Since December 2019, a novel coronavirus, named SARS-CoV-2, has caused a worldwide outbreak of respiratory illness termed COVID-19 (Corona Virus Disease-19).1 Such pandemic has induced governments to promote strict containment measures to reduce the spread of SARS-CoV-2 (Severe Acute Respiratory Syndrome Corona Virus 2), preventing patients from accessing healthcare services and thus impairing their regular follow-up with potential negative consequences on cardiovascular prevention.
In addition, during lockdowns, changes in daily activities, different dietary regimens, and stress might have had an additional impact on the patient’s cardiovascular risk profile and, in particular, on blood pressure (BP) control. Based on these premises, we sought to evaluate the possible occurrence of changes in home BP (HBP) during the COVID-19-related lockdown in a cohort of hypertensive patients regularly followed up by our Hypertension Clinic in Milan, Italy.
Consecutive adult patients with arterial hypertension, as defined by office systolic BP ≥140 mmHg and/or diastolic BP ≥90 mmHg or the presence of antihypertensive treatment, were recruited and followed up by phone. All patients followed up by our Hypertension Unit are routinely instructed to follow the standard rules for HBP monitoring2 and to fill an HBP logbook where the aforementioned rules are present and where HBP values have to be reported. During telephone follow-ups, each patient was asked to report to the investigator at least three morning BP measurements over a period of 2 weeks before and after 22 March 2020 (the day when the Government Decree ordering strict containment measures in the whole Italian country was published). The average of an additional set of at least three HBP measurements, recorded over a 2-week period during the corresponding time window of the previous year, selected because of similar environmental temperature, was taken as reference BP level. Patients who reported changes in antihypertensive treatment over the time period considered in our study were not included in this analysis. Adherence to treatment was systematically assessed during telephone consultations using an analogue scale from 1 to 14. By considering the reference period of the previous year and the 2-week period immediately preceding the lockdown, patients were classified according to their HBP control status in those always uncontrolled (uncontrolled HBP reference and uncontrolled pre-lockdown), those with unstable BP control (controlled HBP reference and uncontrolled pre-lockdown, or vice versa), and those always controlled. Patients were considered controlled if their HBP was <135/85 mmHg.2 Current antihypertensive medication regimen was also recorded. The study was approved by the local Ethics Committee of Istituto Auxologico Italiano (Ref. number 2020_04_21_09).
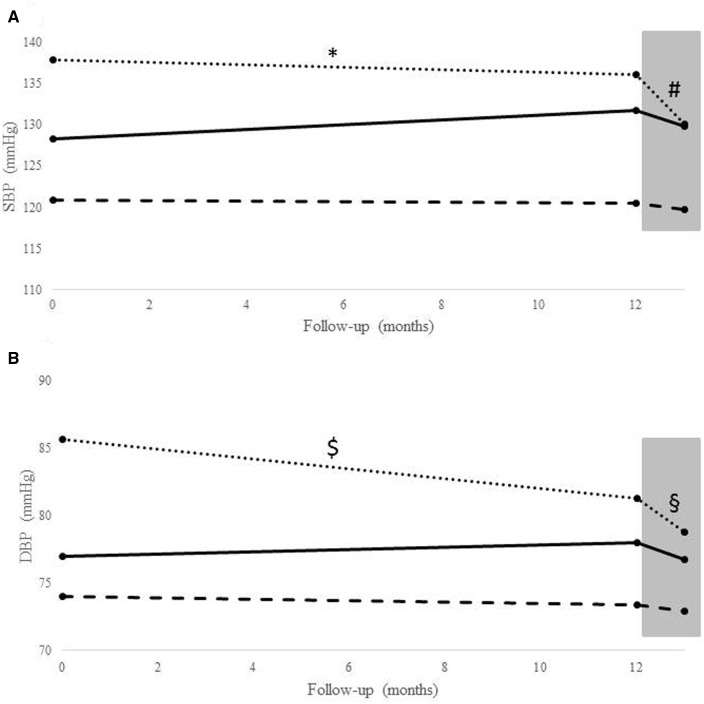
A total of 126 patients were included, whose main characteristics are summarized in Table 1. Adherence to treatment was adequate in all patients enrolled (12.8/14 vs. 13.9/14 days), and no significant body weight variation was seen over the time period of interest. In the whole group, patients during lockdown exhibited lower systolic and diastolic HBP values compared to the pre-lockdown period [123.23 vs. 125.05 mmHg, P = 0.008 for systolic blood pressure (SBP) and 74.45 vs. 75.28 mmHg, P = 0.023 for diastolic blood pressure (DBP)]. In Figure 1, average SBP (A) and DBP (B) values for the above described three different control status groups are shown for the time windows selected during the year before lockdown, in pre-lockdown period and during lockdown. Patients with uncontrolled HBP showed the most consistent drop of systolic [136.06 (8.36) vs. 130 (9.35), P = 0.001] and diastolic [81.30 (6.75) vs. 78.78 (9.25), P = 0.018] HBP from pre-lockdown to lockdown.
Table 1.
Main characteristics of the included patients at the time of inclusion in our study
| Variable | Whole cohort (N = 126) | Uncontrolled (N = 16) | Unstable control (N = 29) | Controlled (N = 81) | P-value |
|---|---|---|---|---|---|
| Baseline characteristics | |||||
| Age (years), median [IQ] | 66 [58–72] | 71 [61–76.5] | 69 [66–77] | 63 [55–70] | 0.003a |
| Male, n (%) | 47 (37.30) | 9 (56.25) | 8 (27.59) | 30 (37.04) | 0.163b |
| BMI (kg/m2), median [IQ] | 26.31 [23.89–28.76] | 28.30 [24.26–31.56] | 26.96 [25.00–30.19] | 26.03 [23.51–28.09] | 0.101a |
| Reference systolic BP, mean (SD) | 124.78 (9.90) | 137.91 (11.14) | 128.31 (8.97) | 120.93 (6.91) | <.0001c |
| Reference diastolic BP, mean (SD) | 76.19 (8.10) | 85.61 (7.31) | 76.98 (8.66) | 74.04 (6.59) | <.0001c |
| Smokers, n (%) | 27 (21.43) | 8 (50.00) | 8 (27.59) | 11 (13.58) | 0.003 b |
| Type II diabetes, n (%) | 23 (18.25) | 2 (12.50) | 7 (24.14) | 14 (17.28) | 0.583b |
| Dyslipidaemia, n (%) | 47 (37.30) | 8 (50) | 16 (55.17) | 23 (28.40) | 0.020b |
| Previous cardiovascular events, n (%) | 19 (15.08) | 3 (18.75) | 5 (17.24) | 11 (13.58) | 0.810d |
| CCB, n (%) | 52 (57.14) | 9 (64.29) | 14 (56.00) | 29 (55.77) | 0.841b |
| Beta-blockers, n (%) | 31 (34.07) | 6 (42.86) | 7 (28.00) | 18 (34.62) | 0.638b |
| Diuretics, n (%) | 39 (42.86) | 7 (50.00) | 14 (56.00) | 18 (34.62) | 0.174b |
| ACE inhibitors, n (%) | 25 (27.45) | 1 (7.14) | 9 (36.00) | 15 (28.85) | 0.145b |
| ARB, n (%) | 44 (48.35) | 10 (71.43) | 11 (44.00) | 23 (44.23) | 0.194d |
| Alpha-blockers, n (%) | 12 (13.19) | 1 (7.14) | 6 (24.00) | 5 (9.62) | 0.217d |
| Number of drugs, n (%) | |||||
| 1 | 25 (27.47) | 1 (7.14) | 8 (32.00) | 16 (30.77) | 0.072d |
| 2 | 32 (35.16) | 8 (57.14) | 4 (16.00) | 20 (38.46) | |
| 3 | 22 (24.18) | 3 (21.43) | 7 (28.00) | 12 (23.08) | |
| 4 | 12 (13.19) | 2 (14.29) | 6 (24.00) | 4 (7.69) | |
ACE, angiotensin-converting enzyme; BMI, body mass index; BP, blood pressure; IQ, interquartile range; SD, standard deviation.
Kruskal–Wallis.
Chi-square test.
ANOVA.
Fisher test.
Figure 1.
Mean values at baseline, pre-lockdown and during lockdown in the three groups for systolic (A) and diastolic (B) blood pressure (dotted line = uncontrolled blood pressure group, solid line = unstable blood pressure control, dashed line = controlled blood pressure group). *P < 0.001 (1 year before lockdown vs. lockdown systolic blood pressure), #P = 0.001 (pre-lockdown vs. lockdown systolic blood pressure), $P = 0.002 (1 year before lockdown vs. lockdown diastolic blood pressure), and §P = 0.018 (pre-lockdown vs. lockdown diastolic blood pressure). Grey box refers to the lockdown period. DBP, diastolic blood pressure.
The main finding of our study is that, during COVID-19 outbreak and associated lockdown, in spite of inability to access healthcare services, HBP was either similar or lower than during both pre-lockdown and a reference period selected over the corresponding time window the year before, these differences being most evident in those with uncontrolled BP.
There are many environmental factors known to influence BP such as physical and emotional challenges and the related stress.3 BP is importantly influenced by sympathetic nervous system activity, which is enhanced in stress-related situations as suggested by the neurogenic component to primary hypertension,4 and reduced in conditions of physical and psychological relaxation. Our results, in particular when considering patients with uncontrolled BP who indeed exhibited a clinically significant decrease in HBP values during lockdown, seem to suggest that the physical and psychological relaxation associated with lockdown prevailed over the COVID-19-related stressors in our cohort of hypertensive patients.
To the best of our knowledge, this is the first study assessing the actual effects of lockdown during COVID-19 on HBP control of hypertensive patients. Recently, considering a neighbouring field, Bonora et al.5 investigated the effects of lockdown on glycaemic control in patients with type 1 diabetes showing that glucose levels were better controlled during lockdown, thus suggesting that slowing down routine daily activities and a more regular and controlled food intake might have beneficial effects on glycaemic control.
Our study has some strengths: (i) important confounders such as medication changes over time and adherence to treatment were taken into account in the analysis and (ii) HBP values pre-lockdown were compared with reference values obtained in the corresponding time window (i.e. during the same season and with similar ambient temperature) of the previous year.
We have also to acknowledge some limitations such as the relatively small sample size, the lack of standardization in use of devices for HBP measurements (although all devices employed had been validated according to international protocols), and the additional possible interference by confounders such as dietary sodium intake, physical activity, sleep quality, and quantity that could not be addressed giving the retrospective nature of the study and the lack of objective monitoring. Moreover, our study was conducted in patients regularly followed up in our Hypertension Centre, highly compliant to BP medications. Therefore, such findings should be replicated in unselected hypertensive patients followed in general practice.
In conclusion, our study reports for the first time the lack of changes or even a reduction in HBP of treated hypertensive patients during lockdown due to COVID-19. Such HBP reduction was most evident in those patients with uncontrolled HBP before lockdown. These results, if confirmed in future larger prospective studies, may have implications for the management of patients with high BP not only during the current pandemic but also in case of future lockdown conditions. More studies on this topic are needed to better characterize predictors of HBP changes during lockdown to optimize the management of those patients more at the risk of uncontrolled BP.
Funding
Funded by the Italian Ministry of Health.
Conflict of interest: none declared.
Contributor Information
Martino F Pengo, Email: m.pengo@auxologico.it, Department of Cardiovascular, Neural and Metabolic Sciences, IRCCS Istituto Auxologico Italiano, Ospedale San Luca, Via Magnasco, 2, 20149, Milan, Italy.
Fabio Albini, “Misuriamo ICT platform”, Milan, Italy.
Giulia Guglielmi, Department of Cardiovascular, Neural and Metabolic Sciences, IRCCS Istituto Auxologico Italiano, Ospedale San Luca, Via Magnasco, 2, 20149, Milan, Italy.
Chiara Mollica, Department of Cardiovascular, Neural and Metabolic Sciences, IRCCS Istituto Auxologico Italiano, Ospedale San Luca, Via Magnasco, 2, 20149, Milan, Italy.
Davide Soranna, IRCCS Istituto Auxologico Italiano, Biostatistics Unit, Milan, Piazza dell'Ateneo Nuovo, 1 - 20126, Italy.
Gaia Zambra, IRCCS Istituto Auxologico Italiano, Biostatistics Unit, Milan, Piazza dell'Ateneo Nuovo, 1 - 20126, Italy.
Antonella Zambon, IRCCS Istituto Auxologico Italiano, Biostatistics Unit, Milan, Piazza dell'Ateneo Nuovo, 1 - 20126, Italy; Department of Statistics and Quantitative Methods, Università di Milano-Bicocca, Milan, Piazza dell'Ateneo Nuovo, 1 - 20126, Italy.
Grzegorz Bilo, Department of Cardiovascular, Neural and Metabolic Sciences, IRCCS Istituto Auxologico Italiano, Ospedale San Luca, Via Magnasco, 2, 20149, Milan, Italy; Department of Medicine and Surgery, University of Milano-Bicocca, Milan, Piazza dell'Ateneo Nuovo, 1 - 20126, Italy.
Gianfranco Parati, Department of Cardiovascular, Neural and Metabolic Sciences, IRCCS Istituto Auxologico Italiano, Ospedale San Luca, Via Magnasco, 2, 20149, Milan, Italy; Department of Medicine and Surgery, University of Milano-Bicocca, Milan, Piazza dell'Ateneo Nuovo, 1 - 20126, Italy.
References
- 1. Zhu N, Zhang D, Wang W, Li X, Yang B, Song J, Zhao X, Huang B, Shi W, Lu R, Niu P, Zhan F, Ma X, Wang D, Xu W, Wu G, Gao GF, Tan W.. A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med 2020;382:727–733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Parati G, Stergiou GS, Asmar R, Bilo G, de Leeuw P, Imai Y, Kario K, Lurbe E, Manolis A, Mengden T, O'Brien E, Ohkubo T, Padfield P, Palatini P, Pickering T, Redon J, Revera M, Ruilope LM, Shennan A, Staessen JA, Tisler A, Waeber B, Zanchetti A, Mancia G; ESH Working Group on Blood Pressure Monitoring. European Society of Hypertension guidelines for blood pressure monitoring at home: a summary report of the Second International Consensus Conference on Home Blood Pressure Monitoring. J Hypertens 2008;26:1505–1526. [DOI] [PubMed] [Google Scholar]
- 3. Parati G, Antonicelli R, Guazzarotti F, Paciaroni E, Mancia G.. Cardiovascular effects of an earthquake: direct evidence by ambulatory blood pressure monitoring. Hypertension 2001;38:1093–1095. [DOI] [PubMed] [Google Scholar]
- 4. Kivimäki M, Steptoe A.. Effects of stress on the development and progression of cardiovascular disease. Nat Rev Cardiol 2018;15:215–229. [DOI] [PubMed] [Google Scholar]
- 5. Bonora BM, Boscari F, Avogaro A, Bruttomesso D, Fadini GP.. Glycaemic Control Among People with Type 1 Diabetes During Lockdown for the SARS-CoV-2 Outbreak in Italy [published online ahead of print, 2020 May 11]. Diabetes Ther 2020;1–‐11. [DOI] [PMC free article] [PubMed] [Google Scholar]