Abstract
Background
Osteoporosis-related fractures are an important public health burden.
Objective
To examine health care costs in Medicare patients with an osteoporosis-related fracture.
Methods
Medicare fee-for-service members with an osteoporosis-related fracture between January 1, 2010, to September 30, 2014 were included. A nonfracture comparator group was selected by propensity score matching. Generalized linear models using a gamma distribution were used to compare costs between fracture and nonfracture cohorts.
Results
A total of 885 676 Medicare beneficiaries had fracture(s) and met inclusion criteria. Average age was 80.5 (±8.4) years; 91% were White, and 94% female. Mean all-cause costs were greater in the fracture vs nonfracture cohort ($47 163.25 vs $16 034.61) overall and for men ($52 273.79 vs $17 352.68). The highest mean costs were for skilled nursing facility ($29 216), inpatient costs ($24 190.19), and hospice care ($20 996.83). The highest incremental costs versus the nonfracture cohort were for hip ($71 057.83 vs $16 807.74), spine ($37 543.87 vs $16 860.49), and radius/ulna ($24 505.27 vs $14 673.86). Total medical and pharmacy costs for patients who experienced a second fracture were higher compared with those who did not ($78 137.59 vs $44 467.47). Proportionally more patients in the fracture versus nonfracture cohort died (18% vs 9.3%), with higher death rates among men (20% vs 11%).
Conclusion and Relevance
The current findings suggest a significant economic burden associated with fractures. Early identification and treatment of patients at high risk for fractures is of paramount importance for secondary prevention and reduced mortality.
Keywords: osteoporosis, fracture, Medicare, male, cost
Introduction
Osteoporosis is characterized by compromised bone strength caused by bone mass loss and bone quality deterioration, resulting in increased fracture risk.1 Based on a 2005-2010 National Health and Nutrition Examination Survey, an estimated 10.2 million adults aged ≥50 years have osteoporosis.2 This number is projected to increase by >30% between 2010 and 2030, based on the aging US population.2 A study of Medicare enrollees demonstrated that although US age-adjusted hip fracture rates declined from 2002 to 2012, the decline subsequently halted, with higher rates in 2013 to 2015.3 Similar findings were reported for commercial and Medicare Advantage enrollees (a decline in overall fracture rate between 2007 and 2013, with a subsequent rise through 2017).4
Potential contributing factors to change in fracture incidence include declining rates of bone mineral density (BMD) testing, osteoporosis diagnosis, and treatment. The majority of women aged ≥50 years with a new low-trauma fracture do not undergo evaluation for osteoporosis or initiate osteoporosis treatment within a year after fracture.5 Indeed, the current national average Medicare STAR rating (a measure of whether female Medicare members aged 67-85 years received BMD testing or were prescribed an osteoporosis drug within 6 months of fracture) for health plans is 2.6 (out of 5), with only 40% of women receiving BMD testing or treatment within 6 months of fracture.6
Limited data exist on the cost of osteoporosis-related fractures. The majority of the studies completed to date include evaluations of commercial and Medicare Advantage enrollees. Documentation of the cost of illness in the Medicare population, which bears the most disease burden, is not well established. Whereas most studies have focused on the cost of a fracture episode, the considerations of downstream costs and impacts on overall cost of care are more relevant from the payer perspective.
It is apparent that inpatient hospitalization is the major cost driver. Christensen et al7 reported hip fracture costs of $38 699 and $33 975 for commercial and Medicare Advantage enrollees, respectively. Rousculp et al8 reported a hip fracture cost of $33 238 and lower rates of $18 000 to $19 000 for other fracture sites, but the data are older. Furthermore, emerging evidence suggests an incremental increase in health care costs associated with subsequent fractures, ranging from $14 100 to $47 351 in 1 year depending on payer and fracture site.9
Most studies have focused on commercial and Medicare Advantage enrollees. Contemporary data in the Medicare population, where most fractures are likely to occur, are needed to efficiently allocate resources. Specifically, quantification of fracture-related costs can help inform decisions on whether to implement osteoporosis-related registries (eg, Own the Bone10) and interventions with up-front costs (eg, fracture liaison services11) that might be offset by downstream cost savings through improved quality of care and avoidance of future fractures. The current study focuses on health care costs in the Medicare fee-for-service (FFS) population and includes mortality assessment, a subanalysis in men (not included in most osteoporosis studies), and a comparison of costs and outcomes in a fracture versus nonfracture cohort.
Methods
This study used Chronic Conditions Data Warehouse for the Medicare FFS population (100% sample for women with osteoporosis [based on diagnosis, fracture, and/or medication use] and a random 5% sample for both sexes, irrespective of osteoporosis) to examine claims that occurred between January 1, 2006, and September 30, 2015. Evidence of osteoporosis included a claim for osteoporotic fracture, diagnosis of osteoporosis indicated on a physician or inpatient encounter, or osteoporosis medication. Diagnoses of osteoporosis could occur on inpatient or outpatient claims and be in any position. The study was approved by the University of Alabama at Birmingham Institutional Review Board and was governed by a data management plan from the Centers for Medicare and Medicaid Services.
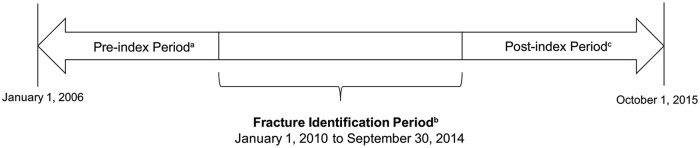
This study included a fracture cohort and a propensity score (PS)-matched nonfracture comparator cohort. To be included in the final sample in either cohort, patients had to meet the following criteria: age ≥65 years at the index date (date of fracture or comparable start of follow-up in the nonfracture cohort, as below) and continuous enrollment in FFS Medicare with medical and pharmacy benefits (ie, Parts A + B + D − C) for ≥1 year before the index date (preindex period), through ≥1 month after the index date (Figure 1). Beneficiaries were excluded if they died within 30 days of the index date or if they had diagnosis code(s) for Paget’s disease of bone (ICD-9: 731.0, 731.1) or malignancy (except nonmelanoma skin cancer) during the preindex period. Patients were censored if these occurred during follow-up. Patients were followed until disenrollment from the health plan, censoring, or September 30, 2015, when the United States transitioned to ICD-10.
Figure 1.
Study design.
a The preindex date is ≥1 year prior to index date (as early as January 1, 2006, for some patients) and is not the same for all patients.
b Index date (date of fracture or comparable start of follow-up in the nonfracture cohort) could occur any time between January 1, 2010, and September 30, 2014, and is not the same for all patients.
c Postindex period (follow-up time after index fracture or comparable start of follow-up in the nonfracture cohort) spans from the day after the index date up to 12 months postindex and is not the same for all patients.
The fracture cohort included patients with evidence of an incident fragility (ie, osteoporosis-related) fracture, including pathological fracture (ie, spine, pelvis, clavicle, humerus, radius/ulna [includes open fractures], carpal/wrist, hip, femur, ankle, and tibia/fibula fracture sites) between January 1, 2010, and September 30, 2014 (the fracture identification period). The preindex period could start as early as January 1, 2006. The index date for the fracture cohort was the episode start date of the first case-qualifying fracture episode during the identification period. Fractures were considered case-qualifying if they occurred during hospitalization (diagnosis in any position on a hospital claim) or during an outpatient visit with a fracture repair procedure code, based on a validated algorithm shown to have accuracy with a positive predictive value that exceeds 90%.12
Comorbidity scores were calculated per Quan-Charlson Comorbidity Index using diagnosis codes in the preindex period and categorized as 0, 1 to 2, 3 to 4, and ≥5. Preindex use of oral corticosteroids was assessed in pharmacy data.
Outcome measures included occurrence of a second fracture, mortality, health care resource utilization (HCRU), and costs (fracture vs nonfracture) by type of event (inpatient vs outpatient) and by fracture site. HCRU was classified as osteoporosis related if there was a diagnosis of osteoporosis, fracture, or aftercare of fracture on the medical claim. For acute inpatient stays and long-term care, counts represented the total number of days of service. The study was done from the payer (Medicare) perspective. The costs were based on the Medicare-allowed amount and did not include coinsurance or other patient-related cost sharing from other perspectives. Costs were adjusted to the 2017 US dollar using the annual medical care component of the Consumer Price Index. All study variables were analyzed descriptively. Time to second fracture analysis was conducted using the Kaplan-Meier method, with cumulative incidence estimated 1, 2, and 3 years after the index fracture.
A nonfracture comparator cohort was created using 1:1 PS matching without replacement. PS models were generated separately for each index year during the identification period. This cohort included patients without evidence of a closed fragility (or osteoporosis-related) fracture during the given year. Patients eligible for the nonfracture cohort had to be without a case-qualifying fracture as of January 1 of each year and no fracture diagnoses (ICD-9: 800-829, 733.1x) at any other time during that calendar year. The index date for the nonfracture cohort was a randomly assigned date in each calendar year where the patient had contact with the health care system (ie, a physician’s visit or hospitalization admission date). Covariates were calculated for the 12-month preindex period and included age, gender, region, medications and comorbidities that increase fall risk, comorbidities that may lengthen fracture healing, and comorbidities associated with fracture, frailty, falls, or worse outcomes. For the male subgroup, additional covariates included polyuria, alcohol use disorder, current/previous smoker, hypogonadism, orthostatic hypotension, testosterone replacement, and additional cardiovascular variables. Multivariable regression analysis on health care cost was conducted using generalized linear models with log-link function and gamma distribution.
Results
A total of 885 676 patients had a fragility fracture and met eligibility criteria. Most were White (91%), female (94%), and aged >75 years (72%). The average age was 80.5 (±8.4) years. The most common fracture sites included the following: spine (n = 239 807, 27.1%), hip (n = 206 298, 23.3%), and radius or ulna (n = 137 939, 15.6%). Nearly 87% of patients (n = 768 328) had a comorbidity or were on medication(s) associated with fall risk or reduced bone strength. The most common comorbidities were diabetes (26.4% [n = 233 764]) and chronic lung disease (21.6% [n = 191 078]); 19.7% (n = 174 339) had oral corticosteroid exposure. Most beneficiaries had more than the required ≥12 months of preindex data available; 81.2% had ≥24 months, and 45.0% had ≥60 months (overall mean ± SD preindex period 54.5 ± 24.0 months). Of all patients with a qualifying fracture, approximately 64.0% (n = 566 556) did not have a diagnosis or treatment of osteoporosis prior to fracture, 15.7% (n = 138 712) were diagnosed but not treated, 7.6% (n = 67 114) were treated but not diagnosed, and 12.8% (n = 113 294) were diagnosed and treated.
Among men, 9876 met eligibility criteria and experienced a fracture. The mean age was 77.9 years (±8.0 SD). In all, 61% were ≥75 years old, and 90.3% were White. The most common fracture sites in men were spine (n = 3046, 30.8%), hip (n = 2637, 26.7%), and radius/ulna (n = 891, 9.0%). On average, male beneficiaries had nearly 5 years of available data in the preindex period. All beneficiaries had ≥12 months’ data, whereas 85.7% had ≥24 months’ data. In the male fracture subgroup, 92.8% (n = 9163) had no osteoporosis diagnosis or treatment at baseline, 2.8% (n = 279) were diagnosed but not treated, 2.3% (n = 227) were treated but not diagnosed, and 2.1% (n = 207) were diagnosed and treated.
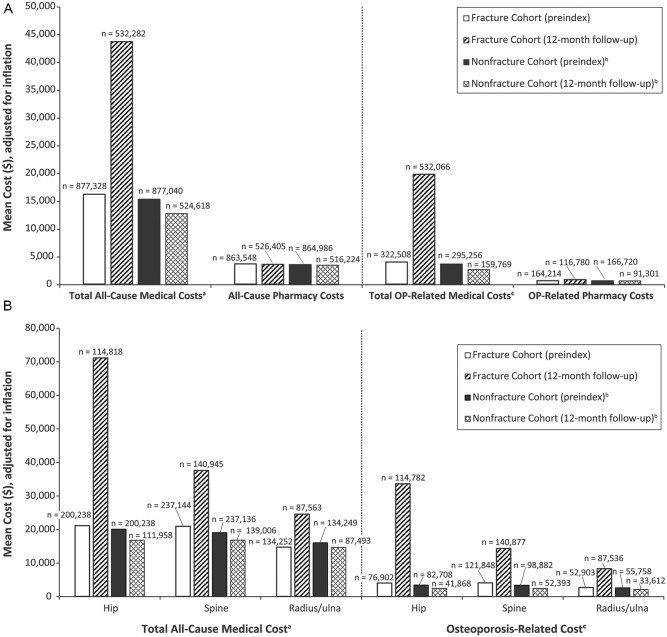
After PS matching, all variables had standard mean differences less than 0.10 (Supplemental Table 1, available online). There was a trend toward increased all-cause and osteoporosis-related HCRU in the year following the index fracture compared with preindex across all care settings for patients who incurred a fracture (Figures 2A and 2B). BMD testing rates during the postindex period were slightly higher in the fracture versus the nonfracture cohort but remained low (6.7% vs 5.3%, 10.8% vs 9.0%, 13.9% vs 12.8%, and 17.0% vs 16.7% in the 3-, 6-, 9-, and 12-month follow-up periods, respectively). Treatment rates at the 3-month follow-up were 15.5% (fracture cohort) versus 13.7% (nonfracture cohort). At the 12-month follow-up, these rates were 24.7% versus 19.0%, respectively.
Figure 2.
Health care resource utilization, percentage of patients: A. Health care costs by cohort (preindex and 12-month follow-up). B. All-cause and osteoporosis-related cost by fracture type.
Abbreviations: OP, Osteoporosis.
a Total medical costs include carrier, durable medical equipment, home health, hospice, inpatient, outpatient, and skilled nursing facility.
b The nonfracture cohort was selected by 1:1 propensity score matching for each index year during the identification period.
c Costs were classified as osteoporosis-related if there was a diagnosis of osteoporosis or fracture in any position on the medical claim.
During follow-up, costs were greater in the fracture cohort, with most occurring within the first 3 months postfracture. Mean total all-cause costs at 3 months were $30 307.28 in the fracture cohort ($34 227.12 for men) and $5342.35 in the nonfracture cohort ($6341.17 for men), a difference of nearly $25 000. Among patients with ≥12 months of follow-up, mean costs were $47 163.25 in the fracture cohort ($52 273.79 for men) and $16 034.61 in the nonfracture cohort ($17 352.68 for men), for a difference of $31 128.64 ($34 921.11 for men).
During the 12-month follow-up, most health care costs in the fracture cohort were for medical services (mean overall: $43 637.00; $48 780.00 for men) versus pharmacy services (mean overall: $3566.07; $3563.56 for men). Within medical services, the highest mean costs were for skilled nursing facility ($29 216.05), inpatient costs ($24 190.19), and hospice care ($20 996.83). For men, the highest mean costs were for inpatient care ($30 198.05), skilled nursing facility ($29 650.61), and hospice care ($20 533.50).
Similar to all-cause costs, osteoporosis-related costs were greater in the fracture cohort, with most occurring within 3 months of follow-up. Mean total osteoporosis-related costs at 3 months were $17 085.64 in the fracture cohort ($34 227.12 for men) versus $1301.30 in the nonfracture cohort ($6341.17 for men). Among patients with ≥12 months of follow-up, mean costs were $19 938.35 ($52 273.79 for men) and $2364.40 ($17 352.68) in the fracture and nonfracture cohorts, respectively. During the 12-month follow-up, mean osteoporosis-related costs in the fracture cohort were mostly for medical services ($19 760.28; men: $48 780.00) versus pharmacy services ($815.26; men: $3563.56). The highest mean medical service costs were for skilled nursing facility ($25 556.86), hospice ($19 540.71), and inpatient ($16 978.07) care overall and for inpatient ($30 198.05), skilled nursing facility ($29 650.61), and hospice ($20 533.50) care for men.
Total all-cause medical and pharmacy costs for 12 months’ follow-up by fracture site were as follows for the fracture cohort and matched nonfracture cohort: hip, $71 057.83 versus $16 807.74; spine, $37 543.87 versus $16 860.49; and radius/ulna, $24 505.27 versus $14 673.86. Osteoporosis-related costs were as follows: hip, $33 542.44 versus $2491.07; spine, $14 282.78 versus $2499.39; and radius/ulna, $8261.36 versus $2143.79.
Among all beneficiaries, 9.1% experienced a second fracture within 1 year, 8.2% between 1 and 2 years (17.3% cumulative 2-year risk), and 6.8% between 2 and 3 years of follow-up (24.1% cumulative 3-year risk; Table 1). Among men, 7.4% experienced a second fracture within 1 year, 6.2% within 1 to 2 years (13.6% cumulative 2-year risk), and 5.7% between 2 and 3 years of follow-up (19.2% cumulative 3-year risk). Proportionally more patients in the fracture versus nonfracture cohort died (18% vs 9.3%), with higher death rate among men (20% vs 11%).
Table 1.
Kaplan-Meier Analysis of Time to Second Fracture, Fracture Cohort.
| Second fracture | Cohort | Time (days) |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0 | 32 | 91 | 182 | 273 | 365 | 730 | 1095 | 1460 | 1825 | |||
| Time without second fracture | Total fracture cohort | Proportion | 1 | 0.9998 | 0.9864 | 0.9605 | 0.9339 | 0.9092 | 0.8272 | 0.7591 | 0.7022 | 0.6507 |
| At risk | 885,676 | 885,676 | 783,615 | 664,141 | 570,470 | 467,941 | 203,688 | 89,249 | 34,696 | 8145 | ||
Total medical and pharmacy costs were $78 137.59 ($76 902.06 for men) for patients who experienced a second fracture during follow-up and $44 525.37 (50 469.58 for men) among those who did not (Table 2).
Table 2.
All-Cause Health Care Cost (Dollars) by Second Fracture Status (12-Month Follow-up).a
| All-cause health care costs |
||||
|---|---|---|---|---|
| Second fracture, n = 32 243b |
Without second fracture, n = 487 333c |
|||
| n | Mean (SD) | n | Mean (SD) | |
| 12-Month follow-up | ||||
| Total medical costs | 32 243 | 74 279 (54 770) | 487 328 | 41 028 (42 858) |
| Carrier | 32 243 | 11 090 (8445) | 487 244 | 6981 (6623) |
| Durable medical equipment | 23 657 | 1163 (2663) | 307 310 | 1034 (2784) |
| Home health | 20 616 | 7939 (5809) | 214 033 | 6520 (5230) |
| Hospice | 2062 | 15 143 (14 935) | 18 501 | 21 881 (18 575) |
| Inpatient | 26 866 | 32 972 (30 824) | 303 983 | 23 166 (26 207) |
| Outpatient | 31 206 | 5940 (7824) | 449 882 | 4137 (6471) |
| Skilled nursing facility | 20 625 | 36 063 (24 594) | 196 348 | 28 376 (20 255) |
| Pharmacy costs | 32 088 | 3878 (4975) | 481 688 | 3539 (5229) |
| Total medical + pharmacy costs | 32 243 | 78 138 (55 335) | 487 333 | 44 525 (43 732) |
Costs are adjusted for inflation using the medical component of the consumer price index for 2015.
Second fracture group includes patients who had a second fracture within 9 months of follow-up.
Without second fracture group includes patients who did not have a second fracture within 12 months of follow-up.
Many factors were significantly associated with both all-cause (Table 3) and osteoporosis-related costs. For total all-cause health care costs, higher Charlson comorbidity score was associated with higher cost, and patients with hip fractures had as high costs as, or higher costs than, patients with fractures of other sites. The variable with the largest cost ratio in the all-cause and osteoporosis-related models was inpatient versus outpatient fracture management (cost ratio all-cause: 2.16; 3.60 osteoporosis related). Another predictor of higher all-cause cost was fractures at multiple sites versus hip only (cost ratio: 1.23; 95% CI = 1.21-1.26).
Table 3.
Multivariable Analysis on All-Cause 12-Month Follow-up Cost After Hip Fracture.
| Estimate | Standard error | Lower 95% CI | Upper 95% CI | χ2 | P value | |
|---|---|---|---|---|---|---|
| Intercept | 30 223.00 | 1392.06 | 27 495.00 | 32 952.00 | 471.38 | <0.0001 |
| Age at time of index fracture (per year) | 293.80 | 16.10 | 262.24 | 325.36 | 332.88 | <0.0001 |
| Sex (male) | 4695.36 | 606.10 | 3507.43 | 5883.30 | 60.01 | <0.0001 |
| Nonwhite race (referent to White) | 9625.16 | 415.40 | 8810.99 | 10 439.00 | 536.88 | <0.0001 |
| Region: northeast | 8295.13 | 363.86 | 7581.98 | 9008.28 | 519.74 | <0.0001 |
| Region: south | 1257.04 | 305.93 | 657.44 | 1856.64 | 16.88 | <0.0001 |
| Region: west | 6411.16 | 394.71 | 5637.56 | 7184.77 | 263.83 | <0.0001 |
| Index year: 2011 | −1928.11 | 352.04 | −2618.10 | −1238.12 | 30.00 | <0.0001 |
| Index year: 2012 | −5933.08 | 365.69 | −6649.81 | −5216.35 | 263.24 | <0.0001 |
| Index year: 2013 | −8586.68 | 374.37 | −9320.43 | −7852.93 | 526.08 | <0.0001 |
| Index year: 2014 | −7426.29 | 422.85 | −8255.05 | −6597.53 | 308.45 | <0.0001 |
| Outpatient index fracture (referent to inpatient) | −26 163.00 | 633.65 | −27 405.00 | −24 921.00 | 1704.78 | <0.0001 |
| History of stroke | 3535.64 | 522.48 | 2511.59 | 4559.68 | 45.79 | <0.0001 |
| History of falls | 2415.63 | 381.15 | 1668.59 | 3162.68 | 40.17 | <0.0001 |
| Mobility impairments | 5779.49 | 360.44 | 5073.03 | 6485.94 | 257.10 | <0.0001 |
| Vision impairments | 1385.35 | 619.40 | 171.35 | 2599.34 | 5.00 | 0.0253 |
| Parkinson disease | 5973.55 | 714.10 | 4573.94 | 7373.17 | 69.98 | <0.0001 |
| Muscle atrophy/weakness/sarcopenia | 2062.85 | 704.09 | 682.86 | 3442.83 | 8.58 | 0.0034 |
| α-Blocker | 4987.31 | 426.00 | 4152.36 | 5822.26 | 137.06 | <0.0001 |
| Anticholinergic antihistamines | 3962.29 | 607.20 | 2772.20 | 5152.39 | 42.58 | <0.0001 |
| Antipsychotic | 4857.56 | 879.19 | 3134.38 | 6580.74 | 30.53 | <0.0001 |
| Barbiturate | 9611.97 | 3750.95 | 2260.24 | 16 964.00 | 6.57 | 0.0104 |
| Benzodiazepine | 801.21 | 445.56 | −72.08 | 1674.50 | 3.23 | 0.0721 |
| β-Blocker | 2112.68 | 268.20 | 1587.02 | 2638.34 | 62.05 | <0.0001 |
| Nonbenzodiazepine, benzodiazepine receptor agonist | 2936.74 | 446.37 | 2061.88 | 3811.61 | 43.29 | <0.0001 |
| Opioids | 2082.89 | 265.25 | 1563.00 | 2602.77 | 61.66 | <0.0001 |
| Proton pump inhibitor | 2860.17 | 273.01 | 2325.07 | 3395.27 | 109.75 | <0.0001 |
| Selective serotonin reuptake inhibitor | 2843.31 | 301.80 | 2251.80 | 3434.82 | 88.76 | <0.0001 |
| Tricyclic antidepressant | 2002.68 | 646.16 | 736.22 | 3269.13 | 9.61 | 0.0019 |
| Vasodilator | 6790.15 | 440.46 | 5926.88 | 7653.43 | 237.66 | <0.0001 |
| Inhaled corticosteroid | 1082.68 | 371.22 | 355.11 | 1810.25 | 8.51 | 0.0035 |
| Diabetes | 5994.30 | 347.93 | 5312.37 | 6676.24 | 296.81 | <0.0001 |
| Renal disease | 10 890.00 | 352.87 | 10 198.00 | 11 581.00 | 952.34 | <0.0001 |
| Liver disease | 7386.35 | 826.28 | 5766.87 | 9005.83 | 79.91 | <0.0001 |
| Rheumatoid arthritis | 3535.20 | 726.00 | 2112.27 | 4958.14 | 23.71 | <0.0001 |
| Arthritis | 1123.85 | 262.34 | 609.67 | 1638.03 | 18.35 | <0.0001 |
| Respiratory diseases | 2874.56 | 289.07 | 2307.99 | 3441.13 | 98.89 | <0.0001 |
| Alzheimer disease | −3134.77 | 463.41 | −4043.04 | −2226.51 | 45.76 | <0.0001 |
| Dementia | 574.76 | 342.42 | −96.38 | 1245.89 | 2.82 | 0.0932 |
| Lung disease | 5687.84 | 348.93 | 5003.95 | 6371.72 | 265.72 | <0.0001 |
| Depression | 2136.73 | 356.60 | 1437.80 | 2835.66 | 35.90 | <0.0001 |
| Cardiovascular disease | 2336.64 | 354.66 | 1641.52 | 3031.76 | 43.41 | <0.0001 |
| Hypothyroidism | 1096.22 | 291.73 | 524.44 | 1667.99 | 14.12 | 0.0002 |
| Obesity | 10 084.00 | 714.22 | 8684.20 | 11 484.00 | 199.34 | <0.0001 |
| Bariatric surgery | −31 708.00 | 10 138.00 | −51 578.00 | −11 838.00 | 9.78 | 0.0018 |
| Charlson score (1 to 2) | 1174.60 | 354.47 | 479.85 | 1869.34 | 10.98 | 0.0009 |
| Charlson score (≥3) | 7674.21 | 409.49 | 6871.62 | 8476.79 | 351.22 | <0.0001 |
Among those with 12 months of follow-up, outpatient management of the index fracture (referent to inpatient) has an estimated cost savings of $28 324 for radius/ulna (95% CI = −28 821 to −27 826; P < 0.0001) and $31 488 for spine (95% CI = −31 967 to 31 010; P < 0.0001).
Discussion
This retrospective cohort study included patients with and without an incident osteoporotic fracture and, importantly, a subgroup analysis of men, a population often overlooked in osteoporosis epidemiology. Rates of prefracture and postfracture treatment and diagnosis were lower than previously reported,5 with appreciably higher underdiagnosis and undertreatment in men when compared with women. Furthermore, a decline in fracture rates between 2007 and 2013 followed by a subsequent rise in 2017 is consistent with previous reports and may be a result of changes in testing and treatment of osteoporosis during this time.3,4
A significant economic burden was observed in the fracture versus nonfracture cohort. Among patients with ≥12 months of follow-up, mean total all-cause health care costs were >3-fold and osteoporosis-related costs were >9-fold higher than the matched, nonfracture cohort. The cost of medical services was 10-fold higher than pharmacy services. A higher economic burden was observed in men.
The higher cost of care in men may be a result of the fact that men tend to have fractures later in life and also have accrued a greater number of comorbid conditions by that time. These comorbidities increase the risk of fracture and having a fracture may exacerbate some of these conditions (ie, cardiovascular disease, chronic obstructive pulmonary disease, etc) and destabilize those that might have previously been well controlled.13 In the current study, this is supported by the higher proportion of men having a 3+ Charlson comorbidity score (46%) than the overall population (17.3%).
This study also illustrated that roughly one-quarter of patients experience a second fracture in the 3 years following index fracture, indicating a higher risk for second fracture in this population than the National Osteoporosis Foundation (NOF) defined treatment threshold (10-year risk of 20%). The implications of this finding are that, in addition to all patients experiencing a hip or vertebral fracture who should be treated for osteoporosis regardless of BMD or other factors, nearly all individuals in this cohort who experienced a fracture at other sites should receive osteoporosis treatment based on NOF guidelines.14 Furthermore, patients who experienced a second fracture had a higher incremental total cost of care compared with those who did not sustain a second fracture, emphasizing the importance of secondary prevention measures, particularly for those with high comorbidity burdens.
Although osteoporosis-related fracture costs were comparable to those previously reported, our findings suggest a higher cost of care for the downstream effects of fracture. Christensen et al7 observed mean 1-year follow-up costs for hip fracture of $38 699 (commercial patients) and $33 975 (Medicare Advantage patients). Similarly, Rousculp et al8 identified 1-year follow-up adjusted predicted health care costs of $33 238 for hip fractures and lower costs for fractures of other sites (ie, $19 672 for hand/fingers and $18 982 for forearm/wrist). Osteoporosis-related costs for hip fracture were comparable in this study ($33 542.44); however, consideration of all-cause costs, which potentially include worsening of existing medical conditions, were significantly higher ($71 057.83). Furthermore, comparison of data with the propensity-matched nonfracture cohort in the current study puts the results in a different perspective ($16 807.74 and $2491.07 for all-cause and osteoporosis-related costs, respectively), highlighting the increased cost burden associated with fracture.
Previous studies have reported a rate of repeat fracture within 1 year of initial fracture between 4% and 17%, depending on the fracture site and population characteristics.9,15 Previous studies also showed that health care costs increase with subsequent fractures, and the increase varies depending on the site of the initial fracture ($47 351, $43 238, and $23 852 for commercial patients with initial hip, clinical vertebral, and nonhip/nonvertebral fractures and $18 645, $19 702, and $19 697 for Medicare patients, respectively).9 The total cost of care is also significantly higher for those experiencing a recurring fracture compared with those without a prior fracture for both Medicare ($34 327 vs $20 790; P < 0.001) and commercial health plan enrollees ($39 501 vs $19 131; P < 0.001).15 Of note, some osteoporosis studies do not consider costs in patients who die in the first year following their fracture and require 12 or more months of follow-up. Those patients are more likely to have severe disease, and excluding their costs is likely to underestimate true fracture-related downstream effects, including the incremental cost of a subsequent fracture. The current study avoided this methodological problem by including costs for patients who died more than 30 days after fracture, a time horizon when fracture-related medical management typically is being considered.
This study has some limitations because of its retrospective nature and inherent features of an administrative claims database. The validated algorithm to identify fractures has high accuracy, but some misclassification may occur. In particular, fractures resulting in neither hospitalization nor repair (eg, a nondisplaced wrist fracture) were not included given potential uncertainties about misclassification and a desire to maximize specificity. Although this approach likely excludes some less-expensive fractures, it nevertheless does not diminish the high cost of the fractures that were studied.12
Diagnosis/treatment groups were determined at preindex, and patients may have been diagnosed and/or treated for osteoporosis shortly after their index fracture, which would not be reflected in preindex assignments. Although differences in BMD testing and treatment for osteoporosis were low after index fracture, BMD testing rates during the postindex period were slightly higher in the fracture cohort compared with the nonfracture cohort. Osteoporosis treatment rates were not different across the fracture and nonfracture cohorts. A high proportion of patients were excluded because of malignancy related to the extended length of the preindex period, even though we required ≥2 outpatient diagnoses. This approach was very sensitive to identification of patients with active malignancies and may limit generalizability of findings.
The study did not evaluate indirect cost of fractures, including productivity loss associated with absenteeism or caregiver burden. Employer and societal perspectives were out of the scope of the current study, which focused on payer perspective. Furthermore, the limited follow-up time of 12-month postfracture is likely to underestimate the total cost, including those related to long-term care. Finally, the estimated cost for skilled nursing facilities has changed because Medicare now pays a lower amount for patient stays that last longer than 20 days, and no payment for stays beyond 100 days. Because of this, the associated costs may be underestimated.16
Despite these limitations, the study has several strengths. First, it provides data on male patients, a subgroup often excluded from burden-of-illness studies in osteoporosis. Documentation of high fracture-related morbidity in men is of importance given the current lack of consensus of screening for men. Second, the study helps place fracture burden in context by comparison of fracture versus nonfracture comparator cohorts. Finally, Medicare data have high generalizability to older individuals in the United States and covers the majority of adults ≥65 years old, a population most likely to suffer from osteoporosis and related fractures. We also were able to study mortality, an outcome that often cannot be reliably estimated from commercially insured or Medicare Advantage enrollees.
Conclusion and Relevance
Using contemporary data, osteoporosis-related fractures are costly, affect overall health status and all-cause costs, and are disproportionately higher for men and patients incurring a subsequent fracture. Given the decline in osteoporosis diagnosis and treatment observed in the past decade as well as poor average performance against Medicare Star measures for osteoporosis management and postfracture quality of care, policies that incentivize timely diagnosis and treatment following a fracture are warranted, including for men, a group that has often been overlooked in osteoporosis management.
Supplemental Material
Supplemental material, AOP-20-0476_Supplemental_Table_1_1 for Economic Burden of Osteoporosis-Related Fractures in the US Medicare Population by Setareh A. Williams, Shanette G. Daigle, Richard Weiss, Yamei Wang, Tarun Arora and Jeffrey R. Curtis in Annals of Pharmacotherapy
Acknowledgments
All listed authors meet the criteria for authorship set forth by the International Committee for Medical Journal Editors. Medical editorial support (Sarah Hummasti, PhD) and graphic services were provided by AOIC, LLC, and were funded by Radius Health, Inc.
Footnotes
Authors’ Note: Data from this study were previously presented at the following meetings:
• Academy of Managed Care & Specialty Pharmacy Annual Meeting, March 25-29, 2019, in San Diego, CA, USA (Medicare fracture baseline characteristics);
• National Conference for Nurse Practitioners, May 14-17, 2019, in Chicago, IL, USA (Medicare fracture baseline characteristics encore);
• International Society of Pharmacoeconomics and Outcomes Research, May 18-22, 2019, in New Orleans, LA, USA (Medicare incidence of OP fractures);
• American College of Rheumatology/The Association of Rheumatology Professionals (ACR/ARP) Annual Meeting, November 8-13, 2019, in Atlanta, GA, USA (Medicare fracture incidence time trends);
• Academy of Managed Care Pharmacy (AMCP) Nexus October 29 to November 1, 2019, in National Harbor, MD, USA (Medicare cost of illness study); and
• EMBRAACE 2020: The American Association of Clinical Endocrinology 29th Annual Scientific & Clinical Congress held virtually (Medicare male subanalysis).
Declaration of Conflicting Interests: The authors declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: SAW, RW, and YW are employees of and own equity stock in Radius Health, Inc; SGD and TA are employees of the School of Public Health (UABMC), which received funding from Radius Health, Inc, for this work; JRC is a consultant for Radius Health, Inc, and Amgen; his institution (UABMC) received grants from Amgen, Lilly, and Radius Health, Inc.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: Funding for this study was provided by Radius Health, Inc.
ORCID iD: Setareh A. Williams  https://orcid.org/0000-0002-6795-2677
https://orcid.org/0000-0002-6795-2677
Supplemental Material: Supplemental material for this article is available online.
References
- 1. Cosman F, de Beur SJ, LeBoff MS, et al. Clinician’s guide to prevention and treatment of osteoporosis. Osteoporos Int. 2014;25:2359-2381. doi: 10.1007/s00198-014-2794-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Wright NC, Looker AC, Saag KG, et al. The recent prevalence of osteoporosis and low bone mass in the United States based on bone mineral density at the femoral neck or lumbar spine. J Bone Miner Res. 2014;29:2520-2526. doi: 10.1002/jbmr.2269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Lewiecki ME, Wright NC, Curtis JR, et al. Hip fracture trends in the United States, 2002 to 2015. Osteoporos Int. 2018;29:717-722. doi: 10.1007/s00198-017-4345-0 [DOI] [PubMed] [Google Scholar]
- 4. Lewiecki EM, Chastek B, Sundquist K, et al. Osteoporotic fracture trends in a population of US managed care enrollees: 2007-2017. Osteoporos Int. 2020;31:1299-1304. doi: 10.1007/s00198-020-05334-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Balasubramanian A, Tosi LL, Lane JM, Dirschl DR, Ho PR, O’Malley CD. Declining rates of osteoporosis management following fragility fractures in the US, 2000 through 2009. J Bone Joint Surg Am. 2014;96:e52. doi: 10.2106/JBJS.L.01781 [DOI] [PubMed] [Google Scholar]
- 6. Centers for Medicare & Medicaid Services. Medicare 2018 part C & D star ratings technical notes. Accessed March 16, 2020. https://www.cms.gov/Medicare/Prescription-Drug-Coverage/PrescriptionDrugCovGenIn/Downloads/2018-Star-Ratings-Technical-Notes-2017_09_06.pdf
- 7. Christensen L, Iqbal S, Macarios D, Badamgarav E, Harley C. Cost of fractures commonly associated with osteoporosis in a managed-care population. J Med Econ. 2010;13:302-313. doi: 10.3111/13696998.2010.488969 [DOI] [PubMed] [Google Scholar]
- 8. Rousculp MD, Long SR, Wang S, Schoenfeld MJ, Meadows ES. Economic burden of osteoporosis-related fractures in Medicaid. Value Health. 2007;10:144-152. doi: 10.1111/j.1524-4733.2006.00161.x [DOI] [PubMed] [Google Scholar]
- 9. Song X, Shi N, Badamgarav E, et al. Cost burden of second fracture in the US health system. Bone. 2011;48:828-836. doi: 10.1016/j.bone.2010.12.021 [DOI] [PubMed] [Google Scholar]
- 10. Own the Bone. What is Own the Bone? Accessed July 9, 2020. https://www.ownthebone.org/OTB/About/What_Is_Own_the_Bone.aspx
- 11. Curtis JR, Silverman SL. Commentary: The five Ws of a fracture liaison service: why, who, what, where, and how? In osteoporosis, we reap what we sow. Curr Osteoporos Rep. 2013;11:365-368. doi: 10.1007/s11914-013-0177-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Wright NC, Daigle SG, Melton ME, Delzell ES, Balasubramanian A, Curtis JR. The design and validation of a new algorithm to identify initial incident recurrent incident fragility fractures in administrative claims data. J Bone Miner Res. 2019;34:1798-1807. doi: 10.1002/jbmr.3807 [DOI] [PubMed] [Google Scholar]
- 13. Curtis JR, Arora T, Matthews RS, et al. Is withholding osteoporosis medication after fracture sometimes rational? A comparison of the risk for second fracture versus death. J Am Med Dir Assoc. 2010;11:584-591. doi: 10.1016/j.jamda.2009.12.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. World Health Organization. FRAX® fracture risk assessment tool. Accessed April 20, 2020. http://www.shef.ac.uk/FRAX/
- 15. Weaver J, Sajjan S, Lewiecki EM, Harris ST, Marvos P. Prevalence and cost of subsequent fractures among US patients with an incident fracture. J Manag Care Spec Pharm. 2017;23:461-471. doi: 10.18553/jmcp.2017.23.4.461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Medicare.gov. Skilled nursing facility (SNF) care. Accessed September 28, 2020. https://www.medicare.gov/coverage/skilled-nursing-facility-snf-care
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, AOP-20-0476_Supplemental_Table_1_1 for Economic Burden of Osteoporosis-Related Fractures in the US Medicare Population by Setareh A. Williams, Shanette G. Daigle, Richard Weiss, Yamei Wang, Tarun Arora and Jeffrey R. Curtis in Annals of Pharmacotherapy