Abstract
Background
Primary diagnosis of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection is based on detection of virus RNA in nasopharyngeal swab samples. In addition, analysis of humoral immunity against SARS-CoV-2 has an important role in viral diagnostics and seroprevalence estimates.
Methods
We developed and optimized an enzyme immunoassays (EIA) using SARS-CoV-2 nucleoprotein (N), S1 and receptor binding domain (RBD) of the viral spike protein, and N proteins from SARS, Middle East respiratory syndrome (MERS), and 4 low-pathogenic human CoVs. Neutralizing antibody activity was compared with SARS-CoV-2 IgG, IgA, and IgM EIA results.
Results
The sensitivity of EIA for detecting immune response in COVID-19 patients (n = 101) was 77% in the acute phase and 100% in the convalescent phase of SARS-CoV-2 infection when N and RBD were used as antigens in IgG and IgA specific EIAs. SARS-CoV-2 infection significantly increased humoral immune responses against the 229E and NL63 N proteins. S1 and RBD-based EIA results had a strong correlation with microneutralization test results.
Conclusions
The data indicate a combination of SARS-CoV-2 S1 or RBD and N proteins and analysis of IgG and IgA immunoglobulin classes in sera provide an excellent basis for specific and sensitive serological diagnostics of COVID-19.
Keywords: COVID-19, SARS-CoV-2, enzyme immunoassay, serology, respiratory infection, antibodies, coronavirus proteins, neutralizing antibodies
A combination of SARS-CoV-2 N and S antigens and measurement of both IgG and IgA increases the sensitivity of serological diagnostics of COVID-19 and provides a basis for accurate estimation of seroprevalence.
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), causing coronavirus disease 2019 (COVID-19), emerged in December 2019 [1] and the outbreak was declared as a pandemic by the World Health Organization in March 2020 [2]. By December 2020, the pandemic had resulted in nearly 3 million deaths and 140 million confirmed cases [3]. SARS-CoV-2 is closely related to 2 coronaviruses causing severe respiratory infections in humans, SARS-CoV and Middle East respiratory syndrome (MERS) CoV, of which SARS-CoV shares 79% sequence identity with SARS-CoV-2 [4]. MERS-CoV, SARS-CoV, and SARS-CoV-2 belong to the genus Betacoronavirus along with 2 other human coronaviruses (HCoVs) OC43 and HKU1, which cause milder respiratory infections. The other 2 low-pathogenic HCoVs, 229E and NL63, belong to the genus Alphacoronavirus [5].
To diagnose an acute SARS-CoV-2 infection, viral RNA is detected in the nasopharyngeal swab sample by quantitative reverse transcription PCR (RT-qPCR). To survey past infections and to assess immunological responses, a variety of serological assays are available [6]. Most of the serological assays are based on the recognition of immunoglobulin G (IgG) and IgM antibodies against SARS-CoV-2 nucleoprotein (N), spike protein (S), or receptor binding domain (RBD) of S, while the detection of IgA antibodies has remained less utilized [7–11]. Antibody assays show remarkable discrepancies [8, 12] and COVID-19 patient sera have been shown to react to low-pathogenic HCoV S antigens [13]. Detailed analysis of serological cross-reactivity against SARS-CoV-2 antigens is still missing and relying on just one of the commonly used viral antigens or immunoglobulin classes may give inaccurate results.
In this study, we have developed SARS-CoV-2 N and S protein-based enzyme immunoassays (EIA) and measured serum anti-SARS-CoV-2 IgG, IgA, and IgM antibodies in COVID-19 patients. We have evaluated the cross-reactivity of antibodies against MERS, SARS, and 4 low-pathogenic HCoV nucleoprotein antigens. We demonstrate that previous infections with low-pathogenic HCoVs can cause some antibody cross-reactivity with the antigens of high-pathogenic coronaviruses and that SARS-CoV-2 infection can boost low-pathogenic HCoV antibody production. For reliable analysis of herd immunity and serodiagnosis of an acute or a recent infection, IgG and IgA antibody determination for both SARS-CoV-2 N and S antigens is necessary.
METHODS
Serum Specimens
COVID-19 patient serum samples (n = 119) were collected from 40 patients at Turku University Hospital (TYKS, Turku, Finland; data treated according to ethical permission HUS/1238/2020) and 61 patients at Helsinki University Hospital (HUS, Helsinki, Finland; data treated according to ethical permissions HUS/32/2018 and HUS/1238/2020). All patients were confirmed to be SARS-CoV-2 RNA-positive with RT-qPCR test from nasopharyngeal swab samples (at TYKS by Corman assay [14]; and at HUSLAB by either Cobas SARS-CoV-2 test on the Cobas 6800 system [Roche Diagnostics], Amplidiag COVID-19 test [Mobidiag], or Corman assay). Paired serum specimens were obtained from 17 patients (1 patient with 3 consecutive samples). Randomly selected control samples (n = 100) were collected in early 2019. A pool of COVID-19–negative control samples was selected from a child serum panel described previously [15] and used as a negative control in EIAs.
Production and Purification of Recombinant Coronavirus Nucleoproteins
Synthetic genes encoding the nucleoproteins of SARS-CoV-2 (GenBank accession NC_045512.2), SARS-CoV (AY278491.2), MERS-CoV (JX869059.2), HCoV-HKU1 (KY674943.1), HCoV-OC43 (MN306053.1), HCoV-229E (KY621348.1), and HCoV-NL63 (KY554967.1) were obtained from GeneArt. Genes were cloned into pBVboost plasmid with N-terminal glutathione S-transferase (GST) and proteins were produced in Spodoptera frugiperda (Sf-9) cells and purified as described earlier [16]. Protein purity and concentration were estimated with sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and Page Blue (Thermo Fischer Scientific) staining with known amounts of bovine serum albumin protein as standards.
Production and Purification of Recombinant SARS-CoV-2 Spike Protein S1 and RBD Antigens
SARS-CoV-2 sequence was obtained from GenBank (MN908947.3). Codon optimized cDNAs representing S1 (amino acid residues 16–541) and RBD (amino acid residues 319–541) were obtained from GeneUniversal. For production of a negative control antigen a cDNA (GeneUniversal) encoding mouse myostatin growth factor proregion (amino acid residues 1–263, with a D76A stabilizing mutation; GenBank accession AAI05675) fused to a GSGGGG linker and a mouse IgG2a Fc part (amino acid residues 238–469) tagged with a C-terminal polyhistidine tail was used to encode proMstn-mFc(IgG2a)-6×His. S1 and RBD cDNAs were subcloned with C-terminal 8×His tag (monomeric) or mouse IgG2a Fc tag into a mammalian expression plasmid vector with a CAG-promoter and internal ribosomal entry site driven puromycin selection gene. Expression plasmids were transfected to human embryonic kidney (HEK293F) and Chinese hamster ovary (CHO-S) cells with Fugene 6 (Promega) and selected for stable cell production with puromycin (Gibco) as described [17]. For large-scale production, the cell lines were adapted to suspension culture in CD OptiCHO medium (Gibco) supplemented with 2 mmol/L Ultraglutamine (Lonza). Cultures were maintained in square-bottom glass flask cultures at 37°C, and subsequently transferred to 33°C for 5–8 days for protein production. Media were harvested, passed through 0.22-µm membrane (Steritop) and recombinant proteins were bound to Protino Ni-NTA columns (Macherey-Nagel) at 4°C, eluted with increasing imidazole concentrations, and dialyzed against phosphate-buffered saline (PBS). Protein purity was evaluated with SDS-PAGE and Page Blue staining. Total protein concentrations were determined with Nanodrop.
Enzyme Immunoassay Assay
EIA was done as described previously for other viral antibody determinations [18]. For coronavirus-specific antibody EIA, 96-well microtiter plates (Nunc Maxisorp, Thermo Fisher Scientific) were coated with 50 µL of GST-N (2.0 µg/mL), RBD-mFc-8×His (4.2 µg/mL), and S1-mFc-6×His (3.0 µg/mL) in PBS at 4°C overnight. GST (0.7 µg/mL) and proMstn-mFc-6×His (4.2 µg/mL) proteins were used as negative control antigens. After coating, the plates were washed once with washing buffer (0.05% Tween-20 in PBS). Serum samples were inactivated at 56°C for 30 minutes, and 100 µL of 1:300 diluted serum specimens in sample buffer (5% swine serum [Biological Industries], 0.1% Tween-20 in PBS), were incubated for 2 hours at 37°C. Horseradish peroxidase-labeled anti-human IgG (1:8000 dilution; Dako), anti-human IgA (1:8000 dilution; Invitrogen), or anti-human IgM (1:4000 dilution; Dako) antibodies in sample buffer was added and incubated for 1 hour at 37°C. TMB One (3, 3’, 5, 5’-tetramethylbenzidine, Kementec Solutions) was used as a substrate and after 20 minutes incubation at room temperature, the reaction was stopped with 0.1 M H2SO4. The absorbance was measured at 450 nm (Victor Nivo, PerkinElmer). The absorbance for negative control antigens was subtracted from the respective sample absorbance and the results were expressed as EIA units using a pool of negative serum samples (given a unit value of 0) and a pool of highly positive serum samples (unit value of 100) as standards. The unit values for SARS-CoV-2 GST-N–based EIA were adopted to other HCoV GST-N–based EIAs because no confirmed negative and highly positive control samples were available for SARS, MERS, and low-pathogenic coronavirus N antibody assays.
Microneutralization Test
Microneutralization test (MNT) was performed as described previously [19] with some modifications. Briefly, serum samples were serially diluted 2-fold from 1:10 to 1:5120 in 2% fetal calf serum in Dulbecco’s Modified Eagle’s Medium in a 96-well cell culture plate and 1000 tissue culture infectious dose 50% (TCID50) of SARS-CoV-2 isolate Finland/1/2020 (GenBank accession number MT020781.2) was added to the serum dilutions. The mixtures were incubated at 37°C for 1 hour, VeroE6 cells (50 000 cells/well) were added, and the plates were incubated at 37°C. Cytopathic effect was observed after 3 days. Serum dilutions were done in triplicates and the neutralizing antibody titer was calculated as a 50% end point of the serum dilution that inhibited the SARS-CoV-2 infection in at least 2 parallel wells.
Statistical Analysis
Receiver operating characteristics (ROC) analysis was done with GraphPad Prism 8 software to determine the cutoff EIA unit values for IgG, IgA, and IgM EIA assays. Statistical differences in antibody levels between the groups were analyzed with 1-way ANOVA followed by Tukey multiple comparisons test. Correlation coefficient determinations and graphs were done with GraphPad Prism 8 software and adjusted P values of <.05 were considered statistically significant.
RESULTS
Optimization of the SARS-CoV-2 N, RBD, and S1 Protein-Based EIAs
SARS-CoV-2 N and S proteins are the most promising candidates for serological assays. N protein is weakly conserved between low-pathogenic and high-pathogenic coronaviruses (24.4%–35.5% amino acid identity; Table 1) whereas the similarity in the S1 and RBD parts of S protein is lower (8.0%–18.3% amino acid identity; Table 1).
Table 1.
Comparison of N, S1, and RBD Protein Amino Acid Sequence Identity
| Protein | SARS-CoV | MERS-CoV | HCoV-229E | HCoV-NL63 | HCoV-OC43 | HCoV-HKU1 |
|---|---|---|---|---|---|---|
| SARS-CoV-2 N | 90.8 | 45.8 | 24.4 | 24.4 | 35.5 | 35.3 |
| SARS-CoV-2 S1 | 59.9 | 7.5 | 16.0 | 9.5 | 14.3 | 15.4 |
| SARS-CoV-2 RBD | 72.4 | 8.0 | 13.8 | 8.0 | 15.7 | 18.3 |
Data are percent amino acid identity.
Abbreviations: HCoV, human coronavirus; MERS-CoV, Middle East respiratory syndrome coronavirus; N, nucleoprotein; RBD, receptor binding domain; S1, spike protein; SARS-CoV, severe acute respiratory syndrome coronavirus.
To select optimal spike-protein antigen(s) for EIA, the performance of RBD and S1 proteins with and without mFc-fusion were analyzed (Supplementary Figure 1). RBD-6×His from Florian Krammer [20] was used as a reference antigen. Reference and our His-tagged RBD antigens showed equal IgG, IgA, and IgM responses, whereas RBD coupled to mFc showed higher antibody binding signals. S1 showed a higher signal than either one of the RBD constructs and coupling of S1 to mFc further increased the antibody signals. Control antigen, proMstn-mFc, showed very low levels of reactivity. Based on these results, RBD and S1 coupled to mFc were chosen for antigens in EIA. The use of mFc fusion in the HEK293 expression system yielded higher production levels of the recombinant protein compared to monomeric molecules or the CHO expression system.
All antigens used in EIA were expressed and purified to a relatively high level (Supplementary Figure 1). To validate our EIA assay, we tested the reactivity of 3 COVID-19 patient paired serum samples and 4 control samples with recombinant SARS-CoV-2 GST-N, S1-mFc, and RBD-mFc proteins. Of the paired serum samples, the first samples were collected within 1 to 4 days after a positive PCR test result and the second samples were collected 2 to 14 days after the first sample collection. All 3 patients showed an increase in the IgG antibody levels between the first and the second samples for all 3 antigens (Figure 1). Negative control antigens, GST, and proMstn-mFc showed a very low level of reactivity. To select an optimal serum dilution for EIA, serum samples were tested in different dilutions for IgG responses for the 3 antigens (Figure 1). The background signals were low in serum dilutions of 1:300 or higher, and therefore the serum dilution of 1:300 was chosen for EIA.
Figure 1.
Validation of SARS-CoV-2 N, S1, and RBD-based enzyme immunoassay. IgG antibody responses against recombinant SARS-CoV-2 GST-N, S1-mFc-6×His, and RBD-mFc-8×His in different serum dilutions of acute and convalescent serum specimens of PCR-positive COVID-19 patients (n = 3) and control individuals (n = 4). GST and proMstn-mFc-6×His were negative control antigens; (A) first sample; (B) second sample. Abbreviations: COVID-19, coronavirus disease 2019; GST, glutathione S-transferase; Ig, immunoglobulin; N, nucleoprotein; OD, optical density; RBD, receptor binding domain; S1, spike protein; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.
Antibody Responses Against SARS-CoV-2 Nucleoprotein in COVID-19 Patients
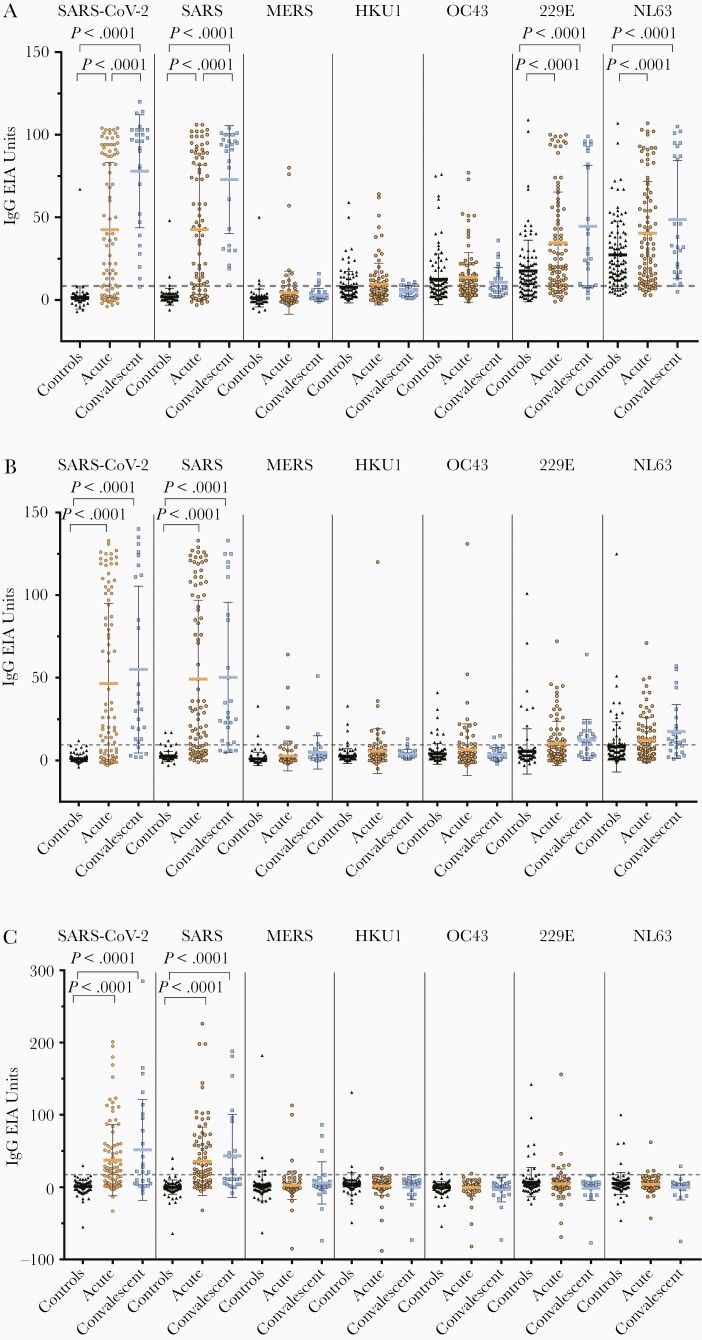
To study antibody responses against SARS-CoV-2 nucleoprotein, we analyzed serum IgG, IgA, and IgM antibody responses in 119 serum samples collected from 101 PCR-confirmed COVID-19 patients. Samples were divided into acute and convalescent phase groups according to the sample collection date (timed by the onset of symptoms or by positive PCR test result when the date of the symptom onset was not available). The acute phase group consisted of 92 samples collected on days 0–13 after the onset of symptoms or positive PCR test result (mean 6 days, SD 4 days, and median 6 days), and the convalescent group consisted of 27 samples collected ≥14 days after the symptom onset or positive PCR test result (range 14–63 days, mean 30 days, SD 16 days, and median 23 days). The timeframes were chosen based on SARS-CoV-2 antibody kinetics with median seroconversion times from 11 to 14 days [10, 21–23]. Cutoff values were determined with ROC curve analysis by choosing 99% specificity for SARS-CoV-2 anti-N EIA of the controls (Supplementary Figure 2).
In the acute phase group, 65% (60/92), 63% (58/92), and 51% (47/92) of the samples had SARS-CoV-2 N protein IgG, IgA, and IgM antibodies, respectively (Figure 2). In the convalescent phase group, the mean IgG antibody levels were significantly higher (P < 0.001) compared to the acute phase group, whereas IgA and IgM antibody levels were only slightly higher compared to the acute phase group. Antibody responses varied in the acute phase group and some samples (1%–3%) had only 1 type of antibodies against SARS-CoV-2 N-protein, while 11 samples had both IgG and IgA antibodies but no detectable IgM antibodies and 2 samples had both IgG and IgM antibodies but no IgA antibodies. In the convalescent phase group, only 1 patient was seronegative for all SARS-CoV-2 N-protein antibodies. This sample was collected at a relatively early stage of the disease (15 days after onset of symptoms).
Figure 2.
IgG, IgA, and IgM antibody responses against human coronavirus nucleoproteins. IgG (A), IgA (B), and IgM (C) antibody levels were determined with EIA in control samples (n = 100) and in PCR-confirmed COVID-19 patient samples collected at an acute phase (n = 92 for SARS-CoV-2 GST-N and n = 91 for other EIAs) and convalescent phase (n = 27 for SARS-CoV-2 GST-N EIA and n = 26 for other EIAs) of the infection. Mean values are shown with SDs. The dotted line indicates the cutoff value, which is based on negative control samples in SARS-CoV-2 GST-N protein antibody determination with data interpretation with ROC analysis (Supplementary Figure 2). P values < .05 are considered statistically significant. Abbreviations: COVID-19, coronavirus disease 2019; EIA, enzyme immunoassay; GST-N, glutathione S-transferase–nucleoprotein; Ig, immunoglobulin; MERS, Middle East respiratory syndrome; PCR, polymerase chain reaction; ROC, receiver operating characteristics; SARS-CoV-2, severe acute respiratory syndrome coronavirus.
Antibody Responses Against Coronavirus Nucleoproteins in Controls and COVID-19 Patients
Next, we evaluated the potential cross-reactivity of SARS-CoV-2 infection-induced antibodies against different coronavirus N proteins. SARS-CoV-2 anti-N IgG, IgA, and IgM antibodies were highly cross-reactive with SARS-CoV N protein but only weakly cross-reactive with MERS-CoV N protein (Figure 2). We also detected weakly cross-reactive IgG, IgA, and IgM antibodies in some of the control group specimens against SARS-CoV (2%–5%) and MERS-CoV (3%–5%) N proteins (Figure 2 and Table 2). The same serum samples had variable levels of antibodies against low-pathogenic HCoV N proteins.
Table 2.
Seroprevalence (%) of IgG, IgA, and IgM Antibodies Against HCoV Nucleoproteins
| Sample | SARS-CoV-2 | SARS-CoV | MERS-CoV | HCoV-HKU1 | HCoV-OC43 | HCoV-229E | HCoV-NL63 |
|---|---|---|---|---|---|---|---|
| IgG | |||||||
| Control | 1.0 | 3.0 | 3.0 | 27.0 | 45.0 | 69.0 | 86.0 |
| Acute | 65.2 | 69.2 | 11.0 | 30.8 | 49.5 | 82.4 | 86.8 |
| Convalescent | 96.3 | 100.0 | 11.5 | 26.9 | 38.5 | 76.9 | 96.2 |
| IgA | |||||||
| Control | 1.0 | 5.0 | 3.0 | 4.0 | 9.0 | 8.0 | 22.0 |
| Acute | 63.0 | 67.0 | 6.6 | 11.0 | 17.6 | 33.0 | 41.8 |
| Convalescent | 81.5 | 84.6 | 11.5 | 7.7 | 7.7 | 61.5 | 65.4 |
| IgM | |||||||
| Control | 1.0 | 2.0 | 5.0 | 3.0 | 1.0 | 9.0 | 6.0 |
| Acute | 51.1 | 51.6 | 5.5 | 1.1 | 1.1 | 7.7 | 3.3 |
| Convalescent | 55.6 | 46.2 | 11.5 | 0.0 | 0.0 | 0.0 | 3.8 |
Seroprevalence was determined by EIAs in control samples (n = 100) and in PCR-confirmed COVID-19 patient samples collected at acute phase (n = 92 for SARS-CoV-2 GST-N and n = 91 for other EIAs) and convalescent phase (n = 27 for SARS-CoV-2 GST-N EIA and n = 26 for other EIAs) of the infection. Abbreviations: COVID-19, coronavirus disease 2019; EIA, enzyme immunoassay; GST-N, glutathione S-transferase–nucleoprotein; HCoV, human coronavirus; Ig, immunoglobulin; MERS-CoV, Middle East respiratory syndrome coronavirus; PCR, polymerase chain reaction; SARS-CoV, severe acute respiratory syndrome coronavirus.
Based on the IgG EIA, in the control group, the seroprevalence of HCoV-HKU1 and HCoV-OC43 was 27% and 45%, respectively, and for HCoV-229E and HCoV-NL63 was 69% and 86%, respectively (Table 2). As expected, IgA and IgM antibodies were less prevalent (seroprevalence of 3%–4% for HCoV-HKU1, 1%–9% for HCoV-OC43, 8%–9% for HCoV-229E, and 6%–22% for HCoV-NL63). Interestingly, N protein-specific IgG antibody levels against HCoV-229E and HCoV-NL63 N-proteins were significantly higher in the acute and especially in the convalescent phase group compared to the control group (Figure 2A). However, significantly higher antibody levels were not observed in IgA or IgM antibodies (Figure 2B and 2C).
Analysis of the paired serum samples of COVID-19 patients revealed an increase in the IgG antibodies against HCoV-229E and HCoV-NL63 N proteins in 6/16 patients (Supplementary Figure 3). Only 1 patient showed an increase in IgG antibodies against HCoV-HKU1 and HCoV-OC43 and 4 patients showed an increase in IgG antibodies against MERS-CoV. The results indicate that SARS-CoV-2 infection can likely induce immunological memory responses as anti-N antibodies against HCoV-229E and HCoV-NL63 but only rarely against HCoV-HKU1 and HCoV-OC43, which are circulating less frequently in the Finnish population.
Antibody Responses Against SARS-CoV-2 S1-mFc and RBD-mFc Proteins
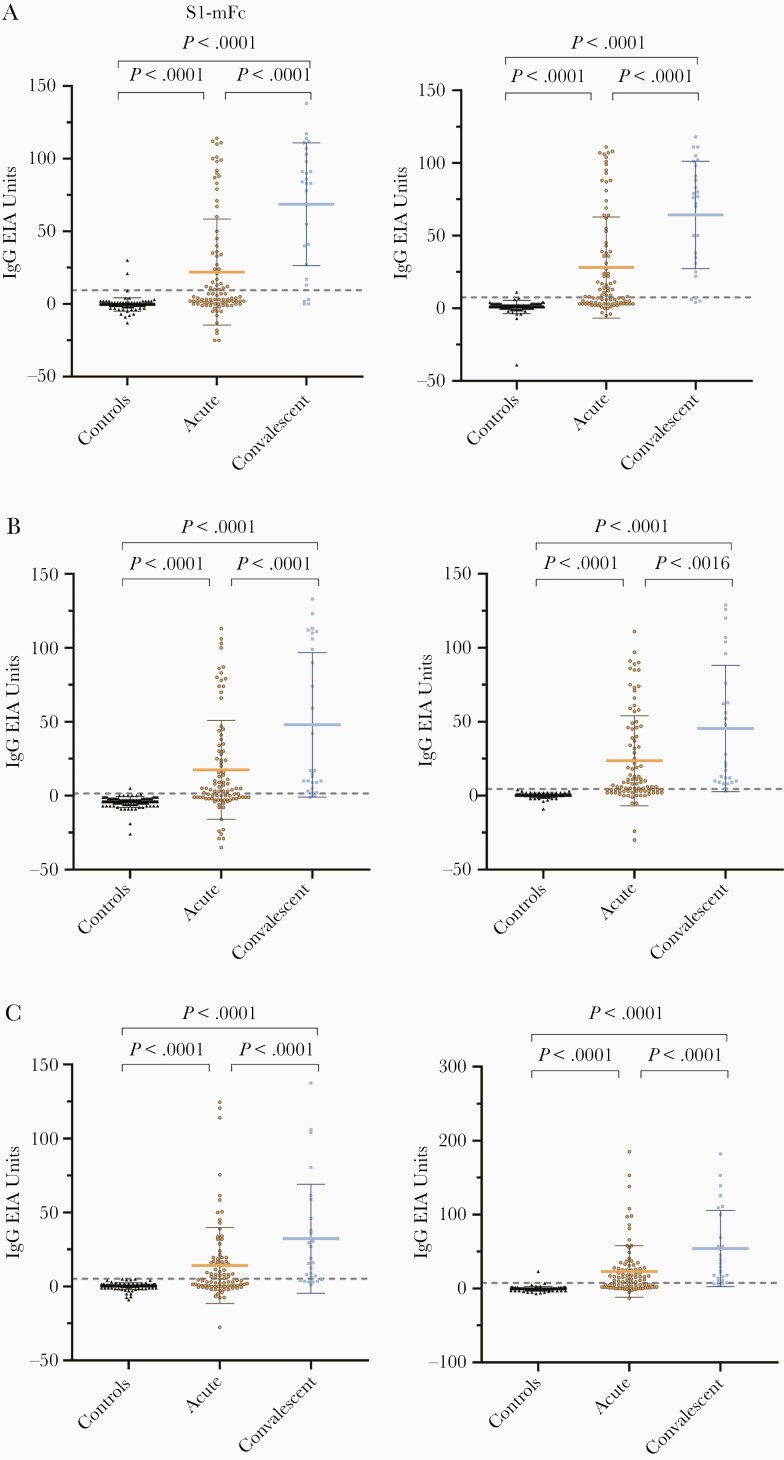
In addition to the N protein-based EIA, serum samples were tested with SARS-CoV-2 S1-mFc and RBD-mFc antibody EIAs. The cutoff units were determined with ROC curve analysis by choosing 98%, 99%, and 100% specificity for anti-S1 IgG, IgA, and IgM EIA, and 99%, 100%, and 99% specificity for anti-RBD IgG, IgA, and IgM EIA, respectively (Supplementary Figure 2). In the acute phase group, IgG antibodies against RBD and S1 were found in 49% (45/92) and 41% (38/92) of samples, respectively. IgA and IgM seropositivity was 65% (56/92) and 58% (53/92) against RBD, and 57% (52/92) and 49% (45/92) against S1, respectively (Table 3). The arithmetic mean IgG, IgA, and IgM antibody levels against RBD and S1 were similar and the mean antibody levels were significantly higher in the convalescent phase group as compared to the acute phase group specimens (Figure 3).
Table 3.
Sensitivity (%) of SARS-CoV-2 N-, S1-, and RBD-Based Enzyme Immunoassays
| Sample | N | S1 | RBD | N + S1 | N + RBD |
|---|---|---|---|---|---|
| COVID-19 patients, acute phase (n = 92) | |||||
| IgG | 65.2 | 41.3 | 48.9 | 67.4 | 67.4 |
| IgA | 63.0 | 56.5 | 65.2 | 69.6 | 75.0 |
| IgM | 51.1 | 48.9 | 57.6 | 57.6 | 65.2 |
| IgG/IgA | 68.5 | 57.6 | 69.6 | 71.7 | 77.2 |
| IgG/IgM | 66.3 | 51.1 | 63.0 | 71.7 | 72.8 |
| IgA/IgM | 66.3 | 57.6 | 73.9 | 71.7 | 80.4 |
| COVID-19 patients, convalescent phase (n = 27) | |||||
| IgG | 96.3 | 85.2 | 85.2 | 96.3 | 96.3 |
| IgA | 81.5 | 92.6 | 96.3 | 96.3 | 100.0 |
| IgM | 55.6 | 74.1 | 88.9 | 81.5 | 88.9 |
| IgG/IgA | 96.3 | 96.3 | 100.0 | 100.0 | 100.0 |
| IgG/IgM | 96.3 | 85.2 | 92.6 | 96.3 | 96.3 |
| IgA/IgM | 81.5 | 96.3 | 100.0 | 96.3 | 100.0 |
Abbreviations: COVID-19, coronavirus disease 2019; Ig, immunoglobulin; N, nucleoprotein; RBD, receptor binding domain; S1, spike protein; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.
Figure 3.
IgG, IgA, and IgM antibody responses against SARS-CoV-2 S1 and RBD domains. Anti-S1 (left) and anti-RBD (right) IgG (A), IgA (B), and IgM (C) antibody levels were determined with EIA in control samples (n = 100) and in PCR-confirmed COVID-19 patient samples collected at acute phase (n = 92) and convalescent phase (n = 27) of the infection. Mean values are shown with SDs. The dotted line indicates the cutoff value, which is based on the control samples in SARS-CoV-2 S1 and RBD protein antibody determination with data interpretation with ROC analysis (Supplementary Figure 2). P values < .05 are considered statistically significant. Abbreviations: COVID-19, coronavirus disease 2019; EIA, enzyme immunoassay; Ig, immunoglobulin; PCR, polymerase chain reaction; RBD, receptor binding domain; ROC, receiver operating characteristics; S1, spike protein; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.
A Combination of IgA and IgG for Both Nucleoprotein and Spike Protein Increases the Sensitivity of the Serological Diagnosis
To analyze whether the EIA results of 3 SARS-CoV-2 antigens correlate, we compared the antibody responses against S1 and RBD to those of N protein-specific responses. Antibody responses against SARS-CoV-2 N and S1 proteins had a moderate to strong positive correlation (IgG r = 0.81, IgA r = 0.67, and IgM r = 0.64) as well as antibody responses against N and RBD proteins (IgG r = 0.85, IgA r = 0.71, and IgM r = 0.66). Antibody responses against S1 and RBD showed a very strong positive correlation (IgG r = 0.96, IgA r = 0.96, and IgM r = 0.95; Supplementary Figure 4).
Next, we analyzed the combined results of anti-N, anti-S1, and anti-RBD IgG, IgA, and IgM EIAs. When the results of anti-N IgA measurement were combined with the results of anti-S1 or anti-RBD IgA measurement, the sensitivity increased in the acute phase group from 63% for anti-N IgA to 70% for anti-N plus S1 IgA and to 75% for anti-N plus RBD IgA combination (Table 3). A combination of anti-N and anti-S1 or anti-RBD IgG antibody data to IgA antibody data increased the sensitivity from 69% for anti-N IgA/IgG to 72% for anti-N plus S1 IgA/IgG combination and to 77% for anti-N plus RBD IgA/IgG combination. Similarly, a combination of anti-N and anti-S1 or anti-RBD IgA and IgM antibody measurement increased the sensitivity in the acute phase group from 66% for anti-N IgA/IgM to 72% for anti-N plus S1 IgA/IgM combination and to 80% for anti-N plus RBD IgA/IgM combination. In the convalescent phase group, a combination of anti-N and anti-RBD IgA showed sensitivity of 100%. Overall, these results indicate that IgA is important in the serological SARS-CoV-2 diagnosis in the acute phase, and both N and S proteins (S1 or RBD) are needed for accurate serological testing.
Correlation of Anti-S1 and Anti-RBD Antibody EIA With the Microneutralization Test
Next, we analyzed the correlation of SARS-CoV-2 N and S protein (S1 and RBD) specific immune responses in EIA with neutralizing antibodies (MNT). Altogether, 119 COVID-19 patient serum specimens were analyzed, of which 68 samples had been determined before [8]. MNTs performed in different laboratories used the same virus isolate and the results correlated very well (n = 18, r2 > 0.9, data not shown). Altogether, anti-S1 and anti-RBD IgG, IgA, and IgM antibody levels correlated strongly with MNT results (r > 0.76) (Figure 4). The highest correlation was with anti-S1 IgG and IgM EIA (r = 0.86). In addition, anti-N IgG, IgA, and IgM antibody levels correlated well with neutralizing antibody titers (r = 0.79, r = 0.65, and r = 0.63, respectively), although the correlation was weaker than with anti-S1 or anti-RBD antibodies.
Figure 4.
Correlation of anti-N, anti-S1, and anti-RBD IgG, IgA, and IgM antibody levels with neutralizing antibody titers. MNT results for COVID-19 PCR-positive patient sera (n = 68, black circles) were obtained from Jääskeläinen et al [8] and the MNT titers of the remaining samples (n = 51, grey circles) were determined as described. MNT titers <40 were marked as 20. Correlations were evaluated with Spearman ranked correlation test and P values < .05 were considered statistically significant. Abbreviations: COVID-19, coronavirus disease 2019; EIA, enzyme immunoassay; Ig, immunoglobulin; MNT, microneutralization test; N, nucleoprotein; PCR, polymerase chain reaction; RBD, receptor binding domain; S1, spike protein; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.
DISCUSSION
Sensitive and specific detection of antibodies against SARS-CoV-2 proteins is critical for early and accurate serological diagnostics and to estimate the rate of past infections or herd immunity in epidemiological studies. Our data support the idea that using both N and S1/RBD antigens in SARS-CoV-2 antibody detection is beneficial. We demonstrated that by combining anti-N and anti-RBD IgA with IgM or IgG EIA measurement, a rather high sensitivity (77%–80%) was reached within the first 2 weeks of SARS-CoV-2 infection. In the convalescent phase, the measurement of anti-N and anti-RBD IgA antibodies resulted in 100% sensitivity. In addition, we show that creating mFc fusion S1 and RBD domains increases the yield in protein production and the signals in EIA, potentially increasing the sensitivity of the assays.
Recent studies have shown that anti-RBD IgA antibodies are short lived and persist up to 2 months after the onset of symptoms [24, 25] supporting the use of IgA antibody determination in identifying an acute/recent SARS-CoV-2 infection. For epidemiological studies, anti-N and anti-S IgG antibodies are important because they have been shown to persist for longer periods of time [24, 26]. Dual measurement of IgA and IgG antibodies provides a reliable assay for determining COVID-19 incidence in low antibody prevalence populations.
The presence of anti-S1 and anti-RBD IgG, IgA, and IgM antibodies in serum correlated well with viral neutralizing activity. Recent studies have reported similar results, and both anti-S1 and anti-RBD IgG and IgM antibodies have been shown to correlate with neutralizing antibody activity [12, 26, 27]. Unlike anti-S1 and anti-RBD antibodies, anti-N antibodies are likely not neutralizing, and indeed we did not find as strong correlation between anti-N antibodies and neutralizing antibody titers as with anti-S1 and anti-RBD antibodies. It is noteworthy that not all anti-S1 and anti-RBD antibodies are neutralizing [28]; however, it may be possible to identify anti-spike antibody levels in EIA that would relatively well correlate with protective immunity, enabling population-level estimates of anti-SARS-CoV-2 immunity.
SARS-CoV-2 infection induced cross-reactive anti-N IgG memory responses, especially against HCoV-229E and HCoV-NL63 in patients who had low or even undetectable preexisting antibodies against corresponding low-pathogenic HCoVs. These responses could be due to cross-reactive epitopes or activation of memory B cells from previous coronavirus infections. Similar results have been observed in SARS patients who experienced an increase in the antibody levels against HCoV-229E, HCoV-NL63, and HCoV-OC43 between an acute and convalescent phase of SARS-CoV infection [29, 30].
We did not detect any significant cross-reactivity against SARS-CoV-2 N and S1/RBD proteins in the serum specimens of the control group, and thus SARS-CoV-2 N, S1, and RBD-based EIA assays had very high specificity (98%–100%). However, we detected some individuals with low-level cross-reactive anti-N IgG, IgA, or IgM antibodies against other high-pathogenic HCoVs, SARS-CoV, and MERS-CoV. This could be due to previous low-pathogenic HCoV infections because antibodies against low-pathogenic HCoVs were highly prevalent in control individuals and the sequence identity between MERS and low-pathogenic HCoV N proteins ranged from 24% to 36% (data not shown). As a whole, our results are well in line with previous studies showing a very low rate of antibody cross-reactivity in pre-COVID-19 pandemic individuals to N and S1 proteins of SARS-CoV-2 [9, 31, 32].
Here we have demonstrated that the dual measurement of IgG and IgA antibodies targeting nucleoprotein and spike protein domains of SARS-CoV-2 provide increased sensitivity in serological testing. Information on the cross-reactivity of HCoVs is crucial for accurate diagnosis and determining the level of preexisting immunity against SARS-CoV-2 infections. The use of other SARS-CoV-2 proteins apart from structural proteins may also provide a particular advantage in increasing the sensitivity of diagnostic tests or seroprevalence studies. In addition, the use of nucleoprotein and immunogenic nonstructural SARS-CoV-2 proteins as antigens in serological assays may provide the means to differentiate natural infection from vaccine-induced immunity, enabling the estimation of true vaccine efficacy against COVID-19 infection. We still have a long way to go for a full understanding of COVID-19 immunity.
Supplementary Data
Supplementary materials are available at The Journal of Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Acknowledgment . The following reagent was produced under HHSN272201400008C and obtained through BEI Resources, NIAID, NIH: spike glycoprotein receptor binding domain (RBD) from SARS-related coronavirus 2, Wuhan-Hu-1, recombinant from HEK293T cells, NR-52306.
Author contributions. P. J., L. K., and I. J. designed the study and analyzed the data. P. J., A. P., O. R., L. K., and I. J. wrote the manuscript. P. J., S. M., and P. K. optimized the assays and performed the experiments. P. J., A. P., K. M., P. K., L. T., H. K., M. R., R. N., and O. R. designed the expression systems, cloned the genes, and expressed and purified the recombinant proteins. P. J., S. M., P. K., M. H., R. L., A. H., P. Ö., and L. K. isolated and grew the viruses and carried out the neutralization assays. A. J., S. K., M. L., K. R., T. V., J. H., and M. W. were responsible for patient data, diagnostics, and sample collection. S. T. provided intellectual contributions throughout the study. All authors edited and approved the manuscript.
Financial support. This work was supported by the Medical Research Council of the Academy of Finland (grant numbers 297329 and 336410); the Jane and Aatos Erkko Foundation (grant numbers 3067-84b53 and 5360-cc2fc); the Finnish Cultural Foundation; and the Sigrid Juselius Foundation.
Potential conflicts of interest. All authors: No reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Zhou P, Yang XL, Wang XG, et al. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature 2020; 579:270–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. World Health Organization. Listings of WHO’s response to COVID-19, 2020. https://www.who.int/news/item/29-06-2020-covidtimeline. Accessed 29 August 2020.
- 3. John Hopkins University. Coronavirus dashboard. https://coronavirus.jhu.edu/map.html. Accessed 15 April 2021.
- 4. Lu R, Zhao X, Li J, et al. Genomic characterisation and epidemiology of 2019 novel coronavirus: implications for virus origins and receptor binding. Lancet 2020; 395:565–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Su S, Wong G, Shi W, et al. Epidemiology, genetic recombination, and pathogenesis of coronaviruses. Trends Microbiol 2016; 24:490–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Food and Drug Administration. EUA authorized serology test performance. 2020. https://www.fda.gov/medical-devices/emergency-situations-medical-devices/eua-authorized-serology-test-performance. Accessed 10 October 2020.
- 7. Liu W, Liu L, Kou G, et al. Evaluation of nucleocapsid and spike protein-based enzyme-linked immunosorbent assays for detecting antibodies. J Clin Microbiol 2020; 58:1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Jääskeläinen AJ, Kuivanen S, Kekäläinen E, et al. Performance of six SARS-CoV-2 immunoassays in comparison with microneutralisation. J Clin Virol 2020; 129:104512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Guo L, Ren L, Yang S, et al. Profiling early humoral response to diagnose novel coronavirus disease (COVID-19). Clin Infect Dis 2020; 71:778–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Long QX, Liu BZ, Deng HJ, et al. Antibody responses to SARS-CoV-2 in patients with COVID-19. Nat Med 2020; 26:845–8. [DOI] [PubMed] [Google Scholar]
- 11. Ma H, Zeng W, He H, et al. Serum IgA, IgM, and IgG responses in COVID-19. Cell Mol Immunol 2020; 17:773–5 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Geurtsvankessel CH, Okba NMA, Bogers S, et al. An evaluation of COVID-19 serological assays informs future diagnostics and exposure assessment. Nat Commun 2020; 11:1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Prévost J, Gasser R, Beaudoin-Bussières G, et al. Cross-sectional evaluation of humoral responses against SARS-CoV-2 spike. Cell Reports Med 2020; 1:100–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Corman VM, Landt O, Kaiser M, et al. Detection of 2019 novel coronavirus (2019-nCoV) by real-time RT-PCR. Eurosurveillance 2020; 25:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kazakova A, Kakkola L, Päkkilä H, et al. Serological array-in-well multiplex assay reveals a high rate of respiratory virus infections and reinfections in young children. mSphere 2019; 4:1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Melén K, Kakkola L, He F, et al. Production, purification and immunogenicity of recombinant Ebola virus proteins—a comparison of Freund’s adjuvant and adjuvant system 03. J Virol Methods 2017; 242:35–45. [DOI] [PubMed] [Google Scholar]
- 17. Hulmi JJ, Oliveira BM, Silvennoinen M, et al. Muscle protein synthesis, mTORC1/MAPK/Hippo signaling, and capillary density are altered by blocking of myostatin and activins. Am J Physiol Endocrinol Metab 2013; 304:E41–50. [DOI] [PubMed] [Google Scholar]
- 18. Kazakova A, Kakkola L, Ziegler T, et al. Pandemic influenza A(H1N1pdm09) vaccine induced high levels of influenza-specific IgG and IgM antibodies as analyzed by enzyme immunoassay and dual-mode multiplex microarray immunoassay methods. Vaccine 2020; 38:1933–42. [DOI] [PubMed] [Google Scholar]
- 19. Haveri A, Smura T, Kuivanen S, et al. Serological and molecular findings during SARS-CoV-2 infection: the first case study in Finland, January to February 2020. Eurosurveillance 2020; 25:1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Amanat F, Stadlbauer D, Strohmeier S, et al. A serological assay to detect SARS-CoV-2 seroconversion in humans. Nat Med 2020; 26:1033–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Ng DL, Goldgof GM, Shy BR, et al. SARS-CoV-2 seroprevalence and neutralizing activity in donor and patient blood. Nat Commun 2020; 11:4698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Zhao J, Yuan Q, Wang H, et al. Antibody responses to SARS-CoV-2 in patients of novel coronavirus disease 2019. Clin Infect Dis 2020; 71:2027–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Yu HQ, Sun BQ, Fang ZF, et al. Distinct features of SARS-CoV-2-specific IgA response in COVID-19 patients. Eur Respir J 2020; 56:7–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Iyer AS, Jones FK, Nodoushani A, et al. Persistence and decay of human antibody responses to the receptor binding domain of SARS-CoV-2 spike protein in COVID-19 patients. Sci Immunol 2020; 5:1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Isho B, Abe KT, Zuo M, et al. Persistence of serum and saliva antibody responses to SARS-CoV-2 spike antigens in COVID-19 patients. Sci Immunol 2020; 5:1–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Wajnberg A, Amanat F, Firpo A, et al. Robust neutralizing antibodies to SARS-CoV-2 infection persist for months. Science 2020; 370:1227–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Premkumar L, Segovia-Chumbez B, Jadi R, et al. The receptor-binding domain of the viral spike protein is an immunodominant and highly specific target of antibodies in SARS-CoV-2 patients. Sci Immunol 2020; 5:1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Li Y, Lai DY, Zhang HN, et al. Linear epitopes of SARS-CoV-2 spike protein elicit neutralizing antibodies in COVID-19 patients. Cell Mol Immunol 2020; 17:1095–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Chan KH, Cheng VC, Woo PC, et al. Serological responses in patients with severe acute respiratory syndrome coronavirus infection and cross-reactivity with human coronaviruses 229E, OC43, and NL63. Clin Diagn Lab Immunol 2005; 12:1317–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Che XY, Qiu LW, Liao ZY, et al. Antigenic cross-reactivity between severe acute respiratory syndrome-associated coronavirus and human coronaviruses 229E and OC43. J Infect Dis 2005; 191:2033–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. van Tol S, Mögling R, Li W, et al. Accurate serology for SARS-CoV-2 and common human coronaviruses using a multiplex approach. Emerg Microbes Infect 2020; 9:1965–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Ng KW, Faulkner N, Cornish GH, et al. Preexisting and de novo humoral immunity to SARS-CoV-2 in humans. Science 2020; 370:1339–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.