Abstract
Background
Children living in sub-Saharan Africa have a high burden of rickets and infectious diseases, conditions that are linked to vitamin D deficiency. However, data on the vitamin D status of young African children and its environmental and genetic predictors are limited. We aimed to examine the prevalence and predictors of vitamin D deficiency in young African children.
Methods
We measured 25-hydroxyvitamin D (25(OH)D) and typed the single nucleotide polymorphisms, rs4588 and rs7041, in the GC gene encoding the vitamin D binding protein (DBP) in 4509 children aged 0–8 years living in Kenya, Uganda, Burkina Faso, The Gambia and South Africa. We evaluated associations between vitamin D status and country, age, sex, season, anthropometric indices, inflammation, malaria and DBP haplotypes in regression analyses.
Results
Median age was 23.9 months (interquartile range [IQR] 12.3, 35.9). Prevalence of vitamin D deficiency using 25(OH)D cut-offs of < 30 nmol/L and < 50 nmol/L was 0.6% (95% CI 0.4, 0.9) and 7.8% (95% CI 7.0, 8.5), respectively. Overall median 25(OH)D level was 77.6 nmol/L (IQR 63.6, 94.2). 25(OH)D levels were lower in South Africa, in older children, during winter or the long rains, and in those with afebrile malaria, and higher in children with inflammation. 25(OH)D levels did not vary by stunting, wasting or underweight in adjusted regression models. The distribution of Gc variants was Gc1f 83.3%, Gc1s 8.5% and Gc2 8.2% overall and varied by country. Individuals carrying the Gc2 variant had lower median 25(OH)D levels (72.4 nmol/L (IQR 59.4, 86.5) than those carrying the Gc1f (77.3 nmol/L (IQR 63.5, 92.8)) or Gc1s (78.9 nmol/L (IQR 63.8, 95.5)) variants.
Conclusions
Approximately 0.6% and 7.8% of young African children were vitamin D deficient as defined by 25(OH)D levels < 30 nmol/L and < 50 nmol/L, respectively. Latitude, age, season, and prevalence of inflammation and malaria should be considered in strategies to assess and manage vitamin D deficiency in young children living in Africa.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12916-021-01985-8.
Keywords: 25-hydroxyvitamin D, Vitamin D deficiency, Africa, Children, Nutrition, Vitamin D binding protein, GC genotype
Background
Vitamin D deficiency is estimated to be common worldwide [1], including in Africa [2]. Vitamin D deficiency is an important public health problem due to its link with a growing number of diseases [1]. Children may be at a higher risk of low 25-hydroxyvitamin D (25(OH)D) levels and related diseases [3] including rickets, infectious diseases and impaired growth and development [1, 3]. Young children living in Africa have a high burden of nutritional rickets [4], infectious diseases, and account for more than half of all under-5-year mortality worldwide [5].
Few studies have investigated the prevalence of vitamin D deficiency in young African children and most have followed a case-control design to determine associations with specific disease conditions [2, 4, 6, 7]. Population-based studies include a study from Nigeria with 218 pre-school children aged between 6 and 35 months [8] and two studies from Tanzania, one with 581 infants aged 6 months [9] and another with 948 HIV-exposed (uninfected) infants [10]. Similarly, little is also known about the risk factors for vitamin D deficiency in African children. A single study found that 25(OH)D levels increased with age in 21 infants of Malawian mothers living with HIV [6], and seasonal variation in vitamin D status has been reported in school children in Algeria (n=435) and South Africa (n=385) [11, 12]. Vitamin D deficiency was associated with severe wasting in 21 young Kenyan children with rickets [4], but was not associated with sex, stunting, underweight or wasting in 581 Tanzanian infants [9]. Studies have reported conflicting findings regarding associations between vitamin D status and inflammation [13, 14]; however, only a single study has been conducted in African children [7]. Similarly, only a few studies have evaluated the relationship between vitamin D status and malaria, with mixed findings [9, 10, 15].
Genetic polymorphisms in the group-specific component gene, GC, in the 4th chromosome that codes for Gc globulin (Gc), also known as the vitamin D binding protein (DBP), have been associated with vitamin D status and many pathophysiological conditions [16]. More than 85% of circulating vitamin D metabolites (including 25(OH)D) are bound to DBP [16]. The combination of two GC SNPs (rs7041 and rs4588) give rise to three major DBP variants with different amino acid and glycosylation characteristics, Gc1f, Gc1s and Gc2, and six DBP haplotypes: Gc1f/1f, Gc1f/1 s, Gc1f/2, Gc1s/1 s, Gc1s/2 and Gc2/2 [16]. The DBP variants have been reported to differ in binding affinity and concentrations [16–18]. The Gc1f allele is most frequent in individuals of African ancestry, while Gc1s is more common in Europeans [17]. Nevertheless, little is known about the genetics of vitamin D and how it is related to vitamin D status in populations living in Africa. To our knowledge, only two small genetic studies in The Gambia (n = 237 and n = 18) have assessed the association between 25(OH)D levels and DBP haplotypes in Africa [19, 20].
Information on the prevalence and predictors of low vitamin D status in young African children is important in guiding public health policy, however, this information is limited in African populations. In the current study, we measured 25-hydroxyvitamin D (25(OH)D) levels in 4509 children living in Kenya, Uganda, Burkina Faso, The Gambia and South Africa and evaluated the prevalence and predictors of vitamin D deficiency.
Methods
Study cohorts
This study included young children living in Kenya (n = 1361), Uganda (n = 1301), Burkina Faso (n = 329), The Gambia (n = 629) and South Africa (n = 889). Details of these cohorts have previously been described [21–25] and are briefly summarised below.
Kilifi, Kenya (3.5° S, 39.9° E)
This is an ongoing community-based cohort aimed at evaluating immunity to malaria in children [21]. Children were followed up from birth to eight years with weekly follow-ups and annual cross-sectional surveys during which anthropometric measurements and blood samples were collected. Levels of 25(OH)D, CRP and malaria parasitemia were measured in plasma samples from a single cross-sectional survey, based on the availability of samples archived at − 80 °C.
Entebbe, Uganda (0.1° N, 32.5° E)
The Entebbe Mother and Baby Study (EMaBS) is a prospective birth cohort study that was originally designed as a randomised controlled trial (ISRCTN32849447) aimed at evaluating the effects of helminths and anthelmintic treatment during pregnancy and early childhood on immunological responses to routine vaccinations and incidence of infections in childhood [22]. Anthropometry and blood samples were collected at birth, and at subsequent annual visits. Laboratory assays were conducted in samples from a single annual visit based on the availability of stored samples archived at – 80 °C.
Banfora, Burkina Faso (10.6° N, 4.8° W)
The VAC050 ME-TRAP Malaria Vaccine trial tested the effectiveness, safety and immunogenicity of a malaria vaccine in children between the ages of six and 17 months [23]. Anthropometry and blood samples were collected at multiple time-points after receipt of the experimental vaccine. Levels of 25(OH)D, CRP and malaria parasitaemia were measured on stored plasma samples archived at – 80 °C.
West Kiang, The Gambia (13.3° N, 16.0° W)
This study included children aged between two and six years recruited from 10 rural villages in the West Kiang region of The Gambia as previously described [25]. Anthropometry and biomarkers were measured in samples from a single cross-sectional survey at the start of the malaria season.
Soweto, South Africa (26.2° S, 27.9° E)
The Soweto Vaccine Response Study included infants of African heritage recruited from vaccine trials [24]. This study used stored plasma samples collected from infants that had received all of their routine Expanded Program on Immunization vaccines. The study was conducted in a non-malaria-endemic region and anthropometry and haemoglobin levels were not measured in this cohort.
Laboratory assays
Assays of 25(OH)D (chemiluminescent microparticle immunoassay, Abbot Architect) and C-reactive protein (CRP) (MULTIGENT CRP Vario assay, Abbot Architect) and α1-antichymotrypsin (ACT) (immunoturbidimetry, Cobas Mira Plus Bioanalyser, Roche) were performed. A verification of the 25(OH)D assay and the comparison of its results with those from an LC/MS method have been published previously [26]. In-house assessments of the assay showed heparinized plasma to give results that were on average 5.1% lower than those obtained on matching serum. The assay’s performance was monitored by 12-hourly quality control checks, with overall CVs that ranged from 2.8% to 7.9% for mean 25(OH)D concentrations ranging from 21 to 116 nmol/L (Additional file 9: Figure S1). Over a 6-month period that spanned the 20-week period of analyses, three sets of external quality assurance (DEQAS) data showed the method to have a mean (SD) bias of − 2.7% (7.6) against the all-laboratory trended values, and one of − 0.4% (7.7) against the target values. Malaria parasitaemia was detected using Giemsa-stained thick and thin blood smears.
Definitions
Vitamin D status was defined using 25(OH)D cut-offs of < 30 nmol/L, < 50 nmol/L, and 50–75 nmol/L, as adapted from the Endocrine Society and the US Institute of Medicine guidelines [27–29]. Inflammation was defined as CRP level > 5 mg/L or ACT > 0.6 g/L [30]. Malaria parasitaemia was defined as the presence of asexual malaria parasites at any density. Height-for-age z-scores (HAZ), weight-for-age z-scores (WAZ), and weight-for-height z-scores (WHZ) were computed using the 2006 WHO child growth standards [31]. Stunting was defined as HAZ < − 2, underweight as WAZ < − 2 and wasting as WHZ < − 2. Season was defined using 3 monthly intervals (1st season, December, January, February; 2nd, March, April, May; 3rd, June, July, August; 4th, September, October, November). In South Africa, the seasons correspond to summer, autumn, winter and spring; in Uganda and Kenya, there are two rainy and two dry seasons; and in Burkina Faso and The Gambia, there is a single rainy and a single dry season, although the timing of the rains is often unpredictable and may vary [12, 32, 33].
Genotyping and SNP quality control
Genomic DNA from study participants were genotyped using genome-wide SNP arrays (see Additional file 1: Supplementary Methods for more details). Two GC SNPs (rs7041 and rs4588) were retrieved from imputed data and their combinations used to classify participants into Gc variants; Gc1f (T and C), Gc1s (G and C) and Gc2 (T and A) and six DBP haplotypes; Gc1f/f (TT, CC), Gc1f/s (TG, CC), Gc1f/2 (TT, CA), Gc1s/s (GG, CC), Gc1s/2 (TG, CA), and Gc2/2 (TT, AA).
Statistical analyses
All statistical analyses were conducted using Stata Statistical Software: Release 15 (College Station, TX: StataCorp LLC) and R version 3.5.1 (https://www.R-project.org/). 25(OH)D levels were natural log (ln)-transformed to normalise their distributions in regression analyses. Medians and geometric means for 25(OH)D levels were used to summarise average 25(OH)D levels for different groups. Between-group differences in median 25(OH)D levels were tested using Wilcoxon rank-sum test (two categories) and Kruskal-Wallis equality-of-populations rank test (more than two categories). Linear and logistic regression analyses were performed to evaluate the association between vitamin D status (ln-25(OH)D levels and 25(OH)D levels of < 50 and between 50 and 75 nmol/L compared to > 75 nmol/L) and country, age, sex, season, stunting, underweight, wasting, inflammation, malaria, and DBP haplotypes and variants. Since few children had 25(OH)D levels < 30 nmol/L, further analyses did not include this group. Multivariable regression analyses were adjusted for age, sex, season, inflammation, and study site, as appropriate.
We further searched PubMed and Embase for published studies that measured serum 25(OH)D levels in healthy children aged 0–8 years in Africa without date of publication or language restrictions. The search strategy is presented in Additional file 2: Table S1. We then carried out meta-analyses of low vitamin D status categories (25(OH)D levels < 50 and < 75 nmol/L) and mean 25(OH)D levels using random effects models (‘meta’ R package).
Role of the funding source
The funders had no role in the study design, data collection, data analysis, data interpretation, or writing of the report. The corresponding authors had full access to all the data and had final responsibility for the decision to submit for publication.
Results
Characteristics of study participants
A total of 4509 infants and children with an age range of 0.2 months to 8 years and a median age of 23.9 months (interquartile range 12.3, 35.9) were included in the study (Table 1). Approximately half (49.1%) of children were female. Overall prevalence of stunting, underweight and wasting was 25.4%, 15.6% and 6.4%, respectively, and varied by country with the highest prevalence observed in Kenyan children. Overall prevalence of inflammation and asymptomatic malaria was 22.8% and 13.5%, respectively, and varied by country with the highest prevalence observed in Burkina Faso (33.9% and 21.1%, respectively) (Table 1).
Table 1.
Characteristics of study participants
| Overall | Kenya | Uganda | Burkina Faso | The Gambia | South Africa | |
|---|---|---|---|---|---|---|
| No. of participants (%) | 4509 (100%) | 1361 (30.1%) | 1301 (28.9%) | 329 (7.3%) | 629 (13.9%) | 889 (19.7%) |
| Median 25(OH)D nmol/L (IQR)a | 77.6 (63.6, 94.2) | 81.0 (66.3, 101.6) | 78.6 (65.1, 94.5) | 78.4 (64.5, 91.3) | 71.2 (59.1, 84.2) | 76.2 (60.6, 91.9) |
| Vitamin D status | ||||||
| 25(OH)D > 150 nmol/l | 79/4509 (1.8%) | 51/1361 (3.7%) | 17/1301 (0.1%) | 4/329 (1.3%) | 1/629 (0.2%) | 6/889 (0.7%) |
| 25(OH)D > 75 nmol/l | 2485/4509 (55.1%) | 815/1361 (59.9%) | 756/1301 (58.1%) | 186/329 (56.5%) | 265/629 (42.1%) | 463/889 (52.1%) |
| 25(OH)D 50–75 nmol/l | 1674/4509 (37.1%) | 464/1361 (34.1%) | 479/1301 (36.8%) | 123/329 (37.4%) | 302/629 (48.0%) | 306/889 (34.4%) |
| 25(OH)D < 50 nmol/l | 350/4509 (7.8%) | 82/1361 (6.0%) | 66/1301 (5.1%) | 20/329 (6.1%) | 62/629 (9.9%) | 120/889 (13.5%) |
| 25(OH)D < 30 nmol/l | 28/4509 (0.6%) | 4/1361 (0.3%) | 5/1301 (0.4%) | 0/329 (0%) | 2/629 (0.3%) | 17/889 (1.9%) |
| Median age (months) | 23.9 (12.3, 35.9) | 19.8 (12.7, 36.8) | 24.1 (23.9, 35.9) | 23.4 (19.7, 26.4) | 46.6 (35.2, 58.7) | 12.0 (11.9, 12.1) |
| Age categories (months) | ||||||
| < 12 | 816/4509 (18.1%) | 300/1361 (22.0%) | 24/1301 (1.8%) | 19/329 (5.8%) | - | 473/889 (53.2%) |
| 12–24 | 1597/4509 (35.4%) | 555/1361 (40.8%) | 440/1301 (33.8%) | 172/329 (52.3%) | 15/629 (2.4%) | 415/889 (46.7%) |
| 24–36 | 1029/450 (22.8%) | 153/1361 (11.2%) | 587/1301 (45.1%) | 138/329 (42.0%) | 150/629 (23.9%) | 1/889 (0.1%) |
| 36–48 | 478/4509 (10.6%) | 146/1361 (10.7%) | 167/1301 (11.8%) | - | 165/629 (26.2%) | - |
| 48+ | 589/4509 (13.1%) | 207/1361 (15.2%) | 83/1301 (6.4%) | - | 299/629 (47.5%) | - |
| Sex: females | 2216/4509 (49.1%) | 671/1361 (49.3%) | 641/1301 (49.3%) | 161/329 (48.9%) | 297/629 (47.2%) | 446/889 (50.2%) |
| Seasoneb | ||||||
| Summer/short rains/dry | 867/4503 (19.3%) | 285/1361 (20.9%) | 331/1296 (25.5%) | 72/329 (21.9%) | - | 179/889 (18.1%) |
| Autumn/dry | 1475/4503 (32.8%) | 896/1361 (65.8%) | 295/1296 (22.8%) | 123/329 (37.4%) | - | 161/889 (18.1%) |
| Winter/long rains | 1361/4503 (30.2%) | 86/1361 (6.3%) | 330/1296 (25.5%) | 129/329 (39.2%) | 536/628 (85.4%) | 280/889 (31.5%) |
| Spring/dry | 800/4503 (17.8%) | 94/1361 (6.9%) | 340/1296 (26.2%) | 5/329 (1.5%) | 92/628 (14.7%) | 269/889 (30.3%) |
| Nutritional statusc | ||||||
| Stunted | 581/2289 (25.4%) | 99/208 (47.6%) | 203/1282 (15.8%) | 103/307 (33.5%) | 176/492 (35.8%) | n/a |
| Underweight | 389/2487 (15.6%) | 102/389 (26.2%) | 103/1296 (8.0%) | 58/309 (18.8%) | 126/493 (25.6%) | n/a |
| Wasted | 147/2285 (6.4%) | 24/205 (11.7%) | 59/1281 (4.6%) | 20/307 (6.5%) | 44/492 (8.9%) | n/a |
| Inflammationd | 1019/4469 (22.8%) | 363/1344 (27.0%) | 306/1285 (23.8%) | 109/322 (33.9%) | 85/629 (13.5%) | 156/889 (17.6%) |
| Malariae | 445/3293 (13.5%) | 227/1082 (20.8%) | 89/1280 (7.0%) | 64/303 (21.1%) | 65/628 (10.4%) | n/a |
South African children were not exposed to malaria
IQR inter-quartile range, n/a not available, 25(OH)D 25-hydroxyvitamin D
aMedians (interquartile ranges) are presented. bSeasons were based on 3 monthly intervals: 1st season, December to February; 2nd season, March to May; 3rd season, June to August; 4th season, September to November. In South Africa, the seasons correspond to summer, autumn, winter and spring, respectively, in Uganda and Kenya there are two rainy and two dry seasons and in Burkina Faso and The Gambia there is a single rainy and dry season. However, timing of the rains is often unpredictable and may vary from these times. cStunted was defined as height-for-age Z score < − 2; underweight as weight-for-age Z score < − 2, wasted as weight-for-height Z score < − 2 (denominator number varied because anthropometry data was not available for South African children). dInflammation as CRP > 5 mg/L or ACT > 0.6 g/L. ACT, but not CRP, was available for The Gambia. eMalaria as the presence of P. falciparum parasites on blood film
Vitamin D status
Overall median 25(OH)D level was 77.6 nmol/L (IQR 63.6, 94.2), and geometric mean 25(OH)D level was 77.0 nmol/L (95% CI 76.3, 77.7). Prevalence of vitamin D deficiency defined by 25(OH)D levels of < 50 nmol/L or < 30 nmol/L were 7.8% (350/4509) and 0.6% (28/4509), respectively (Table 1). A total of 1674 children (37.1%) had 25(OH)D levels between 50 and 75 nmol/L. The prevalence of vitamin D deficiency varied by country, with the highest prevalence observed in South African children (Table 1 and Fig. 1). About 1.8% (79/4509) of children had 25(OH)D levels above 150 nmol/L (51 Kenyan, 17 Ugandan, four Burkinabe, one Gambian and six South African children).
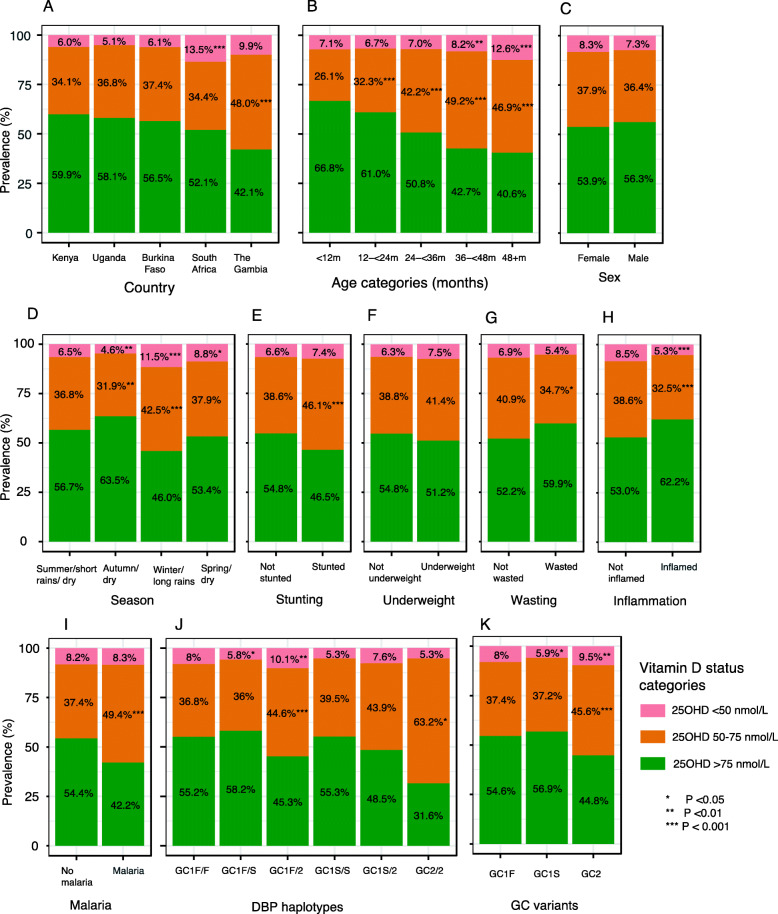
Fig. 1.
Prevalence of vitamin D categories by country (a), age categories (b), sex (c), season (d), stunting (e), underweight (f), wasting (g), inflammation (h), malaria status (i), and vitamin D binding protein (DBP) haplotypes (j) and variants (k). Season was based on 3 monthly intervals. In South Africa the seasons are summer, autumn, winter and spring, in Uganda and Kenya there are two rainy seasons and in Burkina Faso and The Gambia a single rainy season. Stunting was defined as height-for-age Z score < − 2; underweight as weight-for-age Z score < − 2, wasting as weight-for-height Z score < − 2; inflammation as CRP > 5 mg/L or ACT > 0.6 g/L (ACT, but not CRP was available for The Gambia); malaria as presence of P. falciparum parasites on blood film. Prtest (STATA) was used to test the significance in the difference in the proportion of low vitamin status (25(OH)D levels < 50 or 50–75 nmol/L) within each category with the first category as the reference
Vitamin D status is associated with age and season, but not with nutritional status
25(OH)D levels decreased with increasing age across all age groups (Additional file 3: Table S2 and Additional file 10: Figure S2), even after adjustment for potential confounders in multivariable regression analyses (Table 2). Approximately 4.5% of the observed variation (R2 = 0.045) in 25(OH)D levels was explained by age (Table 2). In a multivariable linear regression analysis, study site, age, sex, season and inflammation accounted for 12% (R2 = 0.12) of observed variation in 25(OH)D levels. Each additional year of age increased the odds of 25(OH)D levels < 50 and 50–75 nmol/L by 69% (OR 1.69, [95% CI 1.52, 1.89]) and 43% (OR 1.43, [95% CI 1.34, 1.52]), respectively (Additional file 4: Table S3). Vitamin D deficiency (25(OH)D < 50 nmol/L) was more prevalent during the South African winter and the long rains in sub-Saharan Africa (Fig. 1 and Additional file 4: Table S3). Seasonality explained 3.8% of the variation in 25(OH)D levels (R2 = 0.038) (Table 2).
Table 2.
Environmental factors are associated with vitamin D status
| 25(OH)D levels (nmol/L)a | Unadjusted regression analyses of 25(OH)D levels | Adjusted regression analyses of 25(OH)D levels | Adjusted logistic regression analyses 25(OH)D < 50 nmol/L | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Medians (IQR) | Means (95% CI) | Beta (95% CI) | P | R2 | Beta (95% CI) | P | OR (95% CI) | P | |
| Age in years | - | - | − 0.05 (− 0.06, − 0.04) | < 0.0001 | 0.045 | − 0.07 (− 0.08, − 0.07) | < 0.0001 | 1.69 (1.52, 1.89) | < 0.0001 |
| Sex | |||||||||
| Males | 77.7 (64.1, 94.5) | 77.50 (76.52, 78.49) | Ref. | – | Ref. | – | Ref. | – | |
| Females | 77.2 (63.0, 94.0) | 76.45 (75.43, 77.48) | − 0.01 (− 0.03, 0.01) | 0.15 | 0.0005 | − 0.02 (− 0.03, 0.001) | 0.066 | 1.32 (1.04, 1.68) | 0.021 |
| Country | |||||||||
| Kenya | 81.0 (66.3, 101.6) | 81.85 (80.42, 83.30) | Ref. | – | Ref. | – | Ref. | – | |
| Uganda | 78.6 (65.1, 94.5) | 78.27 (77.02, 79.54) | − 0.04(− 0.07, − 0.02) | 0.0002 | 0.029 | 0.01 (− 0.02, 0.03) | 0.51 | 0.50 (0.34, 0.74) | 0.001 |
| Burkina Faso | 78.4 (64.5, 91.3) | 77.26 (74.98, 79.61) | − 0.06 (− 0.10, − 0.02) | 0.003 | − 0.05 (− 0.08, − 0.01) | 0.012 | 0.81 (0.45, 1.44) | 0.47 | |
| The Gambia | 71.2 (59.1, 84.2) | 72.78 (71.15, 74.45) | − 0.15 (− 0.18, − 0.12) | < 0.0001 | 0.09 (0.05, 0.13) | < 0.0001 | 0.29 (0.17, 0.47) | < 0.0001 | |
| South Africa | 76.2 (60.6, 91.9) | 70.39 (68.95, 71.86) | − 0.12 (− 0.14, − 0.09) | < 0.0001 | − 0.15 (− 0.18, − 0.12) | < 0.0001 | 2.89 (1.94, 4.29) | < 0.0001 | |
| Seasonb | |||||||||
| Summer/short rains/dry | 78.8 (64.8, 95.8) | 78.77 (77.19, 80.39) | Ref. | – | Ref. | – | Ref. | – | |
| Autumn/dry | 83.6 (67.1, 101.1) | 82.72 (81.43, 84.02) | 0.05 (0.02, 0.07) | 0.0002 | 0.038 | 0.03 (0.01, 0.06) | 0.014 | 0.65 (0.40, 0.96) | 0.032 |
| Winter/long rains | 73.3 (60.1, 87.0) | 71.14 (69.96, 72.34) | − 0.10 (− 0.13, − 0.08) | < 0.0001 | − 0.10 (− 0.12, − 0.07) | < 0.0001 | 2.55 (1.75, 3.72) | < 0.0001 | |
| Spring/dry | 76.3 (63.1, 91.4) | 75.18 (73.55, 76.84) | − 0.05 (− 0.08, − 0.02) | 0.002 | − 0.05 (− 0.08, − 0.02) | 0.002 | 1.60 (1.07, 2.38 | 0.022 | |
| Stuntingc | |||||||||
| Not stunted | 77.1 (63.7, 91.0) | 76.13 (75.08, 77.19) | Ref. | – | Ref. | – | Ref. | – | |
| Stunted | 73.1 (61.2, 88.4) | 73.58 (71.89, 75.31) | − 0.03 (− 0.06, − 0.01) | 0.015 | 0.003 | − 0.01 (− 0.04, 0.01) | 0.34 | 1.05 (0.69, 1.58) | 0.82 |
| Underweightd | |||||||||
| Not underweight | 77.2 (63.8, 91.7) | 76.67 (75.72, 77.64) | Ref. | – | Ref. | – | Ref. | – | |
| Underweight | 75.6 (61.9, 91.3) | 74.90 (72.68, 77.18) | − 0.02 (− 0.06, 0.01) | 0.15 | 0.0008 | − 0.004 (− 0.04, 0.03) | 0.81 | 0.88 (0.54, 1.40) | 0.58 |
| Wastinge> | |||||||||
| Not wasted | 76.0 (62.9, 90.6) | 75.32 (74.40, 76.26) | Ref. | – | Ref. | – | Ref. | – | |
| Wasted | 79.2 (63.6, 94.5) | 77.81 (74.45, 81.32) | 0.03 (− 0.02, 0.08) | 0.19 | 0.0008 | 0.04 (− 0.01, 0.08) | 0.12 | 0.64 (0.29, 1.38) | 0.25 |
| Inflammationf | |||||||||
| Without inflammation | 76.4 (62.7, 92.3) | 75.53 (74.74, 76.3) | Ref. | – | Ref. | – | Ref. | – | |
| With inflammation | 81.9 (68.0, 99.5) | 82.11 (80.55, 83.69) | 0.08 (0.06, 0.11) | < 0.0001 | 0.012 | 0.07 (0.05, 0.09) | < 0.0001 | 0.59 (0.43, 0.81) | 0.001 |
| Malariag | |||||||||
| Without malaria | 77.1 (63.1, 92.4) | 76.01 (75.26, 76.77) | Ref. | – | Ref. | – | Ref. | – | |
| With malaria | 71.3 (58.9, 85.4) | 71.63 (69.68, 73.63) | − 0.06 (− 0.09, − 0.03) | 0.0001 | 0.004 | − 0.04 (− 0.07, − 0.01) | 0.02 | 1.02 (0.65, 1.61) | 0.93 |
Correlation coefficients and p values were obtained from linear regression analyses and odds ratios from logistic regression analyses. 25(OH)D levels were ln-transformed in linear regression analyses to make them normally distributed. Covariates in the multivariable linear regression models included age, sex, season, inflammation and study site, as appropriate
25(OH)D 25-hydroxyvitamin D, n/a not available, OR odds ratio, 95% CI 95% confidence interval
aMedians (IQR) and geometric means are presented.bSeason was based on 3 monthly intervals. In South Africa, the seasons were summer, autumn, winter and spring; in Uganda and Kenya, there are two rainy seasons; and in Burkina Faso and The Gambia, there is a single rainy season. cStunting was defined as height-for-age Z score < − 2; dunderweight as weight-for-age Z score < − 2; ewasting as weight-for-height Z score < − 2; finflammation as CRP > 5 mg/L or ACT > 0.6 g/L and gmalaria as presence of P. falciparum parasites on blood film. Analyses by country are presented in Additional file 4: Table S3 (logistic regression) Additional file 5: Table S4 (unadjusted linear regression), and Additional file 6: Table S5 (adjusted linear regression)
Median 25(OH)D levels were lower in stunted children in univariable analyses, but this association was not observed after adjustment for potential confounding factors in multivariable regression models (Table 2). Overall 25(OH)D levels were not associated with sex, underweight or wasting although girls had a 32% higher risk of 25(OH)D levels of <50 nmol/L (Table 2 and Fig. 1), a finding that was mainly observed in The Gambia. Findings are presented by individual countries in Additional file 3: Tables S2, Additional file 4: Table S3, Additional file 5: Table S4 and Additional file 6: Table S5.
25(OH)D levels are higher with inflammation and lower with malaria
Children with inflammation (CRP > 5 mg/L or ACT > 0.6 g/L) had higher median 25(OH)D levels (81.9 nmol/L [IQR 68.0, 99.5]) than those without inflammation (76.4 nmol/L [IQR 62.7, 92.3])) (Table 2 and Additional file 10: Figure S2), a difference that was observed in all countries except Burkina Faso (Additional file 3: Table S2). Inflammation explained 1.2% (R2 = 0.012) of the total variation in 25(OH)D levels (Table 2). Children with inflammation were 42% and 26% less likely to have 25(OH)D levels of < 50 and 50–75 nmol/L, respectively, compared to those with 25(OH)D levels > 75 nmol/L (Additional file 4: Table S3). CRP levels also varied by country (Additional file 11: Figure S3).
Children with asymptomatic malaria parasitaemia had lower median 25(OH)D levels (71.3 nmol/L [IQR 58.9, 85.4]) than those without (77.1 nmol/L (IQR 63.1, 92.4) (Table 2 and Additional file 10: Figure S2). Malaria was further associated with lower vitamin D status in multivariable regression analyses adjusted for potential confounders, although this association was observed only in Kenya (Table 2, Additional file 4: Table S3 and Additional file 6: Table S5). Malaria parasitaemia explained 0.4% of variation in 25(OH)D levels (R2 = 0.004) (Table 2).
Vitamin D binding protein variants are associated with vitamin D status
Overall, the most frequent DBP haplotype was Gc1f/f (69.8%), followed by Gc1f/2 (13.6%), Gc1f/s (13.4%), Gc1s/2 (1.7%), Gc1s/s (1.0%), and least frequent was Gc2/2 (0.6%) (Table 3). The most frequent Gc variant was Gc1f (83.3%), followed by Gc1s (8.5%), and the least frequent was Gc2 (8.2%). Frequencies of DBP haplotypes and variants varied by country, with the highest frequencies of Gc1f, Gc1s and Gc2 observed in South Africa (87.3%), The Gambia (13.1%) and Uganda (10.2%), respectively (Additional file 7: Table S6). Median 25(OH)D levels were lowest in children carrying the Gc2 variant (72.4 nmol/L [IQR 59.4, 86.5]), but did not differ between the Gc1f (77.3 nmol/L [IQR 63.5, 92.8]) and Gc1s variants (78.9 nmol/L [63.8, 95.5]). Median 25(OH)D levels similarly differed by DBP haplotype (Table 3). DBP haplotypes and variants explained 0.9% and 0.4% of the variation in 25(OH)D levels, respectively (Table 3). The Gc2 variant was associated with lower 25(OH)D levels (β = − 0.08 [95% CI − 0.11, − 0.06] and a 69% (OR 1.69 [1.23, 2.31]) increased risk of vitamin D deficiency (25(OH)D levels < 50 nmol/L) in adjusted regression analyses (Table 3). The Gc2 variant was similarly associated with the highest prevalence of vitamin D deficiency (Fig. 1). Country-specific analyses are presented in Additional file 7: Table S6).
Table 3.
Vitamin D binding protein haplotypes and variants are associated with vitamin D status
| Combination of genotypes | DBP Haplotypea | n/total (%) | 25(OH)D levels (nmol/L)c | Unadjusted regression analyses of 25(OH)D levels | Adjusted regression analyses of 25(OH)D levels | Adjusted regression analyses 25(OH)D < 50 nmol/L | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| rs7041 | rs4588 | Medians (IQR) | Means (95% CI) | Beta (95% CI) | P | R2 | Beta (95% CI) | P | OR (95% CI) | P | ||
| TT | CC | Gc1f/f | 2689/3851 (69.8%) | 77.7 (63.6, 93.0) | 76.7 (75.8, 77.6) | Ref | – | 0.009 | Ref | – | Ref | – |
| TG | CC | Gc1f/s | 514/3851 (13.4%) | 79.2 (65.5, 96.0) | 78.7 (76.6, 80.8) | 0.03 (0.004, 0.05) | 0.092 | 0.03 (− 0.0023, 0.05) | 0.077 | 0.71 (0.47, 1.08) | 0.11 | |
| TT | CA | Gc1f/2 | 525/3851 (13.6%) | 72.7 (60.0, 86.8) | 71.6 (69.7, 73.6) | − 0.07 (− 0.10, − 0.04) | < 0.0001 | − 0.08 (− 0.11, − 0.05) | < 0.0001 | 1.79 (1.26, 2.55) | 0.001 | |
| GG | CC | Gc1s/s | 38/3851 (1.0%) | 81.8 (61.0, 95.7) | 77.8 (69.7, 86.9) | 0.01 (− 0.08, 0.11) | 0.78 | 0.01 (− 0.09, 0.10) | 0.85 | 0.73 (0.16, 3.29) | 0.69 | |
| TG | CA | Gc1s/2 | 66/3851 (1.7%) | 74.6 (59.4, 87.6) | 71.7 (66.1, 77.8) | − 0.07 (− 0.14, − 0.01) | 0.081 | − 0.08 (− 0.15, − 0.01) | 0.026 | 1.18 (0.43, 3.30) | 0.75 | |
| TT | AA | Gc2/2 | 19/3851 (0.5%) | 63.9 (52.4, 79.7) | 65.6 (58.0, 74.1) | − 0.16 (− 0.29, − 0.02) | 0.027 | − 0.18 (− 0.37, − 0.04) | 0.009 | 2.34 (0.27, 20.40) | 0.44 | |
| Gc variantb | ||||||||||||
| T | C | Gc1f | 6417/7702 (83.3%) | 77.3 (63.5, 92.8) | 76.5 (75.9, 77.0) | Ref | – | 0.004 | Ref | – | Ref | – |
| G | C | Gc1s | 656/7702 (8.5%) | 78.9 (63.8, 95.5) | 77.9 (76.0, 79.7) | 0.02 (− 0.01, 0.043) | 0.15 | 0.02 (− 0.01, 0.04) | 0.16 | 0.73 (0.52, 1.06) | 0.096 | |
| T | A | Gc2 | 629/7702 (8.2%) | 72.4 (59.4, 86.5) | 71.3 (69.5, 73.1) | − 0.07 (− 0.10, − 0.04) | < 0.0001 | − 0.08 (− 0.11, − 0.06) | < 0.0001 | 1.69 (1.23, 2.30) | 0.001 | |
DBP vitamin D binding protein, Gc group-specific component, IQR inter-quartile range, n/a not available, 25(OH)D 25-hydroxyvitamin D, OR odds ratio, 95% CI 95% confidence interval
a,bDBP haplotypes and Gc variants are based on combination of rs7041 and rs4588 genotypes. cMedian (IQR) and geometric means (95% CI) are presented. Percentage is based on the successfully typed DBP, some participants’ genotype data was not available or failed QC. DBP haplotype and Gc variant frequencies are presented by country in Additional file 7: Table S6
Meta-analysis
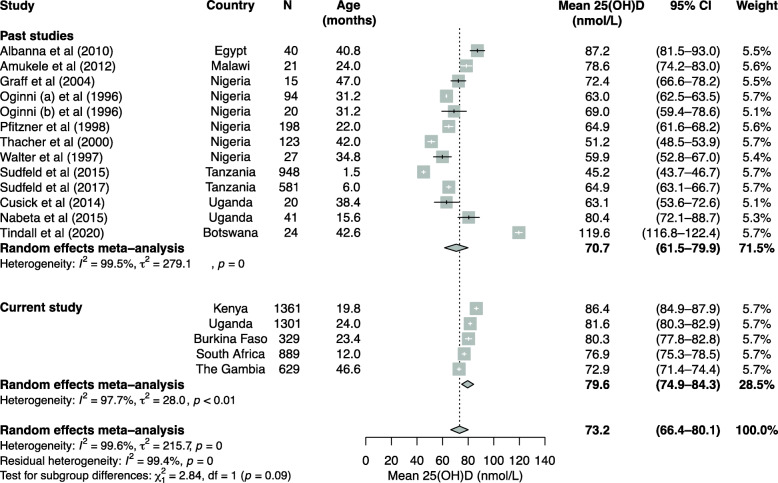
Out of 18 previous studies that assessed the vitamin D status of African children aged 0–8 years (Additional file 8: Table S7), we included a total of 12 studies in the meta-analyses. Six studies were excluded because they lacked estimates of mean 25(OH)D levels, prevalence of vitamin D status (25(OH)D < 50 or < 75 nmol/L) or did not report estimates from children aged between 0 and 8 years. The meta-analyses included 2128 children from five African countries with mean ages ranging from one to 47 months and included estimates from healthy children in 9 case-control and three population-based studies in addition to the current study. Overall, mean 25(OH)D level in young African children was 73.2 nmol/L (95% CI 66.4, 80.1) (Fig. 2) and prevalence of 25(OH)D levels < 50 and < 75 nmol/L was 10.9% (95% CI 6.9, 15.5) and 49.1% (95% CI 40.8, 57.5), respectively (Additional file 12: Figure S4 and Additional file 13: Figure S5). Only a single eligible study reported prevalence defined by 25(OH)D levels < 30 nmol/L [4] precluding meta-analysis. There was high heterogeneity between studies included in the meta-analyses with overall I2 ranging from 95.1% and 99.4% (p < 0.01).
Fig. 2.
Meta-analysis of studies that evaluated 25(OH)D levels in healthy young children in Africa. For case-control studies, we only included mean 25(OH)D levels of healthy controls in the meta-analysis. Means of age in months are presented. Studies that only measured 25(OH)D levels in cord blood, reported only median values, or did not report estimates from young children (aged 0–8 years) separately from older children were excluded from these analyses. Details of the studies are presented in Additional file 8: Table S7
Discussion
Little is known about the vitamin D status of African children. In this study, overall median 25(OH)D level was 77.6 nmol/L (IQR 63.6, 94.2) and prevalence of 25(OH)D cut-offs between 50 and 75, < 50, and < 30 nmol/L was 37.1%, 7.8%, and 0.6%, respectively, in young African children. The prevalence of vitamin D deficiency (25(OH)D levels < 50 nmol/L) was higher in South African children and during the South African winter or the long rainy season in East Africa. Median 25(OH)D levels decreased with increasing age and with inflammation but did not differ by sex or nutritional status after adjustment for potential confounders in multivariable models. Malaria parasitaemia was associated with lower 25(OH)D levels overall and in Kenyan children, but not in Ugandan, Burkinabe or Gambian children. The most common Gc variant was Gc1f (83.3%), followed by Gc1s (8.5%) and Gc2 (8.2%), with Gc2 being associated with the lowest 25(OH)D levels and the highest prevalence of low vitamin D status (25(OH)D levels < 50 and 50–75 nmol/L). In a meta-analysis of the current and previous studies of young African children overall mean 25(OH)D level was 73.2 nmol/L.
Our median 25(OH)D estimate of 77.6 nmol/L is comparable to values reported in previous studies of healthy young children in sub-Saharan Africa including reported levels of 80.4 nmol/L in Uganda, 78.6 nmol/L in Malawi, and 72.4 nmol/L in Nigeria (Fig. 2). However, studies in 198 Nigerian children and 581 Tanzanian infants living in urban areas reported lower mean levels of 64.8 and 64.9 nmol/L, respectively [8, 9]. The prevalence of vitamin D deficiency (25(OH)D levels < 50 nmol/L) was 7.8% overall, with a higher prevalence of 13.5% in South Africa. Previous studies of healthy young children have reported higher prevalence estimates of 13.6% in Kenya, 15.0% in Uganda, and 25.8% in Nigeria [4, 8, 15]. Prevalence estimates were also higher in young children from other continents, including estimates of 15% in the USA, 14% in Japan, and 11% in China [34–36]. Vitamin D deficiency is also more prevalent in northern African countries [2], and the higher vitamin D status observed in our study might be explained by differences in latitude, geography, skin pigmentation, clothing coverage, and religious and cultural practices across Africa [37]. The year-round abundance of sunshine in sub-Saharan Africa may also explain higher vitamin D status although vitamin D supplementation and food fortification is less common. Since vitamin D deficient rickets is rare at levels above 30 nmol/L, the very low prevalence of 25(OH)D levels < 30 nmol/l (0.6%) in the current study suggests that rickets in African children is more likely to be caused by calcium rather than vitamin D deficiency. Conversely, we found very few children with 25(OH)D levels above 150 nmol/L suggesting that even plenty of exposure to sunlight rarely generates these high levels probably because vitamin D production in the skin is highly regulated [1].
In the present study, 25(OH)D levels decreased consistently with age overall and in all countries except The Gambia (Additional file 3: Table S2), perhaps because of older age in the Gambian children and corresponding cultural habits. Age explained 4.5% of the variation in 25(OH)D levels. Previous studies of school children (aged 5–18 years) from South Africa, Ethiopia and Algeria have also reported that 25(OH)D levels decreased with age [11, 38, 39]. However, a single study in Malawian pre-school children reported an increase in 25(OH)D levels with age; however, this study was small (n=21) and included infants of mothers living with HIV in Malawi [6]. Studies from high-income countries have reported an increase in 25(OH)D levels with age, but children in these studies received vitamin D supplementation or fortification [34, 35, 40]. In a meta-analysis of previous studies from Africa, children had higher vitamin D status than adults in African populations [2] possibly due to increased time spent outdoors.
In addition, we found limited evidence of an association between 25(OH)D levels and sex, although overall girls had a 32% (95% CI 1.04, 1.68) increased risk of vitamin D deficiency (25(OH)D < 50 nmol/L) compared to boys. Similar studies from South Africa, China, and Ecuador have not found sex-related differences in 25(OH)D levels [36, 38, 41]. We also found evidence of seasonality in 25(OH)D levels with the strongest effect in South Africa, and more variable effects observed across the sub-Saharan African countries during the rainy season. These findings may be explained by colder winters in South Africa with greater coverage of skin by clothing and more time spent indoors and in East Africa by increased cloud cover and less time spent outdoors during the long rains. Season explained 3.8% of the variation in 25(OH)D levels. These findings agree with previous studies that evaluated the effect of seasonality in vitamin D status in Africa, although many of these studies were in South Africa or northern African countries [2].
We did not find associations between 25(OH)D levels and stunting, underweight, or wasting in adjusted regression analyses. Similarly, studies in young children from Tanzania (n = 948) and Nepal (n = 280) reported that 25(OH)D levels were not associated with stunting, underweight or wasting [10, 42]. However, severe wasting was associated with 25(OH)D levels < 30 nmol/L in 21 Kenyan children with rickets [4]. In addition, Mokhtar and colleagues reported that low 25(OH)D levels (< 42.5 nmol/L) were more common among stunted and underweight Ecuadorian children [41]. The lack of association between vitamin D status and anthropometric indices in the current study suggests that sunlight may be more important than dietary intake in influencing vitamin D status in Africa.
We observed a positive correlation between 25(OH)D levels and markers of inflammation overall and children with inflammation had higher 25(OH)D levels than those without after adjusting for potential confounders. Our findings agree with results from a small case-control study in Egyptian children with sepsis (n = 40, mean age 6 years) and a large community-based study of children (n = 4274, mean age 9.9 years) in England, which reported that 25(OH)D levels increased with increasing levels of CRP and IL-6 [7, 13]. In contrast, a recent meta-analysis of 24 randomised controlled trials reported that vitamin D supplementation had an overall effect of reducing IL6, but not CRP or other inflammatory markers, although many trial participants had medical conditions [14]. A nationally representative survey of 15,167 adults in the USA reported an inverse association between 25(OH)D and CRP levels at 25(OH)D levels < 52 nmol/L and a positive association above this level [43], suggesting that there may be a U-shaped association, which might explain previously mixed findings. In our study, 90% of children had 25(OH)D levels > 52 nmol/L, perhaps explaining the positive association observed between inflammation and 25(OH)D levels. However, the association between inflammation and 25(OH)D may not be clinically relevant since inflammation explained only 1.2% of the observed variation in 25(OH)D levels.
In this study, 25(OH)D levels between 50 and 75 nmol/L were associated with an increased risk of afebrile malaria parasitaemia overall compared to levels > 75 nmol/L. In contrast, a study in western Kenya found no association between 25(OH)D levels and malaria parasitaemia in newborns and their mothers [44]. However, another study in Uganda reported that children with severe malaria had lower 25(OH)D levels than healthy community children [15]. Malaria explained only 0.4% of the variation in 25(OH)D levels in our study, suggesting that this association may not be clinically significant. No clinical trials have yet investigated the effect of vitamin D supplementation on malaria incidence or treatment outcomes [45].
In the current study, we found that Gc1f was the most frequent Gc variant (83.3%) and the Gc1s and Gc2 variants were less frequent (8.5 and 8.2%, respectively). The Gc1f and Gc1s variants were associated with higher 25(OH)D levels compared to the Gc2 variant. In agreement with our study, a study involving 237 Gambian children with similar Gc variant frequencies (Gc1f was 86%, Gc1s 11% and Gc2 3%) reported that Gc1f was associated with higher 25(OH)D levels than the other haplotypes combined [19]. Gc1f, the most frequent Gc variant in Africans, has a higher binding affinity for vitamin D compared to the Gc1s and Gc2 variants which are more frequent in Europeans and Asians [16, 18]. In a study involving adults from The Gambia and the UK (n=36), Gc1f was associated with higher total 25(OH)D levels and shorter 25(OH)D half-life [20]. In addition, DBP levels and genetic polymorphisms have been linked to lower levels of 25(OH)D in black American adults compared with white American adults [17]. In another study involving multi-ethnic children, differences in DBP polymorphisms were associated with lower vitamin D status in African children and reduced response to vitamin D intake compared to Hispanic and Caucasian children [46]. The differences in 25(OH)D levels attributed to DBP polymorphism has led to the suggestion that DBP variants and levels should be considered in the assessment of vitamin D status in different populations [47].
Strengths and limitations
To the best of our knowledge, the current study, which included a total of 4509 children, is the largest study to date to evaluate vitamin D status and its predictors in young African children. The study further evaluated the effect of common genetic polymorphisms encoding the vitamin D binding protein on 25(OH)D levels. However, our findings should be interpreted in the context of some limitations. First, due to the cross-sectional nature of our study, we could not evaluate temporal changes in 25(OH)D and CRP levels or infer the direction of causality for the observed associations. We also did not measure vitamin D binding protein, parathyroid hormone, calcium levels, dietary or supplementary vitamin D intake, or exposure to sunlight, factors which have been shown to influence vitamin D status. DBP may also be a better marker of the effect of inflammation since it is reduced during tissue damage and in inflammation resulting in the reduction of circulating 25(OH)D levels and other vitamin D metabolites [48]. Our study cohorts were also diverse with different ethnicities and ages, and hence may not be generalisable to other age groups or African countries, although cohort-specific data and analyses were presented.
Conclusions
Approximately 0.6% and 7.8% of children in our study had 25(OH)D levels of < 50 or < 30 nmol/L, and approximately one third of children had 25(OH)D levels between 50 and 75 nmol/L. Our data indicate that older children and those who live further from the equator may be at a higher risk of vitamin D deficiency. Stunting, underweight and wasting were not associated with vitamin D deficiency suggesting that sunlight is a more important source of vitamin D than dietary intake in children living in sub-Saharan Africa. Genetic differences in DBP also altered 25(OH)D levels. Further research is required to understand the effects of inflammation and malaria on vitamin D status in Africa and to investigate the causality between vitamin D status and infectious diseases like malaria, which are common in African children. These findings may have important implications for public health strategies involving young children in Africa.
Supplementary Information
Additional file 1: Supplementary methods. (Genotyping and SNP quality control). This file describes the methods used in genotyping and SNP quality control, QC parameters, allocation of vitamin D binding protein Gc variants and haplotypes.
Additional file 2: Table S1. Databases search keywords. This table includes keywords used systematically search PubMed and Embase for articles of studies that measured vitamin D status in young children.
Additional file 3: Table S2. Median 25(OH)D levels by study variable in each country. This is a table of median 25(OH)D levels for each study variable in each country.
Additional file 4: Table S3. Multivariable logistic regression analyses of factors associated with low vitamin D status in each country. This is a table showing odd ratios (with their corresponding 95% CIs and p values) from multivariable logistic regression analyses.
Additional file 5: Table S4. Univariable linear regression of 25(OH)D concentrations by study variables in each country. This is a table of univariable linear regression coefficients (with 95% CI) and p values presented by country.
Additional file 6: Table S5. Multivariable regression of 25(OH)D concentrations by study variables in each country. This is a table of multivariable linear regression coefficients (with 95% CI) and p values presented by country.
Additional file 7: Table S6. Median 25(OH)D levels by vitamin D binding protein haplotype and Gc variant in each country. This is a table of DBP variant and haplotype frequencies and median 25(OH)D levels by country.
Additional file 8: Table S7. Summary characteristics of studies that evaluated the vitamin D status of African children (in alphabetical order). This is a table describing characteristics of past studies that evaluated the vitamin D status of young children in Africa.
Additional file 9: Figure S1. The precision profile of the 25(OH)D assay. This is a scatter plot of coefficient of variation (%) of the 25(OH)D assay used in this study.
Additional file 10: Figure S2. Boxplots of 25(OH)D concentrations by country (A), age categories (B), sex (C), season (D) stunting (E), underweight (F), wasting (G), inflammation (H), malaria (I), vitamin D binding protein (DBP) isotype (J) and Gc variant (K). This is a grid of boxplots of 25(OH)D concentrations for each study variable.
Additional file 11: Figure S3. Boxplots of CRP levels overall and by country. These are boxplots of CRP levels.
Additional file 12: Figure S4. Meta-analysis of studies that estimated the prevalence of 25(OH)D levels < 50 nmol/L in healthy children aged 0–8 years in Africa. This is a meta-analysis plot of prevalence estimates of vitamin D deficiency (defined by 25(OH)D levels < 50 nmol/L) in young children included in the current and previous studies.
Additional file 13: Figure S5. Meta-analysis of studies that estimated the prevalence of 25(OH)D levels < 75 nmol/L in healthy children aged 0–8 years in Africa. This is a meta-analysis plot of prevalence estimates of low vitamin D status (defined by 25(OH)D levels < 75 nmol/L) in young children included in the current and previous studies.
Acknowledgements
We thank colleagues at the KEMRI-Wellcome Trust Research Programme, Jedidah Mwacharo and Barnes Kitsao for assistance in retrieval of archived samples. We also thank the teams at the UVRI/MRC Entebbe Mother and Baby Study, the Malaria Vectored Vaccines Consortium (MVVC) in Burkina Faso, and the Respiratory and Meningeal Pathogens Unit (RMPRU) in Johannesburg, South Africa. We thank the Oxford Genomics Centre at the Wellcome Centre for Human Genetics (funded by Wellcome Trust grant reference 203141/Z/16/Z) for the generation and initial processing of the sequencing data for samples from Kenya and The Gambia. This study is published with the permission of the Office of the Director of KEMRI.
Abbreviations
- 25(OH)D
25-hydroxyvitamin D
- CRP
C-reactive protein
- EMaBS
Entebbe Mother and Baby Study
- HAZ
Height-for-age z-scores
- WAZ
Weight-for-age z-scores
- WHZ
Weight-for-height z-scores
- DBP
Vitamin D binding protein
Authors’ contributions
RMM, TNW, AA and SHA conceived and designed the study. Data was obtained by all the authors and was analysed by RMM, JMM, and SHA. RMM and SHA wrote the first draft of the manuscript. RMM, JMM, AJM, EMW, WK, AWM, FMN, CC, SBS, AD, ABT, SAL, AM, SAM, AMP, MSS, PB, JMP, AME, AA, TNW, SHA contributed to data interpretation, reviewed successive drafts and approved the final version of the manuscript. The sponsors played no role in study design, data collection, data analysis, data interpretation or writing of the report.
Funding
This work was funded by Wellcome [grant numbers SHA 110255, TNW 202800, AJM 106289 and AME 064693, 079110, 095778], and with core awards to the KEMRI-Wellcome Trust Research Programme [203077]. AA is supported by the Intramural Research Program of the National Institutes of Health in the Center for Research on Genomics and Global Health (CRGGH). The CRGGH (1ZIAHG200362) is supported by the National Human Genome Research Institute, the National Institute of Diabetes and Digestive and Kidney Diseases, the Center for Information Technology, and the Office of the Director at the National Institutes of Health. RMM is supported through the Developing Excellence in Leadership, Training and Science (DELTAS) Africa Initiative [DEL-15-003]. The DELTAS Africa Initiative is an independent funding scheme of the African Academy of Sciences (AAS)’s Alliance for Accelerating Excellence in Science in Africa (AESA) and supported by the New Partnership for Africa’s Development Planning and Coordinating Agency (NEPAD Agency) with funding from Wellcome [107769] and the UK government. The Gambian work was supported by the UK MRC (U1232661351, U105960371 and MC-A760-5QX00) and DFID under the MRC/DFID Concordat. The views expressed in this publication are those of the authors and not necessarily those of AAS, NEPAD Agency, Wellcome or the UK government. For the purpose of Open Access, the authors have applied a CC-BY public copyright licence to any author accepted manuscript version arising from this submission. The funders had no role in the study design, data collection, data analysis, data interpretation, or writing of the report.
Availability of data and materials
The data and analyses scripts underlying this article are available in Harvard Dataverse at 10.7910/DVN/MH1LPE and applications for data access can be made through the Kilifi Data Governance Committee cgmrc@kemri-wellcome.org.
Declarations
Ethics approval and consent to participate
Informed written consent was obtained from all children’s parents or guardians before inclusion in the study. Ethical approvals were granted by the Scientific Ethics Review Unit of the Kenya Medical Research Institute (KEMRI/SERU/CGMR-C/046/3257) in Kenya, the Uganda Virus Research Institute (GC/127/12/07/32) and Uganda National Council for Science and Technology (MV625) in Uganda, by Ministere de la Recherche Scientifique et de l’Innovation (reference 2014-12-151) in Burkina Faso, the Gambian Government and the Medical Research Council Ethics Review Committee in The Gambia (874/830), the University of Witwatersrand Human Research Ethics Committee (M130714) in South Africa and in the UK by the London School of Hygiene and Tropical Medicine (A340) and the Oxford Tropical Research Ethics Committees (39-12, 41-12, 42-14, and 1042-13).
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Reagan M. Mogire, Email: reaganmoseti@gmail.com
Sarah H. Atkinson, Email: satkinson@kemri-wellcome.org
References
- 1.Holick MF. Vitamin D deficiency. N Engl J Med. 2007;357(3):266–281. doi: 10.1056/NEJMra070553. [DOI] [PubMed] [Google Scholar]
- 2.Mogire RM, Mutua A, Kimita W, Kamau A, Bejon P, Pettifor JM, Adeyemo A, Williams TN, Atkinson SH. Prevalence of vitamin D deficiency in Africa: a systematic review and meta-analysis. Lancet Glob Health. 2020;8(1):e134–e142. doi: 10.1016/S2214-109X(19)30457-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Weydert JA. Vitamin D in Children's Health. Children (Basel) 2014;1(2):208–226. doi: 10.3390/children1020208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jones KDJ, Hachmeister CU, Khasira M, Cox L, Schoenmakers I, Munyi C, Nassir HS, Hunten-Kirsch B, Prentice A, Berkley JA. Vitamin D deficiency causes rickets in an urban informal settlement in Kenya and is associated with malnutrition. Matern Child Nutr. 2018;14(1):e12452. doi: 10.1111/mcn.12452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kyu HH, Pinho C, Wagner JA, Brown JC, Bertozzi-Villa A, Charlson FJ, Coffeng LE, Dandona L, Erskine HE, Ferrari AJ, et al. Global and National Burden of Diseases and Injuries Among Children and Adolescents Between 1990 and 2013: Findings From the Global Burden of Disease 2013 Study. JAMA Pediatr. 2016;170(3):267–287. doi: 10.1001/jamapediatrics.2015.4276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Amukele TK, Soko D, Katundu P, Kamanga M, Sun J, Kumwenda NI, Taha TE. Vitamin D levels in Malawian infants from birth to 24 months. Arch Dis Child. 2013;98(3):180–183. doi: 10.1136/archdischild-2012-302377. [DOI] [PubMed] [Google Scholar]
- 7.Aydemir G, Cekmez F, Kalkan G, Fidanci MK, Kaya G, Karaoglu A, Meral C, Arziman I, Karademir F, Ayar G, et al. High serum 25-hydroxyvitamin D levels are associated with pediatric sepsis. Tohoku J Exp Med. 2014;234(4):295–298. doi: 10.1620/tjem.234.295. [DOI] [PubMed] [Google Scholar]
- 8.Pfitzner MA, Thacher TD, Pettifor JM, Zoakah AI, Lawson JO, Isichei CO, Fischer PR. Absence of vitamin D deficiency in young Nigerian children. J Pediatr. 1998;133(6):740–744. doi: 10.1016/S0022-3476(98)70143-X. [DOI] [PubMed] [Google Scholar]
- 9.Sudfeld CR, Manji KP, Smith ER, Aboud S, Kisenge R, Fawzi WW, Duggan CP. Vitamin D deficiency is not associated with growth or the incidence of common morbidities among Tanzanian infants. J Pediatr Gastroenterol Nutr. 2017;65(4):467–474. doi: 10.1097/MPG.0000000000001658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sudfeld CR, Duggan C, Aboud S, Kupka R, Manji KP, Kisenge R, Fawzi WW. Vitamin D status is associated with mortality, morbidity, and growth failure among a prospective cohort of HIV-infected and HIV-exposed Tanzanian infants. J Nutr. 2015;145(1):121–127. doi: 10.3945/jn.114.201566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Djennane M, Lebbah S, Roux C, Djoudi H, Cavalier E, Souberbielle JC. Vitamin D status of schoolchildren in Northern Algeria, seasonal variations and determinants of vitamin D deficiency. Osteoporos Int. 2014;25(5):1493–1502. doi: 10.1007/s00198-014-2623-7. [DOI] [PubMed] [Google Scholar]
- 12.Poopedi MA, Norris SA, Pettifor JM. Factors influencing the vitamin D status of 10-year-old urban South African children. Public Health Nutr. 2011;14(2):334–339. doi: 10.1017/S136898001000234X. [DOI] [PubMed] [Google Scholar]
- 13.Williams DM, Fraser A, Sayers A, Fraser WD, Hingorani A, Deanfield J, Davey Smith G, Sattar N, Lawlor DA. Associations of 25-hydroxyvitamin D2 and D3 with cardiovascular risk factors in childhood: cross-sectional findings from the Avon Longitudinal Study of Parents and Children. J Clin Endocrinol Metab. 2012;97(5):1563–1571. doi: 10.1210/jc.2011-2335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mazidi M, Rezaie P, Vatanparast H. Impact of vitamin D supplementation on C-reactive protein; a systematic review and meta-analysis of randomized controlled trials. BMC Nutrition. 2018;4(1):1. doi: 10.1186/s40795-017-0207-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cusick SE, Opoka RO, Lund TC, John CC, Polgreen LE. Vitamin D insufficiency is common in Ugandan children and is associated with severe malaria. Plos One. 2014;9(12):e113185. doi: 10.1371/journal.pone.0113185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bikle DD, Schwartz J. Vitamin D binding protein, total and free vitamin D levels in different physiological and pathophysiological Conditions. Front Endocrinol (Lausanne) 2019;10:317. doi: 10.3389/fendo.2019.00317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Powe CE, Evans MK, Wenger J, Zonderman AB, Berg AH, Nalls M, Tamez H, Zhang D, Bhan I, Karumanchi SA, Powe NR, Thadhani R. Vitamin D-binding protein and vitamin D status of black Americans and white Americans. N Engl J Med. 2013;369(21):1991–2000. doi: 10.1056/NEJMoa1306357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Arnaud J, Constans J. Affinity differences for vitamin D metabolites associated with the genetic isoforms of the human serum carrier protein (DBP) Hum Genet. 1993;92(2):183–188. doi: 10.1007/BF00219689. [DOI] [PubMed] [Google Scholar]
- 19.Braithwaite VS, Jones KS, Schoenmakers I, Silver M, Prentice A, Hennig BJ. Vitamin D binding protein genotype is associated with plasma 25OHD concentration in West African children. Bone. 2015;74:166–170. doi: 10.1016/j.bone.2014.12.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jones KS, Assar S, Harnpanich D, Bouillon R, Lambrechts D, Prentice A, Schoenmakers I. 25(OH)D2 half-life is shorter than 25(OH)D3 half-life and is influenced by DBP concentration and genotype. J Clin Endocrinol Metab. 2014;99(9):3373–3381. doi: 10.1210/jc.2014-1714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bejon P, Williams TN, Liljander A, Noor AM, Wambua J, Ogada E, Olotu A, Osier FH, Hay SI, Farnert A, et al. Stable and unstable malaria hotspots in longitudinal cohort studies in Kenya. Plos Med. 2010;7(7):e1000304. doi: 10.1371/journal.pmed.1000304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Elliott AM, Kizza M, Quigley MA, Ndibazza J, Nampijja M, Muhangi L, Morison L, Namujju PB, Muwanga M, Kabatereine N, Whitworth JA. The impact of helminths on the response to immunization and on the incidence of infection and disease in childhood in Uganda: design of a randomized, double-blind, placebo-controlled, factorial trial of deworming interventions delivered in pregnancy and early childhood [ISRCTN32849447] Clin Trials. 2007;4(1):42–57. doi: 10.1177/1740774506075248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tiono AB, Nebie I, Anagnostou N, Coulibaly AS, Bowyer G, Lam E, Bougouma EC, Ouedraogo A, Yaro JBB, Barry A, et al. First field efficacy trial of the ChAd63 MVA ME-TRAP vectored malaria vaccine candidate in 5-17 months old infants and children. Plos One. 2018;13(12):e0208328. doi: 10.1371/journal.pone.0208328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nunes MC, Cutland CL, Jones S, Hugo A, Madimabe R, Simões EAF, Weinberg A, Madhi SA. Duration of infant protection against influenza illness conferred by maternal immunization. JAMA Pediatr. 2016;170(9):840–847. doi: 10.1001/jamapediatrics.2016.0921. [DOI] [PubMed] [Google Scholar]
- 25.Atkinson SH, Rockett K, Sirugo G, Bejon PA, Fulford A, O'Connell MA, Bailey R, Kwiatkowski DP, Prentice AM. Seasonal childhood anaemia in West Africa is associated with the haptoglobin 2-2 genotype. Plos Med. 2006;3(5):e172. doi: 10.1371/journal.pmed.0030172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hutchinson K, Healy M, Crowley V, Louw M, Rochev Y. Verification of Abbott 25-OH-vitamin D assay on the architect system. Pract Lab Med. 2017;7:27–35. doi: 10.1016/j.plabm.2017.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Holick MF, Binkley NC, Bischoff-Ferrari HA, Gordon CM, Hanley DA, Heaney RP, Murad MH, Weaver CM, Endocrine S. Evaluation, treatment, and prevention of vitamin D deficiency: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2011;96(7):1911–1930. doi: 10.1210/jc.2011-0385. [DOI] [PubMed] [Google Scholar]
- 28.Ross AC, Manson JE, Abrams SA, Aloia JF, Brannon PM, Clinton SK, Durazo-Arvizu RA, Gallagher JC, Gallo RL, Jones G, et al. The 2011 report on dietary reference intakes for calcium and vitamin D from the Institute of Medicine: what clinicians need to know. J Clin Endocrinol Metab. 2011;96(1):53–58. doi: 10.1210/jc.2010-2704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bouillon R, Carmeliet G. Vitamin D insufficiency: Definition, diagnosis and management. Best Pract Res Clin Endocrinol Metab. 2018;32(5):669–684. doi: 10.1016/j.beem.2018.09.014. [DOI] [PubMed] [Google Scholar]
- 30.World Health Organization, Centers for Disease Control . Assessing the iron status of populations: report of a Joint World Health Organization/Centers for Disease Control and Prevention Technical Consultation on the Assessment of Iron Status at the Population Level, Geneva, Switzerland, 6-8 April 2004. 2005. Assessing the iron status of populations: report of a Joint World Health Organization/Centers for Disease Control and Prevention Technical Consultation on the Assessment of Iron Status at the Population Level, Geneva, Switzerland, 6-8 April 2004. [Google Scholar]
- 31.World Health Organisation WHO Child Growth Standards based on length/height, weight and age. Acta Paediatr Suppl. 2006;450:76. doi: 10.1111/j.1651-2227.2006.tb02378.x. [DOI] [PubMed] [Google Scholar]
- 32.Eltahir EAB, Gong C. Dynamics of wet and dry years in West Africa. J Climate. 1996;9(5):1030–1042. doi: 10.1175/1520-0442(1996)009<1030:DOWADY>2.0.CO;2. [DOI] [Google Scholar]
- 33.Philippon N, Camberlin P, Moron V, Boyard-Micheau J. Anomalously wet and dry rainy seasons in Equatorial East Africa and associated differences in intra-seasonal characteristics. Climate Dynamics. 2015;45(7-8):2101–2121. doi: 10.1007/s00382-014-2460-6. [DOI] [Google Scholar]
- 34.TO C, Herreros F, Zhang JH, Ellis BK, Simpson C, Torrealba-Fox E, Kim GJ, Savoye M, Held NA, Cole DE. Demographic, dietary, and biochemical determinants of vitamin D status in inner-city children. Am J Clin Nutr. 2012;95(1):137–146. doi: 10.3945/ajcn.111.018721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nakano S, Suzuki M, Minowa K, Hirai S, Takubo N, Sakamoto Y, Ishijima M, Hoshino E, Tokita A, Shimizu T. Current Vitamin D Status in Healthy Japanese Infants and Young Children. J Nutr Sci Vitaminol (Tokyo) 2018;64(2):99–105. doi: 10.3177/jnsv.64.99. [DOI] [PubMed] [Google Scholar]
- 36.Guo Y, Ke HJ, Liu Y, Fu M, Ning J, Yu L, Xiao Y, Che D, Chen XY, Deng YH, Wu JL. Prevalence of vitamin D insufficiency among children in southern China: A cross-sectional survey. Medicine (Baltimore) 2018;97(25):e11030. doi: 10.1097/MD.0000000000011030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Prentice A, Schoenmakers I, Jones KS, Jarjou LM, Goldberg GR. Vitamin D deficiency and its health consequences in Africa. Clin Rev Bone Miner Metab. 2009;7(1):94–106. doi: 10.1007/s12018-009-9038-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Poopedi MA, Norris SA, Micklesfield LK, Pettifor JM. Does vitamin D status track through adolescence? Am J Clin Nutr. 2015;102(5):1025–1029. doi: 10.3945/ajcn.115.112714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wakayo T, Belachew T, Vatanparast H, Whiting SJ. Vitamin D deficiency and its predictors in a country with thirteen months of sunshine: the case of school children in central Ethiopia. Plos One. 2015;10(3):e0120963. doi: 10.1371/journal.pone.0120963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kunz C, Hower J, Knoll A, Ritzenthaler KL, Lamberti T. No improvement in vitamin D status in German infants and adolescents between 2009 and 2014 despite public recommendations to increase vitamin D intake in 2012. Eur J Nutr. 2018;58(4):1711–1722. doi: 10.1007/s00394-018-1717-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mokhtar RR, Holick MF, Sempertegui F, Griffiths JK, Estrella B, Moore LL, Fox MP, Hamer DH. Vitamin D status is associated with underweight and stunting in children aged 6-36 months residing in the Ecuadorian Andes. Public Health Nutr. 2018;21(11):1974–1985. doi: 10.1017/S1368980017002816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Avagyan D, Neupane SP, Gundersen TE, Madar AA. Vitamin D status in pre-school children in rural Nepal. Public Health Nutr. 2016;19(3):470–476. doi: 10.1017/S136898001500083X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Amer M, Qayyum R. Relation between serum 25-hydroxyvitamin D and C-reactive protein in asymptomatic adults (from the Continuous National Health and Nutrition Examination Survey 2001 to 2006) Am J Cardiol. 2012;109(2):226–230. doi: 10.1016/j.amjcard.2011.08.032. [DOI] [PubMed] [Google Scholar]
- 44.Toko EN, Sumba OP, Daud II, Ogolla S, Majiwa M, Krisher JT, Ouma C, Dent AE, Rochford R, Mehta S. Maternal vitamin D status and adverse birth outcomes in children from rural Western Kenya. Nutrients. 2016;8(12):794. doi: 10.3390/nu8120794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yakoob MY, Salam RA, Khan FR, Bhutta ZA. Vitamin D supplementation for preventing infections in children under five years of age. Cochrane Database Syst Rev. 2016;11:CD008824. doi: 10.1002/14651858.CD008824.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Newton DA, Baatz JE, Kindy MS, Gattoni-Celli S, Shary JR, Hollis BW, Wagner CL. Vitamin D binding protein polymorphisms significantly impact vitamin D status in children. Pediatr Res. 2019;86(5):662–669. doi: 10.1038/s41390-019-0322-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Carter GD, Phinney KW. Assessing vitamin D status: time for a rethink? Clin Chem. 2014;60(6):809–811. doi: 10.1373/clinchem.2013.219386. [DOI] [PubMed] [Google Scholar]
- 48.Yousefzadeh P, Shapses SA, Wang X. Vitamin D binding protein impact on 25-hydroxyvitamin D levels under different physiologic and pathologic conditions. Int J Endocrinol. 2014;2014:981581. doi: 10.1155/2014/981581. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1: Supplementary methods. (Genotyping and SNP quality control). This file describes the methods used in genotyping and SNP quality control, QC parameters, allocation of vitamin D binding protein Gc variants and haplotypes.
Additional file 2: Table S1. Databases search keywords. This table includes keywords used systematically search PubMed and Embase for articles of studies that measured vitamin D status in young children.
Additional file 3: Table S2. Median 25(OH)D levels by study variable in each country. This is a table of median 25(OH)D levels for each study variable in each country.
Additional file 4: Table S3. Multivariable logistic regression analyses of factors associated with low vitamin D status in each country. This is a table showing odd ratios (with their corresponding 95% CIs and p values) from multivariable logistic regression analyses.
Additional file 5: Table S4. Univariable linear regression of 25(OH)D concentrations by study variables in each country. This is a table of univariable linear regression coefficients (with 95% CI) and p values presented by country.
Additional file 6: Table S5. Multivariable regression of 25(OH)D concentrations by study variables in each country. This is a table of multivariable linear regression coefficients (with 95% CI) and p values presented by country.
Additional file 7: Table S6. Median 25(OH)D levels by vitamin D binding protein haplotype and Gc variant in each country. This is a table of DBP variant and haplotype frequencies and median 25(OH)D levels by country.
Additional file 8: Table S7. Summary characteristics of studies that evaluated the vitamin D status of African children (in alphabetical order). This is a table describing characteristics of past studies that evaluated the vitamin D status of young children in Africa.
Additional file 9: Figure S1. The precision profile of the 25(OH)D assay. This is a scatter plot of coefficient of variation (%) of the 25(OH)D assay used in this study.
Additional file 10: Figure S2. Boxplots of 25(OH)D concentrations by country (A), age categories (B), sex (C), season (D) stunting (E), underweight (F), wasting (G), inflammation (H), malaria (I), vitamin D binding protein (DBP) isotype (J) and Gc variant (K). This is a grid of boxplots of 25(OH)D concentrations for each study variable.
Additional file 11: Figure S3. Boxplots of CRP levels overall and by country. These are boxplots of CRP levels.
Additional file 12: Figure S4. Meta-analysis of studies that estimated the prevalence of 25(OH)D levels < 50 nmol/L in healthy children aged 0–8 years in Africa. This is a meta-analysis plot of prevalence estimates of vitamin D deficiency (defined by 25(OH)D levels < 50 nmol/L) in young children included in the current and previous studies.
Additional file 13: Figure S5. Meta-analysis of studies that estimated the prevalence of 25(OH)D levels < 75 nmol/L in healthy children aged 0–8 years in Africa. This is a meta-analysis plot of prevalence estimates of low vitamin D status (defined by 25(OH)D levels < 75 nmol/L) in young children included in the current and previous studies.
Data Availability Statement
The data and analyses scripts underlying this article are available in Harvard Dataverse at 10.7910/DVN/MH1LPE and applications for data access can be made through the Kilifi Data Governance Committee cgmrc@kemri-wellcome.org.