Abstract
Remote ischemic conditioning (RIC) is a noninvasive procedure whereby several periods of ischemia are induced in a limb. Although there is growing interest in using RIC to improve stroke recovery, preclinical RIC research has focused exclusively on neuroprotection, using male animals and the intraluminal suture stroke model, and delivered RIC at times not relevant to either brain repair or behavioral recovery. In alignment with the Stroke Recovery and Rehabilitation Roundtable, we address these shortcomings. First, a standardized session (5-minute inflation/deflation, 4 repetitions) of RIC was delivered using a cuff on the contralesional hindlimb in both male and female Sprague-Dawley rats. Using the endothelin-1 stroke model, RIC was delivered once either prestroke (18 hours before, pre-RIC) or poststroke (4 hours after, post-RIC), and infarct volume was assessed at 24 hours poststroke using magnetic resonance imaging. RIC was delivered at these times to mimic the day before a surgery where clots are possible or as a treatment similar to tissue plasminogen activator, respectively. Pre-RIC reduced infarct volume by 41% compared with 29% with post-RIC. RIC was neuroprotective in both sexes, but males had a 46% reduction of infarct volume compared with 23% in females. After confirming the acute efficacy of RIC, we applied it chronically for 4 weeks, beginning 5 days poststroke. This delayed RIC failed to enhance poststroke behavioral recovery. Based on these findings, the most promising application of RIC is during the hyperacute and early acute phases of stroke, a time when other interventions such as exercise may be contraindicated.
Keywords: cell death, brain repair, neuroprotection
Introduction
There is growing interest in the application of remote ischemic conditioning (RIC) to promote recovery following stroke.1 However, preclinical evidence for RIC as a therapeutic is primarily restricted to the hyperacute poststroke phase,2 a time frame of ongoing cell death versus the later phase of neural repair and behavioral recovery.3 The Stroke Recovery and Rehabilitation Roundtable (SRRR) consortium has emphasized that promising stroke recovery interventions should go through rigorous preclinical evaluation prior to clinical translation, such as replication in both sexes and across different stroke models.4 To date, most RIC studies have not followed preclinical recommendations of the Stroke Treatment Academic Industry Roundtable (STAIR), such as not directly assessing efficacy between sexes and relying almost exclusively on the intraluminal suture, middle cerebral artery occlusion (MCAO) model,2 which has some limitations with respect to human stroke.4 In addition, most studies have delivered RIC at the time of reperfusion, a time frame that encompasses only a portion of the clinical population.2 Early delivery of RIC makes it impossible to distinguish between neuroprotective and neurorestorative effects. This study directly addressed these RIC knowledge gaps by delivering RIC at a variety of times relative to stroke in order to capture the heterogeneity of human stroke presentation. This included delaying delivery of RIC in order to dissociate neuroprotective and neurorestorative effects, performing the first direct comparison of RIC efficacy between sexes, and using an endothelin-1 (ET-1) reperfusion model to confirm efficacy across stroke models.
Material and Methods
Experimental procedures were approved by the University of Ottawa Animal Care Committee in accordance with guidelines of the Canadian Council of Animal Care.
Experiment 1
Male (n = 41) and female (n = 42) Sprague-Dawley rats (250-275 g, Charles River) underwent RIC 18 hours prestroke or 4 hours poststroke (Figure 1A). Rats were randomized into 4 groups: pre-RIC (n = 22), pre-RIC sham (n = 20), post-RIC (n = 21), and post-RIC sham (n = 20). Rats were anesthetized with isoflurane (5% induction, 2%-3% maintenance) for stroke, RIC, and magnetic resonance imaging (MRI) procedures and maintained at a body temperature of 37 ± 0.5 °C. Sham RIC received anesthesia for the same duration and no rats were excluded. Sample size calculations were based on past RIC literature (see Supplemental Material available online).
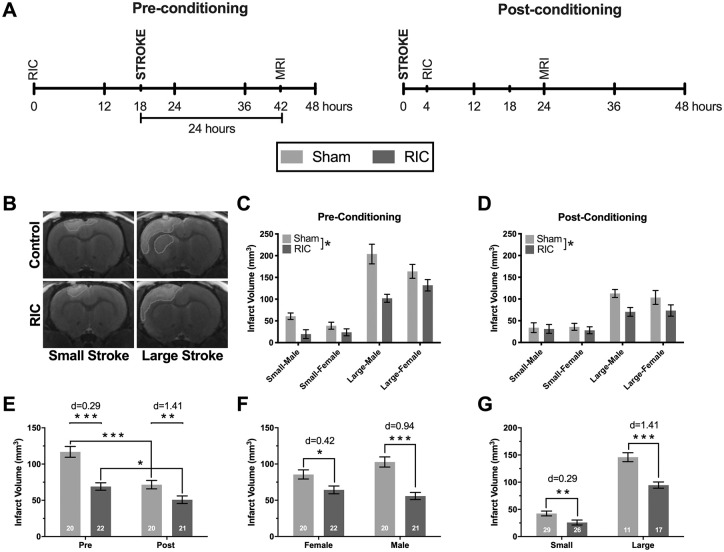
Figure 1.
(A) Experimental timeline for experiment 1. (B) Representative T2-weighted magnetic resonance image (MRI) of small and large strokes in remote ischemic conditioning (RIC) and sham groups. Contour indicates overlapping regions identified as infarct by 2 independent experimenters. (C-D) Both preconditioning and postconditioning reduced infarct volume (main statistical effect), with no higher-order interactions detected between all variables of interest (RIC/control, pre-RIC/post-RIC, male/female, or small/large). (E) RIC reduced infarct volume if delivered 18 hours prestroke or if delivered 4 hours poststroke. (F) RIC reduced infarct volume in both sexes. (G) RIC reduced infarct independent of lesion size.a
a *P < .05, **P < .01, ***P < .001; d, effect sizes by the Cohen d.
Stroke Induction
Two cortical injections of ET-1 (400.0 pmol/µL, 1.0 µL/site) were given, using stereotaxic coordinates relative to bregma: AP 0.0 mm, ML ±2.5 mm, DV (from cortex) −1.7 mm, and AP +2.3 mm, ML ±2.5 mm, DV −1.7 mm.
Remote Ischemic Conditioning
Cuffs (Dispomed Veterinary Instruments; size 1, 3-6 cm) were placed above the knee contralateral to the lesioned hemisphere. A custom automatic pump delivered 4 cycles of 5-minute inflations and deflations to occlude blood flow (~170 mm Hg). Blood flow reduction was verified using laser Doppler imaging and pulse oximeters (Supplementary Material, Figure 1).
MRI and Infarct Analysis
MRI images (22 coronal slices, 800 µm thick) were obtained 24 hours following stroke. Images were acquired with a T2-weighted fast spin echo pulse sequence: 15 axial (transverse) slices (slice thickness = 800 µm; in-plane resolution = 78 µm; echo train length = 8; echo time = 27 ms; scan time = 5 minutes). Two blinded experimenters delineated the infarct region using ImageJ (National Institute of Health), and stroke areas that were identified by both experimenters (overlapping) were used as infarct area. Infarct volumes were calculated by multiplying the sum of infarct areas by slice thickness.
Experiment 2
Female rats underwent chronic RIC beginning 5 days poststroke and continued for 4 weeks. Rats received the same ET-1 stroke as above, were randomized into 2 groups—RIC (n = 20) and sham (n = 20)—and received an MRI 24 hours poststroke (Figure 2A). One rat in the RIC group died during surgery.
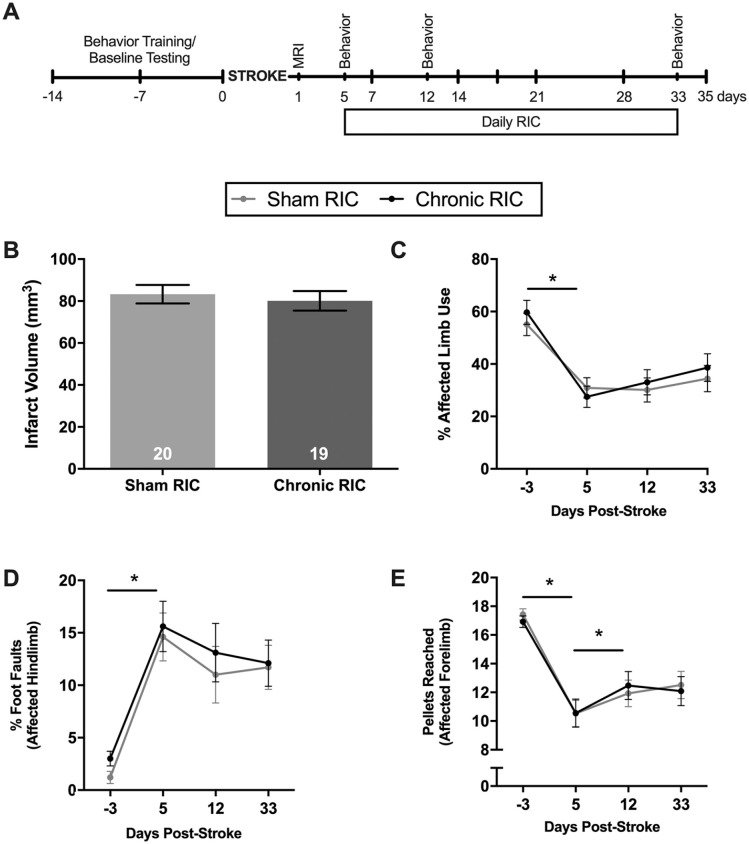
Figure 2.
(A) Experimental timeline for experiment 2. (B) Infarct volumes were similar between groups before chronic RIC administration. (C) Spontaneous use of the affected limb in the cylinder task was reduced following stroke and was not improved with RIC. (D) Stroke increased the number of foot faults on the beam traversal task, which was unaffected by RIC. (E) Pellets reached in the Montoya staircase were reduced following stroke. Performance on the task spontaneously improved from day 5 to 12 and was not improved by RIC.
Abbreviations: MRI, magnetic resonance imaging; RIC, remote ischemic conditioning.
*P < .05.
Behavior
Rats were assessed on skilled reaching (Montoya staircase), spontaneous limb use (cylinder), and gait (beam traversal), at prestroke and at 5, 12, and 33 days poststroke (see Supplemental Methods).
Statistical Analysis
See supplemental materials for detailed statistics. A hierarchical cluster analysis using Ward’s method was used to dichotomously classify stroke sizes as either “large” or “small.” Analysis of variance (ANOVA) was used to analyze infarct volumes (RIC/control, pre-RIC/post-RIC, male/female, small/large). A repeated-measures ANOVA was used to analyze behavioral data. Sidak-corrected t tests were used for post hoc analysis. Pearson effect sizes were calculated by the Cohen d. Significance was P ≤.05. Data are reported as estimated marginal means ± SEM.
Results
Experiment 1
Both preconditioning and postconditioning reduced infarct volume (P < .05; Figures 1B-1D), though the effect was larger with preconditioning than postconditioning (41% vs 29% reduction; Figure 1E). Similarly, RIC reduced infarct volume independent of sex (P < .05); however, RIC was more efficacious in males (46% vs 25% reduction; Figure 1F). When rats were stratified by infarct size, RIC reduced infarct volume in both small (39% reduction) and large (35% reduction) stroke groups (Figure 1G).
Experiment 2
Prior to RIC delivery, groups had similar infarct volumes (P > .05; Figure 2B). When delivered outside the hyperacute poststroke phase (>24 hours), RIC provided no benefit on behavioral recovery (P > .05; Figures 2C-2E).
Discussion
Before RIC is considered as an adjunctive therapy in stroke recovery trials, it is important to recognize that preclinical RIC studies have focused on neuroprotection, utilized primarily male animals and MCAO stroke, delivered RIC at times with limited applicability to brain repair and behavioral recovery, and as a result are at a high risk for potential bias.1,2 Here, we were able to rule out model-specific benefits of RIC, demonstrating that RIC is efficacious in an ET-1 stroke model, which has a different reperfusion and injury profile from MCAO,5 and results in injury volumes more similar to that in humans (4.2% to 14.8% of the injured hemisphere).6 Although others have shown efficacy of RIC in female animals,7,8 our study is the first direct comparison between sexes, where we show that RIC reduces infarct volume to a greater extent in male rats. Most prior studies delivered RIC at reperfusion.2 We show that RIC preconditioning is most efficacious, but delivery of RIC 4 hours poststroke, within the time window for tissue plasminogen activator,9 also significantly reduces stroke injury. Our findings, along with a recent 2-center RIC study that adhered to both STAIR and SRRR guidelines provide compelling evidence for the neuroprotective efficacy of RIC.10 Importantly, we show that when an effective RIC protocol is delivered chronically in female rats, outside the neuroprotective window, there is no benefit on behavioral recovery.11 Nonetheless, it remains possible that RIC could promote recovery in male animals or if paired with task-specific rehabilitation or exercise. However, it appears that the most effective application of RIC is during the hyperacute and very early acute phases of stroke recovery, when patients may be unable to tolerate intensive exercise or rehabilitation protocols.12,13
Supplemental Material
Supplemental material, sj-docx-1-nnr-10.1177_15459683211011224 for Remote Ischemic Conditioning and Stroke Recovery by Matthew W. McDonald, Angela Dykes, Matthew S. Jeffers, Anthony Carter, Ralph Nevins, Allyson Ripley, Gergely Silasi and Dale Corbett in Neurorehabilitation and Neural Repair
Acknowledgments
The authors would like to thank Julian Pitney for his technical expertise. We thank the University of Ottawa Preclinical Imaging Core and Dr Greg Cron for conducting magnetic resonance imaging.
Footnotes
Declaration of Conflicting Interests: The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: DC is a member of the American Society for Neurorehabilitation
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: MWM was supported by scholarships from the Heart and Stroke Foundation Canadian Partnership for Stroke Recovery and the Canadian Vascular Network. This study was supported by grants from the Heart and Stroke Foundation Canadian Partnership for Stroke Recovery and the CIHR Canadian Consortium of Neurodegeneration and Aging awarded to DC.
ORCID iDs: Matthew W. McDonald  https://orcid.org/0000-0002-0171-6102
https://orcid.org/0000-0002-0171-6102
Matthew S. Jeffers  https://orcid.org/0000-0002-4148-2638
https://orcid.org/0000-0002-4148-2638
Dale Corbett  https://orcid.org/0000-0003-0217-4576
https://orcid.org/0000-0003-0217-4576
Supplementary material for this article is available on the Neurorehabilitation & Neural Repair website at http://nnr.sagepub.com/content/by/supplemental-data.
References
- 1. Landman TRJ, Schoon Y, Warlé MC, de Leeuw FE, Thijssen DHJ. Remote ischemic conditioning as an additional treatment for acute ischemic stroke. Stroke. 2019;50:1934-1939. [DOI] [PubMed] [Google Scholar]
- 2. Ripley AJ, Jeffers MS, McDonald MW, et al. Neuroprotection by remote ischemic conditioning in rodent models of focal ischemia: a systematic review and meta-analysis. Transl Stroke Res. Published online January 6, 2021. doi: 10.1007/s12975-020-00882-1 [DOI] [PubMed] [Google Scholar]
- 3. Bernhardt J, Hayward KS, Kwakkel G, et al. Agreed definitions and a shared vision for new standards in stroke recovery research: The Stroke Recovery and Rehabilitation Roundtable Taskforce. Neurorehabil Neural Repair. 2017;31:793-799. [DOI] [PubMed] [Google Scholar]
- 4. Corbett D, Thomas Carmichael S, Murphy TH, et al. Enhancing the alignment of the preclinical and clinical stroke recovery research pipeline: consensus-based core recommendations from the Stroke Recovery and Rehabilitation Roundtable Translational Working Group. Neurorehabil Neural Repair. 2017;31:699-707. [DOI] [PubMed] [Google Scholar]
- 5. Windle V, Szymanska A, Granter-Button S, et al. An analysis of four different methods of producing focal cerebral ischemia with endothelin-1 in the rat. Exp Neurol. 2006;201:324-334. [DOI] [PubMed] [Google Scholar]
- 6. Carmichael ST. Rodent models of focal stroke: size, mechanism, and purpose. NeuroRX. 2005;2:396-409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Hoda MN, Bhatia K, Hafez SS, et al. Remote ischemic perconditioning is effective after embolic stroke in ovariectomized female mice. Transl Stroke Res. 2014;5:484-490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Li S, Hu X, Zhang M, et al. Remote ischemic post-conditioning improves neurological function by AQP4 down-regulation in astrocytes. Behav Brain Res. 2015;289:1-8. [DOI] [PubMed] [Google Scholar]
- 9. Lin K, Lindsay P, Shams T, et al. A summary of the Canadian Stroke Best Practice Recommendations, Sixth Edition (2018): updates relevant to prehospital and emergency medicine providers. Can J Emerg Med. 2018;20:685-692. [DOI] [PubMed] [Google Scholar]
- 10. Basalay MV, Wiart M, Chauveau F, et al. Neuroprotection by remote ischemic conditioning in the setting of acute ischemic stroke: a preclinical two-centre study. Sci Rep. 2020;10:16874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Doeppner TR, Zechmeister B, Kaltwasser B, et al. Very delayed remote ischemic post-conditioning induces sustained neurological recovery by mechanisms involving enhanced angioneurogenesis and peripheral immunosuppression reversal. Front Cell Neurosci. 2018;12:383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. AVERT Trial Collaboration group. Efficacy and safety of very early mobilisation within 24 h of stroke onset (AVERT): a randomised controlled trial. Lancet. 2015;386:46-55. [DOI] [PubMed] [Google Scholar]
- 13. Marzolini S, Robertson AD, Oh P, et al. Aerobic training and mobilization early post-stroke: cautions and considerations. Front Neurol. 2019;10:1187. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-docx-1-nnr-10.1177_15459683211011224 for Remote Ischemic Conditioning and Stroke Recovery by Matthew W. McDonald, Angela Dykes, Matthew S. Jeffers, Anthony Carter, Ralph Nevins, Allyson Ripley, Gergely Silasi and Dale Corbett in Neurorehabilitation and Neural Repair