Abstract
Checkpoint inhibitors offer a promising immunotherapy strategy for cancer treatment; however, due to primary or acquired resistance, many patients do not achieve lasting clinical responses. Recently, the transforming growth factor-β (TGFβ) signaling pathway has been identified as a potential target to overcome primary resistance, although the nonselective inhibition of multiple TGFβ isoforms has led to dose-limiting cardiotoxicities. SRK-181 is a high-affinity, fully human antibody that selectively binds to latent TGFβ1 and inhibits its activation. To support SRK-181 clinical development, we present here a comprehensive preclinical assessment of its pharmacology, pharmacokinetics, and safety across multiple species. In vitro studies showed that SRK-181 has no effect on human platelet function and does not induce cytokine release in human peripheral blood. Four-week toxicology studies with SRK-181 showed that weekly intravenous administration achieved sustained serum exposure and was well tolerated in rats and monkeys, with no treatment-related adverse findings. The no-observed-adverse-effect levels levels were 200 mg/kg in rats and 300 mg/kg in monkeys, the highest doses tested, and provide a nonclinical safety factor of up to 813-fold (based on Cmax) above the phase 1 starting dose of 80 mg every 3 weeks. In summary, the nonclinical pharmacology, pharmacokinetic, and toxicology data demonstrate that SRK-181 is a selective inhibitor of latent TGFβ1 that does not produce the nonclinical toxicities associated with nonselective TGFβ inhibition. These data support the initiation and safe conduct of a phase 1 trial with SRK-181 in patients with advanced cancer.
Keywords: latent TGFβ1, SRK-181, cancer immunotherapy, TGFβ inhibitors
Introduction
Immunotherapy has transformed the treatment of patients with cancer as an increasing number of indications and tumor types are approved for treatment with checkpoint inhibitors. Despite this, a majority of patients do not benefit from a durable clinical response to these therapies due to primary (ie, present before treatment initiation) or acquired resistance mechanisms.1,2 The identification of molecular pathways associated with resistance has therefore become a key focus of cancer immunotherapy. Retrospective analyses of clinical tumor samples have recently implicated transforming growth factor-β (TGFβ) signaling as a potential modulator of primary resistance to PD-(L)1 blockade therapy, particularly in tumors where CD8 T cells are excluded from penetrating the tumor.1-4 These data raise the exciting possibility that TGFβ inhibition may overcome primary anti-PD-(L)1 resistance.
Mammals express 3 closely related TGFβ isoforms, TGFβ1, TGFβ2, and TGFβ3, which bind to and signal through the TGFβ receptor complex.5 Transforming growth factor-β is present on the cell surface or within tissue matrix as latent TGFβ, in which the TGFβ prodomain remains noncovalently associated with the growth factor. Latent TGFβ forms a complex with TGFβ presenting proteins called latent TGFβ-binding protein-1 (LTBP1), LTBP3, glycoprotein A repetitions predominant, or leucine-rich repeat-containing protein-33. For the purpose of this article, these various latent TGFβ complexes are referred to as “latent TGFβ.” Latent TGFβ, when activated, releases the TGFβ growth factor that in turn binds to transmembrane TGFβ type II receptors and recruits the TGFβ type I receptors to form a heterotetrameric receptor complex.6,7 Downstream signaling is then initiated by the activated receptor complex either via the canonical Smad signaling pathway or via Smad-independent, noncanonical pathways. In tumors, active TGFβ signaling promotes an immunosuppressive microenvironment that, in turn, can suppress antitumor immunity and therefore favor tumor progression.8
The apparent critical contribution of TGFβ signaling to an immunosuppressive microenvironment and cancer progression has heightened interest in therapies that inhibit the TGFβ pathway in tumors. These include small-molecule kinase inhibitors, anti-TGFβ growth factor antibodies, ligand traps, and anti-TGFβ receptor antibodies.9-11 To date, however, the majority of these therapies have demonstrated limited clinical success due to a lack of efficacy in clinical trials, an unfavorable safety profile limiting their clinical evaluation, or both.8,12-14
Dose-limiting toxicities noted with TGFβ pathway inhibition have remained a major concern in developing anti-TGFβ therapies. These toxicities include cardiovascular abnormalities, skin lesions, epithelial oral hyperplasia, and gingival bleeding.15-18 Although many of these are either reversible or manageable, the cardiovascular lesions such as inflammation, hemorrhage or hyperplasia in the valves, aortic arch, and associated arteries of the heart are not reversible and therefore continue to be key safety concerns for TGFβ inhibitors.17-19
To date, the majority of TGFβ inhibitors target 2 or all 3 TGFβ isoforms (ie, pan-TGFβ inhibition), and therefore it is unknown if the toxicity profile associated with these drugs can be attributed to a specific isoform.15,18 Recent analysis of RNA-seq data from The Cancer Genome Atlas dataset identified TGFβ1 as the most prevalent TGFβ isoform in a majority of solid tumor types in humans, which suggests that TGFβ1 may be important in the disease state.20 Therefore, we hypothesize that selective targeting of the TGFβ1 isoform may be sufficient to overcome both the primary resistance to anti-PD-(L)1 and some of the dose-limiting toxicities associated with pan-TGFβ pathway inhibition.
To investigate this hypothesis, we developed SRK-181, a fully human antibody of the IgG4/κ subtype that selectively binds to latent TGFβ1 with picomolar affinity but has little to no binding to latent TGFβ2, latent TGFβ3, or any of the active TGFβ growth factors.20 SRK-181 potently inhibits activation of latent TGFβ1 in vitro, and treatment with SRK-181-mIgG1 (ie, the SRK-181 antibody with murine IgG constant domains to minimize immunogenicity) overcomes primary resistance to anti-PD-1 therapy in preclinical mouse tumor models that recapitulate key features of clinical anti-PD-(L)1 resistance.20
Here, we present a comprehensive preclinical assessment of SRK-181 pharmacology, pharmacokinetics (PK), and safety across mice, rats, and cynomolgus monkeys, and describe the dose selection strategy used to initiate and support the ongoing phase 1, first-in-human (FIH) clinical trial with SRK-181 (NCT04291079).
Materials and Methods
Test Article and Vehicle
SRK-181 is a fully human, anti-latent TGFβ1 monoclonal antibody (mAb) of the IgG4/κ isotype that binds to human latent TGFβ1 with high affinity in vitro. The mAb is produced in Expi293 cells transiently transfected with expression vectors encoding for the heavy and light chains of the antibody.20 A mouse chimera of SRK-181 (SRK-181-mIgG) was used in the mouse pharmacology studies and consisted of the human variable domains of SRK-181 fused to the mouse IgG1/κ constant domains.
For the pharmacokinetic studies conducted in mice, rats, and cynomolgus monkeys, SRK-181 (or SRK-181-mIgG1) was supplied at a nominal concentration of 10 mg/mL and stored at −70 °C to −80 °C. The vehicle was citrate-buffered saline and stored at room temperature.
The 4-week rat and monkey toxicology studies were conducted in compliance with US Food and Drug Administration good laboratory practice (GLP) Regulations [21 CFR Part 58]. SRK-181 used in these studies was expressed in a Chinese Hamster Ovary cell line that stably expresses the SRK-181 heavy and light chains and was supplied at a nominal concentration of 50 mg/mL and stored at 2 °C to 8 °C. The vehicle control was stored at room temperature.
In Vitro Pharmacology and Safety Assessments
Sequence Homology for TGFβ1 prodomain across species
To aid in the selection of pharmacologically relevant species for toxicology studies, the homology of the TGFβ1 prodomain and growth factor sequences from human, rat, and cynomolgus monkey were evaluated using sequence data from the National Center for Biotechnology Information protein database (https://www.ncbi.nlm.nih.gov/protein/). Pairwise protein sequence alignment and calculation of sequence identity were performed using EMBOSS Needle (https://www.ebi.ac.uk/Tools/psa/emboss_needle/) on the mature protein sequence without its predicted signal peptide.21
Latent TGFβ1 activation assay
Inhibitory activity of SRK-181 was measured in a cell-based reporter assay as previously described in Martin et al. (2020). Briefly, LN229 human glioblastoma cells (ATCC) were transfected to overexpress human, rat, or cynomolgus monkey latent TGFβ1. About 24 hours after cell transfection, SRK-181 was added to the transfectants together with CAGA12 reporter cells. Approximately 16 to 20 hours after setting up the coculture, the assay was developed using BrightGlo reagent and luminescence read out on a plate reader. Dose–response activities were nonlinearly fit to a 3-parameter log inhibitor versus response model using GraphPad Prism 8 and best-fit IC50 values calculated.
Platelet aggregation, activation, and binding
Human whole blood samples were collected from 2 male and 2 female fasted donors and used for analysis. For the platelet aggregation assay, platelet-rich plasma (PRP) with 200 to 300 × 103 cells/µL was used. Samples were maintained at room temperature on the day of collection until spiked with saline (0.9% NaCl), vehicle control, or SRK-181 (up to a final concentration of 1,000 μg/mL). Samples were brought to 37 °C and ADP, an agonist that initiates changes in platelet shape and aggregation, was added to a final concentration of 10 μM before being loaded into a fixed wavelength aggregometer (Chrono-Log Corporation). Platelet aggregation was then measured by comparing the variation in light transmission through PRP plus ADP with platelet poor plasma for 6 minutes. Data were reported as area under the curve (%/min).
For the platelet activation and binding assays, blood samples were incubated with SRK-181 (up to a final concentration of 1,000 μg/mL) for 15 minutes, followed by addition of ADP for 2 minutes. After the incubations, samples were stained with flow cytometry antibodies for 20 minutes, fixed with paraformaldehyde and acquired using a FACSCanto II flow cytometer. The level of surface expression of the activation marker CD62P (P-Selectin) (mouse anti-human CD62P, BD BioSciences) and binding of SRK-181 were measured on CD61-expressing platelets (mouse anti-human CD61, BD BioSciences) by flow cytometry.
Cytokine release
Peripheral blood mononuclear cells (PBMC) collected from 5 to 8 donors were added to tissue culture wells (200,000/well) precoated with SRK-181, positive control (anti-CD3/anti-CD28 antibody cocktail), or vehicle (IgG) control at concentrations of 0.8 to 100 μg/mL. Cells were then incubated at 37 °C for 48 hours prior to supernatant collection. Supernatant was measured in triplicate by Luminex multiplex assay (Luminex) for interferon γ (IFNγ), interleukin 2 (IL-2), interleukin 1β (IL-1β), tumor necrosis factor α (TNFα), C-C Motif Chemokine Ligand 2 (CCL2), and interleukin 6 (IL-6). Cell culture supernatant was diluted 1:1 in Luminex Assay Buffer (Luminex). Logistically fit standard curves were used to calculate the concentration of each cytokine per well and a minimum of 5 donors were analyzed for each analyte. If variability across triplicates was greater than 10-fold that data point was flagged, and if an analyte had more than 2 flagged data points for a particular donor, then it was removed from analysis for that analyte.
In Vivo Pharmacokinetic, Toxicokinetic, and Toxicology Studies in Mice, Rats, and Cynomolgus Monkeys
A non-GLP single-dose PK study was performed in C57BL/6 mice (10-11 weeks) at Gateway Pharmacology Laboratories. Additional PK and toxicokinetic (TK) studies were conducted in naive young adult Sprague-Dawley rats (7-10 weeks) and cynomolgus monkeys (2-4 years). In rats, the non-GLP PK study was performed at Gateway Pharmacology Laboratories and the GLP toxicology and TK study was conducted at Covance Laboratories. In cynomolgus monkeys, both the non-GLP PK and GLP TK studies were performed at Covance Laboratories. Studies were in compliance with all applicable sections of the Final Rules of the Animal Welfare Act regulations (Code of Federal Regulations, Title 9), the Public Health Service Policy on Humane Care and Use of Laboratory Animals from the Office of Laboratory Animal Welfare, and the Guide for the Care and Use of Laboratory Animals from the National Research Council.
Mouse study
Female mice were group housed (3-5 mice/cage) in polycarbonate cages containing appropriate bedding and water valves in a controlled environment (17.8-26.7 °C; 30 to 70% relative humidity; 12-hour light and dark cycles) and were offered standard rodent chow (PicoLab Rodent Diet 20) and tap water ad libitum. Mice were randomly assigned to treatment groups for all studies. The experimental design of the study is outlined in Table 1.
Table 1.
SRK-181 Non-GLP Single-Dose Pharmacokinetic Studies in C57BL/6 Mice, Sprague-Dawley Rats, and Cynomolgus Monkeys.
| Study | Number of animals | Groups | Dose route/volume | Dose levels | Sample collection for pharmacokinetics |
|---|---|---|---|---|---|
| C57BL/6 mice | 66 females | 16/groupa | IV bolus @ 3 mL/kg | 0.3, 3, 10, and 30 mg/kg | SRK-181-mIgG1– predose and 1, 8, 24, 72, 120, 168, 264, 360, 528, 696, 864, and 1,032 hours postdose |
| Sprague-Dawley rats | 20 females | 4/group | IV bolus @ 3 mL/kg | 0.3, 1, 3, 10, and 30 mg/kg | SRK-181– predose and 1, 8, 24, 72, 120, 168, 264, 360, 528, 696, 864, and 1,032 hours postdose |
| Cynomolgus monkeys | 12 females | 3/group | IV bolus @ 1 or 3 mL/kg | 1, 3, 10, 30 mg/kg | SRK-181– 1, 4, 8, 24, 72, 120, 168, 240, 336, 504, 672, 840, 1,008, and 1,176 hours postdose |
Abbreviations: IV, intravenous; GLP, good laboratory practices.
a Sixteen animals in 0.3 and 3 mg/kg dose groups, 17 animals in 10 and 30 mg/kg dose groups. Four or 5 animals were sampled.
Rat studies
Male and female Sprague-Dawley rats were group housed in polycarbonate cages containing appropriate bedding and water valves in a controlled environment (20-26 °C; 30%-70% relative humidity; 12-hour light and dark cycles; 10 or more air changes per hour) and were offered Certified Rodent Diet #2014C or PicoLab Rodent Diet 20 and tap water ad libitum. Rats were randomly assigned to treatment groups for all studies. The experimental design of each rat study is outlined in Tables 1 and 2.
Table 2.
SRK-181 GLP Multidose Toxicology and Toxicokinetic Studies in the Sprague-Dawley Rats and Cynomolgus Monkeys.
| Study | Number of animals | Groups | Dose route/volume | Dose levelsa | Samples for TK and cytokine analysisb | Samples for Clin pathc and ADA |
|---|---|---|---|---|---|---|
| Sprague-Dawley rats | 81 Males 81 Females |
15/sex/groupd
TK and ADA: 3/sex/group (control) and 6/sex/group (treated) |
IV bolus @ 5.0 mL/kg | 0, 30e, 100, 200 mg/kg once weekly for 4 weeks | Day 1: predose and 1, 24, and 96 hours postdose Day 15: predose and 1 hour postdose Day 22: predose and 1, 24, 96, and 120 hours postdose Day 29: predose and 1 hour postdose Once on days 8, 15, 22, and 29 (recovery phase) |
Clin Path: Day 30 (dosing phase) and day 29 (recovery phase) ADA: Days 1, 15, and 29 (dosing phase): predose and once on days 8, 15, 22, and 29 (recovery phase) |
| Cynomolgus monkeys | 20 Males 20 Females |
4-6/sex/groupf | IV bolus @ 6.0 mL/kg | 0, 30, 100, 300 mg/kg once weekly for 4 weeks | Day 1 and day 22: predose and 1, 24, 48, 96, and 120 hours postdose Day 8 and 15: predose Day 29: predose and 1 hour postdose Once on days 8, 15, 22, and 29 (recovery phase) |
Clin Path: Day 30 (dosing phase) and day 29 (recovery phase) ADA: Days 1, 15, 22, and 29 (dosing phase): predose and once on days 15 and 29 (recovery phase) |
Abbreviations: ADA, antidrug antibody; GLP, good laboratory practices; IV, intravenous; TK, toxicokinetic.
a Control group was dosed with vehicle.
b Cytokine analysis conducted for monkey study only. Samples from days 1 and 22 of the dosing phase (predose and 1 and 24 hours postdose samples only) were sent for cytokine analysis.
c Clinical pathology (clin path) included hematology, serum chemistry, coagulation, and urinalysis.
d 10/sex/group for dosing phase and 5/sex/group for recovery phase (recovery phase day 1 = dosing phase day 30).
e On day 1, animals in 30 mg/kg group received approximately 21 mg/kg dose (∼70.0% of the nominal 30 mg/kg dose).
f 4/sex/group for dosing phase across all dose levels and 2/sex/group for recovery phase in 300 mg/kg dose level only.
Cynomolgus monkey studies
Male and female cynomolgus monkeys were group housed in stainless steel cages in a controlled environment (18-26 °C; 30%-70% relative humidity; 12-hour light and dark cycles; 8 or more air changes per hour with 100% fresh air) and were offered cage enrichment devices and food, Certified Primate Diet #5L4L (PMI Nutrition International Certified LabDiet) 1 to 2 times daily, and tap water ad libitum. Cynomolgus monkeys were randomly assigned to treatment groups for the GLP toxicology study but were not randomized for the PK study, where animals were selected for inclusion based on overall health and body weight. The experimental design of the cynomolgus monkey studies is outlined in Tables 1 and 2.
Safety evaluations
General clinical observations of animals were performed twice daily in both GLP toxicology studies. Cageside observations were conducted 2 to 3 hours postdose to assess acute toxicity in monkeys and once daily in rats. Other observations performed for all toxicology studies included an assessment of food consumption, once daily in monkeys and weekly in rats, and measurement of body weight once weekly. The 4-week GLP studies also included clinical pathology (hematology, serum chemistry, coagulation, and urinalysis) and anatomic pathology (gross and microscopic) evaluations (Table 2). Other safety evaluations performed for the 4-week GLP studies included ophthalmic examinations (both species); and for the monkey only, vital-sign measurements; electrocardiograms; neurologic examinations; and measurements of respiration rate and heart rate. Cytokine levels were also measured in the GLP 4-week monkey study as described below.
In vivo cytokine measurement and analysis
Plasma samples from cynomolgus monkeys were collected from days 1 and 22 of the dosing phase (predose and approximately 1 and 24 hours postdose samples only) and transferred to Myriad RBM, Inc for cytokine analysis. The analytes (n = 22) assayed were CD40 ligand; granulocyte colony-stimulating factor; interleukins-2, 4, 5, 6, 8, 10, 13, 15, 17, and 18; interleukin-1β; interleukin-1 receptor antagonist; interleukin-12 subunit p40; granulocyte-macrophage colony-stimulating factor; IFNγ; macrophage inflammatory protein-1 α and β; monocyte chemotactic protein 1; TNFα; and vascular endothelial growth factor. Samples were run on species-specific assays using the Luminex xMAP technology in accordance with Myriad RBM SOPs.
Raw analyte concentration data, in the form of an MS Excel spreadsheet from Myriad RBM were used to calculate mean concentration values for each target analyte by time point and dose group. When an individual value was less than the assay lower limit of quantitation (LLOQ), a value of 50% of that analyte’s LLOQ was substituted for analysis purposes. Fold changes were calculated for the 1 hour and 24 hour time points for each analyte by taking the average concentration at that time point and dividing by the average predose analyte concentration; however, if all analyte concentrations in the group at a time point were below LLOQ then no fold change was calculated.
Measurement of total SRK-181 concentrations in mouse, rat, and monkey serum (GLP and non-GLP studies)
Serum samples were evaluated for SRK-181 or SRK-181-mIgG1 with an antigen capture ELISA (with isotype-specific detection). The LLOQ for the non-GLP assays were 125, 125, and 250 ng/mL for the mouse, rat, and cynomolgus monkey, respectively, and 75 ng/mL for the optimized and validated GLP assays (rat and monkey). The absorbance versus concentration relationship was regressed using a 4-parameter logistic curve fit (with a weighting factor of 1/Y2 for the validated assays to support the toxicokinetic studies), and concentrations were calculated using GraphPad Prism or Watson LIMS (Thermo) data reduction software.
Measurement of SRK-181 antidrug antibodies in monkey and rat serum (GLP studies)
Antidrug antibodies (ADAs) against SRK-181 were detected using an electrochemiluminescent bridging assay with acid dissociation. Antidrug antibody samples were analyzed in both rat and cynomolgus monkey GLP toxicology studies. The rat ADA assay had a measured sensitivity of 20.2 ng/mL and no significant drug interference from 1.5 mg/mL SRK-181 on the detection of 5,000 ng/mL of positive control antibody. The cynomolgus monkey assay had a measured sensitivity of 4.6 ng/mL and no significant drug interference from 0.2 mg/mL SRK-181 on the detection of 2,000 ng/mL of positive control antibody.
Pharmacokinetics
Pharmacokinetics parameters were calculated in Phoenix WinNonlin (Certara USA Inc.) from the concentration-time data using a noncompartmental analysis method with intravenous (IV) bolus input. Nominal doses and sampling times were used. Concentration values below the LLOQ were treated as zero for descriptive statistics and toxicokinetic analysis. Area under the concentration time curve (AUC) was computed using the linear trapezoidal approximation method. Terminal half-life (t½) was estimated by log-linear regression of the terminal phase of the mean concentration versus time profiles. At least 3 points clearly visible in the terminal phase, an r2 value of at least 0.8, and time interval of at least 3 half-lives (in the GLP toxicity studies only) were required to characterize half-life. Due to the slow elimination relative to the dosing frequency on days 1 and 22 of the dosing phase, estimation of t½, CL, and Vss was limited to the recovery animals on day 29.
Results
Species Selection for Toxicology Studies
As per ICH S6 and S9 guidelines, we conducted the SRK-181 preclinical safety assessment in one rodent and one nonrodent species.22,23 Previously, we have demonstrated that SRK-181 is selective for the TGFβ1 isoform and inhibits the activation of human and mouse latent TGFβ1.20 For the studies described here, we further evaluated in vitro binding and activity to determine whether rat and cynomolgus monkey are pharmacologically relevant rodent and nonrodent species for toxicology studies with SRK-181.
Sequences for TGFβ1 prodomain and growth factor domain share high homology across species
Human and cynomolgus monkey TGFβ1 prodomains share 99.6% sequence identity (a difference in a single amino acid), while the sequence identity between the human and rat TGFβ1 prodomain is 85.9% (Table 3). Similarly, TGFβ1 growth factors in human, cynomolgus monkeys, and rat share high sequence identity (∼99%-100%), increasing the likelihood of equivalent SRK-181 activity across these species.
Table 3.
Percent Sequence Identity of TGFβ1 Prodomain and Growth Factor Across Different Species.
| Species | Prodomain (% Identity) | Growth factor (% Identity) | ||||
|---|---|---|---|---|---|---|
| Human | Rat | Cynomolgus | Human | Rat | Cynomolgus | |
| Human | 85.5% (213/249) | 99.6% (248/249) | 99.1% (111/112) | 100% (112/112) | ||
| Rat | 85.9% (214/249) | 99.1% (111/112) | ||||
Abbreviation: TGF-β, transforming growth factor-β.
Percentages calculated after pairwise sequence alignment using the EMBOSS Needle algorithm.21 Numbers in parentheses represent absolute number of amino acids from specific animal species that align with the human sequence, over total number of amino acids for human TGFβ1.
SRK-181 inhibits the activation of latent TGFβ1 in rat and cynomolgus monkey
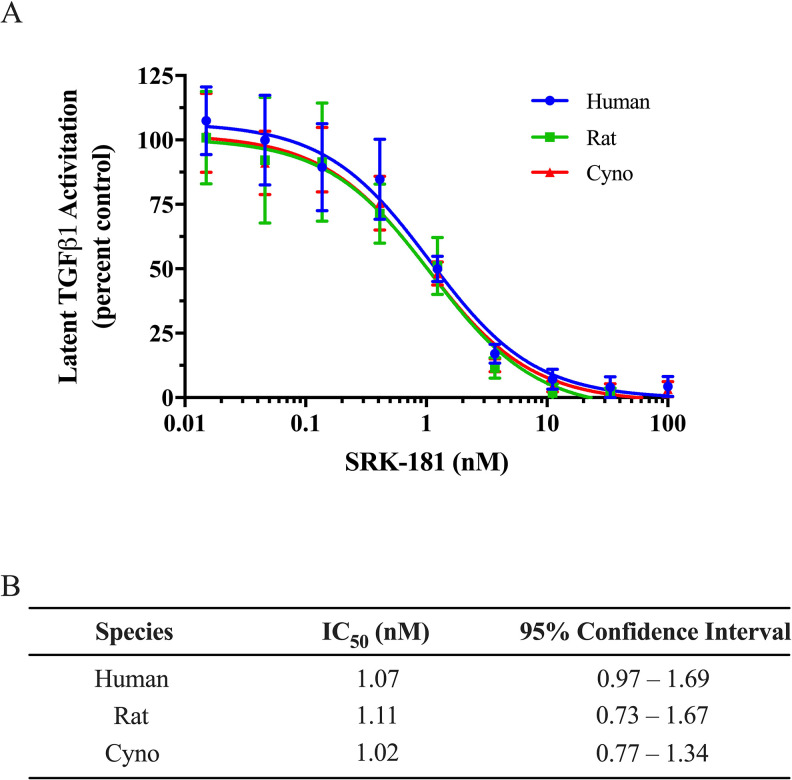
SRK-181 has similar binding affinity to latent TGFβ1 across various species including human, mouse, rat, and cynomolgus monkeys.20 To confirm similar inhibitory activity of SRK-181 across these species, we used a previously described cell-based assay in which species-specific latent TGFβ1 was overexpressed in LN229 cells.20 SRK-181 equivalently inhibits activation of latent TGFβ1 from human, rat, and cynomolgus monkey, with IC50 values between 1.02 and 1.11 nM (Figure 1).
Figure 1.
SRK-181 inhibits activation of latent transforming growth factor-β (TGFβ1) from human, rat, and cynomolgus monkey. (A) LN229 human glioblastoma cells were used to overexpress human (circles), rat (squares), or cynomolgus (triangles) latent TGFβ1 in the extracellular matrix. Latent TGFβ1 activation was measured with CAGA12 reporter cells and expressed as percent. One hundred percent activity was determined in the presence of vehicle control. Data shown are mean ± standard deviation from 2 independent triplicate experiments. Curves are best-fits to a dose–response inhibition model. (B) SRK-181 IC50 values for inhibition of latent TGFβ1 activation, by species.
Based on previous in vitro binding data,20 along with the similarities in sequence homology and demonstrated functional cross-reactivity of inhibitory activity described herein, the rat and cynomolgus monkey were selected as pharmacologically relevant species for the pharmacokinetic and toxicology evaluation of SRK-181.
In Vitro Safety Assessment of SRK-181
SRK-181 has no effect on human platelet aggregation, activation, and binding
Human platelets have been reported to express latent TGFβ1.24 Since SRK-181 binds to and inhibits activation of latent TGFβ1 complexes on human regulatory T (Treg) cells,20 we investigated the potential for SRK-181 to bind to human platelets and determined the impact of SRK-181 on the aggregation and activation of human platelets in vitro.
Platelet aggregometry was performed on PRP prepared from human donor whole blood samples. There was no relevant difference in the extent of platelet aggregation with SRK-181 at 0.01, 0.1, 1, 10, 100, and 1,000 μg/mL as compared to vehicle control (Figure 2A). Platelet activation and SRK-181 binding assessment were determined by measuring surface-level expression of CD62P (P-selectin) and SRK-181 (up to 1,000 μg/mL), respectively, on CD61 expressing platelets. SRK-181 did not induce spontaneous platelet activation or impact ADP-induced platelet activation at any concentration when compared with vehicle control (Figure 2B). Further, there was no evidence of SRK-181 binding to unactivated or activated platelets (Figure 2C). In conclusion, SRK-181 did not affect platelet aggregation, nor did SRK-181 induce platelet activation or bind to platelets.
Figure 2.
SRK-181 has no effect on human platelet aggregation, activation, and binding. (A) Platelet aggregation with ADP agonist was not altered by SRK-181 as compared to vehicle control. (B) Platelet activation with ADP agonist was not affected by increasing concentration of SRK-181, compared with the vehicle control. (C) SRK-181 binding to unactivated or activated platelets was low and did not increase with higher concentrations, as compared to vehicle control. Data shown in panel (A) are the mean of 4 donors ± standard deviation, while data shown in panels (B) and (C) are replicate means from one donor ± standard deviation and are representative of 4 donors.
SRK-181 does not induce cytokine release from healthy human PBMCs
SRK-181 is an immune cell-engaging therapeutic that may have the potential to activate immune cells when administered to patients. Therefore, it is important to determine whether a pro-inflammatory cytokine response is triggered with SRK-181.14,25 A plate-bound cytokine release assay was used to determine the potential for SRK-181 to induce immune cell activation, in which human PBMC were added to tissue culture wells precoated with SRK-181, a positive control (anti-CD3/anti-CD28 antibody cocktail) or vehicle control (IgG) and incubated for up to 48 hours at 37 °C (Figure 3). At all concentrations tested (up to 100 μg/mL SRK-181), measurements of the following cytokines were within 2.5-fold of the response compared to vehicle (IgG) control: IFNγ, IL-2, IL-1β, TNFα, IL-6, and CCL-2. In contrast, the positive control, anti-CD3/anti-CD28 cocktail, produced cytokine responses that were 10- to 1,000-fold above the levels seen with the vehicle (IgG) control. Most donors produced cytokine levels near or below the LLOQ in response to both SRK-181 and IgG control. However, 1 of 8 donors had a non-dose-dependent IL-2 response to SRK-181 of less than 20 pg/mL. No other cytokines were elevated in this donor sample, and there was otherwise no notable response of IL-2 in any other donor. Based on these data, SRK-181 does not induce in vitro cytokine release in healthy human PBMC.
Figure 3.
SRK-181 does not induce cytokine production in healthy human peripheral blood mononuclear cells. Cytokine production in response to SRK-181 (triangles), positive anti-CD3/anti-CD28 control (circles), and vehicle IgG control (squares) was determined in a plate-based cytokine release assay. Summarized results are across 5 to 8 healthy human donors. In some instances, for interleukin (IL)-1β and IL-6, the positive control values were above the upper limit of quantitation (ULOQ) and thus are indicated along the dashed line depicted on the graph. Analytes measured below the limit of quantitation (typically approximately 1-5 pg/mL) were entered as “1” for the purposes of graphing.
In Vivo Safety Assessment of SRK-181 in Rats and Cynomolgus Monkeys
The toxicity profile of SRK-181 has previously been evaluated in a 4-week non-GLP study in rats, wherein SRK-181 was determined to be well tolerated and no SRK-181-related adverse effects were observed up to weekly dose of 100 mg/kg.20 Next, 4-week GLP toxicology studies were performed in both rats and cynomolgus monkeys to confirm the findings and extend the maximum dose administered (experimental designs provided in Table 2).
Four-week Sprague-Dawley rat toxicology study (GLP)
SRK-181 was administered by IV bolus administration at doses of 30, 100, and 200 mg/kg versus vehicle once weekly for 4 weeks (5 doses total) to male and female rats, followed by a 4-week recovery period. All animals survived until scheduled necropsy, which occurred on study day 30 for the main group (one day after last dose) and on day 29 of the recovery phase for the recovery group. There were no SRK-181-related effects on clinical observations, body weight, body weight gain, food consumption, ophthalmic examinations, hematology, coagulation, or urinalysis. At ≥30 mg/kg/wk, minimal increases in total protein were observed in males, and a minimal increase in globulin concentration with a corresponding minimal decrease in mean albumin-globulin (A/G) ratios was observed in both sexes at ≥100 mg/kg/wk. These effects were not considered adverse due to their small magnitude and may have been related to the administration of SRK-181 (an IgG4 immunoglobulin). No SRK-181-related effects were observed on gross macroscopic pathology. Statistically significant organ weight changes at terminal sacrifice were limited to an increase in thymus weights in males administered 200 mg/kg/wk (up to 49%-53% by brain weight or body weight ratio, respectively) and in females administered ≥30 mg/kg/wk (up to 29% to 38% by brain weight and/or body weight ratio, respectively). These changes correlated microscopically with a slight increase in cortical lymphocytes that were morphologically similar to controls. Additional nonadverse SRK-181-related microscopic findings in the main study group consisted of minimal to slight mononuclear cell infiltrates in the Harderian gland of both sexes administered ≥100 mg/kg/wk and in one male administered 30 mg/kg/wk, and an increased incidence and severity of minimal to slight mononuclear cell infiltrates in the prostate of males administered 200 mg/kg/wk. In the recovery group, the SRK-181-related increased thymus weights and some nonadverse microscopic findings persisted (ie, in the thymus and Harderian gland), while the prostate findings showed signs of reversibility.
In conclusion, administration of SRK-181 once weekly by IV injection (5 doses total) was well tolerated in Sprague-Dawley rats at doses of 30, 100, and 200 mg/kg. There were no treatment-related adverse findings observed on any end point evaluated and the no-observed-adverse-effect level (NOAEL) was 200 mg/kg/wk, which was the highest dose tested.
Four-week cynomolgus monkey toxicology study (GLP)
SRK-181 was administered at doses of 30, 100, and 300 mg/kg versus vehicle by IV bolus administration once weekly for 4 weeks (5 doses total) to male and female cynomolgus monkeys, followed by a 4-week recovery period. All animals survived until scheduled necropsy on study day 30 (one day after last dose) for the main study animals and on day 29 of the recovery phase for the recovery group. There were no SRK-181-related effects on clinical observations, body weight, body weight gain, food consumption, ophthalmic and dental examinations, hematology, coagulation, urinalysis, or clinical chemistry, except for minimal decreases in mean A/G ratios in high-dose animals (300 mg/kg/wk); however, this effect lacked histopathological correlates and may have been related to administration of SRK-181 (an IgG4 immunoglobulin). Inter- and intragroup cytokine measurement differences were not considered to be related to SRK-181, as they were highly variable and not dose-responsive. Additionally, no SRK-181-related effects were observed in safety pharmacology end points (ie, ophthalmic examinations, vital-sign measurements, electrocardiograms, neurologic examinations, and measurements of respiration rate and heart rate) or on gross macroscopic or microscopic pathology. A minimal increase in mean heart weights in high-dose (300 mg/kg/wk) males and decreased sex organ weights in some treated groups were observed but were not statistically significant and were not SRK-181 related. The effects on mean heart weight were consistent with normal intergroup variation and lacked histopathological correlates. The effects on sex organ weights were considered secondary to variations in menstrual cyclicity and/or sexual maturity.
In summary, administration of SRK-181 once weekly by IV injection (5 doses total) was well tolerated in cynomolgus monkeys at doses of 30, 100, and 300 mg/kg. There were no SRK-181 treatment-related adverse findings observed on any end point evaluated and the NOAEL was 300 mg/kg/wk, which was the highest dose tested.
SRK-181 Single-Dose PK and Multidose Toxicokinetic Studies
Single-dose PK studies were performed in C57BL/6 mice, Sprague-Dawley rats, and cynomolgus monkeys (experimental designs provided in Table 2; results in Figure 4 and Table 4). In all 3 studies, all animals were systemically exposed to SRK-181. The maximum concentration (Cmax) increased approximately dose proportionally with increased dose, while AUC increased more than dose proportionally. SRK-181 is cross-reactive in the mouse, rat, and cynomolgus monkey; therefore, the observed nonlinear elimination of SRK-181 is likely a result of target-mediated drug disposition.
Figure 4.
Serum concentration time profile for SRK-181 following a single dose. A single intravenous (IV) bolus dose of SRK-181 (or SRK-181-mIgG1, for mice) was administered at (A) 0.3, 3, 10, and 30 mg/kg in female C57BL/6 mice, (B) 0.3, 1, 3, 10, and 30 mg/kg in female SD rats and (C) 1, 3, 10, and 30 mg/kg in female cynomolgus monkeys. In all studies, Cmax increased approximately dose proportionally while area under the concentration time curve (AUC) increased less than dose proportionally, suggesting TMDD. Data shown are mean ± standard deviation. Dose symbols: 0.3 (circles), 1 (downward triangles), 3 (squares), 10 (triangles), and 30 (diamonds) mg/kg.
Table 4.
PK Parameters for SRK-181-mIgG1 in C57BL/6 or SRK-181 in Sprague-Dawley Rats and Cynomolgus Monkeys From Non-GLP Single-Dose PK Studies.
| C57BL/6 mice (female) | n = 4/group/time point | 0.3 mg/kg | 3 mg/kg | 10 mg/kg | 30 mg/kg | |
|---|---|---|---|---|---|---|
| Day 1 | Cmax (µg/mL) | 7.40 | 153 | 233 | 664 | |
| Tmax (h) | 8 | 8 | 1 | 1 | ||
| AUC0-t (h*µg/mL) | 0.10 | 4.71 | 17.4 | 64.1 | ||
| t½ (h) | NC | 33.4 | 46.3 | 74.4 | ||
| Sprague-Dawley rats (female) | n = 4/group | 0.3 mg/kg | 1 mg/kg | 3 mg/kg | 10 mg/kg | 30 mg/kg |
| Day 1 | Cmax (µg/mL) | 6.31 | 20.3 | 78.4 | 263 | 873 |
| Tmax (h) | 1 | 1 | 1 | 1 | 1 | |
| AUC0-t (h*µg/mL) | 221 | 1,230 | 5,830 | 26,000 | 135,000 | |
| t½ (h) | 24 | 43.7 | 48.7 | 37.0 | 31.4 | |
| Cynomolgus monkeys (female) | n = 3/group | 1 mg/kg | 3 mg/kg | 10 mg/kg | 30 mg/kg | |
| Day 1 | Cmax (µg/mL) | 25.2 | 82.2 | 287 | 909 | |
| Tmax (h) | 1 | 1 | 1 | 1 | ||
| AUC0-t (h*µg/mL) | 1,760 | 7,850 | 28,800 | 135,000 | ||
| t½ (h) | 53.8 | 73.9 | 119 | 111 | ||
| CL (mL/h/kg) | 0.535 | 0.363 | 0.307 | 0.223 | ||
| VSS (mL/kg) | 47.1 | 44.5 | 53.7 | 56.3 |
Abbreviations: AUC0-t, area under the curve from time 0 to tlast; CL, clearance; Cmax, maximum observed concentration; NC, not calculated; PK, pharmacokinetics; t½, elimination half-life; Tmax, time of maximum observed concentration; Vss, volume of distribution at steady state.
Single-dose PK study in C57BL/6 mice (non-GLP)
The PK of SRK-181 was evaluated in C57BL/6 female mice following administration of a single IV bolus dose at dose levels of 0.3, 3, 10, and 30 mg/kg (Table 2). SRK-181-mIgG1 Cmax was observed at the first sampling time point of 1 hour in all groups (mean concentrations ranging from 7.4 to 664 µg/mL). SRK-181 attained a half-life (t½) ranging from 33.4 to 74.4 hours (Figure 4 and Table 4).
Single-dose PK study in Sprague-Dawley rats (non-GLP)
The PK of SRK-181 was evaluated in female rats following administration of a single IV bolus dose at dose levels of 0.3, 1, 3, 10, and 30 mg/kg (Table 2). SRK-181 Cmax was observed at the first sampling time point of 1 hour in all groups (mean concentrations ranging from 6.31 to 873 µg/mL). SRK-181 attained a t½ that ranged from 24 to 48.7 hours (Figure 4 and Table 4).
Single-dose PK study in cynomolgus monkeys (non-GLP)
The PK of SRK-181 was further evaluated in female cynomolgus monkeys at dose levels of 1, 3, 10, and 30 mg/kg following administration of a single IV dose (Table 2). Cmax was observed at the first sampling time point of 1 hour in all groups (ranging from 25.2 to 909 µg/mL). SRK-181 attained a t½ ranging from 53.8 to 119 hours. The mean clearance (CL) decreased with increased dose with values ranging from 0.223 to 0.535 mL/h/kg (Figure 4 and Table 4). The volume of distribution at steady state (VSS) values ranged from 44.5 to 56.3 mL/kg and did not exceed the total body water (693 mL/kg) or total blood volume (73.4 mL/kg) in a monkey, indicating SRK-181 was likely confined to the blood and not highly distributed to tissues following administration (Davies 1993). The data from this single-dose cynomolgus monkey PK study were used to allometrically scale human CL.
Multidose TK studies were conducted as part of the GLP toxicology studies in both Sprague-Dawley rats and cynomolgus monkeys administered SRK-181 once weekly for 4 weeks for a total of 5 doses (experimental designs provided in Table 2; results in Table 5 and Figure 5). In both studies, the PK profile across all SRK-181-treated animals confirmed systemic exposure to SRK-181. Additionally, there were no differences in SRK-181 exposure between male and female animals.
Table 5.
PK Parameters for SRK-181 in Sprague-Dawley Rats and Cynomolgus Monkeys From GLP Multidose TK Studies.
| Sprague-Dawley rats (male and female) | Number of animals = 6/sex/group | 30 mg/kga | 100 mg/kg | 200 mg/kg |
|---|---|---|---|---|
| Day 1 | Cmax (µg/mL) | 626 | 2,750 | 5,250 |
| Tmax (h) | 1 | 1 | 1 | |
| AUC0-168 (h*µg/mL) | 61,400 | 252,000 | 506,000 | |
| Day 22 | Cmax (µg/mL) | 1,310 | 4,640 | 9,980 |
| Tmax (h) | 1 | 1 | 1 | |
| AUC0-168 (h*µg/mL) | 162,000 | 559,000 | 1,010,000 | |
| CL (mL/h/kg) | 0.185 | 0.179 | 0.197 | |
| Day 29 | Cmax (µg/mL) | 1,570 | 5,410 | 9,730 |
| Tmax (h) | 1 | 1 | 1 | |
| AUC0-696 (h*µg/mL) | 477,000 | 1,550,000 | 2,910,000 | |
| t½ (h) | 322 | 393 | 356 | |
| CL (mL/h/kg) | 0.145 | 0.147 | 0.157 | |
| Vss (mL/kg) | 54.5 | 60.3 | 62.3 | |
| Cynomolgus monkeys (male and female) |
Number of animals = 4 or 6 /sex/group | 30 mg/kg | 100 mg/kg | 300 mg/kg |
| Day 1 | Cmax (µg/mL) | 688 | 2,290 | 6,470 |
| Tmax (h)b | 1.00 (1.00-1.00) | 1.00 (1.00-1.00) | 1.00 (1.00-1.00) | |
| AUC0-168 (h*µg/mL) | 61,900 | 217,000 | 641,000 | |
| Day 22 | Cmax (µg/mL) | 1,150 | 3,820 | 11,200 |
| Tmax (h)b | 1.00 (1.00-24.0) | 1.00 (1.00-48.0) | 1.00 (1.00-1.00) | |
| AUC0-168 (h*µg/mL) | 121,000 | 454,000 | 1,330,000 | |
| CL (mL/h/kg) | 0.259 | 0.226 | 0.233 | |
| Day 29 | Cmax (µg/mL) | NA | NA | 13,000 |
| Tmax (h)b | NA | NA | 1.00 (1.00-1.00) | |
| AUC0-168 (h*µg/mL) | NA | NA | 1,720,000 | |
| AUC0-696 (h*µg/mL) | NA | NA | 3,660,000 | |
| t½ (h) | NA | NA | 375 | |
| CL (mL/h/kg)c | NA | NA | 0.182 | |
| Vss (mL/kg)c | NA | NA | 69.9 |
Abbreviations: AUC0-168, area under the curve from time 0 to 168 hours; AUC0-696, AUC from 0 to 696 hours; CL, clearance; Cmax, maximum observed concentration; NA, not applicable; PK, pharmacokinetics; t½, elimination half-life; Tmax, time of maximum observed concentration; Vss, volume of distribution at steady state.
a On day 1, animals in 30 mg/kg group were administered approximately 70.0% of the nominal dose level (30 mg/kg/wk). The actual dose level of 21 mg/kg/wk was used to compensate for this event in TK analysis.
b Median (minimum-maximum) values are presented for Tmax.
c Due to the slow elimination relative to the dosing frequency on days 1 and 22, estimation of t½ and Vss was only attempted for recovery animals on day 29.
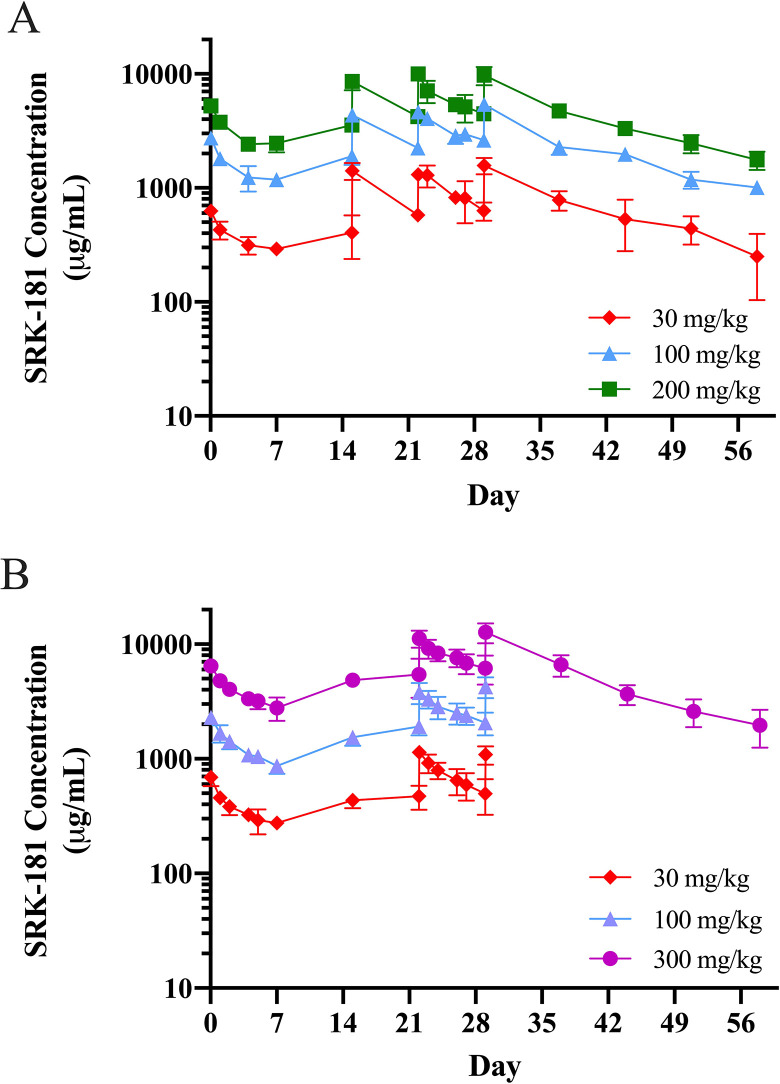
Figure 5.
Serum concentration time profile of SRK-181 following multiple doses. (A) Male and female SD rats were administered an intravenous (IV) bolus dose of SRK-181 at 30, 100, or 200 mg/kg on days 1, 8, 15, 22, and 29. Cmax, area under the concentration time curve (AUC)0-168, and AUC0-696 (on day 29) increased approximately dose proportionally from 30 to 300 mg/kg and there were minimal-to-no differences between sexes. There were no confirmed Antidrug antibody (ADA) positive samples in the 100 or 200 mg/kg SRK-181 treatment groups nor any decrease in exposure consistent with an immune response. (B) Male and female cynomolgus monkeys were administered an IV bolus dose of SRK-181 at 30, 100, or 300 mg/kg on days 1, 8, 15, 22, and 29. Cmax and AUC0-168 increased approximately dose proportionally from 30 to 300 mg/kg, and there were minimal-to-no differences between sexes. There were no confirmed ADA positive samples in any SRK-181 treatment group nor any decrease in exposure consistent with an immune response. Data shown are mean ± standard deviation. Dose symbols: 30 (diamonds), 100 (triangles), 200 (squares), and 300 (circles) mg/kg.
Multidose TK study in Sprague-Dawley rats (GLP)
The PK profile and parameters of SRK-181 were evaluated in the multidose TK study. Female and male rats were administered 5 IV bolus doses of SRK-181 at dose levels of 30, 100, and 200 mg/kg. The PK parameters were determined after day 1, day 22, and day 29. Cmax was observed at the first sampling time point of 1 hour in all groups on day 1, day 22, and day 29. SRK-181 attained a t½ ranging from 322 to 393 hours on day 29. Exposure, as assessed by SRK-181 Cmax, AUC0-168, and AUC0-696, demonstrated a dose-dependent increase on days 1, 22, and 29 and was generally dose proportional (Table 5). Accumulation of SRK-181 was observed after multiple weekly doses of 100 or 200 mg/kg (Figure 5A). Five animals (4 female and 1 male) in the 30 mg/kg dose group displayed reduced exposure consistent with an ADA response. Four of these animals confirmed ADA positive in later intervals; however, this did not impact the interpretation of multidose PK data.
Multidose TK study in cynomolgus monkeys (GLP)
The PK profile and parameters of SRK-181 were evaluated in a multidose TK study. Female and male cynomolgus monkeys were administered 5 IV bolus doses of SRK-181 at dose levels of 30, 100, and 300 mg/kg. The PK parameters were determined after day 1, day 22, and day 29.
SRK-181 Cmax was typically observed at the first sampling time point of 1 hour following dosing in all dosed animals, with the exception of day 22 where 1 animal in each of the 30 and 100 mg/kg groups had a Cmax observed at a later time point. Overall exposure as assessed by Cmax and AUC0-168 increased approximately dose proportionally from 30 to 300 mg/kg (Table 5), suggesting that the target was fully saturated. Animals maintained exposure throughout dosing and recovery periods (Figure 5B). Accumulation (Cmax and AUC) was observed at all dose levels from day 1 to day 22 (day 29 at 300 mg/kg). No SRK-181-treated animals were confirmed ADA positive at any time during the study. During the recovery phase, SRK-181 concentrations declined, with a mean t½ value of 375 hours in the 300 mg/kg/dose group. Mean CL values ranged from 0.182 to 0.259 mL/h/kg. A mean Vss value of 69.9 mL/kg was observed in 300 mg/kg recovery animals on day 29. Similar to the single-dose study, the mean Vss value approximated the total blood volume in a monkey (73.4 mL/kg), indicating SRK-181 may largely reside in the bloodstream following IV administration.26
Selection Strategy for Safe Starting Dose in FIH Clinical Trial
Consistent with both ICH S6 and S9 and regulatory guidance,22,23,27,28 we utilized SRK-181 pharmacology and toxicology assessments to guide the dose selection strategy for the FIH clinical trial. We utilized in vivo pharmacology data to predict the human pharmacological active dose (PAD) and recommend the FIH dose. In addition, we determined the safety margins between the predicted exposure at the recommended FIH dose and the exposure achieved at the NOAELs in toxicology studies.
Predicting the pharmacologically active dose in humans
The pharmacological activity of SRK-181-mIgG1 has been previously described.20 In vivo studies were conducted across 3 different syngeneic tumor models, including the Cloudman S91 melanoma model (S91), MBT-2 bladder cancer model (MBT-2), and EMT-6 breast tumor model (EMT-6). Across all studies, SRK-181-mIgG1, when administered in combination with anti-PD-1, exhibited profound antitumor effects and resulted in reduced tumor burden and improved animal survival. Based on these studies, the pharmacologically active dose for SRK-181-mIgG1 ranged from 3 to 10 mg/kg (Table 6). The estimated average SRK-181-mIgG1 serum exposure (Cavg) achieved at the PAD was determined using the PK parameters obtained from a single-dose PK study in mice (Table 4 and Table 6). The exposures achieved at a PAD of 3 and 10 mg/kg were 28.4 and 86.3 µg/mL, respectively.
Table 6.
Pharmacological Active Dose and Exposure in Mice.
| Tumor model | Dose range (mg/kg) | PADa | Predicted exposure (Cavg) at PADb |
|---|---|---|---|
| Cloudman S91 | 3, 10, and 30 mg/kg | 3 mg/kg | 28.4 μg/mL |
| MBT-2 | 3 and 10 mg/kg | 10 mg/kg | 86.3 μg/mL |
| EMT-6 | 10 mg/kg | 10 mg/kg | 86.3 μg/mL |
Abbreviations: PAD, pharmacological active dose; PK, pharmacokinetics.
a Pharmacological active dose determined from multidose in vivo efficacy studies in Martin 2020.
b Single-dose PK data in mice (Table 4) were used to predict the multidose PK exposure at PAD.
We next calculated the anticipated PAD in humans, assuming similar exposure is necessary to observe pharmacological activity. The PK parameters generated from single-dose PK study in cynomolgus monkeys (Table 4) were used to conduct simple allometric scaling29 and estimate the human CL of 11 mL/h. Based on the estimated human CL, the expected half-life is 11.7 days, which is anticipated to maintain average SRK-181 concentrations of 28.4 to 86.3 µg/mL over a 3-week period. Estimated human CL and predicted human average exposure concentration range were used to calculate the predicted PAD of 2 to 6.1 mg/kg in humans administered SRK-181 every 3 weeks.
Safety margins between recommended FIH starting dose and exposures from safety assessment studies
The predicted PAD of 2 to 6.1 mg/kg was used to guide the FIH dose selection, whereas the exposures achieved in the toxicology studies were utilized to determine the safety margins between the FIH dose and the NOAELs. Since SRK-181 is a mAb (>100 kDa) and will be administered IV, intraspecies normalization of dose-to-weight (mg/kg) was utilized. Due to expected variability in patient tumor load and with the intent to fully characterize the safety, PK, and preliminary efficacy of SRK-181 in humans at multiple dose levels, we applied a safety factor between 2- and 6-fold to the predicted PAD to attain the clinical starting dose of SRK-181 at 1 mg/kg, administered every 3 weeks. The comprehensive toxicology assessment for SRK-181 did not identify any adverse toxicity in the 4-week GLP studies in the rat and cynomolgus monkey, with NOAELs of 200 and 300 mg/kg, respectively. Based on the exposures achieved in these toxicology studies, the nonclinical safety factor for the proposed human starting dose (1 mg/kg) was 139- to 237-fold based on AUC0-168, whereas the safety margins ranged from 624- to 813-fold based on Cmax (Table 7).
Table 7.
Nonclinical Safety Factors for Proposed SRK-181 Human Starting Dose.
| Species | Doses | AUC0-168 (μg*h/mL) | Cmax (μg/mL) | ||
|---|---|---|---|---|---|
| Exposurea | Safety factor | Exposurea | Safety factor | ||
| Monkey | 300 mg/kg | 1,330,000 | 183 | 11,200 | 700 |
| Rat | 200 mg/kg | 1,010,000 | 139 | 9,980 | 624 |
| Predicted humanb | 80 mg (1 mg/kg) | 7,273 | 1 | 16 | 1 |
Abbreviation: AUC, area under the concentration time curve.
a On day 22 of GLP multidose rat and monkey toxicology studies.
b Allometrically scaled human clearance used from single species allometry from monkey, 11 mL/h.
Discussion
Transforming growth factor-β signaling within the tumor microenvironment has recently emerged as a potential primary resistance mechanism to immune checkpoint inhibitor therapies (anti-PD-(L)1 therapy), which only provide a durable response in select patients and cancer types.1,2,30 This observation provides a rationale for combining TGFβ inhibitors with checkpoint inhibitor therapy to potentially overcome this primary resistance mechanism and improve clinical responses in these patients. However, the development of TGFβ inhibitors has been hindered by an inability to identify a therapeutic window that balances pharmacological effects with avoiding on-target toxicities. Transforming growth factor-β inhibitors evaluated to date, which nonselectively block signaling by multiple TGFβ isoforms, have not demonstrated promising clinical data in patients with cancer, as they have failed to achieve the desired efficacy, displayed unfavorable safety profile, or both.8,12-14 The toxicities associated with these molecules include cardiovascular abnormalities, epithelial hyperplasia, gastrointestinal abnormalities, and skin lesions. Each of these toxicities have been well characterized in multiple animal species (eg, rodents, dogs, and cynomolgus monkeys) in studies ranging in duration from 1 to 2 weeks up to 6 months.16-18 Among these toxicities, the irreversible cardiovascular inflammatory lesions, hemorrhage and hyperplasia in heart valves, and arterial lesions that include the aorta and coronary arteries, are of major concern.
Transforming growth factor-β signaling plays a critical role in homeostasis and repair of stress-related injury; however, the exact contribution of specific TGFβ isoforms toward maintaining normal cardiac function is not completely understood. The use of pan-TGFβ inhibitors can produce a cardiovascular disease phenotype similar to that seen with the disruption of the TGFβ pathway in adult cardiovascular disease. For example, genetic mutations in TGFBR1 and TGFBR2 in patients with Loeys-Dietz or Marfan syndromes led to atrial aneurysm and heart valve abnormalities, while a mutation in TGFB3 leads to arrhythmogenic right ventricular cardiomyopathy/dysplasia.31 It is also notable that patients with cardiovascular disease due to a disrupted TGFβ pathway have lesions similar to those observed in TGFβ2 knockout (KO) mice,32 whereas the TGFβ1 KO mice have a substantially different phenotype and display multi-organ inflammation, autoimmunity, and gastrointestinal abnormalities.33,34 Given the role of various TGFβ growth factors in maintaining homeostasis, it is not surprising that nonselective TGFβ signal inhibition leads to on-target toxicities. To circumvent this undesirable safety profile, while achieving the pharmacological benefits of inhibiting TGFβ signaling, we speculated that a targeted TGFβ1 inhibitor such as SRK-181, may have a better safety profile that avoids the cardiovascular toxicities noted with pan-TGFβ inhibitors.
In a recent publication, we confirmed SRK-181 as a potent and highly selective inhibitor of latent TGFβ1 activation and demonstrated that inhibition of the TGFβ1 isoform is sufficient to overcome primary resistance to anti-PD-1 therapies in preclinical tumor models. Furthermore, in a non-GLP pilot toxicology study in rats, we showed an improved preclinical safety profile for SRK-181 as compared to pan-TGFβ inhibition.20 Our current work summarizes follow-up preclinical studies that support the clinical development of SRK-181. These studies provided insight into 3 aspects of SRK-181 nonclinical development: (1) a superior preclinical safety profile of SRK-181 compared to pan-TGFβ inhibitors, (2) favorable preclinical PK properties of SRK-181 allowing for durable and sustained exposures, and (3) a rationale for starting dose selection and dosing interval in the phase 1 clinical trial.
Results from the 4-week GLP toxicology studies with SRK-181 in both rats and cynomolgus monkeys showed that it was well tolerated when administered as an IV bolus injection once weekly for 4 weeks at doses up to 200 mg/kg or 300 mg/kg, respectively. Notably, there were no cardiovascular lesions observed with SRK-181 in either species at any dose tested following up to 4 weeks of treatment, and no other pan-TGFβ inhibition-related toxicities observed, such as epithelial hyperplasia, dental dysplasia, gingivitis, or oral or nasal bleeding. These findings contrast with those published on pan-TGFβ inhibitors, wherein these adverse on-target toxicities occurred and led to animal mortality.16-18,20 Furthermore, there was no evidence that SRK-181 resulted in changes to the cytokine profile in cynomolgus monkeys after multiple doses nor does SRK-181 appear to induce in vitro cytokine release in healthy human PBMC. These preclinical data are promising and in contrast to the uncontrolled cytokine release observed in a phase 1 trial with an anti-TGFβRII receptor mAb.14
Multiple possibilities may explain the superior preclinical safety profile of SRK-181 compared to pan-TGFβ inhibitors. For example, TGFβ2 and TGFβ3 may play a more dominant role in maintaining the normal homeostasis in the heart tissue compared to TGFβ1. It is known that both TGFβ2 and TGFβ3 play an important role in cardiovascular development.35,36 In addition, mutations in the TGFβ2 ligand lead to cardiovascular abnormalities such as valve malformations in humans,37 while congenital heart abnormalities have been noted in TGFβ2 KO mice.32 Another possibility is that all 3 isoforms contribute to TGFβ signaling and that a certain level of basal TGFβ signaling is necessary to maintain cardiac tissue homeostasis. In this case, inhibition of latent TGFβ1 by SRK-181 could induce the desired pharmacological effect within TGFβ1-driven tumors while not completely abrogating TGFβ signaling in cardiac tissue, allowing other TGFβ isoforms to compensate.
Pharmacokinetic properties of SRK-181 were evaluated in single-dose PK and multidose TK studies. SRK-181 exposure following single IV-bolus administration increased more than dose proportionally, with a nonlinear elimination profile noted at lower doses from 0.3 to 3 mg/kg while achieving a relatively linear elimination profile at higher doses of up to 30 mg/kg. The observed nonlinear SRK-181 PK profile may be due to basal levels of latent TGFβ1 expressed in normal animal tissues serving as a sink, leading to faster SRK-181 CL and promoting target-mediated drug disposition. This observation is supported in part by a comprehensive analysis confirming the expression of TGFβ1 in multiple organs across 4 different strains of mice including BALB/c, FVB/N, C57BL/6, and 129S1.38 In multidose TK studies, where doses of 30 to 300 mg/kg SRK-181 were tested, a dose proportional increase in exposure was observed, suggesting that doses above 30 mg/kg reached target saturation in animal species. Importantly, multidose IV-bolus administration of SRK-181 allowed for sustained exposure and drug accumulation over the dosing period in both rat and monkey species. A mean t½ following single dose of SRK-181 ranged from 1 to 2 days in rat and from 2.2 to 4.95 days in cynomolgus monkey, whereas the t½ following multidoses of SRK-181 was up to 15.6 days in cynomolgus monkey. This is in line with the typical half-lives of mAbs administered in humans that range from approximately 11 to 30 days and allows for less frequent dosing of mAbs in humans, such as the proposed Q3W clinical dosing for SRK-181.39
Finally, preclinical studies guided the dose selection strategy in the ongoing phase 1 clinical trial with SRK-181.40 The PAD of SRK-181-mIgG1 in combination with an anti-PD-1 antibody in mouse tumor models ranged from 3 to 10 mg/kg with the average serum exposure at these doses ranging from 28.4 to 86.3 µg/mL, respectively. Assuming similar exposure is necessary to achieve a pharmacological effect in humans, the predicted PAD for SRK-181 in humans was between 2 and 6 mg/kg when administered every 3 weeks. It is important to note that the predicted human PAD is based on animal models, which may not fully represent the possible variability across human disease. For example, we anticipate differences in tumor burden (and therefore target expression) across patients, variability in serum exposure of SRK-181, and variability in SRK-181 exposure within the tumor tissue. For these reasons, the pharmacologically active dose of SRK-181 in humans will be determined in future clinical trials. Nonetheless, the preclinical data supported the selection of the clinical starting dose in accordance with regulatory guidance27,28 and estimating the safety margins when compared with exposures achieved in toxicology studies. Toxicology studies designed to assess the safety of SRK-181 in the rat and cynomolgus monkey identified NOAELs of 200 and 300 mg/kg, respectively, which is notable since these studies dosed above the predicted target saturation and rule out any potential SRK-181 pharmacology-based toxicities. Based on both pharmacology and toxicology data, we selected 1 mg/kg (equivalent to 80 mg based on 80 kg human) administered every 3 weeks as the proposed human starting dose in a phase 1 trial. This dose was 2- to 6-fold lower than the predicted human PAD and provided a nonclinical safety factor of 139- to 813-fold (based on AUC0-168 and Cmax).
In summary, our studies demonstrated that SRK-181 is an effective, targeted, and safe latent TGFβ1 inhibitor with potential therapeutic benefit in the treatment of solid tumors and rare hematological pathologies, for which TGFβ signaling dysregulation has been implicated as a mediator of the disease process. Notably, no adverse effects or TGFβ-associated toxicities were observed in any of the nonclinical studies conducted to date, at doses that are expected to be above target saturation, supporting the hypothesis that selective inhibition of TGFβ1 has a favorable safety profile compared to pan-TGFβ inhibitors. All of the available data in aggregate, including in vitro and in vivo nonclinical pharmacology, PK, and toxicology data with SRK-181 supported the initiation of an ongoing phase 1, FIH, dose-escalation, and dose-expansion study.
Acknowledgments
The authors thank the following Study Directors at Covance for the successful conduct of these studies: Justin Shefchek (Single-dose Cynomolgus Monkey PK Study), Kyle Gelow (Non-GLP Rat PK study and GLP 4-week Rat Study), and Kevin Williams (GLP 4-week Cynomolgus Monkey Study).
Author Contributions: Welsh, B. contributed to conception and design, contributed to analysis and interpretation, drafted manuscript, and critically revised manuscript; Faucette, R. contributed to design, contributed to acquisition, analysis, and interpretation, drafted manuscript, and critically revised manuscript; Bilic, S. contributed to design, contributed to analysis and interpretation, drafted manuscript, and critically revised manuscript; Martin, C. contributed to conception and design, contributed to acquisition, analysis, and interpretation, drafted manuscript, and critically revised manuscript; Schürpf, T. contributed to conception and design, contributed to analysis and interpretation, drafted manuscript, critically revised manuscript; Chen, D. contributed to interpretation and critically revised manuscript; Nicholls, S. contributed to interpretation and critically revised manuscript; Lansita, J. contributed to conception and design, contributed to analysis and interpretation, drafted manuscript, and critically revised manuscript; Kalra, A. contributed to conception and design, contributed to analysis and interpretation, drafted manuscript, and critically revised manuscript. All authors gave final approval and agree to be accountable for all aspects of work ensuring integrity and accuracy.
Declaration of Conflicting Interests: The authors declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: All contributors are either employees or contractors of Scholar Rock. In addition, Dr Kalra and Dr Martin have patent US 63/111,530 pending and Dr Martin and Dr Schürpf have patent WO/2020/014460 pending.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iD: Brian T. Welsh  https://orcid.org/0000-0003-3211-9156
https://orcid.org/0000-0003-3211-9156
Thomas Schürpf  https://orcid.org/0000-0003-3023-1136
https://orcid.org/0000-0003-3023-1136
References
- 1. Hugo W, Zaretsky JM, Sun L, et al. Genomic and transcriptomic features of response to anti-PD-1 therapy in metastatic melanoma. Cell. 2016;165(1):35–44. [published correction appears in Cell. 2017 Jan 26;168(3):542] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Mariathasan S, Turley SJ, Nickles D, et al. TGFβ attenuates tumour response to PD-L1 blockade by contributing to exclusion of T cells. Nature. 2018;554(7693):544–548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Wang JJ, Lei KF, Han F. Tumor microenvironment: recent advances in various cancer treatments. Eur Rev Med Pharmacol Sci. 2018;22(12):3855–3864. [DOI] [PubMed] [Google Scholar]
- 4. Tauriello DVF, Palomo-Ponce S, Stork D, et al. TGFβ drives immune evasion in genetically reconstituted colon cancer metastasis. Nature. 2018;554(7693):538–543. [DOI] [PubMed] [Google Scholar]
- 5. Derynck R, Budi EH. Specificity, versatility, and control of TGF-β family signaling. Sci Signal. 2019;12(570):eaav5183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Dong X, Zhao B, Iacob RE, et al. Force interacts with macromolecular structure in activation of TGF-β. Nature. 2017;542(7639):55–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Hara M, Kirita A, Kondo W, et al. LAP degradation product reflects plasma kallikrein-dependent TGF-β activation in patients with hepatic fibrosis. Springerplus. 2014;3(1):221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Akhurst RJ. Targeting TGF-β signaling for therapeutic gain. Cold Spring Harb Perspect Biol. 2017;9(10):a022301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Herbertz S, Sawyer JS, Stauber AJ, et al. Clinical development of galunisertib (LY2157299 monohydrate), a small molecule inhibitor of transforming growth factor-beta signaling pathway. Drug Des Devel Ther. 2015;9:4479–4499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Morris JC, Tan AR, Olencki TE, et al. Phase I study of GC1008 (fresolimumab): a human anti-transforming growth factor-beta (TGFβ) monoclonal antibody in patients with advanced malignant melanoma or renal cell carcinoma. PLoS One. 2014;9(3):e90353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Varricchio L, Mascarenhas J, Migliaccio AR, et al. AVID200, a potent trap for TGF-β ligands inhibits TGF-β1 signaling in human myelofibrosis. Blood. 2018;132(supplement 1):1791. [Google Scholar]
- 12. Cohn A, Lahn MM, Williams KE, et al. A phase I dose-escalation study to a predefined dose of a transforming growth factor-β1 monoclonal antibody (TβM1) in patients with metastatic cancer. Int J Oncol. 2014;45(6):2221–2231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Voelker J, Berg PH, Sheetz M, et al. Anti-TGF-β1 Antibody therapy in patients with diabetic nephropathy. J Am Soc Nephrol. 2017;28(3):953–962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Tolcher AW, Berlin JD, Cosaert J, et al. A phase 1 study of anti-TGFβ receptor type-II monoclonal antibody LY3022859 in patients with advanced solid tumors. Cancer Chemother Pharmacol. 2017;79(4):673–680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Vitsky A, Waire J, Pawliuk R, et al. Homeostatic role of transforming growth factor-beta in the oral cavity and esophagus of mice and its expression by mast cells in these tissues. Am J Pathol. 2009;174(6):2137–2149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Lonning S, Mannick J, McPherson JM. Antibody targeting of TGF-β in cancer patients. Curr Pharm Biotechnol. 2011;12(12):2176–2189. [DOI] [PubMed] [Google Scholar]
- 17. Stauber AJ, Credille KM, Truex LL, Ehlhardt WJ, Young JK. Nonclinical safety evaluation of a transforming growth factor β receptor I kinase inhibitor in Fischer 344 rats and beagle dogs. J Clin Toxicol. 2014;4(3). [Google Scholar]
- 18. Mitra MS, Lancaster K, Adedeji AO, et al. A potent pan-TGFβ neutralizing monoclonal antibody elicits cardiovascular toxicity in mice and cynomolgus monkeys. Toxicol Sci. 2020;175(1):24–34. [DOI] [PubMed] [Google Scholar]
- 19. Anderton MJ, Mellor HR, Bell A, et al. Induction of heart valve lesions by small-molecule ALK5 inhibitors. Toxicol Pathol. 2011;39(6):916–924. [DOI] [PubMed] [Google Scholar]
- 20. Martin CJ, Datta A, Littlefield C, et al. Selective inhibition of TGFβ1 activation overcomes primary resistance to checkpoint blockade therapy by altering tumor immune landscape. Sci Transl Med. 2020;12(536): eaay8456. [DOI] [PubMed] [Google Scholar]
- 21. Madeira F, Park YM, Lee J, et al. The EMBL-EBI search and sequence analysis tools APIs in 2019. Nucleic Acids Res. 2019;47(W1):W636–W641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Food and Drug Administration. Guidance for Industry: S9 Nonclinical Evaluation for Anticancer Pharmaceuticals. Food and Drug Administration; 2010. [Google Scholar]
- 23. Food and Drug Administration. Guidance for Industry: S6(R1) Preclinical Safety Evaluation of Biotechnology-Derived Pharmaceuticals. Food and Drug Administration; 2012. [Google Scholar]
- 24. Tran DQ, Andersson J, Wang R, Ramsey H, Unutmaz D, Shevach EM. GARP (LRRC32) is essential for the surface expression of latent TGF-beta on platelets and activated FOXP3+ regulatory T cells. Proc Natl Acad Sci U S A. 2009;106(32):13445–13450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Suntharalingam G, Perry MR, Ward S, et al. Cytokine storm in a phase 1 trial of the anti-CD28 monoclonal antibody TGN1412. N Engl J Med. 2006;355(10):1018–1028. [DOI] [PubMed] [Google Scholar]
- 26. Davies B, Morris T. Physiological parameters in laboratory animals and humans. Pharm Res. 1993;10(7):1093–1095. [DOI] [PubMed] [Google Scholar]
- 27. EMEA. Guideline on Strategies to Identify and Mitigate Risks for First-in-Human and Early Clinical Trials with Investigational Medicinal Products. EMEA; 2017. (EMEA/CHMP/SWP/28367/07 Rev. 1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Food and Drug Administration. Guidance for Industry: Estimating the Maximum Safe Starting Dose in Initial Clinical Trials for Therapeutics in Adult Healthy Volunteers. Food and Drug Administration; 2005. [Google Scholar]
- 29. Deng R, Iyer S, Theil FP, Mortensen DL, Fielder PJ, Prabhu S. Projecting human pharmacokinetics of therapeutic antibodies from nonclinical data: what have we learned? MAbs. 2011;3(1):61–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Haslam A, Prasad V. Estimation of the percentage of US patients with cancer who are eligible for and respond to checkpoint inhibitor immunotherapy drugs. JAMA Netw Open. 2019;2(5):e192535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Doetschman T, Barnett JV, Runyan RB, et al. Transforming growth factor beta signaling in adult cardiovascular diseases and repair. Cell Tissue Res. 2012;347(1):203–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Sanford LP, Ormsby I, Gittenberger-de Groot AC, et al. TGFbeta2 knockout mice have multiple developmental defects that are non-overlapping with other TGFbeta knockout phenotypes. Development. 1997;124(13):2659–2670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Shull MM, Ormsby I, Kier AB, et al. Targeted disruption of the mouse transforming growth factor-beta 1 gene results in multifocal inflammatory disease. Nature. 1992;359(6397):693–699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Kulkarni AB, Huh CG, Becker D, et al. Transforming growth factor beta 1 null mutation in mice causes excessive inflammatory response and early death. Proc Natl Acad Sci U S A. 1993;90(2):770–774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Azhar M, Brown K, Gard C, et al. Transforming growth factor beta2 is required for valve remodeling during heart development. Dev Dyn. 2011;240(9):2127–2141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Chakrabarti M, Al-Sammarraie N, Gebere MG, et al. Transforming growth factor beta3 is required for cardiovascular development. J Cardiovasc Dev Dis. 2020;7(2):19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Shimizu C, Jain S, Davila S, et al. Transforming growth factor-beta signaling pathway in patients with kawasaki disease. Circ Cardiovasc Genet. 2011;4(1):16–25. [published correction appears in circ cardiovasc genet. 2011 Apr;4(2):e9. Davila, Sonia [removed]] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Flanders KC, Yang YA, Herrmann M, et al. Quantitation of TGF-β proteins in mouse tissues shows reciprocal changes in TGF-β1 and TGF-β3 in normal vs neoplastic mammary epithelium. Oncotarget. 2016;7(25):38164–38179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Ovacik M, Lin K. Tutorial on monoclonal antibody pharmacokinetics and its considerations in early development. Clin Transl Sci. 2018;11(6):540–552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. SRK-181 Alone or in Combination With Anti-PD-(L)1 Antibody Therapy in Patients With Locally Advanced or Metastatic Solid Tumors (DRAGON). ClinicalTrials.gov Identifier: NCT04291079. Published March 2, 2020. Updated January 15, 2021. Accessed March 3, 2021. https://clinicaltrials.gov/ct2/show/NCT04291079