Abstract
Background
Qatar experienced a severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) epidemic that disproportionately affected the craft and manual worker (CMW) population, who comprise 60% of the total population. This study aimed to assess ever and/or current infection prevalence in this population.
Methods
A cross-sectional population-based survey was conducted during July 26 to September 09, 2020, to assess both anti-SARS-CoV-2 positivity through serological testing and current infection positivity through polymerase chain reaction (PCR) testing. Associations with antibody and PCR positivity were identified through regression analyses.
Results
The study included 2641 participants, 69.3% of whom were <40 years of age. Anti-SARS-CoV-2 positivity was 55.3% (95% CI, 53.3%–57.3%) and was significantly associated with nationality, geographic location, educational attainment, occupation, and previous infection diagnosis. PCR positivity was 11.3% (95% CI, 9.9%–12.8%) and was significantly associated with nationality, geographic location, occupation, contact with an infected person, and reporting 2 or more symptoms. Infection positivity (antibody and/or PCR positive) was 60.6% (95% CI, 58.6%–62.5%). The proportion of antibody-positive CMWs who had a prior SARS-CoV-2 diagnosis was 9.3% (95% CI, 7.9%–11.0%). Only seven infections were ever severe, and only 1 was ever critical—an infection severity rate of 0.5% (95% CI, 0.2%–1.0%).
Conclusions
Six in every 10 CMWs in Qatar have been infected, suggestive of reaching the herd immunity threshold. Infection severity was low, with only 1 in every 200 infections progressing to be severe or critical. Only 1 in every 10 infections had been previously diagnosed, which is suggestive of mostly asymptomatic or mild infections.
Keywords: COVID-19, immunity, Qatar, SARS-CoV-2, seroprevalence
The severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection has spread worldwide, causing disease and mortality as well as social and economic disruptions [1–3]. Qatar, a country in the Arabian Gulf, has experienced a pervasive epidemic, with >55 000 laboratory-confirmed infections per million population as of November 20, 2020 [4, 5].
Most affected by the epidemic were the expatriate craft and manual workers (CMWs) who comprise 60% of the population of Qatar [6]. These workers are typically single men aged 20–49 years, recruited to work in development projects, and living in large shared accommodations [6–9]. Epidemiologic data on this population have indicated large SARS-CoV-2 outbreaks [7, 10, 11] that resembled those in nursing homes [12–14] or influenza outbreaks in regular and boarding schools [15, 16].
This study aimed to assess ever and current infection with SARS-CoV-2, the infection severity rate, and the infection diagnosis (detection) rate in the wider CMW population of Qatar.
METHODS
Study Design and Sampling
A national cross-sectional survey was conducted between July 26 and September 9, 2020, to assess anti-SARS-CoV-2 (antibody) positivity and SARS-CoV-2 polymerase chain reaction (PCR) positivity among CMWs in Qatar. To optimize sample representativeness of the wider CMW population in the absence of a comprehensive listing for CMWs, we devised a sampling strategy based on analysis of the registered users’ database of the Qatar Red Crescent Society (QRCS), the main provider of primary health care for CMWs in the country. QRCS operates 4 geographically distributed centers that were specifically designed to cater to the CMW population across the country. These centers were established over a decade ago and are well known by CMWs, operate long working hours (3 run >24 hours and 1 runs >16 hours), are located in regions where workers live, and provide services that are free of charge or heavily subsidized for enhanced accessibility and affordability. The probability distribution of CMWs by age and nationality in the QRCS database was cross-checked and found to be similar to that of the Ministry of Interior database of expatriate residents [8]. Sex was not considered in the sampling strategy because the vast majority of CMWs (>99%) are men [6].
The overall sample size was determined at 2232 assuming a seroprevalence of 25% (given the large epidemic in Qatar [7, 11]), a margin of error of 2%, and a nonresponse rate of 15%, but was increased to 2658 to ensure that a minimum of 5 individuals were recruited per each age–nationality stratum from each center (for better representation of small groups).
Due to time constraints and operational challenges in directly contacting the CMWs and recruiting them, recruitment was implemented per the above sampling strategy but using systematic sampling of the attendees at these centers during the study duration. By factoring the average number of attendees per day at each of these centers, every fourth attendee visiting each center was invited to participate in this study until the sample size by age and nationality at each center was fulfilled. It was difficult to recruit participants in the small age–nationality strata (such as among younger persons for specific nationalities), and thus toward the end of the study all attendees in these strata (not only every fourth attendee) were approached to participate.
Patient Consent Statement
A written informed consent was collected from all study participants. The study was approved by Hamad Medical Corporation (HMC) and Weill Cornell Medicine-Qatar Institutional Review Boards.
Sample Collection and Handling
An interview schedule inquiring about sociodemographics and history of exposure and symptoms was administered by trained interviewers in the participant’s language of preference. Both informed consent and interview schedule were provided and collected in 9 languages (Arabic, Bengali, English, Hindi, Nepali, Sinhala, Tagalog, Tamil, and Urdu) to cater to the main language groups of CMWs. The study instrument was based on a protocol for SARS-CoV-2 sero-epidemiological surveys developed by the World Health Organization (WHO) [17]. Blood (10 mL) was drawn for serological testing by certified nurses and stored in an ice box before being transported to the HMC Central Laboratory for analysis. Nasopharyngeal and oropharyngeal swabs were also collected by the nurses to assess current infection. National guidelines and standard of care were applied to all identified PCR-positive cases. No action was mandated by national guidelines to those found antibody positive.
Laboratory Methods
Testing for SARS-CoV-2-specific antibodies in the serological samples was performed using an electrochemiluminescence immunoassay, the Roche Elecsys Anti-SARS-CoV-2 (99.5% sensitivity [18], 99.8% specificity [18, 19]; Roche, Switzerland). Interpretation of results was per the manufacturer’s instructions: reactive for optical density cutoff index ≥1.0 and nonreactive for cutoff index <1.0 [19].
PCR testing was performed on aliquots of Universal Transport Medium (UTM) used for collection of nasopharyngeal swabs (Huachenyang Technology, Shenzen, China). Aliquots were extracted on the QIAsymphony platform (QIAGEN, Germantown, Maryland, USA) and tested with real-time reverse transcription PCR (RT-qPCR) using the TaqPath COVID-19 Combo Kit (100% sensitivity and specificity [20]; Thermo Fisher Scientific, Waltham, Massachusetts, USA) on an ABI 7500 FAST (Thermo Fisher, Waltham, Massachusetts, USA), extracted using a custom protocol [21] on a Hamilton Microlab STAR (Hamilton, Reno, Nevada, USA), and tested using the AccuPower SARS-CoV-2 Real-Time RT-PCR Kit (100% sensitivity and specificity [22]; Bioneer, Daejeon, Korea) on an ABI 7500 FAST or loaded directly into the Roche cobas 6800 system and assayed with the cobas SARS-CoV-2 Test (95% sensitivity, 100% specificity [23]; Roche, Basel, Switzerland).
All laboratory testing was conducted at the HMC Central Laboratory following standardized protocols.
Statistical Analysis
Frequency distributions were used to characterize study participants. Absence/presence of symptoms in the 2 weeks preceding the survey (no symptoms, 1 symptom, and 2 or more symptoms) was defined using a composite index score derived by summing up the values for reported symptoms coded as “0” for absence and “1” for presence. Probability weights were applied to adjust for participants’ unequal selection using the CMW population distribution by age group and nationality per the QRCS registered-user database.
Associations with anti-SARS-CoV-2 positivity were explored using the chi-square test and univariable logistic regression analyses. Covariates with P values ≤.2 in the univariable regression analysis were included in the multivariable model. Covariates with P values ≤.05 in the multivariable analysis were considered as showing statistically significant evidence for an association with the outcome. Odds ratios (ORs), adjusted ORs (AORs), 95% CIs, and P values were reported. Associations with PCR positivity were also explored following the above-described methodology.
Antibody test results were subsequently linked to the national SARS-CoV-2 PCR testing and COVID-19 hospitalization and severity database, which includes all PCR testing, hospitalization, and SARS-CoV-2 infection severity classifications, as per the WHO criteria [24], since the start of the epidemic. Relevant epidemiological measures such as prevalence of ever and/or current infection, infection severity rate, and infection diagnosis rate were derived.
RESULTS
The final study sample included 2641 participants (Table 1), with a median age (range) of 35 (18–80) years. Most participants were below 40 years of age (69.3%) and of Indian (29.2%), Bangladeshi (26.2%), or Nepalese (21.6%) origin, representative of the wider CMW population in Qatar [8]. More than 40% had intermediate or lower educational attainment, and another 40% attended high school or vocational training. Over half of the sample consisted of technical and construction workers such as carpenters, crane operators, electricians, foremen, maintenance/air conditioning/cable technicians, masons, mechanics, painters, pipe fitters, plumbers, and welders, while 4.8% held higher professional positions such as architects, designers, engineers, operation managers, and supervisors.
Table 1.
Characteristics of Study Participants and Associations With Anti-SARS-CoV-2 Positivity
| Characteristics | Tested | Anti-SARS-CoV-2 Positive | Univariable Regression Analysis | Multivariable Regression Analysis | ||||
|---|---|---|---|---|---|---|---|---|
| No. (%a) | No. (%b) | Chi-Square P Value | ORc (95% CIc) | P Value | F Test P Valued | AORc (95% CIc) | P Valuee | |
| Age, y | ||||||||
| <29 | 753 (27.5) | 392 (53.2) | .279 | 1.00 | .296 | — | — | |
| 30–39 | 979 (41.8) | 553 (57.5) | 1.19 (0.98–1.45) | .086 | — | — | ||
| 40–49 | 553 (21.5) | 298 (54.4) | 1.05 (0.83–1.32) | .690 | — | — | ||
| 50–59 | 265 (7.5) | 142 (54.9) | 1.07 (0.80–1.44) | .657 | — | — | ||
| 60+ | 91 (1.7) | 42 (47.6) | 0.80 (0.50–1.29) | .356 | — | — | ||
| Nationality | ||||||||
| All other nationalitiesf | 231 (7.5) | 87 (38.2) | <.001 | 1.00 | <.001 | 1.00 | ||
| Filipino | 103 (2.7) | 25 (19.6) | 0.39 (0.22–0.70) | .002 | 0.41 (0.20–0.85) | .016 | ||
| Sri Lankan | 146 (4.8) | 56 (35.5) | 0.89 (0.56–1.40) | .614 | 0.84 (0.46–1.54) | .577 | ||
| Egyptian | 92 (3.2) | 32 (36.2) | 0.92 (0.54–1.56) | .748 | 0.84 (0.40–1.76) | .640 | ||
| Pakistani | 138 (4.9) | 70 (51.9) | 1.75 (1.11–2.73) | .015 | 1.18 (0.63–2.21) | .599 | ||
| Indian | 726 (29.2) | 376 (52.7) | 1.80 (1.31–2.47) | <.001 | 1.37 (0.89–2.09) | .154 | ||
| Nepalese | 570 (21.6) | 345 (59.2) | 2.34 (1.68–3.26) | <.001 | 1.83 (1.17–2.87) | .008 | ||
| Bangladeshi | 635 (26.2) | 436 (70.1) | 3.79 (2.72–5.26) | <.001 | 3.05 (1.93–4.80) | <.001 | ||
| QRCS center (catchment area within Qatar) | ||||||||
| Fereej Abdel Aziz (Doha-East) | 618 (23.4) | 257 (42.5) | <.001 | 1.00 | <.001 | 1.00 | ||
| Zekreet (Northwest) | 238 (2.3) | 125 (52.2) | 1.48 (1.09–2.00) | .012 | 1.46 (1.02–2.09) | .037 | ||
| Hemaila (Southwest; “Industrial Area”) | 981 (42.1) | 554 (57.6) | 1.84 (1.50–2.26) | <.001 | 1.63 (1.16–2.29) | .050 | ||
| Mesaimeer (Doha-South) | 804 (32.2) | 491 (61.7) | 2.18 (1.76–2.71) | <.001 | 1.89 (1.47–2.44) | <.001 | ||
| Educational attainment | ||||||||
| Primary or lower | 633 (24.6) | 377 (60.2) | <.001 | 1.00 | <.001 | 1.00 | ||
| Intermediate | 434 (17.9) | 278 (64.8) | 1.22 (0.94–1.58) | .143 | 1.41 (1.00–1.99) | .049 | ||
| Secondary/high school/vocational | 1102 (44.3) | 599 (55.2) | 0.82 (0.66–1.00) | .054 | 1.10 (0.83–1.47) | .504 | ||
| University | 371 (13.3) | 114 (32.3) | 0.32 (0.24–0.42) | <.001 | 0.70 (0.47–1.06) | .096 | ||
| Occupation | ||||||||
| Professional workersg | 137 (4.8) | 36 (27.8) | <.001 | 1.00 | <.001 | 1.00 | ||
| Food & beverage workers | 93 (3.2) | 26 (29.2) | 1.07 (0.57–2.02) | .827 | 0.71 (0.32–1.61) | .415 | ||
| Administration workers | 82 (3.0) | 25 (31.6) | 1.20 (0.63–2.28) | .573 | 1.40 (0.64–3.07) | .400 | ||
| Retail workers | 171 (6.6) | 67 (40.3) | 1.76 (1.05–2.94) | .030 | 1.58 (0.83–3.01) | .163 | ||
| Transport workers | 435 (16.4) | 227 (53.4) | 2.99 (1.91–4.68) | <.001 | 2.16 (1.20–3.89) | .010 | ||
| Cleaning workers | 105 (4.0) | 55 (54.5) | 3.12 (1.77–5.49) | <.001 | 2.81 (1.32–6.01) | .008 | ||
| Technical and construction workersh | 1329 (52.9) | 862 (65.1) | 4.85 (3.19–7.38) | <.001 | 3.07 (1.77–5.32) | <.001 | ||
| Security workers | 61 (2.3) | 36 (60.1) | 3.92 (2.02–7.61) | <.001 | 3.21 (1.32–7.79) | .010 | ||
| Other workersi | 178 (6.9) | 64 (38.5) | 1.63 (0.98–2.71) | .061 | 1.70 (0.88–3.28) | .114 | ||
| Contact with infected person | ||||||||
| No | 2370 (91.8) | 1283 (55.2) | .257 | 1.00 | .258 | — | — | |
| Yes | 208 (8.2) | 101 (50.9) | 0.84 (0.63–1.13) | .258 | — | — | ||
| Symptoms in the 2 wk preceding the survey | ||||||||
| No symptoms | 2326 (88.0) | 1251 (54.6) | .021 | 1.00 | .021 | 1.00 | ||
| 1 symptom | 173 (6.7) | 107 (65.9) | 1.61 (1.14–2.26) | .006 | 1.15 (0.72–1.85) | .554 | ||
| ≥2 symptoms | 142 (5.5) | 69 (53.3) | 0.95 (0.67–1.36) | .777 | 0.83 (0.51–1.35) | .453 | ||
| Symptoms required medical attention | ||||||||
| No | 2594 (99.0) | 1392 (54.8) | .200 | 1.00 | .205 | — | — | |
| Yes | 25 (1.0) | 17 (68.1) | 1.76 (0.73–4.21) | .205 | — | — | ||
| Symptoms required hospitalization | ||||||||
| No | 2613 (99.9) | 1405 (55.0) | .229 | 1.00 | — | — | — | |
| Yes | 2 (0.1) | 2 (100.0) | Omitted | — | — | — | ||
| Previously diagnosed with infection | ||||||||
| No | 1826 (96.3) | 957 (53.6) | .009 | 1.00 | .012 | 1.00 | ||
| Yes | 65 (3.7) | 45 (70.5) | 2.06 (1.19–3.57) | .010 | 2.06 (1.17–3.63) | .012 | ||
| Total (%, 95% CI) | 2641 (100.0) | 1427 (55.3, 53.3–57.3) | NA | NA | NA | NA | NA | NA |
Abbreviations: AOR, adjusted odds ratio; NA, not applicable; OR, odds ratio; QRCS, Qatar Red Crescent Society; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.
aPercentage of the total sample weighted by age, nationality, and QRCS center. Missing values were excluded from the analysis.
bPercent positive out of those tested, weighted by age, nationality, and QRCS center.
cEstimates weighted by age, nationality, and center.
dCovariates with P values ≤.2 in the univariable analysis were included in the multivariable analysis.
eCovariates with P values ≤.05 in the multivariable analysis were considered as showing statistically significant evidence for an association with anti-SARS-CoV-2 positivity.
fIncludes all other nationalities of craft and manual workers residing in Qatar.
gIncludes architects, designers, engineers, operation managers, and supervisors, among other professions.
hIncludes carpenters, construction workers, crane operators, electricians, foremen, maintenance/air conditioning/cable technicians, masons, mechanics, painters, pipe fitters, plumbers, and welders, among other professions.
iIncludes barbers, firefighters, gardeners, farmers, fishermen, and physical fitness trainers, among other professions.
A total of 1427 participants had detectable SARS-CoV-2 antibodies—a seropositivity of 55.3% (95% CI, 53.3%–57.3%) (Table 1). Seropositivity was independently associated with each of nationality, QRCS center (proxy of catchment area/geographic location), educational attainment, occupation, and previous infection diagnosis in the multivariable regression analysis (Table 1). Still, the differences in seropositivity were overall not considerable, apart from those by nationality, occupation, and geographic location (QRCS center). Compared with all other nationalities, the AOR was 0.41 (95% CI, 0.20–0.85) for Filipinos, 1.83 (95% CI, 1.17–2.87) for Nepalese, and 3.05 (95% CI, 1.93–4.80) for Bangladeshis. Compared with professional workers, the AOR was 2.16 (95% CI, 1.20–3.89) for transport workers, 2.81 (95% CI, 1.32–6.01) for cleaning workers, 3.07 (95% CI, 1.77–5.32) for technical and construction workers, and 3.21 (95% CI, 1.32–7.79) for security workers. No association was found for age, contact with an infected person, symptoms in the 2 weeks preceding the survey, symptoms requiring medical attention, or symptoms requiring hospitalization.
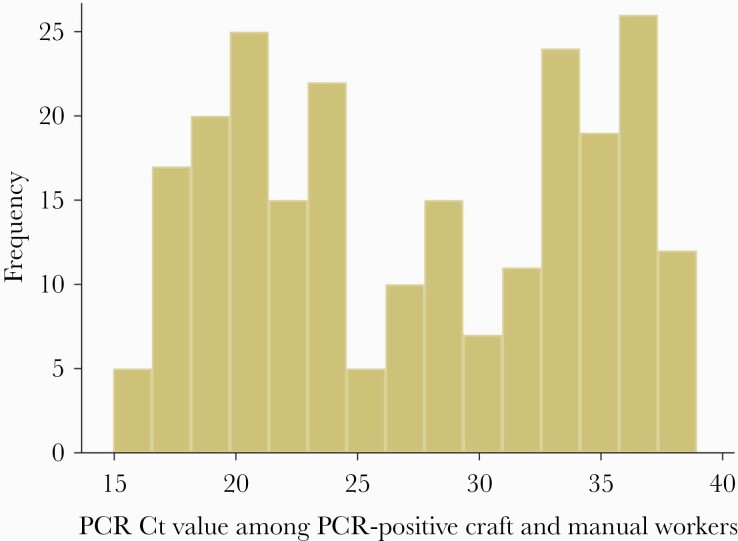
A total of 2092 CMWs consented to PCR testing, of whom 233 had a positive result—a PCR positivity rate of 11.3% (95% CI, 9.9–12.8%) (Table 2). PCR cycle threshold (Ct) values ranged from 15.0 to 38.9, with a median of 27.6 (Figure 1). The Ct value was ≥30 in 41.6% of PCR-positive CMWs, suggesting no active infection [25, 26]. PCR positivity was independently associated with nationality, geographic location (QRCS center), occupation, contact with an infected person, and reporting 2 or more symptoms in the 2 weeks preceding the survey in the multivariable regression analysis, but no association was found for the other variables (Table 2).
Table 2.
Associations With SARS-CoV-2 PCR Positivity
| Characteristics | Tested | SARS-CoV-2 PCR Positive | Univariable Regr ession Analysis | Multivariable Regression Analysis | ||||
|---|---|---|---|---|---|---|---|---|
| No. (%a) | No. (%b) | Chi-Square P Value | OR (95% CI) | P Value | F Test P Valuec | AOR (95% CI) | P Valued | |
| Age, y | ||||||||
| <29 | 634 (29.4) | 82 (13.0) | .292 | 1.00 | .310 | — | — | |
| 30–39 | 780 (42.0) | 77 (10.0) | 0.75 (0.53–1.05) | .090 | — | — | ||
| 40–49 | 408 (20.0) | 47 (11.6) | 0.88 (0.59–1.30) | .525 | — | — | ||
| 50–59 | 200 (7.1) | 24 (12.1) | 0.92 (0.56–1.54) | .762 | — | — | ||
| 60+ | 70 (1.6) | 3 (5.1) | 0.36 (0.10–1.31) | .121 | — | — | ||
| Nationality | ||||||||
| All other nationalitiese | 202 (8.4) | 20 (10.3) | .099 | 1.00 | .110 | 1.00 | ||
| Indian | 549 (27.9) | 49 (8.4) | 0.80 (0.45–1.40) | .428 | 0.74 (0.39–1.39) | .352 | ||
| Sri Lankan | 114 (4.7) | 12 (11.6) | 1.14 (0.53–2.47) | .737 | 0.96 (0.43–2.16) | .931 | ||
| Bangladeshi | 496 (25.9) | 55 (11.4) | 1.12 (0.65–1.95) | .682 | 1.14 (0.60–2.17) | .682 | ||
| Pakistani | 116 (5.2) | 17 (14.7) | 1.50 (0.73–3.06) | .265 | 1.33 (0.57–3.10) | .502 | ||
| Egyptian | 79 (3.5) | 8 (11.4) | 1.12 (0.46–2.70) | .806 | 1.36 (0.55–3.38) | .508 | ||
| Nepalese | 467 (22.2) | 59 (13.3) | 1.34 (0.77–2.33) | .296 | 1.64 (0.87–3.10) | .124 | ||
| Filipino | 69 (2.2) | 13 (20.5) | 2.25 (0.99–5.11) | .053 | 2.90 (1.11–7.59) | .030 | ||
| QRCS center (catchment area within Qatar) | ||||||||
| Fereej Abdel Aziz (Doha-East) | 547 (26.2) | 85 (15.4) | <.001 | 1.00 | <.001 | 1.00 | ||
| Mesaimeer (Doha-South) | 535 (27.0) | 21 (4.0) | 0.23 (0.14–0.37) | <.001 | 0.30 (0.17–0.51) | <.001 | ||
| Zekreet (Northwest) | 186 (2.3) | 16 (8.8) | 0.53 (0.30–0.94) | .029 | 0.62 (0.33–1.15) | .128 | ||
| Hemaila (Southwest; “Industrial Area”) | 824 (44.6) | 111 (13.4) | 0.85 (0.62–1.16) | .310 | 1.10 (0.75–1.60) | .631 | ||
| Educational attainment | ||||||||
| Primary or lower | 502 (24.5) | 60 (12.5) | .638 | 1.00 | .630 | — | — | |
| Intermediate | 347 (18.1) | 45 (12.5) | 1.00 (0.65–1.54) | .991 | — | — | ||
| Secondary/high school/vocational | 870 (44.1) | 93 (11.2) | 0.89 (0.62–1.27) | .509 | — | — | ||
| University | 293 (13.4) | 31 (9.6) | 0.75 (0.46–1.22) | .245 | — | — | ||
| Occupation | ||||||||
| Professional workersf | 107 (4.8) | 15 (13.9) | <.001 | 1.00 | <.001 | 1.00 | ||
| Cleaning workers | 85 (4.1) | 9 (7.9) | 0.53 (0.20–1.41) | .202 | 0.35 (0.11–1.09) | .070 | ||
| Technical and construction workersg | 1041 (52.2) | 86 (8.5) | 0.57 (0.31–1.07) | .081 | 0.43 (0.20–0.93) | .031 | ||
| Security workers | 53 (2.6) | 3 (6.4) | 0.42 (0.11–1.55) | .192 | 0.46 (0.11–1.82) | .266 | ||
| Food & beverage workers | 73 (3.2) | 12 (14.9) | 1.08 (0.44–2.64) | .859 | 0.60 (0.22–1.65) | .325 | ||
| Administration workers | 70 (3.2) | 8 (12.1) | 0.85 (0.33–2.20) | .734 | 0.67 (0.23–1.93) | .457 | ||
| Transport workers | 319 (15.0) | 38 (12.7) | 0.90 (0.46–1.77) | .753 | 0.70 (0.32–1.52) | .364 | ||
| Retail workers | 145 (7.1) | 27 (19.5) | 1.49 (0.72–3.07) | .278 | 1.18 (0.54–2.60) | .673 | ||
| Other workersh | 161 (7.9) | 32 (19.9) | 1.53 (0.76–3.10) | .234 | 1.21 (0.56–2.64) | .625 | ||
| Contact with infected person | ||||||||
| No | 1909 (93.7) | 195 (10.5) | <.001 | 1.00 | <.001 | 1.00 | ||
| Yes | 125 (6.3) | 36 (27.8) | 3.30 (2.14–5.11) | <.001 | 2.96 (1.88–4.65) | <.001 | ||
| Symptoms in the 2 wk preceding the survey | ||||||||
| Asymptomatic | 1856 (88.4) | 189 (10.5) | <.001 | 1.00 | <.001 | 1.00 | ||
| 1 symptom | 125 (6.0) | 15 (11.9) | 1.16 (0.65–2.08) | .615 | 1.09 (0.58–2.05) | .799 | ||
| ≥2 symptoms | 111 (5.5) | 29 (23.5) | 2.63 (1.63–4.24) | <.001 | 2.36 (1.42–3.93) | .001 | ||
| Symptoms required medical attention | ||||||||
| No | 2056 (99.0) | 229 (11.3) | .914 | 1.00 | .914 | — | — | |
| Yes | 19 (0.01) | 2 (12.1) | 1.09 (0.25–4.78) | .914 | — | — | ||
| Symptoms required hospitalization | ||||||||
| No | 2069 (99.9) | 228 (11.1) | .108 | 1.00 | — | — | — | — |
| Yes | 1 (0.01) | 0 (0.0) | Omitted | — | — | |||
| Previously diagnosed with infection | ||||||||
| No | 1428 (98.2) | 135 (9.4) | .152 | 1.00 | .161 | — | — | |
| Yes | 26 (1.9) | 5 (17.6) | 2.06 (0.75–5.63) | .161 | — | — | ||
| Total (%, 95% CI) | 2092 (100.0) | 233 (11.3, 9.9–12.8) | NA | NA | NA | NA | NA | NA |
Abbreviations: AOR, adjusted odds ratio; OR, odds ratio; PCR, polymerase chain reaction; QRCS, Qatar Red Crescent Society; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.
aPercentage of the total sample weighted by age, nationality, and QRCS center. Missing values were excluded from the analysis.
bPercent positive out of those tested weighted by age, nationality, and QRCS center.
cCovariates with P values ≤.2 in the univariable analysis were included in the multivariable analysis.
dCovariates with P values ≤.05 in the multivariable analysis were considered as showing statistically significant evidence for an association with anti-SARS-CoV-2 positivity.
eIncludes all other nationalities of craft and manual workers residing in Qatar.
fIncludes architects, designers, engineers, operation managers, and supervisors, among other professions.
gIncludes carpenters, construction workers, crane operators, electricians, foremen, maintenance/air conditioning/cable technicians, masons, mechanics, painters, pipe fitters, plumbers, and welders, among other professions.
hIncludes barbers, firefighters, gardeners, farmers, fishermen, and physical fitness trainers, among other professions.
Figure 1.
Distribution of PCR Ct values among CMWs identified as SARS-CoV-2 PCR positive during the study period. Abbreviations: CMWs, craft and manual workers; Ct, cycle threshold; PCR, polymerase chain reaction; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.
Table 3 summarizes the key SARS-CoV-2 epidemiological measures assessed in this study. Infection positivity (antibody and/or PCR positive during the study) was assessed at 60.6% (95% CI, 58.6%–62.5%). Of the 1427 antibody-positive CMWs, 131 had a laboratory-confirmed PCR-positive result for SARS-CoV-2 before this study, corresponding to a diagnosis (detection) rate of 9.3% (95% CI, 7.9%–11.0%). The median time between the previous PCR diagnosis and the antibody-positive test was 63 days. Meanwhile, 4 out of the 1214 antibody-negative CMWs, 0.4% (95% CI, 0.1%–1.0%), had been previously diagnosed with the infection before this study. The median time between the previous PCR diagnosis and the antibody-negative test was 28 days. The Ct values and PCR diagnosis date for these individuals were 16.0 on July 23, 22.3 on July 25, 22.8 on June 6, and 28.3 on May 2, 2020, suggesting that the recency of the infection may explain the lack of detectable antibodies for 2 of these 4 individuals.
Table 3.
Key SARS-CoV-2 Epidemiological Measures Assessed in the Study
| Epidemiological Measure | Sample (Denominator) | Positive for Outcome (Numerator) | Estimate (95% CI), %a |
|---|---|---|---|
| Antibody positivity (seropositivity) prevalence | 2641 | 1427 | 55.3 (53.3–57.3) |
| PCR positivity prevalence | 2092b | 233 | 11.3 (9.9–12.8) |
| Infection (antibody and/or PCR) positivity prevalence | 2641 | 1571 | 60.6 (58.6–62.5) |
| Infection diagnosis ratec | 1427 | 131 | 9.3 (7.9–11.0) |
| Antibody-negative CMWs previously PCR-diagnosed with SARS-CoV-2 infection | 1214 | 4 | 0.4 (0.1–1.0) |
| Infection severity rated | 1590e | 8f | 0.5 (0.2–1.0) |
Abbreviations: CMWs, craft and manual workers; PCR, polymerase chain reaction; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.
aEstimates weighted by age, nationality, and center.
bOnly 2092 persons consented to have nasopharyngeal and oropharyngeal swabs.
cProportion of antibody-positive CMWs with a prior SARS-CoV-2 laboratory-confirmed PCR diagnosis.
dNumber of infections ever severe or critical per World Health Organization criteria over total number of laboratory-confirmed infections (antibody and/or PCR positive).
eThis number includes also 4 persons who were antibody negative and PCR negative at the time of the survey but had a PCR-positive result before the survey. This number also includes 15 persons who were antibody negative and PCR negative at the time of the survey but had a PCR-positive result subsequent to the survey at the time of data linking and analysis (October 7, 2020).
fSeven participants in this study had ever had (or progressed to) a severe infection and 1 had a critical infection per World Health Organization infection severity classification [24] at the time of data linking and analysis (October 7, 2020).
Out of the total of 1590 participants with laboratory-confirmed infection (antibody and/or PCR positive), 7 have ever had or progressed to a severe infection (prior, during, or after this study) and 1 has ever had or progressed to a critical infection, as per WHO criteria [24]—an infection severity rate of 0.5% (95% CI, 0.2%–1.0%). All severe and critical infections were hospitalized but cleared their infection; no COVID-19 deaths have been recorded.
DISCUSSION
The above results indicate that the CMW population—a population that constitutes 60% of the population of Qatar—appears to be at or not far from the herd immunity threshold for the SARS-CoV-2 variants circulating in Qatar at the time of this study. Seroprevalence was ~60% in 3 geographic regions, and only lower in Doha East, at 43%. This is to our knowledge the first such evidence for herd immunity, or being near herd immunity, in a majority segment of the population in any country. This conclusion is supported by the fact that no major infection cluster has been identified in any CMW community in Qatar for several months up to the end of 2020, despite the progressive easing of the social and physical distancing restrictions since June 15, 2020 [27]. Meanwhile, large clusters of infection were common in such CMW communities before, around, and shortly after the epidemic peak toward the end of May 2020.
A level of about 60%–70% infection prevalence to reach herd immunity is in concordance with that predicted using the “classical” formula for herd immunity of [28, 29], with R0, the basic reproduction number, being in the range of 2.5–4 [30, 31]. This, however, does not support other evidence arguing that herd immunity for SARS-CoV-2 infection could be reached (without vaccination) at infection levels as low as 15%–20% [32]. Our findings suggest that herd immunity may not be reached before at least half of the population has been infected, even in the presence of heterogeneity in the social contact rate in a given population [10, 29, 32].
A key finding of this study is the low SARS-CoV-2 infection severity rate found in this (relatively young) population, where only 1 in every 200 infections was ever severe or critical as per the WHO infection severity classification [24]. This outcome agrees with the findings of 2 other studies from Qatar, where the infection severity rate has been estimated at 0.25% (95% CI, 0.11%–0.49%) based on antibody and/or PCR laboratory-confirmed infections [10] and at 0.37% (95% CI, 0.37%–0.38%) based on mathematical modeling of the epidemic in the total population [33]—compared with 0.50% (95% CI, 0.22%–0.99%) in this study. These rates are substantially lower than those estimated elsewhere [34], often using early epidemic data, possibly because of insufficient accounting for the large denominator of undiagnosed asymptomatic or mild infections in young persons. These rates are also unlikely to be explained by lower comorbidity levels as disease conditions such as obesity, diabetes, and hypertension are at relatively high prevalence in Qatar [35–38], comparable to what is seen in developed nations.
Notably, despite the large epidemic in Qatar, only 236 COVID-19 deaths have been registered as of November 21, 2020 [5], indicating also a substantially lower infection fatality rate compared with earlier studies [34, 39–41]. An analysis of the severity and fatality of SARS-CoV-2 infection in Qatar suggested the young age structure of the population, potential cross-reactivity to circulating “common cold” coronaviruses, and high-quality standard of health care as reasons behind the low severity [33].
Though the infection was pervasive in this population, there were still some differences in past or current infection prevalence by nationality, catchment area/geographic location (QRCS center), educational attainment, and occupation (Tables 1 and 2). Given the totality of evidence on the Qatar epidemic [7, 10, 27, 33, 42], these differences may be explained by the nature of the shared accommodation (size and density), clustering of social networks by language and/or national background [7], occupational exposures (such as for drivers) [7], or differences in epidemic intensity in different parts of Qatar. Meanwhile, there were no differences in infection prevalence by age.
The study had other notable findings. The study design allowed an empirical estimation of the diagnosis (detection) rate for this population. Out of all detected antibody-positive cases, only 9.3% (95% CI, 7.9%–11.0%) had a documented PCR-confirmed infection before antibody testing in this study, indicating that 9 in every 10 infections were never diagnosed, a finding that agrees with estimates from other settings [39, 43–46]. This outcome supports that most infections were asymptomatic or too mild to be diagnosed, in line with findings of a PCR community survey conducted earlier in Qatar in which 58.5% of those who were PCR positive reported no symptoms in the 2 weeks preceding the survey [7]. Another finding of the present study is that reporting of 2 or more symptoms was predictive of PCR positivity, but not reporting of only 1 symptom (Table 2), a similar finding to that of the earlier PCR community survey [7]. Lastly, a high proportion of those testing PCR positive had a Ct value >30, suggesting that nearly half of the PCR-positive CMWs may have acquired their infection 2–6 weeks earlier, given the common presence of prolonged PCR positivity in infected persons [25, 26].
This study had limitations. While the study design was intended to be based on probability-based sampling of the total CMW population in Qatar, operational challenges and time constraints forced instead a systematic sampling of QRCS attendees supplemented with probability-based weights to generate an estimate that is representative of the wider CMW population. To ensure representation of small age–nationality strata (such as younger persons of specific nationalities), toward the end of the study, all attendees in these strata (not only every fourth attendee) were approached to participate. Operational challenges made it also difficult to track and maintain consistent logs of the response rate by the nurses in these QRCS centers; thus an exact estimate of the response rate could not be ascertained, though it was estimated at >90% for antibody testing and at >70% for PCR testing. An informed consent was obtained from all participants, and thus the purpose of the study could not be masked. However, with only 9.3% of those testing antibody-positive having a record of prior infection, it is unlikely that a previous diagnosis could have appreciably biased participation in the study.
While it is possible that the recruitment scheme may have affected the generalizability of study findings, this is less likely considering that CMWs attend these centers for a range of services beyond illness such as periodic health certifications, vaccinations, refill medications for chronic diseases, and pretravel PCR testing, and that the study’s primary outcome was seroprevalence—a marker of past rather than recent infection. Nevertheless, these limitations may have introduced selection bias for specifically the assessed PCR positivity prevalence and infection severity rate toward higher values, as participants may still have attended the QRCS centers because of current infection symptoms.
The laboratory methods were based on high-quality and validated commercial platforms, such as the Roche platform used for the serological testing [19, 47], one of the best available and most used and investigated commercial platforms with a specificity ≥99.8% [19, 48] and a sensitivity ≥95% [7, 47]. Factoring the less-than-perfect sensitivity and specificity [49] would have increased the measured antibody positivity prevalence to 58.1% instead of 55.3%. History of SARS-CoV-2 testing, hospitalization, and death were extracted through linking participants’ records to the national, centralized, and fully integrated digital health information platforms, and thus it is unlikely that cases/events were missed.
In conclusion, 6 in every 10 CMWs have already been infected with SARS-CoV-2, suggesting that this population is at or not far from herd immunity for the SARS-CoV-2 variants circulating in Qatar at the time of this study. While the prevalence of past or current infection was high, infection severity was low, with only 1 in every 200 infections progressing severe or critical disease. Indeed, most infections must have been asymptomatic or too mild to be diagnosed, as only 1 in every 10 antibody-positive persons had a prior PCR-confirmed SARS-CoV-2 diagnosis.
Acknowledgments
We thank Her Excellency Dr. Hanan Al Kuwari, Minister of Public Health, for her vision, guidance, leadership, and support. We also thank Dr. Saad Al Kaabi, Chair of the System Wide Incident Command and Control (SWICC) Committee for the COVID-19 national healthcare response, for his leadership, analytical insights, and for his instrumental role in enacting data information systems that made these studies possible. We further extend our appreciation to the SWICC Committee and the Scientific Reference and Research Taskforce (SRRT) members for their informative input, scientific technical advice, and enriching discussions. We also thank Dr. Mariam Abdulmalik, CEO of the Primary Health Care Corporation and the Chairperson of the Tactical Community Command Group on COVID-19, as well as members of this committee, for providing support to the teams that worked on the field surveillance. We further thank Dr. Nahla Afifi, Director of Qatar Biobank (QBB), Ms. Tasneem Al-Hamad, Ms. Eiman Al-Khayat, and the rest of the QBB team for their unwavering support in retrieving and analyzing samples and in compiling and generating databases for COVID-19 infection, as well as Dr. Asma Al-Thani, Chairperson of the Qatar Genome Programme Committee and Board Vice Chairperson of QBB, for her leadership of this effort. We also acknowledge the dedicated efforts of the Clinical Coding Team and the COVID-19 Mortality Review Team, both at Hamad Medical Corporation, and the Surveillance Team at the Ministry of Public Health. Last but not least, we thank all participants for their willingness to be part of this study.
Craft and Manual Workers Seroprevalence Study Group. The Craft and Manual Workers Seroprevalence Study Group consists of personnel who have contributed to the implementation of this study.
Qatar Red Crescent Society. Shafeer T. Aerattel, Firoj Ansari, Bennet J. Babu, Ali O. Bakari, Fazil K. Basheer, Muhammed J. Cherikkal, Muhammed R. Chonari, Ahmad S. Darwish, Arvin Dela Cruz, Verlili Z. Dela Cruz, Mark W. Del Carmen, Richie P. Deomampo, Sanu Gopi, Delfin J. R. O. Hortaleza, Robin Joseph, Veerankutty Kadar, Abdul Kareem A. Kalathil, Bigil C. Kandi, Mohammed M. T. Kaniyankandi, Kamarudheen Karimparukuzhiyil, Deelip G. Kurane, Manu Kurungott, Jommel R. C. Lumibao, Walid Mahmoud, Reyaz A. Malik, Jan A. Maxino, Nabeel T. Moosakutty, Hameed N. Nawabjahn, Ryan E. Orio, Mohamed F. Osman, Muhammad H. Ottappilakkool, Vijayakumar Pattakunninmel, Nissar P. Peedika, Suhail T. Puthiyaveettil, Ajith Raghavan, Renjee Ramachandran, Adil S. Sainudheen, Kannan Sassendran, John M. M. Soosai, Harris P. Sseri, Deepu Vallapil, and Patrick J. S. Venzuela.
Ministry of Public Health. Rana A. M. Abdoon, Hind S. M. Ahmed, Ayah M. A. Mahmoud, Omnia O. E. Gismelkhalig, and Farid Shihata.
Community Medicine Department at Hamad Medical Corporation. Khaled M. Ali and Fraih A. A. F. Alsallama.
Financial support. This work was supported by the Ministry of Public Health, Hamad Medical Corporation, and the Biomedical Research Program, the Biostatistics, Epidemiology, and Biomathematics Research Core, and the Clinical Research Core, all at Weill Cornell Medicine–Qatar. The statements made herein are solely the responsibility of the authors. The funders of the study had no role in the study design, data collection, data analysis, data interpretation, or writing of the article.
Potential conflicts of interest. We declare no potential conflicts of interest. All authors: no reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Author contributions. M.H.A. and L.J.A. co-conceived, co-designed, and co-led the study. E.F. led the study logistics and implementation. H.C. developed the study design, managed the databases, performed the data analyses, and wrote the first draft of the article. L.J.A. led the statistical analyses and drafting of the article. All authors contributed to the development of the study protocol, data collection and acquisition, database development, discussion and interpretation of results, and to the writing of the manuscript. All authors read and approved the final manuscript.
Data sharing. All data are available in aggregate form within the manuscript.
Contributor Information
Craft and Manual Workers Seroprevalence Study Group:
Shafeer T Aerattel, Firoj Ansari, Bennet J Babu, Ali O Bakari, Fazil K Basheer, Muhammed J Cherikkal, Muhammed R Chonari, Ahmad S Darwish, Arvin Dela Cruz, Verlili Z Dela Cruz, Mark W Del Carmen, Richie P Deomampo, Sanu Gopi, Delfin J R O Hortaleza, Robin Joseph, Veerankutty Kadar, Abdul Kareem A Kalathil, Bigil C Kandi, Mohammed M T Kaniyankandi, Kamarudheen Karimparukuzhiyil, Deelip G Kurane, Manu Kurungott, Jommel R C Lumibao, Walid Mahmoud, Reyaz A Malik, Jan A Maxino, Nabeel T Moosakutty, Hameed N Nawabjahn, Ryan E Orio, Mohamed F Osman, Muhammad H Ottappilakkool, Vijayakumar Pattakunninmel, Nissar P Peedika, Suhail T Puthiyaveettil, Ajith Raghavan, Renjee Ramachandran, Adil S Sainudheen, Kannan Sassendran, John M M Soosai, Harris P Sseri, Deepu Vallapil, Patrick J S Venzuela, Rana A M Abdoon, Hind S M Ahmed, Ayah M A Mahmoud, Omnia O E Gismelkhalig, Farid Shihata, Khaled M Ali, and Fraih A A F Alsallama
References
- 1.Worldometer. COVID-19 outbreak live update.2020. Available at: https://www.worldometers.info/coronavirus/. Accessed 6 September 2020.
- 2.United Nations. Shared responsibility, global solidarity: responding to the socio-economic impacts of COVID-19.2020. Available at: https://www.un.org/sites/un2.un.org/files/sg_report_socio-economic_impact_of_covid19.pdf. Accessed 16 April 2020.
- 3.McKibbin WJ, Fernando R. The global macroeconomic impacts of COVID-19: seven scenarios. Available at: https://www.brookings.edu/research/the-global-macroeconomic-impacts-of-covid-19-seven-scenarios/. Accessed 25 January 2021.
- 4.Hamad Medical Corporation. SARS-CoV-2 hospitalizations and care. 2020.
- 5.Ministry of Public Health-State of Qatar. Coronavirus disease 2019 (COVID-19).2021. Available at: https://covid19.moph.gov.qa/EN/Pages/default.aspx. Accessed 27 January 2021.
- 6.Planning and Statistics Authority, State of Qatar. Labor force sample survey.2017. Available at: https://www.psa.gov.qa/en/statistics/Statistical%20Releases/Social/LaborForce/2017/statistical_analysis_labor_force_2017_En.pdf. Accessed 1 May 2020.
- 7.Abu-Raddad LJ, Chemaitelly H, Ayoub HH, et al. . Characterizing the Qatar advanced-phase SARS-CoV-2 epidemic. Sci Rep 2021; 11:6233, –48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ministry of Interior, State of Qatar. Population distribution by sex, age, and nationality: results of Kashef database. 2020. Accessed 1 October 2020. [Google Scholar]
- 9.De Bel-Air F. Demography, migration, and labour market in Qatar.2018. Available at: https://www.researchgate.net/publication/323129801_Demography_Migration_and_Labour_Market_in_Qatar-_UPDATED_June_2017. Accessed 1 May 2020.
- 10.Jeremijenko A, Chemaitelly H, Ayoub HH, et al. . Evidence for and level of herd immunity against SARS-CoV-2 infection: the ten-community study. Emerg Infect Dis 2021; 27:1343-52. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Al Kuwari HM, Abdul Rahim HF, Abu-Raddad LJ, et al. . Epidemiological investigation of the first 5685 cases of SARS-CoV-2 infection in Qatar, 28 February-18 April 2020. BMJ Open 2020; 10:e040428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Arons MM, Hatfield KM, Reddy SC, et al. ; Public Health–Seattle and King County and CDC COVID-19 Investigation Team . Presymptomatic SARS-CoV-2 infections and transmission in a skilled nursing facility. N Engl J Med 2020; 382:2081–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Burton JK, Bayne G, Evans C, et al. . Evolution and effects of COVID-19 outbreaks in care homes: a population analysis in 189 care homes in one geographical region of the UK. Lancet Healthy Longevity. 2020; 1:e21–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ladhani SN, Chow JY, Janarthanan R, et al. . Investigation of SARS-CoV-2 outbreaks in six care homes in London, April 2020. EClinicalMedicine 2020; 26:100533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jackson C, Vynnycky E, Hawker J, et al. . School closures and influenza: systematic review of epidemiological studies. BMJ Open 2013; 3:e002149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Glatman-Freedman A, Portelli I, Jacobs SK, et al. . Attack rates assessment of the 2009 pandemic H1N1 influenza A in children and their contacts: a systematic review and meta-analysis. PLoS One 2012; 7:e50228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.World Health Organization. Population-based age-stratified seroepidemiological investigation protocol for COVID-19 virus infection.2020. Available at: https://apps.who.int/iris/handle/10665/331656. Accessed 15 April 2020.
- 18.Muench P, Jochum S, Wenderoth V, et al. . Development and validation of the Elecsys anti-SARS-CoV-2 immunoassay as a highly specific tool for determining past exposure to SARS-CoV-2. J Clin Microbiol 2020; 58:e01694-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.The Roche Group. Roche’s COVID-19 antibody test receives FDA Emergency Use Authorization and is available in markets accepting the CE mark.2020. Available at: https://www.roche.com/media/releases/med-cor-2020-05-03.htm. Accessed 5 June 2020.
- 20.Thermo Fisher Scientific. TaqPath™ COVID‑19 CE‑IVD RT‑PCR Kit instructions for use.2020. Available at: https://assets.thermofisher.com/TFS-Assets/LSG/manuals/MAN0019215_TaqPathCOVID-19_CE-IVD_RT-PCR%20Kit_IFU.pdf. Accessed 2 December 2020.
- 21.Kalikiri MKR, Hasan MR, Mirza F, et al. . High-throughput extraction of SARS-CoV-2 RNA from nasopharyngeal swabs using solid-phase reverse immobilization beads. medRxiv 2020.04.08.20055731 [Preprint]. 11 April 2020. Available at: 10.1101/2020.04.08.20055731. Accessed 4 November 2020. [DOI] [Google Scholar]
- 22.Kubina R, Dziedzic A. Molecular and serological tests for COVID-19 a comparative review of SARS-CoV-2 coronavirus laboratory and point-of-care diagnostics. Diagnostics 2020; 10:434–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.US Food and Drug Administration. Cobas® SARS-CoV-2: qualitative assay for use on the cobas® 6800/8800 Systems.2020. Available at: https://www.fda.gov/media/136049/download. Accessed 2 December 2020.
- 24.World Health Organization. Clinical management of COVID-19.2020. Available at: https://www.who.int/publications-detail/clinical-management-of-covid-19. Accessed 31 May 2020.
- 25.Sethuraman N, Jeremiah SS, Ryo A. Interpreting diagnostic tests for SARS-CoV-2. JAMA 2020; 323:2249–51. [DOI] [PubMed] [Google Scholar]
- 26.Wajnberg A, Mansour M, Leven E, et al. . Humoral response and PCR positivity in patients with COVID-19 in the New York City region, USA: an observational study. Lancet Microbe 2020; 1:e283–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ayoub HH, Chemaitelly H, Seedat S, et al. . Mathematical modeling of the SARS-CoV-2 epidemic in Qatar and its impact on the national response to COVID-19. J Glob Health 2021; 11:05005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Anderson RM, Heesterbeek H, Klinkenberg D, Hollingsworth TD. How will country-based mitigation measures influence the course of the COVID-19 epidemic? Lancet 2020; 395:931–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Britton T, Ball F, Trapman P. A mathematical model reveals the influence of population heterogeneity on herd immunity to SARS-CoV-2. Science 2020; 369:846–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.He W, Yi GY, Zhu Y. Estimation of the basic reproduction number, average incubation time, asymptomatic infection rate, and case fatality rate for COVID-19: meta-analysis and sensitivity analysis. J Med Virol 2020; 92:2543–50.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.MIDAS Online COVID-19 Portal. COVID-19 parameter estimates: basic reproduction number.2020. Available at: https://github.com/midas-network/COVID-19/tree/master/parameter_estimates/2019_novel_coronavirus. Accessed 19 May 2020.
- 32.Aguas R, Corder RM, King JG, et al. . Herd immunity thresholds for SARS-CoV-2 estimated from unfolding epidemics. medRxiv 2020.07.23.20160762 [Preprint]. 16 November 2020. Available at: 10.1101/2020.07.23.20160762. Accessed 30 November 2020. [DOI] [Google Scholar]
- 33.Seedat S, Chemaitelly H, Ayoub H, et al. . SARS-CoV-2 infection hospitalization, severity, criticality, and fatality rates. medRxiv 2020.11.29.20240416 [Preprint]. 30 November 2020. Available at: 10.1101/2020.11.29.20240416. Accessed 30 November 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Salje H, Tran Kiem C, Lefrancq N, et al. . Estimating the burden of SARS-CoV-2 in France. Science 2020; 369:208–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Awad SF, O’Flaherty M, Critchley J, Abu-Raddad LJ. Forecasting the burden of type 2 diabetes mellitus in Qatar to 2050: a novel modeling approach. Diabetes Res Clin Pract 2018; 137:100–8. [DOI] [PubMed] [Google Scholar]
- 36.Mushlin AI, Christos PJ, Abu-Raddad L, et al. . The importance of diabetes mellitus in the global epidemic of cardiovascular disease: the case of the state of Qatar. Trans Am Clin Climatol Assoc 2012; 123:193–207; discussion 207–8. [PMC free article] [PubMed] [Google Scholar]
- 37.Syed MA, Al Nuaimi AS, Latif Zainel AJA, A/Qotba HA. Prevalence of metabolic syndrome in primary health settings in Qatar: a cross sectional study. BMC Public Health 2020; 20:611, –618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Syed MA, Alnuaimi AS, Zainel AJ, A/Qotba HA. Prevalence of non-communicable diseases by age, gender and nationality in publicly funded primary care settings in Qatar. BMJ Nutr Prev Health 2019; 2:20–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ioannidis JP. The infection fatality rate of COVID-19 inferred from seroprevalence data.2020. Available at: https://www.medrxiv.org/content/10.1101/2020.05.13.20101253v1.full.pdf. Accessed 2 July 2020.
- 40.Meyerowitz-Katz G, Merone L. A systematic review and meta-analysis of published research data on COVID-19 infection fatality rates. Int J Infect Dis 2020; 101:138–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hauser A, Counotte MJ, Margossian CC, et al. . Estimation of SARS-CoV-2 mortality during the early stages of an epidemic: a modeling study in Hubei, China, and six regions in Europe. PLoS Med 2020; 17:e1003189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Abu-Raddad LJ, Chemaitelly H, Malek JA, et al. . Assessment of the risk of SARS-CoV-2 reinfection in an intense re-exposure setting. Clin Infect Dis 2020; 14;ciaa1846.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Anand S, Montez-Rath M, Han J, et al. . Prevalence of SARS-CoV-2 antibodies in a large nationwide sample of patients on dialysis in the USA: a cross-sectional study. Lancet 2020; 396:1335-44.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Havers FP, Reed C, Lim T, et al. . Seroprevalence of antibodies to SARS-CoV-2 in 10 Sites in the United States, March 23-May 12, 2020. JAMA Intern Med. 2020; doi: 10.1001/jamainternmed.2020.4130 [DOI] [PubMed] [Google Scholar]
- 45.Wu SL, Mertens AN, Crider YS, et al. . Substantial underestimation of SARS-CoV-2 infection in the United States. Nat Commun 2020; 11:4507, –4517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Stringhini S, Wisniak A, Piumatti G, et al. . Seroprevalence of anti-SARS-CoV-2 IgG antibodies in Geneva, Switzerland (SEROCoV-POP): a population-based study. Lancet 2020; 396:313–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Jahrsdörfer B, Kroschel J, Ludwig C, et al. . Independent side-by-side validation and comparison of 4 serological platforms for SARS-CoV-2 antibody testing. J Infect Dis 2021; 223:796–801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Public Health England. Evaluation of Roche Elecsys AntiSARS-CoV-2 serology assay for the detection of anti-SARS-CoV-2 antibodies.2020. Available at: https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/891598/Evaluation_of_Roche_Elecsys_anti_SARS_CoV_2_PHE_200610_v8.1_FINAL.pdf. Accessed 5 June 2020.
- 49.Sempos CT, Tian L. Adjusting coronavirus prevalence estimates for laboratory test kit error. Am J Epidemiol 2021; 190:109–15. [DOI] [PMC free article] [PubMed] [Google Scholar]