Abstract
Background
Cancer is the second leading cause of mortality worldwide. Integrating different levels of care by implementing screening programmes, extending diagnostic tools and applying therapeutic advances may increase survival. We implemented a cancer fast-track programme (CFP) to shorten the time between suspected cancer symptoms, diagnosis and therapy initiation.
Patients and methods
Descriptive data were collected from the 10 years since the CFP was implemented (2009-2019) at the Clinico-Malvarrosa Health Department in Valencia, Spain. General practitioners (GPs), an oncology coordinator and 11 specialists designed guidelines for GP patient referral to the CFP, including criteria for breast, digestive, gynaecological, lung, urological, dermatological, head and neck, and soft tissue cancers. Patients with enlarged lymph nodes and constitutional symptoms were also considered. On identifying patients with suspected cancer, GPs sent a case proposal to the oncology coordinator. If criteria were met, an appointment was quickly made with the patient. We analysed the timeline of each stage of the process.
Results
A total of 4493 suspected cancer cases were submitted to the CFP, of whom 4019 were seen by the corresponding specialist. Cancer was confirmed in 1098 (27.3%) patients: breast cancer in 33%, urological cancers in 22%, gastrointestinal cancer in 19% and lung cancer in 15%. The median time from submission to cancer testing was 11 days, and diagnosis was reached in a median of 19 days. Treatment was started at a median of 34 days from diagnosis.
Conclusions
The findings of this study show that the interval from GP patient referral to specialist testing, cancer diagnosis and start of therapy can be reduced. Implementation of the CFP enabled most patients to begin curative intended treatment, and required only minimal resources in our setting.
Key words: cancer, early diagnostic, primary care
Highlights
-
•
Our CFP easily connects GPs and hospital specialists.
-
•
Our CFP shortens assessment time in patients with suspected cancer, adding to quality care.
-
•
Our CFP decreases emotional stress in patients without cancer.
Introduction
Cancer remains one of the main causes of morbidity and mortality worldwide, with ∼18.1 million new cases in 2020.1 Current population estimates indicate that the number of new cases will rise during the next two decades to 29.5 million per year by 2040. According to the GLOBOCAN project, the most common cancers in Spain in order of frequency are prostate, colorectal, lung and bladder in males, and breast, colorectal, lung, endometrial and bladder in females.2,3
Early detection dramatically increases the chances of effective treatment,4,5 and recognition of warning symptoms of cancer and taking prompt action leads to early diagnosis. Detection of such symptoms by physicians, nurses and other health care professionals may lead to patient referral to a specialist and early diagnosis, potentially reaching a better outcome. Nonetheless, the correct timing of cancer diagnosis and treatment requires an adequate integration of all available patient care resources.6,7
In Europe, patients often consult their general practitioner (GP) before being diagnosed with cancer,8 although the vast majority of patients seen by GPs present with symptoms that will not ultimately lead to a cancer diagnosis. Patients with symptoms suggestive of cancer therefore need an initial evaluation by their GP, followed by specialized care if they meet criteria for cancer suspicion. Accordingly, the first step in the process requires GPs to distinguish between symptoms that do or do not suggest malignancy.
Symptomatic people who visit their GPs may need further evaluation and/or testing to diagnose or rule out cancer. In order to provide a coordinated, efficient and affordable solution to this issue and improve communication between primary and specialized care, a cancer fast-track programme (CFP) was launched in our health area in 2009. The aim was to promote cooperation between different hospital specialists and GPs and to shorten the time from identification of cancer-related symptoms to diagnosis and initiation of appropriate therapy.9
In this report, we present an assessment after 10 years of follow-up of the CFP, in terms of times from recognition of cancer-related symptoms to diagnosis and initiation of appropriate treatment, types and diagnostic stage of different tumours and time to rule out cancer in suspected patients.
Materials and methods
The programme began in June 2009. This study analysed data from 10 years (June 2009 to July 2019) in the Clinico-Malvarrosa Health Department in Valencia (CMH), an area that includes 33 primary care centres serving a population of around 345 026 inhabitants.10 All data were collected on a systematic, prospective basis.
To accelerate cancer diagnosis and treatment initiation in this setting, six specialists (in tumour types chosen for high incidence or diagnostic delay in our area) met regularly with GPs and the oncology coordinator to discuss cases. Guidelines were initially developed for GPs' use in referring patients with suspected breast, colorectal, cervical, lung or bladder cancers. Patients with growing lateral, cervical and supraclavicular lymph nodes were referred to the Head and Neck Unit, whereas axillary and inguinal lymph nodes were included in the programme for evaluation by the Haematology Department. These criteria, based on clinical practice and medical literature, are shown in Supplementary Figure S1, available at https://doi.org/10.1016/j.esmoop.2021.100148. Concurrently, health guidelines were published to raise public awareness of warning signs and symptoms of the most common tumours which should be brought up in the primary care setting. Patients with high suspicion of cancer or with imaging tests suggestive of cancer were assessed directly at the Oncology Unit.
During the first years of the CFP, the majority of patients referred were for suspected breast cancer (BC; 52%). These patients were evaluated by the breast care specialist, and in most cases imaging test (mammography or breast ultrasound) was requested. With the intent of optimizing resources, from 2017 onwards patients sent to the CFP with suspected BC were referred directly to the breast imaging unit, with subsequent referral to the appropriate specialist as necessary.
During the last 2 years of the programme, the number of specialities in the programme was expanded for several reasons. First, the CFP was proving to function satisfactorily, and second, GPs identified a large number of patients with nonspecific symptoms not included in the programme but which ultimately resulted in cancer diagnosis. Consequently, internal medicine, traumatology and dermatology joined the CFP, and new referral criteria for GPs, such as dysphagia, weight loss and ascites, were also added.
As part of the CFP protocol, GPs identifying patients with suspected cancer sent their record card to the oncology coordinator the same day. The coordinator reviewed these cases and referred them to the appropriate cancer specialist, following previously defined criteria. Patients referred to the CFP with imaging tests suggestive of or high suspicion of cancer, but who otherwise did not meet specific criteria for a particular cancer type, were referred to the Internal Medicine or Clinical Oncology Departments. If the submission did not meet established criteria, the patient was sent back to the GP.
Data from all submitted cases were recorded in a Microsoft Access database (Redmond, WA) to facilitate further evaluation. Statistical analysis was performed using R software (R Foundation for Statistical Analysis, Vienna, Austria).
Results
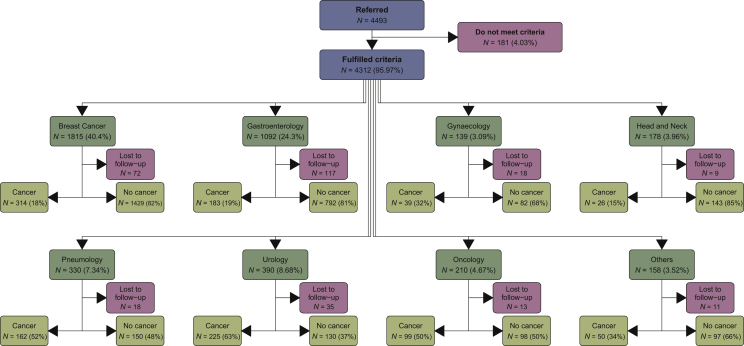
A total of 4493 suspected cancer cases were submitted to the CFP from June 2009 to June 2019, of which 4019 were studied by the corresponding specialist. In all, 181 proposals did not meet established criteria, while another 293 patients were lost to follow-up and did not attend the specialist appointment.
Cancer was confirmed in 1098 patients (27.3% of all specialist-studied proposals). The largest number of proposals were of suspected BC (40.4%), followed by suspected digestive cancer (24.3%).
All proposals and their corresponding specialists are shown in Figure 1.
Figure 1.
Consort diagram showing all referred proposals to different specialist units.
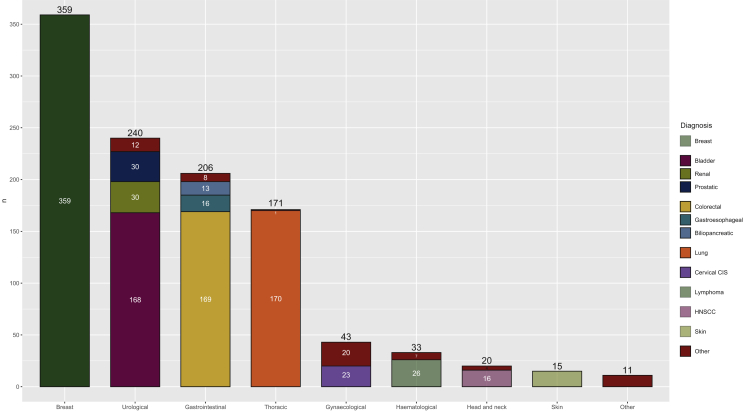
Among all patients assessed in different specialities on the pathway, BC was diagnosed in 359 patients (33%). A total of 244 (68%) women had a localized BC, 80 patients (22%) had a locally advanced BC and 23 patients (7%) had advanced BC. In situ carcinoma was diagnosed in 12 patients (3%).
Out of all BC diagnoses, 223 women (62%) were diagnosed outside the mammography screening target age group in our setting: 54 patients (15%) were younger than 45 and 169 (47%) older than 69, while 136 women (38%) diagnosed were in the screening programme age range (from 45 to 69 years).
Urological carcinoma was confirmed in 240 patients (22%), most of whom were diagnosed with bladder carcinoma (168 patients, 70%), and the majority with localized disease (161 patients), while only 7 patients had advanced disease. A total of 30 patients (13%) were diagnosed with prostate cancer (21 had localized disease and 7 had advanced disease, while the other 2 patients were treated in a different centre with unknown stage). Another 30 patients (13%) were diagnosed with renal carcinoma (24 patients in early stage and 6 patients with advanced cancer). The remaining cases were penile carcinoma (one case, early stage), testicular cancer (seven cases, all localized tumours) and urinary tract carcinoma (four patients; three with localized tumours and one with advanced cancer).
Gastrointestinal tumours were found in 206 patients (19%). The most frequent diagnoses were: colorectal cancer (CRC; 169 patients, 82%), gastroesophageal carcinoma (16 patients, 7%) and biliopancreatic neoplasms (13 patients, 6%). The remaining patients were diagnosed with hepatocellular carcinoma (n = 2), anal carcinoma (n = 3), and neuroendocrine intestinal tumours (n = 3).
Regarding CRC, 116 patients (68%) were diagnosed outside the screening programme age group in our area (50-69 years): 3% were under 50 and 65% were over 70; 53 patients (32%) diagnosed were within the screening age range.
Most CRC patients were diagnosed in an early stage: 121 patients (72%) had localized disease; 20 patients (12%) had locally advanced CRC, whereas 28 patients (16%) were diagnosed at an advanced stage.
Thoracic cancer was diagnosed in 171 patients (15%): 170 patients had lung neoplasm and only 1 patient was diagnosed with pleural mesothelioma. Lung cancer was localized in 42 patients (25%), whereas 39 patients (23%) had locally advanced disease, and 89 (52%) were diagnosed with advanced disease.
Gynaecological malignancy was diagnosed in 43 patients (4%). The most frequent tumour site was the cervix (29 patients, 67%): 20 patients (47%) were diagnosed with in situ carcinoma and 9 patients (21%) with invasive cervix carcinoma (7 had localized disease and 2 locally advanced disease). Seven patients (16%) had ovarian carcinoma (three of which were advanced) and six patients (14%) had localized endometrial carcinoma. Locally advanced vulvar cancer was diagnosed in one patient.
A total of 33 haematological malignancies were diagnosed (3%). Lymphoma was found in 26 patients (79%), 2 had chronic lymphocytic leukaemia and 5 multiple myeloma.
Regarding head and neck malignancies, 20 patients were diagnosed with this type (2%). A total of 16 patients had squamous carcinoma (79%): 3 patients with oropharynx carcinoma, 6 oral cavity carcinoma, 1 patient had laryngeal carcinoma and 6 were diagnosed with cervical metastases from an unknown primary; 12 of those 16 patients had locally advanced carcinoma and 4 were diagnosed with early-stage disease. Three patients were diagnosed with localized salivary gland carcinoma, and one patient with locally advanced nasopharyngeal carcinoma.
Regarding skin tumours, 3 of 15 (20%) had melanoma. The other cases were basal cell carcinoma (n = 8), squamous cell carcinoma (n = 2), Merkel cell carcinoma (n = 1) and porocarcinoma (n = 1).
Other tumour diagnoses were carcinoma of unknown primary origin (n = 5), follicular thyroid carcinoma (n = 1), thymoma (n = 1) and soft-tissue sarcoma (n = 4).
The different diagnoses grouped by body systems are shown in Figure 2.
Figure 2.
Diagnoses by different specialities among all studied patients.
CIS, carcinoma in situ; HNSCC, head and neck squamous cell carcinoma.
The median time from proposal submission to specialist testing was 15 days (interquartile range 8-26 days), and that from submission to a diagnosis was 21 days (interquartile range 12-36 days).
Patients who finally received a cancer diagnosis were assessed by the specialist in a median time of 11 days (interquartile range 7-17 days), and the histopathological diagnosis was achieved at a median of 19 days (interquartile range 11-31 days) from submission. Specific treatment (surgery or chemotherapy) was started at a median of 34 days (interquartile range 16-57 days) from diagnosis.
In patients in whom cancer diagnosis was ultimately ruled out, the median time from referral to specialist assessment was 17 days (interquartile range 10-28 days), and a diagnosis was reached in a median of 22 days (12-38 days) from time of submission.
Table 1 shows waiting times according to the different specialities at submission, in patients who reached oncological diagnosis.
Table 1.
Median time interval from patient referral to first visit at the specialist, diagnosis and start of treatment (patients diagnosed with oncological disease)
| Patients with cancer | From PC to specialist (days), median (interquartile range) | From PC to cancer diagnosis, median (interquartile range) | From cancer diagnosis to treatment, median (interquartile range) |
|---|---|---|---|
| All specialities | 11 (7-17) | 19 (11-31) | 34 (16-57) |
| Breast unit | 13 (9-17) | 17 (11-28) | 30 (19-42) |
| Gastroenterology | 18 (12-28) | 20 (13-29.5) | 48 (26-67) |
| Gynaecology | 15 (10.5-26.5) | 26 (14-37.5) | 31 (0-68) |
| Pneumology | 6 (4-9) | 18 (11-29.75) | 33 (19.5-51.5) |
| Head and neck unit | 7.5 (5.25-10.5) | 9 (6-19.75) | 35 (27-50) |
| Urology | 13 (7-18) | 22 (12-48) | 49 (3-81.5) |
| Oncology | 6 (4-8) | 15 (10.5-22) | 24.5 (8-63.7) |
| Others | 7 (6-14) | 19.5 (9.25-39.5) | 19 (11-34.75) |
Other groups include internal medicine, haematology, dermatology and traumatology.
PC, primary care.
The number of submissions to the CFP was found to increase progressively. During the first 5 years (from July 2009 to June 2014) 1665 patients were referred, whereas 2828 patients were sent to the programme in the last 5 years (from July 2014 to June 2019).
In the case of BC fast-track programme, due to the fact that from 2017 onwards patients were referred directly to breast imaging, times to testing were shorter (median of 9 days from submission to diagnosis) compared with 2016 and earlier (median of 28 days). In other specialties, waiting times remained stable throughout the study. Because of the large number of patients on the breast cancer fast-track programme, overall time intervals were reduced from 2017 onwards.
The number of submissions to the fast-track programme and diagnostic intervals over the years analysed are presented in Table 2.
Table 2.
Total referrals per year; time interval from PC referral to first visit and to diagnosis in patients with cancer
| Year of referral | N | Time from PC referral to first visit | Time from PC referral to diagnosis |
|---|---|---|---|
| 2009 (June-December) | 81 | 17 (11-21) | 30 (18-49) |
| 2010 | 177 | 16 (10-27) | 25 (15-43) |
| 2011 | 378 | 16 (9-27) | 28 (17-46) |
| 2012 | 413 | 18 (11-27) | 29 (20-47) |
| 2013 | 431 | 18 (10-28) | 26 (16-38) |
| 2014 | 350 | 20 (12-30.5) | 29 (18-43.5) |
| 2015 | 456 | 16 (9-28) | 21 (14-33) |
| 2016 | 405 | 19 (13-28.5) | 22 (15-31) |
| 2017 | 480 | 13 (8-21) | 14 (9.25-24) |
| 2018 | 819 | 10 (7-21) | 13 (8-30) |
| 2019 (January-June) | 503 | 12 (7-21) | 13 (8-28) |
PC, primary care.
Discussion
To improve cancer outcomes, most health systems should develop structured strategic programmes.11, 12, 13, 14, 15, 16 Among these programmes, enabling rapid referrals from primary to specialty care for patients highly suspected to have cancer through a CFP is a key one. Known as ‘2 weeks of waiting’, the programme has managed to shorten diagnosis times in patients displaying ‘alarm’ symptoms.
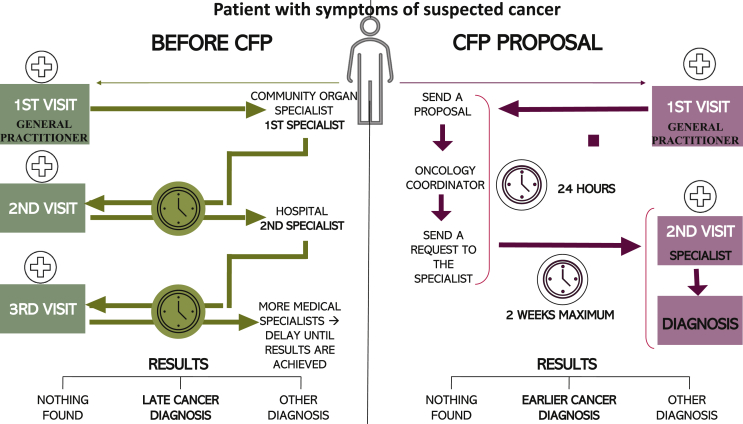
Oncological care development in Spain has been hindered by suboptimal cooperation between the different health care professionals involved in cancer diagnosis, treatment and follow-up, and this may be responsible for most diagnostic delays. Before establishing this programme in our health area, patients consulted their GPs and were then sent to the community organ specialist, where as an intermediate step they were filtered by the specialist before being finally directed to the hospital. Our project stemmed from a perception among GPs and oncologists of a cancer diagnosis delay in some cases. In light of this, we decided to join forces with different specialists at various levels including GPs, in order to improve cancer care in our community. With the creation of the CFP, the community specialist assessment step was omitted, thus significantly reducing time to testing, diagnosis and treatment (Figure 3).
Figure 3.
How delays can occur without a fast-track programme (left) and how they can be reduced (right).
CFP, cancer fast-track programme.
Basic communication and coordination between primary care and specialized medicine are key to a proper functioning of the CFP. GPs face multiple challenges in the symptomatic phase of cancer: symptoms that can be attributed to cancer are very common, but cancer is relatively rare. It is therefore imperative that GPs have the skills and knowledge to determine which patients to refer to the CFP, and accordingly, they need the means available to contact specialized care in case of doubt, so that patients with suspected cancer are not ruled out, while preventing the CFP from becoming overloaded. To this end, our programme facilitated phone contact between GPs and the CFP coordinator, when needed.
CFPs began as early as 1999 in other countries such as the United Kingdom.17. Potter et al.18 found that the BC fast-track programmes increased the number of proposals, but decreased the proportion of cancer diagnoses. However, the percentage of cancers detected in patients referred from outside of the programme were higher, and waiting times for those patients were longer. This underlines the importance of continuous review of fast-track programmes, via regular meetings with GPs, and modifying referral guidelines as needed.
A progressive increase in the number of referrals was seen over time in our programme: the number of referrals doubled between the first five (1665 cases) and last 5 years (2828 cases), reflecting widespread acceptance of the programme by both health care professionals and users. As a result of these good results, since 2018 similar fast-track programmes have been implemented in all hospitals in our region. Likewise, as we mentioned earlier, median times to specialist assessment and pathological diagnosis have improved in both patients who are diagnosed with cancer and those in whom cancer is ruled out (Table 2).
Strengths and limitations
There are several limitations to our study, principally the fact that our referral guidelines are not standardized and are based on the specific needs observed from a retrospective analysis of our Health Department cancer data. Future studies should therefore aim at standardizing these guidelines. Second, many patients with nonspecific symptoms falling outside current cancer criteria guidelines are not referred to the CFP, and most patients ultimately receiving a cancer diagnosis without CFP criteria are eventually diagnosed with later-stage disease because of initially nonspecific symptoms. In light of this, we are currently evaluating the expansion of these criteria to widen GP patient referral and thus reduce time to testing.
In view of the results, one of our next objectives is to improve the time between the diagnosis of cancer and the start of treatment. In most cases, this delay is due to the planning times that the surgery requires.
Our study also has some important implications and strengths: it is so far the largest prospective study conducted in Spain to report a decrease in diagnostic waiting times for patients with suspected cancer, and it is also a sound initiative to set up a collaborative communication between GPs and specialists in the field.
It is important to highlight the role of CFP in those patients who, due to their age, fall outside the age group for the BC and colon cancer screening programmes. In the case of patients diagnosed with BC, 62% did not enter the screening programme with mammography and 68% of those diagnosed in the case of CRC did not meet the screening criteria.
The CFP's impact is not only limited to shortening time to cancer diagnosis, but also extends to rule out cancer in patients suspected to have one, relieving patient anxiety over disease suspicion. Therefore, another of our objective is to quantify the degree of patient satisfaction with the CFP. Finally, since this project was implemented, coordination between GPs and specialists has increased substantially. This key factor contributes to health care quality and potentially reduces patient discomfort, also preventing loss of motivation among professionals and inappropriate use of resources. As a further benefit, this CFP is able to optimize existing resources without additional costs.
In the future, further data analyses will be conducted to examine whether the CFP is truly able to facilitate early-stage diagnosis and to determine whether this early diagnosis can influence cancer survival. Referral criteria for CFP are based on the presence of alarm symptoms, although many patients with cancer do not have such symptoms.19,20 Consequently, a large proportion of patients with cancer are still diagnosed via other diagnostic pathways, in many cases at advanced stages and with lower survival.21 Unlike others,22 our programme also evaluates patients with nonspecific and lower-risk symptoms, which represent the greatest challenge for oncologists. Nonetheless, the vast majority of all referred patients had one or more focal symptoms, including certain red flag symptoms such as change in bowel habits, blood in stool, dysphagia or breast lump. In our setting, patients with nonspecific symptoms such as constitutional syndrome, or who invoke high suspicion of neoplasia in the attending physician despite not meeting referral guideline criteria are evaluated either by the Internal Medicine Service or directly in Medical Oncology.
Conclusion
From symptoms onset to diagnosis, cancer patient care is a dynamic process involving multiple specialists. Cancer symptoms are common to other diseases so we must provide primary care with the tools to make proper diagnoses and avoid unnecessary tests and hospital referrals. The referral process for hospital testing requires adequate communication between GPs, specialists and patients if the diagnostic process is to be started earlier.
It is also important to define optimal waiting times that balance the best results with the lowest resource use and economic burden on the system. By combining all these parameters through the CFP, we have achieved enhanced communication between primary care and specialists, a first visit to the specialist in <2 weeks and consequently improved cancer diagnosis, without overloading the health care system or increasing costs.
Acknowledgements
We thank all patients and GPs.
Funding
None declared.
Disclosure
AC declares institutional research funding from Genentech, Merck Serono, BMS, MSD, Roche, BeiGene, Bayer, Servier, Natera, Lilly, Novartis, Takeda, Astellas, Takeda and FibroGen and serves on the advisory board or receives speaker fees from Amgen, Merck Serono and Roche in the last 5 years. Remaining authors have no conflicts of interest to declare.
Contributor Information
A. Cervantes, Email: andres.cervantes@uv.es.
I. Chirivella Gonzalez, Email: chirivella_isa@gva.es.
Supplementary data
Cancer Fast-Track Programme guidelines.
References
- 1.Sung H., Ferlay J., Siegel R.L. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021:1–41. doi: 10.3322/caac.21660. [DOI] [PubMed] [Google Scholar]
- 2.European Cancer Information System, ECIS Estimaciones de incidencia y mortalidad del cáncer 2018. http://www.redecan.org Available at.
- 3.Sociedad Española de Oncología Médica (SEOM) Las cifras del cáncer en España 2019. http://www.esmo.org Available at.
- 4.Macleod U., Mitchell E.D., Burgess C., Macdonald S., Ramirez A.J. Risk factors for delayed presentation and referral of symptomatic cancer: evidence for common cancers. Br J Cancer. 2009;101(suppl 2):S92–S101. doi: 10.1038/sj.bjc.6605398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mulka O. NICE suspected cancer guidelines. Br J Gen Pract. 2005;55(517):580–581. [PMC free article] [PubMed] [Google Scholar]
- 6.Huebner J., Follmann M. Complementary medicine in guidelines of the German Guideline Program in Oncology: comparison of the evidence base between complementary and conventional therapy. J Cancer Res Clin Oncol. 2013;139(9):1481–1488. doi: 10.1007/s00432-013-1464-7. [DOI] [PubMed] [Google Scholar]
- 7.Morris E.J.A., Whitehouse L.E., Farrell T. A retrospective observational study examining the characteristics and outcomes of tumours diagnosed within and without of the English NHS Bowel Cancer Screening Programme. Br J Cancer. 2012;107(5):757–764. doi: 10.1038/bjc.2012.331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Allgar V.L., Neal R.D. General practitioners' management of cancer in England: secondary analysis of data from the National Survey of NHS Patients-Cancer. Eur J Cancer Care (Engl) 2005;14(5):409–416. doi: 10.1111/j.1365-2354.2005.00600.x. [DOI] [PubMed] [Google Scholar]
- 9.Martínez M.T., González I., Tarazona N. Implementation and assessment of a fast-track programme to improve communication between primary and specialized care in patients with suspected cancer: how to shorten time between initial symptoms of cancer, diagnosis and initiation of treatment. Clin Transl Oncol. 2015;17:167–172. doi: 10.1007/s12094-014-1209-3. [DOI] [PubMed] [Google Scholar]
- 10.http://clinicomalvarrosa.san.gva.es/memorias-departamento Available at.
- 11.Zhou Y., Mendonca S.C., Abel G.A. Variation in ‘fast-track’ referrals for suspected cancer by patient characteristic and cancer diagnosis: evidence from 670 000 patients with cancers of 35 different sites. Br J Cancer. 2018;118:24–31. doi: 10.1038/bjc.2017.381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ingeman M.L., Christensen M.B., Bro F., Knudsen S.T., Vedsted P. The Danish cancer pathway for patients with serious non-specific symptoms and signs of cancer – a cross-sectional study of patient characteristics and cancer probability. BMC Cancer. 2015;15:421. doi: 10.1186/s12885-015-1424-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hamilton W. The CAPER studies: five case-control studies aimed at identifying and quantifying the risk of cancer in symptomatic primary care patients. Br J Cancer. 2009;101:S80–S86. doi: 10.1038/sj.bjc.6605396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Roberts M.J., Campbell J.L., Abel G. Understanding high and low patient experience scores in primary care: analysis of patients' survey data for general practices and individual doctors. Br Med J. 2014;349:g6034. doi: 10.1136/bmj.g6034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Forster A.S., Renzi C., Lyratzopoulos G. Diagnosing cancer in patients with ‘non-alarm’ symptoms: learning from diagnostic care innovations in Denmark. Cancer Epidemiol. 2018;54:101–103. doi: 10.1016/j.canep.2018.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Basta Y.L., Tytgat K.M.A.J., Greuter H.H., Klinkenbijl J.H.G., Fockens P., Strikwerda J. Organizing and implementing a multidisciplinary fast track oncology clinic. Int J Qual Health Care. 2017;29(7):966–971. doi: 10.1093/intqhc/mzx143. [DOI] [PubMed] [Google Scholar]
- 17.Nekhlyudov L., Latosinsky S. The interface of primary and oncology specialty care: from symptoms to diagnosis. J Natl Cancer Inst Monogr. 2010;40:11–17. doi: 10.1093/jncimonographs/lgq001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Potter S., Govindarajulu S., Shere M. Referral patterns, cancer diagnoses, and waiting times after introduction of two week wait rule for breast cancer: prospective cohort study. Br Med J. 2007;335(7614):288. doi: 10.1136/bmj.39258.688553.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Stapley S., Peters T.J., Neal R.D., Rose P.W., Walter F.M., Hamilton W. The risk of pancreatic cancer in symptomatic patients in primary care: a large case–control study using electronic records. Br J Cancer. 2012;106(12):1940–1944. doi: 10.1038/bjc.2012.190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Din N.U., Ukoumunne O.C., Rubin G. Age and gender variations in cancer diagnostic intervals in 15 cancers: analysis of data from the UK Clinical Practice Research Datalink. PLoS One. 2015;10(5):e0127717. doi: 10.1371/journal.pone.0127717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Abel G.A., Shelton J., Johnson S., Elliss-Brookes L., Lyratzopoulos G. Cancer-specific variation in emergency presentation by sex, age and deprivation across 27 common and rarer cancers. Br J Cancer. 2015;112:S129–S136. doi: 10.1038/bjc.2015.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Næser E., Fredberg U., Moller H., Vedsted P. Clinical characteristics and risk of severe disease in patients referred to a diagnostic center: a cohort study. Cancer Epidemiol. 2017;50:158–165. doi: 10.1016/j.canep.2017.07.014. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Cancer Fast-Track Programme guidelines.