Abstract
Objectives
To investigate the association between sex hormones and the severity of coronavirus disease 2019 (COVID-19). Furthermore, associations between sex hormones and systemic inflammation markers, viral shedding and length of hospital stay were studied.
Design and methods
This case–control study included a total of 48 male patients with COVID-19 admitted to an Italian reference hospital. The 24 cases were patients with PaO2/FiO2 <250 mmHg and who needed ventilatory support during hospitalization (severe COVID-19). The 24 controls were selected in a 1:1 ratio, matched by age, from patients who maintained PaO2/FiO2 >300 mmHg at all times and who may have required low-flow oxygen supplementation during hospitalization (mild COVID-19). For each group, sex hormones were evaluated on hospital admission.
Results
Patients with severe COVID-19 (cases) had a significantly lower testosterone level compared with patients with mild COVID-19 (controls). Median total testosterone (TT) was 1.4 ng/mL in cases and 3.5 ng/mL in controls (P = 0.005); median bioavailable testosterone (BioT) was 0.49 and 1.21 in cases and controls, respectively (P = 0.008); and median calculated free testosterone (cFT) was 0.029 ng/mL and 0.058 ng/mL in cases and controls, respectively (P = 0.015). Low TT, low cFT and low BioT were correlated with hyperinflammatory syndrome (P = 0.018, P = 0.048 and P = 0.020, respectively) and associated with longer length of hospital stay (P = 0.052, P = 0.041 and P = 0.023, respectively). No association was found between sex hormone level and duration of viral shedding, or between sex hormone level and mortality rate.
Conclusions
A low level of testosterone was found to be a marker of clinical severity of COVID-19.
Keywords: SARS-CoV-2, Sex hormones, Testosterone, Severity markers, Androgen sensitivity, Gender imbalance
Introduction
Severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) infection is characterized by a huge range of clinical manifestations. Many pathogenetic pathways and virulence mechanisms are still unknown. Nevertheless, it is known that the host’s immune system plays a key role (Blanco-Melo et al., 2020). Notably, age, comorbidities (e.g. diabetes mellitus, obesity), smoking habits and male sex (Rod et al., 2020, Wu et al., 2020) are the fundamental independent risk factors for death from coronavirus disease-19 (COVID-19) (Zhou et al., 2020). A study conducted by Jin et al. (2020) showed that the number of men who died from COVID-19 was 2.4 times higher than the number of women (P = 0.016), with no gender difference in prevalence. The COVID-19 Sex-Disaggregated Data Tracker shows that the COVID-19 fatality rate is higher in men than women in 68 countries (Global Health 5050, n.d.). To explain this discrepancy, behavioural and genetic features have been called into question. The TMPRSS2 receptor is a serine protease expressed on the surface of type II pneumocytes, that contributes to viral penetration into host cells (Jacquinet et al., 2001) and is required for SARS-CoV-2 infectivity (Shirato et al., 2016, Iwata-Yoshikawa et al., 2019). Interestingly, the androgen receptor is the main activator of transcription of the TMPRSS2 gene in humans (Lucas et al., 2014, Stopsack et al., 2020). Moreover, a study conducted by Seeland et al. (2020) showed that oestrogens are protective against severe COVID-19 by downregulating the expression of angiotensin-converting enzyme-2 (ACE-2) receptors. Interestingly, it has been shown that men infected with SARS-CoV-2 had lower serum testosterone levels compared with the general population (Ma et al., 2020). In addition, a recent retrospective study showed that a decline in total testosterone (TT) and calculated free testosterone (cFT) levels was predictive of a poor prognosis in men with COVID-19 (Rastrelli et al., 2021). The present study aimed to test the hypothesis that the prognosis of a patient with COVID-19 can be independently related to blood levels of sex hormones, particularly testosterone and oestradiol.
Objectives
The aim of this study was to investigate the association between sex hormones and the severity of COVID-19. Furthermore, associations between hormones and markers of systemic inflammation, viral shedding and length of hospital stay were studied.
Materials and methods
Study design
A retrospective, case–control study was conducted at the National Institute for Infectious Diseases (INMI) Lazzaro Spallanzani, a large infectious disease research hospital in Rome. Data on patients with COVID-19 have been collected prospectively since March 2020.
In total, 48 male patients, aged between 18 and 65 years, admitted to the hospital with a diagnosis of COVID-19 between 1 March 2020 and 1 May 2020 were included in this study. Infection was documented by a positive result on a qualitative reverse transcriptase polymerase chain reaction (RT-PCR) assay for SARS-CoV-2, performed on a nasopharyngeal swab, at the time of admission. The case group consisted of 24 patients who developed severe pneumonia, the majority of whom had a framework of acute respiratory distress syndrome (ARDS) according to the Berlin definition (Force et al., 2012). Severe pneumonia was defined as the simultaneous presence of: (1) a ratio of arterial oxygen partial pressure to fractional inspired oxygen concentration (PaO2/FiO2) <250 mmHg; and (2) the need for high-flow oxygen ventilatory support by non-intensive ventilation or mechanical ventilation during hospitalization (severe COVID-19).
The control group was selected at random in a 1:1 ratio, matched by age (5-year age groups), among patients who maintained PaO2/FiO2 >300 mmHg at all times and who may have required low-flow oxygen supplementation during hospitalization (mild COVID-19). Of the controls, 83% developed pneumonia (20/24) and 8% did not (2/24). A thoracic computed tomography (CT) scan was not performed in the remaining two patients. The magnitude of pulmonary impairment was calculated by dedicated software (V-Lung Care by GE) using the most affected CT scan for each patient.
All patients received standard care according to the best evidence, local protocols and international guidelines available during the study period.
The primary endpoint of this study was to compare the blood levels of sex hormones between the case and control groups. The secondary endpoints included analysis of the associations between blood levels of sex hormones and (1) markers of systemic inflammation; (2) presence of hyperinflammatory syndrome; (3) percentage of damaged lung volume; (4) length of hospital stay; (5) time to swab clearance; and (6) time to orotracheal intubation (OTI)/death. The association between lung damage and the length of viral swab clearance was also studied.
Procedures
Plasma concentrations of the following parameters were tested for each patient at hospital admission: testosterone, 5α-dihydrotestosterone (DHT), androstenedione (Δ4), oestradiol, prostate-specific antigen (PSA), sex hormone binding globulin (SHBG) and albumin. PSA, SHBG, Δ4 and testosterone levels were measured using a chemiluminescence immunoassay analyser. Oestradiol was measured by direct immunoassay, and DHT was measured by enzyme-linked immunosorbent assay. Approximately 2–3% of TT circulates freely in plasma, binds to SHBG, and weakly binds to non-specific proteins such as albumin. The SHBG-bound fraction is biologically inactive because of the high binding affinity of SHBG for testosterone. The bioavailable testosterone (BioT) includes free testosterone plus testosterone weakly bound to albumin (Vermeulen et al., 1999), and was derived from TT. cFT was determined from TT using the Vermeulen formula (Rastrelli et al., 2021). Hence, an ‘inflammatory phenotype’ was identified, defined as the presence of at least three of the following characteristics in the most impaired blood test collected: (1) lymphocyte count <1000 cells/mm3; (2) ferritin >500 ng/mL; (3) lactate dehydrogenase (LDH) >300 U/L; (4) d-dimer >1000 ng/mL; and (5) C-reactive protein (CRP) >3 mg/dL. The duration of viral shedding was considered to extend from symptom onset to the first negative nasopharyngeal swab test for SARS-CoV-2. The time to OTI/death was calculated from hospital admission to the first occurrence of OTI and/or death.
Statistical analysis
Comparisons between case and control groups were performed using Chi-squared test for categorical variables and the Mann–Whitney U-test for continuous parameters. Correlations between TT, cFT, BioT and selected blood markers for severe disease (i.e. lymphocyte count, neutrophil count, ferritin, LDH, d-dimer, CRP, potassium), as well as the percentage of damaged lung volume and length of hospital stay, were tested using Spearman’s rank correlation coefficient (rho). Plasma hormone levels of patients presenting with or without hyperinflammatory syndrome were compared using the Mann–Whitney U-test.
Finally, Cox regression was used to investigate the association between sex hormone levels and time to viral swab clearance, and sex hormone levels and time to OTI/death. All statistical analyses were performed using Stata Version 15.1 (Stata Corp., College Station, TX, USA).
Results
Patient characteristics
Clinical and demographic characteristics of the study population are shown in Table 1 . The median age was 51 years for the control group and 50 years for the case group. The number of patients without comorbidities was similar in both study groups, but more patients in the case group had multi comorbidities compared with the control group (29.2% vs. 8.3%, respectively; P = 0.035).
Table 1.
Demographic and clinical characteristics of patients.
| Characteristics | Controls n = 24 | Cases n = 24 | P-value |
|---|---|---|---|
| Age, years, median (IQR) | 51 (43–57) | 50 (43–59) | 0.757 |
| Co-existing conditions, n (%) | |||
| COPD | 4 (16.7%) | 2 (8.3%) | 0.383 |
| Type 2 diabetes | 1 (4.2%) | 3 (12.5%) | 0.296 |
| Obesity | 2 (8.3%) | 4 (16.7%) | 0.383 |
| Hypertension | 3 (12.5%) | 7 (29.2%) | 0.155 |
| Number of co-existing conditions, n (%) | |||
| None | 12 (50.0%) | 14 (58.3%) | 0.035 |
| One | 10 (41.7%) | 3 (12.5%) | |
| Two or more | 2 (8.3%) | 7 (29.2%) | |
| Ethnic group: Caucasian, n (%) | 20 (83.3%) | 24 (100%) | 0.037 |
| Worst PaO2/FiO2, median (IQR) | 419 (386–460) | 141 (102–178) | <0.001 |
| Worst peripheral SO2, median (IQR) | 95 (94–95) | 90 (81–92) | <0.001 |
| % of damaged lung, median (IQR) | 12.0% (8.4–21.2%) | 25.1% (15.6–35.0%) | 0.075 |
| Days from symptom onset to hospital admission, median (IQR) | 9 (3–16) | 9 (6–9) | 0.544 |
| Score on ordinal scale | |||
| Hospitalized, not requiring supplemental oxygen, n (%) | 19 (79.2%) | 0 (0%) | <0.001 |
| Hospitalized, requiring supplemental oxygen, n (%) | 5 (20.8%) | 0 (0%) | |
| Hospitalized, receiving non-invasive ventilation or high-flow oxygen devices, n (%) | 0 (0%) | 19 (79.2%) | |
| Hospitalized, receiving invasive mechanical ventilation or ECMO, n (%) | 0 (0%) | 5 (20.8%) | |
| Pneumonia, n (%)a | 20 (83.3%) | 24 (100%) | 0.113 |
| ARDS, n (%) | 0 (0%) | 15 (62.5%) | <0.001 |
| Intensive care unit, n (%) | 0 (0%) | 8 (33.3%) | 0.002 |
| OTI, n (%) | 0 (0%) | 5 (20.8%) | 0.018 |
| Laboratory values at admission, median (IQR) | |||
| Lymphocytes, cells/mm3 | 1715 (1235–2040) | 925 (655–1230) | <0.001 |
| d-dimer, ng/mL | 500 (314–805) | 652 (488–978) | 0.307 |
| CRP, mg/dL | 1.16 (0.66–3.27) | 6.51 (2.93–14.91) | <0.001 |
| Ferritin, pg/mL | 298 (230–539) | 1062 (514–1416) | <0.001 |
| LDH, U/L | 183 (160–219) | 339 (213–398) | <0.001 |
| Neutrophils, ×103/uL | 4.3 (2.4–5.6) | 3.9 (2.8–6.1) | 0.807 |
| Potassium, mmol/L | 3.5 (3.5–3.7) | 3.3 (3–3.4) | 0.046 |
| Received treatment, n (%) | |||
| PI/b | 13 (54.2%) | 22 (91.7%) | 0.003 |
| HCQ | 15 (62.5%) | 21 (87.5%) | 0.046 |
| Remdesivir | 0 (0%) | 2 (8.3%) | 0.149 |
| Immunomodulant therapy | 0 (0%) | 19 (79.2%) | <0.001 |
| Steroids | 1 (4.4%) | 21 (87.5%) | <0.001 |
| Outcome, n (%) | |||
| Discharged | 24 (100%) | 22 (91.7%) | 0.149 |
| Died | 0 (0%) | 2 (8.3%) | |
| Length of stay, days, median (IQR) | 9 (7–12) | 23 (19–33) | <0.001 |
| Viral shedding, days, median (IQR) | 13.5 (9.5–21) | 20 (13–28) | 0.079 |
IQR, interquartile range; PaO2/FiO2, pressure of arterial oxygen to fractional inspired oxygen concentration; SO2, oxygen saturation; ECMO, extracorporeal membrane oxygenation; ARDS, acute respiratory distress syndrome; PI/b, protease inhibitor/boosted; HCQ, hydroxychloroquine; COPD, chronic obstructive pulmonary disease; OTI, orotracheal intubation; CRP, C-reactive protein; LDH, lactate dehydrogenase.
Two patients in the control group did not have a chest computed tomography scan.
The median number of days between symptom onset and hospital admission was 9 days in both groups. The proportion of injured lung was higher in cases compared with controls (25.1% and 12%, respectively; P = 0.075), as reflected in respiratory parameters. The worst PaO2/FiO2 reached during hospitalization was 141 mmHg for the case group and 419 mmHg for the control group (P < 0.001). According to the need for oxygen supplementation, all cases needed oxygen supplementation compared with 20.8% of controls (P < 0.001). Overall, 62.5% of cases developed ARDS, compared with none of the controls (P < 0.001). The majority of patients were treated with hydroxychloroquine and human immunodeficiency virus protease inhibitors. The use of immunomodulant therapies and steroids was highly predominant in cases compared with controls (P < 0.001).
The prognosis and duration of viral shedding were poorer in cases than controls. In fact, 100% (n = 24) of controls were cured and discharged, and none of them were admitted to the intensive care unit (ICU). Conversely, 33.3% (8/24) of cases were admitted to the ICU (P = 0.002). Moreover, 28.8% (5/24) of cases underwent OTI and two cases died (8.3%) (P = 0.149). Cases had median viral shedding of 20 days, compared with 13.5 days for controls (P = 0.079); the median length of hospital stay was 23 days for cases and nine days for controls (P < 0.001).
Laboratory markers of severe disease were far more prevalent in cases: during hospitalization, 91.7% of cases had an hyperinflammed phenotype compared with 13.6% of controls (Table 2 ).
Table 2.
Prevalence of hyperinflammation among controls and cases.
| Controls n = 24 | Cases n = 24 | P-value | |
|---|---|---|---|
| Hyperinflammatory syndromea, n (%) | 3 (13.6%) | 22 (91.7%) | <0.001 |
| Ferritin >500 pg/mL, n (%) | 5 (26.3%) | 21 (95.5%) | <0.001 |
| C-reactive protein >3 mg/dL, n (%) | 9 (40.9%) | 23 (95.8%) | <0.001 |
| Lactate dehydrogenase >300, UI, n (%) | 0 (0%) | 17 (70.8%) | <0.001 |
| d-dimer >1000 ng/mL, n (%) | 7 (36.8%) | 18 (78.3%) | 0.006 |
| Lymphocyte count <1000/mm3, n (%) | 6 (27.3%) | 22 (91.7%) | <0.001 |
Hyperinflammation was defined as the presence of at least three of the following characteristics at the most impaired blood test during hospitalization: (1) lymphocyte count <1000 cells/mm3; (2) ferritin >500 ng/mL; (3) lactate dehydrogenase >300 U/L; (4) d-dimer >1000 ng/mL; and (5) C-reactive protein >3 mg/dL.
Therefore, apart from age, as expected, the case and control groups had different clinical, radiological and laboratory features.
Primary outcome
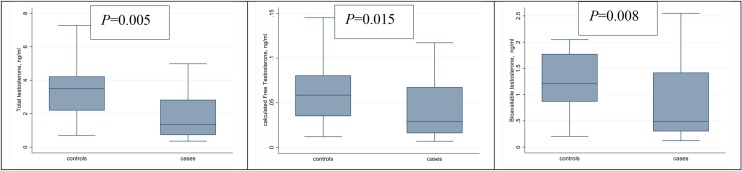
The differences in serum concentrations of sex hormones at hospital admission between cases and controls are shown in Table 3 . Testosterone was the only sex hormone that showed a significant difference between cases and controls. TT, cFT and BioT levels were lower in cases compared with controls (Figure 1). Median TT in cases was 1.4 ng/mL [interquartile range (IQR) 0.7–2.8], compared with 3.5 ng/mL (IQR 2.2–4.2) in controls ( P = 0.005). Median BioT in cases was 0.49 (IQR 0.30–1.42) compared with 1.21 (IQR 0.87–1.78) in controls (P = 0.008). Median cFT in cases was 0.029 ng/mL (IQR 0.016–0.068) compared with 0.058 ng/mL (IQR 0.035–0.081) in controls (P = 0.015). Interestingly, the TT level was so low that secondary transitory hypogonadism, according to the reference values of the Mayo Clinic Laboratories (TT < 240 ng/dL), was present in 66.7% of cases and 29.2% of controls (P = 0.009). Concentration of the other sex hormones did not differ significantly between cases and controls.
Table 3.
Comparison of serum hormone levels between cases and controls at admission.
| Controls n = 24 | Cases n = 24 | P-value | |
|---|---|---|---|
| Total testosterone (ng/mL) | 3.5 (2.2–4.2) | 1.4 (0.7–2.8) | 0.005 |
| Androstenedione (pg/mL) | 1.21 (0.84–1.55) | 0.86 (0.74–1.35) | 0.232 |
| 5α-dihydrotestosterone (pg/mL) | 335 (290–459) | 489 (318–633) | 0.112 |
| Oestradiol (pg/mL) | 30 (25–34) | 28 (25–37) | 0.959 |
| Prostatic serum antigen (ng/mL) | 0.79 (0.41–1.43) | 0.66 (0.44–0.93) | 0.477 |
| Sex hormone binding globulin (nmol/L) | 43.5 (31.1–56.4) | 31.3 (21.6–48.3) | 0.110 |
Values are median (interquartile range).
Figure 1.
Comparison of serum levels of total testosterone, calculated free testosterone and bioavailable testosterone between cases and controls.
Secondary outcomes
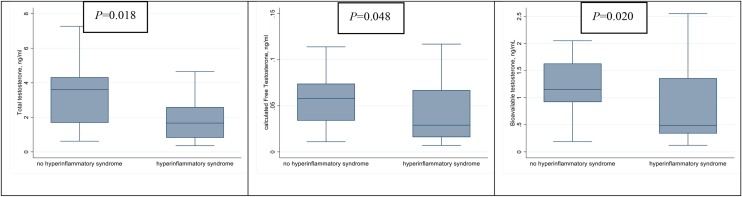
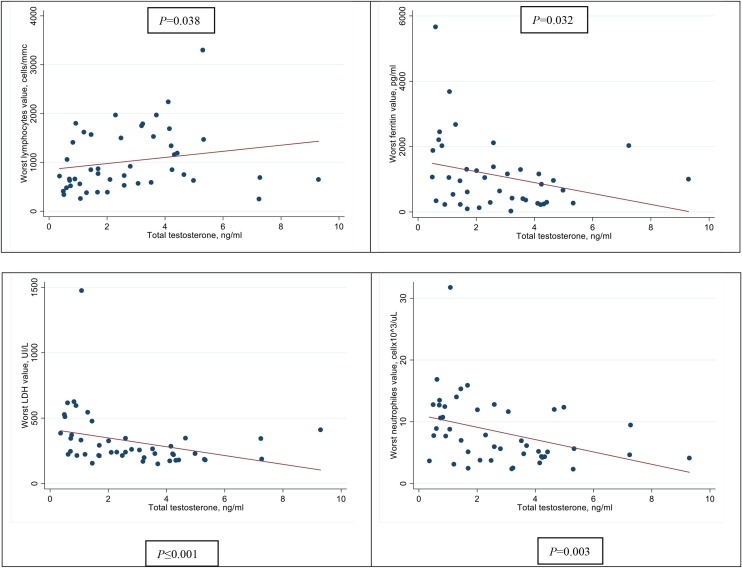
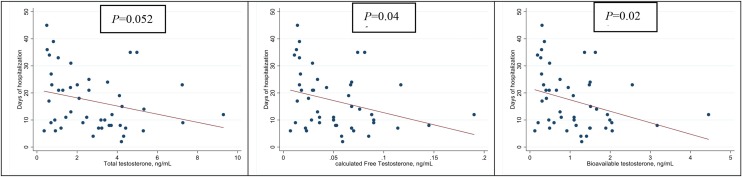
The associations between TT, cFT, BioT and the development of hyperinflammatory syndrome are shown in Figure 2 . As can be seen, median TT was significantly lower in patients who developed hyperinflammatory syndrome compared with those who had a low inflammatory response [1.7 ng/mL (IQR 0.80–2.60) vs. 3.6 ng/mL (IQR 1.70–4.30); P = 0.018]. This was also found for cFT [0.03 pg/mL (IQR 0.02–0.07) vs. 0.06 pg/mL (IQR 0.03–0.07); P = 0.048] and BioT [0.5 ng/mL (IQR 0.3–1.4) vs. 1.2 ng/mL (IQR 0.9–1.60); P = 0.020]. Furthermore, lower levels of TT, cFT and BioT were significantly correlated with some inflammatory markers using the most impaired blood sample for each patient. Lower TT was significantly correlated with lower lymphocyte count (rho = 0.31, P = 0.038); higher ferritin (−0.34, P = 0.032); higher LDH (−0.49, P < 0.001) and higher neutrophil count (rho = −0.43, P = 0.003) (Figure 3 ). Additionally, lower cFT serum concentration was significantly correlated with higher neutrophil count (rho = −0.35, P = 0.016) and higher LDH (rho = −0.42, P = 0.004). Similarly, lower BioT was significantly correlated with higher neutrophil count (rho = −0.39, P = 0.007) and higher LDH (rho = −0.44, P = 0.002). TT, cFT and BioT values showed inverse correlation with length of hospital stay (P = 0.052, P = 0.041 and P = 0.023, respectively) (Figure 4 ).
Figure 2.
Comparison of serum levels of total testosterone, calculated free testosterone and bioavailable testosterone according to presence or absence of hyperinflammatory syndrome.
Figure 3.
Correlation between total testosterone and serum inflammatory markers.
Figure 4.
Correlation between total testosterone, calculated free testosterone, bioavailable testosterone and length of hospital stay.
No association between sex hormone level and risk of OTI/death was detected [hazard ratio (HR) 0.49 per 1 ng/mL of TT (95% CI 0.19–1.28), P = 0.147; HR 0.29 per 1 ng/mL of cFT (95% CI 0.12–7.14), P = 0.105; HR 0.28 per 1 ng/mL of BioT (95% CI 0.03–2.24), P = 0.229]. Interestingly, none of the tested hormones were associated with time to swab clearance [HR per 1 ng/mL of TT 1.16 (95% CI 0.98–1.3), P = 0.078; HR per 1 ng/mL of cFT 1.00 (95% CI 1.00–1.01), P = 0.309; HR per 1 ng/mL of BioT 1.27 (95% CI 0.86–1.86), P = 0.224]. The only parameter that was correlated with the duration of viral clearance was the proportion of healthy lung volume (rho −0.58, P < 0.001).
Discussion
This single-centre, retrospective, case–control study found that low serum testosterone levels were related to severe COVID-19. In contrast, levels of 5α-DHT, Δ4, oestradiol, IGF-1 and PSA were not correlated with disease severity. This study also showed that low TT was significantly related to the hyperinflammatory response (P = 0.018), as well as certain blood severity markers: LDH (rho = −0.49, P < 0.001); ferritin (rho = −0.34, P = 0.032) and lymphocyte count (rho = 0.31, P = 0.038). cFT and BioT presented the same performance but with less consistency. As expected, low TT (rho = −28, P = 0.052), low cFT (rho = −30, P = 0.041) and BioT (rho = −33, P = 0.023) were associated with longer length of hospital stay.
These findings are in accordance with a retrospective study conducted by Rastrelli et al. (2021) of 31 male patients with COVID-19 admitted to a subintensive respiratory department. They showed that both TT and cFT levels showed a significant and progressive decline according to worsening outcomes in men with COVID-19. A steep increase in ICU transfer and mortality risk was observed in men with TT <5 nmol/L or cFT <100 pmol/L. In agreement with the present findings, Rastrelli et al. (2021) found a significant negative correlation between TT and cFT and biochemical risk factors (i.e, neutrophil count, LDH, procalcitonin, CRP, ferritin), and a positive correlation with lymphocyte count.
The mean time for upper respiratory viral clearance was, on average, 6.5 days longer for cases than controls (P = 0.079). Despite this, none of the tested hormones were found to be associated with the duration of viral shedding, although an inverse association trend was shown for TT [HR 1.16 (95% CI 0.98–1.36), P = 0.078]. This may be because the sample size was too small to study the outcome. No correlation was found between the tested hormones and the percentage of damaged lung volume (TT: rho = 0.31, P = 0.065; cFT: rho = 0.26, P = 0.123; BioT: rho = 0.25, P = 0.137).
The only parameter that was found to be correlated with the time for swab clearance was the proportion of healthy lung volume (rho = −0.58, P < 0.001).
Several epidemiological studies have revealed a difference in the severity of COVID-19 by gender, without a prevalence gap (Jacquinet et al., 2001, Hoffmann et al., 2020, Jin et al., 2020). This has been observed previously for severe acute respiratory syndrome coronavirus-1, Middle East respiratory syndrome coronavirus, HCoV-299E, H7N9 and H1N1 viruses (www.genenames.org). The biological explanation may lie primarily in the role of the TMPRSS2 receptor, a serin protease expressed over the surface of type II pneumocytes and other epithelial cells (e.g. gut, prostate, pancreas, lives and kidney). TMPRSS2 receptors play a key role in COVID-19: first, TMPRSS2 primes the SARS-CoV-2 spike protein, activating it for direct virus-cell fusion and reducing viral recognition by neutralizing antibodies; second, TMPRSS2 promotes endocytosis of the virus in the target cell by cleavage, and consequent activation, of ACE2 receptors (Wambier et al., 2020).
Interestingly, the androgen receptor is the main activator of transcription of the TMPRSS2 gene in humans (Lucas et al., 2014, Stopsack et al., 2020; Anon, 2020). Accordingly, androgen sensitivity, determined by haplotypes of the androgen receptor and their expressivity, can explain racial and interindividual susceptibility to severe COVID-19 (Baldassarri et al., 2021). This assumption is supported by the observations that androgenetic alopecia, a phenotypic expression of an androgen-dependent trait, seems to predispose to severe COVID-19 symptoms (CDC COVID-19 Response Team, 2020, Wambier et al., 2020), and that the clinical picture of COVID-19 is less severe before puberty (Shi et al., 2020). Evidence for the risk of infection in patients with prostate cancer receiving androgen deprivation therapy is conflicting (Montopoli et al., 2020). Accordingly, the inhibition of TMPRSS2 activity by Camostat has been studied in 21 clinical trials to date, and anti-androgen treatment with proxalutamide has been shown to improve the prognosis for patients with mild-to-moderate COVID-19 (Cadegiani et al., 2021).
Unexpectedly, a recent study did not show an impactful discrepancy by sex in TMPRSS2 protein expression in human lung secretory cells and club cells. However, higher TMPRSS2 and ACE2 co-expression was detected in male pneumocytes I/II compared with female cells. Therefore, the interaction between the two receptors could be a significant contributor to the sex bias (Song et al., 2020). It has been shown that testosterone enhances ACE2 levels, both transcriptionally and post-translationally (Chanana et al., 2020), while oestrogen underexpresses ACE2 (Jin et al., 2020). In addition, female sex hormones and the immune stimulatory genes present on the X chromosome may impart lesser infectivity and mortality of SARS-CoV-2 in males with complex neuro-endocrino-immunological mechanisms (Chanana et al., 2020). The present data revealed no differences in mean oestrogen blood levels between controls and cases [30 pg/mL (95% CI 25–34) vs. 28 pg/mL (95% CI 25–37), respectively; P = 0.959], but the mechanism could act at an epigenetic level.
It could be hypothesized that patients with more severe COVID-19 would present with higher levels of serum testosterone, but the opposite was found in this study. One of the possible explanations for this finding is that a transient status of primary hypogonadism can develop as a consequence of direct damage of the epithelium of the testis by SARS-CoV-2 (Douglas et al., 2004, Song et al., 2020). Genetic and immunoblot analysis have indicated that ACE2 and TMPRSS2 are highly expressed over the surface of testis cells (Douglas et al., 2004, Song et al., 2020). This theory is also supported by a Chinese study conducted in 81 male patients with COVID-19 which showed a reducing trend in total testosterone in the serum of infected patients compared with the general population, matched for age (Ma et al., 2020). Another possible explanation is that a low level of testosterone could be one of the expressions of the complex interaction between the host and the virus, which, through the effectors of hyperinflammatory syndrome, may alter the central regulation of gonadal function. Ma et al. (2020) found that the ratio of testosterone to luteinizing hormone and the ratio of follicle-stimulating hormone to luteinizing hormone were dramatically decreased in males with COVID-19 as a marker of transient hypogonadism. A decrease in testosterone serum level has been documented in various non-infective acute syndromes, such as myocardial infarction, with an unclear causal mechanism, supporting a pro-thrombotic state (Pugh et al., 2002).
Curiously, in patients with COVID-19, neither semen parameters nor the existence of SARS-CoV-2 in semen were detected. This would represent more straightforward evidence for testes injury caused by SARS-CoV-2. Moreover, the hypothesis of the direct epithelium damage of the testes by SARS-COV-2 needs to be proved (Pan et al., 2020).
Other local mechanisms may co-operate to reduce steroidogenesis during COVID-19. For example, as reported for H7N9 virus (Schroeder et al., 2020), a high local level of some cytokines (e.g. tumour necrosis factor-alpha) may inhibit defined hormonal pathways. It is interesting to note that a low testosterone level may worsen COVID-19 by sustaining pro-thrombotic and pro-inflammatory conditions (Pugh et al., 2002). This is in line with the present finding that a low level of testosterone was correlated with high levels of inflammatory markers.
To summarize, in the authors’ opinion, hypogonadism in most patients with severe COVID-19 may be a transitory status caused by direct damage to the testes by the virus. Taking TT into account, primary hypogonadism was present in 66.7% of cases and 29.2% of controls (P = 0.009). Neither cases nor controls reported symptoms of hypogonadism at hospital admission. These data may be overestimates, considering that TT acheaves its serum peack in the early morning and decreases in the afternoon (Brambilla et al., 2009). In this study 41 patients had a blood sample drawn in the early morning and seven patients had a blood sample drawn in the afternoon (five cases and two controls), with a balance distribution between the two groups (P = 0.220).
The median value of PSA was only slightly lower in cases compared with controls [0.79 (IQR 0.41–1.43) vs. 0.66 (IQR 0.44–0.93), P = 0.477]. This trend is unlikely to be a direct consequence of a pre-existing gonadic insufficiency.
Interestingly, no correlation between sex hormones and time to OTI/death was found. This is likely to be due to the small sample size and the low incidence of the investigated events.
This study has several limitations. First, as it was a retrospective study, the serum sex hormone level before COVID-19 could not be measured. Second, changes in testosterone serum concentration throughout the course of COVID-19 were not studied. Third, it was not possible to measure gonadotropin levels for the few stored plasma samples available. Fourth, the study had a small sample size. To reduce confounders, the authors chose to narrow the patients’ age spectrum and preserve a high difference in clinical presentation between the case and control groups.
This study presents several biases. First, a low testosterone level can be an independent marker for biological immunosenescence. BioT deficiency is jointly correlated with chronic systemic inflammation (Douglas et al., 2004). Second, in patients with COVID-19, other factors such as stress and corticosteroid therapy may also influence the hypothalamo–pituitary–gonadal axis.
In conclusion, this study underlined the dual role of testosterone in COVID-19 as a marker of severe pneumonia and a key pathogenic factor. On one hand, low TT is predictive of severe COVID-19, is related to a longer hospital stay and is associated with a burst in hyper-inflammatory syndrome, probably due to direct damage to the testes by SARS-CoV-2. On the other hand, androgen sensitivity is an important, subjective and inter-racial key factor determining the virulence of SARS-CoV-2.
Further studies are needed to understand the complexity of SARS-CoV-2 pathogenesis.
Conflict of interest statement
None declared.
Funding
This work was supported by Line one — Ricerca Corrente ‘Infezioni Emergenti e Riemergenti’ and Progetto COVID 2020 12371675, both funded by the Italian Ministry of Health.
Ethical approval
The INMI COVID-19 database was approved by the local INMI, Rome Ethical Committee. The INMI COVID-19 database includes epidemiological, demographic, clinical and laboratory data for patients, as well as therapy prescribed for COVID-19. All patients gave informed consent for blood withdrawal (study approved by the Institutional Review Board of INMI Lazzaro Spallanzani, approval number 9/2020) and for using their data for research purposes. The study was performed in accordance with the Declaration of Helsinki.
Authors’ contributions
MC conceived and wrote the paper. PZ contributed to the conceptualization and editing of the manuscript. PL performed statistical analysis and produced the figures. LS and LC performed plasma analysis. VS, EP and FDS performed thoracic CT scans and edited the manuscript. CP, SC, EN, NP, FP, GDO, LM, RG, RB, SC, GI, EG, FV and AA supervised and edited the manuscript. All authors read and approved the final manuscript.
Acknowledgments
Members of the INMI ReCOVeRI Study Group: Maria Alessandra Abbonizio, Amina Abdeddaim, Elisabetta Agostini, Chiara Agrati, Fabrizio Albarello, Gioia Amadei, Alessandra Amendola, Andrea Antinori, Maria Assunta Antonica, Mario Antonini, Tommaso Ascoli Bartoli, Francesco Baldini, Raffaella Barbaro, Barbara Bartolini, Rita Bellagamba, Martina Benigni, Nazario Bevilacqua, Gianluigi Biava, Michele Bibas, Licia Bordi, Veronica Bordoni, Evangelo Boumis, Marta Branca, Rosanna Buonomo, Donatella Busso, Marta Camici, Paolo Campioni, Flaminia Canichella, Maria Rosaria Capobianchi, Alessandro Capone, Cinzia Caporale, Emanuela Caraffa, Ilaria Caravella, Fabrizio Carletti, Concetta Castilletti, Adriana Cataldo, Stefano Cerilli, Carlotta Cerva, Roberta Chiappini, Pierangelo Chinello, Maria Assunta Cianfarani, Carmine Ciaralli, Claudia Cimaglia, Nicola Cinicola, Veronica Ciotti, Stefania Cicalini, Francesca Colavita, Angela Corpolongo, Massimo Cristofaro, Salvatore Curiale, Alessandra D’Abramo, Cristina Dantimi, Alessia De Angelis, Giada De Angelis, Maria Grazia De Palo, Federico De Zottis, Virginia Di Bari, Rachele Di Lorenzo, Federica Di Stefano, Gianpiero D’Offizi, Davide Donno, Francesca Evangelista, Francesca Faraglia, Anna Farina, Federica Ferraro, Lorena Fiorentini, Andrea Frustaci, Matteo Fusetti, Vincenzo Galati, Roberta Gagliardini, Paola Gallì, Gabriele Garotto, Ilaria Gaviano, Saba Gebremeskel Tekle, Maria Letizia Giancola, Filippo Giansante, Emanuela Giombini, Guido Granata, Maria Cristina Greci, Elisabetta Grilli, Susanna Grisetti, Gina Gualano, Fabio Iacomi, Marta Iaconi, Giuseppina Iannicelli, Carlo Inversi, Giuseppe Ippolito, Eleonora Lalle, Maria Elena Lamanna, Simone Lanini, Daniele Lapa, Luciana Lepore, Raffaella Libertone, Raffaella Lionetti, Giuseppina Liuzzi, Laura Loiacono, Andrea Lucia, Franco Lufrani, Manuela Macchione, Gaetano Maffongelli, Alessandra Marani, Luisa Marchioni, Andrea Mariano, Maria Cristina Marini, Micaela Maritti, Annelisa Mastrobattista, Ilaria Mastrorosa, Giulia Matusali, Valentina Mazzotta, Paola Mencarini, Silvia Meschi, Francesco Messina, Sibiana Micarelli, Giulia Mogavero, Annalisa Mondi, Marzia Montalbano, Chiara Montaldo, Silvia Mosti, Silvia Murachelli, Maria Musso, Michela Nardi, Assunta Navarra, Emanuele Nicastri, Martina Nocioni, Pasquale Noto, Roberto Noto, Alessandra Oliva, Ilaria Onnis, Sandrine Ottou, Claudia Palazzolo, Emanuele Pallini, Fabrizio Palmieri, Giulio Palombi, Carlo Pareo, Virgilio Passeri, Federico Pelliccioni, Giovanna Penna, Antonella Petrecchia, Ada Petrone, Nicola Petrosillo, Elisa Pianura, Carmela Pinnetti, Maria Pisciotta, Pierluca Piselli, Silvia Pittalis, Agostina Pontarelli, Costanza Proietti, Vincenzo Puro, Paolo Migliorisi Ramazzini, Alessia Rianda, Gabriele Rinonapoli, Silvia Rosati, Dorotea Rubino, Martina Rueca, Alberto Ruggeri, Alessandra Sacchi, Alessandro Sampaolesi, Francesco Sanasi, Carmen Santagata, Alessandra Scarabello, Silvana Scarcia, Vincenzo Schininà, Paola Scognamiglio, Laura Scorzolini, Giulia Stazi, Giacomo Strano, Fabrizio Taglietti, Chiara Taibi, Giorgia Taloni, Tetaj Nardi, Roberto Tonnarini, Simone Topino, Martina Tozzi, Francesco Vaia, Francesco Vairo, Maria Beatrice Valli, Alessandra Vergori, Laura Vincenzi, Ubaldo Visco-Comandini, Serena Vita, Pietro Vittozzi, Mauro Zaccarelli, Antonella Zanetti and Sara Zito.
References
- Anon. TMPRSS2 transmembrane serine protease 2 [Homo sapiens (human)]. https://www.genenames.org/https://www.ncbi.nlm.nih.gov/gene/7113.
- Baldassarri M., Picchiotti N., Fava F., Fallerini C., Benetti E., Daga S. Shorter androgen receptor polyQ alleles protect against life-threatening COVID-19 disease in European males. EbioMedicine. 2021;65 doi: 10.1016/j.ebiom.2021.103246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanco-Melo D., Nilsson-Payant B.E., Liu W.C., Uhl S., Hoagland D., Moller R. Imbalanced host response to SARS-CoV-2 drives development of COVID-19. Cell. 2020;181 doi: 10.1016/j.cell.2020.04.026. 1036–45e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brambilla D.J., Matsumoto A.M., Araujo A.B., McKinlay J.B. The effect of diurnal variation on clinical measurement of serum testosterone and other sex hormone levels in men. J Clin Endocrinol Metab. 2009;94(3):907–913. doi: 10.1210/jc.2008-1902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cadegiani F.A., McCoy J., Gustavo Wambier C., Vano-Galvan S., Shapiro J., Tosti A. Proxalutamide significantly accelerates viral clearance and reduces time to clinical remission in patients with mild to moderate COVID-19: results from a randomized, double-blinded, placebo-controlled trial. Cureus. 2021;13 doi: 10.7759/cureus.13492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CDC COVID-19 Response Team Coronavirus disease 2019 in children — United States, February 12–April 2, 2020. MMWR Morb Mortal Wkly Rep. 2020;69:422–426. doi: 10.15585/mmwr.mm6914e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chanana N., Palmo T., Sharma K., Kumar R., Graham B.B., Pasha Q. Sex-derived attributes contributing to SARS-CoV-2 mortality. Am J Physiol Endocrinol Metab. 2020;319 doi: 10.1152/ajpendo.00295.2020. E562–E7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douglas G.C., O’Bryan M.K., Hedger M.P., Lee D.K., Yarski M.A., Smith A.I. The novel angiotensin-converting enzyme (ACE) homolog, ACE2, is selectively expressed by adult Leydig cells of the testis. Endocrinology. 2004;145:4703–4711. doi: 10.1210/en.2004-0443. [DOI] [PubMed] [Google Scholar]
- Force A.D.T., Ranieri V.M., Rubenfeld G.D., Thompson B.T., Ferguson N.D., Caldwell E. Acute respiratory distress syndrome: the Berlin definition. JAMA. 2012;307:2526–2533. doi: 10.1001/jama.2012.5669. [DOI] [PubMed] [Google Scholar]
- Global Health 5050. The COVID-19 sex-disaggregated data tracker. Available at: https://globalhealth5050.org/the-sex-gender-and-covid-19-project/the-data-tracker/.
- Hoffmann M., Kleine-Weber H., Schroeder S., Kruger N., Herrler T., Erichsen S. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. 2020;181 doi: 10.1016/j.cell.2020.02.052. 271–80e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwata-Yoshikawa N., Okamura T., Shimizu Y., Hasegawa H., Takeda M., Nagata N. TMPRSS2 contributes to virus spread and immunopathology in the airways of murine models after coronavirus infection. J Virol. 2019;93 doi: 10.1128/JVI.01815-18. e01815–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacquinet E., Rao N.V., Rao G.V., Zhengming W., Albertine K.H., Hoidal J.R. Cloning and characterization of the cDNA and gene for human epitheliasin. Eur J Biochem. 2001;268:2687–2699. doi: 10.1046/j.1432-1327.2001.02165.x. [DOI] [PubMed] [Google Scholar]
- Jin J.M., Bai P., He W., Wu F., Liu X.F., Han D.M. Gender differences in patients with COVID-19: focus on severity and mortality. Front Public Health. 2020;8:152. doi: 10.3389/fpubh.2020.00152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucas J.M., Heinlein C., Kim T., Hernandez S.A., Malik M.S., True L.D. The androgen-regulated protease TMPRSS2 activates a proteolytic cascade involving components of the tumor microenvironment and promotes prostate cancer metastasis. Cancer Discov. 2014;4:1310–1325. doi: 10.1158/2159-8290.CD-13-1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma L., Wen X., Li D., Shi L., Mao Y., Xiong Y. Effect of SARS-CoV-2 infection upon male gonadal function: a single center-based study. medRXiv. 2020 doi: 10.1101/2020.03.21.20037267. preprint. [DOI] [Google Scholar]
- Montopoli M., Zumerle S., Vettor R., Rugge M., Zorzi M., Catapano C.V. Androgen-deprivation therapies for prostate cancer and risk of infection by SARS-CoV-2: a population-based study (N = 4532) Ann Oncol. 2020;31:1040–1045. doi: 10.1016/j.annonc.2020.04.479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan F., Xiao X., Guo J., Song Y., Li H., Patel D.P. No evidence of severe acute respiratory syndrome-coronavirus 2 in semen of males recovering from coronavirus disease 2019. Fertil Steril. 2020;113(6):1135–1139. doi: 10.1016/j.fertnstert.2020.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pugh P.J., Channer K.S., Parry H., Downes T., Jone T.H. Bio-available testosterone levels fall acutely following myocardial infarction in men: association with fibrinolytic factors. Endocr Res. 2002;28:161–173. doi: 10.1081/erc-120015055. [DOI] [PubMed] [Google Scholar]
- Rastrelli G., Di Stasi V., Inglese F., Beccaria M., Garuti M., Di Costanzo D. Low testosterone levels predict clinical adverse outcomes in SARS-CoV-2 pneumonia patients. Andrology. 2021;9:88–98. doi: 10.1111/andr.12821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rod J.E., Oviedo-Trespalacios O., Cortes-Ramirez J. A brief-review of the risk factors for COVID-19 severity. Rev Saude Publica. 2020;54:60. doi: 10.11606/s1518-8787.2020054002481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroeder M., Schaumberg B., Müller Z., Parplys A., Jarczak D., Nierhaus A. Sex hormone and metabolic dysregulations are associated with critical illness in male Covid-19 patients. medRxiv. 2020 preprint. [Google Scholar]
- Seeland U., Coluzzi F., Simmaco M., Mura C., Bourne P.E., Heiland M. Evidence for treatment with estradiol for women with SARS-CoV-2 infection. BMC Med. 2020;18:369. doi: 10.1186/s12916-020-01851-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi Y., Yu X., Zhao H., Wang H., Zhao R., Sheng J. Host susceptibility to severe COVID-19 and establishment of a host risk score: findings of 487 cases outside Wuhan. Crit Care. 2020;24:108. doi: 10.1186/s13054-020-2833-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirato K., Kanou K., Kawase M., Matsuyama S. Clinical isolates of human coronavirus 229E bypass the endosome for cell entry. J Virol. 2016;91:e01387–16. doi: 10.1128/JVI.01387-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song H., Seddighzadeh B., Cooperberg M.R., Huang F.W. Expression of ACE2, the SARS-CoV-2 receptor, and TMPRSS2 in prostate epithelial cells. Eur Urol. 2020;78:296–298. doi: 10.1016/j.eururo.2020.04.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stopsack K.H., Mucci L.A., Antonarakis E.S., Nelson P.S., Kantoff P.W. TMPRSS2 and COVID-19: serendipity or opportunity for intervention? Cancer Discov. 2020;10:779–782. doi: 10.1158/2159-8290.CD-20-0451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vermeulen A., Verdonck L., Kaufman J.M. A critical evaluation of simple methods for the estimation of free testosterone in serum. J Clin Endocrinol Metab. 1999;84:3666–3672. doi: 10.1210/jcem.84.10.6079. [DOI] [PubMed] [Google Scholar]
- Wambier C.G., Vano-Galvan S., McCoy J., Gomez-Zubiaur A., Herrera S., Hermosa-Gelbard A. Androgenetic alopecia present in the majority of patients hospitalized with COVID-19: the “Gabrin sign”. J Am Acad Dermatol. 2020;83:680–682. doi: 10.1016/j.jaad.2020.05.079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu C., Chen X., Cai Y., Xia J., Zhou X., Xu S. Risk factors associated with acute respiratory distress syndrome and death in patients with coronavirus disease 2019 pneumonia in Wuhan, China. JAMA Intern Med. 2020;180:934–943. doi: 10.1001/jamainternmed.2020.0994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou F., Yu T., Du R., Fan G., Liu Y., Liu Z. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395:1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]