Abstract
As the second leading cause of death in the United States, cancer has a considerable impact on society, and one cellular process that is commonly dysregulated in many cancers is the post-translational modification of proteins by the Small Ubiquitin-like Modifier (SUMO; sumoylation). We documented that sumoylation processes are up-regulated in lymphoma tissues in the presence of Latent Membrane Protein-1 (LMP1), the principal oncoprotein of Epstein-Barr virus (EBV). LMP1-mediated dysregulation of cellular sumoylation processes contributes to oncogenesis, modulates innate immune responses, and aids the maintenance of viral latency. Manipulation of protein sumoylation has been proposed for anti-cancer and anti-viral therapies; however, known inhibitors of sumoylation do not only target sumoylation processes. Recently, a specific and selective small-molecule inhibitor of sumoylation (ML-792) was identified; however, nothing is known about the effect of ML-792 on LMP1-mediated dysregulation of cellular sumoylation or the EBV life-cycle. We hypothesized that ML-792 modulates viral replication and the oncogenic potential of EBV LMP1 by inhibiting protein sumoylation. Results showed that ML-792 inhibited sumoylation processes in multiple EBV-positive B cell lines and EBV-positive nasopharyngeal carcinoma cell lines but not in their EBV-negative counterparts. Focusing on its effect on B cells, ML-792 inhibited B-cell growth and promoted cell death at very low doses. ML-792 also modulated LMP1-induced cell migration and cell adhesion, which suggests the abrogation of the oncogenic potential of LMP1. Finally, while higher concentrations of ML-792 were sufficient to induce low levels EBV spontaneous reactivation, they decreased the production of new infectious virus following an induced reactivation and the infection of new cells, suggesting that ML-792 has anti-viral potential. Together, these findings suggest that ML-792 may be a potential therapeutic drug to treat EBV-associated lymphoid malignancies by targeting oncogenesis and the EBV life-cycle.
Keywords: ML-792, Epstein-Barr virus, latent membrane protein-1, sumoylation
1. Introduction
Epstein-Barr virus (EBV), a ubiquitous gamma-herpesvirus, was the first identified human tumor virus (Pagano, et al. 2004, Pagano 2009, Shah and Young 2009). EBV infects approximately 95% of the world population and establishes a life-long latency in the host’s B cells. Primary infection occurs when lytic virus in saliva invades epithelial and naïve B cells in the Waldeyer’s ring (Thorley-Lawson 2015). The linear viral genome circularizes within naïve B cells to form an episome and uses three latency programs, Types I-III, to drive their transformation into resting memory B cells. Each latency program expresses a distinct yet limited viral gene pattern that can be used to identify various epithelial and lymphoid malignancies (Pagano 2009). The principal viral protein important in the sustained proliferation and survival of EBV-infected B cells is Latent Membrane Protein 1 (LMP-1).
LMP-1 is a six-domain, transmembrane signaling protein that mimics the tumor necrosis factor (TNF) receptor family member CD40. The protein’s active C-terminal domain is located in the cytoplasm and consists of three C-terminal activating regions (CTARs1–3) (Li and Chang 2003). CTAR1 and CTAR2 contribute to oncogenesis by activating various cell signaling pathways, resulting in dysregulation of the cell cycle, inhibition of apoptosis, and increased cell migration and proliferation (Lam and Sugden 2003, Li and Chang 2003, Mainou, et al. 2005, Shair, et al. 2007, Soni, et al. 2007, Shair, et al. 2008, Zhang and Huang 2009). Until recently, little was known about the function of CTAR3 (Gires, et al. 1999); however, our lab identified the first function for CTAR3, which is its ability to interact with the enzymatically-active form of the SUMO (small ubiquitin-like modifier)-conjugating enzyme, Ubc9, increasing global levels of sumoylated proteins within the cell (Bentz, et al. 2011). Our subsequent work identified two additional mechanisms by which LMP1 increases levels of sumoylated proteins during EBV latency; induction of the sumo promoters and inhibition of the SUMO protease, SENP2 (Salahuddin, et al. 2019, Selby, et al. 2019).
Protein sumoylation involves the covalent post-translational modification of a protein by SUMO. It is a highly dynamic process that modulates virtually all cellular functions and processes (Kroetz 2005, Kerscher, et al. 2006, Kerscher 2007). Among the four human sumo genes, sumo-1, sumo-2, and sumo-3 are ubiquitously expressed throughout the body (Vertegaal 2007, Zhao 2007, Wilson and Heaton 2008, Lee, et al. 2017, Lowrey, et al. 2017). Following translation, the C-terminal amino acids of the SUMO pro-peptide are removed by a SUMO protease to reveal the SUMO di-glycine motif (Lowrey, et al. 2017). Using the SUMO-activating enzyme (heterodimer of SAE1 and SAE2), the SUMO-conjugating enzyme (Ubc9), and sometimes one of the few identified SUMO-ligases, an isopeptide bond is formed between this C-terminal SUMO di-glycine motif and a specific lysine residue that resides within the conserved ΨKxD/E SUMO motif of a target protein. This covalent bond can be reversed by SUMO proteases (Sentrin-specific proteases or SENPs) (Lowrey, et al. 2017).
Imbalance of protein sumoylation and de-sumoylation is associated with tumorigenesis and pathogenesis (Stewart, et al. 2005, Duan, et al. 2009). Changes in sumoylation can affect numerous cellular functions, such as cell growth, proliferation, apoptosis, DNA repair, genome organization, and cell survival (Kroetz 2005, Kerscher, et al. 2006, Kerscher 2007). Altered expression of the SUMO machinery and increased protein sumoylation is detected in various cancers, including EBV-associated lymphomas (Alarcon-Vargas and Ronai 2002, Stewart, et al. 2005, Katayama, et al. 2007, Duan, et al. 2009, Guo, et al. 2011, Yang, et al. 2013, Zhang, et al. 2013, Salahuddin, et al. 2019). Additionally, increases in protein sumoylation are associated with poor outcomes for patients with hematological malignancies, such as chronic myeloid leukemia, adult T-cell leukemia/lymphoma, and multiple myeloma (Mo, et al. 2005, Moschos, et al. 2010, Chen, et al. 2011, Boulanger, et al. 2019). Therefore, targeting members of the SUMO machinery or inhibiting the sumoylation process are projected to have therapeutic potential (Giorgino, et al. 2000, Mo, et al. 2002, Mo and Moschos 2005, Duan, et al. 2009, Boulanger, et al. 2019).
Because there is only one known SUMO-activating enzyme and one known SUMO-conjugating enzyme, these are the steps in the sumoylation process commonly targeted for inhibition. Use of short hairpin RNAs specific to SAE2 or Ubc9 and knockdown of SAE2 can have therapeutic effects on tumor growth in vivo (He, et al. 2015, Liu, et al. 2015, Yu, et al. 2015). The antibiotics Kerriamycin B and Spectomycin B1 have been documented to inhibit SAE and Ubc9, respectively, (Fukuda, et al. 2009, Hirohama, et al. 2013), but little is known about their mechanism of action, toxicity, and specificity. In addition, several botanical extracts (e.g. glycyrrhizic acid, ginkgolic acid, davidiin, and tannic acid) were shown to bind to and inhibit the interaction between SUMO and the SUMO machinery, resulting in inhibition of the sumoylation processes (Hayakawa, et al. 1985, Fukuda, et al. 2009, Brandt, et al. 2013, Kim, et al. 2014, Takemoto, et al. 2014, Bentz, et al. 2019). However, many of these extracts can induce neurotoxicity and allergic reactions while also targeting multiple different cellular processes (Siegers 1999, Ahlemeyer, et al. 2001, Mahadevan and Park 2008, Liu and Zeng 2009, Berg, et al. 2015, Yao, et al. 2017). In addition to the lack of specificity, these extracts exhibit low potency in their ability to inhibit cellular sumoylation processes (Hayakawa, et al. 1985, Fukuda, et al. 2009, Brandt, et al. 2013, Kim, et al. 2014, Takemoto, et al. 2014, Bentz, et al. 2019).
Recent work has focused on identifying more potent and selective inhibitors of sumoylation processes (He, et al. 2017, Lv, et al. 2018, Li, et al. 2019). ML-792, a methyl sulfumate derivative was found to inhibit SAE activity by binding to the SUMO C-terminal di-glycine motif in an ATP-dependent manner, inhibiting the ability of SUMO to be covalently attached to the target protein (He, et al. 2017). Alternatively, a screen of the NIH Molecular Library Probe Production Center Network program identified COH000, which covalently binds to the SAE and induces the SAE to undergo a conformational change, which locks the enzyme in an inactive confirmation (Lv, et al. 2018, Li, et al. 2019). While both small molecule inhibitors were specific, inhibiting sumoylation while having no effect on other post-translational modifications and exhibited anti-tumor capabilities (He, et al. 2017, Lv, et al. 2018, Li, et al. 2019), we elected to focus our studies on ML-792 due to its unique ability to specifically bind to the SUMO di-glycine motif and its enhanced potency in cells overexpressing MYC (He, et al. 2017). We hypothesized that ML-792 modulates viral replication and the oncogenic potential of EBV LMP1 by inhibiting protein sumoylation.
Here we show that ML-792 can selectively inhibit sumoylation processes in multiple B-cell lines and epithelial cells. The effect of ML-792 was more apparent in proliferating and EBV-positive cells when compared with naïve cells or EBV-negative cells. Using ML-792 to specifically inhibit sumoylation processes also decreased B-cell growth, and induced B-cell death. B-cells treated with higher doses of the small-molecule inhibitor were unable to recover from drug treatment. Interestingly, the effect of ML-792 on the B-cells was more pronounced in LMP1-expressing cells when compared with non-LMP1-expressing cells. Treatment of cells with ML-792 significantly inhibited the oncogenic phenotype associated with EBV LMP1. Our previous work used non-specific inhibitors of sumoylation to investigate the role of sumoylation during the EBV life-cycle (Bentz, et al. 2015, Bentz, et al. 2019), and now we can definitively document that sumoylation is important in the maintenance of EBV latency and lytic replication following induced reactivation. Together, our findings support our proposal that cellular sumoylation processes influence EBV lytic and latent infections and the pathogenesis associated with LMP1 expression.
2. Materials and Methods
2.1. Cells:
All cell lines were maintained at 37°C, 5% CO! and cultured, bi-weekly, for experimentation. Lymphoblastoid cell lines, transformed by wild-type EBV (LCL WT), were obtained from the Lineberger Comprehensive Cancer Center Tissue Culture Facility. Human embryonic kidney cells (HEK 293 cells) containing the EBV Bacterial Artificial Chromosome (HEK 293-EBV cells) were a gift from Wolfgang Hammerschmidt (Munich, Germany) (Gires, et al. 1999). EBV-negative Burkitt’s Lymphoma cells (BL41 Neg), EBV-positive BL41 cells (BL41 Pos), P3HR1 mutant BL41 cells (BL41 Mut), and Raji cells have been previously described (Bentz, et al. 2012, Bentz, et al. 2015, Bentz, et al. 2019, Salahuddin, et al. 2019). GFP-Akata cells are Akata Burkitt lymphoma cells that carry a recombinant EBV genome, where the non-essential BXLF1 genes was replaced with neomycin resistance- and GFP-expression cassettes, were a gift from Rona Scott (LSUHSC, Shreveport, LA, USA) (Birdwell, et al. 2014). The EBV-negative nasopharyngeal carcinoma cell (NPC) line HK1, its EBV-positive counterpart that is infected with the EBV recombinant Akata strain, and the EBV-positive NPC cell line NPC43 were a gift from George Tsao, Hong Kong University, Hong Kong) (Huang, et al. 1980, Lo, et al. 2006, Lin, et al. 2018, Yip, et al. 2018). Human whole peripheral blood was purchased from StemCell Technologies, and peripheral B cells were isolated using the RosetteSep™ Human B cell Enrichment Cocktail followed by Lymphoprep™ gradient in RosetteSep™ tubes (Stem Cell Technologies).
All cells were cultured Cellgro RPMI (Corning) supplemented with 20% fetal bovine serum (FBS; Corning) and antibiotic/antimycotic solution (Corning). HEK 293-EBV cells were grown under selection with Hygromycin B. The GFP-Akata cells and HK1 EBV-positive cells were treated with G418 for selection.
2.2. Cell Treatment:
ML-792, C21H23BrN6O5S or ((1R,2S,4R)-4-((5-(1-(3-bromobenzyl)-1H-pyrazole-3-carbonyl)pyrimidin-4-yl)amino)-2-hydroxycyclopentyl)methyl sulfamate (Cat#407886, Lot#YXM80630), was purchased from MedKoo Biosciences (Morrisville, NC). The provided certificate of analysis stated that its purity, as determined by high-performance liquid chromatography, was ≥ 98.0%. The ML-792 was resuspended in DMSO and stored at −20°C. Subsequent dilutions were performed in Cellgro 1X Dulbecco’s Phosphate-Buffered Saline (DPBS; Corning). Cells were treated with graduated doses of ML-792 (0.001 uM, 0.01 uM, 0.1 uM, 1 uM, and 10 uM) (He, et al. 2017). Equal amounts of DMSO were used as the vehicle control.
In some experiments, peripheral B cells were stimulated with lipopolysaccharides (LPS; 20 μg/ml; Sigma-Aldrich) (Mond and Brunswick 2003, Hawkins, et al. 2013, Salahuddin, et al. 2019).
2.3. Western Blot Analysis:
Whole cell lysates (WCL) were prepared by adding 4X Laemmli sample buffer (Bio-Rad) with 2-Mercaptoethanol (Sigma; final concentration 355 mM) to resuspended cell pellets. Samples were boiled at 100°C for 10 minutes and separated by SDS-PAGE gel electrophoresis. Samples were transferred to PVDF membranes (Bio-Rad) using the Trans-Blot Turbo Transfer System (Bio-Rad). Following transfer, membranes were blocked in milk for one hour at room temperature. Membranes were incubated in Tris-buffered saline with Tween-20 (TBST; Bio-Rad; final concentration 0.1%) with the appropriate primary antibodies overnight at 4°C. Membranes were washed three times with TBST for 10 minutes and then incubated with the appropriate horseradish peroxidase-conjugated secondary antibodies for one hour at room temperature. After three additional 10-minute washes with TBST, membranes were developed in Clarity Western ECL Substrate solution (Bio-Rad) for 5 minutes and images were visualized using the ChemiDoc Touch Imaging System (BioRad). Western blot analyses were performed for experiments performed in triplicate. Representative images
2.4. Densitometric Analysis:
Densitometry was performed using two different methods. First, images were opened in the ImageJ (NIH software). Quantitation of protein levels was performed on each image using a method described in the following website: https://www.lukemiller.org/journal/2007/08/quantifying-Western-blots-without.html (Corpas, et al. 2008, Ning, et al. 2008, Luhtala and Parker 2009, Miller, et al. 2009, Bentz, et al. 2012, Bentz, et al. 2019, Salahuddin, et al. 2019, Selby, et al. 2019). Second, the Bio-Rad Image Lab Software was used, and densitometric analysis was performed following the manufacturers recommended protocol. For both analyses, the relative density was calculated by dividing the densitometry of the protein of interest (SUMO) with the respective loading control (actin). These values were then used to determine the fold change when compared to the control arm of the experiment. When comparing the determined fold changes, results were similar when comparing values calculated from the ImageJ analyses to the values calculated from the Image Lab Software analyses. Results from the ImageJ analyses are shown as the mean ± the standard deviation of experiments performed in triplicate.
2.5. Real-Time PCR:
Intracellular and proteinase K-resistant supernatant DNA was isolated using the DNeasy blood and tissue kit (Qiagen). Quantitative PCR (qPCR) was performed using the Universal SYBR green Supermix (Bio-Rad) ABI7900HT Real-Time PCR system. Relative EBV W-repeat levels (relative to gapdh and actin). Samples were run in triplicate for each experiment, and experiments were performed in triplicate.
2.6. Cell Growth and Death:
Cells were treated with graduated doses of ML-792 or the vehicle control and samples harvested at 0, 24, 48, 72, and 96 hours post-treatment. Trypan blue exclusion assays were performed to determine the number of total, live, and dead cells at each time point using the TC20 Automated Cell Counter (Bio-Rad). In some experiments, cells were collected at 96 hours post-infection, pelleted, and resuspended in fresh media. Cells were allowed to “recover” for 72 hours, and the numbers of total, live, and dead cells were determined.
2.7. Spontaneous Reactivation:
HEK 293-EBV cells or GFP-Akata cells were grown in 6-well dishes (CellStar) and treated with graduated doses of ML-792 or the vehicle control. 48 hours post-treatment, cells and supernatant fluids were collected and DNA was isolated (see above).
2.8. Induced Reactivation:
HEK 293-EBV cells were grown in 6-well dishes (CellStar) and transfected with control- or BZLF/ZTA- and BALF4-expression plasmids. 24 hours post-transfection, cells were treated with graduated doses of ML-792 or the vehicle control. 48 hours post-treatment (72 hours post-transfection), cells and supernatant fluids were gathered for DNA isolation (see above). Supernatant fluids were also collected to determine Raji-units of virus production.
Induced reactivation of GFP-Akata cells was performed by cross-linking cell surface IgG using goat F(ab’)2 fragment to human immunoglobulin G (IgG; MP Biomedicals, 100 μg/ml), as previously described (Molesworth, et al. 2000, Wang, et al. 2009). Four hours post-induction, cells were treated with graduated doses of ML-792 or the vehicle control. 72 hours post-induction, cells and supernatant fluids were gathered for DNA isolation (see above). Supernatant fluids were also collected to determine Raji-units of virus production.
2.9. Raji Superinfection:
Supernatant fluids, from transfected and treated HEK 293-EBV cells or induced and treated GFP-Akata cells, were centrifuged to remove cell debris. Fluids were added to Raji cells, diluting any treatment 1:5. 72 hours post-addition to the Raji cells, fluorescence microscopy was used to determine the number of Green Fluorescent Protein (GFP)-positive Raji cells (Bentz, et al. 2015, Bentz, et al. 2019).
2.10. Modified Scratch Assay:
HEK 293 cells were plated in 6-well dishes and transfected with control- or LMP1-expression constructs using polyethylenimine (PEI 25kDa; Sigma). 0.33 ug of expression construct was diluted in 300 ul Opti-MEM (ThermoFisher). PEI was added, samples were pulsed twice on a vortex, and incubated for 15 minutes at room temperature. The transfection mixture was added drop-wise to the corresponding wells (Ning, et al. 2008, Bentz, et al. 2011, Bentz, et al. 2012, Bentz, et al. 2015, Bentz, et al. 2019, Salahuddin, et al. 2019, Selby, et al. 2019).
Once cells were confluent, a linear scratch was made down the center of each well using a 200 μl pipette tip (VWR). Disrupted cells were removed by two washes with PBS. Fresh RPMI supplemented with 10% FBS was added to the wells and images of each scratch were captured at 20X magnification using the EVOS FLoid to determine the initial scratch width (Fisher). 24 hours post-scratch, ten images were taken of each scratch, and using ImageJ, the migration of cells into each scratch was calculated (Bentz and Yurochko 2008, Bentz, et al. 2011, Bentz, et al. 2019).
2.11. Modified Homotypic Cell Adhesion Assay:
Multiple B-cell lines were resuspended in fresh RPMI supplemented with 10% FBS and treated with graduated doses of ML-792 or its vehicle control. Single-cell suspensions were incubated for 24 hours at 37°C and images of ten random fields of view for each treatment arm were captured using the EVOS FLoid. Using ImageJ, the average cells in each clump of cells in the random fields of view were quantitated (Gregory, et al. 1988).
2.12. Antibodies:
GAPDH- (0411), Ubiquitin- (A-5), PARP-1-HRO (F-2), and UBC9-specific antibodies (67AT1273.95.90) were purchased from Sigma Cruz. β-Actin-(A1978), SAE1- (SAB3500486), SAE2- (SAB3500487), HRP-anti-Mouse IgG- (A9917), and HRP-anti-Rabbit IgG- specific antibodies (A0545) were purchased from Sigma. SUMO-1- (18H8) and SUMO-2/3-specific antibodies (5C11) were purchased from Cell Signaling Technology. LMP1-specific antibodies (CS1–4/ab78113 and D24-G/ab136633) were purchased from Abcam.
2.13. Plasmids:
BZLF1/ZTA- and BALF4-expression plasmids were a gift from Wolfgang Hammerschmidt (Gires, et al. 1999).
2.14. Statistical Analysis:
All experiments were performed in triplicate, and data are shown as the mean ± the standard deviation. Unpaired, two-tailed, Student’s T-tests were used for all statistical analyses, and P-values less than 0.05 were considered to be statistically significant.
3. Results
3.1. ML-792 decreased global levels of sumoylated proteins in proliferating B cells.
To test our hypothesis that ML-792 modulates viral replication and the oncogenic potential of EBV LMP1 by inhibiting protein sumoylation, we started by analyzing global levels of sumoylated proteins in naïve human B cells (Figure 1A), their LPS/mitogen-activated counterparts (Figure 1B), EBV-transformed lymphoblastoid cell lines (LCL; Figure 1C), and the lymphoblast-like Raji cells (Figure 1D). The LCLs and Raji cells were positive for EBV and expressed LMP1 while the naïve B cells and mitogen-blasts lacked the virus and did not express the oncoprotein. Cells were treated with graduated amounts of ML-792, and whole cell lysates were collected 96 hours post-treatment. Western blot analyses were performed to detect free SUMO levels (~12 kDa) and all proteins covalently modified by SUMO, which are represented by the laddering of SUMO-1/2/3-specific, slower migrating bands. Free SUMO and global levels of sumoylated proteins were detectable in the tested B cells, but global levels of sumoylated proteins varied between B cell lines (Figure 1A–1D). In naïve B cells, free SUMO was easily detected but very little laddering of SUMO-specific bands was observed (Figure 1A). ML-792 had no effect on free SUMO levels nor levels of modified proteins in these naïve cells. The laddering of SUMO-specific bands was slightly more evident in LPS-activated B cells (Figure 1B), and while ML-792 decreased global levels of sumoylated proteins in these mitogen-blasts, it had no effect on free SUMO levels. Western blot analyses of the two EBV-positive B cell lines (LCLs and Raji; Figure 1C and 1D) revealed noticeable laddering of sumoylated proteins. Interestingly, free SUMO levels and global levels of modified proteins decreased as ML-792 levels increased in the EBV-positive cells.
Figure 1: ML-792 decreased global levels of sumoylated proteins in proliferating B cells.
A) Naïve peripheral B cells, B) LPS-stimulated peripheral B cells, C) EBV-transformed lymphoblastoid cell lines (LCLs), and D) Raji cells were treated with graduated doses of ML-792. 96 hours post-treatment, cells were harvested and Western blot analyses performed to detect SUMO-1/2/3, Ubiquitin, SAE1, SAE2, Ubc9 and LMP1 levels. Actin was used as a loading control. Representative images of experiments performed in triplicate are shown. F-G) Densitometric analysis was performed on repeat experiments and the fold changes in F) relative SUMO levels and G) relative Ubiquitin levels were determined. Results are shown as the means ± the standard deviation of experiments performed in triplicate.
Densitometric analysis was performed on experiments performed in triplicate (Figure 1E). Results confirmed that ML-792 had no effect on relative SUMO levels in resting naïve B cells. However, ML-792 did inhibit global sumoylation levels in a dose-dependent manner in the examined proliferating B cells. In LPS-stimulated B cells and LCLs, this decrease was statistically significant (P < 0.05) at 0.1 μM of ML-792, while the decrease in levels of sumoylated proteins was significant (P < 0.05) at a lower concentration (0.01 μM) in Raji cells. These findings supported our hypothesis and documented that ML-792 was effective at inhibiting sumoylation in B-cells, but this inhibition only occurred in proliferating B cells and not resting naïve B cells.
3.2. ML-792 Had No Effect on Ubiquitination
Due to the similarities between sumoylation and ubiquitination processes (Kerscher, et al. 2006, Lowrey, et al. 2017) and to establish the specificity of ML-792, we assessed the effect of ML-792 on global levels of ubiquitinated proteins. Western blot analyses indicated that levels of ubiquitinated proteins were not affected by treatment with ML-792 (Figure 1A–1D). Densitometric analysis of repeated experiments confirmed that there were no significant changes in relative levels of ubiquitinated proteins following treatment with ML-792 (Figure 1F). These data demonstrate that ML-792 selectively targets the sumoylation pathway without affecting ubiquitination.
3.3. ML-792 Had No Affect on the SUMO Machinery
Two essential enzymes in the sumoylation process are the SUMO-activating enzyme, which is comprised of SAE1 and SAE2 subunits (E1), and the SUMO-conjugating enzyme, Ubc9 (E2). To elucidate if one mechanism by which ML-792 inhibited global sumoylation levels was by decreasing the expression of the SUMO machinery, we examined levels of these proteins in naïve B cells, mitogen blasts, LCLs, and Raji cells (Figure 1A–1D) following treatment with graduated doses of ML-792. Western blot analyses revealed that SAE1, SAE2, and Ubc9 levels were not affected by treatment with ML-792, which demonstrates that ML-792 does not affect levels of the essential SUMO machinery. Taken together, our findings suggest that ML-792 specifically and selectively target sumoylation processes in proliferating B cells.
3.4. ML-792 inhibited sumoylation at lower concentrations in EBV-positive B cells when compared with EBV-negative B cells.
Next we investigated the effect of ML-792 on a set of paired B cell lines (Calender, et al. 1990, Cherney, et al. 1998, Ning, et al. 2003). BL41 is an EBVnegative Burkitt lymphoma cell line (BL41 neg). This negative cell line was stably infected with wild-type EBV (B95–8; BL41 pos), resulting in a clone that expresses EBV latency-associated genes, including LMP1. The negative cell line was also stably infected with a mutant EBV that lacks EBNA2 (P3HR1; BL41 P3HR1), and because EBV P3HR1 does not express EBNA2 it also does not express LMP1. These three cell lines were treated with graduated amounts of ML-792, and whole cell lysates were collected 96 hours post-treatment. The laddering of SUMO-specific, slower migrating bands was detected in BL41 neg cells (Figure 2A), BL41 pos cells (Figure 2B), and BL41 P3HR1 cells (Figure 2C). As concentrations of ML-792 increased, the global levels of sumoylated proteins decreased. Densitometric analysis of repeated experiments revealed that statistically significant ML-792-mediated decreased levels of sumoylated proteins occurred at lower concentrations in EBV-positive cells (BL41 pos and BL41 P3HR1) when compared with the EBV-negative counterparts (BL41 neg; Figure 2D). When comparing the two EBV-positive cell lines, significant decreases in global levels of sumoylated proteins occurred at lower concentrations of ML-792 in EBV-positive cells expressing LMP1 than EBV-positive cells not expressing LMP1. As observed in the previous set of B cells, ML-792 had no effect on global levels of ubiquitinated proteins or the SUMO machinery in the paired BL41 cell lines (Figure 2A–2C and 2E). These findings led us to propose that ML-792 does inhibit sumoylation processes better in EBV-positive cells when compared with EBV-negative cells.
Figure 2: ML-792 inhibited sumoylation processes at lower concentrations in EBV-positive B cells when compared with EBV-negative B cells.
A) BL41 EBV-negative cells (EBV neg), B) BL41 EBV-positive cells (EBV pos), and C) BL41 P3HR1 cells were treated with graduated doses of ML-792. 96 hours post-treatment, cells were harvested and Western blot analyses performed to detect SUMO-1/2/3, Ubiquitin, SAE1, SAE2, Ubc9, and LMP1 levels. Actin was used as a loading control. Representative images of experiments performed in triplicate are shown. D-E) Densitometric analysis was performed on repeat experiments and the fold changes in D) relative SUMO levels and E) relative Ubiquitin levels were determined. Results are shown as the means ± the standard deviation of experiments performed in triplicate.
3.5. ML-792 inhibited global levels of sumoylated proteins in EBV-positive epithelial cells.
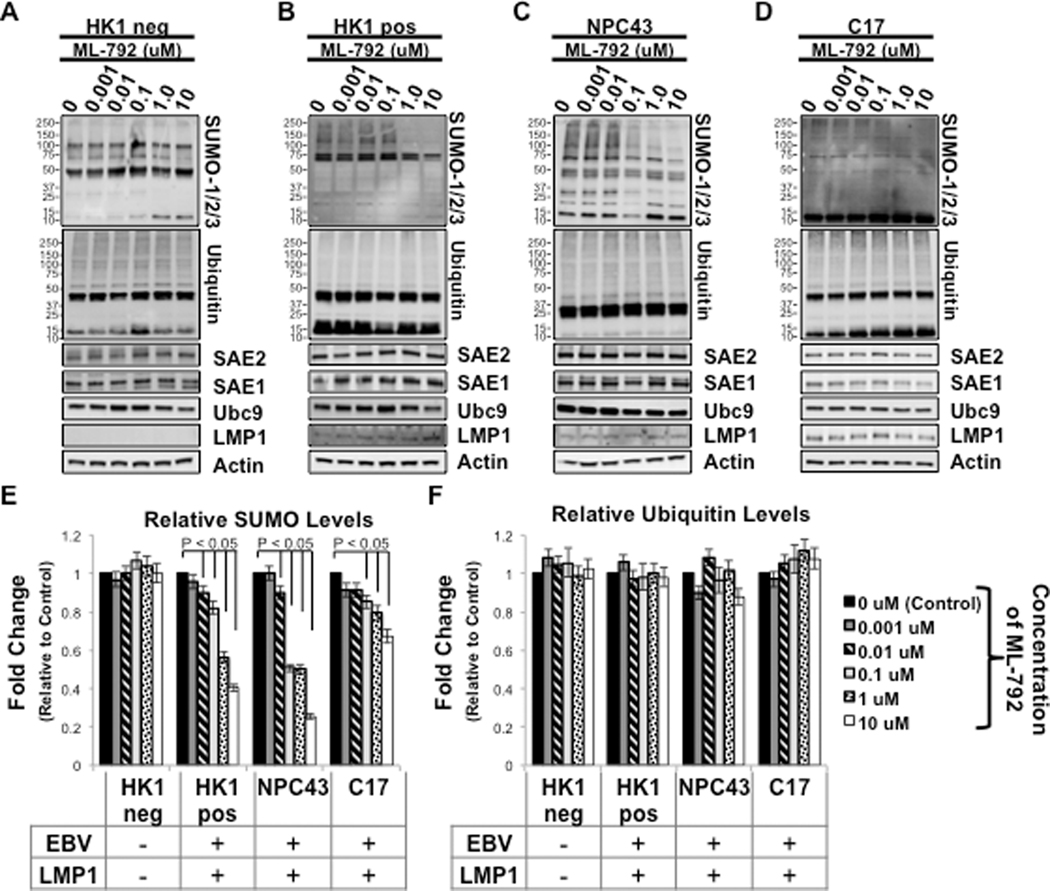
In addition to lymphoid malignancies, latent EBV infection is also associated with various epithelial malignancies, including nasopharyngeal carcinoma (NPC) (Pagano 2009). While our initial interests focused on the effect of ML-792 on B cells, we were curious whether ML-792 had similar effects in epithelial cells, so we tested ML-792 in various NPC cell lines. HK1 is an EBV-negative NPC-derived cell line (HK1 neg), and a clone was made where HK1 cells were infected with EBV (Akata strain; HK1 pos) (Huang, et al. 1980, Lo, et al. 2006, Lin, et al. 2018, Yip, et al. 2018). NPC43 and C17 are two EBV-positive NPC-derived cell line (Huang, et al. 1980, Lo, et al. 2006, Lin, et al. 2018, Yip, et al. 2018). Epithelial cells were treated with graduated amount of ML-792 for 96 hours. Western blot analyses of whole cell lysates revealed global levels of sumoylated proteins and the laddering of sumoylated proteins was detected in each epithelial cellline (Figure 3A–3D). ML-792 had no effect on sumoylation processes in HK1 neg cells (Figure 3A), but did inhibit global levels of sumoylated proteins in the three EBV positive-epithelial cells (Figure 3B–3D). Results from repeat experiments confirmed that no changes in relative SUMO levels were observed in ML-792-treated HK1 neg cells (Figure 3E), and ML-792-mediated inhibition of relative SUMO levels were significant (P < 0.05) in EBV-positive cells at low concentrations (0.01 μM). Similar to what was observed in B cells, ML-792 did not affect global levels of ubiquitinated proteins or expression of the SUMO machinery in epithelial cells (Figure 3A-D and 3F).
Figure 3: ML-792 inhibited global levels of sumoylated proteins in EBV-positive epithelial cells.
A) HK1 EBV-negative (HK1 neg) epithelial cells, B) HK1 EBV-positive (HK1 pos) epithelial cells, C) NPC43 epithelial cells, and D) C17 epithelial cells were treated with graduated doses of ML-792. 96 hours post-treatment, cells were harvested and Western blot analyses performed to detect SUMO-1/2/3, Ubiquitin, SAE1, SAE2, Ubc9, and LMP1 levels. Actin was used as a loading control. Representative images of experiments performed in triplicate are shown. E-F) Densitometric analysis was performed on repeat experiments and the fold changes in E) relative SUMO levels and F) relative Ubiquitin levels were determined. Results are shown as the means ± the standard deviation of experiments performed in triplicate.
These data confirmed that ML-792 specifically inhibits sumoylation processes in different cell lines. The effect of ML-792 was more pronounced in EBV-positive B cells and epithelial cells, indicating that ML-792 can selectively reduce levels of sumoylated proteins in latently infected cells. Interestingly, the levels of a SUMO-positive unidentified protein, approximately 40–50 kDA in size, remained unchanged in LPS-activated B cells, Raji cells, BL41 P3HR1 cells, and NPC43 cells following treatment with ML-792. The level of a similar sized band increased in BL41 EBV neg cells. These findings suggest that the observed effect of ML-792 may be discriminatory to certain cellular proteins.
3.4. ML-792 Inhibited Cell Growth and Induced Cell Death
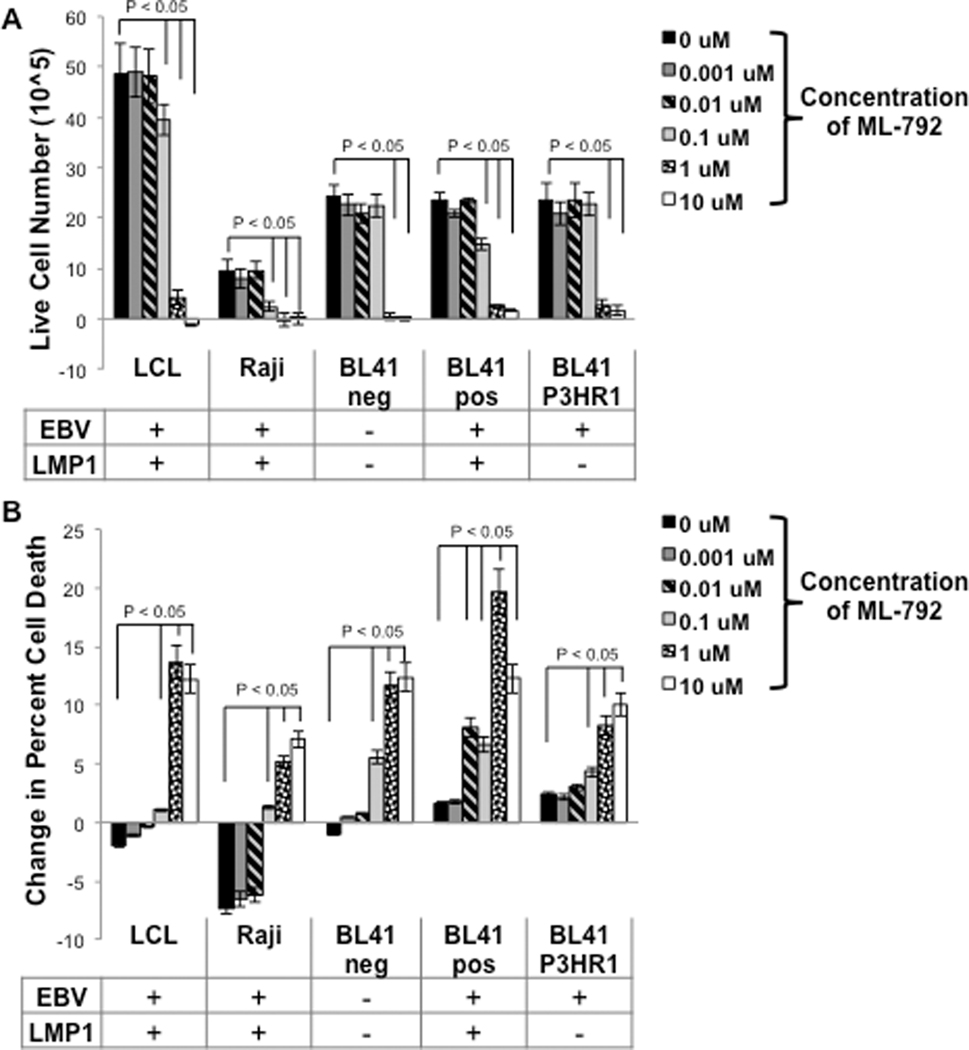
In order to further test our hypothesis, next we wanted to better understand the effect of ML-792 on B cells. Sumoylation plays an important role in regulating various cellular pathways (e.g. cell growth, mitosis, cell maintenance, and cell survival) (Kroetz 2005, Kerscher, et al. 2006, Kerscher 2007), so we examined the affect of the drug on B cell growth and death. Naïve B cells, LPS-stimulated B cells, LCLs, Rajis, BL41 pos cells, BL41 neg cells, and BL41 P3HR1 cells were treated with graduated doses of ML-792. The numbers of live cells and the percentage of dead cells were determined at 24, 48, 72, and 96 hours post-treatment (Supplemental Figure 1 and 2). With the exception of naïve B cells, all tested cell lines exhibited positive cell growth and limited cell death when treated with the vehicle control (DMSO; 0 μM). At higher concentrations, ML-792 inhibited cell growth and increased cell death in all proliferating B cells.
Focusing on B cell growth, the number of population doublings was determined at 96 hours post-treatment (Figure 4A). With the exception of naïve B cells, all control-treated cells went through at least two population doublings. ML-792 decreased the number of population doublings in all proliferating B cells, with the greatest decreases occurring in LMP1-positive cells (LCLs, Raji cells, and BL41 pos cells). In the treated LMP1-positive B cells, the number of population doublings significantly (P < 0.05) decreased when treated with concentrations of ML-792 less than 0.1 μM. The two transformed B cell lines that did not express LMP1 (BL41 neg and BL41 P3HR1) only exhibited significant (P < 0.05) decreases in the number of population doubling at 1 μM of ML-792, suggesting they were not as sensitive to the inhibitor. Interestingly, LPS-stimulated B cells were very sensitive to ML-792 with a significant (P < 0.05) decrease in the number of population doublings occurring at low concentrations (0.01 μM). When comparing growth curves of the B cells following treatment with ML-792 (Supplemental Figure 1) and determining the statistical significance between each experimental arm and the control at each time point (Figure 4B), the LMP1-expresing cells exhibited significant (P < 0.05) decreases in cell number at earlier times and at lower concentrations when compared with BL41 neg cells or BL41 P3HR1 cells. These data reveal that ML-792 inhibits the proliferation of B cells and expression of LMP1 increases the susceptibility of the cells to the drug.
Figure 4: ML-792 decreased B cell growth.
Naïve peripheral B cells, LPS-stimulated peripheral B cells, LCLs, Raji cells, BL41 EBV-negative cells, BL41 EBV-positive cells, and BL41 P3HR1 were treated with graduated doses of ML-792 and the number of live cells was determined daily for four days. A) The number of population doublings was determined 96 hours post-treatment. Results are shown as the means ± the standard deviation. B) Live cell counts were taken every 24 hours and compared to control cells (0 μM or DMSO-treated). Values where the experimental arm is significantly (P < 0.05) less than the control is depicted by (+). No statistical significance is depicted by (−). Results are shown of samples run in triplicate for experiments performed in triplicates.
As stated above, the percentage of cell death was also determined daily for four days (Supplemental Figure 2), and the change in the percentage of cell death was calculated four days post-treatment (Figure 5A). All cells exhibited an increase in cell death over the time of the experiments. ML-792 had no effect on the death of naïve B cells, but all concentrations of the inhibitor did significantly (P < 0.05) increase in the percent of cell death by 96 hours post-treatment in LPS-stimulated B cells. In LMP1-positive B cells, ML-792 significantly increased the change in percentage of cell death at concentrations greater than 0.1 μM. LMP1-negative transformed B cells were less susceptible to ML-792-induced cell death, with significant increases occurring at concentrations over 1 μM. When comparing the percent cell death over time (Supplemental Figure 2), ML-792 induced significant (P < 0.05) increases in the percent cell death at lower concentrations and at earlier times in LMP1-positive B cells when compared with LMP1-negative B cells (Figure 5B). These findings suggest that LMP1-positive B cells are more sensitive to ML-792, and our data support the published work demonstrating that ML-792 affects cancer cell proliferation and viability (He, et al. 2017).
Figure 5: ML-792 induced B cell death.
Naïve peripheral B cells, LPS-stimulated peripheral B cells, LCLs, Raji cells, BL41 EBV-negative cells, BL41 EBV-positive cells, and BL41 P3HR1 were treated with graduated doses of ML-792 and the number of live cells was determined daily for four days. A) The change in the percent of cell death (96 hours compared with 0 hours) was determined. Results are shown as the means ± the standard deviation. B) The percent of cell death was determined every 24 hours and compared to control cells (0 μM or DMSO-treated). Values where the experimental arm is significantly (P < 0.05) greater than the control is depicted by (+). No statistical significance is depicted by (−). Results are shown of samples run in triplicate for experiments performed in triplicates.
3.5. B Cells Were Unable to Recover From High Concentrations of ML-792
Sumoylation can affect a protein’s function even after it is de-sumoylated, so we next determined if the affect of ML-792 remained even after the drug was no longer in the environment. LCLs, Raji, BL41 neg, BL41 Pos, and BL41 P3HR1 cells were treated with graduated doses of ML-792. 96 hours post-treatment, cells were pelleted, resuspended in fresh media. 72 hours after platting cells in fresh media, the number of live and dead cells were determined in order to evaluate if the drug had a prolonged effect on the cells (Figure 6). LCLs, BL41 neg, and BL41 P3HR1 cells displayed significant (P < 0.05) cell growth following removal of ML-792 from the media, which suggested that cells were to recover following treatment with low concentrations of ML-792 (Figure 6A). Interestingly, Raji exhibited very low levels of cell growth during recovery from treatment with ML-792, but similar to the other B-cell lines, the lowest amount of recovery occurred following high levels of drug treatment. Upon examining cell death (Figure 6B), higher concentrations of ML-792, specifically 1 μM, and 10 μM, significantly (P < 0.05) increased cell death even after removal of the inhibitor from the cell environment. These findings suggest that ML-792 can have a sustained effect on multiple B-cell lines and can induce cell death even after the drug is gone from the cellular environment.
Figure 6: B-cells treated with higher concentrations of ML-792 were unable to recover.
LCLs, Raji cells, BL41 neg cells, BL41 pos cells, and BL41 P3HR1 cells were treated with graduated doses of ML-792. 96 hours post-treatment, cells were removed from their media, washed with PBS, and placed in fresh media. Following a 72 hour incubation, A) live cell counts and B) changes in the percent cell death were determined. Results are shown as the means ± the standard deviation of experiments and samples performed in triplicate.
3.6. ML-792 Inhibited Two Common Hallmarks of Oncogenesis
LMP1 is the principal EBV oncoprotein, and in our original study where we identified the first function for LMP1 CTAR3, we documented that LMP1-induced sumoylation affects cellular migration, a hallmark of oncogenesis (Bentz, et al. 2011). Therefore, we were interested if ML-792 could inhibit the oncogenic potential of LMP1. First, we determined if ML-792 would have any effect on the migration of LMP-1 positive cells. HEK 293 cells were transiently transfected with either control- or LMP-1-expression constructs, and once confluent, a modified scratch assay was performed and cell migration into the scratch was quantitated (Figure 7A). As expected, expression of LMP1 significantly (P < 0.05) increased cell migration when compared with control-expressing cells. Treatment of control- and LMP1-expressing cells inhibited cell migration in a dose-dependent manner; however, ML-792-mediated inhibition of cell migration was more pronounced in LMP1-expressing cells. These data demonstrated that ML-792 inhibited cell migration and this inhibition is increased in LMP1-expressing cells.
Figure 7: ML-792 increased cell adhesion and inhibited cell migration.
A) HEK 293 cells were transiently transfected with control- and LMP1-expression constructs. Once confluent, a modified scratch assay was performed and cells were treated with graduated doses of ML-792. Images of the scratch were taken 0 and 24 hours post-scratch using the EVOS Floid. Using Image J, cell migration into the scratch was determined. Results are shown as the means ± the standard deviation of experiments performed in triplicate. Whole cell lysates were collected and immunoblotting performed to detect FLAG-LMP1 and Actin levels. B). LCLs, Raji cells, BL41 neg cells, BL41 pos cells, and BL41 P3HR1 cells were treated with graduated doses of ML-792. 24 hours post-treatment, images were captured for ten random fields of view using the EVOS Floid. The average number of cells per clump was determined.
A second major hallmark of oncogenesis is the dysregulation of cell adhesion. Next, we examined if ML-792 altered B cell homotypic adhesion. Multiple B-cell lines were treated with the vehicle control and graduated doses of ML-792. The average numbers of cells per clump was determined 24 hours post-treatment (Figure 7B). Results showed that the average number of cells per clump significantly (P < 0.05) increased in LCLs, Raji cells, and BL41 pos cells following treatment with ML-792. ML-792 treatment had no effect on cell adhesion in BL41 neg cells or BL41 P3HR1 cells. These data led us to propose that ML-792 could increase cell adhesion in LMP1-expressing cells.
Taken together, these results establish that ML-792 can specifically target sumoylation processes in B cells, most importantly in cells latently infected with EBV, inhibiting cell growth and inducing cell death. The effect of ML-792 on B-cells can be long lived and can abrogate hallmarks of oncogenesis manipulated by LMP1. These findings lead us to propose that ML-792 may have therapeutic potential for the treatment of EBV-associated malignancies.
3.7. ML-792 Induced Low Levels of Spontaneous Reactivation in HEK 293-EBV Cells.
In addition to its anti-oncogenic potential, we were interested in determining if ML-792 had anti-viral properties. We previously documented that glycyrrhizic acid and ginkgolic acid, two natural small molecule inhibitor drugs, weakened EBV latency in HEK 293-EBV cells by inhibiting the sumoylation of the transcriptional co-repressor, KRAB-associated protein-1 (KAP1) (Bentz, et al. 2015, Bentz, et al. 2019), which has been determined to bind to EBV lytic promoters and prevent their activation (Bentz, et al. 2015). Because both botanical extracts can affect other cellular processes, we examined the effect of ML-792 on the spontaneous reactivation of EBV. HEK 293-EBV cells were treated with DMSO (vehicle control) and graduated doses of ML-792 (Figure 9). 48 hours post-treatment genomic DNA (gDNA) was extracted from cells and supernatant fluids. qPCR was performed to determine the fold changes in EBV DNA levels in the supernatant fluids (Figure 8A) and within the cells (Figure 8B). EBV DNA levels in supernatant fluids significantly (P < 0.02) increased when cells were treated with ML-792. Even nanomolar ML-792 treatments induced over a 1.5-fold increase in extracellular viral DNA levels when compared to control-treated cells. While a slight decrease in intracellular EBV levels occurred following ML-792 treatment, these decreases were not significant (P > .5). These data support our published proposal that inhibition of sumoylation results in low levels of EBV spontaneous reactivation (Bentz, et al. 2015, Bentz, et al. 2019).
Figure 9: ML-792 induced low levels of spontaneous reactivation and inhibited EBV induced reactivation in GFP-Akata cells.
A-B) GFP-Akata cells were treated with graduated doses of ML-792. DNA was harvested 48 hours post-treatment from A) supernatant fluids and B) cells. C-E) GFP-Akata cells were induced by cross-linking IgG surface receptors. Four hours post-induction, cells were treated with graduated doses of ML-792. 72 hours post-induction, supernatant fluids and cells were collected. DNA was harvested from C) supernatant fluids and D) cells. qPCR was performed to determine fold changes EBV DNA levels (relative to actin). Results are shown as the means ± the standard deviation of experiments and samples performed in triplicate. E) Western blot analyses were performed to detect ZTA, EA-D, and VCA levels. Actin was used as a loading control. Representative blots are shown for experiments that were performed in triplicate.
Figure 8: ML-792 induced low levels of spontaneous reactivation and inhibited EBV induced reactivation in HEK 293-EBV cells.
A-B) HEK 293-EBV cells were treated with graduated doses of ML-792. DNA was harvested 48 hours post-treatment from A) supernatant fluids and B) cells. C-E) HEK 293-EBV cells were transfected with control- or BZLF1-expression constructs. 24 hours post-transfection, cells were treated with graduated doses of ML-792. 72 hours post-transfection, supernatant fluids and cells were collected. DNA was harvested from C) supernatant fluids and D) cells. qPCR was performed to determine fold changes EBV DNA levels (relative to actin). Results are shown as the means ± the standard deviation of experiments and samples performed in triplicate. E) Western blot analyses were performed to detect ZTA, EA-D, and VCA levels. Actin was used as a loading control. Representative blots are shown for experiments that were performed in triplicate.
3.9. ML-792 Inhibited Induced Lytic Reactivation in HEK 293-EBV Cells
Because we previously determined that glycyrrhizic acid had no effect on EBV DNA levels following induced reactivation while ginkgolic acid inhibited EBV lytic reactivation (Bentz, et al. 2019), we were curious about the effect that ML-792 would have in EBV induced reactivation. HEK 293-EBV cells were transfected to induce reactivation. 24 hours post-transfection, cells were treated with graduated doses of ML-792. Extracellular and intracellular EBV DNA levels were determined 48 hours post-treatment, which was 72 hours post-transfection or post-induction. Results showed that induction of lytic replication resulted in almost at 2500-fold increase in extracellular EBV DNA levels (Figure 8C). ML-792 treatment resulted in significantly (P < 0.05) decreased extracellular EBV DNA levels. Similarly, induced reactivation resulted in significantly (P < 0.05) increased intracellular EBV DNA levels, and this increase was partially, yet significantly (P< 0.05), inhibited by treatment with ML-792 (Figure 8D).
Whole cell lysates were collected to determine if the observed changes in EBV DNA levels corresponded with changes in EBV protein levels (Figure 8E). Lytic EBV genes are expressed in stages. The immediately early genes products are BZLF1 (Zta, Z, or ZEBRA) and BRLF1 (Rta or R), both of which are transactivators. The early genes are involved in replication of the EBV genome, regulating cell metabolism, and modulating the host immune response. The EBV late genes encode for structural proteins. Western blot analyses of whole cell lysates reveal that immediate early (Zta), early (early antigen diffuse; EA-D), and late (viral capsid antigen; VCA) gene products were detected following an induced reactivation (Figure 8E). Surprisingly, treatment with ML-792 following induced reactivation had no effect on the expression of the selected EBV proteins. Taken together, these finding suggest that treatment of induced cells with ML-792 inhibited the replication of EBV DNA but did not affect the translation or transcription of EBV genes.
3.9. ML-792 Affected EBV Spontaneous Reactivation and Induced Lytic Reactivation in GFP-Akata Cells.
To confirm our results, we analyzed EBV reactivation in GFP-Akata cells, which are highly permissive for lytic replication following anti-immunoglobulin G (IgG) cross-linking. First, the ability of ML-792 to mediate the spontaneous induction of EBV was examined. Data showed that only high levels of ML-792 treatment induced small, yet significant (P < 0.05), increases in extracellular and intracellular EBV DNA levels (Figure 9A and 9B), which confirms the data obtained in HEK 293-EBV cells and our previous findings that documented a role for sumoylation processes in EBV spontaneous reactivation (Bentz, et al. 2015, Bentz, et al. 2019). Second, the effect of ML-792 on induced EBV reactivation was determined. Similar to the HEK 293-EBV cells, cross-linking of the surface IgG resulted in significantly (P < 0.001) increased extracellular and intracellular EBV DNA levels (Figure 9C and 9D). Treatment of cells with graduated doses of ML-792 four hours post-induction significantly (P < 0.05) decreased EBV DNA levels when compared with induced, but untreated GFP-Akata cells, which again confirms our data obtained in HEK 293-EBV cells and our previous findings that proposed a function for sumoylation processes in EBV induced reactivation (Bentz, et al. 2019). Third, the effect of ML-792 on select lytic protein levels was determined (Figure 9E). Zta, EA-D, and VCA were all detected in GFP-Akata cells induced by IgG cross-linking and treated with ML-792. Unlike in HEK 293-EBV cells, treatment of the GFP-Akata cells with ML-792 did have an effect levels on select EBV lytic proteins. Both Zta and EA-D levels decreased when cells were treated with low (0.001 μM, and 0.01 μM) and high (1.0 μM and 10 μM) doses of ML-792, but this ML-792-mediated decrease in Zta and EA-D protein levels was not detected when cells were treated with 0.1 μM ML-792. Surprisingly, VCA levels remained unchanged following treatment with ML-792, with only a slight increase in VCA levels being detected when cells were treated with 0.01 μM ML-792. While these findings confirm a role for sumoylation processes in the spontaneous and induced reactivation of EBV, they do suggest that inhibition of sumoylation processes may also have an effect on the expression of EBV lytic proteins.
3.10. ML-792 Decreased Raji Cell Superinfection
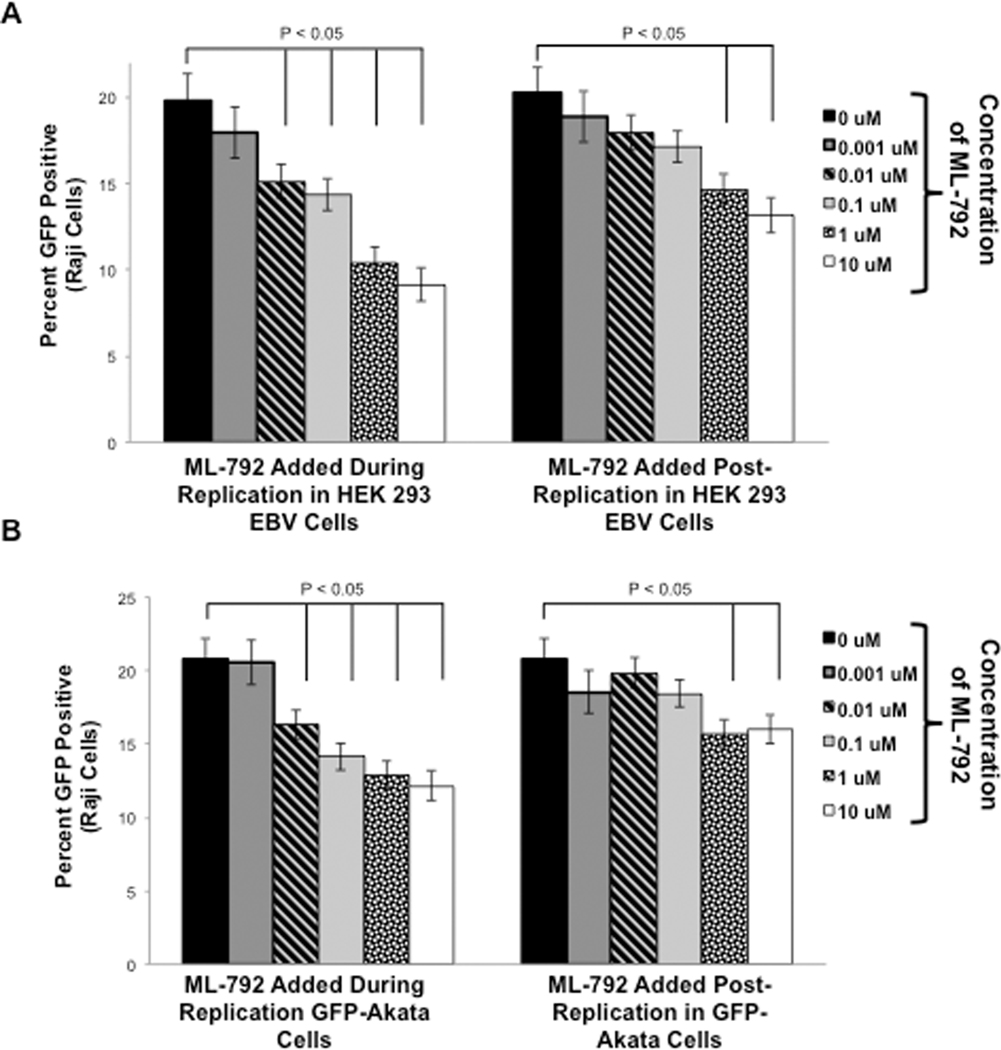
Because ML-792 inhibited EBV induced reactivation, next we determined the ability of any produced virus to super-infect Raji cells. First, the virus produced in HEK 293-EBV cells was examined (Figure 10A). Supernatant fluids were collected from HEK 293-EBV cells that underwent induced reactivation and treated with ML-792 48 hours post-induction. These supernatant fluids were used to super-infect Raji cells and the percentages of GFP-positive Rajis were determined (Figure 10A; ML-792 Added During Replication). As expected, approximately 20% of Raji cells were super-infected with virus under control conditions. Addition of ML-792 during induced reactivation of the virus resulted in a significant (P < 0.05) decrease in GFP-positive Raji cells. Second, the virus produced in GFP-Akata cells was tested (Figure 10B; ML-792 Added During Replication). Similarly, approximately 20% of Raji cells were super-infected with virus under control conditions, and addition of ML-792 during viral replication resulted in a significant (P < 0.05) decrease in the percentages of GFP-positive Raji cells in a dose-dependent manner). These data demonstrate that treatment of cells with ML-792 during viral replication coincides with reduced levels of new infectious virus.
Figure 10: ML-792 decreased Raji cell superinfection.
A) HEK 293-EBV cells were transfected with ZTA-expression constructs and treated with graduated doses of ML-792 24 hours post-transfection. B) Surface IgG receptors were cross-linked on GFP-Akata cells, which were treated with graduated doses of ML-792 four hours post-induction. Supernatant fluids were collected 72 hours post-induction and centrifuged to remove cell debris. Raji cells were superinfected with these supernatant fluids (ML-792 added during replication). In some experiments, supernatant fluids were collected from induced, untreated cells and divided evenly among experimental arms and then treated with ML-792 (ML-792 added after replication). The percent of GFP-positive Raji cells were determined after 72 hours. Results are shown as the means ± the standard deviation of experiments and samples performed in triplicate.
We previously documented that the mere presence of a different sumoylation inhibitor, glycyrrhizic acid, was sufficient to inhibit Raji cell super-infection (Bentz, et al. 2019), and while the observed decrease in Raji cell super-infection corresponded with decreased EBV DNA levels, it was possible that the decreased super-infection was just due to the presence of ML-792 in the supernatant fluids. Therefore, we collected supernatant fluids were from HEK 293-EBV cells that underwent induced reactivation and treated these supernatant fluids were treated with graduated doses of ML-792 prior to the super-infection of Raji cells (Figure 10A; ML-792 Added Post-Replication). Data revealed that addition of ML-792 to supernatant fluids just prior to Raji super-infection still did significantly (P < 0.05) decrease the ability of any virus in the supernatant fluids to penetrate Raji cells. However, this decrease occurred at higher concentrations of ML-792 when the drug was added post-replication when compare with when the drug was added during replication. Similar results were observed when supernatant fluids were collected from GFP-Akata cells that underwent induced reactivation (Figure 10B; ML-792 Added Post Replication). Together, these findings led us to propose that ML-792 inhibited both the production of new infectious virus and the penetrance of any produced virus. In addition, they indicate that ML-792 may have anti-viral potential.
Together, our findings documented that ML-792 inhibited sumoylation processes in multiple cell lines, affecting cell growth, cell survival, cell adhesion, and cell migration. In addition, ML-792 inhibited LMP1-mediated maintenance of EBV latency, EBV induced reactivation, and the ability of EBV to penetrate new cells. Because many of the effects of ML-792 were more pronounced and occurred at lower drug concentrations in LMP1-expressing cells, we propose that ML-792 has therapeutic potential for the treatment of EBV-associated malignancies due to its anti-tumorigenic and anti-viral properties.
4. Discussion
Dysregulation of cellular sumoylation is a hallmark of many cancers (Stewart, et al. 2005, Duan, et al. 2009), which makes it an attractive target for potential anti-cancer therapeutics. Recently, a synthetic small-molecule inhibitor of E1 (SAE), ML-792, was identified as a potent and selective inhibitor that targets the SUMO pathway (He, et al. 2017). Here, we document that ML-792 inhibited global protein sumoylation at nanomolar concentrations in proliferating B cell lines and EBV-positive NPC cell lines, which supports the earlier finding that ML-792 inhibited sumoylation levels in breast, colon, and melanoma cancer cell lines (He, et al. 2017). In addition, we confirmed that ML-792-mediated inhibition of sumoylation can inhibit B cell proliferation and induced cell death. This modulation of cell growth and survival could be sustained even after the removal of ML-792 from the environment. We also demonstrated that ML-792 inhibited the oncogenic potential associated with expression of the principal EBV oncoprotein, LMP1. Investigation into the specific effect on ML-792 on the EBV life-cycle revealed that this specific drug inhibited the maintenance of latency and inhibited induced viral replication, both of which supports previous work from our laboratory (Bentz, et al. 2015, Bentz, et al. 2019). Because we now document ML-792 efficiently inhibited sumoylation processes in EBV-positive B-cell lines and EBV-positive NPC cell lines, we propose that this drug may have therapeutic potential in the treatment of EBV-associated malignancies.
Previously, we documented that LMP1 increased global levels of sumoylation in cells, which correlated to elevated SUMO levels in EBV LMP1-positive lymphoma tissue (Salahuddin, et al. 2019), and also the effectiveness of two botanical extracts, ginkgolic acid and glycyrrhizic acid, in inhibiting LMP1-induced sumoylation (Bentz, et al. 2015, Bentz, et al. 2019). However, both drugs lack specific and selectivity all while inducing neurotoxicity and allergic reactions (Siegers 1999, Ahlemeyer, et al. 2001, Mahadevan and Park 2008, Liu and Zeng 2009, Berg, et al. 2015, Yao, et al. 2017). Recently, selective SUMO inhibitors have been identified (He, et al. 2017, Lv, et al. 2018, Li, et al. 2019). Here, we focus on ML-792, which targets sumoylation by selectively inhibiting SAE (E1) and having no effects on the neddylation activating enzyme or ubiquitination activating enzyme, which are similar to the SUMO-activating enzyme (He, et al. 2017). Supporting this work, we now identified that ML-792 can inhibit global sumoylation levels in proliferating B-cell lines and EBV-positive NPC cells lines without affecting global ubiquitination levels or the levels of the SUMO machinery. ML-792-mediated decreases in levels of sumoylated proteins occurred at lower concentrations in EBV-positive B cells and epithelial cells when compared with their EBV-negative counterparts. In addition, when comparing a set of paired lines, LMP1-positive B cells were even more sensitive to ML-792 than cells infected with a mutant strain of EBV that does not express LMP1. These findings suggest that the presence of the virus and the expression of a latency-associated protein increase the sensitivity of cells to the small molecule inhibitor. Interestingly, in some cell lines (LPS-activated B cells, Raji cells, BL41 neg cells, BL41 P3HR1 cells, and NPC43 cells), the levels of an unidentified, sumoylated protein(s) (40–50 kDA) were constant, or even increased, following treatment with ML-792. While this specific protein, whose sumoylation was not affected by ML-792, has yet to be elucidated, we propose there may be some selectivity in the observed decrease in sumoylation levels. Free and unconjugated SUMO levels were easier to detect in the examined B cells lines when compared with the NPC cell lines. While we expected to observe increases in free SUMO levels as global levels of sumoylated proteins decreased, instead we observed no changes and in some cases decreases in free SUMO levels when cells were treated with higher concentrations of ML-792. It is possible the unconjugated SUMO is somehow marked for destruction in ML-792-treated cells. In addition, it is possible that the detected decreases in free SUMO levels were due to ML-792-induced cell death. Regardless, these results add multiple B cell lines and NPC cells lines to the roster of cancer cell lines susceptible to ML-792.
Not only did ML-792 reduce cell proliferation and increased cell death, but treated B cells cells were unable to recover from the drug when initially treated with higher concentrations of ML-792. These findings suggest that ML-792 can have a sustained effect on cells, which was not examined in the previous study (He, et al. 2017). In our experiments, some cell lines exhibited little cell growth or the ability to recover regardless of drug treatment, but we are uncertain for why this is. However, these data demonstrate that when higher concentrations of ML-792 are used, the drug can have a sustained effect in B cells, even affecting cells after it is gone from the environment. This sustained effect of ML-792 may also occur in other cell types, which would only increase the number of malignancies that could be treated with ML-792.
Because we were interested in the therapeutic potential of ML-792 in the treatment of B-cell lymphomas, we investigated the in vitro effects of ML-792 on some hallmarks of oncogenesis. Based on previous work (Bheda, et al. 2009, Bentz, et al. 2011), we focused on cell adhesion and cell migration, both of which are induced by LMP1-mediated signaling. Treatment with ML-792 significantly decreased LMP1-induced cell migration and increased cell adhesion, which suggests that ML-792 pushed cells towards a more anti-oncogenic phenotype. Based on our findings here and the previous report that ML-792 inhibited anchorage dependent and anchorage independent growth (He, et al. 2017), we propose that ML-792 has a strong in vitro therapeutic effect.
The effect of ML-792 was more significant in LMP1-expressing B cells when compared with non-LMP1-expressing B cells. This difference was the most apparent in LCLs, which imitate EBV-induced malignancies. Interestingly, in the original report on ML-792, the inhibitor had greater effect on myc-over-expressing cells when compared with non-myc-over-expressing cells (He, et al. 2017). Myc helps regulate the cell cycle machinery, and over-expression of myc is one common hallmark in aggressive B-cell lymphomas, including endemic Burkitt’s lymphoma. LMP1 activation of NF-κB can increase myc levels (Dirmeier, et al. 2005), and LCLs and EBV Type III B-cell lymphomas express elevated levels of myc (Korkolopoulou, et al. 1993, Takano, et al. 1997, Faumont, et al. 2009). Therefore, these findings support our proposal that ML-792 could effectively reduce cell growth and survival in EBV LMP1-positive lymphomas.
The observation that LMP1-expressing cells were more sensitive to ML-792, led us to investigate the effect of ML-792 on the EBV life-cycle. We previously documented that LMP1 increases the sumoylation of the co-repressor, KAP1, which binds to EBV OriLyt and immediate early ZTA and RTA promoters and prevents spontaneous viral reactivation, leading us to propose that sumoylation is involved in the maintenance of EBV latency (Bentz, et al. 2015, Bentz, et al. 2019). In our earlier work, we used two inhibitors of the SUMO-E1 (ginkgolic acid and glycyrrhizic acid) and documented that inhibition of sumoylation induces low levels of spontaneous reactivation in HEK 293-EBV cells (Bentz, et al. 2015, Bentz, et al. 2019). Here we confirmed our proposal using a specific inhibitor of SAE (ML-792) and two different viral systems, HEK 293-EBV cells and GFP-Akata cells. Due to the specificity and selectivity of ML-792, we can now definitively state the sumoylation aids the maintenance of EBV latency.
While our laboratory is one of the few investigating sumoylation processes during EBV latency, there are numerous laboratories investigating sumoylation during EBV lytic replication. It is believed that EBV exploits the sumoylation process to create optimum conditions for replication (De La Cruz-Herrera, et al. 2018). Sumoylation of the immediate-early lytic proteins, Zta and Rta, have been documented (Adamson and Kenney 2001, Chang, et al. 2004, Adamson 2005), and sumoylated proteins accumulate following induced reactivation (De La Cruz-Herrera, et al. 2018). In addition, four EBV lytic proteins (SM/EB2, BGLF2, BMRF1, and BVRF2) have been identified to increase global protein sumoylation (De La Cruz-Herrera, et al. 2018). However, the function of sumoylation during lytic replication remains unknown. Coinciding with earlier work (Lin 2003, Lin, et al. 2008), we found that glycyrrhizic acid did not affect EBV induced reactivation while ginkgolic acid inhibited the production of new virus (Bentz, et al. 2019). Our results show that ML-792 inhibits sumoylation, which results in decreased release of new infectious virus and decreased EBV DNA levels in HEK 293-EBV cells transfected with ZTA-expression constructs to induce reactivation. Interestingly, these differences in EBV DNA levels did not correspond with changes in the expression of immediate-early (EBV ZTA), early (Early Antigen Diffuse; EA-D), or late (viral capsid antigen; VCA) protein levels. Taken together, these findings suggest that treatment of induced HEK 293-EBV cells with ML-792 inhibited the replication of EBV DNA but did not affect the transcription or translation of EBV genes. However, in these experiments, induction was performed by transfection of the BZLF1/Zta-expression construct, and to ensure induction occurred, ML-792 was added 24 hours post-transfection. Therefore, it is possible that the artificially elevated Zta protein levels allowed the cells to overcome any effects of ML-792 on transcription and translation but these increased Zta levels were not sufficient to overcome the ability of ML-792 to inhibit viral replication.
To better elucidate the function of ML-792 on the EBV life-cycle, EBV spontaneous and induced reactivation was also assessed in GFP-Akata cells. Using antibodies to cross-link IgG receptors on the cell surface allowed us to treat cells with ML-792 four hours post-induction. Results also indicated that treatment of latently infected cells with ML-792 was sufficient to mediate low levels of spontaneous reactivation and inhibit induced reactivation. However, in the GFP-Akata cells, the decreases in EBV DNA levels did correlate with decreases in expression levels of immediate early and early proteins. Unexpectedly, while ML-792 did decrease EBV DNA and protein levels following induction, this decrease did not occur in a dose-dependent manner. Instead, ML-792 seemed to initially decrease EBV DNA and immediate early and early protein levels following treatment with low (0.001 μM and 0.01 μM) concentrations of ML-792. This was followed by slight gradual increases in EBV DNA levels when treated with higher concentrations of ML-792. EBV protein levels did not correlate with EBV DNA levels at these higher concentrations of ML-792, which suggests there is some kind of dysregulation of the EBV life-cycle occurring. These findings suggest that ML-792 can have anti-viral properties in Akata cells following induced reactivation, and the data highlight differences between two commonly utilized EBV systems. In addition, they insinuate that sumoylation may be an important cellular process during lytic EBV replication. We are unsure of exactly how ML-792 differentially affected EBV DNA and EBV protein levels. Therefore, future work will focus on better elucidating the role of sumoylation during lytic replication.
Recently, we reported that simply the presence of a SUMO inhibitor was sufficient to inhibit the super-infection of Raji cells (Bentz, et al. 2019). Super-infection of Raji cells was significantly decreased when HEK 293-EBV cells and GFP-Akata cells were treated with ML-792 during induced reactivation. Interestingly, detection of viral DNA levels did not correlate with the super-infection of Ragi cells. Instead, while addition of ML-793 did not affect EBV DNA levels in a dose-dependent manner, the inhibitor did decrease the super-infection of Raji cells in a dose-dependent manner. This correlation was also observed when supernatant fluids were treated with ML-792 post-replication. These findings demonstrate that ML-792 has anti-viral properties and show that EBV DNA levels do not always correspond with infectious levels of virus. Finally, our data demonstrate that ML-792 can inhibit EBV attachment and/or the penetrance into a cell, which suggests a potential for ML-792 to inhibit the spread of EBV within the body. However, elucidating the specific effects of ML-792 at each step of the EBV life-cycle.
5. Conclusions
In summary, our current findings suggest a possible therapeutic potential for using ML-792 in the treatment of EBV-associated malignancies. First, this novel inhibitor may be able to inhibit tumorigenesis, decreasing tumor growth and metastasis and increasing cell death within the tumor. Second, ML-792 would limit EBV replication that may be induced during chemotherapeutic interventions to treat the malignancy. However, more studies, especially in vivo experiments, are needed to truly elucidate the effects of ML-792 on EBV-associated malignancies. In addition, there is still more to decipher about the function of sumoylation during EBV-mediated oncogenesis and the EBV life-cycle.
Supplementary Material
A) Naïve peripheral B cells, B) LPS-stimulated peripheral B cells, C) LCLs, D) Raji cells, E) BL41 EBV-negative cells, F) BL41 EBV-positive cells, G) and BL41 P3HR1 were treated with graduated doses of ML-792 and the number of live cells was determined daily at 0, 24, 46, 72, and 96 hours. Results are shown as the means ± the standard deviation of experiments and samples performed in triplicate.
A) Naïve peripheral B cells, B) LPS-stimulated peripheral B cells, C) LCLs, D) Raji cells, E) BL41 EBV-negative cells, F) BL41 EBV-positive cells, G) and BL41 P3HR1 were treated with graduated doses of ML-792 and the percentage of dead cells was determined daily at 0, 24, 46, 72, and 96 hours. Results are shown as the means ± the standard deviation of experiments and samples performed in triplicate.
Acknowledgements
This work was supported by grants from the National Institutes of Health (CA246552 and CA160786) and a Mercer University Provost Seed Grant, which were awarded to G.B. J.P.W. and J.B. efforts on the manuscript were possible due to the Mercer University School of Medicine Summer Scholars Program.
Glossary
- (EBV)
Epstein-Barr virus
- (LMP1)
Latent Membrane Protein-1
- (SUMO)
Small Ubiquitin-like modifier
- (SAE1/SAE2 or the SUMO E1)
SUMO activating enzyme
- (VCA)
viral capsid antigen
- (EA-D)
early antigen-diffuse
Footnotes
Conflict of Interest
The authors declare that they have no competing interests.
References
- Adamson AL (2005). “Effects of SUMO-1 upon Epstein-Barr virus BZLF1 function and BMRF1 expression.” Biochem Biophys Res Commun 336(1): 22–28. [DOI] [PubMed] [Google Scholar]
- Adamson AL and Kenney S. (2001). “Epstein-barr virus immediate-early protein BZLF1 is SUMO-1 modified and disrupts promyelocytic leukemia bodies.” J Virol 75(5): 2388–2399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahlemeyer B, Selke D, Schaper C, Klumpp S. and Krieglstein J. (2001). “Ginkgolic acids induce neuronal death and activate protein phosphatase type-2C.” Eur J Pharmacol 430(1): 1–7. [DOI] [PubMed] [Google Scholar]
- Alarcon-Vargas D. and Ronai Z. (2002). “SUMO in cancer--wrestlers wanted.” Cancer Biol Ther 1(3): 237–242. [DOI] [PubMed] [Google Scholar]
- Bentz GL, Lowrey AJ, Horne DC, Nguyen V, Satterfield AR, Ross TD, et al. (2019). “Using glycyrrhizic acid to target sumoylation processes during Epstein-Barr virus latency.” PLoS One 14(5): e0217578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bentz GL, Moss CR 2nd, Whitehurst CB, Moody CA and Pagano JS (2015). “LMP1-Induced Sumoylation Influences the Maintenance of Epstein-Barr Virus Latency through KAP1.” J Virol 89(15): 7465–7477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bentz GL, Shackelford J. and Pagano JS (2012). “Epstein-Barr virus latent membrane protein 1 regulates the function of interferon regulatory factor 7 by inducing its sumoylation.” J Virol 86(22): 12251–12261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bentz GL, Whitehurst CB and Pagano JS (2011). “Epstein-Barr virus latent membrane protein 1 (LMP1) C-terminal-activating region 3 contributes to LMP1-mediated cellular migration via its interaction with Ubc9.” J Virol 85(19): 10144–10153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bentz GL and Yurochko AD (2008). “Human CMV infection of endothelial cells induces an angiogenic response through viral binding to EGF receptor and beta1 and beta3 integrins.” Proc Natl Acad Sci U S A 105(14): 5531–5536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berg K, Braun C, Krug I. and Schrenk D. (2015). “Evaluation of the cytotoxic and mutagenic potential of three ginkgolic acids.” Toxicology 327: 47–52. [DOI] [PubMed] [Google Scholar]
- Bheda A, Shackelford J. and Pagano JS (2009). “Expression and functional studies of ubiquitin C-terminal hydrolase L1 regulated genes.” PLoS One 4(8): e6764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birdwell CE, Queen KJ, Kilgore PC, Rollyson P, Trutschl M, Cvek U, et al. (2014). “Genome-wide DNA methylation as an epigenetic consequence of Epstein-Barr virus infection of immortalized keratinocytes.” J Virol 88(19): 11442–11458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boulanger M, Paolillo R, Piechaczyk M. and Bossis G. (2019). “The SUMO Pathway in Hematomalignancies and Their Response to Therapies.” Int J Mol Sci 20(16). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandt M, Szewczuk LM, Zhang H, Hong X, McCormick PM, Lewis TS, et al. (2013). “Development of a high-throughput screen to detect inhibitors of TRPS1 sumoylation.” Assay Drug Dev Technol 11(5): 308–325. [DOI] [PubMed] [Google Scholar]
- Calender A, Cordier M, Billaud M. and Lenoir GM (1990). “Modulation of cellular gene expression in B lymphoma cells following in vitro infection by Epstein-Barr virus (EBV).” Int J Cancer 46(4): 658–663. [DOI] [PubMed] [Google Scholar]
- Chang LK, Lee YH, Cheng TS, Hong YR, Lu PJ, Wang JJ, et al. (2004). “Post-translational modification of Rta of Epstein-Barr virus by SUMO-1.” J Biol Chem 279(37): 38803–38812. [DOI] [PubMed] [Google Scholar]
- Chen SF, Gong C, Luo M, Yao HR, Zeng YJ and Su FX (2011). “Ubc9 expression predicts chemoresistance in breast cancer.” Chin J Cancer 30(9): 638–644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cherney BW, Sgadari C, Kanegane C, Wang F. and Tosato G. (1998). “Expression of the Epstein-Barr virus protein LMP1 mediates tumor regression in vivo.” Blood 91(7): 2491–2500. [PubMed] [Google Scholar]
- Corpas FJ, Chaki M, Fernandez-Ocana A, Valderrama R, Palma JM, Carreras A, et al. (2008). “Metabolism of reactive nitrogen species in pea plants under abiotic stress conditions.” Plant & Cell Physiology 49(11): 1711–1722. [DOI] [PubMed] [Google Scholar]
- De La Cruz-Herrera CF, Shire K, Siddiqi UZ and Frappier L. (2018). “A genome-wide screen of Epstein-Barr virus proteins that modulate host SUMOylation identifies a SUMO E3 ligase conserved in herpesviruses.” PLoS Pathog 14(7): e1007176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dirmeier U, Hoffmann R, Kilger E, Schultheiss U, Briseno C, Gires O, et al. (2005). “Latent membrane protein 1 of Epstein-Barr virus coordinately regulates proliferation with control of apoptosis.” Oncogene 24(10): 1711–1717. [DOI] [PubMed] [Google Scholar]
- Duan X, Trent JO and Ye H. (2009). “Targeting the SUMO E2 conjugating enzyme Ubc9 interaction for anti-cancer drug design.” Anticancer Agents Med Chem 9(1): 51–54. [DOI] [PubMed] [Google Scholar]
- Faumont N, Durand-Panteix S, Schlee M, Gromminger S, Schuhmacher M, Holzel M, et al. (2009). “c-Myc and Rel/NF-kappaB are the two master transcriptional systems activated in the latency III program of Epstein-Barr virus-immortalized B cells.” J Virol 83(10): 5014–5027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukuda I, Ito A, Hirai G, Nishimura S, Kawasaki H, Saitoh H, et al. (2009). “Ginkgolic acid inhibits protein SUMOylation by blocking formation of the E1-SUMO intermediate.” Chem Biol 16(2): 133–140. [DOI] [PubMed] [Google Scholar]
- Fukuda I, Ito A, Uramoto M, Saitoh H, Kawasaki H, Osada H, et al. (2009). “Kerriamycin B inhibits protein SUMOylation.” J Antibiot (Tokyo) 62(4): 221–224. [DOI] [PubMed] [Google Scholar]
- Giorgino F, de Robertis O, Laviola L, Montrone C, Perrini S, McCowen KC, et al. (2000). “The sentrin-conjugating enzyme mUbc9 interacts with GLUT4 and GLUT1 glucose transporters and regulates transporter levels in skeletal muscle cells.” Proc Natl Acad Sci U S A 97(3): 1125–1130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gires O, Kohlhuber F, Kilger E, Baumann M, Kieser A, Kaiser C, et al. (1999). “Latent membrane protein 1 of Epstein-Barr virus interacts with JAK3 and activates STAT proteins.” EMBO J 18(11): 3064–3073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregory CD, Edwards CF, Milner A, Wiels J, Lipinski M, Rowe M, et al. (1988). “Isolation of a normal B cell subset with a Burkitt-like phenotype and transformation in vitro with Epstein-Barr virus.” Int J Cancer 42(2): 213–220. [DOI] [PubMed] [Google Scholar]
- Guo WH, Yuan LH, Xiao ZH, Liu D. and Zhang JX (2011). “Overexpression of SUMO-1 in hepatocellular carcinoma: a latent target for diagnosis and therapy of hepatoma.” J Cancer Res Clin Oncol 137(3): 533–541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawkins ED, Turner ML, Wellard CJ, Zhou JH, Dowling MR and Hodgkin PD (2013). “Quantal and graded stimulation of B lymphocytes as alternative strategies for regulating adaptive immune responses.” Nat Commun 4: 2406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayakawa Y, Iwakiri T, Imamura K, Seto H. and Otake N. (1985). “Studies on the isotetracenone antibiotics. II. Kerriamycins A, B and C, new antitumor antibiotics.” J Antibiot (Tokyo) 38(7): 960–963. [DOI] [PubMed] [Google Scholar]
- He X, Riceberg J, Pulukuri SM, Grossman S, Shinde V, Shah P, et al. (2015). “Characterization of the loss of SUMO pathway function on cancer cells and tumor proliferation.” PLoS One 10(4): e0123882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He X, Riceberg J, Soucy T, Koenig E, Minissale J, Gallery M, et al. (2017). “Probing the roles of SUMOylation in cancer cell biology by using a selective SAE inhibitor.” Nat Chem Biol 13(11): 1164–1171. [DOI] [PubMed] [Google Scholar]
- Hirohama M, Kumar A, Fukuda I, Matsuoka S, Igarashi Y, Saitoh H, et al. (2013). “Spectomycin B1 as a novel SUMOylation inhibitor that directly binds to SUMO E2.” ACS Chem Biol 8(12): 2635–2642. [DOI] [PubMed] [Google Scholar]
- Huang DP, Ho JH, Poon YF, Chew EC, Saw D, Lui M, et al. (1980). “Establishment of a cell line (NPC/HK1) from a differentiated squamous carcinoma of the nasopharynx.” International Journal of Cancer 26(2): 127–132. [DOI] [PubMed] [Google Scholar]
- Katayama A, Ogino T, Bandoh N, Takahara M, Kishibe K, Nonaka S, et al. (2007). “Overexpression of small ubiquitin-related modifier-1 and sumoylated Mdm2 in oral squamous cell carcinoma: possible involvement in tumor proliferation and prognosis.” Int J Oncol 31(3): 517–524. [PubMed] [Google Scholar]
- Kerscher O. (2007). “SUMO junction-what’s your function? New insights through SUMO-interacting motifs.” EMBO Rep 8(6): 550–555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerscher O, Felberbaum R. and Hochstrasser M. (2006). “Modification of proteins by ubiquitin and ubiquitin-like proteins.” Annu Rev Cell Dev Biol 22: 159–180. [DOI] [PubMed] [Google Scholar]
- Kim YS, Keyser SG and Schneekloth JS Jr. (2014). “Synthesis of 2’,3’,4’-trihydroxyflavone (2-D08), an inhibitor of protein sumoylation.” Bioorg Med Chem Lett 24(4): 1094–1097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korkolopoulou P, Patsouris E, Pangalis G, Tsenga A, Elemenoglou J, Thomas-Tsangli E, et al. (1993). “A comparative assessment of proliferating cell nuclear antigen, c-myc p62, and nucleolar organizer region staining in non-Hodgkin’s lymphomas: a histochemical and immunohistochemical study of 200 cases.” Hum Pathol 24(4): 371–377. [DOI] [PubMed] [Google Scholar]
- Kroetz MB (2005). “SUMO: a ubiquitin-like protein modifier.” Yale J Biol Med 78(4): 197–201. [PMC free article] [PubMed] [Google Scholar]
- Lam N. and Sugden B. (2003). “LMP1, a viral relative of the TNF receptor family, signals principally from intracellular compartments.” EMBO J 22(12): 3027–3038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JS, Choi HJ and Baek SH (2017). “Sumoylation and Its Contribution to Cancer.” Adv Exp Med Biol 963: 283–298. [DOI] [PubMed] [Google Scholar]
- Li HP and Chang YS (2003). “Epstein-Barr virus latent membrane protein 1: structure and functions.” J Biomed Sci 10(5): 490–504. [DOI] [PubMed] [Google Scholar]
- Li YJ, Du L, Wang J, Vega R, Lee TD, Miao Y, et al. (2019). “Allosteric Inhibition of Ubiquitin-like Modifications by a Class of Inhibitor of SUMO-Activating Enzyme.” Cell Chem Biol 26(2): 278–288 e276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin JC (2003). “Mechanism of action of glycyrrhizic acid in inhibition of Epstein-Barr virus replication in vitro.” Antiviral Res 59(1): 41–47. [DOI] [PubMed] [Google Scholar]
- Lin JC, Cherng JM, Hung MS, Baltina LA, Baltina L. and Kondratenko R. (2008). “Inhibitory effects of some derivatives of glycyrrhizic acid against Epstein-Barr virus infection: structure-activity relationships.” Antiviral Res 79(1): 6–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin W, Yip YL, Jia L, Deng W, Zheng H, Dai W, et al. (2018). “Establishment and characterization of new tumor xenografts and cancer cell lines from EBV-positive nasopharyngeal carcinoma.” Nature communications 9(1): 4663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Xu Y, Pang Z, Guo F, Qin Q, Yin T, et al. (2015). “Knockdown of SUMO-activating enzyme subunit 2 (SAE2) suppresses cancer malignancy and enhances chemotherapy sensitivity in small cell lung cancer.” J Hematol Oncol 8: 67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu ZH and Zeng S. (2009). “Cytotoxicity of ginkgolic acid in HepG2 cells and primary rat hepatocytes.” Toxicol Lett 187(3): 131–136. [DOI] [PubMed] [Google Scholar]
- Lo AK, Lo KW, Tsao SW, Wong HL, Hui JW, To KF, et al. (2006). “Epstein-Barr virus infection alters cellular signal cascades in human nasopharyngeal epithelial cells.” Neoplasia (New York) 8(3): 173–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowrey AJ, Cramblet W. and Bentz GL (2017). “Viral manipulation of the cellular sumoylation machinery.” Cell Commun Signal 15(1): 27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luhtala N. and Parker R. (2009). “LSM1 over-expression in Saccharomyces cerevisiae depletes U6 snRNA levels.” Nucleic Acids Research 37(16): 5529–5536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lv Z, Yuan L, Atkison JH, Williams KM, Vega R, Sessions EH, et al. (2018). “Molecular mechanism of a covalent allosteric inhibitor of SUMO E1 activating enzyme.” Nat Commun 9(1): 5145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahadevan S. and Park Y. (2008). “Multifaceted therapeutic benefits of Ginkgo biloba L.: chemistry, efficacy, safety, and uses.” J Food Sci 73(1): R14–19. [DOI] [PubMed] [Google Scholar]
- Mainou BA, Everly DN and Raab-Traub N. (2005). “Epstein-Barr virus latent membrane protein 1 CTAR1 mediates rodent and human fibroblast transformation through activation of PI3K.” Oncogene 24(46): 6917–6924. [DOI] [PubMed] [Google Scholar]
- Miller RK, Qadota H, Stark TJ, Mercer KB, Wortham TS, Anyanful A, et al. (2009). “CSN-5, a component of the COP9 signalosome complex, regulates the levels of UNC-96 and UNC-98, two components of M-lines in Caenorhabditis elegans muscle.” Molecular Biology of the Cell 20(15): 3608–3616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mo YY and Moschos SJ (2005). “Targeting Ubc9 for cancer therapy.” Expert Opin Ther Targets 9(6): 1203–1216. [DOI] [PubMed] [Google Scholar]
- Mo YY, Yu Y, Shen Z. and Beck WT (2002). “Nucleolar delocalization of human topoisomerase I in response to topotecan correlates with sumoylation of the protein.” J Biol Chem 277(4): 2958–2964. [DOI] [PubMed] [Google Scholar]
- Mo YY, Yu Y, Theodosiou E, Ee PL and Beck WT (2005). “A role for Ubc9 in tumorigenesis.” Oncogene 24(16): 2677–2683. [DOI] [PubMed] [Google Scholar]
- Molesworth SJ, Lake CM, Borza CM, Turk SM and Hutt-Fletcher LM (2000). “Epstein-Barr virus gH is essential for penetration of B cells but also plays a role in attachment of virus to epithelial cells.” J Virol 74(14): 6324–6332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mond JJ and Brunswick M. (2003). “Proliferative assays for B cell function.” Curr Protoc Immunol Chapter 3: Unit 3 10. [DOI] [PubMed] [Google Scholar]
- Moschos SJ, Jukic DM, Athanassiou C, Bhargava R, Dacic S, Wang X, et al. (2010). “Expression analysis of Ubc9, the single small ubiquitin-like modifier (SUMO) E2 conjugating enzyme, in normal and malignant tissues.” Hum Pathol 41(9): 1286–1298. [DOI] [PubMed] [Google Scholar]
- Ning S, Campos AD, Darnay BG, Bentz GL and Pagano JS (2008). “TRAF6 and the three C-terminal lysine sites on IRF7 are required for its ubiquitination-mediated activation by the tumor necrosis factor receptor family member latent membrane protein 1.” Mol Cell Biol 28(20): 6536–6546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ning S, Hahn AM, Huye LE and Pagano JS (2003). “Interferon regulatory factor 7 regulates expression of Epstein-Barr virus latent membrane protein 1: a regulatory circuit.” J Virol 77(17): 9359–9368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pagano J. (2009). EBV Diseases. New York, NY, Springer. [Google Scholar]
- Pagano JS, Blaser M, Buendia MA, Damania B, Khalili K, Raab-Traub N, et al. (2004). “Infectious agents and cancer: criteria for a causal relation.” Semin Cancer Biol 14(6): 453–471. [DOI] [PubMed] [Google Scholar]
- Salahuddin S, Fath EK, Biel N, Ray A, Moss CR, Patel A, et al. (2019). “Epstein-Barr Virus Latent Membrane Protein-1 Induces the Expression of SUMO-1 and SUMO-2/3 in LMP1-positive Lymphomas and Cells.” Sci Rep 9(1): 208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selby TL, Biel N, Varn M, Patel S, Patel A, Hilding L, et al. (2019). “The Epstein-Barr Virus Oncoprotein, LMP1, Regulates the Function of SENP2, a SUMO-protease.” Sci Rep 9(1): 9523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah KM and Young LS (2009). “Epstein-Barr virus and carcinogenesis: beyond Burkitt’s lymphoma.” Clin Microbiol Infect 15(11): 982–988. [DOI] [PubMed] [Google Scholar]
- Shair KH, Bendt KM, Edwards RH, Bedford EC, Nielsen JN and Raab-Traub N. (2007). “EBV latent membrane protein 1 activates Akt, NFkappaB, and Stat3 in B cell lymphomas.” PLoS Pathog 3(11): e166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shair KH, Schnegg CI and Raab-Traub N. (2008). “EBV latent membrane protein 1 effects on plakoglobin, cell growth, and migration.” Cancer Res 68(17): 6997–7005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegers CP (1999). “Cytotoxicity of alkylphenols from Ginkgo biloba.” Phytomedicine 6(4): 281–283. [DOI] [PubMed] [Google Scholar]
- Soni V, Cahir-McFarland E. and Kieff E. (2007). “LMP1 TRAFficking activates growth and survival pathways.” Adv Exp Med Biol 597: 173–187. [DOI] [PubMed] [Google Scholar]
- Stewart MJ, Smoak K, Blum MA and Sherry B. (2005). “Basal and reovirus-induced beta interferon (IFN-beta) and IFN-beta-stimulated gene expression are cell type specific in the cardiac protective response.” J Virol 79(5): 2979–2987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takano Y, Saegusa M, Ikenaga M. and Okayasu I. (1997). “Apoptosis and proliferative activity of non-Hodgkin’s lymphomas: comparison with expression of bcl-2, p53 and c-myc proteins.” Pathol Int 47(2–3): 90–94. [DOI] [PubMed] [Google Scholar]
- Takemoto M, Kawamura Y, Hirohama M, Yamaguchi Y, Handa H, Saitoh H, et al. (2014). “Inhibition of protein SUMOylation by davidiin, an ellagitannin from Davidia involucrata.” J Antibiot (Tokyo) 67(4): 335–338. [DOI] [PubMed] [Google Scholar]
- Thorley-Lawson DA (2015). “EBV Persistence--Introducing the Virus.” Curr Top Microbiol Immunol 390(Pt 1): 151–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vertegaal AC (2007). “Small ubiquitin-related modifiers in chains.” Biochem Soc Trans 35(Pt 6): 1422–1423. [DOI] [PubMed] [Google Scholar]
- Wang FZ, Roy D, Gershburg E, Whitehurst CB, Dittmer DP and Pagano JS (2009). “Maribavir inhibits epstein-barr virus transcription in addition to viral DNA replication.” J Virol 83(23): 12108–12117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson VG and Heaton PR (2008). “Ubiquitin proteolytic system: focus on SUMO.” Expert Rev Proteomics 5(1): 121–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang W, Wang LL, Roehn G, Pearlstein RD, Ali-Osman F, Pan HJ, et al. (2013). “Small ubiquitin-like modifier 1–3 conjugation is activated in human astrocytic brain tumors and is required for glioblastoma cell survival (vol 104, pg 70, 2013).” Cancer Science 104(2): 274–274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao QQ, Liu ZH, Xu MC, Hu HH, Zhou H, Jiang HD, et al. (2017). “Mechanism for ginkgolic acid (15 : 1)-induced MDCK cell necrosis: Mitochondria and lysosomes damages and cell cycle arrest.” Chin J Nat Med 15(5): 375–383. [DOI] [PubMed] [Google Scholar]
- Yip YL, Lin W, Deng W, Jia L, Lo KW, Busson P, et al. (2018). “Establishment of a nasopharyngeal carcinoma cell line capable of undergoing lytic Epstein-Barr virus reactivation.” Laboratory Investigation 98(8): 1093–1104. [DOI] [PubMed] [Google Scholar]
- Yu B, Swatkoski S, Holly A, Lee LC, Giroux V, Lee CS, et al. (2015). “Oncogenesis driven by the Ras/Raf pathway requires the SUMO E2 ligase Ubc9.” Proc Natl Acad Sci U S A 112(14): E1724–1733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H, Kuai X, Ji Z, Li Z. and Shi R. (2013). “Over-expression of small ubiquitin-related modifier-1 and sumoylated p53 in colon cancer.” Cell Biochem Biophys 67(3): 1081–1087. [DOI] [PubMed] [Google Scholar]
- Zhang XN and Huang PC (2009). “Cell survival and death program modulated by LMP1: implication in antitumor immunity.” Ai Zheng 28(8): 831–837. [DOI] [PubMed] [Google Scholar]
- Zhao J. (2007). “Sumoylation regulates diverse biological processes.” Cell Mol Life Sci 64(23): 3017–3033. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
A) Naïve peripheral B cells, B) LPS-stimulated peripheral B cells, C) LCLs, D) Raji cells, E) BL41 EBV-negative cells, F) BL41 EBV-positive cells, G) and BL41 P3HR1 were treated with graduated doses of ML-792 and the number of live cells was determined daily at 0, 24, 46, 72, and 96 hours. Results are shown as the means ± the standard deviation of experiments and samples performed in triplicate.
A) Naïve peripheral B cells, B) LPS-stimulated peripheral B cells, C) LCLs, D) Raji cells, E) BL41 EBV-negative cells, F) BL41 EBV-positive cells, G) and BL41 P3HR1 were treated with graduated doses of ML-792 and the percentage of dead cells was determined daily at 0, 24, 46, 72, and 96 hours. Results are shown as the means ± the standard deviation of experiments and samples performed in triplicate.