Abstract
Background:
Smoking cessation represents an opportunity to reduce both short and long-term effects of smoking on complications after lumbar fusion and smoking-related morbidity and mortality. However, the cost-effectiveness of smoking-cessation interventions prior to lumbar fusion is not fully known.
Methods:
We created a decision-analytic Markov model to evaluate the cost-effectiveness of 5 smoking-cessation strategies (behavioral counseling, nicotine replacement therapy [NRT], bupropion or varenicline monotherapy, and a combined intervention) prior to single-level, instrumented lumbar posterolateral fusion (PLF) from the health payer perspective. Probabilities, costs, and utilities were obtained from published sources. We calculated the costs and quality-adjusted life years (QALYs) associated with each strategy over multiple time horizons and accounted for uncertainty with probabilistic sensitivity analyses (PSAs) consisting of 10,000 second-order Monte Carlo simulations.
Results:
Every smoking-cessation intervention was more effective and less costly than usual care at the lifetime horizon. In the short term, behavioral counseling, NRT, varenicline monotherapy, and the combined intervention were also cost-saving, while bupropion monotherapy was more effective but more costly than usual care. The mean lifetime cost savings for behavioral counseling, NRT, bupropion monotherapy, varenicline monotherapy, and the combined intervention were $3,291 (standard deviation [SD], $868), $2,571 (SD, $479), $2,851 (SD, $830), $6,767 (SD, $1,604), and $34,923 (SD, $4,248), respectively. The minimum efficacy threshold (relative risk for smoking cessation) for lifetime cost savings varied from 1.01 (behavioral counseling) to 1.15 (varenicline monotherapy). A PSA revealed that the combined smoking-cessation intervention was always more effective and less costly than usual care.
Conclusions:
Even brief smoking-cessation interventions yield large short-term and long-term cost savings. Smoking-cessation interventions prior to PLF can both reduce costs and improve patient outcomes as health payers/systems shift toward value-based reimbursement (e.g., bundled payments) or population health models.
Level of Evidence:
Economic Level II. See Instructions for Authors for a complete description of levels of evidence.
Smoking is the leading cause of preventable morbidity and mortality in the U.S., with 500,000 premature deaths each year attributed to smoking and with annual economic costs approaching $300 billion1,2. This has led to the Surgeon General’s endorsement of cost-effective smoking-cessation strategies and the promotion of broader insurance coverage of smoking cessation3. The period prior to elective surgery represents a “teachable moment” that may be especially conducive to, and synergistic with, such interventions in improving short and long-term outcomes. It is estimated that 3.6 million spinal fusions were performed in the U.S. between 2001 and 2010, at a cost of over $287 billion4. Posterolateral fusion (PLF) with instrumentation of the lumbar spine is the most common spinal fusion procedure, with a high societal cost burden5. Consequently, there have been many efforts to improve its value (i.e., reducing the cost relative to the benefits). For example, applying bundled-payment models to lumbar fusion to stimulate efforts to improve value is under discussion, highlighting the importance of reducing costs and complications6–11.
The adverse effects of smoking, mediated by oxidative stress and reduced tissue blood flow12, on orthopaedic procedures that rely on wound-healing and bone growth (e.g., fracture repair, joint arthroplasties, spinal fusion) are well-documented12–19. These include delayed union, nonunion, and infection17,19–21. Smoking cessation, especially in the 4 weeks preceding surgery, was associated with better outcomes, lower morbidity, and the promotion of bone-healing12,20,22–24. For example, structured preoperative smoking-cessation programs were demonstrated to reduce postoperative complications, including infections, with cost savings after total joint arthroplasty25–27. In a retrospective study, Glassman et al. reported a nonunion rate of 17.1% among patients who quit smoking for >6 months following instrumented posterior lumbar fusion compared with 26.5% among patients who continued to smoke24. Recent data also suggest that elective surgery provides a strong impetus for behavioral change, providing an opportunity for intervention using established care pathways28,29.
Reducing preventable costs and complications of lumbar fusion is in the interest of patients, surgeons, health payers, and health systems alike, as incentives to reduce costs and improve outcomes also grow. Smoking cessation represents an opportunity to reduce both the short-term complications of lumbar fusion and long-term morbidity and mortality associated with smoking. However, the value (i.e., benefits in relation to costs) of performing a preoperative smoking-cessation intervention has not been fully determined. A cost-effectiveness analysis of smoking-cessation interventions prior to lumbar fusion would help to define the value, and once demonstrated, can assist in encouraging the implementation of care pathways for smoking cessation to improve patient outcomes and promoting fair compensation for surgeons and health systems that perform such interventions. In this study, we performed a cost-effectiveness analysis of smoking-cessation interventions prior to PLF on multiple time horizons.
Materials and Methods
Decision-Analytic Model
Using TreeAge Pro software, we created a decision-analytic model to compare smoking-cessation interventions versus usual care from the health payer perspective (Fig. 1–A). In the usual-care arm, patients did not receive a formal intervention but could quit smoking on their own. We modeled 5 smoking-cessation interventions: (1) behavioral counseling, consisting of 2 brief counseling sessions (3 to 10 minutes); (2) nicotine replacement therapy (NRT), consisting of the use of nicotine patches and gum (6-week course); (3) bupropion monotherapy (12-week course); (4) varenicline monotherapy (12-week course); and (5) a combined intervention, consisting of 2 brief counseling sessions, 2 long counseling sessions (>10 minutes), and a 6-week course of nicotine patches and gum. We employed Markov modeling to represent transitions between health states, with a cycle length of 1 month (Fig. 1–B). Model validation is presented in the Appendix, including Table S1.
Fig. 1-A.
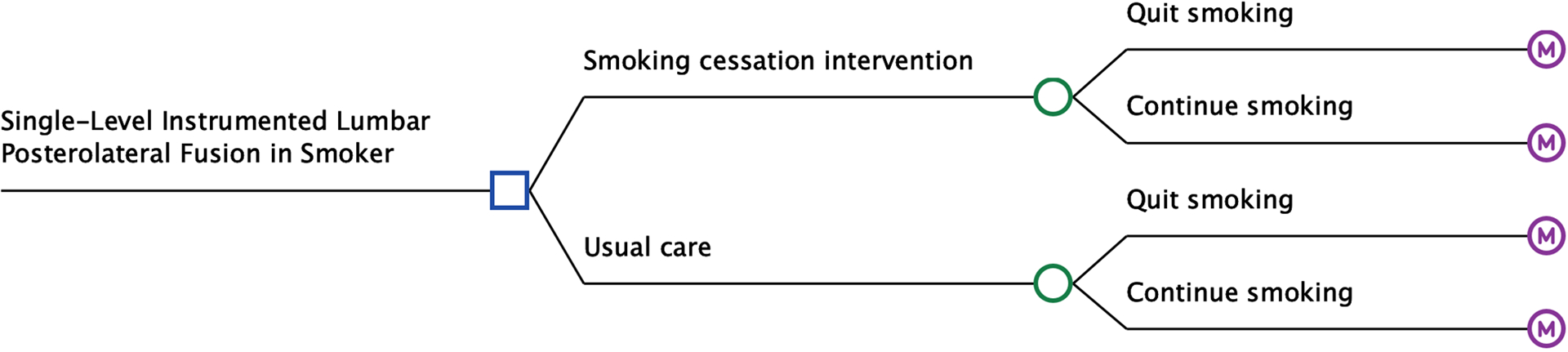
Decision-analytic model. The square represents a decision node, circles represent chance nodes, and the letter “M” represents Markov nodes.
Fig. 1-B.
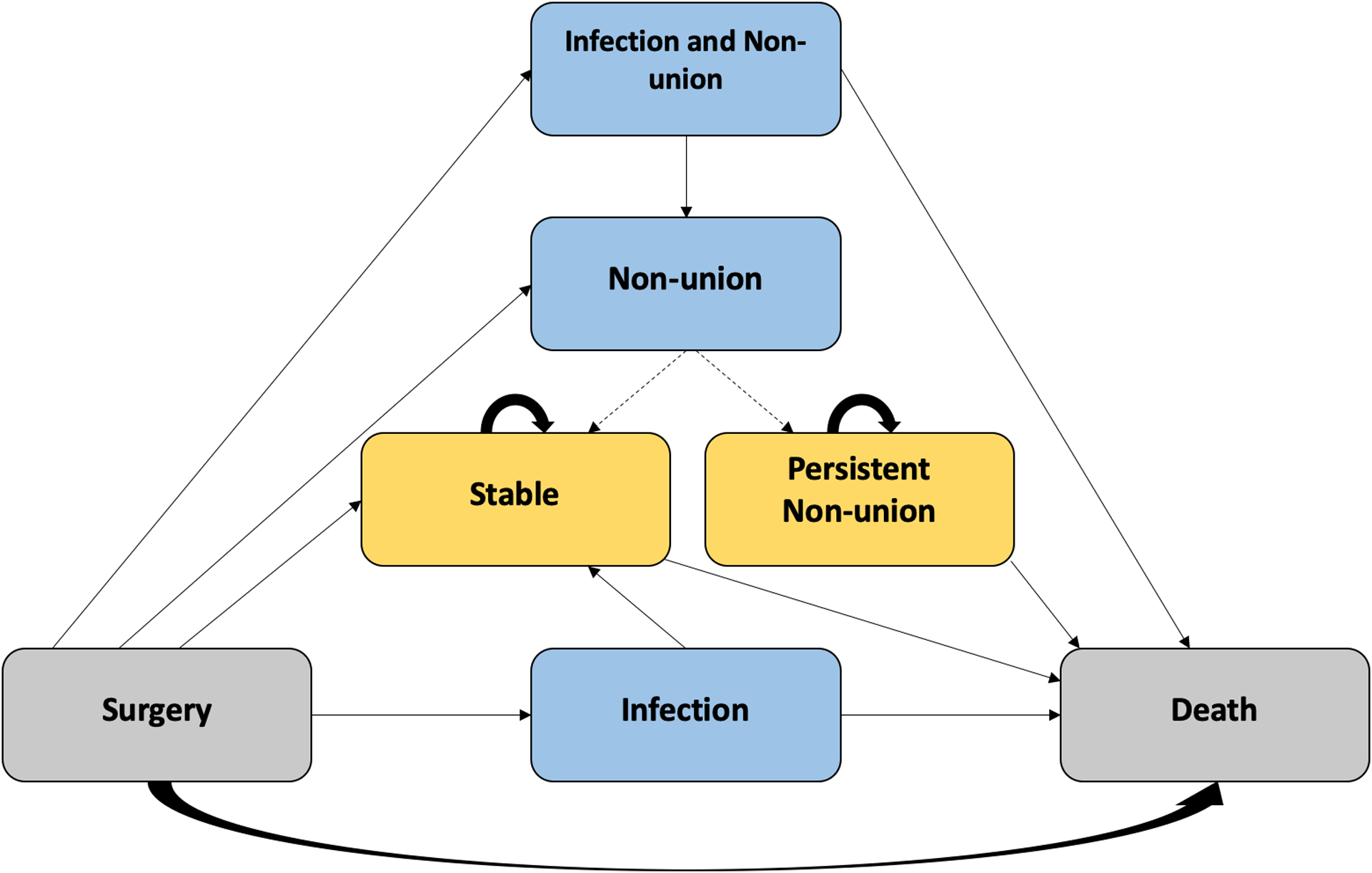
Scheme of a Markov node. Gray boxes represent entry and absorbing states (surgery or death). Blue boxes represent temporary health states (infection and/or nonunion). Gold boxes represent long-term health states (stable postoperatively or persistent nonunion). Arrows signify the possible transitions between health states. A dashed arrow signifies that a transition is cycle-dependent, i.e., does not occur every cycle. Briefly, postoperative health states known to differ between smokers and those who quit smoking are modeled, including deep wound infection, nonunion, comorbid deep wound infection and nonunion, no complications (stable postoperative state), and death. In the model, patients with a deep wound infection are treated with irrigation and debridement, and either return to the stable postoperative state or die from the complication. In patients with a nonunion, revision surgery is attempted after 6 months. If unsuccessful, a second revision is attempted after 6 months. If unsuccessful, patients transition to the persistent nonunion state. Patients with comorbid deep wound infection and nonunion are first treated for their infection.
Our base case is a 45-year-old male smoker undergoing single-level instrumented lumbar PLF. We adopted a lifetime horizon in the base-case analysis, in which the model progresses until all patients are deceased. When published estimates of model inputs differed, we used corresponding values from the same study, where possible, or chose the median value. All costs and benefits were discounted at 3% annually. Several key assumptions were made: (1) for the purposes of this study, we assumed that there were no other short-term differences in outcomes between smokers and those who stopped smoking (“quitters”) than those modeled, (2) smoking relapse rates are negligible (we relaxed this assumption in a sensitivity analysis), (3) the risk of infection attributable to smoking is readily reversible30,31, (4) on the basis of existing evidence32, there were no permanent health effects from a treated deep infection, and (5) patients experiencing complications do not refuse further treatment.
Probabilities
Probabilities with uncertainty estimates were obtained from published sources (Table I). Table S2 (see Appendix) shows ratings of evidence quality for each input. Successful smoking cessation was defined as complete abstinence at 6 months. While rates of nonunion and deep wound infection are known to differ between smokers and those who stop smoking18, it is unknown whether success rates of revision surgery differ. Thus, we assumed that these rates were equal in the base case and varied them in sensitivity analyses. Similarly, we assumed that there was no difference in mortality from deep wound infection or surgery between smokers and those who stopped smoking in the base case. Because of the low incidence of deep wound infections, the odds ratio (OR) was approximated as the relative risk (RR). Other ORs were corrected to RRs using established methods33. We obtained baseline mortality rates from U.S. life tables34. The risk of death was represented as the product of these mortality rates and age/sex-specific hazard ratios (HRs).
TABLE I.
Model Input Parameters*
| Variable | Estimate† | Reference(s) | |
|---|---|---|---|
| Probabilities | Baseline, smoking cessation without intervention | 8% | 27, 54 |
| RR with behavioral intervention alone | 1.66 (1.42–1.94) | 46 | |
| RR with nicotine replacement therapy‡ | 1.55 (1.49–1.61) | 45 | |
| Corrected RR with bupropion monotherapy‡ | 1.71 (1.53–1.90) | 44 | |
| Corrected RR with varenicline monotherapy‡ | 2.50 (2.16–2.90) | 44 | |
| Smoking cessation with combined intervention | 77/120 | 27, 55 | |
| Nonunion | |||
| Smoker | 18/68 | 24 | |
| Quitter | 13/76 | 24 | |
| Deep infection in quitter | 36/1,615 | 56–59 | |
| OR of deep infection in smoker | 2.33 (1.02–5.32) | ||
| Successful correction of nonunion | |||
| First attempt | 71/86 | 60 | |
| Subsequent attempt | 6/15 | 60 | |
| Mortality from deep infection | 1.06% | 59 | |
| Mortality from surgery | 0.5% | 59, 61 | |
| Baseline, age-specific annual mortality | 2017 CDC life tables | 34 | |
| HR for mortality in smoker, male | 2.8 (2.4–3.1) | 62 | |
| HR for mortality in smoker, female | 3.0 (2.7–3.3) | 62 | |
| HR for mortality in 35-year-old quitter, male | 1.1 (1.0–1.4) | 62 | |
| HR for mortality in 35-year-old quitter, female | 1.3 (1.1–1.6) | 62 | |
| HR for mortality in 45-year-old quitter, male | 1.5 (1.2–1.7) | 62 | |
| HR for mortality in 45-year-old quitter, female | 1.6 (1.3–1.9) | 62 | |
| HR for mortality in 55-year-old quitter, male | 1.7 (1.4–2.0) | 62 | |
| HR for mortality in 55-year-old quitter, female | 1.8 (1.5–2.1) | 62 | |
| Annual probability of smoking relapse | 10% | 52 | |
| Costs | Smoking-cessation intervention | ||
| Extended counseling session (>10 min) | $28.83 | CMS | |
| Abbreviated counseling session (3–10 min) | $15.14 | CMS | |
| Nicotine patch (14-day supply) | $19.09 | FSS | |
| Nicotine gum starter kit (110 pieces) | $24.02 | FSS | |
| Varenicline starter pack (0.5 mg × 11, 1 mg × 42) | $254.62 | FSS | |
| Varenicline continuing pack (1 mg × 56) | $254.62 | FSS | |
| Bupropion (150 mg × 60) | $239.13 | FSS | |
| Single-level instrumented posterolateral fusion | $24,095 ($10,862) | 5, 41 | |
| Revision surgery | $41,448 ($13,198) | 5, 40, 41 | |
| Deep infection treatment§ | |||
| Hospital reimbursement | $61,866 ($37,148) | 37–39 | |
| Physician reimbursement | $987.47 | CMS | |
| Baseline annual smoking-attributable health costs (age/sex-specific)§ | |||
| Smoker | See table in reference | 63 | |
| Quitter | See table in reference | 63 | |
| Utilities | Deep infection | 0.459 | 64 |
| Nonunion | 0.552 | 64 | |
| Stable, postoperative | 41 | ||
| Baseline | 0.500 | ||
| Year 1 | 0.621 | ||
| Year 2 | 0.638 | ||
| Year 3 | 0.630 | ||
| Year 4 | 0.646 | ||
| Year 5 | 0.653 | ||
| Stable, long-term (age/sex-specific) | |||
| Moderate smoker# | See table in reference | 42 | |
| Quitter | See table in reference | 42 |
RR = relative risk, OR = odds ratio, CDC = Centers for Disease Control and Prevention, HR = hazard ratio, CMS = Centers for Medicare & Medicaid Services, and FSS = Federal Supply Schedule.
Where available in the literature, uncertainty estimates are provided in parentheses as either standard deviations (e.g., for costs) or ranges (e.g., for RRs and HRs). Range includes either 95% or 99% confidence intervals, as given in the cited literature.
Almost all trials comparing pharmacotherapies included some form of behavioral support, e.g., counseling.
Widely differing costs are reported in the literature. #10 to 19 cigarettes per day.
Costs
Costs were obtained from the 2019 Federal Supply Schedule (FSS)35, Centers for Medicare & Medicaid Services (CMS)36, or published estimates and were inflation-adjusted to current value. Notably, cost estimates for treating deep wound infections after lumbar fusion vary substantially, from as low as $4,067 to considerably higher estimates37,38. Since some studies suggest that deep wound infections lead to a multifold increase in total costs39,40, we used the higher estimate.
Utilities
Utilities were obtained from published sources that used established methods for measuring health-related quality of life (e.g., Short Form-6 Dimensions [SF-6D], EuroQol-5 Dimensions [EQ-5D], and Oswestry Disability Index [ODI]). Postoperative utilities for up to 5 years after single-level instrumented lumbar PLF and long-term age and sex-specific utilities for moderate smokers and those who quit smoking are known41,42. Thus, the stable postoperative health state was modeled with postoperative utility values for 5 years, and then transitioned to long-term utilities for moderate smokers and quitters. The multiplicative method was used for comorbid conditions.
Sensitivity Analyses
For 1-way sensitivity analyses, we varied model inputs within their 95% or 99% confidence intervals (CIs). We varied inputs without measures of uncertainty from 50% to 150% of their base value. We also performed probabilistic sensitivity analyses (PSAs) consisting of 10,000 second-order Monte Carlo simulations, performed with sampling from the uncertainty distributions of model parameters. Table S3 in the Appendix shows the ranges used for model parameters and PSA specifications. Separate PSAs were performed for each smoking-cessation intervention. For costs without uncertainty estimates, we assumed a coefficient of variation equal to 0.5.
Results
In the base-case analysis, the combined smoking-cessation intervention was both less costly and more effective than usual care across all time horizons examined (Table II). The cost savings and effectiveness of behavioral counseling, NRT, and bupropion or varenicline monotherapy at multiple time horizons are shown in Table III. A comparison of various strategies revealed that the combined intervention offered the greatest benefit and the most cost savings (Fig. 2). Thresholds above which the cost of intervention is no longer cost-saving (i.e., the cost-saving threshold) are shown in Table IV. Notably, the immediate costs of bupropion were high relative to its longer-term benefits, such that it was both more effective and more costly than usual care on non-lifetime horizons, with incremental cost-effectiveness ratios of $1,268,075 per quality-adjusted life year (QALY) for the 1-year horizon, $374,705 per QALY for the 2-year horizon, and $12,393 per QALY for the 5-year horizon. We also identified the minimum efficacy thresholds for each individual intervention to be cost-saving (Table IV). Table V shows cost savings by age, sex, and time horizon for the combined smoking-cessation intervention. Younger patients stood to gain more QALYs from smoking cessation than did older patients. For example, the combined intervention resulted in a gain of 1.61 QALYs versus usual care for a 35-year-old male smoker compared with 1.06 QALYs for a 55-year-old male smoker.
TABLE II.
Costs and Effectiveness by Time Horizon for Combined Intervention
| Time Horizon | Strategy | Effectiveness (QALYs) | Cost | Cost Savings |
|---|---|---|---|---|
| Lifetime | Usual care | 12.539 | $125,249 | — |
| Smoking cessation | 13.811 | $90,424 | $34,825 | |
| 1 yr | Usual care | 0.550 | $41,331 | — |
| Smoking cessation | 0.552 | $37,226 | $4,105 | |
| 2 yr | Usual care | 1.137 | $42,858 | — |
| Smoking cessation | 1.142 | $38,073 | $4,785 | |
| 5 yr | Usual care | 2.809 | $46,824 | — |
| Smoking cessation | 2.830 | $40,278 | $6,546 |
TABLE III.
Cost Savings and Effectiveness of Other Smoking-Cessation Interventions by Time Horizon
| Cohort | Time Horizon | Cost Savings* | QALYs Gained* |
|---|---|---|---|
| Behavioral counseling | 1 yr | $384 | |
| 2 yr | $448 | ||
| 5 yr | $615 | ||
| Lifetime | $3,295 | 0.12 | |
| Nicotine replacement therapy | 1 yr | $165 | |
| 2 yr | $218 | ||
| 5 yr | $356 | ||
| Lifetime | $2,573 | 0.10 | |
| Bupropion monotherapy† | Lifetime | $2,844 | 0.13 |
| Varenicline monotherapy | 1 yr | $174 | |
| 2 yr | $320 | ||
| 5 yr | $698 | ||
| Lifetime | $6,769 | 0.27 |
Compared with usual care.
Bupropion monotherapy was more effective but more costly than usual care at the 1, 2, and 5-year time horizons.
Fig. 2.
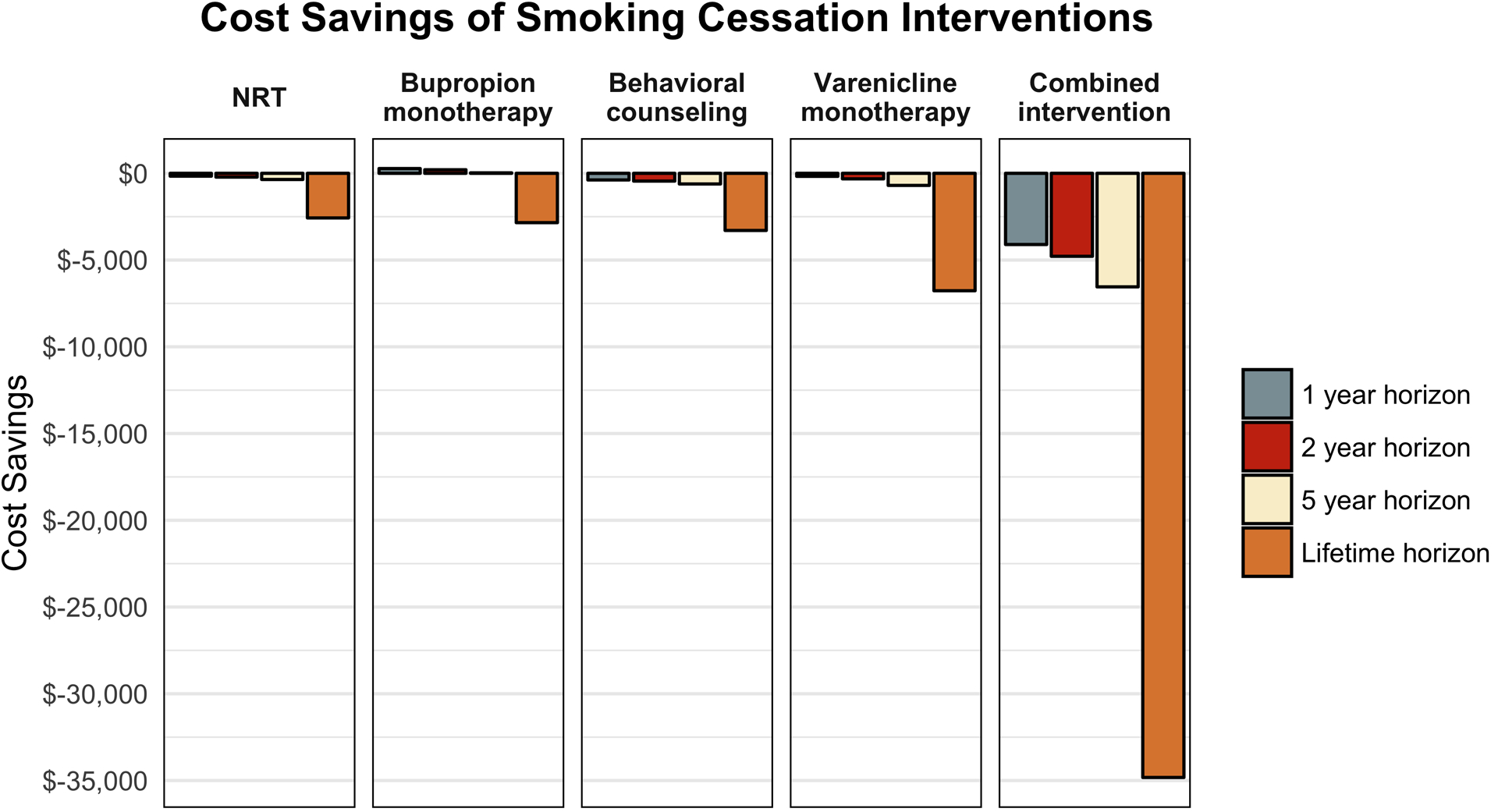
Cost savings of smoking-cessation interventions by time horizon. The combined intervention yielded the greatest cost savings across all time horizons. NRT = nicotine replacement therapy.
TABLE IV.
Cost-Saving and Minimum Efficacy Thresholds for Smoking-Cessation Interventions*
| Intervention | Cost-Saving Threshold | Minimum Efficacy RR Threshold | ||||||
|---|---|---|---|---|---|---|---|---|
| 1 Yr | 2 Yr | 5 Yr | Lifetime | 1 Yr | 2 Yr | 5 Yr | Lifetime | |
| Behavioral counseling | $414 | $478 | $645 | $3,325 | 1.05 | 1.04 | 1.03 | 1.01 |
| Nicotine replacement therapy | $342 | $396 | $534 | $2,750 | 1.28 | 1.25 | 1.18 | 1.04 |
| Bupropion monotherapy | NA | NA | NA | $3,562 | NA | NA | NA | 1.14 |
| Varenicline monotherapy | $938 | $1,084 | $1,462 | $7,532 | 2.23 | 2.06 | 1.79 | 1.15 |
| Combined intervention | $4,370 | $5,050 | $6,811 | $35,090 | 1.43 | 1.37 | 1.27 | 1.05 |
RR = relative risk, and NA = not applicable.
TABLE V.
Cost Savings by Age, Sex, and Time Horizon for the Combined Intervention
| Cohort | Time Horizon | Cost Savings |
|---|---|---|
| 35-yr-old male | 1 yr | $4,111 |
| 2 yr | $4,795 | |
| 5 yr | $6,579 | |
| Lifetime | $28,897 | |
| 35-yr-old female | 1 yr | $4,174 |
| 2 yr | $4,919 | |
| 5 yr | $6,875 | |
| Lifetime | $26,218 | |
| 45-yr-old female | 1 yr | $4,166 |
| 2 yr | $4,907 | |
| 5 yr | $6,837 | |
| Lifetime | $29,213 | |
| 55-yr-old male | 1 yr | $5,596 |
| 2 yr | $7,708 | |
| 5 yr | $13,288 | |
| Lifetime | $44,804 | |
| 55-yr-old female | 1 yr | $5,110 |
| 2 yr | $6,760 | |
| 5 yr | $11,077 | |
| Lifetime | $35,559 |
A 1-way sensitivity analysis of model inputs revealed that the smoking-cessation intervention was always more effective and less costly than usual care, although with varying cost savings, over the lifetime horizon (see Appendix Figures S1 through S5). Intervention efficacy and annual smoking-attributable health costs had large influences on cost savings. At the lowest estimate of intervention efficacy (RR = 1.42), the lifetime cost savings ranged from $1,335 for varenicline monotherapy to $2,069 for behavioral counseling. At the highest estimate of intervention efficacy (RR = 12), the lifetime cost savings ranged from $54,215 for varenicline monotherapy to $54,948 for behavioral counseling. As annual smoking-attributable health costs for smokers were varied from 50% to 150% of their base value, the lifetime cost savings of an intervention ranged from $9,617 to $60,033. Similarly, as the annual smoking-attributable health costs for those who stopped smoking were varied from 50% to 150% of their base value, the lifetime cost savings of an intervention ranged from $44,365 to $25,285. Even when the rate of nonunion for smokers was equal to that of quitters, all smoking-cessation interventions yielded lifetime cost savings.
Our PSA showed that the combined smoking-cessation intervention was consistently more effective and less costly than usual care across 10,000 Monte Carlo simulations (Fig. 3). At the lifetime horizon, the combined smoking-cessation intervention was favored 100% of the time, and the mean cost savings was $34,923 (standard deviation [SD], $4,248). The mean cost savings for behavioral counseling, NRT, bupropion monotherapy, and varenicline monotherapy were $3,291 (SD, $868), $2,571 (SD, $479), $2,851 (SD, $830), and $6,767 (SD, $1,604), respectively. Figure 4 shows the distribution of cost savings over 10,000 Monte Carlo simulations for each smoking-cessation intervention. Finally, when we allowed postoperative relapse of smoking at an annual rate of 10%, the mortality benefit of intervention decreased (Fig. 5), but all interventions were still more effective than usual care. The mean lifetime cost savings for the combined intervention, behavioral counseling, NRT, bupropion monotherapy, and varenicline monotherapy in the model permitting relapse were $9,236 (SD, $3,449), $863 (SD, $388), $571 (SD, $294), $250 (SD, $534), and $1,286 (SD, $914), respectively.
Fig. 3.
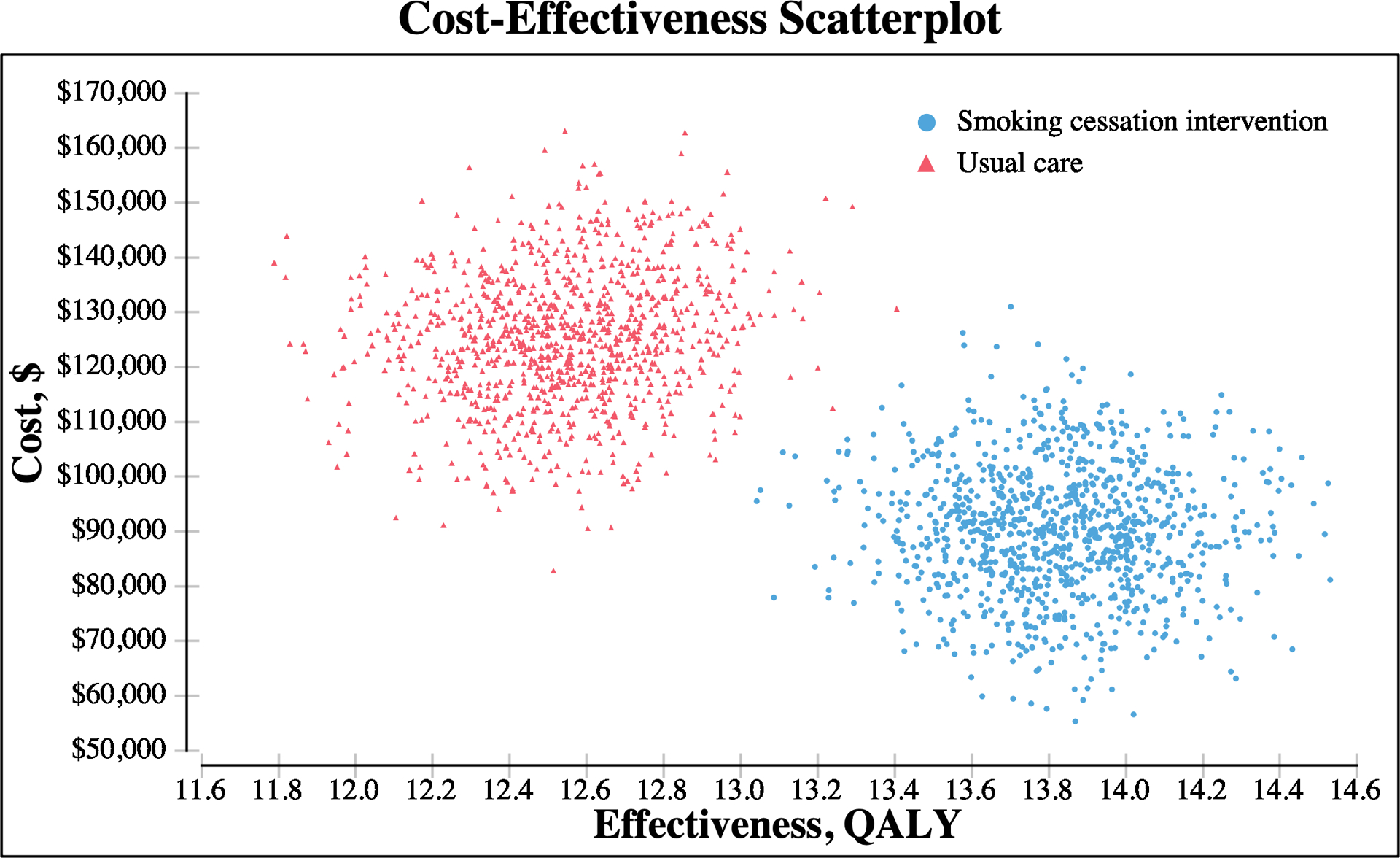
Cost-effectiveness scatterplot of 10,000 Monte Carlo simulations comparing smoking-cessation intervention and usual care. The smoking-cessation intervention was consistently more effective and less costly than usual care over the lifetime horizon.
Fig. 4.
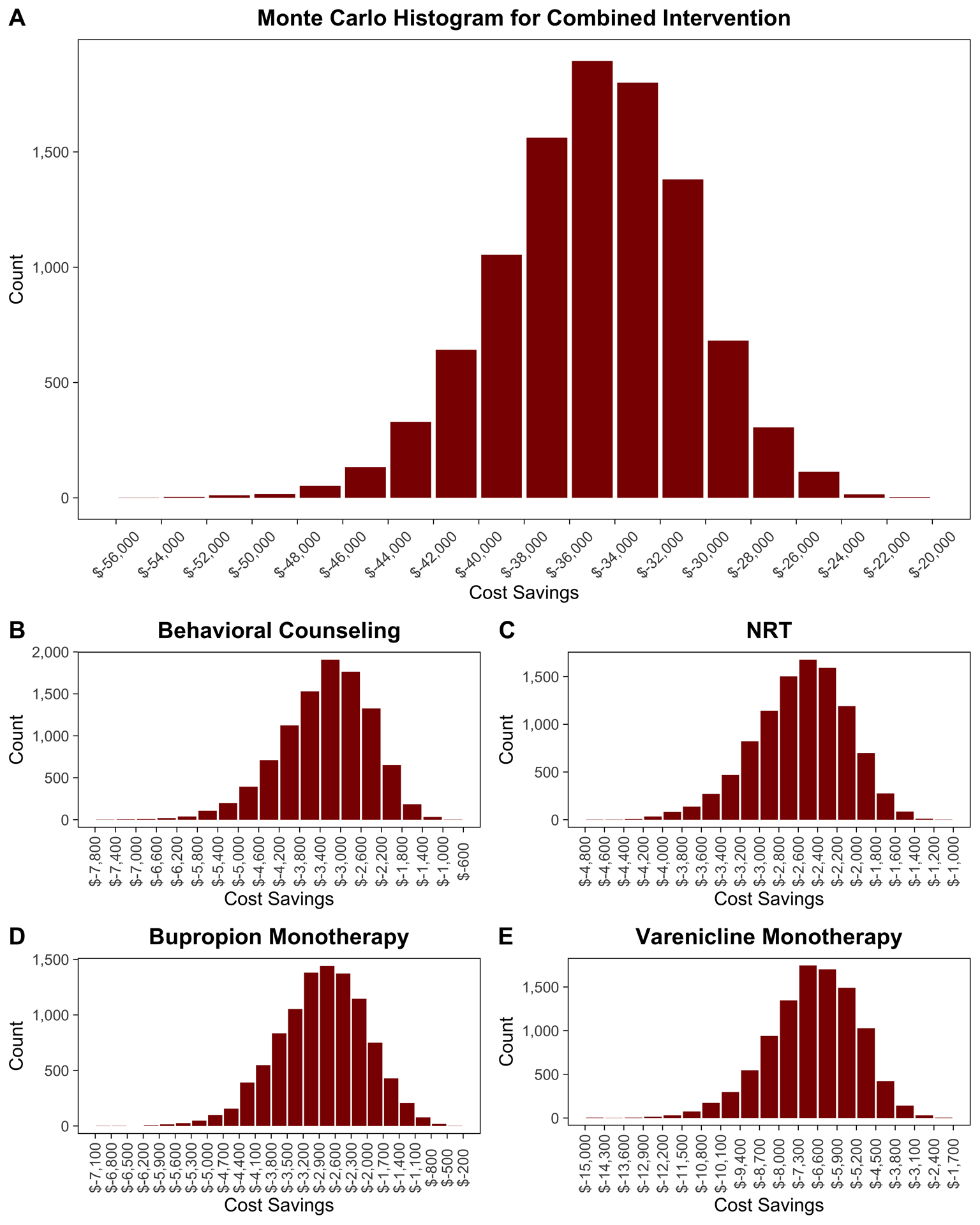
Distributions of cost savings for the combined intervention (Fig. 4-A), behavioral counseling (Fig. 4-B), nicotine-replacement therapy (NRT) (Fig. 4-C), bupropion monotherapy (Fig. 4-D), and varenicline monotherapy (Fig. 4-E) compared with usual care across 10,000 Monte Carlo simulations.
Fig. 5.
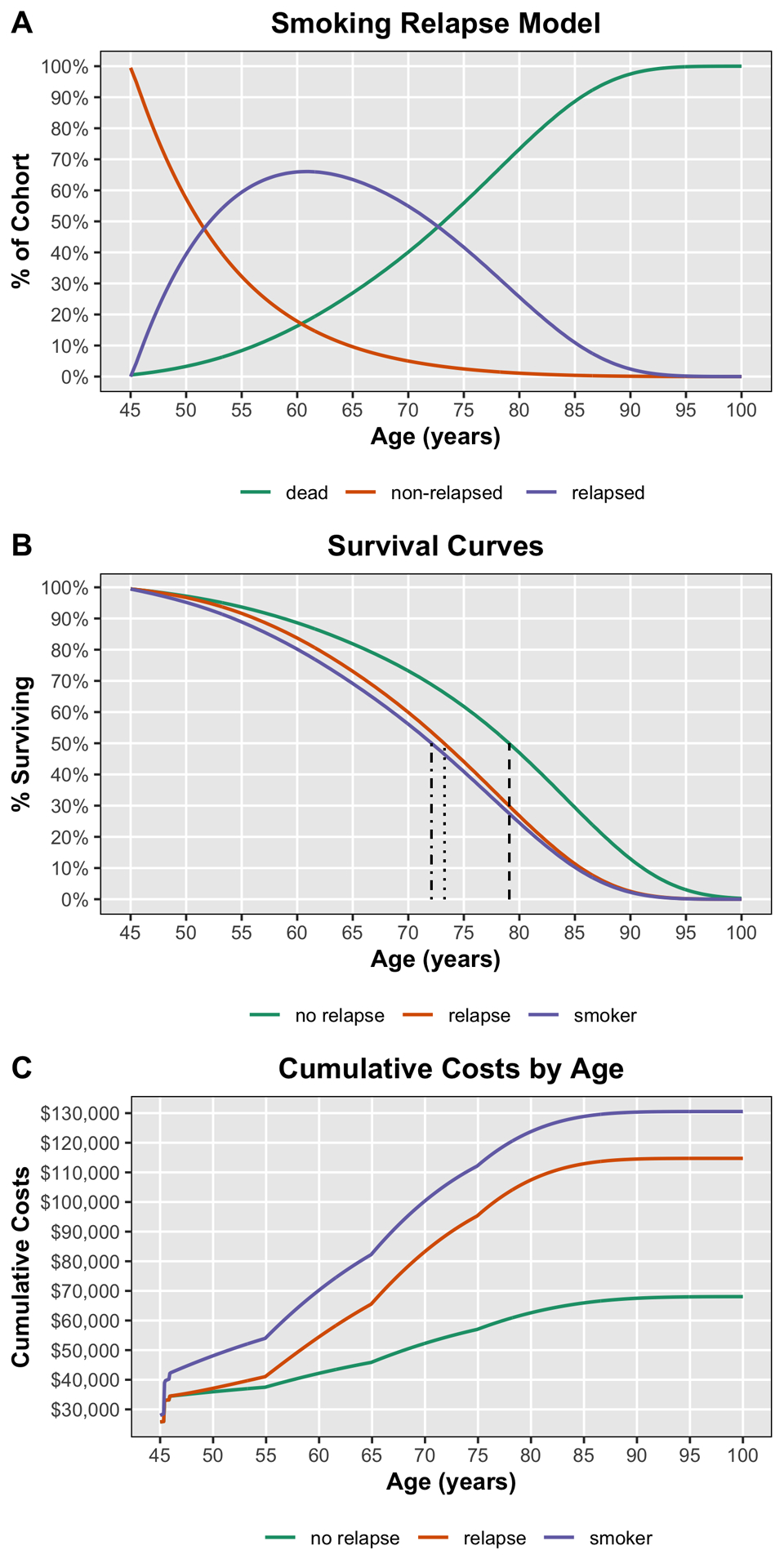
Smoking relapse Markov model for the combined intervention. Fig. 5-A Percentage of cohort in the indicated states (non-relapsed, relapsed, and dead) over a lifespan. Fig. 5-B Survival curves for those who quit smoking and did not relapse (no relapse), quitters who relapsed (relapse), and those who did not quit smoking (smoker). Median survival times were 79.1 years (dashed line), 73.3 years (dotted line), and 72.1 years (dotted-dashed line), respectively. Fig. 5-C Cumulative costs over a lifespan for those who quit smoking and did not relapse (no relapse), quitters who relapsed (relapse), and those who did not quit smoking (smoker).
Discussion
Our findings demonstrate that various smoking-cessation interventions prior to lumbar PLF are both more effective and less costly than usual care across a wide range of time horizons and variation in model inputs. We quantified the cost savings for each smoking-cessation intervention compared with usual care and provided comprehensive estimates of uncertainty using a PSA. Our results support the use of a structured smoking-cessation intervention prior to lumbar fusion, which could yield substantial cost savings of interest to surgeons, health systems, and health payers. These results bolster the case for the development and implementation of lumbar fusion care pathways that include preoperative smoking-cessation interventions.
A recent cost-effectiveness analysis of a smoking-cessation intervention prior to total joint arthroplasty found a net cost savings in the 90-day postoperative period due to avoided periprosthetic infections27. Surgical site infections after spine procedures are likewise considered by CMS to be largely preventable43. As interest increases in bundled payment for lumbar fusion and as health systems increasingly embrace episode-based reimbursement models6,7, the financial burden of surgical complications will likely shift to hospitals and surgeons, stimulating the implementation of cost-effective measures to reduce their incidence. Our results demonstrate that preoperative smoking-cessation interventions could be a valuable tool for accomplishing that goal. Although we chose to use longer time horizons in our study to adequately capture all of the benefits of smoking cessation, the combined smoking-cessation intervention would still have been cost-saving (savings of $952) had we only included the costs and consequences of deep wound infections in our model, which approximates a theoretical 90-day bundle horizon.
Furthermore, our results contain implications for health payers, whose time horizons are on the order of year(s) since they are responsible for the short and potentially long-term health costs of beneficiaries. There was a substantial cost savings for smoking-cessation interventions across all time horizons examined. In other words, the initial cost of the intervention, ranging from $177 for NRT to $764 for varenicline monotherapy, is a relatively small investment leading to large short-term and long-term returns. The sole exception was bupropion monotherapy, which was not cost-saving over the short term. However, its cost-effectiveness ratio for the 5-year time horizon is under accepted cost-effectiveness thresholds. The lifetime cost-saving thresholds shown in Table IV exceed the costs of the interventions themselves by at least 4-fold. Therefore, payers may begin to encourage the use of smoking-cessation interventions prior to lumbar fusion, increase reimbursement for such interventions, and/or direct patients to centers with dedicated programs for preoperative smoking cessation.
There remains variation in the literature regarding the efficacy of smoking-cessation interventions, which likely depend on the characteristics of the study populations. For example, whereas multiple systematic reviews reported modest success rates with various behavioral and pharmacologic interventions44–46, a recent study indicated that elective surgery itself may provide a strong ancillary incentive to quit smoking, with a reported abstinence rate of 64% (9 of 14) 6 months after total joint arthroplasty28. Nevertheless, our results reveal that even interventions with modest success rates over usual care are cost-saving (Table IV). Acknowledging the time constraints of surgeons running busy orthopaedic practices, our sensitivity analysis suggests that offering even brief advice to quit (e.g., a 1-time intervention of <10 minutes in duration, with no follow-up), which has a modest success rate47, could yield large potential cost savings in the health-care system. Moreover, clinical care pathways have been developed to streamline and implement smoking-cessation programs for elective orthopaedic procedures, capitalizing on the unique role of the orthopaedic surgeon. For example, after identifying a current smoker, the surgeon can provide brief counseling and a referral to a smoking-cessation specialist (e.g., clinical psychologist) for subsequent counseling and pharmacotherapy29.
Our study should be interpreted in context of its limitations. First, we did not model a scenario in which PLF is postponed when smoking-cessation attempts are unsuccessful. While highlighting the potential financial attractiveness of a positive approach to smoking cessation (i.e., implementing smoking-cessation programs), our results do not imply that a negative approach (i.e., postponing surgery) would be beneficial and should not be construed as a motive for delaying surgery. Any delay would impose a toll in the form of reduced quality of life and potentially cause worse outcomes that must be balanced against the possible benefits. Also, our model may not contain all relevant outcomes after PLF. For example, we did not include the increased risk of cardiopulmonary complications among smokers, which would further increase the cost savings of smoking cessation38,48–50. Additional high-quality studies on the effects of smoking cessation on outcomes after PLF would better inform future cost-effectiveness analyses. We also did not attempt to quantify alterations in surgical technique that some surgeons might use for smokers, such as increased use of bone morphogenetic protein or circumferential surgery51, since these alternatives may not be consistent between surgeons. Furthermore, our model assumed that all cases of nonunion were treated surgically, whereas some patients may be relatively asymptomatic and treated nonoperatively. Nonetheless, our sensitivity analyses showed that the difference in nonunion rates between smokers and those who stopped smoking was less influential than expected, as smoking cessation was more effective and less costly than usual care even if nonunion rates did not differ. Additionally, definitions of smoking intensity in the literature are inconsistent, and many studies on outcomes do not explicitly quantify it, rendering it difficult to examine whether cost savings vary by smoking intensity. Although the quantity smoked does not appear to affect nonunion rates24, our results should be viewed as an average effect across smokers undergoing PLF since we could not conduct separate analyses by smoking intensity. Finally, although we modeled a 10% annual smoking relapse rate based on meta-analysis data52, another study found lower rates (2% to 4%) that declined over time53. Thus, our base and relapse models represent upper and lower bounds on cost savings.
In conclusion, our study demonstrated that smoking-cessation interventions are more effective and cost-saving compared with usual care over multiple time horizons. Our characterization of the magnitude of cost savings offered by these interventions provides a framework to guide efforts by surgeons, health payers, and health systems to reduce preventable morbidity and health-care costs attributable to smoking after PLF. Smoking-cessation programs as part of care pathways can represent a minimal burden on surgeons and hold potential for long-term cost savings and health benefits.
Supplementary Material
Disclosure:
One author (R.N.K.) was supported by a National Institutes of Health K23AR073307-01 award and an Orthopaedic Research and Education Foundation (OREF) Mentored Clinician Scientist Grant. The funding sources played no role in the investigation. The Disclosure of Potential Conflicts of Interest forms are provided with the online version of the article (http://links.lww.com/JBJS/G151).
Footnotes
Investigation performed at the VOICES Health Policy Research Center, Department of Orthopaedic Surgery, Stanford University, Redwood City, California
Appendix
Supporting material provided by the authors is posted with the online version of this article as a data supplement at jbjs.org (http://links.lww.com/JBJS/G152).
References
- 1.U.S. Department of Health and Human Services. The health consequences of smoking - 50 years of progress. A report of the Surgeon General. U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health; 2014. Accessed 2020 Jul 18. https://www.ncbi.nlm.nih.gov/books/NBK179276/pdf/Bookshelf_NBK179276.pdf [Google Scholar]
- 2.Carter BD, Abnet CC, Feskanich D, Freedman ND, Hartge P, Lewis CE, Ockene JK, Prentice RL, Speizer FE, Thun MJ, Jacobs EJ. Smoking and mortality—beyond established causes. N Engl J Med. 2015. February 12;372(7):631–40. [DOI] [PubMed] [Google Scholar]
- 3.U.S. Department of Health and Human Services. Smoking cessation: a report of the Surgeon General. U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health; 2020. Accessed 2020 Jul 18. https://www.hhs.gov/sites/default/files/2020-cessation-sgr-full-report.pdf [Google Scholar]
- 4.Goz V, Weinreb JH, McCarthy I, Schwab F, Lafage V, Errico TJ. Perioperative complications and mortality after spinal fusions: analysis of trends and risk factors. Spine. 2013. October 15;38(22):1970–6. [DOI] [PubMed] [Google Scholar]
- 5.Saifi C, Cazzulino A, Laratta J, Save AV, Shillingford JN, Louie PK, Pugely AJ, Arlet V. Utilization and economic impact of posterolateral fusion and posterior/transforaminal lumbar interbody fusion surgeries in the United States. Global Spine J. 2019. April;9(2):185–90. Epub 2018 Aug 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dietz N, Sharma M, Alhourani A, Ugiliweneza B, Wang D, Nuño MA, Drazin D, Boakye M. Bundled payment models in spine surgery: current challenges and opportunities, a systematic review. World Neurosurg. 2019. March;123:177–83. Epub 2018 Dec 12. [DOI] [PubMed] [Google Scholar]
- 7.Sullivan R, Jarvis LD, O’Gara T, Langfitt M, Emory C. Bundled payments in total joint arthroplasty and spine surgery. Curr Rev Musculoskelet Med. 2017. June;10(2):218–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ugiliweneza B, Kong M, Nosova K, Huang KT, Babu R, Lad SP, Boakye M. Spinal surgery: variations in health care costs and implications for episode-based bundled payments. Spine. 2014. July 1;39(15):1235–42. [DOI] [PubMed] [Google Scholar]
- 9.Kazberouk A, McGuire K, Landon BE. A survey of innovative reimbursement models in spine care. Spine. 2016. February;41(4):344–52. [DOI] [PubMed] [Google Scholar]
- 10.Martin BI, Lurie JD, Farrokhi FR, McGuire KJ, Mirza SK. Early effects of Medicare’s Bundled Payment for Care Improvement program for lumbar fusion. Spine. 2018. May 15;43(10):705–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bronson WH, Kingery MT, Hutzler L, Karia R, Errico T, Bosco J, Bendo JA. Lack of cost savings for lumbar spine fusions after Bundled Payments for Care Improvement Initiative: a consequence of increased case complexity. Spine. 2019. February 15;44(4):298–304. [DOI] [PubMed] [Google Scholar]
- 12.Truntzer J, Vopat B, Feldstein M, Matityahu A. Smoking cessation and bone healing: optimal cessation timing. Eur J Orthop Surg Traumatol. 2015. February;25(2):211–5. Epub 2014 May 31. [DOI] [PubMed] [Google Scholar]
- 13.Argintar E, Triantafillou K, Delahay J, Wiesel B. The musculoskeletal effects of perioperative smoking. J Am Acad Orthop Surg. 2012. June;20(6):359–63. [DOI] [PubMed] [Google Scholar]
- 14.Patel RA, Wilson RF, Patel PA, Palmer RM. The effect of smoking on bone healing: a systematic review. Bone Joint Res. 2013. June 14;2(6):102–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Møller AM, Pedersen T, Villebro N, Munksgaard A. Effect of smoking on early complications after elective orthopaedic surgery. J Bone Joint Surg Br. 2003. March;85(2):178–81. [DOI] [PubMed] [Google Scholar]
- 16.Al-Hadithy N, Sewell MD, Bhavikatti M, Gikas PD. The effect of smoking on fracture healing and on various orthopaedic procedures. Acta Orthop Belg. 2012. June;78(3):285–90. [PubMed] [Google Scholar]
- 17.Duchman KR, Gao Y, Pugely AJ, Martin CT, Noiseux NO, Callaghan JJ. The effect of smoking on short-term complications following total hip and knee arthroplasty. J Bone Joint Surg Am. 2015. July 1;97(13):1049–58. [DOI] [PubMed] [Google Scholar]
- 18.Jackson KL 2nd, Devine JG. The effects of smoking and smoking cessation on spine surgery: a systematic review of the literature. Global Spine J. 2016. November;6(7):695–701. Epub 2016 Jan 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Andersen T, Christensen FB, Laursen M, Høy K, Hansen ES, Bünger C. Smoking as a predictor of negative outcome in lumbar spinal fusion. Spine. 2001. December 1;26(23):2623–8. [DOI] [PubMed] [Google Scholar]
- 20.Martin CT, Gao Y, Duchman KR, Pugely AJ. The impact of current smoking and smoking cessation on short-term morbidity risk after lumbar spine surgery. Spine. 2016. April;41(7):577–84. [DOI] [PubMed] [Google Scholar]
- 21.Castillo RC, Bosse MJ, MacKenzie EJ, Patterson BM; LEAP Study Group. Impact of smoking on fracture healing and risk of complications in limb-threatening open tibia fractures. J Orthop Trauma. 2005. March;19(3):151–7. [DOI] [PubMed] [Google Scholar]
- 22.Wong J, Lam DP, Abrishami A, Chan MTV, Chung F. Short-term preoperative smoking cessation and postoperative complications: a systematic review and meta-analysis. Can J Anaesth. 2012. March;59(3):268–79. Epub 2011 Dec 21. [DOI] [PubMed] [Google Scholar]
- 23.Sørensen LT. Wound healing and infection in surgery. The clinical impact of smoking and smoking cessation: a systematic review and meta-analysis. Arch Surg. 2012. April;147(4):373–83. [DOI] [PubMed] [Google Scholar]
- 24.Glassman SD, Anagnost SC, Parker A, Burke D, Johnson JR, Dimar JR. The effect of cigarette smoking and smoking cessation on spinal fusion. Spine. 2000. October 15;25(20):2608–15. [DOI] [PubMed] [Google Scholar]
- 25.Møller AM, Villebro N, Pedersen T, Tønnesen H. Effect of preoperative smoking intervention on postoperative complications: a randomised clinical trial. Lancet. 2002. January 12;359(9301):114–7. [DOI] [PubMed] [Google Scholar]
- 26.Hejblum G, Atsou K, Dautzenberg B, Chouaid C. Cost-benefit analysis of a simulated institution-based preoperative smoking cessation intervention in patients undergoing total hip and knee arthroplasties in France. Chest. 2009. February;135(2):477–83. Epub 2008 Aug 21. [DOI] [PubMed] [Google Scholar]
- 27.Boylan MR, Bosco JA 3rd, Slover JD. Cost-effectiveness of preoperative smoking cessation interventions in total joint arthroplasty. J Arthroplasty. 2019. February;34(2):215–20. Epub 2018 Sep 28. [DOI] [PubMed] [Google Scholar]
- 28.Akhavan S, Nguyen LC, Chan V, Saleh J, Bozic KJ. Impact of smoking cessation counseling prior to total joint arthroplasty. Orthopedics. 2017. March 1;40(2):e323–8. Epub 2016 Dec 28. [DOI] [PubMed] [Google Scholar]
- 29.Truntzer J, Comer G, Kendra M, Johnson J, Behal R, Kamal RN. Perioperative smoking cessation and clinical care pathway for orthopaedic surgery. JBJS Rev. 2017. August;5(8):e11. [DOI] [PubMed] [Google Scholar]
- 30.Sorensen LT, Karlsmark T, Gottrup F. Abstinence from smoking reduces incisional wound infection: a randomized controlled trial. Ann Surg. 2003. July;238(1):1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sørensen LT. Wound healing and infection in surgery: the pathophysiological impact of smoking, smoking cessation, and nicotine replacement therapy: a systematic review. Ann Surg. 2012. June;255(6):1069–79. [DOI] [PubMed] [Google Scholar]
- 32.Mok JM, Guillaume TJ, Talu U, Berven SH, Deviren V, Kroeber M, Bradford DS, Hu SS. Clinical outcome of deep wound infection after instrumented posterior spinal fusion: a matched cohort analysis. Spine. 2009. March 15;34(6):578–83. [DOI] [PubMed] [Google Scholar]
- 33.Zhang J, Yu KF. What’s the relative risk? A method of correcting the odds ratio in cohort studies of common outcomes. JAMA. 1998. November 18;280(19):1690–1. [DOI] [PubMed] [Google Scholar]
- 34.Arias E, Xu J. United States Life Tables, 2017. Natl Vital Stat Rep. 2019. June;68(7):1–66. [PubMed] [Google Scholar]
- 35.Department of Veterans Affairs, Federal Supply Schedule Service. Pharmaceutical prices. Accessed 2020 Sep 23. https://www.va.gov/opal/nac/fss/pharmPrices.asp
- 36.Centers for Medicare & Medicaid Services. Physician fee schedule search. Accessed 2020 Sep 23. https://www.cms.gov/apps/physician-fee-schedule/search/search-criteria.aspx
- 37.Emohare O, Ledonio CG, Hill BW, Davis RA, Polly DW Jr, Kang MM. Cost savings analysis of intrawound vancomycin powder in posterior spinal surgery. Spine J. 2014. November 1;14(11):2710–5. Epub 2014 Mar 17. [DOI] [PubMed] [Google Scholar]
- 38.Whitmore RG, Stephen J, Stein SC, Campbell PG, Yadla S, Harrop JS, Sharan AD, Maltenfort MG, Ratliff JK. Patient comorbidities and complications after spinal surgery: a societal-based cost analysis. Spine. 2012. May 20;37(12):1065–71. [DOI] [PubMed] [Google Scholar]
- 39.Calderone RR, Garland DE, Capen DA, Oster H. Cost of medical care for postoperative spinal infections. Orthop Clin North Am. 1996. January;27(1):171–82. [PubMed] [Google Scholar]
- 40.Parker SL, Shau DN, Mendenhall SK, McGirt MJ. Factors influencing 2-year health care costs in patients undergoing revision lumbar fusion procedures. J Neurosurg Spine. 2012. April;16(4):323–8. Epub 2012 Jan 27. [DOI] [PubMed] [Google Scholar]
- 41.Glassman SD, Polly DW, Dimar JR, Carreon LY. The cost effectiveness of single-level instrumented posterolateral lumbar fusion at 5 years after surgery. Spine. 2012. April 20;37(9):769–74. [DOI] [PubMed] [Google Scholar]
- 42.Vogl M, Wenig CM, Leidl R, Pokhrel S. Smoking and health-related quality of life in English general population: implications for economic evaluations. BMC Public Health. 2012. March 19;12(1):203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Milstein A. Ending extra payment for “never events”—stronger incentives for patients’ safety. N Engl J Med. 2009. June 4;360(23):2388–90. [DOI] [PubMed] [Google Scholar]
- 44.Cahill K, Stevens S, Perera R, Lancaster T. Pharmacological interventions for smoking cessation: an overview and network meta-analysis. Cochrane Database Syst Rev. 2013. May 31;5:CD009329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hartmann-Boyce J, Chepkin SC, Ye W, Bullen C, Lancaster T. Nicotine replacement therapy versus control for smoking cessation. Cochrane Database Syst Rev. 2018. May 31;5:CD000146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Stead LF, Buitrago D, Preciado N, Sanchez G, Hartmann-Boyce J, Lancaster T. Physician advice for smoking cessation. Cochrane Database Syst Rev. 2013. May 31;5:CD000165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Aveyard P, Begh R, Parsons A, West R. Brief opportunistic smoking cessation interventions: a systematic review and meta-analysis to compare advice to quit and offer of assistance. Addiction. 2012. June;107(6):1066–73. Epub 2012 Feb 28. [DOI] [PubMed] [Google Scholar]
- 48.Bluman LG, Mosca L, Newman N, Simon DG. Preoperative smoking habits and postoperative pulmonary complications. Chest. 1998. April;113(4):883–9. [DOI] [PubMed] [Google Scholar]
- 49.Mills E, Eyawo O, Lockhart I, Kelly S, Wu P, Ebbert JO. Smoking cessation reduces postoperative complications: a systematic review and meta-analysis. Am J Med. 2011. February;124(2):144–154.e8. [DOI] [PubMed] [Google Scholar]
- 50.Khullar D, Maa J. The impact of smoking on surgical outcomes. J Am Coll Surg. 2012. September;215(3):418–26. Epub 2012 Jul 12. [DOI] [PubMed] [Google Scholar]
- 51.Glassman SD, Dimar JRI 3rd, Burkus K, Hardacker JW, Pryor PW, Boden SD, Carreon LY. The efficacy of rhBMP-2 for posterolateral lumbar fusion in smokers. Spine. 2007. July 1;32(15):1693–8. [DOI] [PubMed] [Google Scholar]
- 52.Hughes JR, Peters EN, Naud S. Relapse to smoking after 1 year of abstinence: a meta-analysis. Addict Behav. 2008. December;33(12):1516–20. Epub 2008 Jun 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Krall EA, Garvey AJ, Garcia RI. Smoking relapse after 2 years of abstinence: findings from the VA Normative Aging Study. Nicotine Tob Res. 2002. February;4(1):95–100. [DOI] [PubMed] [Google Scholar]
- 54.Hughes JR, Gulliver SB, Fenwick JW, Valliere WA, Cruser K, Pepper S, Shea P, Solomon LJ, Flynn BS. Smoking cessation among self-quitters. Health Psychol. 1992;11(5):331–4. [DOI] [PubMed] [Google Scholar]
- 55.Møller AM, Kjellberg J, Pedersen T. [Health economic analysis of smoking cessation prior to surgery—based on a randomised trial]. Ugeskr Laeger. 2006. March 6;168(10):1026–30. Danish. [PubMed] [Google Scholar]
- 56.Schimmel JJP, Horsting PP, de Kleuver M, Wonders G, van Limbeek J. Risk factors for deep surgical site infections after spinal fusion. Eur Spine J. 2010. October;19(10):1711–9. Epub 2010 May 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Weinstein MA, McCabe JP, Cammisa FP Jr. Postoperative spinal wound infection: a review of 2,391 consecutive index procedures. Clin Spine Surg. 2000. October;13(5):422–6. [DOI] [PubMed] [Google Scholar]
- 58.Fang A, Hu SS, Endres N, Bradford DS. Risk factors for infection after spinal surgery. Spine. 2005. June 15;30(12):1460–5. [DOI] [PubMed] [Google Scholar]
- 59.Veeravagu A, Patil CG, Lad SP, Boakye M. Risk factors for postoperative spinal wound infections after spinal decompression and fusion surgeries. Spine. 2009. August 1;34(17):1869–72. [DOI] [PubMed] [Google Scholar]
- 60.Carpenter CT, Dietz JW, Leung KYK, Hanscom DA, Wagner TA. Repair of a pseudarthrosis of the lumbar spine. A functional outcome study. J Bone Joint Surg Am. 1996. May;78(5):712–20. [DOI] [PubMed] [Google Scholar]
- 61.Pumberger M, Chiu YL, Ma Y, Girardi FP, Vougioukas V, Memtsoudis SG. Perioperative mortality after lumbar spinal fusion surgery: an analysis of epidemiology and risk factors. Eur Spine J. 2012. August;21(8):1633–9. Epub 2012 Apr 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Jha P, Ramasundarahettige C, Landsman V, Rostron B, Thun M, Anderson RN, McAfee T, Peto R. 21st-century hazards of smoking and benefits of cessation in the United States. N Engl J Med. 2013. January 24;368(4):341–50. [DOI] [PubMed] [Google Scholar]
- 63.Maciosek MV, Xu X, Butani AL, Pechacek TF. Smoking-attributable medical expenditures by age, sex, and smoking status estimated using a relative risk approach. Prev Med. 2015. August;77:162–7. Epub 2015 Jun 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Schmier JK, Halevi M, Maislin G, Ong K. Comparative cost effectiveness of Coflex® interlaminar stabilization versus instrumented posterolateral lumbar fusion for the treatment of lumbar spinal stenosis and spondylolisthesis. Clinicoecon Outcomes Res. 2014. March 18;6:125–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


