Abstract
Background
With multiple coronavirus disease 2019 (COVID-19) vaccines available, understanding the epidemiologic, clinical, and economic value of increasing coverage levels and expediting vaccination is important.
Methods
We developed a computational model (transmission and age-stratified clinical and economics outcome model) representing the United States population, COVID-19 coronavirus spread (February 2020–December 2022), and vaccination to determine the impact of increasing coverage and expediting time to achieve coverage.
Results
When achieving a given vaccination coverage in 270 days (70% vaccine efficacy), every 1% increase in coverage can avert an average of 876 800 (217 000–2 398 000) cases, varying with the number of people already vaccinated. For example, each 1% increase between 40% and 50% coverage can prevent 1.5 million cases, 56 240 hospitalizations, and 6660 deaths; gain 77 590 quality-adjusted life-years (QALYs); and save $602.8 million in direct medical costs and $1.3 billion in productivity losses. Expediting to 180 days could save an additional 5.8 million cases, 215 790 hospitalizations, 26 370 deaths, 206 520 QALYs, $3.5 billion in direct medical costs, and $4.3 billion in productivity losses.
Conclusions
Our study quantifies the potential value of decreasing vaccine hesitancy and increasing vaccination coverage and how this value may decrease with the time it takes to achieve coverage, emphasizing the need to reach high coverage levels as soon as possible, especially before the fall/winter.
Keywords: coronavirus, COVID-19, vaccination, coverage, rate
Our study quantifies the potential value of decreasing vaccine hesitancy, increasing vaccination coverage, and how this may decrease with the time it takes to achieve coverage, emphasizing the need to reach high coverage levels as soon as possible, especially before fall/winter.
(See the Editorial Commentary by Heaton, on pages 931–3.)
With multiple coronavirus disease 2019 (COVID-19) vaccines available, decision makers need to better understand the epidemiologic, clinical, and economic value of attaining higher coverage levels and the speed at which people are vaccinated in order to set targets for how many people to vaccinate and by when this should be done. Recently, the United States (US) government has stated that the country will have a large enough supply of vaccines to make 90% of all adults eligible for vaccination by 19 April 2021 and offer the vaccine to all adults by 1 May 2021 [1, 2]. However, these statements do not indicate target coverage levels or how quickly vaccinations will occur once the supply is available and people are made eligible. While the US is currently vaccinating at a rate of 1.75 million doses per day, which could result in a 70% coverage by mid-June if maintained [3], multiple obstacles, including vaccine hesitancy and refusal [4–6], anti-vaccine messaging [7–11], issues with vaccination scheduling and rollout [12–14], variations in state policies on who can receive a vaccine [15], and production, supply chain, and other logistical challenges [16–18], could delay how many people ultimately receive the vaccine and when. For example, polls by the Kaiser Family Foundation show vaccine hesitancy decreasing among those who would “wait and see” for vaccination from 39% to 17% (December 2020–March 2021), yet an additional 20% surveyed maintained that they would likely not get the vaccine [6]. A poll by Pew Research Center showed similar improvements in those who would get the vaccine (51%–69% in September 2020–February 2021) [4, 5], yet it is possible that COVID-19 vaccine acceptance levels may remain below the fairly high coverage levels (≥75%) needed to quell the pandemic to a point where social distancing and other nonpharmaceutical interventions (NPIs) can be relaxed [19]. As such, to better understand the epidemiologic, clinical, and economic value of increasing COVID-19 vaccine coverage levels and the rate at which these levels are achieved, we used a computational model of the US population to represent the spread of COVID-19 coronavirus and a range of different COVID-19 vaccination scenarios.
METHODS
Model Structure
Using Microsoft Excel (Microsoft Corporation, Redmond, Washington) with the Crystal Ball add-in (Oracle Corporation, Redwood Shores, California), we adapted a previously described computational model representing the US population (327 167 434 persons), their interactions with each other, COVID-19 coronavirus (ie, severe acute respiratory syndrome coronavirus 2, SARS-CoV-2) spread, its potential health and economic outcomes [19–21], and vaccination. The model advances in discrete, 1-day time steps for 3 years (February 2020–December 2022). On any given day, each individual is in 1 of 5 mutually exclusive SARS-CoV-2 compartments: (1) susceptible (S, not infected and able to become infected); (2) exposed (E, infected, but not able to transmit to others); (3) infectious and asymptomatic (Ia, infected, but without symptoms, and able to transmit to others); (4) infectious and symptomatic (Is, infected, showing symptoms, and able to transmit to others); or (5) recovered/immune (R, not infected and unable to become infected). On day 1, a set number of individuals start in the “Ia” and “Is” compartments (ie, seed coronavirus); the remainder start in the “S” compartment. Each day, individuals randomly interact with each other, and an infectious person can potentially transmit the virus to a susceptible person. Individuals move through the compartments at various rates (Supplementary Appendix). Each symptomatically infected person (ie, COVID-19 case) travels through a probability tree of different sequential age-specific outcomes, accruing relevant costs and health effects (Supplementary Appendix) [19–23].
Since different NPIs (eg, social distancing, wearing face masks, closures, curfews) have been implemented to varying degrees (eg, recommendations and mandates vary by state and local jurisdictions and over time) through the course of the pandemic [24–27], scenarios represented the approximate use and effect of NPIs on a national level through 11 January 2021 [28]. The Supplementary Appendix describes the model data sources, calibration, validation, input parameters, and values.
Vaccination
We represented a vaccine that can prevent infection. Vaccination moves a person into the “V” compartment. After exposure to an infected person, these individuals have a probability of being infected equal to 1 minus the vaccine efficacy. Once an individual is vaccinated, protection lasts throughout the simulation duration (ie, no waning immunity). Vaccination has a probability of causing minor (eg, fever, soreness, headache) and major (eg, Guillain-Barré syndrome, allergic reaction/anaphylaxis, resulting in hospitalization) side effects or events. Once vaccination begins, individuals in the population are vaccinated each day based on the daily vaccination rate to achieve the total vaccination coverage level within a given timeframe. Any individual in the population could be vaccinated, even those who have been already infected. We modeled vaccination onset (eg, time point at which onset of protection occurs) to start on 4 January 2021, which was the first date a person could be fully vaccinated with the 2-dose vaccines available in December 2020 (ie, 21 days after the first dose administered on 14 December 2020 [29]).
Economic Measures
The third-party payer perspective includes direct medical costs (eg, vaccination, hospitalization), while the societal perspective includes direct and indirect (ie, productivity losses due to absenteeism resulting from COVID-19 illness) costs. Hourly wage across all occupations [30] serves as a proxy for productivity losses. Absenteeism results in productivity losses for the symptom duration. All COVID-19 cases accrue productivity losses, regardless of age or employment status, as everyone is assumed to contribute to society. All costs are reported in 2020 values, converting all past and future costs using a 3% annual rate.
For each scenario, we calculated the incremental cost-effectiveness ratio (ICER) as:
where A and B represent vaccination scenarios with different coverage levels (described below) and health effects are measured in quality-adjusted life-years (QALYs) lost due to COVID-19. Each COVID-19 infection loses QALY values based on age-dependent healthy QALY value and infection-specific utility weights for their infection duration. Death results in the loss of the net present value of QALYs for the remainder of an individual’s lifetime [31]. We considered increases in coverage to be cost-effective if the ICER was ≤$50 000/QALY and economically dominant if it saved costs and provided health effects (eg, cost saving).
Experimental Scenarios
We evaluated the epidemiologic, clinical, and economic value of increasing COVID-19 vaccination coverage and decreasing the time it takes to achieve these levels; thus, comparisons were made between various coverage levels and vaccination rates. Experiments consisted of Monte Carlo simulations of 2000 trials, varying parameters throughout their range (Supplementary Appendix Table 1). Scenarios consisted of vaccinating individuals to prevent infection on 4 January 2021 and varied the proportion of the population that had been infected before vaccination onset (20%–30%). This represented a range of total cases that had occurred by this date, capturing varying NPIs use and compliance, as well as underreporting of cases. Different scenarios represented what may happen when varying vaccination coverage (10%–90%) and the time to achieve the coverage level (180–360 days). Sensitivity analyses varied vaccine efficacy (50%–90%) as real-world effectiveness under changing conditions may differ compared to clinical trials. Different scenarios also varied the reproduction number (February 2021–December 2022) representing different possible NPI use and compliance and seasonal differences in transmission (Supplementary Appendix). Additional analyses evaluated the impact of increasing coverage in example states.
RESULTS
Figures 1 and 2 show how varying the vaccination coverage and the days that it took to achieve that coverage level affected the number of cases and deaths and direct medical costs and productivity losses, while Figure 3 shows the impact per person vaccinated. Table 1 reports the differences in these cases and deaths. These figures and table assume effective reproduction number values such that (in the absence of vaccination) cases decrease in February and continue downward in March, then start to go up in September, representing a situation in which NPIs continue with current compliance and a more transmissible variant’s spread is slowed by NPI use, then humidity and temperatures start to decrease in September, increasing transmissibility of the virus. The Supplementary Appendix reports results for selected states as examples.
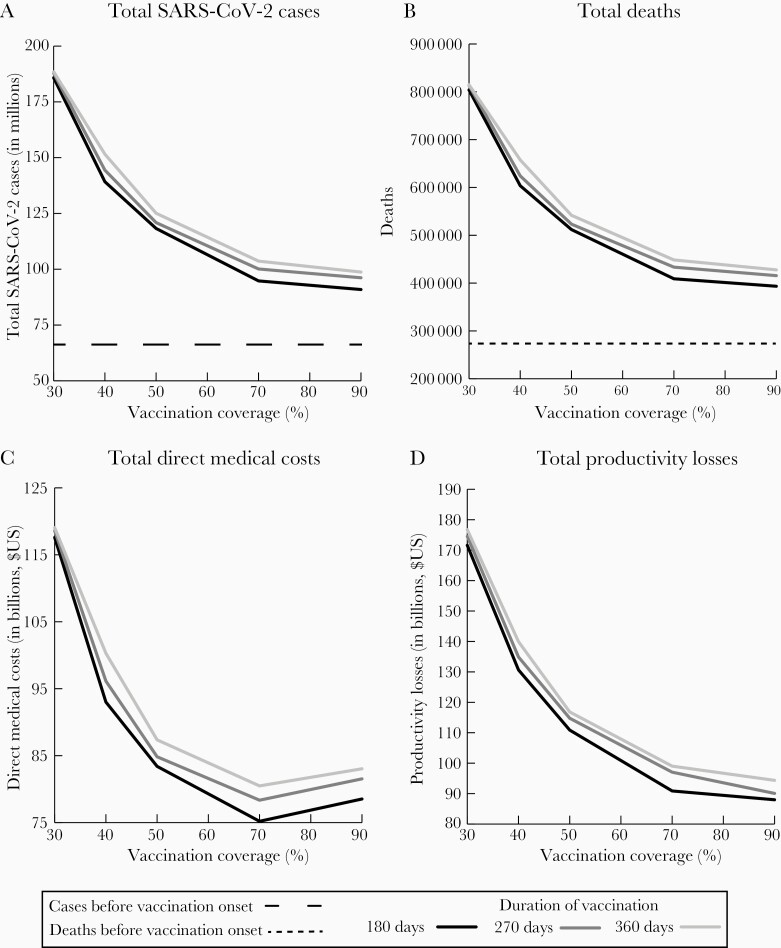
Figure 1.
Impact of increasing vaccination coverage for coronavirus disease 2019 vaccines when 20% of the population has already been infected by vaccination onset (on 4 January 2021) with a 70% vaccine efficacy when varying the days needed to achieve different coverage levels on the number of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) cases (A), the number of deaths (B), direct medical costs (C), and productivity losses due to absenteeism (D). Assumes an $85 vaccination cost. Cases and deaths are simulated through 31 December 2022; scenarios assume reproduction number values such that cases decrease in February and continue downward in March, then start to increase in September, and the following year nonpharmaceutical interventions are discontinued.
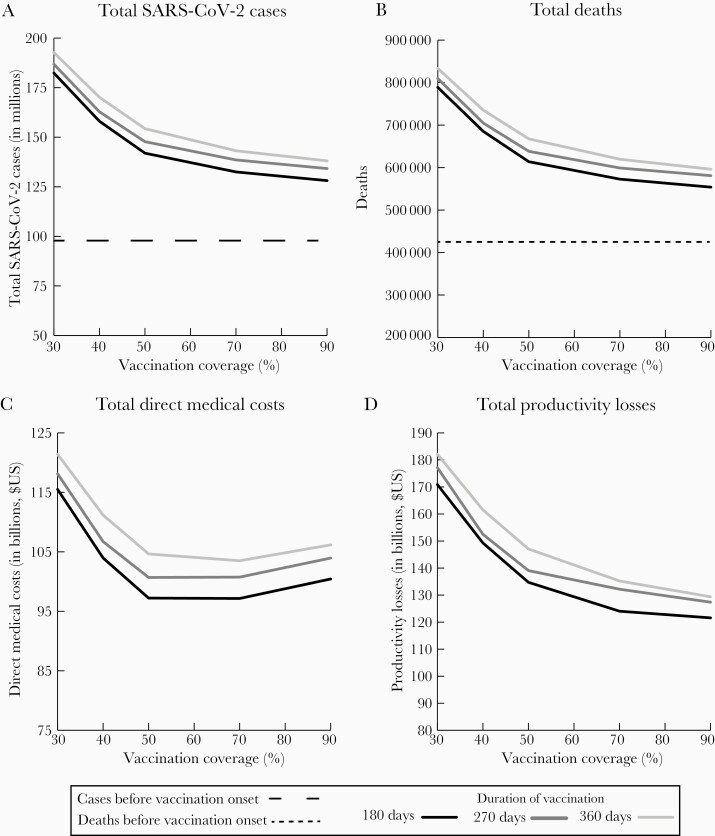
Figure 2.
Impact of increasing vaccination coverage for coronavirus disease 2019 vaccines when 30% of the population has already been infected by vaccination onset (on 4 January 2021) with a 70% vaccine efficacy when varying the days needed to achieve different coverage levels on the number of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) cases (A), the number of deaths (B), direct medical costs (C), and productivity losses due to absenteeism (D). Assumes an $85 vaccination cost. Cases and deaths are simulated through 31 December 2022; scenarios assume reproduction number values such that cases decrease in February and continue downward in March, then start to increase in September, and the following year nonpharmaceutical interventions are discontinued.
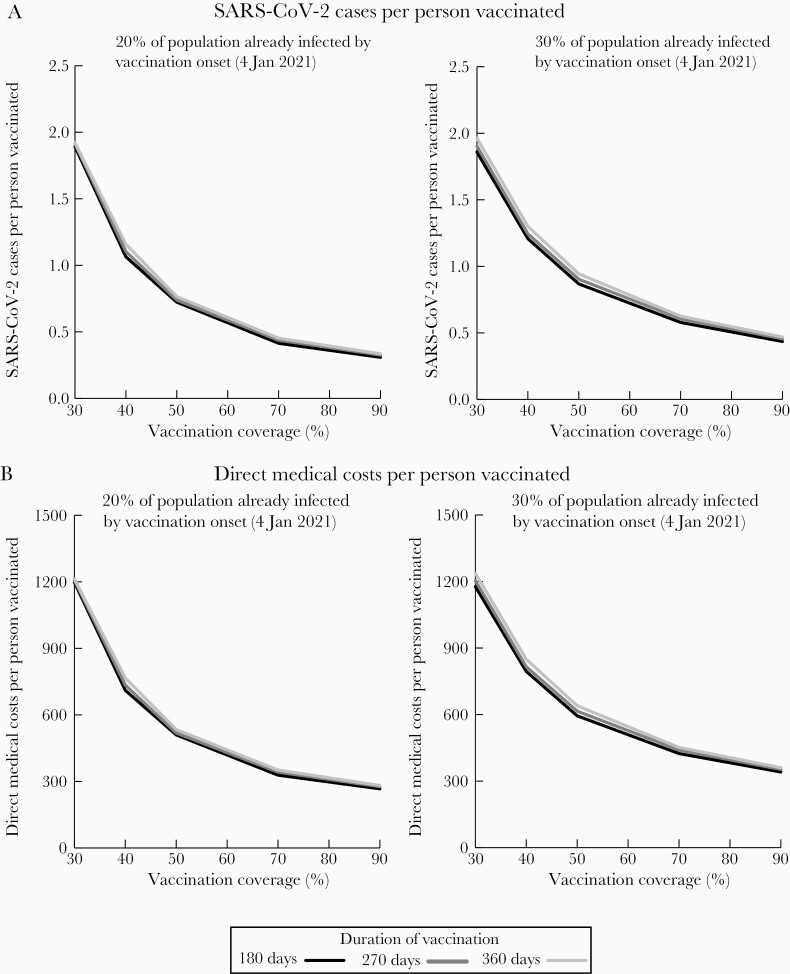
Figure 3.
Impact of increasing vaccination coverage for coronavirus disease 2019 vaccines per person vaccinated with a 70% vaccine efficacy when varying days needed to achieve different coverage levels on the number of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) cases (A) and direct medical costs (B) when different proportions of the population have already been infected by vaccination onset (on 4 January 2021). Assumes an $85 vaccination cost. Cases and costs are simulated through 31 December 2022; scenarios assume reproduction number values such that cases decrease in February and continue downward in March, then start to increase in September, and the following year nonpharmaceutical interventions are discontinued.
Table 1.
Mean Number of Severe Acute Respiratory Syndrome Coronavirus 2 Cases and Deaths Averted When Increasing Vaccination Coverage and Varying the Days Needed to Achieve a Given Vaccination Coverage Level (Vaccine Efficacy of 70%)
| Days Needed to Achieve Vaccination Coverage Level | Increasing Coverage From 30% to 50% | Increasing Coverage From 50% to 70% | Increasing Coverage From 70% to 90% | |||
|---|---|---|---|---|---|---|
| 20% of population had already been infected by vaccination onset | ||||||
| Cases Averted (Millions) | Relative Decrease | Cases Averted (Millions) | Relative Decrease | Cases Averted (Millions) | Relative Decrease | |
| 180 days | 67.5 (66.8–68.3) | 36.3% | 23.5 (22.3–24.7) | 19.9% | 3.9 (2.2–5.5) | 4.1% |
| 270 days | 67.0 (66.4–67.6) | 35.6% | 20.8 (19.8–21.8) | 17.2% | 3.9 (2.6–5.3) | 3.9% |
| 360 days | 63.4 (62.8–64.0) | 33.6% | 21.4 (20.6–22.3) | 17.1% | 4.9 (3.7–6.1) | 4.7% |
| Deaths Averted (Thousands) | Relative Decrease | Deaths Averted (Thousands) | Relative Decrease | Deaths Averted (Thousands) | Relative Decrease | |
| 180 days | 291.8 (287.5–296.2) | 36.3% | 102.9 (97.6–108.3) | 20.1% | 15.6 (8.5–22.7) | 3.8% |
| 270 days | 290.3 (286.4–294.1) | 35.7% | 89.9 (85.2–94.6) | 17.2% | 17.8 (11.5–24.0) | 4.1% |
| 360 days | 272.9 (269.2–276.6) | 33.5% | 93.3 (89.2–97.4) | 17.2% | 20.8 (15.1–26.4) | 4.6% |
| 30% of population had already been infected by vaccination onset | ||||||
| Cases Averted (Millions) | Relative Decrease | Cases Averted (Millions) | Relative Decrease | Cases Averted (Millions) | Relative Decrease | |
| 180 days | 40.4 (39.6–41.2) | 22.2% | 9.5 (8.6–10.3) | 6.7% | 4.4 (3.3–5.4) | 3.3% |
| 270 days | 39.1 (38.2–39.9) | 20.9% | 9.2 (8.4–10.0) | 6.2% | 4.3 (3.4–5.3) | 3.1% |
| 360 days | 38.4 (37.5–39.2) | 19.9% | 11.2 (10.4–11.9) | 7.2% | 5.1 (4.2–5.9) | 3.6% |
| Deaths Averted (Thousands) | Relative Decrease | Deaths Averted (Thousands) | Relative Decrease | Deaths Averted (Thousands) | Relative Decrease | |
| 180 days | 174.9 (170.5–179.4) | 22.2% | 41.0 (36.7–45.4) | 6.7% | 18.8 (13.8–23.9) | 3.3% |
| 270 days | 171.4 (165.7–177.1) | 21.2% | 39.2 (32.3–46.1) | 6.1% | 18.0 (10.6–25.5) | 3.0% |
| 360 days | 165.8 (161.1–170.6) | 19.9% | 48.0 (43.8–52.2) | 7.2% | 23.5 (19.0–27.9) | 3.8% |
Data are presented as mean (95% confidence interval) unless otherwise indicated. Cases and deaths are simulated through 31 December 2022. Scenarios assume reproduction number values in the absence of vaccination such that cases decrease in February and continue downward in March, then start to increase in September, and the following year nonpharmaceutical interventions are discontinued.
Vaccination Protection Onset Starts When 20% of the Population Has Already Been Infected
Impact of Increasing Vaccination Coverage
As Figures 1 and 3 show, achieving 50% coverage in 180 days (eg, by early summer) with a 70% efficacious vaccine resulted in a decrease of 20.9 million cases, 775 980 hospitalizations, and 91 660 deaths and a gain of 977 730 QALYs (about a 15% relative reduction in these outcomes) compared to achieving 40% coverage in 180 days. This saved $9.6 billion in direct medical costs and $19.8 billion in productivity losses. Therefore, every 1% increase in coverage in this range resulted in 2.1 million fewer cases, 77 590 fewer hospitalizations, 9160 fewer deaths, and 97 770 QALYs gained, saving $960.7 million in direct medical costs and $1.9 billion in productivity losses. Dividing by the total population provides the outcomes per person in the population, which equates to a decrease of 0.06 cases and $60 in productivity losses per person. Further increasing coverage to achieve 60% from 50% resulted in a decrease of 11.7 million cases (10% relative reduction), which translates to 1.2 million fewer cases and 5140 fewer deaths and a gain of 52 650 QALYs, saving $410.2 million more in direct medical costs and $1.0 billion in productivity losses per 1% increase in coverage. Increases in coverage between 70% and 90% coverage resulted in higher direct medical costs and were cost-effective, with increases in coverage costing $2978 per QALY gained.
As the figures show, when it took longer to achieve vaccination coverage levels (270 days, by early fall), every 1% increase in coverage between 40% and 50% with a 70% vaccine efficacy resulted in similar incremental reductions in outcomes: 2.3 million fewer cases, 87 990 fewer hospitalizations, 10 090 fewer deaths, and 102 640 QALYs gained (about an 1.6% relative decrease in clinical outcomes), saving $1.1 billion in direct medical costs and $2.0 billion in productivity losses. Further increasing coverage from 50% to 70% resulted in about a 0.9% relative decrease in clinical outcomes (1.0 million fewer cases, 38 470 fewer hospitalizations, 4490 fewer deaths, and 44 890 QALYs gained), saving $324.2 million in direct medical costs and $886.3 million in productivity losses per 1% increase. As Figures 1–3 show, when it took 360 days to achieve vaccination coverage levels, incremental reductions were similar. For example, every 1% increase in vaccination coverage resulted in a 1.8% and 0.9% relative decrease between 40%–50% and 50%–70% coverage, respectively.
With a 90% vaccine efficacy (180 days), achieving 50% coverage compared to 30% coverage resulted in about a 31.8% relative decrease in clinical outcomes (eg, 2.3 million fewer cases per 1% increase in coverage) and saved $2.9 billion in total costs, while increases from 50% to 70% resulted in about a 7.3% relative decrease (eg, 179 330 cases and 756 fewer deaths per 1% increase) and saved $279.9 million. With a 50% vaccine efficacy, increasing coverage from 30% to 50% resulted in a 24.9% relative decrease in clinical outcomes (2.6 million fewer cases and 11 342 fewer deaths per 1% increase in coverage) and saved $69.8 billion, whereas increasing from 50% to 70% coverage also resulted in a 24% relative decrease and saved $55.4 billion.
Impact of Achieving Coverage Levels Faster
The figures also show how shortening the duration in which vaccination coverage levels were achieved from 360 to 270 or 180 days reduced the number of cases, deaths, and costs. For example, reaching a 50% coverage level by 270 days instead of 360 days with a 70% efficacious vaccine decreased cases by 4.2 million and deaths by 18 500, saving $4.5 billion in total costs (Figures 1 and 3). Further shortening to 180 days (vs 270 days) decreased cases by 2.6 million and deaths by 11 300, saving by $5.3 billion in total costs.
Vaccination Protection Onset Starts When 30% of the Population Has Already Been Infected
When 30% of the population had already been infected by vaccination start, increasing coverage resulted in larger incremental gains when increasing between 30% and 50% compared to an increase in coverage between 50% and 90% (Figures 2 and 3; Table 1). For example, when it took 180 days to achieve vaccination coverage levels, by early summer (70% vaccine efficacy), every 1% increase in coverage between 40% and 50% resulted in 1.6 million fewer cases, 60 190 fewer hospitalizations, 7135 fewer deaths, and 78 200 QALYs gained (about a 1% relative decrease in clinical outcomes), saving $674.2 million in direct medical costs and $1.5 billion in productivity losses. When further increasing coverage between 50% and 70%, every 1% increase resulted in 473 917 fewer cases, 17 550 fewer hospitalizations, 2050 fewer deaths, 20 510 QALYs gained, and savings of $3.2 million in direct medical costs and $534.2 million in productivity losses. Increases in coverage were cost saving, except coverages of between 70% and 90%, when increases in coverage cost $6982 per QALY (societal perspective).
When it took 270 days (early fall) to achieve vaccination coverage levels (70% vaccine efficacy), every 1% increase in coverage between 40% and 50% resulted in similar incremental reductions in outcomes (approximately an 0.9% relative reduction), with 1.5 million fewer cases, 56 240 fewer hospitalizations, 6660 fewer deaths, and 77 590 QALYs gained, saving $602.8 million in direct medical costs and $1.3 billion in productivity losses. Further increasing coverage from 50% to 70% resulted in about a 0.3% relative decrease in clinical outcomes (460 360 fewer cases, 16 950 fewer hospitalizations, 1960 fewer deaths, and 16 020 QALYs gained) and saved $343.7 million in productivity losses but cost $2.8 million more in direct medical costs per 1% increase. As Figures 2 and 3 show, trends were similar when it took 360 days (to early winter) to achieve vaccination coverage levels (eg, every 1% increase in vaccination coverage resulted in a 0.9% and 0.3% relative decrease in clinical outcomes between 40%–50% and 50%–70% coverage, respectively).
Figure 4 shows the impact of increasing coverage with different vaccine efficacies. For a 90% efficacious vaccine (180 days), increasing coverage from 30% to 50% resulted in about a 15% relative decrease in clinical outcomes and saved $32.8 billion in total costs ($1.6 billion per 1% increase), while increases between 50% and 70% resulted in about a 4.7% relative decrease in clinical outcomes and saved $188.4 million in total costs ($110.9 million per 1% increase). With a 50% vaccine efficacy, increasing coverage from 30% to 50% resulted in a 18.4% relative decrease in clinical outcomes (eg, 2.1 million fewer cases per 1% increase) and saved $50.7 billion ($2.5 billion per 1% increase), while increasing from 50% to 70% coverage resulted in a 14.1% relative decrease and saved $31.4 billion ($1.6 billion per 1% increase). Increases in coverage resulted in greater gains than increases in efficacy (eg, 70%–90% vaccine efficacy decreases cases by 7.1 million, while increasing coverage from 50% to 70% with a 70% efficacy decreases cases by 9.2 million).
Figure 4.
Impact of increasing vaccination coverage for coronavirus disease 2019 vaccines when varying days needed to achieve different coverage levels on the total number of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) cases when vaccination onset starts once 30% of the population has already been infected with a 50%, 70%, and 90% efficacious vaccine (A), and on the number of SARS-CoV-2 cases per person vaccinated with a 50%, 70%, and 90% efficacious vaccine (B). Cases are modeled through 31 December 2022; scenarios assume reproduction number values such that cases decrease in February and continue downward in March, then start to increase in September, and the following year nonpharmaceutical interventions are discontinued.
Impact of Achieving Coverage Levels Faster
Again, shortening the duration in which vaccination coverage levels were achieved reduced the number of cases, deaths, and total costs (Figures 2–4). For example, reaching a 50% coverage level by 270 days instead of 360 days decreased cases by 6.6 million, hospitalizations by 252 260, deaths by 29 380, direct medical costs by $4.0 billion, and productivity losses by $8.0 billion (70% vaccine efficacy, approximately a 4.4% relative decrease in all outcomes). Further shortening this to 180 days (vs 270 days) decreased cases by 5.8 million, hospitalizations by 215 790, deaths by 24 370, and direct medical costs by $3.5 billion and productivity losses by $4.3 billion (approximately a 3.8% relative decrease in all outcomes).
The Supplementary Appendix presents the results for scenarios in which COVID-19 transmission was higher in February and March.
Discussion
As we have demonstrated, the impact and value of available COVID-19 vaccines will depend heavily on vaccination coverage and the speed at which different coverage levels are achieved. Our results show that even a 1% increase in vaccination coverage can avert several thousands to millions of cases, save thousands of lives, gain thousands of QALYs, and save millions in direct medical costs and productivity losses, depending on the initial coverage level, days to achieve that coverage level, the proportion of the population already infected by vaccination start, and vaccine efficacy. Even if the high coverage levels needed to completely “return to normal” are not achieved, increasing coverage levels still has substantial value with the greatest gains being achieved between 0% and 50% vaccination coverage. Furthermore, the cost savings generated by increasing vaccination coverage represent the amount that could be invested to increase coverage and still break even. The numbers generated can give decision-makers a sense of the value of making vaccines more available and accessible, as well as increasing people’s acceptance of and confidence in COVID-19 vaccines.
Our study also shows how increasing vaccination coverage can be as important as or even more important than having a higher-efficacy vaccine. For example, increasing vaccination coverage from 50% to 70% will prevent 9.2 million cases (70% vaccine efficacy), while going from a 70% efficacious vaccine to 90% will prevent 7.1 million cases (50% coverage). This will be important to consider should the real-world vaccine effectiveness numbers for particular vaccines dip below the ≥90% vaccine efficacy numbers that have been reported in trials. Initial studies report that these vaccines may actually have an 86%–92% effectiveness at preventing infection [32–35]. While the emergence of new variants [36, 37] could further reduce the effectiveness of currently available vaccines [38, 39] and raises concerns about the emergence of future variants adversely affecting vaccine effectiveness, our results show that even with a lower efficacy (eg, 50%), small increases in vaccination can still avert thousands of cases, even at higher coverage levels.
Our results also show how the time it takes to reach different coverage levels can affect the impact and value of the COVID-19 vaccine, showing the importance of achieving high coverage levels as soon as possible, especially by the summer. Even a 90-day delay in achieving 50% coverage (eg, achieving by early fall vs early summer) will result in 5.8 million additional cases, 24 370 more deaths, and $3.5 billion more in direct medical costs. Thus, it will be especially important to achieve high coverage levels before the fall/winter, when relative humidity and temperatures begin to drop, which may be related to an increase in the virus’s transmissibility [40, 41]. This is because the more widespread the virus becomes, the more difficult it will be to prevent its spread, even with vaccination. This underscores not only the importance of getting people vaccinated as quickly as possible (when preexisting immunity is lower and before further spread of variants) but also the importance of using NPIs (eg, social distancing, face mask use) to slow the virus’s spread as much as possible until the vaccines become more widely available and accessible.
While there may be variations in COVID-19 coronavirus spread and NPI use throughout the US, our results show that general trends hold at different scales, such that increases in vaccination coverage in specific states will have similar relative impacts on clinical outcomes and costs as at the national level. Currently, most vaccine deployment decisions are occurring at the state level, and there is wide variation in how many people are being reached in each state, the percentage of people who are hesitant to receive vaccine, and who is able and eligible to receive vaccines [3, 15]. Our results highlight the value of a coordinated national strategy that ensures and supports an adequate supply of vaccines to all states, identifies clear and consistent eligibility guidelines, and provides education and incentives for increasing coverage and doing so faster.
Our findings emphasize the importance of expanding and expediting COVID-19 vaccinations in 2021. They also provide a rationale for working to reach vaccine-hesitant populations and groups in the US, in order to communicate a pro-vaccination agenda.
Limitations
All models, by definition, are simplifications of real life and cannot account for every possible outcome [16]. Model inputs drew from various sources and time points during the pandemic, and new data on SARS-CoV-2 continue to emerge. Since the epidemic course may not be predictable, we explored a range of possible scenarios and parameter values. Our scenarios explore a vaccine protecting against infection based on evidence to date [32–35]; however, the specific efficacy levels for how a vaccine works (eg, reduce severe disease, prevent infection) are still being studied and the data are subject to change. As waning immunity is not yet well understood, our study did not consider natural nor vaccine-induced immunity [42, 43]. Our analyses did not incorporate all costs that vaccines can avert, such as other productivity losses (eg, time required for quarantine), easing other interventions (eg, school closures), or declines in general economic activity (eg, job losses, business closures). Our scenarios assume coverage of the entire population; however, some populations may not be eligible for vaccination (eg, children, immunocompromised). Additionally, we held the daily vaccination rate for each coverage level and duration of time to achieve that coverage; however, in reality, this may vary from day to day.
Supplementary Data
Supplementary materials are available at The Journal of Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Disclaimer. The funders did not have any role in the study design, collection, analysis and interpretation of data, writing of the report, or the decision to submit the report for publication. The authors of this manuscript are responsible for its content, including data analysis. Statements in the manuscript do not necessarily represent the official views of, or imply endorsement by, the National Institutes of Health, the Agency for Healthcare Research and Quality (AHRQ), or the Department of Health and Human Services.
Financial support. This work was supported in part by the City University of New York Graduate School of Public Health and Health Policy; the Agency for Healthcare Research and Quality (AHRQ) (grant number 1R01HS028165-01); and the National Institute of General Medical Sciences as part of the Models of Infectious Disease Agent Study network (grant numbers R01GM127512 and 3R01GM127512-01A1S1).
Potential conflicts of interest. P. J. H. and M. E. B. codirect the Texas Children’s Center for Vaccine Development and with U. S. are co-developers of vaccines against emerging and neglected diseases including coronaviruses such as COVID-19. Baylor College of Medicine nonexclusively licensed a COVID-19 vaccine construct to Biological E, Inc, an India-based manufacturing company. These authors have no financial stakes in any COVID-19 vaccine candidates under development. All other authors report no potential conflicts of interest.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.White House COVID-19 Response Team. Fact sheet: President Biden announces 90% of the adult U.S. population will be eligible for vaccination and 90% will have a vaccination site within 5 miles of home by April 19.https://www.whitehouse.gov/briefing-room/statements-releases/2021/03/29/fact-sheet-president-biden-announces-90-of-the-adult-u-s-population-will-be-eligible-for-vaccination-and-90-will-have-a-vaccination-site-within-5-miles-of-home-by-april-19/. Accessed 31 March 2021.
- 2.The White House . Fact sheet: President Biden to announce all Americans to be eligible for vaccinations by May 1, puts the nation on a path to get closer to normal by July 4th.https://www.whitehouse.gov/briefing-room/statements-releases/2021/03/11/fact-sheet-president-biden-to-announce-all-americans-to-be-eligible-for-vaccinations-by-may-1-puts-the-nation-on-a-path-to-get-closer-to-normal-by-july-4th/. Accessed 31 March 2021.
- 3.New York Times. See how the vaccine rollout is going in your county and state.2021. https://www.nytimes.com/interactive/2020/us/covid-19-vaccine-doses.html. Accessed 3 May 2021.
- 4.Tyson A, Johnson C, Funk C;. Pew Research Center. US public now divided over whether to get covid-19 vaccine. 2020. https://www.pewresearch.org/science/2020/09/17/u-s-public-now-divided-over-whether-to-get-covid-19-vaccine/. Accessed 17 September 2020. [Google Scholar]
- 5.Funk C, Tyson A.. Growing share of Americans say they plan to get a COVID-19 vaccine—or already have. 2021. https://www.pewresearch.org/science/2021/03/05/growing-share-of-americans-say-they-plan-to-get-a-covid-19-vaccine-or-already-have/. Accessed 3 May 2021. [Google Scholar]
- 6.Huetteman E. Covid vaccine hesitancy drops among all Americans, new survey shows.https://khn.org/news/article/covid-vaccine-hesitancy-drops-among-americans-new-kff-survey-shows/. Accessed 31 March 2021.
- 7.Hotez PJ. COVID19 meets the antivaccine movement. Microbes Infect 2020; 22:162–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Olive JK, Hotez PJ, Damania A, Nolan MS. The state of the antivaccine movement in the United States: a focused examination of nonmedical exemptions in states and counties. PLoS Med 2018; 15:e1002578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Malik AA, McFadden SM, Elharake J, Omer SB. Determinants of COVID-19 vaccine acceptance in the US. EClinicalMedicine 2020; 26:100495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Johnson NF, Velásquez N, Restrepo NJ, et al. The online competition between pro-and anti-vaccination views. Nature 2020; 582:230–33. [DOI] [PubMed] [Google Scholar]
- 11.Hotez PJ. America’s deadly flirtation with anti-science and the medical freedom movement. J Clin Invest 2021;131:e149072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zauzmer J, Schneider GS, Cox E. Confusion and chaos: inside the vaccine rollout in D.C., Maryland and Virginia. Washington Post.2021. https://www.washingtonpost.com/local/vaccine-rollout-dc-maryland-virginia/2021/02/07/11d8c4a0-656d-11eb-8c64-9595888caa15_story.html. Accessed 3 May 2021.
- 13.Masters K. Virginia could abandon vaccine scheduling software amid persistent problems. Virginia Mercury, 10 March 2021.
- 14.Laughlin J. Vaccine scheduling software isn’t to blame for rollout problems in Philly and Pa., company founder says. The Philadelphia Inquirer, 11 March 2021.
- 15.Wall Street Journal Staff. How to get a Covid-19 vaccine: a state-by-state guide. Wall Street Journal, 25 March 2021.
- 16.Koons C, Decker S. Inovio tells court supplier is holding covid vaccine “hostage.”https://www.bloomberg.com/news/articles/2020-06-03/inovio-tells-court-supplier-is-holding-covid-vaccine-hostage. Accessed 12 March 2021.
- 17.Centers for Disease Control and Prevention. COVID-19 vaccination program interim playbook for jurisdiction operations, version 2.0. Atlanta, GA: CDC, 2020.
- 18.Michaud J, Kates J, Dolan R, Tolbert J. States are getting ready to distribute COVID-19 vaccines. What do their plans tell us so far?https://www.kff.org/coronavirus-covid-19/issue-brief/states-are-getting-ready-to-distribute-covid-19-vaccines-what-do-their-plans-tell-us-so-far/. Accessed 12 March 2021.
- 19.Bartsch SM, O’Shea KJ, Ferguson MC, et al. Vaccine efficacy needed for a COVID-19 coronavirus vaccine to prevent or stop an epidemic as the sole intervention. Am J Prev Med 2020; 59:493–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bartsch SM, Ferguson MC, McKinnell JA, et al. The potential health care costs and resource use associated with COVID-19 in the United States: a simulation estimate of the direct medical costs and health care resource use associated with COVID-19 infections in the United States. Health Aff 2020;39:927– 35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lee BY, Bartsch SM, Ferguson MC, et al. The value of decreasing the duration of the infectious period of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection. PLoS Comput Biol 2021;17:e1008470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bartsch SM, O’Shea KJ, Wedlock PT, et al. The benefits of vaccinating with the first available COVID-19 coronavirus vaccine. Am J Prev Med 2021; 60:605–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wedlock PT, O’Shea KJ, Conte M, et al. Estimated number of N95 respirators needed for healthcare workers in acute care hospitals during the COVID-19 coronavirus pandemic [manuscript published online ahead of print 11 January 2021]. Infect Control Hosp Epidemiol 2021. doi:10.1017/ice.2020.1418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Institute for Health Metrics and Evaluation. COVID-19 results briefing.http://www.healthdata.org/sites/default/files/files/Projects/COVID/2021/102_briefing_United_States_of_America_4.pdf. Accessed 3 May 2021.
- 25.Crane MA, Shermock KM, Omer SB, Romley JA. Change in reported adherence to nonpharmaceutical interventions during the COVID-19 pandemic, April-November 2020. JAMA 2021; 325:883–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Guy GP Jr, Lee FC, Sunshine G, et al. Association of state-issued mask mandates and allowing on-premises restaurant dining with county-level COVID-19 case and death growth rates—United States, March 1-December 31, 2020. MMWR Morb Mortal Wkly Rep 2021; 70:350–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Institute for Health Metrics and Evaluation. COVID-19 projections—United States of American: social distancing.https://covid19.healthdata.org/united-states-of-america?view=social-distancing&tab=trend. Accessed 12 March 2021.
- 28.Centers for Disease Control and Prevention. United States COVID-19 cases and deaths by state over time.https://data.cdc.gov/Case-Surveillance/United-States-COVID-19-Cases-and-Deaths-by-State-o/9mfq-cb36. Accessed 12 January 2021.
- 29.Centers for Disease Control and Prevention. COVID data tracker: COVID-19 vaccinations in the United States.https://covid.cdc.gov/covid-data-tracker/#vaccinations. Accessed 1 February 2021.
- 30.Bureau of Labor Statistics. Occupational employment statistics: May 2018 national occupational employment and wage estimates, United States.https://www.bls.gov/oes/current/oes_nat.htm. Accessed 9 October 2019.
- 31.Human Mortality Database. Berkeley, CA and Rostock, Germany: University of California, Max Planck Institute for Demographic Research, 2015. [Google Scholar]
- 32.Dagan N, Barda N, Kepten E, et al. BNT162b2 mRNA Covid-19 vaccine in a nationwide mass vaccination setting. N Engl J Med 2021; 384:1412–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hall VJ, Foulkes S, Saei A, et al. COVID-19 vaccine coverage in health-care workers in England and effectiveness of BNT162b2 mRNA vaccine against infection (SIREN): a prospective, multicentre, cohort study [manuscript published online ahead of print 23 April 2021]. Lancet 2021. doi:10.1016/S0140-6736(21)00790-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Thompson MG, Burgess JL, Naleway AL, et al. Interim estimates of vaccine effectiveness of BNT162b2 and mRNA-1273 COVID-19 vaccines in preventing SARS-CoV-2 infection among health care personnel, first responders, and other essential and frontline workers—eight U.S. locations, December 2020-March 2021. MMWR Morb Mortal Wkly Rep 2021; 70:495–500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Keehner J, Horton LE, Pfeffer MA, et al. SARS-CoV-2 infection after vaccination in health care workers in California [manuscript published online ahead of print 23 March 2021]. N Engl J Med 2021. doi:10.1056/NEJMc2101927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Baric RS. Emergence of a highly fit SARS-CoV-2 variant. N Engl J Med 2020; 383:2684–6. [DOI] [PubMed] [Google Scholar]
- 37.Centers for Disease Control and Prevention. Emerging SARS-CoV-2 variants.https://www.cdc.gov/coronavirus/2019-ncov/more/science-and-research/scientific-brief-emerging-variants.html. Accessed 5 January 2021. [PubMed]
- 38.Liu Y, Liu J, Xia H, et al. Neutralizing activity of BNT162b2-elicited serum. N Engl J Med 2021; 384:1466–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rubin R. COVID-19 vaccines vs variants—determining how much immunity is enough. JAMA 2021; 325:1241–3. [DOI] [PubMed] [Google Scholar]
- 40.Merow C, Urban MC. Seasonality and uncertainty in global COVID-19 growth rates. Proc Natl Acad Sci U S A 2020; 117:27456–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Relman D.Rapid expert consultation on SARS-CoV-2 survival in relation to temperature and humidity and potential for seasonality for the COVID-19 pandemic. Washington, DC: The National Academies Press, 2020. [Google Scholar]
- 42.Peeling RW, Wedderburn CJ, Garcia PJ, et al. Serology testing in the COVID-19 pandemic response. Lancet Infect Dis 2020; 20:e245–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Altmann DM, Douek DC, Boyton RJ. What policy makers need to know about COVID-19 protective immunity. Lancet 2020; 395:1527–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.