Abstract
Background
Patients with cirrhosis and ascites experience frequent hospital admissions, leading to poor quality of life and high healthcare costs. Monitoring weight is a component of ascites care and telemonitoring may improve outcomes and costs.
Goals
We aimed to evaluate the cost and outcomes of current care compared to a telemonitoring system for ascites.
Study
We developed a decision-analytic model that examined 100 simulated patients over a 6-month horizon. We compared usual care to a new telemonitoring program, which we estimate costs $50,000/6 months.
Results
The cost of standard of care for 100 patients with cirrhotic ascites over a 6-month period is $167,500 more expensive than telemonitoring. By varying parameter probabilities by ± 10% and outcome costs by ± 20%, we found that standard of care remains more expensive than care with a telemonitoring intervention by $9400 to $340,200 per 6-month period. Standard of care leads to 9 more admissions (range 4 to 12) than a telemonitoring intervention, while telemonitoring leads to 9 more outpatient visits (range 6 to 9) and 28 additional outpatient large volume paracenteses (LVPs) (range 17 to 28). With more and less expensive telemonitoring interventions, standard of care remained more expensive. With 50% adherence to the intervention, standard of care was $89,848 more expensive.
Conclusions
In almost all probability and cost scenarios, a telemonitoring intervention is cost-saving for the management of cirrhotic ascites. Using hospital admissions as a surrogate for quality of care, patient outcomes are improved primarily though more proactive medical intervention and more LVPs.
Supplementary Information
The online version contains supplementary material available at 10.1007/s10620-021-07013-2.
Keywords: Healthcare delivery, Ascites, Telemedicine, Cost-effectiveness, Technology
Introduction
Patients with cirrhosis experience a large number of hospital admissions and at a significant financial cost [1–3]. Ascites is the most common reason for hospitalization [1]. Nearly one-quarter of early hospital readmissions for cirrhosis are preventable. Two of the main reasons for readmission are failure to either arrange outpatient paracentesis for symptomatic ascites or recognize warning signs of over-diuresis [4]. Data are limited, however, supporting interventions that can reduce the burden of hospitalization for patients with ascites [5–9].
Bodyweight is a useful proxy for ascites volume. Monitoring weight is therefore a component of society guidelines for ascites management and ascites treatment trials [10, 11]. Weight telemonitoring may address the two key failures of outpatient ascites management by identifying rapidly increasing or decreasing weight. Single-center studies have generated optimism for weight telemonitoring programs in patients with cirrhotic ascites, but such programs have been limited to narrow populations [7, 12].
In the present COVID-19 era, telemedicine is critical to providing access to timely and safe care. Before undertaking a new intervention with potential incremental costs, data are needed to determine the potential for benefits and global cost saving. We performed an analysis to evaluate the cost and outcomes of current care compared to care enhanced with a telemonitoring system for ascites in place.
Materials and Methods
Study Design Overview
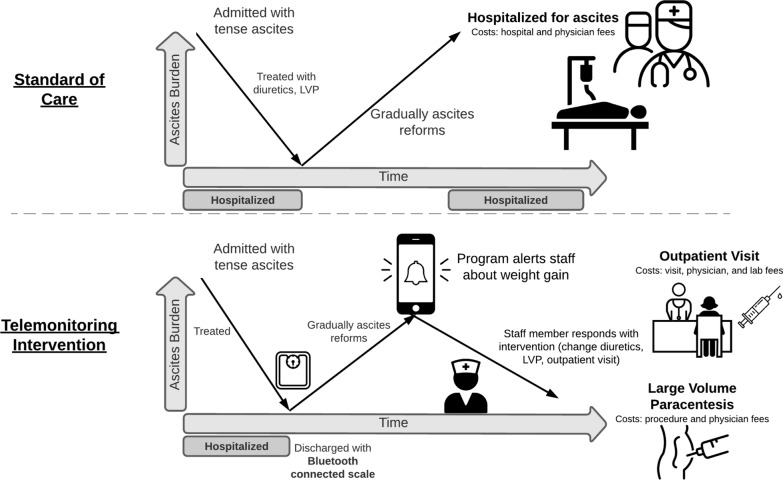
We developed a decision-analytic model for patients with cirrhotic ascites. The model examined 100 simulated patients beginning with a patient’s next encounter for ascites at a transplant referral center and progressed over a 6-month horizon. We compared usual care where patients are managed through a combination of routine visits and urgent presentations for care based on symptoms to a new telemonitoring program. The telemonitoring system tracks patients’ weight remotely through Bluetooth-enabled scales and provides automated, early alerts to providers about weight changes (Fig. 1). An administrative staff member or medical assistant enrolls patients and provides technical support. The outcomes modelled included global costs as well as the number of hospital admissions, office visits, and paracenteses. All outcomes were evaluated from the payer perspective. This study did not use patient identifying data and therefore was exempt from institutional review board review.
Fig. 1.
Potential costs of ascites management
Model Development
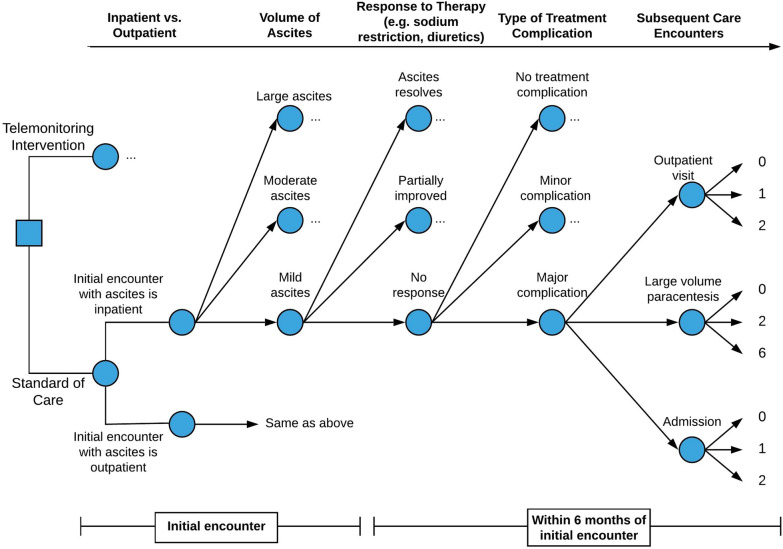
We used a combination of inputs centering on published local experience to develop a decision tree to model care processes and outcomes for patients with cirrhotic ascites (Fig. 2; Supplementary Fig. 1 for comprehensive model). Each patient began the simulation with a health-state stratified according to 4 key elements: location of the first encounter, volume of ascites, response to therapy, and treatment complications. All model inputs are presented in Table 1.
Fig. 2.
Model of care outcomes for patients with cirrhotic ascites. A decision tree modeling the 4 key elements that influence outcomes: location of first encounter, volume of ascites, response to therapy, and treatment complications. Each “…” represents identical branch points from the node below. In other words, much like “mild ascites” can resolve, partially improve, or have no response to treatment, so can moderate and large ascites
Table 1.
Model parameters in standard of care
| Type of parameter | Parameter | Probabilitya | Source | Lowb | Highb | |
|---|---|---|---|---|---|---|
| Location of first encounter | Inpatient | .20 | [13] | .10 | .30 | |
| Outpatient | .80 | .90 | .70 | |||
| Volume of ascites | Inpatient | Mild | .20 | [15] | .30 | .10 |
| Moderate | .60 | .60 | .60 | |||
| Large | .20 | .10 | .30 | |||
| Outpatient | Mild | .40 | .50 | .30 | ||
| Moderate | .50 | .50 | .50 | |||
| Large | .10 | .00 | .20 | |||
| Response to therapy | Mild | Ascites resolves | .90 | [9, 14–16] | 1.00 | .80 |
| Partially improves | .09 | .00 | .09 | |||
| No response | .01 | .00 | .11 | |||
| Moderate | Ascites resolves | .50 | .60 | .40 | ||
| Partially improves | .42 | .40 | .42 | |||
| No response | .08 | .00 | .18 | |||
| Large | Ascites resolves | .20 | .30 | .10 | ||
| Partially improves | .60 | .60 | .60 | |||
| No response | .20 | .10 | .30 | |||
| Type of treatment complication | Ascites resolves | No complication | .80 | [3, 17–19] | .90 | .70 |
| Minor complication | .15 | .10 | .15 | |||
| Major complication | .05 | .00 | .15 | |||
| Partially improves | No complication | .40 | .50 | .30 | ||
| Minor complication | .30 | .30 | .30 | |||
| Major complication | .30 | .20 | .40 | |||
| No response | No complication | .10 | .20 | .00 | ||
| Minor complication | .40 | .40 | .40 | |||
| Major complication | .50 | .40 | .60 | |||
| Subsequent care encountersc | No complication | 0, 1, 2 visits | .20, .60, .20 | [1–5, 11, 13, 20–23] | .30, .60, .10 | .10, .60, .30 |
| 0, 2, 6 LVPs | .80, .15, .05 | .90, .10, .00 | .70, .15, .15 | |||
| 0, 1, 2 admits | .90, .08, .02 | 1.00, .00, .00 | .80, .08, .12 | |||
| Minor complication | 0, 1, 2 visits | .10, .50, .40 | .20, .50, .30 | .00, .50, .50 | ||
| 0, 2, 6 LVPs | .60, .30, .10 | .70, .30, .00 | .50, .30, .20 | |||
| 0, 1, 2 admits | .80, .15, .05 | .90, .10, .00 | .70, .15, .15 | |||
| Major complication | 0, 1, 2 visits | .10, .40, .50 | .20, .40, .40 | .00, .40, .60 | ||
| 0, 2, 6 LVPs | .30, .40, .30 | .40, .40, .20 | .20, .40, .40 | |||
| 0, 1, 2 admits | 0, .50, .50 | 0, .60, .40 | 0, .40, .60 | |||
aProbability of each patient experiencing this event within a 6-month window of the first encounter
b “Low” sensitivity analysis is shifted 10% towards a less expensive outcome. “High” sensitivity analysis is shifted 10% towards a more expensive outcome
c3 probabilities on each row
Standard of Care Probabilities
The probability that a patient’s next encounter for cirrhotic ascites will be inpatient is 20% and outpatient is 80% [13]. Patients can present with mild, moderate, or large-volume ascites, which corresponds with the International Ascites Club grading system I–III [14]. Data from a two-center cohort suggest that the probability of inpatients having ascites that is mild 20%, moderate 60%, and large 20% [15]. Response to low-salt diet and diuretic treatment of ascites can be categorized as partial, complete, or no response. In the same cohort as above, even in those who developed renal failure with ascites treatment, one-quarter had complete response and one-quarter had partial response to ascites treatment [16]. Depending on the patient population, 10–30% of patients have no response to ascites diet and diuretic treatment [9, 14, 15]. Ascites treatment complications were defined as major if they required hospitalization. Recurrent ascites is the most common major complication of ascites treatment, with 23% of patients requiring re-admission for this complication within 90 days of a hospital discharge in a large nationwide cohort [3]. In patients without baseline renal failure, approximately 20% will experience adverse events on combined diuretic therapy, including electrolyte disturbance and renal injury [17]. Complications of large volume paracentesis (LVP) and transjugular intrahepatic portosystemic shunt (TIPS) are uncommon [18, 19].
Telemonitoring Intervention Probabilities
Similar to the standard of care model, we used published data to develop a decision tree to model care and outcomes for patients with cirrhotic ascites enrolled in a telemonitoring intervention. All model inputs are presented in Table 2.
Table 2.
Model parameters with telemonitoring intervention
| Type of parameter | Parameter | Probabilitya | Source | Lowb | Highb | |
|---|---|---|---|---|---|---|
| Location of first encounter | Inpatient | .20 | [13] | .10 | .30 | |
| Outpatient | .80 | .90 | .70 | |||
| Volume of ascites | Inpatient | Mild | .20 | [15] | .30 | .10 |
| Moderate | .60 | .60 | .60 | |||
| Large | .20 | .10 | .30 | |||
| Outpatient | Mild | .40 | .50 | .30 | ||
| Moderate | .50 | .50 | .50 | |||
| Large | .10 | .00 | .20 | |||
| Response to therapy | Mild | Ascites resolves | .92 | [5–9] | 1.00 | .82 |
| Partially improves | .07 | .00 | .07 | |||
| No response | .01 | .00 | .11 | |||
| Moderate | Ascites resolves | .65 | .75 | .55 | ||
| Partially improves | .30 | .35 | .30 | |||
| No response | .05 | .00 | .15 | |||
| Large | Ascites resolves | .35 | .45 | .25 | ||
| Partially improves | .50 | .50 | .50 | |||
| No response | .15 | .05 | .25 | |||
| Type of treatment complication | Ascites resolves | No complication | .82 | [5–9] | .92 | .72 |
| Minor complication | .14 | .08 | .14 | |||
| Major complication | .04 | .00 | .14 | |||
| Partially improves | No complication | .50 | .60 | .40 | ||
| Minor complication | .25 | .25 | .25 | |||
| Major complication | .25 | .15 | .35 | |||
| No response | No complication | .20 | .30 | .10 | ||
| Minor complication | .35 | .35 | .35 | |||
| Major complication | .45 | .35 | .55 | |||
| Subsequent care encounters | No complication | 0, 1, 2 visits | .10, .70, .20 | [5–9] | .20, .70, .10 | .00, .70, .30 |
| 0, 2, 6 LVPs | .70, .20, .10 | .80, .20, .00 | .60, .20, .20 | |||
| 0, 1, 2 admits | .90, .08, .02 | 1.00, .00, .00 | .80, .08, .12 | |||
| Minor complication | 0, 1, 2 visits | .05, .45, .50 | .15, .45, .40 | .00, .40, .60 | ||
| 0, 2, 6 LVPs | .50, .35, .15 | .60, .35, .05 | .40, .35, .25 | |||
| 0, 1, 2 admits | .83, .13, .04 | .93, .07, .00 | .73, .13, .14 | |||
| Major complication | 0, 1, 2 visits | .05, .37, .58 | .15, .37, .48 | .00, .32, .68 | ||
| 0, 2, 6 LVPs | .20, .45, .35 | .30, .45, .25 | .10, .45, .45 | |||
| 0, 1, 2 admits | 0, .58, .42 | .10, .58, .32 | 0, .48, .52 | |||
aProbability of each patient experiencing this event within a 6-month window of the first encounter
b “Low” sensitivity analysis is 10% lower than the predicted probability. “High” sensitivity analysis is 10% higher than the predicted probability
c3 probabilities on each row
One study of 40 patients with enhanced case management and hepatology nursing support post-discharge increased outpatient attendance by 30% but did not reduce readmissions [6]. Another group studied 40 patients with cirrhotic ascites enrolled in an intensive multi-disciplinary outpatient monitoring program, complete with a day hospital and proactive titration of diuretics [9]. By comparison standard of care, 30-day readmission was reduced by nearly one-third (15.4% vs. 42.4%). Another intensive outpatient monitoring program with a day hospital followed 80 patients with cirrhotic ascites and reduced early readmission by one-third (11.3% vs. 29.5%) [5]. Finally, a 25-patient pilot study of a remote weight monitoring program from our group found an 11% readmission rate for ascites, though a much higher rate for all-cause readmission [7]. We predict that a telemonitoring program specifically for patients undergoing ascites management, without day hospital or other ancillary supports, would likely fall somewhere between the outcomes of these four programs: increasing outpatient care utilization by approximately 15% and decreasing inpatient care utilization by approximately 15%.
Outcomes
Outcomes included hospitalization, outpatient visits, and paracenteses. All outcomes were modelled as counts with respect to their contribution to global costs. Cost data were estimated in 2019 US dollars from Medicare reimbursements using diagnosis-related group (DRG) and fee schedule data based on Current Procedural Terminology (CPT) codes for 2019. We found that the average bundled cost of an outpatient visit is $202 and for an inpatient stay is $26,006 (Table 3).
Table 3.
Cost parameters
| Cost parameter | Cost ($) | Source | Low ($)a | High ($)b |
|---|---|---|---|---|
| Itemized costs | ||||
| Hospitalization for cirrhosis complication | 25,633 | FY’18 DRG 432, 433, 434 | 20,506 | 30,759 |
| Inpatient physician fee (initial) | 220 | Medicare fee schedule CPT 99,223 | – | – |
| Inpatient physician fee (subsequent) | 113 | Medicare fee schedule CPT 99,233 | – | – |
| Outpatient visit (subsequent) | 162 | Medicare fee schedule CPT 99,214, 99,215 | – | – |
| Outpatient paracentesis (procedure code) | 347 | Medicare fee schedule CPT 49,083 | – | – |
| Outpatient paracentesis (provider fee) | 1272 | Medicare fee schedule | – | – |
| Laboratory panel (basic metabolic panel, complete blood count, liver biochemistries) | 40 | MGH standard charges | – | – |
| Bundled costsb | ||||
| Outpatient visit | 202 | – | – | – |
| Hospitalization | 26,006 | – | 20,804 | 31,207 |
| LVP | 1619 | – | – | – |
a “Low” sensitivity analysis is 20% lower than the predicted cost. “High” sensitivity analysis is 20% more expensive
bOutpatient visit included one set of labs and subsequent office visit fee. Hospitalization includes DRG cost, 2 initial consultations, and 2 subsequent visits per day for 7 days
In current practice, approximately three-quarters of patients have an outpatient follow visit within 6 months [13]. A large cohort of patients with a new diagnosis of cirrhotic ascites required an average 2.6 LVPs per patient per year [20]. Several large studies report readmission rates of decompensated cirrhosis at approximately 20% at 30 days and 30% at 90 days [1, 3–5, 13, 20–23]. In addition, patients with decompensated cirrhosis are often requiring more than one readmission within a 6-month period [2, 4].
There is insufficient evidence that a telemonitoring intervention would influence rates or timing of liver transplantation or death. Given this, we did not include liver transplantation or death in our model.
Model Analysis
We used Microsoft Excel to construct the model. We included a cost of $50,000 for 6 months of the telemonitoring intervention to cover staffing and supplies. We then performed a sensitivity analysis adjusting parameter probabilities by ± 10% and costs by ± 20%. We also performed sensitivity analyses varying the cost of and adherence to the telemonitoring intervention.
Results
The cost of standard of care for 100 patients with cirrhotic ascites over a 6-month period is $1,221,500 based on the most likely event probabilities, an average bundled outpatient visit cost of $202, outpatient LVP cost of $1,619 each, and admission costs of $26,006 (Table 4). The cost of care with a telemonitoring intervention was $167,500 less expensive than standard of care, using the most likely probabilities of events, the same costs as standard of care for outpatient and inpatient encounters, and a $50,000 cost for 6 months of the telemonitoring intervention. We then varied parameter probabilities by ± 10% and outcome costs by ± 20% and found that standard of care remained more expensive than care with a telemonitoring intervention by $9400–$340,200.
Table 4.
Model outputs for 100 patients in 6 months
| Model | Cost ($) | Admissions (#) | Outpatient visits (#) | Outpatient paracenteses (#) |
|---|---|---|---|---|
| Standard of care | 1,221,500 | 37 | 112 | 105 |
| Telemonitoring interventiona | 1,054,000 | 28 | 121 | 133 |
| Incremental change | − 167,500 | − 9 | + 9 | + 28 |
| Sensitivity analyses | ||||
| Low probability, low cost | ||||
| Standard of care | 225,900 | 7 | 86 | 33 |
| Telemonitoring interventiona | 216,500 | 3 | 95 | 50 |
| Incremental change | − 9400 | − 4 | + 9 | + 17 |
| Low probability, high cost | ||||
| Standard of care | 303,800 | 7 | 86 | 33 |
| Telemonitoring interventiona | 250,500 | 3 | 95 | 50 |
| Incremental change | − 53,300 | − 4 | + 9 | + 17 |
| High probability, low cost | ||||
| Standard of care | 2,088,000 | 77 | 140 | 201 |
| Telemonitoring interventiona | 1,891,100 | 65 | 146 | 225 |
| Incremental change | − 196,900 | − 12 | + 6 | + 24 |
| High probability, high cost | ||||
| Standard of care | 2,954,800 | 77 | 140 | 201 |
| Telemonitoring interventiona | 2,614,600 | 65 | 146 | 225 |
| Incremental change | − 340,200 | − 12 | + 6 | + 24 |
aSalary for a full time a junior administrative staff member to staff the telemonitoring program ($50,000) was added to the cost of the telemonitoring intervention in every analysis
Standard of care led to 9 more admissions in a 6-month period than a telemonitoring intervention, while telemonitoring led to 9 more outpatient visits and 28 additional outpatient LVPs in the same period. We then varied parameter probabilities by ± 10% and standard of care still led to more hospital admissions (range 4 to 12), fewer outpatient visits (range 6 to 9), and fewer outpatient paracenteses (range 17 to 28).
We then performed a sensitivity analysis varying the cost of the telemonitoring intervention. With a less expensive telemonitoring intervention ($25,000/6-month period), standard of care was more expensive than telemonitoring by $192,500 (range $34,400 to $365,200). With a more expensive telemonitoring intervention ($100,000/6-month period), standard of care was more expensive than telemonitoring by $117,500, though with varying parameter probabilities by ± 10% and outcome costs by ± 20% the range included − $40,600 to $290,200.
We then performed a sensitivity analysis varying adherence to the intervention. With 50% adherence to the intervention, modeled using parameter probabilities halfway between those predicted for standard of care and telemonitoring intervention, we found that the standard of care was $89,848 more expensive, led to 5 more admissions, 5 fewer outpatient visits, and 11 fewer LVPs.
Discussion
In this study, we evaluated the potential change in cost and outcomes with utilization of a telemonitoring system for ascites. Compared to current practice, a telemonitoring intervention could save a third-party payer $167,500 over 6 months for every 100 patients and prevent 9 hospital admissions. Even in sensitivity analyses of different probability and cost scenarios, a telemonitoring intervention is likely to be cost-saving and leads to improved outcomes for patients with cirrhosis and ascites.
A telemonitoring intervention reduces inpatient care by expanding opportunities for outpatient care. The few published programs increasing outpatient care monitoring in cirrhosis patients have increased the volume of outpatient care [6, 7, 9]. In our model, a telemonitoring system could increase the number of outpatient visits by 9 per 100 patients (range: 6–9) and LVPs by 28 (range: 17–28) per 100 patients over 6 months. In order for a telemonitoring intervention to succeed, outpatient resources would need to be responsive to the alerts and have the capacity to accommodate this increase in utilization.
Cost savings are not the only potential benefits from a telemonitoring program for ascites. Prior studies have found that patients with cirrhotic ascites and a weight gain or loss of more than 5 lb have a significantly higher rate of readmission [12]. Hospital admissions are associated with poor quality of life broadly as well as specifically in patients with cirrhosis [24]. Any intervention which could reduce hospital admissions would also likely increase quality of life.
Our model accounts for most of the cost of the telemonitoring intervention by including staff salary and smart scales. In our experience, a telemonitoring program can be managed by a junior administrative staff member or medical assistant. The other costs not included in our model are any additional staffing, if needed, to manage the additional outpatient care volume. Finally, programs could offer to provide home smart equipment such as phones or tablets to patients as part of the intervention. We performed a sensitivity analysis with a more expensive telemonitoring intervention costing $100,000 every 6 months, and in most probability and cost scenarios, the telemonitoring intervention remained cost saving.
Healthcare delivery interventions, including telemedicine, do not have perfect adherence in the real world. Even in a sensitivity analysis with 50% adherence, the telemonitoring intervention was cost saving and improved quality outcomes.
Our study used Medicare cost data to approximate the transactional costs from a societal perspective. Costs for each institution will vary and with each patient and payer. Hospitals or systems who receive less than their operational expenses may find the cost savings from a telemedicine intervention even more compelling. In addition, we used the cost of outpatient LVP performed by interventional radiology because that is the most common situation at our institution, though LVP cost may vary depending on location and staff performing this procedure.
Our model is focused on ascites and its potential complications. Competing events such as hepatic encephalopathy, liver transplantation, and death are not included. There is very limited data regarding how telemonitoring would influence those outcomes, and including them would increase uncertainty about model outputs. We also did not compare telemonitoring to other interventions such as increasing frequency of outpatient visits. This model also does not take into account patients with end-stage renal disease or congestive heart failure, in whom a weight monitoring program may not provide as much benefit. Finally, pilot data suggest that providers do not experience alert fatigue with 1–3 months of patient enrollment, but provider adherence may wane with longer enrollment [7]. As preliminary data become available in the future, these outcomes should be included.
In conclusion, care for patients with cirrhotic ascites was enhanced by telemedicine and was less expensive for each scenario analyzed. Using hospital admissions as a surrogate for quality of care, patient outcomes were improved primarily though more proactive medical intervention and more LVPs. Telemedicine is emerging as a critical part of ambulatory care in the present COVID-19 era providing access to timely and safe care this analysis suggests that it is cost effective too.
Supplementary Information
Below is the link to the electronic supplementary material.
Abbreviations
- LVP
Large volume paracentesis
- TIPS
Transjugular intrahepatic portosystemic shunt
- DRG
Diagnosis-related group
- CPT
Current procedural terminology
Declarations
Conflict of interest
Dr. Bloom receives funding from the AASLD Advanced Transplant Hepatology Award and ACG Junior Faculty Award and has consulted for Synlogic Inc. Dr. Tapper receives funding from the National Institutes of Health (K23DK117055, KL2TR002241). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. Dr. Tapper serves on advisory boards for Bausch Health, Rebiotix, and Mallinckrodt, consulted for Axcella, Kaleido, and Novo Nordisk. No other author has relevant conflict of interest.
Footnotes
An editorial commenting on this article is available at 10.1007/s10620-021-07020-3.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Patricia P. Bloom, Email: ppbloom@med.umich.edu
Martin Ventoso, Email: mventoso15@gmail.com.
Elliot Tapper, Email: etapper@med.umich.edu.
Jasmine Ha, Email: jha3@mgh.harvard.edu.
James M. Richter, Email: jrichter@mgh.harvard.edu
References
- 1.Tapper EB, Halbert B, Mellinger J. Rates of and reasons for hospital readmissions in patients with cirrhosis: a multistate population-based cohort study. Clin Gastroenterol Hepatol. 2016;14(8):1181–8.e2. doi: 10.1016/j.cgh.2016.04.009. [DOI] [PubMed] [Google Scholar]
- 2.Bajaj JS, Reddy KR, Tandon P, Wong F, Kamath PS, Garcia-Tsao G, et al. The 3-month readmission rate remains unacceptably high in a large North American cohort of patients with cirrhosis. Hepatology. 2016;64(1):200–208. doi: 10.1002/hep.28414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shaheen AA, Nguyen HH, Congly SE, Kaplan GG, Swain MG. Nationwide estimates and risk factors of hospital readmission in patients with cirrhosis in the United States. Liver Int. 2019;39(5):878–884. doi: 10.1111/liv.14054. [DOI] [PubMed] [Google Scholar]
- 4.Volk ML, Tocco RS, Bazick J, Rakoski MO, Lok AS. Hospital readmissions among patients with decompensated cirrhosis. Am J Gastroenterol. 2012;107(2):247–252. doi: 10.1038/ajg.2011.314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Morales BP, Planas R, Bartoli R, Morillas RM, Sala M, Casas I, et al. HEPACONTROL. A program that reduces early readmissions, mortality at 60 days, and healthcare costs in decompensated cirrhosis. Dig Liver Dis. 2018;50(1):76–83. doi: 10.1016/j.dld.2017.08.024. [DOI] [PubMed] [Google Scholar]
- 6.Wigg AJ, McCormick R, Wundke R, Woodman RJ. Efficacy of a chronic disease management model for patients with chronic liver failure. Clin Gastroenterol Hepatol. 2013;11(7):850–858. doi: 10.1016/j.cgh.2013.01.014. [DOI] [PubMed] [Google Scholar]
- 7.Bloom P, Wang T, Marx M, Tagerman M, Green B, Arvind A, et al. A smartphone app to manage cirrhotic ascites among outpatients: feasibility study. JMIR Med Inform. 2020;8(9):e17770. doi: 10.2196/17770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Siddique SM, Porges S, Lane-Fall M, Mehta SJ, Schweickert W, Kinniry J, et al. Reducing hospital admissions for paracentesis: a quality improvement intervention. Clin Gastroenterol Hepatol. 2019;17(13):2630–3.e2. doi: 10.1016/j.cgh.2019.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Morando F, Maresio G, Piano S, Fasolato S, Cavallin M, Romano A, et al. How to improve care in outpatients with cirrhosis and ascites: a new model of care coordination by consultant hepatologists. J Hepatol. 2013;59(2):257–264. doi: 10.1016/j.jhep.2013.03.010. [DOI] [PubMed] [Google Scholar]
- 10.Runyon BA. Introduction to the revised American Association for the Study of Liver Diseases Practice Guideline management of adult patients with ascites due to cirrhosis 2012. Hepatology. 2013;57(4):1651–1653. doi: 10.1002/hep.26359. [DOI] [PubMed] [Google Scholar]
- 11.Pericleous M, Sarnowski A, Moore A, Fijten R, Zaman M. The clinical management of abdominal ascites, spontaneous bacterial peritonitis and hepatorenal syndrome: a review of current guidelines and recommendations. Eur J Gastroenterol Hepatol. 2016;28(3):e10–e18. doi: 10.1097/MEG.0000000000000548. [DOI] [PubMed] [Google Scholar]
- 12.Thomson M, Volk M, Kim HM, Piette JD. An automated telephone monitoring system to identify patients with cirrhosis at risk of re-hospitalization. Dig Dis Sci. 2015;60(12):3563–3569. doi: 10.1007/s10620-015-3744-3. [DOI] [PubMed] [Google Scholar]
- 13.Seraj SM, Campbell EJ, Argyropoulos SK, Wegermann K, Chung RT, Richter JM. Hospital readmissions in decompensated cirrhotics: factors pointing toward a prevention strategy. World J Gastroenterol. 2017;23(37):6868–6876. doi: 10.3748/wjg.v23.i37.6868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Moore KP, Wong F, Gines P, Bernardi M, Ochs A, Salerno F, et al. The management of ascites in cirrhosis: report on the consensus conference of the International Ascites Club. Hepatology. 2003;38(1):258–266. doi: 10.1053/jhep.2003.50315. [DOI] [PubMed] [Google Scholar]
- 15.Planas R, Montoliu S, Ballesté B, Rivera M, Miquel M, Masnou H, et al. Natural history of patients hospitalized for management of cirrhotic ascites. Clin Gastroenterol Hepatol. 2006;4(11):1385–1394. doi: 10.1016/j.cgh.2006.08.007. [DOI] [PubMed] [Google Scholar]
- 16.Montoliu S, Ballesté B, Planas R, Alvarez MA, Rivera M, Miquel M, et al. Incidence and prognosis of different types of functional renal failure in cirrhotic patients with ascites. Clin Gastroenterol Hepatol. 2010;8(7):616–622. doi: 10.1016/j.cgh.2010.03.029. [DOI] [PubMed] [Google Scholar]
- 17.Angeli P, Fasolato S, Mazza E, Okolicsanyi L, Maresio G, Velo E, et al. Combined versus sequential diuretic treatment of ascites in non-azotaemic patients with cirrhosis: results of an open randomised clinical trial. Gut. 2010;59(1):98–104. doi: 10.1136/gut.2008.176495. [DOI] [PubMed] [Google Scholar]
- 18.Pache I, Bilodeau M. Severe haemorrhage following abdominal paracentesis for ascites in patients with liver disease. Aliment Pharmacol Ther. 2005;21(5):525–529. doi: 10.1111/j.1365-2036.2005.02387.x. [DOI] [PubMed] [Google Scholar]
- 19.Adebayo D, Neong SF, Wong F. Ascites and hepatorenal syndrome. Clin Liver Dis. 2019;23(4):659–682. doi: 10.1016/j.cld.2019.06.002. [DOI] [PubMed] [Google Scholar]
- 20.Le S, Spelman T, Chong CP, Ha P, Sahhar L, Lim J, et al. Could adherence to quality of care indicators for hospitalized patients with cirrhosis-related ascites improve clinical outcomes? Am J Gastroenterol. 2016;111(1):87–92. doi: 10.1038/ajg.2015.402. [DOI] [PubMed] [Google Scholar]
- 21.Peery AF, Crockett SD, Barritt AS, Dellon ES, Eluri S, Gangarosa LM, et al. Burden of gastrointestinal, liver, and pancreatic diseases in the United States. Gastroenterology. 2015;149(7):1731–41.e3. doi: 10.1053/j.gastro.2015.08.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Scaglione SJ, Metcalfe L, Kliethermes S, Vasilyev I, Tsang R, Caines A, et al. Early hospital readmissions and mortality in patients with decompensated cirrhosis enrolled in a large national health insurance administrative database. J Clin Gastroenterol. 2017;51(9):839–844. doi: 10.1097/MCG.0000000000000826. [DOI] [PubMed] [Google Scholar]
- 23.Gaspar R, Rodrigues S, Silva M, Costa-Moreira P, Morais R, Andrade P, et al. Predictive models of mortality and hospital readmission of patients with decompensated liver cirrhosis. Dig Liver Dis. 2019;51(10):1423–1429. doi: 10.1016/j.dld.2019.03.016. [DOI] [PubMed] [Google Scholar]
- 24.Kok B, Whitlock R, Ferguson T, Bailey RJ, Burak KW, Kowalczewski J, et al. Health-related quality of life: a rapid predictor of hospitalization in patients with cirrhosis. Am J Gastroenterol. 2020;115(4):575–583. doi: 10.14309/ajg.0000000000000545. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.