Abstract
Background
Some studies have suggested that women with SARS-CoV-2 infection during pregnancy are at increased risk of adverse pregnancy and neonatal outcomes, but these associations are still not clear.
Objective
This study aimed to determine the association between SARS-CoV-2 infection at the time of birth and maternal and perinatal outcomes.
Study Design
This is a population-based cohort study in England. The inclusion criteria were women with a recorded singleton birth between May 29, 2020, and January 31, 2021, in a national database of hospital admissions. Maternal and perinatal outcomes were compared between pregnant women with a laboratory-confirmed SARS-CoV-2 infection recorded in the birth episode and those without. Study outcomes were fetal death at or beyond 24 weeks’ gestation (stillbirth), preterm birth (<37 weeks’ gestation), small for gestational age infant (small for gestational age; birthweight at the <tenth centile), preeclampsia or eclampsia, induction of labor, mode of birth, specialist neonatal care, composite neonatal adverse outcome indicator, maternal and neonatal length of hospital stay after birth (3 days or more), and 28-day neonatal and 42-day maternal hospital readmission. Adjusted odds ratios and their 95% confidence interval for the association between SARS-CoV-2 infection status and outcomes were calculated using logistic regression, adjusting for maternal age, ethnicity, parity, preexisting diabetes mellitus, preexisting hypertension, and socioeconomic deprivation measured using the Index of Multiple Deprivation 2019. Models were fitted with robust standard errors to account for hospital-level clustering. The analysis of the neonatal outcomes was repeated for those born at term (≥37 weeks’ gestation) because preterm birth has been reported to be more common in pregnant women with SARS-CoV-2 infection.
Results
The analysis included 342,080 women, of whom 3527 had laboratory-confirmed SARS-CoV-2 infection. Laboratory-confirmed SARS-CoV-2 infection was more common in women who were younger, of non-White ethnicity, primiparous, or residing in the most deprived areas or had comorbidities. Fetal death (adjusted odds ratio, 2.21; 95% confidence interval, 1.58–3.11; P<.001) and preterm birth (adjusted odds ratio, 2.17; 95% confidence interval, 1.96–2.42; P<.001) occurred more frequently in women with SARS-CoV-2 infection than those without. The risk of preeclampsia or eclampsia (adjusted odds ratio, 1.55; 95% confidence interval, 1.29–1.85; P<.001), birth by emergency cesarean delivery (adjusted odds ratio, 1.63; 95% confidence interval, 1.51–1.76; P<.001), and prolonged admission after birth (adjusted odds ratio, 1.57; 95% confidence interval, 1.44–1.72; P<.001) were significantly higher for women with SARS-CoV-2 infection than those without. There were no significant differences (P>.05) in the rate of other maternal outcomes. The risk of neonatal adverse outcome (adjusted odds ratio, 1.45; 95% confidence interval, 1.27–1.66; P<.001), need for specialist neonatal care (adjusted odds ratio, 1.24; 95% confidence interval, 1.02–1.51; P=.03), and prolonged neonatal admission after birth (adjusted odds ratio, 1.61; 95% confidence interval, 1.49–1.75; P<.001) were all significantly higher for infants with mothers with laboratory-confirmed SARS-CoV-2 infection. When the analysis was restricted to pregnancies delivered at term (≥37 weeks), there were no significant differences in neonatal adverse outcome (P=.78), need for specialist neonatal care after birth (P=.22), or neonatal readmission within 4 weeks of birth (P=.05). Neonates born at term to mothers with laboratory-confirmed SARS-CoV-2 infection were more likely to have prolonged admission after birth (21.1% compared with 14.6%; adjusted odds ratio, 1.61; 95% confidence interval, 1.49–1.75; P<.001).
Conclusion
SARS-CoV-2 infection at the time of birth is associated with higher rates of fetal death, preterm birth, preeclampsia, and emergency cesarean delivery. There were no additional adverse neonatal outcomes, other than those related to preterm delivery. Pregnant women should be counseled regarding risks of SARS-CoV-2 infection and should be considered a priority for vaccination.
Key words: birth, COVID-19, fetal death, neonatal outcome, obstetrics, preeclampsia, pregnancy, preterm birth, stillbirth
Introduction
COVID-19, caused by SARS-CoV-2, has spread rapidly around the world since the first reported case in late 2019. Studies from registries of pregnant women and single- or multi-center cohorts have reported that pregnant women with COVID-19 are at a greater risk than nonpregnant women of childbearing age with COVID-19 of requiring intensive care unit support and severe morbidity and mortality.1, 2, 3 Delivery may improve maternal condition in women with severe COVID-19, leading to an observed increase in preterm birth and neonatal unit admission for infants of infected mothers.1 , 4, 5, 6 In the general population, advanced age, obesity, minority ethnic origin, socioeconomic deprivation, and comorbidities including diabetes mellitus and hypertensive disease are associated with a higher risk of severe disease, a pattern which is also seen in pregnant women.1 , 7 Neonatal SARS-CoV-2 infection has not been associated with adverse outcomes for the newborn.8
AJOG at a Glance.
Why was this study conducted?
This study aimed to determine the association between SARS-CoV-2 infection and maternal and perinatal outcomes, in the context of universal screening of women giving birth in England.
Key findings
Women who tested positive for SARS-CoV-2 at birth had increased rates of fetal death, preterm birth, preeclampsia, emergency cesarean delivery, and other adverse maternal and neonatal outcomes.
What does this add to what is known?
SARS-CoV-2 infection at the time of birth is associated with a higher rate of fetal death and preterm birth and other adverse maternal and neonatal outcomes. An observed increase in rates of adverse neonatal outcomes was attributed to increased preterm birth.
A recent international registry study demonstrated an increase in adverse maternal and neonatal outcomes for mothers infected with COVID-19 in pregnancy,4 and a study using national data from Sweden demonstrated an increase in adverse neonatal outcomes for infants born to women with SARS-CoV-2 infection, a finding largely mediated by increased rates of preterm birth.9
We aimed to investigate maternal and perinatal outcomes of pregnant women with SARS-CoV-2 infection in England using data available from routinely collected electronic healthcare records.
Materials and Methods
Study design
This study is a national population-based cohort study using Hospital Episode Statistics (HES) data from May 29, 2020, to January 31, 2021. HES contains records of all inpatient admissions to National Health Service (NHS) hospitals in England including data on patient demographics (age, sex, and ethnicity), the admission (date of admission and discharge), and clinical information. On the May 29, 2020, the Royal College of Obstetricians and Gynaecologists recommended universal screening of all women admitted to maternity services with a polymerase chain reaction (PCR) test, in line with recommendations from NHS England to test all hospital admissions.10 , 11
Diagnostic information is coded using the International Classification of Diseases, tenth revision (ICD-10).12 Operative procedures are described using the United Kingdom Office for Population Censuses and Surveys Classification of Surgical Operations and Procedures, fourth revision (OPCS-4).13 Further details about the labor and birth are captured in the episode record (eg, gestational age, birthweight) in supplemental data fields known as the HES “maternity tail.” HES data are sufficiently accurate to be used for research and managerial decision making.14
Cohort selection and outcome definitions
The inclusion criteria were women who had a HES record of a singleton birth between May 29, 2020, and January 31, 2021. HES includes births which occur in NHS hospitals and hospital-associated community care in England. Only 0.3% of births in England in 2020 occurred in non-NHS organizations.15
A maternity episode was defined as any record that contained valid information about mode of birth in either the procedure fields (OPCS-4 codes: R17.1 to R25.9) or the HES maternity tail. Multiple births, which were excluded, were defined as maternity episodes with an ICD code for a multiple birth (Z37.2–Z37.7) or strong evidence of a multiple birth in the maternity tail (more than one distinct birthweight, birth order, and infant recorded in the same birth episode). A neonatal episode was defined as any record that contained a newborn, defined as being less than 1 day of age at episode onset. Maternal and neonatal episodes were linked using encrypted versions of the mother’s and infant’s NHS number (a unique national identifier for each individual NHS user, assigned at birth),16 available in the NHS Birth Notifications data. These data also contained additional information on the birth such as gestational age and birthweight.15 , 17
A woman was classified as having laboratory-confirmed SARS-CoV-2 infection at the time of birth if the ICD-10 code “COVID-19, virus identified” (U07.1) was recorded in the birth episode.18 The test used to confirm infection in NHS hospital admissions is a nasal or throat swab examined using PCR.11
The study outcomes derived for the cohort identified by the maternity episode included fetal death at or beyond 24 weeks’ gestation (stillbirth), preterm birth (less than 37 weeks, liveborn or stillborn), small for gestational age at birth (SGA; defined as birthweight at the <tenth centile using the United Kingdom–World Health Organization pediatric growth charts19), maternal diagnosis with preeclampsia or eclampsia, induction of labor, mode of birth (unassisted vaginal delivery, instrumental vaginal delivery, elective cesarean delivery, and emergency cesarean delivery), maternal length of stay (3 or more days), and 42-day readmission. The study outcomes derived for the linked maternal-neonatal cohort included the provision of specialist neonatal care, neonatal length of stay (3 or more days), 28-day readmission, and a composite neonatal adverse outcome indicator, which includes 16 diagnoses and 7 procedures and has previously been validated in HES.20 The definitions and coding of all study outcomes are specified in Supplemental Table. This dataset does not contain sufficient information to distinguish between antepartum and intrapartum fetal death (stillbirth); in England in 2018 (the latest date for which this information is available), 9 in every 10 stillbirths were antepartum.21
Maternal age was grouped into 5-year periods, with women under 20 and over 40 years being aggregated into single categories. Parity was defined using records of previous births through a “look-back” approach in HES, and handled in 3 categories: primiparous, multiparous without previous cesarean delivery, and multiparous with previous cesarean delivery.22 , 23 Maternal ethnicity was coded using the Office for National Statistics (ONS) categorization system from the 2001 Census and collapsed into 4 groups: White, South Asian, Black, and other stated. Information about preexisting diabetes mellitus and hypertension was available in the diagnosis codes attached to the birth episode, with women assumed not to have the condition if the code was not present. Index of Multiple Deprivation 2019 (IMD) provides an overall measure of multiple deprivation derived from information about income, education, employment, crime, and the living environment. IMD rankings of 32,844 “Lower Super Output Areas,” with typically 1500 inhabitants, were used to categorize women into 5 socioeconomic groups.24
Statistical analysis
Characteristics of women in the cohort with and without laboratory-confirmed SARS-CoV-2 infection at the time of birth were tabulated. Rates of maternal and perinatal outcomes were calculated in women with and without laboratory-confirmed SARS-CoV-2 infection at the time of birth. Adjusted odds ratios (aORs) and their 95% confidence interval (CI) for the association between SARS-CoV-2 infection status and outcomes were calculated using logistic regression, adjusting for maternal age, ethnicity, parity, preexisting diabetes mellitus, preexisting hypertension, and socioeconomic deprivation measured using IMD. Models were fitted with robust standard errors to account for hospital-level clustering. The analysis of the neonatal outcomes was repeated for those born at term (at or beyond 37 weeks’ gestation) because preterm birth has been reported to be more common in pregnant women with SARS-CoV-2 infection.
Data were complete for all variables except maternal ethnicity (89.1% complete) and IMD (99.4% complete). For regression analyses, missing values of ethnicity and IMD were imputed using chained equations to generate 10 datasets; estimates from these datasets were pooled using Rubin’s rules.25 Stata 16 (StataCorp, College Station, TX) was used for all analyses. A P value of less than .05 was assumed to represent statistical significance.
Ethical approval
This study used data collected to evaluate service provision and performance and therefore was exempt from ethical review by the NHS Health Research Authority. The use of personal data without individual consent was approved by the NHS Health Research Authority (16/CAG/0058).
Results
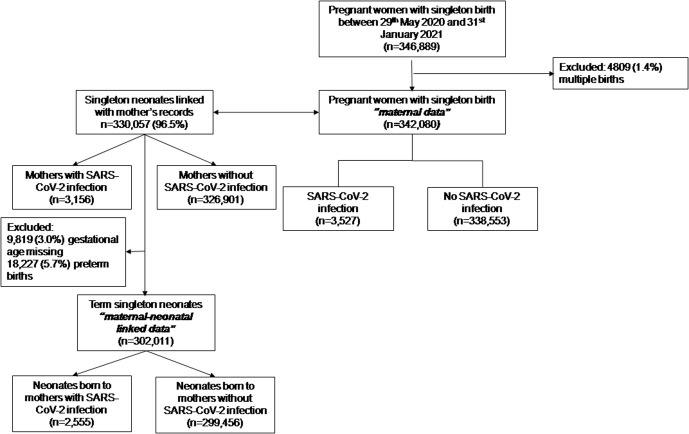
The analysis included 342,080 women with singleton pregnancy who gave birth in England between May 29, 2020, and January 31, 2021, of whom 3527 (10.3 per 1000) were recorded as having laboratory-confirmed SARS-CoV-2 infection (Figure , Table 1 ). Laboratory-confirmed SARS-CoV-2 infection was more likely in younger women, women from non-White ethnicity, those with preexisting diabetes mellitus and preexisting hypertension, and women residing in the most socioeconomically deprived areas (Table 1).
Figure.
Flow chart
Study flowchart.
Gurol-Urganci et al. Maternal and perinatal outcomes of pregnant women with SARS-CoV-2. Am J Obstet Gynecol 2021.
Table 1.
Characteristics and study outcomes of women included in the study
| Pregnant women without laboratory-confirmed SARS-CoV-2 infection at the time of birth | Pregnant women with laboratory-confirmed SARS-CoV-2 infection at the time of birth | P value (chi-squared test) | |
|---|---|---|---|
| Number of births | 338,553 (100) | 3527 (100) | |
| Maternal age, y | <.001 | ||
| ≤19 | 8907 (2.6) | 94 (2.7) | |
| 20–24 | 44,755 (13.2) | 581 (16.5) | |
| 25–29 | 93,051 (27.5) | 1040 (29.5) | |
| 30–34 | 114,639 (33.9) | 1079 (30.6) | |
| 35–39 | 62,451 (18.5) | 587 (16.6) | |
| 40+ | 14,750 (4.4) | 146 (4.1) | |
| Maternal ethnicitya | <.001 | ||
| White | 230,202 (76.3) | 1857 (58.5) | |
| South Asian | 36,834 (12.2) | 768 (24.2) | |
| Black | 13,998 (4.6) | 251 (7.9) | |
| Other | 20,546 (6.8) | 298 (9.4) | |
| Obstetrical history | .13 | ||
| Primiparous | 142,289 (42.0) | 1514 (42.9) | |
| Multiparous with no previous cesarean deliveryb | 156,269 (46.2) | 1634 (46.3) | |
| Multiparous with previous cesarean deliveryb | 39,995 (11.8) | 379 (10.8) | |
| Preexisting diabetes mellitus | 3112 (0.9) | 58 (1.6) | <.001 |
| Preexisting hypertension | 2624 (0.8) | 44 (1.3) | .002 |
| Index of Multiple Deprivationa | <.001 | ||
| 1=least deprived | 50,814 (15.1) | 342 (9.8) | |
| 2 | 57,892 (17.2) | 413 (11.8) | |
| 3 | 65,104 (19.3) | 602 (17.2) | |
| 4 | 75,159 (22.3) | 874 (25.0) | |
| 5=most deprived | 87,703 (26.1) | 1265 (36.2) |
Values are number (percentage) unless indicated otherwise.
IMD, Index of Multiple Deprivation 2019.
Gurol-Urganci et al. Maternal and perinatal outcomes of pregnant women with SARS-CoV-2. Am J Obstet Gynecol 2021.
Ethnicity missing in 37,326 (10.9%) of records, IMD missing in 1912 (0.6%) of records; % may not add to 100 owing to rounding
Cesarean delivery.
Table 2 shows that fetal death was significantly more common in women with laboratory-confirmed SARS-CoV-2 infection at the time of birth (30 of 3527 or 8.5 per 1000) than in those without (1140 of 338,553 or 3.4 per 1000; aOR, 2.21; 95% CI, 1.58–3.11; P<.001). There was also a significant increase in the risk of preterm birth (5.8% in women without laboratory-confirmed SARS-CoV-2 infection; 12.1% in those with laboratory-confirmed SARS-CoV-2 infection; aOR, 2.17; 95% CI, 1.96–2.42; P<.001). Women with laboratory-confirmed SARS-CoV-2 infection were at an increased risk of preeclampsia or eclampsia (3.9% compared with 2.5%; aOR, 1.55; 95% CI, 1.29–1.85; P<.001), and emergency cesarean delivery (27.6% compared with 18.5%; aOR, 1.63; 95% CI, 1.51–1.76; P<.001), with a corresponding reduction in the rate of spontaneous vaginal delivery (49.2% compared with 54.6% in women without laboratory-confirmed SARS-CoV-2 infection; aOR, 0.80; 95% CI, 0.75–0.86). Rates of elective cesarean delivery (10.8% compared with 13.8%; aOR, 0.81; 95% CI, 0.71–0.91; P<.001) were lower in women with laboratory-confirmed SARS-CoV-2 infection than in those without. After birth, women with SARS-CoV-2 infection were at an increased risk of hospital admission lasting 3 days or more (25.8% compared with 17.0%; aOR, 1.57; 95% CI, 1.44–1.72; P<.001) and readmission within 6 weeks after birth (4.3% compared with 3.1%; aOR, 1.39; 95% CI, 1.10–1.76; P=.01) than those without. No significant differences were seen in the rates of SGA (P=.87), induction of labor (P=.40), or instrumental vaginal delivery (P=.20).
Table 2.
Comparison of study outcomes between pregnant women with and without laboratory-confirmed SARS-CoV-2 infection (International Classification of Diseases, tenth revision U07.1) at the time of birth
| Pregnant women without SARS-CoV-2 infection |
Pregnant women with laboratory-confirmed SARS-CoV-2 infection |
Unadjusted OR (95% CI) | P value | Adjusted ORa (95% CI) | P value | |||
|---|---|---|---|---|---|---|---|---|
| Cases/births | % | Cases/births | % | |||||
| Maternal data | ||||||||
| Fetal death | 1140/338,553 | 0.34 | 30/3527 | 0.85 | 2.54 (1.81–3.56) | <.001 | 2.21 (1.58–3.11) | <.001 |
| Preterm birth | 18,572/322,494 | 5.8 | 369/3047 | 12.1 | 2.25 (2.03–2.50) | <.001 | 2.17 (1.96–2.42) | <.001 |
| Small for gestational age | 17,521/320,188 | 5.5 | 191/3009 | 6.4 | 1.17 (1.00–1.37) | .05 | 0.99 (0.84–1.16) | .87 |
| Preeclampsia or eclampsia | 8591/338,553 | 2.5 | 139/3527 | 3.9 | 1.58 (1.32–1.89) | <.001 | 1.55 (1.29–1.85) | <.001 |
| Induction of labor | 96,651/236,822 | 40.8 | 940/2382 | 39.5 | 0.95 (0.82–1.08) | .42 | 0.95 (0.83–1.08) | .40 |
| Elective cesarean delivery | 46,843/338,553 | 13.8 | 380/3527 | 10.8 | 0.75 (0.67–0.85) | <.001 | 0.81 (0.71–0.91) | <.001 |
| Emergency cesarean delivery | 62,479/338,553 | 18.5 | 975/3527 | 27.6 | 1.69 (1.56–1.83) | <.001 | 1.63 (1.51–1.76) | <.001 |
| Instrumental vaginal delivery | 43,393/338,553 | 12.9 | 422/3527 | 12.0 | 0.92 (0.83–1.03) | .14 | 0.93 (0.82–1.04) | .20 |
| Unassisted delivery | 184,989/338,553 | 54.6 | 1734/3527 | 49.2 | 0.80 (0.75–0.86) | <.001 | 0.76 (0.70–0.82) | <.001 |
| Maternal length of stay (3+d) | 55,529/326,248 | 17.0 | 857/3321 | 25.8 | 1.70 (1.55–1.85) | <.001 | 1.57 (1.44–1.72) | <.001 |
| Maternal readmission (42-d) | 8660/281,178 | 3.1 | 78/1818 | 4.3 | 1.41 (1.11–1.78) | .004 | 1.39 (1.10–1.76) | .01 |
| Maternal-neonatal linked data | ||||||||
| E-NAOIb | 16,501/318,073 | 5.2 | 222/2922 | 7.6 | 1.50 (1.32–1.72) | <.001 | 1.45 (1.27–1.66) | <.001 |
| Specialist neonatal care | 35,032/326,901 | 10.7 | 432/3156 | 13.7 | 1.32 (1.04–1.67) | .02 | 1.24 (1.02–1.51) | .03 |
| Neonatal length of stay (3+d) | 58,410/324,665 | 18.0 | 857/3104 | 27.6 | 1.74 (1.62–1.87) | <.001 | 1.61 (1.49–1.75) | <.001 |
| Neonatal readmission (28 d) | 14,259/277,804 | 5.1 | 126/2058 | 6.1 | 1.21 (1.01–1.44) | .04 | 1.18 (0.98–1.41) | .08 |
| Maternal-neonatal linked data of deliveries at term (≥37 wk) | ||||||||
| E-NAOIb | 9970/298,099 | 3.3 | 89/2542 | 3.5 | 1.05 (0.85–1.29) | .45 | 1.03 (0.84–1.27) | .78 |
| Specialist neonatal care | 28,002/299,456 | 9.4 | 294/2555 | 11.5 | 1.26 (0.92–1.73) | .15 | 1.18 (0.90–1.55) | .22 |
| Neonatal length of stay (3+d) | 43,390/297,805 | 14.6 | 534/2530 | 21.1 | 1.56 (1.42–1.74) | <.001 | 1.61 (1.49–1.75) | <.001 |
| Neonatal readmission (28 d) | 12,749/262,437 | 4.9 | 106/1802 | 5.9 | 1.22 (1.02–1.47) | .03 | 1.20 (1.00–1.45) | .05 |
Birth with any of the following: birthweight of <1500 g, gestational age under 32 completed weeks, neonatal death within 28 days, respiratory distress syndrome, seizure, intraventricular hemorrhage (grade 3 or 4), cerebral infarction, periventricular leukomalacia, birth trauma (intracranial hemorrhage paralysis owing to brachial plexus injury, skull or long bone fracture), hypoxic ischemic encephalopathy, necrotizing enterocolitis, sepsis/septicemia, pneumonia, respiratory disease (respiratory failure, primary atelectasis, chronic respiratory disease originating in the perinatal period, bacterial meningitis, resuscitation (intubation/chest compression), mechanical ventilation/continuous positive airway pressure/high flow nasal oxygen, central venous or arterial catheter, pneumothorax requiring intercostal catheter, any intravenous fluids, any body cavity surgical procedure, therapeutic hypothermia.
CI, confidence interval; E-NAOI, neonatal adverse outcome indicator; IMD, Index of Multiple Deprivation 2019; OR, odds ratio.
Gurol-Urganci et al. Maternal and perinatal outcomes of pregnant women with SARS-CoV-2. Am J Obstet Gynecol 2021.
Adjusted for maternal age, ethnicity, socioeconomic deprivation measured by IMD, parity, previous cesarean delivery, diabetes and hypertension
Composite outcome.
Of the 342,080 maternity records, 330,057 (96.5%) were linked to the neonatal record (Figure). Risk of neonatal adverse outcome (aOR, 1.45; 95% CI, 1.27–1.66; P<.001), need for specialist neonatal care (aOR, 1.24; 95% CI, 1.02–1.51; P=.03), and prolonged neonatal admission after birth (aOR, 1.61; 95% CI, 1.49–1.75; P<.001) were all significantly higher for infants with mothers with laboratory-confirmed SARS-CoV-2 infection than those without (Table 2). When the analysis was restricted to pregnancies delivered at term (≥37 weeks), there were no significant differences in neonatal adverse outcome (P=.78), need for specialist neonatal care after birth (P=.22), or neonatal readmission within 4 weeks of birth (P=.05) (Table 2). Term infants born to mothers with laboratory-confirmed SARS-CoV-2 infection had prolonged admission after birth (21.1% compared with 14.6%; aOR, 1.61; 95% CI, 1.49–1.75; P<.001) (Table 2).
Comment
Principal findings
In this population-based study of women giving birth to a singleton infant in England in 2020–2021, we report that women with a record of laboratory-confirmed SARS-CoV-2 infection at the time of birth were more than twice as likely as women without SARS-CoV-2 infection to have fetal death or preterm birth. Women with SARS-CoV-2 infection were also more likely to have preeclampsia and to give birth by emergency cesarean delivery. Both women and their neonates were more likely to have prolonged hospital stay of 3 days or more, and mothers were more likely to be readmitted to hospital in the postnatal period. There was no significant difference in rates of induction of labor, instrumental vaginal delivery, or SGA between women who did and did not have SARS-CoV-2 infection at the time of birth. The composite neonatal adverse outcome and specialist neonatal care were higher in pregnancies with SARS-CoV-2 infection at the time of birth. However, when the analysis was restricted to term deliveries, neonatal outcomes were similar for those born to mothers with and without SARS-CoV-2 infection.
Results in the context of what is known
Our findings concur with those of an ongoing living systematic review which estimates the pooled association between COVID-19 and fetal death at an OR of 2.84 (95% CI, 1.25–6.45),1 with a more recent multinational case-control study which reports an association between COVID-19 and a composite neonatal adverse outcome of a risk ratio of 2.14 (95% CI, 1.66–2.754), and with a recent population-level study reporting an increase in adverse neonatal outcomes for infants born to women with COVID-19 infection.9 However, the systematic review is limited by the size and number of studies available, with only 9 women experiencing a stillbirth in the COVID-19 group of the pooled dataset,1 and the case-control study was unable to report on fetal death alone, instead incorporating it into an adverse outcome including intrauterine or neonatal death, prolonged neonatal stay, or severe neonatal morbidity.4 In the population-level study, as in our study, almost all of the association between maternal COVID-19 infection and adverse neonatal outcome was explained by increased risk of preterm birth.9 In our study, we were not able to stratify preterm birth into spontaneous and indicated or iatrogenic (where birth is initiated by the clinician); other studies have suggested that the increase in preterm birth is caused by indicated delivery to improve maternal condition.1
The key potential bias in our study comes from misclassification of the exposure; this could be caused by selective testing (whether the chance of a woman having been tested for SARS-CoV-2 was dependent on her pregnancy outcome), selective recording (whether the chance of a woman who tested positive had that result recorded in HES was dependent on her pregnancy outcome), or missed cases (women who had SARS-CoV-2 infection but were not recorded as such).
It is unlikely that either selective testing or recording fully explain our results. First, throughout the pandemic, there was a statutory requirement to report cases of SARS-CoV-2 infection in healthcare settings.26 Second, the laboratory-confirmed SARS-CoV-2 infection rate of 1.96% between October 1, 2020, and January 31, 2021 (when national data are available and could be compared), which we observed in all women giving birth in this period is very close to the SARS-CoV-2 infection rate of 1.74% (and within the credible intervals of 1.53% to 1.98%) reported for people between 25 and 35 years old by the ONS for the period October 3, 2020, to January 22, 2021, based on a routine national survey of households27; this provides evidence that universal testing of maternity admissions was fully implemented during this period.28 The slightly higher rate may be attributed to women of childbearing age likely to be living with children and to be required to leave the house to interact with healthcare providers.29
These results provide further evidence that SARS-CoV-2 infection increases the risk of fetal death. The potential mechanisms may be pregnancy specific, including placental disease with reports of abnormal inflammation of the placenta in association with maternal COVID-19.30 , 31 However, the association may also be a more generic consequence of severe maternal illness in pregnancy, given that women who become seriously unwell with other illnesses are known to be at a higher risk of perinatal morbidity and mortality.32
Our findings related to the characteristics of women infected with SARS-CoV-2, and associations with other complications including preeclampsia, preterm birth, cesarean delivery, and adverse neonatal outcomes concur with other studies in the United Kingdom and internationally.1 , 4 Our results regarding length of stay and maternal readmissions are novel, but also relate to the context of care in England, where much of postnatal maternity care is provided in the community.28
Clinical and research implications
The finding that women with a recorded SARS-CoV-2 infection at the time of birth may have an increased risk of fetal death and other adverse maternal and perinatal outcomes concurs with a recent international case-control study4 and will be of particular concern to pregnant women and healthcare professionals. The overall numbers of fetal deaths are too small to impact the overall national rate of stillbirth in the United Kingdom, as seen in provisional national reports for 2020.33 Therefore, it is important to carefully contextualize these findings when counseling pregnant women.
However, this finding should prompt reflection on the treatment of pregnant women infected with SARS-CoV-2 and the relative risks and benefits of vaccination. For pregnant women who have a positive test result for SARS-CoV-2 in the later stages of pregnancy, care should consider the well-being of the baby. At term, acknowledgment of the increased risk of fetal death may prompt discussion of the potential risks of ongoing expectant management of pregnancy, and consideration of an earlier planned birth.
For women earlier in pregnancy, our findings may change the risk-benefit analysis for vaccination. At present, data on the safety and efficacy of COVID-19 vaccination in pregnancy are limited owing to the exclusion of pregnant women in clinical trials,34 although trials are now underway to address this urgent need. This has motivated widespread hesitancy about recommendation of vaccination to all pregnant women, with governments and professional organizations initially recommending offering vaccination to pregnant women at high risk of either occupational exposure or severe disease35 and pregnant women reluctant to take up a vaccine offer.36 In the United States and Israel, where vaccination has been recommended to those at a higher risk, initial data provide a positive signal of safety and efficacy in pregnant women.33 , 37 , 38 Further evidence of a link between SARS-CoV-2 infection and an increased risk of fetal death may motivate prioritization of, and encourage pregnant women to access, vaccination.
Strengths and limitations
The main strengths of this study are its large size and representative nature, covering almost the entire population of births in England during the time period. The use of HES data to understand maternity outcomes is well established and offers rich information about individual women to allow for adjustment for individual risk.23
The principal exposure of SARS-CoV-2 infection is defined using an ICD-10 code recorded if the woman had a laboratory-confirmed infection. The use of ICD-10 codes in this way to understand differences between admissions with and without SARS-CoV-2 infection has been established elsewhere.9 , 39
The use of administrative data including diagnostic and procedure codes to establish exposures and outcomes (including in our study preeclampsia, neonatal adverse outcome, and SARS-CoV-2 status) has inherent limitations because the primary purpose of data recording is for payment rather than clinical research; known limitations include underrecording and misclassification.40 This may particularly affect preeclampsia where there is a variation in diagnostic criteria and thresholds; gestational hypertension may be conflated with preeclampsia.41
Although in our study we were able to adjust for many potential confounders, we had no information on the severity of COVID-19 illness or maternal body mass index in our dataset. Maternal obesity is a risk factor for both severe COVID-19 and fetal death.1 , 42 Therefore, it is possible that the observed association could be partially accounted for by differences between groups of women.
Our results should be strictly interpreted as being related to the result of a test for SARS-CoV-2 at the time of birth, rather than to any infection which occurred during pregnancy. This is an important feature given that some of the observations in women who have a positive test result for SARS-CoV-2, especially the increases in the risk of stillbirth and preterm birth in women with a positive test, may be partly explained by variations in the rate of SARS-CoV-2 infection according to gestational age. This is different from other studies which seek to understand effects on women who are infected with SARS-CoV-2 at any point during their pregnancy and from studies which assess population risks of fetal death measuring both direct and indirect effects.43, 44, 45
Conclusions
Our results demonstrate that women who have laboratory-confirmed infection with SARS-CoV-2 at the time of birth have higher rates of fetal death and preterm birth, preeclampsia and emergency cesarean delivery, and prolonged maternal and neonatal admission after birth than those without SARS-CoV-2 infection. There were no additional adverse neonatal outcomes, other than those related to preterm delivery. These findings should guide the counseling of pregnant women about risks of SARS-CoV-2 infection during pregnancy and indicate that pregnant women should be prioritized for vaccination.
Acknowledgments
All authors interpreted the data. I.G.U. and J.E.J. had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. All authors revised the paper critically for important intellectual content and provided final approval of the submitted manuscript.
Footnotes
The authors I.G.U. and J.E.J. are joint first authors.
The authors J.V.D.M. and A.K. are joint senior authors.
Healthcare Quality Improvement Partnership program grant (National Maternity and Perinatal Audit). The funder had no involvement in the design of the study, interpretation of the data, writing of the manuscript, or approval of the manuscript for publication. The National Maternity and Perinatal Audit is commissioned by the Healthcare Quality Improvement Partnership (HQIP; www.hqip.org.uk) as part of the National Clinical Audit and Patient Outcomes Programme and funded by NHS England and the Scottish and Welsh Governments.
All authors except J.V.D.M., T.D., and E.M. receive full or partial salary funding provided through the Healthcare Quality Improvement Partnership to the Royal College of Obstetricians and Gynaecologists (RCOG). E.M., T.D., J.E.J., and L.W. are members of the RCOG COVID-19 guidance cell which produces clinical guidance and policy documents to support the management of pregnant women during the pandemic in the United Kingdom.
Cite this article as: Gurol-Urganci I, Jardine JE, Carroll F, et al. Maternal and perinatal outcomes of pregnant women with SARS-CoV-2 infection at the time of birth in England: national cohort study. Am J Obstet Gynecol 2021;225:522.e1-11.
Supplementary Data
Appendix
Supplemental Table.
Definitions of study outcomes and their coding in Hospital Episode Statistics
| Outcome | Numerator/coding | Denominator/coding |
|---|---|---|
| Using maternal data: | ||
| Stillbirth (fetal death) | Defined using ICD-10 code (Z37.1) or birth status field (birstat_1=2,3,4) in maternity tail for providers with over 95% data completeness. | All singleton births |
| In the United Kingdom stillbirth is defined as birth without signs of life occurring at or after 24+0 completed gestational weeks, based on estimated due date calculated using universally offered ultrasound scan at 11–13 weeks’ gestation. | This dataset does not contain sufficient information to distinguish between antepartum and intrapartum stillbirth; in England in 2018 (the latest date for which this information is available), 9 in every 10 stillbirths were antepartum.21 | |
| Preterm birth | Defined using gestational age field in HES maternity tail (gestat_1<37) | All singleton births, excluding records missing information on gestational age |
| Small for gestational age | Defined as less than the tenth birthweight centile using the UK-WHO charts.19 Birthweight centiles are calculated using birthweight (birweit_1), gestational age (gestat_1), sex of baby (sexbaby_1) fields in maternity tail | All singleton births, excluding records missing information on gestational age, birthweight or sex of baby |
| Preeclampsia or eclampsia | Defined using the ICD-10 codes O14 (preeclampsia) and O15 (eclampsia) | All singleton births |
| Induction of labor | Defined using the delivery onset field (delonset=3,4,5) from the maternity tail. Failed induction (ICD-10 code O61) is also included in the numerator as this represents intention to treat. | All singleton births, excluding elective cesarean delivery; and records missing information on delivery onset |
| Elective cesarean delivery (ELC) | ELC is defined using OPCS code R17 | All singleton births |
| Emergency cesarean delivery (EMCS) | EMCS is defined using OPCS codes R18/R25.1 | All singleton births |
| Instrumental delivery | Instrumental birth is defined using OPCS codes R21/R22 | All singleton births |
| Unassisted delivery | Unassisted birth is defined using OPCS code R23/R24 | All singleton births |
| Maternal length of stay after birth (3 or more d) | Length of stay is defined as the number of days between date of discharge and date of admission for the birth episode. | All singleton births with nonmissing date of discharge information and date of delivery before January 28, 2021 (to allow for 3-d follow-up) |
| Maternal readmission (42 d) | Maternal readmission is defined as unplanned, overnight readmission to hospital within 42 d of giving birth, excluding those accompanying an unwell baby. Mothers readmitted with the following admission method codes: 21, 22, 23, 24, 28, 2A, 2B, 2D, 31, 32, 82, 83 within 42 days of birth. | All singleton births with nonmissing date of discharge information and date of delivery before December 19, 2020 (to allow for 6-wk follow-up). Women who died before discharge or were not discharged within 42 d of delivery were excluded. |
| Using maternal-neonatal linked data: | ||
| Neonatal specialist care | Neonatal specialist care is defined using the “neocare” variable in HES, and includes values 1=Special care: care given in a special nursery, transitional care ward or postnatal ward, which provides care and treatment exceeding normal routine care; 2 = Level 2 intensive care (high dependency intensive care); and 3 = Level 1 intensive care (maximal intensive care) | All singleton, term births with nonmissing information on neonatal specialist care |
| Neonatal adverse outcome indicator (E-NAOI) | E-NAOI is defined as births with any of the following outcomes: birthweight<1500 g, gestational age under 32 completed weeks, neonatal death within 28 d, respiratory distress syndrome, seizure, intraventricular hemorrhage (grade 3 or 4), cerebral infarction, periventricular leukomalacia, birth trauma (intracranial hemorrhage paralysis owing to brachial plexus injury, skull or long bone fracture), hypoxic ischemic encephalopathy, necrotizing enterocolitis, sepsis/septicemia, pneumonia, respiratory disease (respiratory failure, primary atelectasis, chronic respiratory disease originating in the perinatal period, bacterial meningitis, resuscitation (intubation/chest compression), mechanical ventilation/CPAP/high flow nasal oxygen, central venous or arterial catheter, pneumothorax requiring intercostal catheter, any intravenous fluids, any body cavity surgical procedure, therapeutic hypothermia. Coding of these diagnoses and procedures can be found in Knight et al,20 2018, Supplemental Table. |
All liveborn singleton term births with nonmissing information on gestational age and birthweight |
| Neonatal length of stay after birth (3 or more d) | Length of stay is defined as the number of days between date of discharge and date of admission for the birth episode. | All singleton births with nonmissing date of discharge information and date of birth before January 28, 2021 (to allow for 3-d follow-up) |
| Neonatal readmission (28 d) | Neonatal readmission is defined as unplanned, overnight readmission to hospital within 28 d of birth, excluding those accompanying an unwell mother. Babies readmitted with the following admission method codes: 21, 22, 23, 24, 28, 2A, 2B, 2D, 31, 32, 82, 83 within 28 days of birth. | All singleton neonates with nonmissing date of discharge information and date of birth before January 3, 2021 (to allow for four-week follow-up). Babies who died before discharge or were not discharged within 28 d of birth were excluded. |
CPAP, continuous positive airway pressure therapy; ICD-10, International Classification of Diseases, tenth revision; OPCS, Censuses and Surveys Classification of Surgical Operations and Procedures; UK-WHO, United Kingdom–World Health Organization.
Gurol-Urganci et al. Maternal and perinatal outcomes of pregnant women with SARS-CoV-2. Am J Obstet Gynecol 2021.
References
- 1.Allotey J., Stallings E., Bonet M., et al. Clinical manifestations, risk factors, and maternal and perinatal outcomes of coronavirus disease 2019 in pregnancy: living systematic review and meta-analysis. BMJ. 2020;370:m3320. doi: 10.1136/bmj.m3320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.DeBolt C.A., Bianco A., Limaye M.A., et al. Pregnant women with severe or critical coronavirus disease 2019 have increased composite morbidity compared with nonpregnant matched controls. Am J Obstet Gynecol. 2021;224:510.e1–510.e12. doi: 10.1016/j.ajog.2020.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hantoushzadeh S., Shamshirsaz A.A., Aleyasin A., et al. Maternal death due to COVID-19. Am J Obstet Gynecol. 2020;223:109.e1–109.e16. doi: 10.1016/j.ajog.2020.04.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Villar J., Ariff S., Gunier R.B., et al. Maternal and neonatal morbidity and mortality Among pregnant women with and without COVID-19 infection: The INTERCOVID Multinational Cohort Study. JAMA Pediatr. 2021 doi: 10.1001/jamapediatrics.2021.1050. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vousden N., Bunch K., Morris E., et al. The incidence, characteristics and outcomes of pregnant women hospitalized with symptomatic and asymptomatic SARS-CoV-2 infection in the UK from March to September 2020: a national cohort study using the UK Obstetric Surveillance System (UKOSS) PLoS One. 2021;16 doi: 10.1371/journal.pone.0251123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mullins E., Hudak M.L., Banerjee J., et al. Pregnancy and neonatal outcomes of COVID-19: coreporting of common outcomes from PAN-COVID and AAP-SONPM registries. Ultrasound Obstet Gynecol. 2021;57:573–581. doi: 10.1002/uog.23619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Williamson E.J., Walker A.J., Bhaskaran K., et al. Factors associated with COVID-19-related death using OpenSAFELY. Nature. 2020;584:430–436. doi: 10.1038/s41586-020-2521-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gale C., Quigley M.A., Placzek A., et al. Characteristics and outcomes of neonatal SARS-CoV-2 infection in the UK: a prospective national cohort study using active surveillance. Lancet Child Adolesc Health. 2021;5:113–121. doi: 10.1016/S2352-4642(20)30342-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Norman M., Navér L., Söderling J., et al. Association of maternal SARS-CoV-2 infection in pregnancy with neonatal outcomes. JAMA. 2021 doi: 10.1001/jama.2021.5775. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Royal College of Obstetricians and Gynaecologists Principles for the testing and triage of women seeking maternity care in hospital settings, during the COVID-19 pandemic. 2020. https://www.rcog.org.uk/globalassets/documents/guidelines/2020-05-29-principles-for-the-testing-and-triage-of-women-seeking-maternity-care-in-hospital-settings-during-the-covid-19-pandemic.pdf Available at:
- 11.Public Health England Healthcare associated COVID-19 infections: further action. 2020. https://www.england.nhs.uk/coronavirus/wp-content/uploads/sites/52/2020/06/Healthcare-associated-COVID-19-infections--further-action-24-June-2020.pdf Available at:
- 12.World Health Organization International Statistical Classification of Diseases and Related Health Problems 10th Revision. 2016. https://icd.who.int/browse10/2016/en Available at: Accessed April 15, 2021.
- 13.OPCS classification of interventions and procedures (OPCS-4) https://www.datadictionary.nhs.uk/web_site_content/supporting_information/clinical_coding/opcs_classification_of_interventions_and_procedures.asp Available at:
- 14.Burns E.M., Rigby E., Mamidanna R., et al. Systematic review of discharge coding accuracy. J Public Health (Oxf) 2012;34:138–148. doi: 10.1093/pubmed/fdr054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Provisional births in England and Wales—Office for National Statistics. 2021. https://www.ons.gov.uk/peoplepopulationandcommunity/birthsdeathsandmarriages/livebirths/articles/provisionalbirthsinenglandandwales/2020#:∼:text=Based%20on%20birth%20notification%20data,most%20recent%20peak%20in%202012 Available at:
- 16.national health service. Digital. What is an NHS number? https://www.nhs.uk/using-the-nhs/about-the-nhs/what-is-an-nhs-number/ Available at:
- 17.national health service. Digital. Birth notification service. https://digital.nhs.uk/services/birth-notification-service Available at:
- 18.World Health Organization Emergency use ICD codes for COVID-19 disease outbreak. https://www.who.int/standards/classifications/classification-of-diseases/emergency-use-icd-codes-for-covid-19-disease-outbreak Available at:
- 19.Cole T.J., Williams A.F., Wright C.M., RCPCH Growth Chart Expert Group Revised birth centiles for weight, length and head circumference in the UK-WHO growth charts. Ann Hum Biol. 2011;38:7–11. doi: 10.3109/03014460.2011.544139. [DOI] [PubMed] [Google Scholar]
- 20.Knight H.E., Oddie S.J., Harron K.L., et al. Establishing a composite neonatal adverse outcome indicator using English hospital administrative data. Arch Dis Child Fetal Neonatal Ed. 2019;104:F502–F509. doi: 10.1136/archdischild-2018-315147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Draper E.S., Gallimore I.D., Smith L.K., et al. MBRRACE-UK perinatal mortality surveillance report, UK perinatal deaths for births from January to December 2018. 2020. https://www.npeu.ox.ac.uk/assets/downloads/mbrrace-uk/reports/perinatal-surveillance-report-2018/MBRRACE-UK_Perinatal_Surveillance_Report_2018_-_final_v3.pdf Available at: Accessed April 15, 2021.
- 22.Cromwell D.A., Knight H.E., Gurol-Urganci I. Parity derived for pregnant women using historical administrative hospital data: accuracy varied among patient groups. J Clin Epidemiol. 2014;67:578–585. doi: 10.1016/j.jclinepi.2013.10.011. [DOI] [PubMed] [Google Scholar]
- 23.Knight H.E., Gurol-Urganci I., van der Meulen J.H., et al. Vaginal birth after caesarean section: a cohort study investigating factors associated with its uptake and success. BJOG. 2014;121:183–192. doi: 10.1111/1471-0528.12508. [DOI] [PubMed] [Google Scholar]
- 24.Department for community and Local Government The English indices of Deprivation 2015 statistical release. 2015. https://www.gov.uk/government/statistics/english-indices-of-deprivation-2015 Available at: Accessed April 15, 2021.
- 25.White I.R., Royston P., Wood A.M. Multiple imputation using chained equations: issues and guidance for practice. Stat Med. 2011;30:377–399. doi: 10.1002/sim.4067. [DOI] [PubMed] [Google Scholar]
- 26.COVID-19: infection prevention and control. 2020. https://www.gov.uk/government/publications/wuhan-novel-coronavirus-infection-prevention-and-control Available at:
- 27.Coronavirus (COVID-19) infections in the community in the UK. Office for National Statistics. 2020. https://www.ons.gov.uk/peoplepopulationandcommunity/healthandsocialcare/conditionsanddiseases/datasets/coronaviruscovid19infectionsinthecommunityinengland Available at:
- 28.Royal College of Obstetricians and Gynaecologists Coronavirus (COVID-19) infection and pregnancy. 2021. https://www.rcog.org.uk/en/guidelines-research-services/guidelines/coronavirus-pregnancy/ Available at: Accessed April 15, 2021.
- 29.Forbes H., Morton C.E., Bacon S., et al. Association between living with children and outcomes from covid-19: OpenSAFELY cohort study of 12 million adults in England. BMJ. 2021;372:n628. doi: 10.1136/bmj.n628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schwartz D.A., Baldewijns M., Benachi A., et al. Chronic histiocytic intervillositis with trophoblast necrosis is a risk factor associated with placental infection from coronavirus disease 2019 (COVID-19) and intrauterine maternal-fetal severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) transmission in live-born and stillborn infants. Arch Pathol Lab Med. 2021;145:517–528. doi: 10.5858/arpa.2020-0771-SA. [DOI] [PubMed] [Google Scholar]
- 31.Patberg E.T., Adams T., Rekawek P., et al. Coronavirus disease 2019 infection and placental histopathology in women delivering at term. Am J Obstet Gynecol. 2021;224:382.e1–382.e18. doi: 10.1016/j.ajog.2020.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mengistu T.S., Turner J.M., Flatley C., Fox J., Kumar S. The impact of severe maternal morbidity on perinatal outcomes in high income countries: systematic review and meta-analysis. J Clin Med. 2020;9:2035. doi: 10.3390/jcm9072035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shimabukuro T.T., Kim S.Y., Myers T.R., et al. Preliminary findings of mRNA Covid-19 vaccine safety in pregnant persons. N Engl J Med. 2021 doi: 10.1056/NEJMoa2104983. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dashraath P., Nielsen-Saines K., Madhi S.A., Baud D. COVID-19 vaccines and neglected pregnancy. Lancet. 2020;396:e22. doi: 10.1016/S0140-6736(20)31822-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kalafat E., O’Brien P., Heath P.T., et al. Benefits and potential harms of COVID-19 vaccination during pregnancy: evidence summary for patient counseling. Ultrasound Obstet Gynecol. 2021;57:681–686. doi: 10.1002/uog.23631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Battarbee A.N., Stockwell M.S., Varner M., et al. 2020. Attitudes toward COVID-19 illness and COVID-19 vaccination among pregnant women: a cross-sectional multicenter study during August-December. Available at: Accessed April 15, 2021. [DOI] [PubMed] [Google Scholar]
- 37.Gray K.J., Bordt E.A., Atyeo C., et al. COVID-19 vaccine response in pregnant and lactating women: a cohort study. Am J Obstet Gynecol. 2021 doi: 10.1016/j.ajog.2021.03.023. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Perl S.H., Uzan-Yulzari A., Klainer H., et al. SARS-CoV-2-Specific antibodies in breast milk after COVID-19 vaccination of breastfeeding women. JAMA. 2021;325:2013–2014. doi: 10.1001/jama.2021.5782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jones S.J., Mason N., Palser T., Swift S., Petrilli C.M., Horwitz L.I. Trends in risk-adjusted 28-day mortality rates for patients hospitalized with COVID-19 in England. J Hosp Med. 2021;16:290–293. doi: 10.12788/jhm.3599. [DOI] [PubMed] [Google Scholar]
- 40.De Coster C.D., Quan H., Finlayson A., et al. Identifying priorities in methodological research using ICD-9-CM and ICD-10 administrative data: report from an international consortium. BMC Health Serv Res. 2006;6:77. doi: 10.1186/1472-6963-6-77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Stepan H., Hund M., Andraczek T. Combining biomarkers to predict pregnancy complications and redefine preeclampsia: the Angiogenic-Placental Syndrome. Hypertension. 2020;75:918–926. doi: 10.1161/HYPERTENSIONAHA.119.13763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chu S.Y., Kim S.Y., Lau J., et al. Maternal obesity and risk of stillbirth: a metaanalysis. Am J Obstet Gynecol. 2007;197:223–228. doi: 10.1016/j.ajog.2007.03.027. [DOI] [PubMed] [Google Scholar]
- 43.Pasternak B., Neovius M., Söderling J., et al. Preterm birth and stillbirth During the COVID-19 pandemic in Sweden: a nationwide cohort study. Ann Intern Med. 2021 doi: 10.7326/M20-6367. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Handley S.C., Mullin A.M., Elovitz M.A., et al. Changes in preterm birth phenotypes and stillbirth at 2 Philadelphia hospitals during the SARS-CoV-2 pandemic, March-June 2020. JAMA. 2021;325:87–89. doi: 10.1001/jama.2020.20991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Khalil A., Dadelszen P von, Draycott T., Ugwumadu A., O’Brien P., Magee L. Change in the incidence of stillbirth and preterm delivery during the COVID-19 pandemic. JAMA. 2020;324:705–706. doi: 10.1001/jama.2020.12746. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.