Abstract
Background
High-frequency, rapid-turnaround severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) testing continues to be proposed as a way of efficiently identifying and mitigating transmission in congregate settings. However, 2 SARS-CoV-2 outbreaks occurred among intercollegiate university athletic programs during the fall 2020 semester, despite mandatory directly observed daily antigen testing.
Methods
During the fall 2020 semester, athletes and staff in both programs were tested daily using Quidel’s Sofia SARS Antigen Fluorescent Immunoassay, with positive antigen results requiring confirmatory testing with real-time reverse-transcription polymerase chain reaction. We used genomic sequencing to investigate transmission dynamics in these 2 outbreaks.
Results
In the first outbreak, 32 confirmed cases occurred within a university athletics program after the index patient attended a meeting while infectious, despite a negative antigen test on the day of the meeting. Among isolates sequenced from that outbreak, 24 (92%) of 26 were closely related, suggesting sustained transmission following an initial introduction event. In the second outbreak, 12 confirmed cases occurred among athletes from 2 university programs that faced each other in an athletic competition, despite receipt of negative antigen test results on the day of the competition. Sequences from both teams were closely related and distinct from viruses circulating in the community for team 1, suggesting transmission during intercollegiate competition in the community for team 2.
Conclusions
These findings suggest that antigen testing alone, even when mandated and directly observed, may not be sufficient as an intervention to prevent SARS-CoV-2 outbreaks in congregate settings, and they highlight the importance of vaccination to prevent SARS-CoV-2 outbreak in congregate settings.
Keywords: SARS-CoV-2, antigen testing, genomic epidemiology
Timely reporting of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) test results is critical for controlling transmission through prompt public health action, yet at times during 2020, turnaround times for SARS-CoV-2 test results in the United States have averaged 4 days, with some individuals waiting 10 days or more [1]. While turnaround times in early 2021 have improved, the lag between specimen collection and receipt of a test result continues to represent a window in which the risk of viral spread from SARS-CoV-2–infected individuals is high. Rapid antigen tests, such as Abbott’s BinaxNow COVID-19 Ag Card and Quidel’s Sofia SARS Antigen Fluorescent Immunoassay (FIA), can reduce this lag between testing and reporting of results [2–5]. Because of these qualities, high-frequency, rapid-turnaround SARS-CoV-2 antigen testing has been proposed as a prevention strategy in many congregate settings where SARS-CoV-2 infection risk is elevated [6–8].
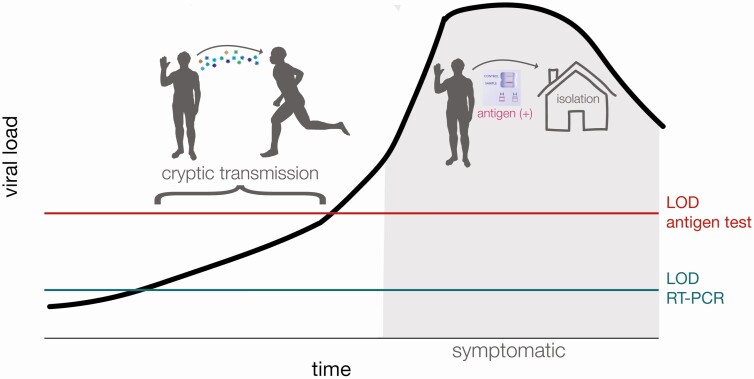
In data submitted for emergency use authorization, the Sofia SARS Antigen FIA antigen test has a reported sensitivity of 97% and specificity of 100% when used in symptomatic patients within 5 days of symptom onset [9, 10]. It therefore follows that serial antigen testing could rapidly identify unvaccinated persons with symptomatic infections enabling rapid isolation of such individuals [2, 4]. Recent studies, however, have found that the Sofia SARS Antigen FIA antigen test was less sensitive (41.2% sensitivity) in asymptomatic individuals [10–13]. Use of this test in asymptomatic patients is not included in the Food and Drug Administration authorization and is considered “off-label” use. Nonetheless, many universities and other congregate settings have used the tests for asymptomatic screening. The potential for false-negative antigen results among asymptomatic patients may present a significant risk, in that a negative result could result in risk disinhibition behavior in an individual who may be infectious during their presymptomatic period, leading to sustained and increased viral spread [14] (Figure 1).
Figure 1.
Graphic abstract of cryptic transmission that could occur when a person is asymptomatic and the amount of virus remains below the limit of detection (LOD) for antigen tests despite the person’s being potentially infectious to others. This is a schematic and is meant to represent general, not quantitative, relationships among these variables.
METHODS
University-Implemented Daily SARS-CoV-2 Antigen Testing for College Athletics
The 2 outbreaks occurred among unvaccinated athletes and staff affiliated with a university’s intercollegiate athletics programs despite daily SARS-CoV-2 testing with Quidel’s Sofia SARS Antigen FIA. Both sports involved in the outbreaks were considered “high risk” by the National Collegiate Athletics Association owing to frequent contact and collision between athletes during play. Students and staff affiliated with the 2 athletics programs began daily antigen testing for SARS-CoV-2 in September 2020. Before September 2020, all athletes were tested with real-time reverse-transcription polymerase chain reaction (RT-PCR) once or twice a week. No students or staff received COVID-19 vaccines, as these were not yet widely available. Outbreak 1 included 133 total individuals, and outbreak 2 included 55 total individuals (32 on team 1 and 23 on team 2).
Daily antigen testing was not required for persons with an RT-PCR–confirmed SARS-CoV-2 infection in the past 3 months or those experiencing symptoms consistent with coronavirus disease 2019 (COVID-19), as symptomatic persons received RT-PCR testing without initial antigen testing. For remaining asymptomatic students and staff, antigen testing was conducted using anterior nasal swab samples that were self-collected each morning under the direct supervision of a nurse. Antigen test results were provided to athletics department medical staff who coordinated exclusion from team activities and confirmatory testing, but they were not reported back to students and staff.
A negative antigen result meant that an individual could engage in all sport-related activities, such as indoor meetings, practices, scrimmages, and intercollegiate competitions. Athletes and staff with positive antigen results were immediately excluded from team activities by department medical staff and subject to confirmatory testing with RT-PCR using the TaqPath COVID-19 Combo Kit (Thermo Fisher Scientific). Students and staff with positive RT-PCR results were excluded from team activities for 21 days and were interviewed by university staff to identify close contacts. Close contacts of RT-PCR–confirmed students or staff were required to self-quarantine for 14 days from the date of last contact per public health guidance [15]. Importantly, contact tracing for student athletes did not include contacts that occurred during practices, competitions, meetings, or other team activities but could include contacts that occurred during social activities or at home (eg, roommates). In addition to daily antigen testing, the athletic programs implemented a physical distancing policy requiring all students and staff to be at least 6 feet apart during meetings, with mandatory mask use during team activities.
Epidemiological Investigation
Confirmed cases of COVID-19 were defined as students or staff affiliated with the 2 athletics programs who received a positive SARS-CoV-2 RT-PCR result during the outbreak period. False-negative antigen results were defined as a negative antigen test result with a positive RT-PCR result collected on the same day. During each outbreak, once the number of confirmed cases reached the threshold established by intercollegiate athletics conference protocols, in-person team activities were suspended, and all students and staff were tested with RT-PCR. Specimens that tested positive by RT-PCR confirmation were used for sequencing analysis.
University names, specific sports, and relevant dates have been removed from the report to protect the privacy of the students and staff involved. We used identifiers (Athletics-##) to denote individuals associated with these outbreaks. Dates are encoded as X-day-YY; X indicates the outbreak investigated, and YY, the day of that outbreak. The first notable event for each outbreak is “day 0”—in outbreak 1, this was a negative antigen test rest for the index case patient (who later tested positive with RT-PCR), and in outbreak 2, this was the date of the first competition between the 2 teams. This activity was reviewed by the Centers for Disease Control and Prevention (CDC) and was conducted in a manner consistent with applicable federal law and CDC policy (see, eg, 45 CFR part 46.102(l)(2), 21 CFR part 56; 42 USC §241(d); 5 USC §552a; 44 USC §3501 et seq).
Laboratory Methods
We obtained a waiver of HIPAA (Health Insurance Portability and Accountability Act) authorization and were approved to obtain the clinical samples along with a limited data set by the Western Institutional Review Board (WIRB 1-1290953-1). Sequences for this study were derived from 42 total nasopharyngeal swab samples collected from outbreak 1 (n = 32); outbreak 2, team 1 (n = 5); and outbreak 2, team 2 (n = 5).
Outbreak 1 Viral RNA Isolation
Nasal swab samples were collected and placed in 3 mL of phosphate-buffered saline. RNA was extracted from 190 µL of sample using the MagMAX Viral/Pathogen II (MVP II) Nucleic Acid Isolation Kit (Thermo Fisher Scientific) and eluted in a volume of 50 µL. After that, 5 µL of RNA was quantitated using a 1-step RT-PCR using a TaqPath COVID-19 Combo Kit (Thermo Fisher Scientific).
Outbreak 2 Viral RNA Isolation
Nasopharyngeal swab samples were received in 3 mL of viral transport medium. Viral RNA was extracted from 100 μL of viral transport medium using the Viral Total Nucleic Acid Purification kit (Promega) on a Maxwell RSC 48 instrument, according to manufacturer guidelines, and was eluted in 50 μL of nuclease-free water.
Library Preparation and Sequencing
Complementary DNA was synthesized using a modified ARTIC Network approach [16]. SARS-CoV-2–specific multiplex PCR for nanopore sequencing was performed, similar to amplicon-based approaches described elsewhere [16, 17]. Amplified product was made compatible for deep sequencing using the 1-pot native ligation protocol with the Oxford Nanopore kit SQK-LSK109 and its native barcodes (EXP-NBD104 and EXP-NBD114) [16]. Up to 24 samples were pooled before being sequenced on a flow cell (FLO-MIN106) using the 24-hour run script.
Processing Raw Oxford Nanopore Technologies Data
Sequencing data was processed using the ARTIC bioinformatics pipeline (https://github.com/artic-network/artic-ncov2019) [15]. Consensus sequences were assembled for samples with >400× coverage. Samples were excluded from analysis if gaps in the consensus sequence totaled ≥20% of the genome. The entire ONT analysis pipeline is available online (https://github.com/gagekmoreno/SARS-CoV-2-in-Southern-Wisconsin).
Phylogenetic Analysis
Phylogenetic analysis was completed using tools implemented in Nextstrain custom builds (https://github.com/nextstrain/ncov) [18, 19]. We used custom python scripts to filter and clean metadata. Sequences names were coded as OB#-T#-A#; OB signifies the outbreak, T represents the team that the sequence came from, and A is the athlete who provided the sample from which the sequence was derived.
Data Availability
Source data after mapping to SARS-CoV-2 reference genome (GenBank MN908947.3) have been deposited in the Sequence Read Archive under bioproject PRJNA614504.
RESULTS
Outbreak 1: Case Linkage to a Single Viral Introduction
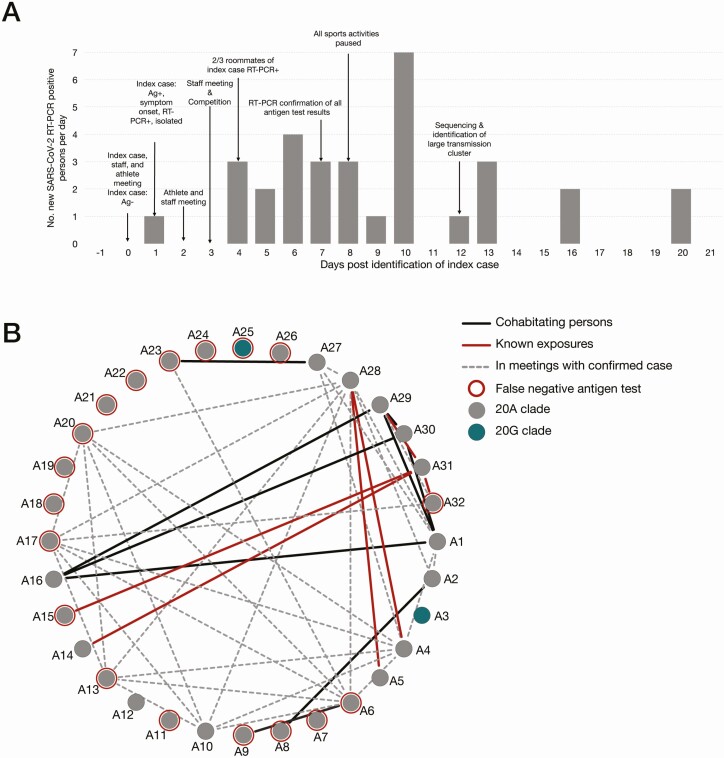
An athlete (Athletics-1) received a negative antigen test result the morning of day 0 and attended an indoor meeting with approximately 10 other student-athletes and staff in which attendees reportedly sat 6 feet apart and wore masks at all times. The following morning (day 1), Athletics-1 received a positive antigen test result followed by RT-PCR swab for confirmation (cycle threshold [Ct], 15.9) and began experiencing symptoms by midafternoon of day 1. During days 3–7, a total of 4 attendees of the initial day 0 meeting began experiencing symptoms and received subsequent positive RT-PCR results. In addition, 3 roommates of Athletics-1 who did not attend the meeting began experiencing symptoms on day 4 and received positive RT-PCR results on days 4 and 5. In-person team activities were suspended on day 8 to prevent additional transmission.
Program-wide RT-PCR and antigen testing was conducted 7 times throughout the outbreak period (days 7, 10, 13–17, and 20). Mass RT-PCR testing identified 21 new SARS-CoV-2 infections among students and staff. Of these, 18 (86%) were negative on contemporaneous rapid antigen tests. Among 11 positive antigen results obtained during mass testing, 4 (36%) were confirmed with RT-PCR and 7 (64%) received negative RT-PCR results. For samples with available Ct values, outbreak 1 included 13 individuals who were antigen negative and RT-PCR positive, with Ct values ranging from 21.3 to 35.3 (average, 29.5). Conversely, there were 9 individuals who were antigen positive and RT-PCR positive, with Ct values ranging from 15.9 to 31.3 (average, 24.9).
Overall, during outbreak 1, a total of 32 individuals (22 students and 10 staff) from the program had laboratory-confirmed SARS-CoV-2 infections (Figure 2A). Of persons with confirmed cases, 4 (13%) were tested by RT-PCR because they were symptomatic, 7 (22%) were antigen positive and received RT-PCR confirmation, and 21 (66%) were positive during mass RT-PCR testing. Contact tracing interviews found that 13 (40%) of 32 confirmed case patients attended a team meeting where someone with confirmed COVID-19 was present and in their infectious period, 6 (13%) had close contact with a roommate with COVID-19, and 8 (25%) had no documented exposures (Table 1).
Figure 2.
Overview of outbreak 1. A, Epidemic curve for confirmed coronavirus disease 2019 cases (n = 32) among students and staff associated with the athletics program during outbreak 1. Abbreviations: Ag, antigen; Ag−, Ag result negative; Ag+, Ag result positive; RT-PCR, reverse-transcription polymerase chain reaction; RT-PCR+, RT-PCR result positive; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2. B, Graphic representation of known interactions between all persons in the athletics program affected by outbreak 1. Solid black lines represent roommates; red lines, confirmed close contact with a positive case as identified through contact tracing interviews; dashed gray lines, persons who attended indoor team meetings together while following physical distancing policies (>6 ft apart and wearing masks); and red circles, persons who received false-negative antigen results.
Table 1.
Characteristics and Exposure Details for Confirmed Coronavirus Disease 2019 Cases During Outbreak 1a
| Characteristics | Cases, No. (%) (n = 32) |
|---|---|
| Program affiliation | |
| Student-athlete | 22 (69) |
| Staff | 10 (31) |
| Symptomatic | 27 (84) |
| Possible contact with confirmed COVID-19 case during exposure period | |
| Housemate | 6 (19) |
| Team meeting | 13 (40) |
| Social gathering (outside of program) | 3 (9) |
| Other | 2 (6) |
| No known exposure | 8 (25) |
| Source of positive RT-PCR result | |
| Symptom-based testing (RT-PCR only) | 4 (13) |
| Antigen-based screening with confirmatory RT-PCR | 7 (22) |
| Mass combined testing (paired RT-PCR and antigen testing) | 21 (66) |
| Antigen positive | 3 (14) |
| Antigen negative | 18 (86) |
Abbreviations: COVID-19, coronavirus disease 2019; RT-PCR, reverse-transcription polymerase chain reaction.
aA total of 1921 antigen and 1012 RT-PCR tests were performed after pause of activities.
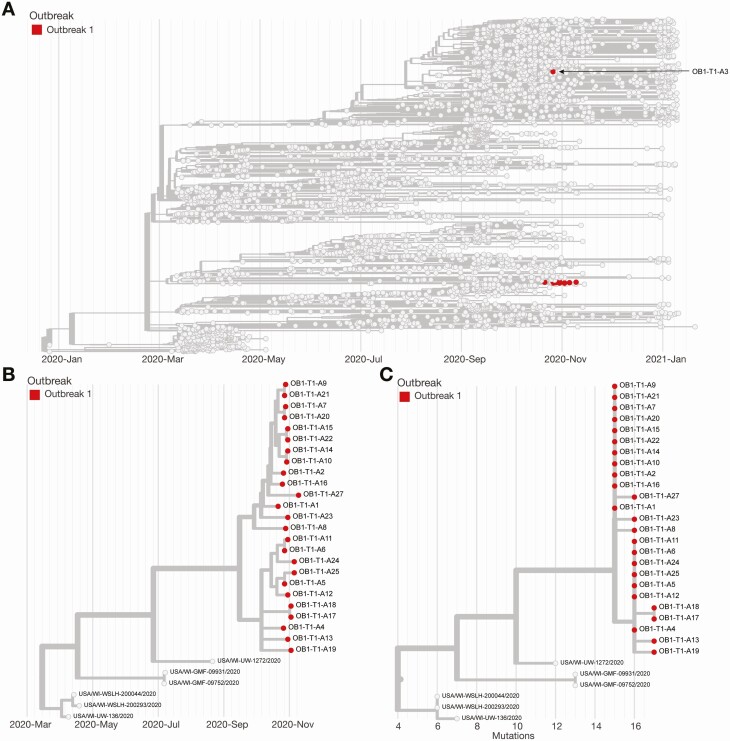
We generated consensus sequences for 26 (81%) of 32 RT-PCR–positive samples [16, 17]. Samples from the remaining 6 RT-PCR–positive individuals in outbreak 1 were not available at the time of sequencing and were excluded from this analysis. We found that 24 (92%) of these 26 genomic sequences cluster tightly in the Nextstrain 20A clade on a time-resolved tree and are separated by 0–2 fixed consensus nucleotide differences (Figure 3). These sequences differ from the closest phylogenetic relative in the same state by 5–7 mutations. The limited diversity of viruses detected in the 24 individuals suggests sustained transmission of SARS-CoV-2 following a single introduction [20–22]. Viruses from Athletics-3 and Athletics-26 did not appear to be part of the primary transmission cluster. The consensus sequence for Athletics-26 was too divergent, differed by 8–10 mutations, and was filtered out of Figure 3. As of day 40 in Outbreak 1, there was no evidence for onward spread within the program originating from Athletics-3 or Athletics-26. The viruses infecting these individuals cluster more closely with sequences seen in the community.
Figure 3.
Phylogeny of outbreak 1. A, Time-resolved phylogenetic tree created using Nextstrain tools and nomenclature showing the team sequences contextualized with all available community sequences (gray) for 25 of 32 confirmed cases (78%) associated with outbreak 1; tips affiliated with outbreak 1 are colored red. B, Zoomed-in time-resolved phylogeny showing that all of these samples are part of the same athletics cluster. C, Divergence tree showing the number of mutations each sequence has relative to Wuhan/WH01/2019 (GenBank MN908947.3), a standard reference comparison sequence.
Outbreak 2: SARS-CoV-2 Transmission During Intercollegiate Competition
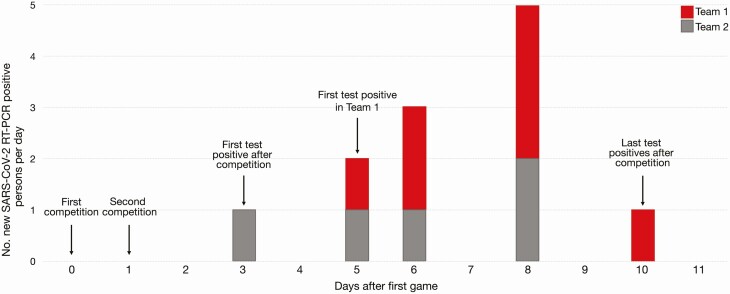
Two teams from different universities engaged in intercollegiate competitions on consecutive days (days 0 and 1). Both teams underwent daily antigen testing and received all negative antigen results in the week preceding the competitions, including both competition days (days 0 and 1). No testing was conducted on day 2. On day 3, an athlete from team 2 received a positive antigen test result, which was confirmed by RT-PCR. No athletes or staff on either team were quarantined from contact with the index athlete that occurred during competition on days 0 and 1. During days 5–10, multiple athletes on both teams began experiencing symptoms and received positive antigen and RT-PCR results. On day 6, all athletes on team 1 were tested with RT-PCR only, with 2 individuals testing positive, and in-person team activities were suspended. Overall, 12 athletes (7 from team 1 and 5 from team 2) had confirmed SARS-CoV-2 infections during this outbreak (Figure 4).
Figure 4.
Overview of outbreak 2. A, Epidemic curve for outbreak 2 showing confirmed coronavirus disease 2019 cases (n = 12) within the 2 intercollegiate teams. Testing was not conducted on day 2. Abbreviations: RT-PCR, reverse-transcription polymerase chain reaction; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.
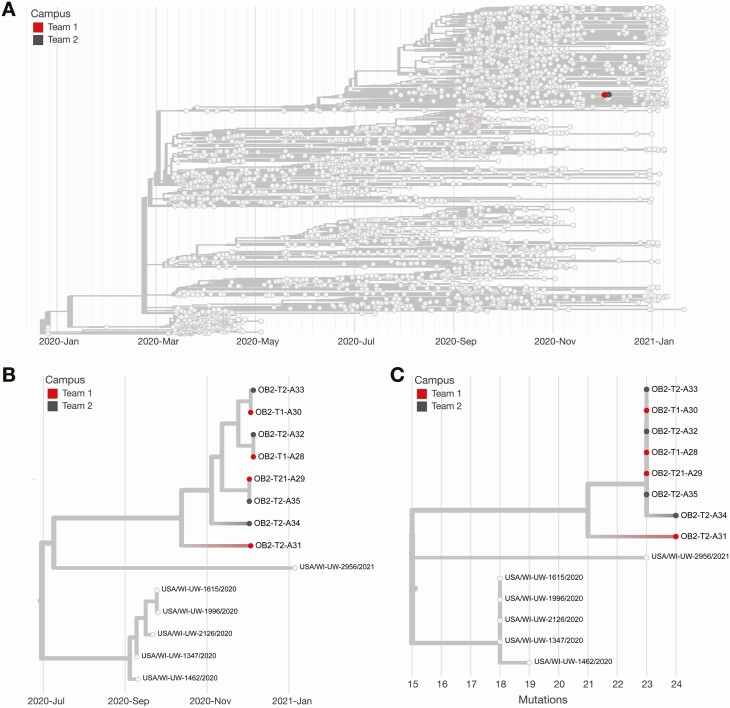
To determine whether the source of these infections could be linked to competition despite negative antigen results on the day of competition, we generated 8 consensus sequences from 10 available samples. All 8 virus sequences (4 from each team) clustered tightly in the 20G clade on a time-resolved tree and were separated by 0–2 fixed consensus nucleotide differences (Figure 5). Given the known epidemiological associations between these teams, this likely represented a single transmission cluster [20–22].
Figure 5.
Phylogeny of outbreak 2. A, Time-resolved phylogenetic tree created using Nextstrain tools and nomenclature showing 8 of 12 available samples (67%) from outbreak 2 sequences contextualized with all available community sequences (light gray). Tips affiliated with team 1 are colored red, and team 2’s sequences are colored dark gray. B, Zoomed-in time-resolved phylogeny showing that all of these samples are part of the same athletics cluster. C, Divergence tree showing the number of mutations each sequence has relative to Wuhan/WH01/2019 (GenBank MN908947.3), a standard reference comparison sequence.
The sequences of the viruses infecting the individuals in outbreak 2 were distinct from those of viruses circulating within the community where outbreak 2 occurred. Sequences in outbreak 2 contained a unique mutation, encoding spike P26Y, which was not otherwise seen in the county where team 1 was located. Given the depth of surveillance community sequencing in team 1’s county available during the outbreak period (approximately 4.7% of test-positive cases), it is unlikely that this unique signature arose independently in the county where team 1 is located.
DISCUSSION
The SARS-CoV-2 testing strategy of daily, directly observed, rapid antigen testing implemented by intercollegiate athletics programs nationwide has been resource intensive, yet its impact on SARS-CoV-2 transmission in this setting has not been evaluated. In the current report, we described 2 outbreaks within intercollegiate athletics programs in which daily antigen testing was unable to interrupt SARS-CoV-2 transmission. Sustained transmission within the program followed when additional exposures from presymptomatic and undetected SARS-CoV-2 infections occurred—at least 13 of the 32 outbreak-associated unvaccinated case patients attended team meetings with individuals who had received negative antigen results yet were in their infectious period.
Transmission within the program was not interrupted until the program implemented serial RT-PCR testing, a strategy that led to identification of 21 new confirmed SARS-CoV-2 infections, 18 of which were negative on contemporaneous antigen tests. Our findings suggest that serial antigen testing as a control strategy may have limited sensitivity for detecting early asymptomatic infections.
Contact tracing during outbreak 1 identified interactions among individuals that may have contributed to at least 21 (66%) of the 32 confirmed cases (Figure 2B). In particular, the team continued to have physically distanced (6-feet-apart) in-person meetings with cloth masks until all in-person team activities were suspended to prevent further spread. Per public health and university guidelines, unvaccinated attendees in these meetings were not quarantined, a step that might have prevented onward transmission during this outbreak. Roommates and household contacts of student-athletes could represent additional sources of infection in outbreak 1, as they were not required to quarantine owing to the large size of the house and the university’s assessment that physical distancing was achievable in this area. Continuing indoor in-person meetings and not quarantining potential contacts represent possible breaches in university’s SARS-CoV-2 mitigation plan that, combined with the limitation of antigen testing, permitted viral spread throughout the team in outbreak 1.
In outbreak 2, we used genomic sequencing to demonstrate that SARS-CoV-2 transmission likely occurred between 2 teams during athletic competition even though both teams received negative antigen results immediately before competition. Supporting evidence for intercollegiate transmission included detection of a unique mutation, encoding spike P26Y, that was common to the samples from both teams and not otherwise seen in the county where team 1 is located. Given these findings, the most parsimonious explanation is that an infection acquired in the community by the unvaccinated index athlete on team 2 was transmitted to other unvaccinated individuals on both teams during the competition. However, it is also possible that unvaccinated athletes on team 1 acquired SARS-CoV-2 infection in the community of team 2, while visiting for competition. This can be seen with Athletics-31, as this viral consensus sequence is closely related to the cluster, but differs by 5 consensus mutations.
The potential for intercollegiate transmission during an athletic competition has important implications for SARS-CoV-2 serial testing strategies and in-competition mitigation protocols. First, antigen testing on the competition dates failed to identify the index case patient, who may have been infectious and exposed other athletes. As in outbreak 1, more sensitive molecular tests could have identified the source case and allowed for exclusion from the competition. Second, this investigation showed that in the absence of widespread vaccination, athletic competition may pose a risk for SARS-CoV-2 transmission, particularly in sports in which direct physical contact occurs. This outbreak occurred during an athletic competition that included contact and collision and is considered “high risk” by the National Collegiate Athletics Association. Despite the short duration of contact between athletes, transmission risk can be exacerbated by heavy breathing and shouting without masking in the absence of vaccination, which regularly occurs in this setting and has been associated with SARS-CoV-2 outbreaks in other athletics competitions [23].
The findings in this report are subject to several limitations. First, we were not able to perform genomic sequencing on all positive samples from these outbreaks (34 of 44 samples were sequenced), because of either high Ct values at RT-PCR or lack of sample availability. Second, contemporaneous antigen and RT-PCR samples in outbreak 1 were not collected as “paired” swab samples (simultaneous swabbing of 2 nares) and may not be comparable to other antigen test evaluations. Similarly, the performance of antigen tests in this context of daily serial testing measured their ability to identify early presymptomatic infections and may not be generalizable to antigen test performance in other settings. Third, there was a lack of testing before readmittance to the team. While probably a rare finding, individuals might still have been infectious after their 21-day quarantine. Similarly, the assumption that individuals who tested positive would remain uninfected for 3 months and were therefore exempt from testing may have been flawed, because reinfection, though uncommon, does occur. Fourth, our ability to determine the source of infections in these outbreaks was limited by incomplete contact tracing data. Undocumented exposures between athletes and staff may have occurred outside of organized team activities that could have caused infections, though the strength of genomic clustering and epidemiological evidence from these investigations suggest that such occurrences were rare.
Among athletics programs and other congregate settings where outbreaks may spread rapidly after introduction of SARS-CoV-2, serial antigen testing alone may not be sufficient to prevent outbreaks. However, serial antigen testing can help to rapidly identify outbreaks as they happen but might fail to detect every positive individual. Instead, they work to detect outbreaks and can inform policy decisions to slow the spread of SARS-CoV-2. In current times, vaccination should be a primary prevention strategy. In settings with low vaccination rates, any robust testing strategy should be supplemented with multilayered prevention strategies that include correct and consistent mask use, physical distancing, increased hand hygiene and disinfection, avoiding crowds and poorly ventilated spaces, and isolation of symptomatic individuals regardless of antigen test result [13, 24–26]. Serial testing with RT-PCR may identify additional cases that were not detected by antigen testing, but the increased sensitivity would have to be balanced with laboratory resources and increased turnaround times.
Notes
Acknowledgments. We gratefully acknowledge Trevor Bedford and the entire Nextstrain team for making Nextstrain phylogenetic tools publicly available and for their commitment to tracking the global spread of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). We also acknowledge the GISAID team for maintaining the largest public repository of SARS-CoV-2 sequence data and metadata.
Disclaimer. The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention. Use of trade names is for identification only and does not imply endorsement by the Centers for Disease Control and Prevention.
Supplement sponsorship. This supplement is supported by the Infectious Diseases Society of America through Cooperative Agreement NU50CK000574 with the U.S. Centers for Disease Control and Prevention.
Financial support. This work was supported by the Wisconsin Partnership Program at the University of Wisconsin School of Medicine and Public Health (coronavirus disease 2019 response grant to T. C. F. and D. H. O.) and the National Library of Medicine (training grant NLM 5T15LM007359 to the Computation and Informatics in Biology and Medicine Training Program [G. K. M.]).
Potential conflicts of interest. All authors: No reported conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Botti-Lodovico Y, Rosenberg E, Sabeti PC. Testing in a pandemic–improving access, coordination, and prioritization. N Engl J Med 2021; 384:197–9. [DOI] [PubMed] [Google Scholar]
- 2. Mak GC, Cheng PK, Lau SS, et al. Evaluation of rapid antigen test for detection of SARS-CoV-2 virus. J Clin Virol 2020; 129:104500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Toptan T, Eckermann L, Pfeiffer AE, et al. Evaluation of a SARS-CoV-2 rapid antigen test: potential to help reduce community spread? J Clin Virol 2021; 135:104713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Porte L, Legarraga P, Vollrath V, et al. Evaluation of a novel antigen-based rapid detection test for the diagnosis of SARS-CoV-2 in respiratory samples. Int J Infect Dis 2020; 99:328–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Prince-Guerra JL, Almendares O, Nolen LD, et al. Evaluation of Abbott BinaxNOW rapid antigen test for SARS-CoV-2 infection at two community-based testing sites—Pima County, Arizona, November 3–17, 2020. MMWR Morb Mortal Wkly Rep 2021; 70:100–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Redditt V, Wright V, Rashid M, Male R, Bogoch I. Outbreak of SARS-CoV-2 infection at a large refugee shelter in Toronto, April 2020: a clinical and epidemiologic descriptive analysis. CMAJ Open 2020; 8:E819–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Patel MC, Chaisson LH, Borgetti S, et al. Asymptomatic SARS-CoV-2 infection and COVID-19 mortality during an outbreak investigation in a skilled nursing facility. Clin Infect Dis 2020; 71:2920–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ghinai I, Woods S, Ritger KA, et al. Community transmission of SARS-CoV-2 at two family gatherings—Chicago, Illinois, February-March 2020. MMWR Morb Mortal Wkly Rep 2020; 69:446–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Food and Drug Administration. Quidel Sofia SARS antigen test emergency use authorization. Available at: https://www.fda.gov/media/137885/download. Accessed 31 January 2021.
- 10. Arnaout R, Lee RA, Lee GR, et al. SARS-CoV2 testing: the limit of detection matters. bioRxiv [Preprint: not peer reviewed]. 4 June 2020. Available from: https://www.biorxiv.org/content/10.1101/2020.06.02.131144v1. [Google Scholar]
- 11. Young S, Taylor SN, Cammarata CL, et al. Clinical evaluation of BD Veritor SARS-CoV-2 point-of-care test performance compared to PCR-based testing and versus the Sofia 2 SARS antigen point-of-care test. J Clin Microbiol 2020; 59:e02338-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kremer R. CDC: UW antigen tests missed nearly 59 percent of COVID-19 cases among asymptomatic individuals.2021. Available at: https://www.wpr.org/cdc-uw-antigen-tests-missed-nearly-59-percent-covid-19-cases-among-asymptomatic-individuals. Accessed 31 January 2021.
- 13. Pray IW, Ford L, Cole D, et al. Performance of an antigen-based test for asymptomatic and symptomatic SARS-CoV-2 testing at two university campuses—Wisconsin, September-October 2020. MMWR Morb Mortal Wkly Rep 2021; 69:1642–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Harapan H, Anwar S, Nainu F, et al. Perceived risk of being infected with SARS-CoV-2: a perspective from Indonesia. Disaster Med Public Health Prep 2020:1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. ARTICnetwork. artic-network/artic-ncov2019. Available at: https://github.com/artic-network/artic-ncov2019. Accessed 31 January 2021.
- 16. Quick J. nCoV-2019 sequencing protocol.2020. Available at: https://www.protocols.io/view/ncov-2019-sequencing-protocol-bbmuik6w.pdf. Accessed 31 January 2021.
- 17. Quick J, Grubaugh ND, Pullan ST, et al. Multiplex PCR method for MinION and Illumina sequencing of Zika and other virus genomes directly from clinical samples. Nat Protoc 2017; 12:1261–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Hadfield J, Megill C, Bell SM, et al. Nextstrain: real-time tracking of pathogen evolution. Bioinformatics 2018; 34:4121–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Sagulenko P, Puller V, Neher RA. TreeTime: maximum-likelihood phylodynamic analysis. Virus Evol 2018; 4:vex042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Popa A, Genger JW, Nicholson MD, et al. Genomic epidemiology of superspreading events in Austria reveals mutational dynamics and transmission properties of SARS-CoV-2. Sci Transl Med 2020; 12:eabe2555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Moreno GK, Braun KM, Riemersma KK, et al. Revealing fine-scale spatiotemporal differences in SARS-CoV-2 introduction and spread. Nat Commun 2020; 11:5558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Braun KM, Moreno GK, Halfmann PJ, et al. Transmission of SARS-CoV-2 in domestic cats imposes a narrow bottleneck. PLoS Pathog 2021; 17:e1009373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Atherstone C, Siegel M, Schmitt-Matzen E, et al. SARS-CoV-2 transmission associated with high school wrestling tournaments—Florida, December 2020–January 2021. MMWR Morb Mortal Wkly Rep 2021; 70:141–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Pray IW, Kocharian A, Mason J, Westergaard R, Meiman J. Trends in outbreak-associated cases of COVID-19—Wisconsin, March-November 2020. MMWR Morb Mortal Wkly Rep 2021; 70:114–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Mack CD, Wasserman EB, Perrine CG, et al. Implementation and evolution of mitigation measures, testing, and contact tracing in the national football league, August 9-November 21, 2020. MMWR Morb Mortal Wkly Rep 2021; 70:130–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Honein MA, Christie A, Rose DA, et al. Summary of guidance for public health strategies to address high levels of community transmission of SARS-CoV-2 and related deaths, December 2020. MMWR Morb Mortal Wkly Rep 2020; 69:1860–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Source data after mapping to SARS-CoV-2 reference genome (GenBank MN908947.3) have been deposited in the Sequence Read Archive under bioproject PRJNA614504.