Supplemental Digital Content is available in the text.
Keywords: atrial fibrillation; catheter ablation; cryosurgery; electrocardiography, ambulatory; pulmonary vein; radiofrequency ablation; recurrence; second-look surgery
Abstract
Background:
Recurrent paroxysmal atrial fibrillation (AF) after catheter ablation is presumably caused by failure to achieve durable pulmonary vein isolation (PVI). The primary methods of PVI are radiofrequency catheter ablation (RF) and cryoballoon catheter ablation (CRYO), but these methods have not been directly compared with respect to PVI durability and the effect thereof on AF burden (% of time in AF).
Methods:
Accordingly, we performed a randomized trial including 98 patients (68% male, 61 [55–67] years) with paroxysmal AF assigned 1:1 to PVI by contact-force sensing, irrigated radiofrequency catheter, or second-generation cryoballoon catheter. Implantable cardiac monitors were inserted ≥1 month before PVI for assessment of AF burden and recurrence, and all patients, irrespective of AF recurrence, underwent a second procedure 4 to 6 months after PVI to determine PVI durability.
Results:
In the second procedure, 152 out of 199 (76%) pulmonary veins (PVs) were found durably isolated after RF and 161 out of 200 (81%) after CRYO (P=0.32), corresponding to durable isolation of all veins in 47% of patients in both groups (P=1.0). Median AF burden before PVI was 5.4% (interquartile range, 0.5%–13.0%) versus 4.0% (0.6%–18.1%), RF versus CRYO (P=0.71), and reduced to 0.0% (0.0%–0.1%) and 0.0% (0.0%–0.5%), respectively (P=0.58)—a reduction of 99.9% (92.9%–100.0%) and 99.3% (85.9%–100.0%; P=0.36). AF burden after PVI significantly correlated to the number of durably isolated PVs (P<0.01), but 9 out of 45 (20%) patients with durable isolation of all veins had recurrence of AF within 4 to 6 months after PVI (excluding a 3-month blanking period).
Conclusions:
PVI by RF and CRYO produce similar moderate to high PVI durability. Both treatments lead to marked reductions in AF burden, which is related to the number of durably isolated PVs. However, for one-fifth of paroxysmal AF patients, complete and durable PVI was not sufficient to prevent even short-term AF recurrence.
Registration:
URL: https://www.clinicaltrials.gov; Unique identifier: NCT03805555.
What Is Known?
In paroxysmal atrial fibrillation (AF) the main ablation strategy is pulmonary vein isolation (PVI) to eliminate AF triggers harbored primarily (>90%) in and around the pulmonary veins. The importance of complete and durable PVI for clinical outcome, however, remains unclear as evidence is derived largely from studies on patients undergoing reablation for clinical AF recurrence.
Radiofrequency catheter ablation (RF) and cryoballoon catheter ablation (CRYO) are the preferred tools for PVI and produce comparable clinical outcome, but the 2 methods’ ability to achieve durable PVI has not been directly compared.
What the Study Adds?
When assessed in a randomized trial with mandatory invasive reassessment, RF and CRYO produced a comparable durability of PVI, with close to 80% of veins remaining isolated after 4-6 months.
When monitored continuously by an implantable cardiac monitor, ablation reduced the burden of AF to a median of 0.002%, corresponding to a median reduction of 99.8%, without differences between the 2 ablation methods.
Though the number of durably isolated veins was significantly related to reductions in AF burden and recurrence, 20% of patients with durable isolation of all veins had recurrence of AF, even within 6 months, highlighting the need for more individualized ablation strategies beyond PVI in addition to more durable PVI.
Pulmonary vein isolation (PVI) is the cornerstone of catheter ablation of atrial fibrillation (AF).1,2 The 2 primary methods to achieve PVI are radiofrequency catheter ablation (RF) and cryoballoon catheter ablation (CRYO). The latter is a more recently established, simpler alternative with shorter procedure time and steeper operator learning curve. Recent trials have shown comparable clinical outcomes,3–6 and studies with mandatory invasive PVI reassessments after CRYO have reported PVI durability similar to analogous RF studies.7–10 However, there has been no head-to-head comparison of the 2 methods’ ability to produce durable PVI. Thus, 2 key questions have not been addressed directly: How do the 2 methods compare in achieving durable PVI? And how does PVI durability relate to clinical outcomes?
To answer these questions, we designed a randomized clinical trial with mandatory reassessment of PVI durability and implantable cardiac monitors (ICM) for AF detection. We aimed to test the hypothesis that RF and CRYO produce comparable durability of PVI and effect on AF burden (% of time in AF).
Methods
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Trial Design
The RACE-AF trial (Radiofrequency versus Cryoballoon Catheter Ablation for Paroxysmal Atrial Fibrillation - Durability of Pulmonary Vein Isolation and Effect on Atrial Fibrillation Burden) is a single-center, prospective, randomized, patient-controlled, clinical trial aiming to assess the durability of PVI and AF burden after RF versus CRYO for paroxysmal AF (PAF) and, additionally, to evaluate the effect of PVI durability on AF recurrence and burden. The trial included insertion of an ICM before ablation and protocol-mandated invasive reassessment of PVI status, irrespective of AF recurrence. The trial was approved by the local ethics committee and data protection agency and conforms to the Declaration of Helsinki. All patients provided written, informed consent.
Patient Population
We recruited consecutive patients with PAF referred for ablation, aged 18 to 75 years with documented PAF and ≥3 AF episodes within the last 3 months. Exclusion criteria included documented atrial flutter or other arrhythmia requiring ablation besides PVI. A full list of inclusion and exclusion criteria can be found in Table I in the Data Supplement.
Follow-Up
After enrollment, patients were block randomized 1:1 to PVI by either contact-force (CF) sensing radiofrequency catheter (RF group) or second-generation cryoballoon catheter (CRYO group). ICMs were implanted ≥ 1 month before the PVI. Office visits were scheduled at 3 months after the procedure. Changes to antiarrhythmic drug therapy were not allowed between enrollment and ablation, and treatment was discontinued at the end of a 3 months blanking period (amiodarone 2 weeks before) if clinically feasible. Reassessment procedures of PVI durability were scheduled at 4 to 6 months after PVI. Follow-up for the current analyses was until the reassessment procedure.
Ablation Procedures
Procedures were performed at a single center that conducts ≈400 AF ablations annually by 3 experienced operators (>1000 AF ablations each). Procedural setup included preprocedural computed tomography scan and transesophageal echocardiography, uninterrupted anticoagulation with warfarin or direct oral anticoagulants, continuous heparinization after the transseptal approach targeting an activated coagulation time of 300 to 350 seconds, general anesthesia and mechanical ventilation (for CRYO procedures either deep sedation or general anesthesia and mechanical ventilation), and multipole esophageal temperature monitoring (CIRCA S-CATH, CIRCA Scientific, CO) restricting ablation to temperatures <39 °C and >12 °C for RF and CRYO, respectively. No patients were excluded on the basis of PV anatomy on preprocedural computed tomography scan.
Implementation of technological advancements introduced over the course of the trial was allowed. The consequence hereof was limited to a shift from radiofrequency application guided by force-time integral to ablation index halfway through the trial (described later). For CRYO, it did not result in any variation from the initial ablation strategy.
The protocol required a waiting period of ≥20 minutes after the last ablation before confirming bidirectional block. PVs were tested for any remaining PV potentials after isolation (entrance block) and high-output pacing inside the PVs was performed to assess for any atrial capture if exit block was not demonstrated by nonconducted automaticity. During confirmation of PVI, standardized fluoroscopy images of lasso position in each PV were obtained as references to ensure that PVI durability was assessed with the same position where isolation was initially confirmed.
Radiofrequency Ablation
RF procedures were performed with a CF sensing, open irrigated, radiofrequency catheter (ThermoCool SmartTouch), a 15 mm circular, multipole mapping catheter (LASSO; both Biosense Webster, CA), a steerable sheath at the operator discretion (Agilis NXT, Abbott Laboratories, IL), and an electroanatomical mapping system (CARTO 3 System v6.0, Biosense Webster, CA). Ipsilateral right and left PVs were encircled in pairs by continuous, point-by-point, wide antral circumferential ablation. Ablation points were applied with 30 W (20–25 W on the posterior wall) and 17 mL/min. 0.9% saline irrigation. Initially there was evidence suggesting that applying a force-time integral target of ≥400 gs was advantageous;11,12 later, an increased benefit was implied by switching to ablation point application guided by an ablation index target of ≥500 anterior and ≥400 posterior, which was implemented halfway through the trial.8,13
Cryoballoon Ablation
CRYO procedures were performed with a 28 mm cryoballoon catheter (Arctic Front Advance), a 20 mm circular mapping catheter (Achieve Advance), and a steerable sheath (FlexCath Advance; all Medtronic, MN). When designing the trial, the second-generation cryoballoon catheter had been introduced and ablation regimens were shifting from the standard of 2×4-minute freezes towards a single 1×3-minute freeze reported to produce similar clinical outcome.14,15 We chose a CRYO delivery protocol of a 1×3-minute freeze as default and a 1×3-minute bonus freeze if time to isolation was >40 seconds, not recordable, or minimal balloon temperature was above −40 °C (−40 °F). Ablation of the right PVs was performed under continuous phrenic nerve pacing with manual palpitation and phrenic nerve compound motor action potential monitoring. Cryoablation was stopped instantly if diaphragmatic contractions weakened, ceased, or if compound motor action potential amplitude decreased.
Reassessment Procedures
Procedural setup was as described in the section on Ablation Procedures. In case of PV reconnection, reconnected veins were reisolated using RF, so reassessment procedures were performed with the catheters and electroanatomical mapping system described for initial RF procedures.
To assess for PV reconnection and the location of gaps, we performed a high-density voltage map with the color display range set to 0.20 to 0.50 mV to accentuate the border zone between healthy tissue and scar for visual identification of gaps. Bidirectional block was assessed as previously described and guided by stored fluoroscopy images from the primary procedure. In case of PV reconnection, we searched for the earliest activation site as a potential location of a gap. The location of gaps was defined by PV reisolation during ablation or, in case of multiple gaps, a clear change in PV activation sequence. Furthermore, adenosine bolus injections in incremental doses until temporary atrioventricular block were used to demask dormant conduction.16 PVs with dormant conduction were not considered durably isolated.
After the assessment of PVI durability (and reisolation of reconnected PVs), induction of extra-PV triggers by incremental isoprenaline-infusion to a heart rate >100 beats per minute, and induction of supraventricular tachyarrhythmias by antegrade and retrograde electrophysiological study was attempted in all patients. Ablation in addition to PVI was at the operators’ discretion based on inducibility, arrhythmic events on ICM monitoring, and the patient’s symptoms.
In case of aberrant PV anatomy, such as a common left trunk, all PVs were assessed individually for durable isolation and are considered individual PVs in the analyses.
Implantable Cardiac Monitors
To compare the effects of the 2 ablation methods and to evaluate the effect of PVI durability on clinical outcomes, all patients had an ICM (Reveal LINQ, Medtronic, MN) implanted at least 1 month before PVI. Through analyses of beat-to-beat variability, the ICM provides a highly sensitive detection of AF episodes ≥2 minutes and therefrom the cumulative burden of AF with an accuracy of 99.4%.17,18 Daily AF burden throughout the monitoring periods was acquired. Protocol-specified ICM setup was optimized for AF monitoring and is presented in Table II in the Data Supplement.
End Points
The primary end point of this randomized comparison of 2 AF ablation methods was the number of durably isolated PVs at the reassessment 4 to 6 months after PVI. Secondary end points included bidirectional block to all PVs at the index procedure, the number of patients with all PVs durably isolated, the number of durably isolated PVs per patient, procedure, ablation, and fluoroscopy time, x-ray exposure, and complications.
Clinical outcome measures from the continuous ICM monitoring included AF recurrence, defined as any electrogram-verified AF episode ≥2 minutes (which is the ICM’s AF detection threshold), AF burden (% of time in AF) before and after PVI, and AF burden reduction, calculated as the difference before and after PVI relative to the baseline (can by definition only be calculated when the baseline AF burden is greater than zero). AF within the blanking period was not considered an end point or included in AF burden. Electrograms were manually adjudicated for AF recurrence, and if the presence/absence of AF was not obvious, a consensus forum with 1 to 2 cardiac electrophysiologists blinded to the randomization provided adjudication. Clinical end points further included direct current cardioversions and class I or III antiarrhythmic drug therapy.
The protocol included a predefined substudy on the effect of PVI durability on AF recurrence and AF burden. These analyses were performed on the pooled data, according to PVI status, regardless of ablation method.
Two PVs not isolated in the index procedure are analyzed and treated as any other vein in the relook analysis.
Statistics
The trial was powered to show noninferiority for the primary end point (the number of durably isolated PVs). Accepting a difference in PVI durability up to 15% as noninferiority and using an α-level of 0.05 and a power of 0.9, the largest sample size required was 191 PVs in each group (if 50% remained isolated). We aimed for 50 patients/200 PVs in each group, anticipated a 5% drop-out, and enrolled 105 patients.
Statistics were calculated using Stata 16 (StataCorp LLC, TX). Normality was tested with the Shapiro-Wilks test. Continuous data were analyzed using the Student t test for normally distributed data and the Mann-Whitney test or the Kruskal-Wallis test for non-normally distributed data. The Spearman rank correlation coefficient was used to compare PVI durability and AF burden. Categorical data were analyzed using the χ2 test. Values are presented as mean±SD or median and interquartile range (Q1–Q3) according to distribution for continuous data and count and percentage for categorical data unless otherwise stated.
Results
A total of 105 patients were enrolled between June 2015 and August 2018. Three patients were excluded, and 4 patients withdrew consent; none were lost to follow-up (Figure 1). The final study population completing both the PVI and the reassessment procedure consisted of 49 patients in each group. Baseline characteristics are presented in Table 1—there were no significant differences between the 2 treatment groups.
Figure 1.
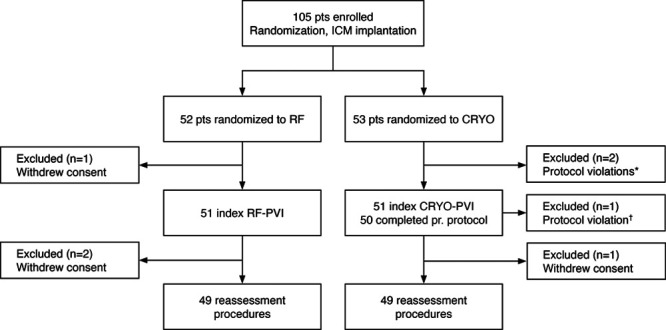
Randomization and patient (pt) flow in the RACE-AF trial. (Radiofrequency versus Cryoballoon Catheter Ablation for Paroxysmal Atrial Fibrillation - Durability of Pulmonary Vein Isolation and Effect on Atrial Fibrillation Burden) *Before pulmonary vein isolation (PVI), the diagnosis of atrial fibrillation was rejected in 2 pts. One did not have symptomatic arrhythmia; the other had ectopic atrial tachycardia. †Protocol was breached as the ablation method was switched from cryoballoon catheter ablation (CRYO) to radiofrequency catheter ablation (RF) to isolate the remaining 3 pulmonary veins in spite of a persistent phrenic nerve palsy. ICM indicates implantable cardiac monitor.
Table 1.
Baseline Characteristics

Index Procedures
Bidirectional block to all PVs was achieved in all 49 RF procedures and in 47 out of 49 (96%) CRYO procedures (P=0.15), corresponding to successful, acute isolation of 199 out of 199 (100%), and 198 out of 200 (99%) PVs, respectively (P=0.16). The failure to isolate 2 PVs in the CRYO group was due to premature abortion of 2 procedures because of a persistent phrenic nerve palsy and an asthma attack during general anesthesia.
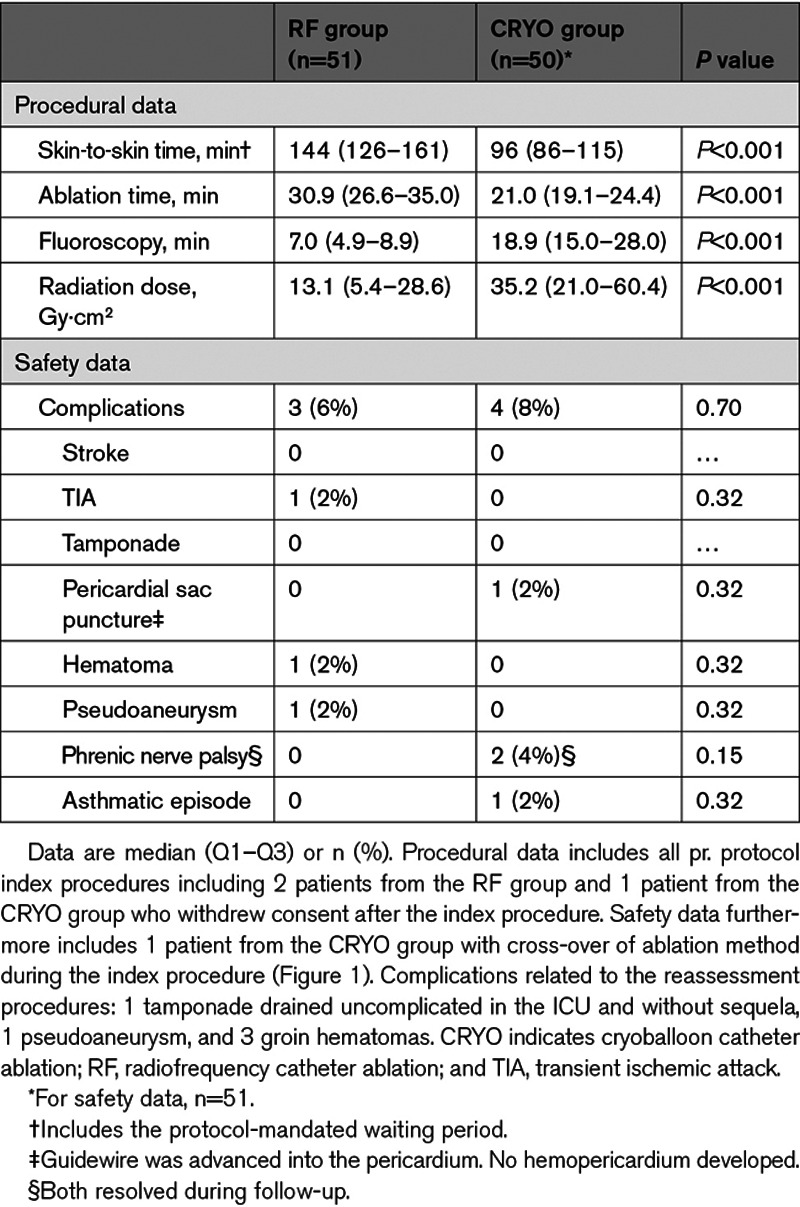
Procedural and safety data are presented in Table 2. Procedure and ablation times were shorter in the CRYO group, however, fluoroscopy time was longer and radiation dose was higher (all P<0.001).
Table 2.
Procedural and Safety End Points Related to the Index Procedure
Primary End Point—Durability of PVI
At the reassessment procedures, 152 out of 199 (76%) PVs were found durably isolated in the RF group and 161 out of 200 (81%) PVs in the CRYO group (P=0.32; Figure 2, top). This corresponded to 23 out of 49 (47%) patients in both groups with all PVs durably isolated (P=1.00). In patients with PV reconnection, the number of durably isolated PVs was similar for both treatment groups (P=0.31; Figure 2, bottom).
Figure 2.

Durability of pulmonary vein isolation after radiofrequency (RF) and cryoballoon (CRYO) ablation. Top, Columns illustrate the number of pulmonary veins in total (left columns), with acute isolation achieved at the index procedures (middle columns) and with durable isolation found at the reassessment procedures (right columns). Bottom, Columns illustrate the number of durably isolated pulmonary veins per patient. In patients with pulmonary vein reconnection, the number of durably isolated pulmonary veins was similar for both treatment groups (P=0.31). NS indicates nonsignificant.
The duration between PVI and the reassessment procedure was 5.0 (4.2–5.7) months in the RF group and 5.1 (4.4–5.7) months in the CRYO group (P=0.59). Patients with PV reconnection did not differ from patients with all PVs isolated on any of the baseline characteristics, but the index procedures were significantly longer: 139 (100–154) minutes in patients with PV reconnection versus 115 (91–138) minutes in patients with all PVs isolated (P=0.01). None of the other procedural parameters, including ablation time, were different between the 2 groups.
Of note, in the RF group, durable isolation was achieved in 73 out of 98 (74%) PVs with radiofrequency application guided by force-time integral versus 79 out of 101 (78%) PVs with application guided by ablation index (P=0.54), and this corresponded to durable isolation of all PVs in 11 out of 24 (46%) versus 12 out of 25 (48%) patients, respectively (P=0.88).
Clinical Outcomes
ICMs were implanted 40 (35–54) days before PVI in the RF group and 42 (35–54) days before PVI in the CRYO group (P=0.66), and in this period, AF burden was 5.41% (0.47%–12.92%) in the RF group and 3.98% (0.61%–18.13%) in the CRYO group (P=0.71). Seventeen patients had no AF in the monitoring period before PVI (RF: 8, CRYO: 9; P=0.79).
After PVI and the 3-month blanking period, AF burden was reduced to 0.00% (0.00%–0.13%) in the RF group and 0.00% (0.00%–0.52%) in the CRYO group (P=0.58), which corresponds to an AF burden reduction of 99.95% (92.90%–100.00%) and 99.34% (85.88%–100.00%), respectively (P=0.36). Individual patient and additional summary data on AF burden are presented in Figure 3. Due to repeated, false annotations of atrial ectopic beats as AF episodes throughout the study period, AF burden could not be acquired in one (RF) patient.
Figure 3.

Atrial fibrillation (AF) burden before and after pulmonary vein isolation (PVI). The line chart illustrates the individual patient’s AF burden before and after PVI (excluding a 3 mo blanking period). Radiofrequency catheter ablation (RF)-patients are shown in red and cryoballoon catheter ablation (CRYO)-patients are shown in blue. The box plots represent the 10th and 90th percentiles (whiskers), the 25th and 75th percentiles (boxes), and the medians (lines inside the boxes). AF burden indicates % of time in AF; and NS, nonsignificant.
Seventeen (35%) patients in both groups had documented AF recurrence after the blanking period. Patients with AF recurrence did not differ from patients without AF on any of the baseline characteristics or procedural parameters. In patients with documented recurrence, AF burden after PVI was 0.51% (0.05%–2.00%) in the RF group and 0.69% (0.25%–1.50%) in the CRYO group (P=0.37), and the reduction in AF burden was 92.95% (82.01%–99.71%) and 85.89% (69.74%–97.47%), respectively (P=0.15).
There was no difference between the treatment groups in the overall use of class I or III antiarrhythmic drugs after the blanking period in terms of starting new treatment or failure to discontinue ongoing treatment per protocol, which concerned 4 out of 49 (8%) patients in the RF group and 2 out of 49 (4%) patients in the CRYO group (P=0.40). Direct current cardioversion after the blanking period occurred in 1 out of 49 (2%) RF patients and 2 out of 49 (4%) CRYO patients (P=0.56).
Relationship Between PVI Durability and AF Burden and Recurrence
AF burden after PVI was significantly related to the number of durably isolated PVs (P<0.01, ρ=−0.31), as was AF burden reduction (P=0.03, ρ=0.25). A similar relationship was found between PVI durability and AF recurrence (Figure 4). AF burden before PVI was not different.
Figure 4.

Pulmonary vein isolation (PVI) status and atrial fibrillation (AF) recurrence. Pie charts illustrate the percentage of patients with AF recurrence according to PVI status. AF recurrence rate was significantly associated with the number of durably isolated pulmonary veins (P<0.01).
In patients with durable isolation of all PVs, AF recurred in 9 out of 45 (20%). In these patients, AF burden after PVI was 0.52% (0.12%–0.92%), and the reduction in AF burden was 95.86% (72.87%–98.73%). Patients with AF recurrence in spite of durable isolation of all PVs did not differ from patients with complete PVI and freedom from AF on any of the baseline characteristics or procedural parameters.
Discussion
This is the first randomized comparison of RF and CRYO for PAF with a protocol-mandated invasive reassessment of PVI durability, and the first study to use continuous ICM monitoring to assess the impact of PVI durability on AF burden. The main result of the study is that CF sensing RF and second-generation CRYO were equally efficient to produce durable PVI, with close to 80% of PVs being durably isolated. The study furthermore found that the 2 ablation methods produced a marked and highly similar reduction in AF burden, and that PVI durability was related to reduction in AF burden and recurrence. However, one-fifth of the patients with durable isolation of all PVs had recurrence of AF already within 4 to 6 months.
Durability of PVI
In the RACE-AF trial—which contains one of the largest sample sizes with mandatory PVI reassessment to date for either ablation method—76% and 81% of PVs were durably isolated after RF and CRYO, respectively. This is comparable to results of previous studies with mandatory reassessment procedures reporting durable isolation of 74% to 93% of PVs after RF7,8 and 73% to 91% after CRYO.9,10
For CRYO, the SUPIR trial10 applied a 2×4-minute freeze regimen in 21 patients and on reassessment found that 91% of PVs were isolated. In another study in 32 patients, 73% of PVs were durably isolated when a 1×3-minute freeze regimen was used.9 Thus, it is possible that a longer freeze duration than the 1×3-minute±bonus freeze regimen used in the RACE-AF trial could produce better PVI durability. However, a recent randomized study with 231 CRYO procedures found similar clinical outcomes with a 2×2- and a 2×4-minute freeze protocol,3 indicating that an optimal freeze dosage to maximize PVI durability and clinical outcome has not been established.
For RF, the PRAISE study8 incorporated ablation index- and interlesion distance-guided ablation in 36 patients and reported durable isolation of 93% of PVs in mandated reassessment. However, reassessment procedures were 2 months after PVI, whereas ours were after 4 to 6 months, raising the possibility that further lesion maturation and reconduction may occur after the second month.
PV reconnection data from reablation has been reported in subsets of patients from the randomized FIRE AND ICE4 and CIRCA-DOSE3 studies, which both report a higher PV reconnection rate as would be expected in a subset with clinical AF recurrence and an indication for reablation (53% and 56% of PVs durably isolated, respectively).19,20 From the FIRE AND ICE trial, significantly fewer PV reconnections were observed after initial CRYO compared to RF (64% versus 46% of PVs durably isolated), where no difference between the ablation methods were observed in the CIRCA-DOSE or the current trial. One explanation may be that only the later trials were restricted to CF sensing radiofrequency and second-generation cryoballoon catheters.
Clinical Outcomes
The standard of reporting AF ablation efficacy as time to first recurrence is debated and AF burden is suggested as a more relevant outcome measure.21–24 Additionally, long-term continuous monitoring with ICM offers the most sensitive AF detection.17,18,25
The only other randomized study to examine the outcome after CF sensing RF and second-generation CRYO using ICM detection of AF before and after ablation, the CIRCA-DOSE study, reported a median reduction in AF burden of 99.3% in the RF group and 99.9% and 98.4% in 2 CRYO groups (nonsignificant),3 where we report 99.9% and 99.3% for RF and CRYO, respectively. Interestingly, similar clinical results after CRYO were reached through 3 different freeze regimens. The recent CLOSE to CURE study26 examined the effect of RF-PVI using ICMs and reported a median AF burden reduction of 100.0%.
All 3 trials above demonstrate a large reduction in AF burden; however, when patients are continuously monitored, less impressive rates of freedom from any AF recurrence are observed; indicating that the former is the realistic goal for the majority of patients with present-day, single procedure, catheter ablation for PAF—not the elimination of all episodes.21
Relationship Between PVI Durability and AF Burden and Recurrence
The comparable clinical outcomes between RF and CRYO seen in earlier studies cannot a priori be assumed to reflect comparable PVI durability. It could theoretically be due to similar PVI durability or to dissimilar PVI durability combined with differential effects on other ablation targets in the PV antrum. We found highly comparable PVI durability and clinical outcomes with the 2 methods which is strongly consistent with the former mechanism.
Although PVI has been the conceptual cornerstone of AF ablation for 2 decades,27 there is little direct evidence regarding the importance of complete and durable PVI for the clinical outcomes of catheter ablation, but a large body of conflicting, indirect evidence. For example, PV reconnection has been reported in 0% to 91% of patients without AF recurrence, and complete and durable PVI has been reported in 0% to 62% of patients with AF recurrence.28–31 The largest study to date with mandatory reassessment procedures, the GAP-AF trial,32 strongly supported the importance of a strategy of complete and durable PVI, but the data also showed that 64% of the patients with durable PVI had recurrence of AF when examined in the blanking period. When examined past the blanking period with continuous monitoring in the present study, we found that 20% of patients with durable PVI had AF recurrence within 4 to 6 months.
Thus, our study adds new insight into this complex relationship: although durable isolation of all PVs is clearly associated with reductions in AF burden and recurrence, it is not sufficient to prevent even short-term AF recurrence in one-fifth of PAF patients. One clinical implication of this is that simply reisolating PVs in reablations in many cases will be insufficient to prevent any recurrence of AF.
Limitations
The main limitation of the trial is first the sample size; however, the study was powered to show noninferiority for the primary end point, and we did not observe any signal suggesting differential outcomes. The patients constituted their own controls in regard to AF outcomes, and although not powered for a noninferiority analysis, the sample size allowed for a single-center design with minimal procedural variation and a strong methodological rigor.
Second, the monitoring periods were relatively short, but this was a practical and ethical compromise to neither excessively delay the initial PVI or a clinically indicated reablation nor lose patients to follow-up before the reassessment procedure wherefrom we obtain data on the primary end point.
Third, the validity of our comparison of PVI durability with clinical outcomes rests on the assumption that PVI status at the time of the reassessment procedure equals the PVI status during the ICM monitoring from the end of the blanking period. Because lesion maturation is thought to be completed within the first 3 months after ablation for both methods, we believe this assumption is justified.1,33–36
The decision to reisolate reconnected PVs in all patients in the repeat procedures precludes some aspects of long-term follow-up in the randomized comparison of RF versus CRYO, but this was an ethical consideration, a means to confirm the primary end point, and will, in turn, allow ICM follow-up on the strategy of mandatory reablation.
Conclusions
The RACE-AF trial demonstrates, that PVI by CF sensing RF and second-generation CRYO are equal treatments to achieve durable PVI. Both treatments lead to a marked reduction in AF burden. However, durable isolation of all PVs was not sufficient to prevent even short-term recurrence of AF in one-fifth of the patients.
Sources of Funding
The study was supported by institutional funds and financial support from Medtronic and Biosense Webster. The funding sources had no part in the trial design or its execution, analyses or interpretation of the data, or submission of the results.
Disclosures
Dr Sørensen has received research grants from Medtronic and Biosense Webster. Dr J. Hansen has received research grants and speaker honoraria from Medtronic and Biosense Webster. Dr Worck has received research grants and speaker honoraria from Biosense Webster. The other authors report no conflicts.
Supplementary Material
Nonstandard Abbreviations and Acronyms
- AF
- atrial fibrillation
- CF
- contact-force
- CRYO
- cryoballoon catheter ablation
- ICM
- implantable cardiac monitor
- PAF
- paroxysmal atrial fibrillation
- PV
- pulmonary vein
- PVI
- pulmonary vein isolation
- RF
- radiofrequency catheter ablation
The Data Supplement is available at https://www.ahajournals.org/doi/suppl/10.1161/CIRCEP.120.009573.
For Sources of Funding and Disclosures, see page 530.
Contributor Information
Arne Johannessen, Email: arne.johannessen@regionh.dk.
René Worck, Email: Rene.Husted.Worck@regionh.dk.
Morten L. Hansen, Email: jim.hansen@regionh.dk.
Jim Hansen, Email: jim.hansen@regionh.dk.
References
- 1.Calkins H, Hindricks G, Cappato R, Kim YH, Saad EB, Aguinaga L, Akar JG, Badhwar V, Brugada J, Camm J, et al. 2017 HRS/EHRA/ECAS/APHRS/SOLAECE expert consensus statement on catheter and surgical ablation of atrial fibrillation. Heart Rhythm. 2017;14:e275–e444.. doi: 10.1016/j.hrthm.2017.05.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hindricks G, Potpara T, Dagres N, Arbelo E, Bax JJ, Blomström-Lundqvist C, Boriani G, Castella M, Dan GA, Dilaveris PE, et al. ; ESC Scientific Document Group. 2020 ESC Guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European Association for Cardio-Thoracic Surgery (EACTS). Eur Heart J. 2021;42:373–498.. doi: 10.1093/eurheartj/ehaa612 [DOI] [PubMed] [Google Scholar]
- 3.Andrade JG, Champagne J, Dubuc M, Deyell MW, Verma A, Macle L, Leong-Sit P, Novak P, Badra-Verdu M, Sapp J, et al. ; CIRCA-DOSE Study Investigators. Cryoballoon or radiofrequency ablation for atrial fibrillation assessed by continuous monitoring: a randomized clinical trial. Circulation. 2019;140:1779–1788.. doi: 10.1161/CIRCULATIONAHA.119.042622 [DOI] [PubMed] [Google Scholar]
- 4.Kuck KH, Brugada J, Fürnkranz A, Metzner A, Ouyang F, Chun KR, Elvan A, Arentz T, Bestehorn K, Pocock SJ, et al. ; FIRE AND ICE Investigators. Cryoballoon or radiofrequency ablation for paroxysmal atrial fibrillation. N Engl J Med. 2016;374:2235–2245.. doi: 10.1056/NEJMoa1602014 [DOI] [PubMed] [Google Scholar]
- 5.Ravi V, Poudyal A, Pulipati P, Larsen T, Krishnan K, Trohman RG, Sharma PS, Huang HD. A systematic review and meta-analysis comparing second-generation cryoballoon and contact force radiofrequency ablation for initial ablation of paroxysmal and persistent atrial fibrillation. J Cardiovasc Electrophysiol. 2020;31:2559–2571.. doi: 10.1111/jce.14676 [DOI] [PubMed] [Google Scholar]
- 6.Wang Y, Wang W, Yao J, Chen L, Yi S. Second-generation cryoballoon vs. contact-force sensing radiofrequency catheter ablation in atrial fibrillation: a meta-analysis of randomized controlled trials. J Interv Card Electrophysiol. 2021;60:9–19.. doi: 10.1007/s10840-020-00893-w [DOI] [PubMed] [Google Scholar]
- 7.Das M, Wynn GJ, Saeed Y, Gomes S, Morgan M, Ronayne C, Bonnett LJ, Waktare JEP, Todd DM, Hall MCS, et al. Pulmonary vein re-isolation as a routine strategy regardless of symptoms: the PRESSURE Randomized Controlled Trial. JACC Clin Electrophysiol. 2017;3:602–611.. doi: 10.1016/j.jacep.2017.01.016 [DOI] [PubMed] [Google Scholar]
- 8.Hussein A, Das M, Riva S, Morgan M, Ronayne C, Sahni A, Shaw M, Todd D, Hall M, Modi S, et al. Use of ablation index-guided ablation results in high rates of durable pulmonary vein isolation and freedom from arrhythmia in persistent atrial fibrillation patients: the PRAISE Study Results. Circ Arrhythm Electrophysiol. 2018;11:e006576. doi: 10.1161/CIRCEP.118.006576 [DOI] [PubMed] [Google Scholar]
- 9.Miyazaki S, Taniguchi H, Hachiya H, Nakamura H, Takagi T, Iwasawa J, Hirao K, Iesaka Y. Quantitative analysis of the isolation area during the chronic phase after a 28-mm second-generation cryoballoon ablation demarcated by high-resolution electroanatomic mapping. Circ Arrhythm Electrophysiol. 2016;9:e003879. doi: 10.1161/CIRCEP.115.003879 [DOI] [PubMed] [Google Scholar]
- 10.Reddy VY, Sediva L, Petru J, Skoda J, Chovanec M, Chitovova Z, Di Stefano P, Rubin E, Dukkipati S, Neuzil P. Durability of pulmonary vein isolation with cryoballoon ablation: results from the Sustained PV Isolation with Arctic Front Advance (SUPIR) Study. J Cardiovasc Electrophysiol. 2015;26:493–500.. doi: 10.1111/jce.12626 [DOI] [PubMed] [Google Scholar]
- 11.Kumar S, Chan M, Lee J, Wong MC, Yudi M, Morton JB, Spence SJ, Halloran K, Kistler PM, Kalman JM. Catheter-tissue contact force determines atrial electrogram characteristics before and lesion efficacy after antral pulmonary vein isolation in humans. J Cardiovasc Electrophysiol. 2014;25:122–129.. doi: 10.1111/jce.12293 [DOI] [PubMed] [Google Scholar]
- 12.Squara F, Latcu DG, Massaad Y, Mahjoub M, Bun SS, Saoudi N. Contact force and force-time integral in atrial radiofrequency ablation predict transmurality of lesions. Europace. 2014;16:660–667.. doi: 10.1093/europace/euu068 [DOI] [PubMed] [Google Scholar]
- 13.Hussein A, Das M, Chaturvedi V, Asfour IK, Daryanani N, Morgan M, Ronayne C, Shaw M, Snowdon R, Gupta D. Prospective use of ablation index targets improves clinical outcomes following ablation for atrial fibrillation. J Cardiovasc Electrophysiol. 2017;28:1037–1047.. doi: 10.1111/jce.13281 [DOI] [PubMed] [Google Scholar]
- 14.Chierchia GB, Di Giovanni G, Sieira-Moret J, de Asmundis C, Conte G, Rodriguez-Mañero M, Casado-Arroyo R, Baltogiannis G, Paparella G, Ciconte G, et al. Initial experience of three-minute freeze cycles using the second-generation cryoballoon ablation: acute and short-term procedural outcomes. J Interv Card Electrophysiol. 2014;39:145–151.. doi: 10.1007/s10840-013-9855-x [DOI] [PubMed] [Google Scholar]
- 15.Ciconte G, de Asmundis C, Sieira J, Conte G, Di Giovanni G, Mugnai G, Saitoh Y, Baltogiannis G, Irfan G, Coutiño-Moreno HE, et al. Single 3-minute freeze for second-generation cryoballoon ablation: one-year follow-up after pulmonary vein isolation. Heart Rhythm. 2015;12:673–680.. doi: 10.1016/j.hrthm.2014.12.026 [DOI] [PubMed] [Google Scholar]
- 16.Macle L, Khairy P, Weerasooriya R, Novak P, Verma A, Willems S, Arentz T, Deisenhofer I, Veenhuyzen G, Scavée C, et al. ; ADVICE trial investigators. Adenosine-guided pulmonary vein isolation for the treatment of paroxysmal atrial fibrillation: an international, multicentre, randomised superiority trial. Lancet. 2015;386:672–679.. doi: 10.1016/S0140-6736(15)60026-5 [DOI] [PubMed] [Google Scholar]
- 17.Mittal S, Rogers J, Sarkar S, Koehler J, Warman EN, Tomson TT, Passman RS. Real-world performance of an enhanced atrial fibrillation detection algorithm in an insertable cardiac monitor. Heart Rhythm. 2016;13:1624–1630.. doi: 10.1016/j.hrthm.2016.05.010 [DOI] [PubMed] [Google Scholar]
- 18.Sanders P, Pürerfellner H, Pokushalov E, Sarkar S, Di Bacco M, Maus B, Dekker LR; Reveal LINQ Usability Investigators. Performance of a new atrial fibrillation detection algorithm in a miniaturized insertable cardiac monitor: results from the Reveal LINQ Usability Study. Heart Rhythm. 2016;13:1425–1430.. doi: 10.1016/j.hrthm.2016.03.005 [DOI] [PubMed] [Google Scholar]
- 19.Kuck KH, Albenque JP, Chun KJ, Fürnkranz A, Busch M, Elvan A, Schlüter M, Braegelmann KM, Kueffer FJ, Hemingway L, et al. ; FIRE AND ICE Investigators. Repeat ablation for atrial fibrillation recurrence post cryoballoon or radiofrequency ablation in the FIRE AND ICE Trial. Circ Arrhythm Electrophysiol. 2019;12:e007247. doi: 10.1161/CIRCEP.119.007247 [DOI] [PubMed] [Google Scholar]
- 20.Cheung CC, Deyell MW, Macle L, Verma A, Champagne J, Leong-Sit P, Novak P, Badra-Verdu M, Sapp J, Khairy P, et al. Repeat atrial fibrillation ablation procedures in the CIRCA-DOSE Study. Circ Arrhythm Electrophysiol. 2020;13:e008480. doi: 10.1161/CIRCEP.120.008480 [DOI] [PubMed] [Google Scholar]
- 21.Calkins H. When it comes to defining the outcomes of catheter ablation of atrial fibrillation, an implantable monitor is a great place to start. Circulation. 2019;140:1789–1791.. doi: 10.1161/CIRCULATIONAHA.119.043155 [DOI] [PubMed] [Google Scholar]
- 22.Chen LY, Chung MK, Allen LA, Ezekowitz M, Furie KL, McCabe P, Noseworthy PA, Perez MV, Turakhia MP; American Heart Association Council on Clinical Cardiology; Council on Cardiovascular and Stroke Nursing; Council on Quality of Care and Outcomes Research; and Stroke Council. Atrial fibrillation burden: moving beyond atrial fibrillation as a binary entity: a scientific statement from the American Heart Association. Circulation. 2018;137:e623–e644.. doi: 10.1161/CIR.0000000000000568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mantovan R, Macle L, De Martino G, Chen J, Morillo CA, Novak P, Calzolari V, Khaykin Y, Guerra PG, Nair G, et al. Relationship of quality of life with procedural success of atrial fibrillation (AF) ablation and postablation AF burden: substudy of the STAR AF randomized trial. Can J Cardiol. 2013;29:1211–1217.. doi: 10.1016/j.cjca.2013.06.006 [DOI] [PubMed] [Google Scholar]
- 24.Van Gelder IC, Healey JS, Crijns HJGM, Wang J, Hohnloser SH, Gold MR, Capucci A, Lau CP, Morillo CA, Hobbelt AH, et al. Duration of device-detected subclinical atrial fibrillation and occurrence of stroke in ASSERT. Eur Heart J. 2017;38:1339–1344.. doi: 10.1093/eurheartj/ehx042 [DOI] [PubMed] [Google Scholar]
- 25.Kirchhof P, Benussi S, Kotecha D, Ahlsson A, Atar D, Casadei B, Castella M, Diener HC, Heidbuchel H, Hendriks J, et al. ; ESC Scientific Document Group. 2016 ESC Guidelines for the management of atrial fibrillation developed in collaboration with EACTS. Eur Heart J. 2016;37:2893–2962.. doi: 10.1093/eurheartj/ehw210 [DOI] [PubMed] [Google Scholar]
- 26.Duytschaever M, De Pooter J, Demolder A, El Haddad M, Phlips T, Strisciuglio T, Debonnaire P, Wolf M, Vandekerckhove Y, Knecht S, et al. Long-term impact of catheter ablation on arrhythmia burden in low-risk patients with paroxysmal atrial fibrillation: the CLOSE to CURE study. Heart Rhythm. 2020;17:535–543.. doi: 10.1016/j.hrthm.2019.11.004 [DOI] [PubMed] [Google Scholar]
- 27.Haïssaguerre M, Jaïs P, Shah DC, Takahashi A, Hocini M, Quiniou G, Garrigue S, Le Mouroux A, Le Métayer P, Clémenty J. Spontaneous initiation of atrial fibrillation by ectopic beats originating in the pulmonary veins. N Engl J Med. 1998;339:659–666.. doi: 10.1056/NEJM199809033391003 [DOI] [PubMed] [Google Scholar]
- 28.De Pooter J, Strisciuglio T, El Haddad M, Wolf M, Phlips T, Vandekerckhove Y, Tavernier R, Knecht S, Duytschaever M. Pulmonary vein reconnection no longer occurs in the majority of patients after a single pulmonary vein isolation procedure. JACC Clin Electrophysiol. 2019;5:295–305.. doi: 10.1016/j.jacep.2018.11.020 [DOI] [PubMed] [Google Scholar]
- 29.Glowniak A, Tarkowski A, Fic P, Wojewoda K, Wojcik J, Wysokinski A. Second-generation cryoballoon ablation for recurrent atrial fibrillation after an index procedure with radiofrequency versus cryo: different pulmonary vein reconnection patterns but similar long-term outcome-Results of a multicenter analysis. J Cardiovasc Electrophysiol. 2019;30:1005–1012.. doi: 10.1111/jce.13938 [DOI] [PubMed] [Google Scholar]
- 30.Jiang RH, Po SS, Tung R, Liu Q, Sheng X, Zhang ZW, Sun YX, Yu L, Zhang P, Fu GS, et al. Incidence of pulmonary vein conduction recovery in patients without clinical recurrence after ablation of paroxysmal atrial fibrillation: mechanistic implications. Heart Rhythm. 2014;11:969–976.. doi: 10.1016/j.hrthm.2014.03.015 [DOI] [PubMed] [Google Scholar]
- 31.Ouyang F, Antz M, Ernst S, Hachiya H, Mavrakis H, Deger FT, Schaumann A, Chun J, Falk P, Hennig D, et al. Recovered pulmonary vein conduction as a dominant factor for recurrent atrial tachyarrhythmias after complete circular isolation of the pulmonary veins: lessons from double Lasso technique. Circulation. 2005;111:127–135.. doi: 10.1161/01.CIR.0000151289.73085.36 [DOI] [PubMed] [Google Scholar]
- 32.Kuck KH, Hoffmann BA, Ernst S, Wegscheider K, Treszl A, Metzner A, Eckardt L, Lewalter T, Breithardt G, Willems S; Gap-AF–AFNET 1 Investigators*. Impact of complete versus incomplete circumferential lines around the pulmonary veins during catheter ablation of paroxysmal atrial fibrillation: results from the Gap-Atrial Fibrillation-German Atrial Fibrillation Competence Network 1 Trial. Circ Arrhythm Electrophysiol. 2016;9:e003337. doi: 10.1161/CIRCEP.115.003337 [DOI] [PubMed] [Google Scholar]
- 33.Fenelon G, Brugada P. Delayed effects of radiofrequency energy: mechanisms and clinical implications. Pacing Clin Electrophysiol. 1996;194 pt 1484–489.. doi: 10.1111/j.1540-8159.1996.tb06520.x [DOI] [PubMed] [Google Scholar]
- 34.Joshi S, Choi AD, Kamath GS, Raiszadeh F, Marrero D, Badheka A, Mittal S, Steinberg JS. Prevalence, predictors, and prognosis of atrial fibrillation early after pulmonary vein isolation: findings from 3 months of continuous automatic ECG loop recordings. J Cardiovasc Electrophysiol. 2009;20:1089–1094.. doi: 10.1111/j.1540-8167.2009.01506.x [DOI] [PubMed] [Google Scholar]
- 35.Liang JJ, Elafros MA, Chik WW, Santangeli P, Zado ES, Frankel DS, Supple GE, Schaller RD, Lin D, Hutchinson MD, et al. Early recurrence of atrial arrhythmias following pulmonary vein antral isolation: Timing and frequency of early recurrences predicts long-term ablation success. Heart Rhythm. 2015;12:2461–2468.. doi: 10.1016/j.hrthm.2015.07.015 [DOI] [PubMed] [Google Scholar]
- 36.Yamashita K, Kholmovski E, Ghafoori E, Kamali R, Kwan E, Lichter J, MacLeod R, Dosdall DJ, Ranjan R. Characterization of edema after cryo and radiofrequency ablations based on serial magnetic resonance imaging. J Cardiovasc Electrophysiol. 2019;30:255–262.. doi: 10.1111/jce.13785 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


