Abstract
Objective
This study aims to evaluate the performance of an antigen-based rapid diagnostic test (RDT) for the detection of the SARS-CoV-2 virus.
Methods
A cross-sectional study was conducted on 677 patients. Two nasopharyngeal swabs and 1 oropharyngeal swab were collected from patients. The RDT was performed onsite by a commercially available immune-chromatographic assay on the nasopharyngeal swab. The nasopharyngeal and oropharyngeal swabs were examined for SARS-CoV-2 RNA by real-time reverse-transcription quantitative polymerase chain reaction (RT-qPCR) assay.
Results
The overall sensitivity of the SARS-CoV-2 RDT was 34.5% and the specificity was 99.8%. The positive predictive value and negative predictive value of the test were 96.6% and 91.5%, respectively. The detection rate of RDT in RT-qPCR positive results was high (45%) for cycle threshold values <25.
Conclusion
The utility of RDT is in diagnosing symptomatic patients and may not be particularly suited as a screening tool for patients with low viral load. The low sensitivity of RDT does not qualify its use as a single test in patients who test negative; RT-qPCR continues to be the gold standard test.
Keywords: RDT, rapid antigen test, RT-qPCR, COVID-19
COVID-19 has influenced every life over the past year. The pandemic has resulted in worldwide lockdowns, bringing life to a standstill. The licensing of vaccines in many countries has brought hope. But until universal immunization is achieved, testing, tracking, and treating continue to be the only tools in our armamentarium to curb the community spread of SARS-CoV-2.1 The current diagnostic method for SARS-CoV-2 infection is focused on the identification of viral genome targets in respiratory specimens by real-time reverse-transcription quantitative polymerase chain reaction (RT-qPCR).2 The molecular tests are the most sensitive and specific methods for the detection of SARS-CoV-2, but the downside is that they are time-consuming and need specialized laboratories with skilled manpower.3 The delay in reporting results can lead to the inadvertent spread of disease in the community. These conditions have prompted the development of antigen-based rapid diagnostic tests (RDTs). The advantage of RDT is the availability of test results within 30 minutes without running any specialized instrument, making it an acceptable point-of-care test that relieves the workload in diagnostic hospitals and laboratories.4 However, the performance of these assays on the ground remains uncertain. The sensitivity of the RDT has been claimed to be between 30.2% and 81.8% across the world.1,5,6 The World Health Organization strongly encourages research into the efficiency and potential diagnostic usefulness of RDT.7
The RDT is being used extensively to detect and trace patients with COVID-19. This study was undertaken to evaluate the performance of antigen-based RDT for the detection of SARS-CoV-2 virus in screening asymptomatic patients and to establish the association between the results of a positive RDT and cycle threshold (Ct) values of RT-qPCR. In addition, this study aimed to understand the Ct value at which the RDT would be able to detect true positives.
Methods
A cross-sectional study was conducted at the largest COVID-19-dedicated hospital (Delhi, India) after clearance from the institutional ethics committee. Adult preoperative or asymptomatic patients seeking health care in the ophthalmology department for eye ailments and who wished to get themselves tested for COVID-19 were included in the study.8 Written informed consent was obtained from participants before enrollment. Two nasopharyngeal swabs (NPS) were collected from patients for testing (for RDT and RT-qPCR). An additional oropharyngeal swab (OPS) was collected from all patients for RT-qPCR. The NPS and OPS were placed together in a 3 mL tube of viral transport medium and were transported to a COVID-19 diagnostic laboratory in the department of microbiology at the same hospital within 4 hours of collection, maintaining the cold chain. The RDT was performed onsite by a trained technician as per the manufacturer’s instructions.
The SARS-CoV-2 antigen was detected by commercially available immune-chromatographic lateral flow assay (PathoCatch/ACCUCARE, Lab Care Diagnostics Private Ltd., Sari Gam, India) approved by the Indian Council of Medical Research (ICMR). Briefly, the nasopharyngeal swab was inserted into a prefilled extraction buffer tube that was provided with the kit for antigen extraction. The swab was squeezed and then removed after 10 to 15 seconds. With the help of a nozzle cap, 2 to 3 drops of the extracted specimen were put on the sample port of the test device. The result was read after 20 to 30 minutes. The appearance of the control line and the detection line was interpreted as a positive result.
For RT-qPCR, the RNA was extracted with the MagNA Pure 96 System (Roche Molecular Systems Inc., Pleasanton, CA). The extracted RNA was examined for SARS-CoV-2 RNA by a COVID-19 real-time PCR assay using an ICMR-approved commercial assay (SD Biosensor Inc., Republic of Korea). This assay utilizes primers and probes for the detection of the E gene and the RdRp (ORF1ab) gene of the SARS-CoV-2 virus. Results were read and interpreted, and Ct values for both genes were recorded. A Ct value up to 32 was known to indicate a positive result for the E/RdRp gene as per validation performed in earlier research.9
The data collected were entered into a Microsoft Excel spreadsheet. The sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV) were expressed as percentages. Cohen’s kappa statistics were used for determining the agreement between the RDT and RT-qPCR while the association of antigen positivity and Ct values was assessed. A receiver operating characteristic (ROC) curve was built to estimate the Ct cutoff value for RDT-positive specimens. A P value <.05 was considered statistically significant. Confidentiality and privacy were ensured at all stages of the study. Results of RDT and RT-qPCR were provided to patients through the ICMR portal.
Results
Between October 27 and November 23, 2020, 677 patients were enrolled in the study. Among these patients, 372 (54.9%) were men and 305 (45.1%) were women. The mean age of study participants was 44.78 ± 16.35 years (range,18–89 years). Thirty patients tested positive by antigen-based RDT. Viral RNA was detected by RT-qPCR in NPS and OPS collected from 84 patients. Concordant results (RT-qPCR+/RDT+) were observed in 29 specimens, whereas discordant results (RT-qPCR+/RDT–) were observed in 55 specimens (Table 1).
Table 1.
Comparison of Results of SARS-CoV-2 Antigen-Based RDT with RT-qPCR (n = 677)
| RDT result | RT-qPCR Result | Total | |
|---|---|---|---|
| Negative | Positive | ||
| Negative | 592 (99.8%) | 55 (65.5%) | 647 |
| Positive | 1 (0.2%) | 29 (34.5%) | 30 |
| Total | 593 | 84 | 677 |
RDT, rapid diagnostic test; RT-qPCR, reverse-transcription quantitative polymerase chain reaction.
The overall sensitivity of the SARS-CoV-2 antigen based RDT was 34.5% (95% confidence interval [CI], 24.5%–45.6%) and the specificity was 99.8% (95% CI, 99.1%–100.0%). The PPV and NPV of the test were 96.6% (95% CI, 80.0%–99.5%) and 91.5% (95% CI, 90.2%–92.6%), respectively. The diagnostic accuracy of the test was found to be 91.7% with moderate agreement between the 2 methods (Cohen’s kappa index = 0.47).
Association of SARS-CoV-2 Antigen-Based RDT Results with Ct Values
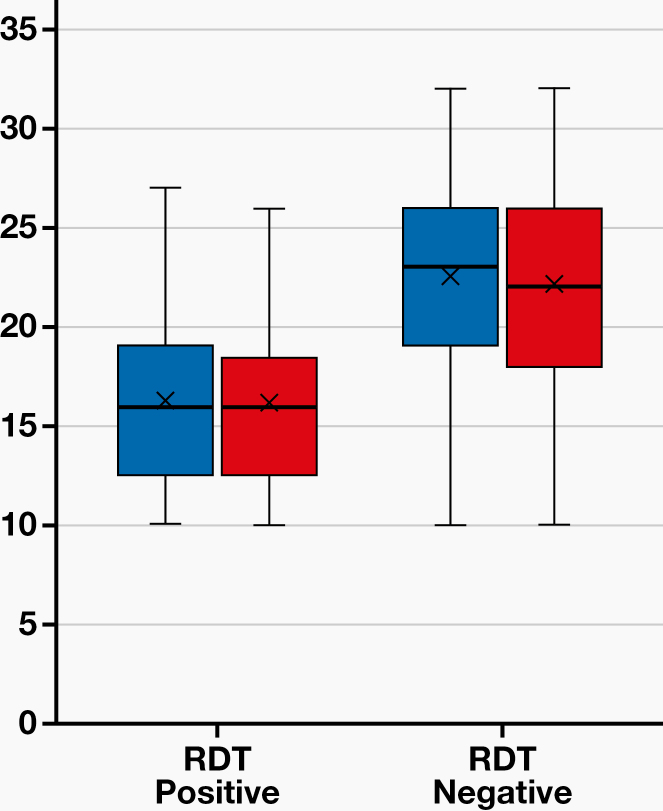
For the 55 specimens that tested positive by RT-qPCR, the Ct values ranged between 10 and 32 (Figure 1). A significant negative association was observed between the RDT results and the Ct values: Lower Ct values were associated with RDT positivity, and vice versa (P <.001; Table 2). The detection rate of the RDT in RT-PCR positive specimens was high (45%) for Ct values <25 (Table 3).
Figure 1.
Association of Ct values with RTD results in positive RT-PCR results. Ct, cycle threshold; RTD, rapid diagnostic test; RT-PCR, reverse-transcription polymerase chain reaction.
Table 2.
Association of Ct Values with RDT Results in Patients with Positive RT-PCR Results
| RDT-Negative (n = 55) | RDT-Positive (n = 29) | P Value | |
|---|---|---|---|
| Ct value (E gene) | 22.62 (21.20–24.04) | 16.24 (14.40–18.08) | <.001* |
| Range | 10–32 | 10–27 | |
| Ct value (RdRp gene) | 22.24 (20.76–23.71) | 16.10 (14.36–17.84) | <.001* |
| Range | 10–32 | 10–26 |
Ct, cycle threshold; RDT, rapid diagnostic test; RT-PCR, reverse-transcription polymerase chain reaction. *Significant at P <.05.
Table 3.
Detection Rate of RDT According to Ct Values in Patients with Positive RT-PCR Results
| Ct Value | RDT-Positive (n) | RDT Detection Rate (%) | |
|---|---|---|---|
| 1 | <25 (n = 60) | 27 | 45 |
| 2 | 25–29 (n = 21) | 2 | 9.5 |
| 3 | >30 (n = 3) | 0 | 0 |
Ct, cycle threshold; RDT, rapid diagnostic test; RT-PCR, reverse-transcription polymerase chain reaction.
Estimation of Ct Cutoff Value for RDT to Detect SARS-CoV-2 Antigen
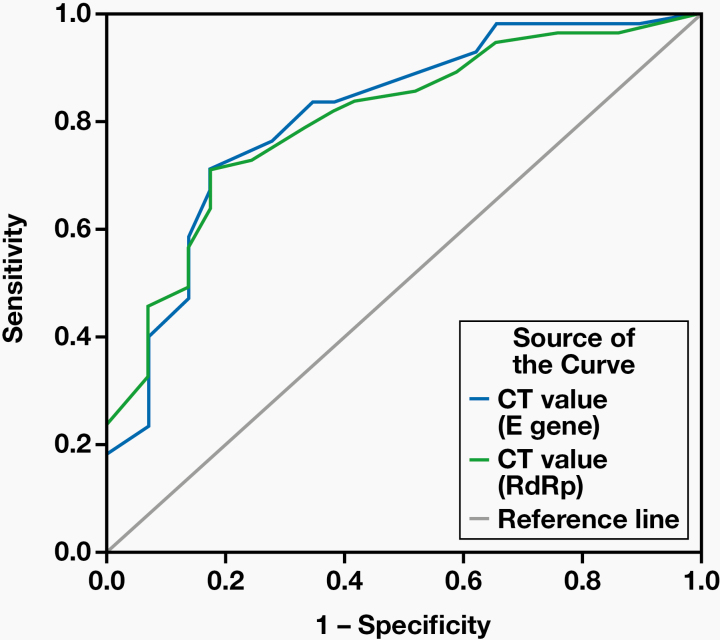
An ROC curve analysis indicated that a Ct value (E gene and RdRp gene) <19.5 best discriminated between RT-qPCR+/RDT+ and RT-qPCR+/RDT– specimens, with a sensitivity and specificity of 70.9% and 82.8%. The area under the curve for the E gene and the RdRp gene were 0.81 (95% CI, 0.71–0.91) and 0.80 (95% CI, 0.71–0.89), respectively (Figure 2).
Figure 2.
ROC curve using Ct values to estimate Ct cutoff value for RDT to detect SARS-CoV-2 antigen. Ct, cycle threshold; RDT, rapid diagnostic test; ROC, receiver operating characteristic.
Discussion
The diagnostic performance of SARS-CoV-2 antigen-based RDT with RT-qPCR as the gold standard was evaluated in our health care setting. The sensitivity of RDT (34.5%) observed in our study was lower than that claimed by the manufacturer (84%). A low sensitivity of an antigen-based assay with false-negative results has been reported previously.4,5,10 The Scohy et al5 evaluation of RDT showed an overall sensitivity of 30.2% for SARS-CoV-2 RT-qPCR–positive specimens. Mak et al4 evaluated RDT using different respiratory specimens such as nasopharyngeal aspirate, throat swab, saliva, and sputum. A very low positivity of RDT was observed in the sputum (11.1 %) of patients who were RT-qPCR–positive, whereas 45.7% of nasopharyngeal aspirate and throat swabs were positive.4
The Ct value indirectly indicates the initial virus concentration in the specimen, so it can be used as a substitute for the viral load. In this study, the lower Ct values were associated with a higher antigen detection rate. The Ct cutoff value for RDT positivity was 19.5, implying that RDT positivity occurred more in specimens with a high viral load. Therefore, there are more likely chances of missing positive specimens with a low viral load if only RDT is used for a diagnosis of COVID-19. Similar observations regarding the association of Ct value with RDT results were seen in previous studies.1,4,11-13 It is understood that antigens are expressed only when the virus is actively replicating.7 This may not be the scenario with patients in the presymptomatic phase or in those seeking routine health care, so the utility of rapid antigen tests is very limited in such patients. A similar situation is also a possibility in patients who are convalescing or asymptomatic. Further, the high sensitivity (70%) and specificity (95%) of RT-qPCR14 enable the test to detect very low levels of the virus, which could be a reason for the detection of a greater number of infections by RT-PCR.
Diagnostics for COVID-19 can be used for the triage of symptomatic individuals in an epidemic or endemic setting, screening of at-risk asymptomatic and symptomatic individuals in an epidemic or endemic setting, confirmatory testing, and testing of patients with previous exposure to SARS-CoV-2.15 Even with its less-than-optimal performance and limitations, the rapid test can still act as an adjunct to RT-qPCR testing.16 It can also be useful in diagnosing symptomatic patients with a high viral load in resource-limited settings. Faster detection enables public health authorities in rapid contact tracing and isolation along with providing specific care.
In a hospital setting, there is a potential risk of disease exposure to health care workers, other uninfected patients, and attendants from an infected patient. The RDT is used as a point-of-care test providing rapid results. Aside from turnaround time, however, the diagnostic accuracy of the test is also paramount. A test with high sensitivity and specificity should be used in such scenarios. The high specificity of RDT offers a quick screening for COVID-19 positivity, especially in patients with a high viral load. The overall sensitivity of RDT in our study was low, so it can give false-negative results and may not be of much help in screening patients if used alone. The challenge is to explore other options and weigh their pros and cons. Cartridge-based nucleic acid amplification tests have a quick turnaround time (30–60 minutes), but these tests require expertise, reagents, and instruments. These tests are also limited by the maximum number of specimens that can be tested per day: 24 to 48 specimens only.
The performance of any test depends upon various epidemiological and technical factors such as clinical manifestations, duration of disease onset to testing, type of specimen, specimen quality, specimen handling and processing techniques, commercial kit, and the batch of the kit used.7 Other factors that can affect the quality of testing are high patient load, fewer staff members, and limited resources. The training of technical staff involved in specimen collection and processing is also very essential and plays a key role in improving the quality of testing.
Conclusion
The high specificity of RDT warrants it as a diagnostic tool in patients with a positive result for SARS-CoV-2 infection. However, its low sensitivity does not qualify its use as a standalone test, especially in asymptomatic or presymptomatic patients, for whom RT-qPCR continues to be the gold-standard test. The utility of RDT is in diagnosing symptomatic patients and may not be particularly suited as a screening tool for patients with a low viral load.
Glossary
Abbreviations
- RDT
rapid diagnostic test
- RT-qPCR
reverse-transcription quantitative polymerase chain reaction
- Ct
cycle threshold
- NPS
nasopharyngeal swab
- OPS
oropharyngeal swab
- ICMR
Indian Council of Medical Research
- PPV
positive predictive value
- NPV
negative predictive value
- ROC
receiver operating characteristic
- CI
confidence interval
References
- 1. Albert E, Torres I, Bueno F, et al. Field evaluation of a rapid antigen test (Panbio™ COVID-19 Ag Rapid Test Device) for COVID-19 diagnosis in primary healthcare centres. Clin Microbiol Infect. 2021;27(3):472.e7–472.e10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Goudouris ES. Laboratory diagnosis of COVID-19. J Pediatr (Rio J). 2021;97(1):7–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Tromberg BJ, Schwetz TA, Pérez-Stable EJ, et al. Rapid scaling up of Covid-19 diagnostic testing in the United States—the NIH RADx initiative. N Engl J Med. 2020;383(11):1071–1077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Mak GC, Cheng PK, Lau SS, et al. Evaluation of rapid antigen test for detection of SARS-CoV-2 virus. J Clin Virol. 2020;129:104500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Scohy A, Anantharajah A, Bodéus M, Kabamba-Mukadi B, Verroken A, Rodriguez-Villalobos H. Low performance of rapid antigen detection test as frontline testing for COVID-19 diagnosis. J Clin Virol. 2020;129:104455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Gupta A, Khurana S, Das R, et al. Rapid chromatographic immunoassay-based evaluation of COVID-19: a cross-sectional, diagnostic test accuracy study and its implications for COVID-19 management in India. Ind J Med Res. Published online October 31, 2020. doi: 10.4103/ijmr.IJMR_3305_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. World Health Organization. Advice on the use of point-of-care immunodiagnostic tests for COVID-19. Published April 8, 2020. https://www.who.int/news-room/commentaries/detail/advice-on-the-use-of-point-of-care-immunodiagnostic-tests-for-covid-19. Accessed March 31, 2021.
- 8.Indian Council of Medical Research. COVID-19: information of testing strategies. https://www.icmr.gov.in/cteststrat.html. Accessed March 31, 2021.
- 9. Siddiqui O, Manchanda V, Yadav A, et al. Comparison of two real-time polymerase chain reaction assays for the detection of severe acute respiratory syndrome-CoV-2 from combined nasopharyngeal-throat swabs. Indian J Med Microbiol. 2020;38(3–4):385–389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Blairon L, Wilmet A, Beukinga I, Tré-Hardy M. Implementation of rapid SARS-CoV-2 antigenic testing in a laboratory without access to molecular methods: experiences of a general hospital. J Clin Virol. 2020;129:104472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Cerutti F, Burdino E, Milia MG, et al. Urgent need of rapid tests for SARS CoV-2 antigen detection: evaluation of the SD-Biosensor antigen test for SARS-CoV-2. J Clin Virol. 2020;132:104654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Lambert-Niclot S, Cuffel A, Le Pape S, et al. Evaluation of a rapid diagnostic assay for detection of SARS-CoV-2 antigen in nasopharyngeal swabs. J Clin Microbiol. 2020;58(8):e00977-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Nagura-Ikeda M, Imai K, Tabata S, et al. Clinical evaluation of self-collected saliva by quantitative reverse transcription-PCR (RT-qPCR), direct RT-qPCR, reverse transcription-loop-mediated isothermal amplification, and a rapid antigen test to diagnose COVID-19. J Clin Microbiol. 2020;58(9):e01438-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Watson J, Whiting PF, Brush JE. Interpreting a Covid-19 test result. BMJ. 2020;369:m1808. [DOI] [PubMed] [Google Scholar]
- 15. Vandenberg O, Martiny D, Rochas O, van Belkum A, Kozlakidis Z. Considerations for diagnostic COVID-19 tests. Nat Rev Microbiol. 2021;19(3):171–183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Chaimayo C, Kaewnaphan B, Tanlieng N, et al. Rapid SARS-CoV-2 antigen detection assay in comparison with real-time RT-PCR assay for laboratory diagnosis of COVID-19 in Thailand. Virol J. 2020;17(1):177. [DOI] [PMC free article] [PubMed] [Google Scholar]