Abstract
Background
Celiac disease is a chronic, small intestinal, immune-mediated enteropathy caused by a permanent intolerance to dietary gluten in genetically predisposed individuals. Clinical studies have found that intestinal cholecystokinin secretion and gallbladder emptying in response to a fatty meal are impaired before celiac patients start the gluten-free diet (GFD).
Design
However, it was never really appreciated whether celiac disease is associated with gallstones because there were very few studies investigating the mechanism underlying the impact of celiac disease on the pathogenesis of gallstones.
Results
We summarize recent progress on the relationship between celiac disease and gallstones and propose that celiac disease is an important risk factor for gallstone formation because defective intestinal cholecystokinin secretion markedly increases susceptibility to cholesterol gallstones via a mechanism involving dysmotility of both the gallbladder and the small intestine. Because GFD can significantly improve the celiac enteropathy, early diagnosis and therapy in celiac patients is crucial for preventing the long-term impact of cholecystokinin deficiency on the biliary and intestinal consequences. When gluten is reintroduced, clinical and histologic relapse often occurs in celiac patients. Moreover, some of the celiac patients do not respond well to GFD.
Conclusions
It is imperative to routinely examine by ultrasonography whether gallbladder motility function is preserved in celiac patients and monitor whether biliary sludge (a precursor of gallstones) appears in the gallbladder, regardless of whether they are under the GFD program. To prevent gallstones in celiac patients, it is urgently needed to investigate the prevalence and pathogenesis of gallstones in these patients.
Keywords: bile salt, cholesterol crystallization, gallbladder motility, lithogenic bile
Introduction
Celiac disease is a chronic autoimmune enteropathy caused by a permanent intolerance to dietary gluten in genetically predisposed individuals [1–3]. The major site of damage is localized on the upper part of small intestine, which typically displays villus atrophy of the mucosa [4]. In the past decades, clinical studies and basic research have extensively investigated the pathogenesis of celiac disease, with a focus mainly on immune-mediated enteropathy that is precipitated by dietary gluten. It was recognized 40 years ago [5] that gallbladder emptying in response to a fatty meal is dramatically reduced because of defective cholecystokinin (CCK) release from the proximal small intestine caused by enteropathy in celiac patients before they start the gluten-free diet (GFD). However, it is still unclear whether these patients display a predisposition to gallstones. Because neither epidemiological investigations on gallstone prevalence rates in celiac patients nor clinical studies on the impact of celiac disease on the pathogenesis of gallstones were reported, it remains largely unknown whether celiac disease is indeed associated with gallstone formation. In this concise review article, we summarize recent progress on the relationship between celiac disease and gallstone disease and propose that celiac disease is an important risk factor for gallstone formation because of impaired intestinal CCK secretion in these patients.
Impaired CCK secretion in patients with celiac disease
CCK is a neuroendocrine peptide hormone that is synthesized and secreted by the I-cells in the mucosal epithelium of the proximal small intestine in response to a meal containing fat and proteins [6]. After CCK is secreted by the I-cells and enters the circulation, it stimulates the contraction of the gallbladder and the relaxation of the Sphincter of Oddi by activating the CCK-1 receptor (CCK-1R) signaling cascade [7]. This promotes the release of concentrated gallbladder bile to the small intestine in where, bile salts, a major lipid component of bile, play a critical role in regulating the digestion and absorption of dietary cholesterol, fat, and fat-soluble vitamins by forming mixed micelles that facilitate transport of these lipids across the unstirred layer of water adjacent to the surface of the apical membrane of enterocytes for uptake through a receptor-mediated mechanism [8]. In addition, CCK modulates gastric emptying and small intestinal motility by stimulating the CCK-2R and the CCK-1R signaling pathways, respectively [9]. The regulatory role of CCK in the gastrointestinal motility could influence the absorption efficiency of nutrient components by the enterocytes [10, 11]. CCK may have an effect on regulating bile flow and hepatic secretion of biliary lipids although further studies are needed to prove it [12].
Compelling evidence has clearly demonstrated that postprandial gallbladder emptying in response to a fatty meal is impaired in untreated celiac patients because of a marked defect in CCK release from the atrophic small intestinal mucosa as found by low CCK concentrations in both plasma and duodenal extracts [5, 13–17]. Another possible explanation is that gallbladder responsiveness to CCK may also be impaired [18]. Further clinical studies found that in the fasting state, the circulating CCK levels are dramatically lower in untreated celiac patients than in healthy control subjects [5]. However, immunocytochemical studies found that the number of CCK-producing endocrine cells, i.e., the I-cells, in jejunal biopsies is significantly higher not only in untreated patients with celiac disease, but also in celiac patients on gluten challenge diet compared to healthy subjects [19–21]. These findings indicate that a fatty meal cannot effectively stimulate CCK secretion by the I-cells in celiac patients and this abnormality is highly attributed to severe villus atrophy, crypt hyperplasia, enterocyte disarray, epithelial cell layer, and intense inflammation of the lamina propria in celiac disease [1]. More importantly, after eating GFD, plasma CCK concentrations are increased in celiac patients and the number of I-cells is comparable between celiac patients on GFD and healthy control subjects [21]. This implies that the physiological function of the intestinal I-cells is not impaired. By contrast, the increased number of I-cells caused possibly by a compensatory mechanism is restored to normal in celiac patients after GFD that leads to histologic improvement in the small intestine.
Obviously, the gallbladder becomes “large, lax, and lazy”, i.e., gallbladder stasis, in celiac patients before starting GFD [22], and this may put them at a high risk of developing biliary sludge and then gallstones. It is well established that under conditions of gallbladder stasis, a large amount of mucin gel, a potent pronucleating agent, is often produced and accumulated in the gallbladder. In addition, gallbladder stasis increases cholesterol absorption by the gallbladder epithelial cells and promotes the conversion of cholesterol to cholesteryl esters for storage in the gallbladder wall, which further worsen impaired gallbladder motor function. More importantly, a longer residence time of cholesterol-supersaturated bile in the gallbladder lumen often leads to rapid cholesterol crystallization and crystal growth and agglomeration into microlithiasis and subsequently macroscopic gallstones not only in patients without celiac disease, but also in celiac patients [23]. These clinical observations support the notion that celiac patients are predisposed to gallstone formation before starting GFD. However, the impact of celiac disease on the formation of gallstones has been overlooked in the past decades. It is imperative to bring up this important issue to physicians for their clinic practice.
Celiac disease is a risk factor for gallstone formation
Because there were very few studies investigating the role of celiac disease in the formation of cholesterol gallstones in the past decades, it was never really appreciated whether celiac disease is a crucial risk factor for gallstone formation. Moreover, little is known about the exact prevalence rate of gallstones in celiac disease because these patients are often recommended immediately starting GFD as soon as a positive diagnosis is made. Another possible explanation is that many celiac patients may not be diagnosed because of their atypical or mild symptoms. In addition, celiac disease was long considered to be a rare disorder, particularly in adults. Because of wide variations in the nature and intensity of clinical presentation, this makes it difficult to early diagnose celiac disease in silent patients. Actually, overt celiac disease is only the emerging peak of the celiac iceberg and most of the patients are under silent or latent conditions [1]. Therefore, the true prevalence of celiac disease remains unknown and may vary according to geographical areas. Furthermore, large efforts have been made in investigating the pathogenesis of celiac disease with a focus on the enteropathy and in preventing it by the GFD program. On the other hand, it is unclear whether some of the gallstone patients that are diagnosed by ultrasonography or have a cholecystectomy due to gallbladder stones are under conditions of silent or latent celiac disease. As a result, the association between celiac disease and gallstones has long been lacking and long been overlooked.
To the best of our knowledge, there was only a clinical survey reporting that gallstone prevalence was 20% in 30 elderly patients with celiac disease that was diagnosed by intestinal biopsy [24], which is markedly higher than that in ordinary Americans (12% of adults). Thus, it is urgently needed to perform more careful epidemiological surveys on gallstone prevalence rates in patients with celiac disease and clinical studies on the impact of celiac disease on gallstone pathogenesis in celiac patients before and after the ingestion of GFD. Of special note is that some of the celiac patients do not respond well to GFD. In addition, when gluten is reintroduced, clinical and histologic relapse often occurs in celiac patients because the intolerance is permanent. Thus, comprehensive prospective and retrospective cohort studies should be performed to investigate the prevalence and pathogenesis of gallstones in subgroups of celiac patients.
Potential lithogenic mechanism of celiac disease
How does celiac disease induce cholesterol-supersaturated bile, a critical factor for gallstone formation? Vuoristo and Miettinen have found that hepatic secretion of biliary cholesterol, phospholipids, and bile salts, as well as bile flow are significantly increased in patients with celiac disease compared to healthy control subjects [25]. More importantly, biliary cholesterol secretion is almost doubled in celiac patients, indicating that hepatic hypersecretion of biliary cholesterol is a primary factor for the formation of supersaturated bile [25]. By studying CCK knockout (KO) mice, an excellent animal model relevant to the biliary characteristic of celiac disease, it is observed that hepatic secretion of biliary cholesterol and phospholipids, but not bile salts, is significantly increased after 56 days of lithogenic diet feeding [26]. These abnormalities enhance biliary lithogenicity, thereby leading to rapid cholesterol crystallization and gallstone formation in CCK KO mice compared to wild-type (WT) mice. Moreover, expression of the sterol transporters Abcg5/g8 that are mainly responsible for hepatic cholesterol secretion is significantly higher in CCK KO mice than in WT mice. However, hepatic expression of Srebp2 and Hmgcr are comparable in CCK KO vs. WT mice, implying that hepatic cholesterol biosynthesis is not a major source for biliary lithogenicity in CCK KO mice.
Where do excess amounts of the cholesterol molecules originate from for inducing supersaturated bile in celiac patients? It has been found that CCK plays a role in the regulation of small intestinal motility because Cck-1r is expressed in the smooth muscle of small intestine, which is a variable known to influence intestinal cholesterol absorption in humans [27]. Indeed, small intestinal transit time is significantly retarded not only in CCK KO mice [26], but also in CCK-1R KO mice [10]. As a result, intestinal cholesterol absorption efficiency is significantly increased in these knockout mice. In parallel with the findings in humans [27], these findings substantiate the concept that the small intestinal transit rate plays a critical role in regulating cholesterol absorption [8]. Thus, retardant small intestinal transit rate augments intestinal cholesterol absorption most likely due to a longer residence time of the sterols in the lumen of small intestine. This, in turn, amplifies cholesterol’s incorporation into mixed micelles and also enhances partitioning of cholesterol monomers out of micelles, rendering them available for intestinal uptake by cholesterol influx sterol transporter, Niemann-Pick C1-like protein 1 (NPC1L1), on the apical membrane of enterocytes. Accumulated evidence has shown that sluggish small intestinal transit rate augments intestinal cholesterol absorption efficiency not only in CCK and CCK-1R KO mice [10, 26], but also in humans with pharmacological intervention [27]. Increased intestinal cholesterol absorption is also a risk factor for the formation of lithogenic bile and gallstones [28]. Aspects related to small intestinal transit time have not examined yet in celiac disease, but intestinal cholesterol absorption efficiency is lower in untreated celiac patients compared to healthy subjects [29], which may be attributable to a mucosal damage in the proximal small intestine, a major site for cholesterol absorption [30]. However, further clinical studies found that because biliary cholesterol secretion is almost doubled in celiac patients [25], intestinal cholesterol absorption in absolute terms is high despite its low fractional absorption [29]. Under these circumstances, a large amount of the cholesterol molecules absorbed from the small intestine is delivered to the liver through the chylomicron remnant pathway for hepatic hypersecretion into bile. This explains, in part, why hepatic cholesterol secretion is high in untreated celiac patients.
The lack of endogenous CCK in mice with disruption of the Cck gene markedly increases fasting and postprandial gallbladder volumes by impairing gallbladder emptying function, leading to gallbladder stasis [26]. This greatly facilitates the crystallization of cholesterol monohydrate crystals and their growth and agglomeration to gallstones [31]. Because impaired gallbladder motor function is strongly linked to gallstone formation [23], these abnormalities could enhance the cholelithogenesis in celiac patients. Moreover, supersaturated bile increases expression of Muc2, Muc5ac, and Muc5b for producing the gel-forming mucins, thus inducing the accumulation of excess mucin gel in the gallbladder. This greatly promotes growth and agglomeration of solid cholesterol monohydrate crystals into microlithiasis. The gallbladder epithelial cells also actively secrete and absorb biliary lipids and water to modify lipid concentrations of bile [23]. In the lithogenic state, gallbladder cholesterol absorption is enhanced so that the accumulation of excess cholesterol in the gallbladder wall is increased. Because gallbladder epithelial cells do not have a capability to synthesize lipoproteins for lipid transport into the circulation, the absorbed cholesterol has to be converted to cholesteryl esters for storage in the mucosa and lamina propria. Excess amounts of cholesterol and cholesteryl esters could disrupt CCK-1R signaling cascade and decouple the G-protein-mediated signal transduction by stiffening the sarcolemmal membrane of the smooth muscle. These alterations further paralyze gallbladder contractility and lead to biliary sludge (a precursor of gallstones) that consists of solid cholesterol monohydrate crystals, calcium bilirubinate granules, or other calcium salts embedded in strands of mucin gel [32]. Biliary sludge can spontaneously disappear and re-form over time, or it can evolve to become gallstones, which is often found in a subgroup of patients who have a predisposition to gallstones [32]. Because the intolerance is permanent in celiac patients, when gluten is reintroduced, clinical and histologic relapse often occurs [33]. Under these circumstances, biliary sludge can disappear and re-form over time in celiac patients. All of these findings imply that impaired gallbladder emptying and increased mucin gel accumulation are two key risk factors for gallstone formation in celiac patients when they suffer from gallbladder stasis. Although an impaired CCK release and gallbladder contractile dysfunction in celiac patients have been recognized 40 years ago, it is unfortunate that no further clinical study was performed to investigate whether biliary sludge was formed by ultrasonography in these patients before starting GFD.
Potential role of CCK-1R agonists in the prevention of gallstones in celiac patients
Removal of gluten from the diet is essential for the treatment of celiac disease [1]. Compelling evidence from clinical studies has clearly demonstrated that after strict adherence to GFD in celiac patients, intestinal CCK release is restored to normal and gallbladder bile is emptied immediately in response to endogenous CCK after a meal [14]. Furthermore, intestinal cholesterol absorption is restored to normal and hepatic cholesterol secretion is reduced markedly [14]. This significant improvement is attributed to a dietary intervention with GFD, leading to well-formed villi and a return of the mucosal architecture toward normal [34, 35]. This indicates that the GFD not only leads to prompt clinical and subsequent histologic improvement, but also could reduce the risk of developing gallstones in celiac patients. It has been found that patients with long-term total parenteral nutrition (TPN) usually suffer from gallbladder stasis that markedly increases the risk of developing biliary sludge and gallstones. A single daily intravenous injection of CCK-8 prevents TPN-induced biliary sludge and gallstones by improving gallbladder contractility in patients [36]. Based on these observations, it is likely that oral non-peptide CCK-1R agonists, i.e., CCK-8 analogues may reduce the risk of developing gallstones in celiac patients. However, further clinical trials are required to support this idea.
Because the detergency of bile acids is obligatory for intestinal cholesterol uptake through micellar solubilization of the intraluminal sterols, the secretion of concentrated gallbladder bile to the small intestine greatly facilitates the digestion and absorption of dietary lipids. If impaired gallbladder contractility is restored by CCK-1R agonists or bile acids such as cholic acid is fed, this may improve the symptoms of maldigestion and malabsorption such as diarrhea, steatorrhea, weight loss, and nutritional deficiencies in celiac patients [37–40]. In addition, improving gallbladder kinetics may restore small intestinal motility and reduce contacting time of dietary gluten with the epithelial cells of the small intestine by accelerating its fecal excretion. This may further alleviate the symptoms of maldigestion and malabsorption in celiac patients even when they are put on the GFD program. A clinical trial should be performed to test the hypothesis that frequent gallbladder emptying through CCK-1R agonists or alternatively gallbladder prokinetic agents restores gallbladder contractility, thereby protecting against gallbladder stasis, biliary sludge, and gallstones in celiac patients.
Conclusions and future directions
The molecular mechanism underlying the critical role of celiac disease in gallstone formation has been largely unknown until now. It is highly likely that the defective CCK release in celiac patients markedly enhances susceptibility to gallstones via a mechanism that involves the dysmotility of both the gallbladder and the small intestine. Because GFD can significantly improve the celiac enteropathy, early diagnosis and therapy in celiac patients is important for preventing the long-term impact of CCK deficiency on the biliary consequences due to the small intestinal mucosal atrophy. Although gallstones could be prevented by strict adherence to GFD, some of the celiac patients do not respond well to GFD and this is still a challenging task for the treatment of celiac disease [1]. Furthermore, it has been found that when gluten is reintroduced, clinical and histologic relapse often occurs because the intolerance is permanent in these patients [33]. Therefore, it is imperative to routinely examine whether gallbladder motor function is impaired and monitor whether biliary sludge is formed in the gallbladder by ultrasonography in patients with celiac disease, regardless of whether or not they are under the GFD program. A special attention should be given to celiac patients under silent or latent conditions because the long-term impact of impaired CCK secretion on gallbladder contractility and gastrointestinal tract transit is often overlooked. CCK-1R agonists should be tested in a cohort study to explore whether they prevent gallstone formation by improving gallbladder contraction function and reducing bile lithogenicity in celiac patients. Moreover, CCK-1R agonists may ameliorate clinical symptoms and subsequently prompt histologic improvement of the small intestinal mucosa caused by dietary gluten by reducing contacting time between gluten and the mucosal surface of the small intestine through accelerating small intestinal transit rate for its fecal excretion. However, further clinical trials are required to support this intriguing idea.
Figure 1.
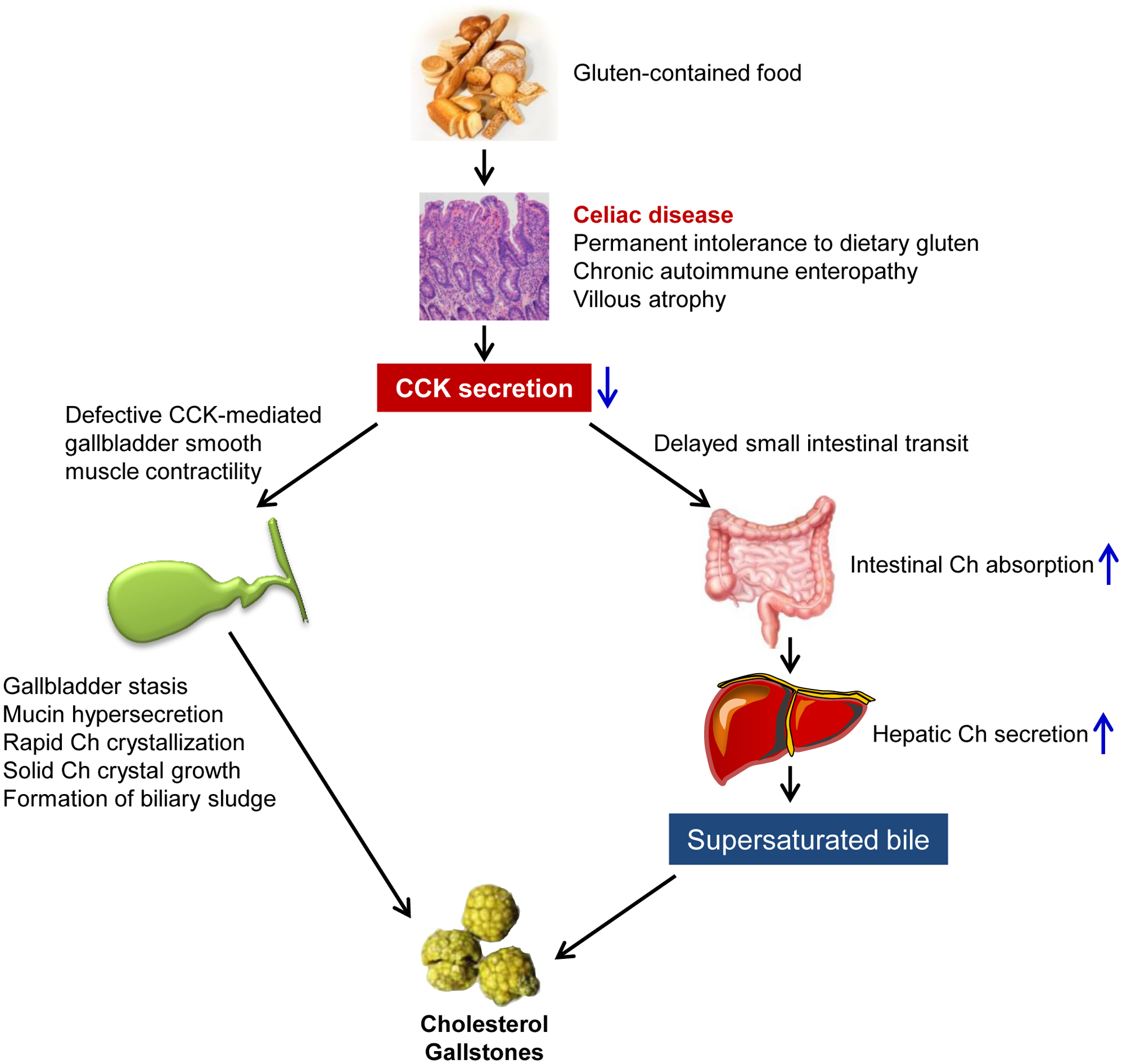
A working model elucidates the mechanism underlying the critical role of celiac disease in cholesterol gallstone formation. Abbreviations: Ch, cholesterol; CCK, cholecystokinin. See text for details.
Acknowledgments:
This work was supported in part by research grants DK101793 and DK106249 (to DQ-HW), both from the National Institutes of Health (US Public Health Service).
Abbreviations:
- ABC
ATP-binding cassette (transporter)
- CCK
cholecystokinin
- CCK-1R
CCK-1 receptor
- CCK-2R
CCK-2 receptor
- CSI
cholesterol saturation index
- GFD
gluten-free diet
- KO
knockout
- NPC1L1
Niemann-Pick C1-like protein 1 (transporter)
- TPN
total parenteral nutrition
- WT
wild-type
Footnotes
Disclosures: Authors have disclosed no conflicts of interest.
REFERENCES
- 1.Kelly CP: Celiac Disease. In: Feldman M, Friedman LS, Brandt LJ, eds. Sleisenger and Fordtran’s Gastrointestinal and Liver Disease. Volume 2. 10 ed: Elsevier Saunders, 2014; 1849–1872. [Google Scholar]
- 2.Choung RS, Ditah IC, Nadeau AM, Rubio-Tapia A, Marietta EV, Brantner TL, Camilleri MJ, et al. Trends and racial/ethnic disparities in gluten-sensitive problems in the United States: findings from the National Health and Nutrition Examination Surveys from 1988 to 2012. Am J Gastroenterol 2015;110:455–461. [DOI] [PubMed] [Google Scholar]
- 3.Rubio-Tapia A, Ludvigsson JF, Brantner TL, Murray JA, Everhart JE. The prevalence of celiac disease in the United States. Am J Gastroenterol 2012;107:1538–1544; quiz 1537, 1545. [DOI] [PubMed] [Google Scholar]
- 4.Green PH, Cellier C. Celiac disease. N Engl J Med 2007;357:1731–1743. [DOI] [PubMed] [Google Scholar]
- 5.Low-Beer TS, Harvey RF, Davies ER, Read AF. Abnormalities of serum cholecystokinin and gallbladder emptying in celiac disease. N Engl J Med 1975;292:961–963. [DOI] [PubMed] [Google Scholar]
- 6.Liddle RA. Cholecystokinin cells. Annu Rev Physiol 1997;59:221–242. [DOI] [PubMed] [Google Scholar]
- 7.Liddle RA, Goldfine ID, Rosen MS, Taplitz RA, Williams JA. Cholecystokinin bioactivity in human plasma. Molecular forms, responses to feeding, and relationship to gallbladder contraction. J Clin Invest 1985;75:1144–1152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang DQ. Regulation of intestinal cholesterol absorption. Annu Rev Physiol 2007;69:221–248. [DOI] [PubMed] [Google Scholar]
- 9.Konturek SJ, Kwiecien N, Obtulowicz W, Kopp B, Oleksy J, Rovati L. Cholecystokinin in the inhibition of gastric secretion and gastric emptying in humans. Digestion 1990;45:1–8. [DOI] [PubMed] [Google Scholar]
- 10.Wang DQ, Schmitz F, Kopin AS, Carey MC. Targeted disruption of the murine cholecystokinin-1 receptor promotes intestinal cholesterol absorption and susceptibility to cholesterol cholelithiasis. J Clin Invest 2004;114:521–528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kirby RJ, Howles PN, Hui DY. Rate of gastric emptying influences dietary cholesterol absorption efficiency in selected inbred strains of mice. J Lipid Res 2004;45:89–98. [DOI] [PubMed] [Google Scholar]
- 12.Krishnamurthy S, Krishnamurthy GT. Biliary dyskinesia: role of the sphincter of Oddi, gallbladder and cholecystokinin. J Nucl Med 1997;38:1824–1830. [PubMed] [Google Scholar]
- 13.Calam J, Ellis A, Dockray GJ. Identification and measurement of molecular variants of cholecystokinin in duodenal mucosa and plasma. Diminished concentrations in patients with celiac disease. J Clin Invest 1982;69:218–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Maton PN, Selden AC, Fitzpatrick ML, Chadwick VS. Defective gallbladder emptying and cholecystokinin release in celiac disease. Reversal by gluten-free diet. Gastroenterology 1985;88:391–396. [DOI] [PubMed] [Google Scholar]
- 15.Hopman WP, Rosenbusch G, Hectors MP, Jansen JB. Effect of predigested fat on intestinal stimulation of plasma cholecystokinin and gall bladder motility in coeliac disease. Gut 1995;36:17–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fraquelli M, Bardella MT, Peracchi M, Cesana BM, Bianchi PA, Conte D. Gallbladder emptying and somatostatin and cholecystokinin plasma levels in celiac disease. Am J Gastroenterol 1999;94:1866–1870. [DOI] [PubMed] [Google Scholar]
- 17.Masclee AA, Jansen JB, Driessen WM, Geuskens LM, Lamers CB. Gallbladder sensitivity to cholecystokinin in coeliac disease. Correlation of gallbladder contraction with plasma cholecystokinin-like immunoreactivity during infusion of cerulein. Scand J Gastroenterol 1991;26:1279–1284. [DOI] [PubMed] [Google Scholar]
- 18.Brown AM, Bradshaw MJ, Richardson R, Wheeler JG, Harvey RF. Pathogenesis of the impaired gall bladder contraction of coeliac disease. Gut 1987;28:1426–1432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sjolund K, Alumets J, Berg NO, Hakanson R, Sundler F. Duodenal endocrine cells in adult coeliac disease. Gut 1979;20:547–552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sjolund K, Alumets J, Berg NO, Hakanson R, Sundler F. Enteropathy of coeliac disease in adults: increased number of enterochromaffin cells the duodenal mucosa. Gut 1982;23:42–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pietroletti R, Bishop AE, Carlei F, Bonamico M, Lloyd RV, Wilson BS, Ceccamea A, et al. Gut endocrine cell population in coeliac disease estimated by immunocytochemistry using a monoclonal antibody to chromogranin. Gut 1986;27:838–843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Low-Beer TS, Heaton KW, Heaton ST, Read AE. Gallbladder inertia and sluggish enterohepatic circulation of bile-salts in coeliac disease. Lancet 1971;1:991–994. [DOI] [PubMed] [Google Scholar]
- 23.Portincasa P, Di Ciaula A, Wang HH, Palasciano G, van Erpecum KJ, Moschetta A, Wang DQ. Coordinate regulation of gallbladder motor function in the gut-liver axis. Hepatology 2008;47:2112–2126. [DOI] [PubMed] [Google Scholar]
- 24.Freeman HJ. Clinical spectrum of biopay-defined celiac disease in the elderly. Can J Gastroenterol 1995;9:42–46. [Google Scholar]
- 25.Vuoristo M, Miettinen TA. Increased biliary lipid secretion in celiac disease. Gastroenterology 1985;88:134–142. [DOI] [PubMed] [Google Scholar]
- 26.Wang HH, Liu M, Portincasa P, Tso P, Wang DQ. Lack of endogenous cholecystokinin promotes cholelithogenesis in mice. Neurogastroenterol Motil 2016;28:364–375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ponz de Leon M, Iori R, Barbolini G, Pompei G, Zaniol P, Carulli N. Influence of small-bowel transit time on dietary cholesterol absorption in human beings. N Engl J Med 1982;307:102–103. [DOI] [PubMed] [Google Scholar]
- 28.Wang DQ, Zhang L, Wang HH. High cholesterol absorption efficiency and rapid biliary secretion of chylomicron remnant cholesterol enhance cholelithogenesis in gallstone-susceptible mice. Biochim Biophys Acta 2005;1733:90–99. [DOI] [PubMed] [Google Scholar]
- 29.Vuoristo M, Miettinen TA. Cholesterol absorption, elimination and synthesis in coeliac disease. Eur J Clin Invest 1982;12:285–291. [DOI] [PubMed] [Google Scholar]
- 30.Leffler D, Schuppan D, Pallav K, Najarian R, Goldsmith JD, Hansen J, Kabbani T, et al. Kinetics of the histological, serological and symptomatic responses to gluten challenge in adults with coeliac disease. Gut 2013;62:996–1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang HH, Portincasa P, Liu M, Tso P, Samuelson LC, Wang DQ. Effect of gallbladder hypomotility on cholesterol crystallization and growth in CCK-deficient mice. Biochim Biophys Acta 2010;1801:138–146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ko CW, Sekijima JH, Lee SP. Biliary sludge. Ann Intern Med 1999;130:301–311. [DOI] [PubMed] [Google Scholar]
- 33.Ciacci C, Cirillo M, Cavallaro R, Mazzacca G. Long-term follow-up of celiac adults on gluten-free diet: prevalence and correlates of intestinal damage. Digestion 2002;66:178–185. [DOI] [PubMed] [Google Scholar]
- 34.Kupper C Dietary guidelines and implementation for celiac disease. Gastroenterology 2005;128:S121–127. [DOI] [PubMed] [Google Scholar]
- 35.Niewinski MM. Advances in celiac disease and gluten-free diet. J Am Diet Assoc 2008;108:661–672. [DOI] [PubMed] [Google Scholar]
- 36.Sitzmann JV, Pitt HA, Steinborn PA, Pasha ZR, Sanders RC. Cholecystokinin prevents parenteral nutrition induced biliary sludge in humans. Surg Gynecol Obstet 1990;170:25–31. [PubMed] [Google Scholar]
- 37.Farrell RJ, Kelly CP. Celiac sprue. N Engl J Med 2002;346:180–188. [DOI] [PubMed] [Google Scholar]
- 38.Vriezinga SL, Schweizer JJ, Koning F, Mearin ML. Coeliac disease and gluten-related disorders in childhood. Nat Rev Gastroenterol Hepatol 2015;12:527–536. [DOI] [PubMed] [Google Scholar]
- 39.Lionetti E, Gatti S, Pulvirenti A, Catassi C. Celiac disease from a global perspective. Best Pract Res Clin Gastroenterol 2015;29:365–379. [DOI] [PubMed] [Google Scholar]
- 40.Verdu EF, Galipeau HJ, Jabri B. Novel players in coeliac disease pathogenesis: role of the gut microbiota. Nat Rev Gastroenterol Hepatol 2015;12:497–506. [DOI] [PMC free article] [PubMed] [Google Scholar]


