Abstract
The hippocampus performs two complementary processes, pattern separation and pattern completion, to minimize interference and maximize the storage capacity of memories. Classic computational models have suggested that the dentate gyrus (DG) supports pattern separation and the putative attractor circuitry in CA3 supports pattern completion. However, recent evidence of functional heterogeneity along the CA3 transverse axis of the hippocampus suggests that the DG and proximal CA3 work as a functional unit for pattern separation, while distal CA3 forms an autoassociative network for pattern completion. We propose that the outputs of these functional circuits, combined with direct projections from entorhinal cortex to CA1, form interconnected, parallel processing circuits to support accurate memory storage and retrieval.
Introduction
The hippocampus is critical for episodic memory storage and retrieval [1]. The classic ‘trisynaptic loop’ model describes the feedforward circuitry of information flow within the hippocampus: the entorhinal cortex projections to the dentate gyrus (DG) via the perforant pathway, DG projections to CA3 via the mossy fibers, and CA3 projections to CA1 via the Schaffer collaterals. However, the connectivity within the hippocampus is much more complex than this standard, textbook description. The entorhinal cortex projects not only to the DG, but also directly to CA1, CA2, and CA3, and there are backprojections from CA3 to the DG [2] and from the subiculum to CA1 [3]. Furthermore, topographic gradients in the projections from the medial entorhinal cortex (MEC) and the lateral entorhinal cortex (LEC) to CA1, from CA3 to CA1, and from CA1 to the subiculum provide the substrate for parallel processing streams along the hippocampal transverse axis [4,5] (Figure 1A) (Similar heterogeneity along the longitudinal axis of the hippocampus has been reviewed extensively and will not be covered in this review [6,7]).
Figure 1:
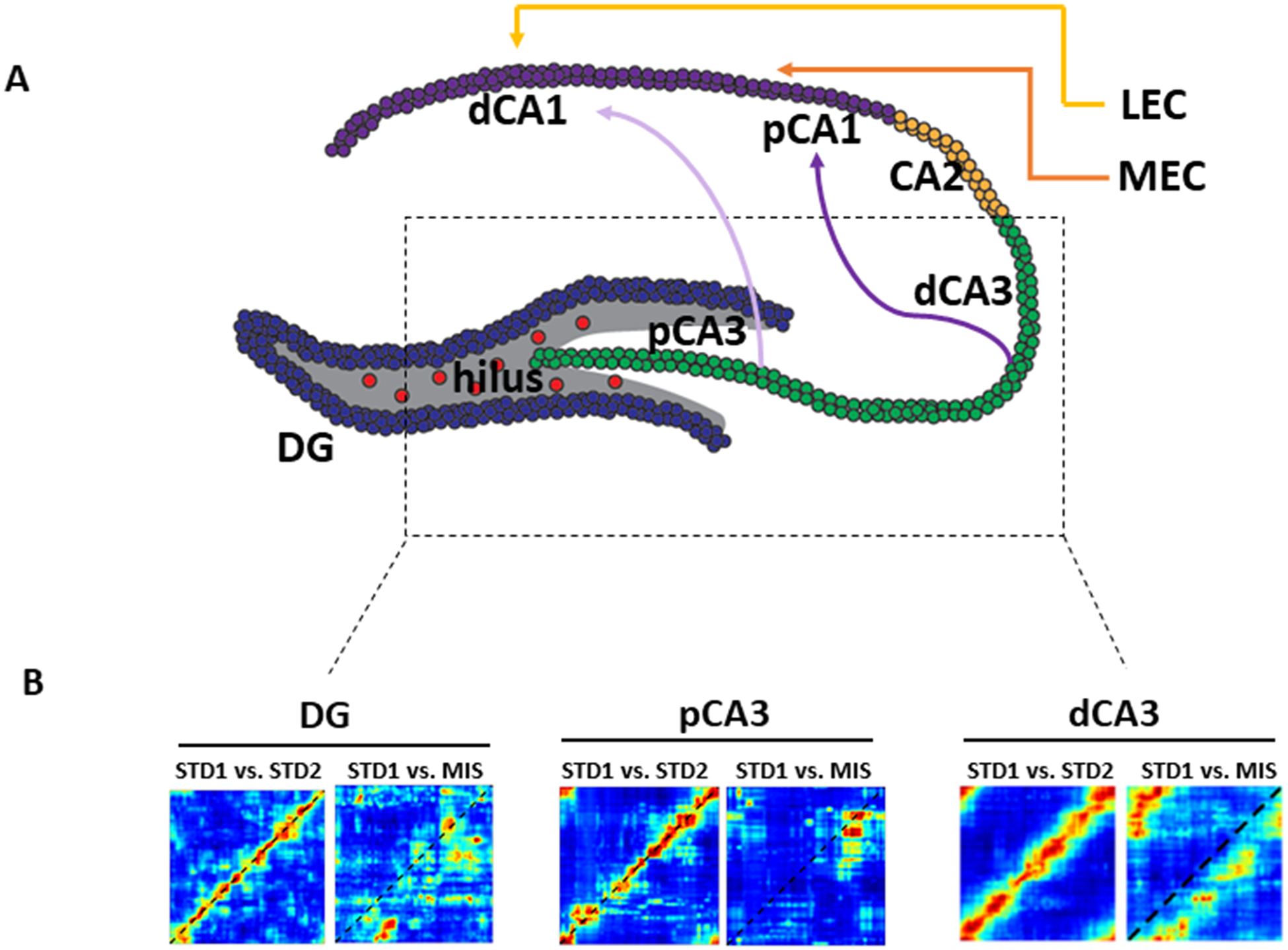
Anatomical and functional organization of DG-CA3 system. A) Topographic organization of parallel processing streams along the hippocampal transverse axis. Proximal CA3 (pCA3, defined as the part of CA3 closer to the DG) projects preferentially to distal CA1 (dCA1, defined as the part of CA1 farthest from the DG along the pyramidal cell layer). Distal CA3 (dCA3), on the other hand, projects preferentially to proximal CA1 (pCA1). pCA1 and dCA1 also receive preferential inputs from the MEC and LEC, respectively. B) Neural population evidence of functional heterogeneity in DG-CA3 system. Cell were recorded in DG and CA3 while animals ran on a circular track with local texture cues and global landmark cues. When two sessions with identical, standard arrangements of local and global cues were compared (STD1 vs. STD2), all 3 areas showed highly correlated representations, as indicated by the heat bands along the main diagonals of the correlation matrices. When local and global cues were in 180° conflict, population activity in DG and pCA3 showed decorrelated representations between the standard and the mismatch sessions (STD1 vs. MIS), as shown by the loss of the high correlation band. In contrast, population activity in dCA3 maintained correlated representations. Thus, pCA3 and DG appear to act as a functional unit for pattern separation, with dCA3 performing the classic computation of pattern completion. Adapted from GoodSmith et al., 2017, 2019; Lee et al., 2015.
Consistent with classic computational models of hippocampal function [8–10], the DG and CA3 have been implicated in two complementary processes, pattern separation and pattern completion [11]. The DG has been proposed to perform pattern separation, the ability to reduce overlap in similar input patterns before storage [10,12–14]. In contrast, CA3, due to its extensive recurrent collateral system, has been proposed to perform pattern completion, the ability to retrieve a full pattern of activity from partial or degraded inputs [9,14,15]). Putative attractor dynamics that underlie pattern completion may also produce related phenomena such as generalization and error correction; in this review we use the term pattern completion generally to refer to any of these phenomena. Although CA3 has classically been modeled as a homogeneous network, there is compelling evidence for anatomical and functional gradients along the CA3 transverse axis (Figure 1B) [17–23]. These gradients suggest that proximal and distal CA3 should perhaps be considered as separate functional components, with proximal CA3 interacting with the DG circuit to support pattern separation and distal CA3 forming an autoassociative network that supports pattern completion.
DG circuit
The DG is unique among hippocampal subfields in its heterogeneity of excitatory cell types. Granule cells (GCs) are the most common cell type in the DG [24]. In addition to the large population of mature GCs, a small number of new GCs are generated throughout life [25]. The extremely sparse activity of GCs [26–34] is consistent with key predictions from Marr’s concept of “expansion recoding” [10,12,15]. In this model (originally proposed by Marr as a theory of cerebellar granule cells), pattern separation in the hippocampus is accomplished by projecting overlapping entorhinal inputs onto a larger, sparsely active GC population. As a very specific set of inputs is required to cause any single GC to fire, small differences in input would lead to distinct (nearly orthogonal) patterns of GC activity. Different environments typically cause robust GC remapping (recruitment of independent place cell populations) (Figure 2) [30–34]; however, in some situations, GCs remap less than mossy cells or CA3 cells [31,32]. Hainmueller and Bartos (2018) suggested that GCs are not very selective to context, as ~ 10% of GCs with place fields imaged in head-fixed mice had similar firing fields in two distinct, virtual contexts. However, the vast preponderance of GC place cells had place fields in only one of the two environments (~90%; Fig 1f of that study), which is overwhelming evidence of global remapping of the two contexts. Likewise, other studies have reported nearly orthogonal DG representations in similar tasks [30,33,35–37].
Figure 2:
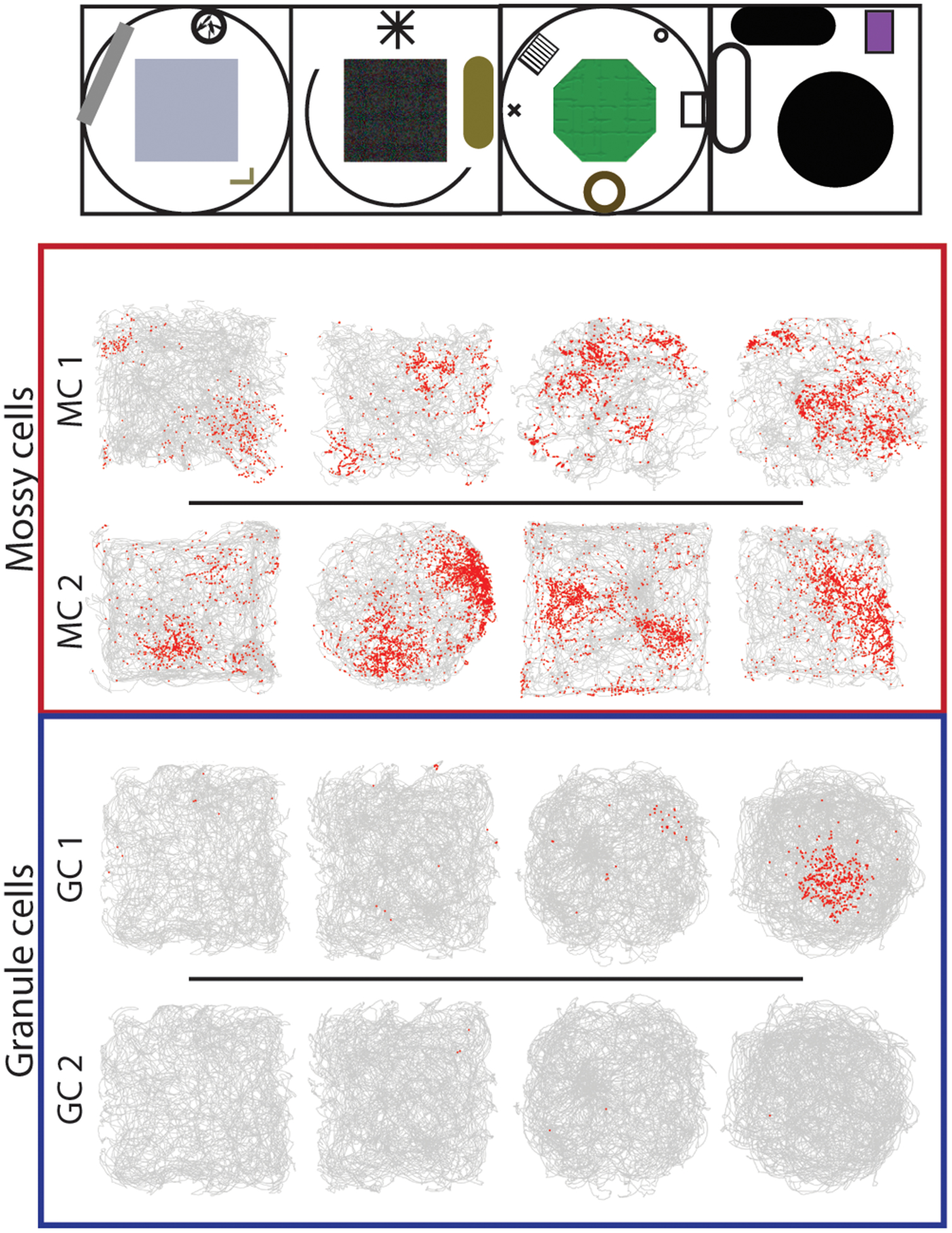
Remapping of granule cells and mossy cells in distinct environments. Activity from two example GCs and mossy cells are shown as an animal explored four distinct contexts (top). Each row is one cell. The trajectory of the rat is plotted in gray, with the locations of spikes overlaid in red. Mossy cells were typically active in multiple environments and had multiple firing fields, while granule cells usually had no place fields in any environment (GC 2), and occasionally had a single field in a single environment (GC 1). This pattern of GC firing supports the orthogonal remapping of GCs expected from expansion recoding models of pattern separation, while the activity of mossy cells could support pattern separation through place field remapping. Adapted from GoodSmith et al., 2017.
As mature GCs are by far the most numerous cell type in the DG, most theories of DG function have focused on these cells [35,36]). However mossy cells of the DG hilus are an integral component of the DG/CA3 circuit, as they mediate a multisynaptic recurrent processing loop in the DG [40] and are the target of proximal CA3 backprojections to DG [2]. Due to the large size and high firing rates of mossy cells relative to granule cells [30,31,41,42], the majority of the active cells recorded extracellularly in the DG are mossy cells [27,37]. Mossy cells may contribute to DG pattern separation by regulating GC sparseness and coordinating activity throughout the DG [43]). In contrast to the “expansion recoding” mechanism of pattern separation, changes in coincident firing across a densely active cell population could also allow for discrimination between environments [35]. Although Leutgeb and colleagues originally attributed this form of pattern separation to granule cells, subsequent work showed instead that the neurons displaying this property were mossy cells [30,31,42], while GCs likely support pattern separation through expansion recoding (Figure 2). To further understand DG function, it will be essential to examine the contributions and interactions of individual cell types within the DG circuit at both the level of population remapping and at the finer timescales at which neural computations occur [45,46].
In addition to a role in pattern separation, the DG has been proposed to be involved in the conjunctive encoding [47,48] or “binding” [49] of the context and content of an experience [50]. Whereas MEC provides the hippocampus with a representation of the animal’s self-location in an allocentric spatial reference frame, LEC provides a representation of the animal’s egocentric relationship to individual items or objects in the environment [51–53]. As the first step in the “trisynaptic loop”, the DG is the first region in the hippocampus to combine these LEC and MEC input streams (although there is considerable anatomical crosstalk between these regions even before the hippocampus [54]. In support of this theory, recent studies have reported representations of object location information in the DG [55,56]. The DG may perform pattern separation on conjunctive context/content representations and relay the output of that computation to CA3.
Heterogeneity in CA3 circuitry
The intrinsic and extrinsic connections along the transverse CA3 axis exhibit a well-organized topography. Proximal CA3 receives mossy fiber inputs from both the upper and the lower blades of the DG; intermediate and distal CA3 receive mossy fiber inputs preferentially from the upper blade [17,57]. The most proximal CA3 receives minimal entorhinal inputs because the perforant path fibers do not extend all the way to the most proximal pyramidal cells buried within the hilus [17,21]. Thus, an increasing gradient of entorhinal inputs from proximal to distal along the transverse axis is mirrored by a decreasing gradient of mossy fiber inputs [58]. Furthermore, proximal CA3 cells do not send recurrent projections to intermediate and distal CA3; rather, they send recurrent projections within the proximal region and backprojections to the hilus. In contrast, intermediate and distal CA3 have a stronger recurrent collateral system and provide associational projections to the entire extent of the transverse axis [2,17,21,58,59].
The presence of such divergent connectivity along the CA3 transverse axis suggests that CA3 may show corresponding functional diversity. There are two types of CA3 principal neurons: the classically described pyramidal cells with thorny excrescences and previously unidentified ‘athorny’ pyramidal cells that lack mossy-fiber input [60]. The athorny cells are located preferentially in distal CA3, and computer simulations suggest that bursting of these cells triggers sharp wave synchronization, a transient network attractor state that may reflect pattern completion computations [60]. Studies of the immediate-early gene Arc suggest that nonspatial information is preferentially processed by proximal CA3 and spatial information is preferentially processed by distal CA3 [19,61,62]. Animals with lesions to proximal CA3 (but not lesions to intermediate/distal CA3) showed similar behavioral deficits as animals with DG lesions in detecting small changes in distance between object locations, suggesting that proximal CA3 and DG may interact to support spatial pattern separation [18]. Under conditions of partial cue changes, Arc expression was similar for both conditions in distal CA3 but distinct in proximal CA3 [20], suggesting a dissociation between proximal and distal CA3 in terms of pattern separation vs. completion. Direct electrophysiological recordings of population ensemble activity confirmed that proximal CA3 reflects pattern separation computations while distal CA3 reflects pattern completion computations [22,23].
Parallel processing loops in the hippocampus
Classic computational models of DG and CA3 assumed anatomical and functional homogeneity within these regions. However, the bidirectional connectivity and functional similarities between DG and proximal CA3 cells [17–23,37] indicate that proximal CA3 may be better considered as part of the DG pattern separation circuit than as part of the autoassociative CA3 pattern completion network [2,18] (Figure 3). In contrast, in agreement with the classic models, the stronger recurrent network in distal CA3 may endow it with the putative attractor dynamics that supports pattern completion following minor changes in input. Increasing input changes can cause a nonlinear shift in CA3 output resulting in pattern separation, as activity settles into a different attractor state or, alternatively, a new stable attractor state is formed [13,14]. As these attractor dynamics vary with changes in recurrent circuitry along the transverse axis, the threshold for pattern separation will also differ along this axis (Figure 3), consistent with rodent studies showing functional gradients along this axis [18,20,22,23]. Human fMRI studies also indicate that DG/CA3 activity is involved in pattern separation [13,63]. These studies did not have the resolution to distinguish DG BOLD signals from CA3 signals. It is puzzling that the putative DG pattern separation signal (from sparsely active GCs) outweighed any putative pattern completion signal from CA3 in these studies. A solution to this puzzle may reside in the anatomical data that proximal CA3 appears to represent a much larger portion of CA3 in humans than rodents [64]. The overabundance of proximal CA3 in humans suggests that the human hippocampus may be biased more heavily toward pattern separation than pattern completion, compared to rodents. Thus, rather than a strictly anatomical division between DG and CA3, it may be more appropriate to divide the DG and CA3 into two interconnected functional computational circuits: a DG/proximal CA3 circuit for pattern separation and a distal CA3 autoassociative network for pattern completion.
Figure 3:
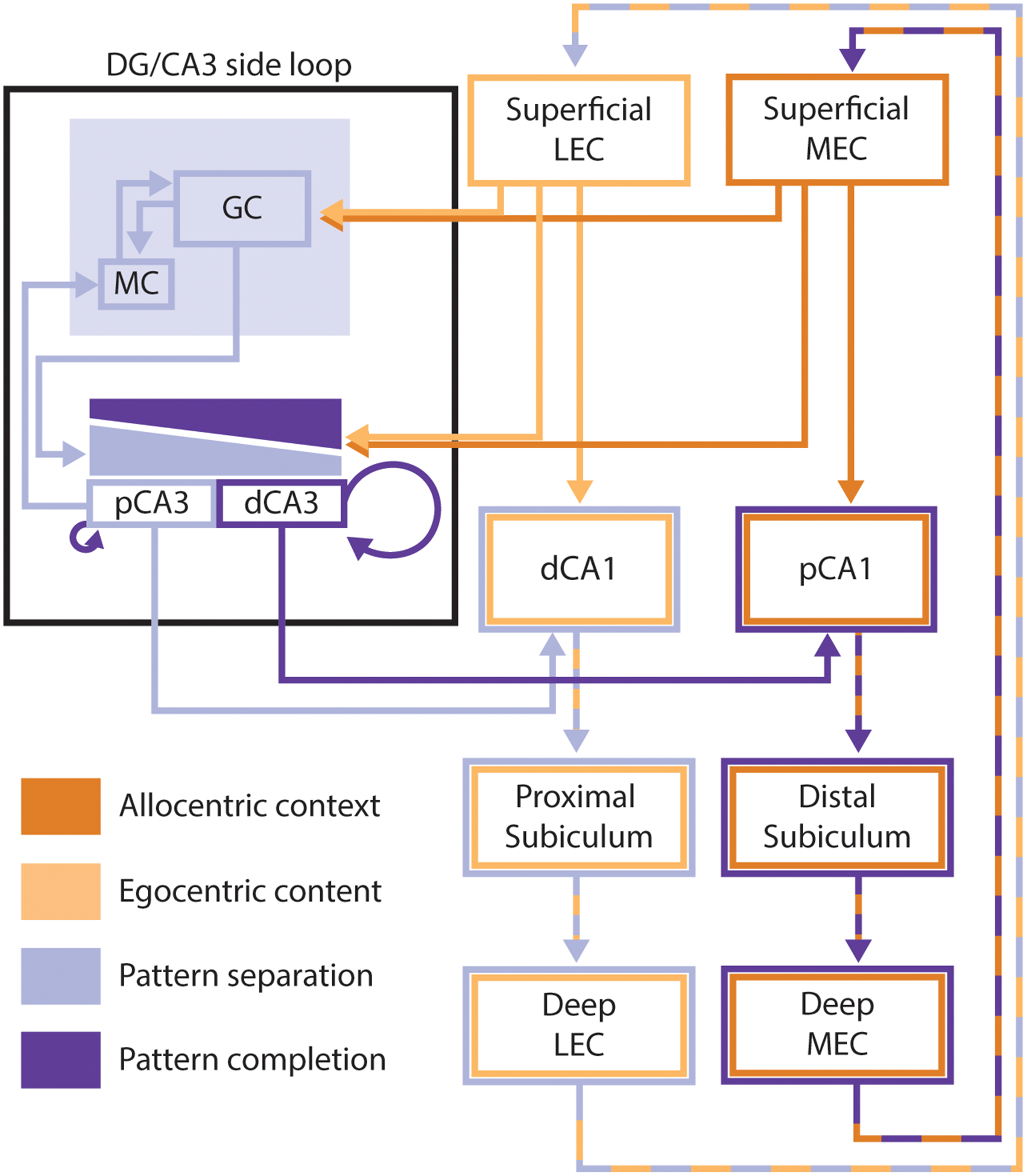
Simplified diagram of the hippocampal circuit. This diagram emphasizes the interacting parallel processing loops within the hippocampus, and how these loops interact with the complementary processes of pattern completion and pattern separation in the DG and CA3. MEC inputs reflecting allocentric contextual information (dark orange) project to distal portions of CA1, while LEC inputs reflecting the egocentric content of an experience (light orange) project to proximal portions of CA1. Both MEC and LEC project directly to the DG. The DG sends mossy fibers to CA3, with stronger inputs to the proximal than distal portion of CA3 (light purple gradient) and proximal CA3 sends backprojections back to DG mossy cells. CA3 also receives direct projections from both MEC and LEC, but this input is weaker at the proximal edge of CA3 than at more distal regions (dark purple gradient). Distal CA3 has a more extensive system of recurrent collaterals than proximal CA3 (proximal CA3 only sends collaterals to proximal CA3 while more distal regions send collaterals throughout CA3). Within the DG/CA3 side loop, the complementary processes of pattern completion and pattern separation occur. Areas and projections reflecting pattern separation (DG, proximal CA3, and communication between these areas) have been marked in light purple, while areas and projections that promote or reflect pattern completion (distal CA3, entorhinal inputs to CA3, recurrent collaterals) have been marked in dark purple. Pattern-separated outputs of proximal CA3 project preferentially to distal CA1 while pattern-completed outputs from distal CA3 project preferentially to proximal CA1. These topographical projections from CA3 to CA1 interact with the parallel input streams from the EC. The output of CA1 is then projected back to the EC (directly and via subiculum) to complete the hippocampal processing loop. Throughout the figure, proximal and distal portions of hippocampal subfields are portrayed as separate areas with entirely distinct inputs and outputs. It is important to keep in mind that, in reality, these areas exhibit gradients from proximal to distal and that the inputs, outputs, and function of these regions vary along those gradients. Further, the gradients of inputs to CA3 from entorhinal cortex and DG are highly simplified, and these gradients are not nearly as uniform as portrayed here.
Pattern separation and completion are often considered as competing processes, with one process “winning” over the other as arbitrated by the CA3 attractor dynamics. However, under most conditions a memory system needs to be able to perform both functions on the same input. For example, you may encounter an old acquaintance who you have not seen in five years. His hair is a bit thinner and grayer, he wears different glasses, and has put on a few pounds. Nonetheless, the CA3 autoassociative network allows you to retrieve your memories of your prior encounters with this individual even though the inputs are altered slightly. However, you also want to create distinct, new memories of the individual you see today, not memories of the younger individual you knew 5 years ago. How can the output of CA3 allow both pattern completion and pattern separation to occur simultaneously? The answer may lie in the anatomical topography of the CA3 outputs to CA1 along the transverse axis. Inputs to CA1 from both CA3 and entorhinal cortex are segregated along the transverse axis (Figure 3). Activity in the distal CA3 pattern completion network and allocentric spatial information from MEC target proximal CA1, whereas pattern-separated, proximal CA3 activity and egocentric sensory information from LEC target distal CA1 [4]. This pattern of connectivity suggests that the hippocampal circuit consists of two parallel processing loops. These loops may reflect distinct computational demands of different input types. LEC-recipient distal CA1 may require pattern-separated inputs from the DG/proximal CA3 circuit to allow for discrimination between two similar egocentric experiences in the same allocentric spatial context. Simultaneously, MEC-recipient proximal CA1 may require pattern-completed distal CA3 inputs to allow similar events to be recognized as occurring in the same spatial context (Figure 3) [65].
Future Questions
There are many questions that need to be addressed to fully understand the hippocampal function in parallel processing loops.
How do neuromodulatory inputs to the DG regulate the balance between pattern separation and pattern completion?
The relative balance between pattern separation and pattern completion may be modified by task demands. The DG receives a number of neuromodulatory inputs, including dopaminergic, serotonergic, noradrenergic, and cholinergic inputs [66,67]. These neuromodulatory inputs (which often preferentially target the hilus) regulate excitability, plasticity, and spike transmission in the DG/CA3 circuit [66] and may influence the balance between pattern completion and pattern separation [13,33]. How these diverse neuromodulatory inputs affect the DG/CA3 circuit to regulate pattern separation and pattern completion remains largely unexplored.
Do the upper and lower blade of the DG make unique contributions to DG processing?
While GCs receive inputs from both MEC and LEC, there are slight differences in the anatomical targets of these entorhinal inputs. The lower blade of the DG receives slightly more MEC input than the upper blade, which receives slightly more LEC inputs [68,69]. Somewhat unexpectedly, Arc expression is increased in the upper blade following spatial exploration, and increased in the lower blade when large landmarks were present in the environment [70,71]. Study of the electrophysiological differences between the DG blades may reveal how these minor differences in input composition affect DG function.
Role of CA2 in parallel processing streams
CA2 occupies a region between CA3 and CA1. In experiments demonstrating pattern separation and pattern completion, CA2 was similar to distal CA3, performing pattern completion [22,23]. Distal CA2 cells fire in phase with the proximal CA1 cells and proximal CA2 cells fire in phase with the distal CA3 cells [72]. These results suggest that the CA2 region is more associated with the distal CA3-proximal CA1 stream, which is associated with the MEC pathway in processing the allocentric spatial representation. However, the topographic organization of inputs and outputs along the CA2 transverse axis is not known. Further studies are necessary to understand the function of CA2 in parallel processing streams.
Conclusion
A full understanding of hippocampal function will require extensive study of the hippocampal circuit beyond the traditional ‘trisynaptic loop’ conceptualization. Functional and anatomical heterogeneity in the hippocampal circuit indicate that one cannot assign a single computational function to each classically defined hippocampal subfield. Hippocampal mossy cells, a historically neglected component of the DG circuit, mediate the extensive communication between the DG and proximal portions of CA3 to form a DG/proximal CA3 circuit to support pattern separation. Meanwhile, direct entorhinal inputs and recurrent circuitry in more distal regions of CA3 form an autoassociative network to support pattern completion. The output of these two functional circuits target CA1 at different portions of the transverse axis. This gradient of projection pairs with a gradient in direct entorhinal projections to CA1 to form parallel and interacting hippocampal processing loops rather than a single feedforward circuit. Further study of how information is processed in this interconnected hippocampal circuit will be essential to fully understand the mnemonic functions of the hippocampus as a whole.
Highlights.
Both granule cells and mossy cells contribute to DG pattern separation
Studies show functional dissociation along the CA3 transverse axis
Similar to DG, proximal CA3 output reflects pattern separation
Distal CA3 output reflects pattern completion
Gradient of CA3 to CA1 projections may reflect computational demands of EC inputs
Acknowledgements
This work was supported by N.I.H. grants PO1 AG009973 and R01 NS039456.
References and recommended reading
Papers of particular interest has been highlighted as:
* of special interest
** of outstanding interest
- 1.Squire LR, Stark CEL, Clark RE: The Medial Temporal Lobe. Annu Rev Neurosci 2004, 27:279–306. [DOI] [PubMed] [Google Scholar]
- 2.Scharfman HE: The CA3 “backprojection” to the dentate gyrus. Prog Brain Res 2007, 163:627–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Xu X, Sun Y, Holmes TC, López AJ: Noncanonical connections between the subiculum and hippocampal CA1. J Comp Neurol 2016, 524:3666–3673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ishizuka N, Cowan WM, Amaral DG: A quantitative analysis of the dendritic organization of pyramidal cells in the rat hippocampus. J Comp Neurol 1995, 362:17–45. [DOI] [PubMed] [Google Scholar]
- 5.Amaral DG, Witter MP: The three-dimensional organization of the hippocampal formation: A review of anatomical data. Neuroscience 1989, 31:571–591. [DOI] [PubMed] [Google Scholar]
- 6.Fanselow MS, Dong H-W: Are the Dorsal and Ventral Hippocampus Functionally Distinct Structures? Neuron 2010, 65:7–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Strange BA, Witter MP, Lein ES, Moser EI: Functional organization of the hippocampal longitudinal axis. Nat Rev Neurosci 2014, 15:655–669. [DOI] [PubMed] [Google Scholar]
- 8.Treves A, Rolls ET: Computational analysis of the role of the hippocampus in memory. Hippocampus 1994, 4:374–91. [DOI] [PubMed] [Google Scholar]
- 9.Marr D: Simple Memory: A Theory for Archicortex. Philos Trans R Soc B Biol Sci 1971, 262:23–81. [DOI] [PubMed] [Google Scholar]
- 10.McNaughton BL, Nadel L: Hebb-Marr Networks and the Neurobiological Representation of Action in Space. In Neuroscience and Connectionist Theory. Edited by Gluck M, Rumelhart D. Hillsdale, NJ: Erlbaum; 1990:1–63. [Google Scholar]
- 11.Rolls ET, Kesner RP: A computational theory of hippocampal function, and empirical tests of the theory. Prog Neurobiol 2006, 79:1–48. [DOI] [PubMed] [Google Scholar]
- 12.Marr D: A theory of cerebellar cortex. J Physiol 1969, 202:437–470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yassa MA, Stark CEL: Pattern separation in the hippocampus. Trends Neurosci 2011, 34:515–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rolls E, Treves A: Neural Networks and Brain Function. Oxford University Press; 1998. [Google Scholar]
- 15.McNaughton BL, Morris RGM: Hippocampal synaptic enhancement and information storage within a distributed memory system. Trends Neurosci 1987, 10:408–415. [Google Scholar]
- 16.McClelland JL, Goddard NH: Considerations Arising From a Complementary Learning Systems Perspective on Hippocampus. Hippocampus 1996, 6:654–665. [DOI] [PubMed] [Google Scholar]
- 17.Witter MP: Intrinsic and extrinsic wiring of CA3: indications for connectional heterogeneity. Learn Mem 2007, 14:705–13. [DOI] [PubMed] [Google Scholar]
- 18.Hunsaker MR, Rosenberg JS, Kesner RP: The role of the dentate gyrus, CA3a,b, and CA3c for detecting spatial and environmental novelty. Hippocampus 2008, 18:1064–73. [DOI] [PubMed] [Google Scholar]
- 19.Nakamura NH, Flasbeck V, Maingret N, Kitsukawa T, Sauvage MM: Proximodistal segregation of nonspatial information in CA3: preferential recruitment of a proximal CA3-distal CA1 network in nonspatial recognition memory. J Neurosci 2013, 33:11506–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Marrone DF, Satvat E, Odintsova IV, Gheidi A: Dissociation of spatial representations within hippocampal region CA3. Hippocampus 2014, 24:1417–1420. [DOI] [PubMed] [Google Scholar]
- 21.Ishizuka N, Weber J, Amaral DG: Organization of intrahippocampal projections originating from CA3 pyramidal cells in the rat. J Comp Neurol 1990, 295:580–623. [DOI] [PubMed] [Google Scholar]
- 22.Lee H, Wang C, Deshmukh SSS, Knierim JJ: Neural Population Evidence of Functional Heterogeneity along the CA3 Transverse Axis: Pattern Completion versus Pattern Separation. Neuron 2015, 87:1093–1105. [DOI] [PMC free article] [PubMed] [Google Scholar]; ** This paper shows physiological evidence of functional heterogeneity along the CA3 transverse axis. Proximal CA3 shows degraded representations in cue-conflict environments whereas distal CA3 maintains coherent representations. Together with Lu et al. (2015), both studies show that CA3 is a functionally graded system with pattern separation at the proximal end and pattern completion at the distal end.
- 23.Lu L, Igarashi KM, Witter MPP, Moser EII, Moser M-B: Topography of Place Maps along the CA3-to-CA2 Axis of the Hippocampus. Neuron 2015, 85:1078–1092. [DOI] [PubMed] [Google Scholar]; ** This paper shows functional gradients along the CA3 transverse axis in the ability of place cells to discriminate changes in the environment. There is a progressive decrease in remapping of place cells along the CA3 transverse axis. Together with Lee et al. (2015), both studies show that CA3 is a functionally graded system with pattern separation at the proximal end and pattern completion at the distal end.
- 24.Amaral DG, Scharfman HE, Lavenex P: The Dentate Gyrus: A Comprehensive Guide to Structure, Function, and Clinical Implications. Elsevier; 2007. [Google Scholar]
- 25.Gonçalves JT, Schafer ST, Gage FH: Adult Neurogenesis in the Hippocampus: From Stem Cells to Behavior. Cell 2016, 167:897–914. [DOI] [PubMed] [Google Scholar]
- 26.Jung MW, McNaughton BL: Spatial selectivity of unit activity in the hippocampal granular layer. Hippocampus 1993, 3:165–82. [DOI] [PubMed] [Google Scholar]
- 27.Neunuebel JP, Knierim JJ: Spatial firing correlates of physiologically distinct cell types of the rat dentate gyrus. J Neurosci 2012, 32:3848–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Danielson NB, Kaifosh P, Zaremba JD, Lovett-Barron M, Tsai J, Denny CA, Balough EM, Goldberg AR, Drew LJ, Hen R, et al. : Distinct Contribution of Adult-Born Hippocampal Granule Cells to Context Encoding. Neuron 2016, 90:101–112. [DOI] [PMC free article] [PubMed] [Google Scholar]; ** By imaging mature and adult-born GCs in head fixed mice, the authors found that adult born GCs were less sparse and less spatially selective than mature GCs. This study was the first to report on the spatial selectivity of adult-born GCs.
- 29.Diamantaki M, Frey M, Berens P, Preston-Ferrer P, Burgalossi A: Sparse activity of identified dentate granule cells during spatial exploration. Elife 2016, 5:1109–1123. [DOI] [PMC free article] [PubMed] [Google Scholar]; * Using juxtacellular recordings, the authors revealed the exceptionally sparse activity of DG GCs. In addition, the authors reported differences in the morphology and waveform shape of silent and active GCs.
- 30.GoodSmith D, Chen X, Wang C, Kim SH, Song H, Burgalossi A, Christian KM, Knierim JJ: Spatial Representations of Granule Cells and Mossy Cells of the Dentate Gyrus. Neuron 2017, 93. [DOI] [PMC free article] [PubMed] [Google Scholar]; ** This study examined the spatial firing properties of DG cell types using tetrode recordings, cell type classification methods, and juxtacellular recordings. Together with Senzai and Buzsaki (2017) and Danielson et al. (2017), the authors reported that mossy cells had multiple fields in most environments (a feature previously attributed to GCs). In contrast, unique populations of GCs were active in distinct environments.
- 31.Senzai Y, Buzsáki G: Physiological Properties and Behavioral Correlates of Hippocampal Granule Cells and Mossy Cells. Neuron 2017, 93:691–704.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]; ** The authors used high density silicon probe recordings and cell type classification to identify the activity of DG cell types. Together with GoodSmith et al., (2017) and Danielson et al. (2017), the study revealed the firing properties of DG mossy cells and granule cells. The authors further reported that GCs remapped less than mossy cells following small changes in the environment.
- 32.Hainmueller T, Bartos M: Parallel emergence of stable and dynamic memory engrams in the hippocampus. Nature 2018, 558:292–296. [DOI] [PMC free article] [PubMed] [Google Scholar]; * By imaging cells in CA1, CA3, and DG as head fixed mice traversed distinct virtual linear tracks, the authors examined how cells throughout the hippocampal circuit remap in a novel environment. While global remapping dominated the GC population, a subpopulation of GCs had place fields in similar locations in both contexts. The location of CA1 and CA3 place fields changed more between sessions and between days than GCs.
- 33.Allegra M, Posani L, Schmidt-Hieber C: The hippocampus as a perceptual map: neuronal and behavioral discrimination during memory encoding. bioRxiv 2019, doi: 10.1101/868794. [DOI] [PMC free article] [PubMed] [Google Scholar]; * This study imaged GCs and CA1 cells during a virtual reality behavioral discrimination task. Small changes in the environment that did not cause behavioral discrimination led to GC, but not CA1, remapping. Larger changes in the environment caused CA1 remapping. Population remapping in CA1, but not DG, was correlated with behavioral performance, suggesting that GCs perform pattern separation after any change in input, while changes in behavioral output depend on CA1 output.
- 34.Hainmueller T, Bartos M: Dentate gyrus circuits for encoding, retrieval and discrimination of episodic memories. Nat Rev Neurosci 2020, 21:1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]; * This review provides a comprehensive summary of recent advances in the study of the DG and explores recent evidence for diverse DG functions beyond pattern separation.
- 35.Leutgeb JK, Leutgeb S, Moser M-B, Moser EI: Pattern separation in the dentate gyrus and CA3 of the hippocampus. Science 2007, 315:961–6. [DOI] [PubMed] [Google Scholar]
- 36.Neunuebel JP, Knierim JJ: CA3 retrieves coherent representations from degraded input: Direct evidence for CA3 pattern completion and dentate gyrus pattern separation. Neuron 2014, 81:416–427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.GoodSmith D, Lee H, Neunuebel JP, Song H, Knierim JJ: Dentate gyrus mossy cells share a role in pattern separation with dentate granule cells and proximal CA3 pyramidal cells. J Neurosci 2019, doi: 10.1523/jneurosci.0940-19.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Schmidt B, Marrone DF, Markus EJ: Disambiguating the similar: the dentate gyrus and pattern separation. Behav Brain Res 2012, 226:56–65. [DOI] [PubMed] [Google Scholar]
- 39.Rolls ET: The mechanisms for pattern completion and pattern separation in the hippocampus. Front Syst Neurosci 2013, 7:74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Scharfman HE: The enigmatic mossy cell of the dentate gyrus. Nat Rev Neurosci 2016, 17:562–575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Henze DA, Buzsáki G: Hilar mossy cells: functional identification and activity in vivo. Prog Brain Res 2007, 163:199–216. [DOI] [PubMed] [Google Scholar]
- 42.Danielson NB, Turi GF, Ladow M, Chavlis S, Petrantonakis PC, Poirazi P, Losonczy A: In Vivo Imaging of Dentate Gyrus Mossy Cells in Behaving Mice. Neuron 2017, 93:552–559.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Myers CE, Scharfman HE: A role for hilar cells in pattern separation in the dentate gyrus: a computational approach. Hippocampus 2009, 19:321–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Scharfman HE, Myers CE: Hilar mossy cells of the dentate gyrus: a historical perspective. Front Neural Circuits 2013, 6:106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.van Dijk MT, Fenton AA: On How the Dentate Gyrus Contributes to Memory Discrimination. Neuron 2018, 98:832–845.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Madar AD, Ewell LA, Jones MV.: Pattern separation of spiketrains in hippocampal neurons. Sci Rep 2019, 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Knierim JJ, Lee I, Hargreaves EL: Hippocampal Place Cells : Parallel Input Streams, Subregional Processing, and Implications for Episodic Memory. Hippocampus 2006, 764:755–764. [DOI] [PubMed] [Google Scholar]
- 48.Kesner RP: An analysis of the dentate gyrus function. Behav Brain Res 2013, 254:1–7. [DOI] [PubMed] [Google Scholar]
- 49.Lee JW, Jung MW: Separation or binding? Role of the dentate gyrus in hippocampal mnemonic processing. Neurosci Biobehav Rev 2017, 75:183–194. [DOI] [PubMed] [Google Scholar]
- 50.Knierim JJ, Neunuebel JP, Deshmukh SS: Functional correlates of the lateral and medial entorhinal cortex: objects, path integration and local – global reference frames. Philos Trans R Soc Lond B Biol Sci 2014, 369:1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lisman JE: Role of the dual entorhinal inputs to hippocampus: a hypothesis based on cue/action (non-self/self) couplets. Prog Brain Res 2007, 163:615–625. [DOI] [PubMed] [Google Scholar]
- 52.Wang C, Chen X, Lee H, Deshmukh SS, Yoganarasimha D, Savelli F, Knierim JJ: Egocentric coding of external items in the lateral entorhinal cortex. Science 2018, 362:945–949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wang C, Chen X, Knierim JJ: Egocentric and allocentric representations of space in the rodent brain. Curr Opin Neurobiol 2020, 60:12–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Burwell RD: The parahippocampal region: corticocortical connectivity. Ann N Y Acad Sci 2000, 911:25–42. [DOI] [PubMed] [Google Scholar]
- 55.Jung D, Kim S, Sariev A, Sharif F, Kim D, Royer S: Dentate granule and mossy cells exhibit distinct spatiotemporal responses to local change in a one-dimensional landscape of visual-tactile cues. Sci Rep 2019, 9:9545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bui AD, Nguyen TM, Limouse C, Kim HK, Szabo GG, Felong S, Maroso M, Soltesz I: Dentate gyrus mossy cells control spontaneous convulsive seizures and spatial memory. Science 2018, windows:787–790. [DOI] [PMC free article] [PubMed] [Google Scholar]; * Epileptic mice were shown to have deficits in object location (but not object identity) learning, and optogenetic inhibition of mossy cells caused the same deficit. This study provides evidence for the importance of mossy cells and the DG in conjunctive object/location memory.
- 57.Claiborne BJ, Amaral DG, Cowan WM: A light and electron microscopic analysis of the mossy fibers of the rat dentate gyrus. J Comp Neurol 1986, 246:435–458. [DOI] [PubMed] [Google Scholar]
- 58.Sun Q, Sotayo A, Cazzulino AS, Snyder AM, Denny CA, Siegelbaum SA: Proximodistal Heterogeneity of Hippocampal CA3 Pyramidal Neuron Intrinsic Properties, Connectivity, and Reactivation during Memory Recall. Neuron 2017, 95:656–672.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]; * This paper shows that there are gradients along the CA3 transverse axis in intrinsic membrane properties and synaptic connectivity. There is a decreasing gradient of mossy fiber strength mirrored by an increasing gradient of entorhinal inputs along the CA3 transverse axis.
- 59.Li X-G, Somogyi P, Ylinen A, Buzsáki G: The hippocampal CA3 network: An in vivo intracellular labeling study. J Comp Neurol 1994, 339:181–208. [DOI] [PubMed] [Google Scholar]
- 60.Hunt DL, Linaro D, Si B, Romani S, Spruston N: A novel pyramidal cell type promotes sharp-wave synchronization in the hippocampus. Nat Neurosci 2018, 21:985–995. [DOI] [PubMed] [Google Scholar]
- 61.Flasbeck V, Atucha E, Nakamura NH, Yoshida M, Sauvage MM: Spatial information is preferentially processed by the distal part of CA3: implication for memory retrieval. Behav Brain Res 2018, 347:116–123. [DOI] [PubMed] [Google Scholar]
- 62.Beer Z, Vavra P, Atucha E, Rentzing K, Heinze HJ, Sauvage MM: The memory for time and space differentially engages the proximal and distal parts of the hippocampal subfields CA1 and CA3. PLoS Biol 2018, 16:e2006100. [DOI] [PMC free article] [PubMed] [Google Scholar]; * This paper shows the segregation of information processing within the parallel processing streams in the hippocampus. Temporal information is processed by the LEC-recipient distal CA1-proximal CA3 stream and spatial information is more strongly processed by the MEC-recipient proximal CA1-distal CA3 stream.
- 63.Berron D, Schütze H, Maass A, Cardenas-Blanco A, Kuijf HJ, Kumaran D, Düzel E: Strong evidence for pattern separation in human dentate gyrus. J Neurosci 2016, 36:7569–7579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Lim C, Blume HW, Madsen JR, Saper CB: Connections of the hippocampal formation in humans: I. The mossy fiber pathway. J Comp Neurol 1997, 385:325–351. [DOI] [PubMed] [Google Scholar]
- 65.Nakazawa Y, Pevzner A, Tanaka KZ, Wiltgen BJ: Memory retrieval along the proximodistal axis of CA1. Hippocampus 2016, doi: 10.1002/hipo.22596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Prince LY, Bacon TJ, Tigaret CM, Mellor JR: Neuromodulation of the Feedforward Dentate Gyrus-CA3 Microcircuit. Front Synaptic Neurosci 2016, 8:32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Swanson, Köhler C, Björklund A: The limbic region. I: The septohippocampal system. In Handbook of Chemical Neuroanatomy. Edited by Hokfelt T, Bjorklund A, Swanson L. Elsevier; 1987:125–277. [Google Scholar]
- 68.Tamamaki N: Organization of the entorhinal projection to the rat dentate gyrus revealed by Dil anterograde labeling. Exp brain Res 1997, 116:250–8. [DOI] [PubMed] [Google Scholar]
- 69.Luna VM, Anacker C, Burghardt NS, Khandaker H, Andreu V, Millette A, Leary P, Ravenelle R, Jimenez JC, Mastrodonato A, et al. : Adult-born hippocampal neurons bidirectionally modulate entorhinal inputs into the dentate gyrus. Science 2019, 364:578–583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Chawla MK, Guzowski JF, Ramirez-Amaya V, Lipa P, Hoffman KL, Marriott LK, Worley PF, McNaughton BL, Barnes CA: Sparse, environmentally selective expression of Arc RNA in the upper blade of the rodent fascia dentata by brief spatial experience. Hippocampus 2005, 15:579–86. [DOI] [PubMed] [Google Scholar]
- 71.Hoang T-H, Aliane V, Manahan-Vaughan D: Novel encoding and updating of positional, or directional, spatial cues are processed by distinct hippocampal subfields: Evidence for parallel information processing and the “what” stream. Hippocampus 2018, 28:315–326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Fernandez-Lamo I, Gomez-Dominguez D, Sanchez-Aguilera A, Oliva A, Morales AV, Valero M, Cid E, Berenyi A, Menendez de la Prida L: Proximodistal Organization of the CA2 Hippocampal Area. Cell Rep 2019, 26:1734–1746.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]


