Abstract
Osteogenesis imperfecta (OI) is a heritable connective tissue disorder with patients exhibiting bone fragility and muscle weakness. The synergistic biochemical and biomechanical relationship between bone and muscle is a critical potential therapeutic target, such that muscle weakness should not be ignored. Previous studies demonstrated mitochondrial dysfunction in the skeletal muscle of oim/oim mice, which model a severe human type III OI. Here, we further characterize this mitochondrial dysfunction and evaluate several parameters of whole body and skeletal muscle metabolism. We demonstrate reduced mitochondrial respiration in female gastrocnemius muscle, but not in liver or heart mitochondria, suggesting that mitochondrial dysfunction is not global in the oim/oim mouse. Myosin heavy chain fiber type distributions were altered in the oim/oim soleus muscle with a decrease (-33 to 50%) in type I myofibers and an increase (+31%) in type IIa myofibers relative to their wildtype (WT) littermates. Additionally, altered body composition and increased energy expenditure were observed oim/oim mice relative to WT littermates. These results suggest that skeletal muscle mitochondrial dysfunction is linked to whole body metabolic alterations and to skeletal muscle weakness in the oim/oim mouse.
1. Introduction
Osteogenesis imperfecta (OI), also referred to as brittle bone disease, is a rare, heritable connective tissue disorder affecting approximately 1 in 15,000 births [1,2]. Roughly 85% of OI cases arise due to mutations in the type I collagen genes, COL1A1 and COL1A2, while the remaining 15% result from mutations in genes involved in posttranslational modification of type I collagen, osteoblast maturation or mineralization [2]. Type I collagen, the most abundant of the collagens, is an important structural protein composing the majority of the organic component of bone. OI is an extremely heterogeneous disease in terms of severity with the most common and striking feature being bone fragility. There are four classical Sillence types of OI: type I is the least severe, type II results in perinatal lethality, type III being the most severe viable form, and type IV results in a moderate phenotype [3]. In addition to bone fragility, clinical manifestations include short stature and craniofacial abnormalities, with more recent investigations also highlighting the presence of an inherent muscle pathology in both mouse models and patients with OI[4–7]. However, current treatment strategies for patients with OI focus primarily on bone fragility, with emphasis on preventing bone resorption (bisphosphonates) and/or surgical rodding interventions[1]. Understanding the mechanisms of muscle weakness in OI could potentiate novel therapies to improve muscle function and ultimately bone strength.
Muscle weakness has been implicated as an inherent part of the pathophysiology of OI through investigation of the osteogenesis imperfecta murine (oim) model[7]. The oim mouse Col1a2 gene contains a nucleotide deletion, leading to a frameshift in the carboxy-terminal end resulting in a nonfunctional proα2(I) collagen. This ultimately leads to production of homotrimeric type I collagen, [α1(I)3], rather than the normal heterotrimeric type I collagen, [α1(I)2 α2(I)][8]. The homozygous (oim/oim) mice model severe human type III OI, exhibiting severe bone fragility, decreased bone mineral density, and small body size compared to wildtype (WT) littermates[8]. Oim/oim mice have significantly reduced muscle size as well as reduced specific contractile generating force [contractile force normalized to muscle cross sectional area][7]. Subsequently, numerous reports have revealed muscle weakness in OI patients, including that 80% of patients with mild/moderate OI experience muscle force deficits [4–6,9]. Because muscle and bone are highly synergistic tissues which “talk” to one another through both chemical signals and physical forces, it is important to understand this muscle weakness in OI in order to exploit this relationship to ultimately improve bone strength through the improvement of muscle function [10–12]. We recently demonstrated that oim/oim hindlimb skeletal muscle exhibits severe mitochondrial dysfunction and altered mitochondrial numbers, which likely contributes to their muscle weakness [13][14].
Evidence of metabolic alterations in OI, though limited, have been observed in both mouse models and the patient population. Previous evidence found that prepubescent OI patients exhibited higher body temperatures and a 45% increase in metabolic rate, but did not show altered respiratory quotients relative to children without OI[15]. Interestingly, they also reported that these changes subsided in patients after puberty, as do the overall fracture rates suggesting a link between hyper-metabolism, fractures, and sex-hormones[15]. More recently, evidence of metabolic alterations were demonstrated in a mouse model of severe OI; 4 week old Col1a1Jrt/+ mice (modeling human OI type IV/III) exhibited sex-dependent changes in metabolism that normalized by puberty (8 weeks of age) [16]. These alterations included increased insulin levels and respiratory exchange rate, in males, as well as improved glucose tolerance in females Additionally, a previous report found evidence that a patient with OI type III/IV, initially suspected to have malignant hyperthermia, was in a hypermetabolic state which was likely related to a primary type I collagen defect [17].
Based on these previous reports of metabolic alterations in both patients and the Col1a1Jrt/+ mouse as well as our recent findings of mitochondrial dysfunction, we sought to investigate the potential of metabolic dysfunction in the oim/oim mouse. The current study provides further investigations of mitochondrial function and metabolism in the oim/oim mouse, by 1) addressing whether the skeletal muscle mitochondrial dysfunction is more global or tissue specific by evaluating cardiac muscle and liver mitochondrial function, 2) evaluating skeletal muscle fiber type distributions, and 3) evaluating several metabolic parameters including measures of whole body energy expenditure and skeletal muscle fatty acid oxidation relative to wildtype littermates. Better characterization and understanding of these alterations provide insight into the skeletal muscle weakness that occurs in OI and the nature of the relationship between mitochondrial function and type I collagen mutations and promise to pave the way for new therapies that will improve muscle function and ultimately bone strength.
2. Methods
2.1. Mice
Col1a2oim mice (stock #001815 (oim)) were originally obtained from Jackson Laboratory (Bar Harbor, ME, USA). All mice were maintained on the C57BL/6 J background and genotyped as previously described [8,18]. Non-breeding male and female wild type (WT) and oim/oim adult mice (13–19 weeks of age) were used for all experiments described, except for an additional subset of 8 week old WT and oim/oim males whose gastrocnemius and quadriceps muscles were used to assess mitochondrial respiration. Age- and sex- matched WT littermates were used as control animals. All mice were given ad libitum access to food (stock chow, Purina Lab Diet 5053) and water. Mice were housed in an AAALAC-accredited facility at the University of Missouri; all experimental manipulations were performed under an approved University of Missouri Animal Care and Use Committee protocol. At the time of sacrifice, a mixture of xylazine/ketamine anesthesia was used followed by cardiac puncture (serum collected for future experiments), and cervical dislocation.
2.2. Mitochondria Isolation, Respiration, and H2O2 Analyses
Mitochondria were isolated from heart, liver, and mixed skeletal muscles including the gastrocnemius and quadriceps muscles (each muscle contains both white/fast-twitch/type II and red/slow-twitch/type I myofibers) following a series of homogenization and differential centrifugation procedures as previously described [19–21]. Mitochondria isolated from heart, liver and gastrocnemius and quadriceps muscles were used to assess mitochondrial respiration via high-resolution respirometry, while mitochondria isolated from gastrocnemius muscle were also used to determine mitochondrial H2O2 production.
High-resolution respirometry was used to measure mitochondrial respiration by the isolated liver, heart, gastrocnemius (gast), and quadriceps (quad) muscle mitochondria using the Oroboros Oxygraph-2k (Oroboros Instruments, Innsbruck, Austria) following a modified protocol[14,19,22]. 20 µL of isolated mitochondria were added to the respiration chambers to measure basal respiration. Steady-state oxygen flux was measured by the addition of 2 mM malate and 5 mM glutamate. Oxygen flux through complex I was measured by the titration of ADP [50 to 125 µM (liver), 25–125 µM (heart), or 125–375 µM (gast and quad)]; through complex I + II by the titration of succinate [5 to 7.5 mM (liver), 10 mM (heart), or 1–5 mM (gast and quad)], and maximal uncoupled respiration was measured by the titration of carbonyl cyanide-4-(trifluoromethoxy) phenylhydrazone (FCCP) [0.125–0.25 µM (liver), 0.25 µM (heart), or 0.125 µM (gast and quad)]. Finally, cytochrome c was added [5 µM (liver and heart) and 10 µM (gast and quad)] to assess the quality of mitochondrial isolation. All measurements were normalized to the mitochondrial protein content.
An Oroboros Oxygraph-2k (Oroboros Instruments, Innsbruck, Austria) equipped with a fluorimeter was used to measure H2O2 production in mitochondria isolated from gastrocnemius muscles. Amplex Red (ThermoFisher Scientific; Waltham, MA) and horseradish peroxidase (HRP) were added to the respiration chamber followed by the addition of 20 uL of isolated mitochondria. 20–70 uM of palmitoyl-CoA was titrated into the respiration chambers and the amount of fluorescence assessed.
2.3. Citrate Synthase Activity
Citrate synthase activity was measured in gastrocnemius, liver, and heart mitochondria as previously described[14,23].
2.4. Skeletal Muscle Mitochondrial Fatty Acid Oxidation
Complete fatty acid oxidation leads to the production of carbon dioxide while incomplete fatty acid oxidation results in the generation of acid soluble metabolite (ASMs). Complete, incomplete, and total [Incomplete (ASM) + Complete (CO2)] oxidation of (1-14C) palmitate were measured in mitochondria isolated from gastrocnemius muscle as well as in whole quadriceps muscle homogenate as previously described [24].
2.5. Myosin Heavy Chain Immunohistochemical Analysis
Skeletal muscles can be characterized according to their oxidative capacity as type I slow twitch fibers and type II fast twitch fibers, also known as red and white muscle, respectively [25]. Furthermore, type II fibers can be further classified as type IIa, type IIb, and type IIx representing a fast oxidative, fast glycolytic and combination, respectively. Due to their increased endurance and oxidative properties, type I fibers tend to have more mitochondria relative to other fiber types[26]. Using myosin heavy chain antibodies against type I, IIa, and IIb fibers, oim/oim and WT soleus (sol) and extensor digitorum longus (EDL) muscles were visualized to determine fiber type composition.
Sol and EDL muscles previously stored in optimal cutting temperature (O.C.T.) compound at −80°C were cut to 12 µm sections. The primary antibodies mouse IgG2b BA.D5 (1:100), mouse IgG1 SC.71 (1:200), and mouse IgM BF.F3 (1:100) (DSHB, University of Iowa, IA) against type I, type IIa, and type IIb fibers respectively were used. Additionally, rabbit IgG anti-laminin antibody (1:200) (ab11575; Abcam) was used to visualize fiber borders. The following Alexa Fluor secondary antibodies were used: 647 goat anti-mouse IgG2b (1:250), 488 goat anti-mouse IgG1 (1:500), 555 goat anti-mouse IgM (1:500), and 350 goat anti-rabbit IgG (1:400) (Invitrogen, IA, USA). Images were captured using the Zeiss Axiovert 200M with fluorescence and ORCA-ER camera and analysis was done using Image J software.
2.6. Glucose Tolerance Testing
Approximately two weeks prior to sacrifice, mice were fasted for six hours and then injected with 2 mg/kg glucose. Duplicate glucose measurements were taken and averaged using the ReliOn™ PRIME (Walmart) glucose monitoring system at the following time points: 0, 15, 30, 60 and 120 minutes.
2.7. Metabolic Chambers and Body Composition Analysis
Approximately one week prior to sacrifice, mice were placed in metabolic monitoring systems (PromethION; Sable Systems International). Mice were singly housed and given ad libitum access to food (stock chow, Purina Lab Diet 5053) and water. All metabolic chamber data are presented as day and night corresponding to the 12-hour diurnal and nocturnal blocks, respectively. Mice were placed in the chamber on the morning of day 1 and allowed to acclimate until the following morning; data from the subsequent 48 hours, 2 full day and night cycles (days 2 and 3), were analyzed and averaged for each animal. Mice were removed from the chambers on day 4. Data regarding oxygen consumption and carbon dioxide production were collected to determine energy expenditure (calculated based on the Weir equation [25]) and respiratory quotient (RQ; calculated as a ratio between oxygen consumption and carbon dioxide utilization [26]) via the PromethION software. RQ gives a sense of fuel utilization with a value of 1 indicative of predominately carbohydrate utilization and a value of 0.7 indicative of predominately fat utilization [26]. Food, water, and body weights were also monitored by the system in addition to activity being measured in three dimensions, in the x, y, and z planes, with the z indicating vertical standing on hindlimbs. All data are presented as absolute values normalized to body weight. Lean body mass and fat mass were determined via EchoMRI™ (Houston, USA) prior to mice being placed in the metabolic chamber.
2.8. Fat Pad Analysis
Following sacrifice, the inguinal white adipose tissue, gonadal white adipose tissue, and subscapular brown adipose tissue were removed and weighed as previously described [27].
2.9. Serum Markers
Blood collected via cardiac puncture sat at room temperature for approximately 15 minutes before being placed on ice. All blood was then centrifuged at room temperature in a tabletop centrifuge (15,000 rpm for 15 minutes) and the serum collected and stored at −80°C. Serum was then used to measure levels of total glycerol, free glycerol, triglycerides and non-esterified fatty acids (NEFA). Serum total glycerol, free glycerol, and triglycerides (Sigma-aldrich: St. Louis, MO; cat TR0100) as well as NEFA (Wako Diagnostics: 999-34691, 995-34791) levels were determined following the manufacturer instructions. The serum triglyceride kit measures the amount of free glycerol in the serum prior to hydrolyzing triglycerides to glycerol and fatty acids at which point the total glycerol is measured again. Triglycerides are determined by subtracting the free glycerol from the total glycerol.
2.10. Statistical Analysis
Statistical analyses were performed using GraphPad Prism 8 program (GraphPad Software, Inc., La Jolla, CA, USA). If not mentioned otherwise, data are presented as mean ± SE. Male and female data were analyzed independently. For all experiments (minus glucose tolerance tests), an unpaired two-tailed Student’s t-test is used for comparisons between two groups if normal distribution of residual was confirmed. For non-normal distributions, Mann-Whitney tests are used for comparisons. For glucose tolerance tests (Figure 7) we performed two-way repeated measures ANOVA with Geisser-Greenhouse correction for sphericity. Differences with p<0.05 were statistically significant.
Figure 7:

oim/oim (blue squares) mice do not exhibit changes in glucose tolerance compared to WT (red circles) littermates. Male (A) and female (B) mice were fasted for six hours, then injected with 2 mg glucose per gram of body weight, and glucose measurements taken at the following time points: 0, 15, 30, 60, and 120 minutes. Data are presented as Means ± SE (n=6 per group).
3. Results
3.1. Mitochondrial respiration, citrate synthase activity and fatty acid oxidation
Previously we demonstrated severe mitochondrial dysfunction in 4-month old male oim/oim gastrocnemius muscle evidenced by reduced gastrocnemius mitochondrial respiration and citrate synthase activity [14]. To determine if this was a global phenomenon or isolated to specific tissues, we investigated mitochondrial respiratory function in the liver and heart as well as gastrocnemius of female oim/oim mice. We found that female oim/oim gastrocnemius mitochondrial respiration was decreased by 27–45% (p=0.05–0.07) with no significant differences in mitochondrial respiration of the female oim/oim liver or heart (Figure 1 A–C) when compared to WT littermates suggesting that mitochondrial dysfunction is tissue specific rather than a global phenomenon. Mitochondrial citrate synthase activity was reduced in female oim/oim gast relative to WT littermates, but did not reach significance. Female liver and heart mitochondrial citrate synthase activity was equivalent between oim/oim and WT littermates (Figure 1D). To further evaluate the skeletal muscle specificity of mitochondrial dysfunction in the oim/oim mouse, we assessed mitochondrial respiration in 2-month old male mitochondria isolated from both gastrocnemius and quadriceps muscles and saw an overall trend of reduced mitochondrial respiration relative to WT counterparts (Figure S1). These findings are consistent with the previous findings in 4-month-old male oim/oim and WT gastrocnemius muscle mitochondria and further support compromised mitochondrial function is a generalized oim/oim skeletal muscle phenomenon that can be present as early as 8 weeks of age. Additionally, we evaluated mitochondrial respiration in heterozygous (+/oim) mice, which exhibit much milder muscle weakness as compared to their oim/oim littermates[7] and found no differences between +/oim gastrocnemius mitochondrial respiration relative to WT littermates (Figure S2).
Figure 1:

Isolated mitochondria from female oim/oim (blue) exhibit reduced (A) gastrocnemius (triangles) mitochondrial respiration, while (B) liver (diamonds) and (C) heart (hearts) mitochondrial respiration remains unchanged as compared to isolated mitochondria from WT (red) littermates. (D) There is no difference in isolated mitochondrial citrate synthase activity between female oim/oim and WT gast (triangles), liver (diamonds), or heart (hearts). Data are presented as Means±SE. *p≤0.05 vs. WT. (n=3–5 per group).
Since skeletal muscle mitochondrial function is reduced in oim/oim mice, we assessed fatty acid oxidation (occurring in the mitochondria) of gast muscle mitochondria and whole quad muscle homogenate. The complete palmitate oxidation to CO2 by gast muscle mitochondria tended to be reduced by 60% (p=0.07) in male oim/oim mice and was reduced by 85% ( p=0.05) in female oim/oim mice relative to WT mice (Figure 2 A), while incomplete and total palmitate oxidation were significantly reduced (p<0.05) in male oim/oim mice only (Figure 2 B,C). Whole quad muscle homogenate palmitate oxidation was not different between oim/oim and WT littermates regardless of sex (Figure 2 D–F).
Figure 2:

Isolated mitochondria from male and female oim/oim gastrocnemius (blue squares) exhibit reduced complete oxidation of [1-14C]-palmitate to CO2 (A), while male oim/oim mitochondria exhibit reduced incomplete oxidation of [1-14C]-palmitate to acid soluble metabolites (ASM) (B) and total oxidation of [1-14C]-palmitate (ASM + CO2) (C) relative to WT (red circles) littermates. Complete (D), incomplete (E), and total oxidation (F) of [1-14C]-palmitate in oim/oim whole quadriceps muscle homogenate relative to WT littermates. Data are presented as Means±SE. *p≤0.05 vs. WT. (n=4–7 per group).
3.2. Myosin heavy chain analysis of soleus and extensor digitorum longus muscle
In WT C57BL/6J soleus (sol) muscle the distribution of myofibers is reported to be as follows: 43% type I, 46% type IIa, and 11% type IIx, while the distribution in WT C57BL/6J extensor digitorum longus (EDL) is: 0% Type I, 11% Type IIa, and 88% type IIb [28,29]. Since oim/oim mitochondria are severely dysfunctional [14], and oxidative capacity is largely dependent on mitochondria [30,31], a change in myofiber type composition of the oim/oim skeletal muscle was hypothesized. The size of oim/oim soleus muscles was reduced as was the total number of myofibers (p=0.10) when compared to WT littermates (Figure 3C). Male and female oim/oim mice displayed a 33–50% decrease in the percentage of slow oxidative type I myofibers and a 31% increase in percentages of fast oxidative type IIa fibers relative to their WT littermates, respectively (Figure 3 A–B, G–H). In the oim/oim male EDL muscle, type IIa myofibers were increased 129% (p=0.16) and type IIb myofibers were decreased 20% (p=0.4) relative to WT littermates, although not reaching significance (Figure 3 D–E, I–J). Total myofiber count was equivalent between oim/oim and WT EDL muscles regardless of sex (Figure 3F).
Figure 3:

Male and female oim/oim soleus muscles (blue squares) exhibit reduced percentages of type I myofibers (A) and type IIa myofibers (B) while the total number of myofibers are not altered (C) compared to WT (red circles) littermates. There were no significant changes between WT and oim/oim type IIa myofibers (D), type IIb myofibers (E), or myofiber count (F) in EDL muscles. Representative images of MyHC staining of male WT and oim/oim soleus (G&H) and EDL (I&J) muscles. Data are presented as Means±SE. *p≤0.05 vs. WT. (n=4–5 per group).
3.3. Mitochondrial H2O2 emission in isolated mitochondria
Due to the potential role of reactive oxygen species in mitochondrial dysfunction[32], we assessed gastrocnemius mitochondrial H2O2 emission. In male oim/oim gastrocnemius mitochondria (Figure 4), we found H2O2 production was reduced by 32% (p=0.06) relative to WT littermates.
Figure 4:
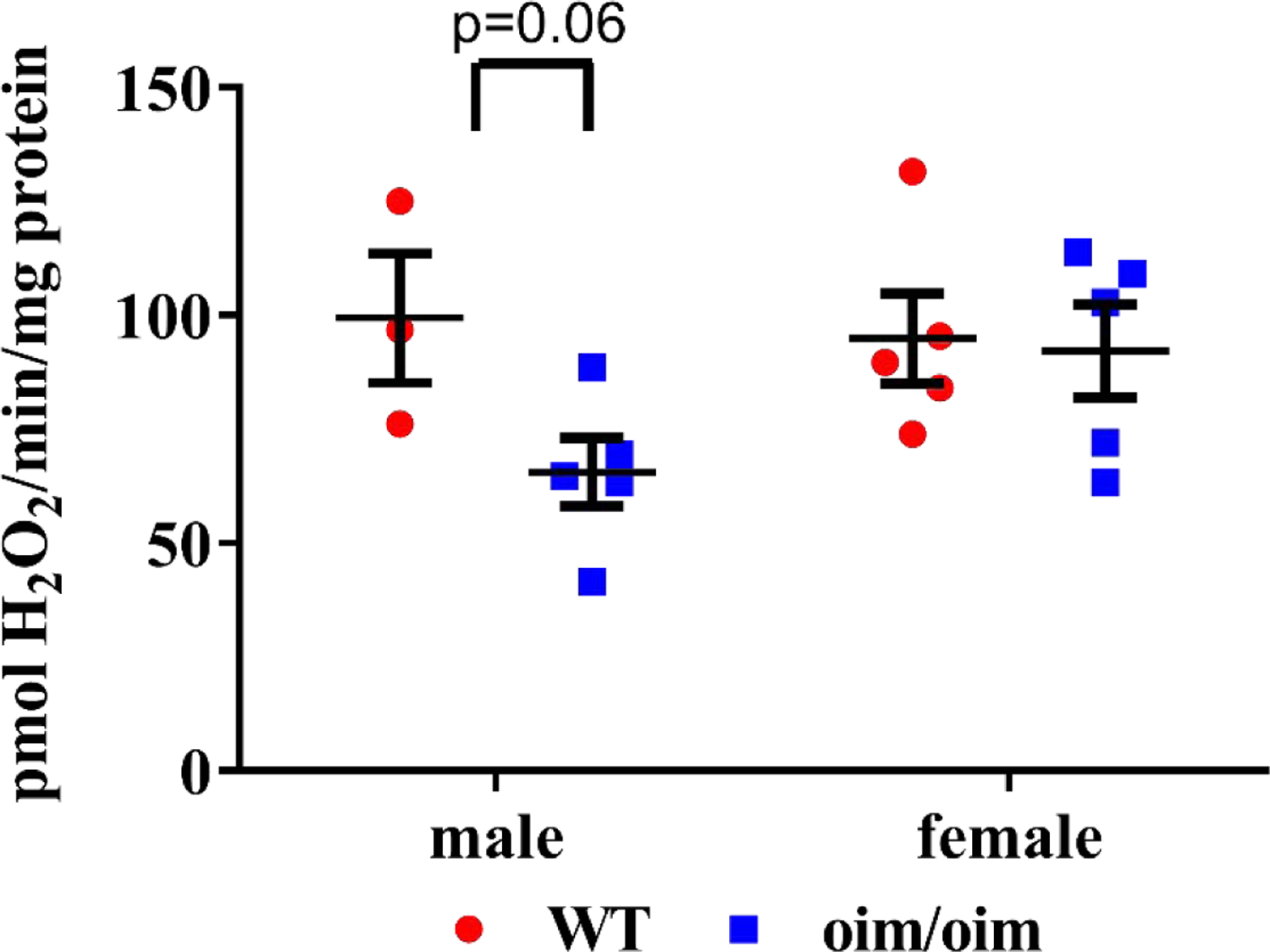
Isolated male oim/oim (blue squares) gastrocnemius mitochondria produced reduced amounts of H2O2 when stimulated with palmitoyl-CoA compared to WT isolated gastrocnemius mitochondria (red circles) littermates. Data are presented as Means±SE. *p≤0.05 vs. WT. (n=3–5 per group).
3.4. Body composition
By EchoMRI, male oim/oim mice were determined to have a 3% (p=0.12) increase in lean mass and a 20% (p=0.07) decrease in fat mass relative to WT littermates (Figure 5 A–B). Female oim/oim mice did not exhibit alterations fat or lean mass relative to female WT littermates. Both male and female oim/oim mice exhibited reduced relative gonadal white adipose tissue (although only reaching significance in female mice) while relative inguinal white adipose tissue was equivalent between oim/oim and WT littermates regardless of sex (Figure 5 C–D). Subscapular brown adipose tissue (BAT) was not different between WT and oim/oim animals.
Figure 5:

Male oim/oim (blue squares) mice exhibit increased percentages of lean mass (A)and reduced percentages of fat mass (B) compared to WT (red circles) littermates. Additionally, relative gonadal fat pad weights were reduced in female oim/oim mice while brown adipose tissue and inguinal adipose depots remained unchanged in oim/oim mice compared to WT littermates (C-D). Data are presented as Means±SE. *p≤0.05 vs. WT. (n=11–17 per group).
3.5. Indirect calorimetry
WT and oim/oim mice were placed in metabolic chambers to determine their whole body energy metabolism; parameters measured included: energy expenditure, activity, and food/water consumption. When normalized to body weight, oim/oim mice displayed significantly increased energy expenditure in light and dark cycles (+9–19%), respectively (Figure 6 A–D). This occurred despite a decrease in physical activity of female and male oim/oim relative to sex-matched WT mice during both light and dark cycles (Figure S3). Additionally, male oim/oim mice consumed ~36% more food per body weight during the 12 hour night cycle compared to their WT littermates (p=0.06; Figure S4).
Figure 6:

When normalized to body weight, both male and female oim/oim (blue squares) mice exhibit increased total (A,C) and average (B,D) energy expenditures for the night (A,B) and day (C,D) cycles when compared to WT (red circles) littermates. Data are presented as Means±SE. *p≤0.05 vs. WT. (n=5–6 per group).
During both day and night cycles, oim/oim animals consumed significantly greater amounts of oxygen per body weight (+9–20%) compared to their WT littermates (Figure S5 A&C), and oim/oim mice produced significantly greater amounts of carbon dioxide per body weight (+6–18%) relative to WT littermates during the night cycle (Figure S5 B&D). The respiratory quotient was not altered in oim/oim mice relative to WT littermates (Figure S5 E&F). Although, high variation and low sample number have likely limited the power of this analysis.
3.6. Glucose Tolerance
Despite reduced skeletal muscle mitochondrial respiration (Figure 1 & S1) and fatty acid oxidation (Figure 2), glucose tolerance was not different in oim/oim mice compared to WT littermates (Figure 7).
3.7. Serum markers
While serum triglycerides and NEFAs did not differ between oim/oim and WT littermates, serum free glycerol was drastically reduced in male oim/oim mice (−68%) relative to WT littermates (Figure 8).
Figure 8:

Male oim/oim mice (blue squares) exhibit reduced total glycerol (A) and free glycerol levels in serum (B) compared to WT red circles) littermates while no differences were present in serum levels of triglycerides (C) and free fatty acids (NEFA) (D) between oim/oim and WT littermates. Data are presented as Means±SE of the genotype values. *p≤0.05 vs. WT. (n=4–10 per group).
4. Discussion
Skeletal muscle weakness is extremely prevalent in OI where roughly 80% of patients with mild to moderate types I and IV OI experience muscle force deficits and even higher levels in more severe patients as muscle weakness in OI correlates with the level of ambulation [4–6,33,34]. Bone and muscles are highly synergistic tissues that talk to one another via both biochemical signaling and mechanotransduction [11,12,35]. Thus, investigating the mechanisms by which skeletal muscle weakness arises is essential.
Previously, we found that skeletal muscle mitochondrial dysfunction was implicated as part of the pathophysiology of OI in the oim/oim mouse model [14]. Here, we further evaluated the impact and potential links between mitochondrial dysfunction, skeletal muscle weakness and whole-body metabolism. We demonstrate that the oim/oim mitochondrial dysfunction appears tissue specific manifesting in skeletal muscle (gastrocnemius and quadriceps) rather than globally as respiration in heart and liver mitochondria were not compromised in oim/oim mice. Additionally, we found alterations in skeletal muscle and whole body parameters related to energy metabolism.
Skeletal muscle, constituting roughly 50% of body mass, is a significant contributor to the body’s resting metabolic rate [36–38]. Yet, despite countless reports of skeletal muscle dysfunction in OI patients, there is a paucity of studies on the role of energy metabolism in OI [4–6]. Reports in prepubescent patients and the Col1a1Jrt/+ mouse suggest a metabolic phenotype in the pathophysiology of OI[15,16]. Reports in prepubescent patients suggest a hyper-metabolic phenotype consisting of increased body temperature, heart rate, and respiratory rates [15] while a more recent report by Boraschi-Diaz et al. implicates whole-body metabolic changes in young mice (4 weeks), including reduced adiposity, increased energy expenditure, increased oxygen consumption, and increased carbon dioxide production, as part of the pathophysiology of OI in the Col1a1Jrt/+ mouse model, containing an 18 amino acid deletion in the triple helical domain of the pro-α1(I) collagen chain [16,39]. Similarly, our observations also implicate a metabolic phenotype in the oim/oim mouse as evidenced by increased relative lean mass and reduced fat mass (Figure 5 A–B), increased energy expenditure despite reduced activity (Figure 6 and Figure S3), and increased oxygen consumption and carbon dioxide production (Figure S5). Although, the metabolic phenotype observed in the Col1a1Jrt/+ mouse was diminished by 8 weeks of age, in the oim mouse this was observed in adult oim/oim mice aged 16–19 weeks.
Despite increased energy expenditures in the oim/oim mouse, we did not observe changes in the respiratory quotient, glucose tolerance, or skeletal muscle fatty acid oxidation suggesting that the oim/oim mice do not exhibit shifts in substrate utilization in response to altered energy metabolism. Additionally, we measured total glycerol, free glycerol, triglyceride, and NEFA levels in serum and found that while serum glycerol was the only marker significantly reduced, triglyceride and NEFA markers also appear slightly decreased in the oim/oim male mouse compared to WT littermates (Figure 8). Glycerol, an important gluconeogenic substrate secreted by white adipose tissue [40,41], was shown to be increased in Swedish men with type II diabetes [42]. The decrease in serum glycerol seen in the oim/oim mouse relative to WT littermates is likely associated with the reduced relative fat mass and may be indicative of upregulated gluconeogenesis, although further studies to better understand the nature of this change are warranted.
Mitochondrial function and content exhibit tissue diversity due to differing cellular needs with cardiac tissue mitochondria having the highest electron transport chain activity relative to either liver or gastrocnemius mitochondria[43–45]. The tissue specific mitochondrial dysfunction observed in the oim/oim mouse may reflect differing tissue response to the type I collagen defect.
Skeletal muscle mitochondrial dysfunction may also reflect the observed change in fiber type distribution observed in the soleus muscle. The soleus muscle, containing the slow oxidative and fast oxidative fibers (type I and IIa, respectively) is considered mitochondria-rich, whereas the extensor digitorum longus (EDL) muscle containing types IIa and IIb fibers (representing fast oxidative and fast glycolytic, respectively), has more variable mitochondrial content[46]. Both male and female oim/oim mice exhibited decreased percentages of type I fibers and increased percentages of type IIa fibers in the soleus muscle relative to WT littermates (Figure 3). Oim/oim male EDL muscles exhibited increased type IIa fibers and decreased IIb fibers relative to WT littermates, (although not reaching significance), while female fiber type distribution was equivalent between WT and oim/oim (Figure 3). The decrease in type I fibers of the soleus muscle may serve as a compensatory mechanism for the dysfunctional mitochondria. Moreover, the contribution of reduced activity levels in oim/oim mice (Figure S3) to fiber type changes in addition to mitochondrial function cannot be discounted, as activity serves as important regulator of fiber type composition and mitochondrial bioenergetics[47,48].
Reduced activity levels in oim/oim mice have been previously reported[49], but the cause(s) of this reduced activity remain unknown. However, the increased prevalence of fractures in the oim/oim mice is potentially an important contributor to decreased activity levels. Specifically, increased long bone fracture rates of oim/oim mice [8] could potentially explain the reduced rearing activity in the z plane that was observed. It is important to note that skeletal muscle weakness in the oim/oim mouse is intrinsic, as demonstrated by the reduced specific contractile force (muscle contractile force normalized to muscle cross sectional area), suggesting that reduced activity alone is not solely responsible for the muscle weakness[7].
In our current study, we observed that skeletal muscle mitochondrial fatty acid oxidation in isolated oim/oim gastrocnemius mitochondria was reduced, but it was not different in whole quadriceps muscle homogenates relative to WT littermates (Figure 2). This suggests that the reduction in mitochondrial fatty acid oxidation may be the result of dysfunctional mitochondria, as mitochondria are the primary site of fatty acid beta-oxidation [50]. The fact that fatty acid oxidation in the whole muscle was not altered suggest that there may be an increased number of mitochondria in the oim/oim skeletal muscle as proposed previously [14], which could lead to a compensation effect. Additionally, we evaluated skeletal muscle mitochondrial ROS production. Although we hypothesized increased hydrogen peroxide as a contributor to mitochondrial dysfunction, we found that hydrogen peroxide emission per amount of mitochondria was reduced in male oim/oim gastrocnemius, which may again be the result of poorer functioning mitochondria (Figure 4). Future studies are warranted to determine the amount of hydrogen peroxide produced within the whole muscle.
In this study, we provide further evidence that the oim/oim mouse exhibits tissue specific skeletal muscle mitochondrial dysfunction independent of heart and liver tissue as evidenced by changes in skeletal muscle mitochondrial respiration, mitochondrial fatty acid oxidation, and mitochondrial ROS production. Additionally, we suggest that a metabolic phenotype exists in the oim/oim mouse that likely contributes further to the pathophysiology of the disease as evidenced by increases in energy expenditure and altered body composition. Further studies are warranted to determine the nature of the relationship between a type I collagen mutation, mitochondrial defects, and metabolic alterations. Understanding these relationships could lead to novel therapeutics addressing the muscle weakness and bone fragility associated with osteogenesis imperfecta.
Limitations
The limitations of this study include the age of the mice, which were primarily from 13 weeks-19 weeks with a subset at 8 weeks. Narrower age range or an earlier prepubescent age may provide additional information concerning the relationship between metabolic alterations and metabolism during the most vulnerable fracture time period [51]. An additional limitation includes small animal number for certain analyses, including metabolic cages and fatty acid oxidations, which had to be balanced against time and fiscal costs.
Supplementary Material
Acknowledgements
We would like to thank Grace M. Meers for her constant help and support with the mitochondrial respiration and fatty acid oxidation experiments. Additionally, we would like to thank the following funding sources: Washington University Institute of Clinical and Translational Sciences (ICTS), Clinical and Translational Research Funding Program Pilot Grant, NIH/National Center for Advancing Translational Sciences (NCATS) grant UL1TR002345, NIH/NIAMS – R21 (1R21AR077813–01), Kansas City Musculoskeletal Diseases (KCMD) Consortium, Collaborative Research for Neuromuscular /Musculoskeletal Disorders pilot grant, University of Missouri, Child Health Research Institute - Leda J. Sears Research Project Awards, University of Missouri Post-baccalaureate Research Education Program, Cherng Summer Scholar Program, Kansas City Area Life Sciences Institute, Patton Trust Research Grant, the Wayne L Ryan Graduate Fellowship, and VA-Merit Grant 101BX003271–04 (RSR). This work was supported with resources and the use of facilities at the Harry S. Truman Memorial Veterans Hospital in Columbia, MO.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures
All authors state they have no conflict of interest.
References
- [1].Morello R, Osteogenesis imperfecta and therapeutics, Matrix Biol 71–72 (2018) 294–312. 10.1016/j.matbio.2018.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Marini JC, Forlino A, Bächinger HP, Bishop NJ, Byers PH, De Paepe A, Fassier F, Fratzl-Zelman N, Kozloff KM, Krakow D, Montpetit K, Semler O, Osteogenesis imperfecta, Nat. Rev. Dis. Prim 3 (2017) 17052. 10.1038/nrdp.2017.52. [DOI] [PubMed] [Google Scholar]
- [3].Sillence DO, Senn A, Danks DM, Genetic heterogeneity in osteogenesis imperfecta, J. Med. Genet 16 (1979) 101–116. http://www.ncbi.nlm.nih.gov/pmc/articles/PMC1012733/. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Veilleux LN, Trejo P, Rauch F, Muscle abnormalities in osteogenesis imperfecta, J. Musculoskelet. Neuronal Interact 17 (2017) 1–7. http://www.ncbi.nlm.nih.gov/pmc/articles/PMC5492314/. [PMC free article] [PubMed] [Google Scholar]
- [5].Veilleux LN, Lemay M, Pouliot-Laforte A, Cheung MS, Glorieux FH, Rauch F, Muscle anatomy and dynamic muscle function in osteogenesis imperfecta type I, J Clin Endocrinol Metab 99 (2014) E356–62. 10.1210/jc.2013-3209. [DOI] [PubMed] [Google Scholar]
- [6].Veilleux LN, Darsaklis VB, Montpetit K, Glorieux FH, Rauch F, Muscle Function in Osteogenesis Imperfecta Type IV, Calcif Tissue Int 101 (2017) 362–370. 10.1007/s00223-017-0287-y. [DOI] [PubMed] [Google Scholar]
- [7].Gentry BA, Ferreira JA, McCambridge AJ, Brown M, Phillips CL, Skeletal muscle weakness in osteogenesis imperfecta mice, Matrix Biol 29 (2010) 638–644. 10.1016/j.matbio.2010.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Chipman SD, Sweet HO, McBride DJ, Davisson MT, Marks SC, Shuldiner AR, Wenstrup RJ, Rowe DW, Shapiro JR, Defective pro alpha 2(I) collagen synthesis in a recessive mutation in mice: a model of human osteogenesis imperfecta, Proc. Natl. Acad. Sci. U. S. A 90 (1993) 1701–1705. http://www.ncbi.nlm.nih.gov/pmc/articles/PMC45947/. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Pouliot-Laforte A, Veilleux LN, Rauch F, Lemay M, Physical activity in youth with osteogenesis imperfecta type I, J. Musculoskelet. Neuronal Interact 15 (2015) 171–176. /pmc/articles/PMC5133720/?report=abstract (accessed October 9, 2020). [PMC free article] [PubMed] [Google Scholar]
- [10].Phillips CL, Jeong Y, Osteogenesis Imperfecta: Muscle–Bone Interactions when Bi-directionally Compromised, Curr. Osteoporos. Rep 16 (2018) 478–489. 10.1007/s11914-018-0456-6. [DOI] [PubMed] [Google Scholar]
- [11].Fricke O, Schoenau E, The ‘Functional Muscle-Bone Unit’: Probing the relevance of mechanical signals for bone development in children and adolescents, Growth Horm. IGF Res 17 (2007) 1–9. 10.1016/j.ghir.2006.10.004. [DOI] [PubMed] [Google Scholar]
- [12].Frost HM, Bone’s mechanostat: A 2003 update, Anat. Rec. Part A Discov. Mol. Cell. Evol. Biol 275A (2003) 1081–1101. 10.1002/ar.a.10119. [DOI] [PubMed] [Google Scholar]
- [13].Jeong Y, Daghlas SA, Xie Y, Hulbert MA, Pfeiffer FM, Dallas MR, Omosule CL, Pearsall RS, Dallas SL, Phillips CL, Skeletal Response to Soluble Activin Receptor Type IIB in Mouse Models of Osteogenesis Imperfecta, J. Bone Miner. Res 0 (2018). 10.1002/jbmr.3473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Gremminger VL, Jeong Y, Cunningham RP, Meers GM, Rector RS, Phillips CL, Compromised Exercise Capacity and Mitochondrial Dysfunction in the Osteogenesis Imperfecta Murine (oim) Mouse Model, J. Bone Miner. Res 34 (2019). 10.1002/jbmr.3732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Cropp GJA, Myers DN, PHYSIOLOGICAL EVIDENCE OF HYPERMETABOLISM IN OSTEOGENESIS IMPERFECTA, Pediatrics 49 (1972) 375. http://pediatrics.aappublications.org/content/49/3/375.abstract. [PubMed] [Google Scholar]
- [16].Boraschi-Diaz I, Tauer JT, El-Rifai O, Guillemette D, Lefebvre G, Rauch F, Ferron M, V Komarova S, Metabolic phenotype in the mouse model of osteogenesis imperfecta, J Endocrinol 234 (2017) 279–289. 10.1530/JOE-17-0335. [DOI] [PubMed] [Google Scholar]
- [17].Porsborg P, Astrup G, Bendixen D, Lund AM, Ørding H, Osteogenesis imperfecta and malignant hyperthermia, Anaesthesia 51 (1996) 863–865. 10.1111/j.1365-2044.1996.tb12619.x. [DOI] [PubMed] [Google Scholar]
- [18].Carleton SM, McBride DJ, Carson WL, Huntington CE, Twenter KL, Rolwes KM, Winkelmann CT, Morris JS, Taylor JF, Phillips CL, Role of Genetic Background in Determining Phenotypic Severity Throughout Postnatal Development and at Peak Bone Mass in Col1a2 Deficient Mice (oim), Bone 42 (2008) 681–694. 10.1016/j.bone.2007.12.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Fletcher JA, Meers GM, Linden MA, Kearney ML, Morris EM, Thyfault JP, Rector RS, Impact of Various Exercise Modalities on Hepatic Mitochondrial Function, Med. Sci. Sports Exerc 46 (2014) 1089–1097. 10.1249/MSS.0000000000000223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Ruiz-Ramírez A, Chávez-Salgado M, Peñeda-Flores JA, Zapata E, Masso F, El-Hafidi M, High-sucrose diet increases ROS generation, FFA accumulation, UCP2 level, and proton leak in liver mitochondria, Am. J. Physiol. Metab 301 (2011) E1198–E1207. 10.1152/ajpendo.00631.2010. [DOI] [PubMed] [Google Scholar]
- [21].Morris EM, Meers GME, Booth FW, Fritsche KL, Hardin CD, Thyfault JP, Ibdah JA, PGC-1α overexpression results in increased hepatic fatty acid oxidation with reduced triacylglycerol accumulation and secretion, Am. J. Physiol. - Gastrointest. Liver Physiol 303 (2012) G979–G992. 10.1152/ajpgi.00169.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Olver TD, Edwards JC, Jurrissen TJ, Veteto AB, Jones JL, Gao C, Rau C, Warren CM, Klutho PJ, Alex L, Ferreira-Nichols SC, Ivey JR, Thorne PK, McDonald KS, Krenz M, Baines CP, Solaro RJ, Wang Y, Ford DA, Domeier TL, Padilla J, Rector RS, Emter CA, Western Diet-Fed, Aortic-Banded Ossabaw Swine: A Preclinical Model of Cardio-Metabolic Heart Failure, JACC Basic to Transl. Sci 4 (2019) 404–421. 10.1016/j.jacbts.2019.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Srere PA, [1] Citrate synthase: [EC 4.1.3.7. Citrate oxaloacetate-lyase (CoA-acetylating)], in: Methods Enzymol, Academic Press, 1969: pp. 3–11. 10.1016/0076-6879(69)13005-0. [DOI] [Google Scholar]
- [24].Rector RS, Thyfault JP, Morris RT, Laye MJ, Borengasser SJ, Booth FW, Ibdah JA, Daily exercise increases hepatic fatty acid oxidation and prevents steatosis in Otsuka Long-Evans Tokushima Fatty rats, Am. J. Physiol. Liver Physiol 294 (2008) G619–G626. 10.1152/ajpgi.00428.2007. [DOI] [PubMed] [Google Scholar]
- [25].Weir JBDB, New methods for calculating metabolic rate with special reference to protein metabolism, J. Physiol 109 (1949) 1–9. https://www.ncbi.nlm.nih.gov/pubmed/15394301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Jequier E, Acheson K, Schutz Y, Assessment of Energy Expenditure and Fuel Utilization in Man, Annu. Rev. Nutr (1987). 10.1146/annurev.nu.07.070187.001155. [DOI] [PubMed]
- [27].Mann A, Thompson A, Robbins N, Blomkalns AL, Localization, identification, and excision of murine adipose depots, J. Vis. Exp (2014). 10.3791/52174. [DOI] [PMC free article] [PubMed]
- [28].Kammoun M, Cassar-Malek I, Meunier B, Picard B, A simplified immunohistochemical classification of skeletal muscle fibres in mouse, Eur. J. Histochem 58 (2014) 2254. 10.4081/ejh.2014.2254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Augusto V, Padovani CR, Rocha Campos GE, Skeletal muscle fiber types in C57Bl6J mice, 2004.
- [30].Willingham TB, McCully KK, In vivo assessment of mitochondrial dysfunction in clinical populations using near-infrared spectroscopy, Front. Physiol 8 (2017) 689. 10.3389/fphys.2017.00689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Conley KE, Jubrias SA, Esselman PC, Oxidative capacity and ageing in human muscle, J. Physiol 526 (2000) 203–210. 10.1111/j.1469-7793.2000.t01-1-00203.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Hood DA, Memme JM, Oliveira AN, Triolo M, Maintenance of Skeletal Muscle Mitochondria in Health, Exercise, and Aging, Annu. Rev. Physiol 81 (2019) 19–41. 10.1146/annurev-physiol-020518-114310. [DOI] [PubMed] [Google Scholar]
- [33].Van Brussel M, Takken T, Uiterwaal CS, Pruijs HJ, Van der Net J, Helders PJ, Engelbert RH, Physical training in children with osteogenesis imperfecta, J Pediatr 152 (2008) 111–6, 116 e1. 10.1016/j.jpeds.2007.06.029. [DOI] [PubMed] [Google Scholar]
- [34].Engelbert RH, Uiterwaal CS, Gerver W-J, van der Net J-J, Pruijs HE, Helders PJ, Osteogenesis imperfecta in childhood: Impairment and disability. a prospective study with 4-year follow-up11No commercial party having a direct financial interest in the results of the research supporting this article has or will confer a benefit on the a, Arch. Phys. Med. Rehabil. 85 (2004) 772–778. 10.1016/j.apmr.2003.08.085. [DOI] [PubMed] [Google Scholar]
- [35].Isaacson J, Brotto M, Physiology of Mechanotransduction: How Do Muscle and Bone “Talk” to One Another?, Clin. Rev. Bone Miner. Metab 12 (2014) 77–85. 10.1007/s12018-013-9152-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Periasamy M, Herrera JL, Reis FCG, Skeletal muscle thermogenesis and its role in whole body energy metabolism, Diabetes Metab. J 41 (2017) 327–336. 10.4093/dmj.2017.41.5.327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Zurlo F, Larson K, Bogardus C, Ravussin E, Skeletal muscle metabolism is a major determinant of resting energy expenditure, J. Clin. Invest 86 (1990) 1423–1427. 10.1172/JCI114857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Rasmussen BB, Phillips SM, Contractile and nutritional regulation of human muscle growth, Exerc. Sport Sci. Rev 31 (2003) 127–131. 10.1097/00003677-200307000-00005. [DOI] [PubMed] [Google Scholar]
- [39].Chen F, Guo R, Itoh S, Moreno L, Rosenthal E, Zappitelli T, Zirngibl RA, Flenniken A, Cole W, Grynpas M, Osborne LR, Vogel W, Adamson L, Rossant J, Aubin JE, First Mouse Model for Combined Osteogenesis Imperfecta and Ehlers-Danlos Syndrome, J. Bone Miner. Res 29 (2014) 1412–1423. 10.1002/jbmr.2177. [DOI] [PubMed] [Google Scholar]
- [40].Ross BD, Hems R, Krebs HA, The Rate of Gluconeogenesis from Various Precursors in the Perfused Rat Liver, 1967. [DOI] [PMC free article] [PubMed]
- [41].Rotondo F, Ho-Palma AC, Remesar X, Fernández-López JA, Romero MDM, Alemany M, Glycerol is synthesized and secreted by adipocytes to dispose of excess glucose, via glycerogenesis and increased acyl-glycerol turnover, Sci. Rep 7 (2017) 1–14. 10.1038/s41598-017-09450-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Mahendran Y, Cederberg H, Vangipurapu J, Kangas AJ, Soininen P, Kuusisto J, Uusitupa M, Ala-Korpela M, Laakso M, Glycerol and fatty acids in serum predict the development of hyperglycemia and type 2 diabetes in Finnish men, Diabetes Care 36 (2013) 3732–3738. 10.2337/dc13-0800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Benard G, Faustin B, Passerieux E, Galinier A, Rocher C, Bellance N, Delage JP, Casteilla L, Letellier T, Rossignolinserm R, Physiological diversity of mitochondrial oxidative phosphorylation, Am. J. Physiol. - Cell Physiol 291 (2006) 1172–1182. 10.1152/ajpcell.00195.2006. [DOI] [PubMed] [Google Scholar]
- [44].Fernández-Vizarra E, Enríquez JA, Pérez-Martos A, Montoya J, Fernández-Silva P, Tissue-specific differences in mitochondrial activity and biogenesis, Mitochondrion 11 (2011) 207–213. 10.1016/j.mito.2010.09.011. [DOI] [PubMed] [Google Scholar]
- [45].Johnson DT, Harris RA, French S, Blair PV, You J, Bemis KG, Wang M, Balaban RS, Tissue heterogeneity of the mammalian mitochondrial proteome, Am. J. Physiol. - Cell Physiol 292 (2007) 689–697. 10.1152/ajpcell.00108.2006. [DOI] [PubMed] [Google Scholar]
- [46].Schiaffino S, Reggiani C, Fiber types in Mammalian skeletal muscles, Physiol. Rev 91 (2011) 1447–1531. 10.1152/physrev.00031.2010. [DOI] [PubMed] [Google Scholar]
- [47].Bogdanis GC, Effects of physical activity and inactivity on muscle fatigue, Front. Physiol 3 May (2012). 10.3389/fphys.2012.00142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Menshikova EV, Ritov VB, Fairfull L, Ferrell RE, Kelley DE, Goodpaster BH, Effects of Exercise on Mitochondrial Content and Function in Aging Human Skeletal Muscle, n.d. [DOI] [PMC free article] [PubMed]
- [49].Jeong Y, Daghlas SA, Kahveci AS, Salamango D, Gentry BA, Brown M, Rector RS, Pearsall RS, Phillips CL, Soluble activin receptor type IIB decoy receptor differentially impacts murine osteogenesis imperfecta muscle function, Muscle Nerve 57 (2017) 294–304. 10.1002/mus.25706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Kunau WH, Dommes V, Schulz H, β-Oxidation of fatty acids in mitochondria, peroxisomes, and bacteria: A century of continued progress, Prog. Lipid Res 34 (1995) 267–342. 10.1016/0163-7827(95)00011-9. [DOI] [PubMed] [Google Scholar]
- [51].Folkestad L, Hald JD, Ersbøll AK, Gram J, Hermann AP, Langdahl B, Abrahamsen B, Brixen K, Fracture Rates and Fracture Sites in Patients With Osteogenesis Imperfecta: A Nationwide Register-Based Cohort Study, J. Bone Miner. Res 32 (2017) 125–134. 10.1002/jbmr.2920. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


