Abstract
Background
Sars-CoV-2 outbreaks resulted in a high case fatality rate in nursing homes (NH) worldwide. It is unknown to which extent presymptomatic residents and staff contribute to the spread of the virus.
Aims
To assess the contribution of asymptomatic and presymptomatic residents and staff in SARS-CoV-2 transmission during a large outbreak in a Dutch NH.
Methods
Observational study in a 185-bed NH with two consecutive testing strategies: testing of symptomatic cases only, followed by weekly facility-wide testing of staff and residents regardless of symptoms. Nasopharyngeal and oropharyngeal testing with RT-PCR for SARs-CoV-2, including sequencing of positive samples, was conducted with a standardised symptom assessment.
Results
185 residents and 244 staff participated. Sequencing identified one cluster. In the symptom-based test strategy period, 3/39 residents were presymptomatic versus 38/74 residents in the period of weekly facility-wide testing (P-value < 0.001). In total, 51/59 (91.1%) of SARS-CoV-2 positive staff was symptomatic, with no difference between both testing strategies (P-value 0.763). Loss of smell and taste, sore throat, headache or myalga was hardly reported in residents compared to staff (P-value <0.001). Median Ct-value of presymptomatic residents was 21.3, which did not differ from symptomatic (20.8) or asymptomatic (20.5) residents (P-value 0.624).
Conclusions
Symptoms in residents and staff are insufficiently recognised, reported or attributed to a possible SARS-CoV-2 infection. However, residents without (recognised) symptoms showed the same potential for viral shedding as residents with symptoms. Weekly testing was an effective strategy for early identification of SARS-Cov-2 cases, resulting in fast mitigation of the outbreak.
Keywords: nursing home, COVID-19, presymptomatic, residents, staff, older people
Key points
Subjective symptoms such as sore throat or loss of smell/taste are hardly reported in SARS-CoV-2 positive nursing home (NH) residents.
Facility wide testing of staff revealed limited presymptomatic or asymptomatic SARS-CoV-2 cases.
Staff did not recognise (self) report or attribute symptoms to SARS-CoV-2 in NH residents and themselves.
Weekly facility wide testing is an effective strategy for early identification of SARS-CoV-2 cases.
Introduction
Worldwide, nursing homes (NHs) are facing outbreaks of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) with high case fatality rates [1, 2]. The current ECDC-guideline recommends expanded viral testing of asymptomatic residents in NHs if a single new case of a SARS-CoV-2 infection is detected, based on data of previous NH outbreaks which suggest an important role for presymptomatic spread of SARS-COV-2 among residents [3–9]. However, it remains unknown to which extent asymptomatic and presymptomatic cases contribute to the spread of SARS-CoV-2. Also, specifically in the NH setting, it remains unclear to what extent asymptomatic cases are truly without symptoms. Sole reliance on symptoms for testing in NHs could be insufficient because self-reporting of complaints is often compromised in residents due to limited ability to communicate (e.g. in residents with dementia) [10]. The Dutch guideline for COVID-19 in NHs states that only residents with possible symptoms of SARS-CoV-2 should be tested [11] and no policy for testing of asymptomatic residents or staff is facilitated in the Netherlands.
Multiple reports have been published about the prevalence of asymptomatic and presymptomatic residents and staff in NHs after the implementation of a facility-wide testing strategy during an outbreak [4, 6, 9, 12, 13]. The prevalence of asymptomatic staff and residents differed from single cases to up to half of the infected cases. Low cycle threshold (Ct) values were found in asymptomatic and presymptomatic cases, suggesting potential of viral shedding [6, 9]. A large registry of 857 Dutch residents with confirmed SARS-CoV-2 showed that 93% of cases expressed any of the symptoms cough, shortness of breath, or fever. A large range of other symptoms were also reported such as fatigue, diminished intake, gastro-intestinal symptoms, malaise or rhinorrhea [14]. However, the presentation of SARS-CoV-2 can be difficult to recognise in NH residents, which can cause delay in testing, isolation and treatment [14, 15]. In addition, during a community-wide outbreak it can be difficult to distinguish residential outbreaks from multiple introductions without sequencing of viruses from cases [16].
Viral spread by presymptomatic or unrecognised symptomatic cases has important implications for Personal Protective Equipment (PPE) use, facility-wide testing and isolation measures in NHs for the prevention of outbreaks. The aim of this study is to analyse the contribution of presymptomatic spread of SARS-CoV-2 in all staff and residents of a NH in the Netherlands by serial weekly point prevalence surveys, PCR and sequencing.
Methods
Setting and study population
The study took place in a 185-bed NH in the province South Holland which provides long-term care and is specialised in dementia care. All residents and staff working during the outbreak were invited to participate in the study. Data were collected retrospectively before May 18th and prospectively from May 18th onwards. NH details are presented in Appendix 1, Supplementary data are available in Age and Ageing online.
SARS-CoV-2 testing and analysis
Two phases in the NH test strategy can be distinguished: First, until May 11th, a symptom-based testing strategy was followed, according to national guidelines: cases were tested when they experienced any symptoms. The only exception of this strategy was at May 6th: at the ward where the outbreak started, all negative residents were tested regardless of symptoms. Second, from May 12th the NH implemented a policy of facility-wide weekly testing in addition to the symptom-based testing strategy, implying SARS-CoV-2 testing of all residents without a previous positive test and regardless of the presence of any symptoms. Staff was tested regardless of symptoms in the week of May 18th and June 1st.
Samples were transported to collaborating laboratories at the end of each test day, where they were tested for SARS-CoV-2 polymerase chain reaction (PCR) targets. Three different laboratories collaborated because of the large number of tests which were conducted: As a result, different PCR platforms were used, however, the used targets were similar (RdRp- gene, E-gene, N- gene; see Appendix 2, Supplementary data are available in Age and Ageing online).
Sequencing, phylogenetic analysis and cluster definition
PCR-positive samples with Ct-value below 32 were selected for sequencing using a SARS-CoV-2 specific amplicon based Nanopore sequencing approach, as previously described [17]. The consensus genome was generated only including positions with a coverage >30 as described previously [18]. Additional details on sequencing methods are provided in Appendix 3, Supplementary data are available in Age and Ageing online.
Data collection
A standardised symptom-assessment form of 16 symptoms was completed by the research team for each assenting resident, using electronic health record review. Staff was invited to complete a first questionnaire electronically (via email) in the week of May 18th (Appendices 4 and 5, Supplementary data are available in Age and Ageing online).
A participant was classified symptomatic if he had at least one new or worsened symptom in the 14 days prior to a positive test result. A participant was classified asymptomatic if no new or worsened symptoms were present and no symptoms would develop in the 14 days following the positive test. Participants were classified pre-symptomatic if they had no symptoms at moment of testing, but developed symptoms in the 2 weeks following a positive test [19].
Analyses
Data are reported as mean/median with range and standard deviations (SD) and counts with percentages as appropriate. Differences between groups were assessed with student’s T-test and Mann–Whitney U for continuous variables and Chi-square test for categorical data. Differences were considered statistically significant at P < 0.05 (two-tailed). All analyses were done using SPSS, version 26 (IBM, Armonk, NY) and Excel.
Ethics
Written information about the study was sent out to residents and their legal representatives at May 18th, with the possibility to opt-out. Health care professionals were asked informed consent for participating in the study prior to digital questionnaire completion. The Medical Ethics Committee of the VU University Medical Centre in Amsterdam reviewed the study protocol and confirmed that the study does not fall under the scope of the Medical Research Involving Human Subjects Act.
Results
At April 29th, when the first resident tested positive for SARS-CoV-2, 185 residents lived and 384 staff worked in the NH. Four legal representatives of residents and 34 staff members declined participation. Baseline characteristics are described in Table 1. Residents who tested positive for SARS-CoV-2 were older and more likely to have cognitive impairment. Staff positive for SARS-CoV-2 consisted mostly of (registered) health care assistants and health-care aids. Appendix 6 (Supplementary data are available in Age and Ageing online) shows the STROBE diagram of participating residents and staff.
Table 1 .
Baseline characteristics residents and staff
| SARS-CoV-2 test results | P-value (95% CI) | ||
|---|---|---|---|
| Residents | Positive N = 113 | Negative N = 68 | |
| Age (median/range) | 85.0 (44–99) | 81.5 (48–100) | 0.001 (−9.009; −2.237) |
| Female n (%) | 82 (72.6) | 50 (73.5) | 0.888 |
| Coexisting conditions | |||
| Pulmonary disease n (%) | 12 (10.6) | 3 (4.4) | 0.142 |
| Cardiovascular disease n (%) | 40 (35.4) | 15 (22.1) | 0.059 |
| Cerebrovascular disease n (%) | 23 (20.4) | 9 (13.2) | 0.224 |
| Diabetes n (%) | 18 (15.9) | 18 (26.5) | 0.085 |
| Cognitive impairment n (%) | 104 (92.0) | 53 (77.9) | 0.007 |
| Reduced kidney function n (%) | 7 (6.2) | 2 (2.9) | 0.329 |
| Obesity n (%) | 5 (4.4) | 8 (11.8) | 0.064 |
| Staffa | Positive N = 56 | Negative N = 188 | |
| Age (median/range) | 43.0 (18–74) | 46.5 (18–74) | 0.853 (−5.764; –3.942) |
| Female n (%) | 47 (83.9) | 175 (93.1) | 0.036 |
| Profession, n (%) | 0.027 | ||
| Health care assistants and aids | 39 (69.6) | 88 (46.8) | |
| Nurse | 3 (5.4) | 11 (5.9) | |
| Physical therapist | 0 | 7 (3.7) | |
| Physician | 0 | 6 (3.2) | |
| Otherb | 14 (24.6) | 76 (40.4) | |
| Reporting contact with Covid-19 suspected or confirmed residents, n (%) | 0.296 | ||
| Yes | 43 (76.8) | 159 (84.6) | |
| No | 5 (8.8) | 8 (4.3) | |
| Unknown | 8 (14.0) | 21 (11.2) | |
a34 Staff members declined participation, 106 staff did not complete the questionnaire.
bStaff working in kitchen, logistics, occupational therapists, psychologists, management.
Introduction of the virus and outbreak
The first positive resident (29 April) had been admitted from 17 to 23 April at the geriatric department of the local hospital with a urosepsis. She had a negative PCR for SARS-CoV-2 and her chest X-ray was classified as CORADS-1, suggesting a very low probability of COVID-19 [20]. On April 29th, she developed a fever and was readmitted to the hospital, where retrospectively an outbreak had occurred, and tested positive for SARS-CoV-2. Previously, three NH staff members tested positive for SARS-CoV-2 in April, but none of them worked in the period they were contagious. Figure 1 shows the timeline with date of onset of COVID-19 for participating residents and staff and the NH policy.
Figure 1 .
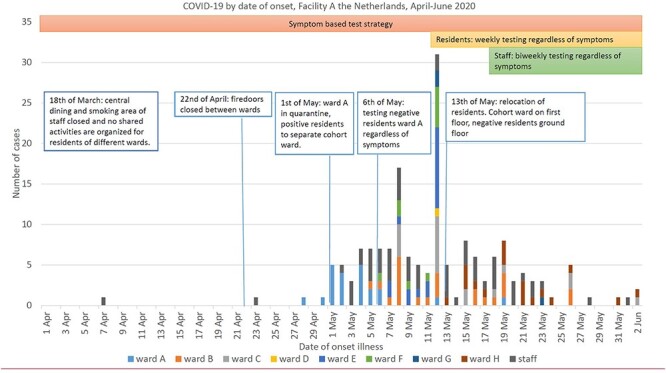
COVID-19 by date of onset and NH policy, shows the date of onset of COVID-19 for participating residents of the different wards and participating staff from the 15th of April until the 2nd of June. Key changes in NH policy for infection prevention and testing are indicated. On May 13th, facility management decided to move all positive tested residents to the first floor of the building, while residents who tested negative were moved to the ground floor of the building. PPE used on the first floor included isolation gown, gloves over the wrists, goggles and a surgical mask; on the ground floor surgical masks and gloves were used.
Sequencing
In total, 53 sequences of residents and NH staff were available. In addition, 7 sequences of the hospital outbreak were generated. All sequences cluster together, also sequences detected at the geriatric department of the hospital outbreak were near identical. Two subclusters appear to be present in sequences of residents and staff, without differences when considering wards where residents lived and staff worked (Figure 2).
Figure 2 .
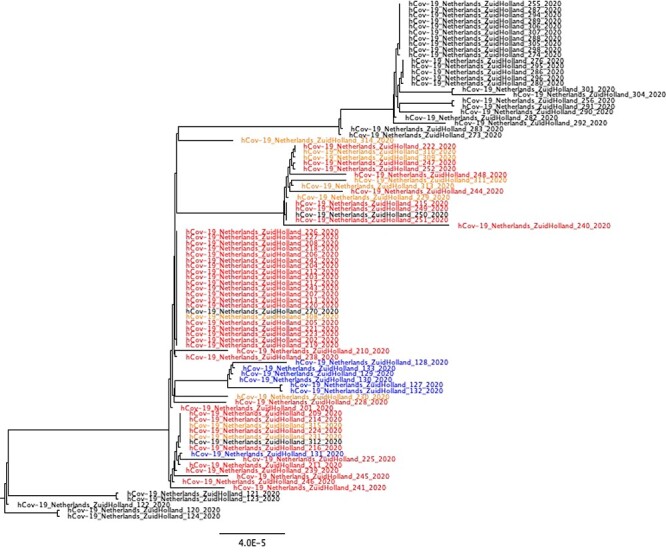
Zoom-in of Dutch phylogenetic tree, with sequences of NH A in red (clients) and orange (employees). Sequences in blue originate from the related hospital outbreak. Sequences in black originate from a Dutch national reference database.
Symptomatic, presymptomatic and asymptomatic cases during symptomatic and weekly testing strategy
Results of the standardised symptom-assessment are presented in Table 2. Except for the symptoms fever and nausea, residents and staff showed different prevalence for all symptoms. Because of retrospective and prospective data collection in staff, we performed sensitivity analyses which compared symptoms in prospective and retrospective questionnaires. Also we repeated comparisons between positive staff and residents only using prospective questionnaires of staff.
Table 2 .
Symptom assessment of residents and staff with a positive SARS-CoV-2 PCR-test
| Symptom assessment n (%) | Residents N = 113 | Staff N = 56 | P-value | ||||
|---|---|---|---|---|---|---|---|
| Symptomatic | 65 (57.5) | 51 (91.1) | <0.001 | ||||
| Presymptomatic | 41 (36.3) | 2 | |||||
| Asymptomatic | 7 (5.2) | 3a | |||||
| Cough | 31 (27.4) | 26 (46.4) | 0.014 | ||||
| Dyspnea | 13 (11.5) | 20 (35.7) | <0.001 | ||||
| Fever | 30 (26.5) | 15 (26.8) | 0.974 | ||||
| Saturation | 27 (23.9) | NA | |||||
| Delirium | 16 (14.2) | NA | |||||
| Chills | 4 (3.5) | 22 (39.3) | <0.001 | ||||
| Malaise | 25 (22.1) | 24 (42.9) | 0.005 | ||||
| Fatigue | 19 (16.8) | 42 (75.0) | <0.001 | ||||
| Myalgia | 2 (1.8) | 26 (46.6) | <0.001 | ||||
| Headache | 5 (4.4) | 36 (64.3) | <0.001 | ||||
| Sore throat | 2 (1.8) | 21 (37.5) | <0.001 | ||||
| Nasal congestion | 15 (13.3) | 34 (60.7) | <0.001 | ||||
| Diarrhoea | 10 (8.8) | 14 (25.0) | 0.005 | ||||
| Nausea | 9 (8.0) | 7 (12.5) | 0.343 | ||||
| Diminished intake | 17 (15.0) | 23 (41.1) | <0.001 | ||||
| Loss of smell or taste | 0 | 27 (48.2) | <0.001 | ||||
| Testing strategy | Symptom based N = 39 | Weekly N = 74 | P-value | Symptom based N = 26 | Weekly N = 30 | P-value | |
| Symptomatic, n (%) | 36 (92.3) | 29 (39.2) | <0.001 | 23 (94.6) | 28 (93.3) | 0.763 | |
| Presymptomatic, n (%) | 3 (7.7) | 38 (51.4) | 1 (3.8) | 1 (3.3) | |||
| Asymptomatic, n (%) | 0 | 7 (9.5) | 2a (7.6) | 1 (3.3) |
aTwo staff members did not complete follow-up questionnaire.
Staff was tested twice regardless of symptoms, while residents were tested four times regardless of symptoms: We performed a third sensitivity analysis where we compared residents/staff who were tested on the dates of the point prevalence surveys regardless of symptoms to residents/staff who were tested at all other dates in the study period. This did not alter results (Appendix 7, Supplementary data are available in Age and Ageing online).
A significant difference in presymptomatic residents was found between the two testing strategies (P-value < 0.001). Before the start of facility-wide weekly testing, 39 residents tested positive: 36 (92.3%) were symptomatic and 3 (7.7%) residents were presymptomatic. The three presymptomatic residents were tested at May 6th when all residents of the ward where the outbreak started were tested regardless of symptoms. In the period of weekly testing, 74 residents tested positive, of which 29 (39.2%) were symptomatic at the time of testing, 38 (51.4%) were presymptomatic and 7(9.5%) were asymptomatic.
A total of 56 staff tested positive and completed the questionnaire: 51 (91.1%) were symptomatic at the moment of testing, 2 (3.9%) were pre-symptomatic and 3 (5.9%) staff members were asymptomatic. No difference in symptomatic, presymptomatic and asymptomatic staff members was found between symptom based or additional weekly testing strategy (P-value 0.763).
Symptom onset and presentation with symptomatic and weekly testing strategy
Until May 11th, 39 residents tested positive and all developed symptoms. Symptoms developed between 6 days before testing and 3 days after testing, with a median of development of symptoms the day before the test (interquartile range 2 days to 1 day before test) (Figure 3A). After the addition of weekly testing regardless of symptoms, 74 residents tested positive of which 67 residents developed symptoms between 11 days before testing and 8 days after testing, with a median of development of symptoms the day of the test (interquartile range 2 days before the test to 3 days after the test) (Figure 3B). The time between onset of symptoms and test date differed significantly between the two testing strategies (P-value = 0.000). With both test strategies, symptomatic residents had symptoms for multiple days without testing.
Figure 3 .
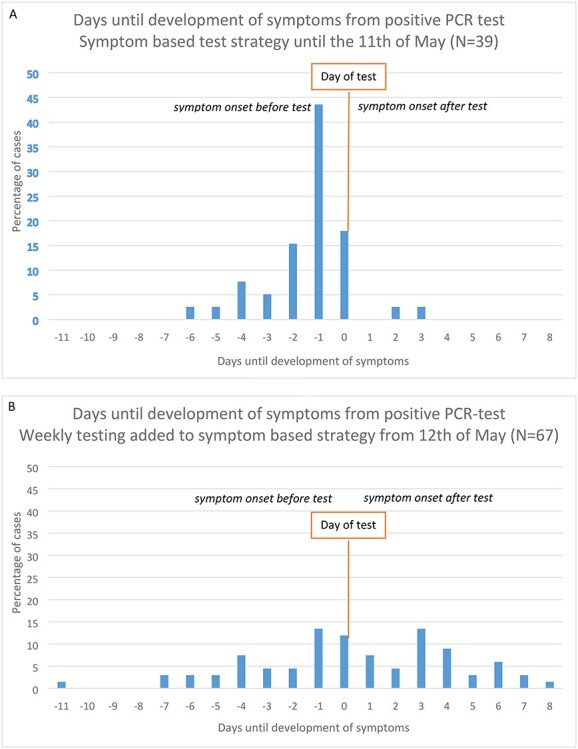
Frequency plot of days until development of symptoms from positive PCR-test of residents. Negative values represent symptomatic residents, while positive values represent presymptomatic residents. The value 0 means that residents developed symptoms at the day of PCR-test: whether the symptoms developed before or after testing determines if they were presymptomatic or symptomatic. (A) symptomatic testing strategy until the 11th of May. (B) Addition of facility-wide weekly testing strategy regardless of symptoms from the 12th of May.
Ct-values
Ct values were available for 97/113 positive residents; the median Ct-value was 21.3 (range 14.5–40). Symptomatic residents (N = 59) had a median Ct-value of 20.8 (range 14.5–38.1), presymptomatic resident (N = 33) had a median Ct-value of 21.3 (range 16.1–40) and asymptomatic resident (N = 5) had a median Ct-value of 20.5 (range 17.3–39.7). There was no difference in Ct-value between these groups (P = 0.624).
Ct-values were available for 38/56 staff members; with a median of 24.6 (range 13.7–38.1). Of one asymptomatic staff Ct-value was 34.6. The two presymptomatic staff members Ct-values were 29.8 and 32.3. Symptomatic staff members (N = 35) had a median Ct-value of 23.7 (range 13.7–38.1).
Discussion
We describe a large SARS-CoV-2-outbreak in a NH which most likely started by an infected resident discharged from a local hospital where SARS-CoV-2 prevailed. The addition of weekly facility-wide testing regardless of symptoms identified 38 (52.7%) presymptomatic residents and 7 (8.1%) asymptomatic residents. These cases were found up to 8 days before symptoms occurred. In staff limited, presymptomatic and asymptomatic cases were identified. The absence of subjective symptoms (such as loss of smell or taste) in residents compared to staff who are infected by the same SARS-CoV-2 strain suggests the under-reporting of symptoms in residents. As such, it is not possible to make a distinction between a/presymptomatic and unrecognised symptomatic residents in this study. However, a/presymptomatic residents have the same high viral load as symptomatic residents, which suggests the same potential for viral shedding. These results support the guidelines of the ECDC and CDC to test asymptomatic residents and staff to identify pre- and asymptomatic cases of SARS-CoV-2.
The high prevalence of presymptomatic cases in residents and the limited registration of subjective symptoms is comparable to other studies [3, 5, 6, 9]. Studies performing facility wide testing regardless of symptoms of staff in NHs with a confirmed COVID-19 case found the same limited number of asymptomatic staff as in our study [3, 4, 13]. To our knowledge, we are the first study reporting on symptoms from residents and staff in a large outbreak of the same virus strain. The large difference between presymptomatic staff and residents found in this study has three possible explanations: First, a large number of residents in this NH are cognitive impaired, which makes it difficult for them to express their symptoms. Second, staff reporting on residents’ symptoms were not aware of all the symptoms related to COVID-19. During the outbreak symptoms of residents were sometimes documented for multiple days, but they were nevertheless not tested. Third, understaffing because of the outbreak could have led to suboptimal symptom registration: mild or subjective symptoms were missed, because staff had to take care of residents they were not familiar to work with, or because of limited time to register symptoms. Understaffing as a risk for under-recognition of new cases is supported by data of Gorges and Li which shows NHs with at least one case, higher nurse aide [21] and total nursing hours [21, 22] are associated with a lower probability of experiencing an outbreak and with fewer deaths.
Our study showed no difference between Ct-values of symptomatic, presymptomatic, or asymptomatic cases in residents, similar to previous studies [6, 9]. All these studies have the same risk of underreporting of (mild) symptoms of SARS-CoV-2 and incorrect classifying residents as pre- or asymptomatic. This suggest that these residents should be treated the same: as possibly infectious. Timely isolation of these residents and PPE could be important interventions to prevent further spread of the virus.
The new approach of mass repeated testing, irrespective of symptoms, in skilled nursing facilities has been advocated since May [23]. After this, studies have been published describing this approach, often resulting in reduced SARS-CoV-2 transmission after the implementation of this testing strategy [24, 25]. However, limited additional cases were found after a weekly testing strategy was implemented in three Dutch NHs after their first cases of SARS-CoV-2 [26]. Possibly, the testing was early in the outbreak and led to rapid isolation, combined with the increased availability of PPE or because cases per capita in the community were very low [26]. Cases per capita in the community have been identified as an important predictor for outbreaks in NHs [21, 27].
The testing of staff regardless of symptoms could be important because previous research showed that health care workers have difficulty in recognising possible COVID-19 symptoms for themselves: 65% reported working while exhibiting symptoms [28]. This is reflected in our results, as we found that almost none of the staff was asymptomatic at the moment of testing, even after the implementation of a testing strategy regardless of symptoms. The WHO advises that syndromic surveillance of health workers for COVID-19 symptoms should be performed before entering the workplace. If human resources and logistics permit it, active syndromic surveillance is recommended. Symptoms that should be monitored at minimum are fever, dry cough, myalgia, arthralgia, fatigue, headache, shortness of breath, anosmia and ageusia [29].
Implementing sequencing, combined with epidemiological information, is important to understand the extent of intramural transmission versus introductions from the community. In addition, transmission clusters and risk factors for transmission can be identified, which can be used to implement infection prevention measures to prevent further spread. Previous research has shown that whole genome sequencing can generate evidence for transmission routes that would not have been identified with traditional epidemiological investigations [16, 17].
Limitations
Not all staff members who tested positive participated in the study. In addition, some staff members had to answer the questionnaire retrospectively, which gives the risk of recall bias. Sensitivity analyses did not alter results.
Further, the difference between symptomatic staff and residents could perhaps be explained by the fact that staff was tested less frequent than residents. This may have contributed partly to the higher proportion of symptomatic staff. In our sensitivity where we compared staff who were tested on the dates of the point prevalence surveys to staff who were tested on other dates during the study period because of symptoms (Appendix 7, Supplementary data are available in Age and Ageing online). Except for loss of smell and taste no difference in symptoms between staff tested regardless of symptoms or outside the point prevalence surveys was observed.
Also, the low rate of reported symptoms in residents could be explained by the high proportion of residents with cognitive impairment. The NH in this study was specialised in psychogeriatric care; in a representative sample of Dutch NH, 59% of residents were diagnosed with cognitive impairment [30].
Last, not all SARS-CoV-2 positive samples were sequenced. However, a lot of time points could be analysed and they show all the same cluster which makes it unlikely that multiple clusters were circulating in the NH.
Conclusion
Our study suggests that a proportion of the presymptomatic cases in NHs are possibly unrecognised symptomatic cases and supports the guideline of the CDC and ECDC that facility-wide testing of residents and staff needs to be undertaken after the first confirmed SARS-CoV-2 case in the facility [7, 8]. If there is limited viral testing capacity, initial testing of (asymptomatic) close contacts is advised [8]. This will identify possible asymptomatic, presymptomatic cases and unrecognised symptomatic cases and prevent further spread of the virus. Sequencing should be performed to discriminate ongoing intramural transmission and multiple introductions. Box 1 summarises the lessons learned during this study.
Box 1.
Lessons learned of SARS-CoV-2 outbreak
Lessons learned
Preparing for an outbreak
Educate staff about all the possible symptoms of COVID-19: Take routine temperature and saturation of residents for reference values. Also sufficient staffing and staff dedicated to a few patients is necessary for early recognition of symptoms. NHs should make protocols with a local laboratory so when an outbreak occurs, rapid testing is possible.
Increasing cases per capita in the population
When cases per capita in the general population of the area are increasing, staff and visitors should wear at least surgical face mask to prevent introduction of the virus. In this outbreak, a resident transferring from another health care facility with a negative SARS-CoV-2 PCR was the index case and presymptomatic at the moment of transfer. Consider quarantine of residents who are admitted, regardless of a recent, negative PCR-test.
During an outbreak
Recognition of start of possible COVID-19 symptoms is very difficult, especially in residents with dementia. Weekly testing during an outbreak identifies presymptomatic or unrecognised symptomatic residents and makes timely isolation and use of PPE possible. We support international recommendations to consider routine testing of staff as soon a positive case of COVID-19 is identified in either staff or residents [29].
Supplementary Material
Contributor Information
Judith H van den Besselaar, Department of Internal Medicine, Section of Geriatric Medicine, Amsterdam Public Health Research Institute, Amsterdam University Medical Center, 1105 AZ Amsterdam, the Netherlands.
Reina S Sikkema, Department of Viroscience, Erasmus Medical Center, 3015 CN Rotterdam, the Netherlands.
Fleur M H P A Koene, Department of Medical Microbiology, Amsterdam University Medical Center, 1105 AZ Amsterdam, the Netherlands; Department of Infectious Diseases, Public Health Laboratory, Public Health Service of Amsterdam, 1018WT Amsterdam, The Netherlands.
Laura W van Buul, Department of Medicine for Older People, Amsterdam Public Health Research Institute, Amsterdam University Medical Center, 1081 BT Amsterdam, The Netherlands.
Bas B Oude Munnink, Department of Viroscience, Erasmus Medical Center, 3015 CN Rotterdam, the Netherlands.
Ine Frénay, Regional Laboratory for Medical microbiology (RLM) Dordrecht- Gorinchem, 3318 AT Dordrecht, The Netherlands.
René te Witt, Eurofins|NMDL-LCPL, 2280 CA Rijswijk, The Netherlands.
Marion P G Koopmans, Department of Viroscience, Erasmus Medical Center, 3015 CN Rotterdam, the Netherlands.
Cees M P M Hertogh, Department of Medicine for Older People, Amsterdam Public Health Research Institute, Amsterdam University Medical Center, 1081 BT Amsterdam, The Netherlands.
Bianca M Buurman, Department of Internal Medicine, Section of Geriatric Medicine, Amsterdam Public Health Research Institute, Amsterdam University Medical Center, 1105 AZ Amsterdam, the Netherlands.
Acknowledgements
We thank all nurses and physician assistants of the participating NH and Public Health Services for their contribution to and/or performance of SARS-CoV-2 testing. We thank the persons from Amsterdam UMC who assisted in questionnaire administration. We thank Ellen Verspuij, Paul van Gennip, Saskia Heugens and Mariëtte van der Spek for their assistance in logistic and administrative aspects of the study. We thank Inge Huijskens of the Regional Laboratory for Medical microbiology (RLM) for her assistance with collecting sequencing data. We thank Anja Schreijer, Menno de Jong, Mariska Petrignani and Joost Wiersinga for their time and discussion for interpreting the data. We thank the laboratories of Regional Laboratory for Medical microbiology (RLM), Eurofins and Sanquin for the performance of SARS-CoV-2 tests.
Declaration of Conflicts of Interest
None.
Declaration of Sources of Funding
This work was supported by the National Institute for Public Health and the Environment (Dutch: RIVM), Bilthoven, the Netherlands.
References
- 1.Comas-Herrera A, Zalakain J, Litwin Cet al. Mortality associated with COVID-19 outbreaks in care homes: early international evidence. Article in LTCcovid. org, International Long-Term Care Policy Network, CPEC-LSE, 26. 2020.
- 2.Yourish L, Ivory S. One-Third of all U.S. Coronavirus Deaths Are Nursing Home Residents or Workers. New York Times, 2020. [Google Scholar]
- 3.Roxby AC, Greninger AL, Hatfield KMet al. Outbreak investigation of COVID-19 among residents and staff of an independent and assisted living community for older adults in Seattle, Washington. JAMA Intern Med 2020; 180: 1101–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Guery R, Delaye C, Brule Net al. Limited effectiveness of systematic screening by nasopharyngeal RT-PCR of medicalized nursing home staff after a first case of COVID-19 in a resident. Med Mal Infect 2020; 50: 748–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dora AV WA, Jatt LP. Universal and serial laboratory testing for SARS-CoV-2 at a long-term care skilled nursing facility for veterans — Los Angeles, California, 2020. Morb Mortal Wkly Rep 2020; 21: 651–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Arons MM, Hatfield KM, Reddy SC, Kimball A, James A, Jacobs JRet al. Presymptomatic SARS-CoV-2 infections and transmission in a skilled nursing facility. New Engl J Med 2020; 382: 2081–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.European Centre for Disease Prevention and Control . Surveillance of COVID-19 in long-term care facilities in the EU/EEA. Stockholm: ECDC, 2020. [Google Scholar]
- 8.CDC . Testing Guidelines for Nursing Homes. Interim SARS-CoV-2 Testing Guidelines for Nursing Home Residents and Healthcare Personnel. In: Centre for Disease Control and Prevention, 2020.
- 9.Kimball A, Hatfield KM, Arons M. Asymptomatic and Presymptomatic SARS-CoV-2 Infections in Residents of a Long-Term Care Skilled Nursing Facility-King County, Washington, 2020. MMWR Morb Mortal Wkly Rep. 2020: 377–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Huffman JC, Kunik ME. Assessment and understanding of pain in patients with dementia. Gerontologist 2000; 40: 574–81. [DOI] [PubMed] [Google Scholar]
- 11.Behandeladvies COVID-19 Acute fase en nazorg . Verenso; 2020. Available from: https://www.verenso.nl/_asset/_public/Thema-en-projecten/Infectieziekten/Covid-19/200825-15-00-COVID-19-behandel-advies-DEFINITIEF.pdf.
- 12.Goldberg SA, Pu CT, Thompson RW, Mark E, Sequist TD, Grabowski DC. Asymptomatic spread of COVID-19 in 97 patients at a skilled nursing facility. J Am Med Dir Assoc 2020; 21: 980–1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Graham NS, Junghans C, Downes Ret al. SARS-CoV-2 infection, clinical features and outcome of COVID-19 in United Kingdom nursing homes. J Infect 2020; 8: 411–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.van Rutten JJS, Loon SM, Joling KJ, van Smalbrugge MS, Buul LW, Hertogh CMPM. Covid-19 in verpleeghuizen. Ned Tijdschr Geneesk 2021; 164: D5173. [PubMed] [Google Scholar]
- 15.D'Adamo H, Yoshikawa T, Ouslander JG.. Coronavirus disease 2019 in geriatrics and long-term care: the ABCDs of COVID-19. J Am Geriatr Soc 2020; 68: 912–7. [DOI] [PubMed] [Google Scholar]
- 16.Voeten HA, Sikkema RS, Damen Met al. Unravelling the modes of transmission of severe acute respiratory syndrome Coronavirus 2 (SARS-CoV-2) during a nursing home outbreak: looking beyond the church super-spread event. Clin Infect Dis 2020: ciaa1664. doi: 10.1093/cid/ciaa1664. [DOI] [PMC free article] [PubMed]
- 17.Oude Munnink BB, Nieuwenhuijse DF, Stein Met al. Rapid SARS-CoV-2 whole-genome sequencing and analysis for informed public health decision-making in the Netherlands. Nat Med 2020; 26: 1405–10. [DOI] [PubMed] [Google Scholar]
- 18.Oude Munnink BB, Nieuwenhuijse DF, Sikkema RS, Koopmans M. Validating whole genome nanopore sequencing, using Usutu virus as an example. J Vis Exp 2020. doi: 10.3791/60906. [DOI] [PubMed] [Google Scholar]
- 19.World Health Organization . Clinical management of COVID-19: interim guidance, 27 May 2020. (No. WHO/2019-nCoV/clinical/2020.5). World Health Organization 2020. https://apps.who.int/iris/handle/10665/332196.
- 20.Prokop M, Everdingen Wv, Vellinga TvRet al. CO-RADS: a categorical CT assessment scheme for patients suspected of having COVID-19—definition and evaluation. Radiology 2020; 296: E97–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gorges RJ, Konetzka RT. Staffing levels and COVID-19 Cases and outbreaks in US nursing homes. J Am Geriatr Soc 2020; 68: 2462–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li Y, Temkin-Greener H, Shan G, Cai X. COVID-19 infections and deaths among Connecticut nursing home residents: facility correlates. J Am Geriatr Soc 2020; 68: 1899–1906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gandhi M, Yokoe DS, Havlir DV. Asymptomatic transmission, the Achilles’ heel of current strategies to control Covid-19. N Engl J Med 2020; 382: 2158–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sanchez GV, Biedron C, Fink LR, Hatfield KM, Polistico JMF, Meyer MPet al. Initial and repeated point prevalence surveys to inform SARS-CoV-2 infection prevention in 26 skilled nursing facilities-Detroit, Michigan, March-May 2020. MMWR Morb Mortal Wkly Rep 2020; 69: 882–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Escobar DJ, Lanzi M, Saberi Pet al. Mitigation of a COVID-19 outbreak in a nursing home through serial testing of residents and staff. Clin Infect Dis 2021; 72: e394–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Buul LW, Besselaar JH, Koene Fet al. Asymptomatic Cases and Limited Transmission of SARS-CoV-2 in Residents and Healthcare Workers in Three Dutch Nursing Homes. Gerontol Geriatr Med 2020; 6:2333721420982800. doi: 10.1177/2333721420982800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shi SM, Bakaev I, Chen H, Travison TG, Berry SD. Risk factors, presentation, and course of coronavirus disease 2019 in a large, academic long-term care facility. J Am Med Dir Assoc 2020; 21: 1378–83.e1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chow EJ, Schwartz NG, Tobolowsky FA, Zacks RLT, Huntington-Frazier M, Reddy SCet al. Symptom screening at illness onset of health care personnel with SARS-CoV-2 infection in King County, Washington. JAMA 2020; 323: 2087–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.World Health Organization. Prevention, identification and management of health worker infection in the context of COVID-19. World Health Organization. 2020. Report No.: WHO/2019-nCoV/HW_infection/2020.1. [Google Scholar]
- 30.Rutten JJS, Loon AM, Kooten J, Buul LW, Joling KJ, Smalbrugge Met al. Clinical suspicion of COVID-19 in nursing home residents: symptoms and mortality risk factors. J Am Med Dir Assoc 2020; 21: 1791–7.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


