Supplemental Digital Content is available in the text.
Keywords: heart failure, hospitalizations, iron, outcomes, prognosis, survival
Abstract
Background:
Iron deficiency (ID) has a prevalence of ≈40% to 50% among patients in heart failure (HF) with reduced ejection fraction and is associated with worse prognosis. Several trials demonstrated that intravenous ferric carboxymaltose leads to early and sustained improvement in patient-reported outcomes and functional capacity in patients with HF with reduced ejection fraction with ID, yet morbidity and mortality data are limited.
Methods:
The objective of the HEART-FID trial (Ferric Carboxymaltose in Heart Failure With Iron Deficiency) is to assess efficacy and safety of ferric carboxymaltose compared with placebo as treatment for symptomatic HF with reduced ejection fraction with ID. HEART-FID is a multicenter, randomized, double-blind, placebo-controlled trial enrolling ≈3014 patients at ≈300 international centers. Eligible patients are aged ≥18 years in stable chronic HF with New York Heart Association functional class II to IV symptoms, ejection fraction ≤40%, ID (ferritin <100 ng/mL or ferritin 100–300 ng/mL with a transferrin saturation <20%), and documented HF hospitalization or elevated N-terminal pro-brain natriuretic peptide. Consented patients are assigned to ferric carboxymaltose or placebo at baseline, with repeated visits/assessments every 6 months for additional study drug based on hemoglobin and iron indices for the trial duration. The primary end point is a hierarchical composite of death and HF hospitalization at 12 months and change from baseline to 6 months in the 6-minute walk test distance.
Conclusions:
The HEART-FID trial will inform clinical practice by clarifying the role of long-term treatment with intravenous ferric carboxymaltose, added to usual care, in ambulatory patients with symptomatic HF with reduced ejection fraction with ID.
Registration:
URL: https://www.clinicaltrials.gov; Unique identifier: NCT03037931.
See Editorial by Ponikowski and Jankowska
Heart failure (HF) is a serious medical condition, with an estimated 38 million patients affected worldwide.1,2 Among patients who have HF with reduced ejection fraction (HFrEF), there is a 40% to 50% estimated prevalence of iron deficiency (ID) defined as ferritin <100 ng/mL or ferritin 100 to 300 ng/mL and transferrin saturation <20% (Figure I in the Data Supplement).3–6 ID has been associated with worse signs and symptoms of HF, impaired health-related quality of life (HRQOL), and adverse outcomes independent of baseline anemia.7–9 Several studies have shown that patients in HFrEF with ID receiving intravenous ferric carboxymaltose (FCM) experienced an early and sustained improvement in New York Heart Association functional class, disease-specific and generic HRQOL, and functional capacity as assessed by change in 6-minute walk test (6-MWT) distance and peak oxygen consumption.10–12 In response, guidelines for HF management from the American College of Cardiology/American Heart Association/Heart Failure Society of America were updated and recommend that intravenous iron supplementation be considered to improve HRQOL and functional status in patients with symptomatic HFrEF with ID anemia.13,14 Similarly, other international guidelines have recommended this treatment be considered for ID anemia, if not ID, in patients with HFrEF.15,16
Despite positive results from European studies of intravenous FCM in patients with HFrEF and ID, several knowledge gaps in the existing evidence base require further investigation. Although clinical trials to date have not been adequately powered to evaluate morbidity and mortality in chronic HFrEF, pooled data from published trials suggest that treatment may lead to improvements in clinical outcomes.17 Accordingly, adequately powered cardiovascular outcomes trials are needed to evaluate the impact of intravenous iron repletion in chronic HFrEF with ID on hospitalization and mortality over an extended follow-up. In addition, although prior studies have shown that a dosing regimen of intravenous FCM over a period of up to 36 weeks resulted in a marked improvement in iron stores and an early and sustained benefit on surrogate end points (eg, 6-MWT distance, peak oxygen consumption, quality of life and New York Heart Association functional class), additional data are needed to inform on the long-term efficacy and safety of an iron repletion strategy that involves repeat dosing for patients with chronic HFrEF who continue to remain iron deficient or may experience recurrent ID after initial repletion. Finally, prior studies have enrolled patients in Europe, with only limited clinical and research experience with intravenous iron supplementation in this HFrEF population in North America and other geographic regions.
Thus, the HEART-FID trial (Randomized Placebo-Controlled Trial of Ferric Carboxymaltose as Treatment for Heart Failure With Iron Deficiency) aims to evaluate the impact of long-term therapy with intravenous FCM compared with matching placebo on a hierarchical composite of death, HF hospitalizations in the first 12 months, and change in 6-MWT distance in patients with symptomatic HFrEF with ID.
Study Design
The data that support the rationale of this article are available from the corresponding author upon reasonable request.
Overview
A multicenter, randomized, double-blind, placebo-controlled trial was designed to assess the efficacy and safety of intravenous FCM in the treatment of symptomatic patients in HFrEF with ID (Figure). Approximately 3014 patients will be enrolled from ≈300 participating centers in North America, Australia, New Zealand, and Eastern Europe. Patients will be randomized 1:1 to receive intravenous FCM or matching placebo. Eligible patients are ≥18 years of age with chronic HF with New York Heart Association functional class II to IV symptoms on maximally tolerated background therapy for ≥2 weeks before randomization. Other key inclusion criteria are EF ≤40% within 24 months or ≤30% within 36 months of screening, hemoglobin >9.0 g/dL and <13.5 g/dL (females) or <15.0 g/dL (males), ferritin <100 ng/mL or 100 to 300 ng/mL with a transferrin saturation <20%, and a documented HF hospitalization within 12 months of enrollment or elevated N-terminal-pro-brain natriuretic peptide within 90 days of randomization (Table I in the Data Supplement). Importantly, the trial includes those with and without anemia. Study patients must be willing and able to perform a 6-MWT at the time of randomization. Patients are excluded from participation if they have a known hypersensitivity to any component of FCM, a history of acquired iron overload, received intravenous iron therapy or a blood transfusion (within 3 months), or have active gastrointestinal bleeding or a screening ferritin <15 ng/mL without an appropriate medical evaluation within the past 3 months.
Figure.
HEART-FID study (Randomized Placebo-Controlled Trial of Ferric Carboxymaltose as Treatment for Heart Failure With Iron Deficiency) overview. After an initial screening period of up to 28 days, eligible participants are randomized in a 1:1 ratio to receive ferric carboxymaltose or placebo. Study drug administration occurs on days 0 and 7, with additional study visits at 3-month intervals and additional dosing every 6 mo, as applicable. Δ6MWT distance indicates change in distance walked on the 6-minute walk test; and HF, heart failure.
Treatment Protocol and Follow-Up
The study protocol will be conducted in 3 phases: screening, baseline/initial treatment, and follow-up (Table II in the Data Supplement). Patients who have signed the informed consent form will undergo the following clinical evaluations to confirm study eligibility: demographics and medical history, left ventricular EF assessment (historical values may be used if performed within 24 months of the screening visit or within 36 months if EF ≤30%), central laboratory evaluations (hematology, iron indices, and brain natriuretic peptide/N-terminal-pro-brain natriuretic peptide), concomitant medications, and review of inclusion/exclusion criteria. If all eligibility criteria are verified, qualified patients may proceed to the day 0 visit and be randomized.
At the beginning of the treatment phase (day 0), blinded study personnel will verify all inclusion/exclusion criteria, perform a focused physical examination, draw blood samples for central laboratory tests (hematology, iron indices, and N-terminal-pro-brain natriuretic peptide), review concomitant medications, complete a point-of-care urine pregnancy test (if appropriate), and obtain a baseline 6-MWT distance. Blinded study staff will obtain baseline vital signs including heart rate, blood pressure, and body temperature before beginning study drug infusion. Unblinded study staff will prepare and administer FCM or matching placebo to patients who are blinded to the treatment. Consistent with the US label for FCM (currently approved for ID anemia), the dosing is based on weight (≥50 versus <50 kg), is given in 2 doses separated by 7 days and does not involve use of Ganzoni’s formula—which remains theoretical and may not be applicable to all disease states or clinical scenarios. FCM will be supplied in 15 mL vials containing 750 mg and given either as a continuous infusion or a slow intravenous injection at a rate of 2 mL (100 mg) per minute for a total dose of 750 mg (estimated infusion time ≈7.5 minutes) for patients weighing >50 kg. For patients weighing <50 kg, the dose of FCM will be adjusted to 15 mg/kg. Placebo dosing volume will be adjusted for weight to maintain blinding. Blinded study staff will obtain postadministration vital signs immediately after and again 30 minutes after completing study drug infusion. Blinded study personnel will monitor patients for adverse and serious adverse events during and following study drug administration. Patients will return on day 7±2 for the second dose of study medication following the same procedure.
Participants are blinded to the content of the study drug for the duration of the trial. During administration, patients either wear an opaque mask covering their eyes or a drape is used to prevent the patient from viewing the infusion. In addition, sites are required to prespecify and maintain both blinded and unblinded study staff with delineation of the different roles and procedures. Unblinded site personnel prepare, document, and administer FCM or placebo, and ensure that participants and all blinded staff are not able to observe the preparation, administration, or clean-up. During the period of study drug administration, the blinded personnel are not with the patient or in a location that could result in the blind being inadvertently broken. However, the principal investigator or designee are available in the event of an emergency, and the need for adverse event assessment. All blinded study personnel will be blinded to the post-treatment iron indices and serum phosphorous laboratory results, as the values may break the blind.
All patients will be contacted in person or via telephone at day 90±14 to assess potential clinical end points (death or hospitalizations) and adverse/serious adverse events. In addition, all patients will receive additional doses of study drug every 6 months (eg, day 180±7 and day 187±7; day 360±7 and day 367±7) for the duration of the trial. Patients will return 4 to 20 days before every 6-month visit to have blood drawn by blinded staff for central laboratory tests. The FCM treatment group is dosed based on hemoglobin levels (<13.5 g/dL [females] and <15.0 g/dL [males]) and iron studies (ferritin <100 ng/mL or 100–300 ng/mL with a transferrin saturation <20%). Patients randomized to the FCM group not meeting these prespecified laboratory criteria will receive matching placebo during follow-up.
Blinded staff will administer the 6-MWT at both the 6- and 12-month study visits. All participants will complete an end-of-study visit once the last participant has reached 12 months of follow-up and the prespecified number of clinical outcome events has occurred (N=771 participants with a cardiovascular death or HF hospitalization). At the final study visit, blinded staff will perform a focused physical examination, draw blood samples for central laboratory tests, review concomitant medications, and assess for potential clinical end points and adverse/serious adverse events.
Study End Points
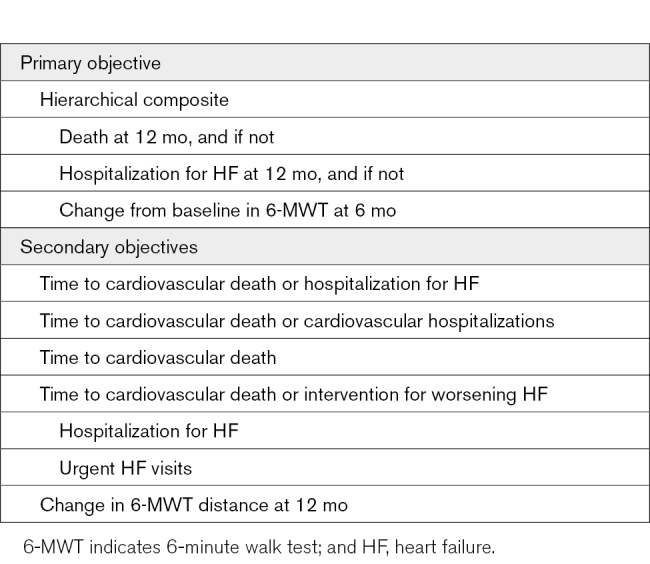
The primary end point is a hierarchical composite including death, hospitalization for HF, and change in 6-MWT distance (Table). Death and hospitalizations for HF will be assessed at 12 months, while change from baseline in 6-MWT distance will be evaluated at 6 months. This end point was chosen to investigate the 12-month experience for patients in terms of survival and total number of hospitalizations for HF while also allowing each participant to contribute to the primary end point (even if he/she does not experience a clinical event) through inclusion of 6-month 6-MWT distance. Secondary end points include cardiovascular death, cardiovascular hospitalizations, hospitalizations for HF, urgent HF visits, and change in 6-MWT distance at 12 months. A blinded and independent clinical event committee will adjudicate the cause of hospitalizations and deaths. Mode of death will be classified as cardiovascular or noncardiovascular. Cardiovascular death includes mortality due to HF, sudden cardiac death, acute myocardial infarction, stroke, and other cardiovascular events. Hospitalization is defined as a nonelective hospital admission for medical therapy with a duration that extends over a change in calendar date. Cardiovascular hospitalizations include admissions for HF, acute myocardial infarction, stroke, and other cardiovascular events. Hospitalization for HF is defined as admission for worsening signs or symptoms of HF resulting in the augmentation of oral medications or administration of intravenous therapies, mechanical or surgical intervention, or provision of ultrafiltration, hemofiltration, or dialysis specifically for management of persistent or worsening HF.
Table.
Study End Points
Substudy of Serum Phosphate Homeostasis
Given that FCM is associated with short-term, transient hypophosphatemia of unclear clinical significance,18 a substudy was developed to further characterize serum phosphorus levels in patients who have HFrEF with ID after dosing with FCM. Approximately 110 study patients will be enrolled in the substudy. Each study patient will have additional blood samples collected for analysis (Table II in the Data Supplement). All blood samples will be analyzed at the central laboratory.
Statistical Considerations
The intention-to-treat population will consist of all patients randomized to a treatment group in the study regardless of compliance with the study medication. All analyses involving the intention-to-treat population will include data from patients as randomized. The intention-to-treat population will be the primary population of all efficacy analyses. The per-protocol population will be a subset of the intention-to-treat population excluding patients who complied with the randomized treatment for <50% of follow-up. In cases of medication error, treatment assignments in the per-protocol analysis will be analyzed according to the actual treatment received.
The primary outcome follows a hierarchical scale of clinical severity comprising (1) death at 12 months, (2) number of hospitalizations for HF at 12 months, or (3) change from baseline in 6-MWT distance at 6 months. Each patient from the active treatment group will be ranked for comparison with each patient from the control group based on the 12-month experience for death and HF hospitalizations and 6-month results for change in 6-MWT distance to determine treatment response per the following hierarchy:
-
Death
—If both patients die, the one who survives longer is better off.
—If one dies and one does not die, the one who survives is better off.
—If neither dies, examine hospitalizations for HF.
-
Hospitalizations for HF
—The patient with fewer hospitalizations is better off.
—If neither has been hospitalized for HF or the number of HF hospitalizations is equal, compare change from baseline in 6-MWT distance.
-
Change in 6-MWT distance
—The one with the higher change from baseline in 6-MWT distance is better off.
These components were chosen and ordered based on clinical relevance and prior clinical research.19 The main comparison will be conducted using the Wilcoxon-Mann-Whitney test. The analysis of the composite end point, the hierarchical composite including death, hospitalization for HF, and change in 6-MWT distance, will be conducted along the lines of those outlined in Finkelstein and Schoenfeld20 and use a similar hierarchical approach as that used in the analysis of the PARTNER (Placement of Aortic Transcatheter Valves) and EMPHASIS-HF (Eplerenone in Mild Patients Hospitalization and Survival Study in Heart Failure) trials, in which a coprimary end point of the hierarchical composite of death or repeat hospitalization was used.21,22 The null hypothesis being tested is that a randomly chosen patient in the treatment group is equally likely to be ranked better or worse than a randomly chosen patient in the control group. The 2-sided alternative is that the patient is not equally likely to be ranked better or worse.
For missing data, the primary analysis will rely on a multiple imputation model, with Markov chain Monte Carlo algorithm based on the totality of observed data. One exception to this rule will be individuals unable to complete the 6-MWT at 6 months will have their value imputed as the worst observed change in 6-MWT distance.
Secondary outcomes, based on time-to-first event, will be compared using the Cox proportional hazards model. Mean change in 6-MWT distance from baseline to 12 months will be compared using linear regression adjusting for baseline value of 6-MWT distance.
The study design allows for sufficient power for both the primary and top secondary outcomes. Numerical simulations based on multivariate normal vectors were conducted to estimate power for the primary treatment comparison based on the following assumptions about events rates described in Table III in the Data Supplement.
With 3014 patients (1507 per arm) and 2.5% annual loss to follow-up for clinical outcomes and 15% of individuals with missing 6-MWT at 6 months (unable to perform or lost to follow-up), projected simulations estimate 90% power at an overall 2-sided significance level of 0.01, accounting for one interim analysis.
For the top secondary composite (time to cardiovascular death or hospitalization for HF), there was an assumed event rate of 0.0128 per month in the control arm which represents a conservative 75% discounting of the event rate obtained by the FCM meta-analysis.23 The anticipated hazard ratio was set at 0.80 (20% reduction). Uniform enrollment was assumed over the period of 30 months, with an anticipated minimum follow-up of 12 months, anticipated maximum follow-up of 42 months, and monthly loss to follow-up of 0.0021 (2.5% annualized). With these assumptions, 1500 per study arm (3000 total) provides 90% power to reject the null hypothesis of no difference between treatment arms when tested at an overall 2-sided level of significance α=0.05, accounting for the interim analysis. This results in a total of 771 participants with an event necessary to achieve the desired power for this top secondary end point; the trial will continue until this number of participants with an event has occurred. Cardiovascular death or hospitalization for HF will be analyzed as time-to-first event. The primary and top secondary outcome will be tested sequentially, and thus, no multiplicity adjustment is necessary.
One interim analysis is planned to determine if an early stopping for an overwhelming efficacy should be recommended. This analysis will be conducted after 2250 (75%) participants have been enrolled. Significance level will be set at 0.0001 for this analysis, resulting in an adjusted significance level for the final analysis of 0.0099 for the primary end point and 0.0499 for the first secondary end point, preserving the overall significance at 0.01 and 0.05, respectively.
Study recruitment is projected to be completed in September of 2021 with last follow-up through September 2022 with planned reporting in the fourth quarter of 2022 or first quarter of 2023.
Funding and Study Organization
The trial is sponsored by American Regent, Inc, a Daiichi Sankyo Group company, with trial coordination provided by the Duke Clinical Research Institute (Durham, NC) in collaboration with academic partners at the Canadian VIGOUR Centre (CVC, Edmonton, Canada), South Australian Health & Medical Research Institute (SAHMRI, Adelaide, Australia), and Green Lane Coordinating Centre (GLCC; Auckland, New Zealand). Trial expansion to Eastern Europe, led by KCR (Warsaw, Poland), will include study sites in Bulgaria, Czech Republic, Georgia, Hungary, Poland, Russia, and Ukraine. Overall responsibility for the oversight and management of the trial lies with an assigned Steering Committee comprising academic investigators from the United States, Canada, Australia, New Zealand, and Eastern Europe who are experts in their field, as well as sponsor representatives. The Data Safety Monitoring Board includes specialists in HF and an independent statistician responsible for active surveillance of safety data including all adverse and serious adverse events. Members of the Steering Committee and Data Safety Monitoring Board are listed in Table IV in the Data Supplement.
Ethical Considerations
The HEART-FID trial is designed to comply with the Declaration of Helsinki and Good Clinical Practice Guidelines. The institutional review board at each participating center independently approved the protocol, and written informed consent will be obtained from all study patients before enrollment.
Discussion
The HEART-FID trial is a multicenter, randomized, double-blind trial designed to assess the efficacy and safety of intravenous FCM versus placebo in the treatment of symptomatic patients in HFrEF with ID. The study rationale is based on epidemiological data and mechanistic studies,7–9 as well as modest-sized clinical trials primarily from Europe that were designed to evaluate the response to intravenous FCM in patients in HFrEF with ID.10,11,24,25 Prior to the recently published AFFIRM-AHF trial, previously published trials of iron repletion in HFrEF with ID have been underpowered for clinical events and, therefore, unable to comprehensively evaluate treatment-related mortality and morbidity. Notably, the HEART-FID trial has a novel hierarchical composite end point of mortality and morbidity (hospitalizations and change in the 6MWT distance as a measure of functional status) and will provide an understanding of ongoing FCM repletion needs in the HFrEF with ID patient population.
Several aspects of the symptomatic HFrEF with ID study population merit mention. Eligible patients with HFrEF require comorbid ID confirmed by laboratory studies. Epidemiological evidence supports the fact that ID is common among patients with HFrEF and that iron repletion is associated with improvements in health outcomes, HRQOL, and functional capacity independent of baseline hemoglobin concentrations.10,11 Given the laboratory criteria for ID (defined as ferritin <100 ng/mL or ferritin 100–300 ng/mL with a transferrin saturation <20%), valuable information will be obtained for patients with HFrEF with ID and ID anemia. HEART-FID is currently the largest randomized, double-blind, placebo-controlled trial to assess recurrent subsequent iron repletion requirements for HFrEF with ID after initial FCM treatment. In addition to characterizing iron retreatment patterns, the study findings will provide insights on postexposure safety parameters, including hypersensitivity and anaphylactic reactions as well as adverse events of special interest such as changes in serum phosphate levels.26,27
Prior trials in support of HEART-FID were not powered a priori to assess clinical events, yet findings from the CONFIRM-HF (Ferric Carboxymaltose Evaluation on Performance in Patients with Iron Deficiency in Combination with Chronic Heart Failure) trial were that FCM compared with placebo was associated with a 60% reduction in hospitalizations for HF over 12 months of follow-up.11 A subsequent meta-analysis of multiple clinical trials reported that FCM-treated patients experienced a significant reduction in the composite of all-cause mortality or hospitalizations for HF.23 In contrast, the 24-week EFFECT-HF (Effect of Ferric Carboxymaltose on Exercise) trial of 174 study participants who received FCM, compared with standard of care, was associated with more hospitalizations (37 versus 21), including hospitalizations for HF, other cardiovascular-related events, and noncardiovascular events.12 Together, these studies provide a foundation for the HEART-FID trial design to definitively assess the efficacy and safety of FCM in patients with symptomatic HFrEF with ID.
Importantly, HEART-FID is one of several IV iron studies evaluating clinical outcomes in patients with HFrEF (and mid-range EF) as have been recently reviewed.17 The AFFIRM-AHF (Study to Compare Ferric Carboxymaltose With Placebo in Patients With Acute Heart Failure and Iron Deficiency) trial28 has recently reported the topline result in a sample of 1132 patients recruited during acute HF (after stabilization) with left ventricular ejection fraction <50%.29 The study evaluating FCM in this patient population missed statistical significance on its composite primary end point of reducing the risk of total HF hospitalizations and cardiovascular death. A prespecified sensitivity analysis considering the impact of the COVID-19 pandemic, revealed a statistically significant difference in favor of FCM on cardiovascular mortality and hospitalization for heart failure. The 2 other large ongoing trials are FAIR-HF-2 (target N=1200, chronic HFrEF with left ventricular ejection fraction ≤45%, FCM, event-driven with primary end point of recurrent hospitalizations for HF and CV death) and IRONMAN (target N=1300 from the United Kingdom, chronic HFrEF with left ventricular ejection fraction<45%, iron [III] isomaltoside, 120-weeks with primary end points of CV death or hospitalization for worsening HF—first and recurrent). HEART-FID, which will be the largest of these trials, focuses on the chronic HFrEF population and is the only one with a primary end point that includes functional status.
Several challenges and potential limitations of the study warrant acknowledgment. First, as a double-blind trial, there is a potential for unblinding, given that FCM is an intravenous brown-colored drug while normal saline is the placebo comparator. Rigorous procedures have been implemented at all sites, including either masking the patient or covering the infusing intravenous tubing as well as having both blinded and unblinded site staff for study drug administration and follow-up assessment of laboratory study parameters. Second, the anticipated findings for the HEART-FID trial will be limited to patients with HFrEF and not be generalizable to patients with HF with preserved EF.
In conclusion, ID is highly prevalent in patients with HFrEF and is associated with increased risk of CV morbidity and mortality. Several nonregistrational studies in HFrEF with ID study populations reported an early and sustained improvement in signs and symptoms of HF, disease-specific, and generic HRQOL, and functional capacity after FCM exposure. The design, execution, and planned analysis of HEART-FID as a registrational trial will inform on the efficacy and safety of FCM as treatment for patients with symptomatic HFrEF with ID and its therapeutic role in this patient population.
Acknowledgments
Geoffrey Mukwaya, Nicole Blackman.
Sources of Funding
HEART-FID (Randomized Placebo-Controlled Trial of Ferric Carboxymaltose as Treatment for Heart Failure With Iron Deficiency) is funded by American Regent, Inc (Shirley, NY), a Daiichi Sankyo Group company.
Disclosures
All authors have received research support from American Regent, Inc. Dr Ambrosy has received modest reimbursement for travel from American Regent, Inc, and Novartis. He receives salary support from contracts between his employer and the National Institute on Aging and Amarin Pharma. J.A. Ezekowitz has received research grants from Amgen, AstraZeneca, Bayer, Bristol-Myers Squibb, eko.ai, Merck, Novartis, Sanofi, Servier, and Ortho-Biotech/Johnson & Johnson and has equity in eko.ai. Dr Lewis has received research support or consultancy fees from Novartis, Abbott, Bayer, Merck, AstraZeneca, Cyclerion, Cytokinetics, Applied Therapeutics, Amgen, Pfizer, and Relypsa. Dr Butler is a consultant for Abbott, Adrenomed, Amgen, Applied Therapeutics, Arena, Array, AstraZeneca, Bayer, Boehringer Ingelheim, Bristol-Myers Squib, CVRx, G3 Pharma, Janssen, LivaNova, Medtronic, Merck, Novartis, NovoNordisk, Relypsa, Roche, V-Wave Limited, and Vifor. Y.W. Wong has received honoraria/consultancy for Novartis, AstraZeneca, Medtronic, and Pfizer. Dr De Pasquale has received speaking and consulting fees from AstraZeneca, Biotronic, Boehringer Ingelheim, Bristol-Myers Squibb, Lilly, Novartis, Servier, and Vifor. He has served as a consultant for AstraZeneca, Boehringer Ingelheim, Novartis, Otsuka, St Jude, and Vifor. Dr Troughton has received grant funding and modest consulting fees from Merck and Roche Diagnostics. Dr O’Meara or her institution has received financial support for clinical trials from Amgen, AstraZeneca, Bayer, Boehringer-Ingelheim, Merck, and Novartis. She has served as a consultant or speaker for Amgen, Boehringer-Ingelheim, Novartis, and AstraZeneca. Dr Rockhold has received research funding from AstraZeneca, Alzheimer's Drug Discovery Foundation, Eidos, Bristol Myers Squibb, Duke Clinical Research Institute, National Institutes of Health (NIH), Patient-Centered Outcomes Research Institute, and ReNeuron. He has participated in data safety monitoring boards for Aldeyra, AstraZeneca, Complexa, KLSMC, Merck Research Laboratories, Novartis, Novo Nordisk, Rhythm Pharma, Lilly, Sanofi, UCB, and Tolerion. He has received fees for consultancy/honoraria from California Institute for Regenerative Medicine, Eidos Therapeutics, Janssen, Merck KGaA, Phathom Pharma, Icosavax, Pfizer, and Sarepta Bio and served on scientific advisory boards for Athira Pharma, DataVant, the European Medicines Agency, Spencer Health Solutions and ownership interest in HunterRockhold, Inc. He also has an equity interest in GlaxoSmithKline, Athira, Spencer and DataVant. Dr Samsky receives a salary supported by an NIH T32 training grant (HL069749). Drs Dugan and Mundy are employees of American Regent, Inc, a Daiichi Sankyo Group company. Dr Hernandez has received research support from AstraZeneca, GlaxoSmithKline, Merck, Novartis, and Verily and has consulted for Amgen, AstraZeneca, Bayer, Boehringer Ingelheim, Boston Scientific, Merck, and Novartis. Dr Mentz received research support and honoraria from Abbott, American Regent, Amgen, AstraZeneca, Bayer, Boehringer Ingelheim/Eli Lilly, Boston Scientific, Cytokinetics, Fast BioMedical, Gilead, Innolife, Medtronic, Merck, Novartis, Relypsa, Respicardia, Roche, Sanofi, Vifor, and Windtree Therapeutics.
Supplemental Materials
Figure I
Tables I–IV
Supplementary Material
Nonstandard Abbreviations and Acronyms
- 6MWT
- 6-minute walk test
- FCM
- ferric carboxymaltose
- HEART-FID
- Randomized Placebo-Controlled Trial of Ferric Carboxymaltose as Treatment for Heart Failure With Iron Deficiency
- HF
- heart failure
- HFrEF
- heart failure with reduced ejection fraction
- HRQOL
- health-related quality of life
- ID
- iron deficiency
This article was sent to Kenneth B. Margulies, MD, Senior Guest Editor, for review by expert referees, editorial decision, and final disposition.
The Data Supplement is available at https://www.ahajournals.org/doi/suppl/10.1161/CIRCHEARTFAILURE.120.008100.
For Sources of Funding and Disclosures, see page 602.
Contributor Information
Robert J. Mentz, Email: robert.mentz@duke.edu.
Andrew P. Ambrosy, Email: andrew.p.ambrosy@kp.org.
Justin A. Ezekowitz, Email: jae2@ualberta.ca.
Gregory D. Lewis, Email: glewis@partners.org.
Javed Butler, Email: jbutler4@umc.edu.
Yee Weng Wong, Email: YeeWeng.Wong@health.qld.gov.au.
Carmine G. De Pasquale, Email: carmine.depasquale@sa.gov.au.
Richard W. Troughton, Email: richard.troughton@cdhb.health.nz.
Eileen O’Meara, Email: eileen.omeara@umontreal.ca.
Frank W. Rockhold, Email: frank.rockhold@duke.edu.
Jyostna Garg, Email: jyotsna.garg@duke.edu.
Marc D. Samsky, Email: marc.samsky@duke.edu.
Dianne Leloudis, Email: dianne.leloudis@duke.edu.
Michael Dugan, Email: MDugan@americanregent.com.
Linda M. Mundy, Email: LMundy@americanregent.com.
References
- 1.Ambrosy AP, Fonarow GC, Butler J, Chioncel O, Greene SJ, Vaduganathan M, Nodari S, Lam CSP, Sato N, Shah AN, et al. The global health and economic burden of hospitalizations for heart failure: lessons learned from hospitalized heart failure registries. J Am Coll Cardiol. 2014;63:1123–1133. doi: 10.1016/j.jacc.2013.11.053 [DOI] [PubMed] [Google Scholar]
- 2.Braunwald E. The war against heart failure: the Lancet lecture. Lancet. 2015;385:812–824. doi: 10.1016/S0140-6736(14)61889-4 [DOI] [PubMed] [Google Scholar]
- 3.Jankowska EA, Malyszko J, Ardehali H, Koc-Zorawska E, Banasiak W, von Haehling S, Macdougall IC, Weiss G, McMurray JJ, Anker SD, et al. Iron status in patients with chronic heart failure. Eur Heart J. 2013;34:827–834. doi: 10.1093/eurheartj/ehs377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jankowska EA, von Haehling S, Anker SD, Macdougall IC, Ponikowski P. Iron deficiency and heart failure: diagnostic dilemmas and therapeutic perspectives. Eur Heart J. 2013;34:816–829. doi: 10.1093/eurheartj/ehs224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ezekowitz JA, McAlister FA, Armstrong PW. Anemia is common in heart failure and is associated with poor outcomes: insights from a cohort of 12 065 patients with new-onset heart failure. Circulation. 2003;107:223–225. doi: 10.1161/01.cir.0000052622.51963.fc [DOI] [PubMed] [Google Scholar]
- 6.Ezekowitz JA, McAlister FA, Armstrong PW. The interaction among sex, hemoglobin and outcomes in a specialty heart failure clinic. Can J Cardiol. 2005;21:165–171 [PubMed] [Google Scholar]
- 7.Okonko DO, Mandal AK, Missouris CG, Poole-Wilson PA. Disordered iron homeostasis in chronic heart failure: prevalence, predictors, and relation to anemia, exercise capacity, and survival. J Am Coll Cardiol. 2011;58:1241–1251. doi: 10.1016/j.jacc.2011.04.040 [DOI] [PubMed] [Google Scholar]
- 8.Comín-Colet J, Enjuanes C, González G, Torrens A, Cladellas M, Meroño O, Ribas N, Ruiz S, Gómez M, Verdú JM, et al. Iron deficiency is a key determinant of health-related quality of life in patients with chronic heart failure regardless of anaemia status. Eur J Heart Fail. 2013;15:1164–1172. doi: 10.1093/eurjhf/hft083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Núñez J, Comín-Colet J, Miñana G, Núñez E, Santas E, Mollar A, Valero E, García-Blas S, Cardells I, Bodí V, et al. Iron deficiency and risk of early readmission following a hospitalization for acute heart failure. Eur J Heart Fail. 2016;18:798–802. doi: 10.1002/ejhf.513 [DOI] [PubMed] [Google Scholar]
- 10.Anker SD, Comin Colet J, Filippatos G, Willenheimer R, Dickstein K, Drexler H, Lüscher TF, Bart B, Banasiak W, Niegowska J, et al. ; FAIR-HF Trial Investigators. Ferric carboxymaltose in patients with heart failure and iron deficiency. N Engl J Med. 2009;361:2436–2448. doi: 10.1056/NEJMoa0908355 [DOI] [PubMed] [Google Scholar]
- 11.Ponikowski P, van Veldhuisen DJ, Comin-Colet J, Ertl G, Komajda M, Mareev V, McDonagh T, Parkhomenko A, Tavazzi L, Levesque V, et al. ; CONFIRM-HF Investigators. Beneficial effects of long-term intravenous iron therapy with ferric carboxymaltose in patients with symptomatic heart failure and iron deficiency†. Eur Heart J. 2015;36:657–668. doi: 10.1093/eurheartj/ehu385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.van Veldhuisen DJ, Ponikowski P, van der Meer P, Metra M, Böhm M, Doletsky A, Voors AA, Macdougall IC, Anker SD, Roubert B, et al. ; EFFECT-HF Investigators. Effect of ferric carboxymaltose on exercise capacity in patients with chronic heart failure and iron deficiency. Circulation. 2017;136:1374–1383. doi: 10.1161/CIRCULATIONAHA.117.027497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yancy CW, Jessup M, Bozkurt B, Butler J, Casey DE, Jr, Drazner MH, Fonarow GC, Geraci SA, Horwich T, Januzzi JL, et al. 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on practice guidelines. Circulation. 2013;128:e240–327 [DOI] [PubMed] [Google Scholar]
- 14.Yancy CW, Jessup M, Bozkurt B, Butler J, Casey DE, Jr, Colvin MM, Drazner MH, Filippatos GS, Fonarow GC, Givertz MM, et al. 2017 ACC/AHA/HFSA focused update of the 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology/American Heart Association Task Force on clinical practice guidelines and the Heart Failure Society of America. Circulation. 2017;136:e137–e161. doi: 10.1161/CIR.0000000000000509 [DOI] [PubMed] [Google Scholar]
- 15.Ponikowski P, Voors AA, Anker SD, Bueno H, Cleland JGF, Coats AJS, Falk V, González-Juanatey JR, Harjola VP, Jankowska EA, et al. ; ESC Scientific Document Group. 2016 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure: the task force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC) developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur Heart J. 2016;37:2129–2200. doi: 10.1093/eurheartj/ehw128 [DOI] [PubMed] [Google Scholar]
- 16.Ezekowitz JA, O’Meara E, McDonald MA, Abrams H, Chan M, Ducharme A, Giannetti N, Grzeslo A, Hamilton PG, Heckman GA, et al. 2017 comprehensive update of the Canadian Cardiovascular Society guidelines for the management of heart failure. Can J Cardiol. 2017;33:1342–1433. doi: 10.1016/j.cjca.2017.08.022 [DOI] [PubMed] [Google Scholar]
- 17.von Haehling S, Ebner N, Evertz R, Ponikowski P, Anker SD. Iron deficiency in heart failure: an overview. JACC Heart Fail. 2019;7:36–46. doi: 10.1016/j.jchf.2018.07.015 [DOI] [PubMed] [Google Scholar]
- 18.Wolf M, Rubin J, Achebe M, Econs MJ, Peacock M, Imel EA, Thomsen LL, Carpenter TO, Weber T, Brandenburg V, et al. Effects of iron isomaltoside vs ferric carboxymaltose on hypophosphatemia in iron-deficiency anemia: two randomized clinical trials. JAMA. 2020;323:432–443. doi: 10.1001/jama.2019.22450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Maurer MS, Elliott P, Merlini G, Shah SJ, Cruz MW, Flynn A, Gundapaneni B, Hahn C, Riley S, Schwartz J, et al. Design and rationale of the phase 3 ATTR-ACT clinical trial (tafamidis in transthyretin cardiomyopathy clinical trial). Circ Heart Fail. 2017;10:e003815. doi: 10.1161/CIRCHEARTFAILURE.116.003815 [DOI] [PubMed] [Google Scholar]
- 20.Finkelstein DM, Schoenfeld DA. Combining mortality and longitudinal measures in clinical trials. Stat Med. 1999;18:1341–1354. doi: 10.1002/(sici)1097-0258(19990615)18:11<1341::aid-sim129>3.0.co;2-7 [DOI] [PubMed] [Google Scholar]
- 21.Leon MB, Smith CR, Mack M, Miller DC, Moses JW, Svensson LG, Tuzcu EM, Webb JG, Fontana GP, Makkar RR, et al. ; PARTNER Trial Investigators. Transcatheter aortic-valve implantation for aortic stenosis in patients who cannot undergo surgery. N Engl J Med. 2010;363:1597–1607. doi: 10.1056/NEJMoa1008232 [DOI] [PubMed] [Google Scholar]
- 22.Pocock SJ, Ariti CA, Collier TJ, Wang D. The win ratio: a new approach to the analysis of composite endpoints in clinical trials based on clinical priorities. Eur Heart J. 2012;33:176–182. doi: 10.1093/eurheartj/ehr352 [DOI] [PubMed] [Google Scholar]
- 23.Anker SD, Kirwan BA, van Veldhuisen DJ, Filippatos G, Comin-Colet J, Ruschitzka F, Lüscher TF, Arutyunov GP, Motro M, Mori C, et al. Effects of ferric carboxymaltose on hospitalisations and mortality rates in iron-deficient heart failure patients: an individual patient data meta-analysis. Eur J Heart Fail. 2018;20:125–133. doi: 10.1002/ejhf.823 [DOI] [PubMed] [Google Scholar]
- 24.Melenovsky V, Hlavata K, Sedivy P, Dezortova M, Borlaug BA, Petrak J, Kautzner J, Hajek M. Skeletal muscle abnormalities and iron deficiency in chronic heart failure an exercise 31P magnetic resonance spectroscopy study of calf muscle. Circ Heart Fail. 2018;11:e004800. doi: 10.1161/CIRCHEARTFAILURE.117.004800 [DOI] [PubMed] [Google Scholar]
- 25.Charles-Edwards G, Amaral N, Sleigh A, Ayis S, Catibog N, McDonagh T, Monaghan M, Amin-Youssef G, Kemp GJ, Shah AM.Effect of iron isomaltoside on skeletal muscle energetics in patients with chronic heart failure and iron deficiency. FERRIC-HF II randomized mechanistic trial. Circulation. 2019;139:2386–2398. doi: 10.1161/CIRCULATIONAHA.118.038516 [DOI] [PubMed] [Google Scholar]
- 26.Wolf M, Koch TA, Bregman DB. Effects of iron deficiency anemia and its treatment on fibroblast growth factor 23 and phosphate homeostasis in women. J Bone Miner Res. 2013;28:1793–1803. doi: 10.1002/jbmr.1923 [DOI] [PubMed] [Google Scholar]
- 27.Van Wyck DB, Mangione A, Morrison J, Hadley PE, Jehle JA, Goodnough LT. Large-dose intravenous ferric carboxymaltose injection for iron deficiency anemia in heavy uterine bleeding: a randomized, controlled trial. Transfusion. 2009;49:2719–2728. doi: 10.1111/j.1537-2995.2009.02327.x [DOI] [PubMed] [Google Scholar]
- 28.Ponikowski P, Kirwan BA, Anker SD, Dorobantu M, Drozdz J, Fabien V, Filippatos G, Haboubi T, Keren A, Khintibidze I, et al. Rationale and design of the AFFIRM-AHF trial: a randomised, double-blind, placebo-controlled trial comparing the effect of intravenous ferric carboxymaltose on hospitalisations and mortality in iron-deficient patients admitted for acute heart failure. Eur J Heart Fail. 2019;21:1651–1658. doi: 10.1002/ejhf.1710 [DOI] [PubMed] [Google Scholar]
- 29.Nasdaq. Vifor Pharma announces outcome of AFFIRM-AHF topline data. Accessed September 24, 2020. https://www.nasdaq.com/press-release/vifor-pharma-announces-outcome-of-affirm-ahf-topline-data-2020-09-24
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.



