Abstract
Background
The severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) pandemic poses an urgent need for the development of effective therapies for coronavirus disease 2019 (COVID-19).
Methods
We first tested SARS-CoV-2–specific T-cell (CοV-2-ST) immunity and expansion in unexposed donors, COVID-19–infected individuals (convalescent), asymptomatic polymerase chain reaction (PCR)–positive subjects, vaccinated individuals, non–intensive care unit (ICU) hospitalized patients, and ICU patients who either recovered and were discharged (ICU recovered) or had a prolonged stay and/or died (ICU critical). CoV-2-STs were generated from all types of donors and underwent phenotypic and functional assessment.
Results
We demonstrate causal relationship between the expansion of endogenous CoV-2-STs and the disease outcome; insufficient expansion of circulating CoV-2-STs identified hospitalized patients at high risk for an adverse outcome. CoV-2-STs with a similarly functional and non-alloreactive, albeit highly cytotoxic, profile against SARS-CoV-2 could be expanded from both convalescent and vaccinated donors generating clinical-scale, SARS-CoV-2–specific T-cell products with functional activity against both the unmutated virus and its B.1.1.7 and B.1.351 variants. In contrast, critical COVID-19 patient-originating CoV-2-STs failed to expand, recapitulating the in vivo failure of CoV-2–specific T-cell immunity to control the infection. CoV-2-STs generated from asymptomatic PCR-positive individuals presented only weak responses, whereas their counterparts originating from exposed to other seasonal coronaviruses subjects failed to kill the virus, thus disempowering the hypothesis of protective cross-immunity.
Conclusions
Overall, we provide evidence on risk stratification of hospitalized COVID-19 patients and the feasibility of generating powerful CoV-2-ST products from both convalescent and vaccinated donors as an “off-the shelf” T-cell immunotherapy for high-risk patients.
Keywords: coronavirus 2, COVID-19, T-cell responses, adoptive immunotherapy, virus-specific T cells
Polyclonal severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2)–specific–specific T-cell products with a safe and strong cytotoxic profile against SARS-CoV-2–presenting targets, as well as SARS-CoV-2 variants, can be generated from both coronavirus disease 2019 convalescent or vaccinated donors to be used as adoptive therapy of high-risk patients.
The emergence of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has led to the devastating outbreak of coronavirus disease 2019 (COVID-19). Despite that several repurposed or novel agents have been evaluated as COVID-19 treatment, no proven therapeutic strategy exists [1].
Similar to the essential role in viral clearance of the related virus SARS-CοV T cells shown to persist for >10 years after exposure [2], T-cell responses play a significant role in recovering from SARS-CoV-2 [3, 4]. The power of T cells is clearly emphasized in the transplant setting, where the adoptive transfer of graft- or third-party donor–derived virus-specific T cells (VSTs) into immunocompromised recipients successfully controls adenovirus, cytomegalovirus, Epstein-Barr virus, BK virus, JC virus, and human herpesvirus 6, conferring only minimal risk of graft-vs-host disease [5–8]. VSTs, ex vivo expanded from seropositive donors, and targeting multiple viral antigens and a plethora of epitopes via stimulation with overlapping peptides, provide the benefits of strong cytotoxic potential and minimization of immune evasion by viral mutants.
We here provide the rationale toward the development of a SARS-CoV-2–specific T-cell (CoV-2-ST) bank from convalescent donors as T-cell immunotherapy against severe COVID-19. Since it is still unclear whether vaccination will provide a similar to natural SARS-CoV-2 infection T-cell “training,” extending also to emerging variants, we compared convalescent donor–derived CoV-2-STs (Conv-CoV-2-STs) with vaccinated donor–derived CoV-2-STs (Vac-CoV-2-STs) as regards phenotype and functionality, against the unmutated virus and both the British B.1.1.7 and the South African B.1.351 variants.
MATERIALS AND METHODS
Study Approval
The protocol and informed consent forms were approved by the Institutional Review Board of the George Papanikolaou Hospital.
Participants
The study subjects were unexposed donors with no COVID-19 history or contact with affected individuals, vaccinated subjects, asymptomatic polymerase chain reaction (PCR) SARS-CoV-2–positive subjects, and SARS-CoV-2–infected individuals, with ≥1 month recovery before sampling (convalescent), non–intensive care unit (ICU) hospitalized patients, ICU-recovered patients, or ICU-critical patients having a prolonged/complicated stay (>25 days) and/or who died (Supplementary Table 1).
Enzyme-Linked Immunospot Assay
Circulating CoV-2-STs were measured posthospitalization or ICU admission weekly. Peripheral blood mononuclear cells (PBMCs) or T-cell products were pulsed with spike, B.1.1.7 or B.1.351 or Nuclecapsid (NCAP) protein, and the secretion of interferon gamma (IFN-γ) or tumor necrosis factor alpha (TNF-α) was measured by enzyme-linked immunospot assay (ELISpot). Spot-forming cells (SFCs) were counted on Eli.Scan ELISpot scanner (A.EL.VIS; Eli.Analyse software V6.2.SFC). Cell response was considered positive if the total SFCs against antigens tested, were ≥30 per 5 × 105 PBMCs or 2 × 105 CoV-2-STs.
CoV-2-ST Generation
PBMCs pulsed with 1 μg/mL of spike and NCAP pepmixes were cultured as described previously [5, 6, 9], in G-Rex10, in media supplemented with 10 ng/mL interleukin 7 and 400 U/mL interleukin 4 until day 9–11.
Statistical Analysis
Results are expressed as mean ± standard error of the mean. Differences between data sets were analyzed using nonparametric Kruskal-Wallis test for multiple comparisons or Mann-Whitney test or 2-tailed Student t test for 2-group comparisons. T-cell numbers and disease severity were correlated using a linear regression model and Pearson correlation. The outcome in association with CoV-2-ST absolute number or expansion was assessed by receiver operating characteristic (ROC) curve analysis. Statistical analysis was performed using GraphPad Prism software. P values ≤.05 were considered significant.
RESULTS
SARS-CoV-2 Boosts Long-Lasting T-Cell Immunity
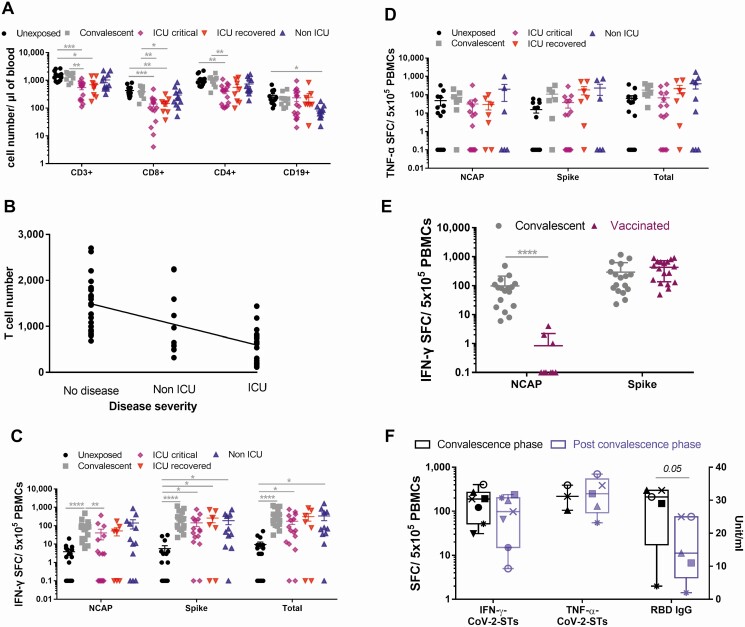
ICU-recovered or ICU-critical individuals exhibited profound T-cell lymphopenia over unexposed and convalescent donors and marginally more severe over non-ICU patients, suggesting that T-cell numbers inversely correlate with disease severity (Pearson r = –0.6163; Figure 1A and 1B). Circulating T lymphocytes of critical ICU patients were functionally impaired, demonstrating lower activation, higher levels of exhaustion, and a varying differentiation status, with decreased memory and naive subpopulations and elevated percentages of terminally differentiated effector T cells, than unexposed- or convalescent-donor derived T cells (Supplementary Figure 1). Strong SARS-CoV-2–specific responses were observed in convalescent donors against both NCAP and spike antigens, suggesting that COVID-19 boosts T-cell immunity. Previous exposure to other seasonal coronaviruses could interpret that 3 of 16 and 8 of 15 unexposed donors elicited negligible/low to moderate SARS-CoV-2–specific responses against Spike and NCAP (Figure 1C and 1D), respectively. Irrespective of the varying magnitude of T-cell immunity after natural infection, responses against spike were abundant over NCAP (Supplementary Figure 2). Expectedly, vaccinated donors with BNT162b2 encoding the spike protein showed almost exclusively, circulating spike-specific CoV-2-STs (Figure 1E). CoV-2-STs persisted for at least 8 months postinfection in the majority of convalescents, in whom, however, spike-specific immunoglobulin G showed clear reduction over time (Figure 1F).
Figure 1.
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) T-cell immunity in healthy individuals, vaccinated subjects, and patients with SARS-CoV-2 infection. A, Absolute lymphocyte counts in unexposed donors (dots; n = 14), convalescent donors (squares; n = 10) and coronavirus disease 2019 (COVID-19) patients stratified by disease severity to intensive care unit (ICU) critical (rhombuses; n = 16), ICU recovered (inverted triangles; n = 8), and non-ICU (triangles; n = 12). Differences between data sets were analyzed using Kruskal-Wallis test. *P ≤ .036; **P ≤ .0054; ***P ≤ .0002. B, Pearson correlation analysis of the absolute T-cell number correlation with disease severity (n = 58), P < .0001. C and D, Interferon gamma (IFN-γ; C) and tumor necrosis factor alpha (TNF-α; D) secretion of peripheral blood mononuclear cells of unexposed donors (dots; n = 15–16), convalescent donors (squares; n = 17 and n = 7, respectively) and COVID-19 patients stratified by disease severity to ICU-critical (rhombuses; n = 16), ICU-recovered (inverted triangles; n = 7), and non-ICU (triangles; n = 12 and n = 9, respectively). Each dot represents an individual donor. Differences between data sets were analyzed using Kruskal-Wallis test. *P ≤ .04; **P ≤ .0085; ***P ≤ .0006; ****P < .0001. E, IFN-γ secretion of peripheral blood mononuclear cells of convalescent donors (dots; n = 17) and vaccinated donors (triangles; n = 11). Each dot represents an individual donor. Differences between data sets were analyzed using Mann-Whitney test. ****P < .0001. F, IFN-γ– and TNF-α–producing circulating SARS-CoV-2–specific T cells and immunoglobulin G levels in individual donors during convalescence phase (dark colored boxes; n = 3-7) and postconvalescence phase (light colored boxes; n = 5-7). Differences between data sets were analyzed using a 2-tailed Student t test. *P ≤ .04. Abbreviations: CoV-2-ST, severe acute respiratory syndrome coronavirus 2–specific T cell; ICU, intensive care unit; IFN-γ, interferon gamma; IgG, immunoglobulin G; NCAP, Nucleocapsid protein; PBMC, peripheral blood mononuclear cell; RBD, receptor-binding domain; SFC, spot-forming cell; TNF-α, tumor necrosis factor alpha.
T-Cell Responses and Clinical Outcome
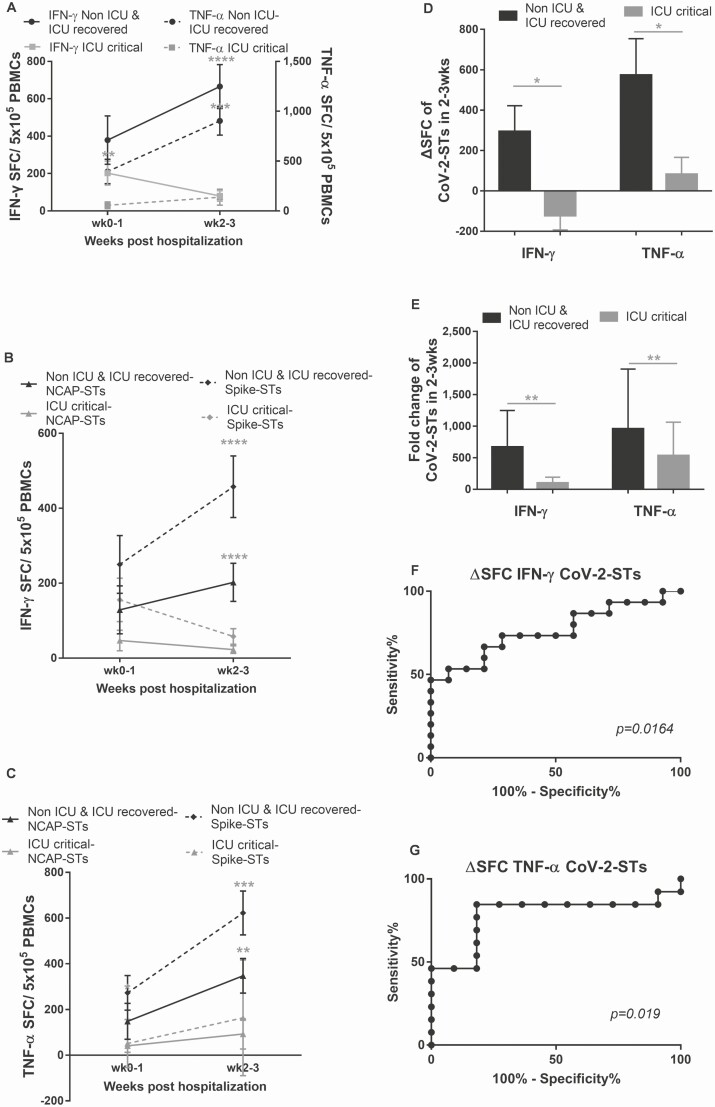
Following the temporal evolution of CoV-2-STs for up to 2 weeks postadmission, we observed that the majority of patients unable to expand their CoV-2-STs in vivo failed to control the infection and either had a prolonged/complicated ICU stay or died (ICU critical), whereas patients with CoV-2-ST rebounds, cleared the infection and were discharged (non-ICU and ICU recovered) (Figure 2A–C). The latter presented significant expansion of CoV-2 T-cell immunity over baseline as opposed to ICU-critical patients (Figure 2D and 2E).
Figure 2.
Kinetics of endogenous circulating severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2)–specific T cells (CoV-2-STs) in patients with coronavirus disease 2019 (COVID-19). A, The expansion of interferon gamma (IFN-γ)– and tumor necrosis factor alpha (TNF-α)–secreting endogenous CoV-2-STs in response to SARS-CoV-2 antigens postadmission to the clinic or intensive care unit (ICU) is associated with a favorable disease outcome (IFN-γ Non-ICU and ICU recovered, n = 14; IFN-γ ICU critical, n = 14; TNF-α Non-ICU/ICU recovered, n = 14; TNF-α ICU critical, n = 14). Differences between data sets were analyzed using Mann-Whitney test. **P = .0093; ***P = .0002; ****P < .0001. B and C, The expansion of IFN-γ (B)– and TNF-α (C)–secreting endogenous CoV-2-STs in response to NCAP or spike antigens postadmission to the clinic or ICU is associated with a favorable disease outcome (IFN-γ Non-ICU and ICU recovered, n = 14; IFN-γ ICU critical, n = 14; TNF-α Non-ICU/ICU recovered, n = 14; TNF-α ICU critical, n = 14). Differences between data sets were analyzed using Mann-Whitney test. **P = .0032; ***P = .0006; ****P < .0001. D and E, Δ spot-forming cells instead of units (ΔSFC; D) or fold change (E) of IFN-γ– and TNF-α–secreting circulating CoV-2-STs postadmission to the clinic or ICU (Non-ICU and ICU recovered, n = 15; ICU critical, n = 14). Differences between data sets were analyzed using Mann-Whitney test. *P ≤ .0184; **P ≤ .0061. F and G, Receiver operating characteristic curves of the ΔSFC of circulating IFN-γ (n = 29; F)– and TNF-α (n = 24; G)–secreting CoV-2-STs showing the predictive power of COVID-19 favorable outcome (high probability to self-control the infection). Abbreviations: CoV-2-ST, severe acute respiratory syndrome coronavirus 2–specific T cell; ICU, intensive care unit; IFN-γ, interferon gamma; NCAP, nucleocapsid protein; PBMC, peripheral blood mononuclear cell; SFC, spot-forming cell; TNF-α, tumor necrosis factor alpha.
The magnitude of CoV-2-ST expansion 2 weeks postadmission (ΔSFC), rather than baseline CoV-2-STs, was predictive of the patient outcome by ROC curve analysis; a threshold of ΔSFC >35 and ΔSFC >101 of IFN-γ– and TNF-α–secreting CoV-2-STs, respectively, could predict with high sensitivity and specificity a favorable outcome (Figure 2F and 2G; Supplementary Figure 3; Supplementary Table 2), suggesting that the magnitude of CoV-2-ST expansion could serve as a risk stratification tool.
Generation of CoV-2-STs From SARS-CoV-2–Convalescent, –Vaccinated, -Asymptomatic or –Unexposed Donors
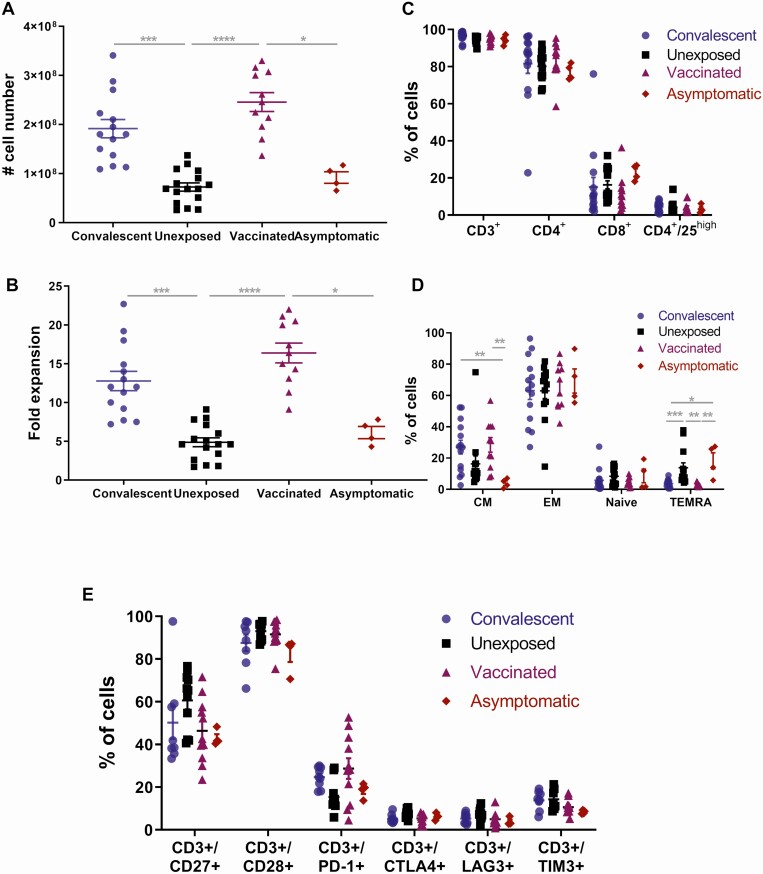
To generate CoV-2-STs for adoptive immunotherapy (AI), donor PBMCs were stimulated with pepmixes spanning NCAP and spike antigens and cultured as described elsewhere [5, 6, 9, 10]. Convalescent (Conv-) or vaccinated (Vac-) donor–derived T cells robustly expanded upon antigen exposure, providing multiple clinical-scale doses per T-cell product, whereas virus-naive or asymptomatic donor–derived CoV-2-STs had considerably lower expansion (Figure 3A and 3B).
Figure 3.
Generation and phenotypic characterization of severe acute respiratory syndrome coronavirus 2–specific T cells from convalescent, unexposed, vaccinated, and asymptomatic donors. A and B, Absolute cell numbers (A) and fold expansion (B) of T-cell products generated after a 10-day culture from convalescent (dots; n = 14), unexposed (squares; n = 16), vaccinated (triangles; n = 11), and asymptomatic (rhombuses; n = 4) individuals. C–E, Immunophenotype of T-cell products generated after a 10-day culture from convalescent (dots; n = 14), unexposed (squares; n = 16), vaccinated (triangles; n = 11), and asymptomatic (rhombuses; n = 4) individuals. Each dot represents a single T-cell product. Differences between data sets were analyzed using Kruskal-Wallis test. *P ≤ .02; **P ≤ .009; ***P ≤ .001; ****P < ·0001. Abbreviations: CM, central memory; EM, effector memory; TEMRA, terminally differentiated effector memory expressing CD45RA.
Characterization of CoV-2-ST Products
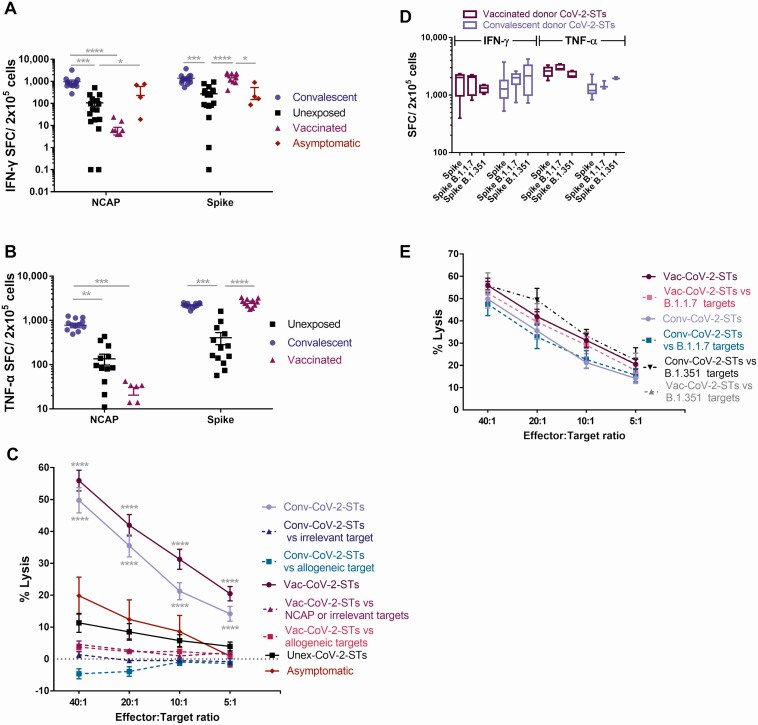
Conv- or Vac-CoV-2-STs were predominantly CD4+ but also CD8+ T cells, expressing memory and only at a minimum regulatory T-cell markers (Figure 3C) and presenting an activated and nonexhausted profile (Figure 3D and 3E). After reexposure to initial stimuli, Conv-CoV-2-ST products showed robust specificity against both targeted antigens, with dominant responses against spike (Figure 4A and 4B; Supplementary Figure 4). Not unexpectedly, Vac-CoV-2-STs induced strong specificity only against spike, similar to Conv-CoV-2-ST spike specificity, whereas uninfected or asymptomatic donor–derived CoV-2-ST-products presented significantly milder, albeit specific, responses. Notably, the specificity of same donor Conv-CoV-2-STs, at convalescence (2 months) and postconvalescence (8 months), was almost identical (Supplementary Figure 5), further supporting persistent SARS-CoV-2 T-cell immunity.
Figure 4.
Functional characterization of severe acute respiratory syndrome coronavirus 2–specific T cells (CoV-2-STs) from convalescent, unexposed, vaccinated, and asymptomatic donors. A and B, Interferon gamma (IFN-γ) and tumor necrosis factor alpha (TNF-α) secretion of the CoV-2-STs generated from convalescent (dots; n = 14), unexposed (squares; n = 16), vaccinated (triangles; n = 11), and asymptomatic (rhombuses; n = 4) individuals upon stimulation with their initial stimuli. Each dot represents a single T-cell product. Differences between data sets were analyzed using Kruskal-Wallis test. *P ≤ .04; **P ≤ .0043; ***P ≤ .0007; ****P < .0001. C, Percentage of killing of autologous, peptide-pulsed, or allogeneic unpulsed Phytohemagglutinin (PHA) blasts by CoV-2-STs. Differences between data sets were analyzed using Kruskal-Wallis test vs the respective unexposed donor condition or allogeneic unpulsed PHA blasts or irrelevant-peptide condition. ****P < .0001. D, IFN-γ and TNF-α secretion of the CoV-2-STs generated from convalescent (light colored boxes; n = 3-14) and vaccinated (dark colored; n = 4-11) individuals upon stimulation with spike antigen of SARS-CoV-2 or its B.1.1.7 and B.1.351 variants. E, Percentage of killing of autologous, unmutated spike-, B.1.1.7 or B.1.351 spike-pulsed PHA blasts by CoV-2-STs. Abbreviations: Conv, convalescent donor–derived T-cell product; CoV-2-ST, severe acute respiratory syndrome coronavirus 2–specificT cell; IFN-γ, interferon gamma; NCAP, Nucleocapsid; SFC, spot-forming units; TNF-α, tumor necrosis factor alpha; Unex, unexposed individual–derived T-cell product; Vac, vaccinated donor–derived T-cell product.
To further functionally characterize CoV-2-STs, a cytotoxicity assay against autologous antigen-pulsed phytohemagglutinin (PHA) blasts was performed in representative products. Convalescent- and vaccinated-donor cell products induced strong, specific, and comparable lysis of SARS-CoV-2–pulsed PHA blasts whereas they were noncytolytic against irrelevant (influenza)–pulsed PHA blasts. Expectedly, NCAP-pulsed lysis was induced only by Conv-CoV-2-STs (Figure 4C). Interestingly, unexposed or asymptomatic donor–derived CoV-2-STs, although specific, were noncytotoxic or barely cytotoxic, respectively. SARS-CoV-2 functional responses of CoV-2-ST products were mapped by HLA-restricted viral epitopes (Supplementary Table 3; Supplementary Figure 6).
To recapitulate the in vivo performance of CoV-2-STs in patients with severe COVID-19, cell products were also representatively produced from 2 patients, ICU-1 with a dismal outcome and ICU-2 who recovered. Although either CoV-2-ST product was specific and cytotoxic against SARS-CoV-2–pulsed autologous PHA blasts, only ICU-2 CoV-2-STs could proliferate upon specific stimulation without expressing exhaustion markers (Supplementary Figure 7), thus confirming the in vivo inability of critical patients to expand T cells and control the infection.
Alloreactivity would be an important safety issue in the present context. Co-culture of Conv- or Vac-CoV-2-STs with allogeneic PHA blasts resulted in very low cell lysis (Figure 4C), underlying the specific-only cytotoxic potential of CoV-2-ST products.
Conv- and Vac-CoV-2-STs Against B.1.1.7 and B.1.351 variants
To address the efficacy of CoV-2-ST products against the British B.1.1.7- and the South African B.1.351-SARS-CoV-2 variants, generated cells were pulsed with mutated virus peptides. Both Conv-CoV-2 and Vac-CoV-2-STs presented robust IFN-γ and TNF-α responses and strong cytotoxicity against the B.1.1.7-pulsed, B.1.351-pulsed and the unmutated SARS-CoV-2-cell targets (Figure 4D and 4E), implicating effective cytotoxic potential in vivo against the virus and its variants.
DISCUSSION
As the mortality due to COVID-19 continues to increase, developing therapeutic modalities against SARS-CoV-2 remains mandatory. Reasonably, vaccination has generated great optimism [11–14]; however, specific and effective therapeutic approaches are lacking. Even after herd immunity is achieved, vaccine breakthrough cases will exist, emerging mutations may escape antibody binding, vaccine deniers will be vulnerable, and immunocompromised patients will always at risk for severe COVID-19.
Based on the safety and the high response rates of posttransplant AI with donor- or third party–derived VSTs against, most commonly, the human herpesvirus (HHV) family [5–8], and by leveraging a previous protocol to generate multivirus- [5, 6], Aspergillus fumigatus– [9], and multipathogen-specific T cells [10, 15], we explored the possibility of producing CoV-2-STs from COVID-19 convalescent donors and, for first time, BNT162b2-vaccinated donors. Furthermore, by monitoring the endogenous CoV-2-ST kinetics, we could identify appropriate candidates for T-cell immunotherapy, that is, patients at high risk for an adverse outcome.
We here confirmed the observed lymphopenia [16, 17] in severely affected patients and the skewing of surviving T cells toward an ineffective differentiation status of exhausted or/and terminally differentiated cells, at the expense of functional memory and naive subpopulations.
T-cell immunity plays a major role in COVID-19 resolution [18], but whether protective memory provides long-lasting immunity, as with the related SARS-CoV [2], is still unclear. By contrast, there is increasing evidence that antibody-based immunity wanes over time [19, 20]. Herein, further supporting recent findings [21], we demonstrate that the majority of recovered donors maintained CoV-2-ST responses for ≥8 months postinfection, suggesting that this branch of immunity is not compromised, whereas decreasing antibodies in the same donors implicated a rather short-lived humoral immunity [19, 22]. It remains to be proven, however, whether T-cell immunity after natural infection or vaccination could protect from reinfection long-term.
A major finding in our study was the association of immune features with disease outcome. Non-ICU or ICU-recovered patients developed high magnitude SARS-CoV-2 T-cell expansion, in contrast to ICU-critical patients who failed to expand CoV-2-STs and in their majority, died. The kinetics and breadth of SARS-CoV-2 T-cell response over time could predict outcome. Indeed, ROC analysis based on the expansion of endogenous CoV-2-STs—rather than baseline CoV-2-STs—defined threshold levels predicting outcome. Thieme et al [23] reported that critical COVID-19 patients elicited powerful SARS-CoV-2 T-cell responses not associated with virus clearance. This observation based on the mean frequency of CoV-2-STs in measured samples at different time-points, from our point of view, cannot mirror the magnitude of SARS-CoV-2 T-cell response. In our study, the expansion of endogenous CoV-2-STs was a major indicator of a favorable outcome, discriminating patients with solid immunity from those having low probability to recover. The in vivo performance of endogenous CoV-2-STs was also correlated with their failure or success to expand ex vivo and an exhausted or active phenotype, respectively. T-cell exhaustion implies that checkpoint inhibitors could reverse the CoV-2-ST anergic phenotype to a functional one and be used as a COVID-19 therapeutic approach. However, several studies on the outcomes of COVID-19 patients, with or without cancer, receiving checkpoint inhibitors remain controversial and inconclusive [24, 25].
Given that no curative therapy exists for COVID-19, adoptive transfer of immunity has emerged as a promising alternative. In this context, convalescent plasma did not reduce mortality over placebo [26], probably reflecting the inherent heterogeneity of plasma therapy providing different immune signatures, and the waning antibody-mediated immunity in convalescent plasma donors [20].
We here pursued the generation of Conv- and Vac-CoV-2-STs as a feasibility study for future establishment of a CoV-2-ST cell bank and adoptive transfer of T-cell immunity. Unlike plasma, in which antibodies concentration decreases postinfusion, memory CoV-2-STs expand and proliferate, proving a longer-lasting effect.
Conv-CoV-2-STs presented a polyclonal mixture with dominant CD4+ immune signatures and a memory, nonexhaustion phenotype [27, 28], while exerted specific and strong cytolytic activity against SARS-CoV-2 without inducing alloreactivity, thus implicating in vivo efficacy and safety. Moreover, we report for first time the feasibility of generating Vac-CoV-2-STs sharing similar functional features with Conv-CoV-2-STs. Expectedly, due to active immunization with the spike-encoding vaccine, Vac-CoV-2-STs presented single-antigen specificity, albeit comparable cytotoxicity to Conv-CoV-2-STs. Consequently, vaccinated individuals, apart from being potentially protected from future reinfection, may also serve as donors for AI. Given, however, that infected subjects are exposed to a plethora of SARS-CoV-2 antigens whereas BNT162b2-vaccinated donors only to spike, Conv-CoV-2-STs may, at least theoretically, have greater potential to conquer immune escape mutations over active immunization or AI with Vac-CoV-2-STs. Nevertheless, at least for the British and the South African variants, both Conv- or Vac-CoV-2-STs presented strong specificity and cytotoxicity, demonstrating that both natural infection and BNT162b2 vaccination provide coverage against either mutation. We also investigated whether CoV-2-ST products could be generated from asymptomatic or unexposed individuals harboring small quantities of circulating CoV-2-STs, presumably as response to other endemic coronaviruses [4, 29, 30]. Asymptomatic and unexposed donor–derived CoV-2-STs over Conv-CoV-2-STs were expanded but at lower frequencies, secreted moderate to low levels of cytokines upon antigen encounter and, importantly, presented moderate or no cytotoxicity, respectively. Despite the limited number of asymptomatic individuals, the observed lower cytotoxic potential of asymptomatic donor–derived CoV-2-STs over Conv-CoV-2-STs suggested a weaker overall immune response in this cohort [31, 32]. Moreover, the lack of cytotoxicity of unexposed donor–derived CoV-2-STs, strongly opposes the hypothesis based on studies assessing specificity only, that preexisting cross-reactive immunity against endemic coronaviruses may provide protection against COVID-19 [27, 33].
SARS-CoV-2 is a new virus to humanity which so far, does not seem to evolve to latency; several cases initially thought as reactivations were reinfection from another virus version or fluctuating laboratory results around the detection threshold. However, in immunocompromised patients, prolonged viral shedding has been described.
The rationale of investigating AI as a COVID-19 therapeutic approach, out of the transplantation context, is challenging, albeit highly justified. Even if SARS-CoV-2 does not perform as a latent virus, the COVID-19–associated lymphopenia will provide a permissive microenvironment and a “therapeutic window” for the retention and expansion of infused, partially HLA-matched, CoV-2-STs and, consequently, virus elimination. Eventually, the patient’s recovered immune system will reject the partially matched CoV-2-STs, however at a time that their presence may not be necessary. Importantly, hematopoietic cell transplant recipients infused with recovered or vaccinated graft donor–derived CoV-2-STs will maintain long-term protection against SARS-CoV-2 reactivation/reinfection. The different tropism between HHVs and SARS-CoV-2 may also create scepticism for T-cell immunotherapy against COVID-19. SARS-CoV-2, although prevalent in the lung epithelium, usually leads to viremia and systemic invasion [34] as occurs with HHVs and has been described for other respiratory viruses [35, 36]. Despite different kinetics and cellular receptors, both latent HHVs and SARS-CoV-2 show broad and similar organotropism including the lung, intestine, kidneys, liver, heart, and immune-privileged territories, like the brain [37–39]. VSTs accumulate at organ sites of virus-induced inflammation controlling disease (colitis, hemorrhagic cystitis/nephritis, lymphoma, pneumonia), even crossing sanctuary sites (progressive multifocal leukoencephalopathy, encephalitis, retinitis) [5, 6, 40, 41]. Finally, our findings, including detection of reactive T cells in the circulation of convalescent COVID-19 donors who successfully cleared SARS-CoV-2 and the relevance of patient endogenous CoV-2-ST expansion in controlling SARS-CoV-2 infection, strongly support the idea of AI with CoV-2-STs for high-risk COVID-19 patients.
In the current imperfect landscape of COVID-19 therapeutics, our findings suggest that suboptimal or failed expansion of endogenous CoV-2-STs, identifies patients at high risk for an adverse outcome for whom an off-the-shelf, Conv-CoV-2ST, or Vac-CoV-2-ST cell product targeting SARS-CoV-2 via a shared HLA may represent an effective treatment. Whether this intervention will fulfill expectations remains to be answered in clinical trials, some of which have already started (ClinicalTrials.gov identifiers NCT04401410, NCT04457726, and NCT04351659) or are close to initiation, including one in our center (EudraCT identifier 2021-001022-22).
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Supplementary Material
Notes
Author contributions. Conceptualization: A. P. and E. Y. Methodology: P. G. P., D. C., K. K., A. G., A. I., E. G., C. G., M. B., E.-G. E., P. T., D.-C. L., A. F., I. K., M. T., S. D. B., G. K., A. K. B., E. S., and A. P. Formal analysis: E. G., A. P., and E. Y. Investigation: P. G. P., D. C., K. K., A. G., A. I., E. G., C. G., M. B., E.-G. E., P. T., D.-C. L., A. F., I. K., M. T., S. D. B., G. K., A. K. B., E. S., and A. P. Resources: A. A. and E. Y. Original draft preparation: A. P. and E. Y. Manuscript review and editing: P. G. P., A. P., and E. Y. Visualization: A. P. and E. Y. Supervision: A. A., A. P., and E. Y. Project administration: A. A., A. P., and E. Y. Funding acquisition: A. A. and E. Y. Verification of underlying data: P. G. P., A. P., and E. Y. All authors had full access to all the data in the study and accept responsibility to submit for publication. All data associated with this study are available in the main text or the Supplementary Materials.
Financial support. This work was supported by the Institute of Applied Biosciences, Centre for Research and Technology Hellas, Thessaloniki, Greece, and institutional resources.
Potential conflicts of interest. The authors: No potential conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Stasi C, Fallani S, Voller F, Silvestri C. Treatment for COVID-19: an overview. Eur J Pharmacol 2020; 889:173644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Li CK, Wu H, Yan H, et al. T cell responses to whole SARS coronavirus in humans. J Immunol 2008; 181:5490–500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Andersen KG, Rambaut A, Lipkin WI, Holmes EC, Garry RF. The proximal origin of SARS-CoV-2. Nat Med 2020; 26:450–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Le Bert N, Tan AT, Kunasegaran K, et al. SARS-CoV-2-specific T cell immunity in cases of COVID-19 and SARS, and uninfected controls. Nature 2020; 584:457–62. [DOI] [PubMed] [Google Scholar]
- 5. Papadopoulou A, Gerdemann U, Katari UL, et al. Activity of broad-spectrum T cells as treatment for AdV, EBV, CMV, BKV, and HHV6 infections after HSCT. Sci Transl Med 2014; 6:242ra83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Tzannou I, Papadopoulou A, Naik S, et al. Off-the-shelf virus-specific T cells to treat BK virus, human herpesvirus 6, cytomegalovirus, Epstein-Barr virus, and adenovirus infections after allogeneic hematopoietic stem-cell transplantation. J Clin Oncol 2017; 35:3547–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kaeuferle T, Krauss R, Blaeschke F, Willier S, Feuchtinger T. Strategies of adoptive T -cell transfer to treat refractory viral infections post allogeneic stem cell transplantation. J Hematol Oncol 2019; 12:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. O’Reilly RJ, Prockop S, Hasan A, Doubrovina E. Therapeutic advantages provided by banked virus-specific T-cells of defined HLA-restriction. Bone Marrow Transplant 2019; 54:759–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Papadopoulou A, Alvanou M, Koukoulias K, et al. Clinical-scale production of Aspergillus-specific T cells for the treatment of invasive aspergillosis in the immunocompromised host. Bone Marrow Transplant 2019; 54:1963–72. [DOI] [PubMed] [Google Scholar]
- 10. Papadopoulou A, Koukoulias K, Alvanou M, et al. Multipathogen-specific T cells against viral and fungal infections. Bone Marrow Transplant 2021; doi:10.1038/s41409-020-01210-9. [DOI] [PubMed] [Google Scholar]
- 11. Baden LR, El Sahly HM, Essink B, et al. ; COVE Study Group . Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N Engl J Med 2021; 384:403–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Polack FP, Thomas SJ, Kitchin N, et al. ; C4591001 Clinical Trial Group . Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N Engl J Med 2020; 383:2603–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ramasamy MN, Minassian AM, Ewer KJ, et al. ; Oxford COVID Vaccine Trial Group . Safety and immunogenicity of ChAdOx1 nCoV-19 vaccine administered in a prime-boost regimen in young and old adults (COV002): a single-blind, randomised, controlled, phase 2/3 trial. Lancet 2021; 396:1979–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Sadoff J, Le Gars M, Shukarev G, et al. Interim results of a phase 1–2a trial of Ad26.COV2.S Covid-19 vaccine. N Engl J Med 2021; doi:10.1056/NEJMoa2034201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Koukoulias K, Papadopoulou A, Papayanni PG, et al. Νοn-transplantable cord blood units as a source for adoptive immunotherapy of leukemia and a paradigm of circular economy in medicine. Br J Haematol 2021; doi: 10.1111/bjh.17464 [DOI] [PubMed] [Google Scholar]
- 16. De Biasi S, Meschiari M, Gibellini L, et al. Marked T cell activation, senescence, exhaustion and skewing towards TH17 in patients with COVID-19 pneumonia. Nat Commun 2020; 11:3434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 2020; 395:497–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Chen Z, John Wherry E. T cell responses in patients with COVID-19. Nat Rev Immunol 2020; 20:529–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Cañete PF, Vinuesa CG. COVID-19 makes B cells forget, but T cells remember. Cell 2020; 183:13–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Terpos E, Mentis A, Dimopoulos MA. Loss of anti–SARS-CoV-2 antibodies in mild Covid-19. N Engl J Med 2020; 383:1695. [DOI] [PubMed] [Google Scholar]
- 21. Dan JM, Mateus J, Kato Y, et al. Immunological memory to SARS-CoV-2 assessed for up to 8 months after infection. Science 2021; 371:eabf4063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Seow J, Graham C, Merrick B, et al. Longitudinal observation and decline of neutralizing antibody responses in the three months following SARS-CoV-2 infection in humans. Nat Microbiol 2020; 5:1598–607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Thieme CJ, Anft M, Paniskaki K, et al. Robust T cell response toward spike, membrane, and nucleocapsid SARS-CoV-2 proteins is not associated with recovery in critical COVID-19 patients. Cell Rep Med 2020; 1:100092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Garassino MC, Ribas A. At the crossroads: COVID-19 and immune-checkpoint blockade for cancer. Cancer Immunol Res 2021; 9:261–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Pezeshki PS, Rezaei N. Immune checkpoint inhibition in COVID-19: risks and benefits. Expert Opin Biol Ther 2021; doi:10.1080/14712598.2021.1887131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Simonovich VA, Burgos Pratx LD, Scibona P, et al. A randomized trial of convalescent plasma in Covid-19 severe pneumonia. N Engl J Med 2020; 384:619–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Bonifacius A, Tischer-Zimmermann S, Dragon AC, et al. COVID-19 immune signatures reveal stable antiviral T cell function despite declining humoral responses. Immunity 2021; 54:340–54.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Keller MD, Harris KM, Jensen-Wachspress MA, et al. SARS-CoV-2-specific T cells are rapidly expanded for therapeutic use and target conserved regions of the membrane protein. Blood 2020; 136:2905–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Grifoni A, Weiskopf D, Ramirez SI, et al. Targets of T cell responses to SARS-CoV-2 coronavirus in humans with COVID-19 disease and unexposed individuals. Cell 2020; 181:1489–501.e15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Mateus J, Grifoni A, Tarke A, et al. Selective and cross-reactive SARS-CoV-2 T cell epitopes in unexposed humans. Science 2020; 370:89–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Reynolds CJ, Swadling L, Gibbons JM, et al. Discordant neutralizing antibody and T cell responses in asymptomatic and mild SARS-CoV-2 infection. Sci Immunol 2020; 5:eabf3698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Mazzoni A, Maggi L, Capone M, et al. Cell-mediated and humoral adaptive immune responses to SARS-CoV-2 are lower in asymptomatic than symptomatic COVID-19 patients. Eur J Immunol 2020; 50:2013–24. [DOI] [PubMed] [Google Scholar]
- 33. Swadling L, Maini MK. T cells in COVID-19—united in diversity. Nat Immunol 2020; 21:1307–8. [DOI] [PubMed] [Google Scholar]
- 34. Grant PR, Garson JA, Tedder RS, Chan PKS, Tam JS, Sung JJY. Detection of SARS coronavirus in plasma by real-time RT-PCR. N Engl J Med 2003; 349:2468–9. [DOI] [PubMed] [Google Scholar]
- 35. Waghmare A, Campbell AP, Xie H, et al. Respiratory syncytial virus lower respiratory disease in hematopoietic cell transplant recipients: viral RNA detection in blood, antiviral treatment, and clinical outcomes. Clin Infect Dis 2013; 57:1731–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Choi SM, Xie H, Campbell AP, et al. Influenza viral RNA detection in blood as a marker to predict disease severity in hematopoietic cell transplant recipients. J Infect Dis 2012; 206:1872–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Alpalhão M, Ferreira JA, Filipe P. Persistent SARS-CoV-2 infection and the risk for cancer. Med Hypotheses 2020; 143:109882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Zhu N, Wang W, Liu Z, et al. Morphogenesis and cytopathic effect of SARS-CoV-2 infection in human airway epithelial cells. Nat Commun 2020; 11:3910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Hui KPY, Cheung MC, Perera RAPM, et al. Tropism, replication competence, and innate immune responses of the coronavirus SARS-CoV-2 in human respiratory tract and conjunctiva: an analysis in ex-vivo and in-vitro cultures. Lancet Respir Med 2020; 8:687–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Muftuoglu M, Olson A, Marin D, et al. Allogeneic BK virus-specific T cells for progressive multifocal leukoencephalopathy. N Engl J Med 2018; 379:1443–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. O’Reilly RJ, Prockop S, Hasan AN, Koehne G, Doubrovina E. Virus-specific T-cell banks for ‘off the shelf’ adoptive therapy of refractory infections. Bone Marrow Transplant 2016; 51:1163–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.