Abstract
Background
People with autoimmune or inflammatory conditions taking immunomodulatory/suppressive medications may have higher risk of novel coronavirus disease 2019 (COVID-19). Chronic disease care has also changed for many patients, with uncertain downstream consequences.
Methods
We included participants with autoimmune or inflammatory conditions followed by specialists at Johns Hopkins. Participants completed periodic surveys querying comorbidities, disease-modifying medications, exposures, COVID-19 testing and outcomes, social behaviors, and disruptions to healthcare. We assessed whether COVID-19 risk is higher among those on immunomodulating or suppressive agents and characterized pandemic-associated changes to care and mental health.
Results
In total, 265 (5.6%) developed COVID-19 over 9 months of follow-up (April–December 2020). Patient characteristics (age, race, comorbidity, medications) were associated with differences in social distancing behaviors during the pandemic. Glucocorticoid exposure was associated with higher odds of COVID-19 in models incorporating behavior and other potential confounders (odds ratio [OR]: 1.43; 95% confidence interval [CI]: 1.08, 1.89). Other medication classes were not associated with COVID-19 risk. Diabetes (OR: 1.72; 95% CI: 1.08, 2.73), cardiovascular disease (OR: 1.68; 95% CI: 1.24, 2.28), and kidney disease (OR: 1.76; 95% CI: 1.04, 2.97) were associated with higher odds of COVID-19. Of the 2156 reporting pre-pandemic utilization of infusion, mental health or rehabilitative services, 975 (45.2%) reported disruptions therein, which disproportionately affected individuals experiencing changes to employment or income.
Conclusions
Glucocorticoid exposure may increase risk of COVID-19 in people with autoimmune or inflammatory conditions. Disruption to healthcare and related services was common. Those with pandemic-related reduced income may be most vulnerable to care disruptions.
Keywords: autoimmune disease, COVID-19, glucocorticoids, immune-modulating medications
A large study of people with autoimmune or inflammatory conditions finds that glucocorticoid exposure may increase risk of coronavirus disease 2019 (COVID-19). The study also suggests that in this population, those with pandemic-related reduced income or employment were most vulnerable to care disruptions.
In people with autoimmune or inflammatory conditions, there is concern that immunomodulatory medications used to treat these conditions may increase the risk of developing novel coronavirus disease 2019 (COVID-19) based on their mechanisms of action and data from earlier studies [1–7]. Most existing COVID-19 studies failed to account for social distancing behaviors. Such behavior differences may confound (or impact in unexpected ways) estimates of medication-associated risks (eg, individuals taking stronger immune-modulating/suppressive medications may perceive higher COVID-19 risk and thus more strictly practice social distancing or refrain from in-person socialization). Controlling for behavior is a critical component in analyses assessing COVID-19 risk associated with exposure to immunomodulatory/suppressive medications.
Beyond COVID-19 risk concerns, the pandemic has altered care in many chronic conditions. For example, many patients have discontinued, lowered, or delayed their medication or refrained from obtaining critical safety laboratory tests [8, 9]. Others experienced disruptions to infusion, rehabilitative, homecare, or mental health services. Currently, limited research has assessed 1) which patients are most vulnerable to care disruption and 2) the downstream effects of these disruptions on disease outcomes. The impact of such changes may be very significant, as they may affect the majority of patients with autoimmune or inflammatory conditions, not only those who develop COVID-19.
We sought to assess whether risk for COVID-19 is higher among those on immunomodulating or suppressive agents (after accounting for behavior) and to quantify and identify consequences of pandemic-associated changes to longitudinal outpatient care and mental health in this population. To do so, we established COVID-19 Risk with Immune-modulating Medication Study (COVID-RIMS), a cohort study of nearly 5000 individuals with a variety of autoimmune and inflammatory conditions managed as outpatients by specialists at the Johns Hopkins Medical Institutions.
METHODS
Study Population
We established the COVID-RIMS study in April 2020. Eligible participants had autoimmune or inflammatory conditions (included diagnoses listed in Supplementary Table 1) and had been seen by relevant specialists at Johns Hopkins within the past 3 years. Participants had at least 2 ICD-10 codes associated with a particular disorder in the electronic medical record at 2 separate visits prior to study initiation. Patients were invited to participate in COVID-RIMS through an individualized e-mail link.
Survey Assessments
From April through June 2020, COVID-RIMS participants answered weekly online questionnaires, and from July 2020 onward, they completed surveys on an approximately monthly basis. Surveys queried COVID-19 testing and results, participant characteristics (including demographic, medication, and comorbidity), behaviors, mental health, and pandemic-associated changes to healthcare; a more detailed description is provided in the Supplementary methods.
Assessment of COVID-19 Disease
In each survey, participants were asked several COVID-19-related questions including whether 1) a health provider ever suspected them of having COVID-19, 2) they had tested positive for COVID-19, or 3) had received a positive COVID-19 serology assessment. In addition, Johns Hopkins also maintains a COVID-19 registry in which all tests, results, and COVID-19 outcomes performed within the state of Maryland or District of Columbia (via the Chesapeake Regional Information System for our Patients [CRISP], a health information exchange resource for Maryland/District of Columbia) are automatically uploaded into a database designed for research; we linked participants in COVID-RIMS with this registry to allow for maximum case capture. Participants with self-reported positive COVID-19 tests or serum antibody testing, self-reported healthcare provider suspected COVID-19 (but were never tested), and those who had tested positive in CRISP were included as cases. We performed sensitivity analyses excluding individuals with suspected COVID-19 from the case definition.
Statistical Analysis
Initial analyses compared demographic characteristics of invited participants versus those who agreed to participate. We evaluated non-medication associated risk factors for COVID-19 (see Supplementary methods) using logistic regression, as exact timing of infection was not always clear. Primary analyses assessed the association between exposure to immunomodulatory agent classes and odds of COVID-19 using multivariable-adjusted logistic regression models. A full list of individual medications considered, and their associated class, are included in the Supplementary methods. We also assessed COVID-19 risk associated with medication classes stratified by disorders and individual medication exposures for medication classes or medications in which at least 10 COVID-19 cases were recorded. We then evaluated predictors of interruptions to care using logistic regression models. Finally, we assessed how depression and anxiety symptoms changed longitudinally and evaluated predictors of higher overall symptom burden using mixed effects models. Statistical calculations were performed with R software, version 3.6.2 [10].
RESULTS
Of the invited 22 516 eligible patients, 4666 (20.9%) agreed to participate and completed at least one follow-up survey as of December 2020. Multiple sclerosis (MS), Sjogren’s syndrome, and rheumatoid arthritis were the most common qualifying conditions represented, with 878, 741, and 545 individuals, respectively (Supplementary Table 1). COVID-RIMS participants were more likely to be female (76.7% vs 72.3%), white (83.1% vs. 63.2%) and have higher socioeconomic status (SES) (mean ADI [SD] 23.7 [20.4] vs 31.2 [24.4]) relative to nonresponders (Supplementary Table 2). COVID-RIMS participants completed weekly (and later monthly) surveys during follow-up for a total of 10 surveys (baseline + 9 follow-up surveys); a median of 8 (interquartile range [IQR]: 5–9) follow-up surveys were completed.
Table 1 summarizes responder characteristics overall and by COVID-19 status; 2187 (46.7%) report having been tested at least once for COVID-19, and 265 (5.7%) reported positive COVID-19 results during follow-up. For all responders, 4161 (89.1%) reported ever being treated with an immune-modulating/suppressing medication, 167 (3.6%) were smokers, 1344 (28.8%) were obese, and 2736 (58.6%) had at least 1 comorbidity potentially associated with more severe COVID-19 disease or COVID-19 related hospitalization (eg, hypertension, diabetes, cardiovascular disease [CVD], lung diseases, chronic kidney disease [CKD], stroke, cancer); of the medical comorbidities considered, hypertension (30.8%) and CVD (18.8%) were most common. Some characteristics (male sex, Black race, comorbidity burden, exposure to more immune modulating agents) and behaviors (working onsite, in-person socialization, changes to employment related to COVID-19 or capacity to pay for disorder-associated costs) were associated with an increased odds of ever being tested for COVID-19 (Supplementary Table 3).
Table 1.
Characteristics of COVID-RIMS Participant by COVID-19 Status
| Overall | No COVID-19 | COVID-19+ | P valuea | |
|---|---|---|---|---|
| N | 4666 | 4401 | 265 | |
| Age | 55.10 (13.77) | 55.28 (13.77) | 52.19 (13.46) | <.001 |
| Male sex | 1086 (23.3) | 1036 (23.5) | 50 (18.9) | .094 |
| Smoker | 167 (3.6) | 159 (3.6) | 8 (3.0) | .737 |
| Race | .212 | |||
| White | 3877 (83.1) | 3662 (83.2) | 215 (81.1) | |
| Asian | 125 (2.7) | 122 (2.8) | 3 (1.1) | |
| Black or African American | 447 (9.6) | 413 (9.4) | 34 (12.8) | |
| Other | 171 (3.7) | 161 (3.7) | 10 (3.8) | |
| Unknown | 45 (1.0) | 42 (1.0) | 3 (1.1) | |
| Hispanic or Latino | 146 (3.1) | 136 (3.1) | 10 (3.8) | .661 |
| In person socializing over follow-up | 2737 (58.7) | 2559 (58.1) | 178 (67.2) | .005 |
| In person socializing at baseline | 851 (18.2) | 798 (18.1) | 53 (20.0) | .495 |
| Employment status change due to COVID-19 pandemic | 548 (11.7) | 498 (11.3) | 50 (18.9) | <.001 |
| Working onsite | 1138 (24.4) | 1041 (23.7) | 97 (36.6) | <.001 |
| Change in ability to pay for care associated costs | 608 (13.0) | 546 (12.4) | 62 (23.4) | <.001 |
| BMI | 29.51 (7.45) | 29.47 (7.39) | 30.23 (8.33) | .116 |
| Comorbidity | ||||
| Stroke | 159 (3.4) | 150 (3.4) | 9 (3.4) | 1.000 |
| Asthma | 748 (16.0) | 696 (15.8) | 52 (19.6) | .120 |
| Cardiovascular disease (CVD) | 877 (18.8) | 814 (18.5) | 63 (23.8) | .040 |
| Hypertension | 1437 (30.8) | 1362 (30.9) | 75 (28.3) | .402 |
| Chronic kidney disease (CKD) | 195 (4.2) | 178 (4.0) | 17 (6.4) | .086 |
| Diabetes | 283 (6.1) | 261 (5.9) | 22 (8.3) | .150 |
| Cancer | 544 (11.7) | 515 (11.7) | 29 (10.9) | .783 |
| Lung | 520 (11.1) | 485 (11.0) | 35 (13.2) | .318 |
| Number of comorbidities | 1.02 (1.14) | 1.01 (1.13) | 1.14 (1.22) | .079 |
| Number of autoimmune conditions | 1.33 (0.66) | 1.33 (0.65) | 1.40 (0.83) | .083 |
| Ever treated with immune modulating medication | 4161 (89.2) | 3915 (89.0) | 246 (92.8) | .062 |
| Changes to care | ||||
| Any disruption in care/services | 975 (45.2) | 903 (44.6) | 72 (55.0) | .026 |
| Delay in infusion | 341 (29.4) | 315 (28.7) | 26 (41.9) | .038 |
| Delay in rehab services | 623 (57.6) | 575 (57.2) | 48 (63.2) | .373 |
| Delay in mental health services | 211 (28.9) | 189 (27.9) | 22 (40.7) | .065 |
| Delay in home care services | 65 (25.6) | 58 (24.7) | 7 (36.8) | .371 |
Abbreviations: BMI, body mass index; COVID-19, coronavirus disease 2019; COVID-RIMS, COVID-19 Risk with Immune-modulating Medication Study.
a P values are derived from univariate generalized linear models using a univariate test for differences between COVID-19 cases versus those with no reported evidence of COVID-19.
Some patient characteristics were associated with social distancing behavior differences. Younger individuals, men, non-White individuals, those with lower SES, those with greater comorbidity burden, and those with exposure to more immune modulating/suppressing agents were less likely to socialize in person (Supplementary Table 4). For example, individuals exposed to 3+ immune modulating/suppressing agents in the past year were 20% less likely to socialize in person relative to those who did not report using such medications in the past year (odds ratio [OR]: 0.80; 95% confidence interval [CI]: .65, .97). In contrast, individuals working on site were over 2-fold more likely to report socializing in person (OR: 2.28; 95% CI: 1.96, 2.65). Results were consistent when evaluating predictors of not wearing a mask over follow-up (Supplemental Table 4).
Factors Associated With Increased Odds of Contracting COVID-19
Younger age, comorbidity burden, working onsite, in-person socialization, and having a pandemic-associated change in ability to pay for disorder-associated costs were each associated with increased odds of COVID-19 in multivariable-adjusted models (Table 2). Diabetes (OR: 1.72; 95% CI: 1.08, 2.73), CVD (OR: 1.68; 95% CI: 1.24, 2.28), or CKD (OR: 1.76; 95% CI: 1.04, 2.97) were individually associated with COVID-19 odds in multivariable-adjusted models (Supplementary Table 5). Results were similar when stratified by disorder; there was no significant heterogeneity when pooling results across disorder for estimated ORs.
Table 2.
Association Between Participant Characteristics and COVID-19 Risk in COVID-RIMS Participants
| Univariate | Multivariablea | |||
|---|---|---|---|---|
| OR (95% CI) | P value | OR (95% CI) | P value | |
| Age (per 10 years) | 0.85 (.78, .93) | .0004 | 0.86 (.77, .95) | .004 |
| Male sex | 0.76 (.55, 1.04) | .08 | 0.86 (.61, 1.19) | .361 |
| Race | ||||
| White | 1.00 [ref] | 1.00 [ref] | ||
| Asian | 0.42 (.13, 1.33) | .14 | 0.42 (.13, 1.33) | .139 |
| Black/African American | 1.40 (.96, 2.04) | .08 | 1.30 (.88, 1.94) | .188 |
| Other | 1.06 (.55, 2.03) | .87 | 1.03 (.53, 2.00) | .938 |
| Unknown | 1.22 (.37, 3.96) | .74 | 1.31 (.40, 4.34) | .654 |
| Low SES (<25th percentile of ADI) | 1.48 (1.03, 2.13) | .04 | 1.37 (.93, 2.01) | .111 |
| Number of autoimmune/inflammatory conditions | 1.16 (.98, 1.37) | .09 | 1.15 (.96, 1.36) | .125 |
| Ever exposed to an immune modulating agent | 1.61 (1.00, 2.59) | .05 | 1.45 (.82, 2.54) | .201 |
| Number of autoimmune immune modulating agents exposed to in past year | ||||
| 0 | 1.00 [ref] | 1.00 [ref] | ||
| 1 | 1.08 (.79, 1.47) | .65 | 0.96 (.67, 1.37) | .827 |
| 2 | 1.32 (.92, 1.90) | .13 | 1.10 (.74, 1.65) | .637 |
| 3+ | 1.54 (1.05, 2.24) | .03 | 1.15 (.76, 1.76) | .507 |
| Obesity | 0.88 (.67, 1.17) | .38 | 0.81 (.61, 1.09) | .161 |
| Number of comorbiditiesb | 1.10 (.99, 1.22) | .08 | 1.17 (1.04, 1.31) | .008 |
| Working onsite | 1.87 (1.44, 2.42) | <.0001 | 1.65 (1.25, 2.19) | <.0001 |
| Current smoker | 0.83 (.40, 1.71) | .61 | 0.75 (.36, 1.56) | .435 |
| Socializing in person at baseline | 1.13 (.83, 1.54) | .445 | 1.44 (1.09, 1.90) | .011 |
| Socializing in person at any point in follow-up | 1.47 (1.13, 1.92) | .004 | 0.99 (.71, 1.37) | .94 |
| Change in employment status due to COVID-19 | 1.82 (1.32, 2.51) | .0003 | 1.35 (.96, 1.91) | .089 |
| Change in ability to pay for disorder associated costs | 2.16 (1.60, 2.91) | <.0001 | 1.80 (1.30, 2.48) | <.0001 |
Abbreviations: CI, confidence interval; COVID-19, coronavirus disease 2019; COVID-RIMS, COVID-19 Risk with Immune-modulating Medication Study; OR, odds ratio; SES, socioeconomic status.
aMutually adjusts for all variables included in the table.
bIncludes diabetes, cardiovascular disease (CVD), lung disease (chronic obstructive pulmonary disease [COPD], interstitial lung disease, pulmonary hypertension), stroke, asthma, chronic kidney disease (CKD), hypertension, cancer.
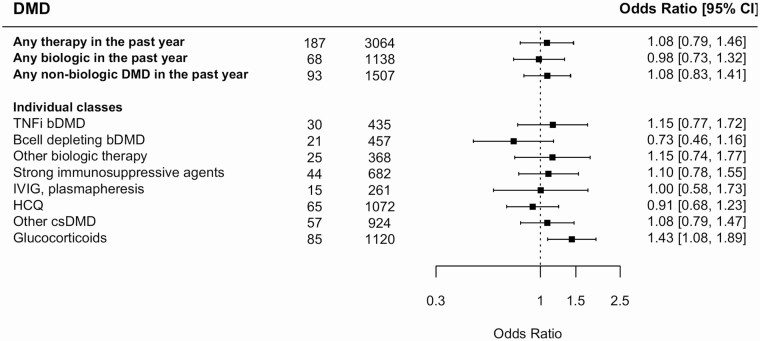
When considering immune medications, glucocorticoid use in the past year was associated with 43% increased odds of COVID-19 (Figure 1; OR: 1.43; 95% CI: 1.08, 1.89) in multivariable-adjusted models. With respect to glucocorticoid dose (sum of prednisone, methylprednisolone, and dexamethasone exposure), individuals reporting daily prednisone equivalent doses of 0.5 to 10 mg/day or ≥10 mg/day had respective ORs of COVID-19 of 1.65 (95% CI: 1.14, 2.41) and 1.48 (95% CI: 1.00, 2.19) relative to those without exposure. (Supplementary Figure 1). Results were relatively consistent in analyses stratified by disorder (Supplementary Table 6). Beyond glucocorticoids, other medication classes didn’t appear to be associated with COVID-19 risk. In sensitivity analyses, when we reclassified sphingosine-1-phosphate inhibitors as strong (rather than conventional) immunosuppressive agents or reclassified mycophenolate mofetil, azathioprine, or mercaptopurine as conventional (rather than strong) disease-modifying drugs (DMD), the findings were unaltered. Likewise, removing natalizumab or vedolizumab from biologic therapies (since they may not affect peripheral immune responses) also didn’t alter findings. Except for prednisone use in the past year (OR: 1.67; 95% CI: 1.21, 2.30), we did not find strong evidence of higher odds of COVID-19 associated with other individual medications (Supplementary Table 7).
Figure 1.
Association between immune-modulating or suppressive medications* and risk of COVID-19. Odds ratios are adjusted for age, sex, race, SES, working on site, in person socialization habits (at baseline and during follow-up), smoking status, number of comorbidities, number of autoimmune or inflammatory condition diagnoses, and current smoking status. Abbreviations: CI, confidence interval; COVID-19, coronavirus disease 2019; DMD, disease modifying drug; SES, socioeconomic status; TNF, tumor necrosis factor.*For individuals medications included in each medication class, please refer to Supplementary Table 7.
Assessment of risk factors for COVID-19 were consistent when excluding individuals suspected of having, but not tested for, COVID-19 (50 individuals, leaving 215 COVID-19+ cases eligible for analyses). Results were also similar when stratifying by number of surveys completed (<median, ≥median) to account for potential differences in risk factor distribution between those with more or less complete data.
Interruptions to Care or Services
Of the 2156 individuals who reported receiving infusions, rehabilitative or mental health services prior to the pandemic, 942 (45.2%) experienced an interruption to any services during the pandemic; 341 of 1158 (29.4%) delayed infusions, 623 of 1081 (57.6%) had an interruption in rehabilitative service, and 211 of 731 (28.8%) had interrupted mental healthcare (Table 3 and Supplementary Table 8). Those who experienced a change in their ability to pay for disorder-associated costs were nearly 2-fold more likely to have an interruption in services. Similarly, COVID-19-related changes to employment (eg, furlough or termination) were also associated with increased risk of service interruption (OR: 1.34; 95% CI: 1.02, 1.76). Those with moderate to severe anxiety (as defined as >1 SD above the mean T-scores from PROMIS measures) were 53% more likely to report an interruption in services (OR: 1.53; 95% CI: 1.20, 1.94). Men were less likely to experience an interruption in services (OR: 0.79; 95% CI: .64, .99). Findings were generally similar when considering specific reasons for disruption to services (eg, infusions, rehabilitative services, or mental health).
Table 3.
Association Between Patient Characteristics and Disruption to Routine Healthcare or Related Services
| Any Disruption in Servicesa (975 of 2156) | ||||
|---|---|---|---|---|
| Univariate | Multivariableb | |||
| OR (95% CI) | P value | OR (95% CI) | P value | |
| Age, per 10 years | 1.01 (.95, 1.08) | .659 | 1.03 (.96, 1.11) | .355 |
| Male sex | 0.73 (.59, .89) | .003 | 0.79 (.64, .99) | .039 |
| Low SES | 0.99 (.74, 1.33) | .970 | 0.94 (.69, 1.29) | .718 |
| Race | ||||
| White | 1.00 [ref] | … | 1.00 [ref] | … |
| Asian | 1.12 (.60, 2.10) | .725 | 1.14 (.59, 2.20) | .706 |
| Black/African American | 1.12 (.83, 1.52) | .449 | 1.03 (.75, 1.43) | .843 |
| Other | 1.33 (.85, 2.10) | .209 | 1.23 (.77, 1.97) | .388 |
| Unknown | 0.82 (.34, 2.03) | .675 | 0.82 (32, 2.10) | .677 |
| Number of comorbiditiesc | 1.10 (1.03, 1.19) | .008 | 1.07 (.99, 1.16) | .097 |
| Obesity | 0.87 (0.72, 1.04) | .13 | 0.89 (.73, 1.08) | .229 |
| Moderate to severe anxiety | 1.64 (1.32, 2.03) | <.0001 | 1.53 (1.20, 1.94) | .0004 |
| History of depression | 1.06 (.88, 1.27) | .556 | 0.92 (.75, 1.13) | .443 |
| Number of autoimmune diagnoses | 1.12 (.99, 1.27) | .073 | 1.06 (.93, 1.21) | .374 |
| Ever treated with an immune-modulating medication | 1.03 (.77, 1.37) | .853 | 0.99 (.73, 1.35) | .95 |
| Working onsite | 0.71 (.57, .87) | .001 | 0.71 (.57, 0.90) | .004 |
| In person socializing over follow-up | 0.88 (.70, 1.10) | .263 | 0.95 (.75, 1.20) | .667 |
| In person socializing at baseline | 0.80 (.68, .96) | .014 | 0.86 (.71, 1.04) | .114 |
| COVID-19 infection | 1.52 (1.06, 2.16) | .022 | 1.35 (0.93, 1.96) | .115 |
| Change in ability to pay for disorder associated costs | 2.26 (1.80, 2.84) | <.0001 | 1.96 (1.54, 2.50) | <.0001 |
| Change in employment status due to COVID-19 | 1.48 (1.15, 1.90) | .002 | 1.34 (1.02, 1.76) | .034 |
Abbreviations: CI, confidence interval; COVID-19, coronavirus disease 2019; OR, odds ratio.
aIncludes any disruption to infusions, mental health, or rehabilitative services.
bMutually adjusts for all variables included in the table.
cIncludes diabetes, cardiovascular disease (CVD), lung disease (chronic obstructive pulmonary disease [COPD], interstitial lung disease, pulmonary hypertension), stroke, asthma, chronic kidney disease (CKD), hypertension, cancer.
Overall Burden of Anxiety and Depression
Over follow-up, participants reported average anxiety and depressive symptom T-scores of 51.8 [9.5] and 49.9 [8.5], respectively. Younger participants, men, and Black/African Americans tended to report a lower burden of anxiety and depression over time (eg, lower T-scores), whereas previous self-reported previous physician-diagnosed depression was associated with substantially higher anxiety and depression symptom burden (Table 4). Several pandemic-associated factors were also associated with higher symptom levels. For example, pandemic-associated changes in ability to pay for disorder-associated costs were associated with 2.17 (1.33, 3.01) points higher anxiety and 2.45 (1.70, 3.20) points higher depressive symptom T-scores. COVID-19 related changes to employment were also associated with small increases in anxiety and depressive symptoms, though these changes may not be clinically meaningful (for anxiety: 0.73 points higher; 95% CI: .10, 1.35; for depression: 0.80; 95% CI: .25, 1.34).
Table 4.
Patient Characteristics Associated With Overall Anxiety and Depressive Symptom Burden
| Anxiety | Depression | |||
|---|---|---|---|---|
| Characteristic | Mean Difference in Symptomsa (95% CI) | P value | Mean Difference in Symptomsa (95% CI) | P value |
| Age, per 10 years | −1.37 (−1.64, −1.10) | <.0001 | −0.68 (−.92, −.43) | <.0001 |
| Male sex | −3.44 (−4.19, −2.68) | <.0001 | −1.06 (−1.75, −.38) | .002 |
| Race | ||||
| white | ||||
| Asian | −1.54 (−3.34, .26) | .093 | −0.52 (−2.15, 1.12) | .536 |
| Black/African American | −1.84 (−2.94, −.73) | .001 | −1.11 (−2.11, −.11) | .03 |
| Other | 0.17 (−1.49, 1.82) | .844 | 0.06 (−1.45, 1.57) | .936 |
| Unknown | 2.30 (−1.03, 5.62) | .176 | 1.45 (−1.57, 4.48) | .346 |
| Low SES | 0.81 (−.31, 1.94) | .156 | 0.83 (−.20, 1.85) | .114 |
| Number of comorbiditiesb | 0.29 (−.04, .63) | .089 | 0.26 (−.04, .57) | .094 |
| History of depression | 4.89 (4.09, 5.70) | <.0001 | 5.97 (5.24, 6.70) | <.0001 |
| Obesity | 0.14 (−.55, .84) | .686 | −0.07 (−.70, .56) | .83 |
| Change in immune-modulating therapy | 0.09 (−.44, .62) | .735 | −0.02 (−.49, .45) | .942 |
| In person socialization | −0.42 (−.75, −.09) | .014 | −0.42 (−.71, −.13) | .004 |
| Working onsite | −0.20 (−.68, .28) | .413 | −0.28 (−.71, .14) | .187 |
| Change in ability to pay for disorder-associated costs | 2.17 (1.33, 3.01) | <.0001 | 2.45 (1.70, 3.20) | <.0001 |
| Change in employment status due to COVID-19 | 0.73 (.10, 1.35) | .023 | 0.80 (.25, 1.34) | .004 |
| COVID-19 infection | −0.38 (−1.68, .91) | .562 | −0.84 (−2.02, .34) | .164 |
Abbreviations: CI, confidence interval; COVID-19, coronavirus disease 2019; OR, odds ratio.
aMean difference in T-scores for anxiety and depression are estimated from a mixed effect model allowing for multiple assessments per person and also adjusting for follow-up time (categorically as month of follow-up). All estimates are adjusted simultaneously for all other variables included in the table.
bIncludes diabetes, cardiovascular disease (CVD), lung disease (chronic obstructive pulmonary disease [COPD], interstitial lung disease, pulmonary hypertension), stroke, asthma, chronic kidney disease (CKD), hypertension, cancer.
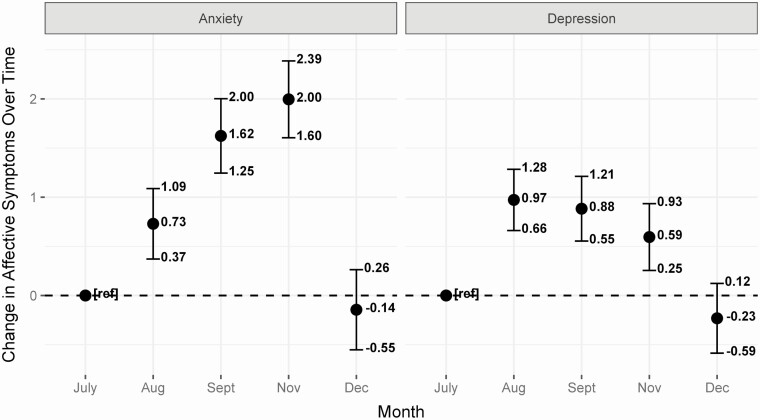
Over the course of follow-up, the burden of depression and anxiety symptoms changed non-linearly. Peak anxiety levels occurred in November (Figure 2; 2.00 points higher; 95% CI: 1.60, 2.39). Depressive symptoms peaked slightly in August (0.97 points higher; 95% CI: .66, 1.28), although this difference may not be clinically meaningful. Longitudinal changes in anxiety and depression were similar across different conditions and across different medication classes.
Figure 2.
Change in anxiety (left) and depression (right) occurring over the course of study follow-up. Mean differences are adjusted for age, sex, race, SES, working on site, in person socialization, number of comorbidities, number of autoimmune or inflammatory condition diagnoses, COVID-19 infection, change in employment due to COVID-19, and changes in ability to pay for disorder-associated costs. Abbreviations: COVID-19, coronavirus disease 2019; SES, socioeconomic status.
Discussion
Herein we assessed risk factors for COVID-19 in a large cohort of patients with known autoimmune or inflammatory conditions in a well-phenotyped prospective registry at Johns Hopkins. Adherence to in person socialization recommendations was non-uniformly distributed. Consistent with existing evidence, individuals working onsite or socializing in-person had higher odds of contracting SARS-CoV-2 and developing COVID-19 disease. In addition, diabetes, CKD, and CVD were associated with higher COVID-19 odds. With respect to immunomodulatory/suppressive medications, any glucocorticoid use in the past year was associated with increased odds of COVID-19, but other medications classes or individual medications themselves didn’t appear to be associated with COVID-19 risk. Our results were consistent in sensitivity analyses, where we varied both definitions for medication exposure and COVID-19 case definitions. Beyond COVID-19 disease, general interruptions to healthcare were common; individuals with changes in their ability to pay for disorder-associated costs as well as those who experienced a COVID-19-related change to employment were most vulnerable to care disruptions. Overall, these findings suggest that as the COVID-19 pandemic continues to cause considerable morbidity and mortality, people with autoimmune or inflammatory disorders may be particularly vulnerable to impacts it has on maintaining healthcare access.
Exposure to glucocorticoids may increase odds of COVID-19, with a potential non-linear association between total glucocorticoid dose and COVID-19 risk. Glucocorticoids were among the most common medications used in this population, and many previous studies suggest a link between chronic glucocorticoid exposure and infection risk in people with autoimmune or inflammatory disorders as well worse COVID-19 outcomes in rheumatology patients [11–15]. Although it’s likely our sample size and number of COVID-19 cases precluded us from identifying the precise COVID-19 risk associated with all DMDs considered, strong risk signals were not noted for more broad classifications of many of the common DMDs herein. In the MS population, some prior studies had indicated an association between anti-CD20 therapies and risk of COVID-19; these studies did not account for social distancing behaviors, which we observed were non-uniformly distributed across patients [1, 2, 7, 16]. At the same time, the populations studied (in Europe) may have other characteristics that underlie the different outcomes. Most publications evaluating risk in other autoimmune disease evaluated risk of more severe disease rather than illness as a whole [8, 17, 18]. With the success of recent COVID-19 vaccine trials, a critical next step will be to determine if and how common immunomodulating/suppressive medications or specific medication classes (eg, B-cell depleting therapies) affect vaccination response, as has been shown for other vaccines [19, 20].
Notably, nearly half of participants who reported receiving infusion, rehabilitative, or mental health services reported a pandemic-related disruption to care. These results set the stage for future studies in this cohort assessing the downstream consequences of these changes to disease-specific outcomes, especially as certain subgroups of patients (eg, those with changes to household income) may be particularly vulnerable to these potential effects.
Finally, we also note nonlinear changes in the burden of depression and anxiety. The sharp decrease in the trend of symptom burden between November and December could be related to announcements of the success of large COVID-19 vaccine effectiveness trials, which occurred in this period. It is also worth noting that the burden of mental health comorbidities is generally higher in many autoimmune and inflammatory disease populations relative to the general population [21–25]; extended periods of social isolation may exaggerate symptoms of depression and anxiety in an already vulnerable population, an important observation for providers to remember when caring for such individuals.
Our study has a number of important strengths. Our study is relatively distinct from prior studies of individuals taking immune medications, which have largely focused on risk factors for poor COVID-19 outcomes. We included a large population of nearly 5000 individuals with autoimmune or inflammatory conditions who are already followed by specialists at a large health system and thus may limit some biases related to right censoring inherent to studies including only hospitalized patients. Our study was also longitudinal and included information from patients collected at 10 different time points over the course of follow-up. We also included assessments of social distancing behavior in analyses to ensure that any observed differences in medication-associated risks were not driven by differences in behavior. Finally, surveys included information related to disruptions to care that affected a substantial proportion of participating patients. To date, most reports have focused on recommendations from providers, whereas we collected such information on the observed burden in patients, which we found to be non-uniform.
Important limitations of our study are worth noting. First, we lacked detailed information on exact timing of COVID-19 infection, so we could not incorporate this information into analyses or assess potential time-varying variables. Future studies using this cohort will restrict to the subset of cases for which timing of infection is well delineated. We also could miss potential COVID-19 cases if participants were lost to follow-up or were not tested for COVID-19 in Maryland/District of Columbia, although follow-up was relatively complete (median 8 of 9 follow-up surveys). Furthermore, we used self-reported medications, comorbidities, COVID-19 testing, and result status. We also note that certain participant characteristics and behaviors were associated with being tested for COVID-19, which could have confounded our ability to detect certain risk factors for COVID-19.
We also didn’t collect detailed information on glucocorticoid exposure duration or medication dosages (beyond glucocorticoids), which could possibly lead to misclassification. We have no measure of activity of autoimmune or inflammatory conditions prior to the pandemic, which has been shown to affect COVID-19 outcomes, and it’s possible glucocorticoid use is an indicator for disease activity rather than a causally associated with COVID-19 [15]. Lower SES individuals may have more limited access to biologic medications or infusions services, and glucocorticoid use could serve as a surrogate for low SES. Therefore, although we adjusted for SES using the ADI (a neighborhood-level indicator), it’s also possible that participant-level contributors to SES were not accounted for and could have confounded the observed association. We also identified eligible participants using diagnostic codes, which may inaccurately identify patients. Nonetheless, we required ≥2 codes for a specific disease/condition from providers in specific specialty departments to reduce potential misclassification. Results are also derived from individuals with email addresses who receive care at Johns Hopkins and responded to our initial survey invitation and may not apply to the larger group of patients or more broadly to people with autoimmune disease. Responders were more likely to be White and have higher SES, so it’s possible our results have underestimated the impact of the pandemic in vulnerable groups.
CONCLUSION
Our findings are in line with existing research studies suggesting that exposure risks are strong risk-factors for contracting COVID-19. Other risk factors include a high comorbidity burden or a previous exposure to glucocorticoids. Disruption to healthcare and important related services were common, and non-universally distributed across patients. Those with pandemic-related changes to income (largely those with lower SES) may be a particular vulnerable subgroup, but providers should be mindful of potential delays of infusion therapies and disruption to care in general caused by COVID-19.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Acknowledgments. We would like to acknowledge the following COVID-RIMS investigators for their contributions to the study: Brendan Antiochos, Peter Calabresi, Laura Cappelli, Reezwana Choudhury, Jonathan Chrispin, Andrea Corse, Dana DiRenzo, Mark Donowitz, Matthew Elrick, Lisa Fox, Thomas Grader Beck, Regina Greco, Lindsey Hayes, Laura Hummers, Mohammed Khoshnoodi, Michael Kornberg, Nicholas Maragakis, Justin McArthur, Brett McCray, Joanna Melia, Zsuzsanna McMahan, Joanna Melia, John Miller, Brett Morrison, Lyle Ostrow, Alyssa Parian, Florin Selaru, Philip Seo, George Stojan, Harikrishna Tandri, Fredrick Wigley, and Huimin Yu.
Financial support. K. C. F. is supported by grant 1K01MH121582-01 from the National Institutes of Health (NIH)/ National Institute of Mental Health (NIMH) and grant TA-1805–31136 from the National MS Society (NMSS). C. A. M. is supported by grant K23AR075898-02 from NIH/NIAMS.
Potential conflicts of interest. K. C. F. reports salary support (NIH/ NIMH 1K01MH121582-01, NMSS TA-1805–31136) during the conduct of the study. K. C. F. reports receiving NIH/NICHD grant number HD101056 during the last 36 months. C. A. M. reports receiving grant number NIAMS K23AR075898 during the conduct of the study; C. A. M. also reports grants/contracts from Bill and Melinda Gates Foundation and Jerome L Greene Foundation, during the last 36 months. E. S. S. has served on scientific advisory boards for Alexion, Viela Bio, and Genetech, and has received speaker honoraria from Viela Bio and Biogen; E. S. S. also reports grants/contracts from NIH and National Multiple Sclerosis Society, during the last 36 months. A. H. has received grants from NIH, Foundation for Peirpheral Neuropathy, and Dr Miriam and Sheldon G. Adelson Medical Research Foundation. He has received free medication from Chromadex for an investigator initiated clinical trial. He receives royalties for editorial duties from Annals of Clinical and Translational Neurology and Experimental Neurology. A.-M. O. has research grants from Amgen, Celgene, and Jerome L Greene Foundation, is site principal investigator (PI)/received grants/contracts for clinical trials sponsored by Abbvie, Amgen, Eli Lilly, and Novartis, all for contracted research, to John Hopkins University; and received consulting fees from Janssen, Novartis, Lilly, Pfizer, and UCB; holds leadership/fiduciary role with Medical Board National Psoriasis Foundation, all during the last 36 months. L. C.-S. reports receiving grants/contracts from Bill and Melinda Gates Foundation as Co-Investigator for COVID-19 CTA: Autoimmunity and COVID-19, during the last 36 months. A. N. B. is site PI for a clinical trial sponsored by Viela Bio and has served as a consultant for Abbvie, Bristol Myers Squibb and Novartis. He receives royalties from UpToDate. J. J. P. has received research grants for clinical trials from Pfizer Corporation. She is site PI for a clinical trial sponsored by Kezar. She has served as consultant for/received consulting fees from Alexion, Riovant, and EMD Serono. She received royalties from UpToDate. P. B. has received research support from GSK, EMD-Serono, Genentech and Amylyx pharmaceuticals, paid to their institution, and received honoraria from GSK, EMD-Serono and MedDay pharmaceuticals. S. D. N. has received consultant fees for scientific advisory boards from Biogen, Genentech, Celgene, EMD Serono, Novartis, Greenwich Biosciences, is an advisor for Autobahn Therapeutics and BioIncept, a clinical adjudication committee member for a medDay Pharmaceuticals clinical trial and has received research funding (paid directly to institution) from Biogen, Novartis, and Genentech. S. D. N. reports serving as a Steering Committee Member for North American COVID-19 Infections in Multiple Sclerosis and related diseases (COViMS) Registry Taskforce (unpaid); as a Steering Committee Member for National MS Society COVID-19 Vaccine Guidance Work Group (unpaid); as a Steering Committee Member for Johns Hopkins Outpatient Neurology COVID-19 Taskforce (unpaid), all during the last 36 months. T. E. L. has served as a consultant and site PI for a clinical trial sponsored by Orphazyme. He has served as a consultant for Third Rock Ventures, Dren Bio, and Aavogen, and receives royalties from UpToDate. B. J. S. reports being a member/on Scientific Advisory Board for Foundation for Sarcoidosis Research (unpaid), during the last 36 months. S. S. has received consulting fees from Medical Logix for the development of CME programs in neurology and has served on scientific advisory boards for Biogen, Genzyme, Genentech Corporation, EMD Serono and Celgene. He is the PI of investigator-initiated studies funded by Genentech Corporation and Biogen Idec (Investigator initiated study grant), received support from the Race to Erase MS foundation, and was the site investigator of a trial sponsored by MedDay Pharmaceuticals (Site PI for clinical trial). He has received equity compensation/holds stock or stock options for consulting from JuneBrain LLC, a retinal imaging device developer. He is the President Elect of the International MS Visual System Consortium, and a program committee member for Americas Committee for Treatment and Research in MS and is Editor for Multiple Sclerosis Journal—Experimental, Translational and Clinical. E. S. C. reports receiving grant NIH / NHLBI R01 HL136681 and grants for Novartis research site for: CMK389: A subject and investigator blinded, randomized, placebo-controlled, repeat-dose, multicenter study to investigate efficacy, safety, and tolerability of CMK389 in patients with chronic pulmonary sarcoidosis. E. S. C. also reports serving on scientific advisory board for Foundation for Sarcoidosis Research, www.stoparcoidosis.org (unpaid), all during the last 36 months. A. C. G. reports receiving grants as a Co-Investigator for Identifying Novel Biological Pathways for Gout by Integrating DNA Methylation and Genetics (grant number R01 AR073178–01). A. C. G. reports receiving $1000 honorarium as part of CME Educational Program and $750 for serving as NIH/NIAMS DSMB Chair, all during the last 36 months. C. O. B. has received research grants from BMS, paid to their institution/unrelated to current work, and served as a consultant to/received consulting fees from AbbVie, BMS, Eli Lilly, Gilead, Janssen, Moderna, Pfizer, Regeneron, Sanofi. He receives royalties from UpToDate (unrelated to current work). He serves on Participation on a Data Safety Monitoring Board/Advisory Board for Moderna, unrelated to current work, all during last 36 months. A. A. S. has research grants for clinical trials from Eicos Sciences, Inc, Medpace LLC and Kadmon Corp, in scleroderma, (unrelated to COVID-19). She receives royalties from UpToDate (unrelated to COVID-19). E. M. M. has received grants from Biogen (site PI of sponsored and investigator-initiated studies), is site PI for studies sponsored by Biogen and Genentech, has received free medication for a clinical trial from Teva, and receives royalties for editorial duties from UpToDate; is Steering Committee Member for MS PATHS (unpaid) for Biogen, during the last 36 months. All other authors report no potential conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Sormani MP. An Italian programme for COVID-19 infection in multiple sclerosis. Lancet Neurol 2020. Available at: https://www.thelancet.com/journals/laneur/article/PIIS1474-4422(20)30147-2/abstract. Accessed 21 May 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Zabalza A, Cárdenas‐Robledo S, Tagliani P, et al. . COVID-19 in multiple sclerosis patients: susceptibility, severity risk factors and serological response. Eur J Neurol n/a. Available at: https://onlinelibrary.wiley.com/doi/abs/10.1111/ene.14690. Accessed 20 January 2021. [DOI] [PubMed] [Google Scholar]
- 3. Doran MF, Crowson CS, Pond GR, O’Fallon WM, Gabriel SE. Frequency of infection in patients with rheumatoid arthritis compared with controls: a population-based study. Arthritis Rheum 2002; 46:2287–93. [DOI] [PubMed] [Google Scholar]
- 4. Wijnands JMA, Zhu F, Kingwell E, et al. . Disease-modifying drugs for multiple sclerosis and infection risk: a cohort study. J Neurol Neurosurg Psychiatry 2018; 89:1050–6. [DOI] [PubMed] [Google Scholar]
- 5. Monti S, Balduzzi S, Delvino P, Bellis E, Quadrelli VS, Montecucco C. Clinical course of COVID-19 in a series of patients with chronic arthritis treated with immunosuppressive targeted therapies. Ann Rheum Dis 2020; 79:667–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Slimano F, Baudouin A, Zerbit J, et al. . Cancer, immune suppression and coronavirus disease-19 (COVID-19): need to manage drug safety (French Society for Oncology Pharmacy [SFPO] guidelines). Cancer Treatment Rev 2020; 88. Available at: https://www.cancertreatmentreviews.com/article/S0305-7372(20)30101–8/abstract. Accessed 20 January 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Sormani MP, De Rossi N, Schiavetti I, et al. ; Musc-19 Study Group . Disease-modifying therapies and coronavirus disease 2019 severity in multiple sclerosis. Ann Neurol 2021; 89:780–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Zen M, Fuzzi E, Astorri D, et al. . SARS-CoV-2 infection in patients with autoimmune rheumatic diseases in northeast Italy: a cross-sectional study on 916 patients. J Autoimmun 2020; 112:102502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Moss BP, Mahajan KR, Bermel RA, et al. . Multiple sclerosis management during the COVID-19 pandemic. Mult Scler 2020; 112:1352458520948231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. R Core Team. R: a language and environment for statistical computing. Vienna, Austria. 2019. Available at: https://www.R-project.org/. [Google Scholar]
- 11. Fardet L, Petersen I, Nazareth I. Common infections in patients prescribed systemic glucocorticoids in primary care: a population-based cohort study. PLoS Med 2016; 13. Available at: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4878789/. Accessed 24 April 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Luna G, Alping P, Burman J, et al. . Infection risks among patients with multiple sclerosis treated with fingolimod, natalizumab, rituximab, and injectable therapies. JAMA Neurol 2020; 77:184–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Dixon WG, Abrahamowicz M, Beauchamp ME, et al. . Immediate and delayed impact of oral glucocorticoid therapy on risk of serious infection in older patients with rheumatoid arthritis: a nested case-control analysis. Ann Rheum Dis 2012; 71:1128–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Ethgen O, de Lemos Esteves F, Bruyere O, Reginster JY. What do we know about the safety of corticosteroids in rheumatoid arthritis? Curr Med Res Opin 2013; 29:1147–60. [DOI] [PubMed] [Google Scholar]
- 15. Strangfeld A, Schäfer M, Gianfrancesco MA, et al. . Factors associated with COVID-19-related death in people with rheumatic diseases: results from the COVID-19 Global Rheumatology Alliance physician-reported registry. Ann Rheumat Dis 2021. Available at: https://ard.bmj.com/content/early/2021/01/26/annrheumdis-2020–219498. Accessed 1 March 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Louapre C, Collongues N, Stankoff B, et al. . Clinical characteristics and outcomes in patients with coronavirus disease 2019 and multiple sclerosis. JAMA Neurol 2020; 77:1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Akiyama S, Hamdeh S, Micic D, Sakuraba A. Prevalence and clinical outcomes of COVID-19 in patients with autoimmune diseases: a systematic review and meta-analysis. Ann Rheumat Dis 2020. Available at: https://ard.bmj.com/content/early/2020/10/13/annrheumdis-2020–218946. Accessed 20 January 2021. [DOI] [PubMed] [Google Scholar]
- 18. Emmi G, Bettiol A, Mattioli I, et al. . SARS-CoV-2 infection among patients with systemic autoimmune diseases. Autoimmun Rev 2020; 19:102575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Bar-Or A, Calkwood JC, Chognot C, et al. . Effect of ocrelizumab on vaccine responses in patients with multiple sclerosis: the VELOCE study. Neurology 2020; 95:e1999–2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Bingham CO 3rd, Looney RJ, Deodhar A, et al. . Immunization responses in rheumatoid arthritis patients treated with rituximab: results from a controlled clinical trial. Arthritis Rheum 2010; 62:64–74. [DOI] [PubMed] [Google Scholar]
- 21. Bernardinello N, Alfieri V, Turatto C, et al. . Prevalence of depression in sarcoidosis: a comparison with asthmatic and healthy controls. Eur Respir J 2019; 54. Available at: https://erj.ersjournals.com/content/54/suppl_63/PA1952. Accessed 20 January 2021. [Google Scholar]
- 22. Zhang CK, Hewett J, Hemming J, et al. . The influence of depression on quality of life in patients with inflammatory bowel disease. Inflamm Bowel Dis 2013; 19:1732–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Koçer B, Tezcan ME, Batur HZ, et al. . Cognition, depression, fatigue, and quality of life in primary Sjögren’s syndrome: correlations. Brain Behav 2016; 6. Available at: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5167008/. Accessed 20 January 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Feinstein A, Magalhaes S, Richard JF, Audet B, Moore C. The link between multiple sclerosis and depression. Nat Rev Neurol 2014; 10:507–17. [DOI] [PubMed] [Google Scholar]
- 25. Peterson S, Piercy J, Blackburn S, Sullivan E, Karyekar CS, Li N. The multifaceted impact of anxiety and depression on patients with rheumatoid arthritis. BMC Rheumatol 2019; 3:43. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.