D, Kreutzweiser DP, Krupke C, Liess M and McField M, 2015. Effects of neonicotinoids and fipronil on non‐target invertebrates. Environmental Science and Pollution Research, 22, 68–102. 10.1007/s11356-017-0341-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pisa L, Goulson D, Yan EC, Gibbons D, Sánchez‐Bayo F, Mitchell E, van der Sluijs J, MacQuarrie C, Giorio C, Long EY, McField M, Bijleveld van Lexmond M and Bonmatin JM, 2017. An update of the world integrated assessment (WIA) on systemic insecticides. Part 2: impacts on organisms and ecosystems. Environmental Science and Pollution Research. 10.1007/s11356-017-0341-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potts SG, Biesmeijer JC, Kremen C, Neumann P, Schweiger O and Kunin WE, 2010. Global pollinator declines: trends, impacts and drivers. Trends in Ecology and Evolution, 25, 345–353. 10.1016/j.tree.2010.01.007 [DOI] [PubMed] [Google Scholar]
- Ramsey M‐T, Bencsik M, Newton MI, Reyes M, Pioz M, Crauser D, Simon Delso N and Le Conte Y, 2020. The prediction of swarming in honeybee colonies using vibrational spectra. Scientific Reports, 10, 9798. 10.1038/s41598-020-66115-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Requier F, Odoux JF, Tamic T, Moreau N, Henry M, Decourtye A and Bretagnolle V, 2015. Honey bee diet in intensive farmland habitats reveals an unexpectedly high flower richness and a major role of weeds. Ecological Applications, 25, 881–890. [DOI] [PubMed] [Google Scholar]
- Requier F, Odoux JF, Henry M, Decourtye A and Bretagnolle V, 2016. The carry‐over effects of pollen shortage decrease the survival of honey bee colonies in farmlands. Journal of Applied Ecology, 54, 1161–1170. 10.1111/1365-2664.12836 [DOI] [Google Scholar]
- Requier F, Rome Q, Villemant C and Henry M, 2020. A biodiversity‐friendly method to mitigate the invasive Asian hornet's impact on European honey bees. Journal of Pest Science, 93, 1–9. 10.1007/s10340-019-01159-9 [DOI] [Google Scholar]
- Ribière M, Ball B and Aubert M, 2008. Natural history and geographical distribution of honey bee viruses. In: Aubert M, Ball B, Fries I, Moritz R, Milani N and Bernardinelli I (eds.). Virology and the Honey Bee. Office for Official Publications of the European Communities, Brussels. pp. 15–84. [Google Scholar]
- Rinderer TE, de Guzman LI, Delatte GT, Stelzer JA, Lancaster VA, Kuznetsov V, Beaman L, Watts R and Harris JW, 2001. Resistance to the parasitic mite Varroa destructor in honey bees from far‐eastern Russia. Apidologie, 32, 381–394. [Google Scholar]
- Rinderer TE, Harris JW, Hunt GJ and de Guzman LI, 2010. Breeding for resistance to Varroa destructor in North America. Apidologie, 21, 409–424. [Google Scholar]
- Rinkevich FD, Margotta JW, Pittman JM, Danka RG, Tarver MR, Ottea JA and Healy KB, 2015. Genetics, synergists, and age affect insecticide sensitivity of the honey bee, Apis mellifera . PLoS ONE, 10, 2–13. 10.1371/journal.pone.0139841 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson GE, 1992. Regulation of division of labor in insect societies. Annual Review of Entomology, 37, 637–665. [DOI] [PubMed] [Google Scholar]
- Rosenkranz P, 1999. Honey bee (Apis mellifera L.) tolerance to Varroa jacobsoni Oud. in South America. Apidologie, 30, 159–172. [Google Scholar]
- Rumkee JCO, Becher MA, Thorbek P, Kennedy PJ and Osborne JL, 2015. Predicting honeybee colony failure: using the BEEHAVE model to simulate colony responses to pesticides. Environmental Science and Technologies, 49, 12879–12887. 10.1021/acs.est.5b03593 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell S, Barron AB and Harris D, 2013. Dynamic modelling of honey bee (Apis mellifera) colony growth and failure. Ecological Modelling, 265, 158–169. 10.1016/j.ecolmodel.2013.06.005 [DOI] [Google Scholar]
- Ruttner F, 1988. Biogeography and Taxonomy of Honeybees. Springer Verlag, Heidelberg, Berlin, New York. 284 pp. [Google Scholar]
- Schmickl T and Crailsheim K, 2007. HoPoMo: a model of honeybee intracolonial population dynamics and resource management. Ecological Modelling, 204, 219–245. 10.1016/j.ecolmodel.2007.01.001 [DOI] [Google Scholar]
- Schmitt W, Auteri D, Bastiansen F, Ebeling M, Liu C, Luttik R, Mastitsky S, Nacci D, Topping C and Wang M, 2016. An example of population‐level risk assessments for small mammals using individual‐based population models. Integrated Environment Assessment and Management, 12, 46–57. 10.1002/ieam.1640 [DOI] [PubMed] [Google Scholar]
- Schmolke A, Abi‐Akar F and Hinarejos S, 2019. Honey bee colony‐level exposure and effects in realistic landscapes: an application of BEEHAVE simulating clothianidin residues in corn pollen. Environmental Toxicology and Chemistry, 38, 423–435. 10.1002/etc.4314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmolke A, Abi‐Akar F, Roy C, Galic N and Hinarejos S, 2020. Simulating honey bee large‐scale colony feeding studies using the BEEHAVE Model—Part I: model validation. Environmental Toxicology and Chemistry. 10.1002/etc.4839 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeley TD, 1985. Honeybee Ecology, A Study of Adaptation in Social Life. Princeton University Press, Princeton. [Google Scholar]
- Seeley , 1989. The honey bee colony as a superorganism. American Scientists, 77, 546–553. [Google Scholar]
- Sgolastra F, Medrzycki P, Bortolotti L, Maini S, Porrini C, Simon‐Delso N and Bosch J, 2020. Bees and pesticide regulation: lessons from the neonicotinoid experience. Biological Conservation, 241. 10.1016/j.biocon.2019.108356 [DOI] [Google Scholar]
- Sheppard S and Meixner D, 2003. Apis mellifera pomonella, a new honey bee subspecies from Central Asia. Apidologie, 34, 367–375. 10.1051/apido:2003037 [DOI] [Google Scholar]
- Sibly R, Akçakaya H, Topping C and O'Connor R, 2005. Population‐level assessment of risks of pesticides to birds and mammals in the UK. Ecotoxicology, 14, 863–876. [DOI] [PubMed] [Google Scholar]
- Simon‐Delso N, San Martin G, Bruneau E and Hautier L, 2018. Time‐to‐death approach to reveal chronic and cumulative toxicity of a fungicide for honeybees not revealed with the standard ten‐day test. Scientific Reports, 8, 7241. 10.1038/s41598-018-24746-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siviter H, Brown MJF and Leadbeater E, 2018. Sulfoxaflor exposure reduces bumblebee reproductive success. Nature, 561, 109–112. 10.1038/s41586-018-0430-6 [DOI] [PubMed] [Google Scholar]
- Skarbek CJ, Kobel‐Lamparski A and Dormann CF, 2021. Trends in monthly abundance and species richness of carabids over 33 years at the Kaiserstuhl, southwest Germany. Basic and Applied Ecology, 50, 107–118. 10.1016/j.baae.2020.11.003 [DOI] [Google Scholar]
- Søvik E, Perry CJ, LaMora A, Barron AB and Ben‐Shahar Y, 2015. Negative impact of manganese on honeybee foraging. Biology Letters, 11, 20140989. 10.1098/rsbl.2014.0989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sperandio G, Simonetto A, Carnesecchi E, Costa C, Hatjina F, Tosi S and Gilioli G, 2019. Beekeeping and honey bee colony health: a review and conceptualization of beekeeping management practices implemented in Europe. Science of the Total Environment, 696. 10.1016/j.scitotenv.2019.133795 [DOI] [Google Scholar]
- Spurgeon D, Hesketh H, Lahive E, Svendsen C, Baas J, Robinson A, Horton A and Heard M, 2016. Chronic oral lethal and sub‐lethal toxicities of different binary mixtures of pesticides and contaminants in bees (Apis mellifera, Osmia bicornis and Bombus terrestris). EFSA supporting publication 2016;EN‐1076, 66 pp. 10.2903/sp.efsa.2016.en-1076 [DOI]
- Stabentheiner A, Kovac H and Brodschneider R, 2010. Honeybee colony thermoregulation–regulatory mechanisms and contribution of individuals in dependence on age, location and thermal stress. PLoS ONE, 29. 10.1371/journal.pone.0008967 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinhauer NA, Rennich K, Wilson ME, Caron DM, Lengerich EJ, Pettis JS, Rose R, Skinner JA, Tarpy DR, Wilkes JT and van Engelsdorp D; for the Bee Informed Partnership, 2014. A national survey of managed honey bee 2012–2013 annual colony losses in the USA: results from the Bee Informed Partnership. Journal of Apicultural Research, 53, 1–18. 10.3896/IBRA.1.53.1.01 [DOI] [Google Scholar]
- Strange JP, Garnery L and Sheppard WS, 2007. Persistence of the Landes ecotype of Apis mellifera in southwest France: confirmation of a locally adaptive annual brood cycle trait. Apidologie, 38, 259–267. [Google Scholar]
- Struye MH, Mortier HJ, Arnold G, Miniggio C and Borneck R, 1994. Microprocessor‐controlled monitoring of honeybee flight activity at the hive entrance. Apidologie, 25, 384–395. [Google Scholar]
- Tarek H, Hamiduzzaman MM, Morfin N and Guzman‐Novoa E, 2018. Sub‐lethal doses of neonicotinoid and carbamate insecticides reduce the lifespan and alter the expression of immune health and detoxification related genes of honey bees (Apis mellifera). Genetics and Molecular Research, 17, gmr16039908. 10.4238/gmr16039908 [DOI] [Google Scholar]
- Thompson H, 2012. Interaction between pesticides and other factors in effects on bees. Supporting Publications 2012:EN‐340, 204 pp.
- Thorbek P and Topping C, 2005. The influence of landscape diversity and heterogeneity on spatial dynamics of agrobiont linyphiid spiders: an individual‐based model. BioControl, 50, 1–33. 10.1007/s10526-004-1114-8 [DOI] [Google Scholar]
- Thorbek P, Campbell PJ and Thompson HM, 2017a. Colony impact of pesticide‐induced sublethal effects on honeybee workers: a simulation study using BEEHAVE. Environmental Toxicology and Chemistry, 36, 831–840. [DOI] [PubMed] [Google Scholar]
- Thorbek P, Campbell PJ, Sweeney PJ and Thompson HM, 2017b. Using BEEHAVE to explore pesticide protection goals for European honeybee (Apis mellifera L.) worker losses at different forage qualities. Environmental Toxicology and Chemistry, 36, 254–264. 10.1002/etc.3504 [DOI] [PubMed] [Google Scholar]
- Tison L, Hahn M‐L, Holtz S, Rößner A, Greggers U, Bischoff G and Menzel R, 2016. Honey bees’ behavior is impaired by chronic exposure to the neonicotinoid thiacloprid in the field. Environmental Science and Technology, 50, 7218–7227. 10.1021/acs.est.6b02658 [DOI] [PubMed] [Google Scholar]
- Tong L, James CN and Tosi S, 2019. Combined nutritional stress and a new systemic pesticide (flupyradifurone, Sivanto®) reduce bee survival, food consumption, flight success, and thermoregulation. Chemosphere, 237. 10.1016/j.chemosphere.2019.124408 [DOI] [PubMed] [Google Scholar]
- Topping CJ and Luttik R, 2017. Simulation to aid in interpreting biological relevance and setting of population‐level protection goals for risk assessment of pesticides. Regulatory Toxicology and Pharmacology, 89, 40–49. 10.1016/j.yrtph.2017.07.011 [DOI] [PubMed] [Google Scholar]
- Topping CJ, Hansen TS, Jensen TS, Jepsen JU, Nikolajsen F and Odderskær P, 2003. ALMaSS, an agent‐based model for animals in temperate European landscapes. Ecological Modelling, 167, 65–82. 10.1016/S0304-3800(03)00173-X [DOI] [Google Scholar]
- Topping C, Sibly R, Akçakaya H, Smith GC and Crocker DR, 2005. Risk assessment of UK skylark populations using life‐history and individual‐based landscape models. Ecotoxicology, 14, 925–936. 10.1007/s10646-005-0027-3 [DOI] [PubMed] [Google Scholar]
- Topping C, Dalquist T and Nabe‐Nielsen J, 2008. Incorporating realism into ecological risk assessment—an ABM approach. Ecological models in support of regulatory risk assessments of pesticides: Developing a strategy for the future. SETAC, Pensacola, Florida. [Google Scholar]
- Topping CJ, Dalkvist T, Forbes VE, Grimm V and Sibly RM, 2009. The potential for the use of agent‐based models in ecotoxicology. In: Devillers J (eds.). Ecotoxicology Modeling. Emerging Topics in Ecotoxicology: Principles, Approaches and Perspectives 2. Springer. pp. 205–235. [Google Scholar]
- Topping CJ and Lagisz M, 2012. Spatial dynamic factors affecting population‐level risk assessment for a terrestrial arthropod: an agent‐based modeling approach. Human and Ecological Risk Assessment, 18, 168–180. 10.1080/10807039.2012.632292 [DOI] [Google Scholar]
- Topping CJ, Kjær LJ, Hommen U, Høye TT, Preuss TG, Sibly RM and van Vliet P, 2014. Recovery based on plot experiments is a poor predictor of landscape‐level population impacts of agricultural pesticides. Environmental Toxicology and Chemistry, 33, 1499–1507. 10.1002/etc.2388 [DOI] [PubMed] [Google Scholar]
- Topping CJ, Craig PS, de Jong F, Klein M, Laskowski R, Manachini B, Pieper S, Smith R, Sousa JP, Streissl F, Swarowsky K, Tiktak A and van der Linden T, 2015. Towards a landscape scale management of pesticides: environmental risk assessment using changes in modelled occupancy and abundance to assess long‐term population impacts of pesticides. Science of the Total Environment, 537, 159–169. 10.1016/j.scitotenv.2015.07.152 [DOI] [PubMed] [Google Scholar]
- Topping CJ, Dalby L and Skov F, 2016. Landscape structure and management alter the outcome of a pesticide ERA: evaluating impacts of endocrine disruption using the ALMaSS European Brown Hare model. Science of the Total Environment, 541, 1477–1488. 10.1016/j.scitotenv.2015.10.042 [DOI] [PubMed] [Google Scholar]
- Topping CJ and Weyman GS, 2018. Rabbit population landscape‐scale simulation to investigate the relevance of using rabbits in regulatory environmental risk assessment. Environmental Modeling & Assessment, 23, 415–457. 10.1007/s10666-017-9581-3 [DOI] [Google Scholar]
- Topping CJ, Aldrich A and Berny P, 2020. Overhaul environmental risk assessment for pesticides. Science, 367, 360–363. 10.1126/science.aay1144 [DOI] [PubMed] [Google Scholar]
- Torres DJ, Ricoy UM and Roybal S, 2015. Modeling honey bee populations. PLoS ONE, 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tosi S, Nieh JC, Brandt A, Colli M, Fourrier J, Giffard H, Hernández‐López J, Malagnini V, Williams GR and Simon‐Delso N, in press. Long‐term field‐realistic exposure to a next‐generation pesticide, flupyradifurone, impairs honey bee behaviour and survival. Communications Biology. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tosi S and Nieh JC, 2017. A common neonicotinoid pesticide, thiamethoxam, alters honey bee activity, motor functions, and movement to light. Scientific Reports, 7, 15132. 10.1038/s41598-017-15308-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tosi S and Nieh JC, 2019. Lethal and sublethal synergistic effects of a new systemic pesticide, flupyradifurone (Sivanto®), on honeybees. Proceedings of the Royal Society B, 286, 20190433. 10.1098/rspb.2019.0433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tosi S, Démares FJ, Nicolson SW, Medrzycki P, Pirk CW and Human H, 2016. Effects of a neonicotinoid pesticide on thermoregulation of African honey bees (Apis mellifera scutellata). Journal of Insect Physiology, 93–94, 56–63. 10.1016/j.jinsphys.2016.08.010 [DOI] [PubMed] [Google Scholar]
- Tosi S, Nieh JC, Sgolastra F, Cabbri R and Medrzycki P, 2017a. Neonicotinoid pesticides and nutritional stress synergistically reduce survival in honey bees. Proceedings of the Royal Society B, 284, 1711. 10.1098/rspb.2017.1711 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tosi S, Burgio G and Nieh JC, 2017b. A common neonicotinoid pesticide, thiamethoxam, impairs honey bee flight ability. Scientific Reports, 7, 1201. 10.1038/s41598-017-01361-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tosi S, Costa C, Vesco U, Quaglia G and Guido G, 2018. A 3‐year survey of Italian honey bee‐collected pollen reveals widespread contamination by agricultural pesticides. Science of the Total Environment, 615, 208–218. 10.1016/j.scitotenv.2017.09.226 [DOI] [PubMed] [Google Scholar]
- Tsvetkov N, Samson‐Robert O, Sood K, Patel HS, Malena DA, Gajiwala PH, Maciukiewicz P, Fournier V and Zayed A, 2017. Chronic exposure to neonicotinoids reduces honey bee health near corn crops. Science, 356, 1395–1397. 10.1126/science.aam7470 [DOI] [PubMed] [Google Scholar]
- Valido A, Rodríguez‐Rodríguez MC and Jordano P, 2019. Honeybees disrupt the structure and functionality of plant‐pollinator networks. Scientific Report, 9, 4711. 10.1038/s41598-019-41271-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Gestel CAM, Jonker MJ, Kammenga JE, Laskowski R and Svendsen C, 2011. Mixture Toxicity. Linking Approaches from Ecological and Human Toxicology. SETAC Press, Pensacola, USA. 320 pp. ISBN 9781439830086. [Google Scholar]
- Vandame R and Belzunces LP, 1998. Joint actions of deltamethrin and azole fungicides on honey bee thermoregulation. Neuroscience Letters, 251, 57–60. 10.1016/S0304-3940(98)00494-7 [DOI] [PubMed] [Google Scholar]
- Von der Leyen U, 2019. Mission letter to Frans Timmermans, Executive Vice‐President‐designate for the European Green Deal. Brussels, 10 September 2019, 6 pp. Available online: https://ec.europa.eu/commission/sites/beta-political/files/mission-letter-margrethe-vestager_2019_en.pdf
- Wagner DL, 2019. Insect declines in the anthropocene. Annual Review of Entomology, 65, 1–24. 10.1146/annurev-ento-011019-025151 [DOI] [PubMed] [Google Scholar]
- Weiss ES, Anderson RM and Lasker RD, 2002. Making the most of collaboration: exploring the relationship between partnership synergy and partnership functioning. Health Education & Behavior, 29, 683–698. [DOI] [PubMed] [Google Scholar]
- Wendling C, 2014. Incorporating social sciences in public risk assessment and risk management organisations. European Journal of Risk Regulation, 5, 7–13. 10.1017/S1867299X00002907 [DOI] [Google Scholar]
- Wilson DS and Sober E, 1989. Reviving the superorganism. Journal of Theoretical Biology, 136, 337–356. 10.1016/S0022-5193(89)80169-9 [DOI] [PubMed] [Google Scholar]
- Winfree R, Aguilar R, Vázquez DP, LeBuhn G and Aizen MA, 2009. A meta‐analysis of bees’ responses to anthropogenic disturbance. Ecology, 90, 2068–2076. [DOI] [PubMed] [Google Scholar]
- Winston ML, 1987. The Biology of the Honey Bee. Harvard University Press, Cambridge. [Google Scholar]
- Withrow JM and Tarpy DR, 2018. Cryptic ‘royal’ subfamilies in honey bee (Apis mellifera) colonies. PLoS ONE, 13. 10.1371/journal.pone.0199124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu YY, Luo QH, Hou CS, Wang Q, Dai PL, Gao J, Liu YJ and Diao QY, 2017. Sublethal effects of imidacloprid on targeting muscle and ribosomal protein related genes in the honey bee Apis mellifera L. Scientific Reports, 7, 15943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu‐Smart J and Spivak M, 2016. Sub‐lethal effects of dietary neonicotinoid insecticide exposure on honey bee queen fecundity and colony development. Scientific Reports, 6, 32108. 10.1038/srep32108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaluski R, Justulin LA and de Oliveira Orsi R, 2017. Field‐relevant doses of the systemic insecticide fipronil and fungicide pyraclostrobin impair mandibular and hypopharyngeal glands in nurse honeybees (Apis mellifera). Scientific Reports, 7, 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Technical report: Systems based approach honey bees


