Abstract
OBJECTIVE:
To evaluate whether there is an increased risk of neurologic diagnoses in children who are HIV-exposed but uninfected (CHEU) exposed in utero to specific antiretroviral medications.
DESIGN:
Prospective cohort study of CHEU enrolled from 2007–2017.
METHODS:
We evaluated children for neurologic case status, including microcephaly, febrile seizures, seizure disorders, ophthalmologic disorders, and other neurologic disorders. Adjusted relative risks (aRRs) were estimated for the association between in utero antiretroviral exposure and neurologic case using log-binomial regression, accounting for potential confounders. Sensitivity analyses were conducted to evaluate robustness of findings.
RESULTS:
Among 3747 eligible CHEU, 231 (6.2%) met neurologic case criteria (95% CI:5.4%, 7.0%). Most eligible children (86%) were exposed in utero to combination antiretroviral regimens. In adjusted models, children exposed to efavirenz at any time during pregnancy had higher risk of neurologic case status (aRR=1.53, 95% CI:0.94, 2.51). This association was stronger when comparing efavirenz exposure at conception to no exposure during pregnancy (aRR=1.92, 95% CI:1.09,3.36) and considering follow-up and case diagnosis only through age 2 (aRR=2.14, 95% CI:1.11,4.12). Children exposed to didanosine at conception and during the first trimester had increased risk of neurologic case status (aRR=2.28, 95% CI:1.07,4.87 and aRR=2.02, 95% CI:1.01,4.04, respectively), compared to didanosine-unexposed children. Children with dolutegravir exposure had some suggestion of increased risk of neurologic case (aRR=2.43, 95% CI:0.75,7.84), which was observed consistently across several sensitivity analyses.
CONCLUSIONS:
Efavirenz and didanosine exposure during pregnancy were associated with higher risk of neurologic abnormalities in CHEU, and dolutegravir exposure showed some suggestive associations which warrant further monitoring.
Keywords: HIV, pediatrics, antiretroviral agents, long term adverse effects, neurologic manifestations
Introduction
With an estimated 1.3 million women living with HIV giving birth annually, it is critical to assess the safety of in utero antiretroviral (ARV) exposure to the fetus.(1–3) There have long been concerns regarding the potential teratogenicity of ARV medications, in particular efavirenz (EFV).(4–7) Multiple studies have now shown that in utero EFV exposure is not associated with an increased risk of central nervous system congenital anomalies (8, 9); however, data on newer ARVs are limited. Indeed the recent experience with peri-conception dolutegravir (DTG) exposure and neural tube defects has highlighted the importance of continued surveillance for potential adverse effects from in utero ARV exposure.(10–12)
Most studies of ARV safety in pregnancy have focused on outcomes at or soon after birth; however, not all adverse effects are evident at birth and data on the risk of more subtle neurologic diagnoses are limited.(13–16) Studies with follow-up beyond one month of life have not demonstrated an increased risk of neurologic diagnoses in children who are HIV-exposed but uninfected (CHEU), but have limited follow-up to the first few years of life or less.(8, 9, 17, 18) (19) Thus, concerns remain regarding adverse neurologic outcomes from possible mitochondrial dysfunction or other mechanisms in children exposed in utero to ARVs. As new ARVs are introduced in pregnancy, the safety of in utero exposure remains a critical public health issue.
The Surveillance Monitoring for ART Toxicities (SMARTT) Study is a prospective observational study established by the Pediatric HIV/AIDS Cohort Study (PHACS) network to identify potential adverse effects of in utero ARV exposure to CHEU.(20) The aim of this analysis was to determine the prevalence of neurologic diagnoses among CHEU and to evaluate whether there is an increased risk of neurologic diagnoses associated with in utero exposure to specific ARVs.
Methods
Study Population
The PHACS SMARTT study includes two cohorts of CHEU: the Static cohort (children enrolled from 2007–2009 ages 1 to 12 years who had participated in prior studies with available pregnancy and birth data) and the Dynamic cohort (enrolled during gestation or within 1 week after birth).(20) The SMARTT study is conducted at 22 clinical research sites in the U.S. including Puerto Rico and enrollment and follow-up are ongoing, with youth followed to age 18 years. The protocol was approved by Institutional Review Boards at each site and at the Harvard T.H. Chan School of Public Health. Written informed consent was obtained from the parent or legal guardian of each enrolled child.
Maternal and child clinical diagnoses and dates of prenatal ARV use were obtained from medical records and participant interview at study entry; retrospectively for the Static cohort. Birth characteristics (gestational age, birth weight, and delivery mode) were abstracted and maternal HIV disease characteristics during pregnancy were collected, including plasma HIV viral load (VL), absolute CD4 lymphocyte (CD4) cell counts, and CD4%. Information on substance use during pregnancy was obtained by self-reported questionnaire, including alcohol, tobacco, marijuana, opiates, and other substances.(21) After enrollment, children and their mothers or caregivers were followed at annual study visits. A complete physical examination was conducted on the child including anthropometric assessments (height, weight, head circumference [HC]), and any new diagnoses or illnesses that occurred since the prior study visit were abstracted from the medical chart or interview. Caregivers completed questionnaires on household composition, education and income levels, history of psychiatric diagnoses or substance use, and other information related to family environment at all study visits.
A study “trigger” for potential neurologic diagnoses was defined as a febrile or afebrile seizure, microcephaly, or other neurologic or ophthalmologic disorders, as previously described.(20) Children meeting any of these triggers either at enrollment (retrospectively for the Static cohort) or at an annual study visit were referred to an appropriate specialist (neurologist or ophthalmologist) for clinical evaluation. The results of the triggered evaluations were reviewed and children classified as cases or non-cases by the SMARTT Review Panel (SRP), a group of clinical and epidemiological experts, using a predefined “neurologic case” definition and blinded to the specific ARV regimens mothers used during pregnancy. The following diagnoses did not require a follow-up assessment by a specialist for confirmation: microcephaly, febrile seizures and diagnoses that were recorded at two or more consecutive study visits (e.g., persisted for ≥2 years). Neurologic diagnoses determined to be secondary to events occurring after birth (e.g. post-natal meningitis, trauma) were not included as cases. A small percentage of participants met a trigger but did not have follow-up assessments needed to determine case status and were considered unevaluable for case determination (Figure 1). Children were deemed eligible for inclusion in this analysis if they enrolled by April 1, 2017 and had a study visit for neurologic trigger assessment by August 1, 2017.
Figure 1.

Derivation of Study Population for Evaluating Incidence of Neurologic Diagnoses and Associations with in utero Antiretroviral Exposures
Outcome measures
As the purpose of this study was to identify any potential signal of a neurological adverse event associated with in utero ARV exposure, the primary outcome of neurologic case was broadly defined and included all of the diagnoses chosen a priori to be included in the definition of a neurologic trigger. Microcephaly was determined based on HC measurements obtained after 6 months of age and was defined as a HC Z-score <−2 for children up to age 3 years based on the Centers for Disease Control and Prevention (CDC) growth curves, and less than the 2nd percentile according to annual thresholds based on criteria published by Nellhaus for children over 3 years.(22) We accounted for prematurity for children up to age 2 years by subtracting the gestational age in weeks from 40, and adjusting the age at HC measurement by this difference. Children were classified as a neurologic case if they met the criteria at any time during follow-up, while those never having any of the diagnoses of interest were considered to not have the outcome.
Exposure measures
The primary exposure of interest was in utero ARV exposure. Children were classified according to exposure to individual ARV agents, by trimester of maternal ARV initiation, and by pre-conception exposure versus never exposed during gestation. The reference group was children unexposed to the specific ARV agent of interest, either overall or within the time period (trimester or pre-conception) of interest. For example, for 1st trimester exposure the exposed groups were defined as children of mothers who initiated the ARV agent of interest during the first trimester whereas the unexposed (reference) group were children who were not exposed to the ARV agent during gestation. Individual ARV agents were evaluated among a targeted at-risk subset of participants, based on the calendar year in which the ARV had been approved. Specifically, models were restricted to children born after 2007 for darunavir and raltegravir, after 2011 for rilpivirine, and after 2013 for DTG, and elvitegravir. These restrictions allowed a more appropriate comparison to contemporaneous regimens in use at the time each child was born. All other ARV drugs were evaluated in the full SMARTT cohort.
Potential confounders
We identified potential confounders using directed acyclic graphs (DAGs) based on previous literature and our own study findings. These potential confounders included maternal factors (age, race, ethnicity, chronic health conditions, obstetrical complications and substance use), birth cohort (<2011, 2011–2014, 2015–2017), and family/household factors (socioeconomic status, household income level and caregiver education level). Since maternal ARV use has been demonstrated to be associated with adverse birth outcomes and maternal health measures in pregnancy, preterm birth, low birthweight, cesarean section, maternal HIV VL and CD4 count were not considered as potential confounders (eg., not considered for inclusion in adjusted models) since they may be on the causal pathway between in utero ARV exposure and adverse neurologic outcomes. However, descriptive summaries of neurologic case status by these measures were provided.
Statistical analysis
The percent classified as neurologic cases and the corresponding exact 95% confidence intervals (CIs) were estimated under the binomial distribution. Demographic and maternal characteristics were compared between cases and non-cases using Fisher’s exact test for binary characteristics and Chi-Square tests for categorical characteristics. Log-binomial regression analysis was used to obtain relative risks (RRs) for associations with in utero ARV exposure. Due to the large number of ARV exposures evaluated, covariates included in the final adjusted models for case status were selected based on their association with neurologic case and potential role as a confounder based on the DAGs described above. Missing indicator approaches were used to account for missing information on covariates. A significance level of p=0.05 was employed, but conclusions were based on consistency across primary and multiple sensitivity analyses.
Sensitivity analyses:
A number of sensitivity analyses were conducted to assess the robustness of findings. As follow-up of the cohort is currently ongoing, children born in earlier years generally had more follow-up time during which a neurologic case could occur. We conducted analyses to account for this possible bias: 1) by restricting analysis to children enrolled in the dynamic cohort; 2) by restricting follow-up to the first two years of life (i.e. excluding events after age 2 and children enrolled after age 2); and 3) by fitting Poisson regression models for the incidence rate ratios (IRRs) accounting for person-time of follow-up by each participant. For the first two sensitivity analyses noted above, we utilized modified Poisson regression to obtain estimated RRs and their corresponding 95% CIs.(23) A sensitivity analysis adjusting for preterm birth was also conducted to confirm that associations between ARV exposure and neurologic case were not solely due to prematurity. Sensitivity analyses were also conducted to account for clustering of children within clinical research sites and to account for clustering of children within families (e.g. the same mother or caregiver). Finally, we conducted a sensitivity analysis excluding children with major congenital anomalies based on review by the SRP and outside experts.
Results
Characteristics of study population
A total of 3758 SMARTT participants had enrolled as of April 1, 2017 and had at least one study visit by August 1, 2017. Among these, 11 had an indeterminate neurologic case status and were excluded from analyses, yielding 3747 evaluable participants; their characteristics are shown in Table 1. Just under half were female (48%), 68% Black, and 31% Hispanic. Self-reported tobacco and alcohol use during pregnancy were relatively common (17% and 8%, respectively), and 8% of mothers reported marijuana use during pregnancy; however, reported cocaine/opiates use was relatively rare (3%).
Table 1.
Demographic and Maternal Characteristics of SMARTT study participants by Neurologic Case Status, 2007–2017
| Characteristic | Neurologic Case Status | Total (N=3747) |
P-Value | |
|---|---|---|---|---|
| No (N=3516) |
Yes (N=231) |
|||
| Child Characteristics | ||||
| Duration of follow-up (years), Median (IQR) | 4.2 (1.3, 6.9) | 5.9 (4.1, 7.9) | 4.3 (1.4, 7.0) | <0.001 |
| Birth Cohort | <0.001 | |||
| < 2002 | 367 (10%) | 24 (10%) | 391 (10%) | |
| 2002–2006 | 582 (17%) | 48 (21%) | 630 (17%) | |
| 2007–2010 | 1,075 (31%) | 97 (42%) | 1,172 (31%) | |
| 2011–2014 | 960 (27%) | 51 (22%) | 1,011 (27%) | |
| 2015–2017 | 532 (15%) | 11 (5%) | 543 (14%) | |
| Female sex | 1,681 (48%) | 122 (53%) | 1,803 (48%) | 0.15 |
| Race/origin | 0.021 | |||
| White | 916 (26%) | 70 (30%) | 986 (26%) | |
| Black/African American | 2,397 (68%) | 141 (61%) | 2,538 (68%) | |
| Other | 25 (1%) | 5 (2%) | 30 (1%) | |
| Not reported | 178 (5%) | 15 (6%) | 193 (5%) | |
| Latino/Hispanic | 1,082 (31%) | 86 (37%) | 1,168 (31%) | 0.048 |
| Birth characteristics | ||||
| Low birth weight (< 2500g) | 566 (16%) | 81 (35%) | 647 (17%) | <0.001 |
| Preterm birth (< 37 weeks gestation) | 633 (18%) | 71 (31%) | 704 (19%) | <0.001 |
| Cesarean delivery | 1,899 (54%) | 142 (61%) | 2,041 (54%) | 0.056 |
| Caregiver/Household Characteristics | ||||
| Annual household income < $20,000 | 2,328 (66%) | 157 (68%) | 2,485 (66%) | 0.64 |
| Caregiver not high school graduate | 1,135 (32%) | 92 (40%) | 1,227 (33%) | 0.030 |
| Maternal Characteristics | ||||
| Age and Marital Status | ||||
| <25 years at birth of child | 1,072 (30%) | 67 (29%) | 1,139 (30%) | 0.61 |
| Single, never married | 2,284 (65%) | 145 (63%) | 2,429 (65%) | 0.42 |
| Maternal Immunologic and Virologic Health During Pregnancy | ||||
| VL > 1000 copies/mL at delivery | 463 (13%) | 22 (10%) | 485 (13%) | 0.11 |
| VL > 1000 copies/mL earliest available in pregnancy | 1,662 (47%) | 115 (50%) | 1,777 (47%) | 0.49 |
| CD4 < 250 cells/mm3 at delivery | 479 (14%) | 37 (16%) | 516 (14%) | 0.28 |
| CD4 < 250 cells/mm3 early in pregnancy | 620 (18%) | 41 (18%) | 661 (18%) | 0.93 |
| Maternal Substance Use During Pregnancy | ||||
| Cocaine/opiates | 84 (2%) | 11 (5%) | 95 (3%) | 0.047 |
| Marijuana use | 263 (7%) | 23 (10%) | 286 (8%) | 0.20 |
| Alcohol use | 286 (8%) | 24 (10%) | 310 (8%) | 0.27 |
| Tobacco use | 587 (17%) | 65 (28%) | 652 (17%) | <0.001 |
| Maternal Sexually Transmitted Infections During Pregnancy | ||||
| Gonorrhea | 87 (2%) | 6 (3%) | 93 (2%) | 0.83 |
| Chlamydia | 280 (8%) | 19 (8%) | 299 (8%) | 0.99 |
| Trichomonas | 370 (11%) | 26 (11%) | 396 (11%) | 0.99 |
| Syphilis | 92 (3%) | 6 (3%) | 98 (3%) | 0.99 |
| Any of above | 668 (19%) | 43 (19%) | 711 (19%) | 0.80 |
Combination ARV regimen defined as at least 3 ARV drugs from at least two drug classes
Percentages add up to more than 86% because some combination regimens included agents from 2 or more of the following ARV drug classes: PI, NNRTI, and II
IQR=interquartile range (25th, 75th percentiles), PI=protease inhibitor, NNRTI=non-nucleoside reverse transcriptase inhibitor, II=integrase inhibitor, VL= HIV viral load.
P-value calculated using Wilcoxon rank sum test for continuous characteristics, Fisher’s exact test for binary characteristics and a Chi-Square test for categorical characteristics. Data were unavailable for some participants for: ethnicity (n=3), birthweight (n=26), gestational age (n=45), delivery mode (n=56), household income (n=255), caregiver education (n=44), maternal ARV regimen (n=94), maternal age at delivery (n=40), marital status (n=44), maternal VL (n=170), maternal CD4 (n=178), substance use during pregnancy (n=218), and sexually transmitted infections (n=148).
Case status and neurologic diagnoses
Of the 3747 participants included, 231 (6.2%; exact 95% CI:5.4%, 7.0%) met criteria for being a neurologic case over a median follow-up of 4.3 years (interquartile range [IQR]:1.4, 7.0). The most common neurologic diagnoses were microcephaly (25.1%), febrile seizure (17.6%), eye-related abnormalities including esotropia, exotropia, strabismus, ptosis, nystagmus, ambylopia, and optic nerve abnormalities (16.5%) and non-febrile seizure (13.5%). A full list of the neurologic diagnoses is provided in Supplemental Table 1. The median age of first neurologic diagnosis was 2.1 years (IQR:1.1, 5.0).
Demographic and maternal characteristics by neurologic case status
Children were more likely to be a neurologic case if they were born in earlier years (≤ 2010), of white race or Hispanic ethnicity, with low caregiver education, and if their mother reported use of tobacco, alcohol, marijuana, or cocaine/opiates during pregnancy (Table 1). In multivariable models, tobacco use during pregnancy emerged as the substance most strongly associated with neurologic case status and, due to high rates of concurrent tobacco use, other substances (alcohol, marijuana, cocaine/opiates) were no longer related to case status after accounting for tobacco use. Low caregiver education was also no longer associated with case status in multivariable models after adjusting for the other demographic characteristics and sociodemographic measures. The final adjusted model included Hispanic ethnicity, tobacco use during pregnancy, and birth cohort (2011–2014 and 2015–2017 vs. pre-2011).
Association of in utero antiretroviral exposures with neurologic case status
In adjusted models, exposure to EFV was associated with a 53% increased risk of neurologic case (adjusted RR (aRR)=1.53, 95% CI:0.94, 2.51) (Table 2, Figure 2). There was a stronger association for EFV exposure at conception (aRR=1.92, 95% CI:1.09, 3.36) (Supplemental Table 2). In addition, didanosine (ddI) exposure at conception (aRR=2.28, 95% CI:1.07, 4.87) was associated with an increased risk of being a neurologic case. DTG exposure was associated with over two-fold increased risk of being a neurologic case (aRR=2.43, 95% CI:0.75, 7.84). The magnitude of association for DTG was stronger for exposure during the first trimester (aRR=2.95, 95% CI:0.79, 11.01) and at conception (aRR=3.47, 95% CI:0.74, 16.36).
Table 2.
Association of in utero ARV Exposures with Neurologic Case Status in SMARTT Cohort
| Antiretroviral Drug or Class | Event Rates | Estimated Relative Risks | ||||||
|---|---|---|---|---|---|---|---|---|
| N | % Exposed |
Cases, exposed |
Cases, unexposed |
Unadjusted RR (95% CI) |
P-value | Adjusted RR (95% CI) |
P-value | |
| By ARV Drug Class or Regimen | ||||||||
| HAART | 3623 | 86.0 | 192/3116 (6.2%) | 33/507 (6.5%) | 0.95 (0.65, 1.37) | 0.77 | 1.30 (0.86, 1.97) | 0.22 |
| NRTIs | 3653 | 97.4 | 218/3558 (6.1%) | 8/95 (8.4%) | 0.73 (0.36, 1.47) | 0.38 | 0.88 (0.39, 1.99) | 0.77 |
| NNRTIs | 3653 | 18.6 | 42/678 (6.2%) | 184/2975 (6.2%) | 1.00 (0.72, 1.39) | 0.99 | 1.20 (0.86, 1.66) | 0.28 |
| PIs | 3653 | 69.9 | 166/2553 (6.5%) | 60/1100 (5.5%) | 1.19 (0.89, 1.60) | 0.24 | 1.09 (0.80, 1.49) | 0.57 |
| IIs | 2492 | 15.7 | 13/391 (3.3%) | 122/2101 (5.8%) | 0.57 (0.32, 1.01) | 0.056 | 0.88 (0.48, 1.61) | 0.68 |
| Nucleoside Reverse Transcriptase Inhibitors (NRTIs) | ||||||||
| Lamivudine | 3653 | 61.5 | 146/2245 (6.5%) | 80/1408 (5.7%) | 1.14 (0.88, 1.49) | 0.32 | 0.89 (0.67, 1.18) | 0.42 |
| Abacavir | 3653 | 17.7 | 38/647 (5.9%) | 188/3006 (6.3%) | 0.94 (0.67, 1.32) | 0.72 | 0.92 (0.65, 1.30) | 0.63 |
| Stavudine | 3653 | 2.8 | 7/104 (6.7%) | 219/3549 (6.2%) | 1.09 (0.51, 2.32) | 0.82 | 0.91 (0.43, 1.95) | 0.81 |
| Didanosine | 3653 | 2.5 | 9/92 (9.8%) | 217/3561 (6.1%) | 1.61 (0.82, 3.13) | 0.16 | 1.36 (0.67, 2.77) | 0.40 |
| Emtricitabine | 3653 | 39.1 | 70/1427 (4.9%) | 156/2226 (7.0%) | 0.70 (0.53, 0.92) | 0.011 | 0.99 (0.73, 1.34) | 0.93 |
| Tenofovir DF | 3653 | 41.6 | 79/1520 (5.2%) | 147/2133 (6.9%) | 0.75 (0.58, 0.98) | 0.037 | 1.00 (0.75, 1.34) | 0.97 |
| Zidovudine | 3653 | 61.5 | 149/2247 (6.6%) | 77/1406 (5.5%) | 1.21 (0.93, 1.58) | 0.16 | 0.90 (0.67, 1.20) | 0.48 |
| Non-nucleoside Reverse Transcriptase Inhibitors (NNRTIs) | ||||||||
| Efavirenz | 3653 | 4.5 | 15/166 (9.0%) | 211/3487 (6.1%) | 1.49 (0.91, 2.46) | 0.12 | 1.53 (0.94, 2.51) | 0.090 |
| Nevirapine | 3653 | 6.8 | 21/250 (8.4%) | 205/3403 (6.0%) | 1.39 (0.91, 2.14) | 0.13 | 1.23 (0.79, 1.92) | 0.36 |
| Rilpivirine | 1261 | 19.2 | 4/242 (1.7%) | 44/1019 (4.3%) | 0.38 (0.14, 1.07) | 0.066 | 0.47 (0.17, 1.31) | 0.15 |
| Protease Inhibitors (PIs) | ||||||||
| Atazanavir | 3653 | 18.8 | 38/685 (5.5%) | 188/2968 (6.3%) | 0.88 (0.62, 1.23) | 0.44 | 1.02 (0.72, 1.44) | 0.90 |
| Darunavir | 2492 | 13.0 | 14/325 (4.3%) | 121/2167 (5.6%) | 0.77 (0.44, 1.34) | 0.36 | 0.89 (0.51, 1.57) | 0.69 |
| Indinavir | 3653 | 1.3 | 1/47 (2.1%) | 225/3606 (6.2%) | 0.34 (0.05, 2.43) | 0.28 | 0.30 (0.04, 2.12) | 0.23 |
| Lopinavir/r | 3653 | 27.7 | 69/1012 (6.8%) | 157/2641 (5.9%) | 1.15 (0.87, 1.51) | 0.33 | 1.05 (0.80, 1.39) | 0.73 |
| Nelfinavir | 3653 | 16.3 | 46/594 (7.7%) | 180/3059 (5.9%) | 1.32 (0.96, 1.80) | 0.084 | 1.02 (0.73, 1.42) | 0.90 |
| Saquinavir | 3653 | 2.3 | 8/85 (9.4%) | 218/3568 (6.1%) | 1.54 (0.76, 3.12) | 0.23 | 1.39 (0.68, 2.83) | 0.37 |
| Integrase Inhibitors (IIs) | ||||||||
| Dolutegravir | 782 | 12.0 | 4/94 (4.3%) | 17/688 (2.5%) | 1.72 (0.58, 5.12) | 0.33 | 2.43 (0.75, 7.84) | 0.14 |
| Elvitegravir | 782 | 13.8 | 2/108 (1.9%) | 19/674 (2.8%) | 0.66 (0.15, 2.82) | 0.57 | 0.70 (0.16, 3.04) | 0.63 |
| Raltegravir | 2492 | 8.0 | 7/199 (3.5%) | 128/2293 (5.6%) | 0.63 (0.29, 1.35) | 0.23 | 0.71 (0.33, 1.53) | 0.38 |
Relative risks estimated by log-binomial regression for full SMARTT cohort and by modified Poisson regression for other subgroups.
Adjusted model includes Hispanic ethnicity, tobacco use during pregnancy, and birth cohorts (2011–2014 and 2015–2017 vs <2011).
Models restricted to children born after 2007 for darunavir, raltegravir, and IIs (as drug class), after 2011 for rilpivirine, and after 2013 for dolutegravir and elvitegravir; all other ARV drugs are evaluated in the full SMARTT cohort.
Figure 2. Adjusted relative risks for associations of in utero ARV exposures with neurologic case, based on any exposure during pregnancy and first trimester exposures.

Adjusted relative risks (aRRs) and their confidence intervals (CIs) were estimated by log-binomial regression for full SMARTT cohort and by modified Poisson regression for other subgroups. Adjusted models include Hispanic ethnicity, tobacco use during pregnancy, and birth cohorts (2011–2014 and 2015–2017 vs <2011. Models restricted to children born after 2007 for darunavir and raltegravir, after 2011 for rilpivirine, and after 2013 for dolutegravir and elvitegravir; all other ARV drugs were evaluated in the full SMARTT cohort.
Sensitivity analyses
Analyses restricting to those enrolled at birth, accounting for clustering within research site and within the same family (same mother or caregiver), considering only the first two years of life, excluding children with major congenital anomalies, accounting for person years of follow-up and adjusting for prematurity for associations between EFV, ddI or DTG exposure (any exposure in pregnancy and 1st trimester exposure only) with neurologic case status are shown in Figure 3. Results of the unadjusted analyses and sensitivity analyses for all ARVs are provided in Supplemental Tables 3–10. Of note, the magnitude of association between in utero EFV exposure and neurologic case status was similar to that seen in the primary analyses across all sensitivity analyses and was statistically significant when restricting to the first two years of life for any EFV exposure or first trimester exposure. The magnitude of association between in utero DTG exposure and neurologic case status also remained similar across all sensitivity analyses to that seen in the primary analyses and was statistically significant for first trimester DTG exposure after accounting for person years of follow-up and after accounting for clustering within site.
Figure 3. Sensitivity Analyses for Associations of Efavirenz, Dolutegravir, and Didanosine with Neurologic Case Status.
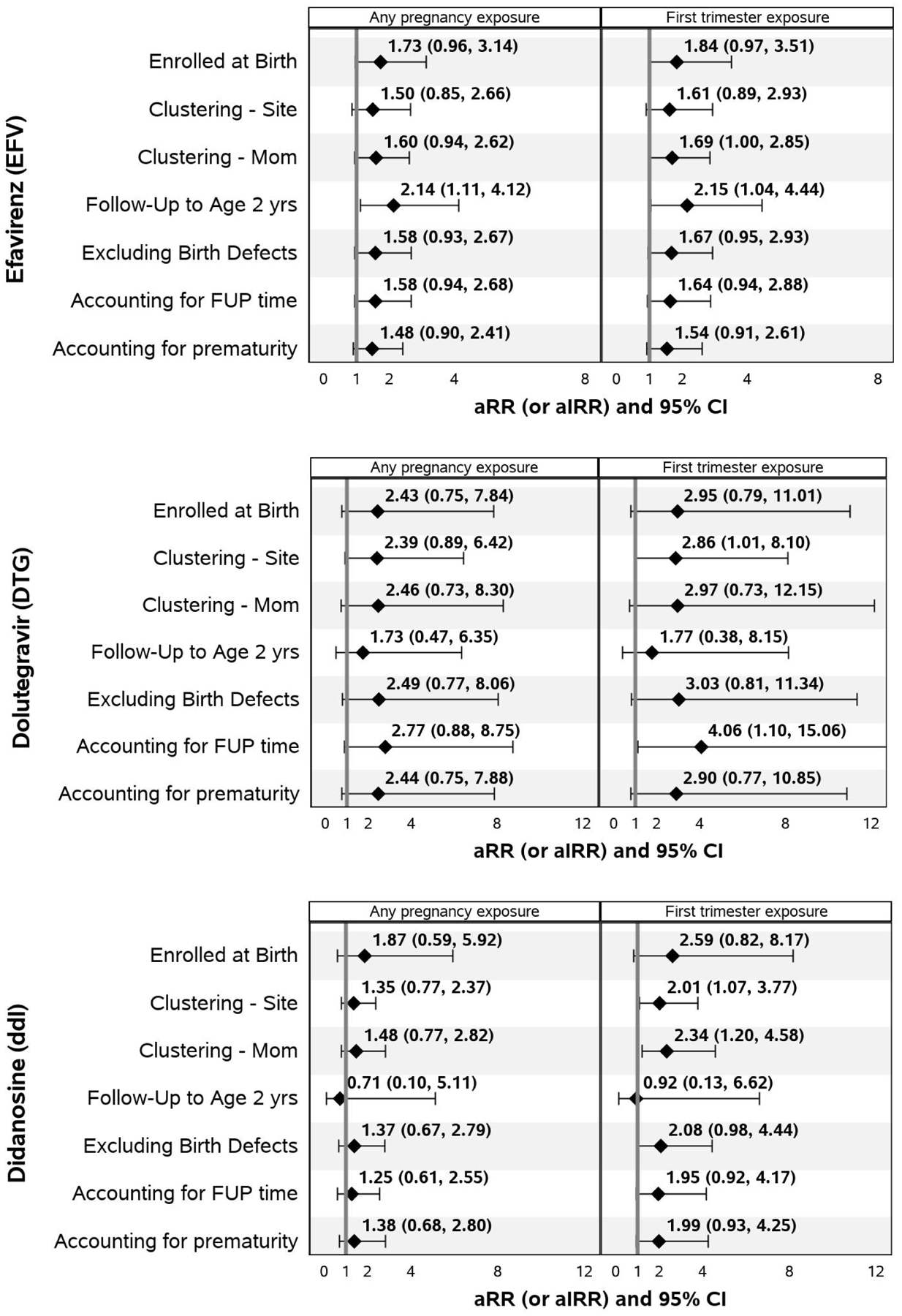
Adjusted relative risks (aRRs) are based on modified Poisson regression models and adjusted incidence rate ratios (aIRRs) are based on standard Poisson regression models accounting for person time; both types of models adjust for Hispanic ethnicity, tobacco use during pregnancy, and birth cohort (2011–2014 and 2015–2017 vs <2011). Models accounting for clustering within site or within the same mother/family are fit using generalized estimating equation models with an assumed exchangeable correlation structure. FUP=follow-up.
Discussion
We observed a high percentage of children who developed one or more neurologic diagnoses in our cohort compared to previous studies (6.2% vs. 0.3–2%).(13, 14, 16, 24) This may be in part because our study was designed to detect a broad range of adverse neurologic events, including microcephaly and eye-related abnormalities, which previous studies have not included, and because our study included longer follow-up of infants and children.
Our findings demonstrate that infants with in utero EFV exposure had over 50% increased risk of having a neurologic diagnosis. This association persisted in multiple sensitivity analyses. In utero DTG exposure was associated with an increased risk of having a neurologic diagnosis but the associations were not statistically stable except in specific sensitivity analyses due to the small number of exposed cases. The data on the association between in utero ARV exposure and neurologic diagnoses in CHEU have been conflicting; however, studies to date have been limited by their duration of follow-up, sample size and ability to adjust for confounders. The French Perinatal Cohort Study (FPCS) found that CHEU exposed to a non-nucleoside reverse transcriptase inhibitor combination, most commonly zidovudine + lamivudine, were more likely to be classified as having mitochondrial dysfunction compared to CHEU exposed to zidovudine monotherapy (RR=2.5; 95% CI:1.0, 6.5) with the majority of cases characterized by neurologic symptoms (86%); however, this analysis did not adjust for potential confounders.(14) A follow-up study of the FPCS found that CHEU exposed to EFV in utero had a higher risk of neurologic congenital anomalies but this was only statistically significant in a secondary analysis when applying a broader classification of neurologic congenital anomalies.(5) The IMPAACT 219/219C study demonstrated an increased risk of mitochondrial dysfunction associated with third trimester initiation of lamivudine alone and in combination with zidovudine.(16) In addition, ddI exposure during the peri-conception period and during the first trimester was found to be associated with an increased risk of having a neurologic diagnosis.
Subsequent studies in other cohorts have failed to confirm this potential increased risk of neurologic diagnoses associated with in utero ARV exposure; however, most of these studies have been limited by either their duration of follow-up, restricted outcome definition to only cases that resulted in death, or limited ability to compare various ARV regimens.(17, 18, 24–29) Experience with other medications has demonstrated the importance of long-term follow-up when evaluating the safety of medications administered in pregnancy.(19) Indeed, results from the FPCS have shown that symptoms of mitochondrial dysfunction are usually not present in the first few months of life. We followed children in our cohort for a median of 4 years allowing us to detect potential safety signals later in childhood. The median age of first confirmation of neurologic case status was 2.1 years, suggesting that a large proportion of cases would be missed if follow-up were restricted to the first few months of life.
Additionally, when conducting analyses of drug safety in pregnancy it is crucial to have the correct comparator groups. Comparing ARV-exposed to ARV-unexposed CHEU raises issues of confounding by indication (e.g. worse maternal HIV disease status leading to ARV initiation in the ARV-exposed group in earlier years of SMARTT). Comparing one ARV-exposed group to another can raise issues of confounding by historical bias if the years of exposure differ greatly between the two groups. Given our large sample size and the high proportion of in utero ARV-exposure in our cohort, we were able to address these issues by restricting our analyses to the specific time-period when the ARV of interest was approved for use.
Febrile seizures was one of the most commonly encountered neurologic diagnosis in our study (17.6%) with an overall prevalence of 1.3%. The prevalence in our cohort is lower than that found in the general U.S. population (2–5%); however, this does not necessarily negate an association with specific ARV exposures. Indeed, the FPCS observed a higher cumulative risk of febrile seizures by 18 months in CHEU with in utero and/or postnatal ARV exposure compared to CHEU without ARV exposure (11.0 vs. 4.1 per 1000, p=0.02).(15) Their study observed no association with specific ARV exposures, but was conducted early in the epidemic when the number of ARVs available was limited.
Our study had several limitations. First, as mentioned above, in an observational study, both known and unknown confounders could be present. We adjusted for several confounders and restricted the analyses to the time-period when the ARV of interest was approved for use. Additionally, we systematically identified and confirmed our neurological cases blinded to ARV exposure. As many participants enrolled after birth, we may have missed some major neurologic congenital anomalies that were detected on prenatal ultrasound and subsequently resulted in either spontaneous or therapeutic abortion. Missing cases due to pregnancy termination would have biased our findings towards the null and excluding children with major congenital anomalies did not change the magnitude of the associations seen in our study. Our analysis was implemented as a screening study rather than a test of any single hypothesis, with a goal of identifying potential signals of toxicity. We recognize that this approach involves multiple comparisons and may increase the risk of false positive findings. At the same time, we did not want to fail to detect true associations. Key to this screening strategy is an emphasis on consistency and robustness of findings across multiple sensitivity analyses, along with the need to confirm any potential associations in further more focused studies. We also acknowledge that sensitivity analyses conducted to examine different exposure periods do not reflect independent analyses, given the overlap in ARV-exposed cases between time periods of interest; these time-specific risk estimates must be interpreted cautiously, but can help inform whether the effect of ARV exposure differs by timing. Another limitation was that smaller numbers of children were exposed to newer ARV agents, which limited our ability to detect statistically significant signals for these agents; our data suggest a potential association between DTG and neurologic case status emphasizing the importance of continued surveillance of CHEU exposed to DTG and other new ARV agents. Finally, in an attempt to be inclusive and not miss any potential safety signals our definition of neurologic case included all potential neurologic and ophthalmologic diagnoses, which precludes us from making any conclusions about causal effects and potential long-term consequences. Despite the broad outcome measure, we feel that our results are important as they demonstrate potential safety concerns associated with specific in utero ARV exposures. Given the fact that our outcome measure represents different etiologies with potentially different causal mechanisms and severity of presentation, it is important that our findings be confirmed with larger sample sizes within specific exposure groups of interest.
In conclusion, we found that in utero EFV and ddI exposure were associated with higher risks of neurologic abnormalities in infancy and childhood. There was a suggestion of an association between in utero DTG exposure and higher risk of neurologic abnormalities in infancy and childhood but this association was not statistically stable due to the recent introduction of DTG and the small numbers of cases to date. The recommended antiretroviral therapy (ART) in pregnancy has recently been called into question by reports of increased risk of neural tube defects in CHEU exposed to DTG.(10) Statistical modelling suggests that despite this increased risk of neural tube defects DTG-based ART in pregnancy would avert more maternal deaths than EFV-based ART.(30) Our findings highlight the importance of continuing to proactively evaluate the safety of ART in pregnancy and the need for long-term follow-up of CHEU.(31) Further study is needed to confirm our findings in other cohorts, as well as to determine the clinical implications of the neurologic diagnoses and investigate the underlying mechanisms of the associations seen in our study.(32, 33)
Supplementary Material
Acknowledgements:
We thank the children and families for their participation in PHACS, and the individuals and institutions involved in the conduct of PHACS. The study was supported by the Eunice Kennedy Shriver National Institute of Child Health and Human Development with co-funding from the National Institute on Drug Abuse, the National Institute of Allergy and Infectious Diseases, the Office of AIDS Research, the National Institute of Mental Health, the National Institute of Neurological Disorders and Stroke, the National Institute on Deafness and Other Communication Disorders, the National Institute of Dental and Craniofacial Research, and the National Institute on Alcohol Abuse and Alcoholism, through cooperative agreements with the Harvard T.H. Chan School of Public Health (HD052102) (Principal Investigator: George Seage; Program Director: Julie Alperen) and the Tulane University School of Medicine (HD052104) (Principal Investigator: Russell Van Dyke; Co-Principal Investigator: Ellen Chadwick; Project Director: Patrick Davis). Data management services were provided by Frontier Science and Technology Research Foundation (PI: Suzanne Siminski), and regulatory services and logistical support were provided by Westat, Inc (PI: Julie Davidson).
The following institutions, clinical site investigators and staff participated in conducting PHACS SMARTT in 2017, in alphabetical order: Ann & Robert H. Lurie Children’s Hospital of Chicago: Ellen Chadwick, Margaret Ann Sanders, Kathleen Malee, Scott Hunter; Baylor College of Medicine: William Shearer, Mary Paul, Norma Cooper, Lynnette Harris; Bronx Lebanon Hospital Center: Murli Purswani, Emma Stuard, Mahboobullah Mirza Baig, Alma Villegas; Children’s Diagnostic & Treatment Center: Ana Puga, Dia Cooley, Patricia A. Garvie, James Blood; New York University School of Medicine: William Borkowsky, Sandra Deygoo, Marsha Vasserman; Rutgers - New Jersey Medical School: Arry Dieudonne, Linda Bettica, Juliette Johnson; St. Jude Children’s Research Hospital: Katherine Knapp, Kim Allison, Megan Wilkins, Jamie Russell-Bell; San Juan Hospital/Department of Pediatrics: Nicolas Rosario, Lourdes Angeli-Nieves, Vivian Olivera; SUNY Downstate Medical Center: Stephan Kohlhoff, Ava Dennie, Ady Ben-Israel, Jean Kaye; Tulane University School of Medicine: Russell Van Dyke, Karen Craig, Patricia Sirois; University of Alabama, Birmingham: Marilyn Crain, Paige Hickman, Dan Marullo; University of California, San Diego: Stephen A. Spector, Kim Norris, Sharon Nichols; University of Colorado, Denver: Elizabeth McFarland, Emily Barr, Christine Kwon, Carrie Chambers; University of Florida, Center for HIV/AIDS Research, Education and Service: Mobeen Rathore, Kristi Stowers, Saniyyah Mahmoudi, Nizar Maraqa, Laurie Kirkland; University of Illinois, Chicago: Karen Hayani, Lourdes Richardson, Renee Smith, Alina Miller; University of Miami: Gwendolyn Scott, Sady Dominguez, Jenniffer Jimenez, Anai Cuadra; Keck Medicine of the University of Southern California: Toni Frederick, Mariam Davtyan, Guadalupe Morales-Avendano, Janielle Jackson-Alvarez; University of Puerto Rico School of Medicine, Medical Science Campus: Zoe M. Rodriguez, Ibet Heyer, Nydia Scalley Trifilio.
Conflicts of Interest and Source of Funding:
EGC holds stock in Abbot and AbbVie. All other authors report no conflicts of interest. The Pediatric HIV/AIDS Cohort Study (PHACS) was supported by the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) with co-funding from the National Institute of Dental and Craniofacial Research (NIDCR), the National Institute of Allergy and Infectious Diseases (NIAID), the National Institute of Neurological Disorders and Stroke (NINDS), the National Institute on Deafness and Other Communication Disorders (NIDCD), Office of AIDS Research (OAR), the National Institute of Mental Health (NIMH), the National Institute on Drug Abuse (NIDA), and the National Institute on Alcohol Abuse and Alcoholism (NIAAA), through Cooperative agreements with the Harvard T.H. Chan School of Public Health (HD052102) and the Tulane University School of Medicine (HD052104).
Footnotes
Note: The conclusions and opinions expressed in this article are those of the authors and do not necessarily reflect those of the National Institutes of Health or U.S. Department of Health and Human Services.
References:
- 1.UNAIDS. Pregnant Women Needing ARV for PMTCT [cited 2019 October 8]. Available from: http://aidsinfo.unaids.org/.
- 2.Bailey H, Zash R, Rasi V, Thorne C. HIV treatment in pregnancy. Lancet HIV. 2018;5(8):e457–e67. [DOI] [PubMed] [Google Scholar]
- 3.Slogrove AL, Clayden P, Abrams EJ. Toward a universal antiretroviral regimen: special considerations of pregnancy and breast feeding. Curr Opin HIV AIDS. 2017;12(4):359–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sibiude J, Le Chenadec J, Bonnet D, Tubiana R, Faye A, Dollfus C, et al. In utero exposure to zidovudine and heart anomalies in the ANRS French perinatal cohort and the nested PRIMEVA randomized trial. Clin Infect Dis. 2015;61(2):270–80. [DOI] [PubMed] [Google Scholar]
- 5.Sibiude J, Mandelbrot L, Blanche S, Le Chenadec J, Boullag-Bonnet N, Faye A, et al. Association between prenatal exposure to antiretroviral therapy and birth defects: an analysis of the French perinatal cohort study (ANRS CO1/CO11). PLoS Med. 2014;11(4):e1001635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fundaro C, Genovese O, Rendeli C, Tamburrini E, Salvaggio E. Myelomeningocele in a child with intrauterine exposure to efavirenz. AIDS. 2002;16(2):299–300. [DOI] [PubMed] [Google Scholar]
- 7.De Santis M, Carducci B, De Santis L, Cavaliere AF, Straface G. Periconceptional exposure to efavirenz and neural tube defects. Arch Intern Med. 2002;162(3):355. [DOI] [PubMed] [Google Scholar]
- 8.Williams PL, Crain MJ, Yildirim C, Hazra R, Van Dyke RB, Rich K, et al. Congenital anomalies and in utero antiretroviral exposure in human immunodeficiency virus-exposed uninfected infants. JAMA Pediatr. 2015;169(1):48–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ford N, Mofenson L, Shubber Z, Calmy A, Andrieux-Meyer I, Vitoria M, et al. Safety of efavirenz in the first trimester of pregnancy: an updated systematic review and meta-analysis. AIDS. 2014;28 Suppl 2:S123–31. [DOI] [PubMed] [Google Scholar]
- 10.Zash R, Makhema J, Shapiro RL. Neural-Tube Defects with Dolutegravir Treatment from the Time of Conception. The New England journal of medicine. 2018;379(10):979–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zash R, Holmes L, Diseko M, Jacobson DL, Brummel S, Mayondi G, et al. Neural-Tube Defects and Antiretroviral Treatment Regimens in Botswana. The New England journal of medicine. 2019;381(9):827–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Raesima MM, Ogbuabo CM, Thomas V, Forhan SE, Gokatweng G, Dintwa E, et al. Dolutegravir Use at Conception - Additional Surveillance Data from Botswana. The New England journal of medicine. 2019;381(9):885–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Blanche S, Tardieu M, Rustin P, Slama A, Barret B, Firtion G, et al. Persistent mitochondrial dysfunction and perinatal exposure to antiretroviral nucleoside analogues. Lancet. 1999;354(9184):1084–9. [DOI] [PubMed] [Google Scholar]
- 14.Barret B, Tardieu M, Rustin P, Lacroix C, Chabrol B, Desguerre I, et al. Persistent mitochondrial dysfunction in HIV-1-exposed but uninfected infants: clinical screening in a large prospective cohort. AIDS. 2003;17(12):1769–85. [DOI] [PubMed] [Google Scholar]
- 15.Landreau-Mascaro A, Barret B, Mayaux MJ, Tardieu M, Blanche S, French Perinatal Cohort Study G. Risk of early febrile seizure with perinatal exposure to nucleoside analogues. Lancet. 2002;359(9306):583–4. [DOI] [PubMed] [Google Scholar]
- 16.Brogly SB, Ylitalo N, Mofenson LM, Oleske J, Van Dyke R, Crain MJ, et al. In utero nucleoside reverse transcriptase inhibitor exposure and signs of possible mitochondrial dysfunction in HIV-uninfected children. AIDS. 2007;21(8):929–38. [DOI] [PubMed] [Google Scholar]
- 17.Petra Study Team. Efficacy of three short-course regimens of zidovudine and lamivudine in preventing early and late transmission of HIV-1 from mother to child in Tanzania, South Africa, and Uganda (Petra study): a randomised, double-blind, placebo-controlled trial. Lancet. 2002;359(9313):1178–86. [DOI] [PubMed] [Google Scholar]
- 18.Spaulding AB, Yu Q, Civitello L, Mussi-Pinhata MM, Pinto J, Gomes IM, et al. Neurologic Outcomes in HIV-Exposed/Uninfected Infants Exposed to Antiretroviral Drugs During Pregnancy in Latin America and the Caribbean. AIDS Res Hum Retroviruses. 2016;32(4):349–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Goodman A, Schorge J, Greene MF. The long-term effects of in utero exposures--the DES story. The New England journal of medicine. 2011;364(22):2083–4. [DOI] [PubMed] [Google Scholar]
- 20.Williams PL, Hazra R, Van Dyke RB, Yildirim C, Crain MJ, Seage GR 3rd, et al. Antiretroviral exposure during pregnancy and adverse outcomes in HIV-exposed uninfected infants and children using a trigger-based design. AIDS. 2016;30(1):133–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tassiopoulos K, Read JS, Brogly S, Rich K, Lester B, Spector SA, et al. Substance use in HIV-Infected women during pregnancy: self-report versus meconium analysis. AIDS and behavior. 2010;14(6):1269–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nellhaus G Head circumference from birth to eighteen years. Practical composite international and interracial graphs. Pediatrics. 1968;41(1):106–14. [PubMed] [Google Scholar]
- 23.Zou G A modified poisson regression approach to prospective studies with binary data. Am J Epidemiol. 2004;159(7):702–6. [DOI] [PubMed] [Google Scholar]
- 24.Brogly SB, Foca M, Deville JG, Mirochnick M, Scott GB, Mofenson LM, et al. Potential confounding of the association between exposure to nucleoside analogues and mitochondrial dysfunction in HIV-uninfected and indeterminate infants. J Acquir Immune Defic Syndr. 2010;53(1):154–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.European Collaborative Study. Exposure to antiretroviral therapy in utero or early life: the health of uninfected children born to HIV-infected women. J Acquir Immune Defic Syndr. 2003;32(4):380–7. [DOI] [PubMed] [Google Scholar]
- 26.Nucleoside exposure in the children of HIV-infected women receiving antiretroviral drugs: absence of clear evidence for mitochondrial disease in children who died before 5 years of age in five United States cohorts. J Acquir Immune Defic Syndr. 2000;25(3):261–8. [DOI] [PubMed] [Google Scholar]
- 27.Bulterys M, Nesheim S, Abrams EJ, Palumbo P, Farley J, Lampe M, et al. Lack of evidence of mitochondrial dysfunction in the offspring of HIV-infected women. Retrospective review of perinatal exposure to antiretroviral drugs in the Perinatal AIDS Collaborative Transmission Study. Ann N Y Acad Sci. 2000;918:212–21. [DOI] [PubMed] [Google Scholar]
- 28.Dominguez K, Bertolli J, Fowler M, Peters V, Ortiz I, Melville S, et al. Lack of definitive severe mitochondrial signs and symptoms among deceased HIV-uninfected and HIV-indeterminate children < or = 5 years of age, Pediatric Spectrum of HIV Disease project (PSD), USA. Ann N Y Acad Sci. 2000;918:236–46. [DOI] [PubMed] [Google Scholar]
- 29.Lindegren ML, Rhodes P, Gordon L, Fleming P, Perinatal Safety Review Working G, State, et al. Drug safety during pregnancy and in infants. Lack of mortality related to mitochondrial dysfunction among perinatally HIV-exposed children in pediatric HIV surveillance. Ann N Y Acad Sci. 2000;918:222–35. [PubMed] [Google Scholar]
- 30.Dugdale CM, Ciaranello AL, Bekker LG, Stern ME, Myer L, Wood R, et al. Risks and Benefits of Dolutegravir- and Efavirenz-Based Strategies for South African Women With HIV of Child-Bearing Potential: A Modeling Study. Ann Intern Med. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hoffman RM, Mofenson LM. Decision-Making in a Time of Uncertainty: Dolutegravir for Reproductive-Age Women. Ann Intern Med. 2019. [DOI] [PubMed] [Google Scholar]
- 32.van de Wijer L, Garcia LP, Hanswijk SI, Rando J, Middelman A, Ter Heine R, et al. Neurodevelopmental and behavioral consequences of perinatal exposure to the HIV drug efavirenz in a rodent model. Transl Psychiatry. 2019;9(1):84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schomaker M, Davies MA, Cornell M, Ford N. Assessing the risk of dolutegravir for women of childbearing potential. Lancet Glob Health. 2018;6(9):e958–e9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


