One year into the coronavirus disease 2019 (COVID-19) epidemic, data regarding a more detailed risk profile and treatment aspects of patients with immune-mediated kidney diseases and anti-neutrophil cytoplasmic antibody–associated vasculitis (AAV) have remained scarce. The International Registry of COVID infection in glomerulonephritis reported 40 patients with primary glomerulonephritis including AAV. Compared with 80 hospitalized controls, mortality and acute kidney rates were significantly higher in patients with glomerulonephritis [1]. A UK and Ireland Vasculitis Society analysis of 65 vasculitis patients found associations between comorbid respiratory disease and prescription of glucocorticoids with severe outcomes (Supplementary data, Table S1) [2].
Some treatment data are available from rheumatology registry studies. Immunosuppression and outcomes of 694 COVID-19 patients with inflammatory rheumatic and musculoskeletal diseases was reported from France. Older age, comorbidities including chronic kidney disease (CKD) and the use of glucocorticoids ≥10 mg/day or equivalent (prednisone) or rituximab were associated with COVID-19 severity [3]. In a recent publication from the COVID-19 Global Rheumatology Alliance of 3729 severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2)-infected patients, older age, male sex, CKD and other comorbidities were associated with COVID-19-related death, which occurred in 10.5%. Disease-specific factors such as moderate/high disease activity, a diagnosis of vasculitis and drugs such as rituximab were associated with mortality (Supplementary data, Table S1) [4]. In neither of these studies were differences between induction or maintenance treatment reported. In a real-life setting, rituximab has frequently been postponed during the pandemic to mitigate the risk of severe SARS-CoV-2 infections. Twenty-one of 206 studied patients with AAV had their rituximab maintenance treatment postponed. Relapses or flares of disease occurred in 12 of these cases. The disruption of care was highlighted, as this may have contributed to delayed relapse diagnosis [5]. These recent findings underline that rituximab-treated patients are at risk of developing severe COVID-19, but effective treatment needs to be maintained to reduce the risk of disease relapse in AAV.
RITUXIMAB AND INDUCTION/MAINTENANCE THERAPY
The impact of the pandemic has varied between countries and regions. Risk profiles of patient groups and therapies have emerged and become more apparent with cases of COVID-19 prior to and after AAV diagnosis, and in some cases concurrently. How to best treat new or relapsing AAV needs consideration since the pandemic is not over. Glucocorticoid therapy as induction should preferably be dosed in the range of 0.5 mg/kg/day or equivalent (prednisone) and reduced to <10 mg/day as soon as possible. Rituximab may also constitute an additional risk. However, it is essential to not delay effective treatment of new AAV and rapidly progressive glomerulonephritis and to treat relapses adequately. Cyclophosphamide or mycophenolatemofetil induction, or even methotrexate, depending on kidney function, may be alternatives [6].
Whether patients with rituximab maintenance therapy without or on a low dose of corticosteroids have lower COVID-19-associated risks needs to be studied further. Concurrently, rituximab has been discussed as a potential therapy in severe COVID-19. Some patients produce antibodies that functionally block the production of interferon-stimulated gene-expressing cells, which are associated with milder disease forms [7].
RITUXIMAB AND COVID-19 VACCINES
COVID-19 vaccines are the most promising approach to rein in the current pandemic. It is nevertheless possible that COVID-19 vaccine efficacy and response rates are lower in patients with immunosuppressive therapy and CKD than in the pivotal studies published so far [8]. There may be a concern of vaccines provoking disease relapses, but immunization against influenza does not appear to increase the relapse rate in patients with AAV [9]. The use of anti-CD20 monoclonal antibodies such as rituximab is of particular concern, as it induces rapid and prolonged B-cell depletion, abrogating humoral immunity and thereby vaccine response. In rheumatoid arthritis (RA) patients, influenza vaccination 1–2 months after rituximab did not exhibit an immunoglobulin M (IgM) or IgG response compared with a measurable IgG response in rituximab-treated RA subjects 6–10 months before vaccination [10]. Similar concerns have been raised by the haematology and neurology communities, leading to suggestions for an optimal time window to vaccinate individuals treated with B-cell-depleting therapies that will allow immunity to new infections [11].
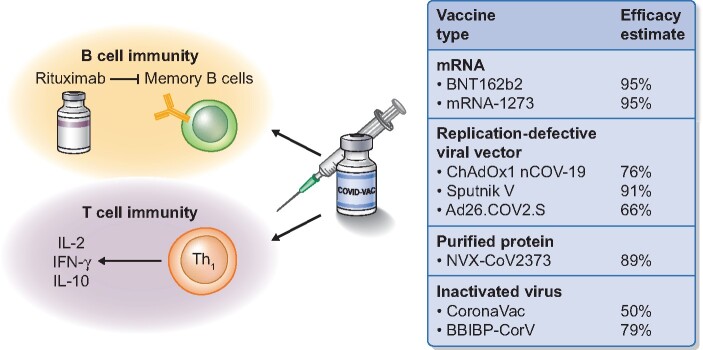
‘Vaccine readiness’ needs to take two scenarios into account: induction of remission in patients presenting with a new diagnosis or relapse and maintenance of remission. In patients with a new diagnosis or relapse of AAV, an alternative therapeutic approach to rituximab may be chosen to achieve remission. During the maintenance phase, we suggest that the time window before SARS-CoV-2 vaccine administration should be a minimum of 6 months after rituximab. AAV patients have been shown to have a considerably longer time to B-cell repopulation after rituximab when compared with RA and other connective tissues diseases [12]. In addition, previous investigations have indicated that the production of neutralizing antibodies and specific vaccination responses are blunted until B cells repopulate (Figure 1), which may argue for measurements of B cells before vaccines are scheduled, as it may vary between individuals. Again, the time frame after the last vaccine dose should be 4 weeks before B-cell-depletion therapy is again given. We also suggest that antibody response and potentially cellular immunity should be monitored regularly with an appropriate assay and that booster doses should be considered if serology responses decline or SARS-CoV-2 variants of concern with reduced effectiveness for current vaccines emerge.
FIGURE 1.
Rituximab therapy blunts humoral vaccine response by depleting CD20, which is expressed from the early pre-B-cell stage to the mature B-cell stage (including memory B cells). Effects on T-cell immunity are unclear, but remain likely unchanged following anti-CD20-depleting therapy. The most promising vaccine platforms include messenger RNA vaccines, replication-defective viral vector vaccines, purified protein vaccines and inactivated virus vaccines. The efficacy estimates are either published Phase 3 trial results or based on press releases. Notably, most COVID-19 vaccine candidates have been tested in a ‘healthy’ population and patients with autoimmune diseases and receiving immunosuppressive measures have been excluded from major trials.
Vaccine ‘rollout’ to this vulnerable patient group has been slow in a number of countries, including those of the European Union. Most will tailor maintenance treatment and likely delay additional doses of rituximab. Again, temporary application of less-effective maintenance agents (e.g. azathioprine) might be considered, with a potential switch to rituximab once an appropriate immune response is ensured. Finally, little is known about SARS-CoV-2 immunity in AAV patients after having experienced COVID-19, and even less if this will be affected by rituximab. Repeated serology monitoring and a readiness to administer booster doses may be a reasonable approach.
FUNDING
The authors received no funding in relation to this article.
SUPPLEMENTARY DATA
Supplementary data are available at ndt online.
CONFLICT OF INTEREST STATEMENT
A.B. reports personal fees from AstraZeneca, ChemoCentryx, Merck/MSD, Vifor and AbbVie. A.K. reports personal fees from Novartis, Terumo BCT, Miltenyi Biotech, Vifor Pharma and Alexion. F.A. and M.S. report no conflicts of interest. F.C.F. reports personal fees from Genentech, Janssen, Chemocentryx, Alnylam, Retrophin, Morphysis, Alexion, GlaxoSmithKline and Takeda. D.R.W.J. reports personal fees from AstraZeneca, Chemocentryx, GlaxoSmithKline, InflaRx and Vifor. V.T. reports personal fees from Callitidas, Travere and Omeros. W.M.S. reports personal fees from Terumo BCT.
Supplementary Material
REFERENCES
- 1.Waldman M, Soler MJ, García-Carro C. et al. Results from the IRoc-GN international registry of patients with COVID-19 and glomerular disease suggest close monitoring. Kidney Int 2021; 99: 227–237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rutherford MA, Scott J, Karabayas M. et al. Risk factors for severe outcomes in patients with systemic vasculitis & COVID-19: a bi-national registry-based cohort study. Arthritis Rheumatol 2021; doi: 10.1002/art.41728 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.FAI2R/SFR/SNFMI/SOFREMIP/CRI/IMIDIATE Consortium and contributors. Severity of COVID-19 and survival in patients with rheumatic and inflammatory diseases: data from the French RMD COVID-19 cohort of 694 patients. Ann Rheum Dis 2020; 80: 527–538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Strangfeld A, Schäfer M, Gianfrancesco MA. et al. Factors associated with COVID-19-related death in people with rheumatic diseases: results from the COVID-19 Global Rheumatology Alliance physician-reported registry. Ann Rheum Dis 2021; 80: 930–942 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kant S, Morris A, Ravi S. et al. The impact of COVID-19 pandemic on patients with ANCA associated vasculitis. J Nephrol 2021; 34: 185–190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gapud EJ, Kronbichler A, Gauckler P. et al. Immunotherapy for ANCA-associated vasculitis during the COVID-19 pandemic. Eur J Rheumatol 2020; 7(Suppl 2): S121–S128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Combes AJ, Courau T, Kuhn NF. et al. Global absence and targeting of protective immune states in severe COVID-19. Nature 2021; 591: 124–130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Windpessl M, Bruchfeld A, Anders HJ. et al. COVID-19 vaccines and kidney disease. Nat Rev Nephrol 2021; 17: 291–293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Stassen PM, Sanders JS, Kallenberg CG. et al. Influenza vaccination does not result in an increase in relapses in patients with ANCA-associated vasculitis. Nephrol Dial Transplant 2008; 23: 654–658 [DOI] [PubMed] [Google Scholar]
- 10.Westra J, van Assen S, Wilting KR. et al. Rituximab impairs immunoglobulin (Ig)M and IgG (subclass) responses after influenza vaccination in rheumatoid arthritis patients. Clin Exp Immunol 2014; 178: 40–47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Baker D, Roberts CAK, Pryce G. et al. COVID-19 vaccine-readiness for anti-CD20-depleting therapy in autoimmune diseases. Clin Exp Immunol 2020; 202: 149–161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Thiel J, Rizzi M, Engesser M. et al. B cell repopulation kinetics after rituximab treatment in ANCA-associated vasculitides compared to rheumatoid arthritis, and connective tissue diseases: a longitudinal observational study on 120 patients. Arthritis Res Ther 2017; 19: 101. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.