Abstract
Background
Because of the increased risk for severe coronavirus disease 2019 (COVID-19), the Advisory Committee on Immunization Practices (ACIP) initially prioritized COVID-19 vaccination for persons in long-term care facilities (LTCF), persons aged ≥65 years, and persons aged 16–64 years with high-risk medical conditions when there is limited vaccine supply. We compared characteristics and severe outcomes of hospitalized patients with COVID-19 in the United States between early and later in the pandemic categorized by groups at higher risk of severe COVID-19.
Methods
Observational study of sampled patients aged ≥18 years who tested positive for severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and admitted to one of 14 academic hospitals in the United States during March–June and October–December 2020. Demographic and clinical information were gathered from electronic health record data.
Results
Among 647 patients, 91% met ≥1 of the following risk factors for severe COVID-19 [91% March-June (n = 434); 90% October–December (n = 213)]; 19% were LTCF residents, 45% were aged ≥65-years, and 84% had ≥1 high-risk condition. The proportion of patients who resided in a LTCF declined significantly (25% vs 6%) from early to later pandemic periods. Compared with patients at lower risk for severe COVID-19, in-hospital mortality was higher among patients at high risk for severe COVID-19 (20% vs 7%); these differences were consistently observed between March–June and October–December.
Conclusions
Most adults hospitalized with COVID-19 were those recommended to be prioritized for vaccination based on risk for developing severe COVID-19. These findings highlight the continued urgency to vaccinate patients at high risk for severe COVID-19 and monitor vaccination impact on hospitalizations and outcomes.
Keywords: SARS-CoV-2, United States, COVID-19, hospitalization, vaccination
The Advisory Committee on Immunization Practices (ACIP) recommended a phased approach to coronavirus disease 2019 (COVID-19) vaccination, given the initial limited supply of vaccine. To protect patients at increased risk for severe acute respiratory syndrome coronavirus 2 (SARS-COV-2) infection or severe COVID-19, ACIP recommended prioritizing vaccination to long-term care facility (LTCF) residents, persons aged ≥75 years, persons aged 65–74 years, and persons aged 16–64 years with high-risk medical conditions, along with frontline healthcare personnel and other essential workers [1]. The increased risk for severe disease with increasing age is well established [2]. Through the week ending 2 January 2021, the cumulative COVID-19-associated hospitalization rate among persons aged ≥65 was 1122 per 100 000 population, double the rate among those aged 50–64 years (526 per 100 000) [2]. Previous studies have also shown that hospitalization rates are higher among those with underlying conditions such as obesity, diabetes, and chronic kidney disease [3–6]. Evidence from SARS-CoV-2 vaccine trials suggests that vaccination may provide the greatest protection against severe COVID-19 illness [7, 8]. Thus, prioritizing groups at higher risk of severe illness from COVID-19 for vaccination is important for reducing hospitalizations and deaths.
Using data from adults hospitalized for COVID-19 at 14 academic medical centers during 2 periods of the pandemic (March–June and October–December 2020), our study had two objectives. First, we assessed whether demographic and baseline health characteristics among patients differed between early (March–June) and later (October–December) periods as overall characteristics of adults diagnosed with SARS-CoV-2 has changed markedly over time in the United States with a shift toward increasing infections within younger age groups, whereas classification of high-risk conditions for hospitalization in the United States were characterized early during the pandemic [5, 9]. Second, we assessed severe outcomes categorized by groups at higher risk of severe COVID-19 and examined whether outcomes for patients with COVID-19 improved over time.
METHODS
In a prospective study at 14 US academic health centers in the Influenza and other Viruses in the acutely ill (IVY) Network, which performs epidemiological and vaccine effectiveness evaluations on influenza and SARS-CoV-2, hospitalized patients aged ≥18 years who had a positive reverse transcription-polymerase chain reaction (RT-PCR) test result for SARS-CoV-2 were sampled at 2 time points [10]. Sites participating in the IVY Network include: University of Washington (Washington), Oregon Health and Sciences University (Oregon), University of California Los Angeles and Stanford University (California), Hennepin County Medical Center (Minnesota), Vanderbilt University Medical Center (Tennessee), The Ohio State University (Ohio), Wake Forest University (North Carolina), Montefiore Medical Center (New York), Beth Israel Deaconess Medical Center and Baystate Medical Center (Massachusetts), UCHealth University of Colorado Hospital (Colorado), Intermountain Medical Center (Utah), and Johns Hopkins University (Maryland).
For the initial time period, we used site-specific random sampling to select a subset of hospitalized patients from each site with positive SARS-CoV-2 testing during March–June 2020 for the purpose of understanding the clinical epidemiology of COVID-19 in different settings and regions early during the pandemic [10]. For the second time period, October–December 2020, as part of efforts to prepare for SARS-CoV-2 vaccine effectiveness studies through active surveillance, we prospectively sampled from hospitalized patients who had a positive RT-PCR test result for SARS-CoV-2 and 1or more signs or symptoms associated with an acute respiratory illness. These signs or symptoms included fever, feverishness, cough, sore throat, myalgias, shortness of breath, chest pain, loss of smell, loss of taste, pulse oximetry on room air <94% or need for supplemental oxygen, or findings consistent with pneumonia on chest imaging. Patients in the intensive care unit (ICU) and non-ICU in both time periods were selected for this analysis. During both time periods, patients sampled represented a subset of hospitalized patients across network sites.
Trained site personnel with extensive clinical research experience abstracted data from the electronic health record on patient demographics, high-risk medical conditions [Supplementary Table 1], and clinical outcomes using a standardized case report form. High-risk medical conditions associated with increased risk for severe COVID-19 were defined by the Centers for Disease Control and Prevention (CDC) as cancer; chronic kidney disease; chronic obstructive pulmonary disease (COPD); heart conditions (eg, heart failure, coronary artery disease, or cardiomyopathies); immunocompromised state from solid organ transplant; obesity (body mass index [BMI] ≥ 30 kg/m2); sickle cell disease; smoking; diabetes mellitus; and pregnancy [1]. Criteria defined by ACIP to prioritize those at increased risk for severe COVID-19 were LTCF residents, persons aged ≥75 years, persons aged 65–74 years, and persons aged 16–64 years with high-risk medical conditions [1]. Hospitalized patients were followed for up to 30 days after admission to record severe in-hospital outcomes. Severe outcomes were defined as receipt of noninvasive ventilation including bilevel positive pressure ventilation or continuous positive pressure ventilation, receipt of invasive mechanical ventilation, receipt of vasopressor support, receipt of dialysis/renal replacement therapy, receipt of extracorporeal membrane oxygenation, incident stroke, venous thromboembolic event (deep vein thrombosis or pulmonary embolism), or death. Missing height or weight information (needed to calculate BMI for obesity determination) were not imputed, and patients with missing weight or height were excluded from the obesity analysis. Detailed information on employment history, including healthcare-related work or other essential work, was not available for either time period.
Characteristics of patients during the two time periods, as well as of those meeting one or more versus no criteria for increased risk of severe COVID-19, were compared using chi-squared or Fisher’s exact test for categorical variables. Significance was defined as P < .05. Analyses were performed using SAS version 9.4 (Cary, North Carolina).
The study was reviewed by the CDC and was conducted consistent with applicable federal law and CDC policy (See, eg, 45 C.F.R. part 46.102(l)(2), 21 C.F.R. part 56; 42 U.S.C. Sect. 241(d); 5 U.S.C. Sect. 552a; 44 U.S.C. Sect. 3501 et seq.)
RESULTS
Among 647 hospitalized patients included in the analysis, 434 (67%) were admitted during 31 March 31 to 4 June (including 172 [40%] admitted to the ICU during hospitalization) and 213 (33%) admitted during 31 October to 31 December (including 95 [45%] admitted to the ICU during hospitalization) as part of active surveillance and enrolment [Table 1]. Of the 647 hospitalized patients, 45% were aged ≥65 years, 19% resided in a LTCF (eg, nursing home, assisted living home or other facility) and 84% had ≥1 high-risk medical condition recognized by CDC for severe COVID-19. The most common medical conditions included obesity (48%), diabetes mellitus (38%), heart disease (23%), chronic kidney disease (18%), and COPD (10%). Participants had none (9%), 1 (47%), 2 (32%), or all 3 (12%) criteria for increased risk for severe COVID-19.
Table 1.
Demographic and Baseline Health Characteristics of Hospitalized Adults Testing Positive for SARS-CoV-2 by RT-PCR, by Time Period—14 Academic Medical Centers, United States, March–June and October–December 2020
| Characteristica | No. (%) | |||
|---|---|---|---|---|
| Total N = 647 | March–June N = 434 | Oct–Dec N = 213 | P-valueb | |
| Female sex | 286/645 (44) | 201/433 (46) | 85/212 (40) | 0.13 |
| Age group (years) | n = 642 | n = 429 | n = 213 | 0.086 |
| 18–49 | 157 (24) | 114 (27) | 43 (20) | |
| 50–64 | 198 (31) | 127 (30) | 71 (33) | |
| 65–74 | 147 (23) | 89 (21) | 58 (27) | |
| 75+ | 140 (22) | 99 (23) | 41 (19) | |
| Race/ethnicity | n = 632 | n = 419 | n = 213 | <0.001 |
| White, non-Hispanic | 254 (40) | 119 (28) | 135 (63) | |
| Black, non-Hispanic | 135 (21) | 103 (25) | 32 (15) | |
| Other, non-Hispanicc | 56 (9) | 50 (12) | 6 (3) | |
| Hispanic | 187 (30) | 147 (35) | 40 (19) | |
| Residence before admission | n = 628 | n = 424 | n = 204 | <0.001 |
| Community residence | 490 (78) | 299 (71) | 191 (94) | |
| Long-term care facility | 117 (19) | 105 (25) | 12 (6) | |
| Nursing home | 80 (13) | 74 (17) | 6 (3) | |
| Assisted living home | 15 (2) | 11 (3) | 4 (2) | |
| Rehab facility | 22 (4) | 20 (5) | 2 (1) | |
| Other residence | 21 (3) | 20 (5) | 1 (1) | |
| High-risk medical conditions | n = 643 | n = 434 | n = 209 | |
| Obesity (BMI ≥ 30 kg/m2) | 280/583 (48) | 159/374 (43) | 121/209 (58) | <0.001 |
| Diabetes mellitus | 243 (38) | 165 (38) | 78 (37) | 0.73 |
| Heart disease (excluding hypertension) | 146 (23) | 105 (24) | 41 (20) | 0.19 |
| Chronic kidney disease | 114 (18) | 79 (18) | 35 (16) | 0.58 |
| COPD | 65 (10) | 43 (10) | 22 (11) | 0.81 |
| Currently use tobacco | 43/604 (7) | 32/406 (8) | 11/198 (6) | 0.28 |
| Cancer | 26 (4) | 21 (5) | 5 (2) | 0.14 |
| Immunocompromised from solid organ transplant | 11 (2) | 5 (1) | 6 (3) | 0.12 |
| Currently pregnant | 14/647 (2) | 14/434 (3) | 0/213 (0) | 0.008 |
| Sickle cell disease | 5 (1) | 2 (0.5) | 3 (1) | 0.34 |
| No. of ACIP criteria met for increased risk for severe COVID-19d | n = 618 | n = 409 | n = 209 | <0.001 |
| 0 | 56 (9) | 35 (9) | 21 (10) | |
| 1 | 292 (47) | 189 (46) | 103 (49) | |
| 2 | 197 (32) | 119 (29) | 78 (37) | |
| 3 | 73 (12) | 66 (16) | 7 (3) | |
Abbreviations: ACIP, Advisory Committee on Immunization Practices; BMI, body mass index; COPD, chronic obstructive pulmonary disease; IQR, interquartile range; RT-PCR, reverse transcription-polymerase chain reaction; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.
a Among 647 hospitalized patients with SARS-CoV-2 infection, some had missing data; denominators used to calculate proportions of respondents with select characteristics excluded patients who had missing data.
b χ 2 or Fisher exact test were used for comparisons between the 2 time periods. P-values for the variables with multiple subcategories compare different distributions between 2 time periods.
c Other non-Hispanic included 7 persons who reported being American Indian/Alaska Native, 21 Asian, 4 Native Hawaiian/Other Pacific Islander, and 18 Other.
d ACIP criteria of increased risk for severe coronavirus disease 2019 (COVID-19) include: long-term care facility residents, persons aged ≥65 years, and persons aged 16–64 years with high-risk medical conditions associated with increased risk for severe COVID-19 defined by CDC (cancer; chronic kidney disease; chronic obstructive pulmonary disease; heart conditions [eg, heart failure, coronary artery disease, or cardiomyopathies]; immunocompromised state from solid organ transplant; obesity; sickle cell disease; smoking; diabetes mellitus; and pregnancy) [1].
Compared with March–June, a higher percentage of patients hospitalized during October–December were non-Hispanic White (63% vs 28%; 15% vs 25% were non-Hispanic Black; 19% vs 35% were Hispanic) and resided in the community before admission (94% vs 71%; 6% vs 25% resided in a LTCF) [Table 1]. The distribution of age and sex were similar between periods. The percentage of patients with a medical condition that increased the risk for severe COVID-19 was generally similar in the March-June and October-December cohorts. However, obesity was more prevalent in the October–December cohort (58% vs 43%).
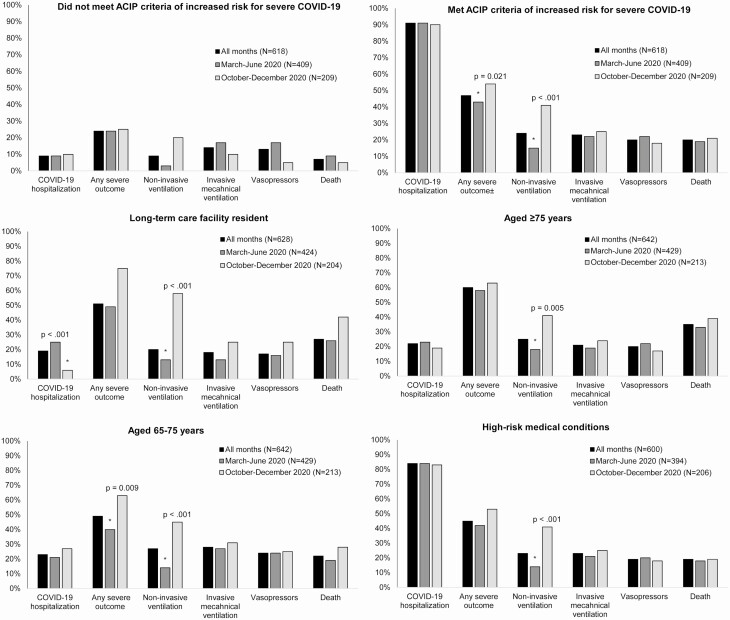
During both time periods, 91% (562 of 618 with complete information on age, place of residence before admission, and underlying conditions) of hospitalized patients met at least 1 of the criteria for increased risk for severe COVID-19, with similar percentages during October–December (90%) and March–June (91%) [Figure 1, Supplementary Table 2]. Excluding obesity, a prevalent condition in the study population, 80% (333 of 418) and 74% (155 of 210) during the early and later period, respectively, met the criteria for increased risk for severe COVID-19.
Figure 1.
Outcomes among hospitalized adults testing positive for SARS-COV-2 by ACIP priority group for vaccine allocation and by time period—14 Academic Medical Centers, United States, March–June and October–December 2020.
*Denotes a significant P-value. χ 2 or Fisher exact test were used for statistical testing of categorical variables. ACIP criteria of increased risk for severe COVID-19 include: long-term care facility residents, persons aged ≥65 years, and persons aged 16–64 years with high-risk medical conditions associated with increased risk for severe COVID-19 defined by CDC (cancer; chronic kidney disease; chronic obstructive pulmonary disease; heart conditions [eg, heart failure, coronary artery disease, or cardiomyopathies]; immunocompromised state from solid organ transplant; obesity; sickle cell disease; smoking; diabetes mellitus; and pregnancy) [1]. Some severity subgroups were missing data (from 4% to 6%): any severe outcome (March–June: 26, October–December: 3), noninvasive ventilation (March–June: 34, October–December: 4), invasive mechanical ventilation (March–June: 29, October–December: 3), and vasopressors (March–June: 32, October–December: 2). Denominators used to calculate proportions of respondents with select outcomes in the specified group excluded patients who had missing data. Any severe outcome was defined as: noninvasive ventilation, invasive mechanical ventilation, vasopressor support, dialysis/renal replacement therapy, extracorporeal membrane oxygenation, stroke, venous thromboembolic event, or death. Abbreviations: ACIP, Advisory Committee on Immunization Practices; CDC, Centers for Disease Control and Prevention; COVID-19, coronavirus disease 2019; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.
Overall, in-hospital mortality was 18% (n = 117), similar between early and later periods (18% vs 19%, respectively) and highest among persons aged ≥75 years (35%) (Supplementary Table 2). Mortality was significantly higher among patients who met at least 1 criterion for increased risk for severe COVID-19 compared with patients who did not meet these criteria (20% vs 7%, respectively; P = .021). Of the 562 who met at least 1 criterion for increased risk for severe COVID-19, 47% had a severe outcome documented compared with 24% of patients who did not meet the criteria. Between March–June and October–December, significant increases were observed in the percentage of patients who had any severe outcome (41% vs 51%; P = .015), which was driven by an increasing percentage of patients who received non-invasive ventilation from the early to the later time period (13% vs 39%; P < .001). Use of invasive mechanical ventilation was similar between periods (22% vs 24%).
DISCUSSION
This study characterizes persons hospitalized with COVID-19 in the context of the ACIP’s recommended prioritization of initial vaccine allocations during an early and later period in the pandemic, using a sample of adults representing a broad cross-section of the US population from a network of academic hospitals. The majority (91%) of the patients hospitalized with COVID-19 during March–June 2020 and October–December 2020 met criteria for increased risk for severe COVID-19, and, if vaccinated, many of the hospitalizations might have been averted. Almost 20% of patients who met 1 or more COVID-19 vaccine priority criteria died in hospital, despite improvements in COVID-19 management over time, including likely benefits from the use of systemic steroids in patients with respiratory failure [11]. These findings from a sample of patients are comparable to published data on cross-sectional cohorts from other US hospitals [5, 12].
Patients residing in LTCFs continue to be an important priority group for COVID-19 vaccination because of high risk of infection, severe disease, and death [6]. The slight decline in patients from LTCFs between the 2 time periods in our sites may have been due to rapid interventions and improved mitigation efforts following multiple LTCF outbreaks early in the pandemic, such as serial facility-wide testing requirements in nursing homes, and a subsequent reduction of infection in this population [13, 14]. The higher proportion of hospitalized patients from the community during October-December might be a result of increasing COVID-19 cases among younger age groups in the community [9]. Thus, although ensuring high vaccination rates in those at increased risk of severe COVID-19 is important, rapidly expanding access to vaccination in the community is urgently needed once there is wider availability of vaccine. As this network of health centers continues to conduct active post-vaccination surveillance for vaccine effectiveness against severe COVID-19, these data provide a useful baseline at each of the network sites to monitor changes in epidemiology.
Some limitations must be considered. First, distribution of patients by site differed between the early and late cohort with higher cases from New York and Massachusetts hospitals in the Spring and Tennessee in the Fall [Supplementary Table 3]. This could have influenced some of the differences that were observed between cohorts such as differences in race/ethnicity between periods. In the New York and Massachusetts sites, which represented 47% of the early cohort, 25% were non-Hispanic Black, 32% were non-Hispanic White, and 37% were Hispanic; however, in the Tennessee site, which represented 44% of the later cohort, 11% were non-Hispanic Black, 73% were non-Hispanic White, and 14% were Hispanic. Despite differences in enrollment by site during the 2 time periods, we consistently found that most patients met 1 or more COVID-19 vaccine priority criteria, and most had medical conditions associated with higher risk of severe outcomes. Second, although not a criterion in the early cohort, 100% of the later cohort included patients who had ≥1 sign or symptom of a COVID-like illness because of expanded testing to all hospitalized patients; 90% of patients in the early cohort had ≥1 sign or symptom of a COVID-like illness. Similar results were found in a sensitivity analysis for ICU admission and severe outcomes excluding those without COVID-like signs or symptoms. We included outcome measures that were clinically meaningful and reflected disease severity, including noninvasive and invasive mechanical ventilation. Use of noninvasive mechanical ventilation increased substantially from the first to the second time period, which might reflect concerns about risk of lung injury from invasive ventilation in adults with COVID-19, availability of ventilators, or additional factors.
CONCLUSION
We characterized clinical outcomes of hospitalized COVID-19 patients in the context of COVID-19 vaccine priority groups in a multistate sample of patients representing different regions of the United States at 2 time points in the pandemic. A vast majority of patients with a COVID-19-associated hospitalization during March–June were in groups considered to be at increased risk for severe COVID-19 who are recommended to be prioritized for vaccination during the phased allocation period, with no differences seen over the later October–December time period despite significant changes in the age distribution and other characteristics of COVID-19 cases in the United States over time. Widespread vaccination of persons in these groups will have a great impact on COVID-19 hospitalizations and deaths in the United States and reduce burden on the health care system. This study demonstrates the importance of increasing vaccination coverage among these groups. Finally, monitoring the impact of vaccination in high-risk priority populations will provide support for the effectiveness of COVID-19 vaccines in preventing hospitalization among patients predisposed to adverse outcomes.
Supplementary Material
Notes
Acknowledgments. Influenza and Other Viruses in the Acutely Ill (IVY) Network investigators.
Financial support. This work was supported by CDC contract 75D30120C07637 to Dr Wesley Self of Vanderbilt University Medical Center, Nashville, Tennessee. REDCap was funded by UL1TR000445 from National Center for Advancing Translational Sciences in the National Institutes of Health.
Disclaimer. The findings and conclusions in this report are those of the authors and do not necessarily represent the views of the CDC.
Supplement sponsorship. This supplement is supported by the Infectious Diseases Society of America through Cooperative Agreement NU50CK000574 with the U.S. Centers for Disease Control and Prevention.
Potential conflicts of interest. D. J. H. reports grants from Baxter, Cytovale, and Biomerieux and consulting fees from CytoVale. A. K. reports grants from United Therapeutics, Reata Pharmaceuticals, Johnson & Johnson, and the National Institutes of Health (NIH). C. J. L. reports contracts and grants from CDC, NIH, Department of Defense (DoD), Marcus Foundation, and data analysis and study coordination contracts with Abbvie, Entergrion, Endpoint Health, and BioMerieux. C. J. L. reports a patent issued for risk stratification in septic shock. M. N. reports grants from CDC, NIH, and Agency for Healthcare Research and Quality (AHRQ), consulting fees from Regeneron. S. B. reports grants from CDC, Sedana, Janssen, NIH and DoD, consulting fees from Hamilton, Faron, and New York University (NYU), and book royalties from Oxford University and Brigham Young University. I. P. reports grants from CDC, National Institute of General Medical Studies, Janssen, National Heart, Lung, and Blood Institute and Intermountain Research and Medical Foundation, and other payments from Regeneron and Asahi Kaeei Pharma. D. H. reports grants from CDC and other payments from Incyte Corporation. T. R. reports grants from CDC and consulting fees from Cumberland Pharmaceuticals and Cytovale. S. C. reports speaker fees from La Jolla Pharmaceuticals and consulting fees from PureTech Health. C. G. reports grants from Campbell Alliance, CDC, NIH, the Food and Drug Administration (FDA), AHRQ, and Sanofi, and consulting fees from Pfizer, Merck, and Sanofi-Pasteur. J. C. reports a grant from NIH. D. C. F. reports consulting fees from Cytovale, Faraday Pharmaceuticals and Foresee Pharmaceuticals. W. S. reports a grant from the CDC. All other authors report no conflict of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Dooling K, Marin M, Wallace M, et al. . The advisory committee on immunization practices’ updated interim recommendation for allocation of COVID-19 vaccine—United States, December 2020. MMWR Morb Mortal Wkly Rep 2021; 69:1657–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. CDC. COVID-NET: laboratory-confirmed COVID-19–associated hospitalizations.https://gis.cdc.gov/grasp/COVIDNet/COVID19_3.html. Accessed 29 January 2021.
- 3. Ko JY, Danielson ML, Town M, et al. . COVID-NET surveillance team. risk factors for COVID-19-associated hospitalization: COVID-19-Associated Hospitalization Surveillance Network and Behavioral Risk Factor Surveillance System. Clin Infect Dis 2021; 72:e695–03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Kim L, Garg S, O’Halloran A, et al. . Risk factors for intensive care unit admission and in-hospital mortality among hospitalized adults identified through the U.S. Coronavirus Disease 2019 (COVID-19)-Associated Hospitalization Surveillance Network (COVID-NET). Clin Infect Dis 2021; 72:e206–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Garg S, Kim L, Whitaker M, et al. . Hospitalization rates and characteristics of patients hospitalized with laboratory-confirmed Coronavirus disease 2019–COVID-NET, 14 States, March 1–30, 2020. MMWR Morb Mortal Wkly Rep 2020; 69:458–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Centers for Medicare and Medicaid Services. COVID-19 nursing home data. Baltimore, MD: US Department of Health and Human Services, Centers for Medicare & Medicaid Services; 2020. [Google Scholar]
- 7. Baden LR, El Sahly HM, Essink B, et al. ; COVE Study Group . Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N Engl J Med 2021; 384:403–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Polack FP, Thomas SJ, Kitchin N, et al. ; C4591001 Clinical Trial Group . Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N Engl J Med 2020; 383:2603–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Boehmer TK, DeVies J, Caruso E, et al. . Changing age distribution of the COVID-19 pandemic–United States, May–August 2020. MMWR Morb Mortal Wkly Rep 2020; 69:1404–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Tenforde MW, Billig Rose E, Lindsell CJ, et al. ; CDC COVID-19 Response Team . Characteristics of adult outpatients and inpatients with COVID-19–11 academic medical centers, United States, March–May 2020. MMWR Morb Mortal Wkly Rep 2020; 69:841–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. RECOVERY Collaborative Group, Horby P, Lim WS, et al. . Dexamethasone in hospitalized patients with COVID-19: preliminary report. N Engl J Med 2021; 384:693–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Killerby ME, Link-Gelles R, Haight SC, et al. ; CDC COVID-19 Response Clinical Team . Characteristics associated with hospitalization among patients with COVID-19–metropolitan Atlanta, Georgia, March–April 2020. MMWR Morb Mortal Wkly Rep 2020; 69:790–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Hatfield KM, Reddy SC, Forsberg K, et al. . Facility-wide testing for SARS-CoV-2 in nursing homes–Seven U.S. Jurisdictions, March–June 2020. MMWR Morb Mortal Wkly Rep 2020; 69:1095–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Sanchez GV, Biedron C, Fink LR, et al. . Initial and repeated point prevalence surveys to inform SARS-CoV-2 infection prevention in 26 skilled nursing facilities–Detroit, Michigan, March–May 2020. MMWR Morb Mortal Wkly Rep 2020; 69:882–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.