Abstract
Background
The medium- and long-term effects of severe acute respiratory syndrome coronavirus 2 infection on survivors are unknown. In the current study, we assessed the medium-term effects of coronavirus disease 2019 (COVID-19) on survivors of severe disease.
Methods
This is a retrospective, case series of 200 patients hospitalized across 3 large Birmingham hospitals with severe-to-critical COVID-19 infection 4–7 months from disease onset. Patients underwent comprehensive clinical, laboratory, imaging, lung function tests (LFTs), and quality of life and cognitive assessments.
Results
At 4–7 months after disease onset, 63.2% of patients reported persistent breathlessness; 53.5%, significant fatigue; 37.5%, reduced mobility; and 36.8% pain. Serum markers of inflammation and organ injuries that persisted at hospital discharge had normalized on follow-up, indicating no sustained immune response causing chronic maladaptive inflammation. Chest radiographs showed complete resolution in 82.8%, and significant improvement or no change in 17.2%. LFTs revealed gas transfer abnormalities in 80.0% and abnormal spirometric values in 37.6% of patients. Compared with patients who did not experience breathlessness, those who did had significantly higher incidences of comorbid conditions and residual chest radiographic and LFT abnormalities (P < .01 to all). For all parameters assessed and persisting symptoms there were no significant differences between patients in hospital wards and those in intensive treatment units. All patients reported a significantly reduced quality of life in all domains of the EQ-5D-5L quality-of-life measures.
Conclusions
A significant proportion of severely ill patients with COVID-19 still experience symptoms of breathlessness, fatigue, pain, reduced mobility, depression and reduced quality of life 4–7 months after disease onset. Symptomatic patients tend to have more residual chest radiographic and LFT abnormalities.
Keywords: SARS-CoV-2, coronavirus
A case series of 200 patients hospitalised with severe-to-critical COVID-19 found persisting breathlessness and fatigue in over half of cases 4-7 months post disease-onset, with higher comorbidities, abnormal chest radiographic evidence and LFT without enduring evidence of active disease.
Coronavirus disease 2019 (COVID-19), caused by the severe acute respiratory syndrome (SARS) coronavirus (CoV) 2 (SARS-CoV-2), is a new illness with a global distribution and highly variable case fatality rate. At the time of writing, the World Health Organization estimates that approximately 137 million people have been infected worldwide and that there have been 2.9 million deaths [1]. Little is yet known about the medium- and long-term sequelae of severe COVID-19. Even individuals not admitted to the hospital are reporting a prolonged and debilitating set of symptoms after their acute episode, sometimes labeled as “long COVID” [2]. Patients hospitalized with COVID-19 most commonly experience pneumonitis and in many cases multiorgan involvement, requiring supplemental oxygen, invasive ventilation, and organ support [3].
While most survivors will have a full recovery, some may experience chronic physical, mental health, and social issues. Data from previous CoV (SARS-CoV and Middle East respiratory syndrome CoV) outbreaks indicate that 20%–40% of survivors experience medium- and long-term complications [4, 5]. In a 2020 report describing 143 patients with COVID-19 at a mean follow-up of 2 months, a high proportion still reported fatigue (53.5%), dyspnea (43.4%), joint pain (27.3%), or chest pain (21.7%) [6]. Other short-term studies have confirmed this observation [7].
The recovery trajectories are likely to be heterogeneous and may be influenced by different factors, including severity of the acute COVID-19 illness, duration of hospitalization, preexisting comorbid conditions, and patient age, sex, and ethnicity. There is an urgent need to follow up these patients carefully to elucidate the natural course of the disease, recognize and manage disease sequelae, support patients and care providers, and determine the driving mechanisms. Here, we report follow-up details, at 4–7 months after disease onset, in severely to critically ill patients seen in our multidisciplinary COVID-19 follow-up clinic.
METHODS
Study Setting and Participants
This study included 200 severely to critically ill patients with laboratory-confirmed SARS-CoV-2 infection, who were admitted for treatment in 3 large hospitals of the University Hospitals Birmingham National Health Service Foundation Trust, Birmingham, United Kingdom, between 2 March and 30 May 2020, and later invited to a follow-up clinic. Details of the multidisciplinary and multiprofessional clinic and inclusion and exclusion criteria were published elsewhere [8]. In brief, inclusion criteria included hospital admission for >3 days, with fraction of inspired oxygen >40% for >6 hours, new stroke, pulmonary embolism, deep venous thrombosis, delirium, elevated high-sensitivity troponin levels, residual acute kidney injury, or tachycardia (pulse rate >100/min) at discharge. Patients with mild to moderate disease who did not meet any of the above disease severity criteria, had a frailty score ≥6 at admission, or were discharged to residential or nursing care facilities were excluded.
The Clinical Frailty Scale (CFS) was adopted by the United Kingdom’s National Institute for Health and Care Excellence (NICE) in response to the COVID-19 outbreak to assess risk of outcome and ration the limited intensive treatment unit (ITU) capability [9]. This study was carried out as part of contemporaneous clinical service evaluation and registered with the local audit authority.
Data Collection
Demographic and clinical information was obtained from patient records and included the dates and spectrum of COVID-19 symptoms, inpatient treatment modalities, treatment on a ward or ITU, and any COVID-19–related complications. Furthermore, frailty was assessed using CFS scores (range, 1–7), compared with scores at admission. To assess subjective recovery from COVID-19 illness, participants were also asked a binary question regarding their perception of having returned to their state of health before disease onset. Using a Visual Analogue Scale (VAS), patients were asked to grade their overall recovery compared with their pre–COVID-19 state of health, on a scale ranging from 0% to 100%.
In addition, the EQ-5D-5L questionnaire was used to assess mobility, personal care, usual activities, pain, and anxiety/depression. The Medical Research Council dyspnea scale was used to assess the extent of breathlessness, and the Montreal Cognitive Assessment to screen for cognitive impairment.
Laboratory, Chest Radiographic, and unction Assessments
Follow-up blood tests included full blood cell count, coagulation profiles, serum biochemistry (including renal, liver, and bone profiles and measurement of ferritin, lactate dehydrogenase, C-reactive protein, and markers of cardiac injury [high-sensitive troponin, B-type natriuretic peptide, and creatine kinase]). SARS-CoV-2 antibody and throat swab specimens were obtained for SARS-CoV-2 polymerase chain reaction reexamination at follow-up. Spirometric parameters included forced expiratory volume in first second of expiration (FEV1), forced vital capacity (FVC), slow vital capacity, peak expiratory flow, and FEV1/FVC, and the single-breath carbon monoxide gas transfer test was used to measure the gas transfer coefficient (transfer factor of the lung for carbon monoxide [TLCO]). Values were considered abnormal if <80% of predicted or more than −1.64 standardized residual.
Routine chest radiography and high-resolution computed tomography (CT) was performed, per standard practice, and images were classified using the British Society of Thoracic imaging criteria [10], as follows: “Resolved” was defined as normal findings, no significant persisting COVID-19 changes, or return to the pre–COVID-19 baseline; “significantly improved,” as resolution of ≥50% of abnormalities (extent and/or density of opacification); “not significantly improved or unchanged,” as resolution of <50% abnormalities (extent and/or density of opacification); and “worsening,” as deteriorating alveolar opacity or as development of fibrosis, even with improvement in alveolar opacity.
Statistical Analysis
Continuous variables were described using mean values with standard deviations (SD) for parametric data and median values with interquartile range for nonparametric data. Categorical variables were reported as frequency with percentage. The mean differences between 2 groups were evaluated using Student t tests. Distributions of nonparametric data were compared using Mann-Whitney U tests for comparisons between 2 groups and Kruskal-Wallis test for comparisons between 3 groups. Associations between 2 groups were determined using χ 2 or Fisher exact tests, as appropriate. Statistical analyses were performed using SPSS software, version 27 (IBM). Statistical significance was set at P < .05.
RESULTS
Demographic and Clinical Characteristics
A total of 200 severely to critically ill patients with COVID-19 infection were assessed in the COVID-19 follow-up clinic at a mean (SD) of 143.4 (42.4) days after onset of the first COVID-19 symptoms. Of these, 87 patients (43.5%) required ITU admission, and 81 (40.5%) required invasive mechanical ventilation. Demographic and clinical characteristics of the study participants are summarized in Table 1. Their mean age (SD) was 56.5 (13.2) years, 62.5% were men, and 52% belonged to black, Asian, and other minority ethnic groups.
Table 1.
Demographic and Clinical Characteristics of Study Participants
| Characteristic | Participants, No. (%)a |
|---|---|
| Demographics (n = 200) | |
| Age, mean (SD) y | 56.5 (13.2) |
| ITU admission | 87 (43.5) |
| Sex | |
| Male | 125 (62.5) |
| Female | 75 (37.5) |
| Ethnicity | |
| White | 96 (48.0) |
| Asian or Asian British | 73 (36.5) |
| Black or Black British | 20 (10.0) |
| Other ethnic group | 11 (5.5) |
| Comorbid conditions (n = 170) | |
| Obesity | 80 (47.1) |
| Hypertension | 77 (45.3) |
| Diabetes mellitus | 65 (38.2) |
| Asthma | 38 (22.4) |
| Anxiety/depression | 21 (12.4) |
| Immunosuppression | 13 (7.6) |
| Cardiac disease | 24 (14.1) |
| Chronic kidney disease (stage III or above) | 14 (8.2) |
| Active cancer | 8 (4.7) |
| COPD | 9 (5.3) |
| Stroke | 8 (4.7) |
| Neuromuscular disorders | 7 (4.1) |
| Symptoms at admission (n = 157) | |
| Cough | 119 (75.8) |
| Dyspnea | 123 (78.3) |
| Fever | 106 (67.5) |
| Myalgia | 38 (24.2) |
| Tiredness | 45 (28.7) |
| Headache | 24 (15.3) |
| Diarrhea | 23 (14.6) |
| Loss of smell | 17 (10.8) |
| Loss of taste | 17 (10.8) |
| Additional complications during hospitalization (n = 200) | |
| Mechanical ventilation | 81 (40.5) |
| ITU-related delirium | 47 (23.5) |
| Acute kidney injury | 26 (13.0) |
| Arrhythmia | 14 (7.0) |
Abbreviations: COPD, chronic obstructive pulmonary disease; ITU, intensive treatment unit; SD, standard deviation.
aData represent no. (%) of participants, unless otherwise specified.
Comorbid conditions were present in nearly half of all patients, with obesity the most common (47.1%), followed by hypertension (45.3%), diabetes (38.2%), and asthma (22.4%). The mean duration of hospital stay (SD) was 22.7 (18.4) days. At admission, more than three-quarters of patients presented with dyspnea (78.3%) and cough (75.8%). Other common symptoms were fever (67.5%), tiredness (28.7%), myalgia (24.2%), headache (15.3%), diarrhea (14.6%), loss of taste (10.8%), and loss of smell (10.8%). ITU-related delirium occurred during hospitalization in 23.5% of patients, and stage III acute kidney injury in another 13%.
Ongoing Symptoms and Changes in CFS
At 4–7 months after disease onset, none of the patients had residual fever or any signs or symptoms of acute illness. More than half of still experienced some breathlessness (63.2%) and fatigue (53.5%); other common symptoms were reduced mobility (37.5%) and pain (36.8%). A transient cough was still present in 17.4% of patients. More than 20% of patients reported anxiety or low mood, sometimes associated with intrusive thoughts or flashbacks. Moreover, those who had psychological problems before contracting COVID-19 (12.4%) had a worsening of their symptoms during hospitalization and after discharge. In 12.5% of patients, some cognitive impairment was noted, mainly in concentration and short-term recall (Table 2).
Table 2.
Persistent Symptoms and Chest Radiographic and Lung Function Findings at Follow-up
| Symptoms and Findings | Patients, No. (%)a |
|---|---|
| Duration of symptoms (from onset to follow-up), mean (SD), d | |
| Total | 143.4 (42.4) |
| Ward patients | 124.4 (38.1) |
| ITU patients | 162.3 (38.1) |
| Symptoms at follow-up (n = 144) | |
| Breathlessness | |
| None at present—only the first few days/weeks after discharge | 53 (36.8) |
| Current breathlessness | |
| Any | 91 (63.2) |
| Only with strenuous exercise | 36 (25.0) |
| When hurrying on the level or up a slight hill | 25 (17.4) |
| When walking at own pace on the level surface | 30 (20.8) |
| Cough | |
| Any | 25 (17.4) |
| Transient cough before sleep | 20 (13.9) |
| Cough that can affect night sleep | 5 (3.5) |
| Fatigue | 77 (53.5) |
| Reduced mobility | 54 (37.5) |
| Pain | 53 (36.8) |
| Psychological issues | 29 (20.1) |
| Cognitive difficulties | 18 (12.5) |
| Sleeping disturbances | 21 (14.6) |
| Loss of smell | 10 (6.9) |
| Loss of appetite | 12 (8.3) |
| Loss of taste | 9 (6.3) |
| Follow-up chest radiographic findings (n = 180) | |
| Complete resolution | 149 (82.8) |
| Partial resolution | 29 (16.1) |
| No change | 2 (1.1) |
| Follow-up LFT findings (n = 85) | |
| Normal spirometric values | 53 (62.4) |
| Abnormal spirometric values | 32 (37.6) |
| Abnormal gas transfer | 68 (80.0) |
Abbreviations: ITU, intensive treatment unit; LFT, lung function test; SD, standard deviation.
aData represent no. (%) of patients, unless otherwise specified.
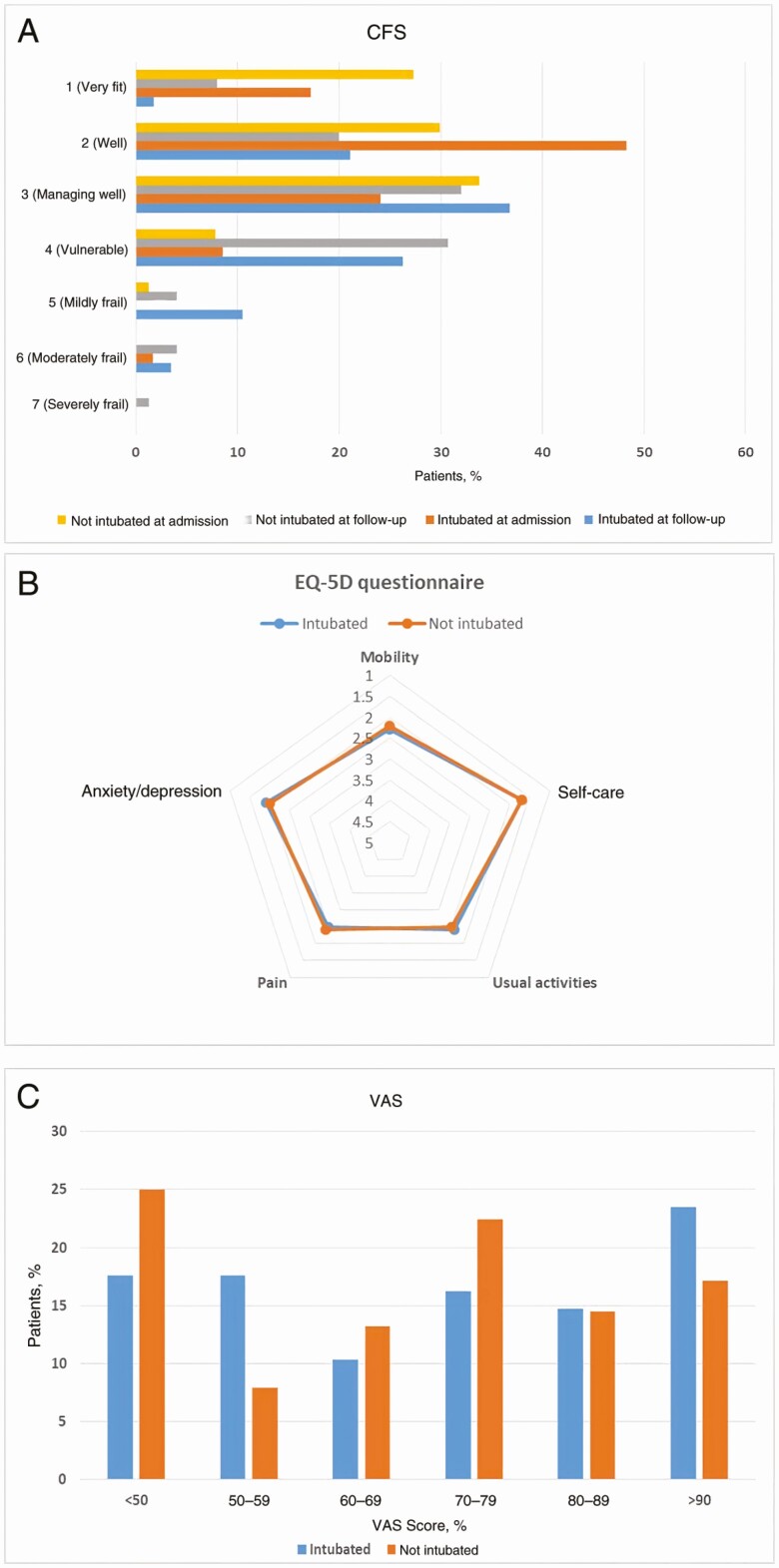
With the CFS, at admission 98% of patients scored between 1 (very fit) and 4 (vulnerable), and 68% of patients scored very fit or well (score, 1 or 2). In contrast, only 26.5 % scored 1 or 2 at follow-up, and 12.2 % became frail (score, ≥5). There was no significant difference between patients with and those without mechanical ventilatory support (Figure 1A).
Figure 1.
A, Clinical Frailty Scale (CFS) assessed at follow-up in 58 intubated and 77 nonintubated patients. B, Quality-of-life (EQ-5D-5L) questionnaire evaluated at follow-up in 75 intubated and 77 nonintubated patients. In this questionnaire, each domain is scored on a 5-point scale, where 1 indicates no problem; 2, slight problem; 3, moderate problem; 4, severe problem; and 5, unable to do. C, Visual Analogue Scale (VAS) evaluated at follow-up in 68 intubated and 76 nonintubated patients. There were no significant differences in CFS, EQ-5D-5L, and VAS scores between the 2 study groups.
Further analysis of a similar patient cohort admitted during the second COVID-19 wave in the United Kingdom, using identical clinical indices 3 months after hospital discharge, revealed no significant difference in the persistence of symptoms (see Supplementary Material). The second wave cohorts were all treated with dexamethasone and/or remdesivir, according to updated national guidance.
Self-Reported Quality-of-Life Measures
A high proportion of patients reported a significantly reduced quality of life (EQ-5D-5L in all 5 domains) (Figure 1B). However, no significant differences were found in any domain according to ITU or ward treatment (Figure 1B). As shown in Figure 1C, patients’ VAS grading of their overall recovery showed that only 17% reported a return to their pre–COVID-19 state of health. In addition, more than half reported a score <70%.
Laboratory, Imaging, and Lung Function Findings
At follow-up (Table 2), all patients underwent follow-up chest radiography, which showed complete resolution in 82.8% and partial resolution or no change in 17.2%. Lung function tests (LFTs) revealed mild abnormal gas transfer in 80% of patients and abnormal spirometric values in 37.6%. The abnormal features were consistent with restrictive lung disease, with the predominance of abnormalities in the TLCO, vital capacity, and alveolar volume.
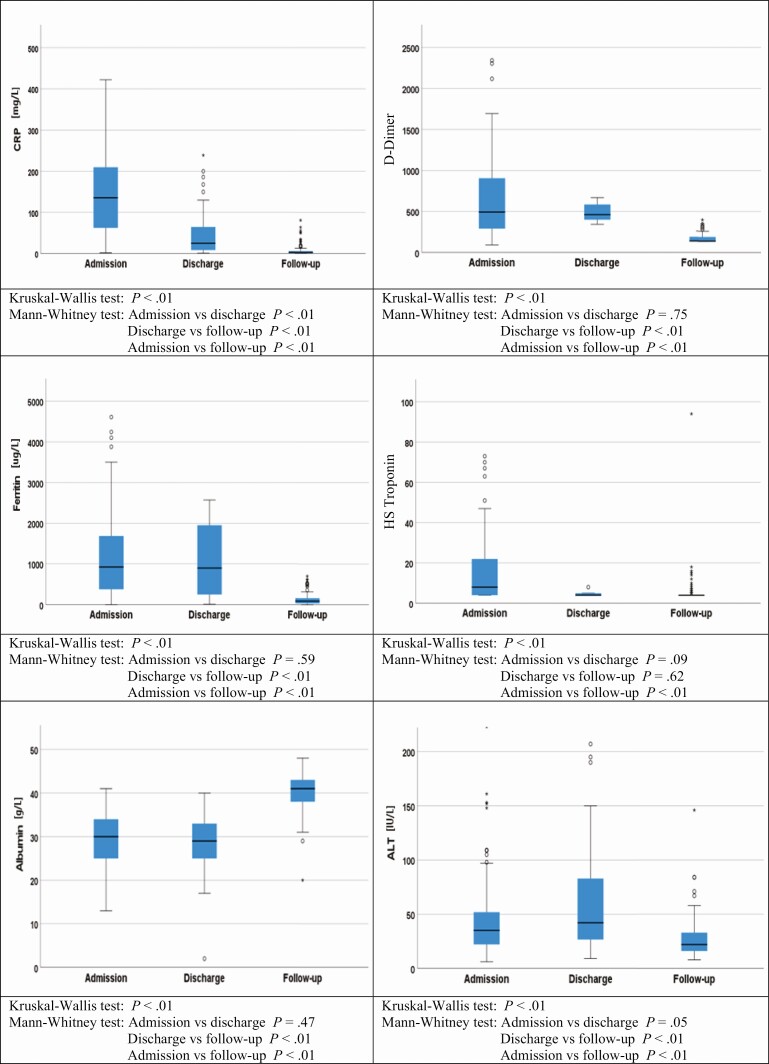
Figure 2 and Supplementary Table 2 show the distribution of blood tests results at admission, before discharge, and at follow-up. At a mean (SD) of 22.7 (18.4) days prior to discharge, a significant proportion of patients still had deranged inflammatory/coagulation and organ injury markers, such as C-reactive protein, serum ferritin, D-dimer, high-sensitivity troponin, albumin, and alanine aminotransferase. At follow-up all the blood marker levels had normalized (P < .01) (Supplementary Table 3). Brain natriuretic peptide levels were assessed in all patients during follow-up, and 7 patient had levels >400 ng/L (normal value, <400 ng/L). Among them, 4 patients had a history of ischemic heart disease or heart failure before admission.
Figure 2.
Inflammatory and organ injury markers levels at admission, discharge, and follow-up. Abbreviations: ALT, alanine aminotransferase; CRP, C-reactive protein; HS, high-sensitivity.
Breathlessness, Findings of LFTs and Chest Radiography, and COVID-19 Markers
Persistent breathlessness was a key finding in our cohort, so we performed a subanalysis. Seventy-six patients (38.2%) experienced new-onset symptoms of significant breathlessness (Medical Research Council dyspnea score ≥2). Table 3 shows clinical characteristics, LFT results, and chest radiographic findings in patients with or without shortness of breath. Patients with breathlessness tend to have higher rate of comorbid conditions and LFT and chest radiographic abnormalities (P < .01). In addition, of the 13 symptomatic patients who underwent high-resolution chest CT, 12 (92%) had patchy reticulation seen within the lungs, indicating mild focal fibrosis at follow-up. We found no significant difference in blood markers for inflammation or organ injury, nor in the state of their acute illness between the 2 groups (Supplementary Table 4). Persistent cardiac dysfunction was considered in all symptomatic patients, and their 12-lead resting electrocardiograms and BNP values showed no significant differences between the 2 groups.
Table 3.
Comorbid Conditions and Chest Radiographic and Lung Function Findings in Patients With or Without Shortness of Breath at Follow-up
| Comorbid Conditions and Findings | Patients, No. (%) | P Value | |
|---|---|---|---|
| No Shortness of Breath (n = 53) |
Shortness of Breath (n = 91) |
||
| Comorbid conditions | |||
| Obesity | 24 (45.3) | 46 (50.5) | <.01 |
| Hypertension | 21 (39.6) | 49 (53.8) | <.01 |
| Diabetes mellitus | 20 (37.7) | 37 (40.7) | <.01 |
| Asthma | 9 (16.9) | 22 (24.2) | <.01 |
| Coronary heart disease | 2 (3.8) | 9 (9.9) | <.01 |
| Other cardiac disorder | 1 (1.9) | 7 (7.7) | <.01 |
| COPD | 0 (0) | 8 (8.8) | <.01 |
| Interstitial lung disease | 0 (0) | 2 (2.2) | <.01 |
| Follow-up chest radiographic findings | |||
| Complete resolution | 48 (90.6) | 72 (79.1) | <.01 |
| Partial resolution | 5 (9.4) | 19 (20.9) | |
| Follow-up LFT findings | |||
| Normal spirometric values | 51 (96.2) | 46 (50.5) | <.01 |
| Abnormal spirometric values | 2 3 (8) | 45 (49.5) |
Abbreviations: COPD, chronic obstructive pulmonary disease; LFT, lung function test.
Discussion
The medium- and long-term sequelae of COVID-19 remain largely unknown. We found that just over half of patients who survived severe COVID-19 illness had persistent symptoms that interfered with activities of daily living and quality of life 4–7 months after their critical illness. Of those who were symptomatic, biochemical, hematological, and immunological findings had normalized during the follow-up periods. Chest radiographic and LFT findings were near normal to normal in most patients. However, patients with significant breathlessness tended to have high rate of comorbid conditions, residual chest radiographic abnormalities, and abnormal LFT results.
As reported above, breathlessness (63.2%) and fatigue (53.5%) were the symptoms most commonly reported by participants. However, the rate of significant breathlessness (38.2%) in our severely ill patients followed up for 7 months is lower than that reported in an earlier study (45%–53%) 2–3 months after disease onset [6, 7], which suggests that dyspnea caused by COVID-19 may improve over time. On the other hand, Huang et al [11] described the clinical follow-up in a cohort of 1733 adults with COVID-19, and 6 months after illness onset 76% of those patients reported ≥1 persistent symptom.
It is noteworthy that patents with persistent breathlessness tended to have a significantly more residual chest radiographic and an LFT abnormalities. In addition, there is an early indication of focal fibrosis on chest CT scans. Respiratory compromise in survivors of other CoV infections (SARS-CoV) was a key persisting symptom at 6 months [12]. Similarly, the impairment is mainly restrictive, with the predominance of abnormalities in the TLCO, vital capacity, and alveolar volume, compared with the FEV1, thereby suggesting parenchymal infiltrative damage during acute insult that might lead to pulmonary fibrosis. Furthermore, the absence of an inflammatory signature associated with persistent breathlessness is a striking positive finding, suggesting that there is no enduring evidence of active disease. Our study has excluded cardiac causes for shortness of breath.
Postviral fatigue is well recognized [13], and fatigue has been reported in 40% of individuals 1 year after initial infection with SARS-CoV-1 [14]. Consistent with this observation, we observed a high proportion of our patients still reporting fatigue after up to 7 months of follow-up. Fatigue is a complex symptom, overlapping with physical, mental, and social well-being and having a significant impact on an individual’s daily function. We observed an increased tendency to fatigue in patients who also experienced pain symptoms and had preexisting anxiety/depression. Longitudinal studies will be needed to assess the trajectory and persistence of fatigue as a form of chronic postviral fatigue syndrome. The other common persistent symptoms are myalgia and arthralgia. These are more notable in patients who were placed in a prone position during ITU admissions. In line with previous reports [15], we have observed a high-level of self-reported symptoms of anxiety, low mood, and depression among survivors. This is amplified in survivors with preexisting chronic disease or previous anxiety.
The semiquantitative CFS is a simple tool to assess patients’ frailty (diminished physiologic reserve), and scores has been shown to correlate with morbidity and mortality rates [16]. At the peak of the pandemic, all hospital-admitted patients were risk stratified, using the CFS as a tool to escalate therapy. Reflecting the national criteria used, 98% of survivors on their hospital admission using a 7-point grading scale scored ≤4, indicating a range between very fit and living with very mild frailty. However, at follow-up after 4–7 months of illness, 65% of patient described as very fit or well (scale, 1 or 2) progressed down on the frailty scale.
Similarly, the proportion of patients at the bottom of the scale has increased by 4-fold. Furthermore, 12.2% of the patients had scores of ≥5. This observation after 4–7 months of viral illness in a cohort with a mean age of 56.5 years and normal cognition is alarming, because frailty confers vulnerability that is highly associated with adverse health outcomes, increased falls, hospitalization, and death. There is also an additional burden for a person living with frailty, including impaired quality of life, unemployment, and loneliness. The functional disability appears to be out of proportion up to 7 months after recovery and may be due to additional factors, such as muscle deconditioning, multiorgan involvement of COVID-19, inflammation-related musculoskeletal complications, critical illness–related neuropathy or myopathy, and psychological factors. Further study is warranted to better understand the CFS trajectory in COVID-19 survivors.
The clinical course of severely ill patients with COVID-19 was characterized by arrays of increased inflammatory markers and grossly deranged biochemical, hematological, and imaging abnormalities. Often patients were discharged from the hospital as soon as they had shown clinical improvement rather than waiting for the normalization of abnormal markers. While it is clear that the extent of acute inflammatory response plays an important role in determining clinical outcomes during acute illness, reports of a chronic inflammatory response are also emerging [17, 18]. At mean duration of 22 days after discharge, abnormalities persisted in significant proportions of patients, in hemoglobin, C-reactive protein, ferritin, D-dimer, albumin, and alanine aminotransferase. At follow-up, all parameters had normalized. This finding presents no evidence to suggest a sustained response leading to chronic maladaptive inflammation that may be accompanied by further tissue damage.
Experience from earlier studies of SARS survivors has raised concerns about the long-term impact on the survivors’ quality of life [5]. Our patients reported a significantly reduced quality of life in all domains of the EQ-5D-5L. In contrast to the well-defined post-ITU syndrome, patients admitted to the ITU have an equal reduction in almost all reported domains with patients who were not admitted to ITU. Given that up to half of the study participants still report experiencing breathlessness, fatigue, myalgia, and anxiety, this finding may not be surprising. In addition, this reduced quality of life could potentially also be influenced by the severity of the acute illness, interventions including the need for mechanical ventilation, length of hospital stay, comorbid conditions, rehabilitation input in the community, ethnicity, and socioeconomic factors. Importantly, the decrement in both physical and emotional health may lead to unemployment, reduced household income, loss in self-confidence, and further deterioration in health.
The underlying mechanism of “long COVID” is unknown but is likely to be multifactorial and might include the residual effects of viral infection, ongoing complex immune inflammatory response, and the propensity for thromboembolic phenomena in targeted organ microcirculations, leading to endothelialitis [19–21]. Whether such a complex and varied postacute COVID-19 illness is transient and self-limiting or has the potential to lead to long-term sequelae will only become apparent with longer-term follow-up and further research.
This study has some limitations. First, it is a single-city, retrospective study with a relatively small sample, which curtails the generalizability of our findings and estimates of the prevalence of post–COVID-19 sequelae. However, this is the first study of the largest cohort of severely ill patients and longest follow-up to undertake holistic assessment of physical and mental well-being and comprehensive investigations of patients with COVID-19 after hospital discharge. Our findings of persistent symptoms 4–7 months after illness onset underscore the need for a larger longitudinal study, as currently planned by the Public Health England national consortium [21]. Secondly, our study cohort consists mostly of patients with severe-to-critical illness and patients discharged back to their area of residence before admission. Given the sheer number of hospital admissions with COVID-19, the follow-up clinic was set to cater to the cohort of the most severely ill. This may underestimate the extent of post–COVID-19 sequelae in the wider population.
The strength of our study lies in the larger number of severely ill patients and the longer follow-up to, with comprehensive assessment. In addition, we have developed a service delivering clinical care that is holistic, integrated, coordinated, person-centered, multidisciplinary, and multiprofessional.
In summary, most COVID-19 survivors had good recovery from their illness and complete resolution of laboratory and imaging abnormalities. A significant proportion of patients continue to experience ongoing symptoms of breathlessness, fatigue, pain, and reduced quality of life 4–7 months after disease onset. The proportion of patients with residual radiographic and LFT abnormalities is small but significant and will warrant ongoing follow-up to detect developing pulmonary fibrosis.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Acknowledgments. The authors thank all of the patients who have sought to help others understand the impact of coronavirus disease 2019 (COVID-19). They also thank Lynn Willetts for helping us set up the COVID-19 follow-up clinic, Kelly Watkins, Heather Small, Liz Wadsworth, and Adam Botkia for coordinating the clinic, and Dr James Stockley for coordinating and measuring the results of all lung function tests.
Disclaimer. This work uses data provided by patients and collected by the NHS as part of their care and support at University Hospitals Birmingham NHS Foundation Trust. It has been approved by University Hospitals Birmingham NHS Foundation Trust, Clinical Audit Registration & Management System and the COVID-19 research facilitation group under application reference CARMS-16371.
Potential conflicts of interest. All authors: No reported conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest.
References
- 1. World Health Organization. COVID-19 dashboard. Available at: https://www.who.int/covid-19. Accessed 24 November 2020.
- 2. Alwan NA, Attree E, Blair JM, et al. From doctors as patients: a manifesto for tackling persisting symptoms of COVID-19. BMJ 2020; 370:m3565. [DOI] [PubMed] [Google Scholar]
- 3. Zhou F, Yu T, Du R, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet 2020; 395:1054–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Hui DS, Joynt GM, Wong KT, et al. Impact of severe acute respiratory syndrome (SARS) on pulmonary function, functional capacity and quality of life in a cohort of survivors. Thorax 2005; 60:401–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ahmed H, Patel K, Greenwood DC, et al. Long-term clinical outcomes in survivors of severe acute respiratory syndrome and Middle East respiratory syndrome coronavirus outbreaks after hospitalization or ICU admission: a systematic review and meta-analysis. J Rehabil Med 2020; 52:jrm00063. [DOI] [PubMed] [Google Scholar]
- 6. Carfì A, Bernabei R, Landi F; Gemelli Against COVID-19 Post-Acute Care Study Group . Persistent symptoms in patients after acute COVID-19. JAMA 2020; 324:603–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Mandal S, Barnett J, Brill SE, et al. Long-COVID; a cross-sectional study of persisting symptoms, biomarker and imaging abnormalities following hospitalization for COVID-19. Thorax 2020; 0:1–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Geberhiwot T, Madathil S, Gautam N. After care of survivors of COVID-19—challenges and a call to action. JAMA Health Forum doi: 10.1001/jamahealthforum.2020.0994. Published 26 August 2020. [DOI] [PubMed] [Google Scholar]
- 9. National Institute for Health and Care Excellence (NICE ). COVID-19 rapid guideline: managing COVID-19 [NG191]. 20 March 2020. Available at: https://www.nice.org.uk/guidance/ng191. Accessed 15 May 2020.
- 10. British Society of Thoracic Imaging. COVID-19 chest x-ray classification. Available at: https://www.bsti.org.uk/media/resources/files/BSTI_COVID_CXR_proforma_v.3-1.pdf. https://www.bsti.org.uk/covid-19-resources/covid-19-bsti-reporting-templates/x. Accessed 24 June 2020.
- 11. Huang C, Huang L, Wang Y, et al. 6-Month consequences of COVID-19 in patients discharged from hospital: a cohort study. Lancet 2021; 397:220–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ng CK, Chan JW, Kwan TL, et al. Six month radiological and physiological outcomes in severe acute respiratory syndrome (SARS) survivors. Thorax 2004; 59:889–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Hickie I, Davenport T, Wakefield D, et al. ; Dubbo Infection Outcomes Study Group . Post-infective and chronic fatigue syndromes precipitated by viral and non-viral pathogens: prospective cohort study. BMJ 2006; 333:575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Lam MH, Wing YK, Yu MW, et al. Mental morbidities and chronic fatigue in severe acute respiratory syndrome survivors: long-term follow-up. Arch Intern Med 2009; 169:2142–7. [DOI] [PubMed] [Google Scholar]
- 15. Hoogendijk EO, Afilalo J, Ensrud KE, Kowal P, Onder G, Fried LP. Frailty: implications for clinical practice and public health. Lancet 2019; 394:1365–75. [DOI] [PubMed] [Google Scholar]
- 16. Bagshaw SM, Stelfox HT, McDermid RC, et al. Association between frailty and short- and long-term outcomes among critically ill patients: a multicentre prospective cohort study. CMAJ 2014; 186:E95–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Zhao YM, Shang YM, Song WB, et al. Follow-up study of the pulmonary function and related physiological characteristics of COVID-19 survivors three months after recovery. EClinicalMedicine 2020; 25:100463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Mazza MG, De Lorenzo R, Conte C, et al. ; COVID-19 BioB Outpatient Clinic Study group . Anxiety and depression in COVID-19 survivors: role of inflammatory and clinical predictors. Brain Behav Immun 2020; 89:594–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Verdecchia P, Cavallini C, Spanevello A, Angeli F. The pivotal link between ACE2 deficiency and SARS-CoV-2 infection. Eur J Int Med 2020; 76:14–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Wichmann D, Sperhake JP, Lütgehetmann M, et al. Autopsy findings and venous thromboembolism in patients with COVID-19: a prospective cohort study. Ann Intern Med 2020; 173:268–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Ackermann M, Verleden SE, Kuehnel M, et al. Pulmonary vascular endothelialitis, thrombosis, and angiogenesis in Covid-19. N Engl J Med 2020; 383:120–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. NIHR Leicester Biomedical Research Centre. PHOSP-COVID: Post-HOSPitalisation COVID-19 study—a national consortium to understand and improve long-term health outcomes. Available at: https://www.leicesterbrc.nihr.ac.uk/themes/respiratory/research/phosp-covid/. Accessed 31 October 2020.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.