Abstract
The microbiota-gut-brain axis is a bidirectional signaling mechanism between the gastrointestinal tract and the central nervous system. The complexity of the intestinal ecosystem is extraordinary; it comprises more than 100 trillion microbial cells that inhabit the small and large intestine, and this interaction between microbiota and intestinal epithelium can cause physiological changes in the brain and influence mood and behavior. Currently, there has been an emphasis on how such interactions affect mental health. Evidence indicates that intestinal microbiota are involved in neurological and psychiatric disorders. This review covers evidence for the influence of gut microbiota on the brain and behavior in Alzheimer disease, dementia, anxiety, autism spectrum disorder, bipolar disorder, major depressive disorder, Parkinson’s disease, and schizophrenia. The primary focus is on the pathways involved in intestinal metabolites of microbial origin, including short-chain fatty acids, tryptophan metabolites, and bacterial components that can activate the host’s immune system. We also list clinical evidence regarding prebiotics, probiotics, and fecal microbiota transplantation as adjuvant therapies for neuropsychiatric disorders.
Keywords: Prebiotics, probiotics, microbiota-gut-brain axis, neuroinflammation, neuropsychiatric disorders
Introduction
The intestinal microbiota influences the brain and may be involved in neuropsychiatric disorders, partially by modulating the availability of circulating tryptophan, serotonin, kynurenine, and short-chain fatty acids (SCFA), as well as blood-brain barrier (BBB) permeability and activation of peripheral immune cells and brain glial cells. The gut-brain axis involves a biochemical signaling pathway between the gastrointestinal (GI) tract and the central nervous system (CNS). When GI structure is compromised, the functionality of the protective barrier is impaired, leading to increased intestinal permeability and, consequently, penetration by substances that can alter physiological functions.1 These events lead to activation of the innate immune response, resulting in chronically high levels of inflammation mediators that are known to trigger diseases, including a broad spectrum of psychiatric diseases.2 It has been suggested that the processes of intestinal dysbiosis and neurological deficits are linked through chronic low inflammation,3 including direct inflammatory stimulation, the production of pro-inflammatory mediators, and the loss of immune-regulatory function.4 Neuropsychiatric disorders and inflammation are strictly linked.5 Bipolar disorder, major depressive disorder (MDD), and schizophrenia patients have high plasma levels of proinflammatory cytokines, inflammation inducers (e.g., damage-associated molecular patterns), activated sensors (e.g., toll-like receptors [TLRs] and inflammasome), increased levels of acute-phase proteins (e.g., C-reactive protein), and adhesion molecules in their blood and cerebrospinal fluid.6-8 An association has been demonstrated between inflammation and the clinical progression of neuropsychiatric disorders.5
In recent decades, the trillions of microorganisms in the intestines have been shown to regulate the gut-brain axis. Several lines of research have shown that the intestinal microbiota affects the brain. First, studies on germ-free animals have shown that the brain is affected by the absence of microorganisms.9,10 Second, behavioral changes occurred in animals treated with specific microorganisms (Lactobacillus), demonstrating that the intestinal microbiota also affect animal behavior.11,12 Third, modulation of the gut-brain axis and behavioral changes were observed in individuals with infectious diseases.13-15 Fourth, preclinical and clinical studies have shown that antibiotics affect the brain and the enteric nervous system.16,17 Thus, given that they influence the brain and behavior, understanding the complex processes that occur in the gut and microbiota is crucial.
Gut microbiota: the effects of prebiotics, probiotics, and the synthesis of tryptophan metabolites
Gut microbiota
The gut microbiota encompasses a compact and diverse ecosystem that contains approximately 1,000 to 5,000 different species, of which 99% belong to the phyla Firmicutes, Bacteroidetes, Proteobacteria, and Actinobacteria.18 This community comprises more than 100 trillion microbial cells inhabiting the small and large intestine, which is estimated to be 10 times the number of all other cells in the human body. Similarly, there are nearly 3.3 million gut microbial genes, which is 150 times the number of genes in the human genome.19 SCFAs are the essential gut-microbial derived metabolites formed from dietary and environmental compounds, including acetate, propionate, and butyrate,20 as well as tryptophan metabolites, which include indole-3-acetic acid, indole 3-propionic acid, indole-3-acetaldehyde, indole acrylic acid, and indole-3-aldehyde21 (for more details, see Box 1).
Box 1. The essential gut-microbial derived metabolites and their effect.
| Metabolites/producers | Molecular target and/or effect on host |
|---|---|
| Short-chain fatty acids | |
| Acetate | |
| Akkermansia muciniphila | AhR agonists that block the production of pro-inflammatory cytokines and chemokines.20 Microglia homeostasis via AhR in experimental study.22 |
| Bacteroides spp. | |
| Bifidobacterium spp. | |
| Prevotella spp. | |
| Ruminococcus spp. | |
| Butyrate | |
| Anaerostipes spp. | AhR agonists that block the production of pro-inflammatory cytokines and chemokines.20 Intracellular butyrate inhibits the activity of HDACs.23 Butyrate-producing gut bacteria decrease gut permeability and inflammation.24 Microglia homeostasis via AhR in an experimental study.22 Suppressed lysolecithin-induced demyelination and enhanced remyelination in an in vitro study.25 |
| Coprococcus catus | |
| C. comes | |
| C. eutactus | |
| Eubacterium rectale | |
| E. hallii | |
| Faecalibacterium prausnitzii | |
| Roseburia spp. | |
| Propionate | |
| Bacteroides spp. | AhR agonists that block the production of pro-inflammatory cytokines and chemokines.20 Intracellular propionate inhibits HDAC activity.23 Propionate regulates microglia homeostasis.22 |
| Phascolarctobacterium succinatutens | |
| Dialister spp. | |
| Veillonella spp. | |
| Tryptophan metabolites | |
| Indole-3-acetic acid | |
| Bacteroides spp. | AhR ligands26 decrease the production of pro-inflammatory cytokines.21 Indole-3-acetic acid affects the severity of intestinal inflammation.27 |
| Bifidobacterium adolescentis | |
| B. longum | |
| B. pseudologum | |
| Clostridium spp. | |
| Enterobacter cloacae | |
| Indole-3-aldehyde | |
| Lactobacillus acidophilus | AhR ligands.26 Indole-3-aldehyde increases AhR-dependent IL-22 transcription,28 maintaining intestinal homeostasis. Indole-3-aldehyde activates cell lymphoids28 and confers resistance against pathogens.29 |
| L. reuteri | |
| Indole 3-propionic acid | |
| Clostridium spp. Peptostreptococcus spp. | AhR ligands.26 Indole 3-propionic acid is a free radical scavenger.30 Indole 3-propionic acid protects against amyloid β in Alzheimer disease.31 Indole 3-propionic acid causes better insulin secretion and sensitivity and decreased type 2 diabetes.32 |
| Indole acrylic acid | |
| Clostridium sporogenes Peptostreptococcus spp. | AhR ligands.26 Indole acrylic acid have anti-inflammatory function and increase the intestinal epithelial barrier.33 |
AhR = aryl hydrocarbon receptor; HDAC = histone deacetylase; IL-22 = interleukin-22.
Prebiotics
According the International Scientific Association for Probiotics and Prebiotics, prebiotic is defined as “a substrate that is selectively utilized by the host microorganisms, conferring a health benefit.” A typical diet of prebiotics includes inulin (sugar beets, leeks, and asparagus), fructooligosaccharides (bananas, onions, chicory root, garlic, asparagus, and leeks), galactooligosaccharides (lentils, beans, cashews, and pistachios), resistant starch (green banana, beans, peas, and lentils), and soluble fiber (fruits, vegetables, oats, beans, peas, and lentils). The health properties of prebiotics include benefits to the GI tract, such as inhibition of pathogens and immune stimulation; benefits to the cardiac metabolism, such as lower blood lipid levels and insulin resistance effects; and benefits to mental health, such as metabolites that influence brain function, decrease the BBB permeability, decrease neuroinflammation, etc.34 Studies have shown that prebiotic supplementation reduces stress responsiveness, anxiety, and depressive-like behavior, increases brain-derived neurotrophic factor (BDNF) expression, and improves cognition.35,36 In clinical studies, prebiotic supplementation increased SCFA levels,37 improved social behavior symptoms and sleep patterns in autism spectrum disorder (ASD) patients,38 and reduced anxiety scores in irritable bowel syndrome patients.39
Probiotics
Probiotics are defined as “live microorganisms that, when dispensed in suitable amounts, provide a health benefit for the host” (p. 2).40 Although commensal microorganisms in the gut are often the source of probiotic strains, until these strains are isolated, identified, and a credible case presented for their health effects, they cannot be considered probiotics.41 Preclinical studies have demonstrated that chronic administration of probiotics can reduce anxiety- and depressive-like behavior and can normalize associated physiological outputs, such as corticosterone, noradrenaline, and BDNF levels, as well as immune function.11,12,42,43 The probiotic Bifidobacterium infantis was administered in an animal maternal separation model and, compared to placebo, resulted in normalized immune response, reversed behavioral deficits, and restored basal noradrenaline concentrations in the brain stem.42 In a mouse model, Lactobacillus rhamnosus ingestion was found to regulate emotional behavior and central gamma-aminobutyric acid receptor expression via the vagus nerve.12 A clinical study demonstrated that ingesting Bifidobacterium longum 1714 was associated with reduced stress reduction and memory improvement in healthy volunteers.44
In another clinical trial, a double-blind, placebo-controlled, randomized parallel-group study involved daily administration of probiotics for 30 days. Volunteers were evaluated with the Hopkins Symptom Checklist, the Hospital Anxiety and Depression Scale, the Perceived Stress Scale, the Coping Checklist, and with urinary free cortisol levels. Sub-chronic administration of probiotics alleviated psychological distress in terms of global severity index, somatization, depression, anger, hostility, global Hospital Anxiety and Depression Scale and Coping Checklist (problem-solving) scores, as well as urinary free cortisol levels. When taken in combination, Lactobacillus helveticus R0052 and B. longum R0175 had beneficial psychological effects in healthy volunteers.43
Probiotic metabolites (short-chain fatty acids: acetate, butyrate, and propionate)
Soluble fiber, protein, and peptides, which are not degraded in the upper gut by digestive enzymes, are metabolized by gut microbiota in the cecum and colon. Their main products are SCFAs, including acetate, propionate, and butyrate.20 Butyrate is the principal energy source for colonocytes and protects against colorectal cancer and inflammation by inhibiting histone deacetylases (EC 3.5. 1.98).45
Luminal acetate and propionate are endogenous ligands of two G protein-coupled receptors, GPR41 and GPR43, which can modulate inflammation; they also increase the production of glucagon-like peptide-1 and peptide tyrosine-tyrosine, which affect satiety and intestinal transit.46 SCFAs also regulate the permeability of the BBB. In a preclinical study, colonization of germ-free mice with Clostridium tyrobutyricum (a butyrate producer) or with Bacteroides thetaiotaomicron (an acetate and propionate producer) decreased BBB permeability and was associated with increased occludin protein expression in the frontal cortex and hypothalamus.47 In an animal model, intraperitoneal and intravenous administration of sodium butyrate prevented BBB breakdown and promoted angiogenesis and neurogenesis.48,49 Although germ-free mice presented microglia defects with altered cell proportions, leading to impaired innate immune response, microbiota recolonization restored microglia properties, which demonstrates that SCFAs regulate microglia homeostasis.22
Probiotics in tryptophan metabolite synthesis
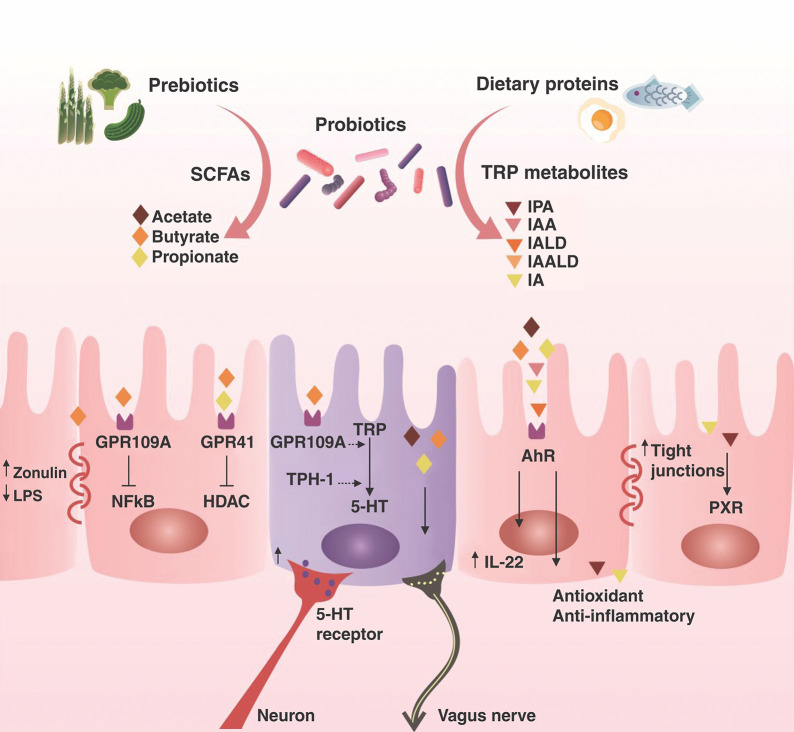
The essential amino acid tryptophan is a common constituent of protein-based foods, including eggs, fish, meat, and cheese. Tryptophan metabolism follows three essential pathways in the GI tract: first, gut microbiota can metabolize tryptophan into ligands of aryl hydrocarbon receptor (AhR); second, the kynurenine pathway; and third, the serotonin (5-hydroxytryptamine) pathway in enterochromaffin cells via tryptophan hydroxylase 1.26 Lactobacillus reuteri can use tryptophan as an energy source, producing the AhR agonist indole-3-aldehyde as a metabolic product. Other tryptophan metabolites also are produced by commensal microbiota, and their metabolites are AhR agonists, including indole-3-acetic acid, indole-3-acetyl aldehyde, indole-3-aldehyde, tryptamine, and 3-methylindol.50,51 These AhR agonist metabolites can cross the BBB and activate AhR in astrocytes and microglial cells (the resident macrophages of the brain and spinal cord, acting as the primary source of immune defense in the CNS).52 Thus, AhR activation suppresses the transcription factor nuclear factor-κB (NF-κB), blocking the production of pro-inflammatory cytokines and chemokines. AhR activation also promotes the expression of microglial transforming growth factor-alpha, which acts on astrocytes to suppress their pro-inflammatory activity.51,53 The concept of neuroinflammation is characterized by microglial cell activation and the presence of peripheral infiltrating leukocytes in the CNS parenchyma.52 When microglial cells are in homeostasis, they do not produce pro-inflammatory mediators. Consequently, there are no infiltrating peripheral immune cells in the brain and neuroinflammation does not occur. For more details about probiotics and their metabolites in the CNS, refer to Figure 1.
Figure 1. The function of SCFAs and TRP metabolites in the gut. Gut microorganisms ferment dietary fibers, producing SCFAs. SCFAs inhibit HDAC. Butyrate presents an effect on G protein-coupled receptor 109A, decreasing inflammation and increasing the synthesis of zonulin, a consequence of preventing gut permeability. Intestinal microbiota can convert TRP from protein-based foods into metabolites with different functions in the host, including decreased gut permeability, AhR activation, increasing serotonin synthesis, and activating the vagus nerve. 5HT = 5-hydroxytryptamine; AhR = aryl hydrocarbon receptor; HDAC = histone deacetylase; IA = indole acrylic acid; IAA = indole-3-acetic acid; IAALD = indole-3-acetaldehyde; IALD = indole-3-aldehyde; IL-22 = interleukin-22; IPA = indole-3-propionic acid; LPS = lipopolysaccharide; NF-κB = transcription factor nuclear factor-κB; PXR = pregnane X receptor; SCFAs = short-chain fatty acids; TRP = tryptophan; TPH-1 = tryptophan hydroxylase 1.
Potential communication pathways between gut microbiota and the brain
The vagus nerve
The vagus nerve is the principal constituent of the parasympathetic nervous system, and it is the most direct intermediary pathway between the gut and the brain (80% afferent and 20% efferent fibers). Afferent fibers in the vagus nerve do not cross the epithelial layer of the digestive wall and are not in direct communication with the gut luminal microbiota.54 Enteroendocrine cells interact with vagal afferents either directly, through the release of serotonin, which activates 5-hydroxytryptamine-3 receptors in vagal afferent fibers, or through gut hormones. Enteroendocrine cells comprise approximately 1% of the total epithelial cell population. Notably, these cells express TLRs, such as TLR1, TLR2, and TLR4, which mediate the sensing of microorgarnisms.55
Additionally, enteroendocrine cells identify signals from Gram-negative bacteria by detecting lipopolysaccharide (LPS) through TLR4, by identifying signs from Gram-positive bacteria, and by detecting peptidoglycan through TLR2; these cells also have SCFA receptors. Consequently, enteroendocrine cells can detect bacterial compounds and have an indirect effect on vagal afferent fibers by regulating GI motility, secretion, and food intake. For instance, the microbiota can sense the vagus nerve through direct mechanisms, including TLR4, which is activated by LPS. LPS also directly activates vagal afferent fibers at the nodose ganglia level (inferior ganglion of the vagus nerve).54 SCFAs produced by the microbiota activate vagal afferent fibers by different mechanisms, depending on the compound. Interestingly, the results of a Swedish register-based matched cohort study suggested that truncal vagotomies could have a protective effect against Parkinson’s disease.56
Immune system and bacterial compounds
There is a high concentration of GI tract cells in the immune system, and these immune cells are in constant communication with the trillions of microbial cells that inhabit the small and large intestine.18 Epithelial goblet cells secrete protective viscous mucins, which form mucus layers, and host-microbiota interaction occurs in this interface. The intestinal immune system maintains tolerance to commensals and immunity to pathogenic bacteria, and the imbalance between the host immune system and microbiota modulates inflammation and can contribute to several diseases. Several bacterial compounds, such as peptidoglycan, lipoteichoic acid (a constituent of the cell wall of Gram-positive bacteria), LPS (a constituent of the cell wall of Gram-negative bacteria), flagellum (motility), pilus (which mediates the attachment of bacteria to cells), DNA, and cell wall fragments57-59 are considered pathogen-associated molecular patterns.60,61 Pathogen-associated molecular patterns are recognized by pattern-recognition receptors and non-pattern-recognition receptors, which are essential constituents of the immune system.62,63 The sensing of pathogen-associated molecular patterns by immune receptors triggers a cascade of signaling pathways that activate several transcription factors and stimulate the production of pro-inflammatory mediators. These mediators include cytokines, chemokines, and antimicrobial peptides, which are required for the elimination of invading pathogens.64 This host-immune response increases intestinal permeability, facilitating the passage of substances into the bloodstream, causing a systemic inflammatory reaction that leads to an increase in BBB permeability, which triggers microglial cell activation.65
Several animal models of neuropsychiatric disorders have administered LPS intraperitoneally or intracerebrally as an inductor of disease. Repeated, intermittent, or single exposure to LPS has been used to model depressive-like behavior in animals.66,67 Maternal immune activation can be produced by LPS, which, in a rat model, has triggered an immune response in pregnant mothers and fetuses and behavioral impairment in surviving adults.68 A clinical study evaluated GI inflammatory markers in the bloodstream of patients with a recent history of suicide attempts (actual, aborted, or interrupted). The study included 90 schizophrenia patients, 72 bipolar disorder patients, 48 MDD patients, and 72 healthy controls and found that recent suicide attempters had higher antibody levels to yeast mannan from Saccharomyces cerevisiae, the food antigen gliadin, and bacterial LPS than the healthy group.69 Another study reported finding bacterial LPS in post-mortem brain lysates from the hippocampus and superior temporal lobe neocortex of Alzheimer disease patients.70 A distinctive microbiota composition that favored a pro-inflammatory environment was found in the GI tract of Parkinson’s patients.71
Tryptophan metabolism
Tryptophan is an essential aromatic amino acid that can be metabolized by microorganisms in the GI tract into several molecules, including indole-3-aldehyde, indole-3-acetic acid, indole-3-propionic acid, indole-3-acetaldehyde, and indole acrylic acid, which are AhR ligands.26 Gut microbiota affects the brain and may be involved in neuropsychiatric disorders by modulating circulating tryptophan levels. Agonists derived from tryptophan cross the BBB to activate AhR in astrocytes and microglia cells. AhR activation suppresses pro-inflammatory NF-κB signaling, which interferes with transcriptional factors associated with the recruitment of inflammatory monocytes via chemokine production.51
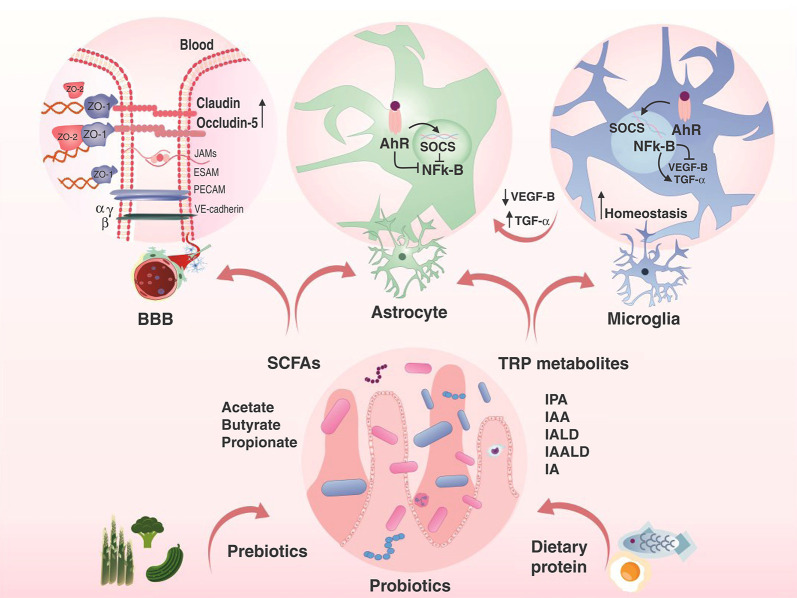
Additionally, AhR activation increases the expression of suppressor of cytokine signaling 2 and indirectly blocks NF-κB signaling. A proteomic study comparing post-mortem hippocampal tissue from schizophrenia patients, bipolar disorder patients, and healthy controls found that the hippocampi of schizophrenia patients had prominent abnormalities in 14-3-3 proteins (proteins that impact many aspects of brain function, including neural signaling, neuronal development, and neuroprotection) and in AhR signaling. In contrast, the hippocampi of bipolar disorder patients displayed marked changes in glucose metabolism.72 Another study found an association between AhR-related gene variants and ASD severity73 (for more details, see Figure 2).
Figure 2. The microbiota-gut-brain axis. SCFAs can increase the expression of claudin and occludin, which decreases BBB permeability. SCFAs and TRP metabolites can prevent astrocyte and microglial cell activation by blocking pro-inflammatory transcript factors, which leads to homeostasis in the brain. AhR = aryl hydrocarbon receptor; BBB = blood-brain barrier; IA = indole acrylic acid; IAA = indole-3-acetic acid; IAALD = indole-3-acetaldehyde; IALD = indole-3-aldehyde; IPA = indole-3-propionic acid; NF-κB = transcription factor nuclear factor-κB; SCFAs = short-chain fatty acids; SOCS = suppressors of cytokine signaling; TGF-α = transforming growth factor-alpha; TRP = tryptophan; VEGF-β = vascular endothelial growth factor-beta.
Short-chain fatty acids
The primary source of SCFAs is microbial fiber fermentation, while fermented foods can be a secondary source. Endogenous sources of SCFAs include host metabolism of long-chain fatty acids, pyruvate to acetate, and the breakdown of proteins by the microbiota. SCFA levels in human feces generally conform to a 60:20:20 ratio (∼ 60 g/kg acetate, ∼ 10 to 20 g/kg propionate, and ∼ 3 to 30 g/kg butyrate, respectively). SCFAs are metabolized in cells via the citric acid cycle (Krebs cycle) to produce energy. All SCFAs present inhibitory effects on histone deacetylase, while butyrate affects specific receptors (GPR43/FFAR2; GPR41/FFAR3; GPR109a/HCAR2), and transporters (MCT1/SLC16A1; SMCT1/SLC5A8), which has led to the use of butyrate as an experimental drug in neuropsychiatric disorders.74
In several neuropsychiatric animal models, the administration of butyrate, 4-phenylbutyrate, or butyrate-producing bacteria has positive effects on brain physiology and function.75 In an Alzheimer model, butyrate treatment increased contextual fear learning, spatial learning, hippocampal synaptophysin, and neural plasticity.75 In animal models of psychosis and drug abuse, animals treated with butyrate decreased cocaine self-administration.76 In mania and bipolar disorder models, animals treated with butyrate presented decreased locomotor activity and increased activity of mitochondrial respiratory chain complexes in the prefrontal cortex, hippocampus, striatum, and amygdala.77 In an animal depressive-like behavior model, decreased depressive-like behavior, increased prefrontal cortical ten-eleven translocation methylcytosine dioxygenase-1, and increased BDNF levels were found in the butyrate treatment group.78 In an ASD model, butyrate treatment increased long-term memory, hippocampal CA1 dendritic spine density, and histone acetylation79 (Figure 2).
The microbiota-gut-brain axis and neuropsychiatric disorders
A large body of accumulated evidence points to the involvement of gut microbiota in several neuropsychiatric disorders. See Tables 1 and 2 for more details.
Table 1. Selected studies about prebiotics and probiotics administration in patients diagnosed with neuropsychiatric disorders.
| Author | Study design | Sample | Bacterial species intervention | Main findings |
|---|---|---|---|---|
| Kazemi et al.80 | Double-blind controlled clinical trial | MDD probiotic group (n=38); 27 F/11 M; age 36.15±7.85 MDD prebiotic group (n=36); 27 F/9 M; age 37.35±7.97 MDD placebo group (n=36); 24 F/12 M; age 36±8.47 | Probiotic: L. helveticus and B. longum (≥10 × 109 CFU) | Probiotic supplementation resulted in lower BDI scores (17.39-9.1) than the prebiotic group (19.72-14.14) and the placebo group (18.18-15.55). |
| Liu et al.81 | Randomized, double-blind, placebo-controlled trial | ASD probiotic group (n=36); 36 M; age 10.11±2.34 ASD placebo group (n=35); 35 M; age 9.91±2.33 | L. plantarum PS128 (3 × 1010 CFU) | Probiotic treatment ameliorated opposition/defiance behaviors, and total SNAP-IV scores for younger children (aged 7 to 12) improved significantly compared with the placebo group. Several others elements were also improved in the probiotic group after 28 days of treatment. |
| Reininghaus et al.82 | Cohort study | Euthymic BD probiotic group (n=27); 11 F/16 M; age 50.7±12.2 | B. bifidum W23, B. lactis W51, B. lactis W52, L. acidophilus W22, L. casei W56, L. paracasei W20, L. plantarum W62, L. salivarius W24, and L. lactis W19 (7.5 billion organisms per 1 portion, 3 g) | GI problems were prevalent in more than half of the patients upon inclusion. One-third of the patients reported positive changes (reduced flatulence and more comfortable and frequent bowel movements). Although the patients presented reduced cognitive reactivity to sad mood, significant symptom reduction was found in manic symptom scales. |
| Severance et al.83 | Longitudinal, double-blinded, and placebo-controlled | SCZ probiotic group (n=30); 8 F/22 M; age 44.66±11.4 SCZ placebo group (n=26); 11 F/15 M; age 48.11±9.6 | Bifiform balance: L. rhamnosus strain GG (109 CFUs), B. animalis subspecies (109 CFUs) | An association was found between C. albicans seropositivity and worse positive psychiatric symptoms. However, probiotics administration normalized C. albicans antibody levels. |
| Tamtaji et al.84 | Randomized, double-blind, and controlled clinical trial | AD selenium probiotic group (n=27); age 78.5±8.0 AD selenium group (n=26); age 78.8±10.2 AD placebo group (n=26); age 76.2±8.1 | L. acidophilus, B. bifidum, and B. longum (2 × 109 CFU/g) | Twelve weeks of probiotic and selenium co-supplementation in AD patients improved cognitive function (reflected in increased MMSE scores) and had favorable results for specific inflammation and oxidative stress markers, such as high-sensitivity-CRP, TAC, and GSH, compared to selenium-only and placebo AD groups. |
| Tamtaji et al.85 | Randomized, double-blind, placebo-controlled clinical trial | PD probiotic group (n=30); age 68.2±7.8 PD placebo group (n=30); age 67.7±10.2 | L. acidophilus, B. bifidum, L. reuteri, and L. fermentum (each 2 x 109 CFU/g) | Twelve weeks of probiotic treatment by PD patients had a beneficial impact on MDS-UPDRS scores. |
| Tankou et al.86 | Cohort study | MS probiotic group (n=9); age 50±10 Healthy control group (n=13); age 35�14 | L. paracasei, L. plantarum, L. acidophilus, and L. delbruckeii subspecies bulgaricus, B. longum, B. infantis, and B. breve, Streptococcus thermophilus (3,600 billion CFU/g for each strain) | Probiotic treatment was associated with an increased abundance of many taxa, especially Lactobacillus, Streptococcus, and Bifidobacterium species. Probiotics also induced an anti-inflammatory peripheral immune response characterized by a decreased frequency of intermediate monocytes and decreased mean fluorescence intensity of CD80 in classical monocytes, as well as decreased human leukocyte HLA-DR MFI in dendritic cells. |
AD = Alzheimer disease; ASD = autism spectrum disorder; BD = bipolar disorder; BDI = Beck Depression Inventory; CFU = colony-forming unit; CRP = C-reactive protein; F = female; FMT = fecal microbiota transplant; GI = gastrointestinal; GSH = glutathione; HLA-DR = human leukocyte antigen-antigen D related; HLA-DR = human leukocyte antigen-antigen D related; M = male; MDD = major depression disorder; MDS-UPDRS = Movement Disorders Society-Unified Parkinson's Disease Rating Scale; MDS-UPDRS; Movement Disorders Society-Unified Parkinson's Disease Rating Scale; MFI = mean fluorescence intensity; MMSE = mini-mental state examination; MS = multiple sclerosis; PD = Parkinson’s disease; SCZ = schizophrenia; SNAP-IV = Swanson, Nolan, and Pelham-IV-Taiwan version; TAC = total antioxidant capacity.
Table 2. Selected studies about fecal microbiota transplantation and neuropsychiatric disorders.
| Title | Study designer | Sample | Main findings |
|---|---|---|---|
| Zhao et al.87 | Case report | Patient (n=1); M; age 9-year-old Healthy donor (n=1); M; age 14-year-old | Eight weeks after treatment, the patient’s YGTSS score reduced from 31 to 5, his motor severity score reduced from 16 to 5, and his vocal severity score reduced from 15 to 0 (i.e., from severe to mild). |
| Cai et al.88 | Case report | MDD patient (n=1); F; age 79-year-old Healthy donor (n=1); M; age 6-year-old | Four days after the FMT, the patient felt less sleepy, her appetite improved, and she was more talkative. Two weeks after FMT, the patient was able to live independently and her weight increased. Six months after FMT, the patient’s weight had returned to normal, her constipation symptoms improved, and her PHQ-9 score decreased from 21 to 4 (normal). |
| Kang et al.89 | Open-label clinical trial | ASD patients (n=18); age 7 to 16-years-old | The patients presented an 80% reduction of GI symptoms after treatment, including improvements constipation, diarrhea, indigestion, and abdominal pain symptoms. Clinical assessments showed that behavioral ASD symptoms improved significantly and remained improved 8 weeks after treatment ended. |
| Kang et al.90 | Follow-up study of a clinical trial | ASD patients (n=18); age 7 to 16-year-old | Two years after the FMT, most GI symptom improvements continued, and autism-related symptoms improved even more. The changes in gut microbiota found at the end of treatment continued in follow-up, demonstrating the long-term safety and efficacy of FMT as a potential therapy for children with ASD who have GI problems. |
ASD = autism spectrum disorder; F = female; FMT = fecal microbiota transplantation; GI = gastrointestinal; M = male; MDD = major depression disorder; PHQ = Patient Health Questionnaire; YGTSS = Yale Global Tic Severity Scale.
Alzheimer disease and dementia
Alzheimer disease is an irreversible type of dementia that triggers brain disorders and causes memory and thinking problems, which eventually affect activities of daily living. The characteristic brain pathology includes amyloid plaque deposition and hyperphosphorylation of tau protein in the brain.91 Alzheimer disease can be categorized as familial (5% of all cases) or sporadic (95%).74 Infection plays a role in late-onset disease pathology in sporadic Alzheimer disease. Several studies have suggested that infectious agents, including viruses, parasites, bacteria, and fungi, are trigger factors for the development of Alzheimer disease pathology.18,92,93 The infection hypothesis is not a novel idea; Aloysius Alzheimer (1864-1915) himself proposed that microorganisms could be involved in progression of the disease.94,95
Recently, a probiotic combination containing B. longum and Lactobacillus spp. improved cognitive function and metabolic status in Alzheimer disease patients.96 Probiotic and selenium co-supplementation for 12 weeks in Alzheimer patients led to improved cognitive function (i.e., higher Mini-Mental State Exam scores) and favorable results for specific markers of inflammation and oxidative stress in comparison with selenium-only or placebo groups of Alzheimer patients.84 In an explorative intervention study using probiotic supplementation in with Alzheimer dementia patients, multispecies probiotic treatment influenced gut bacteria composition and tryptophan metabolism in serum. After treatment, these patients showed lower intestinal permeability (reflected in lower fecal zonulin concentrations) and more abundant Faecalibacterium prausnitzii (a microorganism that produces SCFAs) than controls.97 Taken together, these results indicate the potential efficacy of probiotics in improving cognitive function in Alzheimer patients and healthy populations.
Anxiety
Most of the evidence demonstrating an association between anxiety and the microbiota-gut-brain axis comes from preclinical studies. Some probiotic administration studies have found improvements in specific anxiety measures. A meta-analysis of randomized controlled trials evaluated the efficacy of probiotics for anxiety behavior and found no significant difference between probiotics and placebo in alleviating anxiety symptoms.98 In a placebo-controlled study in stressed adults, 12 weeks of treatment with Lactobacillus plantarum reduced stress and anxiety symptoms, as well as total Depression Anxiety Stress Scales-42 scores. Plasma cortisol levels, as well as plasma pro-inflammatory cytokines, were lower in individuals who received probiotics than placebo. Compared to the placebo and young adult (< 30 years old) groups, L. plantarum treatment improved cognitive and memory function in healthy adults > 30 years old, including primary attention, emotional cognition, and associated learning. The administration of probiotics may modulate the serotonin pathway by decreasing the plasma levels of dopamine β-hydroxylase, tyrosine hydroxylase, indoleamine 2,3-dioxygenase, and tryptophan 2,3-dioxygenase. Probiotics was observed to increase the levels of tryptophan hydroxylase-2 and 5-hydroxytryptamine receptor-6 in the patients’ plasma.99
Autism spectrum disorder
Several studies have demonstrated the role of GI microbiota in ASD symptomatology. Approximately 70 to 80% of children with ASD also have GI disorders, such as bloating, constipation, and diarrhea, which indicates that there is a relationship between ASD, gut physiology, and altered microbiota.100,101 Children with ASD tend to eat fewer vegetables than other children of the same age. Their fiber intake is also inadequate since they tend to eat more energy-dense foods.102 The microbiota of children with ASD differs from that of other children regarding species and bacterial metabolites, such as SCFAs, etc. These children have fewer genera of beneficial bacteria, such as Bifidobacterium spp., along with a greater abundance of potentially pathogenic genera, such as Desulfovibrio and Clostridium.18 A recent study identified Clostridium perfringens and its toxin genes in the gut of children with ASD, associating this toxin with GI-related illnesses.103 In a placebo-controlled trial, probiotic treatment had a positive effect on oppositional-defiant behavior, as well as on total Swanson, Nolan, and Pelham, version IV scale scores for younger children with ASD (aged 7 to 12).81 After treatment with fecal microbiota transplantation (FMT), GI symptom rating scale scores were reduced by approximately 80%, including significant improvements in constipation, diarrhea, indigestion, and abdominal pain symptoms. Clinical assessment showed that behavioral ASD symptoms improved and remained better 8 weeks after treatment.89 Two years after FMT treatment,89 most GI symptom improvements continued, and autism-related symptoms had improved even more. The changes in gut microbiota found at the end of treatment continued in follow-up, demonstrating the long-term safety and efficacy of FMT as a potential therapy for children with ASD who have GI problems.90 A study by Buffington et al.104 reported that a maternal high-fat diet induces shifts in microbial ecology that increase the risk of neurodevelopmental disorders and negatively impact the social behavior of offspring.
Bipolar disorder
In a stool microbiome analysis, bipolar disorder patients had a decreased abundance of Firmicutes and Faecalibacterium spp. compared to and healthy controls, which also correlated with self-reported symptom severity.105 A recent probiotic clinical intervention containing Lactobacillus spp. and Bifidobacterium lactis strains significantly reduced psychiatric rehospitalizations in individuals recently discharged after a manic episode. This effect was increased in individuals who had elevated systemic inflammation at baseline.106
A pilot study evaluated the effects of probiotic treatment on GI symptoms in a cohort of euthymic bipolar disorder patients. GI problems were prevalent in more than half of the patients upon inclusion. Approximately one-third of the patients had positive changes (reduced flatulence and more frequent bowel movements) during treatment. Although the patients presented reduced cognitive reactivity to sad mood, significant symptom reduction was found in the manic symptom scales.82
Major depressive disorder (MDD)
Disturbances in the equilibrium of the gut-microbiota axis have been associated with the pathophysiology of depression. In a recent study, 110 depressed patients were randomized to receive probiotics (B. longum and L. helveticus), prebiotics (galactooligosaccharide), or placebo for 8 weeks. After treatment, Beck Depression Inventory scores were improved in MDD patients, although prebiotic supplementation had no effect.80 In a case report, an older woman diagnosed with depression was treated with FMT. On the fourth day after treatment, she felt less sleepy, had a better appetite, and was more talkative. After 2 weeks, she was able to live independently and her weight increased. Six months later, her weight had returned to normal, constipation symptoms had improved, and her Patient Health Questionnaire-9 score decreased from 21 to 4.88 In a cohort of healthy older adults, treatment with Lactobacillus casei (added to a milk drink) triggered improved mood ratings, and the greatest benefits were observed in those whose mood was initially poor.107 Furthermore, in a placebo-controlled clinical trial that included 40 MDD patients, probiotic treatment including Lactobacillus acidophilus, L. casei, and Bifidobacterium bifidum resulted in improved depression scores.108 A recent open-label study in patients with treatment-resistant depression found that the probiotic Clostridium butyricum, in combination with antidepressants, led to a significant improvement in depression symptoms.109
Parkinson’s disease
In the course of Parkinson’s disease, the enteric nervous system and parasympathetic nerves are among the first and most frequent structures to be affected by alpha-synuclein pathology. Scheperjans et al.110 provided the first evidence of an association between the microbiota-gut-brain axis and Parkinson’s disease by comparing the fecal microbiome of Parkinson’s patients with 72 healthy controls. They found that the prevalence of Prevotellaceae was 77.6% lower in Parkinson's disease patients than in healthy controls, as well as that the relative abundance of Enterobacteriaceae was positively associated with postural instability and gait difficulty.110 Another study found that Parkinson’s patients have intestinal dysbiosis and reduced serum LPS-binding protein levels. LPS-binding protein opsonizes LPS, and acute LPS concentrations in the bloodstream increase serum levels of LPS-binding protein, whereas chronic LPS invasion somewhat decreases serum levels of LPS-binding protein.111 In a placebo-controlled clinical trial, Parkinson’s patients were treated with probiotics (Lactobacillus acidophilus, B. bifidum, L. reuteri, and Lactobacillus fermentum), and the Movement Disorders Society-Unified Parkinson’s Disease Rating Scale was administered pre- and post-intervention. Compared with placebo, probiotic treatment decreased Movement Disorders Society-Unified Parkinson’s Disease Rating Scale scores, reduced high-sensitivity C-reactive protein, decreased oxidative damage, and increased enzymatic defense.85 Moreover, in a Swedish register-based matched cohort study, Liu et al. suggested that truncal vagotomy had potential protective effect against the development of Parkinson’s disease.56
Schizophrenia
A recent study found differences in gut microbiome composition between chronic schizophrenia patients and healthy controls. Schizophrenia patients had lower levels of the phylum Proteobacteria, higher levels of the genus Anaerococcus, and lower levels of Haemophilus spp., Sutterella spp., and Clostridium spp. than healthy controls. Schizophrenia patients had an abundance of Ruminococcaceae, which is associated with less severe negative symptoms, as well as Bacteroides, which is associated with more severe depressive symptoms, and Coprococcus sp., which is associated a higher risk of coronary heart disease.112 This trial included 60 patients with chronic schizophrenia and treated them with vitamin D and probiotic co-supplementation. The treatment was associated with a significant improvement in Positive and Negative Syndrome Scale scores, increased total antioxidant capacity, decreased malondialdehyde, and high sensitivity C-reactive protein levels compared to placebo.113 In an open-label single-arm study, all participants received Bifidobacterium breve for 4 weeks, which improved anxiety and depressive symptoms in schizophrenia patients.114 A placebo-controlled longitudinal pilot study evaluated the effects of probiotic administration on yeast antibody levels, as well as the association between probiotics, antibody levels, and bowel discomfort in schizophrenia patients. It was found that probiotic treatment significantly reduced Candida albicans antibodies in men, but not women. Probiotic treatment has been found to help normalize C. albicans antibody levels and C. albicans-associated gut discomfort in many men.115 Additionally, mice that received schizophrenia microbiome fecal transplants had lower glutamate, higher glutamine, and higher gamma-aminobutyric acid in the hippocampus and displayed schizophrenia-relevant behaviors.115
Conclusion
This article has presented a review of current evidence about microbiota-gut-brain axis activity through different pathways, such as the immune system, the vagus nerve, and microbial metabolites, such as SCFAs and tryptophan metabolites. We also showed evidence from preclinical and clinical studies (Tables 1 and 2) that prebiotics, probiotics, and FMT may be involved in several neuropsychiatric disorders, and that the composition of the intestinal microbiota plays an essential role in the physiology and pathophysiology of these disorders.
The complexity of microbiota-host interactions and their relationship with the diseases studied herein requires further investigation of SCFAs and tryptophan metabolites and their functional implications at different stages of life. This will help clarify how the microbiota-gut-brain axis interferes in health and disease, which could refine our goals and lead to therapeutic interventions.
Disclosure
The authors report no conflicts of interest.
Acknowledgements
The authors would like to thank UTHealth, the Universidade do Extremo Sul Catarinense (UNESC), the Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq), and the Fundação de Amparo à Pesquisa e Inovação de Santa Catarina (FAPESC). TB has received grants from the Alzheimer’s Association® (AARGDNTF-19-619645).
Footnotes
How to cite this article: Generoso JS, Giridharan VV, Lee J, Macedo D, Barichello T. The role of the microbiota-gut-brain axis in neuropsychiatric disorders. Braz J Psychiatry. 2021;43:293-305. http://dx.doi.org/10.1590/1516-4446-2020-0987
References
- 1.Turner JR. Intestinal mucosal barrier function in health and disease. Nat Rev Immunol. 2009;9:799–809. doi: 10.1038/nri2653. [DOI] [PubMed] [Google Scholar]
- 2.Gallo RL, Hooper LV. Epithelial antimicrobial defence of the skin and intestine. Nat Rev Immunol. 2012;12:503–16. doi: 10.1038/nri3228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rogers GB, Keating DJ, Young RL, Wong ML, Licinio J, Wesselingh S. From gut dysbiosis to altered brain function and mental illness: mechanisms and pathways. Mol Psychiatry. 2016;21:738–48. doi: 10.1038/mp.2016.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Neufeld KM, Kang N, Bienenstock J, Foster JA. Reduced anxiety-like behavior and central neurochemical change in germ-free mice. Neurogastroenterol Motil. 2011;23:255–64. doi: 10.1111/j.1365-2982.2010.01620.x. [DOI] [PubMed] [Google Scholar]
- 5.Sethi R, Gómez-Coronado N, Walker AJ, Robertson OD, Agustini B, Berk M, et al. Neurobiology and therapeutic potential of cyclooxygenase-2 (COX-2) inhibitors for inflammation in neuropsychiatric disorders. Front Psychiatry. 2019;10:605. doi: 10.3389/fpsyt.2019.00605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Modabbernia A, Taslimi S, Brietzke E, Ashrafi M. Cytokine alterations in bipolar disorder: a meta-analysis of 30 studies. Biol Psychiatry. 2013;74:15–25. doi: 10.1016/j.biopsych.2013.01.007. [DOI] [PubMed] [Google Scholar]
- 7.Inoshita M, Numata S, Tajima A, Kinoshita M, Umehara H, Nakataki M, et al. A significant causal association between C-reactive protein levels and schizophrenia. Sci Rep. 2016;6:26105. doi: 10.1038/srep26105. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 8.Köhler CA, Freitas TH, Maes M, de Andrade NQ, Liu CS, Fernandes BS, et al. Peripheral cytokine and chemokine alterations in depression: a meta-analysis of 82 studies. Acta Psychiatr Scand. 2017;135:373–87. doi: 10.1111/acps.12698. [DOI] [PubMed] [Google Scholar]
- 9.Nishino R, Mikami K, Takahashi H, Tomonaga S, Furuse M, Hiramoto T, et al. Commensal microbiota modulate murine behaviors in a strictly contamination-free environment confirmed by culture-based methods. Neurogastroenterol Motil. 2013;25:521–8. doi: 10.1111/nmo.12110. [DOI] [PubMed] [Google Scholar]
- 10.Huo R, Zeng B, Zeng L, Cheng K, Li B, Luo Y, et al. Microbiota modulate anxiety-like behavior and endocrine abnormalities in hypothalamic-pituitary-adrenal axis. Front Cell Infect Microbiol. 2017;7:489. doi: 10.3389/fcimb.2017.00489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bercik P, Denou E, Collins J, Jackson W, Lu J, Jury J, et al. The intestinal microbiota affect central levels of brain-derived neurotropic factor and behavior in mice. Gastroenterology. 2011;141:599–609. doi: 10.1053/j.gastro.2011.04.052. 699.e1-3. [DOI] [PubMed] [Google Scholar]
- 12.Bravo JA, Forsythe P, Chew MV, Escaravage E, Savignac HM, Dinan TG, et al. Ingestion of Lactobacillus strain regulates emotional behavior and central GABA receptor expression in a mouse via the vagus nerve. Proc Natl Acad Sci U S A. 2011;108:16050–5. doi: 10.1073/pnas.1102999108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Thabane M, Simunovic M, Akhtar-Danesh N, Garg AX, Clark WF, Collins SM, et al. An outbreak of acute bacterial gastroenteritis is associated with an increased incidence of irritable bowel syndrome in children. Am J Gastroenterol. 2010;105:933–9. doi: 10.1038/ajg.2010.74. [DOI] [PubMed] [Google Scholar]
- 14.Lyte M, Varcoe JJ, Bailey MT. Anxiogenic effect of subclinical bacterial infection in mice in the absence of overt immune activation. Physiol Behav. 1998;65:63–8. doi: 10.1016/s0031-9384(98)00145-0. [DOI] [PubMed] [Google Scholar]
- 15.Gaykema RP, Goehler LE, Lyte M. Brain response to cecal infection with Campylobacter jejuni: analysis with Fos immunohistochemistry. Brain Behav Immun. 2004;18:238–45. doi: 10.1016/j.bbi.2003.08.002. [DOI] [PubMed] [Google Scholar]
- 16.Verdu EF, Bercik P, Huang XX, Lu J, Al-Mutawaly N, Sakai H, et al. The role of luminal factors in the recovery of gastric function and behavioral changes after chronic Helicobacter pylori infection. Am J Physiol Gastrointest Liver Physiol. 2008;295:G664–70. doi: 10.1152/ajpgi.90316.2008. [DOI] [PubMed] [Google Scholar]
- 17.Champagne-Jorgensen K, Mian MF, Kay S, Hanani H, Ziv O, McVey Neufeld KA, et al. Prenatal low-dose penicillin results in long-term sex-specific changes to murine behaviour, immune regulation, and gut microbiota. Brain Behav Immun. 2020;84:154–63. doi: 10.1016/j.bbi.2019.11.020. [DOI] [PubMed] [Google Scholar]
- 18.Cryan JF, O'Riordan KJ, Cowan CS, Sandhu KV, Bastiaanssen TF, Boehme M, et al. The microbiota-gut-brain axis. Physiol Rev. 2019;99:1877–2013. doi: 10.1152/physrev.00018.2018. [DOI] [PubMed] [Google Scholar]
- 19.Yu Y, Raka F, Adeli K. The role of the gut microbiota in lipid and lipoprotein metabolism. J Clin Med. 2019 Dec 17;8(12):pii: E2227. doi: 10.3390/jcm8122227. doi: http://10.3390/jcm8122227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Koh A, De Vadder F, Kovatcheva-Datchary P, Backhed F. From dietary fiber to host physiology: short-chain fatty acids as key bacterial metabolites. Cell. 2016;165:1332–45. doi: 10.1016/j.cell.2016.05.041. [DOI] [PubMed] [Google Scholar]
- 21.Krishnan S, Ding Y, Saedi N, Choi M, Sridharan GV, Sherr DH, et al. Gut microbiota-derived tryptophan metabolites modulate inflammatory response in hepatocytes and macrophages. Cell Rep. 2018;23:1099–111. doi: 10.1016/j.celrep.2018.03.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Erny D, Hrabě de Angelis AL, Jaitin D, Wieghofer P, Staszewski O, David E, et al. Host microbiota constantly control maturation and function of microglia in the CNS. Nat Neurosci. 2015;18:965–77. doi: 10.1038/nn.4030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Louis P, Hold GL, Flint HJ. The gut microbiota, bacterial metabolites and colorectal cancer. Nat Rev Microbiol. 2014;12:661–72. doi: 10.1038/nrmicro3344. [DOI] [PubMed] [Google Scholar]
- 24.Morkl S, Lackner S, Meinitzer A, Mangge H, Lehofer M, Halwachs B, et al. Gut microbiota, dietary intakes and intestinal permeability reflected by serum zonulin in women. Eur J Nutr. 2018;57:2985–97. doi: 10.1007/s00394-018-1784-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chen T, Noto D, Hoshino Y, Mizuno M, Miyake S. Butyrate suppresses demyelination and enhances remyelination. J Neuroinflammation. 2019;16:165. doi: 10.1186/s12974-019-1552-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Agus A, Planchais J, Sokol H. Gut microbiota regulation of tryptophan metabolism in health and disease. Cell Host Microbe. 2018;23:716–24. doi: 10.1016/j.chom.2018.05.003. [DOI] [PubMed] [Google Scholar]
- 27.Lamas B, Richard ML, Leducq V, Pham HP, Michel ML, Da Costa G, et al. CARD9 impacts colitis by altering gut microbiota metabolism of tryptophan into aryl hydrocarbon receptor ligands. Nat Med. 2016;22:598–605. doi: 10.1038/nm.4102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zelante T, Iannitti RG, Cunha C, De Luca A, Giovannini G, Pieraccini G, et al. Tryptophan catabolites from microbiota engage aryl hydrocarbon receptor and balance mucosal reactivity via interleukin-22. Immunity. 2013;39:372–85. doi: 10.1016/j.immuni.2013.08.003. [DOI] [PubMed] [Google Scholar]
- 29.Rooks MG, Garrett WS. Gut microbiota, metabolites and host immunity. Nat Rev Immunol. 2016;16:341–52. doi: 10.1038/nri.2016.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Karbownik M, Reiter RJ, Garcia JJ, Cabrera J, Burkhardt S, Osuna C, et al. Indole-3-propionic acid, a melatonin-related molecule, protects hepatic microsomal membranes from iron-induced oxidative damage: relevance to cancer reduction. J Cell Biochem. 2001;81:507–13. [PubMed] [Google Scholar]
- 31.Chyan YJ, Poeggeler B, Omar RA, Chain DG, Frangione B, Ghiso J, et al. Potent neuroprotective properties against the Alzheimer beta-amyloid by an endogenous melatonin-related indole structure, indole-3-propionic acid. J Biol Chem. 1999;274:21937–42. doi: 10.1074/jbc.274.31.21937. [DOI] [PubMed] [Google Scholar]
- 32.de Mello VD, Paananen J, Lindström J, Lankinen MA, Shi L, Kuusisto J, et al. Indolepropionic acid and novel lipid metabolites are associated with a lower risk of type 2 diabetes in the Finnish Diabetes Prevention Study. Sci Rep. 2017;7:46337. doi: 10.1038/srep46337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gao J, Xu K, Liu H, Liu G, Bai M, Peng C, et al. Impact of the gut microbiota on intestinal immunity mediated by tryptophan metabolism. Front Cell Infect Microbiol. 2018;8:13. doi: 10.3389/fcimb.2018.00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gibson GR, Hutkins R, Sanders ME, Prescott SL, Reimer RA, Salminen SJ, et al. Expert consensus document: The International Scientific Association for Probiotics and Prebiotics (ISAPP) consensus statement on the definition and scope of prebiotics. Nat Rev Gastroenterol Hepatol. 2017;14:491–502. doi: 10.1038/nrgastro.2017.75. [DOI] [PubMed] [Google Scholar]
- 35.Burokas A, Arboleya S, Moloney RD, Peterson VL, Murphy K, Clarke G, et al. Targeting the microbiota-gut-brain axis: prebiotics have anxiolytic and antidepressant-like effects and reverse the impact of chronic stress in mice. Biol Psychiatry. 2017;82:472–87. doi: 10.1016/j.biopsych.2016.12.031. [DOI] [PubMed] [Google Scholar]
- 36.Savignac HM, Corona G, Mills H, Chen L, Spencer JP, Tzortzis G, et al. Prebiotic feeding elevates central brain derived neurotrophic factor, N-methyl-D-aspartate receptor subunits and D-serine. Neurochem Int. 2013;63:756–64. doi: 10.1016/j.neuint.2013.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Baxter NT, Schmidt AW, Venkataraman A, Kim KS, Waldron C, Schmidt TM. Dynamics of human gut microbiota and short-chain fatty acids in response to dietary interventions with three fermentable fibers. mBio. 2019 Jan 29;10(1):pii: e02566–18. doi: 10.1128/mBio.02566-18. doi: http://10.1128/mBio.02566-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Grimaldi R, Gibson GR, Vulevic J, Giallourou N, Castro-Mejía JL, Hansen LH, et al. A prebiotic intervention study in children with autism spectrum disorders (ASDs) Microbiome. 2018;6:133. doi: 10.1186/s40168-018-0523-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Azpiroz F, Dubray C, Bernalier-Donadille A, Cardot JM, Accarino A, Serra J, et al. Effects of scFOS on the composition of fecal microbiota and anxiety in patients with irritable bowel syndrome: a randomized, double blind, placebo controlled study. Neurogastroenterol Motil. 2017 Feb;29(2) doi: 10.1111/nmo.12911. doi: http://10.1111/nmo.12911. Epub 2016 Jul 31. [DOI] [PubMed] [Google Scholar]
- 40.World Health Organization; Food and Agriculture Organization of the United Nations. Probiotics in food: health and nutritional properties and guidelines for evaluation. Joint FAO/WHO expert consultation on evaluation of health and nutritional properties of probiotics in food including powder milk with live lactic acid bacteria. Rome: WHO/FAO; 2006. [Google Scholar]
- 41.Hill C, Guarner F, Reid G, Gibson GR, Merenstein DJ, Pot B, et al. Expert consensus document. The International Scientific Association for Probiotics and Prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat Rev Gastroenterol Hepatol. 2014;11:506–14. doi: 10.1038/nrgastro.2014.66. [DOI] [PubMed] [Google Scholar]
- 42.Desbonnet L, Garrett L, Clarke G, Kiely B, Cryan JF, Dinan TG. Effects of the probiotic Bifidobacterium infantis in the maternal separation model of depression. Neuroscience. 2010;170:1179–88. doi: 10.1016/j.neuroscience.2010.08.005. [DOI] [PubMed] [Google Scholar]
- 43.Messaoudi M, Lalonde R, Violle N, Javelot H, Desor D, Nejdi A, et al. Assessment of psychotropic-like properties of a probiotic formulation (Lactobacillus helveticus R0052 and Bifidobacterium longum R0175) in rats and human subjects. Br J Nutr. 2011;105:755–64. doi: 10.1017/S0007114510004319. [DOI] [PubMed] [Google Scholar]
- 44.Allen AP, Hutch W, Borre YE, Kennedy PJ, Temko A, Boylan G, et al. Bifidobacterium longum 1714 as a translational psychobiotic: modulation of stress, electrophysiology and neurocognition in healthy volunteers. Transl Psychiatry. 2016;6:e939. doi: 10.1038/tp.2016.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Flint HJ, Scott KP, Louis P, Duncan SH. The role of the gut microbiota in nutrition and health. Nat Rev Gastroenterol Hepatol. 2012;9:577–89. doi: 10.1038/nrgastro.2012.156. [DOI] [PubMed] [Google Scholar]
- 46.Samuel BS, Shaito A, Motoike T, Rey FE, Backhed F, Manchester JK, et al. Effects of the gut microbiota on host adiposity are modulated by the short-chain fatty-acid binding G protein-coupled receptor, Gpr41. Proc Natl Acad Sci U S A. 2008;105:16767–72. doi: 10.1073/pnas.0808567105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Braniste V, Al-Asmakh M, Kowal C, Anuar F, Abbaspour A, Tóth M, et al. The gut microbiota influences blood-brain barrier permeability in mice. Sci Transl Med. 2014;6:263ra158. doi: 10.1126/scitranslmed.3009759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yoo DY, Kim W, Nam SM, Kim DW, Chung JY, Choi SY, et al. Synergistic effects of sodium butyrate, a histone deacetylase inhibitor, on increase of neurogenesis induced by pyridoxine and increase of neural proliferation in the mouse dentate gyrus. Neurochem Res. 2011;36:1850–7. doi: 10.1007/s11064-011-0503-5. [DOI] [PubMed] [Google Scholar]
- 49.Kim HJ, Leeds P, Chuang DM. The HDAC inhibitor, sodium butyrate, stimulates neurogenesis in the ischemic brain. J Neurochem. 2009;110:1226–40. doi: 10.1111/j.1471-4159.2009.06212.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gutierrez-Vazquez C, Quintana FJ. Regulation of the immune response by the aryl hydrocarbon receptor. Immunity. 2018;48:19–33. doi: 10.1016/j.immuni.2017.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rothhammer V, Mascanfroni ID, Bunse L, Takenaka MC, Kenison JE, Mayo L, et al. Type I interferons and microbial metabolites of tryptophan modulate astrocyte activity and central nervous system inflammation via the aryl hydrocarbon receptor. Nat Med. 2016;22:586–97. doi: 10.1038/nm.4106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Dokalis N, Prinz M. Resolution of neuroinflammation: mechanisms and potential therapeutic option. Semin Immunopathol. 2019;41:699–709. doi: 10.1007/s00281-019-00764-1. [DOI] [PubMed] [Google Scholar]
- 53.Muku GE, Murray IA, Espin JC, Perdew GH. Urolithin a is a dietary microbiota-derived human aryl hydrocarbon receptor antagonist. Metabolites. 2018 Nov 29;8(4):pii: E86. doi: 10.3390/metabo8040086. doi: http://10.3390/metabo8040086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bonaz B, Bazin T, Pellissier S. The vagus nerve at the interface of the microbiota-gut-brain axis. Front Neurosci. 2018;12:49. doi: 10.3389/fnins.2018.00049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bogunovic M, Davé SH, Tilstra JS, Chang DT, Harpaz N, Xiong H, et al. Enteroendocrine cells express functional Toll-like receptors. Am J Physiol Gastrointest Liver Physiol. 2007;292:G1770–83. doi: 10.1152/ajpgi.00249.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Liu B, Fang F, Pedersen NL, Tillander A, Ludvigsson JF, Ekbom A, et al. Vagotomy and Parkinson disease: a Swedish register-based matched-cohort study. Neurology. 2017;88:1996–2002. doi: 10.1212/WNL.0000000000003961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Mitchell AM, Mitchell TJ. Streptococcus pneumoniae: virulence factors and variation. Clin Microbiol Infect. 2010;16:411–8. doi: 10.1111/j.1469-0691.2010.03183.x. [DOI] [PubMed] [Google Scholar]
- 58.Kim KS. Current concepts on the pathogenesis of Escherichia coli meningitis: implications for therapy and prevention. Curr Opin Infect Dis. 2012;25:273–8. doi: 10.1097/QCO.0b013e3283521eb0. [DOI] [PubMed] [Google Scholar]
- 59.Barichello T, Fagundes GD, Generoso JS, Elias SG, Simoes LR, Teixeira AL. Pathophysiology of neonatal acute bacterial meningitis. J Med Microbiol. 2013;62:1781–9. doi: 10.1099/jmm.0.059840-0. [DOI] [PubMed] [Google Scholar]
- 60.Kumar S, Ingle H, Prasad DV, Kumar H. Recognition of bacterial infection by innate immune sensors. Crit Rev Microbiol. 2013;39:229–46. doi: 10.3109/1040841X.2012.706249. [DOI] [PubMed] [Google Scholar]
- 61.Heckenberg SG, Brouwer MC, van de Beek D. Bacterial meningitis. Handb Clin Neurol. 2014;121:1361–75. doi: 10.1016/B978-0-7020-4088-7.00093-6. [DOI] [PubMed] [Google Scholar]
- 62.Sellner J, Täuber MG, Leib SL. Pathogenesis and pathophysiology of bacterial CNS infections. In: Roos KL, Tunkel AR, editors. Handbook of clinical neurology. v. 96. Philadelphia: Elsevier; 2010. pp. 1–16. [DOI] [PubMed] [Google Scholar]
- 63.Mook-Kanamori BB, Geldhoff M, van der Poll T, van de Beek D. Pathogenesis and pathophysiology of pneumococcal meningitis. Clin Microbiol Rev. 2011;24:557–91. doi: 10.1128/CMR.00008-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Iwasaki A, Medzhitov R. Regulation of adaptive immunity by the innate immune system. Science. 2010;327:291–5. doi: 10.1126/science.1183021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Abdel-Haq R, Schlachetzki JC, Glass CK, Mazmanian SK. Microbiome-microglia connections via the gut-brain axis. J Exp Med. 2019;216:41–59. doi: 10.1084/jem.20180794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Rodrigues FT, de Souza MR, Lima CN, da Silva FE, Costa DV, Dos Santos CC, et al. Major depression model induced by repeated and intermittent lipopolysaccharide administration: long-lasting behavioral, neuroimmune and neuroprogressive alterations. J Psychiatr Res. 2018;107:57–67. doi: 10.1016/j.jpsychires.2018.10.003. [DOI] [PubMed] [Google Scholar]
- 67.Gu M, Li Y, Tang H, Zhang C, Li W, Zhang Y, et al. Endogenous omega (n)-3 fatty acids in fat-1 mice attenuated depression-like behavior, imbalance between microglial M1 and M2 phenotypes, and dysfunction of neurotrophins induced by lipopolysaccharide administration. Nutrients. 2018 Sep 21;10(10):pii: E1351. doi: 10.3390/nu10101351. doi: http://10.3390/nu10101351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Simoes LR, Sangiogo G, Tashiro MH, Generoso JS, Faller CJ, Dominguini D, et al. Maternal immune activation induced by lipopolysaccharide triggers immune response in pregnant mother and fetus, and induces behavioral impairment in adult rats. J Psychiatr Res. 2018;100:71–83. doi: 10.1016/j.jpsychires.2018.02.007. [DOI] [PubMed] [Google Scholar]
- 69.Dickerson F, Adamos M, Katsafanas E, Khushalani S, Origoni A, Savage C, et al. The association between immune markers and recent suicide attempts in patients with serious mental illness: a pilot study. Psychiatry Res. 2017;255:8–12. doi: 10.1016/j.psychres.2017.05.005. [DOI] [PubMed] [Google Scholar]
- 70.Zhao Y, Jaber V, Lukiw WJ. Secretory products of the human GI tract microbiome and their potential impact on Alzheimer's disease (AD): detection of lipopolysaccharide (LPS) in AD hippocampus. Front Cell Infect Microbiol. 2017;7:318. doi: 10.3389/fcimb.2017.00318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Pietrucci D, Cerroni R, Unida V, Farcomeni A, Pierantozzi M, Mercuri NB, et al. Dysbiosis of gut microbiota in a selected population of Parkinson's patients. Parkinsonism Relat Disord. 2019;65:124–30. doi: 10.1016/j.parkreldis.2019.06.003. [DOI] [PubMed] [Google Scholar]
- 72.Schubert KO, Focking M, Cotter DR. Proteomic pathway analysis of the hippocampus in schizophrenia and bipolar affective disorder implicates 14-3-3 signaling, aryl hydrocarbon receptor signaling, and glucose metabolism: potential roles in GABAergic interneuron pathology. Schizophr Res. 2015;167:64–72. doi: 10.1016/j.schres.2015.02.002. [DOI] [PubMed] [Google Scholar]
- 73.Fujisawa TX, Nishitani S, Iwanaga R, Matsuzaki J, Kawasaki C, Tochigi M, et al. Association of aryl hydrocarbon receptor-related gene variants with the severity of autism spectrum disorders. Front Psychiatry. 2016;7:184. doi: 10.3389/fpsyt.2016.00184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Stilling RM, van de Wouw M, Clarke G, Stanton C, Dinan TG, Cryan JF. The neuropharmacology of butyrate: the bread and butter of the microbiota-gut-brain axis? Neurochem Int. 2016;99:110–32. doi: 10.1016/j.neuint.2016.06.011. [DOI] [PubMed] [Google Scholar]
- 75.Fischer A, Sananbenesi F, Wang X, Dobbin M, Tsai LH. Recovery of learning and memory is associated with chromatin remodelling. Nature. 2007;447:178–82. doi: 10.1038/nature05772. [DOI] [PubMed] [Google Scholar]
- 76.Chandrasekar V, Dreyer JL. The brain-specific neural zinc finger transcription factor 2b (NZF-2b/7ZFMyt1) suppresses cocaine self-administration in rats. Front Behav Neurosci. 2010;4:14. doi: 10.3389/fnbeh.2010.00014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Moretti M, Valvassori SS, Varela RB, Ferreira CL, Rochi N, Benedet J, et al. Behavioral and neurochemical effects of sodium butyrate in an animal model of mania. Behav Pharmacol. 2011;22:766–72. doi: 10.1097/FBP.0b013e32834d0f1b. [DOI] [PubMed] [Google Scholar]
- 78.Wei Y, Melas PA, Wegener G, Mathe AA, Lavebratt C. Antidepressant-like effect of sodium butyrate is associated with an increase in TET1 and in 5-hydroxymethylation levels in the Bdnf gene. Int J Neuropsychopharmacol. 2014 Oct 31;18(2):pii: pyu032. doi: 10.1093/ijnp/pyu032. doi: http://10.1093/ijnp/pyu032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Takuma K, Hara Y, Kataoka S, Kawanai T, Maeda Y, Watanabe R, et al. Chronic treatment with valproic acid or sodium butyrate attenuates novel object recognition deficits and hippocampal dendritic spine loss in a mouse model of autism. Pharmacol Biochem Behav. 2014;126:43–9. doi: 10.1016/j.pbb.2014.08.013. [DOI] [PubMed] [Google Scholar]
- 80.Kazemi A, Noorbala AA, Azam K, Eskandari MH, Djafarian K. Effect of probiotic and prebiotic vs placebo on psychological outcomes in patients with major depressive disorder:aA randomized clinical trial. Clin Nutr. 2019;38:522–8. doi: 10.1016/j.clnu.2018.04.010. [DOI] [PubMed] [Google Scholar]
- 81.Liu YW, Liong MT, Chung YE, Huang HY, Peng WS, Cheng YF, et al. Effects of Lactobacillus plantarum PS128 on children with autism spectrum disorder in Taiwan: a randomized, double-blind, placebo-controlled trial. Nutrients. 2019 Apr 11;11(4):pii: E820. doi: 10.3390/nu11040820. doi: http://10.3390/nu11040820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Reininghaus EZ, Wetzlmair LC, Fellendorf FT, Platzer M, Queissner R, Birner A, et al. Probiotic treatment in individuals with euthymic bipolar disorder: a pilot-study on clinical changes and compliance. Neuropsychobiology. 2020;79:71–9. doi: 10.1159/000493867. [DOI] [PubMed] [Google Scholar]
- 83.Severance EG, Gressitt KL, Stallings CR, Katsafanas E, Schweinfurth LA, Savage CL, et al. Probiotic normalization of Candida albicans in schizophrenia: a randomized, placebo-controlled, longitudinal pilot study. Brain Behav Immun. 2017;62:41–5. doi: 10.1016/j.bbi.2016.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Tamtaji OR, Heidari-Soureshjani R, Mirhosseini N, Kouchaki E, Bahmani F, Aghadavod E, et al. Probiotic and selenium co-supplementation, and the effects on clinical, metabolic and genetic status in Alzheimer's disease: a randomized, double-blind, controlled trial. Clin Nutr. 2019;38:2569–75. doi: 10.1016/j.clnu.2018.11.034. [DOI] [PubMed] [Google Scholar]
- 85.Tamtaji OR, Taghizadeh M, Daneshvar Kakhaki R, Kouchaki E, Bahmani F, Borzabadi S, et al. Clinical and metabolic response to probiotic administration in people with Parkinson's disease: a randomized, double-blind, placebo-controlled trial. Clin Nutr. 2019;38:1031–5. doi: 10.1016/j.clnu.2018.05.018. [DOI] [PubMed] [Google Scholar]
- 86.Tankou SK, Regev K, Healy BC, Cox LM, Tjon E, Kivisakk P, et al. Investigation of probiotics in multiple sclerosis. Mult Scler. 2018;24:58–63. doi: 10.1177/1352458517737390. [DOI] [PubMed] [Google Scholar]
- 87.Zhao H, Shi Y, Luo X, Peng L, Yang Y. The effect of fecal microbiota transplantation on a child with Tourette syndrome. Case Rep Med. 2017;2017:6165239. doi: 10.1155/2017/6165239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Cai T, Shi X, Yuan LZ, Tang D, Wang F. Fecal microbiota transplantation in an elderly patient with mental depression. Int Psychogeriatr. 2019;31:1525–6. doi: 10.1017/S1041610219000115. [DOI] [PubMed] [Google Scholar]
- 89.Kang DW, Adams JB, Gregory AC, Borody T, Chittick L, Fasano A, et al. Microbiota transfer therapy alters gut ecosystem and improves gastrointestinal and autism symptoms: an open-label study. Microbiome. 2017;5:10. doi: 10.1186/s40168-016-0225-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Kang DW, Adams JB, Coleman DM, Pollard EL, Maldonado J, McDonough-Means S, et al. Long-term benefit of microbiota transfer therapy on autism symptoms and gut microbiota. Sci Rep. 2019;9:5821. doi: 10.1038/s41598-019-42183-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Serrano-Pozo A, William CM, Ferrer I, Uro-Coste E, Delisle MB, Maurage CA, et al. Beneficial effect of human anti-amyloid-beta active immunization on neurite morphology and tau pathology. Brain. 2010;133:1312–27. doi: 10.1093/brain/awq056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Torres L, Robinson SA, Kim DG, Yan A, Cleland TA, Bynoe MS. Toxoplasma gondii alters NMDAR signaling and induces signs of Alzheimer's disease in wild-type, C57BL/6 mice. J Neuroinflammation. 2018;15:57. doi: 10.1186/s12974-018-1086-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Kumar DK, Choi SH, Washicosky KJ, Eimer WA, Tucker S, Ghofrani J, et al. Amyloid-beta peptide protects against microbial infection in mouse and worm models of Alzheimer's disease. Sci Transl Med. 2016;8:340ra72. doi: 10.1126/scitranslmed.aaf1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Hill JM, Clement C, Pogue AI, Bhattacharjee S, Zhao Y, Lukiw WJ. Pathogenic microbes, the microbiome, and Alzheimer's disease (AD) Front Aging Neurosci. 2014;6:127. doi: 10.3389/fnagi.2014.00127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Giridharan VV, Masud F, Petronilho F, Dal-Pizzol F, Barichello T. Infection-induced systemic inflammation is a potential driver of Alzheimer's disease progression. Front Aging Neurosci. 2019;11:122. doi: 10.3389/fnagi.2019.00122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Akbari E, Asemi Z, Daneshvar Kakhaki R, Bahmani F, Kouchaki E, Tamtaji OR, et al. Effect of probiotic supplementation on cognitive function and metabolic status in Alzheimer's disease: a randomized, double-blind and controlled trial. Front Aging Neurosci. 2016;8:256. doi: 10.3389/fnagi.2016.00256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Leblhuber F, Steiner K, Schuetz B, Fuchs D, Gostner JM. Probiotic supplementation in patients with Alzheimer's dementia: an explorative intervention study. Curr Alzheimer Res. 2018;15:1106–13. doi: 10.2174/1389200219666180813144834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Liu B, et al. Efficacy of probiotics on anxiety-A meta-analysis of randomized controlled trials. Depress Anxiety. 2018;35:935–45. doi: 10.1002/da.22811. [DOI] [PubMed] [Google Scholar]
- 99.Chong HX, Yusoff NA, Hor YY, Lew LC, Jaafar MH, Choi SB, et al. Lactobacillus plantarum DR7 alleviates stress and anxiety in adults: a randomised, double-blind, placebo-controlled study. Benef Microbes. 2019;10:355–73. doi: 10.3920/BM2018.0135. [DOI] [PubMed] [Google Scholar]
- 100.Stephen AM, Champ MM, Cloran SJ, Fleith M, van Lieshout L, Mejborn H, et al. Dietary fibre in Europe: current state of knowledge on definitions, sources, recommendations, intakes and relationships to health. Nutr Res Rev. 2017;30:149–90. doi: 10.1017/S095442241700004X. [DOI] [PubMed] [Google Scholar]
- 101.Lefter R, Ciobica A, Timofte D, Stanciu C, Trifan A. A descriptive review on the prevalence of gastrointestinal disturbances and their multiple associations in autism spectrum disorder. Medicina (Kaunas) 2019 Dec 27;56(1):pii: E11. doi: 10.3390/medicina56010011. doi: http://10.3390/medicina56010011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Ristori MV, Quagliariello A, Reddel S, Ianiro G, Vicari S, Gasbarrini A, et al. Autism, gastrointestinal symptoms and modulation of gut microbiota by nutritional interventions. Nutrients. 2019 Nov 18;11(11):pii: E2812. doi: 10.3390/nu11112812. doi: http://10.3390/nu11112812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Alshammari MK, AlKhulaifi MM, Al Farraj DA, Somily AM, Albarrag AM. Incidence of Clostridium perfringens and its toxin genes in the gut of children with autism spectrum disorder. Anaerobe. 2019;61:102114. doi: 10.1016/j.anaerobe.2019.102114. [DOI] [PubMed] [Google Scholar]
- 104.Buffington SA, Di Prisco GV, Auchtung TA, Ajami NJ, Petrosino JF, Costa-Mattioli M. Microbial reconstitution reverses maternal diet-induced social and synaptic deficits in offspring. Cell. 2016;165:1762–75. doi: 10.1016/j.cell.2016.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Evans SJ, Bassis CM, Hein R, Assari S, Flowers SA, Kelly MB, et al. The gut microbiome composition associates with bipolar disorder and illness severity. J Psychiatr Res. 2017;87:23–9. doi: 10.1016/j.jpsychires.2016.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Dickerson F, Adamos M, Katsafanas E, Khushalani S, Origoni A, Savage C, et al. Adjunctive probiotic microorganisms to prevent rehospitalization in patients with acute mania: a randomized controlled trial. Bipolar Disord. 2018;20:614–21. [Google Scholar]
- 107.Benton D, Williams C, Brown A. Impact of consuming a milk drink containing a probiotic on mood and cognition. Eur J Clin Nutr. 2007;61:355–61. doi: 10.1038/sj.ejcn.1602546. [DOI] [PubMed] [Google Scholar]
- 108.Akkasheh G, Kashani-Poor Z, Tajabadi-Ebrahimi M, Jafari P, Akbari H, Taghizadeh M, et al. Clinical and metabolic response to probiotic administration in patients with major depressive disorder: a randomized, double-blind, placebo-controlled trial. Nutrition. 2016;32:315–20. doi: 10.1016/j.nut.2015.09.003. [DOI] [PubMed] [Google Scholar]
- 109.Miyaoka T, Kanayama M, Wake R, Hashioka S, Hayashida M, Nagahama M, et al. Clostridium butyricum MIYAIRI 588 as adjunctive therapy for treatment-resistant major depressive disorder: a prospective open-label trial. Clin Neuropharmacol. 2018;41:151–5. doi: 10.1097/WNF.0000000000000299. [DOI] [PubMed] [Google Scholar]
- 110.Scheperjans F, Aho V, Pereira PA, Koskinen K, Paulin L, Pekkonen E, et al. Gut microbiota are related to Parkinson's disease and clinical phenotype. Mov Disord. 2015;30:350–8. doi: 10.1002/mds.26069. [DOI] [PubMed] [Google Scholar]
- 111.Hasegawa S, Goto S, Tsuji H, Okuno T, Asahara T, Nomoto K, et al. Intestinal dysbiosis and lowered serum lipopolysaccharide-binding protein in Parkinson's disease. PLoS One. 2015;10:e0142164. doi: 10.1371/journal.pone.0142164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Nguyen TT, Kosciolek T, Maldonado Y, Daly RE, Martin AS, McDonald D, et al. Differences in gut microbiome composition between persons with chronic schizophrenia and healthy comparison subjects. Schizophr Res. 2019;204:23–9. doi: 10.1016/j.schres.2018.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Ghaderi A, Banafshe HR, Mirhosseini N, Moradi M, Karimi MA, Mehrzad F, et al. Clinical and metabolic response to vitamin D plus probiotic in schizophrenia patients. BMC Psychiatry. 2019;19:77. doi: 10.1186/s12888-019-2059-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Okubo R, Koga M, Katsumata N, Odamaki T, Matsuyama S, Oka M, et al. Effect of bifidobacterium breve A-1 on anxiety and depressive symptoms in schizophrenia: a proof-of-concept study. J Affect Disord. 2019;245:377–85. doi: 10.1016/j.jad.2018.11.011. [DOI] [PubMed] [Google Scholar]
- 115.Zheng P, Zeng B, Liu M, Chen J, Pan J, Han Y, et al. The gut microbiome from patients with schizophrenia modulates the glutamate-glutamine-GABA cycle and schizophrenia-relevant behaviors in mice. Sci Adv. 2019 Feb 6;5(2):eaau8317. doi: 10.1126/sciadv.aau8317. doi: http://10.1126/sciadv.aau8317. eCollection 2019 Feb. [DOI] [PMC free article] [PubMed] [Google Scholar]