Abstract
The coronavirus disease 2019 (COVID-19) pandemic and associated public health responses have disrupted daily living activities with economic and health consequences globally. We observed transient decreases in human immunodeficiency virus (HIV) clinic visit adherence and food security among persons living with HIV early in the pandemic, and an increase in viral suppression later in the pandemic.
Keywords: HIV, East Africa, West Africa, COVID-19 pandemic, food security
The coronavirus disease 2019 (COVID-19) pandemic is an unprecedented public health crisis. Governmental responses have varied in stringency and enforcement, sometimes including handwashing, physical distancing, face covering, lockdowns, curfews, and/or suspension of large gatherings and public transportation.
In sub-Saharan Africa, policies must precariously balance public health and economic consequences in settings with a high reliance on informal economic activity [1], insufficient social safety net systems [2], and densely populated cities or cramped living conditions that impede physical distancing and hygiene [3]. Failure to contain the COVID-19 pandemic could overwhelm underresourced African healthcare systems [4] and obstruct healthcare access for other conditions due to interrupted supply chains, healthcare provider redeployment or illness, and disrupted transportation [5]. People living with human immunodeficiency virus (PLWH) could be particularly vulnerable to such disruptions [6], with the potential for lifelong consequences if they lead to viral failure and emergent drug resistance. Financial, food security, and social losses could contribute to emotional distress, reduced mental well-being, unhealthy coping strategies, and noncompliance with mitigation measures [7, 8].
We assessed human immunodeficiency virus (HIV) care and food security in the context of the COVID-19 pandemic in 4 African countries.
METHODS
Since 2013, the African Cohort Study (AFRICOS) has prospectively enrolled individuals aged 15 years or older with and without HIV, in an approximate 5:1 ratio, at 12 clinics across 5 HIV care programs in Tanzania; Uganda; South Rift Valley, Kenya; Kisumu West, Kenya; and Nigeria [9]. Participants living with HIV were randomly selected from client lists, were new clinic enrollees, or were recruited from previous studies. Those without HIV were recruited from HIV testing activities or via serodiscordant partnership with an AFRICOS enrollee. The study was approved by institutional review boards at all participating institutions. All participants provided written informed consent.
At enrollment and biannual follow-up visits, participants underwent clinical assessment and socio-behavioral questionnaire administration by trained study staff. Self-reported variables included sex, age, education, employment status, marital status, and distance from the clinic. Food security was evaluated by asking participants if they had enough food to eat in the past 12 months and whether they had cut or reduced in size 1 or more meals per day on average because there was not enough food or money for food.
For PLWH, antiretroviral therapy (ART) history was extracted from medical records and viral load was assessed at each visit. Adherence to ART was defined as no self-reported missed doses of ART in the past 30 days. HIV clinic visit adherence was defined as no self-reported missed clinic visits in the past 6 months. During COVID-19, a recently added question was used to evaluate multi-month dispensing, defined by self-report of receiving at least 3 months of ART at last prescription, which is standard at all AFRICOS clinics for eligible PLWH. Depression was defined by a score of 16 or greater on the 20-item Center for Epidemiological Studies–Depression (CES-D) scale [10].
Study activities were paused on 19 March 2020 to establish safety procedures for streamlined telephonic and, subsequently, in-person visits. The first telephonic visit was on 7 May 2020 and abbreviated in-person visit on 26 May 2020; reopening dates varied by program.
The main comparison of interest was between study visits before and during the COVID-19 pandemic among participants with both types of visits. The pre–COVID-19 period was 1 January 2019 to 19 March 2020 and the COVID-19 period was 7 May 2020 to 28 February 2021. The median post–COVID-19 visit date, 7 September 2020, was used to split the COVID-19 period into early and late phases to assess potential differences over the course of the pandemic. Logistic regression with generalized estimating equations, clustered by participant to account for repeated measures, was used to estimate odds ratios (ORs) and 95% confidence intervals (95% CIs) comparing HIV care and food security before and during the pandemic. Models were stratified by HIV status and adjusted for age, sex, and program.
To evaluate for selection bias due to differential study participation during COVID-19, we described differences between participants with and without missed study visits in the COVID-19 period. Participants were classified as missing visits during COVID-19 if the last attended visit was before 19 March 2020 and more than 90 days had elapsed since a scheduled visit after study activities resumed. Study visits were not considered missed if scheduled when study activities were paused. Pearson’s chi-square and Wilcoxon rank-sum tests were used to compare these 2 groups, stratified by HIV status, using data from participants’ most recent visit before COVID-19.
All analyses were restricted to participants with a pre–COVID-19 visit on or after 1 January 2019 to reflect the population actively engaged with study visits. Analyses were performed using Stata 16.0 (StataCorp, College Station, TX).
RESULTS
Between 1 January 2019 and 28 February 2021, 2666 participants were actively engaged with AFRICOS, including 2280 (85.5%) PLWH and 386 (14.5%) participants without HIV. Of these, 1447 (63.5%) PLWH and 228 (59.1%) participants without HIV had visits before and during COVID-19 (Supplementary Table 1).
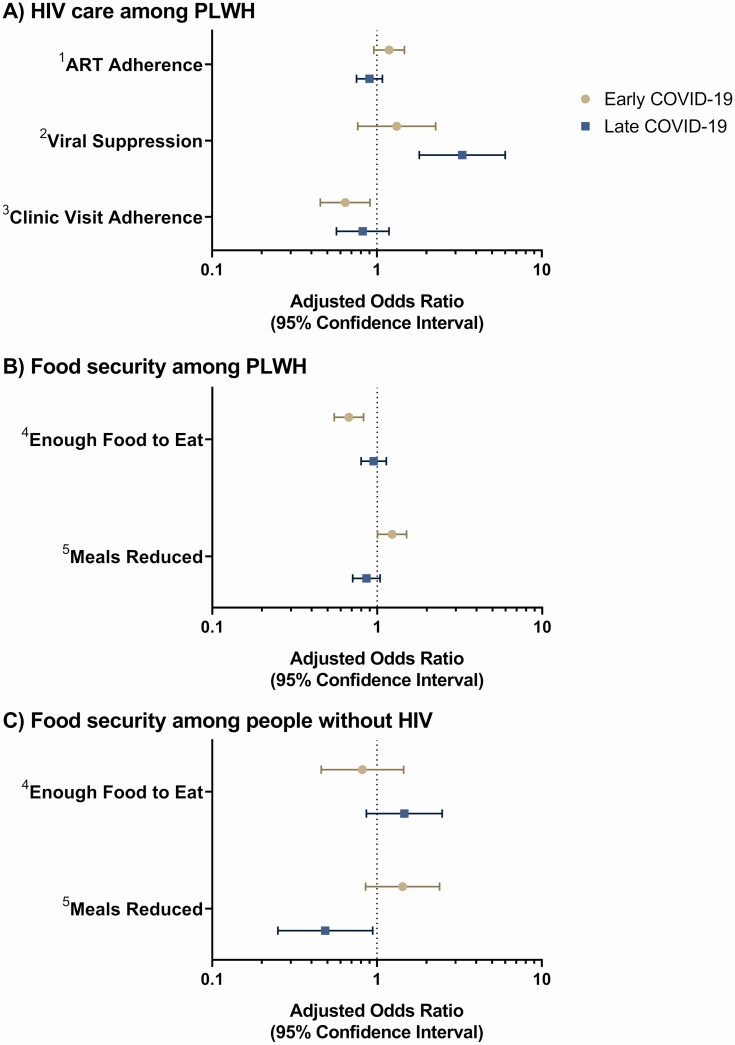
During the early COVID-19 period, PLWH were less likely to be adherent to HIV clinic visits (adjusted OR [aOR]: .64; 95% CI: .45–.91); however, this association was not significant in the late COVID-19 period (aOR: .82; 95% CI: .57–1.19). Although no significant associations of COVID-19 with ART adherence were observed, PLWH were more likely to be virally suppressed in the late COVID-19 period as compared with before the pandemic (aOR: 3.29; 95% CI: 1.81–6.00) (Figure 1A, Supplementary Tables 3–7).
Figure 1.
Adjusted ORs for associations between early and late COVID-19 periods and (A) HIV care among PLWH, (B) food security among PLWH, and (C) food security among people without HIV. The main comparison of interest was between study visits before and during the COVID-19 pandemic, among participants with both types of visits. The pre–COVID-19 period was 1 January 2019 to 19 March 2020 and the COVID-19 period was 7 May 2020 to 28 February 2021. The median post–COVID-19 visit date, 7 September 2020, was used to split the COVID-19 period into early and late phases to assess potential differences over the course of the pandemic. Logistic regression with generalized estimating equations, clustered by participant to account for repeated measures, was used to estimate ORs and 95% CIs comparing HIV care and food security before and during the pandemic. Models were stratified by HIV status and adjusted for age, sex, and program. Although all available data were utilized, only a subset of PLWH, those attending an in-person visit during COVID-19, had viral load data available during the COVID-19 period (n = 1392 [96.2%] with available viral load data pre–COVID-19 and n = 871 [60.2%] with viral load data available during COVID-19). Note: 1 participant seroconverted between their study visit before COVID-19 and their study visit during COVID-19 and was omitted from all analyses. 1ART adherence: defined as no self-reported missed doses of ART in the past 30 days. 2Viral suppression: defined as a viral load <1000 copies/mL after being on ART for ≥6 months. 3Clinic visit adherence: defined as no self-reported missed HIV clinic visits in the past 6 months; different than AFRICOS study visits. 4Enough food to eat: defined as having enough food to eat in the past 12 months. 5Meals reduced: defined as ≥1 meals cut or reduced in size per day on average because there was not enough food or money for food. Abbreviations: AFRICOS, African Cohort Study; ART, antiretroviral therapy; CI, confidence interval; COVID-19, coronavirus disease 2019; HIV, human immunodeficiency virus; OR, odds ratio; PLWH, people living with HIV.
Participants living with HIV were less likely to have had enough food to eat (aOR: .67; 95% CI: .55–.83) and more likely to have reduced the number or size of meals (aOR: 1.23; 95% CI: 1.00–1.51) in the early COVID-19 period; however, these associations did not remain significant in the late COVID-19 period (Figure 1B).
Participants without HIV were less likely to have reduced the number or size of meals in the late COVID-19 period as compared with before the pandemic (aOR: .49; 95% CI: .25–.94) (Figure 1C), but no significant associations were observed with regard to having enough food (Supplementary Tables 8 and 9).
Among 1181 PLWH with available data, 1047 (88.7%) had a 3 or more months’ supply of ART. Multi-month dispensing was similar for males and females (467 [90.7%] vs 580 [87.1%]; P = .06) but was more common among participants aged 30 and older compared with those younger than 30 years old (967 [90.5%] vs 80 [70.8%]; P < .001).
A total of 649 participants, including 546 (24.1%) PLWH and 103 (26.9%) participants without HIV did not present for any scheduled study visits during COVID-19 (Supplementary Table 2). As compared with PLWH who did not miss study visits during COVID-19, PLWH who missed study visits were, at their last visit before the pandemic, more likely to live 10+ km from the study clinic (60.1% vs 47.2%; P < .001), less likely to be employed (27.3% vs 40.7%; P < .001), less likely to have reported missed doses of ART (6.2% vs 11.0%; P < .01), and less likely to have a viral load less than 1000 copies/mL (87.5% vs 91.6%; P = .09). Among people without HIV, those who missed visits were more likely to be female (70.9% vs 50.4%; P < .001) and unemployed (68.9% vs 50.7%; P < .01).
DISCUSSION
In this cohort, we observed a transient decrease in HIV clinic visit adherence and food security among PLWH early in the COVID-19 pandemic and an increase in viral suppression later in the pandemic.
The COVID-19 pandemic and mitigation measures have highlighted the interconnectedness between food systems, socioeconomics, and health [11]. Consistent with other recent studies, we observed a temporary decrease in food security among PLWH early in the COVID-19 pandemic when control measures were most stringent [12, 13]. Although this association was temporary, the long-term effects of this period of heightened food insecurity remain unknown; food assistance for those affected by COVID-19 should remain a priority.
While ART adherence was unaffected by COVID-19, HIV clinic adherence temporarily decreased early in the pandemic. A mixed-methods study conducted in Uganda similarly observed a reduction in HIV clinic attendance associated with mobility reductions due to lockdowns and curfews. Likewise, they did not observe negative effects of COVID-19 on ART adherence [14]. Community-based HIV care and increased time at home may promote social support and foster environments conducive to routinized ART adherence. These systems should be strengthened even as the pandemic comes to an end.
It should be noted that participants enrolled in a clinic-based cohort study may have unique access to care during the pandemic and, although these clinic populations are typical of their regions, may not be representative of the general population. Participants who missed AFRICOS study visits during the pandemic were demographically and clinically different than those who did not miss study visits, potentially introducing selection bias into our analyses. Clinic-level heterogeneity, including differences in resumption of study activities during COVID-19, could have influenced the observed effects of the pandemic on study participants but could not be fully explored in these analyses.
The COVID-19 pandemic temporarily reduced food security and HIV clinic visit adherence among PLWH in this cohort. While ART adherence was unaffected and viral suppression increased, additional longitudinal data are needed to better understand any long-term effects of COVID-19.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Acknowledgments. The authors thank the study participants, local implementing partners, and hospital leadership at Kayunga District Hospital, Kericho District Hospital, AC Litein Mission Hospital, Kapkatet District Hospital, Tenwek Mission Hospital, Kapsabet District Hospital, Nandi Hills District Hospital, Kisumu West District Hospital, Mbeya Zonal Referral Hospital, Mbeya Regional Referral Hospital, Defence Headquarters Medical Center, and the 68th Nigerian Army Reference Hospital. The authors also thank the AFRICOS Study Group.
Disclaimer. The views expressed are those of the authors and should not be construed to represent the positions of the US Army or the Department of Defense. The investigators have adhered to the policies for protection of human subjects as prescribed in Army Regulation 70–25.
Financial support. This work was supported by the President’s Emergency Plan for AIDS Relief via a cooperative agreement between the Henry M. Jackson Foundation for the Advancement of Military Medicine, Inc, and the US Department of Defense (cooperative agreement number W81XWH-18–2–0040).
Potential conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
AFRICOS Study Group. The US Military HIV Research Program Headquarters team: Danielle Bartolanzo, Alexus Reynolds, Katherine Song, Mark Milazzo, Leilani Francisco, Shauna Mankiewicz, Steven Schech, Badryah Omar, Tsedal Mebrahtu, Elizabeth Lee, Kimberly Bohince, Jaclyn Hern, Kara Lombardi, Michelle Imbach, and Leigh Anne Eller; AFRICOS Uganda team: Michael Semwogerere, Prossy Naluyima, Godfrey Zziwa, Allan Tindikahwa, Hilda Mutebe, Cate Kafeero, Enos Baghendaghe, William Lwebuge, Freddie Ssentogo, Hellen Birungi, Josephine Tegamanyi, Paul Wangiri, Christine Nabanoba, Phiona Namulondo, Richard Tumusiime, Ezra Musingye, Christina Nanteza, Joseph Wandege, Michael Waiswa, Evelyn Najjuma, Olive Maggaga, Isaac Kato Kenoly, and Barbara Mukanza; AFRICOS South Rift Valley, Kenya team: Rither Langat, Aaron Ngeno, Lucy Korir, Raphael Langat, Francis Opiyo, Alex Kasembeli, Christopher Ochieng, Japhet Towett, Jane Kimetto, Brighton Omondi, Mary Leelgo, Michael Obonyo, Linner Rotich, Enock Tonui, Ella Chelangat, Joan Kapkiai, Salome Wangare, Zeddy Bett Kesi, Janet Ngeno, Edwin Langat, Kennedy Labosso, Joshua Rotich, Leonard Cheruiyot, Enock Changwony, Mike Bii, Ezekiel Chumba, Susan Ontango, Danson Gitonga, Samuel Kiprotich, Bornes Ngtech, Grace Engoke, Irene Metet, Alice Airo, and Ignatius Kiptoo; AFRICOS Kisumu, Kenya team: Valentine Sing’oei, Winne Rehema, Solomon Otieno, Celine Ogari, Elkanah Modi, Oscar Adimo, Charles Okwaro, Christine Lando, Margaret Onyango, Iddah Aoko, Kennedy Obambo, Joseph Meyo, and George Suja; AFRICOS Abuja, Nigeria team: Yakubu Adamu, Nnamdi Azuakola, Mfreke Asuquo, Abdulwasiu Bolaji Tiamiyu, Afoke Kokogho, Samirah Sani Mohammed, Ifeanyi Okoye, Sunday Odeyemi, Aminu Suleiman, Lawrence Umeji, Onome Enas, Miriam Mbachu, Ijeoma Chigbu-Ukaegbu, Wilson Adai, Felicia Anayochukwu Odo, Rabi Abdu, Rosemary Akiga, Helen Nwandu, CHisara Okolo, Ndubuisis Okeke; AFRICOS Lagos, Nigeria team: Zahra Parker, Asogwa Ugochukwu Linus, Concilia Amaka Agbaim, Tunde Adegbite, Nkenchiere Harrison, Adewale Adelakun, Ekeocha Chioma, Victoria Idi, Rachel Eluwa, Jumoke Nwalozie, Igiri Faith, Blessing Okanigbuan, Achugwo Emmanuel, Nkiru Nnadi, Ndubuisi Rosemary, Uzoegwu Amaka Natalie, Obende Theresa Owanza, Falaju Idowu Francis, Jacintal Elemere, Obilor Ifeoma Lauretta, Edward Akinwale, and Inalegwu Ochai; AFRICOS Mbeya, Tanzania team: Lucas Maganga, Samoel Khamadi, John Njegite, Connie Lueer, Abisai Kisinda, Jaquiline Mwamwaja, Faraja Mbwayu, Gloria David, Mtasi Mwaipopo, Reginald Gervas, Doroth Mkondoo, Nancy Somi, Paschal Kiliba, Gwamaka Mwaisanga, Johnisius Msigwa, Hawa Mfumbulwa, Peter Edwin, and Willyhelmina Olomi.
Contributor Information
AFRICOS Study Group:
Danielle Bartolanzo, Alexus Reynolds, Katherine Song, Mark Milazzo, Leilani Francisco, Shauna Mankiewicz, Steven Schech, Badryah Omar, Tsedal Mebrahtu, Elizabeth Lee, Kimberly Bohince, Jaclyn Hern, Kara Lombardi, Michelle Imbach, Leigh Anne Eller, Michael Semwogerere, Prossy Naluyima, Godfrey Zziwa, Allan Tindikahwa, Hilda Mutebe, Cate Kafeero, Enos Baghendaghe, William Lwebuge, Freddie Ssentogo, Hellen Birungi, Josephine Tegamanyi, Paul Wangiri, Christine Nabanoba, Phiona Namulondo, Richard Tumusiime, Ezra Musingye, Christina Nanteza, Joseph Wandege, Michael Waiswa, Evelyn Najjuma, Olive Maggaga, Isaac Kato Kenoly, Barbara Mukanza, Rither Langat, Aaron Ngeno, Lucy Korir, Raphael Langat, Francis Opiyo, Alex Kasembeli, Christopher Ochieng, Japhet Towett, Jane Kimetto, Brighton Omondi, Mary Leelgo, Michael Obonyo, Linner Rotich, Enock Tonui, Ella Chelangat, Joan Kapkiai, Salome Wangare, Zeddy Bett Kesi, Janet Ngeno, Edwin Langat, Kennedy Labosso, Joshua Rotich, Leonard Cheruiyot, Enock Changwony, Mike Bii, Ezekiel Chumba, Susan Ontango, Danson Gitonga, Samuel Kiprotich, Bornes Ngtech, Grace Engoke, Irene Metet, Alice Airo, Ignatius Kiptoo, Valentine Sing’oei, Winne Rehema, Solomon Otieno, Celine Ogari, Elkanah Modi, Oscar Adimo, Charles Okwaro, Christine Lando, Margaret Onyango, Iddah Aoko, Kennedy Obambo, Joseph Meyo, George Suja, Yakubu Adamu, Nnamdi Azuakola, Mfreke Asuquo, Abdulwasiu Bolaji Tiamiyu, Afoke Kokogho, Samirah Sani Mohammed, Ifeanyi Okoye, Sunday Odeyemi, Aminu Suleiman, Lawrence Umeji, Onome Enas, Miriam Mbachu, Ijeoma Chigbu Adai, Wilson Ukaegbu, Felicia Anayochukwu Odo, Rabi Abdu, Rosemary Akiga, Helen Nwandu, CHisara Okolo, Ndubuisis Okeke, Zahra Parker, Asogwa Ugochukwu Linus, Concilia Amaka Agbaim, Tunde Adegbite, Nkenchiere Harrison, Adewale Adelakun, Ekeocha Chioma, Victoria Idi, Rachel Eluwa, Jumoke Nwalozie, Igiri Faith, Blessing Okanigbuan, Achugwo Emmanuel, Nkiru Nnadi, Ndubuisi Rosemary, Uzoegwu Amaka Natalie, Obende Theresa Owanza, Falaju Idowu Francis, Jacintal Elemere, Obilor Ifeoma Lauretta, Edward Akinwale, Inalegwu Ochai, Lucas Maganga, Samoel Khamadi, John Njegite, Connie Lueer, Abisai Kisinda, Jaquiline Mwamwaja, Faraja Mbwayu, Gloria David, Mtasi Mwaipopo, Reginald Gervas, Doroth Mkondoo, Nancy Somi, Paschal Kiliba, Gwamaka Mwaisanga, Johnisius Msigwa, Hawa Mfumbulwa, Peter Edwin, and Willyhelmina Olomi
References
- 1. Wegerif MCA. “Informal” food traders and food security: experiences from the Covid-19 response in South Africa. Food Secur 2020; 12:797–800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Barasa E, Mothupi CM, Guleid F, et al. Health and socio-economic impacts of physical distancing for Covid-19 in Africa. 2020. Available at: https://www.aasciences.africa/sites/default/files/2020-05/DFIDReport-RapidReviewofPhysicalDistancinginAfrica-19052020-compressed.pdf. Accessed 9 April 2021.
- 3. Wilkinson A, Ali H, Bedford J, et al. Local response in health emergencies: key considerations for addressing the COVID-19 pandemic in informal urban settlements. Environ Urban 2020; 32:503–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Kirigia JM, Barry SP. Health challenges in Africa and the way forward. Effic Heal Syst Units Africa A Data Envel Anal 2013; 3:37–41. [Google Scholar]
- 5. Siedner MJ, Kraemer JD, Meyer MJ, et al. Access to primary healthcare during lockdown measures for COVID-19 in rural South Africa: an interrupted time series analysis. BMJ Open 2020; 10:e043763. doi: 10.1136/bmjopen-2020-04376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Doherty M. What we know about HIV and COVID‐19.2020. Available at: https://www.iasociety.org/HIV-Programmes/Cross-cutting-issues/COVID-19-and-HIV-Webinars. Accessed 9 April 2021.
- 7. Yue JL, Yan W, Sun YK, et al. Mental health services for infectious disease outbreaks including COVID-19: a rapid systematic review. Psychol Med 2020; 50:2498–513. doi: 10.1017/S0033291720003888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Pfefferbaum B, North CS. Mental health and the Covid-19 pandemic. N Engl J Med 2020; 383:510–2. [DOI] [PubMed] [Google Scholar]
- 9. Esber AL, Coakley P, Ake JA, et al. Decreasing time to antiretroviral therapy initiation after HIV diagnosis in a clinic-based observational cohort study in four African countries. J Int AIDS Soc 2020; 23:e25446. doi: 10.1002/jia2.25446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Lewinsohn PM, Seeley JR, Roberts RE, Allen NB. Center for Epidemiologic Studies Depression Scale (CES-D) as a screening instrument for depression among community-residing older adults. Psychol Aging 1997; 12:277–87. [DOI] [PubMed] [Google Scholar]
- 11. Ekumah B, Armah FA, Yawson DO, et al. Disparate on-site access to water, sanitation, and food storage heighten the risk of COVID-19 spread in sub-Saharan Africa. Environ Res 2020; 189:109936. Available at: http://www.sciencedirect.com/science/article/pii/S0013935120308318. Accessed 9 April 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Quaife M, van Zandvoort K, Gimma A, et al. The impact of COVID-19 control measures on social contacts and transmission in Kenyan informal settlements. BMC Med 2020; 18:316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Egger D, Miguel E, Warren SS, et al. Falling living standards during the COVID-19 crisis: quantitative evidence from nine developing countries. Sci Adv 2021; 7:eabe0997. Available at: http://advances.sciencemag.org/content/7/6/eabe0997.abstract. Accessed 9 April 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Linnemayr S, Jennings Mayo-Wilson L, Saya U, et al. HIV care experiences during the COVID-19 pandemic: mixed-methods telephone interviews with clinic-enrolled HIV-infected adults in Uganda. AIDS Behav 2021; 25:28–39. Available at: https://pubmed.ncbi.nlm.nih.gov/32918641. Accessed 9 April 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.