Abstract
Coronavirus disease 2019 (COVID-19) caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has been led to a pandemic emergency. So far, different pathological pathways for SARS-CoV-2 infection have been introduced in which the excess release of pro-inflammatory cytokines (such as interleukin 1 β [IL-1β], IL-6, and tumor necrosis factor α [TNFα]) has earned most of the attentions. However, recent studies have identified new pathways with at least the same level of importance as cytokine storm in which endothelial cell (EC) dysfunction is one of them. In COVID-19, two main pathologic phenomena have been seen as a result of EC dysfunction: hyper-coagulation state and pathologic angiogenesis. The EC dysfunction-induced hypercoagulation state seems to be caused by alteration in the levels of different factors such as plasminogen activator inhibitor 1 (PAI-1), von Willebrand factor (vWF) antigen, soluble thrombomodulin, and tissue factor pathway inhibitor (TFPI). As data have shown, these thromboembolic events are associated with severity of disease severity or even death in COVID-19 patients. Other than thromboembolic events, pathologic angiogenesis is among the recent findings. Furthermore, over-expression/higher levels of different proangiogenic factors such as vascular endothelial growth factor (VEGF), hypoxia-inducible factor 1 α (HIF-1α), IL-6, TNF receptor super family 1A and 12, and angiotensin-converting enzyme 2 (ACE2) have been found in the lung biopsies/sera of both survived and non-survived COVID-19 patients. Also, there are some hypotheses regarding the role of nitric oxide in EC dysfunction and acute respiratory distress syndrome (ARDS) in SARS-CoV-2 infection. It has been demonstrated that different pathways involved in inflammation are generally common with EC dysfunction and angiogenesis. Altogether, considering the common possible upstream pathways in cytokine storm, pathologic angiogenesis, and EC dysfunction, it seems that targeting these molecules (such as nuclear factor κB) could be more effective in the management of patients with COVID-19.
Abbreviations: COVID-19, Coronavirus disease 2019; ARDS, acute respiratory distress syndrome; EC, endothelial cell; IL-1β, interleukin 1β; IL-6, interleukin 6; TNF-α, tumor necrosis factor α; NF-κB, nuclear factor κB; PAMPs, pathogen-associated molecular patterns; PRRs, pattern recognition receptors; ADP, adenosine diphosphate; TXA2, thromboxane A2; ATP, adenosine triphosphate; NO, nitric oxide; PGI2, prostaglandin I2; COX-2, cyclooxygenase-2; TFPI, tissue factor pathway inhibitor; AT, antithrombin; PAI-1, plasminogen activator inhibitor-1; HIF-1 α, hypoxia-inducible factor 1α; VEGF, vascular endothelial growth factor; FLT1, VEGF receptor 1; NOS, nitric oxide synthase; Ang2, angiopoietin; Tie-2, Ang receptor; ACE2, angiotensin-converting enzyme 2; TIMP-1, tissue inhibitor of metalloproteinases 1; ICAM-1, intracellular adhesion molecule; ENG, endoglin; FGF-1, fibroblast growth factor 1; FLT-3L, Fms-related tyrosine kinase 3 ligand; eNOS, endothelial nitric synthase; VCAM-1, vascular cell adhesion molecule-1
Keywords: Coronavirus disease 2019, Endothelial cells, Coagulation, Angiogenesis, Inflammation, Cytokine storm
Graphical abstract
1. Introduction
Coronavirus disease 2019 (COVID-19) is nowadays known to be probably the most important issue in health care systems worldwide. This disease with manifestations ranging from asymptomatic to critically ill state in the patients has been seen in different age categories (Norooznezhad et al., 2020). The main finding in these patients is pneumonia which most patients with greater severities could turn into acute respiratory distress syndrome (ARDS) (Wang et al., 2020). According to the studies, non-survivor patients with COVID-19 had exhibited sepsis (100%), respiratory failure (98%), acute respiratory distress syndrome or ARDS (93%), and septic shock (70%); all being evidence of a multi-organ involvement in these patients (Zhou et al., 2020). Thus, what we should expect is a complex systemic physiopathology for this disease. So far, different pathological pathways have been described in COVID-19 among which inflammation has earned most of the credit due to its unbridled form termed cytokine storm in critically ill patients (Hantoushzadeh and Norooznezhad, 2020a; Tay et al., 2020). Certain studies, however, have bolded different other pathways such as hypercoagulation state as well as pathologic intussusceptive angiogenesis led by endothelial cells (ECs) dysfunction in patients with COVID-19, especially the non-survivor cases (Ackermann et al., 2020; Libby and Lüscher, 2020).
The aim of the current study is thus to review the latest information on the role of EC dysfunction and connect the pieces of this puzzle as far as data are available (Fig. 1).
Fig. 1.
A brief overview of endothelial cell functions.
2. Inflammation and COVID-19
Although the main focus of this study is not on inflammation, due to certain phases where inflammation affects ECs (Capitão and Soares, 2016) and its undeniable role in COVID-19 it will be shortly reviewed. As already mentioned, cytokine storm is one of the most important aspects of COVID-19 pathogenesis in critically ill patients which could lead to poor clinical outcomes and even death. Different pro-inflammatory cytokines such as interleukin 1β (IL-1β), interleukin 6 (IL-6), and tumor necrosis factor α (TNF-α) are now known to participate in the COVID-19-induced cytokine storm (Hantoushzadeh and Norooznezhad, 2020a; Ragab et al., 2020). These pro-inflammatory cytokines could be produced by different cells such as macrophages, epithelial cells, and ECs as a response to different conditions including the innate immune system activation (Ragab et al., 2020). Clinical evaluations have shown the increased cytokine levels of IL-1β, IL-7, IL-9, IL-10, and TNF-α in patients with confirmed COVID-19 (Huang et al., 2020). Moreover, a study on patients with COVID-19 affirmed significantly elevated IL-6 levels in non-survivor cases of the disease compared to survived individuals (Ruan et al., 2020). Most of the mentioned factors are expressed greatly depends on the activation of nuclear factor κB (NF-κB). This major transcription factor in turn could be induced through the binding of pathogen-associated molecular patterns (PAMPs) to the pattern recognition receptors (PRRs) (Ragab et al., 2020). Also, it could be triggered by IL-1β and TNF-α through the canonical pathway (Lawrence, 2009). In a study on isolated lung tissues, NF-kB was observed to be significantly higher in cases with ARDS compared to the controls (Fudala et al., 2011). As data have shown, there is a close association between inflammation and ECs which would be explained in the following sections.
3. Endothelial cells: coagulation and angiogenesis
3.1. Endothelial cells
ECs cover all the blood vessels inner surface and consist 600–700 g of the total body weight in an adult enabling them to be considered as a gland. Other than the well-known responsibilities such as regulating the exchange between the inner and outer sides of vessels, ECs are involved in many other physiological processes. Maintenance of vascular function for keeping blood fluidity, regulating/participating of/in inflammatory responses, preventing pathological hemorrhage and holding the tissue hemostasis, and prevention of unwanted vascular occlusive states (thrombosis) are among the mentioned phenomena (Aird, 2015; Ince et al., 2016; van Hinsbergh, 2012).
3.2. Endothelial cells and coagulation
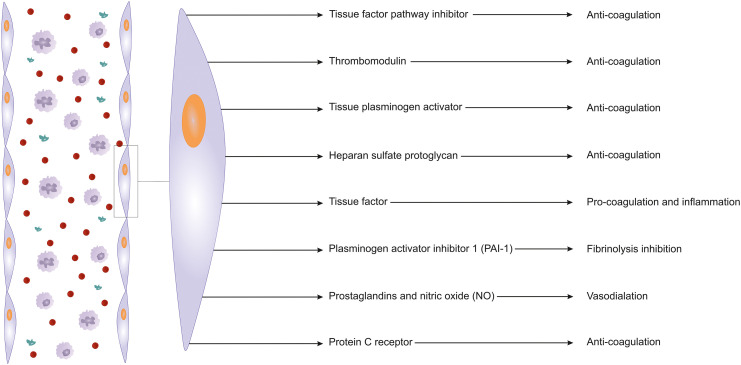
As discussed above, among the responsibilities of ECs is the maintenance of vascular function for blood fluidity along with the prevention of thromboembolic events. Under certain conditions, clot formation (coagulation) should occur for hemostasis. For this aim, ECs play a dual role in the coagulation system of the human body. This means that they participate in the clot production process while at the same time being crucial to the prevention of any thromboembolic event in other sites of the body and maintaining the blood fluidity based on the condition (Aird, 2015; Rajendran et al., 2013; van Hinsbergh, 2012). Herein, we focus on the role of ECs in the prevention of unnecessary coagulative events.
In a case of vascular damage, platelets face the platelet-activator agents such as thrombin, adenosine diphosphate (ADP), and thromboxane A2 (TXA2). Exposure of platelets to these factors in the inner layer of the vessel would cause platelet aggregation and vasocompression. This phenomenon is necessary to face the problem in the damaged site. However, non-damaged sites in the other areas should be kept safe from the coagulation cascade. ECs of damage-free areas prevent activation of the coagulation cascade through different pathways. Using the help of a surface enzyme named ecto-ATPase they induce the degradation of adenosine triphosphate (ATP) and ADP. Upon the decreased levels of ADP, this substance can no longer participate in the platelet activation process (Di Minno et al., 1983; Kumar et al., 2015; Lordan et al., 2021). Also, nearby damaged-free ECs cause vasodilation and inhibit platelet aggregation through the synthesis and release of nitric oxide (NO) and prostaglandin I2 (PGI2). PGs are produced in the ECs following the action of cyclooxygenase (COX-2) on the arachidonic acid substrate (Landmesser et al., 2004; Mitchell et al., 2008). Among other potent target pathways of the coagulation cascade in damage-free areas, could be inhibiting thrombin (crucial to clot formation) synthesis. In healthy ECs, through the anti-thrombin and C protein as well as and tissue factor pathway inhibitor (TFPI), thrombin synthesis could be prevented to finally limit the coagulation cascade. TFPI, identified as an inhibitor of factor Xa and factor VIIa, is mostly is secreted from endothelial cells and placed on the surface of these cells; however, it is also detectable in plasma in small amounts while bound to low-density lipoprotein. Also, through an anti-serine protease activity, heparan sulfate bound to antithrombin (AT) A1 neutralizes different coagulative factors such as thrombin, factor IX, and factor X. Moreover, on the surface of ECs, thrombomodulin (CD141) catches thrombin and this complex (at the presence of endothelial protein C receptor) not only decreases the enzymatic activity/affinity of thrombin for fibrinogen but also inactivates the factor V and factor VIII (Ellery and Adams, 2014; Maroney and Mast, 2012; Mast, 2016; Yau et al., 2015). Moreover, protein S which acts as a cofactor for activated C protein helps to inactivate factor V and factor VIIIa. Also, it has been suggested that protein S synthesis occurs in ECs and its free not-C protein-dependent form could exert an inhibitory effect on prothrombinase and tenase (Cramer et al., 2010; Dahlbäck, 1997).
ECs are not defenseless against the clot formation and are able to lysis them through fibrinolysis. These cells are responsible for the synthesis of most tissue plasminogen activator (tPA) amounts. However, it has been shown that about 40% of tPA is attached to its inhibitor, plasminogen activator inhibitor-1 (PAI-1). Thus, any increase in PAI-1 levels seems to lead to a hyper-coagulation state (Binder et al., 2002; Cesari et al., 2010) (Fig. 2).
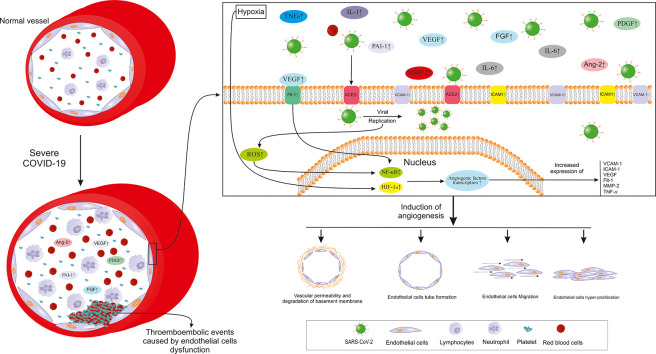
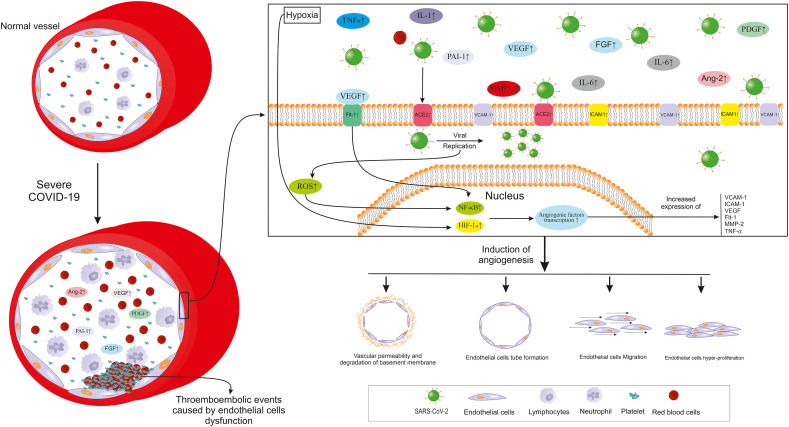
Fig. 2.
Important pathways involved in angiogenesis and endothelial dysfunction in severe COVID-19. SARS-CoV-2: Severe acute respiratory syndrome coronavirus 2; IL: Interleukin; TNF-α: Tumor necrosis factor α; VEGF: Vascular endothelial growth factor; PDGF: Platelet-derived growth factor; FGF: Fibroblast growth factor; PAI-1: Plasminogen activator inhibitor 1; Flt-1: VEGF receptor 1; Ang-2: Angiopoietin 2; MMP-2: Matrix metalloproteinase 2; ROS: Reactive oxygen species; NF-κB: Nuclear factor κ B; HIF-1α; Hypoxia-inducible factor 1.
3.3. Endothelial cells and angiogenesis
Angiogenesis, new blood vessel formation from their pre-existing microvascular structures, could be seen in a few physiologic (Mohammadi et al., 2017; Rezazadeh et al., 2020) but more pathologic conditions (Li et al., 2018; Norooznezhad and Norooznezhad, 2017; Rasouli et al., 2018). There are many triggering factors such as inflammation, hypoxia, and oxidative stress which activate the angiogenesis cascade (Jahani et al., 2020). Angiogenesis is divided into two main categories including sprouting angiogenesis and intussusceptive angiogenesis (Díaz-Flores et al., 2020). It consists of several steps which most important ones include degradation of the basement membrane, proliferation, migration, and tube formation of ECs affected by pro-angiogenic factors (Plank and Sleeman, 2003). The hypoxia is introduced as the most eminent trigger of angiogenesis activating transcription factors such as hypoxia-inducible factor 1α (HIF-1α). This, in turn, induces the expression of some pro-angiogenic factors; the most important one being vascular endothelial growth factor (VEFG). Under such a situation, different pro-angiogenic and pro-inflammatory factors/cytokines involved in angiogenesis are expressed. The very well-known pro-angiogenic factors expressed under hypoxia are VEFG, VEGF receptor 1 or VEGFR1 (FLT1), transforming growth factor β (TGF-β), cycloxygenase-2 (COX-2), endothelin, nitric oxide synthase (NOS), angiopoietin 2 (Ang-2), and Tie-2 (Ang receptor). Moreover, some pro-inflammatory cytokines/chemokines such as IL-6 and IL-8 are among the pro-angiogenic factors (Harris, 2002; Plank and Sleeman, 2003). Interestingly, NF-κB, as a very crucial transcription factor in inflammation and especially cytokine storm, could cause the expression of IL-1β, IL-6, and TNF-α which are also involved in angiogenesis (Hantoushzadeh and Norooznezhad, 2020b). The oxidative stress occurring during many pathological conditions such as inflammation, could trigger angiogenesis through direct pathways or upon producing active oxidants. The oxidative stress-induced angiogenesis mostly drives by VEGF (Kim and Byzova, 2014). Notable numbers of the mentioned factors such as VEGF (Lee et al., 2007), IL-1β, and IL-6 (Karakike and Giamarellos-Bourboulis, 2019; Lamertz et al., 2018; Turnquist et al., 2020) not only affect ECs or other related targets but are also could act through positive feedback (by acting as an auto/paracrine agent) for themselves or other factors (Karakike and Giamarellos-Bourboulis, 2019; Lamertz et al., 2018; Lee et al., 2007; Turnquist et al., 2020).
4. Endothelial cells, coagulation, angiogenesis, and COVID-19
4.1. Endothelial cell invasion by the virus
As previously described, COVID-19 could cause an inflammatory status that negatively affects endothelium, also indirectly, leading to its dysfunction. There are cell surface proteoglycans and (glycol)proteins such as heparan sulfate proteoglycans and serine transmembrane protein 2 (TMPRSS2) mentioned as crucial agents for virus-cell interaction (Barbosa et al., 2021; Evans et al., 2020). For SARS-CoV-2 it has been affirmed that the virus could infect the ECs through the angiotensin-converting enzyme 2 (ACE2) and impair their function, known as the direct pathway (Tousoulis et al., 2012; Varga et al., 2020). It has been shown that subdomain I of ACE2 (but not subdomain II) interacts with SARS-CoV spike protein which this interaction helps the fusion of the virus to the host. Interestingly, studies have shown 10 to 20 folds of affinity in SARS-CoV-2 spike to this protein compared to the other members of the SARS-CoV family (Maiuolo et al., 2020). Moreover, it has been revealed that TMPRSS2 is a crucial factor to SARS-CoV-2 cell entrance through cleavage of viral spike (Hoffmann et al., 2020) which this serine protease also has been shown to be expressed on the EC surface in microvessels vessels (Aimes et al., 2003).
4.2. Angiogenesis, endothelial dysfunction, and coagulation in COVID-19
Other than inflammatory pathways, the role of angiogenesis and impaired coagulation caused by EC dysfunction has been studied in COVID-19 (Bernard et al., 2020). According to a study, autopsy investigations on the lungs from non-survivors with COVID-19 as well as non-survivors with H1N1 influenza and normal controls (biodemographic matched cases) proved pieces of evidence of microvascular injuries (endothelialitis) and pathologic inflammation-induced intussusceptive angiogenesis. Other than angiogenesis, microangiopathy and thrombotic events were also higher in patients with COVID-19 compared to influenza patients and control groups. Evaluating the angiogenic-related gene expression patterns in patients with COVID-19 showed the over-expression of pro-angiogenic factors such as platelet-derived growth factor (PDGF), vascular endothelial growth factor (VEGF), VEGF receptor 1 (Flt-1), matrix metalloproteinase 2 (MMP-2), tissue inhibitor of metalloproteinases 1 (TIMP1), hypoxia-inducible factor 1 α (HIF-1α), intracellular adhesion molecule 1 (ICAM-1), TNF receptor super family 1A (TNFRSF1A), TNFRSF12A and IL-6 (Ackermann et al., 2020). As spoken of earlier, ACE2 is now identified as a host receptor for SARS-CoV-2 (Varga et al., 2020). Other than the mentioned (pro-)angiogenic factors being elevated, evaluation of autopsies showed increased ACE2 expression (ACE2 positive cells) in COVID-19 patients compared to influenza and control groups. Thus, it seems that endothelial dysfunction especially pathologic angiogenesis and impaired coagulation are among the most important role-playing pathways in COVID-19 pathogenesis (Ackermann et al., 2020). Also, as previously mentioned, VEGF, one of the most important factors involved in angiogenesis, shows elevated levels in patients with COVID-19 compared to healthy controls (Huang et al., 2020). Upon binding its specific receptor, uPAR (CD87), urokinase plasminogen activator (uPA) facilitates the transformation of plasminogen to the plasmin which further initiates a cascade of proteolytic phenomenon finally resulting in the degradation of extracellular components. The activation of this pathway eventually ends with pro-angiogenic and pro-inflammatory cytokines release (D'Alonzo et al., 2020). Interestingly, it has been shown that in patients with sepsis, uPAR levels could predict ARDS. Also, its plasma levels were associated with the inflammatory state, disease severity, and mortality (Chen et al., 2019). Also, it has been suggested that antiphospholipid antibodies might have role coagulation events of COVID-19, however, this is still a controversial issue (Huertas et al., 2020). Moreover, another study on sera of patients with COVID-19 evaluated angiogenic cytokines in four groups: healthy control, COVID-19 hospital admitted, COVID-19 survived ICU admitted, and COVID-19 non-survived patients. They showed that angiopoietin 2 (Ang-2) and plasminogen activator inhibitor 1 (PAI-1) were significantly higher in non-survivors compared to survived ICU patients. In the survived ICU patients, levels of Ang-2, endoglin (ENG), fibroblast growth factor 1 (FGF-1), Fms-related tyrosine kinase 3 ligand (FLT-3L), and PAI-1 were significantly higher than the ward admitted COVID-19 patients. Furthermore, sera levels of VEGF-A, PDGF-AA, PDGF-AB/BB, PAI-1 were significantly higher in the ward admitted COVID-19 patients compared to the healthy controls (Pine, 2020).
PAI-1, on the other hand, was detected extremely increased upon worsening of COVID-19 patients' clinical status (Pine, 2020). Thrombosis and vascular clots were observed in up to 71.4% of non-survived COVID-19 patients which seems to be due to a disrupted balance between coagulative and anti-coagulative agents (Tang et al., 2020). Also, it has been shown that 31% of ICU admitted COVID-19 patients had thrombotic complications (Klok et al., 2020). During inflammation, endothelial cells are activated and express tissue factors which in turn trigger the extrinsic coagulation pathway finally leading to vascular endothelium facing a hypercoagulable state (Del Turco et al., 2007). Also, another hypothesis regarding the COVID-19-induced hypercoagulative state is the direct infection of endothelial cells with SARS-CoV-2 which results in endothelial dysfunction and, therefore, the mentioned condition (Amraei and Rahimi, 2020; Mestas and Ley, 2008). A study has shown that in critically ill patients with COVID-19, levels of IL-6, VEGF, tPA, TFPI, and D-dimer were significantly higher than non-critical patients (White et al., 2021). Moreover, it has been shown that mortality in ICU admitted and non-ICU admitted COVID-19 was significantly correlated with vWF antigen and soluble thrombomodulin levels (Goshua et al., 2020). Also, it has been revealed that thrombin generation was a finding compared to the healthy controls (Campello, 2021). The other study on severe and non-severe cases with COVID-19 who were compared with hospitalized controls has shown elevated D-dimer, agonist-induced ADP release (30- to 90-fold higher), thrombopoietin, platelet factor 4, and soluble P-selectin levels in COVID-19 patients. Also, it was found that platelets of patients with COVID-19 were much hyperactive when compared to the controls (Comer et al., 2021).
The host immune response is directly linked to the rate of leukocyte migration to the tissues. The extravasation of these cells from the bloodstream into the target tissue is facilitated by activated ECs and expressed adhesion molecules such as vascular cell adhesion molecule-1 (VCAM-1) and ICAM-1. Thus, these receptors are crucial to the inflammatory responses to pathogens by the immune system (Mestas and Ley, 2008). It has been shown that both VCAM-1 (Schlesinger and Bendas, 2015) and ICAM-1-mediated leukocyte adhesion (Becker et al., 1999) are involved in pathologic angiogenesis. IL-1, released from the immune cells, not only induces other pro-inflammatory cytokines expression (e.g., TNF-α) but also acts as an autocrine agent increasing its levels via positive feedback. This in turn could cause capillary leakage followed by pneumonitis. This scenario is usually detected in activated endothelial cells expressing and secreting IL-1 under inflammatory conditions (Libby and Lüscher, 2020). Also, the pro-inflammatory roles of these cytokines in angiogenesis and coagulation were discussed before.
EC dysfunction has been detected in many infectious conditions including sepsis and septic shock (Page and Liles, 2013). As mentioned, sepsis is the leading step in the pathogenesis of COVID-19 and 100% of non-survivors presented sepsis (Zhou et al., 2020). Via its biomarkers, EC dysfunction could predict the severity of sepsis as well as prognosis of the patients as the hall marks. These biomarkers are not only defined for sepsis but are somehow of the same predictor value in some other diseases such as diabetes mellitus, heart failure, and chronic kidney disease (Leite et al., 2020). As known, such diseases have also has been listed among the comorbidities of COVID-19 reported by the Center for Disease Control and Prevention (CDC) (Center for Disease Control and Prevention, 2021). Among these factors, Ang1, Ang2, VEGF, sFlt-1, von Willebrand Factor, thrombomodulin have been suggested as biomarkers for sepsis in regard to endothelial function (Page and Liles, 2013).
4.3. Nitric oxide, endothelial cell dysfunction, and COVID-19
Excessive release of pro-inflammatory cytokines (following the cytokine storm), hypoxia (ARDS), and immune system activation could bring about ECs dysfunction. Nitric oxide (NO), produced by endothelial nitric synthase (eNOS) is a crucial agent in vascular hemostasis, inflammation, and oxidative stress. NO acts as a vasodilator and a regulator of thrombosis and cell growth which can reduce inflammation through inhibition of pro-inflammatory cytokines expression (by affecting NF-κB) and certain cellular adhesion molecules. Also, NO could decrease platelet adherence and aggregation, and downregulate neutrophil secretion and aggregation (Tousoulis et al., 2012; Vallance and Hingorani, 1999). Interestingly, it has been shown that NO could inhibit the replication cycle of SARS-CoV. Thus, it could be possible that it affects the SARS-CoV-2 replication cycle as well (Keyaerts et al., 2004). Clinically speaking, during ARDS, NO could increase tissue oxygenation and decrease pulmonary hypertension through increasing vascular dilation. Due to the alteration in eNOS activity upon aging, diabetes, obesity, and chronic obstructive pulmonary disease, eNOS activity as well as levels of NO might be reduced probably causing vascular inflammation and endothelial cell dysfunction (Adusumilli et al., 2020). It should be noted that the mentioned underlying conditions are among the most important co-morbidities in patients with COVID-19 (Amraei and Rahimi, 2020). Moreover, Gattinoni et al. have stated that the hypoxemia in the patients with COVID-19 and ARDS is far different from the ARDS definition in the Berlin criteria. They have hypothesized that this condition might be resulted by loss of lung perfusion regulation and hypoxic vasoconstriction (Gattinoni et al., 2020). Interestingly, it has been revealed that in the severe endothelial dysfunction and abolition of the NO release, NO agonists such as histamine and thrombin instead of vasodilation induce vasoconstriction which is mediated by perivascular smooth muscle cells (Tousoulis et al., 2012). All and all, it seems that endothelial dysfunction somehow could affect the NO system and cause the mentioned issues which require more studies.
4.4. Endothelial cell dysfunction and COVID-19 comorbidities
According to the reports from the CDC, patients with some underlying conditions such as diabetes, obesity, cardiovascular disease, chronic kidney disease, cancer, chronic lung diseases, pregnancy, and many other ones are at higher risk for the severe type of COVID-19 (Center for Disease Control and Prevention, 2021). Interestingly, angiogenesis and impaired endothelial cell function has been observed in many of the suggested lists by CDC. As it has been mentioned above, impairment in NOS system could alter the function of ECs and is usually detected in the diabetes, atherosclerosis (Eelen et al., 2015), cancer (Pacher et al., 2007), chronic kidney disease (Fu et al., 2015), obesity (Engin, 2017), smoking (Messner and Bernhard, 2014) and cardiovascular diseases (Brassington et al., 2019). Also, pathologic angiogenesis with increased levels of pro-angiogenic factors has been seen in many of comorbidities such as cancers (Gupta and Qin, 2003) and obesity (Nijhawans et al., 2020).
5. NF-κB as a possible therapeutic option
There could be a notable number of treatment options for endothelial dysfunction which herein, NF-κB would be shortly discussed. As mentioned, different pathways are involved in the pathogenesis of EC dysfunction in COVID-19. Thus, different treatments targeting one or more of them could be useful in the treatment of EC dysfunction. NF-κB is responsible for the expression of different pro-inflammatory cytokines involved in cytokine storm and is one of these targets. Also, NF-κB increases the expression of PAF-1 expression which itself could increase the possibility of clot formation (Elkhodary, 2020). Other than PAF-1 and inflammation, NF-κB seems to be an important target for the inhibition of angiogenesis (De Martin et al., 2000). There are three suggestive stages for inhibition of NF-κB. I) Blockage of the initiating (incoming) signal stimulates the NF-κB pathway which terminates the whole pathway at the first step. II) Targeting and blocking a specific agent in the cascade during the cytoplasmic step in the NF-κB activation pathway. III) Blocking the nuclear activity of NF-κB which could be consist of different other sub-pathways all reviewed by Gilmore and Herscovitch (Gilmore and Herscovitch, 2006). Of the most common and successful treatments targeting NF-κB (as well as other pathways) in COVID-19 is dexamethasone. This medication which is a potent corticosteroid could be useful in the treatment of EC dysfunction in patients with COVID-19 through affecting NF-κB. It has been shown that treatment with this medication could upregulate IκBα which is a blocker of the signaling pathway of NF-κB (Kandasamy, 2021). Dexamethasone also reduces the feedback between some pro-inflammatory cytokines such as IL-6 with NF-κB (Hariharan et al., 2021).
6. Conclusion and future perspectives
COVID-19 is a global issue and despite the quite notable studies performed so far, our knowledge on the pathophysiology of this disease is not yet enough. There are different pathologic pathways involved in this disease some of which are not fully understood or have been highlighted just recently. EC dysfunction is a very crucial involved pathology in COVID-19 which leads to different poor outcomes. This condition could be caused by direct infection of SARS-CoV-2 or as a consequence of other conditions such as systemic inflammation. Different cytokines/growth factors are expressed upon the activation of certain transcriptional factors are involve in these pathways and in turn cause or boost the expression of each other. We hope future investigations result in finding the most sensitive and also specific biomarker(s) of endothelial dysfunction with an early-predictive value for sepsis in the infectious diseases. Also, there are treatments such as dexamethasone that seems to affect the upper pathways and cut the string connecting to other downstream pathways. However, we could expect treatments with more accuracy on EC dysfunction and angiogenesis to be developed and examined as the time passes and knowledge increases in this field. Thus, the authors strongly suggest considering those possible treatments targeting those in common transcriptional factors between the mentioned pathways for further studies.
Role of the funding source
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Ackermann M., et al. Pulmonary vascular endothelialitis, thrombosis, and angiogenesis in Covid-19. N. Engl. J. Med. 2020;383:120–128. doi: 10.1056/NEJMoa2015432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adusumilli N.C., et al. Harnessing nitric oxide for preventing, limiting and treating the severe pulmonary consequences of COVID-19. Nitric Oxide. 2020;103:4–8. doi: 10.1016/j.niox.2020.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aimes R.T., et al. Endothelial cell serine proteases expressed during vascular morphogenesis and angiogenesis. Thromb. Haemost. 2003;89:561–572. [PubMed] [Google Scholar]
- Aird W.C. Endothelium and haemostasis. Hamostaseologie. 2015;35:11–16. doi: 10.5482/HAMO-14-11-0075. [DOI] [PubMed] [Google Scholar]
- Amraei R., Rahimi N. COVID-19, renin-angiotensin system and endothelial dysfunction. Cells. 2020;9 doi: 10.3390/cells9071652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbosa L.C., et al. Endothelial cells and SARS-CoV-2: an intimate relationship. Vasc. Pharmacol. 2021;137 doi: 10.1016/j.vph.2021.106829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker M.D., et al. In vivo significance of ICAM-1--dependent leukocyte adhesion in early corneal angiogenesis. Invest. Ophthalmol. Vis. Sci. 1999;40:612–618. [PubMed] [Google Scholar]
- Bernard I., et al. Endothelium infection and dysregulation by SARS-CoV-2: evidence and caveats in COVID-19. Viruses. 2020;13 doi: 10.3390/v13010029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binder B.R., et al. Plasminogen activator inhibitor 1: physiological and pathophysiological roles. News Physiol. Sci. 2002;17:56–61. doi: 10.1152/nips.01369.2001. [DOI] [PubMed] [Google Scholar]
- Brassington K., et al. New frontiers in the treatment of comorbid cardiovascular disease in chronic obstructive pulmonary disease. Clin. Sci. (Lond.) 2019;133:885–904. doi: 10.1042/CS20180316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campello E. Thrombin generation in patients with COVID-19 with and without thromboprophylaxis. Clin. Chem. Lab. Med. 2021 doi: 10.1515/cclm-2021-0108. [DOI] [PubMed] [Google Scholar]
- Capitão M., Soares R. Angiogenesis and inflammation crosstalk in diabetic retinopathy. J. Cell. Biochem. 2016;117:2443–2453. doi: 10.1002/jcb.25575. [DOI] [PubMed] [Google Scholar]
- Center for Disease Control and Prevention, C . vol. 2021. 2021. People With Certain Medical Conditions. [Google Scholar]
- Cesari M., et al. Plasminogen activator inhibitor-1 (PAI-1): a key factor linking fibrinolysis and age-related subclinical and clinical conditions. Cardiovasc. Ther. 2010;28:e72–e91. doi: 10.1111/j.1755-5922.2010.00171.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen D., et al. Serum plasminogen activator urokinase receptor predicts elevated risk of acute respiratory distress syndrome in patients with sepsis and is positively associated with disease severity, inflammation and mortality. Exp. Ther. Med. 2019;18:2984–2992. doi: 10.3892/etm.2019.7931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Comer S.P., et al. COVID-19 induces a hyperactive phenotype in circulating platelets. PLoS Biol. 2021;19 doi: 10.1371/journal.pbio.3001109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cramer T.J., et al. Factor V is an anticoagulant cofactor for activated protein C during inactivation of factor Va. Pathophysiol. Haemost. Thromb. 2010;37:17–23. doi: 10.1159/000315141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahlbäck B. Factor V and protein S as cofactors to activated protein C. Haematologica. 1997;82:91–95. [PubMed] [Google Scholar]
- D’Alonzo D., et al. COVID-19 and pneumonia: a role for the uPA/uPAR system. Drug Discov. Today. 2020;25:1528–1534. doi: 10.1016/j.drudis.2020.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Martin R., et al. The transcription factor NF-kappa B and the regulation of vascular cell function. Arterioscler. Thromb. Vasc. Biol. 2000;20:E83–E88. doi: 10.1161/01.atv.20.11.e83. [DOI] [PubMed] [Google Scholar]
- Del Turco S., et al. Parallel decrease of tissue factor surface exposure and increase of tissue factor microparticle release by the n-3 fatty acid docosahexaenoate in endothelial cells. Thromb. Haemost. 2007;98:210–219. [PubMed] [Google Scholar]
- Di Minno G., et al. The role of ADP secretion and thromboxane synthesis in factor VIII binding to platelets. Blood. 1983;62:186–190. [PubMed] [Google Scholar]
- Díaz-Flores L., et al. Participation of intussusceptive angiogenesis in the morphogenesis of lobular capillary hemangioma. Sci. Rep. 2020;10:4987. doi: 10.1038/s41598-020-61921-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eelen G., et al. Endothelial cell metabolism in normal and diseased vasculature. Circ. Res. 2015;116:1231–1244. doi: 10.1161/CIRCRESAHA.116.302855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elkhodary M.S.M. Treatment of COVID-19 by controlling the activity of the nuclear factor-kappa B. CellBio. 2020;9:109–121. [Google Scholar]
- Ellery P.E., Adams M.J. Tissue factor pathway inhibitor: then and now. Semin. Thromb. Hemost. 2014;40:881–886. doi: 10.1055/s-0034-1395153. [DOI] [PubMed] [Google Scholar]
- Engin A. Endothelial dysfunction in obesity. Adv. Exp. Med. Biol. 2017;960:345–379. doi: 10.1007/978-3-319-48382-5_15. [DOI] [PubMed] [Google Scholar]
- Evans P.C., et al. Endothelial dysfunction in COVID-19: a position paper of the ESC Working Group for Atherosclerosis and Vascular Biology, and the ESC Council of Basic Cardiovascular Science. Cardiovasc. Res. 2020;116:2177–2184. doi: 10.1093/cvr/cvaa230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu J., et al. Glomerular endothelial cell injury and cross talk in diabetic kidney disease. Am. J. Physiol. Ren. Physiol. 2015;308:F287–F297. doi: 10.1152/ajprenal.00533.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fudala R., et al. Increased levels of nuclear factor κB and Fos-related antigen 1 in lung tissues from patients with acute respiratory distress syndrome. Arch. Pathol. Lab. Med. 2011;135:647–654. doi: 10.5858/2009-0660-OAR1.1. [DOI] [PubMed] [Google Scholar]
- Gattinoni L., et al. COVID-19 does not lead to a “typical” acute respiratory distress syndrome. Am. J. Respir. Crit. Care Med. 2020;201:1299–1300. doi: 10.1164/rccm.202003-0817LE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilmore T.D., Herscovitch M. Inhibitors of NF-kappaB signaling: 785 and counting. Oncogene. 2006;25:6887–6899. doi: 10.1038/sj.onc.1209982. [DOI] [PubMed] [Google Scholar]
- Goshua G., et al. Endotheliopathy in COVID-19-associated coagulopathy: evidence from a single-centre, cross-sectional study. Lancet Haematol. 2020;7:e575–e582. doi: 10.1016/S2352-3026(20)30216-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta M.K., Qin R.Y. Mechanism and its regulation of tumor-induced angiogenesis. World J. Gastroenterol. 2003;9:1144–1155. doi: 10.3748/wjg.v9.i6.1144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hantoushzadeh S., Norooznezhad A.H. Inappropriate antibiotic consumption as a possible cause of inflammatory storm and septic shock in patients diagnosed with coronavirus-19 disease (COVID-19) Arch. Med. Res. 2020;51:347–348. doi: 10.1016/j.arcmed.2020.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hantoushzadeh S., Norooznezhad A.H. Possible cause of inflammatory storm and septic shock in patients diagnosed with (COVID-19) Arch. Med. Res. 2020;51:347–348. doi: 10.1016/j.arcmed.2020.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hariharan A., et al. The role and therapeutic potential of NF-kappa-B pathway in severe COVID-19 patients. Inflammopharmacology. 2021;29:91–100. doi: 10.1007/s10787-020-00773-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris A.L. Hypoxia—a key regulatory factor in tumour growth. Nat. Rev. Cancer. 2002;2:38–47. doi: 10.1038/nrc704. [DOI] [PubMed] [Google Scholar]
- van Hinsbergh V.W. Endothelium—role in regulation of coagulation and inflammation. Semin. Immunopathol. 2012;34:93–106. doi: 10.1007/s00281-011-0285-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffmann M., et al. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. 2020;181:271–280.e8. doi: 10.1016/j.cell.2020.02.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang C., et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huertas A., et al. Endothelial cell dysfunction: a major player in SARS-CoV-2 infection (COVID-19)? Eur. Respir. J. 2020;56 doi: 10.1183/13993003.01634-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ince C., et al. The endothelium in sepsis. Shock. 2016;45:259–270. doi: 10.1097/SHK.0000000000000473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jahani M., et al. Regenerative medicine and angiogenesis; challenges and opportunities. Adv. Pharm. Bull. 2020;10:490–501. doi: 10.34172/apb.2020.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kandasamy M. NF-κB signalling as a pharmacological target in COVID-19: potential roles for IKKβ inhibitors. Naunyn Schmiedeberg’s Arch. Pharmacol. 2021;394:561–567. doi: 10.1007/s00210-020-02035-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karakike E., Giamarellos-Bourboulis E.J. Macrophage activation-like syndrome: a distinct entity leading to early death in sepsis. Front. Immunol. 2019;10:55. doi: 10.3389/fimmu.2019.00055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keyaerts E., et al. Inhibition of SARS-coronavirus infection in vitro by S-nitroso-N-acetylpenicillamine, a nitric oxide donor compound. Int. J. Infect. Dis. 2004;8:223–226. doi: 10.1016/j.ijid.2004.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim Y.W., Byzova T.V. Oxidative stress in angiogenesis and vascular disease. Blood. 2014;123:625–631. doi: 10.1182/blood-2013-09-512749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klok F.A., et al. Incidence of thrombotic complications in critically ill ICU patients with COVID-19. Thromb. Res. 2020;191:145–147. doi: 10.1016/j.thromres.2020.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar N.G., et al. Fibrinolytic activity of endothelial cells from different venous beds. J. Surg. Res. 2015;194:297–303. doi: 10.1016/j.jss.2014.09.028. [DOI] [PubMed] [Google Scholar]
- Lamertz L., et al. Soluble gp130 prevents interleukin-6 and interleukin-11 cluster signaling but not intracellular autocrine responses. Sci. Signal. 2018;11 doi: 10.1126/scisignal.aar7388. [DOI] [PubMed] [Google Scholar]
- Landmesser U., et al. Endothelial function: a critical determinant in atherosclerosis? Circulation. 2004;109:Ii27–33. doi: 10.1161/01.CIR.0000129501.88485.1f. [DOI] [PubMed] [Google Scholar]
- Lawrence T. The nuclear factor NF-κB pathway in inflammation. Cold Spring Harb. Perspect. Biol. 2009;1 doi: 10.1101/cshperspect.a001651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S., et al. Autocrine VEGF signaling is required for vascular homeostasis. Cell. 2007;130:691–703. doi: 10.1016/j.cell.2007.06.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leite A.R., et al. Novel biomarkers for evaluation of endothelial dysfunction. Angiology. 2020;71:397–410. doi: 10.1177/0003319720903586. [DOI] [PubMed] [Google Scholar]
- Li T., et al. Tumor angiogenesis and anti-angiogenic gene therapy for cancer. Oncol. Lett. 2018;16:687–702. doi: 10.3892/ol.2018.8733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Libby P., Lüscher T. COVID-19 is, in the end, an endothelial disease. Eur. Heart J. 2020;41:3038–3044. doi: 10.1093/eurheartj/ehaa623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lordan R., et al. Platelet activation and prothrombotic mediators at the nexus of inflammation and atherosclerosis: potential role of antiplatelet agents. Blood Rev. 2021;45 doi: 10.1016/j.blre.2020.100694. [DOI] [PubMed] [Google Scholar]
- Maiuolo J., et al. The contribution of endothelial dysfunction in systemic injury subsequent to SARS-Cov-2 infection. Int. J. Mol. Sci. 2020;21 doi: 10.3390/ijms21239309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maroney S.A., Mast A.E. Platelet tissue factor pathway inhibitor modulates intravascular coagulation. Thromb. Res. 2012;129(2):21–Suppl 2. doi: 10.1016/j.thromres.2012.02.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mast A.E. Tissue factor pathway inhibitor: multiple anticoagulant activities for a single protein. Arterioscler. Thromb. Vasc. Biol. 2016;36:9–14. doi: 10.1161/ATVBAHA.115.305996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messner B., Bernhard D. Smoking and cardiovascular disease: mechanisms of endothelial dysfunction and early atherogenesis. Arterioscler. Thromb. Vasc. Biol. 2014;34:509–515. doi: 10.1161/ATVBAHA.113.300156. [DOI] [PubMed] [Google Scholar]
- Mestas J., Ley K. Monocyte-endothelial cell interactions in the development of atherosclerosis. Trends Cardiovasc. Med. 2008;18:228–232. doi: 10.1016/j.tcm.2008.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell J.A., et al. Role of nitric oxide and prostacyclin as vasoactive hormones released by the endothelium. Exp. Physiol. 2008;93:141–147. doi: 10.1113/expphysiol.2007.038588. [DOI] [PubMed] [Google Scholar]
- Mohammadi M.H., et al. Evaluation of wound healing in diabetic foot ulcer using platelet-rich plasma gel: a single-arm clinical trial. Transfus. Apher. Sci. 2017;56:160–164. doi: 10.1016/j.transci.2016.10.020. [DOI] [PubMed] [Google Scholar]
- Nijhawans P., et al. Angiogenesis in obesity. Biomed. Pharmacother. 2020;126 doi: 10.1016/j.biopha.2020.110103. [DOI] [PubMed] [Google Scholar]
- Norooznezhad A.H., Norooznezhad F. Cannabinoids: possible agents for treatment of psoriasis via suppression of angiogenesis and inflammation. Med. Hypotheses. 2017;99:15–18. doi: 10.1016/j.mehy.2016.12.003. [DOI] [PubMed] [Google Scholar]
- Norooznezhad A.H., et al. Primary symptoms, comorbidities, and outcomes of 431 hospitalized patients with confirmative RT-PCR results for COVID-19. Am. J. Trop. Med. Hyg. 2020;103:834–837. doi: 10.4269/ajtmh.20-0512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pacher P., et al. Nitric oxide and peroxynitrite in health and disease. Physiol. Rev. 2007;87:315–424. doi: 10.1152/physrev.00029.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Page A.V., Liles W.C. Biomarkers of endothelial activation/dysfunction in infectious diseases. Virulence. 2013;4:507–516. doi: 10.4161/viru.24530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pine AB. Circulating markers of angiogenesis and endotheliopathy in COVID-19. Pulm. Circ. 2020;10(1) doi: 10.1177/2045894020966547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plank M., Sleeman B. Tumour-induced angiogenesis: a review. J. Theor. Med. 2003;5:137–153. [Google Scholar]
- Ragab D., et al. The COVID-19 cytokine storm; what we know so far. Front. Immunol. 2020;11:1446. doi: 10.3389/fimmu.2020.01446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajendran P., et al. The vascular endothelium and human diseases. Int. J. Biol. Sci. 2013;9:1057–1069. doi: 10.7150/ijbs.7502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasouli H., et al. Comparative in vitro/theoretical studies on the anti-angiogenic activity of date pollen hydro-alcoholic extract: highlighting the important roles of its hot polyphenols. BioImpacts. 2018;8:281–294. doi: 10.15171/bi.2018.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rezazadeh D., et al. Autologous amniotic membrane: an accelerator of wound healing for prevention of surgical site infections following cesarean delivery. Med. Hypotheses. 2020;137 doi: 10.1016/j.mehy.2019.109532. [DOI] [PubMed] [Google Scholar]
- Ruan Q., et al. Clinical predictors of mortality due to COVID-19 based on an analysis of data of 150 patients from Wuhan, China. Intensive Care Med. 2020;46:846–848. doi: 10.1007/s00134-020-05991-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlesinger M., Bendas G. Vascular cell adhesion molecule-1 (VCAM-1)—an increasing insight into its role in tumorigenicity and metastasis. Int. J. Cancer. 2015;136:2504–2514. doi: 10.1002/ijc.28927. [DOI] [PubMed] [Google Scholar]
- Tang N., et al. Abnormal coagulation parameters are associated with poor prognosis in patients with novel coronavirus pneumonia. J. Thromb. Haemost. 2020;18:844–847. doi: 10.1111/jth.14768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tay M.Z., et al. The trinity of COVID-19: immunity, inflammation and intervention. Nat. Rev. Immunol. 2020;20:363–374. doi: 10.1038/s41577-020-0311-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tousoulis D., et al. The role of nitric oxide on endothelial function. Curr. Vasc. Pharmacol. 2012;10:4–18. doi: 10.2174/157016112798829760. [DOI] [PubMed] [Google Scholar]
- Turnquist C., et al. Cytokine storms in cancer and COVID-19. Cancer Cell. 2020;38:598–601. doi: 10.1016/j.ccell.2020.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vallance P., Hingorani A. Endothelial nitric oxide in humans in health and disease. Int. J. Exp. Pathol. 1999;80:291–303. doi: 10.1046/j.1365-2613.1999.00137.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varga Z., et al. Endothelial cell infection and endotheliitis in COVID-19. Lancet. 2020;395:1417–1418. doi: 10.1016/S0140-6736(20)30937-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L., et al. Coronavirus disease 2019 in elderly patients: characteristics and prognostic factors based on 4-week follow-up. J. Inf. Secur. 2020;80:639–645. doi: 10.1016/j.jinf.2020.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White D., et al. Evaluation of COVID-19 coagulopathy; laboratory characterization using thrombin generation and nonconventional haemostasis assays. Int. J. Lab. Hematol. 2021;43:123–130. doi: 10.1111/ijlh.13329. [DOI] [PubMed] [Google Scholar]
- Yau J.W., et al. Endothelial cell control of thrombosis. BMC Cardiovasc. Disord. 2015;15:130. doi: 10.1186/s12872-015-0124-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou F., et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395:1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]