Abstract
Aims:
Our goal was to expand the spectrum of clinico-radiologic characteristics and the possible therapeutic choices in patients with tumefactive demyelinating lesions (TDLs).
Methods:
A retrospective analysis of 50 patients with at least one TDL was performed at an academic neurology center (2008–2020).
Results:
Our cohort comprised mostly women (33/50) with a mean age of 38 years at TDL onset. The mean follow-up time was 76 months. The mean Expanded Disability Status Scale score at TDL onset and at the latest neurological evaluation was 3.7 and 2.3, respectively. We subcategorized the patients into seven groups based mainly on the clinical/radiological findings and disease course. Group A included patients presenting with a Marburg-like TDL (n = 4). Groups B and C comprised patients presenting with monophasic (n = 7) and recurrent TDLs (n = 12), respectively. Multiple sclerosis (MS) patients who subsequently developed TDL (n = 16) during the disease course were categorized as Group D. Group E comprised patients who initially presented with TDL and subsequently developed a classical relapsing–remitting MS without further evidence of TDL (n = 5). Groups F (n = 2) and G (n = 4) involved MS patients who developed TDL during drug initiation (natalizumab, fingolimod) and cessation (interferon, fingolimod), respectively. Regarding long-term treatments applied after corticosteroid administration in the acute phase, B-cell-directed therapies were shown to be highly effective especially in cases with recurrent TDLs. Cyclophosphamide was spared for more aggressive disease indicated by a poor response to corticosteroids and plasma exchange failure.
Conclusion:
Tumefactive central nervous system demyelination is an heterogenous disease; its stratification into distinct groups according to different phenotypes can establish more efficient treatment strategies, thus improving clinical outcomes in the future.
Keywords: classification, demyelinating diseases, MRI, multiple sclerosis, tumefactive
Introduction
Tumefactive demyelinating lesions (TDLs) are defined as large demyelinating lesions (approximately ⩾2 cm in diameter) that can present as tumor-like space-occupying lesions, with or without mass effect, perilesional edema, and characteristic radiographic appearances, such as open ring-enhancement on T1-weighted (T1w) images.1,2 It is well known that TDLs can emerge in the context of multiple sclerosis (MS) and appear during the disease course, or as the initial presenting radiographic feature.3–5 Interestingly, specific disease-modifying drugs (e.g. fingolimod, natalizumab) used in MS have been associated with the occurrence of TDLs, especially after drug initiation or cessation.6–9 There are limited cases describing the presence of large demyelinating lesions with an aggressive clinical presentation during natalizumab therapy that were not progressive multifocal leukoencephalopathy (PML).8–10 Moreover, various pathological entities such as atypical MS variants (Marburg’s type, Balo’s concentric sclerosis, Schilder’s disease, and acute disseminated encephalomyelitis) have been shown to present with tumefactive lesions, with overlapping clinical presentation, and distinct immunological signatures.11–13 Nevertheless, it is still controversial whether tumefactive CNS lesions represent a variant of MS or a unique form of an idiopathic isolated demyelinating disease.14–16 A substantial number of studies have considered TDLs as an heterogeneous group of demyelinating disorders that extend from an isolated monophasic disease (isolated TDL) to a recurrent form of the disease (recurrent TDL), with or without the classical clinico-radiological MS features.17,18 Limited data exist regarding factors or biomarkers associated with the risk of relapse following TDL presentation or the conversion to definite MS.
In this study, we aimed to extend the clinical spectrum of TDLs described so far. Therefore, we retrospectively analyzed the demographics, clinico-radiological, and therapeutic characteristics and the outcomes of patients with tumor-like lesions at disease onset and patients with TDL during the MS disease course. We included patients who had other foci of demyelination, fulfilling or not MS criteria, and associated or not with drug cessation or initiation.
Methods
In a single academic center, we retrospectively evaluated data from patients who had at least one clinical episode related to one or more large demyelinating plaques on brain magnetic resonance imaging (MRI), either as the first clinical event or during the follow-up period of their MS course. Brain biopsy was not an inclusion criterion for this study. Patients with a previous demyelinating event (i.e. confirmed MS) were also included. We excluded patients with Balo-like lesions, as they represent a distinct clinical and radiological disease entity described by our research team,19 as well as pediatric MS patients, and patients with acute demyelinating encephalomyelitis and neuromyelitis optica (NMO). Other exclusion criteria were the presence of peripheral nervous system involvement, other autoimmune comorbidities or systemic diseases, prior exposure to radiation, and radiological or clinical data indicative of an ensuing neoplastic or paraneoplastic process. Based on the aforementioned criteria, 50 patients were identified between 2008 and 2020. All retrospective study procedures were performed under protocols approved by our centers’ ethics committee of Eginition Hospital (12360/2.12.2019). Written informed consent was obtained from all recruited research participants.
Clinical and demographic data
The demographic and clinical data included age at disease onset, age at TDL presentation, sex, and family history of demyelinating disease. Neurological findings at TDL onset were classified as pyramidal, sensory, brainstem, cerebellar, optic neuritis, visual field defects, bowel/bladder/sexual dysfunction, acute cognitive changes, global aphasia, diplopia, and epileptic seizures. We also evaluated: (a) the total follow-up time from disease onset, (b) the number of clinical attacks during the follow-up, (c) the type of disease course (relapsing–remitting, secondary progressive, monophasic), (d) relapse occurrence, (e) treatment during the acute phase (first-line treatments: corticosteroids and/or additional plasma exchange; second-line treatments: immunosuppressive therapeutic intervention, with either first cycle of rituximab or monthly doses of cyclophosphamide, less than 6 months from disease initiation) and long-term treatments during follow-up (chronic treatment, immunomodulatory and/or immunosuppressive therapeutic interventions). Finally, we compared the Expanded Disability Status Scale (EDSS) score at the TDL onset, after acute treatment (<3 months from TDL onset) and at the latest neurological evaluation.
MRI acquisition and analysis
The study protocol included brain MRI scans in all patients, on 1.5 or 3 TESLA scanners, using T1w pre- and post-gadolinium infusion, fluid attenuation inversion recovery, and T2-weighted (T2w) sequences. TDLs were defined as lesions with size ⩾2 cm in diameter, with or without perilesional edema, mass effect, and/or contrast enhancement. We recorded specific radiological parameters at TDL occurrence: the size of all TDLs, number and localization, presence of a mass effect, edema, presence of T2w hyperintensity, fulfillment of the Barkhof criteria,20 periventricular white matter involvement, presence of longitudinally extensive transverse myelitis (extensive involvement of the spinal cord, with abnormal T2 signal traversing at least three vertebral body segments in length), spinal involvement, and brain atrophy on MRI. We further categorized the enhancement pattern at TDL occurrence (homogeneous gadolinium enhancement, heterogeneous gadolinium enhancement, closed ring, open ring, nodular, and punctuate) as well as after acute or chronic treatment (whenever data were available). Enhancement patterns were defined according to the study of Lucchinetti et al. as homogenous (uniform and solid enhancement throughout the lesion); open-ring (whenever the open part was layered toward the gray matter); closed ring (when having a complete clear circular border line); heterogeneous (with variable and complex pattern of enhancement). In indicated patients, more specific enhancement patterns could be observed and included: nodular with distinct areas of enhancement each > 2mm among non-enhancing areas and punctate enhancement characterized by distinct areas of enhancement each <2 mm.21 Repeat neuroimaging was available in 31/50 of patients. Missing participant data on follow-up MRI was either due to the monophasic demyelinating disease without recurrence or due to the unavailability of patients for a second follow-up MRI scan in our neuroimaging facility.
Biological and histological analysis
Cerebrospinal fluid (CSF) test included white blood cell (WBC) count, total protein level, glucose level, IgG index (the normal IgG index reference was <0.65), and oligoclonal band (OCB) evaluation. When available, we reviewed the histological data of brain biopsies performed in stereotactic conditions (available biopsies: n = 6, Supplemental material Table 5 online). We also included in the study the presence of anti-myelin oligodendrocyte glycoprotein (MOG), anti-Aquaporin 4 (AQ4), and antinuclear (ANA) antibodies.
Statistical analysis
We compared variables among the five subpopulations of patients (Marburg-like TDL, monophasic TDL, recurrent TDL, MS→TDL, and TDL→MS). Mann–Whitney test was used for quantitative variables. A p-value <0.05 was considered as the threshold of statistical significance. Analyses and image construction were performed using GraphPad-software.
Results
Demographic and clinical cohort features
The main demographic and clinical features of all patients included in our study are shown in Table 1 and Supplemental Tables 1 and 2. Analysis of patients’ past medical and family histories did not indicate any potential preceding triggering factors or presence of comorbid autoimmune conditions; however, a family history of MS was found in seven patients (14%). Sixty-six patients were women with a mean age of 34.44 (SD ± 10.60) years at disease initiation and 38.12 (SD ± 10.67) years at TDL presentation. The mean follow-up time of our patients from disease onset was 76.44 (SD ± 73.24) months. At TDL presentation, neurological signs of pyramidal involvement were observed in almost all cases (94%), whereas sensory involvement was evident in approximately 60% of the cases. Patients also manifested visual deficits (hemianopsia or cortical blindness, 18%), optic neuritis (8%), brainstem involvement (14%), and cerebellar involvement (28%). Global aphasia and epileptic seizures occurred in two (4%) and three (6%) cases, respectively (Table 1, Supplemental Tables 1 and 2, Supplemental Figure 1). TDL was the first neurological event in 28 of the 50 (56%) patients analyzed, while the others had another prior demyelinating event or already carried a diagnosis of MS. MS criteria during the total follow-up were fulfilled in 43 patients. The mean number of clinical relapses was 2.42 (SD ± 1.162) and only 10 patients underwent only one clinical attack. After the first attack with TDL, 24% (12/50) of patients developed new TDLs during the follow-up with a mean number of clinical attacks of 3.25 (SD ± 1.485) (Table 1). Among patients with TDL with a previous MS diagnosis, 13 cases were relapsing–remitting MS (RRMS; 81%), whereas three cases were categorized as secondary-progressive MS (19%). The mean duration between the disease onset (MS) and the development of the first TDL was 75.63 ± 75.49 months. When patients with a prior history of MS developed TDLs they were either on therapy (56%) or treatment-naïve (44%). Drugs used for MS treatment before TDL appearance were interferons (31%), fingolimod (6%), natalizumab (6%), and glatiramer acetate (25%) for a period of more than 12 months.
Table 1.
Clinico-radiological characteristics of the subgroups of patients with TDL.
| Parameter analyzed | A: Marburg-like TDL n = 4 |
B: Monophasic TDL n = 7 |
C: Recurrent TDL n = 12 |
D: MS →TDL n = 16 |
E: TDL →MS n = 5 |
Statistical differences |
|---|---|---|---|---|---|---|
| Clinical and biological characteristics of the subgroups | ||||||
| Demographics | ||||||
| Age at disease initiation, years, mean (± SD) | 41 (12.36) | 34.14 (13.23) | 33.42 (11.41) | 34.06 (7.629) | 39 (15.60) | NS |
| Age at TDL presentation, years, mean (± SD) | 41 (12.36) | 37.71 (9.759) | 34.92 (11.91) | 40.63 (9.186) | 39 (15.60) | NS |
| Sex, female (%) | 4/4 (100) | 5/7 (71) | 7/12 (58) | 11/16 (69) | 3/5 (40) | NS |
| Positive family history of demyelinating disease (%) | 0/4 (0) | 1/7 (14) | 0/12 (0) | 4/16 (25) | 0/5 (0) | NS |
| General clinical data | ||||||
| Follow-up from disease onset, months, mean (± SD) | 57 (56.70) | 68.57 (33.99) | 54.25 (43.03) | 104.9 (106.6) | 29.6 (18.96) | B versus D; p = 0.0278, E versus D; p = 0.0377 |
| Number of clinical attacks during follow-up, mean (± SD) | 1.25 (0.5000) | 1 (0.000) | 3.25 (1.485) | 2.563 (0.8921) | 2 (0.000) | A versus C; p = 0.0192, A versus D; p = 0.0043, A versus E; p = 0.0476, B versus C; p = 0.0003, B versus D; p < 0.0001, B versus E; p = 0.0013 |
| MS criteria fulfilled during follow-up (%) | 2/4 (50) | 2/7 (29) | 12/12 (100) | 16/16 (100) | 5/5 (100) | NS |
| EDSS score at first tumefactive attack, mean (± SD) | 3.5 (1.732) | 3.357 (1.864) | 3.792 (1.658) | 3.719 (1.366) | 2.4 (0.9618) | NS |
| CSF studies | ||||||
| WBC, cells/μL, mean (± SD) | 0 (0.000) | 12.6 (9.555) | 1.286 (1.254) | 7.8 (7.510) | 11.5 (16.26) | B versus C; p = 0.0189, C versus D; p = 0.0169 |
| Protein, mg/dL, mean (± SD) | 35 (0.000) | 50.6 (10.88) | 41.29 (11.40) | 45.9 (29.77) | 26 (14.14) | NS |
| Glucose, mg/dL, mean (± SD) | 70 (0.000) | 62.2 (9.654) | 61.14 (8.915) | 62.2 (10.65) | 65.5 (0.7071) | NS |
| IgG index, mean (± SD) | 0.4 (0.000) | 0.996 (0.3743) | 0.5229 (0.09517) | 0.871 (0.4021) | 0.855 (0.4596) | B versus C; p = 0.0101, C versus D; p = 0.0452 |
| Oligoclonal bands, (%) | 0/1 (0) | 5/6 (83) | 4/10 (40) | 11/12 (92) | 1/3 (33) | C versus D; p = 0.0201 |
| MRI characteristics of the subgroups | ||||||
| A. Number of TDLs at disease onset with TDL | ||||||
| Solitary (%) | 4/4 (100) | 5/7 (71) | 9/12 (75) | 13/16 (81) | 5/5 (100) | NS |
| Number of TDLs, mean (± SD) | 1.000 (0.000) | 1.286 (0.4880) | 1.333 (0.6513) | 1.25 (0.5774) | 1 (0.000) | NS |
| B. Radiological characteristics at disease onset with TDL | ||||||
| Size of all TDLs, cm, mean (± SD) | 7.488 (2.196) | 3.771 (1.363) | 3.338 (0.7699) | 2.813 (0.5968) | 2.482 (0.4187) | A versus B; p = 0.0212, A versus C; p = 0.0011, A versus D; p = 0.0004, A versus E; p = 0.0159, C versus E; p = 0.0357 |
| Presence of mass effect (%) | 3/4 (75) | 0/7 (0) | 1/12 (8) | 1/16 (6) | 0/5 (0) | A versus B; p = 0.0242, A versus C; p = 0.0269, A versus D; p = 0.0134, A versus E; p = 0.0476 |
| Presence of edema (%) | 3/4 (75) | 2/7 (29) | 3/12 (25) | 5/16 (31) | 0/5 (0) | A versus E; p = 0.0476 |
| Presence of T2w hyperintensity (%) | 4/4 (100) | 3/7 (43) | 4/12 (33) | 11/16 (69) | 1/5 (20) | A versus E; p = 0.0476 |
| Barkhof criteria (%) | 0/4 (0) | 2/7 (29) | 6/12 (50) | 16/16 (100) | 3/5 (60) | A versus D; p = 0.0035, B versus D; p = 0.0006, C versus D; p = 0.0025, D versus E; p = 0.0476 |
| Spine MRI involvement (%) | 1/4 (25) | 2/7 (29) | 6/12 (50) | 10/16 (63) | 2/5 (40) | NS |
| Presence of LETM (%) | 0/4 (0) | 0/7 (0) | 2/12 (17) | 2/16 (13) | 0/5 (0) | NS |
| PVWM involvement (%) | 2/4 (50) | 4/7 (57) | 8/12 (67) | 16/16 (100) | 3/5 (60) | A versus D; p = 0.0316, B versus D; p = 0.0198, C versus D; p = 0.0242, D versus E; p = 0.0476 |
| Atrophy (%) | 0/4 (0) | 1/7 (14) | 3/12 (25) | 10/16 (63) | 2/5 (40) | NS |
| C. Enhancement pattern of disease onset with TDL | ||||||
| Presence of GD+ in TDL (%) | 4/4 (100) | 4/7 (57) | 10/12 (83) | 15/16 (94) | 4/5 (80) | NS |
| Homogeneous GD+ (%) | 1/4 (25) | 1/7 (14) | 2/12 (17) | 1/16 (6) | 0/5 (0) | NS |
| Heterogeneous GD+ (%) | 3/4 (75) | 2/7 (29) | 0/12 (0) | 5/16 (31) | 1/5 (20) | A versus C; p = 0.0071 |
| Closed ring, percentage (%) | 0/4 (0) | 0/7 (0) | 4/12 (33) | 6/16 (38) | 0/5 (0) | NS |
| Open ring (%) | 0/4 (0) | 0/7 (0) | 4/12 (33) | 1/16 (6) | 3/5 (60) | B versus E; p = 0.0455, D versus E; p = 0.0276 |
| Nodular (%) | 1/4 (25) | 1/7 (14) | 1/12 (8) | 1/16 (6) | 0/5 (0) | NS |
| Punctuate (%) | 0/4 (0) | 0/7 (0) | 1/12 (8) | 2/16 (13) | 0/5 (0) | NS |
| D. Radiological characteristics after acute treatment | ||||||
| Size of all TDLs, cm, mean (± SD) | 2.125 (3.005) | 3.147 (0.8418) | 2.052 (1.049) | 1.773 (0.9438) | 1.75 (0.3820) | B versus D; p = 0.0315 |
| Enhancement (%) | 0/2 (0) | 1/3 (33) | 1/9 (11) | 1/10 (10) | 0/3 (0) | |
| E. Radiological characteristics at last follow-up | ||||||
| Size of all TDLs, cm, mean (± SD) | 6.565 | NA | NA | 1.082 (0.8824) | 1.195 (0.3182) | NS |
| Enhancement (%) | 1/2 (50) | NA | NA | 0/6 (0) | 0/2 (0) | NS |
| Treatment strategies in the subgroups | ||||||
| First line treatment after TDL | ||||||
| Corticosteroids (%) | 4/4 (100) | 7/7 (100) | 12/12 (100) | 16/16 (100) | 5/5 (100) | NS |
| Additional plasma exchange (%) | 0/4 (0) | 2/7 (29) | 3/12 (25) | 1/16 (6) | 0/5 (0) | NS |
| Second line treatment | ||||||
| A. Immunomodulatory therapeutic approach | ||||||
| Glatiramer acetate (%) | 2/4 (50) | 1/7 (14) | 5/12 (42) | 2/16 (13) | 1/5 (20) | NS |
| Teriflunomide (%) | 0/4 (0) | 0/7 (0) | 0/12 (0) | 2/16 (13) | 0/5 (0) | NS |
| Fingolimod, percentage (%) | 0/4 (0) | 0/7 (0) | 0/12 (0) | 1/16 (6) | 1/5 (20) | NS |
| Natalizumab (%) | 0/4 (0) | 0/7 (0) | 1/12 (8) | 1/16 (6) | 1/5 (20) | NS |
| Alemtuzumab (%) | 0/4 (0) | 0/7 (0) | 1/12 (8) | 2/16 (13) | 1/5 (20) | NS |
| B. Immunosuppressive therapeutic approach | ||||||
| Cyclophosphamide (%) | 4/4 (100) | 3/7 (43) | 6/12 (50) | 3/16 (19) | 0/5 (0) | A versus D; p = 0.0072, A versus E; p = 0.0079 |
| Rituximab (%) | 1/4 (25) | 3/7 (43) | 6/12 (50) | 5/16 (31) | 0/5 (0) | C versus D; p = 0.0441 |
| Azathioprine (%) | 0/4 (0) | 1/7 (14) | 0/12 (0) | 1/16 (6) | 0/5 (0) | NS |
| Mitoxandrone (%) | 1/4 (25) | 0/7 (0) | 1/12 (8) | 1/16 (6) | 0/5 (0) | NS |
| Clinical response to treatment strategies | ||||||
| EDSS after acute treatment, mean (± SD) <3 months from disease initiation | 2.875 (0.8539) | 2.214 (1.468) | 2.542 (0.8649) | 2.375 (1.466) | 1.3 (0.4472) | A versus E; p = 0.0238, C versus E; p = 0.0134 |
| EDSS at last follow up, mean (± SD) | 3.75 (4.193) | 2.071 (1.170) | 2.5 (1.523) | 2.281 (1.653) | 1.2 (0.4472) | C versus E; p = 0.0498 |
NS = non-statistically significant difference among subgroup analysis for multiple comparisons for all of the p > 0.05.
CSF, cerebrospinal fluid; EDSS, Expanded Disability Status Scale; GD+, gadolinium enhancement; LETM, longitudinal extensive transverse myelitis; MRI, magnetic resonance imaging; MS, multiple sclerosis; PVWM, periventricular white matter; SD, standard deviation; T2w, T2 weighted; TDL, tumefactive demyelinating lesion; WBC, white blood cell.
MRI findings in TDLs
Examples of patients’ MRI are shown in Figures 1–5 and Supplemental Figures 3 and 4 . The mean largest diameter of TDLs on MRI was 3.45 (SD ± 1.56) cm. Edema surrounded the lesions in 14 cases (29%) and a mass effect was evident in six cases (12%). The presence of T2w hyperintensity inside the TDL was prominent in 29 cases (57%) (Table 1 and Supplemental Tables 1 and 2). Regarding topography, lesions were most common in the parietal (31 patients, 62%) and frontal lobes (26 patients, 52%) and less in the temporal (13 patients) and occipital lobes (10 patients), and the cerebellum (five patients) (Supplemental Figure 2). At the initial brain MRI of the tumefactive attack, solitary TDLs were observed in 40 patients (80%), whereas 10 patients presented with multiple TDLs. The mean number of TDL lesions in our cohort was 1.3 (SD 0.74). Of all patients, 67% fulfilled the radiological Barkhof criteria at TDL onset (Table 1 and Supplemental Tables 1 and 2). Interestingly, in patients initially presenting with TDL, the radiological Barkhof criteria were fulfilled in 29% of those with monophasic TDL, in 50% of patients with subsequent TDL attacks, and in 60% of patients that eventually developed classical MS (without evidence of further TDL attacks) (Table 1). On T1w sequences, gadolinium enhancement was observed in 43 patients (86%), with a closed ring and open ring enhancement in 13 (26%) and 10 (20%) patients, respectively. Other gadolinium enhancement patterns observed were homogeneous (10%), heterogeneous (26%), nodular (8%), and punctuate (6%). Spinal lesions were observed in 27 of 50 (53%) patients (five with longitudinally extensive lesions, seronegative in AQ4, MOG antibodies) (Table 1 and Supplemental Tables 1 and 2). MRIs were available in 31 out of 50 patients for analysis of TDL characteristics after acute treatment with either corticosteroids or plasma exchange. At this time point, the mean largest diameter of the previous TDLs was 1.967 (SD ± 1.116) cm (marked reduction in mean size compared with initial TDL size) and residual gadolinium enhancement was observed only in three patients (Table 1 and Supplemental Tables 1 and 2). After chronic treatment, during the last follow-up, the mean diameter of the previous TDLs was 1.703 (SD ± 1.938) cm and none of the patients had any gadolinium enhancement.
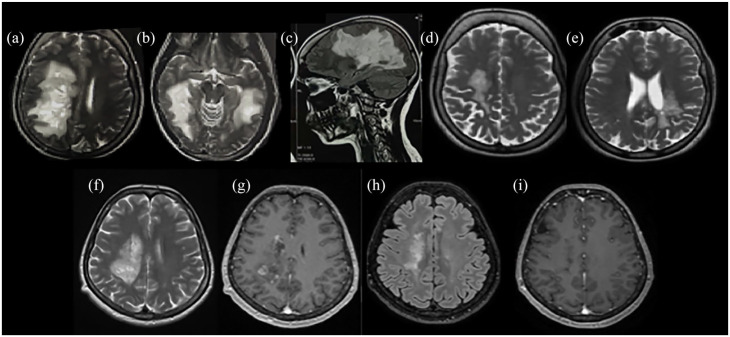
Figure 1.
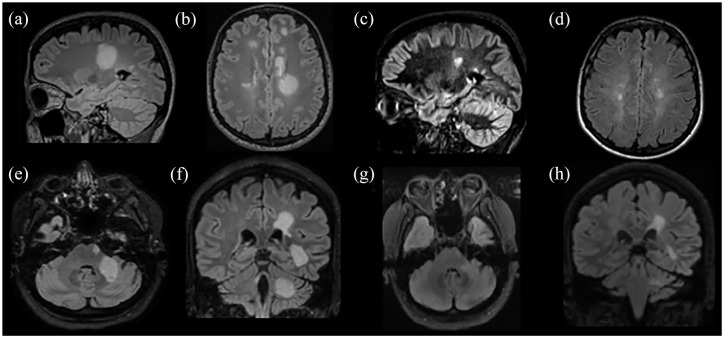
Radiological characteristics of two patients presented with Marburg-like tumefactive lesions. Bilateral Marburg-like tumefactive lesions of a patient at disease onset [(a)–(c)], after chronic treatment with mitoxantrone, cyclophosphamide, and glatiramer acetate showing significant resolution of the lesions [(d) and (e)]. This patient, 10 years after disease onset has an EDSS score of 1. A second patient with Marburg-like demyelination in the right centrum semiovale at onset [(f)] with Gd+ [(g)] and after three monthly cycles of cyclophosphamide. A significant resolution of the tumefactive lesion with no Gd+ was observed after three monthly cycles of cyclophosphamide [(h) and (i)] with EDSS score 1.5.
T2-weighted images: (a), (b), (d) to (f). FLAIR images: (c), (h). T1-weighted contrast-enhanced images: (g), (i).
EDSS, Expanded Disability Status Scale; FLAIR, fluid-attenuated inversion recovery; Gd+, gadolinium enhancement.
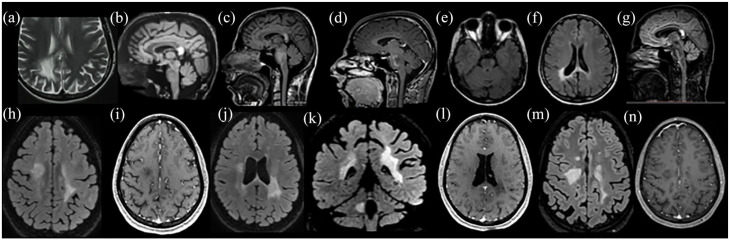
Figure 2.
Radiological characteristics of two patients presented with tumefactive lesions with recurrent disease course with TDL (recurrent TDL). (a)–(g): Patient 1 presented with two simultaneous TDLs at initial TDL attack; in the right parietal lobe [(a)] and in the splenium of the corpus callosum [(b)]. On the second attack, a new TDL was observed in the pons [(c)] with Gd+ [(d)], another brain area distinct from the initial attack. Two years after rituximab and cyclophosphamide treatment all TDLs were significantly reduced in size [(e)–(g)]. A brain biopsy was performed in the persistent lesion in the splenium of the corpus callosum, which was indicative of demyelination. (h)–(n): Patient 2 presented with two TDLs at the initial tumefactive attack in the right corona radiata [(h)] with subtle Gd+ [(i)] and in the left parietal-temporal region [(j) and (k)] with no Gd+ [(l)]. Two years after, a second clinical attack occurred due to an increase in size of the initial TDL at the right centrum semiovale [(m)] without Gd+ [(n)]. The patient initially received intravenous cyclophosphamide cycles that were discontinued due to treatment failure and switched to rituximab after the second clinical attack resulting in disease remission.
T2-weighted image: (a). FLAIR images: (b), (c), (e) to (h), (j), (k), (m). T1-weighted contrast-enhanced images: (d), (i), (l), (n).
FLAIR, fluid-attenuated inversion recovery; Gd+, gadolinium enhancement; TDL, tumefactive demyelinating lesion.
Figure 3.
Representative examples of MS patients presented with tumefactive lesions after fingolimod cessation and after fingolimod or natalizumab initiation. Patient 1 developed five TDLs [(a) and (b)] 5 months after fingolimod cessation with both closed and open ring Gd+ pattern [(c) and (d)]. Patient 2 presented with two TDLs [(e)] with heterogeneous Gd+ [(f)] 3 months after natalizumab initiation in the right frontal and right temporal lobes. MRI of a third patient with a TDL in the left middle cerebellar peduncle [(g)] 1 month after fingolimod initiation with subtle Gd+ [(h)].
FLAIR images: (a), (b), (e). T2-weighted image: (g). T1-weighted contrast-enhanced images: (c), (d), (f), (h).
FLAIR, fluid-attenuated inversion recovery; Gd+, gadolinium enhancement; MRI, magnetic resonance imaging; TDL, tumefactive demyelinating lesion.
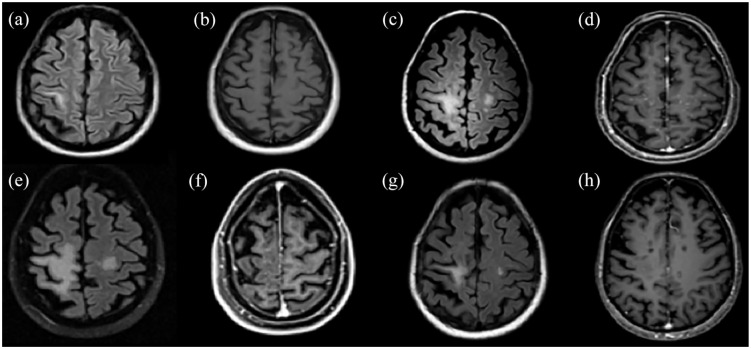
Figure 5.
A paradigm of successfully treated TDL with rituximab in a patient with MS during chronic treatment with natalizumab. (a) and (b) The patient presented for the first time TDL-like lesion (non-PML) in the right precental gyrus with no Gd+ during natalizumab treatment. (c) and (d) After natalizumab withdrawal and IVMP course, a significant MRI activity with enlargement of the TDL (non-PML) and multiple bilateral hemispheric punctuate Gd+ lesions were noted. (e) and (f) Further increase in size of the TDL with a little reduction of the punctuate Gd+ lesions following three monthly cycles of cyclophosphamide (cyclophosphamide failure, 9 months after TDL-like lesion appearance). (g) and (h) Remarkable reduction of the TDL (non-PML) with slight remaining Gd+ post-rituximab initiation.
FLAIR images: (a), (c), (e), (g). T1-weighted contrast-enhanced images: (b), (d), (f), (h).
Gd+, gadolinium enhancement; IVMP, intravenous methylprednisolone; MRI, magnetic resonance imaging; MS, multiple sclerosis; PML, progressive multifocal leukoencephalopathy; TDL, tumefactive demyelinating lesion.
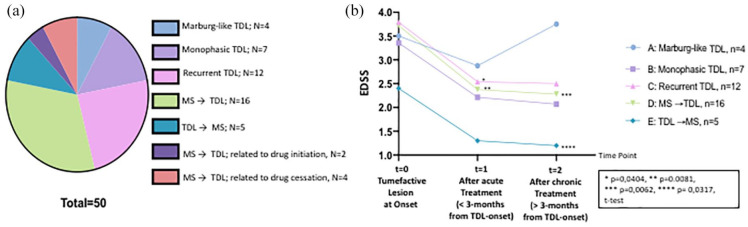
Figure 6.
Stratification of patients in subgroups. (a) Illustrative graph with different colors showing the various subgroups of patients included in the present study. Group A consisted of patients presented with Marburg-like TDL (n = 4). Groups B and C consisted of patients presented with monophasic (n = 7) and recurrent TDL (n = 12), respectively. Group D consisted of MS patients that subsequently developed TDL (n = 16) during MS disease course. Group E comprised patients initially presenting with TDL and subsequently developing MS without evidence of TDL (n = 5). Groups D (n = 2) and F (n = 4) involved patients with MS who developed TDL during drug initiation (natalizumab, fingolimod) and cessation, (interferon/possible, fingolimod) respectively. (b) Graph showing the EDSS score of each subgroup analyzed at TDL onset, after acute treatment (less that 3 months of disease initiation), and after the last follow-up. Statistically significant differences in the EDSS scores among the various time points in each subgroup are depicted in the figure. GraphPad was applied for image making and statistical analysis.
EDSS, Expanded Disability Status Scale; MS, multiple sclerosis; TDL, tumefactive demyelinating lesion.
Biological findings
CSF analysis showed an increased IgG index (mean, 0.83; SD ± 0.44) in patients with TDLs (11 out of 26 patients tested presented high IgG index, 42%), with a mean WBC count of 6.73 (SD ± 8.13) cells/μL and mean protein level of 42.62 (SD ± 20.97) mg/dL. OCBs were found in 24/35 (68.6%) of patients tested. A low titer of ANA antibodies (>1:80) was detected in four patients, whereas anti-MOG and AQ4 antibodies were negative (Table 1 and Supplemental Table 1).
Outcome profiles of patients presenting with TDLs
Details of follow-up and treatment strategies in our cohort are summarized in Table 1 and Supplemental Table 1. By the end of the follow-up, 17 patients had significantly improved (>50% reduction in the EDSS score) and 31 were stable, whereas two had worsened. One patient died after a disease course of 2 years due to the neurological consequences of the TDL (previously reported by our research team).22 For the purposes of our study, we assigned an EDSS score of 10 to this patient. The mean EDSS score of the cohort at the TDL onset and at the end of the follow-up was 3.65 (SD ± 1.51) and 2.32 (SD ± 1.76), respectively.
Stratification in subgroups
Based mainly on literature reviews, the clinical/radiological data of our patients, and considering the disease course during the total follow-up time, we classified our patients in seven major subgroups (Figure 6).21 Group A consisted of patients presenting with Marburg-like TDL (n = 4) (Figure 1). Groups B and C consisted of patients presenting with monophasic (n = 7) and recurrent TDLs (n = 12), respectively (Figure 2 and Supplemental Figure 3). Group D consisted of MS patients who subsequently developed TDL (n = 16) during the disease course (Figure 5 and Supplemental Figure 4). Group E comprised patients initially presenting with TDL and subsequently developing classical RRMS without further evidence of TDL (n = 5). Group D (n = 2) and F (n = 4) involved patients with MS who developed TDL during drug initiation (natalizumab, fingolimod) and cessation, (interferon/possible, fingolimod), respectively (Figure 3).
Differences observed among the subgroups regarding clinical, radiological, and biological data are presented in Table 1, Supplemental Tables 2 and 3, and Figure 6. Subgroup analysis showed that patients with monophasic TDL compared with those with recurrent TDL had higher WBC count in the CSF analysis and a higher IgG index, but the presence of OCBs did not differ significantly among groups (Table 1). Moreover, a pattern of open ring contrast enhancement was more prominent in patients with TDL who subsequently developed MS compared with patients with monophasic TDLs (Table 1). The frequency of positive OCB in patients with monophasic TDL was 83%, whereas in patients with TDL at disease initiation who subsequently developed classical MS it was 33% (no statistical significance between these subgroups due to the small number of data). The frequency of positive OCB was very high (90%) in the group of MS patients who subsequently developed TDL (92%), compared with the recurrent TDL group (40%), and that difference was statistically significant (p = 0.02) (Table 1).
Progression to MS
Of the patients (n = 24) who initially presented with TDL (Marburg variant excluded), seven (29%) had monophasic TDL (71% female), 12 (50%) had recurrent TDLs (58% female), and five (21%) developed classical MS without the emergence of further TDLs (40% female) during the total follow-up time. Patients with recurrent TDLs eventually fulfilled the MS diagnostic criteria (MacDonald) during the follow-up time (mean time: 54.25 months; SD ± 43.03). The mean follow-up time of cases with monophasic TDL was 68.57 (SD ± 33.99) months and of cases with TDL who subsequently developed MS 29.6 (SD ± 18.96) months. Longer follow-up periods will reveal the progression of TDL to definite MS. We compared patients with TDL at initial presentation who did not convert to MS (n = 10) with those with TDL who eventually developed MS (as defined by the MacDonald criteria, including patients with recurrent TDL) (TDL converters; n = 18). Non-TDL converters exhibited larger TDLs (p = 0.01) in brain MRI analysis with prominent heterogeneous gadolinium enhancement pattern (p = 0.0126) and less frequent open ring enhancement pattern (p = 0.0593), as well as a greater number of WBC count (p = 0.0256) and protein level (p = 0.0663) in CSF analysis, compared with TDL converters. There were no significant differences between groups regarding sex, OCB presence, and EDSS score (Supplemental Table 4).
TDL onset in MS patients related to drug initiation or cessation
We identified two patients who developed TDLs after natalizumab and fingolimod initiation. The time after drug initiation was 3 and 1 months from natalizumab and fingolimod, respectively. Moreover, four patients developed TDL after drug cessation and specifically one after interferon withdrawal (11 months from the last dose, possible association due to long time interval, but a causal relationship cannot be totally excluded) and three after fingolimod withdrawal (5.75 months; mean time from the last dose). The clinical, biological, and radiological features of these patients are summarized in Supplemental Table 3 and characteristic images are depicted in Figure 3.
Treatments used in the cohort
Regarding acute treatment after TDL appearance, all patients received high doses of corticosteroids (100%), whereas only 7/50 (14%) were placed on additional plasma exchange therapy. The mean EDSS score after the acute treatment was 2.35 (SD ± 1.196). In the total cohort of TDL patients, chronic treatment involved both immunomodulatory drugs, such as glatiramer acetate (22%), teriflunomide (4%), fingolimod (4%), natalizumab (18%), and alemtuzumab (8%), as well as immunosuppressive drugs, such as cyclophosphamide (34%), rituximab (28%), azathioprine (4%), and mitoxantrone (6%). Sixteen patients (32%) received more than one treatment agent during follow-up as the first could not totally control disease activity and progression (change to another immunomodulatory or immunosuppressive therapy, or change from an immunomodulatory to immunosuppressive treatment) (Table 1). We did not find any significant correlation among the radiographic characteristics of tumefactive lesions and the various treatments used or any correlation with demographic or regional data. Decisions regarding therapeutic strategies after first-line treatment were made based on various factors such as lesion characteristics (size and presence of edema or mass effect), the initial response to corticosteroids, fulfillment of the Barkhof criteria, presence of OCBs, and suggestions retrieved from the literature for tumefactive MS (expert opinions/small case series). We stratified our patients with initial TDL presentation in two subgroups, based on the presence or not of concomitant lesions typical of MS (the radiological Barkhof criteria were applied to this categorization). In Group 1, patients with isolated TDLs who did not fulfill the Barkhof criteria were treated with intense immunosuppressant therapy; cyclophosphamide: eight cases out of 16 (50%) and rituximab: four cases out of 16 (25%). Of the patients in Group 1, 43% were placed on glatiramer acetate for maintenance therapy. Patients presenting with a TDL and more typical MS lesions simultaneously, fulfilling the radiological Barkhof criteria or having a prior history of MS (Group 2; n = 12), were mainly treated with rituximab (50%) and, to a lesser extent, with cyclophosphamide (40%), while also other less immunosuppressive therapies, such as glatiramer acetate (16%), were applied. A limited number of cases were treated with natalizumab (n = 1) and alemtuzumab (n = 1). Patients who presented initially with isolated TDLs and during follow-up developed more typical MS relapses without further TDLs (classical RRMS) were treated with chronic maintenance therapies with MS disease-modifying drugs (e.g. natalizumab, glatiramer acetate). An interesting case presented with an atypical (non-PML) TDL during chronic treatment for MS with natalizumab and was eventually treated with rituximab (Figure 5).
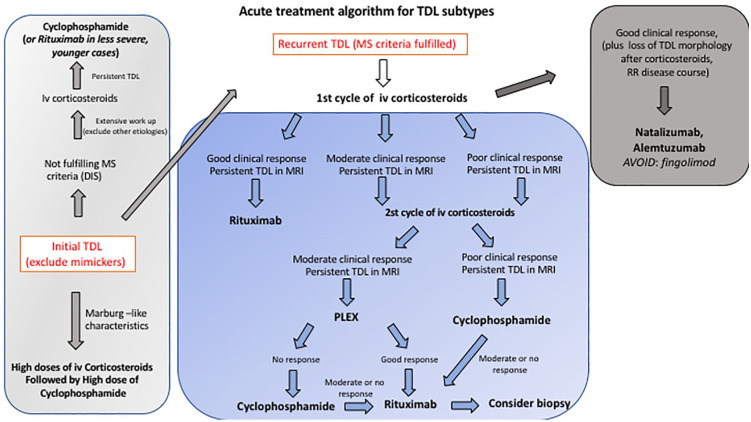
A distinct disease subgroup in our cohort was that of recurrent TDLs (Figure 2). We noticed a variable and incomplete response to first-line treatment with steroids. For better therapeutic results, acute treatment with either cyclophosphamide (50%) or rituximab (50%) was initiated after first-line treatment with corticosteroids. Good radiological and clinical response to corticosteroids was a factor favoring further B cell depletion therapy with rituximab. Patients with moderate or no response to steroid therapy were treated with either plasmapheresis (n = 3) or monthly doses of cyclophosphamide (n = 4). Patients who responded partially to plasmapheresis were eventually successfully treated with cyclophosphamide (n = 2). One patient that did not respond well to steroids but eventually responded to plasmapheresis was placed on rituximab (Figure 4). Three patients with high disease activity were placed during the follow-up in both cyclophosphamide and subsequent rituximab therapy. Drugs used in the interim between attacks were mostly glatiramer acetate and only two case received natalizumab and alemtuzumab respectively (Table 1). Suggested treatment algorithm used in the present study is depicted in Figure 7. This algorithm represent our clinical experience, and validation in larger cohorts is warranted for better therapeutic decisions.
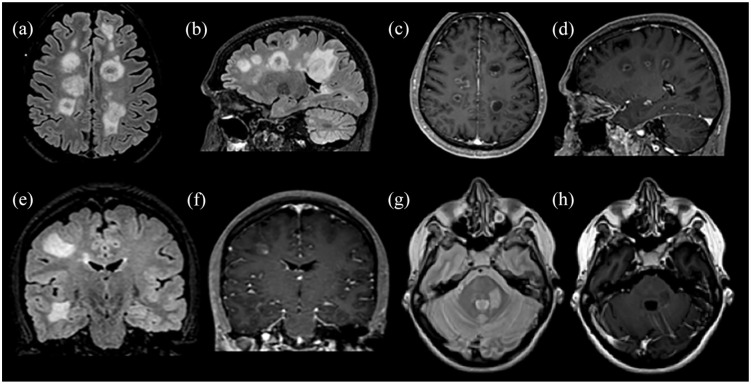
Figure 4.
Paradigms of successfully treated patients with TDL after alemtuzumab or rituximab. (a) and (b) Images represent a patient with a TDL in the left centrum semiovale, treated in the acute phase with IVMP. Subsequently, the patient developed a typical MS and received 6 months later the first course of alemtuzumab; an excellent clinical and radiological response 1 year after was noted with also a significant decrease in the TDL size [(c) and (d)]. The second patient presented with multiple TDLs in the left cerebral hemisphere and left middle cerebellar peduncle [(e) and (f)]; responded well to rituximab courses with no further clinical attacks [(g) and ( h)].
FLAIR images: (a), (b), (d) to (h). Double inversion recovery (DIR) image: (c).
Gd+, gadolinium enhancement; IVMP, intravenous methylprednisolone; MS, multiple sclerosis; TDL: tumefactive demyelinating lesion.
Figure 7.
Suggested treatment algorithm for patients initially presented with TDL depending on data from this manuscript.
DIS, dissemination in space; i.v., intravenous; MRI, magnetic resonance imaging; MS, multiple sclerosis; PLEX, plasma exchange therapy; RR, relapsing–remitting; TDL, tumefactive demyelinating lesion.
Discussion
Our current series spans the spectrum of tumefactive demyelination from an isolated demyelinating syndrome, like clinically isolated syndrome, to a demyelinating syndrome with atypical radiological and clinical findings (Marburg variant), to a recurrent form of demyelination with TDL, and finally to classical MS.13 One of the largest case series of biopsy-proven tumefactive demyelination reported that 14% of patients exhibited a monophasic course, whereas 70% of patients eventually developed definite MS, with a median time to relapse of 4.8 years.21 Other studies have shown that approximately 50% of patients initially diagnosed with TDL finally converted to definite MS, with a mean time to conversion shown to be 8 months and presence of OCBs at TDL onset to confer a high probability for MS diagnosis during follow-up.23 In the study by Tremblay et al., the percentage of patients exhibiting relapse was 29% when they presented with solitary TDL and 33% in multifocal lesions at initial presentation.24 Siri et al. described three possible TDL evolutions over time: conversion to MS (31%), isolated TDL (63%), and recurrent TDL (6%).18
In our study, among patients who initially presented with TDL (n = 28), approximately 36% did not have any further demyelinating episodes and thus were classified as monophasic TDL, 43% had recurrent TDL lesions, and 18% developed classical MS without the emergence of further TDLs during the follow-up. Nevertheless, a limitation that should be considered is that further patients with TDL may evolve to MS or recurrent TDLs in the future. Importantly, from patients (n = 7) that during the follow-up exhibited monophasic disease course (Marburg-like disease excluded), only two fulfilled MacDonald criteria, so a considerable number of patients did not show any signs of dissemination in space. Regarding the severity of the overall clinical course of TDLs, we showed that our patients, in agreement with previous reports, exhibited a favorable disease course (mean reduction in the EDSS score from disease initiation to last follow-up; 36%) with only a few exceptions (n = 2) and one death.25,26 We believe that the appropriate therapeutic decisions, as described below, beyond disease-inherent factors, contributed to this benign overall clinical course.
In our cohort, patients with isolated TDLs who did not fulfill the MS diagnostic criteria were treated with a more intense immunosuppressant therapy [mainly placed on cyclophosphamide (eight out of 16 cases) and fewer on rituximab (four cases)]. A considerable number of such cases were placed on glatiramer acetate for maintenance therapy (43%). Patients presenting with TDL and more typical MS lesions simultaneously, fulfilling the radiological Barkhof criteria or having a prior history of MS, were mainly treated with rituximab (11 out of 27 cases; 41%) and to a lesser extent with cyclophosphamide (seven out of 27; 26%), while other less immunosuppressive therapies, such as glatiramer acetate (15%), teriflunomide (7%), and azathioprine (4%), were also applied. Patients not fulfilling the radiological Barkhof criteria or without prior history of MS were treated mainly with cyclophosphamide (nine out of 17, 53%) and treated less with rituximab (four out of 17, 24%), glatiramer acetate (seven out of 17, 41%), or azathioprine (6%) during the total follow-up period.
Recurrent TDL represents a clinical/radiological syndrome that has not been thoroughly described before due to the limited numbers of cases.18,22,27–30 It is unclear whether such cases belong to a separate subset of demyelinating diseases of the CNS or represent a variant of MS. Further clinical evidence on natural history and optimal treatment is warranted. Herein, we present for the first time in the literature 12 patients with recurrent TDL and try to identify any distinct features of this disease entity. These patients, who all fulfilled MS criteria during the total follow-up time, exhibited the highest number of clinical attacks compared with the other disease subgroups. Of these patients, 40% had positive OCBs. Our analysis did not reveal any difference in the presence of OCB among patients with monophasic TDL and those with recurrent TDL. In accordance with previous observations showing that approximately 50% of tumefactive MS patients were resistant to steroid therapy and developed fulminant attacks, we noticed a variable and incomplete response to first-line treatment with steroids.31 For that reason, subsequent plasmapheresis and/or immunosuppression was immediately initiated to achieve further clinical and radiological improvement. The decision on the immunosuppressant agent was based on the magnitude of response to steroids or plasmapheresis. Generally, a good response to either steroids or plasmapheresis prompted us to continue with rituximab, whereas in patients with the incomplete response we further treated them with cyclophosphamide. Within the first 3 months of such treatment, patients exhibited optimal therapeutic benefits and maintained stable during the follow-up. Herein, we also report one case with recurrent TDL in which the disease was controlled by alemtuzumab treatment. This patient with positive OCB presented with recurrent TDL that was initially treated with natalizumab, but further attacks with TDL and seropositivity for John Cunningham (JC) virus led to the switch to alemtuzumab. Of note, TDLs of this patient during attacks were very responsive to corticosteroid treatment (significant reduction in size, no more fulfilling TDL criteria) and the disease course was typical relapsing–remitting, like classical MS. So, we believe that second-line disease modifying therapies used in classical MS, with the exception of fingolimod, under specific disease settings and with high clinical vigilance, could be applied to patients with highly active relapsing TDL fulfilling MS diagnosing criteria and optimal response to first-line treatment with corticosteroids, as previously described by others in small case series.32–35 The overall clinical response considering the EDSS score during the last visit was benign (only one patient was resistant to cyclophosphamide, rituximab, and mitoxantrone). There was a statistically significant reduction in the EDSS score (~33%) after acute treatment (measured at the first 3 months from TDL onset) with high doses of corticosteroids and subsequent immunosuppression with either rituximab or cyclophosphamide that was maintained stable with the subsequent chronic immunomodulatory or immunosuppressant therapies. The disease pathogenesis and the ensuing immune mechanisms associated with recurrent TDL are still elusive and further studies are needed to strengthen our results.
There are inadequate data regarding TDLs arising during long term treatment with immune modifying drugs in MS.36 We could not identify any predisposing factor for TDL emergence. Interestingly, a rebound syndrome can occur as an increase in disease activity after treatment cessation in MS, especially when discontinuing natalizumab or fingolimod. We found three cases who developed TDLs as a rebound after fingolimod cessation with a mean time lapse between treatment withdrawal and rebound found to be 5.75 months. Acute treatment for rebound included a high dose of steroids (3/3) and plasma exchange (1/3). Treatment after fingolimod cessation included initially high doses of corticosteroids and subsequent administration of rituximab (1/3), natalizumab (1/3), and cyclophosphamide (1/3) with a mean reduction in the EDSS score of 31% at the last follow-up. Regarding pathophysiological aspects, fingolimod withdrawal has been associated with deregulated sphingosine-1-phosphate signaling on the astrocytes.37 Interestingly, sphingosine-1-phosphate signaling is ubiquitously expressed in cells of the innate immune system and their response after fingolimod cessation is obscure.38 Other groups have suggested sustained lymphopenia as a risk factor for the rebound, but such results have not been verified in larger studies yet.39 We also identified one patient who developed TDLs after fingolimod initiation. In the literature, there are various hypotheses regarding TDL emergence in MS patients after fingolimod initiation. One is the predominance in selected patients of CD8+ effector T cells in the CSF that release perforin, another is the transient increase in T helper 17 (Th17) cells producing IL-17 and Th1-cells producing interferon-γ, and the last hypothesis suggests the overactivation of the innate immune system (overt macrophage activation and vasogenic edema) due to drug-related impairment of adaptive immune mechanisms.33,40 We could not identify any specific risk factors or clinical/radiological features that would increase susceptibility to TDL with fingolimod use at either initiation or cessation.
Finally, we described an interesting case presenting an atypical TDL during chronic treatment with natalizumab for MS. Natalizumab exerts an immune modulatory effect on the periphery affecting preferentially circulating B cells (increases the number of mature B cells and pre-B cells) and, as known, exacerbates NMO disorders.41,42 TDL lesions during natalizumab treatment have been exceptionally described in the literature, some of them with fatal outcomes and the presence of anti-natalizumab antibodies.8,9,43 We decided to withdraw natalizumab and intravenous methylprednisolone courses were started. JC virus polymerase chain reaction resulted negative in the CSF sample in repeated measurements. Anti-natalizumab antibodies were not tested. Despite corticosteroid treatment, significant MRI activity with enlargement of the lesion (that involved both white matter and subcortical U-fibers) and multiple bilateral hemispheric punctuate gadolinium-enhanced lesions were noted. Immune reconstitution inflammatory syndrome (IRIS) after natalizumab treatment interruption not associated with PML has been described in limited cases.44,45 We considered that the underlying mechanism for the exacerbation after natalizumab withdrawal in our case was a non-PML IRIS-like syndrome. Three monthly cycles of cyclophosphamide could not prevent disease progression. However, a remarkable reduction in the TDL was observed after post-rituximab initiation and the disease is still in remission for years.
In the limitations of our study are included the relatively small sample size of each subgroup and its retrospective design. While a strength of our study is the long follow-up study, our results regarding treatment strategies may not be generalizable to all cases. More studies are warranted to strengthen our conclusions regarding therapeutic protocols.
Conclusions
TDLs still represent a challenging scenario for clinicians. In the present study, we compared patients with TDL at initial presentation who did not convert to MS (36%) with TDL patients who eventually developed (64%). Non-TDL converters exhibited larger TDL lesions in brain MRI analysis, with prominent heterogeneous gadolinium enhancement pattern and less frequent open ring enhancement pattern compared with TDL converters. We present an interesting disease subgroup of patients who exhibit recurrent TDLs that might be a distinct group in the inflammatory demyelinating diseases spectrum. We also present patients with MS that developed TDL during a relapse or during drug initiation or cessation. Finally, we provide a therapeutic algorithm that is mainly based on the observed clinical and radiologic response to treatment options at various time points. Our suggested treatment approach requires close monitoring of patients with repeated MRI to better understand the nature of the disease. We noticed a variable and incomplete response to first-line treatment with steroids in patients with recurrent TDL. Plasma exchange is used as a second-line treatment in corticosteroid-resistant TDL relapses. Good radiological and clinical response to corticosteroids and/or plasma exchange therapy is a factor favoring further B-cell depletion strategies. B-cell directed therapies such as rituximab are highly effective in patients with recurrent TDL lesions, and provide long and stable improvement. Cyclophosphamide was reserved for more aggressive cases with poor response to corticosteroids and after plasma exchange failure. Second-line disease-modifying therapies used in classical MS, with the exception of fingolimod, could be applied to patients with highly active relapsing TDL and optimal clinical and radiological response to first-line treatment. To date, there are no established treatment guidelines for TDL and most knowledge arises from small case series and expert opinions. Further, multicenter, larger, prospective studies of patients with TDL lesions are necessary for a better understanding of these disease entities and for generation of therapeutic algorithms. The pathogenetic mechanisms of tumefactive MS are elusive and further functional and phenotypic experiments are needed to investigate whether such disease represents a distinct demyelinating disease or a subset of MS patients with specific radiological features.
Footnotes
Conflict of interest statement: AGV, GV, PT, EG, AD, EP, LS has nothing to disclose. DT has received research grants and lecture fees from Sanofi Genzyme, Biogen, Teva, and Novartis. JST has shares in the research and diagnostic laboratory of Tzartos NeuroDiagnostics. He has also received travel grants from Genzyme, Genesis Pharma, Teva, and Novartis and advisory boards from Genesis Pharma, Novartis, and Teva. MA has received research grants from Biogen, Merck-Serono, Novartis, Teva, Bayer, and Genzyme, as well as lecture-fees from Novartis, Teva, Biogen, and Genzyme. EA has received research grants from Biogen, Merck-Serono, Novartis, and Sanofi Aventis, as well as lecture-fees from Teva. GK has received research grants from Genesis Pharma and Teva, consultation fees, advisory board remuneration and honoraria from Genzyme, Genesis Pharma, Teva, and Novartis. MEE has received travel grants and consulting fees from Biogen, Novartis, Teva, Genzyme, and Merk. CK has received grants and honoraria from: Bayer, Biogen, Genesis Pharma, Merck-Serono, Novartis, Sanofi Genzyme, Teva.
Funding: This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Data availability: The datasets generated for this study are available on reasonable request to the corresponding author.
ORCID iDs: Aigli G. Vakrakou  https://orcid.org/0000-0002-7712-7216
https://orcid.org/0000-0002-7712-7216
Dimitrios Tzanetakos  https://orcid.org/0000-0001-8925-3327
https://orcid.org/0000-0001-8925-3327
Supplemental material: Supplemental material for this article is available online.
Contributor Information
Aigli G. Vakrakou, 1st Department of Neurology, Medical School of Athens, National & Kapodistrian University, Aeginition Hospital, 72 Vasilissis Sofias Ave, Athens, 11528, Greece.
Dimitrios Tzanetakos, Demyelinating Diseases Unit, 1st Department of Neurology, School of Medicine, Eginition Hospital, National and Kapodistrian University of Athens, Athens, Greece.
Maria-Eleptheria Evangelopoulos, Demyelinating Diseases Unit, 1st Department of Neurology, School of Medicine, Eginition Hospital, National and Kapodistrian University of Athens, Athens, Greece.
Theodore Argyrakos, Department of Pathology, Evaggelismos Hospital, Athens, Greece.
John S. Tzartos, Demyelinating Diseases Unit, 1st Department of Neurology, School of Medicine, Eginition Hospital, National and Kapodistrian University of Athens, Athens, Greece
Maria Anagnostouli, Demyelinating Diseases Unit, 1st Department of Neurology, School of Medicine, Eginition Hospital, National and Kapodistrian University of Athens, Athens, Greece.
Elissavet Andreadou, Demyelinating Diseases Unit, 1st Department of Neurology, School of Medicine, Eginition Hospital, National and Kapodistrian University of Athens, Athens, Greece.
Georgios Koutsis, Demyelinating Diseases Unit, 1st Department of Neurology, School of Medicine, Eginition Hospital, National and Kapodistrian University of Athens, Athens, Greece.
Georgios Velonakis, Research Unit of Radiology, 2nd Department of Radiology, National and Kapodistrian University of Athens, Athens, Greece.
Panagiotis Toulas, Research Unit of Radiology, 2nd Department of Radiology, National and Kapodistrian University of Athens, Athens, Greece.
Elias Gialafos, Demyelinating Diseases Unit, 1st Department of Neurology, School of Medicine, Eginition Hospital, National and Kapodistrian University of Athens, Athens, Greece.
Antonios Dimitrakopoulos, Demyelinating Diseases Unit, 1st Department of Neurology, School of Medicine, Eginition Hospital, National and Kapodistrian University of Athens, Athens, Greece.
Erasmia Psimenou, Department of Clinical Therapeutics, National and Kapodistrian University of Athens, Athens, Greece.
Leonidas Stefanis, Demyelinating Diseases Unit, 1st Department of Neurology, School of Medicine, Eginition Hospital, National and Kapodistrian University of Athens, Athens, Greece.
Constantinos Kilidireas, Demyelinating Diseases Unit, 1st Department of Neurology, School of Medicine, Eginition Hospital, National and Kapodistrian University of Athens, Athens, Greece.
References
- 1. Hardy TA, Chataway J. Tumefactive demyelination: an approach to diagnosis and management. J Neurol Neurosurg Psychiatry 2013; 84: 1047–1053. [DOI] [PubMed] [Google Scholar]
- 2. Hardy TA. Pseudotumoral demyelinating lesions: diagnostic approach and long-term outcome. Curr Opin Neurol. 2019; 32: 467–474. [DOI] [PubMed] [Google Scholar]
- 3. Xia L, Lin S, Wang ZC, et al. Tumefactive demyelinating lesions: nine cases and a review of the literature. Neurosurg Rev 2009; 32: 171–179; discussion 179. [DOI] [PubMed] [Google Scholar]
- 4. Aringer M, Costenbader K, Daikh D, et al. 2019 European League Against Rheumatism/American College of Rheumatology classification criteria for systemic lupus erythematosus. Arthritis Rheumatol 2019; 71: 1400–1412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Wattamwar PR, Baheti NN, Kesavadas C, et al. Evolution and long term outcome in patients presenting with large demyelinating lesions as their first clinical event. J Neurol Sci 2010; 297: 29–35. [DOI] [PubMed] [Google Scholar]
- 6. Croteau D, Tobenkin A, Brinker A, et al. Tumefactive multiple sclerosis in association with fingolimod initiation and discontinuation. Mult Scler 2020: 1352458520938354. [DOI] [PubMed] [Google Scholar]
- 7. Algahtani H, Shirah B, Alassiri A. Tumefactive demyelinating lesions: a comprehensive review. Mult Scler Relat Disord 2017; 14: 72–79. [DOI] [PubMed] [Google Scholar]
- 8. Debs R, Maillart E, Fahed R, et al. Extensive brain demyelinating lesions under natalizumab: the role of anti-natalizumab antibodies. Neurology 2015; 85: 1630–1632. [DOI] [PubMed] [Google Scholar]
- 9. Moghadasi AN, Baghbanian SM. Tumefactive demyelinating lesions in a patient with multiple sclerosis receiving natalizumab. Acta Neurol Belg 2019; 119: 137–139. [DOI] [PubMed] [Google Scholar]
- 10. Twyman C, Berger JR. A giant MS plaque mimicking PML during natalizumab treatment. J Neurol Sci 2010; 291: 110–113. [DOI] [PubMed] [Google Scholar]
- 11. Hardy TA, Reddel SW, Barnett MH, et al. Atypical inflammatory demyelinating syndromes of the CNS. Lancet Neurol 2016; 15: 967–981. [DOI] [PubMed] [Google Scholar]
- 12. Ayrignac X, Carra-Dalliere C, Labauge P. Atypical inflammatory demyelinating lesions and atypical multiple sclerosis. Rev Neurol 2018; 174: 408–418. [DOI] [PubMed] [Google Scholar]
- 13. Hardy TA, Tobin WO, Lucchinetti CF. Exploring the overlap between multiple sclerosis, tumefactive demyelination and Baló’s concentric sclerosis. Mult Scler 2016; 22: 986–992. [DOI] [PubMed] [Google Scholar]
- 14. Eckstein C, Saidha S, Levy M. A differential diagnosis of central nervous system demyelination: beyond multiple sclerosis. J Neurol 2012; 259: 801–816. [DOI] [PubMed] [Google Scholar]
- 15. Rovira A. Tumefactive idiopathic inflammatory demyelinating lesions: a diagnostic challenge. Mult Scler 2014; 20: 634–635. [DOI] [PubMed] [Google Scholar]
- 16. Weinshenker BG. Tumefactive demyelinating lesions: characteristics of individual lesions, individual patients, or a unique disease entity? Mult Scler 2015; 21: 1746–1747. [DOI] [PubMed] [Google Scholar]
- 17. Altintas A, Petek B, Isik N, et al. Clinical and radiological characteristics of tumefactive demyelinating lesions: follow-up study. Mult Scler 2012; 18: 1448–1453. [DOI] [PubMed] [Google Scholar]
- 18. Siri A, Carra-Dalliere C, Ayrignac X, et al. Isolated tumefactive demyelinating lesions: diagnosis and long-term evolution of 16 patients in a multicentric study. J Neurol 2015; 262: 1637–1645. [DOI] [PubMed] [Google Scholar]
- 19. Tzanetakos D, Vakrakou AG, Tzartos JS, et al. Heterogeneity of Baló’s concentric sclerosis: a study of eight cases with different therapeutic concepts. BMC Neurol 2020; 20: 400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Barkhof F, Filippi M, Miller DH, et al. Comparison of MRI criteria at first presentation to predict conversion to clinically definite multiple sclerosis. Brain 1997; 120: 2059–2069. [DOI] [PubMed] [Google Scholar]
- 21. Lucchinetti CF, Gavrilova RH, Metz I, et al. Clinical and radiographic spectrum of pathologically confirmed tumefactive multiple sclerosis. Brain 2008; 131: 1759–1775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Vakrakou AG, Tzanetakos D, Argyrakos T, et al. Recurrent fulminant tumefactive demyelination with Marburg-like features and atypical presentation: therapeutic dilemmas and review of literature. Front Neurol 2020; 11: 536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Sánchez P, Meca-Lallana V, Barbosa A, et al. Tumefactive demyelinating lesions of 15 patients: clinico-radiological features, management and review of the literature. J Neurol Sci 2017; 381: 32–38. [DOI] [PubMed] [Google Scholar]
- 24. Tremblay MA, Villanueva-Meyer JE, Cha S, et al. Clinical and imaging correlation in patients with pathologically confirmed tumefactive demyelinating lesions. J Neurol Sci 2017; 381: 83–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Brod SA, Lindsey JW, Nelson F. Tumefactive demyelination: clinical outcomes, lesion evolution and treatments. Mult Scler J Exp Transl Clin 2019; 5: 2055217319855755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Balloy G, Pelletier J, Suchet L, et al. Inaugural tumor-like multiple sclerosis: clinical presentation and medium-term outcome in 87 patients. J Neurol 2018; 265: 2251–2259. [DOI] [PubMed] [Google Scholar]
- 27. Häne A, Bargetzi M, Hewer E, et al. Recurrent tumefactive demyelination without evidence of multiple sclerosis or brain tumour. J Neurol 2011; 258: 318–320. [DOI] [PubMed] [Google Scholar]
- 28. Omerhodžic’ I, Džurlic’ A, Lisica D, et al. Relapsing tumefactive demyelination: a case report. Acta Med Acad 2018; 47: 193–198. [DOI] [PubMed] [Google Scholar]
- 29. Rissardo JP, Caprara ALF. Management of recurrent tumefactive multiple sclerosis: case report and literature review. Asian J Neurosurg 2018; 13: 893–896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Bhargava A, Pujar GS, Banakar BF, et al. Recurrent tumefactive demyelination: an unusual presentation. J Pediatr Neurosci 2015; 10: 55–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Nagappa M, Taly AB, Sinha S, et al. Tumefactive demyelination: clinical, imaging and follow-up observations in thirty-nine patients. Acta Psychiatr Scand 2013; 128: 39–47. [DOI] [PubMed] [Google Scholar]
- 32. Nakamura M, Itani K, Miyake K, et al. Natalizumab is effective for the treatment of relapsing-remitting tumefactive multiple sclerosis. Intern Med 2017; 56: 211–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Sánchez P, Meca-Lallana V, Vivancos J. Tumefactive multiple sclerosis lesions associated with fingolimod treatment: report of 5 cases. Mult Scler Relat Disord 2018; 25: 95–98. [DOI] [PubMed] [Google Scholar]
- 34. Baroncini D, Annovazzi P, Guaschino C, et al. Long-term remission of tumefactive relapsing multiple sclerosis after alemtuzumab rescue treatment in an adolescent patient. Mult Scler Relat Disord 2020; 41: 102061. [DOI] [PubMed] [Google Scholar]
- 35. La Puma D, Llufriu S, Sepúlveda M, et al. Long-term follow-up of immunotherapy-unresponsive recurrent tumefactive demyelination. J Neurol Sci 2015; 352: 127–128. [DOI] [PubMed] [Google Scholar]
- 36. Siffrin V, Muller-Forell W, von Pein H, et al. How to treat tumefactive demyelinating disease? Mult Scler 2014; 20: 631–633. [DOI] [PubMed] [Google Scholar]
- 37. Giordana MT, Cavalla P, Uccelli A, et al. Overexpression of sphingosine-1-phosphate receptors on reactive astrocytes drives neuropathology of multiple sclerosis rebound after fingolimod discontinuation. Mult Scler 2018; 24: 1133–1137. [DOI] [PubMed] [Google Scholar]
- 38. Bryan AM, Del Poeta M. Sphingosine-1-phosphate receptors and innate immunity. Cell Microbiol 2018; 20: e12836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Sepúlveda M, Montejo C, Llufriu S, et al. Rebound of multiple sclerosis activity after fingolimod withdrawal due to planning pregnancy: analysis of predisposing factors. Mult Scler Relat Disord 2020; 38: 101483. [DOI] [PubMed] [Google Scholar]
- 40. Pilz G, Harrer A, Wipfler P, et al. Tumefactive MS lesions under fingolimod: a case report and literature review. Neurology 2013; 81: 1654–1658. [DOI] [PubMed] [Google Scholar]
- 41. Krumbholz M, Meinl I, Kümpfel T, et al. Natalizumab disproportionately increases circulating pre-B and B cells in multiple sclerosis. Neurology 2008; 71: 1350–1354. [DOI] [PubMed] [Google Scholar]
- 42. Kleiter I, Hellwig K, Berthele A, et al. Failure of natalizumab to prevent relapses in neuromyelitis optica. Arch Neurol 2012; 69: 239–245. [DOI] [PubMed] [Google Scholar]
- 43. Svenningsson A, Dring AM, Fogdell-Hahn A, et al. Fatal neuroinflammation in a case of multiple sclerosis with anti-natalizumab antibodies. Neurology 2013; 80: 965–967. [DOI] [PubMed] [Google Scholar]
- 44. Sepúlveda M, Llufriu S, Blanco Y, et al. Intense immunosuppression for the treatment of an immune reconstitution inflammatory syndrome-like exacerbation after natalizumab withdrawal: a case report. J Neurol 2015; 262: 219–221. [DOI] [PubMed] [Google Scholar]
- 45. Miravalle A, Jensen R, Kinkel RP. Immune reconstitution inflammatory syndrome in patients with multiple sclerosis following cessation of natalizumab therapy. Arch Neurol 2011; 68: 186–191. [DOI] [PubMed] [Google Scholar]